Disorders of the Thyroid Gland
Authors
INTRODUCTION
Following diabetes mellitus, thyroid disorders are the most common endocrine problems encountered in clinical practice. With the advent of more precise diagnostic tests, the diagnosis of thyroid disease can be made before classic symptoms become evident.
HYPOTHYROIDISM
Hypothyroidism is defined as the constellation of symptoms and signs resulting from insufficient synthesis of thyroid hormone. The clinical spectrum of hypothyroidism is wide and can range from absent signs and symptoms to the most severe form of hypothyroidism, myxedema coma. The term myxedema,sometimes used as a synonym for hypothyroidism, is used to define the presence of mucinous edema that can be recognized clinically in various tissues. Because many patients with hypothyroidism do not show such marked clinical changes, the term myxedema should be applied only to those with severe disease and specific tissue alterations.
Hypothyroidism may occur as a result of intrinsic disorders of the thyroid gland (primary hypothyroidism), lack of normal stimulation of the thyroid gland due to hypothalamic or pituitary disease (secondary hypothyroidism), or peripheral resistance to thyroid hormone, a rare but intriguing syndrome (Table 1).1 More than 95% of cases of hypothyroidism are due to primary thyroid disease. The diagnosis in most cases is readily available with the determination of serum thyroid-stimulating hormone (TSH) in the presence of low free thyroxine (FT4) concentrations. Serum TSH is elevated in cases of primary hypothyroidism and within normal limits in patients with secondary hypothyroidism. Other causes of low FT4 in the presence of normal TSH concentrations, including non-thyroidal illness (also known as sick euthyroid syndrome) are discussed briefly below.
Table 1. Etiology of hypothyroidism
| Primary hypothyroidism |
| Destruction or loss of thyroid tissue |
| Primary idiopathic hypothyroidism or primary thyroid atrophy |
| Post-131I ablation |
| Postsurgical ablation |
| Postexternal radiation |
| Congenital hypothyroidism, arising from sporadic thyroid aplasia or dysplasia or maternal hypothyroidism |
| Thyroid hormone biosynthetic defects |
| Iodine deficiency or excess |
| Use of antithyroid drugs |
| Inherited enzymatic defects |
| Goiter |
| Chronic autoimmune (Hashimoto's) thyroiditis |
| Endemic goiter (iodine deficiency) |
| Infiltrative disease |
| Goitrogen ingestion from foodstuffs or exposure from environmental toxicants |
| Secondary hypothyroidism |
| Pituitary disease |
| Tumor |
| Infarction |
| Trauma |
| Infiltrative disorders |
| Medications |
| Hypothalamic disease |
| Generalized thyroid hormone resistance |
| Transient hypothyroidism |
| Subacute thyroiditis |
| Silent thyroiditis |
| Postpartum thyroid dysfunction |
| Drugs (lithium, iodine, amiodarone, interferon-α) |
| Autoimmune chronic thyroiditis |
Classification
Primary hypothyroidism
Primary hypothyroidism may result from the destruction of thyroid tissue, biosynthetic defects in thyroxine (T4), or intrinsic disease of the thyroid gland with formation of goiter.2 From epidemiologic studies in the United States, Europe, and Japan, the prevalence of hypothyroidism ranges from 0.06 to 1.2% in women and 0.13 to 0.4% in men.3 In the United States, overt hypothyroidism and subclinical hypothyroidism are present in 0.3% and 4.3% of the population, respectively; overall, hypothyroidism is more common in women and the elderly,4 particularly in individuals with positive thyroid autoantibodies.
Subclinical hypothyroidism Subclinical hypothyroidism, in which the serum TSH is the only abnormal thyroid function test (i.e. the peripheral thyroid hormone concentrations are normal) is a risk factor for the development of overt hypothyroidism. In a follow up survey of the large United Kingdom-based Whickham study, an elevated serum TSH (defined as >2 mIU/L) was associated with an 8-fold risk of developing hypothyroidism among women over 20 years, which was increased to 38-fold in those also with positive thyroid autoantibodies.5 The incidence of progression to overt hypothyroidism is higher in women, due to the higher prevalence of thyroid antibodies in this group. Among subclinically hypothyroid individuals with only mildly elevated serum TSH concentrations and negative thyroid antibodies, there is a high rate of conversion back to euthyroidism.6
The most common causes of subclinical hypothyroidism are Hashimoto's thyroiditis resulting in hypothyroidism during its early stages, post-radioactive iodine ablation of the thyroid, and partial thyroidectomy. Subclinical hypothyroidism may also be diagnosed in patients taking insufficient thyroid hormone replacement therapy for the treatment of overt primary hypothyroidism. Medications such as lithium, saturated solution of potassium iodide (SSKI), iodine-rich amiodarone, and interferon-α may also cause mild thyroid dysfunction, particularly in patients with chronic autoimmune thyroid disease.
Some studies have shown subtle abnormalities of organ dysfunction, particularly cardiac, in patients with subclinical hypothyroidism.7 Elevations in cholesterol and low-density lipoprotein cholesterol levels and decreases in high-density lipoprotein levels have been reported in some studies.8 Subclinical hypothyroidism is associated with decreased right atrial and ventricular function and mechanics, which improve but do not entirely return to normal even after 1 year of maintaining euthyroidism with levothyroxine treatment.9 In one small double-blind randomized study, patients with subclinical hypothyroidism showed improvement of abnormal myocardial contractility after receiving levothyroxine therapy.10 Thyroid antibody status does not appear to influence cardiovascular risk in patients with subclinical hypothyroidism.11
Secondary hypothyroidism
Secondary hypothyroidism results from an impairment of TSH secretion from the pituitary resulting in decreased production of thyroid hormone. TSH deficiency may be accompanied by hyposecretion of other pituitary hormones (i.e. adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and growth hormone (GH)). Specific types of secondary hypothyroidism include: (1) pituitary hypothyroidism (e.g. pituitary tumor, postpartum pituitary necrosis); and (2) hypothalamic hypothyroidism (e.g. hypothalamic tumors, head injury, granulomatous disease, isolated TSH-releasing-hormone (TRH) deficiency).
Generalized thyroid hormone resistance This is a rare disorder characterized by reduced target tissue responsiveness to circulating free thyroid hormone concentrations (not discussed in this chapter).1
Transient hypothyroidism syndrome Although most hypothyroid patients require thyroid replacement therapy for life, some individuals may have only transient hypothyroidism. In this situation, the patient may experience a period of hypothyroidism during the course of subacute thyroiditis, painless thyroiditis, postpartum thyroid dysfunction, or following the discontinuation of thyroid therapy in euthyroid patients. Transient hypothyroidism also has been reported in some patients receiving supraphysiologic iodine and additionally, lithium therapy. There may be few or no clinical symptoms of hypothyroidism, but the diagnosis can be confirmed with thyroid function tests. In most of these patients, thyroid hormone levels will return to normal spontaneously, and thyroid replacement hormone medication is not indicated unless the hypothyroidism is severe or the patient is symptomatic.
However, patients with transient hypothyroidism should be followed closely, as the hypothyroidism may recur and can become permanent, such as in some cases of postpartum thyroid dysfunction.12 Resolution of hypothyroidism has been reported in a group of patients with chronic thyroiditis as a result of the disappearance of TSH-receptor-blocking antibodies (TRAb).13 This mechanism would also explain reports of spontaneous Graves’ disease, which developed among eight patients with longstanding Hashimotos’ hypothyroidism found to have positive titers of both thyroid stimulating and blocking antibodies.14
Clinical manifestations
Chronic thyroid failure develops over a period of many months or years, and many of the initial manifestations (e.g., fatigue, constipation, dry skin, weight gain) are not specific for hypothyroidism, but are frequent complaints in the general population. Mental and physical fatigue are among the first symptoms of most patients with hypothyroidism; this may go unnoticed for some time or may be attributed to other reasons, such as aging.15 With progression of hypothyroid disease, cold intolerance, slow speech, hoarseness, periorbital edema, and puffiness of the hands and feet become common complaints.
Thyroid failure may involve many organ systems, and the degree of involvement depends on several factors, the most important perhaps being chronicity of hypothyroidism. Changes in the central nervous system are manifested by a decrease in mental activity, lethargy, and loss of mental concentration. Sleepiness at the end of the day, hearing loss, and mental depression may be prominent manifestations. Paresthesias of the fingers as a consequence of carpal tunnel syndrome may be the first and only manifestation in some hypothyroid patients. The relaxation phase of the deep tendon reflexes is delayed, and this has become a characteristic sign of the disease, although it is present only in patients with advanced symptoms. Hypothyroid patients may present with arthralgia and stiffness of the small joints of the hands, and muscle cramps are not infrequent complaints. On physical examination, the skin may be rough, scaly and thickened and cold to the touch. Hair becomes coarse and brittle, and loss of axillary, pubic, and scalp hair is not unusual in severe cases. Features of periorbital edema may be evident when compared against previous pictures of the patient covering a span of several years. Bradycardia is noticed in some patients, and the electrocardiogram (ECG) may show a low voltage with nonspecific ST abnormalities. Occasionally, a patient may demonstrate cardiomegaly due to pericardial effusion or cardiomyopathy.
Gynecologic and obstetric abnormalities are not infrequent in hypothyroid women. Menorrhagia is commonly reported, although the true incidence is unknown. Anovulation is said to be common, although pregnancy with normal fetal outcome is not an uncommon event. If hypothyroidism is not promptly corrected, however, there is a higher risk of pregnancy-induced hypertension and premature delivery.16 Given the importance of adequate thyroid hormone levels during pregnancy, women on thyroid hormone replacement require an increase in levothyroxine dosage during pregnancy.17, 18 In the postpartum woman with galactorrhea-amenorrhea, primary hypothyroidism should be considered as one of the differential diagnoses.19
Laboratory diagnosis
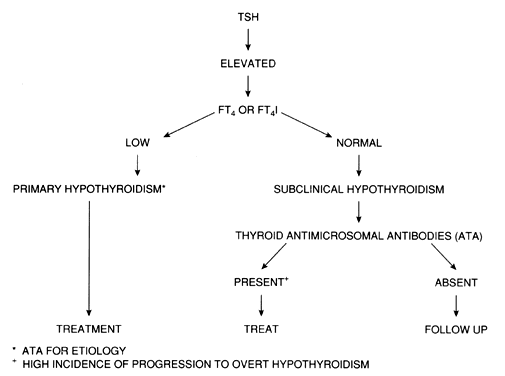
A decrease in the serum free thyroxine (FT4) concentration or its estimated FT4 index (FT4I) is characteristic of almost all forms of hypothyroidism, regardless of the specific subtype. Serum TSH concentration is elevated in patients with primary hypothyroidism, and this measurement is the most sensitive test for the diagnosis of primary thyroid failure.20 Serum TSH levels may be elevated in patients with a normal FT4I, and this condition has been called “decreased thyroid reserve,” or subclinical hypothyroidism. It may represent the first indication of primary hypothyroidism in the majority of cases (see below).
A low serum FT4I in the presence of normal serum TSH concentrations is diagnostic of secondary hypothyroidism in ambulatory patients. In hospitalized patients with intercurrent illness and abnormal thyroid function tests, underlying non-thyroidal illness must be ruled out.21
Other biochemical abnormalities seen in hypothyroid patients include elevations in serum cholesterol and triglycerides,22 elevations in serum creatinine phosphokinase, and nonspecific electrocardiographic abnormalities. It may be helpful to obtain serum thyroid antibody concentrations to confirm the cause of hypothyroidism; they are elevated in most patients with chronic thyroiditis. Although thyroid ultrasonography may demonstrate a characteristic pattern to support the diagnosis of chronic thyroiditis, it is usually unnecessary in patients with hypothyroidism. Radioisotope studies are not indicated in evaluating hypothyroid patients.
Screening
Screening for primary hypothyroidism is not presently recommended for the general adult non-pregnant population in the US.24 However, obtaining a serum TSH concentration may be helpful in evaluating patients with the following high risk factors for the development of hypothyroidism:
- Autoimmune thyroiditis
- Goiter
- Previous treatment for hyperthyroidism
- Previous high-dose head or neck radiation therapy
- Current lithium, amiodarone, or interferon-alpha therapy
- Type 1 diabetes mellitus and other autoimmune diseases (e.g., pernicious anemia, idiopathic thrombocytopenia)
- Postpartum state, particularly in combination with a goiter.
Other risk factors include sleep apnea and hyperlipidemia in adults and failure to grow properly in children. Because the incidence of subclinical hypothyroidism is increased after the age of 50, particularly in women, screening postmenopausal women may be also reasonable (Fig. 1). Given the important of normal thyroid function during pregnancy, the American Thyroid Association and Endocrine Society recommend selected screening (i.e. a case-finding approach) in pregnant women with risk factors.25, 26
Myxedema coma
Myxedema coma is a rare clinical situation and defined as the most severe form of hypothyroidism which constitutes a life-threatening emergency. It can arise in untreated patients with longstanding myxedema exacerbated by stressful situations, such as infection. Occasionally, the use of narcotics and analgesics can precipitate myxedema coma in patients with overt hypothyroidism. A potential cause of hypothyroid coma is the discontinuation of thyroid therapy in hypothyroid patients previously taking thyroid hormone replacement therapy; lack of thyroid hormone for more than 2 weeks can produce severe hypothyroidism. Clinically, patients present with progressive stupor and coma, seizures, hypotension, hypoventilation, and hypothermia, and respiratory acidosis and hyponatremia may be present. A diagnostic scoring system for grading myxedema coma has been recently proposed.27
Because the mortality is high, aggressive treatment with thyroid hormone replacement is indicated and should be initiated urgently. An initial dose of 4 µg/kg ideal body weight of levothyroxine, followed by 100 µg 24 hours later and 50 µg/day thereafter, should be administered.28 Triiodine (T3) can also be co-administered at 10 µg every 8–12 hours until the patient is able to tolerate oral levothyroxine.28 Concomitant with levothyroxine administration, 50–100 mg IV hydrocortisone every 6–8 hours should be administered every 8 hours, as many patients with such severe hypothyroidism may also have relative adrenal insufficiency, or the hypothyroidism may be due to pituitary or hypothalamic disease.28 Assisted ventilation may be required, and fluid replacement should be given cautiously.
Treatment
Before thyroid hormone replacement therapy is initiated in hypothyroid patients, a careful diagnosis between primary and secondary hypothyroidism is imperative. In patients with secondary hypothyroidism, a complete evaluation of the hypothalamic–pituitary axis should be made to rule out potential deficiency of other pituitary hormones, as isolated TSH deficiency is uncommon.
Several preparations of thyroid hormone replacement are available, and principles to be considered before therapy is started include:
1. Most patients will need lifelong replacement therapy;
2. Therapy should be started with a low dosage, especially in the elderly and those with heart problems, and gradually increased to the full replacement dosage;
3. The goal of any treatment is to achieve euthyroidism, both clinically and biochemically.
Treatment of overt primary hypothyroidism
Because the hormonal content in synthetic levothyroxine is more reliably standardized than that of desiccated thyroid hormone extract, it is considered the mainstay of thyroid hormone replacement therapy in patients with overt primary hypothyroidism. With very few exceptions, a recent survey of US endocrinologists showed that levothyroxine is the current preferred therapy among.29 Additionally, thyroxine (T4) is deiodinated to triiodothyronine (T3) in extrathyroidal tissues in humans, and thus, more closely simulates normal physiology. If desiccated thyroid hormone extract is used, it should be noted that the T4/T3 ratio varies by brand and whether it is of porcine or bovine origin.30
Levothyroxine is generally well-tolerated; after administration as a single oral daily dose, approximately 62–82% is absorbed.31 The half-life of levothyroxine ranges from 6 to 9 days.32 Some medications (e.g. ferrous sulfate, cholestyramine, sucrosulfate, iron, calcium, aluminum hydroxide) and food, such as expresso coffee, have been reported to decrease thyroid absorption in the gastrointestinal tract if taken concomitant with thyroid hormone therapy.30, 31 Malabsorption, such as that due to celiac sprue and inflammatory bowel disease, and hypertrophic gastritis also may produce a decrease in T4 absorption by the gastrointestinal tract.31 If the patient is unable to take an oral preparation (i.e. myxedema coma), an intravenous levothyroxine dose should be initiated at 70% of their estimated oral dose requirement.30
Normal serum TSH concentrations are the goal of levothyroxine therapy. The recommended replacement oral dose is approximately 1.6 µg/kg ideal body weight/day.30, 33 The physician should be aware of the potential differences in the levothyroxine content among several generic thyroid preparations available today, although the US Food and Drug Administration requires a maximum difference potency of 12.5% for all generic preparations.34 For most patients, full replacement is achieved by 6–8 weeks. The serum TSH level is determined 4–6 weeks after therapy is initiated, and the dose is adjusted accordingly. Thereafter, patients should be seen at yearly intervals, at which time the dose may be adjusted depending on serum TSH results.
It should also be noted that elderly patients generally require smaller doses of levothyroxine. In older patients without known heart disease, the recommended initial dose is 50 µg levothyroxine daily. In those with coronary heart disease, levothyroxine should be started at 12.5–25 µg/day.30
Treatment of subclinical hypothyroidism
Individuals with subclinical hypothyroidism in which the serum TSH is ≥10 mIU/L should generally be recommended to begin thyroid hormone replacement therapy.30 However, because the degree of hypothyroidism is mild and most individuals with subclinical hypothyroidism are asymptomatic, treatment is controversial for those with TSH ≤10 mIU/L. As the rate of progression to overt hypothyroidism in patients with positive antibodies is over 3-fold that of those with negative antibodies over 2 years,6 it is advisable to start thyroid therapy in patients with subclinical hypothyroidism and positive thyroid antibodies. Treatment is also imperative in women of childbearing age with subclinical hypothyroidism, given the importance of euthyroidism for neurodevelopment of the fetus during pregnancy.23
Treatment of secondary hypothyroidism
In patients with secondary hypothyroidism, serum TSH concentrations are uninterpretable and thus, normal FT4 levels should be targeted. Oral levothyroxine should be initiated at1.6 µg/kg ideal body weight/day.30
Long-term thyroid hormone therapy
Individuals with primary hypothyroidism treated with long-term levothyroxine therapy should have serum TSH maintained within normal limits by monitoring performed yearly.30
Other uses of thyroid hormone
Although thyroid hormone is used primarily as replacement therapy for hypothyroidism, it is also used for other conditions in euthyroid persons, such as nodular goiters,35 and empirically for the treatment of obesity, chronic fatigue, hypercholesterolemia,36 excessive daytime somnolence,37 and infertility.38 In recent years, thyroid hormone receptor-β selective agonists have been proposed to be potentially therapeutic in metabolic disorders,39, 40 but use their future application may be limited due to their associated toxicity in animal models.41
Risks of suppressive levothyroxine therapy
However, higher doses of levothyroxine are intentionally used in patients with thyroid cancer, in whom a lower serum TSH is targeted. Particularly in older individuals, this suppressive thyroid hormone therapy has been associated with adverse risks to cardiac and bone health and should be weighed against the benefits of thyroid cancer management. Levothyroxine suppressive therapy is associated with increased rates of atrial fibrillation,42 heart rate, and left ventricular mass.43 Hyperthyroidism due to intrinsic thyroid disease or excessive thyroid dosage affects bone resorption and bone formation44 and increases the risk of hip fracture.45 In postmenopausal women, these adverse effects persist even among those who receive adequate calcium and vitamin D supplementation, in whom the benefit of alendronate therapy is dampened by long-term thyroid suppression.46
THYROIDITIS
Hashimoto's thyroiditis (chronic lymphocytic or autoimmune) is one of the most common causes of goiter and primary hypothyroidism, and observed much more frequently in females than males (4:1). The diagnosis is confirmed by clinical findings and elevated serum thyroid antibody titers. The patient may present with a goiter that is firm in consistency, has a tendency to be lobulated, and affects both lobes of the thyroid gland. Although biopsy is unnecessary, histology would demonstrate diffuse lymphocytic infiltration, obliteration of thyroid follicles, and fibrosis.49 Patients with chronic thyroiditis and their families have an increased incidence of other autoimmune diseases, such as pernicious anemia, insulin-dependent diabetes mellitus, vitiligo, Sjögren's syndrome, rheumatoid arthritis, chronic active hepatitis, and adrenal insufficiency.
The natural course of goiter is to remain unchanged or to enlarge gradually over many years if left untreated. Hashimoto’s thyroiditis is a risk factor for the development of hypothyroidism, and exposure to excess iodine in patients with chronic thyroiditis may trigger the development of hypothyroidism or hyperthyroidism.50 Although reports have been inconsistent, there does not appear to be an increased incidence of papillary thyroid carcinoma among individuals with Hashimoto’s thyroiditis.51
Patients with chronic thyroiditis may develop transient thyroid dysfunction during pregnancy and in the postpartum period. They may have mild symptoms of hyperthyroidism 4–6 weeks postpartum, with enlargement of the thyroid gland and subsequent hypothyroidism at 3–5 months postpartum. These patients will return to the euthyroid state 5–10 months after delivery without undergoing specific treatment. It has been proposed that these changes are due to changes in the immune response during pregnancy.52
Treatment of euthyroid chronic thyroiditis with thyroid hormone is used by some clinicians to decrease goiter size, although this is controversial and not widely practiced. In long-standing goiter that is firm on palpation, therapy is usually not effective in reducing its size as the gland is generally fibrotic. In the usual patient not treated with thyroid hormone, they are followed yearly to detect any trends toward hypothyroidism. These patients should be advised to have their thyroid tests checked during pregnancy and in the postpartum period, as there is some risk for development of thyroid dysfunction. Surgery is justified only in the unusual case of local pressure symptoms (e.g. dysphagia, cough, respiratory distress).
Subacute thyroiditis
Subacute thyroiditis is an acute, self-limiting viral infection affecting the thyroid gland resulting in leakage of preformed thyroid hormone (i.e. thyrotoxicosis).53 On pathologic examination, the gland is enlarged and characterized by an inflammatory reaction involving the capsule. Polymorphs, lymphocytes, and foreign-body giant cells are recognized. Women are affected twice as often as men. The disease is preceded by a protracted phase of low-grade fever, malaise, and muscle aches for 2–3 weeks, followed by neck pain that often radiates to the angle of the jaw and to the ear on the involved side. Some patients may complain of dysphagia. The thyroid gland is tender to palpation and enlarged, and sometimes one of the lobes is as hard as stone. Over several days, it returns to normal as the opposite side enlarges and becomes tender. Mild signs of hyperthyroidism may develop in some patients. The course of the disease varies from a few weeks to several months, and there is eventual spontaneous recovery. Patients may experience a period of hypothyroidism before final recovery to euthyroidism.54
Diagnostic findings include elevation in serum FT4I and FT3I and, in most cases, suppression of TSH concentrations. There may also be a slight elevation in the thyroid antibody titer. Diagnosis is confirmed by an elevation in the serum erythrocyte sedimentation rate and a low 24-hour radioiodine 131I thyroid uptake. In the early phase of the disease, treatment consists of analgesics. In patients in whom analgesic medication is ineffective, the use of corticosteroids (e.g. 40 mg prednisone daily in divided doses) is very effective in controlling symptoms, particularly neck pain.(54) Prednisone dosage may be reduced after a few days and discontinued by 3–6 weeks in most cases. If a patient has significant hyperthyroid symptoms, 10–40 mg propranolol three times per day is effective in controlling the symptoms. Antithyroidal drug therapy is not indicated in the treatment of the thyrotoxic phase of subacute thyroiditis, as the underlying etiology is not increased thyroid hormone production.
A similar clinical picture without thyroid pain has been described. This has been termed painless thyroiditis or silent thyroiditis;53 its relationship to the classic subacute thyroiditis is not clear. Clinically, more than 90% of patients with silent thyroiditis have manifestations of thyrotoxicosis with elevations in serum T4 and T3 and suppressed TSH concentrations. Similarly, the 24-hour 131I thyroid uptake is low. The most common symptoms are palpitations, nervousness, weakness, and heat intolerance. Eye signs typical of Graves' disease are absent, although approximately one-third have lid lag or lid retraction. The thyroid gland is enlarged and nontender. Recovery from hyperthyroidism usually takes 3–5 months. Many of these patients go through a period of transient hypothyroidism lasting a few weeks. The course is similar to patients with postpartum thyroid dysfunction.54
MULTINODULAR GOITER
Multinodular goiter can be defined as an enlargement of the thyroid gland resulting from excessive growth and structural or functional transformation of one or several areas within the normal thyroid tissue. The pathogenesis of nodular transformation is not known, although chronic TSH stimulation in thyroid glands with abnormal follicular growth pattern plays an important role, as does iodine deficiency in endemic areas.55 However, the etiology of sporadic nodular goiter is unclear, and many associations have been proposed, including smoking, genetics, and other risk factors.56 The prevalence of nodular goiter differs whether it is detected by palpation (2–6%), ultrasonography (19–35%), or autopsy studies (8–65%).57
The slow natural history of nontoxic multinodular goiter suggests that diffuse enlargement of the thyroid may begin in adolescence with the gradual appearance of nodularities. These nodules grow slowly, over several decades, without producing significant clinical signs or symptoms. They are encountered more often in women and the elderly. Some goiters will remain very stable, whereas others will continue to grow steadily or may regress.58 In some patients, thyroid antibodies may be elevated, indicating that some of these goiters are related to chronic lymphocytic thyroiditis (Hashimoto's thyroiditis).
Most commonly, the diagnosis of a nontoxic nodular goiter is made during a routine physical examination. Inspection of the neck is an important part of the thyroid examination, as nodules and asymmetry of the thyroid gland can be demonstrated easily in this way, particularly as the patient swallows gulps of water. The consistency of the nodules varies widely, from soft to hard. The patient may be unaware of the goiter. Very seldom, compression or local symptoms are the presenting complaint; occasionally, rapid growth of the nodule or sudden, acute pain indicating hemorrhage into a nodule causes the patient to seek help from the physician. Serum thyroid function tests are usually normal; however, if the sensitive TSH test is suppressed in the presence of normal peripheral serum thyroid hormone concentrations, the possibility of an autonomous thyroid nodule(s) should be considered. Thyroid nuclear 131I scanning is helpful in defining the presence of an autonomous or “hot” nodule within a multinodular goiter. Although no therapy is usually required, such patients should be followed closely for the development of overt hyperthyroidism.
Two possible conditions related to the natural history of multinodular goiter deserve discussion: (1) the development of hyperthyroidism; and (2) an underlying thyroid malignancy.59 Thyrotoxicosis in patients with multinodular goiters may develop insidiously. In others, exposure to iodine excess may inadvertently result in thyrotoxicosis,50 with sources of supraphysiologic iodine exposure to include iodinated contrast dyes, topical antiseptics, dietary supplements (e.g. iodine or kelp tablets), and amiodarone, an iodine-containing antiarrhythmic drug.60 Therefore, patients with known nodular goiters should be followed and thyroid tests should be performed at regular intervals, as it may not always be possible to recognize this complication clinically. Ultrasound may be indicated to follow the progression of size of the gland. Additionally, nodular goiter may be a predisposing risk factor for the development of thyroid carcinoma.61 Carcinoma should be suspected when the thyroid scan shows hypofunctioning nodules or if there has been a recent increase in the size of one of the nodules; fine needle aspiration biopsy should be performed in such cases.
In some cases, levothyroxine therapy has proved effective in suppressing further goiter growth, although it is unusual to obtain complete regression. It should be kept in mind, however, that giving thyroid therapy to patients with multinodular goiter can lead to hyperthyroidism in patients with autonomous nodules. Therefore, thyroid therapy for multinodular goiter should be used only in patients with normal serum TSH values and only to decrease goiter size; this practice is not considered routine.62
The indication for surgical therapy in multinodular goiter varies from center to center, but it is widely accepted that surgery should be performed only by highly trained surgeons and ideally in facilities equipped with the ability to intraoperatively examine frozen sections. Thyroid surgery is indicated for a goiter producing associated symptoms, for a suspicion of malignancy, or for cosmetic reasons.
SOLITARY THYROID NODULE
As in the case of multinodular goiters, the finding of a single thyroid nodule is usually incidental during a routine physical examination. For the nodule to be palpable, it is usually at least 1 cm in diameter.
Clinical manifestations
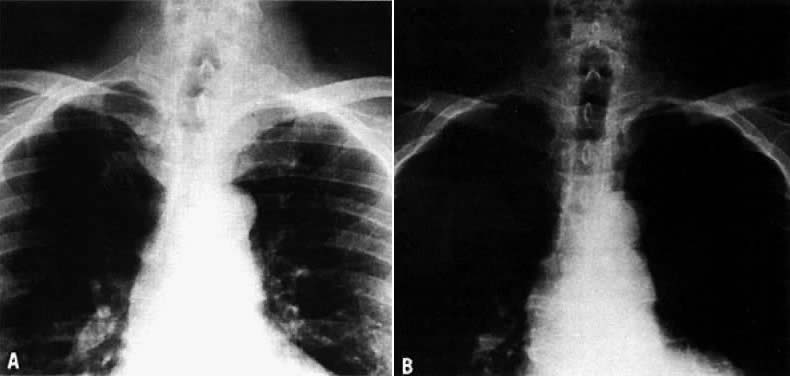
On physical examination, an asymptomatic neck mass is the most common presenting sign. Very seldom does the patient have local symptoms, such as dysphagia, hoarseness, and occasionally acute neck pain due to bleeding into the nodule. The majority of patients with thyroid nodules are clinically and biochemically euthyroid. Cervical lymph nodes, cord paralysis, pain, or dysphagia may suggest an associated thyroid carcinoma. Larger thyroid nodules may produce deviation of the trachea, as demonstrated by X-ray or computed tomography (CT) imaging. A thyroid cyst can produce a deviation of the trachea that resolves after aspiration of the fluid (Fig. 2).
Evaluation
The possibility of an underlying thyroid malignancy should be considered upon detection of a thyroid nodule, particularly in young persons and older men.61 Other risk factors include a history of irradiation to the head or neck during infancy or childhood or rapid nodule growth. A familial basis for papillary thyroid cancer has been proposed as another risk factor.63
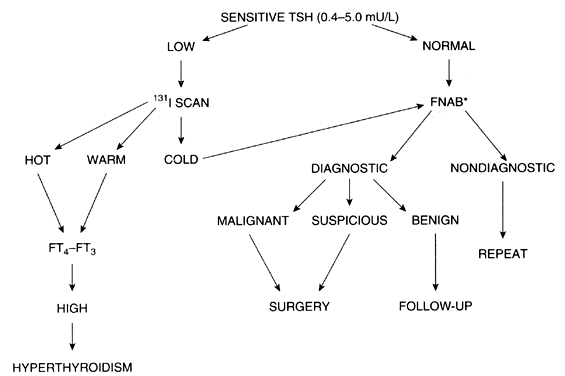
The evaluation of a single thyroid nodule is controversial, and several approaches have been recommended.64 The usual approach is the determination of thyroid function as the initial test,65 because the presence of thyroid dysfunction in the presence of a single thyroid nodule is almost always an indication of a benign process, although exceptions occur, particularly in “cold” nodules in the presence of Graves' disease. The next step suggested is a thyroid scan to determine whether the nodule is hypofunctioning (i.e. cold) or hyperfunctioning (i.e. warm, hot). as hyperfunctioning nodules have a <1% chance of an underlying malignancy.
Fine-needle aspiration biopsy is indicated for hypofunctioning nodules. The rate of false negative cytology results is lowest among experienced cytopathologists; overall rates range from 7 to 13%.66 Improvement in the sensitivity and specificity of the cytological interpretation of a thyroid nodule has resulted in a higher rate of excision of cancerous nodules and a decline in surgical intervention of noncancerous nodules. In most series reported, 65% of the nodules are benign, 8% are malignant, and the remainder are either insufficient for analysis due to an inadequate biopsy or indeterminate.67 The introduction of Bethesda system for classifying thyroid cytopathology has allowed for the standardization of results,68 although it remains not universally used and findings have been different across various series. Additionally, in recent years, the availability of molecular expression profile testing of fine needle aspiration biopsy samples has allowed additional risk stratification of thyroid nodules.69
A less common approach to the evaluation of thyroid nodules is preferred in some centers, with fine needle aspiration biopsy the first test performed for a single or predominant thyroid nodule.64 If the diagnosis is malignancy, surgical excision is indicated; the type of surgery (lobectomy vs. subtotal or total thyroidectomy) depends on the experience and criteria of the surgeon; a full discussion is beyond the scope of this review. Following surgery, thyroid suppression with levothyroxine is initiated, and consideration is made regarding 131I therapy.65 The long-term prognosis for thyroid cancer patients depends on the type of tumor, presence of local or distant metastasis, and other factors. The authors of this review’s approach to the evaluation of solitary thyroid nodules is presented in Fig. 3.
Treatment
For lesions that are benign on cytologic examination, long-term thyroid suppression therapy has been classically recommended; however, observations show that it is effective only in a minority of patients.70 It is the senior author’s practice to use thyroid suppression therapy for 3–6 months in benign nodules and to continue it if there has been a significant reduction in the size of the nodule. To avoid the potential complications of osteoporosis and subclinical hyperthyroidism, however, the serum TSH should be kept between 0.02 and 0.4 mIU/L. Other recent therapeutic modalities that have been developed include percutaneous laser ablation and ethanol ablation.71 If a large benign thyroid nodule is excised by partial thyroidectomy, thyroid hormone replacement therapy may still be required if postsurgical hypothyroidism develops.
Most malignant tumors of the thyroid are papillary or follicular carcinoma, although mixed lesions are not unusual. Undifferentiated carcinoma occurs in the elderly, and the prognosis is poor. Medullary thyroid carcinoma accounts for approximately 2% of thyroid cancer; it may occur alone or as part of the syndrome of multiple endocrine neoplasia in association with pheochromocytomas, parathyroid adenomas, and mucosal neuromas.72 In patients suspected of having this type of tumor, it is useful to measure serum calcitonin levels, which are characteristically elevated.
HYPERTHYROIDISM
Thyrotoxicosis is a general term used to define excess serum thyroid hormone concentrations arising from multiple potential etiologies, whereas the term hyperthyroidism should be reserved to characterize excess thyroid hormone produced specifically by the thyroid gland. The clinical manifestations of thyrotoxicosis vary depending on the patient's age, duration of the disease, complications, abnormalities in other systems, and the cause of the syndrome. The most common etiology of hyperthyroidism is Graves' disease; other causes are listed in Table 2. In addition to accurately diagnosing thyrotoxicosis, it is important to identify its etiology, as each is associated with different natural histories and may require different forms of therapy.
Table 2. Etiologies of thyrotoxicosis
| Most common |
| Graves' disease |
| Toxic adenoma |
| Toxic multinodular goiter |
| Less common |
| Subacute thyroiditis |
| Thyrotoxicosis factitia |
| Iodine-induced thyrotoxicosis |
| Chronic autoimmune thyroiditis |
| Silent (painless) thyroiditis |
| Unusual cases |
| Trophoblastic hyperthyroidism |
| Struma ovarii |
| Thyroid carcinoma |
| Pituitary thyrotropin-stimulating hormone (TSH) hypersecretion |
| Hyperthyroxinemia from hyperemesis gravidarum |
Graves' disease
Graves' disease, also known as Basedow's disease, exophthalmic goiter, or diffuse toxic goiter, is of autoimmune etiology and characterized by diffuse goiter, biochemical hyperthyroidism, infiltrative ophthalmopathy, pretibial myxedema, and acropachy. Only a minority of patients, however, display all of these manifestations; hyperthyroidism and goiter with some degree of eye involvement is the characteristic presentation. In addition, infiltrative ophthalmopathy and, rarely, infiltrative dermopathy may be present without concurrent hyperthyroidism (i.e. euthyroid Graves' disease). Graves’ disease occurs more frequently in predisposed families and could be associated with other autoimmune disorders, similar to the epidemiology of chronic thyroiditis.
Clinical manifestations
The most common clinical manifestations include nervousness, increased sweating, hypersensitivity to heat, palpitations, fatigue, weight loss, shortness of breath, muscle weakness, and eye complaints. In some patients, edema of the lower extremities, frequent bowel movements without diarrhea, anorexia, and eye complaints may be the initial manifestations. The time course may be insidious over a few months or of rapid onset over a few weeks. In older persons, the disease may have few presenting symptoms, such as severe weakness, wasting of muscles, and atrial fibrillation (e.g. masked hyperthyroidism).
On physical examination, the thyroid gland tends to be bilaterally enlarged 2–4 times the normal size, firm, and painless. In a minority of patients, particularly the elderly, a palpable goiter may be absent. A thrill and bruit may be appreciated in patients with large thyroid glands. Retraction of the eyelids leads to widening of the palpebral fissure, and this may produce the lid lag typical of hyperthyroidism, regardless of the cause. On close observation, a tremor of the lids may be seen. Changes in the nails are seen commonly as separation of the distal margin of the nail from the nail bed (i.e. onycholysis). The skin is warm, moist, and smooth. Tremor of the hands and tongue is sometimes easily detected. Vitiligo is present in less than 5% of pediatric patients with Graves' disease.74 Tachycardia is the most frequent cardiovascular manifestation and usually presents as palpitations; tachycardia during sleep may be helpful in distinguishing tachycardia of thyrotoxic origin from that caused by anxiety. Cardiac arrhythmias (particularly in the form of atrial fibrillation) are common in the elderly. Edema of the lower extremities may be seen in patients with thyrotoxicosis unrelated to congestive heart failure; it resolves after the hyperthyroidism is treated. In women, menstrual function may be disturbed, and oligohypomenorrhea, anovulation, and sometimes amenorrhea are not uncommon. Changes in menstrual function could be related to alterations in estrogen metabolism in patients with thyrotoxicosis. However, pregnancy in hyperthyroid women, particularly when the thyrotoxicosis is not severe, is not an uncommon event.
Graves’ opthalmopathy The ophthalmopathy of Graves' disease is a disorder of complex etiology and variable clinical presentation.75 It may precede, accompany, or follow hyperthyroidism, or it may be seen in the absence of biochemical thyroid dysfunction. The clinical presentation varies from minimal disease to severe infiltrative disease. The most common symptoms include mild pain or eye discomfort, lacrimation, photophobia, and blurring of vision. Patients may complain of diplopia, or it may be discovered for the first time on careful physical examination. The most common signs are periorbital edema, lid edema, lid lag, conjunctival edema and injection, and exophthalmos. Exophthalmos is probably the most common manifestation and is usually bilateral, but often slightly asymmetric. Unilateral exophthalmos may be present; this was present in 13.5% of patients undergoing initial evaluation for Grave’s disease in one Italian study.76 Graves’s disease is the most common etiology of both unilateral and bilateral exophthalmos in adults.77 Cigarette smoking is a known risk factors for Graves’ ophthamopathy (GO); treatments used to treat GO include local measures and observation, selenium therapy, glucocorticoids, decompression surgery, and in recent trials, rituximab and other immunomodulators.78, 79, 80
Pretibial myxedema
Pretibial myxedema, or infiltrative dermopathy, is an uncommon manifestation of Graves' disease, but which often coexists with severe orbitopathy.81 The lesion is usually localized in the pretibial area and appears as a violaceous induction of the skin or, at times, as localized nodular areas (Fig. 4). In some patients, spontaneous remission may occur. However, in most patients, the disease must be treated because of the severity of the symptoms and potential complications of limb enlargement and impaired function. Local topical corticosteroid application and compression stockings should be used. Thyroid acropachy may also be present and describes an unusual presentation of clubbing of the digits and osteoarthropathy.
Fig. 4. Pretibial myxedema (nodular form)
Diagnosis
The diagnosis is confirmed by a suppressed serum TSH and elevation in FT4I or FT3I concentrations. Occasionally, the FT4I is within normal limits despite clinical symptoms of thyrotoxicosis, but the serum T3 concentration should be elevated (i.e. T3 toxicosis). Once the diagnosis is made, the second step is to determine the cause of hyperthyroidism. When the clinical diagnosis of Graves' disease is obvious (e.g. diffuse goiter, infiltrative ophthalmopathy, high ratio of T3:T4, and infiltrative dermopathy), no other tests are indicated. In other situations in which the cause of hyperthyroidism is not readily apparent, a thyroid nuclear uptake and scan and determination of a TSH-receptor antibody titer may be important tools.
TSH-receptor antibody
In the early 1960s, Adams and colleagues identified a substance in the serum of some patients with Graves' disease that was able to stimulate the thyroid gland of the guinea pig;73 because of its prolonged effect, this substance was named long-acting thyroid-stimulating (LATS) hormone. This observation and those of others seemed to indicate that LATS was a thyroid-stimulating antibody occurring in the blood of some patients with Graves' disease and possibly causing hyperthyroidism. It is now recognized that this substance is a TSH-receptor antibody and may be further classified into activating antibodies (i.e. thyroid stimulating antibodies can be helpful to confirm Graves’ disease), blocking antibodies, or neutral antibodies. Several commercially available techniques are used for its determination, but the clinical indications are few (Table 3).
Table 3. Clinical use of TSH-receptor antibody (TRAb)
| Pregnancy |
| Prediction of neonatal hyperthyroidism |
| Diagnosis of fetal hyperthyroidism |
| Euthyroid Graves' ophthalmopathy |
| Euthyroid Graves' dermopathy |
Treatment
There are three principal forms of treatment for hyperthyroidism due to Graves' disease. Antithyroid drug therapy (with or without beta-adrenergic antagonist medications), 131I therapy, and surgery are all very effective in controlling symptoms, and all various treatment options are to be discussed with the patient. However, the indications for choosing a specific treatment can depend on the physician's experience and preference.82
Antithyroidal drugs (thionamides)
Thionamides have been available for the treatment of hyperthyroidism since the early 1940s.83 The most commonly used drugs in the US are methimazole and propylthiouracil, which block thyroid hormone synthesis by different mechanisms.84 In addition, propylthiouracil acts extrathyroidally as a potent inhibitor of the type I 5-deiodinase, which converts T4 to T3.85 During pregnancy, propylthiouracil should be used during the first trimester, then switched to methimazole at the beginning of the second trimester until delivery, given the slightly increased risks of various congenital malformations with propylthiouracil and of maternal fulminant hepatic failure with methimazole.25, 26, 30 Carbimazole is comparable to methimazole and used in many other parts of the world.
Initial divided doses of 100–150 mg propylthiouracil every 8 hours or methimazole (up to 30 mg a day), are expected to produce euthyroidism in 4–8 weeks. After the euthyroid state is achieved, the amount of medication is decreased and adjusted according to the clinical picture and the determination of serum T4 and T3 concentrations; serum TSH concentrations may continue to be suppressed and should not be used as the primary marker of therapy. Treatment with this regimen for 6–24 months should produce remission in approximately half of patients,86 their mechanism of action may be in part a combination of the medications’ immunosuppressive effects and/or their actions in restoring euthyroidism.87 Although the recommended duration of antithyroidal therapy is up to 18 months due to the risk of potential adverse effects in these medications,84 long-term use has been reported with some benefits,88 particularly in children with Graves’ disease.89, 90 The “block and replace” approach, which utilizes a combination of antithyroidal drugs and levothyroxine therapy, has also been used by some clinicians.91
Prognostic factors which appear to be associated with increased remission rates while using antithyroidal therapy include small goiters, mild symptoms, and short-term duration of the disease. However, side-effects of various types can be seen with both methimazole and propylthiouracil. The most common side-effects of both medications are pruritus and mild skin rashes, which are treated with antihistamines and switching to a different thionamide. The most dangerous complication is agranulocytosis, which occurs in fewer than 0.4% of patients treated, but is associated with mortality.84 Because this complication can occur suddenly, frequent routine blood counts are not useful in predicting an idiosyncratic response. The patient should be aware of this possible complication and alert their physician if a severe fever and/or sore throat develop, which can be noted as instructions on the prescription. If the diagnosis of agranulocytosis is confirmed by laboratory testing, the patient should be hospitalized and treated with antibiotics. The use of granulocytosis-stimulating factors appears to shorten the period of recovery in selected individuals.92
Beta-adrenergic antagonist medications
Beta-blockers (i.e. 20–80 mg propranolol three to four times daily for a few days) have been historically used in the treatment of symptoms of hyperthyroidism with good effect. Propranolol does not, however, play a role in the long-term treatment of thyrotoxicosis. It should be used in conjunction with antithyroid drugs only until symptoms improve and the antithyroid drug becomes effective, which is normally 7–14 days. Other beta-blocker agents, such as atenolol, are as effective as propranolol and may be easier to take given their once daily dosing.
Radioiodine therapy
131I therapy for Graves’ disease has been used since the early 1940s and is an effective therapeutic choice, although those with severe hyperthyroidism and larger goiters are less likely to respond.93 In patients with mild symptoms of hyperthyroidism, 131I may be given as the only treatment; if needed, beta-blockers can be also used for controlling hyperthyroid symptoms for a few weeks before and after treatment. In more symptomatic patients and in the elderly, it is advisable to control hyperthyroidism with antithyroid drugs before radiotherapy is given. The antithyroid drug can be discontinued 3 or 4 days before radiotherapy, although this is not always necessary. Radiotherapy is contraindicated in pregnancy and, given after 8–10 weeks of gestation, may produce congenital hypothyroidism. Women of reproductive age should receive the treatment in the first 10 days of the onset of menses or after a negative pregnancy test. For Graves’ disease in general, the usual dose of 131I is usually less than 20 mCi; the most common complication is permanent hypothyroidism.
Ophthalmopathy associated with Graves’ disease may be exacerbated with the use of 131I therapy.94 Thus, it is recommended that radiotherapy be postponed until the ophthalmopathy is stable or that concurrent glucocorticoid therapy be given at the time of 131I administration. Cigarette smoking also increases the risk of worsened thyroid eye disease upon 131I therapy,94 and thus, patients should be counseled to quit.
There is also a potential risk of secondary malignancies following radioiodine therapy; this association remains controversial and has not been definitively proven. In a meta-analysis of two large multicenter centers, the use of radioiodine was associated with a very modest increase (RR 1.19) of secondary primary malignancies.95
Surgery
There are few indications for subtotal thyroidectomy in the management of patients with Graves' disease, which include patients refusing 131I therapy and those with large goiters or a hypofunctioning nodule. It also has been used in the first half of pregnancy to avoid the use of antithyroid drugs or in the rare pregnant patient who is allergic to both antithyroid medications. Permanent hypothyroidism is a potential complication, and patients should be followed up with thyroid tests on a regular basis. Relapses of hyperthyroidism can occur up to 30 years after the original surgery, hence the need for long-term follow-up in these patients.96 As thyroid antibodies traverse the placenta easily, thus fetal Graves’ disease should be monitored for in pregnant women with a history of Graves’ disease treated previously with thyroidectomy.97
Other causes of hyperthyroidism
Toxic adenoma
In patients with a toxic adenomas (also known as a hot nodule or autonomously functioning nodule), thyrotoxicosis is caused by overproduction of thyroid hormones due to a single autonomous thyroid nodule. On physical examination, a single nodule may be palpable. The clinical symptoms are generally mild in most patients, and signs of Graves' disease (e.g. exophthalmopathy and dermopathy) are characteristically absent. The diagnosis is confirmed by a thyroid nuclear scan showing a warm or hyperfunctioning nodule and low radioiodine uptake in the rest of the thyroid gland. Occasionally, a hyperfunctioning nodule may be incidentally detected on a thyroid nuclear scan in a patient with normal thyroid tests. It is possible for some of these euthyroid nodules to progress to toxic adenomas. Most of them, however, remain euthyroid or may regress over many years. Radiotherapy is the therapy of choice in the majority of patients with a toxic adenoma. Antithyroid medications can be used to control hyperthyroidism, but are not a definitive treatment; instead, they may be used preoperatively if thyroid surgery is chosen as the treatment option for a toxic adenoma.
Toxic multinodular goiter
The occurrence of hyperthyroidism in patients with multinodular goiter is seen mainly in older patients who have had a long-standing goiter or those who reside in an area of relative iodine deficiency, in whom supraphysiologic iodine exposure or therapy may trigger this development.50 It may also occur in patients who have changed their diet from low to high iodine intake. Similar to a toxic adenoma, 131I therapy or thyroid surgery are the preferred treatment options.
Subacute thyroiditis and silent thyroiditis
Patients with both subacute and silent thyroiditis may present with symptoms of hyperthyroidism during the initial phase of these disorders. The clinical history, physical examination, and a low 131I thyroid uptake are helpful toward the diagnosis. The usual course, over approximately 9 months in total, is a period of hyperthyroidism, followed by hypothyroidism, then restoration to euthyroidism. Beta-blockers may be used to treat the symptoms of hyperthyroidism if needed; antithyroidal drugs are not indicated, given that the underlying etiology of thyroiditis is not increased thyroid hormone production.
Iodine-induced thyrotoxicosis (Jod-Basedow phenomenon)
The administration of supraphysiologic iodine can result in thyrotoxicosis as a result of the Jod-Basedow phenomenon.50 Risk factors include patients with underlying thyroid disease, such as multinodular goiters, endemic goiter, or a previous history of Graves' disease, although it can also develop in individuals with normal, intact thyroid glands.60 Sources of supraphysiologic iodine exposure include iodinated contrast dyes, topical antiseptics, dietary supplements (i.e. kelp), and amiodarone, an iodine-containing antiarrhythmic drug.60 The symptoms can be mild to severe; both serum FT4 and FT3 concentrations are elevated, and characteristically the 24-hour 131I uptake is suppressed due to the exogenous iodine exposure. Treatment consists of removing the source of iodine if possible, and if symptoms are severe, a beta-blocker may be initiated.
Thyrotoxicosis factitia
In thyrotoxicosis factitia, exogenous thyroid hormone exposure may be intentional or unintentional to result in thyrotoxicosis.98 In many circumstances, these patients have personality disorders and deny the use of exogenous thyroid medication. One epidemic of thyrotoxicosis during the late 1980s in the midwest US stemmed from the inadvertent inclusion of bovine thyroid gland parts in a slaughterhouse producing ground beef.99
The diagnosis of thyrotoxicosis factitia can be sometimes difficult to confirm, but thyroid function tests in the hyperthyroid range, together with a suppressed 24-hour 131I thyroid uptake, are suggestive of the condition.98 Determination of serum thyroglobulin levels is helpful in the differential diagnosis, as suppressed values are characteristic of patients with the condition.98
Thyroid-stimulating hormone (TSH)-dependent hyperthyroidism
TSH-dependent hyperthyroidism (i.e. excess TSH secretion) is a rare form of thyrotoxicosis in which serum TSH concentrations are elevated, usually due to a TSH-secreting pituitary tumor. Abnormalities in TSH dynamics have been suggested as the cause of this syndrome: there is an increase in the α-subunit of TSH in patients with a demonstrable pituitary tumor, whereas there is suppression of the α-subunit and elevation of the β-subunit in patients without a pituitary tumor.100 Surgery remains the optimal treatment option for TSH-secreting pituitary tumors, which if successful, offers a low chance of recurrence.101
Thyroid carcinoma
Hyperthyroidism due to thyroid carcinoma is rare, occurs is less than 2% of thyroid cancer cases,102 and may be secondary to a hot autonomous thyroid cancerous nodule or to metastatic follicular carcinoma. This is a rare condition which has been described as mostly case reports in the literature.103, 104
Molar pregnancy and choriocarcinoma
Hyperthyroidism may occur in patients with hydatidiform moles, as first recognized by Tisné and colleagues in 1955.105 The syndrome is caused by excess levels of human chorionic gonadotropin, which bind weakly to the TSH receptor to result in TSH suppression.106 Thus, in most patients, clinical signs of hypermetabolism are absent in the presence of abnormally high circulating thyroid hormones. The thyroid gland is generally not enlarged or is only slightly increased in size. Although most cases of gestational trophoblastic disease are not associated with thyrotoxicosis, cases of severe thyrotoxicosis complicated by life-threatening situations have been reported.107
Choriocarcinoma can produce hyperthyroidism by a similar mechanism, due to elevated human chorionic gonadotropin concentrations. As in the case of molar pregnancy, the patient may be clinically euthyroid despite having high levels of circulating thyroid hormones.
The thyrotoxicosis in both conditions resolves quickly after evacuation of the mole. It can be controlled just before surgery with iodine therapy (i.e. saturated solution of potassium iodide), propranolol, or antithyroid drugs. In cases of choriocarcinoma, it is advisable to treat the hyperthyroidism while chemotherapy is concurrently administered.
Struma ovarii
Struma ovarii is a rare condition that should be suspected in hyperthyroid women with a palpable ovarian lesion or ascites in the presence of a low 131I uptake in the neck area.108 It is defined as a teratoma, usually located in the ovary, which contains mostly thyroid tissue. Although most cases are benign, struma ovarii can also be malignant, but following resection, rarely recurs and demonstrates a 25-year survival rate of 84%.109
Thyroid storm
Thyroid crisis or storm is a medical emergency with a high mortality rate of between 20 and 30%.110 It often occurs in patients with history of thyrotoxicosis who present as the first manifestation of recurrent hyperthyroidism. It is usually triggered by infection, acute illness, or trauma, or after subtotal thyroidectomy or other operations. Patients develop marked hyperthermia, severe hypermetabolic symptoms, and alterations of the central nervous system; a diagnostic scoring system for thyroid storm has been proposed by Burch and Wartofsky.111
Treatment consists of IV fluids, alcohol sponge baths to reduce temperature, oxygen, antithyroid therapy, iodide, and beta-blockers. It is important that antithyroid medication should be initiated prior to iodide in order to prevent further stimulation of thyroid hormone synthesis and exacerbation of the hyperthyroidism. Propylthiouracil is the preferred antithyroid drug, as in addition to suppressing the synthesis of new thyroid hormone by the gland, it decreases T3 generation from T4 in the circulation.110 Within 1 hour of propylthiouracil administration, IV sodium iodide (1 gram over a 24-hour period) or the oral saturated solution of potassium iodide (SSKI) as 5 drops every 8 hours can be given. The iodine-rich radiographic contrast agent, ipodate sodium or iopanoic acid (1 g/day by mouth), can also be used as the source of iodine, although it is no longer readily available. Treatment with both propylthiouracil (with the IV formulation continued for control of tachycardia) and iodine is continued until a satisfactory response has occurred; iodine is then gradually withdrawn over the following 2–3 weeks. Historically, 100 mg IV hydrocortisone every 8 hours or equivalent amounts of other glucocorticoids have been used empirically in the treatment of thyroid storm, as corticosteroids are also able to suppress peripheral conversion of T4 to T3.112 After the treatment of thyroid storm, definite therapy for the source of thyrotoxicosis by either thyroidectomy or 131I is imperative.
Transient thyrotoxicosis of hyperemesis gravidarum (pregnancy-induced thyrotoxicosis)
As serum thyroid function tests in women with severe hyperemesis gravidarum may be in the hyperthyroid range, the ability to diagnosis this and confirm that underlying hyperthyroidism is not present may be difficult.106 Patients report severe nausea and vomiting and frequently require hospitalization, due to a mean weight loss of 5 kg.113, 114 In addition, because severe nausea and vomiting could be an early manifestation of hyperthyroidism, the physician is presented with a difficult task. The detection of TSH-receptor antibodies could be helpful as a marker to differentiate transient thyrotoxicosis of hyperemesis gravidarum from Graves' disease.
Tachycardia is common, but other symptoms of thyrotoxicosis are usually absent. In most cases, the symptoms abate after a few days of hospitalization, and serum thyroid function tests return to normal before 20 weeks' gestation. If the thyroid function tests do not normalize spontaneously in a short period of time, or if the patient has other signs and symptoms of thyrotoxicosis, treatment with antithyroid drugs should be instituted.
Postpartum thyroid dysfunction
Postpartum thyroiditis has been reported to occur in 5.4% of all women after delivery.12 Women with a history of other autoimmune thyroid disease are at increased risk for developing postpartum thyroiditis. The clinical course is similar to that for patients with subacute thyroiditis: transient hyperthyroidism, followed by a hypothyroid phase, and finally complete recovery to euthyroidism. However, approximately a quarter of women will eventually develop permanent hypothyroidism.115 The differential diagnosis of postpartum thyroiditis may be difficult at times, as patients with a previous history of hyperthyroidism due to Graves' disease may experience recurrence of thyrotoxicosis after delivery. However, recurrence of hyperthyroidism due to Graves' disease is accompanied by an elevation in 131I thyroid uptake, rather than a low uptake characteristic of postpartum thyroiditis.
In most cases, the symptoms of postpartum thyroiditis are mild, and spontaneous recovery is expected. Treatment is conservative, and if symptoms are severe, consists primarily of beta-blockers during the hyperthyroid phase and thyroid hormone replacement in the hypothyroid phase; often times, no therapy is required.
Funding
This work was supported by NIH K23HD068552 (AML).
REFERENCES
Visser WE, van Mullem AA, Visser TJ, Peeters RP. Different causes of reduced sensitivity to thyroid hormone: diagnosis and clinical management. Clinical endocrinology. 2013;79(5):595. |
|
Brent GA, Davies TF. Hypothyroidism and thyroiditis. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams Textbook of Endocrinology. 12 ed. Philadelphia: Elsevier Saunders; 2011. p. 407. |
|
Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. The Medical clinics of North America. 2012;96(2):203. |
|
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). The Journal of clinical endocrinology and metabolism. 2002;87(2):489. |
|
Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43(1):55. |
|
Somwaru LL, Rariy CM, Arnold AM, Cappola AR. The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. The Journal of clinical endocrinology and metabolism. 2012;97(6):1962. |
|
Staub JJ, Althaus BU, Engler H, Ryff AS, Trabucco P, Marquardt K, et al. Spectrum of subclinical and overt hypothyroidism: effect on thyrotropin, prolactin, and thyroid reserve, and metabolic impact on peripheral target tissues. The American Journal of Medicine. 1992;92(6):631. |
|
Duntas LH, Wartofsky L. Cardiovascular risk and subclinical hypothyroidism: focus on lipids and new emerging risk factors. What is the evidence? Thyroid : official journal of the American Thyroid Association. 2007;17(11):1075. |
|
Tadic M, Ilic S, Celic V. Right ventricular and right atrial function and deformation in patients with subclinical hypothyroidism: a two- and three-dimensional echocardiographic study. Eur J Endocrinol. 2014;170(1):77-85. |
|
Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-Thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Annals of Internal Medicine. 1984;101(1):18. |
|
Collet TH, Bauer DC, Cappola AR, Asvold BO, Weiler S, Vittinghoff E, et al. Thyroid Antibody Status, Subclinical Hypothyroidism, and the Risk of Coronary Heart Disease: An Individual Participant Data Analysis. The Journal of clinical endocrinology and metabolism. 2014:jc20141250. |
|
Stagnaro-Green A, Pearce E. Thyroid disorders in pregnancy. Nature reviews. Endocrinology. 2012;8(11):650. |
|
Takasu N, Yamada T, Takasu M, Komiya I, Nagasawa Y, Asawa T, et al. Disappearance of thyrotropin-blocking antibodies and spontaneous recovery from hypothyroidism in autoimmune thyroiditis. The New England journal of medicine. 1992;326(8):513. |
|
Takasu N, Yamada T, Sato A, Nakagawa M, Komiya I, Nagasawa Y, et al. Graves' disease following hypothyroidism due to Hashimoto's disease: studies of eight cases. Clinical endocrinology. 1990;33(6):687. |
|
Parsaik AK, Singh B, Roberts RO, Pankratz S, Edwards KK, Geda YE, et al. Hypothyroidism and risk of mild cognitive impairment in elderly persons: a population-based study. JAMA neurology. 2014;71(2):201. |
|
Leung AS, Millar LK, Koonings PP, Montoro M, Mestman JH. Perinatal outcome in hypothyroid pregnancies. Obstet Gynecol. 1993;81(3):349. |
|
Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. The New England journal of medicine. 2004;351(3):241. |
|
Yassa L, Marqusee E, Fawcett R, Alexander EK. Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. The Journal of clinical endocrinology and metabolism. 2010;95(7):3234. |
|
Kroese JM, Grootendorst AF, Schelfhout LJ. Postpartum amenorrhoea-galactorrhoea associated with hyperprolactinaemia and pituitary enlargement in primary hypothyroidism. The Netherlands journal of medicine. 2004;62(1):28. |
|
Hershman JM, Pittman JA, Jr. Utility of the radioimmunoassay of serum thyrotrophin in man. Annals of Internal Medicine. 1971;74(4):481. |
|
Farwell AP. Nonthyroidal illness syndrome. Current opinion in endocrinology, diabetes, and obesity. 2013;20(5):478. |
|
Willard DL, Leung AM, Pearce EN. Thyroid function testing in patients with newly diagnosed hyperlipidemia. JAMA internal medicine. 2014;174(2):287. |
|
Papi G, Uberti ED, Betterle C, Carani C, Pearce EN, Braverman LE, et al. Subclinical hypothyroidism. Current opinion in endocrinology, diabetes, and obesity. 2007;14(3):197. |
|
Force USPST. Recommendation Summary 2014. Available from: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/thyroid-disease-screening. |
|
Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081-125. |
|
De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of Thyroid Dysfunction during Pregnancy and Postpartum: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2012;97(8):2543. |
|
Popoveniuc G, Chandra T, Sud A, Sharma M, Blackman MR, Burman KD, et al. A diagnostic scoring system for myxedema coma. Endocr Pract. 2014;20(8):808-17. |
|
Wartofsky L. Myxedema coma. In: Braverman LE, Braverman LEaCDS, editors. Wener & Ingbar's The Thyroid: A Fundamental and Clinical Text2013. p. 600-5. |
|
Burch HB, Burman KD, Cooper DS, Hennessey JV. A 2013 survey of clinical practice patterns in the management of primary hypothyroidism. The Journal of clinical endocrinology and metabolism. 2014;99(6):2077. |
|
Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid : official journal of the American Thyroid Association. 2012;22(12):1200. |
|
Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best practice & research.Clinical endocrinology & metabolism. 2009;23(6):781. |
|
U.S. Food and Drug Administration DoHaHS, Center for Drug Evaluation and Research. Guidance for industry: levothyroxine sodium tablets—in vivo pharmacokinetic and bioavailability studies and in vitro dissolution testing. 2000. |
|
Roos A, Linn-Rasker SP, van Domburg RT, Tijssen JP, Berghout A. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Archives of Internal Medicine. 2005;165(15):1714. |
|
Bolton S. Bioequivalence studies for levothyroxine. The AAPS journal. 2005;7(1):E47. |
|
Puzziello A, Carrano M, Angrisani E, Marotta V, Faggiano A, Zeppa P, et al. Evolution of benign thyroid nodules under levothyroxine non-suppressive therapy. Journal of endocrinological investigation. 2014. |
|
Geng H, Zhang X, Wang C, Zhao M, Yu C, Zhang B, et al. Even mildly elevated TSH is associated with an atherogenic lipid profile in postmenopausal women with subclinical hypothyroidism. Endocrine research. 2014. |
|
Shinno H, Ishikawa I, Yamanaka M, Usui A, Danjo S, Inami Y, et al. Effect of levothyroxine on prolonged nocturnal sleep time and excessive daytime somnolence in patients with idiopathic hypersomnia. Sleep medicine. 2011;12(6):578. |
|
Scoccia B, Demir H, Kang Y, Fierro MA, Winston NJ. In vitro fertilization pregnancy rates in levothyroxine-treated women with hypothyroidism compared to women without thyroid dysfunction disorders. Thyroid : official journal of the American Thyroid Association. 2012;22(6):631. |
|
Pantos C, Mourouzis I, Malliopoulou V, Paizis I, Tzeis S, Moraitis P, et al. Dronedarone administration prevents body weight gain and increases tolerance of the heart to ischemic stress: a possible involvement of thyroid hormone receptor alpha1. Thyroid : official journal of the American Thyroid Association. 2005;15(1):16. |
|
Grover GJ, Mellstrom K, Malm J. Therapeutic potential for thyroid hormone receptor-beta selective agonists for treating obesity, hyperlipidemia and diabetes. Current vascular pharmacology. 2007;5(2):141. |
|
Shoemaker TJ, Kono T, Mariash CN, Evans-Molina C. Thyroid hormone analogues for the treatment of metabolic disorders: new potential for unmet clinical needs? Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2012;18(6):954. |
|
Abonowara A, Quraishi A, Sapp JL, Alqambar MH, Saric A, O'Connell CM, et al. Prevalence of atrial fibrillation in patients taking TSH suppression therapy for management of thyroid cancer. Clinical and investigative medicine.Medecine clinique et experimentale. 2012;35(3):E152. |
|
Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2010;20(2):135. |
|
Tournis S, Antoniou JD, Liakou CG, Christodoulou J, Papakitsou E, Galanos A, et al. Volumetric Bone Mineral Density and Bone Geometry Assessed by Peripheral Quantitative Computed Tomography in Women with Differentiated Thyroid Cancer under TSH-suppression. Clinical endocrinology. 2014. |
|
Abrahamsen B, Jorgensen HL, Laulund AS, Nybo M, Brix TH, Hegedus L. Low Serum Thyrotropin Level and Duration of Suppression as a Predictor of Major Osteoporotic Fractures - The OPENTHYRO Register Cohort. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014. |
|
Panico A, Lupoli GA, Fonderico F, Marciello F, Martinelli A, Assante R, et al. Osteoporosis and thyrotropin-suppressive therapy: reduced effectiveness of alendronate. Thyroid : official journal of the American Thyroid Association. 2009;19(5):437. |
|
Krugman LG, Hershman JM, Chopra IJ, Levine GA, Pekary E, Geffner DL, et al. Patterns off recovery of the hypothalamic-pituitary-thyroid axis in patients taken of chronic thyroid therapy. The Journal of clinical endocrinology and metabolism. 1975;41(1):70. |
|
Molitch ME, Spare SV, Arnold GL, Fawaz K, Scally J, Zack N. Letter: Pituitary-thyroid function after cessation of prolonged thyroid suppression. The New England journal of medicine. 1976;295(4):231. |
|
Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmunity reviews. 2014;13(4-5):391. |
|
Leung AM, Braverman LE. Consequences of excess iodine. Nature reviews.Endocrinology. 2014;10(3):136. |
|
Jankovic B, Le KT, Hershman JM. Clinical Review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? The Journal of clinical endocrinology and metabolism. 2013;98(2):474. |
|
Balucan FS, Morshed SA, Davies TF. Thyroid autoantibodies in pregnancy: their role, regulation and clinical relevance. J Thyroid Res. 2013;2013:182472. |
|
Samuels MH. Subacute, silent, and postpartum thyroiditis. The Medical clinics of North America. 2012;96(2):223. |
|
Pearce EN, Farwell AP, Braverman LE. Thyroiditis. The New England journal of medicine. 2003;348(26):2646. |
|
Carlé A, Krejbjerg A, Laurberg P. Epidemiology of nodular goitre. Influence of iodine intake. Best Pract Res Clin Endocrinol Metab. 2014;28(4):465-79. |
|
Knudsen N, Brix TH. Genetic and non-iodine-related factors in the aetiology of nodular goitre. Best practice & research.Clinical endocrinology & metabolism. 2014;28(4):495. |
|
Dean DS, Gharib H. Epidemiology of thyroid nodules. Best practice & Research. Clinical endocrinology & metabolism. 2008;22(6):901. |
|
Quadbeck B, Pruellage J, Roggenbuck U, Hirche H, Janssen OE, Mann K, et al. Long-term follow-up of thyroid nodule growth. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2002;110(7):348. |
|
Brito JP, Yarur AJ, Prokop LJ, McIver B, Murad MH, Montori VM. Prevalence of thyroid cancer in multinodular goiter versus single nodule: a systematic review and meta-analysis. Thyroid : official journal of the American Thyroid Association. 2013;23(4):449. |
|
Pramyothin P, Leung AM, Pearce EN, Malabanan AO, Braverman LE. Clinical problem-solving. A hidden solution. The New England journal of medicine. 2011;365(22):2123. |
|
Bahn RS, Castro MR. Approach to the patient with nontoxic multinodular goiter. The Journal of clinical endocrinology and metabolism. 2011;96(5):1202. |
|
Hegedus L, Bonnema SJ. Approach to management of the patient with primary or secondary intrathoracic goiter. The Journal of clinical endocrinology and metabolism. 2010;95(12):5155. |
|
Oakley GM, Curtin K, Pimentel R, Buchmann L, Hunt J. Establishing a familial basis for papillary thyroid carcinoma using the Utah Population Database. JAMA otolaryngology-- head & neck surgery. 2013;139(11):1171. |
|
Paschke R, Hegedus L, Alexander E, Valcavi R, Papini E, Gharib H. Thyroid nodule guidelines: agreement, disagreement and need for future research. Nature reviews.Endocrinology. 2011;7(6):354. |
|
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167. |
|
Giles WH, Maclellan RA, Gawande AA, Ruan DT, Alexander EK, Moore FD, Jr., et al. False Negative Cytology in Large Thyroid Nodules. Annals of surgical oncology. 2014. |
|
Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111(5):306. |
|
Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid : official journal of the American Thyroid Association. 2009;19(11):1159. |
|
Eszlinger M, Hegedus L, Paschke R. Ruling in or ruling out thyroid malignancy by molecular diagnostics of thyroid nodules. Best practice & research.Clinical endocrinology & metabolism. 2014;28(4):545. |
|
Vermiglio F, Lo Presti VP, Violi MA, Moleti M, Castagna MG, Finocchiaro MD, et al. Changes in both size and cytological features of thyroid nodule after levothyroxine treatment. Clinical endocrinology. 2003;59(3):347. |
|
Gharib H, Hegedus L, Pacella CM, Baek JH, Papini E. Clinical review: Nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. The Journal of clinical endocrinology and metabolism. 2013;98(10):3949. |
|
Schlumberger M, Bastholt L, Dralle H, Jarzab B, Pacini F, Smit JW, et al. 2012 European thyroid association guidelines for metastatic medullary thyroid cancer. European thyroid journal. 2012;1(1):5. |
|
Adams D, Purvis H, Sirett N. Response of hypophysectomized mice to injections of human serum containing long acting thyroid stimulator. Endocrinology. 1961;68:154. |
|
Prindaville B, Rivkees SA. Incidence of vitiligo in children with Graves' disease and Hashimoto's thyroiditis. Int J Pediatr Endocrinol. 2011;2011(1):18. |
|
Piantanida E, Tanda ML, Lai A, Sassi L, Bartalena L. Prevalence and natural history of Graves' orbitopathy in the XXI century. J Endocrinol Invest. 2013;36(6):444-9. |
|
Strianese D, Piscopo R, Elefante A, Napoli M, Comune C, Baronissi I, et al. Unilateral proptosis in thyroid eye disease with subsequent contralateral involvement: retrospective follow-up study. BMC Ophthalmol. 2013;13:21. |
|
Kamminga N, Jansonius NM, Pott JW, Links TP. Unilateral proptosis: the role of medical history. Br J Ophthalmol. 2003;87(3):370-1. |
|
Bartalena L. Prevention of Graves' ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26(3):371-9. |
|
Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of Graves ophthalmopathy. Med Clin North Am. 2012;96(2):311-28. |
|
Minakaran N, Ezra DG. Rituximab for thyroid-associated ophthalmopathy. Cochrane Database Syst Rev. 2013;5:CD009226. |
|
Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6(5):295-309. |
|
Bartalena L. Diagnosis and management of Graves disease: a global overview. Nat Rev Endocrinol. 2013;9(12):724-34. |
|
Marino M, Chiovato L, Pinchera A. In: DeGroot LJ, Jameson JL, editors. Endocrinology Adult and Pediatric: The Thyroid Gland, Volume 2. Philadelphia: Elsevier Saunders; 2010. |
|
Cooper DS. Antithyroid drugs. The New England journal of medicine. 2005;352(9):905. |
|
Geffner DL, Azukizawa M, Hershman JM. Propylthiouracil blocks extrathyroidal conversion of thyroxine to triiodothyronine and augments thyrotropin secretion in man. J Clin Invest. 1975;55(2):224-9. |
|
Abraham P, Avenell A, Park CM, Watson WA, Bevan JS. A systematic review of drug therapy for Graves' hyperthyroidism. Eur J Endocrinol. 2005;153(4):489-98. |
|
Laurberg P. Remission of Graves' disease during anti-thyroid drug therapy. Time to reconsider the mechanism? Eur J Endocrinol. 2006;155(6):783-6. |
|
Rajput R, Goel V. Indefinite antithyroid drug therapy in toxic Graves' disease: What are the cons. Indian J Endocrinol Metab. 2013;17(Suppl 1):S88-92. |
|
Ohye H, Minagawa A, Noh JY, Mukasa K, Kunii Y, Watanabe N, et al. Antithyroid drug treatment for Graves' disease in children: a long-term retrospective study at a single institution. Thyroid. 2014;24(2):200-7. |
|
Léger J, Gelwane G, Kaguelidou F, Benmerad M, Alberti C, Group FCGDS. Positive impact of long-term antithyroid drug treatment on the outcome of children with Graves' disease: national long-term cohort study. J Clin Endocrinol Metab. 2012;97(1):110-9. |
|
Vaidya B, Wright A, Shuttleworth J, Donohoe M, Warren R, Brooke A, et al. Block & replace regime versus titration regime of antithyroid drugs for the treatment of Graves' disease: a retrospective observational study. Clin Endocrinol (Oxf). 2014;81(4):610-3. |
|
Tajiri J, Noguchi S. Antithyroid drug-induced agranulocytosis: how has granulocyte colony-stimulating factor changed therapy? Thyroid. 2005;15(3):292-7. |
|
Allahabadia A, Daykin J, Sheppard MC, Gough SC, Franklyn JA. Radioiodine treatment of hyperthyroidism-prognostic factors for outcome. J Clin Endocrinol Metab. 2001;86(8):3611-7. |
|
Stan MN, Bahn RS. Risk factors for development or deterioration of Graves' ophthalmopathy. Thyroid. 2010;20(7):777-83. |
|
Sawka AM, Thabane L, Parlea L, Ibrahim-Zada I, Tsang RW, Brierley JD, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009;19(5):451-7. |
|
Hegele RA, Volpé R. Relapse of Graves' disease 23 years after treatment with radioactive iodine (131I). J Clin Lab Immunol. 1985;18(2):103-5. |
|
Laurberg P, Bournaud C, Karmisholt J, Orgiazzi J. Management of Graves' hyperthyroidism in pregnancy: focus on both maternal and foetal thyroid function, and caution against surgical thyroidectomy in pregnancy. Eur J Endocrinol. 2009;160(1):1-8. |
|
Leung A, Safer J. Thyrotoxicosis of extrathyroid origin. In: Braverman L, DS C, editors. Werner & Ingbar's Thyroid: A Fundamental and Clinical Text. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 429-33. |
|
Hedberg CW, Fishbein DB, Janssen RS, Meyers B, McMillen JM, MacDonald KL, et al. An outbreak of thyrotoxicosis caused by the consumption of bovine thyroid gland in ground beef. The New England journal of medicine. 1987;316(16):993. |
|
Kourides IA, Ridgway EC, Weintraub BD, Bigos ST, Gershengorn MC, Maloof F. Thyrotropin-induced hyperthyroidism: use of alpha and beta subunit levels to identify patients with pituitary tumors. J Clin Endocrinol Metab. 1977;45(3):534-43. |
|
Malchiodi E, Profka E, Ferrante E, Sala E, Verrua E, Campi I, et al. Thyrotropin-secreting pituitary adenomas: outcome of pituitary surgery and irradiation. J Clin Endocrinol Metab. 2014;99(6):2069-76. |
|
Gabriele R, Letizia C, Borghese M, De Toma G, Celi M, Izzo L, et al. Thyroid cancer in patients with hyperthyroidism. Horm Res. 2003;60(2):79-83. |
|
Basaria S, Salvatori R. Thyrotoxicosis due to metastatic papillary thyroid cancer in a patient with Graves' disease. Journal of endocrinological investigation. 2002;25(7):639. |
|
Kasagi K, Takeuchi R, Miyamoto S, Misaki T, Inoue D, Shimazu A, et al. Metastatic thyroid cancer presenting as thyrotoxicosis: report of three cases. Clinical endocrinology. 1994;40(3):429. |
|
Tisne L, Barzelatto J, Stevenson C. [Study of thyroid function during pregnancy-puerperal state with radioactive iodine]. Bol Soc Chil Obstet Ginecol. 1955;20(8-9):246-51. |
|
Labadzhyan A, Brent GA, Hershman JM, Leung AM. Thyrotoxicosis of Pregnancy. J Clin Transl Endocrinol. 2014;1(4):140-4. |
|
Walkington L, Webster J, Hancock BW, Everard J, Coleman RE. Hyperthyroidism and human chorionic gonadotrophin production in gestational trophoblastic disease. Br J Cancer. 2011;104(11):1665-9. |
|
Gunasekaran S, Kopecka E, Maung KH, England RJ. Struma ovarii and the thyroid surgeon. J Laryngol Otol. 2012;126(8):858-60. |
|
Robboy SJ, Shaco-Levy R, Peng RY, Snyder MJ, Donahue J, Bentley RC, et al. Malignant struma ovarii: an analysis of 88 cases, including 27 with extraovarian spread. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2009;28(5):405. |
|
Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am. 2006;35(4):663-86, vii. |
|
Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am. 1993;22(2):263-77. |
|
Carroll R, Matfin G. Endocrine and metabolic emergencies: thyroid storm. Ther Adv Endocrinol Metab. 2010;1(3):139-45. |
|
Goodwin TM, Montoro M, Mestman JH. Transient hyperthyroidism and hyperemesis gravidarum: clinical aspects. American Journal of Obstetrics and Gynecology. 1992;167(3):648. |
|
Leylek OA, Cetin A, Toyaksi M, Erselcan T. Hyperthyroidism in hyperemesis gravidarum. Int J Gynaecol Obstet. 1996;55(1):33-7. |
|
Stagnaro-Green A. Postpartum thyroiditis. Best Pract Res Clin Endocrinol Metab. 2004;18(2):303-16. |