Pediatric Gynecology
Authors
INTRODUCTION
The subspecialized area of pediatric and adolescent gynecology is an integral part of gynecologic care. In most instances it is the first gynecologic encounter that a female will experience. It is of the utmost importance that this be a positive experience in an effort to set the stage for all future gynecologic care. This chapter addresses the history and physical examination process and basic tenets of the more common gynecologic problems identified in the pediatric patient. The importance of an adequate examination cannot be overemphasized. The clinician should remain cognizant of both the psychological effects and the physical problem at hand. For instance, with precocious puberty, an understanding of the psychological effects the patient and parents experience is intrinsic to the evaluation process. The problem may prove to be life threatening, such as congenital adrenal hyperplasia, and the importance of the obstetrician/gynecologist identifying this and providing appropriate management is paramount.
EXAMINATION
General Principles
It is important to obtain the history from the parent or parents, with the patient also being integrally involved in the process as age-appropriate. The key idea is to develop rapport and communication; it is extremely important for the patient to develop trust in the healthcare provider. Investing in education before the examination process will pay appropriate dividends with respect to patient cooperation and overall assessment of the problem at hand. The “show and tell” concept is often helpful in achieving an adequate evaluation. Identification of office personnel (i.e, medical assistant or nurse who spends time with the patient and parent before the physical examination) sets the stage for a successful evaluation. Familiarity with the instrumentation to be used for the examination process (e.g., otoscope, cotton swab) may allay the patient's anxiety. Emphasis should be placed on the nonthreatening nature of the equipment. Often only inspection of the vulvovaginal area is adequate. Both parent and patient should be reassured that if the evaluation becomes uncomfortable or painful, the procedure will cease.
For the vast majority of patients, evaluation can be accomplished without sedation, except for the rare circumstance when it is needed for an adequate evaluation. In general, parents and patients should be reassured that an "internal" exam is frequently not required. However, if the child requires some degree of sedation, pediatric conscious sedation may be used when trained personnel are available or with an examination under anesthesia. As the pediatric patient approaches puberty, the option of having an examination without the parent present should be offered. The physical presence of an assistant (i.e., medical assistant or nurse) must be emphasized.
Positioning and Initial Assessment
The overall assessment should be approached as for any physical examination; that is, the skin should be assessed and the presence of any lesions or disfigurements noted. The child should not be restrained during the examination process. Once again, a spirit of cooperation (i.e., “show and tell”) is often helpful in initiating the examination process. Giving the child a sense of control over the examination and making a commitment not to cause discomfort during the examination represent the optimal approach.
Breast Examination
The breast examination in the neonate frequently reveals evidence of maternal estrogen effect in that palpable breast tissue is noted. In addition, a milky discharge can be expressed. This discharge usually abates within several days postpartum. Massaging of the breast tissue is discouraged because it can lead to abscess formation, especially with staphylococcal organisms.
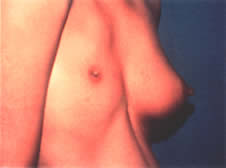
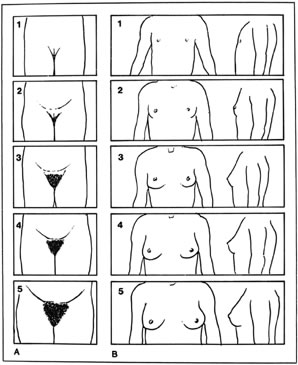
Thelarche on average begins at around 9 years of age in white girls and slightly earlier in African American girls. The median age of onset is 9.8 years.1 Asymmetric breast development is not uncommon, especially in the early stages of breast formation (Fig. 1). Tanner staging of breasts is encouraged (Fig. 2).
Abdominal Examination
Inspection for any skin lesions is the initial approach to evaluation of the abdomen. Assessment of bowel sounds is followed by palpation for masses. The ovaries in the prepubertal child are located at the pelvic brim; thus, enlargement of an adnexum presents as an intra-abdominal mass.2 Ultrasonography is a useful complement to the examination process to identify reproductive tract abnormalities.
Pelvic Examination
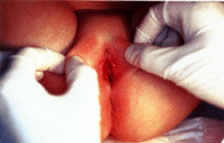
The ideal positioning for good visualization of the external genitalia is in the supine position with the buttocks on the end of the gynecologic examination table. The legs are placed in a “frog-leg” position. If there is a problem with cooperation, the child can be placed on the parent's lap or the parent may sit in a chair holding the child. If further visualization is needed, the patient can go into a knee-chest position and asked to hold their breath and push their stomach out (ie. Valsalva). The Valsalva maneuver may assist visualization in both positions. Often visualization of the entire vaginal canal up to and including identification of the cervix is possible. A hand-held mirror can be used so that the patient can be further involved in the examination process. In the “frog-leg” position, the clinician should gently grasp the labia and move them laterally and downward in an effort to assess the introitus and the lower third of the vagina (Fig. 3). Either an otoscope or a colposcope often facilitates this segment of the examination. Tanner staging of pubic hair should occur (see Fig. 1); identification of any discharge, edema, erythema, or congenital anomalies should be made. In addition, evidence of lacerations, abrasions, bruises, or scarring may indicate sexual abuse, independent of the chief complaint provided by the parent or guardian.
Forceful manipulation of the labia is discouraged, and any pain or discomfort merits re-evaluation of the technique being used for evaluation. The hymenal diameter, configuration, and symmetry are important. Documentation of the size and configuration of the clitoris and any urethral abnormalities should occur.
An external light source such as a vaginoscope, hysteroscope, or otoscope is especially helpful in ruling out the presence of a foreign body, trauma, neoplasm, or other congenital anomaly.
Recurrent vaginal discharge and bleeding are the most common historical complaints voiced by the parent or guardian, but a clinician must remain open to an extensive differential. A wet preparation is useful in assessing vaginal discharge.
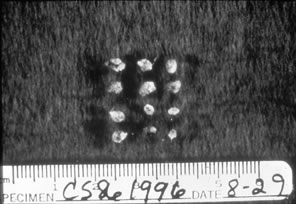
A speculum is rarely if ever necessary in assessing the pediatric patient. If a foreign body is suspected, irrigation of the vaginal area with body-temperature saline frequently lavages out the foreign body. The most common foreign body is rolled toilet paper, and this is easily amenable to irrigation (Fig. 4).
A rectal examination should be considered under specific circumstances, especially if a pelvic-abdominal mass is suspected (Table 1).
Table 1. Tips for getting through the pediatric gyn exam
Obtain the patient's cooperation; this is necessary for teachable moments and adequate examinations.
Give her as much control as possible.
Give a realistic description of what to expect in regards to what will happen and when during the exam.
Describe what she might feel and talk with her about it.
Explain who will be in the room.
Define the information that is to be obtained.
Special aspects of the prepubertal female's examination
Inform the child that the genital examination is sanctioned.
Involve the child by giving them a hand-held mirror and magnification device.
Be open to different types of positioning for the exam. Consider the child with the feet in the stirrups, with the feet on the examiner's lap, or sitting on the parent's lap, with the child's legs droped over the parent's thighs.
Attempt both the supine and knee-chest positions.
Be knowledgeable about the various visualization methods to see the vestibule.
There is only a limited need to use instruments.
Special aspects of the peripubertal genital examination
Understand the impact of estrogen.
Choose the proper speculum width after the introitus is evaluated.
First Pelvic Examination
At what age should a virginal adolescent have her first pelvic examination? This topic has been addressed by many authorities in the field. Current recommendation is to perform a speculuum exam with Pap smear 3 years after coitarche or age 21 for a virginal patient. Evaluations for sexually transmitted infections (STI), however should be routine in any sexually active young woman regardless of coitarche. Options for STI testing may include urine, vaginal or cervical specimens. In a sexually active patient, if a Pap smear is not indicated, the clinician will decide whether visualization of the external genitalia, vagina or cervix are indicated. Bear in mind that a patient may not be "sexually active" but may have been the victim of childhood sexual abuse. In these patients, a Pap smear may be warranted if the event was 3 years or greater. Counseling sessions with adolescent patients may allow the clinician to address preventive health aspects, sexuality and establish rapport in a nonthreatening manner.
NORMAL GENITAL ANATOMY
Newborn Infant
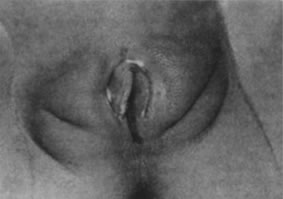
The typical appearance of the newborn female genitalia is primarily due to the effects of maternal hormones acquired transplacentally. The labia majora are full and the labia minora thickened. The hymenal folds appear thick and redundant. The vaginal mucosa is pink and moist, with an acidic pH (Fig. 5). Physiologic leukorrhea is usually present. The normal decline of maternal estrogen in the first week of life can stimulate vaginal bleeding, creating parental anxiety and concern. It should be explained that this is a normal process resulting from maternal hormone effects and usually resolves in 7–10 days. Circulating maternal estrogen also causes breast tissue development and in some cases nipple discharge in the newborn infant. Thus, the infant may be more susceptible to breast inflammation and infection. As the maternal hormone levels decrease, the labia majora lose their fullness, the labia minora and hymen become thinner and flatter, and the breast tissue decreases.3
Prepubertal Girls
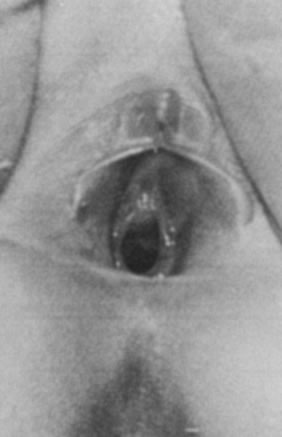
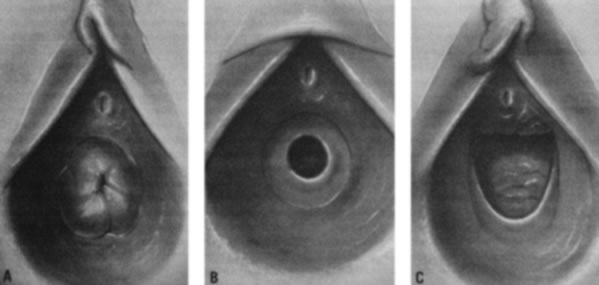
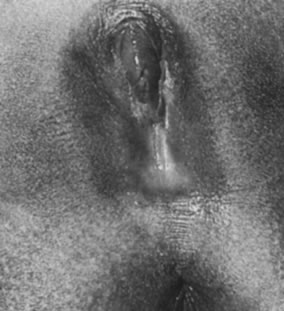
As the infant matures, the labia decrease in size. This leaves the vaginal vestibule less protected from bacteria and external irritants, especially when the child is in the sitting or squatting position. The vaginal mucosa becomes thin and relatively atrophic (Fig. 6). The vaginal pH is neutral or alkaline, and secretions become minimal. By 18–24 months of age, the hymen becomes thin and translucent, with smooth edges. By this time, normal variations in the shape of the vaginal orifice can be seen.4 For example, the hymen may appear annular, crescentic, or fimbriated (Fig. 7). In addition, the perineum, perivaginal tissues, and pelvic supporting structures become relatively rigid and inelastic, increasing the likelihood of tearing as a result of trauma. In addition, before the onset of puberty, the ovaries are positioned above the pelvic brim. Thus, ovarian disorders in young children present more commonly with abdominal pain rather than pelvic complaints.
Peripubertal Girls
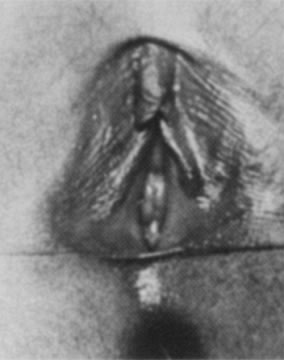
Most of the secondary sex characteristics that develop during puberty are due to the effects of ovarian estrogen (Fig. 8). The mons pubis becomes full and midline abdominal hair forms. The labia majora and the labia minora thicken, becoming softer and more rounded. The clitoris enlarges slightly, and the urethra becomes more prominent. The hymen also thickens as its central opening enlarges. The vaginal mucosa also thickens and softens. Vaginal secretions increase, making the mucosa moist. It also takes on a pink appearance, and pH levels decrease. Perineal and pelvic tissues become more elastic and the ovaries move into the pelvis. In the months preceding menarche, increasing estrogen levels stimulate physiologic leukorrhea, a white discharge containing mature epithelial cells, which often becomes a concern to patients and their parents. They should be reassured that this is a normal part of development and does not signify infection.
As androgens increase, facial acne and body odor develop. The first physical sign of puberty is breast development, followed by pubic hair development, a maximal rate of linear growth, and then menarche (see Fig. 2).
LABIAL AGGLUTINATION
Labial adhesions have been reported to occur in 1.4% of infants.3 Other authors4 have found that labial fusion was most frequently seen in infants and young children, with a peak incidence of 3.3% in children age 13–23 months (Table 2). The etiology appears to be related to the hypoestrogenic state. Inflammation may also be a factor. The labia minora become adherent, and when there is incomplete fusion it usually involves the posterior aspect of the labia. In extreme cases, a small aperture just below the clitoris may be the only open area. Trauma may be enhanced by minor friction injury that denudes the delicate hypoestrogenic surface of the labia. Adherence occurs with re-epithelialization. The condition is uncommon in the neonate and individuals once past puberty as the increased estrogen level occurs. It may again be observed in the postmenopausal patient when a hypoestrogenic state returns.
Table 2. Age range of children with labial adhesion
Reference | Age Range | Peak Incidence |
Capraro and Greenberg5 | 2 months–14 years | 24 months |
Leung et al.4 | 3–48 months | 13–27 months |
Nag6 | 3–90 months | 24–60 months |
Aribarg7 | 2–24 months | Not quoted |
Khanam et al.8 | 3–48 months | 12–24 months |
Jenkinson and Mackinnon9 | 10–22 months | Not quoted |
Berkowitz et al.10 | Sexually abused children 2.9–3% | |
Muram11 | 3–36 months | Not quoted |
Physical examination shows a thin lucent vertical line within the central area of the labia. Inquiry should be made about any urinary tract signs and symptoms because urinary tract infections can be associated with labial adhesions. The differential diagnosis includes lichen sclerosus, child sexual abuse, and less common causes, such as childhood cicatricial pemphigoid.
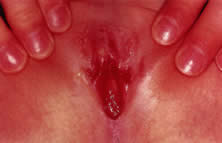
On physical examination, gentle traction should be applied to the labia, but this should be accomplished in a manner that is not traumatic to the child (Fig. 9). Any discomfort should lead to cessation of efforts to separate the labia.
The parent should be reassured that this will be a self-limited problem once puberty occurs. Until that time, recurrence of labial adhesions once separation occurs is not uncommon. As long as there is no urinary tract-associated problem, estrogen cream should be applied on a fingertip over the adhesions once or twice a day for 2–4 weeks. Potential side effects include transient vulvar hyperpigmentation, and rarely is there systemic absorption of estrogen, resulting in signs of precocity. Perineal hygiene is important and every effort should be made to prevent secondary infection. Once separation occurs, application of a preparation such as A&D ™ or zinc oxide ointment nightly is helpful. The ointment can be applied with a cotton-tipped applicator with gentle pressure over the adhesion area.12, 13
Successful treatment with topical estrogen occurs in 88% to 90% of patients.7, 8, 9, 10, 11, 12, 14, 15, 16 Recurrence occurs in 20–100% of patients.5, 7, 8, 14 Many clinicians have considered labial adhesions as “physiologic fusion”; this implies that treatment with observation alone is appropriate. One series9 of 10 patients age 13–22 months showed spontaneous resolution in all patients within 18 months, with no treatment other than reassurance. Biopsies are rarely if ever indicated. If there is acute urinary retention, a pelvic mass is usually identified with a distended bladder; ultrasound is often helpful in establishing this diagnosis. This is an emergency requiring catheterization and bladder decompression. Examination under anesthesia may be necessary to identify the urethral meatus and sharp dissection may be necessary to discern the meatus. After surgery, estrogen cream is applied to the vulvar area for 7–10 days to promote healing and prevent recurrence of the adhesion and urinary retention.15 Other clinicians advocate use of Xylocaine gel on a cotton-tipped applicator to the line of fusion, and thus separation may be possible in an office setting with certain patients. Patients who do not respond to topical agent application should be re-evaluated.16 A recent retrospective study showed that Betamethasone 0.05% cream appeared to be a safe and effective treatment as well.17
VULVOVAGINITIS
Pathophysiology and Clinical Presentation
Vulvitis is defined as inflammation of the labia majora, labia minora, clitoris, and introitus; vaginitis is inflammation of the vaginal mucosa. Pediatric vulvovaginitis, involving the vulvar and vaginal tissues, is a very common diagnosis made by the primary care provider, who often refers the patient to a specialist when initial treatment is unsuccessful. Thus, it is important to understand the pathophysiology, know the various etiologies as they relate to the clinical presentation, and establish a methodologic approach to the evaluation of vulvovaginitis.
Because of anatomic and behavioral factors, the prepubertal girl is at increased risk for vulvovaginitis. First, with the absence of pubic hair and labial fat pads, the vaginal vestibule and vulva are less protected from external irritants, especially when squatting or sitting. Second, the skin of the vulva and vaginal mucosa is thinner, more sensitive, and thus more easily irritated by trauma as well as chemical, environmental, and allergic exposures. Third, the vaginal cavity has a neutral pH (6.5–7.5),18 is warm and moist, and has unestrogenized epithelium that lacks both lactobacilli and glycogen. All of these factors facilitate bacterial growth. Lastly, prepubertal children tend to have poor hygiene in terms of perineal cleansing and hand washing; this can lead to autoinoculation with fecal bacteria or less commonly from organisms associated with an infected urinary or respiratory tract.19, 20, 21
Pediatric vulvovaginitis typically presents as vaginal itching with associated excoriation, vaginal discharge that may be malodorous, generalized vulvar, vaginal, or perianal discomfort, or pain or dysuria.22, 23 Parents may also report staining, odor, or color on the child's underwear. The history is very important in narrowing the etiology and directing treatment. Parents should be asked about the onset, timing, and duration of symptoms, previous home therapies and medications used (including prescription and over-the-counter oral and topical therapies), and prior laboratory tests or evaluative procedures. The possibility of sexual abuse should be assessed, along with a detailed review of the developmental, behavioral, and psychosocial history. The child's past medical or surgical history should be evaluated for other skin infections, dermatoses, or allergies. Family history of chronic illness, allergies, and contact sensitivities should also be assessed. A list of possible acute or chronic irritant exposures such as bubble baths, cleaning agents and techniques (e.g. use of washcloths), lotions, powders, fabric softeners, and hair products that may have leaked into the bathwater should be investigated.24
The child should have a complete physical examination documenting pubertal stage as well as evidence of chronic disease or other skin abnormalities. With the patient in the frog-leg or knee–chest position, the perineum and vulva can be examined for the presence of erythema, discharge, odor, and edema. An otoscope or colposcope can aid the examiner by providing focused light and magnification. Vaginal discharge when present can vary from copious to minimally dried secretions.
It is often helpful to examine the vaginal discharge, but obtaining the specimen from a child can be challenging. Saline instilled into the vagina can be “recollected” as it accumulates in the lower vagina and vestibule. More commonly, the discharge can also be collected directly with a thin dry or saline-moistened bacteriostatic swab, being very careful not to touch the sensitive hymenal tissue.25, 26 Having the child perform a Valsalva maneuver (e.g., cough) may aid in this procedure. Topical anesthetics should be used with caution because of initial burning; this may upset the child, prohibiting further examination. An exception to this is EMLA cream. It is usually used without discomfort but must be applied 30-60 minutes in advance of the evaluation, specimen collection or even biopsy in older cooperative children. Once the sample is collected, it should be examined with saline wet mount inspection, potassium hydroxide (KOH), Gram stain, vaginal pH, and cultures.
Based on the history, physical examination, and laboratory evaluation, the causes of pediatric vulvovaginitis are most easily classified into noninfectious (or nonspecific) and infectious (or specific) groups, with the latter subclassified into nonsexually and sexually transmitted infections19, 20 (Table 3).
Table 3. Prepubertal vulvovaginitis
Noninfectious
Chemical irritants (e.g., bubble baths, perfumes, soaps, and hair products in the bathwater)
Allergic contact
Poor hygiene
Poor perineal aeration
Foreign body
Urologic anatomic abnormalities
Infectious
Nonsexually transmitted
Bacteria: group A beta-hemolytic streptococcus, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus viridans, Shigella sonnei, methicillin-resistant Staphylococcus aureus
Viral: adenovirus, varicella zoster, echovirus, non-sexually acquired human immunodeficiency virus
Fungal: Candida albicans
Helminths: Enterobius vermicularis
Sexually transmitted
Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas, Mycoplasma hominis, Ureaplasma urealyticum, herpesvirus (1 and 2), human papilloma virus, human immunodeficiency virus, molluscum contagiosum, Treponema pallidum
Noninfectious Vulvovaginitis
One of the most common causes of noninfectious vulvovaginitis, usually referred to as nonspecific vulvovaginitis, is poor perineal hygiene. On examination, patients usually have mild, nonspecific vulvar inflammation and may have stool or pieces of toilet paper on the perineal tissue as well as soiled underwear. Treatment consists mainly of hygiene education and supportive care. The clinician should recommend that the child have sufficient opportunities to urinate, uses a front-to-back wiping technique with soft, white, unscented toilet paper, and washes her hands regularly, especially after bathroom use. Undergarments should be 100% cotton, loose fitting, and cleaned or rinsed thoroughly with mild hypoallergenic unscented detergent without fabric softener. Mild hypoallergenic perfume-free and dye-free cleansers used to wash the perineal area gently leave the skin more moisturized than regular soap. Cleaning agents should never be applied with a washcloth, which can exacerbate areas of irritation or transfer infectious organisms to that area. After bathing, the perineal area should be air-dried or patted dry with a towel, avoiding rubbing.
In most patients, acute nonspecific vulvovaginitis of any cause can be treated symptomatically with frequent sitz baths containing baking soda or colloidal oatmeal or with wet compresses of Burrow's solution. For extremely severe cases where other etiologies have been excluded, a 1% hydrocortisone cream can be used once or twice a day for up to 2 weeks for itching, or a 1–2-week course of estrogen cream can be used to facilitate healing of excoriation.
Another common cause of vulvovaginitis is excessive or prolonged exposure to moisture combined with poor aeration of the perineal tissues. Predisposing factors to this form of vulvovaginitis include obesity, wearing tight or synthetic undergarments, and exposure to long periods of wet undergarments (e.g., enuresis, swimsuits). Physical examination may show nonspecific inflammation to severe excoriation, which may be associated with secondary bacterial infections, most commonly due to Staphylococcus aureus. Sitz baths and proper techniques of perineal cleansing and drying should be reviewed. Patients should wear loose-fitting cotton undergarments and might find sleeping without undergarments more comfortable.
Allergic vulvovaginitis or contact dermatitis may present with pruritus as the most prominent symptom. Acute or chronic offending agents are usually topical creams, lotions, perfumed soaps, toilet paper, and poison ivy. With chronic exposure, the vulva may develop cracks or fissures and eventually a lichenified appearance. Eosinophils in the vaginal fluid may be found in cases resulting from allergic reactions.27 Chemical irritants from bubble baths, laundry detergents, soaps, and fabric softeners produce a similar clinical picture. Treatment consists of removal of the offending agent, hygiene education, sitz baths, and a brief (up to 2 weeks maximum) course of 1% hydrocortisone cream.20
Vaginal foreign bodies can also present as nonspecific vulvovaginitis in the prepubertal child. Possible symptoms include profuse, persistent, foul-smelling discharge that may be blood stained (Table 4).
Table 4. Bloody genital discharge
Infectious vulvovaginitis: group A beta-hemolytic streptococcus, Haemophilus influenzae, Shigella sonnei, Shigella flexneri
Foreign body
Sexual abuse
Trauma
Urethral prolapse
Exogenous hormone exposure
Lichen sclerosus
Tumor
Because superinfection is common, antibiotics may provide temporary relief, followed by the recurrence of symptoms. Thus, recurrent symptoms requiring repeated trials of medication accompanied by the above history might warrant vaginoscopy to rule out a foreign body. Although foreign objects made of firm material may be palpable on rectal examination, a child may not allow this procedure to be completed. In addition, because foreign bodies may be multiple or fragmented, irrigation is usually needed to loosen and remove all debris. Objects can also become embedded and encased by granulation tissue, which may cause erosion or perforation into the bladder or bowel tissue. Thus, to evaluate a child fully for the presence of a vaginal foreign object, an examination under anesthesia may be necessary. It is important as always with the diagnosis of vulvovaginitis, but especially with foreign bodies, to keep a high index of suspicion for sexual abuse. Removal of the offending agent, most commonly toilet paper, is curative. For developmental or behavioral reasons, some patients may find that using bulkier items (e.g., disposable wipes) to clean themselves is helpful in preventing recurrences.
Anatomic disorders such as ectopic ureter, urethral prolapse, and fistulas are rare causes that can present as vulvovaginitis. Instead of opening into the trigone of the bladder, an ectopic ureter opens elsewhere, usually along the urethra. It can also open into the vagina or in the area of the vaginal vestibule, where an additional meatal opening around the urethral meatus is seen. It is highly associated with other congenital anomalies such as dilatation and duplicated systems. Patients typically present with a history of incontinence and a constantly wet perineum. With the contralateral ureter being normal, these children can accumulate urine in the bladder and thus have normal voiding habits. Although the diagnosis can be made prenatally, these patients may not be diagnosed until adulthood because they can be easily misdiagnosed as having primary enuresis or stress incontinence. A voiding cystourethrogram confirms the diagnosis, ultrasound can identify any associated müllerian structural anomalies, and a renal scan will detect functioning. Surgical correction is necessary for this condition.20, 28
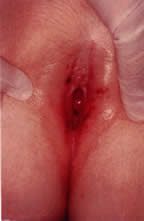
Patients with vesicovaginal fistulas also usually have a history of a constantly wet perineum and show nonspecific vulvovaginitis and excoriation on physical examination as a result of the continuous presence of urine. Presence of feculent vaginal discharge suggests a rectovaginal fistula. Patients with urethral prolapse usually present with blood staining on their underwear. Other symptoms include frank vaginal bleeding with accompanying vulvar pain or dysuria. This problem is often precipitated by activities that increase intra-abdominal pressure (e.g., coughing, straining with bowel movements, crying). Examination typically reveals an everted, red, circular mass at the external urethral meatus (Fig. 10). It is most common in African-American children younger than 10 years of age.19, 20, 28 Hypothesized factors that place young children at increased risk include the combination of weak attachments of the different layers of urethral muscle and episodes of increased intra-abdominal pressure.29 Medical treatment of urethral prolapse includes sitz baths, or estrogen cream two or three times a day for 2–4 weeks. Surgical excision is considered when it does not resolve or continues to recur.
Infectious Vulvovaginitis, Nonsexually Transmitted
Vaginal discharge is a more prominent finding associated with infectious causes of vulvovaginitis than with noninfectious causes. It is best to make the diagnosis of infection based on culture rather than treating empirically so that appropriate antibiotics can be prescribed and a definitive diagnosis given. Also, the recovery of certain organisms may prompt a sexual abuse evaluation that may not have been performed otherwise. There are limited data about the normal vaginal flora in the prepubertal child. Organisms cultured from prepubertal asymptomatic “control” subjects have included Bacteroides species, lactobacilli, Staphylococcus epidermidis, and other enteric organisms.30, 31 Although not well studied, antibiotic treatment may be warranted when these organisms are found in cultures of patients with symptoms that do not resolve with supportive care.
The poor hygiene habits of the young child commonly promote autoinoculation of respiratory, gastrointestinal, or urinary pathogens, while the unprotected, unestrogenized prepubertal vaginal tissues support their growth. Bacterial respiratory pathogens, such as group A beta-hemolytic streptococci (GAS), Streptococcus pneumoniae, and Haemophilus influenzae can cause a purulent vaginal discharge with an associated vulvovaginitis with or without other symptoms.32 GAS in particular can have a dramatic appearance, with severe vulvovaginal erythema, edema, and discharge; it may be or not be associated with a concurrent scarlet fever and a positive throat culture. It is also associated with desquamation that can take place a few weeks later. Bacterial cultures of the perineum can confirm the diagnosis.25, 32, 33 GAS vulvovaginitis can be treated with amoxicillin 40 mg/kg divided three times a day for 10 days. Methicillin-resistant Staphylococcus aureus (MRSA) is an increasingly common community-acquired pathogen and antibiotic choice should be influenced by the possibility of this infecting organism. A family history of infection with MRSA may be helpful when considering empiric therapy. MRSA is usually sensitive to clindamycin. Culture results and bacterial sensitivities should direct the antibiotic choice.
Viral pathogens usually present as ulcerative lesions in the vaginal area (Fig. 11). Offending viral pathogens include adenovirus, varicella, echovirus, Epstein-Barr virus, and herpesvirus 1 and 2. A viral culture is necessary for a definitive diagnosis. A direct florescent antibody evaluation of a swab of the lesion may give an immediate indication as to whether or not HSV is present while awaiting culture results. Presumptive treatment with acyclovir should be considered while awaiting cultures. Gastrointestinal pathogens such as Shigella can produce an acute or chronic vaginal discharge that is bloody, purulent, and foul-smelling, with associated vulvovaginal erythema. It is sometimes associated with diarrhea in the patient or family members. Culture of the vaginal discharge is diagnostic, and sensitivities should be obtained so that appropriate systemic antibiotics can be administered.34, 35
Although intestinal infestation with Enterobius vermicularis (pinworms) usually causes perianal itching, vulvovaginitis has also been reported. Because of close proximity, the pinworms may crawl into the vagina (or be transferred by scratching), bringing eggs and attached enteric organisms. Physical examination may show vaginal discharge, nonspecific inflammation, and excoriation from scratching. The diagnosis is confirmed by observation of pinworm ova and/or adults with a saline wet mount or with Scotch tape testing, which is best done when the patient is asleep during the night, when the worms emerge to feed. Patients are treated with mebendazole; empiric treatment of the entire family is given to avoid reinfection.
Candidal vulvovaginitis, although very common after puberty, is extremely rare in healthy prepubertal children and is often overdiagnosed and overtreated.23 Predisposing factors include recent antibiotic use, poor perineal aeration, inflammatory skin conditions such as seborrheic dermatitis, and chronic diseases such as diabetes mellitus and immunodeficiency syndromes. Pruritus and dysuria are the most common complaints, along with the presence of diffuse vulvar erythema, thick cheesy vaginal discharge, excoriation from scratching, and the presence of white plaques on the vaginal mucosa. “Satellite lesions” and erythematous prominence in the creases are characteristic of candidal rashes, especially in those who wear diapers. A patient with a candidal infection will have a low vaginal pH, and budding yeast and pseudohyphae can be seen with a saline wet mount and KOH preparation. Treatment consists of topical or oral antifungal agents such as fluconazole; the area should be kept as dry as possible. Persistent candidal infections, especially in the prepubertal child, should prompt investigation for the presence of diabetes or HIV or other immunodeficiency syndromes.19, 20
Infectious Vulvovaginitis, Sexually Transmitted
Organisms associated with sexual transmission that are found in prepubertal girls require an evaluation for sexual abuse. Practitioners should always have a high index of suspicion and incorporate questions about sexual abuse into the routine history. They should also know their local mandated reporting laws and should perform a thorough physical examination as well as an extensive psychosocial history. When sexual abuse is discovered, referral to a child abuse specialist in the community should be made to ensure proper and complete care, especially because the collection of evidence may vary from the office guidelines. Acute injury and infection require immediate attention and possible referral to an emergency department. Cultures should always be obtained for gonorrhea and chlamydia before treatment. Antibiotic choices and doses vary with age, weight, and pregnancy and are outlined in the “American Academy of Pediatrics Red Book.”36
The lack of estrogenized epithelium in this age group may inhibit sexually transmitted organisms from extending upward into the pelvis. Thus, symptoms typically include “lower tract” symptoms of pruritus, dysuria, vaginal discharge, and odor. Physical findings consist of vulvovaginitis, external lesions, and vaginal discharge. Genital infection with Neisseria gonorrhoeae is essentially pathognomonic for sexual abuse. It is usually associated with a purulent thick yellow discharge along with vulvar erythema, edema, and excoriation and inguinal lymphadenopathy. Diagnosis is confirmed with a cervical or vaginal culture or with nucleic acid amplification testing (NAAT) of a cervical, vaginal or urine specimen. The patient is treated with ceftriaxone, cefixime, or azithromycin for penicillin allergic patients. Chlamydia trachomatis can infect the atrophic vaginal squamous cells of a prepubertal child as well, causing a similar vulvovaginitis with pruritus and a vaginal discharge. The presence of C. trachomatis is also indicative of sexual abuse. It can also be acquired perinatally; infants born to mothers with chlamydia have been shown to have asymptomatic carriage for up to 18 months.37 As with gonorrhea, cervical or vaginal culture or NAAT of a cervical, vaginal or urine specimen will confirm the diagnosis. The patient should be treated with azithromycin or doxycycline.
Trichomonas vaginalis is diagnosed either by culture or more easily by observing the organisms with a saline wet mount. There is the presence of a fishy odor (positive “whiff”) when potassium hydroxide is added to the sample of vaginal discharge. It is treated with metronidazole. Sexual abuse should be suspected.
Bacterial vaginosis can be associated with sexual abuse but has been reported in “control” subjects.38 It is diagnosed by the presence of a positive “whiff”, when KOH is added to a specimen, a higher-than-normal vaginal pH, and the presence of “clue cells”, which are vaginal epithelial cells covered with bacteria observed with a saline wet mount. Treatment consists of metronidazole.
Herpes simplex virus (HSV) produces vesicular lesions with a resultant ulcerative vulvovaginitis and usually includes inguinal lymphadenopathy and systemic symptoms. Both serotypes may produce genital lesions and both should alert the clinician to the possibility of child abuse. HSV can be treated with acyclovir.
Human papilloma virus (HPV) causes condyloma acuminata, or painless, soft, moist, granular, and friable lesions that predominate in the vaginal vestibule and perianal area. The lesions can become secondarily infected and produce pruritus, pain, and discharge. Therapy consists of cryotherapy, serial applications of TCA, podophyllin or imiquimod cream. Older children (11 and above) may be offered immunization against high risk types of HPV associated with cervical dysplasia as well as those associated with genital warts.
Molluscum contagiosum are waxy, centrally umbilicated lesions 2–5 mm in diameter. Treatment can include imiquimod cream or curettage.
Vulvovaginitis from syphilis is usually due to the manifestation of secondary syphilis, which includes a rash over the perineum and inner thighs and development of condyloma lata on the vulva and anus. Serologic and cerebrospinal fluid testing confirms the diagnosis and establishes the therapy.20, 39
NONINFECTIOUS DERMATOLOGIC CONDITIONS
Lichen Sclerosus
Lichen sclerosus is a condition that affects all areas of the body in both sexes and in all ages. Its etiology is unknown, but it is believed to be associated with autoimmune disease.40, 41 Evidence currently links lichen sclerosus to specific HLA types; a familial incidence is well recognized and significant.42 Attention has also been focused on Borrelia burgdorferi, but further delineation is needed43, 44 (Fig. 12). It is primarily a condition seen after menopause but is also common in the hypoestrogenic prepubertal state. It appears as white lesions.45 In children the average age of onset is 5 years.46 It presents as tiny, pink-white papules that coalesce to plaques with a wrinkled, atrophic “cigarette paper” surface, with fine follicular keratinous plugs, which become prominent. An hourglass or figure-of-eight appearance is not uncommon. An edematous hyalinized area in the dermis with red cells with an underlying band of inflammatory cells is often noted on the biopsy sample. A clinical appearance of ivory-colored, flat-topped polygonal papules, which coalesce into plaques, reflects the dermatopathology. Purpuric speckling and frank ecchymosis are common, and the Koebner effect is well recognized.47
|
The usual complaint is vulvar irritation and pruritus that results in an itch/scratch cycle, and ultimately there may be destruction of the vulvar architecture with scarring, labial agglutination, and kraurosis.45
Management includes perineal hygiene and reduction of trauma (i.e, scratching of the involved area). Depending on the symptomatology, the use of a potent topical corticosteroid in the form of clobetasol propionate 0.05%, applied to the affected area on a daily basis for approximately 2 weeks, has been advocated.47, 48 Therapies have also included betamethasone dipropionate 0.05% applied three times a day for 3 weeks, then twice daily until the appearance of the vulva becomes normal. This may require 1 to 6 months of therapy.49 As symptoms improve, the corticosteroid preparation may be reduced to 1% to 2% hydrocortisone ointment or cream applied daily to the affected area on an as-needed basis. If the diagnosis is not clear-cut, a vulvar biopsy is recommended. Twice-yearly examinations after puberty have been advocated but are not uniformly accepted. There is a low but potential long-term risk of malignancy.50
The question of sexual abuse in association with Lichen sclerosus has been addressed. The concern is whether there is mistaken accusations of abuse that may act as a "trigger" for the onset of Lichen sclerosus possibly via the Koebner phenomenon. 51 As has been reported, both sexual abuse and Lichen sclerosus are frequently seen by gynecologists; the two conditions may not be mutually exclusive. 52
Lichen simplex is a thickening of the skin in response to scratching. The skin becomes “lichenified” and thus is thickened in response to repeated irritation. There may also be an element of psoriasis, eczema, or lichen sclerosus. The lesions clinically appear as pale with increased skin margin markings also noted. Histologically, the skin is hyperkeratotic and acanthotic, with dermal inflammation. The condition responds to applications of topical corticosteroids (1% to 2% hydrocortisone) until the underlying factors causing the problem are eliminated; antihistamines are also helpful. An effort must be made to break the itch/scratch cycle.
Lichen Planus
Although rare in the premenarchal child in general, and quite uncommonly manifested as vulvar involvement, lichen planus has been reported in the pediatric patient. Lichen planus is categorized by angular, violaceous demarcated flap papules. Koebner's phenomenon results in a striking linear distribution along sites that are traumatized by scratching. The more common sites are in the flexor surfaces of the wrists, the forearms, and the inner thighs. Vulvar lesions are less distinctive and may resemble leukoplakia, with a variable degree of excoriation, maceration, or verrucous thickening. The buccal mucosa may exhibit a lacy pattern of minute white papules. Biopsy verifies the diagnosis. Exacerbations and recurrences allow for only limited success with most treatment plans, which primarily involve topical and intralesional corticosteroid administration as well as antihistamines. Squamous cell carcinoma may arise in long-standing hypertropic vulvar lichen planus. Careful follow-up with biopsy as indicated is recommended.53
Psoriasis
Psoriasis is a papulosquamous skin disease caused in part by a marked increase in epidermal cell turnover, with excessive cell proliferation. It affects 1% to 3% of the total population, with 2% of cases starting by 2 years of age.54 It can be inherited, with up to one third of patients having a family member with the disease.55 The exact cause of psoriasis is unknown, but the changes seen are thought to be the result of a T-cell lymphocyte immune response that stimulates cytokines and other mediators to produce inflammation.56
Psoriasis can develop in the genital area, and this may be the only affected area. Typically, however, similar lesions are present elsewhere on the body in characteristic symmetric locations: extensor surfaces of the extremities, lumbosacral area, scalp, and anogenital area. Psoriatic lesions appear as well-demarcated erythematous papules or plaques that have adherent silvery or gray scales. Arthritis is an associated finding in up to 5% to 7% of patients and occurs up to 2 years after the onset of skin findings.55 With vaginal psoriasis, the scaly appearance may be absent if the vagina is moist enough, but it usually occurs as scaly well-defined patches.26 Removal of scales produces the characteristic punctate bleeding points known as the Auspitz sign, resulting from the rupture of capillaries high in the papillary dermis. Another phenomenon characteristic of psoriatic lesions is the Koebner phenomenon, an abnormal reaction of the skin in which local injury or trauma produces an extension of the lesion.55
Treatment starts with educating the parents about the disease course, which includes remissions and exacerbations of variable onset and duration, and the potential for emotional impact. The mainstay of therapy consists of topical treatment. Emollients aid in promoting moisture, and tar preparations can decrease epidermal proliferation via their anti-inflammatory effects.55, 57 Topical corticosteroids can produce dramatic results even at low potencies. However, topical corticosteroids should be used cautiously, especially in the genital area, because of the increased potential for side effects. Higher potencies should be reserved for severe exacerbations, tapering soon to a lower dose.57, 58 In addition, treatment should be limited to 2-week courses with at least 2 weeks in between. Referral to a dermatologist should be made for patients with complicated and extensive involvement.
Seborrheic Dermatitis
Seborrheic dermatitis is characterized by erythematous, scaly, crusting, or greasy-appearing eruptions that occur in places that have high concentrations of sebaceous glands, mainly the scalp, face, neck, axillae, and intertriginous areas. Infantile seborrheic dermatitis can occur as soon as the second week of life as a result of the effects of transplacentally acquired maternal hormones. It may persist up to 12 months of age and resolves spontaneously. Pruritus is not a major feature of seborrheic dermatitis except when it occurs in the groin area, where excessive scratching can be severe and lead to secondary bacterial infections. Its early onset, greasy, scalelike appearance, predilection for the scalp and intertriginous areas, and general absence of pruritus distinguish this disorder from atopic dermatitis and psoriasis.
Seborrhea is treated with softening of the scales with selenium sulfide preparations, lubricants, and antifungal agents. Referral to a dermatologist may be necessary for refractory cases or if the diagnosis is unclear.55
Atopic Dermatitis
Atopic dermatitis is one of the most common skin disorders seen in infants and children, affecting 10% to 15% of the childhood population. It is a chronic disorder that is often seen with allergies and asthma. Although the exact pathophysiology is unknown, there is evidence that the primary immunologic abnormality is altered T-cell immune function. In addition, the skin of patients with atopic dermatitis is structurally abnormal, leaving a compromised barrier against external irritants.
There are three basic forms of atopic dermatitis that are classified based on the age of the patient. The infantile form begins between 2 and 6 months of age and is characterized by erythema, papules, vesicles, and intense pruritus on the face, trunk, or extremities. Atopic dermatitis in this age group usually spares the diaper area because of the moist nature of that environment. Childhood atopic dermatitis occurs in the prepubertal years and classically involves the wrists, ankles, and antecubital and popliteal fossas. The lesions are usually more dry and can eventually become lichenified. Patients can also have “wet” lesions that can be erythematous, edematous, oozing, and weepy. When the vulva is involved, pruritus is common, as is an increased chance of secondary infections with bacteria, most commonly GAS, or with fungus.
Treatment starts with environmental control of possible offending allergic agents, such as food or environmental allergens. Lubricants should be used frequently to moisten and rehydrate dry areas. Wet compresses with Burrow's solution can also be used to soothe acutely inflamed skin. To reduce the chance of secondary infection, patients should be instructed to keep the child's fingernails short and clean. Systemic antihistamines can be helpful to help control severe pruritus.59, 60 Topical corticosteroids have been the mainstay in treating atopic dermatitis but should be used with extreme caution in the genital area because of possible permanent adverse effects, such as skin atrophy, immune suppression, telangiectasias, striae, and pigment changes.55 These adverse effects are dependent on the duration, potency, and location of use, especially in intertriginous areas. For severe or refractory cases, referral to a dermatologist may be necessary.
OBSTRUCTIVE VAGINAL DISORDERS
Obstructive disorders of the vagina are divided into longitudinal or transverse fusion problems. With respect to longitudinal fusion problems, septate vagina is a situation in which the vagina is duplicated and is asymptomatic. On occasion, dyspareunia secondary to narrowing of the vagina may require removal of the septum.
A transverse septum can create an outflow tract obstruction in the vagina. This presents in the adolescent with severe dysmenorrhea; in addition, there may be vaginal “swelling” indicative of hematocolpos.
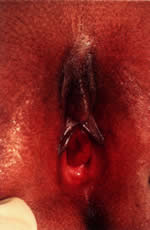
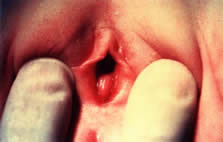
Disorders of transverse fusion are more common; the most common is imperforate hymen. The differential for an imperforate hymen includes labial adhesions. In the pediatric patient, microperforate hymen (Fig. 13)and imperforate hymen are the most commonly encountered problems. The imperforate hymen is noted in the neonate with the Valsalva maneuver (crying) and is associated with bulging at the introitus. The obstruction is associated with a mucocolpos distention of the hymenal membrane.
In the girl age 9 or older with normal secondary sexual development with cyclic pelvic and/or abdominal pain, imperforate hymen should be considered. The accumulation of the endometrial fluid in the form of hematocolpos, hematometra, hematosalpinx, and often associated endometriosis secondary to retrograde menstruation occurs. Vulvar examination identifies a tense, bulging, bluish membrane at the introitus. The blue discoloration reflects the menstrual fluid. After the diagnosis is made, a stellate incision is made into the membrane and any redundant segments are removed. No additional surgery is necessary, and once the obstruction is relieved there is cessation of the hematocolpos and hematometra as well as endometriosis.
Correction of an imperforate hymen in the prepubertal patient is appropriate but is usually avoided during the first 6 months of life because of increased anesthesia risks.61 Authors advocate waiting until the premenarchal period for surgical intervention, in part because estrogenization may facilitate the healing process.
The transverse vaginal septum can occur at the junction of the middle and upper thirds of the vagina. Magnetic resonance imaging or sonography can be used to identify the presence of the septum. An intravenous pyelogram should be obtained or other renal ultrasonographic assessment performed to exclude renal abnormalities such as pelvic kidney before surgical intervention is undertaken to correct the vaginal septum.62 Surgical resection of the septum relieves any associated obstruction.
The incidence of a transverse vaginal septum, representing a disorder of vertical fusion, is 1 in 80,000 females.63 Surgical resection is indicated to relieve the outflow of tract obstruction. Reanastomosis between the upper and lower vaginal mucosa is recommended. If this is not feasible, use of a transfundal uterine sound placed through the cervix along the vaginal septum allows safe excision and resection. Mucosal undermining and mobilization or Z-plasty techniques may facilitate a primary reanastomosis. The space so created maintains its patency by placement of a hollow mold until the patient becomes sexually active.64
AMBIGUOUS GENITALIA
Ambiguous genitalia in a neonate is a life-threatening, true emergency. Appropriate evaluation is paramount. Adrenal hyperplasia must be ruled out because it is potentially fatal. If adrenal failure is unrecognized, vomiting and diarrhea may progress quickly to dehydration, shock, and death.
Inability to answer the delivery room question “Is it a boy or a girl?” is distressing to a parent. Although parents may pressure the physician to assign a gender in the delivery room, it is better to delay than to be forced to change the sex assignment later, resulting in parental confusion about whether the final sex of rearing is actually the correct one. A sensitive approach is critical. It is better to explain that the infant's sexual development is “incomplete” than to use terms with negative connotations such as “hermaphrodite.” The American Academy of Pediatrics in a recent consensus statement on the management of intersex disorders proposes the term “disorders of sex development”.65 A multidisciplinary approach is best.
The search for a cause begins with history, especially with regard to medications taken during pregnancy that have possible masculinizing effects (Table 5). Danocrine, testosterone, and certain 19-nortestosterone derivatives and drugs such as P-450 inhibitors taken for seizure disorders during pregnancy all have been implicated. A review of systems may uncover signs of endogenous maternal androgen excess, such as acne or hirsutism, that point to a virilizing ovarian tumor.66, 67 A family history of a previously affected fetus or prior neonatal death may be revealed in cases of virilizing adrenal hyperplasia, which is inherited as an autosomal recessive gene.
Table 5. Ambiguous genitalia in neonates
Partially virilized 46,XX: Female pseudohermaphrodite
- Congenital adrenal hyperplasia
21-Hydroxylase deficiency
11-β-Hydroxylase deficiency
3-β-Hydroxysteroid dehydrogenase deficiency - Maternal drug intake
Testosterone
Ethisterone
Norethindrone
Oral contraceptives
Danocrine - Maternal ovarian tumor
Luteoma of pregnancy
Sertoli Leydig cell tumor
Leydig cell tumor - Maternal adrenal tumor
Incompletely virilized 46,XY: Male pseudohermaphrodite
- Müllerian duct inhibitory factor defect
- Impaired androgenization
Testicular feminization
5-α-Reductase deficiency - Testosterone biosynthesis defects
20,22-Desmolase deficiency
3-β-Hydroxysteroid dehydrogenase deficiency
17-α-Hydroxylase deficiency
17,20-Desmolase deficiency
17-Hydroxysteroid dehydrogenase deficiency
Stepwise evaluation of the genital system is appropriate. The full length and width of the genital tubercle are measured. At times it is possible to decide whether the phallus represents a clitoris or a penis. A clitoris has two folds that extend laterally to the labia minora, whereas a penis has a midline ventral frenulum. The urethral meatus may occupy various positions, from the perineal area or urogenital sinus to a mild hypospadias.
The inguinal canals and labioscrotal folds are palpated for the presence of gonads. Rarely a normal ovary may herniate, but these ovaries appear just outside the inguinal ring and do not migrate into the labioscrotal folds.68 Palpable masses in the lower inguinal or scrotal areas are virtually always testicles; their absence justifies a provisional diagnosis of virilizing adrenal hyperplasia until proven otherwise. This anatomy does not rule out the possibility of a 46,XY infant with undescended intra-abdominal testes; however, true bilateral cryptorchidism is extremely rare and not usually associated with ambiguous genitalia.68 The extent of the Müllerian system presence may be defined in several ways. A rectal examination may reveal a uterus, particularly during the newborn period, when it may be somewhat hypertrophied from the recent effects of maternal estrogen. Further confirmation can be obtained by urogenital sonography or ultrasonography.
During diagnostic evaluation, immediate blood samples should be taken from the newborn and sent for karyotype, electrolytes, 17-hydroxyprogesterone, androstenedione, and dehydroepiandrosterone sulfate (DHEAS) (Table 6). Maternal blood levels of testosterone and DHEAS may also be helpful in diagnosing an ovarian or adrenal neoplasm; if elevated, ultrasonography is in order.69 The most frequent enzyme defect in congenital adrenal hyperplasia is 21-hydroxylase. It is often associated with virilization of a 46,XX infant, as in 11-β-hydroxylase deficiency. In both, serum levels of 17-hydroxyprogesterone will be greatly increased. Of course, signs of adrenal failure in a hyperkalemic, hyponatremic infant verify the diagnosis of adrenal hyperplasia, regardless of the steroid pattern. Electrolytes should be closely monitored during the first 7 to 10 days because it may take this long before metabolic derangements develop.
Table 6. Laboratory workup: ambiguous genitalia in neonates
Maternal
Serum testosterone
Serum DHEAS (dihydroepiandrosterone sulfate)
Pelvic/abdominal ultrasonography
Newborn
Karyotype
Serum electrolytes
Serum 17-hydroxyprogesterone
Serum androstenedione
Serum DHEAS (dihydroepiandrosterone sulfate)
In-depth counseling with the parents is best managed by a team involving a pediatrician, reproductive endocrinologist, geneticist, surgeon, and psychologist. A high degree of anxiety is inherent in the determination of sex of rearing, and irrevocable trauma may be inflicted on the patient and family if gender assignment is reversed at a later date. Reconstructive surgical procedures may be limited by the adequacy of the phallus, regardless of gonadal sex, so this may influence the final decision. Successful psychological development and adjustment of the affected child is highly influenced by the support and acceptance of the parents. Education and ongoing support of the parents through diagnosis and treatment is of critical importance.
PRECOCIOUS PUBERTY
Precocious puberty is defined as the onset of puberty before age 8 in girls. However, recent data show puberty to be starting as much as 1 year earlier in white girls and 2 years earlier in African-American girls.70 Until more prospective studies are performed with comparable evaluative methods, girls with signs of puberty before age 8 should be investigated. Signs of precocious puberty include breast or pubic hair development, in addition to an increased rate of linear growth or early bone maturation. These children may not follow the sequence of normal pubertal development. They can be classified as having isosexual precocity, in which there is progression of normal female development, or contrasexual precocity, in which male androgen effects predominate.
Premature thelarche is the early development of isolated breast tissue without other signs of puberty. It can be confused with the first sign of precocious puberty. Premature adrenarche is the early development of isolated pubic hair, axillary hair, and body odor, also without other pubertal signs. These two conditions are not usually associated with an increased rate of linear growth.
Precocious puberty is divided into two main etiologic categories: gonadotropin releasing hormone (GnRH)-dependent or GnRH-independent (Table 7). GnRH-dependent precocious puberty, also termed central or true precocious puberty, refers to an early activation of the hypothalamic–pituitary–gonadal axis, which controls the process of puberty. Precocious puberty is much more common in girls and is usually idiopathic. However, life-threatening causes of early puberty must be excluded. The most common cause of nonidiopathic precocity is central nervous system (CNS) pathology. CNS tumors, most commonly hypothalamic hamartomas, as well as other CNS processes such as trauma, encephalitis, meningitis, hydrocephalus, neurofibromatosis,71 and abnormal skull development from rickets can produce GnRH-dependent precocious puberty. Other neurologic abnormalities such as spina bifida often are associated with early puberty. Other etiologies include ectopic gonadotropin production, most commonly hCG-producing chorioepitheliomas and dysgerminomas of the ovary or liver as well as severe, long-standing hypothyroidism.
Table 7. Precocious puberty classification
GnRH-dependent, central, or true precocious puberty
- Idiopathic
- Central nervous system lesions
—Cranial trauma
—Encephalitis, meningitis
—Rickets
—Tumors: hypothalamic hamartomas, craniopharyngioma, optic glioma, suprasellar teratoma, astrocytoma
—Hydrocephalus
—Neurofibromatosis type I - Gonadotropin-producing tumors
—Ovary, liver, mediastinum
- Prolonged untreated primary hypothyroidism
- GnRH-independent processes
GnRH-independent, peripheral or pseudoprecocious puberty
- Ovarian cyst
- Ovarian tumor: granulosa cell, theca cell, gonadoblastoma, teratoma, chorioepithelioma, lipid cell tumor
- Feminizing adrenal tumor
- Congenital adrenal hyperplasia
- Drug ingestion: synthetic estrogens, oral contraceptives, anabolic steroids
- McCune–Albright syndrome
With GnRH-independent precocious puberty, also known as peripheral or pseudoprecocious puberty, sex steroids are secreted independently of pituitary gonadotropin. Most of these cases are due to estrogen-secreting tumors of the ovary, most commonly granulosa cell, that are frequently palpable on the abdominal examination.72 Large estrogen-secreting ovarian luteal or follicular cysts are also usually palpable on the abdominal examination of a patient with early puberty. Other etiologies include congenital adrenal hyperplasia and feminizing adrenal tumors.
GnRH-independent precocity is also associated with genetic syndromes such as McCune-Albright (polyostotic fibrous dysplasia), characterized by multiple cystic bone lesions, café-au-lait spots, and sexual precocity. In this syndrome, premature menarche may be the first pubertal sign, with skeletal anomalies developing later in life.73
Almost every cause of GnRH-independent precocious puberty can secondarily activate the hypothalamic-pituitary-gonadal axis, resulting in additional GnRH-dependent effects.74
The diagnostic evaluation begins with a detailed history of the rate of pubertal progression (thelarche, pubarche), acne, body odor, vaginal discharge or bleeding, growth rate, past medical history, including history of head trauma or CNS infection, and family history of medical problems, including early unexplained deaths (which could have been due to salt-losing congenital adrenal hyperplasia). A detailed physical examination should be done that includes growth progression and percentiles and abdominal, skin, neurologic, and external genitalia examinations, as well as an assessment of Tanner stage. The clinical scenario dictates the extent of the evaluation and should always start with a radiographic evaluation to determine bone age. Other tests that may be useful include brain magnetic imaging, abdominal/pelvic computed tomography or ultrasound, and serum levels of follicle-stimulating hormone, luteinizing hormone, hCG, thyroid function tests, prolactin, DHEAS, testosterone, estradiol, progesterone, and 17-hydroxyprogesterone. Consultation with an endocrinologist can help to orchestrate, evaluate and interpret tests is recommended. The activity of the hypothalamic–pituitary–gonadal axis is determined by a GnRH stimulation test: serum luteinizing hormone levels are measured, 100 micrograms of GnRH is administered subcutaneously, and then another serum luteinizing hormone measurement is obtained 40 minutes later. Patients with GnRH-dependent processes will have serum levels of luteinizing hormone above the pubertal range. Elevated levels of 17-OH progesterone and adrenal androgens suggest a diagnosis of congenital adrenal hyperplasia, which must be confirmed with an ACTH stimulation test75 (Table 8).
Table 8. Precocious puberty: diagnostic evaluation
Physical examination
Growth velocity and percentiles
Tanner staging
Abdominal, neurologic, genital, and skin examination
Palpation of thyroid
Evidence of androgen excess (hirsuitism, clitoromegaly)
Radiologic
X-ray for bone age
Brain MRI
Abdominal or pelvic CT or ultrasound
Serologic
Follicle-stimulating hormone
Luteinizing hormone
Human chorionic gonadotropin
Thyroid-stimulating hormone
Free T4
Dehydroepiandrosterone sulfate
Testosterone
Estradiol
Progesterone
17-hydroxyprogesterone
Prolactin
Testing
Gonadotropin releasing hormone stimulation test
ACTH stimulation test
Management goals of precocious puberty include excluding life-threatening etiologies, delaying the progression of secondary sexual development and bone maturation, maximizing final adult height, and screening for possible emotional and psychosocial effects of early puberty. GnRH agonists are the mainstay of treatment for precocious puberty; however, if a specific etiology is identified, treatment should be tailored to managing that process first.76 For CNS tumors, excision, irradiation, chemotherapy, and watchful waiting may be indicated. In certain CNS tumors, such as hypothalamic hamartomas, pubertal effects can be controlled with GnRH agonists. Any suspicion of abdominal neoplasm or ovarian cysts that may be large enough to undergo torsion or rupture usually requires surgical exploration and removal.72, 74 Most patients with idiopathic true precocious puberty have a final adult height of less than 5 feet because of the effects of estrogen causing early epiphyseal closure. GnRH agonists are most frequently used to delay this process and have been shown to significantly reduce the rate of growth and to delay the progression of secondary sexual characteristics. The effectiveness of exogenous GnRH administration can be monitored by measuring serum estradiol levels, which should remain in prepubertal ranges (less than 10 pg/mL), or by a negative GnRH stimulation test.75
The patient should be treated until the epiphyses close or until appropriate pubertal versus chronologic age is achieved. Idiopathic precocious puberty is not associated with premature menopause or impaired fertility. Because the chronologic age does not match the pubertal age, the patient may suffer emotional effects either from looking different from her peers or from experiencing social, intellectual, and sexual expectations. These concerns should be addressed, as well as the possible need for contraception.
REFERENCES
Kaplowitz P. Pubertal development in girls: secular trends. Curr Opinion Obstet Gynecol 18(5): 487-91, 2006 |
|
Muram D: Pediatric and adolescent gynecology. In Pernoll ML, Benson RC (eds): Current Gynecologic and Obstetric Diagnosis and Treatment, pp 563–585. Norwalk, Appleton & Lange, 1987 |
|
Christensen E, Oster J: Adhesions of labia minora (synechia vulvae) in childhood. Acta Paediatr Scand 60: 709, 1971 |
|
Leung AK, Robson WL, Tay-Uyboco J: The incidence of labial fusion in children. J Paediatric Child Health 29: 235, 1993 |
|
Capraro VJ, Greenberg H: Adhesions of labia minora: A study of 50 patients. Obstet Gynecol 39: 65, 1972 |
|
Nag RN: Labial adhesion in children. Indian J Pediatr 9: 33, 1972 |
|
Aribarg A: Topical oestrogen therapy for labial adhesions in children. Br J Obstet Gynaecol 82: 424, 1975 |
|
Khanam W, Chogtu L, Mir Z, Shawl F: Adhesion of the labia minora: A study of 75 cases. Aust NZ J Obstet Gynaecol 17: 176, 1977 |
|
Jenkinson SD, Mackinnon AE: Spontaneous separation of fused labia minora in prepubertal girls. Br Med J 289: 160, 1984 |
|
Berkowitz C, Elvik S, Logan M: Labial fusion in prepubescent girls: a marker for sexual abuse? Am J Obstet Gynecol 156: 16, 1987 |
|
Muram D: Labial adhesions in sexually abused children. JAMA 259: 252, 1988 |
|
Droegemueller W, Herbst A, Mishell D et al: Comprehensive Gynecology, 1st edn. St. Louis, CV Mosby, 1987 |
|
Pokorny SP: Configuration of the prepubertal hymen. Am J Obstet Gynecol 157: 950, 1987 |
|
Muram D: Treatment of labial adhesions in prepubertal girls. Adolesc Pediatr Gynecol 12: 67, 1999 |
|
Stovall T, Muram D: Urinary retention secondary to labial adhesions. Adolesc Pediatr Gynecol 1: 203, 1988 |
|
Mroueh J, Muram D: Common problems in pediatric gynecology, new developments. Curr Opinion Obstet Gynecol 11: 463, 1999 |
|
Myers JB, Sorensen CM, Wisner BP et al: Betamethasone cream for the treatment of pre-pubertal labial adhesions. J Pediatr Adolesc Gynecol 2006; 19(6): 407-11 |
|
Singleton A: Vaginal discharge in children and adolescents. Evolution and management: a review. Clin Pediatr 19: 799, 1980 |
|
Murray PJ, Davis HW, Hamp M: Pediatric and adolescent gynecology. In Zitelli BJ, Davis HW (eds): Atlas of Pediatric Physical Diagnosis, 5th edn, pp 525–561. St. Louis, Mosby-Wolfe, 2007 |
|
Slupik R: Pediatric gynecology. Clinical gynecology. In Sciarra J (ed.): Gynecology and Obstetrics, pp 1–19. Philadelphia, Lippincott Williams & Wilkins, 1991 |
|
Cox R: Haemophilus influenzae: An underrated cause of vulvovaginitis in young girls. J Clin Pathol 50: 765, 1997 |
|
Stylianopoulos J, Hogg G, Grover S: Vulvovaginitis: Clinical features, aetiology, and microbiology of the genital tract. Arch Dis Child 81: 64, 1999 |
|
Jaquiery A, Stylianopolis A, Hogg G, Grover S: Vulvovaginitis: Clinical features, aetiology, and microbiology of the genital tract. Arch Dis Child 81: 64, 1999 |
|
Brown J: Hair shampooing technique and pediatric vulvovaginitis. Pediatrics 83: 146, 1989 |
|
Emans S, Goldstein D: The gynecologic examination of the prepubertal child with vulvovaginitis: Use of the knee-chest position. Pediatrics 65: 758, 1980 |
|
Rau FJ, Muram D: Vulvovaginitis. In Sanfillipo J (ed.): Pediatric and Adolescent Gynecology, 2d edn, pp 199–215. Philadelphia, WB Saunders, 2001 |
|
Moraes P: Allergic vulvovaginitis induced by house dust mites: A case report. J Allergy Clin Immunol 101: 557, 1998 |
|
Starr N: Pediatric gynecology urologic problems. Clin Obstet Gynecol 40: 181, 1997 |
|
Lowe F, Hill G, Jeffs R, Brandler C: Urethral prolapse in children: Insights into etiology and management. J Urol 135: 100, 1986 |
|
Paradise J, Campos J, Friedman H, Frishmuth G: Vulvovaginitis in premenarchal girls: Clinical features and diagnostic evaluation. Pediatrics 70: 193, 1982 |
|
Gardner J: Comparison of the vaginal flora in sexually abused and nonabused girls. J Pediatr 120: 872, 1992 |
|
Dhar V, Roker K, Adhami Z, McKenzie S: Streptococcal vulvovaginitis in girls. Pediatric Dermatol 10: 366, 1993 |
|
Mogielnicki N, Schwartzman J, Elliot J: Perineal group A streptococcal disease in a pediatric practice. Pediatrics 106: 276, 2000 |
|
Bogaerts J, Lepage P, De Clercq A et al: Shigella and gonococcal vulvovaginitis in prepubertal central African girls. Pediatric Infect Dis J 11: 890, 1992 |
|
Smith R, McNamara J, Ladd M: Shigella and child abuse. Pediatrics 78: 953, 1986 |
|
American Academy of Pediatrics, Pickering LD (ed.): 2007 Red Book: Report of the Committee on Infectious Diseases, 25th edn. Elk Grove Village, IL, American Academy of Pediatrics, 2007 |
|
Schachter J, Grossman M, Sweet R et al: Prospective study of perinatal transmission of Chlamydia trachomatis. JAMA 255: 3374, 1986 |
|
Bartley D, Morgan L, Rimsza M: Gardnerella vaginalis in prepubertal girls. Am J Dis Child 141: 1014, 1987 |
|
Sobel J: Current concepts: Vaginitis. N Engl J Med 337: 1896, 1997 |
|
Ridley CM: Lichen sclerosus et atrophicus. Br Med J 295: 1295, 1987 |
|
Meyrick Thomas R, Ridley C, McGibbon D, Black M: Lichen sclerosus et atrophicus and autoimmunity: A study of 350 women. Br J Dermatol 118: 41, 1998 |
|
Purcell K, Spence L, Simpson P et al: HLA antigens in lichen sclerosus at atrophicus. Arch Dermatol 126: 1043, 1990 |
|
Orss S, Sanchez J, Taboas J: Spirochetal forms in the dermal lesions of morphea and lichen sclerosus et atrophicus. Am J Dermatol Pathol 12: 357, 1990 |
|
Paul J, Wojnarowska F, Winsey S, Marren P, Welsh K: Lichen sclerosus premenarche: Autoimmunity and immunogenetics. Br J Dermatol 142: 481, 2000 |
|
Friedrich E: Vulvar Disease, 2nd edn. Philadelphia, WB Saunders, 1983 |
|
Redmond C, Cowell C, Krafchick B: Genital lichen sclerosus in prepubertal girls. Adolesc Pediatr Gynecol 1: 177, 1988 |
|
Ridley CJ: Vulvar disorders. In Sanfilippo J, Muram D, Lee P, Dewhurst J (eds): Pediatric Adolescent Gynecology, p 217. Philadelphia, WB Saunders, 1994 |
|
Lorenz B, Kaufman RH, Kutzner SK: Lichen sclerosus. Therapy with clobetasol propionate. J Reprod Med 43: 790, 1998 |
|
Fischer G, Rogers M: Treatment of childhood vulvar lichen sclerosus with potent topical corticosteroids. Pediatric Dermatol 14: 235, 1997 |
|
Clark J, Muller S: Lichen sclerosus et atrophicus in children. Arch Dermatol 95: 476, 1967 |
|
Todd P, Halpern S, Kirby J, Pembroke A: Lichen sclerous and the Koebner phenomenon. Clin Exp Dermatol 1994;19:262-3 |
|
Powell J: Paediatric vulval disorders. J Obstet Gynaecol 2006; 26(7): 596-602 |
|
Williams T, Callen J, Owen L: Vulvar disorders in the prepubertal female. Pediatr Ann 15: 588, 1986 |
|
Farber E, Jacobs A: Infantile psoriasis. Am J Dis Child 131: 1266, 1977 |
|
Hurwitz S: Clinical pediatric dermatology, 2nd edn. Philadelphia, WB Saunders, 1993 |
|
Linden K, Weinstein G: Psoriasis: Current perspectives with an emphasis on treatment. Am J Med 107: 595, 1999 |
|
Burden A: Management of psoriasis in childhood. Clin Exp Dermatol 24: 341, 1999 |
|
Katz H: Topical corticosteroids. Dermatol Clin 13: 805, 1995 |
|
Klein P, Clark R: An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol 135: 1522, 1999 |
|
Fleischer A: Treatment of atopic dermatitis: Role of tacrolimus ointment as a topical noncorticosteroidal therapy. J Allergy Clin Immunol 104: S126, 1999 |
|
Gregory G: Action of pediatric anesthesia. In Gregory G (ed): Pediatric Anesthesia, 2nd edn, p 15. New York, Churchill Livingstone, 1989 |
|
Edmonds DK: Sexual developmental anomalies and vary construction: upper and lower tracts. In Sanfilippo J, Muram D, Lee P, Dewhurst J (eds): Pediatric Adolescent Gynecology, p 555. Philadelphia, WB Saunders, 1994 |
|
Rock J, Azziz R: Genital anomalies in childhood. Clin Obstet Gynecol 30: 682, 1987 |
|
Rock J: Surgical correction of uterovaginal anomalies. In Sciarra JJ (ed.): Gynecology and Obstetrics, Vol 1. Chicago, Harper & Row, 1984 |
|
Lee PA, Houk CP, Ahmed SF, Hughes IA; International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006; 118(2): e488-500. Comment in: Pediatrics. 2006 Aug;118(2):814-15 |
|
Reindollar R, Tho S, McDonough P: Abnormalities of sexual differentiation: Evaluation and management. Clin Obstet Gynecol 30: 697, 1987 |
|
Lee M, Donahoe P: The Infant With Ambiguous Genitalia, 6th edn. St. Louis: CV Mosby, 1997 |
|
Lippe B: Ambiguous genitalia and pseudohermaphroditism. Pediatr Clin North Am 26: 91, 1979 |
|
Reindollar R, Tho S, McDonough P: Abnormalities of sexual differentiation: Evaluation and management. Clin Obstet Gynecol 30: 697, 1987 |
|
Kaplowitz P, Oberfield S: Reexamination of the age limit for defining when puberty is precocious in girls in the United States: Implications for evaluation and treatment. Pediatrics 104: 936, 1999 |
|
Doron C, Shohat M, Metzker A, Dickerman Z: Growth, puberty, and endocrine functions in patients with sporadic or familial neurofibromatosis type 1: A longitudinal study. Pediatrics103:1257, 1999 |
|
Cronje H, Niemand I, Bam R, Woodruff J: Granulosa and theca cell tumors in children: a report of 17 cases and literature review. Obstet Gynecol Survey 53: 240, 1998 |
|
Jones K: McCune-Albright syndrome. In Jones KL (ed.): Smith's Recognizable Patterns of Human Malformation, p 510. Philadelphia, WB Saunders, 1997 |
|
Merke D, Cutler G: Evaluation and management of precocious puberty. Arch Dis Child 75: 269, 1996 |
|
Speroff L, Glass RH, Kase NG (eds): Clinical Gynecologic Endocrinology & Infertility, 5th edn. Baltimore, Williams & Wilkins, 1989 |
|
Conn P, Crowley W: Gonadotropin-releasing hormone and its analogs. Annu Rev Med 45: 391, 1994 |