Vaginal Cancer
Authors
INTRODUCTION
Primary cancers of the vagina are rare. The estimated number of new cases in the United States in 2008 was 2210, with 790 estimated deaths (3% of all gynecologic malignancies).1 Most vaginal cancers are of squamous cell origin (approximately 93%); adenocarcinomas account for less than 5%.2, 3 Vaginal carcinoma comprises a heterogeneous group of tumors: endodermal sinus tumor4 and embryonal rhabdomyosarcoma (sarcoma botryoides) present in childhood;5 clear-cell adenocarcinoma typically presents in adolescence;6, 7, 8 and squamous cell carcinoma, adenocarcinoma, melanoma, and sarcoma predominate in adult life.2, 9, 10 Most malignancies found in the vagina, however, are metastatic. They may be the first manifestation of an occult neoplasm, commonly arising from an endometrial, cervical, or vulvar carcinoma. Up to 14% of endometrial carcinomas metastasize to the vagina, presumably by retrograde spread through submucosal lymphatics. Infrequently, vaginal metastases originate from malignancies of the ovary, rectum, kidney, or choriocarcinoma.2, 3, 11
By definition, primary vaginal neoplasms arise in the vagina and do not involve the external os of the cervix superiorly or the vulva inferiorly (the former tumors are classified as cervical and the latter as vulvar cancers).10 Growth limited to the urethra should be classified as a urethral primary. Vaginal cancers are staged according to criteria set forth by the International Federation of Gynecology and Obstetrics (FIGO)12, 13 (Table 1).
Table 1. FIGO staging system for vaginal carcinoma: 2001
| FIGO stage | Disease extent |
| O | Carcinoma in situ |
| I | Carcinoma limited to vaginal wall |
| II | Carcinoma extending into subvaginal tissues, not extending to pelvic wall |
| III | Extension to pelvic wall |
| IV | Extension beyond the true pelvis or involvement of the mucosa of bladder or rectum. Bullous edema alone does not allow a case to be allocated as stage IV |
| IVa | Invasion of the bladder or rectal mucosa, or extension beyond the true pelvis |
| IVb | Distant spread |
(Source: Beller U, Sideri M, Maisonneuve P et al. Carcinoma of the vagina, J Epidemiol Biostat 2001;6:141)
VAGINAL ANATOMY, EMBRYOLOGY, AND ROUTE OF DISSEMINATION
The vagina is a fibromuscular tube that forms the distal portion of the female reproductive tract, extending from the uterus to the vulva. The vagina averages 7 cm in length and is located posterior to the base of the bladder and urethra and anterior to the rectum. The lower three-quarters of the posterior wall are in contact with the rectum, whereas the upper one-quarter is separated from the rectosigmoid by the pouch of Douglas. At the superior end of the vagina, the cervix projects through the vaginal wall, creating a circular sulcus that entirely surrounds the cervix, termed the vaginal fornices.14
The vaginal wall consists of three layers: mucosa, muscularis, and adventitia. Grossly, the mucosal membrane has numerous infoldings, or rugae, separated by transverse folds. This mucosal surface is lined by stratified, nonkeratinizing squamous epithelium. The vaginal mucosa does not contain glands and is lubricated by transudation across the mucosa and by cervical secretions. Epithelial changes are minimal in response to the reproductive cycle. The muscularis layer is composed of smooth muscle fibers arranged circularly on the inner portion and longitudinally on the thicker outer portion. This muscular layer encircles a highly vascular submucosa that contains a venous plexus and loose connective tissue. A vaginal sphincter is formed by skeletal muscle at the introitus. The adventitia is a thin, outer connective tissue layer abundantly supplied with elastic fibers and further venous and lymphatic plexuses that merges with the outer connective tissue layer of adjacent organs.
The vagina is supported by external fibrous and muscular tissue, the central parts of which coalesce to blend with the pelvic floor musculature. The most important of these vaginal supports are the cardinal ligaments cranially and the perineal body caudally. The paired vaginal arteries arise from the adjacent internal iliac arteries or uterine vessels. The vaginal arteries lie lateral to the vagina and anastomose with the uterine, inferior vesical, and middle rectal arteries. The longitudinal anterior and posterior azygos arteries develop from the lateral and anastomotic branches that join in the midline. Branches of the internal pudendal arteries supply the distal vagina. The vaginal venous plexus communicates with the vesical, hemorrhoidal, and uterine plexuses and eventually drains into the hypogastric vein.14
Vaginal lymphatics form a meshed net in the mucosal membrane and anastomose with lymphatic vessels in the muscularis.14 The lymphatics of the upper vagina communicate with those of the cervix and drain into the pelvic nodes. The posterior vaginal wall is defined by lymphatics that anastomose with the rectal lymphatic system. Lymph from the posterior vagina is channeled to the deep pelvic nodes, such as the inferior gluteal, sacral, and rectal nodes. The anterior wall is drained by lymphatics that flow to the nodes of the lateral pelvic walls. The lymphatics of the distal third of the vagina drain to inguinal and pelvic nodes.
Vaginal carcinoma spreads primarily by local tissue invasion and lymphatic permeation with embolization, similar to cervical and vulvar malignancies, although hematogenous spread can occur. The vagina has a fine network of capillaries throughout the mucosa and the muscularis that anastomose freely and join laterally into major drainage channels. The lymphatic system of the vagina has many variations, which may account for some treatment failures.14 Once the preferred routes of drainage are obstructed by tumor, the very extensive anastomotic connections may account for the often bizarre and widespread nature of metastases. The tumor may locally infiltrate the paracolpium, parametrium, bladder, and rectovaginal septum. The proximity of the cervix and vulva make it inevitable that a significant number of vaginal cancers become classified as cervical or vulvar primaries.
The developmental origin of the vagina remains controversial. Three origins of the vagina have been proposed:
- Epithelium derived from the müllerian ducts;
- Epithelium derived from müllerian and wolffian ducts; and
- Urogenital sinus derivation.6, 7, 8
Vaginal embryogenesis is a complex process, because three epithelial layers interact over a limited area.
The occurrence of lower genital tract anomalies and clear-cell carcinomas in women exposed to diethylstilbestrol (DES) in utero stimulated further interest in determining the developmental origin of the vagina.15 At 4 weeks of development, the müllerian (paramesonephric) duct system begins as an invagination of the coelomic epithelium in the urogenital fold just lateral to the cranial end of the mesonephric ducts. The paired müllerian ducts extend caudally, fuse in the midline, and reach the urogenital sinus by 7 weeks of gestational age. The septum formed by fusion of the müllerian ducts disappears, forming a single cavity: the uterovaginal canal.
The simple columnar epithelium of the vagina is transformed into a stratified epithelium of polygonal cells. The vaginal plate arises at the urogenital sinus and exhibits progressive cephalad growth into the uterovaginal canal, thereby obliterating the vaginal lumen. The vaginal plate then capitates, leaving behind the stratified squamous epithelium of the vagina. In utero exposure to DES results in hormonally mediated persistence of müllerian glandular epithelium (adenosis) within the vagina.6, 7, 8
EPIDEMIOLOGY
Most cancers found in the vagina (80–90%) are metastatic from other primary sites, involving the vagina by direct extension or by lymphatic or hematogenous routes. Primary carcinoma of the vagina is rare; it represents only 1–3% of all gynecologic malignancies.2, 3, 11, 13 There has been a decrease in the incidence of primary vaginal tumors. This decrease suggests either the benefits of early detection by Papanicolaou smears or the application of more rigid diagnostic criteria that have eliminated primary cancers arising from adjacent organs such as the cervix, vulva, and endometrium.8, 11, 15 Risk factors associated with vaginal carcinoma include low socioeconomic level, a history of genital warts, vaginal discharge/irritation, previous abnormal results on Pap smear, early hysterectomy, and vaginal trauma.13, 16, 17, 18, 19, 20 Possible etiologic agents, including cervical fluids, cellular debris, and irritants such as chemical douches, feminine hygiene preparations, and retained tampons, have not been found to be causative agents.
Human papillomavirus (HPV) has been implicated in the pathogenesis of vaginal cancer, much like cervical carcinoma.16, 17, 18, 19, 20 Waggoner and colleagues21 found HPV 31 DNA exclusively in the clear-cell adenocarcinomas that contained HPV sequences. A larger, population-based study of patients with vaginal intraepithelial neoplasia (VAIN) III and invasive vaginal squamous cell cancers22 found similar risk factors to those related to cervical cancer. Compared with controls, these patients were more likely to have multiple sexual partners, early age at first intercourse, and to be current smokers. HPV DNA was detected in more than 80% of patients with VAIN III and 60% of patients with invasive cancers. Antibodies to HPV 16 were strongly related to the risk for cancer.22
In 1971, Herbst and associates first reported the correlation between clear-cell adenocarcinoma of the vagina and cervix and the use of DES.8 The incidence of clear-cell adenocarcinoma in women prenatally exposed to DES is estimated to be between 0.14 and 1.4 cases per 100,000.6, 7, 8, 15 The median age of these DES-exposed patients at tumor diagnosis was 19 years. An association was found between the risk for vaginal cancer and the time of first exposure to DES. The greatest risk for vaginal cancer from exposure to DES was found to occur during the first 16 weeks of pregnancy. This risk decreased when exposure began at 17 weeks or later.7 A question that remains unanswered is whether DES exposure is related to vaginal and cervical squamous cell cancers, which typically present later in life. To address this issue, Hatch and colleagues performed a retrospective cohort study of 3899 DES-exposed women versus 1374 controls.23 They found a relative risk of 2.1 (95% confidence interval: 1.2–3.8) for high-grade dysplasia of the vagina or cervix with DES exposure. The low incidence of invasion precluded statistical analysis regarding cancer.
CLINICAL PRESENTATION
Vaginal carcinoma often presents as either an ulcerative or exophytic lesion, with the exophytic or fungating lesion being more common.24 The vaginal skin can be undermined by the growing cancer, producing thickening and rigidity of the vagina. This phenomenon is more commonly associated with clear-cell carcinoma. Early infiltration of the submucosa is a frequently observed feature, and local tissue reaction and induration tend to give the impression of significant extension, even in small tumors. More than half of vaginal carcinomas occur in the posterior wall of the upper third of the vagina. The second most common site is the anterior wall of the lower third of the vagina.24, 25
The prognosis is better for patients in whom the tumor occupies the upper third of the vagina, because the pattern of spread is similar to that of cervical carcinoma. Lower vaginal tumors have a tendency to spread by the pelvic and inguinal lymphatics, making management more complicated. The incidence of clinically positive nodes at initial diagnosis ranges from 5.3 to 20%, depending on the anatomic location of the lesion. Involvement of inguinal nodes is more common if the lesion involves the lower third of the vagina. Therefore, the site of the carcinoma and the disease stage must be considered to optimize patient management.
Abnormal vaginal bleeding is a presenting symptom in 50–75% of patients with primary vaginal tumors. It most commonly occurs as random bleeding or postcoital spotting; however, antepartum bleeding has been described.26 Vaginal discharge is frequent. Less common presenting symptoms are dysuria or pelvic pain.
DIAGNOSTIC EVALUATION
As for any suspected cancer, a complete history and physical examination should be performed. It is critical to uncover any signs or symptoms that could indicate a primary tumor elsewhere in the patient. Pelvic assessment should include a rectovaginal and a detailed speculum examination. Frick and coworkers27 reported that at least 19% of cases are missed on initial examination. This is particularly true when the lesion is small and situated in the lower two thirds of the vagina, where it may be covered by the blades of the speculum (if not rotated to visualize the anterior and posterior walls). Definitive diagnosis is made by biopsy of a gross lesion, which can usually be performed in the office. Examination under anesthesia (with biopsy and clinical staging ± cystoscopy and proctoscopy) is useful for locally advanced tumors and for certain patients who are debilitated, very elderly, or have vaginal stenosis. Primary cancer of the vagina is a diagnosis of exclusion. If there is evidence of a primary tumor in an adjacent organ, then the vaginal cancer diagnosis cannot be made.
Clear-cell adenocarcinomas often grow into the submucosa and are not detected on cytologic smear. Early squamous cancers, however, can be detected in this manner. Colposcopy, with the use of Lugol's solution and/or acetic acid, is useful for identification and biopsy of a lesion. Multicentric disease can only be fully appreciated with the aid of careful colposcopy.
Radiological evaluation can be useful in determining the extent of local disease and detecting metastases.28, 29, 30 Chest x-ray is simple and inexpensive and can be followed by computed tomography (CT) or magnetic resonance imaging (MRI) if clinically indicated. MRI appears to have a superior ability to differentiate tumor from fibrosis,29, 30, 31 which can be difficult clinically. The recent encouraging data on the use of positron-emission tomography (PET) in cervix cancer32 would also seem to apply to many patients with vaginal carcinoma. However, these data are limited.28, 33
STAGING
Cancers are clinically staged according to the FIGO staging system (Table 1).12 In situ carcinomas (VAIN III) are superficial tumors that have not penetrated the basement membrane of the mucosal layer. They tend to be multicentric. Stage I lesions, although confined to the mucosa, may spread to involve more than one vaginal wall. Many practicing clinicians subdivide stage II disease into IIa (subvaginal) and IIb (parametrial), as proposed by Perez et al.13 Although those tumors with parametrial involvement appear to behave more aggressively, this subclassification is not officially part of the FIGO staging system. Enlarged inguinal lymph nodes should be evaluated by fine-needle aspiration to determine the stage and the radiation treatment ports. Most patients initially present with stage I or stage II disease (Table 2).
Table 2. Approximate stage distribution of primary vaginal carcinomas
| Stage | Percentage |
| I | 26 |
| II | 34 |
| III | 25 |
| IV | 15 |
The close proximity of the bladder and rectum to the vaginal wall can produce difficulties in staging. This results in a fine distinction between stage I and stage IV disease. Therefore, staging is best performed by gynecologic and radiation oncologists, often with the use of local anesthesia and occasionally regional or general anesthesia. At that time, additional biopsy specimens of the vagina should be obtained to delineate the margins of abnormal vaginal mucosa. Careful evaluation of the cervix is mandatory to rule out a primary cervical tumor. If there is a concomitant malignant lesion of the same histology in the cervix, the lesion must be classified as a primary cervical carcinoma and staged accordingly.
PRIMARY CANCER TYPES AFFECTING THE VAGINA
Treatment and prognosis for vaginal squamous cell carcinomas are discussed later, whereas those for all other histologies are discussed in this section.
Squamous cell carcinoma
Squamous cell carcinoma is the most common primary vaginal malignancy but constitutes only 1% of all gynecologic cancers (Table 3). The mean age of patients with vaginal squamous cell carcinoma initially diagnosed is 60 years (range 25–98).10, 13, 20 More than 75% of these lesions occur in patients older than age 50 years.13 The cause of squamous cell carcinoma of the vagina is becoming clearer. Recent interest has focused on the association between HPV infection and multifocal carcinoma of the lower female genital tract.24 Between 20 and 60% of cases of vaginal cancer have been associated with HPV DNA, usually type 16.16, 17, 18, 21, 22, 34
Table 3. Approximate percentages of primary vaginal malignancies according to histology
| Histology | Percentage |
| Squamous cell | 88 |
| Adenocarcinoma | 5 |
| Sarcoma | 3 |
| Melanoma | 3 |
| Small cell | 1 |
VAIN has been proposed as a precursor of vaginal cancer, although the true malignant potential of VAIN is unknown. The “HPV field-effect” hypothesis also has merit, because patients with VAIN commonly had cancer in other genital organs. Women with primary vaginal carcinoma have a 20% chance for previous invasive cervical cancer and a 70% chance for previous cervical intraepithelial neoplasia (CIN).18, 35 The median interval between the diagnosis of cervical cancer and the diagnosis of vaginal cancer is 14 years, with a range of approximately 6–28 years. Sixteen per cent of these patients have a history of previous pelvic radiation.10, 19
It is often difficult to accurately determine the spread into subvaginal tissues, particularly the spread of anterior and posterior lesions. Therefore, differences in clinical observations are common. This is reflected in the wide range of reported stage distributions and survival rates per stage. The best data estimate that approximately 25% of patients present with stage I, 33% with stage II, 25% with stage III, and 15% with stage IV disease (Table 2).10, 13, 36, 37, 38
The most common site for squamous cell carcinoma is the upper third of the vagina. Fifty-one per cent of these lesions are found to arise from the upper third, 19% from the middle third, and 30% from the distal third. Fifty-seven per cent of tumors originate from the posterior wall, 27% from the anterior wall, and 16% from the lateral wall.35, 36, 37, 38
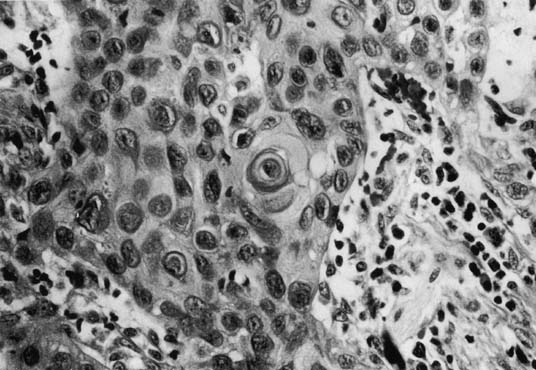
Tumor size can range from an occult lesion to a large mass measuring more than 10 cm in diameter.38 Grossly, these tumors may be polypoid fungating masses or indurated, ulcerative plaques. On microscopic examination, the carcinoma consists of malignant squamous cells infiltrating from the vaginal epithelium and extending between the submucosa (Fig. 1). Cords of malignant cells advance into the submucosa and are usually surrounded by a large inflammatory reaction. Initially, local extension is into the submucosa, with later spread into the paracolpium and parametrium.39, 40 Squamous cell carcinomas are usually moderately differentiated and nonkeratinizing. The degree of histologic differentiation, based on the amount of keratinization and number of squamous pearls, does not constitute a sound basis for assessing prognosis.24 The major prognostic factors are the stage of the tumor at the time of diagnosis and the size.17
|
Verrucous carcinoma is a distinctive variant of well-differentiated squamous cell carcinoma that rarely occurs in the vagina.41, 42 This tumor typically presents as a relatively large, well-circumscribed, soft, cauliflower-like mass. Microscopically, it exhibits a papillary growth pattern with marked hyperkeratosis and broad, bulbous pegs of acanthotic epithelium that push into the underlying stroma. Cytologic features of malignancy are lacking. Because of its well-differentiated character, the microscopic diagnosis of invasive carcinoma may be difficult, especially if the biopsy specimens are superficial. Verrucous carcinoma can recur locally after surgery, but it rarely metastasizes. This behavior difference justifies treating this carcinoma as a distinct tumor entity.
Adenocarcinoma
Approximately 5% of primary vaginal malignancies are adenocarcinomas (Table 3). Whenever this diagnosis is considered, it is necessary to rule out metastatic lesions from the bowel, uterus, or ovary. The most common variant is the clear-cell adenocarcinoma, which can occur spontaneously and in women with in utero exposure to DES.6, 7, 8, 15, 43 Primary non-DES-related adenocarcinoma of the vagina is rare and occurs predominately in postmenopausal women.
DES was used extensively in the late 1940s and early 1950s to maintain high-risk pregnancies, such as those in women with a past history of abortion, diabetes, or multiple gestation.44, 45, 46 Approximately 5% of all pregnant women in the United States during the late 1940s and early 1950s used DES. In 1953, Dieckmann and colleagues44 reported that DES offered no improvement in fetal outcome, and its use gradually decreased until it was discontinued by the Food and Drug Administration in 1971. In the same year, Herbst and associates7 reported seven young women (aged 15–22 years) who presented to Vincent Memorial Hospital (Boston, MA, USA) between 1966 and 1969 with a diagnosis of clear-cell carcinoma or endometrioid-type adenocarcinoma with intrauterine exposure to DES. The Registry for Hormonal Transplacental Carcinogenesis and the Registry for Research on Hormonal Transplacental Carcinogenesis were established to correlate clinical and pathologic data on these unusual cancers.47
Many DES-exposed women have unusual vaginal epithelial changes such as adenosis.43, 48 Vaginal adenosis is a condition in which müllerian-type glandular epithelium is present after vaginal development is complete. It most commonly involves the anterior wall and upper third of the vagina, and the classical gross appearance is that of red, velvety, grape-like clusters.7, 8, 15 The process may involve the surface epithelium or glands in the superficial stroma. Microscopically, the glandular epithelium can be composed of any of the müllerian epithelial cell types, but cervical-type mucus cells are most common. The glands within the lamina propria may be lined by tuboendometrial-type epithelium, which exists in approximately 25% of cases and is more common in the lower vagina. Robboy and associates48 suggested that atypical vaginal adenosis and atypical cervical ectropion of the tuboendometrial type are precursors of adenocarcinoma.
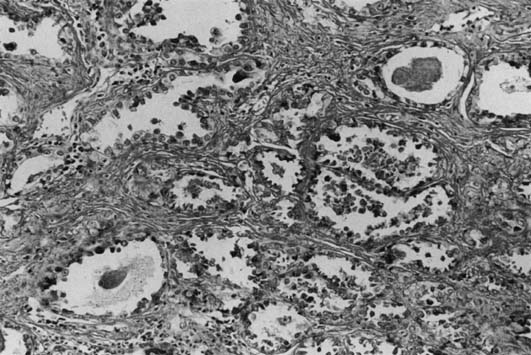
Most DES-associated tumors have occurred in women between 17 and 21 years (median age 19 years).6, 7 Clear-cell carcinoma develops in 0.1% of exposed women. The DES-associated clear-cell cervical adenocarcinomas have a predilection for the ectocervix. Most vaginal carcinomas arise on the anterior wall, usually in the upper third, corresponding to the most common site of adenosis. The tumors vary greatly in size (range 1–30 cm). Most of the tumors are exophytic and superficially invasive.49 The larger tumors are polypoid and nodular, and the smaller tumors appear flat or ulcerated with a granular or indurated surface. Small tumors can be easily missed on colposcopic examination if confined to the lamina propria and if covered by intact, normal epithelium. These small tumors are usually asymptomatic and are detected only by palpation and directed biopsies as part of a thorough examination for a DES-exposed patient. Microscopically, they exhibit three basic histologic patterns: tubulocystic (most common); papillary; and solid.43 The tumor cells are cuboidal or columnar with clear cytoplasm and a distinct cell membrane, or they are the hobnail-type with large, atypical, protruding nuclei rimmed by a small amount of cytoplasm (Fig. 2). Glycogen is also found to be abundant.
Treatment can involve surgical intervention and radiation therapy.7, 49, 50, 51, 52, 53, 54 For stage I clear-cell adenocarcinoma in the typical young patient, surgery may be considered to preserve ovarian function.6, 7, 8, 10, 14, 15 Surgery for vaginal clear-cell adenocarcinoma requires a radical hysterectomy and vaginectomy with reconstruction. The vaginectomy is performed only to the level required to obtain an adequate margin. Local excision appears inferior to radical surgery. The role of chemotherapy has not been determined.
The overall recurrence rate for clear-cell carcinoma approaches 21%, with the lungs, supraclavicular lymph nodes, and pelvis being the most common areas.7 Such cancers appear to have recurrence patterns different from those of squamous cell carcinomas, with a greater tendency to develop metastases in these distant sites.50, 51, 52, 53, 54 Although most recur within 3 years, late relapse of more than 19 years has been reported.7, 49, 50, 51, 52, 53, 54 Recurrent disease can be treated with radiation, surgery, or chemotherapy if widely metastatic. For central recurrences after surgery, pelvic radiation with external and interstitial therapy has been used. Pelvic exenteration appears to be more successful for patients with clear-cell carcinoma than for those with squamous cell carcinoma.50
Small-cell carcinoma
Small-cell carcinoma can occur in the vagina in pure form or associated with squamous or glandular elements. A high proportion of small-cell carcinoma cases show ultrastructural or immunocytochemical evidence of neuroendocrine differentiation. Scully and associates55 first reported a vaginal small-cell carcinoma confirmed by immunohistochemical staining for neuroendocrine features in 1984. Histologically, these tumors are small and round, with a rim of sparse cytoplasm around the nuclei. Growth patterns include sheets, ribbons, rosettes, and palisades. The tumors are reactive for neuron-specific enolase and cytokeratin. Peters and co-workers56 reported that patients with small-cell tumors (five patients) had a mean age of 61 years.
Optimal therapy for small-cell carcinoma has yet to be determined. Therapeutic decisions are based on the examination results of patients with cervical or lung cancer. Because small-cell carcinoma is associated with early metastases, aggressive treatment with radiation for local control combined with systemic multiagent chemotherapy is recommended.57, 58, 59
Melanoma
Primary malignant melanoma is the second most common cancer of the vagina (5% of all primary vaginal tumors) and has the worst prognosis of all vaginal malignancies.10, 36 The incidence is estimated to be 0.03 per 100,000 women per year. Symptoms have usually been present for 3–6 months before a biopsy is performed. A recent review of 26 patients treated more than 30 years confirmed the previously cited clinicopathologic features.60 Most patients are white, with a mean age of 60 years (range 38–90 years). The most common presenting symptom is vaginal bleeding, followed by a palpable vaginal mass.
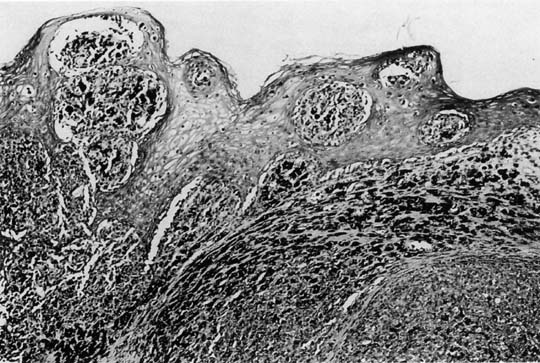
The lesions are polypoid–nodular in most cases with ulceration and a thickness greater than 3 mm.10, 36 They are typically located in the distal third of the anterior vagina and appear pigmented black or dark blue. The disease is usually locally advanced on initial examination. Microscopically, these tumors are similar to those in other sites (Fig. 3). Poorly differentiated lesions that are difficult to distinguish from sarcomas or squamous cell carcinomas can be identified by their distinctive ultrastructural features or by immunohistochemical staining patterns. S-100 is the most sensitive immunohistochemical marker for these tumors, and is used along with MART-1 and HMB-45.60, 61 Tumor depth according to the method of Breslow62 should be assessed, because depth of tumor invasion is the best predictor of survival.
|
Multifocal lesions are observed in 30% of patients, and nodal or distant spread is found in 20%. Sixty-six per cent of patients (6/9) in one study63 had more than one focus of melanoma, either at initial diagnosis or at follow-up. The melanocytes probably come from aberrant melanocyte migration or melanocyte metaplasia. These tumors have poorer prognoses than cutaneous melanomas, with 5-year survival rates of 5–21% compared with 48%.64, 65, 66, 67, 68 The most frequent site of recurrence is local; the most common distant site is the lung.66
The only potentially curative treatment for vaginal melanoma is surgery. Most investigators have found no long-term survival difference between treatment with radical surgery and treatment with conservative therapy. Van Nostrand and colleagues in 199469 reported that radical surgery gave patients a significant improvement in the 2-year survival rate if the initial lesion was smaller than 1 cm2 (48% vs. 20%). However, the overall 5-year survival was similar. The resection must include all obvious disease, and conservative to ultraradical operations have been used.69, 70, 71 Regardless of the type of operation, positive histologic margins for melanoma or melanoma in situ result in a higher incidence of local failure and decreased survival rates. Irradiation alone, or in combination with surgery, has been successful in treating some patients.72
Local recurrences (usually within 2 years of initial treatment) can develop regardless of the type of surgical procedure. Recurrences can be treated with reoperation or radiation therapy. The exact role of chemotherapy in the adjuvant setting and for metastatic disease has not been determined.60, 73
Endodermal sinus tumor
Extraovarian endodermal sinus tumors (EST) are extremely rare and generally originate in the vagina or cervix of young girls. EST of the vagina was first recognized in 1965. Because of the tumor's resemblance to the endodermal sinus structures of the rat placenta, which derive from the yolk sac endoderm, the names EST and yolk sac tumor were proposed.74 Most cases have been reported in patients younger than age 3 years.74, 75, 76, 77 A bloody vaginal discharge is the typical clinical presentation. The disease appears to be locally aggressive and capable of metastasizing by hematogenous and lymphatic pathways. Grossly, the tumor appears as a polypoid mass that distends the vaginal lumen and may protrude through the introitus. The tumor may also appear as a sessile thickening of the vaginal wall with mucosal ulceration. Schiller-Duval bodies can be diagnosed on microscopic examination but are found in only a minority of cases. Alpha-fetoprotein can be demonstrated by immunoperoxidase staining, and serum levels can usually be used as a marker of disease.
Previously, untreated patients died within 2–4 months of presentation; however, with more effective chemotherapy, the prognosis has improved significantly. Copeland and co-workers78 reported on six patients with EST of the vagina and cervix who received excisional surgery with adjuvant vincristine, actinomycin-D, and cyclophosphamide (VAC) chemotherapy. They found that four of the six patients had been disease-free for 2–23 years. The Maligne Keimzelltumoren Study Group from Germany reviewed 14 patients with vaginal EST.79 The protocol consisted of neoadjuvant cisplatin-based chemotherapy followed by surgical removal of tumor (if necessary) and further cisplatin-based chemotherapy. In 10 children, the residual mass was completely resected, whereas in the remaining four only vaginoscopy was necessary, because no visible tumor remained. All children were free of disease after a median follow-up of 76 months (range 9–150). Primary chemotherapy was also successful in the treatment of three children, as reported by Handel and colleagues.80
Embryonal rhabdomyosarcoma
Embryonal rhabdomyosarcoma is a malignant tumor of the rhabdomyoblasts characterized by two structural variances:
- Asolid form;
- A multicystic grape-like form referred to as sarcoma botryoides.5 Sarcoma botryoides is a highly malignant tumor and is the most common malignant tumor of the vagina in infants and children.81, 82, 83
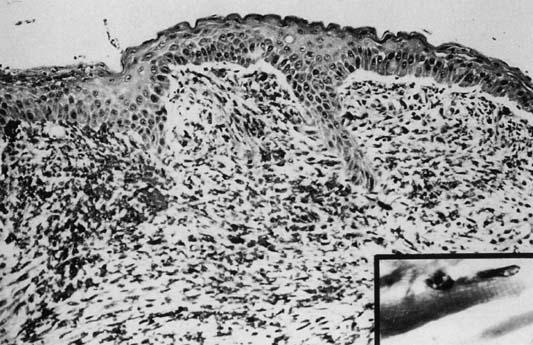
Ninety per cent occur in children younger than age 5 years. Although variable in size and location, rhabdomyosarcomas have a characteristic gross appearance consisting of multiple gray-red, translucent, edematous, grape-like masses that fill and protrude from the vagina. The tumors are typically found in the vagina during infancy and early childhood, in the cervix during the reproductive years, and in the uterine corpus during the rare postmenopausal patient. Microscopically, there is a continuous zone of dense round or spindle cells (they can be layered) immediately beneath the intact vaginal epithelium. The neoplastic cells are immature rhabdomyoblasts that surround blood vessels. Elsewhere, the tumor is composed of small, dark cells that are sparsely distributed in the myxoid stroma. Some cells may show cytologic evidence of differentiation by intensely staining eosinophilic cytoplasm with cross-striation (Fig. 4).
The tumor invades adjacent structures and metastasizes to local lymph nodes and distant sites by lymphatic and hematogenous dissemination. Lymph node metastasis exceeds 25% at initial presentation. Previously, the overall 5-year survival rate was approximately 35%. Local excision combined with multiagent chemotherapy for early-stage disease has significantly improved survival.2, 82, 83, 84, 85 Neoadjuvant chemotherapy along with postoperative radiation therapy have also improved outcomes, even in older patients.85, 86
Other sarcomas and rare vaginal primaries
Leiomyosarcomas,87 spindle cell sarcomas, angiosarcomas,88 alveolar soft-part sarcomas,89, 90, 91 fibrosarcomas, neurofibrosarcomas, and mixed mesodermal tumors of the vagina occur predominantly in older patients but are rare (2% of all malignant vaginal tumors).87 Leiomyosarcomas are the most common of the adult vaginal sarcomas. These tumors usually develop along the vaginal sidewall and present as a lump that may cause problems with micturition, defecation, or intercourse. Because they are usually subdermal in location, bleeding and discharge are late symptoms.
Alveolar soft-part sarcoma of the genital tract is extremely rare, constituting 0.5–1% of all soft-tissue sarcomas.89, 90 Patients are treated with radiation therapy after local excision and, occasionally, chemotherapy. Christopherson in 195291 described alveolar soft-part sarcoma microscopically as dense, fibrous trabeculae of varying thickness dividing the tumor into compact compartments of irregular size. Current periodic acid-Schiff (PAS) staining reveals various amounts of intracellular glycogen and characteristically PAS-positive, diastase-resistant crystalline material. These crystals are diagnostic features of this tumor.
Hemangiopericytoma has been reported to develop as an ulcerative mass on the vaginal epithelium, with bleeding occurring both spontaneously and after trauma.92 This tumor is characterized by proliferation of capillaries surrounded by a cell population derived from the myoepithelial pericyte. It can be mistakenly diagnosed as leiomyosarcoma.
The genital tract, especially the vagina, is an infrequent site for metastatic lymphomas, and it is rare for the vagina to be a primary site. These tumors can occur in premenopausal and postmenopausal patients.93 Bleeding is the most common presenting symptom, with pain and dyspareunia also occurring. Masses may be noted on routine pelvic examination.
THERAPY FOR VAGINAL SQUAMOUS CELL CARCINOMA
General concepts
Radiation therapy and surgical intervention have been used as primary treatment for carcinoma of the vagina.94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105 Treatment choices have been determined by extent of disease and by individual and institutional preferences. Radiation therapy is considered the treatment of choice because of excellent tumor control and good functional results. Surgical procedures may be reserved for the treatment of radiation failures, nonepithelial tumors, and early-stage disease in young, sexually active women. Limited surgery alone has quality-of-life advantages, including ovarian preservation and a lower risk for vaginal stenosis, and even the possibility of fertility in young patients.94
Principles of radiation treatment include the application of adequate tumoricidal doses to the cancer and the primary routes of dissemination (if at risk). For tumors of the middle and upper vagina, the pelvic lymph nodes must be included in any treatment field. The inguinal nodes must also be included for tumors of the lower vagina. In an effort to deliver substantial dose to the primary site while minimizing dosage applied to nearby organs, brachytherapy is commonly used.95, 96, 97 This can be delivered via interstitial implants or with a simple vaginal cylinder.98 Low-dose rate and high-dose rate systems are available.99 The final decision on treatment apparatus and dose rate is determined by many factors, including tumor volume, location, availability of technology, and physician preference.
Definitive conservative surgical treatment is a rarely used option. It is often difficult to obtain adequate tumor-free margins because of proximity of the bladder and rectum. Sacrifice of the whole vagina, or at least a significant part of the vagina, must be accepted. Replacement with skin grafts or other plastic surgical procedures can never be regarded as a return to normalcy. Distal vaginal tumors treated surgically may require inguinal lymph node dissection. Sentinel node evaluation has been demonstrated in cases of vaginal melanoma,100, 101 but not extensively in squamous cell vaginal cancer. Surgical intervention is also useful as an adjunct to radiation therapy for more advanced lesions and to treat tumors that do not respond to radiation. Davis and colleagues102 reported that the Mayo Clinic surgical experience yielded a 5-year survival rate of 82%. Patients with central recurrences or persistence of tumor after radiotherapy are candidates for exenterative surgery.
Stage I
Stage I carcinoma of the vagina is primarily treated with radiation therapy.95, 103, 104, 105 Lesions of the vagina that involve the upper vaginal fornices, particularly the posterior and lateral fornices, allow the option of surgical treatment involving a radical hysterectomy, pelvic lymphadenectomy, partial or total vaginectomy, or radical trachelectomy with partial vaginectomy.94
In patients with stage I lesions, it is important to individualize radiation therapy techniques to obtain optimal subsequent vaginal function. Superficial tumors can be treated with cylinder brachytherapy alone to the entire vagina (6000–7000 cGy mucosal dose and an additional 2000–3000 cGy mucosal dose to the tumor area).95, 103, 104, 105, 106
Larger lesions may require an interstitial implant to selectively increase the tumor dose and limit excessive radiation to the entire vaginal mucosa.95, 98, 104, 105, 106, 107, 108 A dose of 6000–6500 cGy is delivered to the entire vaginal mucosa, and an additional 1500–2000 cGy is calculated 0.5 cm beyond the plane of the implant to the gross tumor, with the involved vaginal mucosa receiving an estimated 8000–10,000 cGy.95, 103, 104, 105, 106, 107, 108
Use of external beam radiation in stage I disease should be considered to supplement intracavitary and interstitial therapy for bulky lesions, marked infiltration, or poor differentiation. The whole pelvis is treated with 1000–2000 cGy. Additional split pelvic radiation to a total dose of 4500–5000 cGy to the parametria should be delivered.95, 96, 103, 104, 105, 106, 107, 108
Stage II
Patients with stage IIa13 disease have significantly improved survival compared with patients with more advanced disease. Perez and associates109 suggest that these patients should be treated with a greater external radiation dose: 2000 cGy to the whole pelvis and an additional parametrial dose with a midline block, for a total dose of 4500–5000 cGy (in comparison with stage I patients). A combination of interstitial and intracavitary therapy can also be used to deliver 5000–6000 cGy 0.5 cm beyond the deep margin of the tumor in addition to the whole pelvis dose for gross disease. Neoadjuvant chemotherapy, followed by radical surgery has also been utilized with success in some centers.110
Advanced disease: stages III and IV
For advanced disease (including IIb13), Perez recommends a 4000 cGy dose to the whole pelvis and a 5500–6000 cGy total parametrial dose (with midline shielding) have been given in combination with interstitial and intracavitary insertions to deliver a total vaginal mucosal dose of 7500–10,000 cGy; the total dose to a depth of 0.5 cm from the vaginal mucosa is 6000–8000 cGy.95, 97, 98, 103, 104, 105, 106, 107, 108, 109, 111, 112, 113
Treatment of vaginal recurrence
Tabata and colleagues40 noted that all relapses in their patients with stage 0–II tumors were local. Poor outcome in patients with advanced-stage disease stemmed from either local persistence or new distant spread. The authors highlight the importance of aggressive local control. Isolated recurrences can be effectively treated with surgery after postradiation local failure. The surgical procedure can range from a wide local excision or partial vaginectomy to a pelvic exenteration.50, 95
Radiation is the treatment of choice after primary surgical therapy has failed. The Syed-Nesbitt template used for interstitial brachytherapy in a patient treated for recurrent vaginal clear-cell adenocarcinoma is shown in Figure 5A. An orthogonal radiograph obtained for treatment planning is shown in Figure 5B. Figure 5C demonstrates the isodose plan. This patient received interstitial iridium (Ir) 192 after external beam radiation therapy.
PROGNOSIS FOR VAGINAL SQUAMOUS CELL CARCINOMA
With adequate therapy, the survival rates of patients with squamous cell carcinoma of the vagina are comparable with those reported for cervical cancer. The most important predictor of survival is stage10, 36 (Table 4). The overall 5-year survival rate is 80%. The 5-year survival rates for FIGO stage I tumors range from 66 to 90%.
Table 4. Five-year overall survival rates for primary vaginal carcinoma
| Stage | Percentage |
| I | 80 |
| II | 70 |
| III | 31 |
| IV | 17 |
Regarding stage II disease, Kirkbride and co-workers103 reported a 5-year survival rate of 82% for patients treated with radiation therapy. Stock and colleagues95 reported a significant improvement in disease-free survival (DFS) in patients treated with surgery (53%) compared with that of those treated with radiation therapy (31%). The variation in survival ranged from 31 to 82% and was dependent on many factors, such as tumor stage (IIA vs. IIB), lesion size, histologic type and grade, tumor location, patient age, treatment modality, and radiation dose. Older patients with large, poorly differentiated tumors have a significantly worse prognosis when compared with other patients within the same disease stage.
The 5-year survival rate for patients with more advanced disease (stage III and stage IV) ranges from 0 to 62%. Lee and associates105 found that the single most important predictor of pelvic control was overall treatment time. They found that if the entire course of radiotherapy was completed within 9 weeks (63 days), the pelvic control rate was 67% for stage IVA and 97% for all stages. However, the pelvic control rate was only 54% if treatment time extended beyond 9 weeks (p = 0.0003).
SUMMARY
Malignant tumors of the vagina are rare and commonly present with abnormal vaginal bleeding. It is a diagnosis of exclusion. If there is evidence of a primary tumor in an adjacent organ, then the vaginal cancer diagnosis cannot be made. Major histologic subtypes include squamous cell carcinoma, adenocarcinoma (typically clear cell), small-cell carcinoma, melanoma, endodermal sinus tumor, and sarcoma (particularly embryonal rhabdomyosarcoma). Radiation therapy is generally considered the treatment of choice because of excellent tumor control and good functional results. Surgical procedures may be reserved for the treatment of radiation failures, nonepithelial tumors, and early-stage disease in young, sexually active women. FIGO stage, patient age, lymph node status, histologic grade, and radiation treatment time are predictors of survival.10, 105
REFERENCES
Jemal A, Siegel R, Ward E et al: Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. Epub 2008 Feb 20. |
|
Cramer D, Cutler S. Incidence and histopathology of malignancies of the female genital organs in the United States. Am J Obstet Gynecol 1979;118:443 |
|
Henson D, Tarrone R. An epidemiologic study of cancer of the cervix, vagina, and vulva based on the Third National Cancer Survey in the United States. Am J Obstet Gynecol 1977;129:525 |
|
Arora M, Shrivastav RK, Jaiprakash MP. A rare germ-cell tumor site: Vaginal endodermal sinus tumor. Pediatr Surg Int 2002;18:521 |
|
Copeland LJ, Gershenson DM, Saul PB et al. Sarcoma botryoides of the female genital tract. Obstet Gynecol 1985;66:262 |
|
Herbst AL, Robboy SJ, Scully RE et al. Clear-cell adenocarcinoma of the vagina and cervix in girls: Analysis of 170 registry cases. Am J Obstet Gynecol 1974;119:713 |
|
Herbst AL, Cole P, Norusis MJ. Epidemiologic aspects and factors related to survival in 384 registry cases of clear cell adenocarcinoma of the vagina and cervix. Am J Obstet Gynecol 1979;135:875 |
|
Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. N Engl J Med 1971;284:878 |
|
Diakomanolis E, Rodolakis A, Stefanidis K et al. Primary invasive vaginal cancer. Report of 12 cases. Eur J Gynaecol Oncol 2002;23:573 |
|
Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecologic system: An analysis of 1973–87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol 1994;10:31 |
|
Jemal A, Thomas A, Murray T et al. Cancer Statistics 2002. CA-A Cancer J Clin 2002;52:23 |
|
Beller U, Sideri M, Maisonneuve P et al. Carcinoma of the vagina. J Epidemiol Biostat 2001;6:141 |
|
Perez CA, Arneson AN, Galakatos A et al. Malignant tumors of the vagina. Cancer 1973;31:36 |
|
Plentl AA, Friedman EA. Lymphatic systems of the female genitalia: The Morphologic Basis of Oncologic Diagnosis and Therapy. pp 51-74, Philadelphia: WB Saunders; 1971 |
|
Herbst AL, Scully RE. Adenocarcinoma of the vagina in adolescence. Cancer 1970;25:745 |
|
Crum CP, Roche JK. Papilloma virus-related genital neoplasia: Present and future prevention. Cancer Detect Prev 1990;14:465 |
|
Campion MU, Cuzick J, McCance DJ et al. Progressive potential of mild cervical atypia: Prospective, cytological, colposcopic, and virological study. Lancet 1986;2:237 |
|
Benedet JL, Saunders BH. Carcinoma in-situ of the vagina. Am J Obstet Gynecol 1984;148:695 |
|
Lenehan PM, Meffe F, Lickrish GM. Vaginal intraepithelial neoplasia: Biologic aspects and management. Obstet Gynecol 1986;68:333 |
|
Rutledge F. Cancer of the vagina. Am J Obstet Gynecol 1967;97:635 |
|
Waggoner SE, Anderson SM, Van Eyck S et al. Human papillomavirus detection and P53 expression in clear-cell adenocarcinoma of the vagina and cervix. Obstet Gynecol 1994;84:404 |
|
Daling JR, Madeleine MM, Schwartz SM et al. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol Oncol 2002;84:263 |
|
Hatch EE, Herbst AL, Hoover RN et al. Incidence of squamous neoplasia of the cervix and vagina in women exposed prenatally to diethylstilbestrol (United States). Cancer Causes and Control 2001;12:837 |
|
Wilkinson EJ. Pathology of the vagina. Curr Opin Obstet Gynecol 1991;3:553 |
|
Stryker JA. Radiotherapy for vaginal cancer: A 23-year review. Br J Radiol 2000;73:1200 |
|
Steed HL, Pearcey RG, Capstick V et al. Invasive squamous cell carcinoma of the vagina during pregnancy. Obstet Gynecol 2002;100:1105 |
|
Frick HC, Jacox HW, Taylor HC. Primary carcinoma of the vagina. Am J Obstet Gynecol 1968;101:695 |
|
Soper JT. Radiologic imaging in gynecologic oncology. Clin Obstet Gynecol 2001;44:485 |
|
Chen YCF, Hricak H, Thurnher S et al. Vagina: Evaluation with MR imaging. II. Neoplasms Radiology 1988;169:175 |
|
Ebner F, Kressel HY, Mintz MC et al. Tumor recurrence versus fibrosis in the female pelvis: Differentiation with MR at 1.5T. Radiology 1988;166:333 |
|
Taylor MB, Dugar N, Davidson SE et al. Magnetic resonance imaging of primary vaginal carcinoma. Clin Radiol 2007;62(6):549-55 |
|
Grigsby PW. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol 2001;19:3745 |
|
Husain A, Akhurst T, Larson S et al. A prospective study of the accuracy of 18Fluorodeoxyglucose positron emission tomography (18FDG PET) in identifying sites of metastasis prior to pelvic exenteration. Gynecol Oncol 2007;106(1):177-80 |
|
Merino MJ. Vaginal cancer: The role of infectious and environmental factors. Am J Obstet Gynecol 1991;165:1255 |
|
Eddy GL, Marks RD, Miller MC III et al. Primary invasive vaginal carcinoma. Am J Obstet Gynecol 1994;165:282 |
|
Manetta A, Gutrecht EL, Berman ML et al. Primary invasive carcinoma of the vagina. Obstet Gynecol 1990;62:699 |
|
Rubin SC, Young J, Mikuta JJ. Squamous carcinoma of the vagina: Treatment, complications and long-term follow-up. Gynecol Oncol 1985;20:346 |
|
Pride GL, Schwultz AE, Chuprevich TW et al. Primary invasive squamous cell carcinoma of the vagina. Obstet Gynecol 1979;53:218 |
|
Tjalma WAA, Monaghan JM, Lopes AB et al. The role of surgery in invasive squamous carcinoma of the vagina. Gynecol Oncol 2001;81:360 |
|
Tabata T, Takeshima N, Nishida H et al. Treatment failure in vaginal cancer. Gynecol Oncol 2002;84:309 |
|
Anderson ES, Sorenson IM. Verrucous carcinoma of the female genital tract: Report of a case and review of the literature. Gynecol Oncol 1988;30:427 |
|
Crowthe ME, Lowe DG, Sheperd JH. Verrucous carcinoma of the female genital tract: A review. Obstet Gynecol Surv 1988;43:263 |
|
Robboy SJ, Kaufman RH, Prat J. Pathologic findings in young women enrolled in the National Cooperative Diethylstilbestrol Adenosis (DESAD) Project. Obstet Gynecol 1979;53:309 |
|
Dieckmann WJ, Davis M, Rynkiewicz L et al. Does administration of diethylstilbestrol during pregnancy have therapeutic value? Am J Obstet Gynecol 1953;66:1062 |
|
Smith OW, Smith G et al. Increased excretion of pregnanediol in pregnancy from DES with special reference to the prevention of pregnancy accidents. Am J Obstet Gynecol 1946;51:411 |
|
Smith OW. Diethylstilbestrol in the prevention and treatment of complications of pregnancy. Am J Obstet Gynecol 1948;56:821 |
|
Adenocarcinoma Registry. Am J Obstet Gynecol 1972;113:718 |
|
Robboy SJ, Young RH, Welch WR et al. Atypical vaginal adenosis and cervical ectropion – association with clear cell adenocarcinoma in diethylstilbestrol-exposed offspring. Cancer 1984;54:869 |
|
Herbst AL. Clear cell adenocarcinoma and the current status of DES-exposed females. Cancer 1981;48:484 |
|
Senekjian EK, Frey K, Herbst AL. Pelvic exenteration and clear cell adenocarcinoma of the vagina and cervix. Gynecol Oncol 1989;34:413 |
|
Burks RT, Schwartz AM, Wheeler JE et al. Late recurrence of clear-cell adenocarcinoma of the vagina and cervix: Case report. Obstet Gynecol 1990;76:525 |
|
Jones WB, Tan LK, Lewis JL. Late recurrence of clear cell adenocarcinoma of the vagina and cervix: a report of three cases. Gynecol Oncol 1993;54:266 |
|
Goodman A, Sullinger JC, Rice LW et al. Clear cell adenocarcinoma of the vagina: A second primary in the diethylstilbestrol-exposed woman? Gynecol Oncol 1991;43:173 |
|
Fishman DA, Williams S, Small W et al. Late recurrence of vaginal clear cell adenocarcinoma. Gynecol Oncol 1996;62:128 |
|
Scully RE, Aguirre P, DeLellis RA. Argyrophilic, serotonin, and peptide hormones in the female genital tract and its tumors. Int J Gynecol Path 1984;3:51 |
|
Peters WA III, Kumar NB, Morley GW. Carcinoma of the vagina. Cancer 1985;55:892 |
|
Joseph RE, Enghardt MH, Doering DL et al. Small cell neuroendocrine carcinoma of the vagina. Cancer 1992;70:784 |
|
Chafe W. Neuroepithelial small cell carcinoma of the vagina. Cancer 1989;64:1948 |
|
Kaminski JM, Anderson PR, Han AC et al. Primary small cell carcinoma of the vagina. Gynecol Oncol 2003;88:451 |
|
Gupta D, Malpica A, Deavers MT et al. Vaginal melanoma: A clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Path 2002;26:1450 |
|
Primary malignant melanoma of the vagina. Frumovitz M, Etchepareborda M, Sun CC, Soliman PT, Eifel PJ, Levenback CF, Ramirez Obstet Gynecol. 2010;116(6):1358. |
|
Breslow A. Prognosis in cutaneous melanoma: Tumor thickness as a guide to treatment. Pathol Ann 1980;1:1 |
|
Lotem M, Anteby S, Peretz T et al. Mucosal melanoma of the female genital tract is a multifocal disorder. Gynecol Oncol 2003;88:45 |
|
Ariel I. Malignant melanoma of the female genital system. A report of 48 patients and review of the literature J Surg Oncol 1981;16:371 |
|
Morrow C, Disaia P. Malignant melanoma of the female genitalia: A clinical analysis. Obstet Gynecol Surv 1976;31:85 |
|
Weinstrock MA. Malignant melanoma of the vulva and vagina in the United States: Patterns of incidence and population-based estimates of survival. Am J Obstet Gynecol 1994;171:1225 |
|
Ragnarsson-Olding B, Johansson H, Rutguist LE et al. Malignant melanoma of the vulva and vagina. Cancer 1993;71:1893 |
|
Heller DS, Moom YM, Koulos J et al. Vulva and vaginal melanoma—A clinicopathologic study. J Reprod Med 1994;39:945 |
|
Van Nostrand KM, Lucci JA, Schell M et al. Primary vaginal melanoma: Improved survival with radical pelvic surgery. Gynecol Oncol 1994;55:234 |
|
Chang A, Casey M, Flannery J et al. Malignant melanoma of the vagina: A report of 19 cases. Obstet Gynecol 1980;55:720 |
|
Borazjani G, Prem K, Okagaki T et al. Primary malignant melanoma of the vagina: A clinicopathological analysis of 10 cases. Gynecol Oncol 1990;37:264 |
|
Peru E, Nagele F, Czerwenka K et al. Primary malignant melanoma of the vagina: long-term remission following radiation therapy. Gynecol Oncol 1998;70:23 |
|
Beg MH, Muchmore JH, Carter RD et al. Vaginal melanoma and the role of regional chemotherapy. J Surg Oncol 1993;53:133 |
|
Vawter GF. Carcinoma of the vagina in infancy. Cancer 1965;18:1479 |
|
Young RH, Scully RE. Endodermal sinus tumor of the vagina: A report of nine cases and reviews of the literature. Gynecol Oncol 1984;18:380 |
|
Kohorn EI, McIntosh S, Lytton B et al. Endodermal sinus tumor of the infant vagina. Gynecol Oncol 1985;20:196 |
|
Andersen WA, Sabio H, Durso N et al. Endodermal sinus tumor of the vagina. Cancer 1985;56:1025 |
|
Copeland LJ, Sneige N, Ordonez NG et al. Endodermal sinus tumor of the vagina and cervix. Cancer 1985;55:2558 |
|
Mauz-Korholz C, Harms D, Calamins G et al. Primary chemotherapy and conservative surgery for vaginal yolk-sac tumor. Maligne Keimzelltumoren Study Group. Lancet 2000;355:625 |
|
Handel LN, Scott SM, Giller RH et al. New perspectives on therapy for vaginal endodermal sinus tumors. J Urol 2002;168:687 |
|
Hilgers RD, Malkasian GD, Soule EH. Embryonal rhabdomyosarcoma (botryoid type) of the vagina: A clinicopathologic review. Am J Obstet Gynecol 1970;107:484 |
|
Friedman M, Peretz BA, Nissenbaum M et al. Modern treatment of vaginal embryonal rhabdomyosarcoma. Obstet Gynecol Surv 1986;41:614 |
|
Davos I, Abell MR. Sarcoma of the vagina. Obstet Gynecol 1976;4:32 |
|
Hicks ML, Piver MS. Conservative surgery plus adjuvant therapy for vulvovaginal rhabdomyosarcoma, diethylstilbestrol clear cell adenocarcinoma of the vagina, and unilateral germ cell tumors of the ovary. Obstet Gynecol Clin North Am 1992;19:219 |
|
O'Connell ME, Hoskin PJ, Mayles WP et al. Intravaginal iridium-192 in the management of embryonal rhabdomyosarcoma. Clin Oncol (R Coll Radiol) 1991;3:236 |
|
Hahlin M, Jaworski RC, Wain GU et al. Integrated multimodality therapy for embryonal rhabdomyosarcoma of the lower genital tract in the postpubertal female. Gynecol Oncol 1998;70:141 |
|
Peters WA III, Kumar NB, Andersen WA et al. Primary sarcoma of the adult vagina: A clinicopathologic study. Obstet Gynecol 1985;65:699 |
|
Morimura Y, Hashimoto T, Soeda S et al. Angiosarcoma of vagina successfully treated with interleukin-2 therapy and chemotherapy: A case report. J Obstet Gynaecol Res 2001;27:231 |
|
Carinelli SG, Guidiu MN, Broschi D et al. Alveolar soft part sarcoma of the vagina. Tumori 1990;76:77 |
|
Chang HC, Hseuh S, Ho YS et al. Alveolar soft part sarcoma of the vagina – A case report. J Reprod Med 1994;39:121 |
|
Christopherson WM, Foote FW Jr, Stewart FW. Alveolar soft part sarcoma: Structurally characteristic tumors of uncertain histogenesis. Cancer 1952;5:100 |
|
Hiura M, Nagai N. Vaginal hemangiopericytoma: A light microscopic and ultrastructural study. Gynecol Oncol 1985;21:376 |
|
Harris NL, Scully RE. Malignant lymphoma and granulocytic sarcoma of the uterus and vagina: A clinicopathologic analysis of 27 cases. Cancer 1984;53:2530 |
|
Matthews KS, Numnum TM, Conner MG et al. Fertility-sparing radical abdominal trachelectomy for clear cell adenocarcinoma of the upper vagina: a case report. Gynecol Oncol 2007;105(3):820-2 |
|
Stock RG, Chen ASJ, Seski J. A 30-year experience in the management of primary carcinoma of the vagina: Analysis of prognostic factors and treatment modalities. Gynecol Oncol 1995;56:45 |
|
Nanavati PJ, Fanning J, Hilgers RD et al. High dose-rate brachytherapy in primary stage I and II vaginal cancer. Gynecol Oncol 1993;51:67 |
|
Stock RG, Mychkzak B, Armstrong JG et al. The importance of brachytherapy technique in the management of primary carcinoma of the vagina. Int J Radiat Oncol Biol Phys 1992;24:747 |
|
Tewari KS, Cappuccini F, Puthawala AA et al. Primary invasive carcinoma of the vagina: Treatment with interstitial brachytherapy. Cancer 2001;91:758 |
|
Tyree WC, Cardenes H, Randall M et al. High-dose-rate brachytherapy for vaginal cancer: Learning from treatment complications. Int J Gynecol Cancer 2002;12:27 |
|
Nakagawa S, Koga K, Kugu K et al. The evaluation of the sentinel node successfully conducted in a case of malignant melanoma of the vagina. Gynecol Oncol 2002;86:387 |
|
Abramova L, Parekh J, Irvin WP et al. Sentinel node biopsy in vulvar and vaginal melanoma: Presentation of six cases and a literature review. Ann Surg Oncol 2002;9:840 |
|
Davis KP, Stanhope CR, Garton GR et al. Invasive vaginal carcinoma: Analysis of early-stage disease. Gynecol Oncol 1991;131:36 |
|
Kirkbride P, Fyles A, Rawlings GA et al. Carcinoma of the vagina – Experience at the Princess Margaret Hospital (1974–1989). Gynecol Oncol 1995;56:435 |
|
Leminen A, Forss M, Lehtovirta P. Therapeutic and prognostic considerations in primary carcinoma of the vagina. Acta Obstet Gynecol Scand 1995;74:379 |
|
Lee WR, Marcus RB Jr, Somebeck MD et al. Radiotherapy alone for carcinoma of the vagina: The importance of overall treatment time. Int J Radiat Oncol Biol Phys 1994;29:983 |
|
Roberts WS, Hoffman MI, Kavanaugh JJ et al. Further experience with radiation therapy and concomitant intravenous chemotherapy in advanced carcinoma of the lower female genital tract. Gynecol Oncol 1991;43:233 |
|
Kucera H, Vaura N. Radiation management of primary carcinoma of the vagina: Clinical and histopathological variables associated with survival. Gynecol Oncol 1991;40:12 |
|
Dixit S, Singhal S, Baboo HA. Squamous cell carcinoma of the vagina: A review of 70 cases. Gynecol Oncol 1993;48:80 |
|
Perez CA, Camel HM, Galakatol A et al. Definitive irradiation in carcinoma of the vagina: Evaluation of long-term results. Int J Radiat Oncol Biol Phys 1988;15:1283 |
|
Neoadjuvant chemotherapy followed by radical surgery in patients affected by vaginal carcinoma. Benedetti Panici P, Bellati F, Plotti F, Di Donato V, Antonilli M, Perniola G, Manci N, Muzii L, Angioli R Gynecol Oncol. 2008;111(2):307. |
|
Spirtos NM, Doshi BP, Kapp DL et al. Radiation therapy for carcinoma of the vagina: The Stanford University experience. Gynecol Oncol 1987;26:19 |
|
Reddy S, Lee MS, Graham JE et al. Radiation therapy in primary carcinoma of the vagina. Gynecol Oncol. 1987;26:19-24. |
|
Manetta A, Gutrecht EL, Berman ML et al. Primary invasive carcinoma of the vagina. Obstet Gynecol 1990;76:639 |