Episiotomy
Authors
INTRODUCTION
Management of the perineum is an important component of vaginal birth. In current obstetric practice, incision of the perineal body and vagina to enlarge the vaginal opening and facilitate delivery is referred to as an episiotomy.
Episiotomy was once the most frequently performed operation in obstetrics.1 In 1979, episiotomy was performed in 62.5% of all vaginal deliveries in the United States, and in nulliparous women, the episiotomy rate rose to 80%. Since that time, the routine use of episiotomy has been increasingly questioned. In 2004 the rate of episiotomy with all vaginal births was 24.5%.2 The use of episiotomy has been said to decrease trauma to the fetus, decrease the frequency of extensive perineal tears, and protect the maternal soft tissues, yet disagreement persists about its actual effectiveness.
HISTORICAL PERSPECTIVE
The origin of episiotomy is difficult to determine, but one of the first to describe it was a midwife, Sir Fielding Ould. In 1742, in his Treatise of Midwifery in Three Parts, he recommended the procedure for those cases in which the external vaginal opening is so tight that labor is dangerously prolonged.3 The first report of the procedure in the United States was 110 years later in a journal entitled The Stethoscope and Virginia Medical Gazette. Taliaferro cut a small mediolateral episiotomy to facilitate delivery in young eclamptic women.4 For these women, episiotomy was used to facilitate an unusually difficult labor. The use of episiotomy was expanded in 1921, when DeLee published a paper entitled “The Prophylactic Forceps Operation.” In this publication, he recommended the use of forceps with a mediolateral episiotomy, which he believed saved the fetal brain from injury, preserved the integrity of the pelvic floor, and restored the parturient canal to “near perfect.”5
DeLee believed labor to be disease producing and therefore to be a “decidedly pathologic process.”5 Historically, physicians have been trained to intervene in disease processes, including protecting the mother from the morbidity of the birthing process. It was on this basis that numerous modalities to support the perineum, as well as to incise it, have been described. In the 1920s, a shift to hospital deliveries occurred and with it an increase in operative procedures. A 1915 mail survey of prominent obstetricians indicated that few physicians routinely used episiotomy.6, 7 By 1938, Diethelm asserted that the indications for episiotomy were well established and needed no defense.8 This opinion supported the increasing trend of the use of episiotomy. The desire to maximize maternal comfort and safety, to improve infant outcomes, and to facilitate the delivery process came together at a time when technological intervention and hospital-based delivery were prevalent.6
Couples have now become more involved in the decision-making process surrounding the birth of their infant and have questioned the routine use of technology during labor and delivery. Along with many other “routine” practices, the use of episiotomy has become contoversial.
ANATOMY
The perineal body is one of the areas of the human body that is not well understood. In actuality, it is simply a mass of dense connective tissue. When examining the area between the anus and the vagina, the following structures can be found in the midline:
Vaginal mucosa
Dense connective tissue
External anal sphincter
Internal anal sphincter
Anal wall
The levator ani, bulbocavernosus, and transverse vaginal muscles all have attachments to or near the perineal body but do not actually cross the midline. If the perineal body is transected and not repaired, the important connections between the two sides of each of these structures are lost. The continuity of structures across the midline in the perineal body can be appreciated by feeling the ridge that is palpable just inside the hymenal ring as the perineum is distended. This probably represents the attachment of structures from both sides through their midline perineal body attachments.
The structural importance of the pelvic floor can be appreciated by looking at its position relative to the abdominal and pelvic contents. If the abdominopelvic cavity is thought of as having a barrel shape, the lid of the barrel is the respiratory diaphragm and the floor is the pelvic diaphragm. Like the respiratory diaphragm, the pelvic diaphragm is a muscle stretched across a relatively circular opening in the pelvis. It is composed of the levator ani and coccygeus muscles. It is in the shape of a fan that has its apex at the coccyx and its opposite edge attached to the pubic bones and the pelvic walls. Its muscle fibers form a series of slings that begin ventrally and loop around the back of the rectum, attaching to its wall and the wall of the vagina. The effect of their activity is to pull the rectum and perineal body toward the pubic bones and to squeeze the lumina of the pelvic viscera closed, occluding any opening in the pelvic floor.
In addition to the levator ani muscles, the perineal membrane (urogenital diaphragm) spans the pelvic outlet. Its broad sheets of connective tissue attach the perineal body to the ischiopubic rami and suspend it. The perineal membrane does not traverse the perineal body as an identifiable structure but is attached to either side of it. The ability of the urogenital diaphragm to suspend the pelvic floor is dependent on a continuity to the two sides, connected through an intact perineal body.
Two changes occur in the pelvic floor during delivery of the fetal head: distention of the vagina and introitus and descent of the perineal body. These are interrelated. Because the fetal head is relatively larger than the introitus, it pushes the pelvic floor in front of it. Once the elastic limit of the tissues is reached in descent, the introitus is dilated by the fetal head. The greater the disproportion between the head and the opening, the greater the downward force exerted on the structures that suspend the dilating introitus.
EPISIOTOMY AND SPONTANEOUS DELIVERY
The indications for episiotomy are varied and based largely on clinical opinion. The suggested maternal benefits are: (1) a reduction in the likelihood of severe perineal lacerations and obstetric anal sphincter injury; and (2) preservation of pelvic floor muscle function and a reduced risk of fecal and urinary incontinence.9 Potential benefits for the fetus include: (1) cranial protection, especially for the premature infant; and (2) less fetal acidosis. Episiotomy may also be necessary for maneuvers during shoulder dystocia and for operative vaginal delivery.10
There are conflicting data regarding the relationship between episiotomy and severe perineal tears. The controversy may be partly due to comparing unlike with unlike. For example, midline episiotomy, which is more commonly practiced in the United States, adversely affects the rate of anal sphincter damage, although mediolateral episiotomy reduces such risk.11, 12, 13 Furthermore, not all mediolateral episiotomies are actually mediolateral, and the angle of episiotomy affects the incidence of anal sphincter injury.14 A study carried out by Eskandar and Shet in 2009 reported that the use of mediolateral episiotomy protected against severe perineal trauma (OR 0.35, 95% CI 0.08–1.4).15 In comparison, Hudelist et al. reported a significant risk increase in sphincter trauma with midline episiotomy (OR 4.68, 95% CI 2.09–11.55). They also stated that midline episiotomies should be avoided in combination with vaginal operative delivery as it was likely that they would substantially increase the rate of anal sphincter injuries.16
Fritel et al. investigated pelvic floor disorders 4 years after first delivery in two groups of women who delivered in two French hospitals – one with a restrictive episiotomy policy and one where episiotomy was routinely used. They found that there was no difference in the prevalence of urinary incontinence, perineal pain or pain during intercourse between women in the restrictive and routine groups. Anal incontinence was less prevalent in the restrictive group. The difference was significant for flatus but not for fecal incontinence.17 Carroli et al., in a systematic review using severe vaginal and perineal trauma as a primary outcome, found no evidence to support a policy of routine episiotomy.18 The authors stated that there was a need to properly evaluate whether midline or mediolateral technique provides the best outcome.
Pomeroy in 1918 advocated episiotomy as a tool to shorten the second stage of labor.19 Indications for a shortened second stage include significant maternal cardiac disease or any other medical reason to minimize intra-abdominal pressure changes, a prolonged second stage of labor, fetal distress, and breech presentation of a fetus.
Episiotomy has been especially advocated in the delivery of premature infants. Lobb investigated the use of episiotomy in very low birth weight infants and found that when babies of similar weight and age were considered, the routine use of episiotomy appeared to hold no advantage.20 Shortening the second stage of labor rather than opening the birth canal is perhaps the more critical contribution of episiotomy in the delivery of premature infants.
When an infant is in distress or potentially in distress during the later portion of the second stage of labor, episiotomy could be expected to minimize the length of time it is compromised. This beneficial effect may be lost in studies that investigate large populations of normal individuals, in whom the length of the second stage makes little difference. Janni et al. evaluated the effects of the duration of labor on fetal distress and maternal perinatal morbidity. A prolonged duration of the second stage was not associated with low Apgar scores 5 and 10 min postpartum (p = 0.76 and p = 0.38, respectively), a higher incidence of umbilical artery pH levels of <7.20 (p = 0.60), nor with an increased rate of admission to the neonatal intensive unit (p = 0.24).21 Saunders et al. carried out a retrospective analysis to investigate the relation between the duration of the second stage of labor and subsequent early neonatal and maternal morbidity. They could show no such relation between time spent in the second stage of labor and the frequency of low Apgar scores or the rate of admission to special care baby unit.22
Although increasing perineal space would seem beneficial for prevention and management of shoulder dystocia, there are no data to support this notion. Nocon et al. reported that episiotomy did not improve the outcome with respect to neonatal injury.23 In addition, Gurewitsch et al. suggested that the addition of episiotomy to fetal manipulation in severe shoulder dystocia conferred no benefit in averting neonatal brachial plexus injury.24 However, episiotomy is still advocated in practice guidelines merely to facilitate additional maneuvers.25
EPISIOTOMY AND OPERATIVE DELIVERY
A restrictive approach to episiotomy at the time of operative vaginal delivery has become more common over recent years. There is currently, however, no conclusive evidence to support this approach. A survey of obstetricians in the United Kingdom and Ireland, where mediolateral episiotomy is preferred, reported restrictive use of episiotomy with both non-rotational (86%) and rotational vacuum (72%) delivery. In contrast, the majority of respondents reported a routine approach for non-rotational mid-cavity forceps (73%) and for rotational forceps (80%).26 The authors commented that the wide variation in the approach to episiotomy at the time of operative vaginal delivery and the lack of evidence for its role supported the need for further research. A study carried out in the United States by Robinson et al. reported that, in forceps delivery, the use of episiotomy was not associated with a difference in the rate of significant perineal trauma (RR 1.2, 95% CI 0.8–1.9). With vacuum delivery, an increased rate of significant trauma was seen when episiotomy was used (RR 3.7, 95% CI 1.2–11.2). However, in this study, midline episiotomy was used and rates of anal sphincter damage would be expected to be higher with this approach.27 Several other studies, both prospective and retrospective, have subsequently produced conflicting results regarding the benefit of episiotomy at the time of operative vaginal delivery with regard to reducing the risk of anal sphincter injury.13, 28, 29 A randomized controlled trial of routine versus restrictive use of episiotomy at the time of operative delivery was undertaken by Macleod and Murphy using perineal trauma involving the anal sphincter as the primary outcome measure. Routine use of episiotomy was not associated with a statistically significant difference in anal sphincter tears when compared with restrictive use (OR 0.72, 95% CI 0.28–1.87). Routine use was associated with a slightly higher incidence of postpartum hemorrhage but this was not statistically significant. There was no reduction in adverse neonatal outcomes with routine use of episiotomy. The authors concluded that a definitive randomized controlled trial with a large sample size would be necessary to conclusively inform clinical practice and that obstetricians should continue to use their clinical judgment when undertaking operative episiotomy in the context of vaginal delivery.26
CONTRAINDICATIONS
Contraindications to episiotomy are few. Relative contraindications include abnormalities of the perineum. Inflammatory bowel disease, lymphogranuloma venereum, severe perineal scarring, and perineal malformation are some to consider. Coagulation disorders have been suggested as a contraindication, but an episiotomy would be preferable to a cesarean section or a complex laceration if a shortened second stage of labor is all that is needed. If there is any doubt that vaginal delivery can be achieved, as in a trial of instrumental delivery, episiotomy should be delayed until the operator is confident that delivery is imminent. The most important contraindication to episiotomy would be a patient's refusal for the procedure to be performed.30
TIMING
When in the delivery process an episiotomy should be performed is controversial.31 DeLee, in hope of preventing subsequent pelvic relaxation, recommends midline episiotomy when the fetal head begins to part the levator ani pillars and just begins to stretch the fascia between them.32 The area of the fetal scalp visible at the introitus at this time is about 4 cm. To minimize soft tissue damage, the incision must be made before the supporting structures have been damaged to the extent that they cannot recover.33 Gainey believed that the absence of visible tears did not guarantee that the pelvic floor was left undamaged, again emphasizing early episiotomy.34
When an episiotomy is done to prevent lacerations, it is best performed when the fetus is expected to deliver within the next three or four contractions. An episiotomy performed close to the time of delivery will prevent excessive blood loss. Episiotomy, whether midline or mediolateral, is associated with increased maternal blood loss at the time of delivery.35
TECHNIQUES
Midline episiotomy
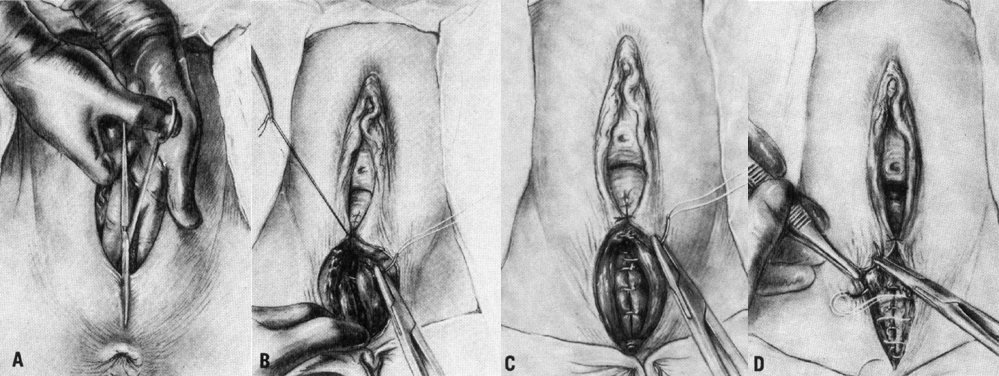
The midline episiotomy incision is made in the perineal body from the midline of the hymenal ring through the connective tissue that unites the bulbocavernous muscle, the superficial transverse perineal muscles, and the perineal membrane (urogenital diaphragm).33 The incision is made down to but not including the anal sphincter (Fig. 1A). The vagina should be incised 3–4 cm above the hymenal ring, with the incision entering the rectovaginal space. The risk of vaginal lacerations is thus avoided. Scissors or a scalpel is used with care to avoid injury to the fetus.
The depth of the incision is limited to the distance between the vagina and the anal sphincter and thus poses restrictions on the amount of enlargement of the birth canal. If more room is needed, extension into the rectum either spontaneously or intentionally is inevitable. Intentional extension of the incision to involve the anorectal area is referred to as an episioproctotomy. It is not surprising that a midline episiotomy is not elected when the perineal body is short or when the infant is thought to be very large. Opinion varies about whether a mediolateral incision is preferable to episioproctotomy. Mediolateral episiotomy, because it can be extended to incise the levator ani (which episioproctotomy does not), provides more room for delivering impacted shoulders or for managing breech delivery.
Mediolateral episiotomy
The type of episiotomy used depends on the judgment of the physician and often the region of practice. Midline incisions are believed to be less painful than mediolateral, but when properly repaired there is little difference. Mediolateral incisions are only rarely extended into the rectum and anal sphincter, and are often used when more room is required for the delivery process.
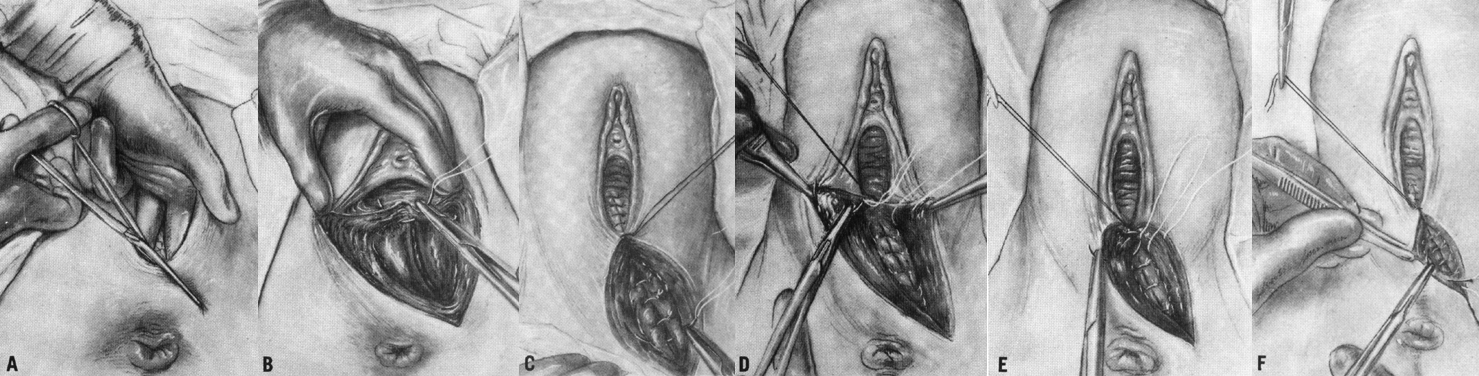
Mediolateral episiotomy extends from the posterior fourchette mediolaterally towards the patient's ischial tuberosity (Fig. 2A). In Europe and the United Kingdom, right mediolateral episiotomy is preferred. The same structures are separated as with the midline incision, and the ischiorectal fossa is exposed. In addition, when extra room is needed for a difficult delivery, a mediolateral incision has the advantage that it can be extended through the levator ani muscles, expanding the outlet. This additional room is not available with a midline incision, which when extended cannot alleviate resistance from these muscles and their fascia.
It is important to begin the incision at the midline. If the episiotomy is begun in a lateral position on the vaginal outlet, the Bartholin's duct may be incised; at least theoretically, this error could lead to subsequent cyst formations.30 Quilligan and Zuspan advise that the mediolateral episiotomy be performed as a two-step procedure. The first incision is made in the soft tissues of the fourchette and the vagina, followed by incision of the perineum, extending in the mediolateral direction. They believe that this allows for an optimal anatomical approximation at the time of repair.36 Following a case–control study investigating the relationship between the angle of mediolateral episiotomy and the incidence of anal sphincter injury, Eogan et al. recommended that the angle of the episiotomy should be as large as possible in order to reduce the incidence of anal sphincter injury.37
The question of which type of episiotomy is more beneficial remains unanswered. A recent systematic review concluded that all the published trials comparing midline with mediolateral episiotomy were of poor methodological quality and therefore uninterpretable.38
REPAIR
Repair of the median incision is often deferred until the placenta has delivered and inspection of the cervix and vaginal canal has been performed. Such delay provides adequate exposure for repair of vaginal and cervical lacerations, if present, and manual removal of the placenta, if necessary. Midline episiotomies may bleed briskly at the time of the incision, but after delivery of the fetus there is remarkably less bleeding. This has been believed to be due to a change in the venous congestion of the perineal tissues at the time of delivery.30
Before repairing an episiotomy the vagina and perineum should be examined systematically for any extension of the episiotomy and for any separate lacerations. A per rectum examination should be carried out to exclude anal sphincter injury. Effective episiotomy repair requires knowledge of perineal anatomy and surgical technique. Effective analgesia, adequate lighting, visualization, and assistance are all prerequisites. In recent years, the techniques and material used in perineal repair have been investigated. Whatever the method of repair, the following principles seem evident. Take care to explore the entire extent of the episiotomy to avoid fistulas that can be created by an incomplete repair. Avoid causing ischemia and trauma to the tissues and restore to the midline all of the tissues that have been separated. The perineum should be reconstructed in layers after adequate anesthesia has been obtained. The vaginal wall is closed first with a continuous suture that starts 1 cm above the apex of the incision, including any retracted blood vessels, which may otherwise result in hematoma formation. The closure continues to the hymenal ring. Each bite should include the rectovaginal fascia so that posterior vaginal support is maintained.33 The operator should avoid placing sutures in the mucocutaneous portion of the fourchette in order to avoid postpartum dyspareunia. The perineal body may be repaired with either interrupted or continuous sutures. Continuous attention to anatomical approximation is important. In the case of mediolateral episiotomy, more tissue may appear to be present laterally than medially. A continuous subcuticular stitch is used for skin closure. In some cases, the skin edges may be well approximated after repair of the deep tissues and may not need to be closed separately. This technique is associated with less perineal pain postpartum.39
When the repair is complete, the vagina and perineum should be examined systematically to ensure that the repair is complete, that no suture material extends through the rectal mucosa and that there are no swabs or tampons left in the vagina. It is also important to repeat the rectal examination to exclude an anal sphincter injury that may not have been evident initially.
A randomized controlled trial comparing continuous with interrupted perineal repair with standard or rapidly absorbed sutures carried out in 2002 found that the continuous technique of repair was associated with less pain at 10 days than the interrupted method. This difference persisted up to 12 months after delivery. The authors also reported less pain on walking at 10 days in the group where rapidly absorbed polyglactin 910 suture material was used compared with standard polyglactin 910.40 Similarly, a systematic review of continuous versus interrupted sutures for repair of episiotomy or second degree tears carried out in 2007 showed that continuous suture techniques compared with interrupted sutures for perineal closure (all layers or perineal skin only) are associated with less pain for up to 10 days postpartum (RR 0.70, 95% CI 0.64–0.76). Subgroup analysis showed that there is a greater reduction in pain when continuous suturing techniques are used for all layers (RR 0.65, 95% CI 0.60–0.71). There was an overall reduction in analgesia use associated with the continuous subcutaneous technique versus interrupted stitches for repair of perineal skin (RR 0.70, 95% CI 0.58–0.84).41 The most recent systematic review of absorbable materials for perineal repair, carried out in 2009, again provided significant evidence that synthetic absorbable suture material such as polyglactin 910 and polyglycolic acid is associated with less short-term pain, a reduction in the use of analgesia, and less wound dehiscence, but with the need for more suture removal than catgut.42
A small number of studies have evaluated skin adhesive for perineal skin repair after episiotomy. Bowen et al. compared enbucrilate tissue adhesive with subcuticular suturing with polyglycolic acid and reported that using enbucrilate tissue adhesive for skin closure resulted in less pain on micturition, walking, and defecation when compared with subcuticular Dexon but there was no significant difference when lying or sitting. The time taken for the wound and for sexual intercourse to become pain free was significantly less in the enbucrilate group.43 Mota et al., however, reported no significant differences in short-term (7 days) and long-term (30 days) perineal pain or return of sexual activity between women who had skin adhesive and women who had subcuticular suturing with rapidly absorbable Polyglactin 910. They also noted that although total duration of wound repair is less when skin adhesive is used, these devices are more expensive than suture material.44
CARE OF EPISIOTOMY
Daily attention should be directed to the episiotomy. Discomfort should progressively abate. Any evidence of infection is then promptly acted on to avoid such serious complications as necrotizing fasciitis. An episiotomy is a wound, and its care parallels that of any other wound. The perineum needs to be kept clean and dry. Unlike most wounds, cleanliness is made difficult by defecation, micturition, and lochia. A squeeze bottle of water to irrigate the perineum may be helpful for maintaining cleanliness as well as for providing comfort.
Several studies have investigated the effect of cooling treatments such as ice packs, cold gel pads, and iced baths. A systematic review was carried out to evaluate the effectiveness of such treatment compared with no treatment and with non-cooling treatments such as witch hazel, pulsed electromagnetic energy (PET), and hydrocortisone/pramoxine foam. Although one randomized controlled trial reported improvement in perineal pain 24–72 hours after birth with ice-packs compared with no treatment, this did not achieve statistical significance. The authors of this review found no conclusive evidence to support the use of cooling treatments and recommended further evaluation.45 Simple cooling treatments such as ice-packs and gel pads, however, are inexpensive and readily available and may increase women's satisfaction with their general perineal care.
Many patients with perineal incisions or lacerations require analgesics for several days after delivery. The requirements for a good postpartum analgesic are that it be rapid acting and highly effective. It should also allow new mothers to be free of pain but alert and should be safe for patients who are still experiencing pain but are ready to be discharged.46 When perineal pain is mild, paracetamol is the most common analgesic used.47 Chou et al. carried out a systematic review on the efficacy of paracetamol as a single dose in the early postpartum period and concluded that women who received paracetamol at a dose of either 500–650 mg or 1000 mg had less pain at 4 hours than those who received placebo. The higher (1000 mg) dose appeared to be slightly more effective.48 Non-steroidal anti-inflammatory agents may also be used and may have a role in reducing swelling as well as their analgesic effect. A randomized controlled trial carried out by Dodd et al. reported that diclofenac suppositories were effective in reducing perineal pain 24 hours after birth for women while walking, sitting, and on opening their bowels when compared with placebo. This effect was not sustained 48 hours after birth.49 This was similar to the findings of a systematic review which stated that rectal analgesia appears effective for reducing moderate pain from perineal trauma in the short term (within 24 hours). This review also reported less analgesia use compared with placebo up to 48 hours after birth. No side-effects with the use of rectal analgesia were reported in any of the studies analysed.50
COMPLICATIONS
As with any surgical procedure, episiotomy is not without risk. Extension of an episiotomy to involve deeper structures, excessive blood loss, and infection are some of the immediate complications of episiotomy. Dehiscence of the wound and dyspareunia may occur after discharge from the hospital. Complications with episiotomy in association with operative vaginal delivery were investigated by Macleod and Murphy28. They concluded that the use of episiotomy at the time of operative vaginal delivery was associated with an increased risk of postpartum hemorrhage, perineal infection, and a greater use of analgesia on day 10 after birth compared with women who did not have an episiotomy.
Extension of the episiotomy involving the anal sphincter or rectum has been discussed. It has been reported to be increased with midline episiotomy. Rarely the rectum may be unexpectedly incised or perforated when an episiotomy is performed or during the repair. Special attention to the rectal mucosa after the repair is critical in detection of this complication. If suture material is palpated, the sutures should be removed to prevent rectovaginal fistula formation.
Excess blood loss can occur with episiotomy either at the time of the episiotomy or with hematoma formation after the repair. Thacker and Banta51 estimate that 10% of women who undergo an episiotomy lose at least 300 ml more blood than if they did not have the procedure. Such claims have not been investigated, but the blood loss does not justify blood banking services on a routine basis. Ozdegermenci et al. carried out a randomized trial to investigate the effect of the timing of repair of a mediolateral episiotomy on the amount of blood lost. Women who underwent repair of their episiotomy prior to delivery of the placenta compared with after had slightly less blood loss, although there was no effect of hemoglobin concentration or hematocrit.52 Postpartum hemorrhage should raise the question of incomplete episiotomy repair and should be thoroughly investigated.30 Increasing perineal pain and a decreasing hematocrit are signs of possible hematoma formation. The episiotomy should be opened, the clots evacuated, and bleeding points ligated. If the bleeding source cannot be identified, a drain can be placed to ensure drainage and a pack placed.
Infection rates in episiotomy wounds are surprisingly low. Necrotizing fascitis is a rare but potentially fatal complication of episiotomy. Shy and Eschenbach reported three deaths from 1969 to 1977 from this cause.53 More recently, a case of methicillin-resistant Staphylococcus aureus necrotizing pneumonia arising from an episiotomy site was reported.54
If healing is delayed, either from infection, hematoma, or perhaps use of steroids or anticoagulants, breakdown may occur. Dehiscence of an episiotomy occurs less than 2% of the time. The area becomes subjectively more painful and appears red and swollen. Patients may be febrile and may have oozing from the site. Treatment involves debridement of any necrotic tissue and broad-spectrum antibiotics. These wounds are usually allowed to heal by secondary intention, although there are some reports of good results after early repair of dehiscence.55
Dyspareunia is another potential complication of episiotomy but has not often been discussed in the literature. Scarring of the perineum can also be a reason for long-term dyspareunia. The mediolateral episiotomy has been cited more often as a cause, but both approaches can cause discomfort. Robson and Kumar noted soreness and dyspareunia at the episiotomy site in British women; the incidence at 3, 6, and 12 months was 40%, 18%, and 8%, respectively.56 Ejegard et al. also found that episiotomy during a first birth was a risk factor for dyspareunia 12–18 months postpartum.57 A relatively rare complication of endometriosis in an episiotomy scar has been reported.58 A tender nodule producing cyclic symptoms at the site of an episiotomy is highly suggestive of this phenomenon. Malignant change of endometriosis in episiotomy scars has also been reported.59
CONCLUSION
ACKNOWLEDGMENTS
We wish to acknowledge the work of Karen D. Bartscht, MD, MPH and John O. DeLancy, MD, authors of the previous version of this chapter.
REFERENCES
Pritchard JA, M. P., Gant NF (1985). William's Obstetrics. Norwalk, Appleton-Century-Crofts. 17. |
|
Frankman EA, W. L., Bunker CH, Lowder JL (2009). "Episiotomy in the United States: has anything changed?" Am J Obstet Gynecol 200(5). |
|
Ould F: A Treatise of Midwifery. Dublin, Nelson and Connor 1742. |
|
Taliaferro RM: Rigidity of soft parts: Delivery effected by incision in the perineum. Stethoscope Va Med Gazette 2: 383, 1852 |
|
DeLee JB: The prophylactic forceps operation. Am J Obstet Gynecol 1: 34, 1921 |
|
Thacker SB, Banta HA: Benefits and risks of episiotomy: An interpretative review of the English language literature, 1860–1980. Obstet Gynecol Surv 38: 322, 1979 |
|
Williams JT: Episiotomy. Boston Med Surg J 173: 946, 1915 |
|
Diethelm MW: Episiotomy: Technique of repair. Ohio Med 34: 1107, 1938 |
|
Carrolli G, M. L. (2009). "Episiotomy for Vaginal Birth." The Cochrane Collaboration(1): 1 - 50. |
|
ACOG 2006 |
|
Coats, P. M., K. K. Chan, et al.. (1980). "A comparison between midline and mediolateral episiotomies." Br J Obstet Gynaecol 87(5): 408-412 |
|
Signorello, L. B., B. L. Harlow, et al.. (2000). "Midline episiotomy and anal incontinence: retrospective cohort study." BMJ 320(7227): 86-90. |
|
de Leeuw J, d. W. C., Bruinse H, Kuiken J (2008). "Mediolateral episiotomy reduces the risk for anal sphincter injury during operative vaginal delivery." BJOG 115: 104-108. |
|
Eogan M, D. L., O'Connell PR, O'Herlihy C (2006). "Does the angle of episiotomy affect the incidence of anal sphincter injury?" BJOG 113: 190-194. |
|
Eskandar, O. and D. Shet (2009). "Risk factors for 3rd and 4th degree perineal tear." J Obstet Gynaecol 29(2): 119-122. |
|
Hudelist G, M. H., Gorti M (2008). "The role of episiotomy in instrumental delivery: is it preventative for severe perineal injury?" J Obstet Gynaecol 25(5): 469 - 473 |
|
Fritel X, S. J., Fauconnier A, Bertrand V, Levet C,and Pigné A (2008). "Pelvic floor disorders 4 years after first delivery: a comparative study of restrictive versus systematic episiotomy." BJOG 115(2): 247 - 252. |
|
Carrolli G, M. L. (2009). "Episiotomy for Vaginal Birth." The Cochrane Collaboration(1): 1 - 50. |
|
Pomeroy, R. (1918). "Shall we cut and reconstruct the perineum for every primipara?" Am J Obstet Dis Women Child 78(211 |
|
Lobb MO, D. S., Cooke RWI (1986). "The influence of episiotomy on the neonatal survival and incidence of periventricular haemorrhage in very-low-birth-weight infants." Eur J Obstet Gynecol Reprod Biol 22. |
|
Janni W, S. B., Peschers U, Huber S, Strobl B, Hantschmann P, Uhlmann N, Dimpfl T, Rammel G, Kainer F. (2002). "The prognostic impact of a prolonged second stage of labor on maternal and fetal outcome." Acta Obstet Gynaecol Scand March (81)(3): 214-221. |
|
Saunders NS, P. C., Wadsworth J. (1992). "Neonatal and maternal morbidity in relation to the length of the second stage of labour." Br J Obstet Gynaecol May 99(5): 381 - 385. |
|
Nocon JJ, M. D., Thomas LJ, Hansell RS (1993). "Shoulder dystocia: an analysis of risks and obstetric maneuvers." Am J Obstet Gynecol June 168(6 (pt 1)): 1732 - 1737. |
|
Gurewitsch ED, D. M., Stallings SP, Moore PL, Agarwal S, Allen LM, Allen RH. (2004). "Episiotomy versus fetal manipulation in managing severe shoulder dystocia: a comparison of outcomes." Am J Obstet Gynecol Sept 191(3): 911-916. |
|
RCOG (2005). Shoulder Dystocia. London, Royal College of Obstetricians and Gynaecolgists. Guideline No 42. |
|
Macleod, M. and D. J. Murphy (2008). "Operative vaginal delivery and the use of episiotomy--a survey of practice in the United Kingdom and Ireland." Eur J Obstet Gynecol Reprod Biol 136(2): 178-183. |
|
Robinson JN, N. E., Cohen AP, McElrath TF, Lieberman ES. (1999). "Episiotomy, operative vaginal delivery, and significant perineal trauma in nulliparous women." Am J Obstet Gynecol 181: 1180-1184. |
|
Macleod, M., B. Strachan, et al.. (2008). "A prospective cohort study of maternal and neonatal morbidity in relation to use of episiotomy at operative vaginal delivery." BJOG 115(13): 1688-1694. |
|
Yousef R, R. U., Macleod M, Murphy DJ (2005). "Cohort study of maternal and neonatal morbidity in relation to the use of episiotomy at instrumental vaginal delivery." BJOG 112: 941-945. |
|
Varner, M. (1986). "Episiotomy: Techniques and Indications." Clin Obstet Gynecol 29. |
|
Pomeroy, R. (1918). "Shall we cut and reconstruct the perineum for every primipara?" Am J Obstet Dis Women Child 78(211). |
|
DeLee, J. (1920). "The prophylactic forceps operation." Am J Obstet Gynecol 1: 34-44. |
|
Wilson, J. (1987). "Prophylactic episiotomy to minimize soft tissue damage." Infect Surg 7. |
|
Gainey, H. (1943). "Postpartum observation of pelvic tissue damage:Further studies." Am J Obstet Gynecol 70. |
|
Combs CA, M. E., Laros RK Jr. (1991). "Factors associated with postpartum haemorrhage with vaginal birth." Obstet Gynecol 77: 69 - 76. |
|
Quilligan EJ, Z. F. (1988). Douglass-Stromme Operative Obstetrics. Norwalk, Appleton-Century-Crofts. |
|
Eogan M, D. L., O'Connell PR, O'Herlihy C (2006). "Does the angle of episiotomy affect the incidence of anal sphincter injury?" BJOG 113: 190-194. |
|
Carrolli G, M. L. (2009). "Episiotomy for Vaginal Birth." The Cochrane Collaboration(1): 1 - 50. |
|
Kettle C, J. R. (2003). "Continuous versus interrupted sutures for perineal repair. ." Cochrane Database Syst Rev. 1. |
|
Kettle, C., R. K. Hills, et al.. (2002). "Continuous versus interrupted perineal repair with standard or rapidly absorbed sutures after spontaneous vaginal birth: a randomised controlled trial." Lancet 359(9325): 2217-2223. |
|
Kettle, C., R. K. Hills, et al.. (2007). "Continuous versus interrupted sutures for repair of episiotomy or second degree tears." Cochrane Database Syst Rev(4): CD000947. |
|
Kettle, C., T. Dowswell, et al.. (2010). "Absorbable suture materials for primary repair of episiotomy and second degree tears." Cochrane Database Syst Rev 6: CD000006. |
|
Bowen, M. L. and M. Selinger (2002). "Episiotomy closure comparing enbucrilate tissue adhesive with conventional sutures." Int J Gynaecol Obstet 78(3): 201-205. |
|
Mota R, C. F., Amaral A, Oliveira F, Costa Santos C, Ayres-De-Campos D (2009). "Skin adhesive versus subcuticular suture for perineal skin repair after episiotomy - a randomised controlled trial." Acta Obstet Gynaecol Scand 88: 660-666. |
|
East CE, B. L., Henshaw NE, Marchant P, Wallace K (2009). "Local cooling for relieving pain from perineal trauma sustained during childbirth (Review)." The Cochrane Collaboration. |
|
Harrison RF, B. M., Reed JV (1987). "A review of postepisiotomy pain and its treatment." Curr Med Res Opin 10 |
|
Sleep J, G. A., Ed. (1989). Relief of perineal pain and discomfort after childbirth. Effective Care in Pregnancy and Childbirth. Oxford, Oxford University Press. |
|
Chou D, a. E., Gyte GML, Gulmezoglu AM (2010). "Paracetamol/acetominophen (single administration) for perineal pain in the early postpartum period (Review)." The Cochrane Collaboration(3). |
|
Dodd, J. M., H. Hedayati, et al.. (2004). "Rectal analgesia for the relief of perineal pain after childbirth: a randomised controlled trial of diclofenac suppositories." BJOG 111(10): 1059-1064. |
|
Hedayati H, P. J., Crowther CA (2009). "Rectal analgesia for pain from perineal trauma following childbirth (Review)." The Cochrane Collaboration(1). |
|
Thacker SB, B. H. (1979). "Benefits and risks of episiotomy: An interpretative review of the English language literature." Obstet Gynecol Surv 38. |
|
Ozdegirmenci, O., S. Erkaya, et al.. (2010). "Does early repair of episiotomy decrease postpartum blood loss: a randomized clinical trial." J Matern Fetal Neonatal Med 23(4): 308-310. |
|
Shy KK, E. D. (1979). "Fatal perineal cellulitis from an episiotomy site." Obstet Gynecol 54(3): 292-298. |
|
Rotas M, M. S., Liu C, Minkhoff H (2007). "Fatal perineal cellulitis from an episiotomy site." Obstet Gynecol 109(2 pt 2): 533-536. |
|
Uygur D, Y. N., Kis S, Sipahi T (2004). "Early repair of episiotomy dehiscence." Aust N Z J Obstet Gynaecol 44(3): 244-246 |
|
Robson KM, K. R. (1981). "Maternal sexuality." BJOG 88. |
|
Ejegard, H., E. L. Ryding, et al.. (2008). "Sexuality after delivery with episiotomy: a long-term follow-up." Gynecol Obstet Invest 66(1): 1-7. |
|
Hambrick E, A. H., Smith D (1979). "Perineal endometrioma in episiotomy incisions: Clinical features and management." Dis Colon Rectum 22. |
|
Chene, G., C. Darcha, et al.. (2007). "Malignant degeneration of perineal endometriosis in episiotomy scar, case report and review of the literature." Int J Gynecol Cancer 17(3): 709-714. |