Biologic Effects of Nuclear Magnetic Resonance
Authors
INTRODUCTION
The intent of this chapter is to acquaint the reader with current opinions concerning bioeffects of nuclear magnetic resonance (NMR) and its clinical implentation, magnetic resonance imaging (MRI). MRI has the desirable feature of not involving ionizing radiation; therefore, at first glance it may appear entirely safe. However, a variety of interaction mechanisms exist between magnetic and electromagnetic fields and tissue that should be considered in evaluating safety. These effects are categorized and discussed in terms of observable thresholds. It is emphasized that while numerous bioeffects have been reported in the literature, studies were often contradictory or inconclusive in establishing the nature of the effect. For this reason, we will discuss only those effects that are reasonably well established or are believed to have the greatest potential of biohazard. In addition, safety guidelines established by government agencies are reviewed, and emphasis is placed on how these guidelines impact routine clinical magnetic resonance imaging. Recently there is increased concern raised regarding safety of gadolinium-based MRI contrast agents and risk of developing nephrogenic systemic fibrosis (NSF) in patients with poor renal function. There is evidence associating the development of NSF in patients with renal failure with some of the FDA-approved gadolinium-based agents. Accordingly, the FDA issued an alert and recommedations for exposure of patients with acute or chronic severe renal insuffiency.
BACKGROUND
It is beneficial to review fundamentals of MRI to introduce which aspects of the procedure have the potential of adverse effects.
At the heart of all MRI systems is a strong static magnetic field. When placed in the magnetic field,1 hydrogen nuclei (protons), which themselves have a magnetic property, will align parallel or antiparallel with the magnetic field direction. The slight excess of protons aligned parallel to the field cumulatively produces a macroscopically measurable quantity known as magnetization. A means to measure magnetization is by the NMR process wherein a radiofrequency (rf) pulse is applied causing the magnetization to be tipped away from the direction defined by the static magnetic field. Once the radiofrequency wave is turned off, the magnetization will relax back to its original orientation, releasing energy in the form of an NMR/MRI signal. The minute magnetic resonance signal is detected by a radiofrequency pickup coil (may be the same coil used for transmission of the radiofrequency pulse), digitized, and processed by a computer.
Contained within the NMR/MRI signal is a wealth of information about the tissue. Signal strength is dependent on concentration of protons, frequency content of the signal is dependent on the chemical environment of the protons (e.g., protons in water versus fat have a slightly different signal frequency), and signal persistence and recovery rate yield information about the physical environment (e.g., bound versus free water) such as an excess of water in edematous tissue. Typically, MRI systems forfeit chemical information to generate tomographic maps of the protons' signal intensity emanating from the body. Spatial encoding of the source of the magnetic resonance signal involves rapid alterations of the magnetic field strengths across the body as the radiofrequency pulses prepare the magnetization to yield its signal. These field gradient transients alter the resonant frequency of the protons as a function of position in the body. Rapidly switched magnetic fields across the body are supplied by pulse gradient coils which are themselves electromagnets installed within the strong static magnet. In fact, pulsing magnets within a magnet are the source of loud acoustic noise generated during an MRI exam. For our interest in bioeffects, greater detail of the imaging sequence is not required.2 As a preface to our discussion of bioeffects, it is sufficient to say that MR imaging requires exposure of the patient to (1) a strong static magnetic field, (2) radiofrequency pulses, and (3) time-varying magnetic field gradients. Possible biohazards are usually grouped within these three categories.3
EFFECTS OF STATIC MAGNETIC FIELDS
Human MRI systems operate at magnetic field strengths between 0.1 tesla (T) and 7 T, although clinical MRI systems operate at or below 3 T. For a reference, the earth's magnetic field is approximately 50 microtesla (10–6). Whole-body magnets designed specifically to maintain a high field strength over the large volume of the body are either permanent magnets, resistive electromagnets, or, most commonly, superconductive magnets. Optimal field strength for MRI remains an open issue; however, systems operating at 0.15–0.7 T, 1 T, 1.5 T, and 3 T are popular for clinical work. High field-strength units may have advantages in signal to noise over low field strength systems and offer the potential of spectroscopy. However, as field strength increases so does radiofrequency power deposition in the body,4 as we shall see later.
By classic studies, it was believed there were no direct adverse effects of static magnetic field strengths up to 2 T.5, 6, 7 This was based on studies in which behavioral and important physiological processes of mammals exposed to fields up to 2 T were monitored. Thanks to engineering advances in magnet design, much higher field strengths are now achievable even for human imaging. Subsequent biosafety studies have led the FDA to raise the threshold for "significant risk" of diagnostic devices to 8 T in adults and children (>1month old) and 4 T for neonates and infants (<1 month).8, 9, 10
An indirect, but very real, danger associated with high magnetic fields is the attractive force on ferromagnetic objects, which may become projectiles causing injury or death as they are drawn to the bore of the magnet.11 Also, ferromagnetic implants, protheses, heart valves, and surgical and aneurysm clips are a concern since they may become displaced under the pull of the magnet.12 The list of biomedical implants is extensive and ever growing, thus simple classification of broad types of devices as MRI "safe" or "not safe" is not possible. Fortunately, most bioimplants/devices have undergone individualized testing for ferromagnetic force, as well as other potential adverse interaction with the MRI (see below). Print and online resources provided Frank Schellock provide what many consider the most extensive and up-to-date summary of MRI compatibility status of devices listed according to function, vendor, and model number.13, 14
MRI scanning has been performed on patients with pacemakers, although as a general rule persons with cardiac pacemaker implants should not be allowed near the magnet.15, 16, 17 Some of these devices contain a reed relay switch that can be activated externally by a relatively low magnetic field. Tests have demonstrated that fields as low as 1.7 mT can activate the relay, causing a change in pace mode from synchronous to asynchronous. The 0.5-mT limit (sometimes referred to as 5 Gauss limit) has been established as a conservative perimeter within which persons with pacemakers and uncontrolled traffic should not be allowed.
Another observable effect of high magnetic fields is the force on ionic currents flowing in blood.18 This force is perpendicular to the direction of flow, which causes a separation of charge across the blood vessel diameter. The resulting electric potential is measurable on the electrocardiogram signal in animal studies. but it appears to be completely reversible and to have no adverse physiological effect.19
TIME-VARYING MAGNETIC FIELDS
As mentioned, pulsed magnetic field gradients are essential in an imaging sequence for spatial localization of the NMR signal within the body. The peak additional magnetic field strength provided by the gradients is several orders of magnitude weaker than the static magnetic field and therefore presents no additional risk in terms of magnetic field strength. However, the temporal rate of change of the magnetic field is high.20 Gradient pulses may have field changes as high as 60 T/sec, although they are generally in the range of 5–20 T/sec for conventional MRI. The response of any conductive body in the presence of changing magnetic fields is to oppose the field change with internal electric currents. using the conductivity of tissue and a 10-cm-diameter current path, the current density induced by a 1-T/sec field change would be 1 microamp/cm2.11
An observable effect of induced current densities of this magnitude is the visual sensation of flashing lights known as magnetophosphenes.21 Studies indicate that duration and frequency of the magnetic field change play an important role in the threshold at which this sensation is induced. For example, 1.3-T/sec, 2-msec pulses will invoke magnetophosphenes, yet much higher field rate changes will not if the pulse duration is short.22
Another bioeffect observed in animal studies of tissue currents induced by time-varying magnetic fields is direct neuromuscular stimulation.23, 24 Commercial MRI systems typically operate well below the perceptible threshold of pain derived from population studies. That is, half of the study subjects sensed peripheral nerve stimulation (PNS) at switching fields of 60 T/sec, and most clinical MRI systems operate at 20 T/sec or lower.25 The threshold for induction of cardiac stimulation requires very high switching magnetic fields, roughly an order of magnitude greater than those generated in clinical MRI systems.
RADIOFREQUENCY FIELDS
The patient is exposed to short radiofrequency fields to stimulate magnetized protons to yield an MRI signal. A by-product of this process is deposition of radiofrequency energy in the body as heat.26 In addition to transmitted radiofrequency power level, several factors influence the amount of tissue heating; these include radiofrequency coil design, electric properties of tissue, pulse sequence, and size and shape of the body part being scanned. The body's ability to dispose of additional heat is an important consideration in evaluating safety. Tissues having low thermoregulatory blood supply, such as the testes and lens of the eye, may be more susceptible to damage by radiofrequency power deposition. Excessive localized heating may also occur in areas near conductive metals such as metallic implants and in constricted current paths such as the armpits and groin.
When a radiofrequency pulse is applied, the body experiences an oscillating magnetic field. Just as with a pulsed magnetic field gradient, the body responds to the oscillating magnetic field with internal electric currents that generate heat by resistive losses. The measure of power deposition is the specific absorption rate (SAR), defined as the amount of power deposited per kilogram of tissue.26 It can be shown that power deposition in whole-body NMR increases with current path radius (body size), frequency of the radiofrequency pulses, and pulse duty cycle.4 In MRI the frequency of radiofrequency pulses is proportional to the static magnetic field strength. Consequently, tissue heating is a greater concern in high field MRI systems. This is particularly true in body imaging, in which high-frequency radiofrequency penetrates less deeply into the body so high-powered radiofrequency pulses are required.
The total radiofrequency power absorbed by the body can be determined empirically using the following equation:7
![]()
where P is the root mean square power input to the radiofrequency coil, Qp is the coil quality factor with the patient in the coil, Qe is the quality factor with the coil empty, T is the duration of the radio-frequency pulse, and t-1 is the number of radiofrequency pulses per second. For the body average SAR, Vp represents the total body mass, or as a more conservative and accurate measure Vp is only the tissue mass contained within the coil. The quality factor is an electrical quantity indicating the ratio of inductance to resistance. The quantity (1 – Qp/Qe) represents the fraction of total resistive heat losses that is deposited in the patient.
Usually an imaging sequence requires more than one type of radiofrequency pulse. For example, the popular two-echo spin-echo sequence requires two of the more powerful 180° radiofrequency pulses for each 90° radiofrequency pulse. The total SAR is the sum of contributions from each type of pulse. An SAR calculation for a medium-field-strength two-echo spin-echo sequence is shown below:
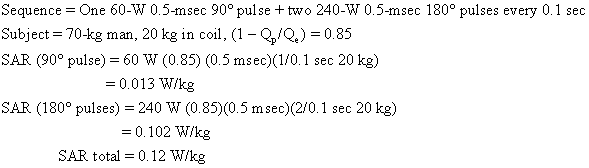
As we shall see, this body average SAR is well below guideline limits.
The power requirements for head scanning is one tenth or less that of body scanning; therefore, it is not limited by tissue heating.
Peak SAR refers to the maximum power deposited per kilogram averaged over any 1 g of tissue. Estimating peak SAR involves models of tissue geometry and electric characteristics.4 For cylindrical and spherical models peak SAR (at the skin surface) is two to three times the average SAR. It is emphasized, however, that actual peak SAR may be significantly higher depending on body geometry and actual electric properties.
The temperature rise of tissue for given SAR and exposure time (texp) is given by the equation
![]()
where C is the heat capacity of tissue (0.83 kcal/kg °C).7 Tissue exposed to 2 W/kg for 20 minutes would be expected to rise in temperature by 0.7°C. Temperature increases of this magnitude are not observed clinically owing to the body's thermoregulatory system (acquisition time for MRI sequences varies from subminute to several tens of minutes).
Currently, static magnetic field strength and time-varying magnetic field gradients do not place limits on routine NMR procedures for reasons of patient safety. Field strengths in excess of 3 T are technically difficult to achieve on whole-body NMR systems and may not offer advantages in image quality or diagnostic value. Also, magnetic field gradient pulses currently used are believed to be within safe limits and are adequate for virtually all magnetic resonance procedures. However, as new techniques are developed that require stronger gradient pulses, such as rapid scanning, further careful investigation of safety of time-varying magnetic fields is warranted. The remaining issue of radiofrequency power deposition does restrict the type of pulse sequences that are usable in body scanning at high magnetic field strengths. Considerable effort has gone into radiofrequency coil design and modes of radiofrequency polarization to minimize the percentage of protocols that exceed safety guidelines. In the event the selected protocol exceeds radiofrequency power deposition limits, the examination may be partitioned into two or more acquisition sequences that individually do not exceed power deposition limits. The body's ability to continually transfer heat to the environment allows many pulse sequences to be applied sequentially as long as no one sequence exceeds the guidelines. This is a fundamental distinction between radiofrequency power deposition in MRI and ionizing radiation dosage in x-ray procedures, in which the total examination dose is the primary concern.
Often tissue is not the only conductive material in the MRI bore. For example, ECG leads, temperature or other monitor leads, conductors within biomedical implants and MRI reciever coil cables themselves can absorb radiofrequency energy and cause significant focal heating. Large conductor loops are particularly prone to absorb energy, thus eliminating non necessary wires/conductors/MRI-compatible monitoring equipment, and careful positioning of conductors within the bore to limit inductive pick of radiofrequency energy are essential for safe MRI scanning. Fortunately, the majority of biomedical implant devices are considered safe in terms of radiofrequency heating, but again one is referred to Shellock's resources to confirm MRI compatibility of a given device.13, 14
GUIDELINES FOR OPERATION OF CLINICAL NMR DEVICES
Table 1 summarizes the threshold limits of significant risk for static magnetic field, time-varying fields, and SAR values as determined by the US Food and Drug Administration10
Table 1. FDA threshold of significant risk – guidelines for clinical NMR
Static magnetic field (telsa) | Time-varying magnetic field* | SAR average** (watts/kg) | SAR peak*** | |
8 (adult & children) 4 (infants age <1 month) | 20 | 4 whole body | 8 head and torso |
*or any time rate of change sufficient to produce severe discomfort or painful nerve stimulation
**for any 15-minute interval for whole body, any 10-minute inerval for head
***for any 5-minute interval
GADOLINIUM-BASED MRI CONTRAST AGENTS
MRI and its use of gadolium-based contrast agents has enjoyed the perception of relative low adverse risk for over two decades and over 100 million contrast agent doses administered world-wide. In May 2007 the Food and Drug Administration (FDA) issued a "black box" warning to call attention to reports of nephrogenic systemic fibrosis (NSF) in patients with renal failure following administration of gadolinium-based contrast agents. NSF is a debilitating and potentially fatal desease that involves hardening/stiffening of skin, muscle and internal organs. The gadolinium-based agents in question are cleared by the kidneys, thus compromised renal function allows prolonged exposure to the agent which may result in increased dissassociation of gadolium and is chelate. All five of the FDA-approved agents were included in the warning, despite evidence that NSF cases were not distributed in proportion to the number of administrations by each brand. Moreover, the incidence of NSF directly resultant from these agents has been difficult to quantify. As of March 12, 2007, by one review of the literature a total of 74 NSF cases suspected from gadolinium-based MRI agents 27; although the FDA MedWatch database has reports of 236 cases in its files. While the numerical risk of developing NSF from an injection of gadolinium-based contrast material as part of an MRI scan is very low, the FDA recommends the injection should not be performed unless the patient has adequate renal function by a glomerular filtration rate (GFR) > 30 mL/min/1.73 m2.
OPINIONS OF RISK TO THE CONCEPTUS IMPOSED BY MRI
As is true of other imaging modalities, risk to the human embryo or fetus as imposed by MRIprocedures is an important issue that must be considered by physicians ordering examinations. It is the policy of many MRI facilities not to image pregnant women. This conservative posture is not based on scientific evidence of biohazards, but rather is a precautionary measure. Institutions that do perform MRI of the pregnant abdomen usually do not do so during the first trimester. The NRPB recommends that women in their first trimester be excluded from MRI even though there is no evidence of damage to the embryo.6
Recent animal studies of the effects of NMR/MRI fields on the developing fetus did not demonstrate adverse bioeffects28, 29 unless very high power deposition levels were used.30 These results, coupled with growing experience with MRI, leads one to conclude that routine MRI procedures are probably safe for humans and their unborn. The American College of Radiology issued a position on this matter in its Radiology Practice Standards in 1997. In it, the ACR recommends that MRI may be used for pregnant patients if other non ionizing imaging is not adequate. In scanning pregnant patients, however, each case should be considered for its medical need vs risk by the referring physician in consultation with a radiologist. It is further recommended verbal and written consent be obtained from the patient.
REFERENCES
Price, R.R., The AAPM/RSNA physics tutorial for residents. MR imaging safety considerations. Radiological Society of North America. Radiographics, 1999. 19(6): p. 1641-51. |
|
Pykett IL: NMR imaging in medicine. Sci Am 246 (6): 78, 1982 |
|
Price, R.R., The AAPM/RSNA physics tutorial for residents. MR imaging safety considerations. Radiological Society of North America. Radiographics, 1999. 19(6): p. 1641-51. |
|
Bottomley PA, Edelstein WA: Power deposition in whole-body NMR imaging. Med Phys 8 (4): 510, 1981 |
|
Bureau of Radiological Health: Guidelines for evaluating electromagnetic exposure risk for trials of clinical NMR systems. Department of Health and Human Services, US Public Health Service, Food and Drug Administration, 1982 |
|
National Radiological Protection Board: Revised guidance on acceptable limits of exposure during nuclear magnetic resonance clinical imaging. Br J Radiol 56:974, 1983 |
|
Budinger TF: Nuclear magnetic resonance (NMR) in vivo studies: Known thresholds for health effects. J Comput Assist Tomogr 5 (6): 800, 1981 |
|
Kangarlu, A., et al., Cognitive, cardiac, and physiological safety studies in ultra high field magnetic resonance imaging. Magn Reson Imaging, 1999. 17(10): p. 1407-16. |
|
Schenck, J.F., Safety of strong, static magnetic fields. J Magn Reson Imaging, 2000. 12(1): p. 2-19. |
|
http://www.fda.gov/cdrh/ode/guidance/793.pdf |
|
de Kerviler, E., et al., [Risks associated with MRI: safety rules, incidents, and accidents]. J Radiol, 2005. 86(5 Pt 2): p. 573-8. |
|
New PFJ, Rosen BR, Brady TJ et al: Potential hazards and artifacts of ferromagnetic and nonferromagnetic surgical and dental materials and devices in nuclear magnetic resonance imaging. Radiology 147: 139, 1983 |
|
Reference Manual for Magnetic Resonance Safety, Implants, and Devices: 2008 Edition (600 pages; ISBN 978-0-9746410-4-1) |
|
http://www.mrisafety.com/ |
|
Pavlicek W, Geisinger M, et al: The effects of nuclear magnetic resonance on patients with cardiac pacemakers. Radiology 147: 149, 1983 |
|
Roguin, A., et al., Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace, 2008. 10(3): p. 336-46 |
|
Kalin, R. and M.S. Stanton, Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol, 2005. 28(4): p. 326-8. |
|
Kolin A: Improved apparatus and technique for electromagnetic determination of blood flow. Rev Sci Instrum 23: 236, 1952 |
|
Beischer DE: Vectorcardiogram and aortic blood flow of squirrel monkeys (Saimiri sciureus) in a strong superconductive electromagnet. In Barnothy MF (ed): Biological Effects of Magnetic Fields, Vol 2. New York, Plenum Press, 1969 |
|
Gore JC, McDonnell MJ, Pennock JM, Stanbrock HS: An assessment of the safety of rapidly changing magnetic fields in the rabbit: Implications for NMR imaging. Magn Reson Imag 1: 191, 1982 |
|
Barlow HB, Kohn HI, Walsh EG: Visual sensation aroused by magnetic fields. Am J Physiol 148: 372, 1947 |
|
Budinger TF, Cullander C, Bordaw R: Switched magnetic field thresholds for the induction of magnetophosphenes (abstr). Proceedings of the 3rd Annual Meeting of the Society of Magnetic Resonance in Medicine, pp 118 and 119, 1984 |
|
Watson AB, Wright JS, Joughman J: Electrical thresholds for ventricular fibrillation in man. Med J Aust 1: 1179, 1973 |
|
Roy OZ: Summary of cardiac fibrillation for 60 Hz currents and voltages applied directly to the heart. Med Biol Eng Cornput 18: 657, 1980 |
|
Reilly, J.P., Peripheral nerve stimulation by induced electric currents: exposure to time-varying magnetic fields. Med Biol Eng Comput, 1989. 27(2): p. 101-10 |
|
American National Standard Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 300 kHz to 100 kHz (C95.1–1982). ANSI, New York, 1982 |
|
Thomsen, H.S., P. Marckmann, and V.B. Logager, Enhanced computed tomography or magnetic resonance imaging: a choice between contrast medium-induced nephropathy and nephrogenic systemic fibrosis? Acta Radiol, 2007. 48(6): p. 593-6. |
|
McRobbie D, Foster MA: Pulsed magnetic field exposure during pregnancy and implication for NMR foetal imaging: A study with mice. Magn Resort Imag 3: 231, 1985 |
|
Heinrichs WL, Fong P, Moseley ME et al: Analysis of teratogenesis and reproductive toxicity in Balb/c mice after midpregnancy MRI or MRS exposure (abstr). Proceedings of the 4th Annual Meeting of the Society of Magnetic Resonance in Medicine, p 922, 1985 |
|
O'Conner ME: Mammalian teratogenesis and radiofrequency fields. Proc IEEE 68: 56– 60, 1980 |

