Laparoscopy in Gynecologic Oncology
Authors
INTRODUCTION
Since the introduction of the laparoscope by Jacobaeus1 in 1910, its usefulness in the surgical arena has been met with skepticism by traditional surgeons. Despite the skepticism, potential advantages afforded by this minimally invasive technique motivated the development and evaluation of the laparoscope in an expanding number of surgical applications. Numerous technologic advances were critical to this evolution. During the first half of this century, the development of various lens systems and endoscopes by Kalk,2 the development of cold light by Fourestier and coworkers,3 and the introduction of fiberoptics by Hopkins and Kapany4 all contributed to the modern endoscope. Palmer5,6 in the 1940s reported the creation of a pneumoperitoneum and the control of intra-abdominal pressure, leading to wider acceptance of the laparoscope as a diagnostic tool.
The potential of the laparoscope as an operative tool was shown in 1962 when Palmer7 performed the first tubal fulguration. The development of laparoscopic instruments for grasping, cutting, irrigating, ligating, and performing endocoagulation for hemostasis by Semm and Mettler8 in the 1970s extended the applications of operative laparoscopy beyond tubal sterilization, with laparoscopic adnexectomies, ovarian cystectomies, and resection of endometriosis cysts being reported. Gomel9 further broadened the indications for operative laparoscopy by showing its value and safety when performing salpingolysis, fimbrioplasty, and salpingostomy. Despite the efforts of these and other pioneer surgeons, laparoscopic surgery was still limited by the technology. Rapid advancements in laparoscopic instrumentation and technology occurred in the 1980s that included the development of a safe peritoneal insufflator, laparoscopic instruments to manipulate pelvic organs and control bleeding, and the development of the videoscope. In 1989, Reich and coworkers10 reported the first laparoscopic-assisted vaginal hysterectomy. Laparoscopy is now often used in the diagnosis and surgical management of benign gynecologic conditions.
Attempts to integrate laparoscopic techniques into the treatment of patients with gynecologic malignancies were met with an appropriate demand to show feasibility and then equivalency to traditional surgical approaches. In 1975, Rosenoff and coworkers11 first reported using the laparoscope for staging ovarian cancer. Subsequently, Smith and colleagues,12 Spinelli and colleagues,13 and Ozol and colleagues14 used the laparoscope as a diagnostic tool in second-look operations. The slow integration of laparoscopy into gynecology oncology, other than as a diagnostic tool, was because of the belief that radical surgery could not be performed adequately or safely. Other factors that discouraged the use of laparoscopy among gynecologic oncologists were the inability to completely evaluate the abdominal cavity due to adhesions from previous operations, the loss of tactile sense, and technical concerns such as the ability to control hemorrhage during radical surgery and lymph node dissection. Despite these concerns, many pioneering surgeons have shown the feasibility of using a minimally invasive approach in the treatment of women with gynecologic malignancies. Initially, the role of laparoscopy was limited to staging procedures. Dargent15 in 1987 was the first to propose the use of the laparoscope to evaluate the lymph node status, and subsequently Querlue and coworkers16 in 1991 reported their experiences performing laparoscopic pelvic lymph node dissections. Since then, laparoscopy applications have broadened, and instrumentation and surgical techniques have been refined. However, laparoscopic surgery still has not been established as the standard treatment for gynecologic malignancies. A growing body of literature is available to evaluate the feasibility, benefits, limitations, cost, thoroughness, and complications of laparoscopic procedures, although more studies are needed before the value of laparoscopy is proved. This chapter summarizes the current literature pertaining to the use of laparoscopy in the treatment of patients with gynecologic malignancies. In addition, surgical techniques are described in detail and photographs provided to help the reader better understand how equivalent procedures can be achieved using minimally invasive techniques.
LAPAROSCOPY AND ENDOMETRIAL CANCER
In 1988, the International Federation of Gynecology and Obstetrics determined that endometrial cancer should be staged surgically. The Gynecologic Oncology Group (GOG) in Protocol 33 showed that even patients with grade 1 and grade 2 lesions without myometrial invasion, as well as those with grade 3 lesions (stage IA) can have pelvic lymph node metastasis, and patients with all grades of endometrial cancer with myometrial invasion can have both pelvic and aortic lymph node metastases.17 The incidence of lymph node metastases in this landmark study was underestimated as only unilateral; right-sided aortic lymph node sampling was required for patient entry; the superior boundary of the dissection was the inferior mesenteric artery (IMA), not the renal vessels; and if a sample was submitted that was devoid of nodal tissue, it was categorized as negative instead of nonevaluable. More important, pathology in this study was evaluated retrospectively as related to lymph node metastases and myometrial invasion, minimizing the usefulness of the results reported to guide clinical practice. The retrospectively reported relationship between depth of invasion and grade of the cancer and lymph node metastases might not be an accurate reflection of information known to the surgeons intraoperatively, when a decision to complete surgical staging must be made. If only lymph node metastases had been correlated to the intraoperative assessment of grade and depth of invasion and other noted shortcomings corrected, then the results of this study could serve as a reasonable basis to determine candidacy for surgical staging. Recent analysis of GOG Protocol 99, in which patients with all grades of endometrial cancer, negative lymph nodes, and any myometrial invasion were randomly assigned with one group of patients receiving whole pelvic radiation and another, no further treatment, showed there was no significant difference in survival between the two groups.18 This further supports the position that all patients should undergo surgical staging as patients with histologically negative lymph nodes, regardless of the grade of lesion or depth of invasion, can avoid unnecessary whole pelvis radiation. The morbidity or mortality associated with complete surgical staging has not been shown to be increased over a similar group of patients with endometrial cancer undergoing hysterectomy alone.19 Hospital stays associated with surgical staging have been reported to be between 6 and 10 days.20 In integrating minimally invasive surgical techniques into the treatment of patients with endometrial cancer, it was thought that potential benefits might include reduced morbidity, shortened hospital stay, and reduced costs. Incumbent on those introducing this procedure was to show its feasibility and then compare it with laparotomy. Retrospective studies comparing patients with endometrial cancer treated laparoscopically with those patients undergoing laparotomy appear to have statistically significant benefits of shorter hospitalization and earlier return to normal activity, which may lead to a cost benefit without compromising the adequacy of the surgical staging (Table 1).
TABLE 1. Comparison of Surgery Treatment for Early Stage Endometrial Cancer: Laparotomy Versus Laparoscopy
Authors | Type of Procedure and Number of Patients | Cost | Average Hospital Stay (days) | Average Number of LNs Removed |
| |
|
|
|
| PLN | PALN | Number of Patients Requiring Conversion from LSC to LAP |
Spirtos, 199622 | LSCN = 13 LAPN = 17 | $19,362 ± $3675* $13,809 ± $3560* | 2.4* 6.3* | 20 22 | 8 7 | 0 |
Boike, 199623 | LSCN = 20 LAPN = 21 | NA | 2.7* 5.9* | 14.5 13.3 | 5.8 4.7 | 3 |
Hidelbaugh, 199724 | LSCN = 29 LAPN = 64 | $11,038 ($6403---17,104) $9369 ($6720---23,537) | 2.3 5.1 | 7 15 | 14 Not performed | 3 |
Holub, 199825 | LSCN = 11 LAPN = 26 | N/A | 4.2 7.7 | 9 12 | Not performed Not performed | 3 |
LSC, laparoscopy; LAP, laparotomy; N, number of patients; LNs, lymph nodes; PLN, pelvic lymph node; PALN, para-aortic lymph node.
*Statistically significant.
Laparoscopic pelvic and aortic lymph node sampling can be performed with vaginal hysterectomy or laparoscopic-assisted vaginal hysterectomy or complete laparoscopic hysterectomy with bilateral salpingo-oophorectomy. The ability to perform a complete laparoscopic hysterectomy offers an advantage in a patient in whom there is no uterine descensus, a narrow vaginal arch, or small introitus.
Morbidity, lymph node counts, and operative time associated with laparoscopic lymph node dissection and hysterectomy in the treatment of endometrial cancer are summarized (Table 2). The yield of lymph node appears to be adequate. Operative time appears to decrease as surgeons gained more experience. Intraoperative morbidity included bowel, urinary tract, and vascular injuries. Postoperative complications included bowel obstruction, bowel herniation (10- or 12-mm ports), bleeding, cuff dehiscence, and deep venous thrombosis. There was no associated mortality reported. The average hospital stay was approximately 2.5 days. These studies were not randomly assigned, and therefore comments comparing these results with those associated with laparotomy are premature.
TABLE 2. Literature Review of Laparoscopic Lymphadenectomy for Treatment of Early Stage Endometrial Cancer and its Associated Morbidity
Authors | n | Type of Hysterectomy Performed | Average Number of LNS Removed | Number of Positive LNs Detected | Average Operative time (min) | EBL (mL) | Morbidity | ||
|
|
| PLN | PALN |
|
|
| Intraoperative | Postoperative |
Childers26 | 53 | LAVH | NI | NI | 1 PLN | NI | <200 | 1 pneumothorax | 2 bowel obstruction |
Spirtos21 | 35 | LAVH | 20.8 | 7.9 | 2 PLN 1 PALN | 193 (70---440) | 100* | 1 caval injury, 5 converted to laparotomy | 2 DVT, 2 bowel obstruction |
Melendez27 | 60 | LAVH | NI | NI | NI | 240 | 2 entertomy, 2 cystotomy, 2 conversion to laparotomy | NI |
|
Possover28 | 41 | LAVH | 19.4 | 5.1 | 2 PLN 1 PALN | 177.9 | NI | None | 3 urinary tract infections |
LAVH, laparoscopic-assisted vaginal hysterectomy; LN, lymph node; PLN, pelvic lymph node; PALN, para-aortic lymph node; NI, not indicated.
*Excluded patient with caval injury.
Important in achieving the benefits of laparoscopic staging are proper patient selection, availability of state-of-the-art equipment, a dedicated surgical team, and surgical experience. Spirtos and Childers both initially reported obesity as a limiting factor in successful completion of the para-aortic lymph node dissection.21,26 Spirtos and coworkers initially used a Quetelet index (weight in kilograms/height in square meters) of 30 or less as an eligibility criterion for patients undergoing laparoscopic aortic lymph node sampling. Initial GOG feasibility trials had similar restrictions. Other relative contraindications unique to laparoscopy include previous whole abdominal radiation therapy and multiple prior surgeries, particularly if the retroperitoneal spaces have undergone exploration and dissection. For lest there be a misunderstanding, surgery in these patients, regardless of the technique used, should be undertaken with caution.
The GOG recognized the importance of evaluating the feasibility of these techniques and opened two protocols to accrual for patients with uterine cancers. The first protocol was opened to establish the feasibility of performing aortic and pelvic lymphadenectomies along with a laparoscopically assisted vaginal hysterectomy bilateral salpingooophorectomy in patients with endometrial cancer. The second protocol was opened to establish the feasibility of performing laparoscopic staging in patients with incompletely staged cancers of the uterus. Final analysis of these two protocols has not been completed, but suffice it to say that preliminary results were sufficient enough to allow the opening of a prospective, randomly assigned protocol comparing surgery and the quality of life associated with it in patients treated laparoscopically versus traditional laparotomy-treated patients. There are no weight limitations in this protocol; therefore, hopefully, given the sample size, the limits of this procedure will be defined more clearly.
Another role for laparoscopy in the management of endometrial cancer is to complete staging in those patients having only undergone hysterectomy. Childers and colleagues29 described 16 patients with either higher grade lesions or more myometrial invasion than thought to be present at the time of initial hysterectomy who underwent laparoscopic restaging. The interval to restaging averaged just more than 6 weeks. All patients were restaged successfully without laparotomy being necessary. Two patients were found to have positive lymph nodes, and appropriate treatment was then undertaken. Just as important, the completion of surgical staging in patients showing no evidence of extrauterine disease can minimize the use of radiation therapy, which does not impact overall survival.
Finally, there is no long-term survival data for patients undergoing laparoscopic surgery for endometrial cancer, but recently Magrina and colleagues30 reported equivalent 3-year survival in patients undergoing laparoscopy and laparotomy in their series of patients.
LAPAROSCOPIC MANAGEMENT OF CERVICAL CANCER
The surgical treatment of early cervical cancer consists of two components: a radical hysterectomy and lymphadenectomy. Because the extent of the lymph node involvement is one of the most important prognostic factors, evaluation of these lymph nodes is an integral part of the management of cervical cancer. Laparoscopic techniques have been adapted successfully in the management of early cervical cancer to complete both the lymphadenectomy and the hysterectomy.
The feasibility of endoscopic surgery in the treatment of early-stage cervical cancer was first shown by Dargent15 in 1987. He reported his experience on 10 patients undergoing laparoscopy to determine the presence of metastatic disease in the pelvic lymph nodes. Successful sampling of the external iliac and obturator lymph nodes was confirmed at laparotomy immediately after endoscopic surgery. Subsequently, Querlue and coworkers16 proposed pretreatment laparoscopic staging for patients with stage I and II cervical cancer to assist in treatment planning. In their series, 39 patients underwent laparoscopic pelvic lymphadenectomy, and an average of 8.7 nodes was removed. Lymph node metastasis was detected in five of the 39 patients, who were subsequently offered radiation therapy as the primary treatment. Of the 34 patients with negative nodes, two patients had radical vaginal hysterectomy and 32 patients had Wertheim abdominal radical hysterectomy. No further metastatic lymph nodes were detected during the abdominal hysterectomy in these 32 patients. Both Dargent and Querlue and coworkers limited their laparoscopic lymphadenectomy to pelvic lymph nodes. Childers and coworkers31 subsequently described their experience with both laparoscopic pelvic and para-aortic lymphadenectomy. Their study of 18 patients with cervical cancer included eight with stage Ib disease who were candidates for radical hysterectomy. Three of eight patients were discovered to have positive lymph nodes at the time of laparoscopy and instead were treated with radiation therapy. The remaining five patients underwent abdominal radical hysterectomy, in whom an average of 31 lymph nodes were removed laparoscopically, and only two of the five patients had residual lymph nodes found at the time of the laparotomy. Overall, 91% of the lymph nodes normally removed at laparotomy were removed at laparoscopy. One patient in particular had 11 residual lymph nodes, including one parametrial node positive for microscopic disease. The authors attributed this high residual lymph node count to the patient's weight and early experience with this procedure. The mean operative time was less than 2 hours and the average hospitalization stay for laparoscopic lymphadenectomy was 1.5 days. There were no major complications.
One major concern when introducing a new surgical technique such as laparoscopic lymphadenectomy for cervical cancer is showing its adequacy or equivalence when compared with laparotomy, the current standard of care. Incomplete dissection of pelvic lymph nodes may leave residual microscopic disease, which may worsen the prognosis of the patient. Fowler and coworkers32 addressed this concern in their series of 12 patients with stage Ib cervical cancer who underwent laparoscopic pelvic lymphadenectomy followed immediately by laparotomy. Two of these 12 patients also underwent a right-sided para-aortic lymphadenectomy. The overall yield of lymph nodes obtained laparoscopically was 75% compared with laparotomy. However, there was a clear improvement in lymph node yield in the latter six patients compared with the first six patients, suggesting that there is a learning curve associated with this procedure. Most important, there was no residual metastatic disease detected at the time of laparotomy. More recently, Schlaerth and coworkers33 evaluated the adequacy of laparoscopic pelvic and aortic lymph node sampling in cervical cancer. They reported despite obtaining average pelvic and aortic lymph node counts of 31 and 11, respectively, in the series, the most common lymph node group left unresected were those lateral to the common iliac vessels. These early reports suggest that laparoscopic pelvic lymphadenectomy in the management of cervical cancer is feasible and safe.
Laparoscopy lymphadenectomy has been advocated both as an adjunct to the radical vaginal hysterectomy, laparoscopically assisted radical vaginal hysterectomy and complete laparoscopic radical hysterectomy. Canis and colleagues34 in 1990 and Nezhat and colleagues35 in 1992 were the first to describe laparoscopic radical vaginal hysterectomies; however, the procedures were limited to a type II radical hysterectomy based on Piver's classifications.36 More recently, Spirtos and colleagues37 reported their experience with a type III radical hysterectomy and pelvic and para-aortic lymphadenectomy performed entirely by the laparoscopic approach. Their series consisted of 10 patients with stages IA2 and IB cervical cancer. The average operative time was 253 minutes with a decrease in operative time in the last set of five patients compared with the first five patients. Unlike earlier reports by Canis and Nezhat, Spirtos provided evidence of the radicality of the hysterectomy by reporting measurements of the parametria and vaginal cuff margin that was removed. There were no major intraoperative or postoperative complications. The average blood loss was 300 mL with no patients requiring blood transfusion. The average length of hospitalization was 3.2 days.
Several authors have also described a laparoscopically assisted radical vaginal hysterectomy. Querlue38 reported a pilot study of eight patients who underwent a laparoscopically assisted radical vaginal hysterectomy for early-stage cervical cancer. In his procedure, the ureteric tunnel is dissected laparoscopically and the cardinal ligaments are divided vaginally. The radicality of hysterectomy was tailored to the size of the tumor. There were no major urinary tract complications and two patients required blood transfusion for a blood loss over 1000 mL. The average operative time including the laparoscopic lymphadenectomy was 4½ hours. A 1- and 3-year follow-up on the eight patients indicated that all but one patient remained disease-free and one patient required a posterior exteneration for a recurrence involving the rectum. Dargent and Mathevet39 described a laparoscopic-assisted radical vaginal hysterectomy in which division of parametria was performed laparoscopically and dissection of the ureter was performed vaginally. Kadar40 described a series of eight patients with stage Ia2 to stage IIa cervical cancer treated with laparoscopically assisted radical vaginal hysterectomy. The first two patients were complicated by a transected ureter, and the subsequent six patients, however, did not have major urinary tract injuries; four of the six patients received blood transfusion. The operative time was not reported. These reports suggest that it is feasible and relatively safe to apply laparoscopic techniques to the radical hysterectomy portion of cervical cancer. However, there is minimal long-term survival data comparing the laparoscopic radical hysterectomy or the laparoscopicassisted vaginal hysterectomy to the traditional abdominal approach. More recently, Spirtos and colleagues41 have reported a 4% recurrence rate on a 3-year follow-up of 78 patients with early-stage cervical cancer who were treated with total laparoscopic type III radical hysterectomy with bilateral pelvic lymphadenectomy.
ROLE OF LAPAROSCOPIC SURGERY IN ADVANCED CERVICAL CANCER
The standard therapy for advanced stage cervical cancer is combined pelvic radiation and chemotherapy. The benefit of incorporating extended field radiation is less well-defined. The rationale for extended field radiation is that as many as 30% of patients with advanced stage cervical cancer have metastatic disease to the para-aortic lymph nodes.42 Typically, extensive clinical staging is performed using noninvasive techniques such as computed tomography, magnetic resonance imaging, and lympangiogram to document metastatic disease in the para-aortic lymph nodes. However, clinical staging has been reported to underestimate the extent of disease in 29% to 48% of patients with advanced disease.43,44,45 Because the proportion of patients without metastatic para-aortic lymph nodes in stage III disease can be as high as 70%, most patients are subjected to extended field radiation unnecessarily.46 Therefore, surgical staging has been advocated in advanced stage cervical cancer to document the extent of disease and assist in treatment planning. Traditionally, this constitutes sampling of pelvic and para-aortic lymph nodes via celiotomy either transperitoneally or extraperitoneally.47,48,49 One of the disadvantages to surgical staging is that the transperitoneal approach, when followed by radiation, is associated with significant bowel adhesions and enteric morbidity. Although the extraperitoneal approach results in fewer postradiation bowel complications, it still requires a major incision and prolonged hospitalization. Thus, it is easy to understand why, in this group of patients, the use of laparoscopy for pretreatment staging might result in significant patient benefit. If laparoscopy is used for pretreatment surgical staging, it is imperative that the para-aortic lymph nodes be sampled. More extensive studies are required to show that there is a clear advantage of laparoscopic pretreatment staging over the conventional pretreatment celiotomy.
Laparoscopy may have other applications in the management of cervical cancer. Plante and Roy50 have reported using laparoscopy to rule out intra-abdominal disease or metastatic disease before an exenterative procedure, although transposition of ovaries laparoscopically certainly can be achieved while avoiding unnecessary laparotomy.51
Technique of Total Laparoscopic Type III Radical Hysterectomy and Pelvic and Aortic Lymphadenectomy
PREOPERATIVE PREPARATION.
It is essential to retract the bowel away from the pelvis into the upper abdomen. This greatly improves the visual exposure of the operative field and minimizes the potential for inadvertent bowel injury. Several strategies for achieving this are used:
- Mechanical bowel preparation begins 48 hours before surgery with a clear liquid diet. At 24 hours before surgery, patients ingest 4 L of balanced electrolyte solution. Fleet's enemas are given on the morning of surgery until clear. This preparation eliminates bowel contents and decreases active peristalsis during the procedure; thus, manipulating the intestines becomes easier and surgical exposure is enhanced.
- An epidural anesthetic is used for splanchnic sympathetic blockade. The resultant parasympathetic override causes additional contraction of the bowel. In addition, less-general anesthetic agents are needed during surgery.
- Nitrous oxide can accumulate in the bowel and increase distension. Thus, the anesthesiologist is instructed to avoid its use after induction.
- An oral gastric tube is passed into the stomach after intubation and before any laparoscopic instruments are placed. This decreases the distention of the upper gastrointestinal tract.
OPERATING ROOM PERSONNEL.
Because this surgery is very dependent on complex machinery and instruments, it is important to have a dedicated team that allows surgery to proceed smoothly. This team must possess expertise for problem solving and on-the-spot equipment maintenance. The scrubbed technician directly participates in the procedure by manipulating the cervix and uterus; by wiping, cleaning, and adjusting the laparoscope during the case; and by handling all sterile troubleshooting measures. This individual can reduce operative times significantly by staying actively involved in the case. The scrubbed technician typically is positioned between the patient's legs with the instrument table just behind him or her. The circulating nurse should also be part of the laparoscopic team and be responsible for stocking necessary supplies. This nurse must be trained and responsible for all nonsterile troubleshooting measures.
INSTRUMENTATION AND EQUIPMENT.
Operating room tables, whether manual or electric, need to provide a Trendelenburg position of approximately 30 to 40 degrees. However, most operating tables do not have this capability. Allen stirrups (Allen Medical Systems, Bedford Heights, OH) are used to secure the lower extremities with the knees bent at right angles and the legs separated. The hips are not flexed. The arms are tucked to the patient's sides, and padded shoulder braces are used at the surgeon's discretion.
Virtually all dissection is performed with a 10-mm argon beam coagulator (ABC) (Conmed Corp, Utica, NY). Because the use of argon gas can lead to high intra-abdominal pressures, one trocar vent is left opened while the ABC is being used. Thus, either one or two high-flow carbon dioxide insufflators, instilling at least 30 L/min, are necessary to maintain adequate pneumoperitoneum during the procedure. Laparoscopic pieces of equipment needed include four 12-mm trocars, two 5-mm graspers with serrated jaws, 5-mm and 10-mm toothed graspers, and two flat-surfaced graspers (used for suturing). The video system includes a Stryker 888 three-chip camera and a Q-5000 light source (Stryker Endoscopy, Santa Clara, CA). Both gauze Raytec sponges (Johnson and Johnson Medical, Arlington, TX) and vaginal “baby” laparotomy tapes with radio-opaque markings can be inserted through a trocar into the peritoneum; these are counted systematically by the operating room staff. Disposable laparoscopic devices required include a LIGACLIP clip applier (Ethicon Endo-surgery, Cincinnati, OH), an EndoGIA vascular stapler (US Surgical, Norwalk, CT) with six to eight reloads, as well as an EndoStitch (US Surgical) suturing device for closing the vagina and an EndoClose device (US Surgical) to close the trocar sites. Finally, a general instrument set with vascular instruments is in the room or immediately outside the door for possible conversion to laparotomy.
SURGICAL TECHNIQUE.
Initially in our experience, radical pelvic surgery using minimally invasive techniques was limited to patients with a Quetelet index of less than 30.52 We currently use an index of 40 as a general limit for radical hysterectomies, but this is not the sole factor in determining whether a patient should undergo laparoscopic radical hysterectomy.
After epidural and general anesthetics are administered, the oral gastric tube is placed. The placement of ureteral stents is optional at this time. Historically, the arguments against placing ureteral stents include (1) increased anesthesia time and (2) the stiffness of the stent, which makes the ureteral dissection more difficult to perform. In our 6-year experience, a concern has arisen that the improved visualization afforded by performing this procedure laparoscopically may result in vascular compromise of the ureter. We hypothesize that periureteral blood vessels, not typically visible to the naked eye during a standard laparotomy, are now clipped or cauterized when dissecting the ureter through the tunnel. This results, on occasion, in an apparent color change of the distal ureter. Whether this change in color is clinically significant is a moot point, as it seems safer to place bilateral ureteral stents prophylactically and remove them in 6 to 12 weeks. Since beginning this practice, our patients have had no ureterovaginal fistulae, and the dissection has not proved as difficult as anticipated.
In addition, placing the stent after the ureter is dissected off of the attached peritoneum can be more difficult because of the loss of countertraction from the surrounding tissue. This has necessitated two “open cystostomies” to pass ureteral stents after having completed the radical hysterectomy laparoscopically. Without question, if stent placement were to be considered, one would be well-advised to place them before surgery. We recently have begun inserting 5-French Infravision ureteral catheters (Stryker Endoscopy), which, when attached to an infrared light source, are easily visualized in the operative field. The bladder is catheterized and an intrauterine sound is placed and taped to a single-toothed tenaculum attached to the cervix.
Four 12-mm trocars typically are used in this procedure. The lateral port sites are located approximately 1 cm medial to the iliac crests, and midline ports are placed at the symphysis pubis and 2 to 3 cm above the umbilicus. A fifth port can be placed for additional retraction or placement of the laparoscopic stapler; this can be positioned either 8 to 10 cm above the umbilicus or just lateral to the periumbilical port. If previous surgical scars are present on the anterior abdominal wall, the procedure is started by placing a Verres needle in the left upper quadrant, just beneath the left costal margin in the midclavicular line. The Verres needle is then replaced with a 2- or 5-mm trocar after insufflating the peritoneum to approximately 14 to 15 mmHg. Using a smaller laparoscope, the surgeon explores the peritoneal cavity for adhesions to the periumbilical region. If present, they are sharply lysed with laparoscopic scissors that are inserted through a trocar placed close to one of the four prospective trocar sites. Vascular adhesions are dissected with the ABC.
Once the anterior adhesions are removed, the periumbilical trocar is placed and the camera is attached to a 10-mm 0-degree laparoscope. The patient is then placed in Trendelenburg, followed by placement of the lateral ports. The suprapubic port is placed while graspers placed through the lateral ports are used to elevate the anterior abdominal wall to prevent tenting of the peritoneum. All trocars are secured to the skin with 0-Vicryl (Ethicon) suture.
In general, if the operating surgeon is right-handed, he or she should stand on the left side of the patient when working in the pelvis and on the right side when operating on the upper abdomen or performing the aortic lymph node dissection or both. The opposite positions are recommended for the left-handed surgeon. We also recommend that when using the ABC, it should be used through a midline port and held in the dominant hand with a grasper in the nondominant hand, just as a surgeon would use scissors in the dominant hand during surgery. The assistant is responsible for the camera and should concentrate on maintaining the tip of the ABC or scissors into center of the video screen while using a grasper in the opposite hand to provide countertraction at the point of dissection. Two video monitors are placed at 45-degree angles at the foot of the bed to allow the surgeon and assistant to stand in a natural, comfortable position. The operating table is also dropped to the lowest position to reduce any additional stress on the surgeon's shoulders during the procedure.
The “baby” laparotomy sponges are placed intra-abdominally at the beginning of the surgery. These are used to absorb small amounts of blood and lymph fluid throughout the case. If any significant bleeding is encountered, the sponge can be used to tamponade the affected area. The sponge also aids with the suction device to prevent unwanted tissue from being aspirated. In addition to using sponges as noted, the liberal use of the ABC, Trendelenburg position, and the modest increases in intraperitoneal pressure result in a surprisingly dry operative field, which is no small part of the success of laparoscopic radical surgery.
Once the trocars are placed, systemic inspection of the peritoneal cavity is performed. The order of the inspection is as follows: (1) diaphragm; (2) liver and gallbladder; (3) spleen; (4) omentum; (5) small and large intestine, which are inspected as they are packed into the upper right quadrant; and (6) the reproductive organs.
DEVELOPMENT OF THE PARAVESICAL AND PARARECTAL SPACES.
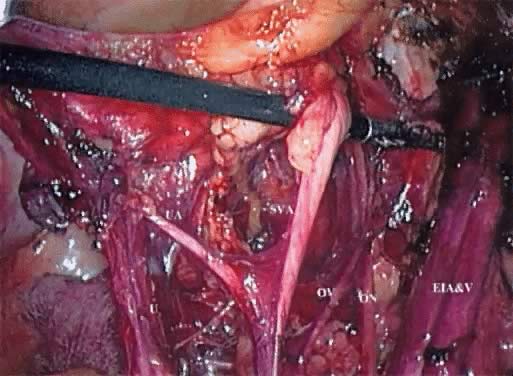
With the camera in the periumbilical port, the round ligament is transected at the pelvic side wall with the ABC by use of traction/countertraction. The peritoneum is then incised in an inferior direction. After ensuring the superior vesical artery is medial and the external iliac vein is lateral to the intended field of dissection, the surgeon uses the tip of the ABC to develop the paravesical space bluntly down to the pelvic floor. Small blood vessels are coagulated at this time, and care should be taken to identify the accessory obturator vein, if present, because bleeding from this site can be difficult to control laparoscopically. The paravesical space is bounded by the bladder medially, the cardinal ligament and uterine artery posteriorly, the pubis anteriorly, and the obturator internus laterally.
The peritoneal incision is extended in a cephalad direction along the psoas muscle and parallel to the ovarian vessels to a point well past the pelvic brim. The ABC is used to first “spray” the peritoneal surface before incising it to control any troublesome small peritoneal vessels. The ureter is easily identified as it crosses to the medial peritoneum, and the ovarian vessels can then be transected at a point that allows for either conservation or resection of the ovary/Fallopian tube. With the ureter in view, incising the medial peritoneum just posterior to the ovarian vessels from the pelvic brim to the lateral aspect of the uterus allows an endoscopic stapler placed through the suprapubic port to staple and transect this pedicle.
With the uterus displaced superiorly and to the opposite side of the patient, the ABC is used to coagulate perforators between the ureter and the hypogastric vessels, and the pararectal space is entered. Occasionally, these perforating vessels measure more than 3 mm and should be clipped with the LIGACLIP instead of coagulated. Care should be taken to press the instrument toward the bleeding vessel because activating the LIGACLIP often causes it to retract slightly and the clip is then not optimally placed to achieve hemostasis. Blunt dissection with the ABC is performed carefully down to the levator ani muscle. In developing this space, traction is best directed at 90 degrees to the iliac vessels. This allows the operating surgeon the best opportunity to recognize perforating vessels and either clip or cauterize them before they become problematic. The pararectal space is bounded by the uterosacral ligament medially, the sacrum posteriorly, the hypogastric vessels and piriformis muscle laterally, and the cardinal ligament and uterine vessels anteriorly. When these steps are completed, an adequate assessment of the parametrium can be performed, either with a blunt laparoscopic probe or on manual rectovaginal examination, while the paravesical and pararectal spaces are opened and laparoscopic observation is possible.
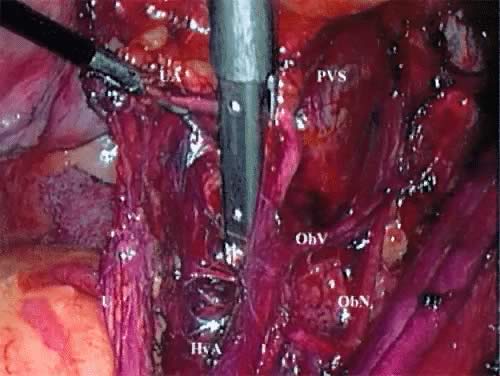
These spaces are similarly developed on the opposite side of the pelvis. If there is no evidence of extracervical disease, the radical hysterectomy and pelvic lymphadenectomy can then be performed. The superior vesical artery is isolated away from its attachments, retracted laterally, and followed proximally from the lateral aspect of the bladder. The middle and inferior vesical arteries are identified and the uterine vessels are isolated, with the ABC developing a space between the superior vesical artery and the uterine artery (Fig. 1). The EndoGIA (U.S. Surgical) is then passed through the suprapubic port and placed across the lateral parametrium. One jaw of the stapler can be visualized extending into the paravesical space with the other jaw in the pararectal space. The stapler is pressed laterally against the hypogastric vessels and superior vesicle artery. After it is ensured that the obturator nerve is free laterally and the ureter is free medially, the stapler is activated, transecting the upper cardinal ligament, including the uterine vessels (Fig. 2).
PELVIC LYMPHADENECTOMY.
For the pelvic lymphadenectomy, the surgeon continues to stand on the patient's left side. Beginning with the nodal tissue overlying the anterior and medial aspect of the common iliac and external iliac artery/vein, lymph-node-bearing tissue is resected using the tip of the ABC as a dissector and coagulator. The contralateral grasper can gently retract the common iliac vessels medially while the ipsilateral grasper elevates these nodes. The dissection is continued distally until the circumflex iliac vein is encountered, serving as the distal boundary of the dissection. Any perforating vessel in this region can be controlled with either the ABC or clip application.
A plane is carefully created between the external iliac vessels and the psoas muscle by retracting these vessels medially. The ABC is then used to coagulate any perforators in this space. The obturator fossa is entered laterally, and attachments of the lymph node bundle to the psoas muscle are incised. The obturator nerve can be identified easily with this approach, and the tip of the ABC is placed just above the nerve and is used to bluntly strip the nodal tissue off the nerve. Using the inferior border of the external iliac vein as a landmark, the nodal tissue can now be retracted medially, and the ABC is used to coagulate the nodal tissue just beneath the vein. This dissection begins at the bifurcation of the internal and external iliac vessels and is performed distally to the point that the obturator nerve exits the pelvis. At the bifurcation of the iliac vessels, the bundle of nodes often are “tethered” to this area. This is caused by attachment and continuation of the nodal bundle lateral and proximal to the bifurcation of the common iliac vessels. Often at this point, the posterior divisions of the hypogastric vein, including the lateral sacral and inferior gluteal veins, are visible. The ABC is of particular value at this point. There is little space for surgical maneuvering because large nerves and fixed veins are present. By using the ABC as a blunt dissector and then with short bursts of coagulating current, the surgeon can complete the lymphadenectomy with minimal risk of neural or vascular injury. The remaining pelvic lymph nodes at this point lie along the medial aspect of the common iliac and hypogastric arteries. Again, these nodes are grasped and the ABC is used to coagulate and dissect this tissue off the vessels.
DEVELOPMENT OF THE RECTOVAGINAL SEPTUM AND RESECTION OF THE UTEROSACRAL/CARDINAL LIGAMENTS.
The proximal pelvic ureter is bluntly separated from the posterior leaf of the broad ligament. Care should be taken to identify the periureteral tissue, which may be as much as 1 cm anterior to the ureter. Dissection of this tissue along with the ureter minimizes bothersome bleeding and may reduce devascularization of the ureter. The ureter is pushed posteriorly and laterally in a distal direction to the uterine vessels. The posterior peritoneum can be incised down toward the uterosacral ligament. Working from a lateral-to-medial direction, the plane between the sigmoid colon serosa/mesentery and peritoneum is developed using short bursts of the ABC current. This point is not often addressed, but we have found that by beginning this dissection laterally, there is little difficulty in identifying this plane and separating the rectum from the vagina. The peritoneum over the posterior uterosacral ligament can then be incised safely. The rectovaginal septum is entered and the rectum is dissected off of the posterior aspect of the vagina. Again, small amounts of argon gas are insufflated in this region by tapping the foot pedal in very short bursts; this infuses the argon without deploying the unipolar current and greatly facilitates opening this space in a relatively bloodless fashion. A gauze Raytec sponge also assists in bluntly developing this space.
The surgeon transects the uterosacral ligaments at the level appropriate to the individual case. The stapler is passed through the ipsilateral post, and the uterosacral ligament is divided with one or two purchases of the stapler on each side. Alternatively, if a fifth port is in place, the laparoscope is placed through the upper port and the stapler passed through the periumbilical port. The ureter must be visualized and retracted laterally before the stapler is closed and activated. The cardinal ligaments are similarly transected with the stapler; again, the extent of the resection can be individualized. It should be stressed that we attempt to maximize the amount of the cardinal and uterosacral ligaments resected before the ureteral dissection. In our opinion, this approach shortens the operative time by facilitating the surgeon's ability to place the uterus on sufficient traction and thereby delineating the dissectional planes important to this portion of the procedure.
DEVELOPMENT OF THE VESICOVAGINAL SPACE AND URETERAL DISSECTION.
The ABC is used to develop the vesicovaginal space by pressing the tip on the underside of the peritoneal edge near its reflection from the bladder onto the cervix. The pedal is tapped as described above. The ABC then transects the vesicovaginal peritoneum, and an Endoscopic Blunt Tip Dissector (Ethicon Endo-surgery) is used to separate the bladder from the cervix and upper vagina. Small blood vessels are coagulated with the ABC.
The ureteral dissection is now performed. As the scrubbed technician elevates the uterus to the side opposite the dissection, the assistant surgeon retracts the ureter laterally and somewhat posteriorly. The surgeon then grasps the transected uterine artery and places medial traction on it. The ABC is used to coagulate and dissect the periarterial tissue over the ureter. The vesicouterine sheath is next incised while the ureter continues to be placed on posterolateral traction. A helpful maneuver in developing this space is placing the tip of the ABC directly on top of the ureter and retracting firmly in a distal and anterior direction. The ureter can then be rolled and retracted laterally. In attempting to complete this dissection with unipolar surgical instruments such as shears, the surgeon needs to exercise exceptional care because unintentional, if not unavoidable, contact can be made with the ureter. The position and magnification provided by the laparoscope/camera are great assets here in safely completing the ureteral dissection. Occasionally, a vessel in the area measuring greater than 3 mm is encountered and requires clips to be used for hemostasis.
RESECTION OF THE UPPER VAGINA.
Before the medial aspect of the parametria and paracolpos is resected, the bladder is dissected away from the distal vagina using the ABC. The surgeon places her or his nondominant hand into the anterior vaginal fornix and tents the vagina anteriorly. This maneuver not only facilitates the dissection by increasing the countertraction; it also assists in determining the extent of dissection needed to achieve adequate vaginal margins. The EndoGIA (U.S. Surgical) is again used to transect the paravaginal tissue. The stapler is passed through the suprapubic port to establish the lateral margin and then from the lateral port to establish the distal margin of resection. Consecutive purchases with the stapler are taken until the vagina is encountered. When the bladder has been satisfactorily advanced off of the top of the vagina, an anterior colpotomy is performed either with the ABC or with laparoscopic scissors connected to unipolar current. This is one of the few instances during this procedure in which the ABC may cause extra char to the tissue. Once the anterior colpotomy is performed, grasping the cervix laparoscopically and placing upward traction on it allows for rapid completion of this step. The sound and tenaculum are removed at this time. Although the simplest step conceptually, this is often the most difficult step because the maintenance of the pneumoperitoneum becomes problematic once the colpotomy is started. Prior solutions included packing the vagina with a moistened laparotomy sponge or a partially filled surgical glove or using a balloon/manipulator, all placed into the vagina. With the advent of the 30 L/min insufflator, we are able to maintain adequate pneumoperitoneum by closing all open trocar vents and having the scrubbed technician manually approximate the labia majora. The specimen is now completely freed and easily removed by grasping it with a tenaculum brought through the vagina.
CLOSURE OF THE VAGINAL CUFF.
Laparoscopic vaginal cuff closure is accomplished with an EndoStitch suturing device placed through the suprapubic port. A 48-inch 0 or 2-0 Polysorb suture is cut to approximately 12 inches, and a small loop is tied to the end of the suture. The flat-surfaced graspers are passed through the lateral ports; they are more effective in grasping the suture and providing tension on the suture. The first stitch incorporates both sides of the vaginal angle opposite to the surgeon, with the needle passed through the looped end. With the assistant pulling the end of the suture laterally and slightly posteriorly, the vagina is closed in a running fashion and tied laparoscopically.
AORTIC LYMPHADENECTOMY.
An aortic lymphadenectomy can be performed based on surgeon preference. We typically do this procedure first after placing and securing the laparoscopic trocars. This is because the bowel is least active immediately after induction of anesthesia and can be retracted easiest into the upper quadrants. The right-handed surgeon stands on the patient's right side for this part of the procedure. The video monitors are placed at the head of the table at 45 degrees to the patient's clavicles, and the laparoscope is placed through the suprapubic port. We reported a significant decrease in our operating times when the video monitors were thus positioned for the aortic lymphadenectomy.21
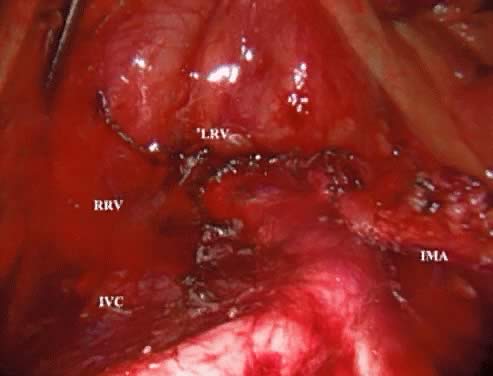
A peritoneal incision is made with the ABC by grasping the peritoneum over the proximal right common iliac artery and aortic bifurcation and extending it superiorly to the level of the IMA. The argon gas helps to dissect this retroperitoneal space and identify any small perforating vessels. Continuing the incision across the midline just above the IMA mobilizes the duodenum. The assistant places a grasper through the left lateral trocar and elevates the third portion of the duodenum by pushing it superiorly and laterally. A gauze Raytec sponge can be placed posterior to the duodenum to assist in elevating it. These maneuvers provide excellent exposure of the vena cava, lower aorta, and right common iliac vessels (Fig. 3).
After the right ureter is identified, the lymph-node-bearing tissue overlying the distal vena cava and right common iliac artery is grasped and the ABC is used to coagulate small perforators entering the nodal fat pad as well as the small lymphatics. The dissection continues in the interspace between the vena cava and aorta. The nodal tissue is retracted superiorly and laterally to the right. This provides an excellent way to identify a small vein commonly found, which drains the fat pad into the vena cava. Once identified, this vessel can almost always be coagulated or clipped. When using the ABC or any unipolar source, the surgeon should take care to apply the current to the vessel nearest to the nodal side as contrasted with the vena cava side.
The first step in removing the left aortic lymph nodes is identifying the origin of the IMA and then incising the peritoneum distally, which parallels the IMA into the pelvis. This incision allows for elevation of the IMA away from the aorta. Using the ABC bluntly, this dissection is performed until the left ureter and psoas muscle is identified as the lateral border of the dissection. The surgeon gently elevates and pushes the IMA and the attached mesentery to the left with a grasper in the right lateral port, and the assistant surgeon grasps the nodal bundle lateral to the aorta. Using the ABC, the aorta is retracted to the right, and the lymphatic attachments are coagulated and resected.
CLOSURE OF THE TROCAR SITES.
While the trocars are still in place, an EndoClose is passed on either side of the trocar with a 0-Polysorb suture loaded in the carrier. The suture should be passed though the fascia and not the skin. Once the sutures are placed, each trocar is closely removed while reapproximating the fascia. This is confirmed by digital examination. In our opinion, the last trocar to be removed should be the suprapubic because it is located in the least likely location for bowel herniation as the incision virtually abuts the pubis. The laparoscope allows for direct inspection of the closure of the other trocar sites.
PLACEMENT OF DRAINS.
If placement of a pelvic drainage system is planned, the top (nondrain) end of a 12- to 15-mm Jackson-Pratt drain is easily passed through either of the lateral ports, grasped from the opposite port, and pulled through to the appropriate length on the opposite side. The drain end can then be placed to the desired site of drainage with the laparoscopic graspers. The sutures closing the fascial defects should be placed before the drains are placed to avoid pulling the drain through the skin when the trocar is pulled out. This can lead to a problem in re-establishing the pneumoperitoneum as well as closing the trocar site. Then, as the drains are pulled through, the trocar can be removed and the suture knotted. The skin can then be either stapled or sutured with 4-0 Polysorb suture.
LAPAROSCOPY AND ADNEXAL MASSES
The treatment of presumably benign adnexal masses using laparoscopic techniques has become routine. The role of laparoscopy in the management of malignant adnexal masses is less well-defined. In an Australian-wide study that looked at 54,198 laparoscopies, 16,601 on adnexal masses, 108 were subsequently found to be malignant. This accounted for 0.65% of all adnexal masses.53 Other studies have found a much higher incidence. In a survey addressing the laparoscopic management of ovarian cysts, Blanc and coworkers54 reported that 20% of the adnexal masses were found to be malignant despite having a benign preoperative clinical profile in addition to having a benign intraoperative appearance. Patients with advanced ovarian carcinomas that present with markedly elevated CA-125 and massive ascites are routinely identified and appropriately treated. The use of laparoscopic techniques in patients with complex or persistent adnexal mass without ascites with a slightly elevated CA-125 is controversial. The potential pitfall is the intraoperative rupture of a malignant mass, resulting in upstaging and potentially worsening the prognosis. Therefore, it is important to make every effort to identify malignant masses before surgery to appropriately inform and consent the patient before proceeding to exploratory laparotomy or laparoscopy. Laparoscopy is not contradicted, based on the risk of intraoperative rupture, as laparotomy does not eliminate that risk nor has it been shown in a prospective fashion to even reduce it.
In an attempt to identify malignant adnexal masses from benign adnexal masses, serum blood tests such as CA-125 and pelvic ultrasound gray-scale technique and color Doppler flow imaging studies have been used. Unfortunately, there is no preoperative testing that yields a positive or negative predictive value of 100%. If such tests were available, consultation regarding the risk, benefits, and complications associated with both laparotomy and laparoscopy would allow patients to make a well-informed decision regarding the surgical options available.
CA-125
CA-125 is a 200-kD glycoprotein that is found on the surface of ovarian cancer cells. The monoclonal antibody used in the assay originally was raised against the antigen OVCA 433, which was prepared from a cell line of a papillary serous cystadenocarcinoma.55,56 The normal physiologic function is unknown, but it is found on the surfaces of cells that line the peritoneum, pleura, pericardium, bronchus, endometrium, Fallopian tube, and endocervix. CA-125 is elevated in up to 90% of patients with stages II, III, and IV epithelial carcinomas of the ovary. Sensitivity is poor in the early stages of ovarian cancer with only approximately 50% of women with stage I epithelial ovarian carcinoma having an elevated CA-125.57
The use of CA-125 blood test to distinguish an adnexal mass from benign or malignant has limitations.58 Using an upper limit of 35 IU/mL, Einhorn and coworkers59 showed in their study of 100 women with palpable mass that a preoperative CA-125 has a sensitivity for malignant disease of 78% (18/22), a specificity of 95% (73/77), and a positive predictive value of 82%. Serum CA-125 sampling also has a poor specificity for ovarian cancer. Using a cutoff of 35 IU/mL, Bast and coworkers56 showed that 6.3% of patients with benign disease have an elevated serum level, and CA-125 is elevated in 29% of patients with nongynecologic cancers, which include pancreatic, lung, breast, colorectal, and miscellaneous nongastrointestinal cancers. More recent articles have described elevated serum levels in association with benign processes such as endometriosis, pregnancy, pelvic inflammatory disease,60 tuberculosis, hematomas,61 and fibroids.62 Generally, these levels are in the range between 35 and 100 IU/mL.
The primary clinical application of this test is to follow patients with advanced ovarian cancer as a means to assess response to chemotherapy during and after its administration. Patients with stage III and stage IV ovarian cancer having a normalization of CA-125 (less than 35 IU/mL) after cytoreductive surgery and two cycles of chemotherapy have an improved median survival compared with patients whose serum levels remain elevated (42 months versus 13 months).63,64
Transvaginal Ultrasound
Transvaginal ultrasound alone also has limitations as a screening test. Three large studies have reported on the limitation of using ultrasound as a screening tool for ovarian cancer as summarized in Table 3. A total of 11,283 perimenopausal and postmenopausal women underwent either transabdominal or transvaginal ultrasound; 5% of women who had screened positive for suspicious adnexal masses underwent exploration and only 2% of women were found to have ovarian cancer with the majority having stage I disease. Thus, nearly 40 patients underwent exploration to detect one ovarian cancer. Transvaginal color and pulsed Doppler assessment of adnexal tumor vascularity has been studied in the hopes of decreasing the number of false-positives obtained from gray-scale transvaginal ultrasound screening. The theory is that malignant tumors have increased angiogenesis to support their growth. Therefore, malignant tumors are more likely to exhibit a diffuse vessel arrangement located in the center, septa, or papillary projection of the mass and have a low-resistance index (RI = peak systolic minus diastolic Doppler shift over peak systolic Doppler shift) below 0.41. Benign lesions, conversely, have a single peripheral vessel with a high resistance index (more than 0.40). However, several studies have shown no benefit over traditional gray-scale ultrasound.70,71
TABLE 3. Summary of Ultrasound Screening for Ovarian Cancer
Authors | No. of Patients Screened (Age) | Type of Ultrasound | No. Surgery Performed | No. Ovarian Cancers Found | No. Stage 1 | PPV |
Campbell, 198965 | 5749 (18---79 yr) | TAS | 326 | 9 | 5 | 1.5 |
DePreist, 199266 | 776 (33---90 yr) | TVS, MI | 44 | 3 | 2 | 6.8 |
Bourne, 199367 | 1601 (17---79 yr) | TVS, CDI | 61 | 6 | 5 | 9.8 |
Karlan, 199368 | 597 (35---80 yr) | TVS, CDI | 19 | 1 | 1 | 5.3 |
Muto, 199369 | 386 (20--->60 yr) | TVS, CI | 38 | 0 | 0 | -- |
Total | 11,283 |
| 486 | 19 | 13 |
|
TAS, transabdominal ultrasound; TVS, transvaginal ultrasound; MI, morphology index; CDI, color doppler index; PPV, positive predictive value
To distinguish malignant from benign adnexal masses, Sassone and coworkers72 proposed a scoring system based on morphology of ovarian lesion as seen on transvaginal ultrasound. The morphologic variables analyzed were inner wall structure whether it was smooth, irregular, papillary projection, or solid component; the thickness of the wall; the presence of septation within the cyst; and the echogenicity of the cyst. Using this scoring system, they were able to distinguish benign from malignant masses with a sensitivity of 100%, specificity of 83%, negative predictive value of 100%, and positive predictive value of only 37%. The majority of the false-positive findings were associated with benign teratomas as often these masses have similar morphologic appearance to malignant masses on transvaginal ultrasound. The population screened consisted of premenopausal women, which may also account for this finding.
To distinguish malignant from benign adnexal masses in the preoperative diagnosis of pelvic masses, Jacobs and coworkers73 incorporated CA-125 value, morphology of sonographic findings, and menopausal status and derived a formula to calculate the relative malignancy index, defined as the product of ultrasound score (U)  menopausal score (M)
menopausal score (M) 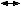 CA-125 level. Ultrasound score (U) was assigned as either 0, 1, or 3 based on sonographic features suggestive of malignancy: multiloculated cysts; evidence of solid component; evidence of ascites; and bilateral lesions. A score (U) of 0, 1, or 3 was assigned if none of the malignancy features were absent, if one feature was present, or if more than one feature was present, respectively. A score (M) of 1 or 3 was given to premenopausal and postmenopausal status, respectively. Incorporating a cutoff level of 200 yielded a sensitivity, specificity, and positive predictive value of 100%, 83%, and 37%, respectively. Roman and coworkers74 reported similar results when incorporating pelvic examination, tumor marker level, and transvaginal ultrasound findings. Using logistic regression analysis, the probability of having ovarian cancer was calculated. In postmenopausal women with CA-125 levels greater than 100 U/mL and suspicious ultrasound morphology, the probability of finding cancer was nearly 90%. In premenopausal women, the size of the mass and the ultrasound were found to be better predictors of pelvic malignancy than CA-125 value. This is not surprising, as this group of women had a higher prevalence of endometriosis, which may cause an elevation of CA-125, thus giving rise to higher false-positive values. Based on these studies, the following algorithm (Fig. 4) should be used as part of the laparoscopic management of adnexal masses.
CA-125 level. Ultrasound score (U) was assigned as either 0, 1, or 3 based on sonographic features suggestive of malignancy: multiloculated cysts; evidence of solid component; evidence of ascites; and bilateral lesions. A score (U) of 0, 1, or 3 was assigned if none of the malignancy features were absent, if one feature was present, or if more than one feature was present, respectively. A score (M) of 1 or 3 was given to premenopausal and postmenopausal status, respectively. Incorporating a cutoff level of 200 yielded a sensitivity, specificity, and positive predictive value of 100%, 83%, and 37%, respectively. Roman and coworkers74 reported similar results when incorporating pelvic examination, tumor marker level, and transvaginal ultrasound findings. Using logistic regression analysis, the probability of having ovarian cancer was calculated. In postmenopausal women with CA-125 levels greater than 100 U/mL and suspicious ultrasound morphology, the probability of finding cancer was nearly 90%. In premenopausal women, the size of the mass and the ultrasound were found to be better predictors of pelvic malignancy than CA-125 value. This is not surprising, as this group of women had a higher prevalence of endometriosis, which may cause an elevation of CA-125, thus giving rise to higher false-positive values. Based on these studies, the following algorithm (Fig. 4) should be used as part of the laparoscopic management of adnexal masses.
LAPAROSCOPIC MANAGEMENT OF THE ADNEXAL MASS
A thorough preoperative evaluation including pelvic examination, transvaginal ultrasound, and appropriate tumor markers should be performed as part of a workup for an adnexal mass. However, regardless of the preoperative assessment undertaken indicating a benign condition, some patients with malignant masses undergo laparoscopy. All suspicious masses should be evaluated intraoperatively with frozen section, and the potential for exploration should be considered. Preoperative bowel preparation should be considered, particularly when a high index of suspicion exists before surgery.
Use of sound surgical principles is imperative. A thorough initial assessment of the abdominal cavity should be performed as in a laparotomy. Several authors have recommended that when there is a suspicious adnexal mass, the primary trocar should be introduced in the midline. In this manner, if the lesion is found to be malignant or there is gross intra-abdominal disease and the case is converted to a laparotomy, the trocar puncture site may be resected at the time of the abdominal incision.
Aspiration of cysts should be avoided. An adnexal mass that is large or involves significant dense adhesions may pose a higher risk of rupturing. Meticulous surgical techniques must be applied to minimize this risk and the consequences of cyst rupture. Endobags should be used to prevent spillage of the cyst fluid content into the abdominal cavity. In cases in which the mass is too large or adherent to perform the resection without rupture, a transvaginal colpotomy may be used either to remove the intact mass or to rupture and decompress the mass while minimizing the risk of intraperitoneal spill. Using this approach, Teng and coworkers75 reported the successful removal of a dermoid cyst with a mean diameter of 10 cm. The risk of rupturing a malignant ovarian cyst laparoscopically will never be eliminated as is the case for masses managed via laparotomy. We recommend performing frozen section intraoperatively, and, based on preoperative discussion with the patient, surgical staging should be completed using laparoscopic techniques or via laparotomy.
Rupture of a Malignant Ovarian Mass
The effect of rupturing an early-stage malignant ovarian mass on a patient's prognosis remains controversial. Table 4 summarizes pertinent studies to date that have tried to determine whether rupture of malignant ovarian cyst is an independent poor prognostic factor. It is difficult to interpret the data as the majority of the patients did not have adequate surgical staging, thus probably underestimating the stage of disease in some patients. Based on these studies, tumor grade is the most important prognostic factor for early-stage ovarian cancer. The prognosis associated with intraoperative rupture is poorly understood. There currently is no study that clearly defines the risks of rupturing an early-stage malignant cyst. It is equally unlikely that any study to answer this question in a prospective, randomly assigned fashion will ever be undertaken. Perhaps it is not the rupture of the cyst at the time of laparoscopy but rather the delay in the patient receiving treatment that results in the poor outcomes. Maiman and coworkers82 and Lehner and coworkers83 attributed metastatic disease found in patients undergoing laparoscopic treatment of early ovarian cancer to a delay in definitive treatment. Maiman and coworkers82 found in their survey that in patients who underwent laparoscopic excision of adnexal mass found subsequently to have ovarian cancer, treatment was delayed on average by 4.8 weeks. Although they did not specifically address whether the delay in therapy contributed to poor prognosis, it was inferred that treatment delay may have contributed to worsening prognosis. The concept of delay in treatment is particularly thought-provoking because intraperitoneal absorption of tumor cells could take place over as little as 9 hours.84 Thus, if there was tumor spillage at the time of laparoscopy, delay in treatment or insufficient irrigation of the abdominal cavity may explain the poor prognosis. Nasu and coworkers85 found that survival associated with stage IC (ascites) was better than survival associated with stage Ic (rupture) with the probability of 5-year survival being 87.5% and 63.5%, respectively. It is not clear whether there is a difference between rupture occurring at the time of surgery and longstanding rupture found at the time of surgery. However, it would be prudent to do everything reasonable to avoid rupture during open or closed procedures. If rupture occurs, then vigorous irrigation should be undertaken. The physician should also consent the patient for a possible full staging procedure and have the capabilities to proceed with a full staging procedure if a malignancy is encountered. If the patient cannot be staged immediately, then planned restaging should be undertaken as soon as possible. The GOG currently has a protocol (9302) open to accrual for incompletely staged ovarian cancer patients to determine the feasibility of completing surgical staging laparoscopically.
TABLE 4. Summary of Various Prognositic Factors in Early-Stage Ovarian Cancer
Authors | n | Grade | Factors | Other Significant Findings | ||
|
|
| Rupture | Adhesions | Ascites |
|
Webb, 197276 | 271 | NA | S | S | NA | Capsular excresence |
Smith, 197977 | 281 | S | NS | NA | S | -- |
Sevelda, 198978 | 204 | S | NS | NA | NA | -- |
Dembo, 199079 | 519 | S | NS | NS | S | Age |
Sjovall, 199480 | 394 | S | NS | NA | S | -- |
Sainz de la Cuesta, 199481 | 79 | S | S | NA | NA | Type of surgery |
S, significant; NA, not analyzed; NS, not significant.
Trocar Site Implantation
The risk of cutaneous metastasis secondary to laparoscopy has been of great concern to surgeons since it was first reported in 1993.86 This phenomenon has been described repeatedly with adenocarcinomas. More recently, other histologic tumor metastases, including squamous cell carcinoma, have also been documented as spreading through trocar sites.87 Childers and coworkers88 reviewed a total of 557 laparoscopic procedures performed by gynecologic oncologists over a 4-year period and reported 0.2% of patients (1/437) had a malignant implantation develop at the abdominal wall trocar site. The majority of those in the case study had ovarian cancer with intraperitoneal disease, thus suggesting that tumor cells were implanted at the time of specimen removal. Kruitwagen and Swinkels89 studied the effect on survival of abdominal wall metastasis at trocar or puncture sites after laparoscopy or paracentesis in women with ovarian cancer. This retrospective study reviewed the records of 219 patients. In seven (16%) of 43 patients who had undergone laparoscopy and three (10%) of 30 who had undergone paracentesis before primary debulking, abdominal wall metastasis had developed at the entry site. All metastasis occurred in women with stage IIIC through IV diseases, including ascites. This study was unable to show a statistically significant decrease in survival.
Nevertheless, the trocar site implantation of tumor is a real phenomenon. Thus, if a tumor spillage occurs at the time of removal of unsuspected malignant ovarian cysts, Childers and colleagues88 advocate irrigating all abdominal puncture sites to decrease the potential of abdominal wall tumor implantation.
LAPAROSCOPY AND OVARIAN CANCER
Despite all precautions, there inevitably are situations in which a presumed benign adnexal mass is removed and subsequently diagnosed as malignant. For example, the false-negative rate of frozen sections is between 1% and 6%.90,91 These patients often have incomplete surgical staging. In this setting, laparoscopic surgery may have a more significant role in the management of early ovarian cancer. An obvious advantage to laparoscopic surgery for a patient with incomplete staging is that the recuperation period is shorter than for conventional surgical staging via laparotomy. A shorter recuperation is especially attractive in the event that adjuvant treatment may be required. However, laparoscopic surgical staging of early ovarian cancer must be proved to be as thorough and effective as conventional laparotomy. The staging procedure must be able to assess the areas at high risk for metastasis such as mesentery of bowel, the omentum, the diaphragmatic peritoneum, and the pelvic and para-aortic lymph nodes. In fact, early reports on laparoscopic surgical staging of ovarian cancer found a false-negative rate of approximately 36% when the retroperitoneal space was inaccessible.92 One of the limitations of laparoscopic surgical staging for ovarian cancer is the inability to evaluate the inferior portion of the right diaphragm. The ability to perform complete laparoscopic omentectomy and pelvic lymph node dissection can be achieved without difficulty. The ability to assess and dissect the para-aortic lymph nodes adequately, particularly at the level of the renal vessels on the left side, was not shown in early reports. However, more recent experiences have shown that this area is accessible and an adequate dissection should be performed (Fig. 5). The feasibility of laparoscopic surgical staging in early ovarian cancer either during primary surgery for an adnexal mass or during a secondary surgery when incomplete staging occurred in the initial surgery is shown in three series. Querlue and LeBlanc93 describe adequate laparoscopic staging procedures on nine patients, four of whom had presumed stage I ovarian cancer. The importance of this initial work was that both pelvic and para-aortic lymph nodes were sampled and an infracolic omentectomy was performed. There were no complications and the average hospitalization was 2.8 days. Childers and coworkers92 performed laparoscopic staging on 14 patients with apparent stage I ovarian cancer, and eight (57%) of the 14 patients were upstaged: two patients to stage Ic based on peritoneal washings, three patients to stage II based on metastatic disease on the pelvic biopsies, and three patients to stage IIIc based on metastatic disease found on the para-aortic lymph nodes. There were two complications that consisted of vascular injury, including one to the vena cava, which was repaired laparoscopically without incident.91 Pomel and coworkers94 described 10 patients who underwent laparoscopic surgical staging for early ovarian cancer. They described pelvic and paracolic gutter peritoneal biopsies, pelvic and para-aortic lymph node sampling, and infracolic omentectomy as part of their laparoscopic surgical staging procedures. However, in all three reports, the authors performed an infracolic omentectomy rather than complete omentectomy, and an obvious concern is whether occult disease may remain undetected. The extent of the omentectomy needed for staging purposes is debated regardless of the surgical technique used. Nevertheless, these results are encouraging, and the authors have shown that laparoscopic surgical staging is a feasible and relatively safe procedure. Larger prospective trials are needed to evaluate the adequacy of laparoscopy compared with laparotomy for the surgical staging of early ovarian cancer. Currently, the GOG is undertaking a study (GOG 9302) evaluating laparoscopic surgical staging for patients with incompletely staged ovarian cancer.
A potential role of laparoscopic surgery in the treatment of advanced stage ovarian cancer is in second-look procedures. The value of second-look surgery as part of the standard treatment for ovarian cancer is debatable; however, laparoscopic surgery may be most applicable to this procedure when it is performed. In 1975, Rosenoff and coworkers95 initially described the use of the laparoscope as a diagnostic tool to evaluate the response of patients with ovarian cancer to chemotherapy. Numerous reports subsequently documented that laparoscopy was useful for detecting disease while avoiding the higher morbidity associated with second-look laparotomy. However, the false-negative rate of laparoscopy compared with that of conventional second-look laparotomy was high in these initial reports, ranging from 20% to 55%.96,97 More recent reports suggest that with modern endoscopic instrumentation and techniques, laparoscopy is comparable to laparotomy in detecting persistent disease. The major advantages of second-look laparoscopy are decreased blood loss, shorter hospitalization and recovery time, and decreased financial cost.98,99 The disadvantage of second-look laparoscopy is the risk of major bowel injuries, which have been reported to be between 2% and 14%.100,101 Placing the trocar in the upper left quadrant or using a 1.5-mm needle laparoscope may minimize the risk of bowel injury.102
Laparoscopy probably has little role in the cytoreductive surgery for advanced stage ovarian cancer, in which bulky tumors are often present intraperitoneally on the omentum, small and large bowel, and there is carcinomatosis on the diaphragmatic peritoneum and large bulky pelvic and para-aortic lymph nodes. It is well-documented that optimal cytoreductive surgery offers a survival advantage for the patient with advanced stage ovarian cancer, particularly if the residual tumor volume is microscopic. Extensive cytoreductive surgery may include modified posterior exteneration, large and small bowel resections, diaphragm peritoneal stripping, splenectomy, and debulking of large retroperitoneal lymph nodes, particularly the para-aortic lymph nodes. These procedures are technically challenging and time-consuming in a conventional laparotomy, and it is difficult to imagine that laparoscopic cytoreductive surgery can achieve the same results as those of laparotomy. However, some investigators recently have proposed treating advanced stage ovarian cancer with neoadjuvant chemotherapy followed by laparoscopic debulking. The rationale is that the neoadjuvant chemotherapy offers immediate relief of pain and ascites while reducing both the extent of surgery required to achieve optimal debulking and the associated morbidity. Atlas and coworkers103 reported their results on 11 patients who received neoadjuvant chemotherapy followed by laparoscopic cytoreductive surgery to treat advanced stage ovarian cancer. The 11 patients were selected based on a significant tumor response to chemotherapy. These authors were able to achieve an optimal level of residual disease (less than 2 cm). There was no significant morbidity, although one individual required a blood transfusion. This report should be viewed with caution primarily because long-term survival data are lacking and the role of neoadjuvant chemotherapy in the management of ovarian cancer is still investigational.
In summary, laparoscopy has a role in the surgical management of early and advanced ovarian cancers. The preliminary experience of pioneer laparoscopic surgeons has shown that laparoscopic surgical staging and second-look procedures are feasible and relatively safe. However, there is a tremendous learning curve in achieving the technical expertise required for these complicated laparoscopic procedures. Finally, more prospective trials are needed to answer the critical issue of survival benefit.
MANAGEMENT OF COMPLICATIONS ASSOCIATED WITH LAPAROSCOPIC SURGERY IN GYNECOLOGIC MALIGNANCY
Rare, but more serious, complications reported include vascular injury, bowel obstruction, and trocar site recurrence.104 The most common bleeding complication results usually from transecting a perforator from the anterior aspect of the vena cava. This can often be managed by applying pressure to the area with a 4  4 sponge, which can be placed easily through a 12-mm trocar. An additional suprapubic (12-mm) port should be placed just to the left of the midline as this will allow the hemaclips to be applied with ease as the caval defect can be reapproximated vertically and the clips applied along the vertical axis. If, on reinspection, there remains brisk bleeding that is not controlled easily, then a laparotomy should be performed. The incision should extend from approximately 5 to 6 cm above the umbilicus to the pubis to guarantee that access to the vena cava above and below the defect is gained. Appropriate intraoperative consultation is also advised at this point.
4 sponge, which can be placed easily through a 12-mm trocar. An additional suprapubic (12-mm) port should be placed just to the left of the midline as this will allow the hemaclips to be applied with ease as the caval defect can be reapproximated vertically and the clips applied along the vertical axis. If, on reinspection, there remains brisk bleeding that is not controlled easily, then a laparotomy should be performed. The incision should extend from approximately 5 to 6 cm above the umbilicus to the pubis to guarantee that access to the vena cava above and below the defect is gained. Appropriate intraoperative consultation is also advised at this point.
Small bowel entrapment and subfascial herniation of the small intestine have been observed, even in situations in which the fascia has been closed, resulting in bowel obstruction. Most often, this presents itself within 1 week of surgery. The most frequent site of occurrence is in the laterally placed 12-mm trocar sites. There usually is some tenderness at the site of the bowel herniation. Radiologic studies may be helpful but not diagnostic. An awareness of this complication occurring usually hastens the diagnosis. Surgical management can be undertaken laparoscopically or through a traditional laparotomy. If laparoscopic repair is to be attempted, an open laparoscopy technique is recommended. It has been our experience that the bowel usually can be retracted from the trocar site without the need for resection. Once the bowel has been retracted, it should be examined throughout its peritoneal course to ensure that no other segment is obstructed or injured. Finally, the trocar sites should be closed with care being taken to reapproximate the fascia and as well as the skin and subcutaneous tissues. We have not had a fascial herniation or obstruction at this site since using an Endoclose device (U.S. Surgical).
A rare potential complication related to the laparoscopic procedure in a patient with endometrial cancer is recurrence at a port site. There have been a total of 19 cases of metastases at the port site insertion for gynecologic malignancy, and the majority of them are secondary to advanced ovarian cancer. However, recently, Wang and coworkers104 reported one case of port site metastases occurring 6 months after the original laparoscopic surgical procedure for an advanced stage endometrial cancer. They attributed it to over-manipulation of the pathologic specimen during the laparoscopic procedure. Certainly, it is conceivable that such a complication could occur in an advanced stage endometrial cancer or where occult metastatic lymph node disease is removed through the port site; thus, a consideration can be made to remove all the lymph nodes through an endopouch to possibly minimize this risk.
REFERENCES
Jacobaeus HC: Ueber die Moeglichkeit die Zystokopie bei Untersachung seroeser Hoehlungen anzuwenden. Muench Med Wochenschr 57:2, 90, 1910 |
|
Kalk H: Erfahrungen mit der Laparoskopie. Z Klin Med 111: 303, 1929 |
|
Fourestier N, Gladu A, Vulmiere J: Perfectionnements a l'endoscopie medicale. Realization bronchoscopique. Presse Med 60:1, 292, 1952 |
|
Gunning JE: History of laparoscopy. In JM Phillips (ed): Laparoscopy, p 13. Baltimore, Williams & Wilkins, 1977 |
|
Palmer R: Technique et instrumentation de la coelioscopie gynecologique. Gynecol Obstet (Paris) 46: 420, 1947 |
|
Palmer R: LA Coelioscopie. Brux Med 28: 305, 1948 |
|
Palmer R: Essais de sterilisation tubaire celioscopique par electrocoagulation isthmique. Bull Fed Soc Gynecol Obstet Lang Fr 14: 298, 1962 |
|
Semm K, Mettler L: Technical progress in pelvic surgery via operative laparoscopy. Am J Obstet Gynecol 138: 121, 1980 |
|
Gomel V: Operative laparoscopy; time for acceptance. Fertil Steril 52: 1, 1989 |
|
Reich H, DeCaprio J, McGlynn F: Laparoscopic hysterectomy. J Gynecol Surg 5: 213, 1989 |
|
Rosenoff SH, Devita VT, Hubbard S et al: Peritoneoscopy in the staging and follow-up of ovarian cancer. Semin Oncol 2: 223, 1975 |
|
Smith WG, Day TG, Smith JP: The use of laparoscopy to determine the results of chemotherapy for ovarian cancer. J Reprod Med 18: 257, 1977 |
|
Spinelli P, Luini A, Pizetti P et al: Laparoscopy in staging and restaging of 95 patients with ovarian cancer. Tumori 62: 493, 1976 |
|
Ozol RF, Fisher RI, Anderson T et al: Peritoneoscopy in the management of ovarian cancer. Am J Obstet Gynecol 140: 611, 1981 |
|
Dargent D: A new future for Schauta's Operation through pre-surgical retroperitoneal pelviscopy? European J Gynecol Oncol 8: 292, 1987 |
|
Querlue D, LeBlanc E, Castelain B: Laparoscopic pelvic lymphadnectomy in the staging of early carcinoma of the cervix. Am J Obstet Gynecol 164: 579, 1991 |
|
Creasman WT, Morrow CP, Bundy BM et al: Surgical pathological study of endometrial cancer. A gynecologic oncology group study. Cancer 60: 2035, 1987 |
|
Roberts JA, Brunetto VL, Keys HM et al: Phase III randomized study of surgery versus surgery plus adjunctive radiation therapy in intermediate risk adenocarcinoma. Gynecol Oncol 68: 73, 1998 |
|
Homsley HD, Kadar N, Barrett RJ et al: Selective pelvic and para-aortic lymphadenectomy does not increase morbidity in the surgical stage of endometrial carcinoma. Am J Obstet Gynecol 167: 1225, 1992 |
|
Clark-Pearson DL, Cliby WA, Dodge R et al: Morbidity and mortality associated with selective pelvic and paraaortic lymph node dissection in the surgical staging of endometrial adenocarcinoma. Presented at the 24th annual meeting of SGO Feb 7–10, 1993 |
|
Spirtos NM, Schlaerth JB, Spirtos TW et al: Laparoscopic bilateral and paraaortic lymph node sampling: An evolving technique. Am J Obstet Gynecol 173: 105, 1995 |
|
Spirtos NM, Schlaerth JB, Spirtos TW et al: Cost and quality-of-life analyses of surgery for early endometrial cancer: Laparotomy versus laparoscopy. Am J Ob Gyn 171: 1795, 1996 |
|
Boike G, Lurain J, Burke J: A comparison of laparoscopic management of endometrial cancer with traditional laparotomy. Abstract presented at the 23rd annual meeting of the Western Association of Gyencologic Oncology. Gyn Oncol 38: 400, 1995 |
|
Hidelburrgh DA, Orr RK: J Reprod Med 42:482, 1997 |
|
Holub Z, Voracek J, Shomani A: A comparison of laparoscopic surgery and open procedure in endometrial cancer. Eur J Gynaecol Oncol 19: 294, 1998 |
|
Childers JM, Brezechiffa PR, Hatch KD et al: Laparoscopically assisted surgical staging of endometrial cancer. Gynecol Oncol 51: 53, 1993 |
|
Melendez TD, Childers JM, Nour M et al: Laparoscopic staging of endometrial cancer: The learning experience. JSLS 1: 49, 1997 |
|
Possover M, Krause N, Plaul K et al: Laparoscopic para-aortic and pelvic lymphadnectomy: experience with 150 patients and review of the literature. Gynecol Oncol 71: 19, 1998 |
|
Childers JM, Spirtos NM, Brainard P et al: Laparoscopic staging of the patient with incompletely staged early adenocarcinoma of the endometrium. Obstet Gynecol 83: 597, 1994 |
|
Magrina J, Mitone NF, Weaver AL et al: Laparoscopic lymph node dissection and vaginal of LAVH for endometrial cancer: Morbidity and survival. Am J Ob Gyn 181: 376, 1999 |
|
Childers JM, Hatch KD, Surqit EA: The role of laparoscopic lymphadenectomy in the management of cervical carcinoma. Gynecol Oncol 47: 38, 1992 |
|
Fowler JM, Carter JR, Carlson JW et al: Lymph node yield from laparoscopic lymphadenectomy in cervical cancer: A comparative study. Gynecol Oncol 51: 187, 1992 |
|
Schlaerth JB, Spirtos NM, Boike GM et al: Laparoscopic retroperitoneal lymphadenectomy followed by laparotomy in women with cervical cancer. Gynecol Oncol 72: 443, 1999 |
|
Canis M, Age G, Waittiez A et al: Vaginally assisted laparoscopic radical hysterectomy. J Gynecol Surg 8: 103, 1992 |
|
Nezhat CR, Burrell MO, Nezhat FR et al: Laparoscopic radical hysterectomy with paraaortic and pelvic lymph node dissection. Am J Obstet Gynecol 166: 864, 1992 |
|
Piver MS, Rutledge F, Smith JP: Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol 44: 265, 1974 |
|
Spirtos NM, Schlaerth JB, Kimball RE et al: Laparoscopic radical hysterectomy (type III) with aortic and pelvic lymphadenectomy. Am J Obstet Gynecol 174: 1763, 1996 |
|
Querlue D: Laparoscopically assisted radical vaginal hysterectomy. Gynecol Oncol 51: 248, 1993 |
|
Dargent D, Mathevet P: Hysterectomie elargie laparoscopico-vaginale. J Gynecol Obstet Biol Reprod 21: 709, 1992 |
|
Kadar N: Laparoscopic vaginal radical hysterectomy: An operative technique and its evolution. Gynecol Endosc 3: 109, 1994 |
|
Spirtos NM, Eisenkop SM, Schlaerth JB et al: Laparoscopic radical hysterectomy (Type III) with aortic and pelvic lymphadenectomy: Surgical morbidity and intermediate follow-up. Gynecol Oncol 76: 232, 2000 |
|
Lagasse LD, Creasman WT, Shingelton HM et al: Results and complications of operative staging in cervical cancer: Experience of the Gynecology Oncology Group. Gynecol Oncol 9: 90, 1980 |
|
Henriksen E: The lymphatic spread of the carcinoma of cervix and the body of the uterus: A study of 420 necropsies. Am J Obstet Gynecol 58: 924, 1949 |
|
Averette HE, Dudan RC, Ford JH: Exploratory celiotomy for surgical staging of cervical cancer. Am J Obstet Gynecol 13: 1090, 1972 |
|
LaPolla JP, Schlaerth JB, Gaddis O et al: The influence of surgical staging on the evaluation and treatment of patients with cervical carcinoma. Gynecol Oncol 24: 194, 1986 |
|
Berman M, Keys H, Creasman W et al: Survival and patterns of recurrence in cervical cancer metastatic to periaortic lymph nodes: A Gynecology Oncology Group study. Gynecol Oncol 19: 8, 1984 |
|
Berman ML, Lagasse LD, Watring WG et al: The operative evaluation of patients with cervical carcinoma by an extraperitoneal approach. Obstet Gynecol 50: 658, 1977 |
|
Ballon SC, Berman ML, Lagasse LD et al: Survival after extraperitoneal pelvic and paraaortic lymphadenectomy and radiation therapy in cervical carcinoma. Obstet Gynecol 57: 90, 1981 |
|
Berman ML, Lagasse LD, Ballon SC et al: Modification of radiation therapy following operative evaluation of patients with cervical carcinoma. Gynecol Oncol 6: 328, 1978 |
|
Plante M, Roy M: The use of laparoscopy in determining eligibility for pelvic exenteration inpatients with recurrent cervical cancer: Gynecol Oncol 59:401, 1995 |
|
Clough KB, Goffinet F, Labib A et al: Laparoscopic unilateral ovarian transposition prior to irradiation. Cancer 77: 2638, 1996 |
|
Khosla I, Lowe CR: Indices of obesity derived from body weight and height. Br J Prev Med Soc 21: 122, 1967 |
|
Wenzl R, Lehner R, Husslein P et al: Laparoscopic surgery in cases of ovarian malignancies: An Australia-wide survey. Gynecol Oncol 1996:63, 57–61 |
|
Blanc B, Boubli L, D'ercole, Nicoloso E et al: Laparoscopic management of malignant ovarian cysts: A 78 case national survey. Part 1: Pre-operative and laparoscopic evaluation. Eur J Obstet Reprod Biol 56: 177, 1994 |
|
Davis HW, Zurawski VR, Bast RC et al: Characterization of the CA-125 Antigen associated with epithelial ovarian carcinomas. Cancer Res 46: 6143, 1986 |
|
Bast RC Jr, Klug TL, St John E et al: A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 309: 883, 1983 |
|
Berek JS, Bast RC Jr: Ovarian cancer screening: The use of serial complimentary tumor markers to improve sensitivity and specificity for early detection. Cancer 76: 2092, 1995 |
|
Vasilev S, Schlaerth JB, Campeau J et al: Serum CA-125 levels in preoperative evaluation of pelvic masses. Obstet Gynecol 71: 751, 1988 |
|
Einhorn N, Bast RC, Knapp RC et al: Preoperative evaluation of serum CA-125 levels in patients with primary epithelial ovarian cancer. Obstet Gynecol 67: 414, 1986 |
|
Moley KH, Massad LS, Mutch DG: Pelvic inflammatory disease. Correlation of severity with CA-125 levels. J Reprod Med 41:341 1996 |
|
Eddy GL Sr: Pelvic hematoma following angiography: Another cause of elevated CA-125. Gynecol Oncol 65: 370, 1997 |
|
Ghamande SA, Elenou B, Hamid AM: High levels of CA-125 in a case of a parasitic leiomyoma presenting as an abdominal mass. Gynecol Oncol 61: 297, 1996 |
|
Ferrero JM, Largillier R, Ramaioli A et al: Prognostic value of early normalization of CA-125 during chemotherapy in Stage III and IV ovarian tumors. Bull Cancer 84: 722, 1997 |
|
Gadducci A, Zola P, Landoni F et al: Serum half-life of Ca-125 during early chemotherapy as an independent prognostic variable for patients with advanced epithelial ovarian cancer: Results of a multicentric Italian study. Gynecol Oncol 58: 42, 1995 |
|
Campbell S, Bhan V, Royston P et al: Transabdominal ultrasound screening for early ovarian cancer. Br Med J 299: 1363, 1989 |
|
DePriest PD, van Nagell JR, Gallion H et al: Ovarian cancer screening in asymptomatic postmenopausal women. Gynecol Oncol 51: 205, 1993 |
|
Bourne TH, Campbell S, Reynolds KM et al: Screening for early familial ovarian cancer with transvaginal ultrasonography and colour blood flow imaging. Br Med J 306: 1025, 1993 |
|
Karlan BY, Raffel LJ, Crevenlovic G et al: A multidisciplinary approach to the early detection of ovarian carcinoma: Rationale, protocol design, and early results. Am J Obstet Gynecol 169: 494, 1993 |
|
Muto MG, Cramer DW, Brown DL et al: Screening for ovarian cancer: The preliminary experience of a familial ovarian cancer. Gynecol Oncol 51: 12, 1993 |
|
Predanic M, Vlahos N, Pennisi J et al: Color and pulsed Doppler sonography, grey-scale imaging, and serum CA-125 in the assessment of adnexal disease. Obstet Gynecol 88: 283, 1996 |
|
Kohkichi H, Toshiyuki H et al: A critical evaluation of transvaginal Doppler studies, transvaginal sonography, magnetic resonance imaging, and CA-125 in detecting ovarian cancer. Obstet Gynecol 80, Dec 1992 |
|
Sassone AM, Timor-Tristh IE, Artner A et al: Transvaginal sonographic characterization of ovarian disease: Evaluation of a new score system to predict ovarian malignancy. Obstet Gynecol 78: 70, 1991 |
|
Jacobs I, Oram D, Fairbanks J et al: A risk of malignancy index incorporating CA-125, ultrasound, and menopausal status for accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol 97: 922, 1990 |
|
Roman LD, Muderspach LI, Stein SM et al: Pelvic examination, tumor marker level, and gray-scale and Doppler sonography in the prediction of pelvic cancer. Obstet Gynecol 89: 493, 1997 |
|
Teng FY, Muzsnai D, Perez R et al: A comparative study of laparoscopy and colpotomy for the removal of ovarian dermoid cysts. Obstet Gynecol 87: 1009, 1996 |
|
Webb MJ, Decker DG, Mussey E: Factors influencing survival in Stage I ovarian cancer. Am J Obstet Gynecol 116, 222:1973 |
|
Smith JP, Day TG: Review of ovarian cancer at the University of Texas Systems Cancer Center, M.D. Anderson Hospital and Tumor Institute. Am J Ob Gyn 135, 984:1979 |
|
Sevelda P, Dittrich C, Salzer H: Prognostic value of the rupture of the capsule in stage I epithelial ovarian carcinoma. Gynecol Oncol 35: 321, 1989 |
|
Dembo A, Davy M, Stenwig A et al: Prognostic factors in patients with stage I epithelial ovarian carcinoma. Obstet Gynecol 1990:75, 263–273 |
|
Sjovall K, Nilsson B, Einhorn N: Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer 4: 333, 1994 |
|
Sainz de la Cuesta R, Goff BA, Fuller AF et al: Prognostic importance of intraoperative rupture of malignant ovarian epithelial neoplasms. Ob Gyn 84: 1, 1994 |
|
Maiman M, Seltzer V, Boyce J: Laparoscopic excision of ovarian neoplasm subsequently found to be malignant. Obstet Gynecol 77: 563, 1991 |
|
Lehner R, Wenzl R, Heinzl H et al: Influence of delayed staging laparotomy after laparoscopic removal of ovarian cases later found malignant. Obstet Gynecol 92: 967, 1998 |
|
Raybuck H, Allen L, Hamus S: Absorption of serum from the peritoneal cavity. Am J Phys 199: 1021, 1960 |
|
Nasu K, Hirota Y, Kawano Y et al: Characterization of intraperitoneal rupture of epithelial ovarian cancer at early stage. Nippon Sanka Fujinaka Gakkar Zasshi 47: 907, 1995 |
|
Gleeson NC, Nicosia SV, Mark JE et al: Abdominal-wall metastases from ovarian carcinoma after laparoscopy. Am J Obstet Gynecol 69: 522, 1993 |
|
Naumann RW, Spencer S: An umbilical metastasis after laparoscopy for squamous cell carcinoma of the cervix. Gynecol Oncol 64: 507, 1997 |
|
Childers JM, Aqua KA, Surwit EA et al: Abdominal-wall tumor implantation after laparoscopy for malignant conditions. Obstet Gynecol 84: 765, 1994 |
|
Kruitwagen RFP, Swinkels BM: Incidence and effect on survival of abdominal wall metastasis at trocar or puncture sites following laparoscopy of paracentesis in women with ovarian cancer. Gynecol Oncol 60: 233, 1996 |
|
Twaalfhoven FCM, Peters AA, Trimbos JB et al: The accuracy of frozen section diagnosis of ovarian tumors. Gynecol Oncol 41: 189, 1991 |
|
Obiakor I, Maiman M, Mittal K et al: The accuracy of frozen section in diagnosis of ovarian neoplasm. Gynecol Oncol 43: 61, 1991 |
|
Childers JM, Lang J, Surwit EA et al: Laparoscopic surgical staging of ovarian cancer. Gynecol Oncol 59: 25, 1995 |
|
Querlue D, LeBlanc E: Laparoscopic infrarenal paraaortic lymph node dissection for restaging of carcinoma of the ovary of Fallopian tube. Cancer 73: 1467, 1994 |
|
Pomel C, Provencher D, Dauplat J et al: Laparoscopic staging of early ovarian cancer. Gynecol Oncol 58: 301, 1995 |
|
Rosenoff SH, Young RC, Anderson T et al: Peritoneoscopy: A valuable staging tool in ovarian carcinoma. Ann Intern Med 83: 37, 1973 |
|
Xygakis AM, Politis GS, Michalas SP et al: Second-look laparoscopy in ovarian cancer. J Reprod Med 29: 583, 1984 |
|
Ozols RF, Fisher RI, Anderson T et al: Peritoneoscopy in the management of ovarian cancer. Am J Obstet Gynecol 40: 611, 1981 |
|
Abu-Rustum N, Barakat RR, Siegel PL et al: Second-look operation for epithelial ovarian cancer: Laparoscopy or laparotomy? Obstet Gynecol 88: 549, 1996 |
|
Casey AC, Farais-Eisner R, Pisani AL et al: What is the role of reassessment laparoscopy in the management of gynecologic cancers in 1995? Gynecol Oncol 60: 454, 1996 |
|
Berek JS, Griffiths CT, Leventhal JM: Laparoscopy for second-look evaluation in ovarian cancer. Obstet Gynecol 58: 192, 1981 |
|
Berek JS, Hacker NF: Laparoscopy in the management of patients with ovarian carcinoma. Clin Obstet Gynecol Saunders, 10: 213, 1983 |
|
Childers JM, Brzechffa PR, Surwit EA: Laparoscopy using the left upper quadrant as the primary trocar site. Gynecol Oncol 50: 221, 1993 |
|
Atlas I, Childers JM, Surwit EA: Laparoscopic cytoreductive surgery after chemotherapy for advanced ovarian cancer. J Am Assoc Gynecol Laparosc 3: S2, 1996 |
|
Wang PH, Yen MS, Yuan CC et al: Port site metastasis after laparoscopic assisted vaginal hysterectomy for endometrial cancer: Possible mechanisms and prevention. Gynecol Oncol 66: 151, 1997 |