Indications and Patient Selection for Preimplantation-Related Chromosome Abnormalities
Authors
INTRODUCTION
Preimplantation genetic diagnosis was originally proposed to detect single gene mutations in gametes and embryos before implantation and to transfer unaffected embryos to prospective parents determined to be at high reproductive risk for genetic disease. The singular value of preimplantation diagnosis was to avoid the possibility of having to consider terminating an affected pregnancy following prenatal diagnosis. Certain families also considered undertaking preimplantation genetic diagnosis in the belief that they could avoid the obstetric risks associated with the invasive procedures of chorionic villus sampling (CVS) and amniocentesis.
Initially, preimplantation genetic diagnosis was the preferred option for couples at risk for passing on an X-linked disorder for which no specific molecular diagnosis was available. Therapy previously available to them included fetal sex determination and selective abortion of all males, of which on average 50% would not have been affected. A second group of couples were those that had suffered repeated terminations of pregnancy with or without any specific diagnosis. Couples at risk for transmitting single gene mutations (e.g. cystic fibrosis) formed the largest set of prospective parents attracted to preimplantation diagnosis. Finally, there were couples with moral and religious objections to pregnancy termination that preferred genetic selection before implantation intended to provide them a reasonable chance of a normal pregnancy at the outset.
PREIMPLANTATION CHROMOSOME DIAGNOSIS
Performing preimplantation diagnosis has two technical components: removal of cells from the oocyte or embryo and the genetic diagnosis itself. Biopsy can be carried out on cells from the oocyte (polar body biopsy) and from the preimplantation embryo (blastomere biopsy). Both approaches require artificial reproductive procedures (in vitro fertilization [IVF]) to generate oocytes and embryos in vitro. Polar body biopsy involves removal of the first and second polar bodies. Blastomere biopsy involves aspiration of 1 or 2 cells (preferably 2 cells) from the cleavage stage embryo 3 days after insemination. Preimplantation diagnosis for genetic disease must be sensitive and rapid because genetic information is derived from 1 or 2 cells and human embryos must be transferred within 5 days of aspiration (blastocyst stage) to achieve implantation and pregnancy.
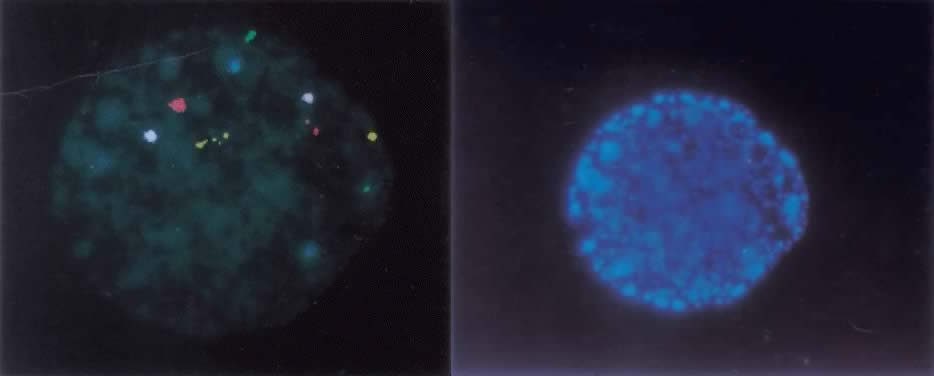
The ideal method of studying chromosomes has been by karyotyping. Cells are arrested in metaphase by preventing spindle fiber formation, fixed in an acidified solution, and then treated to enzymatic digests (e.g. trypsin) so that chromosomes could be banded and that numeric and structural chromosome aberrations detected. To obtain metaphase chromosomes from polar bodies and cleavage-stage human embryos has been problematic, because these cells could not be cultured for extended periods of time (i.e. 2 to 3 days) and few cells were suitable for karyotyping. When metaphase chromosomes were obtained, the chromosomes were short and difficult to band. With the introduction of interphase fluorescent in situ hybridization, or FISH, examination of the chromosomes of every cell within an embryo became possible. FISH uses DNA probes that bind to specific regions of the target chromosomes. FISH probes directly labeled with different colored fluorochromes were developed which bind to the centromeric, or α satellite, regions of the X, Y, and 18 chromosomes, because each of their centromeres contained unique repeat sequences. After the nuclei chromatin had been denatured to separate the double-stranded DNA into single strands, the probes were then hybridized to their complementary strand. Because the fluorochromes were bound to the probes, a fluorescent dot was seen on the chromosome. Nuclei derived from sperm, oocytes, polar bodies, or blastomeres can be FISHed within two hours.1,2 For some chromosomes, however, centromeric sequences were not specific (e.g. the α satellite probe for chromosomes 13 and 21 cross hybridized and distinguishing the two chromosomes was not possible). When the number of signals indicated a numeric chromosome aberration, it was not possible to determine which chromosome was involved. In response to this technical problem, unique-sequence, single-copy FISH probes were introduced. Because they were specific for a single gene (i.e. locus specific), these probes were also chromosome specific. A significant disadvantage is that a limited number of chromosomes, usually no more than five, can be FISHed at one time, due to their close proximity within the interphase nucleus (Fig. 1).
Chromosome aberrations occurring during gametogenesis, fertilization, and preimplantation embryonic development are in large part responsible for implantation failure, spontaneous abortion, stillbirth, and live birth with congenital malformations. Only 30% of human embryos during natural cycle conception are estimated to be able to implant themselves and become viable.3 More than 20% of these embryos in natural cycle pregnancies are believed lost before the third week of gestation.4 The rate of chromosome aberrations in spontaneously aborted fetuses following IVF was not dissimilar to that in natural cycle conceptions and exceeded 60%.5 Karyotyping of human preimplantation embryos following IVF revealed that 37% were chromosomally abnormal.6 Numeric chromosome aberrations were present in 56.5% of arrested preimplantation embryos, using FISH probes for only three chromosomes (X, Y, and 18).7 Because only 5% of 7- and 8-week-old embryos were found to be chromosomally abnormal,8 most embryos with a chromosome aberration are presumed to be lost before being recognized as a clinical pregnancy.
This chapter reviews the origin and characteristics of chromosome aberrations in the preimplantation period with particular emphasis on their implications for the treatment of infertility by IVF and the preimplantation diagnosis of chromosome disorders.
INFERTILITY AND PREIMPLANTATION DIAGNOSIS
With the routine application of intracytoplasmic sperm injection (ICSI) in IVF programs, men with severe male factor infertility can now procreate. It is likely that preimplantation diagnosis will become an integral component when artificial reproductive technologies are applied in such cases for the following reasons.
Prospective paternal parents participating in IVF programs because of male factor infertility require karyotyping, because chromosome aberrations were present in 17% of azospermic men and 7% of oligospermic men (reviewed in a paper by De Braekeleer and Dao9). Chromosome analysis of blastomeres, either by karyotyping or FISH analysis, also should be considered for embryos conceived from sperm of men with severe male factor infertility. FISH analysis of sperm from oligospermic men has demonstrated an increased level of numeric chromosome aberrations, particularly sex chromosome aneuploidy.10 It has been hypothesized that they occur as a consequence of gonadal mosaicism. This hypothesis was recently supported by an investigation of nondisjunction in mitotic (diploid) and meiotic spermatogenic cells in testicular tissue samples of infertile men with impaired spermatogenesis of unknown etiology.11 Incidence of sex chromosome aneuploidy in germ cells was considerably higher than any previous studies both in fertile and infertile men (29.1% and 43%, respectively). The relative ratio of normal to aneuploid nuclei in diploid spermatogenic cells was 300% lower in cells of men with impaired spermatogenesis compared with men with normal spermatogenesis, confirming earlier reports of increased incidence of sex chromosome aneuploidy in germ cells of men with severely impaired spermatogenesis. Moreover, this study provided direct evidence that aneuploidy not only arises in meiotic spermatogenic cells but also earlier during mitotic cell division of spermatogonia.11 Not expected, therefore, are reports that sex chromosomal aberrations increase more than fivefold in prenatal fetuses conceived by ICSI, when compared with the expected rate.12,13,14 When applying IVF and ICSI for male factor infertility, embryonic analysis by blastomere biopsy would seem prudent to ensure the transfer of embryos with normal chromosome complements. To be complete, genetic analysis of men with severe male factor infertility not only must include identifying cytogenetic abnormalities (6% to 18% of cases) but Y-chromosome microdeletions (18% to 22%), mutations in the androgen receptor (8% to 14%) and cystic fibrosis transmembrane conductance regulator (CGTR) (3% to 10%), and mitochondrial deletions (5% to 7%).15
ADVANCED MATERNAL AGE AND PREIMPLANTATION DIAGNOSIS
It has been proposed that the preimplantation embryos of women undergoing IVF should be tested on a routine basis with FISH for clinically important chromosome syndromes, namely, trisomies 13, 16, 18, 21, and 22 as well as for numeric changes in the X and Y chromosomes. This approach may have particular benefit to women of advanced maternal age (35 years of age and older) because pregnancy rates following IVF rapidly decline and spontaneous abortion rates markedly increase for older women. By selecting for transfer only chromosomally normal embryos based on results of FISH analyses, it has been inferred that implantation rates would increase, spontaneous abortion rates would decrease, and that there would be considerable improvement in the overall “take home baby” rate. Moreover, the need for prenatal diagnosis, a standard of care for women of advanced maternal age, would in time be eliminated, if preimplantation chromosome diagnosis proved accurate. This possibility also may be attractive to older women who do not want to risk pregnancy loss as a consequence of undergoing the invasive procedures of amniocentesis or chorionic villous sampling.16
Chromosome aberrations present in human oocytes appears to account for the relatively low success rate of IVF and, in turn, further justifies the proposal that all embryos should be tested before transfer, especially in the case of older women. Human oocytes that failed to fertilize properly after in vitro insemination are important in determining the level of chromosome aberrations developing as a consequence of oogenesis. Early studies indicated that the incidence of aneuploidy ranged from 2% to 14.5%, with a weighted mean estimated to be 13%.17 Much higher incidences of chromosome aberrations have been reportedly recently. In one study, 39% in uncleaved oocytes without a polar body were chromosomally abnormal (17% unbalanced due to predivision of the chromatids and 21.5% diploid) and 21.5% of oocytes with a polar body.18 In another study of inseminated oocytes with no pronucleus formation, 58.7% were chromosomally abnormal.19 A wide range exists in the number of chromosome aberrations identified in human oocytes. This variation results from various laboratory and biologic factors: technical factors resulting in chromosome loss and poor chromosome morphology, differences in the age and/or reproductive histories of oocyte donors, and the morphology of the oocytes undergoing chromosome analysis.
The frequency and distribution of trisomies associated with advancing maternal age were similar in oocytes as in first trimester spontaneous abortions.20 For example, in case of oocytes rejected for IVF and embryo transfer, the distribution of chromosome aberrations was that trisomy 16 was the most frequent, followed by chromosomes 21 and 22, and then chromosomes 13 to 15 and the X chromosome, a pattern similar to that of spontaneous abortions. The overall frequency of potential trisomies after fertilization was 7%, which is actually higher than the 4% estimated at conception based on spontaneous abortion data. For trisomy 16, the potential frequency after fertilization was 2.1%, again higher than the estimated frequency of 1.3% based on spontaneous abortion data. Both these differences were explained based on the presumed loss of trisomy conceptions before clinically recognized pregnancies end in spontaneous abortion.20
The ages of those bearing oocytes with chromosome abnormalities were significantly increased, compared with the ages of those with chromosomally normal oocytes.20 There was one exception, trisomy 16, which was no different from that of spontaneous abortions in which maternal age in case of trisomy 16 was significantly less than maternal ages in cases of trisomy conceptions involving other chromosomes.21,22 Considerable increase in ages of women bearing double trisomy oocytes also parallels the spontaneous abortion data.23 This association of increasing frequency of chromosome aberrations with advancing maternal age has profound impact on the potential success of IVF and justifies the proposal that embryos of women routinely undergo preimplantation diagnosis for the clinically important autosomal and sex chromosomal aneuploidies.
Approximately 60% of human conceptions fail because of the presence of a chromosome aberration.5 Karyotyping of human preimplantation embryos has demonstrated a spectrum of chromosomally abnormal cells and embryos composed of chromosome mosaicism, aneuploidy, and ploidy.5,17,24,25 Chromosome mosaicism involving normal and either aneuploid or polyploid cells appeared to be the most common karyotypic abnormality. An early study found that 16% of normally fertilized embryos were chromosomally abnormal because of diploid/haploid or diploid/triploid mosaicism.26 One study found a much higher rate, with 30% of normally fertilized embryos chromosomally abnormal due to aneuploidy (19%), chromosome mosaicism (2%), structural chromosome rearrangement (1%) and polyploidy (7%).25 FISH technology allowed analysis of each blastomere of an embryo and, not unexpectedly, the level of numeric chromosome aberrations detected was considerably higher than that identified by conventional karyotyping. For example, in one study of morphologically normal embryos monitored for only three chromosomes (X, Y, and 1), 62% were chromosomally abnormal (aneuploid, 4%; abnormal mosaic, 4%; diploid mosaic [majority diploid with a few being aneuploid, haploid, or tetraploid]).27 Claims of improved pregnancy outcomes have been made following screening for aneuploidy in embryos.28 Because the number of FISH probes applied is usually limited to 5 to 7, a positive effect of screening on pregnancy outcome has been questioned. No current valid data demonstrate the efficacy of this approach. Prospective, randomized, multicenter, experimental trials have yet to be conducted.
Genetic analysis of the first and second polar bodies also has been clinically applied to avoid age-related aneuploidy in women of advanced maternal age (i.e. 35 years of age and older). The incidence of aneuploidy for chromosomes 13, 21, 16 or 18, X and Y ranged from 12%, when the first polar body was noted during biopsy,29 to 40% for chromosomes 13, 18 and 21, when the first and/or second polar body was analyzed.30 Claims of improved implantation and outcome have not been proven, primarily because of the study design and limited number of available embryos. In addition, several disadvantages of polar body biopsy are inherent, especially that analysis of the first and second polar bodies would not address the 10% to 25% of chromosome aberrations of paternal and postygotic origin.
STRUCTURAL CHROMOSOME REARRANGEMENTS
Carriers of structurally altered chromosomes, such as inversions and translocations, represent a high-risk population for which preimplantation chromosome diagnosis continues to prove exceedingly useful. Carriers of balanced chromosome rearrangements, although physically and mentally normal, experience recurrent adverse pregnancy outcomes as a consequence of forming unbalanced gametes. In most cases, this chromosomal imbalance in zygotes is incompatible with embryonic viability expressed in the form of arrested embryos, failed implantation, or spontaneous abortion. By selecting chromosomally balanced oocytes or embryos, IVF success rates would be expected to improve for carriers of structurally altered chromosomes. Preimplantation chromosome diagnosis may be particularly beneficial to carriers of inversions or translocations who have experienced infertility, repeated pregnancy loss, and infants born with birth defects secondary to an unbalanced chromosome complement.
The level of normal gametes produced by carriers highlights their reproductive dilemma. In one series of four carriers of balanced translocations, 25 of 33 embryos, or 76%, were either unbalanced or otherwise chromosomally abnormal.31 In two couples with robertsonian translocations (t13;14 and t13;21), combined results showed that only 13% of embryos were normal and 87% were chromosomally abnormal.32 For certain reciprocal translocations, chromosome imbalance in oocytes and embryos reached levels higher than 90% as a consequence of 3:1 segregation.33 A strong negative selection process must exist against chromosomally unbalanced gametes and preimplantation embryos: the risk of an infant with an unbalanced chromosome complement being born to a balanced translocation carrier actually is considerably lower than the potential risk at the time of conception. Carriers of robertsonian translocations involving chromosomes 14 and 21 have a 33% theoretic risk of an infant's being born with Down syndrome; however, the actual risk is 10% to 15% for a maternal parent and less than 5% for a paternal parent.
Several approaches have been applied to address the needs of such patients. Because first polar body chromosomes remain in the metaphase stage for several hours after oocyte retrieval, FISH with chromosome-paints has been used in preconception genetic diagnosis of translocations of maternal origin.34 FISH analysis has also been applied to interphase chromosomes of blastomeres and selecting embryos before transfer has resulted in normal or chromosomally balanced infants.31 By combining probes that label the ends of chromosomes (termed “telomere” probes) with centromeric probes, any translocation can be characterized. With preimplantation diagnosis, the low pregnancy rate and high frequency of spontaneous abortion can now be effectively reversed in translocation carriers.
RECURRENT PREGNANCY LOSS AND PREIMPLANTATION DIAGNOSIS
There is preliminary evidence that couples experiencing recurrent pregnancy loss are at increased risk for generating gametes and embryos with chromosome aberrations.35 A rather extreme example of this are cases of prospective parents in whom the nuclei of blastomeres from an embryo randomly show different chromosome complements. These embryos were characterized as exhibiting “chaotic” division. The occurrence of chaotically dividing embryos was patient related because some patients demonstrated repeated IVF cycles of “chaotic” embryos, whereas other patients were completely free of this type of chromosome anomaly.27 Chaotically dividing embryos are very unlikely to implant. At present, the morphologic appearance of chaotically dividing cells cannot be distinguished morphologically from blastomeres with a normal chromosome complement, and, therefore, there is a question as to the usefulness of preimplantation chromosome diagnosis in these cases. These chaotically dividing cells may represent mutations in normal cell-cycle mitotic check-points and that failure of these checkpoints to be activated during early cleavage effectively eliminates the possibility of implantation and further embryonic development.
PROBLEMS AND SOLUTIONS IN PREIMPLANTATION CHROMOSOME DIAGNOSIS
Preimplantation chromosome diagnosis must still be considered an experimental approach to the prevention of genetic disease, despite technical advancements over the past 2 decades. The initial goal of preimplantation diagnosis addressed the needs of families at risk for mendelian disorders. Over the past 15 years, the number of centers providing preimplantation diagnosis has continually increased and at the beginning of this millennium exceeds 50. With the introduction of FISH technology, clinical issues related to chromosome aberrations have begun to be addressed. The ability of the IVF embryologist to culture embryos to the blastocyst stage in the laboratory before transfer has increased IVF pregnancy rates to 50%. The challenge to the clinical geneticist is whether the application of preimplantation chromosome diagnosis can effectively enhance the implantation rate while reducing the spontaneous abortion rate for women of advanced maternal age, for prospective parents who have experienced repeated pregnancy loss, and for carriers of chromosome aberrations.
There are significant disadvantages inherent in conducting preimplantation chromosome diagnosis with both polar body biopsy of oocytes and blastomere biopsy of embryos. Polar body biopsy only addresses maternally derived chromosome aberrations and requires that the first as well as the second polar body be analyzed to characterize the genotype of the oocyte correctly. This approach obviously cannot be applied to men with severe male factor infertility having a chromosomal basis or to males requiring preimplantation diagnosis because of numeric or structurally altered chromosomes in their complement. The presence of chromosome mosaicism in preimplantation embryos represents a significant potential for misdiagnosis both of single genes and chromosome aberrations. Chromosome mosaicism appears to be a normally occurring event in developing human embryos, particularly the diploid/haploid and diploid/tetraploid types. For preimplantation chromosome diagnosis to be successful, the biopsied cells must be representative of the entire embryo. However, it has been firmly established that preimplantation embryos are mosaic and patterns of mosaicism and chaotic embryos are clearly problematic. For example, trisomy cells have been observed in diploid embryos and normal cell cells have been observed in chromosomally abnormal embryos. Understanding the molecular basis for chromosome mosaicism and chaotic embryos ultimately may contribute to being able to “stabilize” the chromosome complement of preimplantation embryos and provide accurate definition of the karyotype as normal or abnormal.
Methods of detecting chromosome imbalance in the polar body and preimplantation embryo continue to develop. In consideration of the frequency of chromosome aberrations in the gametes of older women, men with male factor infertility, and carriers of structurally altered chromosomes, reliable uncomplicated methods are needed. Multicolor FISH probes for chromosomes 13, 16, 18, 21, 22, X, and Y have been used to screen polar bodies and embryos before transfer in women of advanced maternal age (see Fig. 1). However, it has yet to be demonstrated that screening with a limited set of FISH probes will enhance the “take home baby” rate. The availability of telomere probes for each chromosome will considerably reduce the expense and time required to provide preimplantation chromosome diagnosis for patients carrying chromosome translocations. It is clear that what remains needed for high-risk couples and older women having routine IVF treatment is a complete genome screen. Possible approaches being investigated include comparative genome hybridization (CGH) onto normal metaphase chromosomes following whole genome amplification from single polar bodies or blastomeres36,37 as well as a conventional chromosome analysis that heretofore has eluded cytogenetic investigation.
CGH is only capable of detecting deletions and duplications of whole chromosomes or segments of chromosomes. DNA isolated from single cells (e.g. polar body or blastomere) is labeled with a fluorochrome of one color and mixed with reference DNA labeled with a different color. The mixture is then hybridized to the chromosomes in cells of a normal male after which intensity ratio profiles are calculated for each of the 46 chromosomes. Based on these color ratios, chromosome aberrations involving trisomy, monosomy, and partial gain or loss of chromosome material can be identified. The problem with applying CGH to preimplantation diagnosis is that the technique currently requires up to 6 days to complete and is subject to the technical difficulties inherent in conducting molecular analyses on single cells.
Recently, karyotyping of human blastomeres was accomplished by using the metaphase-inducing factors present in unfertilized eggs.38 Karyotyping of human oocytes by chromosomal analysis of the second polar body has also been described.39 A rapid technique for karyotyping of embryos and polar bodies would have enormous application in the field of preimplantation chromosome diagnosis and would affect the rate of successful pregnancy in women of advanced maternal age, carriers of chromosomal rearrangements, and couples with infertility and recurrent pregnancy loss. Eventually, conventional chromosome analysis will be replaced by molecular approaches using DNA “chip” technology that will be rapid, accurate, and applicable to a wide-range of reproductive genetic challenges.
REFERENCES
Harper JC, Coonen E, Ramaekers FCS et al: Identification of the sex of human preimplantation embryos in two hours using an improved spreading method and fluorescent in situ hybridisation using directly labelled probes. Hum Reprod 9: 721, 1994 |
|
Harper JC, Delhanty JDA: FISH in preimplantation diagnosis. In: Walker J (ed): Methods in Molecular Biology, Molecular Diagnosis of Genetic Disease. New Jersey: Humana Press, 1996 |
|
Lidley M: Life and death before birth. Nature 280: 635, 1979 |
|
Wilcox AJ, Weinberg CR, O'Connor JF et al: Incidence of early loss of pregnancy. N Engl J Med 319: 189, 1988 |
|
Plachot M: Chromosome analysis of spontaneous abortions after IVF: A European Survey. Hum Reprod 5: 425, 1989 |
|
Plachot M, Veiga A, Montagut J et al: Are clinical and biological IVF parameters correlated with chromosomal disorders in early life: A multicentric study. Hum Reprod 5: 627, 1988 |
|
Munne S, Grifo J, Cohen J et al: Chromosome abnormalities in human arrested preimplantation embryos: A multiple-probe FISH study. Am J Hum Genet 55: 150, 1994 |
|
Burgoyne PS, Holland K, Stephens R: Incidence of numerical chromosome abnormalities in human pregnancy estimated from induced and spontaneous abortion data. Hum Reprod 6: 555, 1991 |
|
De Braekeleer M, Dao TN: Cytogenetic studies in male infertility, a review. Hum Reprod 6: 245, 1991 |
|
Moosani N, Pattinson HA, Carter MD et al: Chromosomal analysis of sperm from men with idiopathic infertility using sperm karyotyping and fluorescence in situ hybridisation. Fertil Steril 64: 811, 1995 |
|
Huang WJ, Lamb DJ, Kim ED et al: Germ-cell nondisjunction in testes biopsies of men with idiopathic infertility. Am J Hum Genet 64: 1638, 1999 |
|
Bonduelle M, Wilikens A, Buysse A et al: Prospective follow-up study of 877 children born after intracytoplasmic sperm injection (ICSI), with ejaculated epididymal and testicular spermatozoa and after replacement of cryopreserved embryos obtained after ICSI. Hum Reprod 11: 131, 1996 |
|
In't Veld PA, Brandenburg H, Verhoeff A et al: Sex chromosomal abnormalities and intracytoplasmic sperm injection. Lancet 346: 773, 1995 |
|
Liebaers I, Bonduelle M, Van Assche E: Sex chromosome abnormalities after intracytoplasmic sperm injection. Lancet 346: 1095, 1995 |
|
de Kretser DM: Male infertility. Lancet 349: 787, 1997 |
|
Reubinoff B, Shushan A: Preimplantation diagnosis in older patients, To biopsy or not to biopsy? . Hum Reprod 11: 2071, 1996 |
|
Zenses MT, Casper RF: Cytogenetics of human oocytes, zygotes, and embryos after in-vitro fertilization. Hum Genet 88: 367, 1992 |
|
Benkhalifa M, Menezo Y, Janny L et al: Cytogenetics of Uncleaved Oocytes and arrested zygotes in IVF programs. J Assist Reprod Genet 13: 140, 1996 |
|
Almeida PA, Bolton VN: The relationship between chromosomal abnormalities in the human oocyte and fertilization in vitro. Hum Reprod 9: 343, 1994 |
|
Angell R: First-meiotic-division nondisjunction in human oocytes. Am J Hum Genet 61: 23, 1997 |
|
Hassold TJ, Jacobs PA: Trisomy in man. Annu Rev Genet 18: 69, 1984 |
|
Risch N, Stein Z, Kline J et al: The relationship between maternal age and chromosome size in autosomal trisomy. Am J Hum Genet 39: 68, 1986 |
|
Reddy KS: Double trisomy in spontaneous abortions. Hum Genet 101: 339, 1997 |
|
Angell R: Chromosome abnormalities in human preimplantation embryos. In: Development of preimplantation embryos and their environment, p. 181. New York: Alan R. Liss, 1989 |
|
Jamieson ME, Coutss JRT, Connor JM: The chromosome constitution of human preimplantation embryos fertilised in vitro. Hum Reprod 9: 709, 1994 |
|
Plachot M, Junca AM, Mandelbaum J et al: Timing of in-vitro fertilization of cumulus-free and cumulus-enclosed human oocytes. Hum Reprod 1: 237, 1986 |
|
Delhanty JDA, Harper JC, Ao A et al: Multicolour FISH detects frequent chromosomal mosaicism and chaotic division in normal preimplantation embryos from fertile patients. Hum Genet 99: 755, 1997 |
|
Gianaroli L, Magli MC, Ferraretti AP et al: Preimplantation genetic diagnosis increases the implantation rate in human in vitro fertilization by avoiding the transfer of chromosomally abnormal embryos. Fertil Steril 68: 1128, 1997 |
|
Munne S, Dailey T, Sultan KM et al: The use of first polar bodies for preimplantation diagnosis of aneuploidy. Mol Hum Reprod 1: 1014, 1995 |
|
Verlinsky Y, Cieslak J, Ivakhnenko et al: Preimplantation diagnosis of common aneuploidies by the first- and second-polar body FISH analysis. J Assist Reprod Genet 15: 285, 1998 |
|
Munne S, Fung J, Cassel MJ, et al: Preimplantation genetic analysis of translocations: case-specific probes for interphase cell analysis. Hum Genet 102: 663, 1998 |
|
Conn CM, Harper JC, Winston RML et al: Infertile couples with Robertsonian translocation: preimplantation genetic analysis of embryos reveals chaotic cleavage divisions. Hum Genet 102: 117, 1998 |
|
Conn CM, Cozzi J, Harper JC et al: Preimplantation genetic diagnosis for couples at high risk of Down syndrome pregnancy owing to parental translocation or mosaicism. J Med Genet 36: 45, 1999 |
|
Munne S, Dailey T, Scott R et al: First pregnancies after preconception diagnosis of translocations of maternal origin. Fertil Steril 69: 675, 1998 |
|
Magli MC, Gianaroli L, Munne S et al: Incidence of chromosomal abnormalities from a morphologically normal cohort of embryos in poor-prognosis patients. J Assist Reprod Genet 15: 297, 1998 |
|
Kallioniemi A, Kallioniemi OP, Rutovitz D et al: Comparative genomic hybridization for molecular cytogenetic analysis of solid tumor. Science 258: 818, 1992 |
|
Wells D, Sherlock JK, Handyside AH et al: Detailed chromosomal and molecular genetic analysis of single cells by whole genome amplification and comparative genomic hybridization. Nucleic Acid Res 27: 1214, 1999 |
|
Willadsen S, Levron J, Munne S et al: Rapid visualization of metaphase chromosomes in single human blastomeres after fusion with in-vitro matured bovine eggs. Hum Reprod 14: 470, 1999 |
|
Verlinsky Y, Evsikov S: Karyotyping of human oocytes by chromosomal analysis of the second polar bodies. Mol Hum Reprod 5: 89, 1999 |