This chapter should be cited as follows:
Kasaven LS, Galazis N, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.419763
The Continuous Textbook of Women’s Medicine Series – Gynecology Module
Volume 10
Ultrasound in gynecology
Volume Editors:
Professor Antonia Testa, Agostino Gemelli University Hospital, Rome, Italy
Professor Simona Maria Fragomeni, Agostino Gemelli University Hospital, Rome, Italy

Chapter
Intraoperative Gynecological Ultrasound
First published: January 2024
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Ultrasound within the field of gynecology has played an imperative role in the diagnosis of various conditions. Real-time ultrasonography provides a useful adjunct to many minor gynecological procedures, including oocyte collection and embryo transfer in assisted reproductive technology (ART), aspiration of ovarian cysts or pelvic abscesses, insertion and removal of intrauterine devices and guiding endometrial biopsies. As such, advancement in technology of this non-invasive and cost-effective resource has enabled wider application of the modality across the speciality, with many gynecologists now skilled in carrying out the above procedures through office-based ultrasound. However, the use of intraoperative ultrasound (IOUS) is not as widely reported in gynecological surgery, despite it being broadly implemented across other surgical specialties.
INTRAOPERATIVE ULTRASOUND
IOUS was pioneered by a general surgeon in 1979, when the diagnosis of biliary calculi was confirmed during cholecystectomy.1 It was particularly effective in the resection of hepatocellular carcinoma associated with cirrhosis, in which lesions < 5 cm identified during pre-assessment were missed intraoperatively in 50% of patients, due to the lesions being too small to be identified or not palpable intraoperatively.2 Given that tumors as small as 3–5 mm can be detected by IOUS, this modality is deemed to be more accurate at diagnosing metastasis than computerized tomography (CT), percutaneous ultrasound and surgical exploration, with sensitivities and specificities of greater than 90% in screening for liver metastasis in colorectal cancer patients.3,4,5 Furthermore, in 5–10% of surgeries performed during colorectal cancer surgery, IOUS identified new occult liver metastatic lesions.4,5,6 The ability for IOUS to determine the margins of cryosurgical ablation has further revolutionized the treatment of tumor deposits deemed previously unresectable.7 Consequently, approximately 30–50% of operations performed for the surgical management of hepatic tumors have been altered as a result of IOUS.8,9,10,11 In the 1980s, IOUS was also introduced during neurosurgery, with evidence suggesting that utilization of the tool depicted more accurate localization and characterization of intracranial masses.12 It has since also been used to resect pancreatic cancers and thyroid tumors, and has been used in breast surgery.13
Despite the vast array of evidence and applications of IOUS in other surgical specialties, the implementation of this technique in gynecology remains in its infancy, particularly with regards to complex gynecological surgery. In a recent systematic review of 45 studies in which IOUS was utilized in gynecological surgery, it was predominantly in the context of hysteroscopic or laparoscopic surgery, using the following ultrasound modalities: transabdominal ultrasound (TAUS), transvaginal ultrasound (TVUS), laparoscopic ultrasound (LUS), transrectal ultrasound (TRUS) or contact ultrasound (CUS) during open surgery.14 We describe herein the application of IOUS used in gynecological procedures thus far.
Laparoscopic myomectomy
The use of IOUS for laparoscopic myomectomy was first described in 2004.15 As it was not feasible at the time of laparoscopy to palpate the myoma for resection, a sterile laparoscopic ultrasound transducer was introduced through the 10–12-mm trocar, with an adjustable head allowing for 90° movement in four directions to maximize tissue contact. The ultrasound transducer allowed for localization of the myoma to determine the incision site for hysterotomy, in the absence of serosal distortion visible to the naked eye, thus minimizing healthy tissue injury.15
Since then, there has been significant evidence to suggest that the use of IOUS is safe and effective in the management of leiomyomas and fibroids.16,17,18,19 In particular, IOUS can facilitate the screening and laparoscopic resection of deep intramural myomas within the myometrium through either TVUS or endoscopic probes, potentially avoiding a laparotomy.20 It also improves surgical technique by accurately assessing the number, location and dimension of the fibroids, negating the requirement for expensive preoperative imaging.21
Regarding open myomectomy, IOUS has also demonstrated superior outcomes, with lower rates of residual myomas observed postoperatively, thus less risk of recurrence requiring further surgery, when compared to without IOUS.22 Furthermore, submucosal myomas with an intramural component of more than 50% are associated with increased risk of intraoperative complications during hysteroscopic resection, necessitating the requirement for open surgery.23 Through the use of IOUS, however, it may be feasible to use laparoscopy to treat fibroids classified as Munro types 2, 3 and 4,24 particularly when conventional laparoscopy may be associated with an endometrial defect following enucleation of the myoma.
Hysteroscopy and transcervical surgery
Operative hysteroscopy is often used for the resection of endometrial polyps, leiomyomata and adhesions, and for septoplasty. The introduction of IOUS has been successfully demonstrated during hysteroscopic metroplasty of a uterine septum, in which complete resection was reported in 100% of cases undergoing surgery, and assessment during follow-up identified a regular endometrial cavity.25,26,27 Absence of ultrasound guidance was associated with an increased rate of uterine perforation.28 In women of reproductive age, IOUS used during the resection of intrauterine septa was also associated with favorable reproductive outcomes. This was demonstrated by a study of 34 patients presenting with primary subfertility secondary to the pathology, in which 14 spontaneous conceptions resulting in 13 livebirths following the procedure were achieved.29 Various studies have also since described outcomes following the use of IOUS during hysteroscopic resection of fibroids and reported complete excision of the tissue in all cases performed.30,31
Ultrasound guidance is also effective during instrumentation of the uterus in cases of Asherman’s syndrome, in which intrauterine adhesions significantly impair visualization of the cavity, thereby reducing the risk of uterine perforation.32 An abdominal ultrasound transducer can be used intraoperatively in the presence of a full bladder, to guide the surgeon during dilation, in cases of a tortuous or stenosed cervix, aiding the surgeon to manipulate hysteroscopic instruments.
IOUS with TAUS, TVUS or TRUS during transcervical surgery has also been observed in the surgical management of cornual ectopic pregnancies.33,34 In one particular case of IOUS used to guide complete resection of a cervical ectopic in a heterotopic pregnancy, the intrauterine pregnancy was preserved, which subsequently resulted in a livebirth at term.35
ART surgical procedures
Within ART, the implementation of ultrasound-guided embryo transfer has significantly increased implantation and clinical pregnancy rates, when compared to the clinical touch blind method performed in the absence of ultrasound.36,37 Moreover, dilation and curettage procedures performed with continuous ultrasound guidance are associated with a significantly lower complication rate (RR, 0.23; CI, 0.08–0.67), including reduced intraoperative blood loss, procedure time, analgesic requirement, postoperative bleeding and infection rate.38
Vaginal surgery
IOUS has been effectively used during vaginal pelvic surgery using TAUS or TVUS in a study of 29 patients to aid posterior culdotomy, whilst improving access to the pouch of Douglas.39 No complications were reported amongst the cohort, contrary to the 162 patients who had the procedure in the absence of ultrasound guidance and experienced six complications, including three rectal injuries, one ureteric and two retroperitoneal fluid collections.39
Fertility-sparing surgery
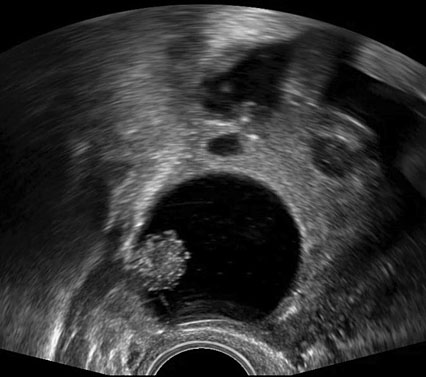
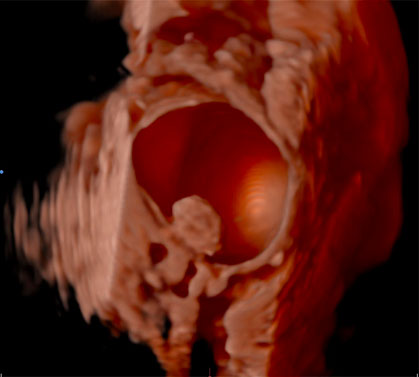
The use of IOUS has been described in the context of fertility-sparing surgery, the aim of which is to resect gynecological pathology, whilst preserving reproductive potential and ability to conceive. In the management of borderline ovarian tumors (BOTs) for example, conventional methods to treat primary or recurrent disease have included performing an oophorectomy to ensure complete resection of pathology. Through surveillance monitoring with ultrasound, it is possible to identify recurrences presenting as small intraovarian deposits, too small to be visualized at laparoscopy (Figures 1).40 Although it is feasible to manage such patients conservatively and operate only once the lesion is large enough to be seen at laparoscopy,41 it is possible that delaying intervention may later cause upstaging of disease, subjecting women to unnecessary invasive surgery. Recurrent serous BOTs (sBOTs) also increase in size at a rate of approximately 0.06 mm per month, and therefore, the time it takes for the lesion to be deemed adequate enough to perform laparoscopic resection, may delay women’s future plans for pregnancy.41 IOUS using TVUS and TRUS modalities have been successfully used to treat a series of seven women presenting with a first recurrence of sBOT, who wished to preserve their fertility.40 The women in the series all underwent ultrasound-guided ovarian wedge resection of lesions between 12 mm and 37 mm, which were too deeply embedded within ovarian tissue to be seen laparoscopically.40 Six of the seven women operated on did not develop recurrence of disease during a follow-up period of 1–20 months, thus demonstrating that safe and complete resection of non-benign intraovarian lesions is feasible.40 Ultrasound-guided ovarian wedge resection has since been used in the management of primary BOT, with again no reported recurrence of disease identified at follow-up, particularly in cases in which the procedure was not performed in combination with other methods of fertility-sparing surgery.42 Recurrent BOT has also been successfully resected using LUS.43 The advantages of using LUS over TVUS or TRUS in the case reported were described as closer proximity of the probe to the lesion, thereby reducing abdominal or vaginal wall inference and risk of infection, whilst also not having to change the laparoscopic position of the patient.43
(a) |
(b) |
1
Two-dimensional (a) and three-dimensional (b) preoperative transvaginal ultrasound images of recurrent serous borderline ovarian tumor.
Intraoperative LUS has also been used to perform fertility-sparing surgery for the treatment of Herlyn-Werner-Wunderlich syndrome, in which direct contact of the LUS probe with the uterus provided enhanced visualization of the origin of a longitudinal vaginal septum, which the surgeon was then able to excise through a transvaginal approach. Thus, the use of LUS in this case was imperative in both the diagnosis and treatment of a complex uterine malformation, with excellent surgical outcomes reported.44
Open gynecological surgery
Further use of IOUS in fertility-sparing surgery includes a case report on the application of CUS during open surgery to resect a placental site trophoblastic tumor in a patient wishing to preserve her fertility. The use of IOUS enabled accurate hysterotomy, minimizing the excision of healthy tissue whilst preserving the woman’s fertility, thus avoiding the need for hysterectomy which would render the woman infertile. The patient subsequently went on to conceive naturally and delivered at term.45
IOUS in open surgery has also been performed in a woman at 14 weeks' gestation who presented with abdominal pain secondary to the diagnosis of a fundal intramural subserous myoma measuring 157 × 90 mm, as well as other smaller myomas, causing compression of the gestational sac. A convex transducer placed directly onto the uterine serosa intraoperatively, guided the surgeon with respect to location of the myoma, its relationship with the endometrial cavity and the optimal site for incision, whilst also providing information on fetal viability.46
Radical debulking surgery
IOUS can be used in complex gynecological procedures for diagnostic purposes, particularly when suspicious lesions are identified on palpation during open surgery. This was observed in a woman who underwent bilateral adnexectomy, hysterectomy, omentectomy and peritoneal biopsies for bilateral sBOT.47 During the procedure, the lumboaortic region appeared enlarged on palpation and was thought to be a lymph node. IOUS assessment of the lesion identified a hypoechoic lymph node in the precaval region measuring 9.6 mm, with an irregular echo structure, no color Doppler flow and presence of calcification.47 The lymph node was subsequently isolated and excised surgically based on the ultrasound findings, and identified as adenocarcinoma on frozen section analysis. As such, the surgical procedure was altered by including para-aortic and bilateral iliac lymphadenectomies.47 Histology confirmed the lymph node was a metastasis of low-grade serous carcinoma, which could have been missed in the absence of the information provided from the IOUS.47 A similar case has been reported in which two enlarged cardiophrenic lymph nodes were effectively resected during primary debulking surgery for advanced ovarian cancer, that was only identified by IOUS following initial abdominal cytoreduction.48
Improved visualization of pelvic anatomy
IOUS can also be used during laparoscopic surgery to identify pelvic anatomy and avoid tissue injury to adjacent structures. This has been demonstrated in two cases in which cystic adenomyosis was safely resected using TVUS with LUS guidance, consequently resolving symptoms of severe dysmenorrhea in both cases.49,50 Furthermore, TAUS during laparoscopy has helped retrieve a lost intrauterine device (IUD) identified in the abdominal cavity during preoperative imaging, but not seen laparoscopically.51 Use of the IOUS confirmed the presence of a tubular hyperechoic structure in the abdominal cavity, precisely locating the IUD within the bowel loops, thus avoiding the conversion to laparotomy to retrieve the device.51
IOUS has been reported to provide excellent diagnostic accuracy when characterizing adnexal masses. In one study of 58 women, the accuracy of characterizing ovarian cysts was significantly higher following laparoscopic ultrasonography than preoperative TVUS (83.8% vs 73.5%, respectively, P < 0.05), when compared with histological diagnosis.52 Accuracy in diagnosis was particularly high in differentiating endometriomas or dermoid cysts from other benign pathology.52
EVIDENCE-BASED PRACTICE
Indications for use of IOUS
The primary indications for the use of IOUS include: (1) to acquire new information not otherwise available; (2) to complement or replace intraoperative radiography; (3) to confirm completion of an operation; and (4) to guide surgical procedures.
IOUS provides real-time imaging and intraoperative exploration, not previously available during preoperative assessment. This aims to accurately reassess the location of previously diagnosed lesions, exclude previously suspected pathology, assess for new lesions, establish the anatomic structures surrounding the pathology such as blood vessels, before performing tissue dissection/resection to ensure optimization of the surgical approach, and minimize intraoperative complications. Given the improved diagnostic accuracy of IOUS, it also has the potential to negate the requirement for the use of contrast during preoperative imaging assessment. It provides a useful tool for assessing complete excision of lesions, preventing the requirement for further surgery. IOUS also guides procedures requiring surgical tissue dissection or intervention, such as needle or cannula insertion during the aspiration of pelvic cysts or abscesses, or during probe placement.
For the purpose of this chapter, we describe the application of IOUS performed either transabdominally during hysteroscopic or transcervical (without hysteroscopic guidance) surgery, or transvaginally or transrectally during laparoscopic surgery.
Indications for the use of IOUS during operative hysteroscopy include the resection of intrauterine pathology, such as complete or partial uterine septum, submucous or intramural fibroids, uterine synechiae (Asherman’s syndrome), atypical placental site nodules, osseous metaplasia of the endometrium and surgical management of miscarriage (Table 1). Indications for transcervical surgery without hysteroscopy, include dilating a stenosed cervical canal after conization or trachelectomy or the treatment of cervical or cornual ectopic pregnancy. IOUS in this context aims to reduce the risks of uterine perforation or incomplete resection of the lesion.
1
Indications for IOUS.
Gynecological procedure | Indication |
Operative hysteroscopy |
|
Transcervical surgery |
|
Operative laparoscopy |
|
Vaginal pelvic surgery |
|
Open surgery |
|
The indications for IOUS during operative laparoscopic surgery include resection of pelvic lesions not visualized laparoscopically, such as small BOTs and ovarian cysts, or when pelvic adhesions obscure visualization of the ovaries (Table 1). During extensive adhesiolysis of the pelvic side walls, ultrasound can improve access to the lesion and minimize risk of injury to the ureter or pelvic vasculature. When treatment of ovarian pathology is required, IOUS should also be used to delineate diseased pathology from healthy ovarian tissue, particularly in women of reproductive age, in order to optimize preservation of fertility where possible. The advantages and disadvantages of IOUS are listed in (Table 2).
2
Advantages and disadvantages of IOUS.
Advantages |
|
Disadvantages |
|
Contraindications
A well-known complication associated with the utilization of distention media during gynecological procedures, for example, during hysteroscopic surgery, is fluid overload, with or without electrolyte imbalance,53 the incidence of which is reportedly less than 5% secondary to operative hysteroscopy.54 Despite the risks of systemic absorption of isotonic saline distension fluid, through retrograde passage of fluid through the Fallopian tubes, endometrium or via vasculature and sinuses open during resection of uterine tissue, especially when the intrauterine pressure is greater than the pressure in the venous sinus or blood vessels,53 there have been no reported incidences of fluid overload following the use of intraoperative ultrasound during laparoscopic surgery. This is unsurprising given that a fluid deficit of 2500 ml is the threshold required to define fluid overload when using isotonic solutions, and more than 1000 ml when using hypotonic solutions in women of reproductive age.55 Given that only 500 ml of isotonic saline is required as a distention medium during IOUS laparoscopic surgery, it is therefore unlikely to result in this complication. In the context of hysteroscopic surgery, the use of intraoperative ultrasound does not impact the volume of fluid media required during operative surgery, therefore, the < 5% risk of fluid overload associated with operative hysteroscopic procedures remains the same.
Transabdominal ultrasound for hysteroscopic or transcervical (without hysteroscopic guidance) surgery
For TAS for hysteroscopic or transcervical (without hysteroscopic guidance) surgery, a curvilinear or phased-array probe may be used. Both sagittal (longitudinal) and transverse views of the uterus should be obtained. TAUS is usually operated at lower frequencies of 3.5 MHz, allowing for deeper signal penetration, although with poorer resolution when compared to probes used for TVUS.56 The patient is positioned in the dorsal lithotomy position. The preferred positioning of the sonographer is to stand at the right side of the patient, with the surgeon sitting between the legs of the patient. This allows for the sonographer to obtain adequate visualization of the cervix and uterus, whilst not confined to a space shared with the primary surgeon. TAUS allows for improved flexibility to image a larger field of view when compared to TVUS. The urinary bladder should be filled with 300 ml of saline solution to displace the uterus superiorly, for improved transabdominal views. During transcervical surgery, in the absence of hysteroscopy, the uterus is instilled with 30 ml of saline through the external os, to distend the uterine cavity and delineate anatomy from the pathology to be resected. The urinary bladder should be emptied at the end of the procedure.
Laparoscopic, transvaginal or transrectal ultrasound during laparoscopic surgery
IOUS for laparoscopy incorporates B-mode ultrasound with high-frequency transducers. The higher frequency allows for greater resolution, despite penetrating less deeply. Most general surgeons use transducer frequencies between 7 and 8 MHz. This allows for the smallest of lesions to be detected, such as a 1-mm calculi, 2-mm cyst or a 3–5-mm tumor.2 The use of color Doppler also allows for faster recognition of blood vessels from other pelvic structures.
The type of transducers used in IOUS have typically included linear, curvilinear (convex) and phased (sector) array. Linear 7.5-MHz probes, in particular, are the preferred choice of transducer used in general surgery. In the context of IOUS within gynecology, it is important that the probe can be manipulated in small and narrow operating fields, thus high-resolution transvaginal probes with three-dimensional (3D) imaging are frequently used, allowing detailed examination of pelvic anatomy. Laparoscopic probes are configured with linear or convex linear array transducers placed on the tip of a long shaft.57 The shaft is < 10 mm in diameter and 35–45 cm in length to allow a wide examination field from a single port. Such transducers have a frequency range of 5–10 MHz.
The two techniques for scanning intraoperatively include contact and standoff. Contact scanning requires the probe to have direct contact with the organ/tissue surface to create a transcutaneous ultrasound effect. It allows for the examination of target lesions located deep within tissue (away from the probe). Standoff scanning, however, requires that the probe is positioned 1–2 cm away from the surface of the structure and immersed in saline.2 It is particularly useful for the examination of the surface area of pelvic organs, or to examine superficial structures. As most gynecological procedures require examination of surface areas, the latter technique is predominantly used, with the ultrasound transducer inserted transvaginally or transrectally (when instrumentation of the uterus is required), to allow the surface of the pathology to remain immersed in saline, whilst maintaining an adequate distance from the probe.
IOUS in management of surgical resection of ovarian or pelvic pathology
For management of surgical resection of ovarian or pelvic pathology, the patient is placed in the Trendelenburg or lithotomy position. Laparoscopic entry and peritoneal insufflation of carbon dioxide (CO2) is performed to achieve a pneumoperitoneum. CO2 has a hyperechoic effect on ultrasound which can cause suboptimal ultrasound views. Following laparoscopic entry and assessment of the operating field, 500 ml of normal saline (0.9%) is therefore instilled into the pelvis for transmission of ultrasound and enhancement of the ultrasound image quality, by acting as a conducting agent. This remains within the pelvis during the course of the operation. An assistant with competence in pelvic ultrasound scanning inserts a probe with a sterile cover either transrectally or transvaginally, to provide real-time ultrasound images of the ovary, uterus, pelvic organs and pathology to be resected. As the probe is sterile, it can be used repeatedly throughout the operation. It is essential that IOUS is performed early in the operation to ensure newly diagnosed lesions can be identified, so that imaging can guide the surgeon, such that no residual pathology remains postoperatively. For this reason, the target organs are systematically scanned in the longitudinal and transverse views, with sliding, rotation and angulating of the probe encouraged. In the context of ovarian pathology, a non-traumatic instrument is then used to stabilize the ovary +/− pathology (e.g. ovarian cyst, BOT lesion within the ovary), whilst correlating between the laparoscopic and ultrasound images (Figure 2).
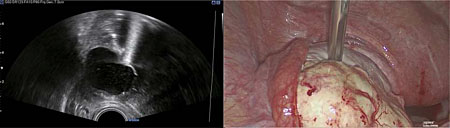
2
Correlation between ultrasound and laparoscopic findings, using a non-traumatic instrument to facilitate identification of the recurrent serous borderline ovarian lesion during laparoscopy.
Diathermy can be used to demarcate the surface of the lesion on the ovary to be removed (Figure 3). The resection of ovarian pathology is then performed under continuous ultrasound guidance, above the level of the saline solution, ensuring the surgeon can differentiate between healthy ovarian tissue and ovarian pathology (Figure 4). It is imperative that communication between the sonographer and surgeon remains throughout the procedure, to co-ordinate the laparoscopic and ultrasound views. IOUS is then used to ensure that pathology has been excised completely (Figures 5 and 6) and the saline fluid is aspirated prior to closure of the abdomen following removal of the pathology through laparoscopic specimen retrieval bags, followed by routine laparoscopic closure of the abdomen (Videos 1 and 2).
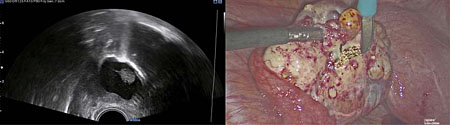
3
Diathermy used to demarcate the area of incision of the ovary to guide ovarian wedge resection performed laparoscopically.
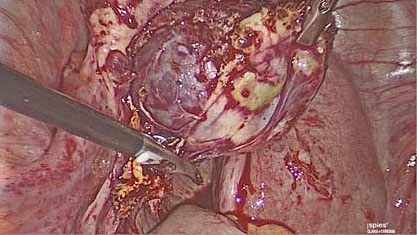
4
Ovarian wedge resection performed laparoscopically.
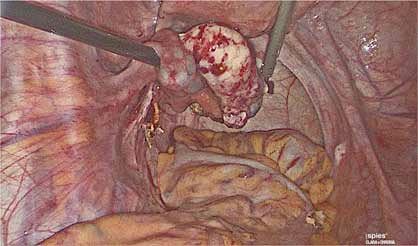
5
Healthy ovarian tissue after ovarian wedge resection of recurrent serous borderline ovarian tumor.
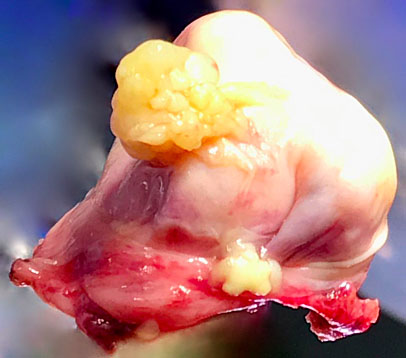
6
Macroscopic appearance of serous borderline ovarian lesion resected laparoscopically.
1
Ultrasound-guided ovarian wedge resection of a recurrent serous borderline ovarian tumor.
2
Ultrasound-guided ovarian wedge resection of a recurrent serous borderline ovarian tumor.
Postoperative care
The postoperative management of patients undergoing IOUS does not differ from that of patients undergoing conventional operative hysteroscopy or laparoscopy. The patient can eat and drink after the operation and adequate analgesia should be administered. The patient is encouraged to mobilize and pass urine and may be able to go home the same day, depending on the complexity of the procedure performed.
FUTURE WORK
Future work will consist of developing ultrasound technology and instrumentation, and the incorporation of this intraoperatively. This will focus mainly on developing user-friendly scanners and probes for surgeons, improved resolution and deeper sound penetration, harmonic imaging with contrast agents in the context of color Doppler imaging and refinement of 3D images to improve diagnostic accuracy.
Given that IOUS is deemed a low-cost modality, which is readily accessible, in addition to the fact that most training programs require trainees to achieve competence in minimal access surgery and TVUS through objective structured assessment of technical skills,14 the use of IOUS is considered a proficiency that can be reproducible and implemented nationally, rather than a skill limited to specialist tertiary units. Training and education on IOUS, therefore, should be encouraged and may be enhanced through the use of computer-based ultrasound simulation.
Further high-quality prospective studies assessing the efficacy of IOUS in gynecological surgery are also required, as opposed to reported experience and case reports only. As such, the authors of this chapter have designed a prospective two-armed randomized controlled trial comparing laparoscopic ovarian cystectomy for the management of benign ovarian cysts, with ultrasound-guided laparoscopic ovarian cystectomy (Trial Registration: NCT05032846).58 The aim of the trial is to determine the efficacy of IOUS as an adjunct of fertility-sparing surgery. The primary outcome will be to determine the difference in anti-Müllerian hormone (pmol/L) and antral follicle count measured at 3 and 6 months postoperatively, compared to the preoperative baseline. Secondary outcomes will include assessment of various surgical and histopathological findings, such as duration of hospital stay (days), duration of surgery (minutes), presence of intraoperative cyst rupture (yes/no), presence of ovarian tissue within the resected specimen (yes/no) and the grade of follicles excised (0–4). The findings of this study will determine whether IOUS is able to optimize healthy ovarian tissue preservation during minimally invasive surgery for benign ovarian pathology. Conclusions will be disseminated to clinicians and used to provide informative counseling to patients to ensure they can make well-informed decisions regarding their future fertility, before deciding to undergo laparoscopic surgery for benign gynecological pathology.
SUMMARY
Evidence thus far suggests that the use of IOUS within gynecological surgery, including operative hysteroscopy and laparoscopy for benign and premalignant conditions, is associated with favorable surgical and reproductive outcomes. However, further high-quality studies are required, particularly to support the effectiveness of IOUS as an adjunct of fertility-sparing surgery in women of reproductive age. IOUS is considered a low-cost and non-invasive tool that can be widely implemented by gynecologists who are skilled in ultrasound scanning.
PRACTICE RECOMMENDATIONS
- Evidence thus far suggests that IOUS in gynecological surgery for benign and premalignant pathology is safe and effective.
- Published studies include case reports and anecdotal experience describing the use of IOUS during laparoscopic and open myomectomy, reproductive surgical procedures, vaginal surgery and fertility-sparing surgery. There are few high-quality studies to support the use of IOUS in gynecological surgery.
- The four main indications for the use of IOUS include: (1) to acquire new information not otherwise available; (2) to complement or replace intraoperative radiography; (3) to confirm completion of an operation; and (4) to guide surgical procedures.
- IOUS is predominantly used during operative hysteroscopy or transcervical surgery using TAUS and during operative laparoscopy using LUS, TVUS or TRUS.
- There are no reported contraindications for the use of IOUS.
- A curvilinear or phased-array probe is used during TAUS for operative hysteroscopic surgery or transcervical (without hysteroscopic guidance) surgery with a frequency of 3.5 MHz.
- Linear LUS, TVUS or TRUS probes with a high frequency (7.5 MHz) are commonly used during laparoscopic surgery.
- Standoff scanning is the preferred method during gynecological laparoscopy for assessment of surface area lesions. This requires instillation of normal saline (0.9%) into the pelvis for transmission of ultrasound and enhancement of image quality, by acting as a conducting agent.
- IOUS minimizes intraoperative complications such as uterine perforation, injury to vascular structures and surgical tissue dissection, operating time and requirement for postoperative analgesia, and possibly preserves fertility by minimizing dissection and resection of healthy ovarian tissue.
- Future work will consist of developing ultrasound technology and instrumentation, and the incorporation of this intraoperatively.
- Further education and training in IOUS is required to widen applicability of this approach.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Sigel B, Spigos DG, Donahue PE, Pearl R, Popky GL, Nyhus LM. Intraoperative ultrasonic visualization of biliary calculi. Curr Surg 1979;36:158–9. | |
Machi J, Oishi AJ, Furumoto NL, Oishi RH. Intraoperative ultrasound. Surg Clin North Am. 2004 Aug;84(4):1085-111, vi-i. doi: 10.1016/j.suc.2004.04.001. PMID: 15261754. | |
Machi J, Isomoto H, Yamashita Y, Kurohiji T, Shirouzu K, Kakegawa T. Intraoperative ultrasonography in screening for liver metastases from colorectal cancer: comparative accuracy with traditional procedures. Surgery. 1987 Jun;101(6):678-84. PMID: 3296262. | |
Russo A, Sparacino G, Plaja S, Cajozzo M, La Rosa C, Demma I, Bazan P. Role of intraoperative ultrasound in the screening of liver metastases from colorectal carcinoma: initial experiences. J Surg Oncol. 1989 Dec;42(4):249-55. doi: 10.1002/jso.2930420410. PMID: 2687585. | |
Stone MD, Kane R, Bothe A Jr, Jessup JM, Cady B, Steele GD Jr. Intraoperative ultrasound imaging of the liver at the time of colorectal cancer resection. Arch Surg. 1994 Apr;129(4):431-5; discussion 435-6. doi: 10.1001/archsurg.1994.01420280109014. PMID: 8154969. | |
Olsen AK. Intraoperative ultrasonography and the detection of liver metastases in patients with colorectal cancer. Br J Surg. 1990 Sep;77(9):998-9. doi: 10.1002/bjs.1800770913. PMID: 2207593. | |
Ravikumar TS, Kane R, Cady B, Jenkins R, Clouse M, Steele G Jr. A 5-year study of cryosurgery in the treatment of liver tumors. Arch Surg. 1991 Dec;126(12):1520-3; discussion 1523-4. doi: 10.1001/archsurg.1991.01410360094015. PMID: 1842183. | |
Machi J. Intraoperative and laparoscopic ultrasound. Surg Oncol Clin N Am. 1999 Jan;8(1):205-26. PMID: 9824369. | |
Castaing D, Emond J, Kunstlinger F, Bismuth H. Utility of operative ultrasound in the surgical management of liver tumors. Ann Surg. 1986 Nov;204(5):600-5. doi: 10.1097/00000658-198611000-00015. PMID: 3021072; PMCID: PMC1251346. | |
Gozzetti G, Mazziotti A, Bolondi L, Cavallari A, Grigioni W, Casanova P, Bellusci R, Villanacci V, Labò G. Intraoperative ultrasonography in surgery for liver tumors. Surgery. 1986 May;99(5):523-30. PMID: 3010481. | |
Bismuth H, Castaing D, Garden OJ. The use of operative ultrasound in surgery of primary liver tumors. World J Surg. 1987 Oct;11(5):610-4. doi: 10.1007/BF01655836. PMID: 2823488. | |
Rubin JM, Dohrmann GJ. Intraoperative neurosurgical ultrasound in the localization and characterization of intracranial masses. Radiology. 1983 Aug;148(2):519-24. doi: 10.1148/radiology.148.2.6867352. PMID: 6867352. | |
Sigel B, Coelho JC, Nyhus LM, Velasco JM, Donahue PE, Wood DK, Spigos DG. Detection of pancreatic tumors by ultrasound during surgery. Arch Surg. 1982 Aug;117(8):1058-61. doi: 10.1001/archsurg.1982.01380320042011. PMID: 7103724. | |
Galazis N, Saso S, Sorbi F, Jones B, Landolfo C, Al-Memar M, Ben-Nagi J, Smith JR, Yazbek J. Intraoperative Ultrasound during Fertility-Sparing Surgery: A Systematic Review and Practical Applications. Gynecol Obstet Invest. 2020;85(2):127-148. doi: 10.1159/000505689. Epub 2020 Jan 22. PMID: 31968340. | |
Lin PC, Thyer A, Soules MR. Intraoperative ultrasound during a laparoscopic myomectomy. Fertil Steril. 2004 Jun;81(6):1671-4. doi: 10.1016/j.fertnstert.2003.10.049. PMID: 15193493. | |
Miller CE. Myomectomy. Comparison of open and laparoscopic techniques. Obstet Gynecol Clin North Am. 2000 Jun;27(2):407-20. doi: 10.1016/s0889-8545(00)80031-5. PMID: 10857130. | |
Mais V, Ajossa S, Guerriero S, Mascia M, Solla E, Melis GB. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996 Feb;174(2):654-8. doi: 10.1016/s0002-9378(96)70445-3. PMID: 8623802. | |
Seracchioli R, Rossi S, Govoni F, Rossi E, Venturoli S, Bulletti C, Flamigni C. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000 Dec;15(12):2663-8. doi: 10.1093/humrep/15.12.2663. PMID: 11098042. | |
Rossetti A, Sizzi O, Soranna L, Cucinelli F, Mancuso S, Lanzone A. Long-term results of laparoscopic myomectomy: recurrence rate in comparison with abdominal myomectomy. Hum Reprod. 2001 Apr;16(4):770-4. doi: 10.1093/humrep/16.4.770. PMID: 11278231. | |
Urman B, Boza A, Ata B, Aksu S, Arslan T, Taskiran C. Intraoperative endoscopic ultrasound guidance for laparoscopic excision of invisible symptomatic deep intramural myomas. J Obstet Gynaecol. 2018 Jan;38(1):85-89. doi: 10.1080/01443615.2017.1327515. Epub 2017 Aug 1. PMID: 28764594. | |
Moro F, Bitonti G, Mascilini F, Testa AC, Scambia G. Intraoperative transvaginal ultrasound examination during myomectomy. J Ultrasound. 2019 Mar;22(1):109-110. doi: 10.1007/s40477-018-0310-9. Epub 2018 Jul 17. PMID: 30019287; PMCID: PMC6430292. | |
Shimanuki H, Takeuchi H, Kikuchi I, Kumakiri J, Kinoshita K. Effectiveness of intraoperative ultrasound in reducing recurrent fibroids during laparoscopic myomectomy. J Reprod Med. 2006 Sep;51(9):683-8. PMID: 17039695. | |
Chang CY, Chang YT, Chien SC, Yu SS, Hung YC, Lin WC. Factors associated with operative hysteroscopy outcome in patients with uterine adhesions or submucosal myomas. Int J Gynaecol Obstet. 2010 May;109(2):125-7. doi: 10.1016/j.ijgo.2009.11.018. Epub 2010 Jan 22. PMID: 20096833. | |
Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011 Apr;113(1):3-13. doi: 10.1016/j.ijgo.2010.11.011. Epub 2011 Feb 22. PMID: 21345435. | |
Coccia ME, Becattini C, Bracco GL, Scarselli G. Ultrasound-guided hysteroscopic management of endometrial osseous metaplasia. Ultrasound Obstet Gynecol. 1996 Aug;8(2):134-6. doi: 10.1046/j.1469-0705.1996.08020134.x. PMID: 8883319. | |
Mullesserill BT, Dumesic DA, Damario MA, Session DR. Ultrasound-guided unification of noncommunicating uterine cavities. JSLS. 2003 Apr-Jun;7(2):155-7. PMID: 12856848; PMCID: PMC3015487. | |
Wang JH, Xu KH, Lin J, Chen XZ. Hysteroscopic septum resection of complete septate uterus with cervical duplication, sparing the double cervix in patients with recurrent spontaneous abortions or infertility. Fertil Steril. 2009 Jun;91(6):2643-9. doi: 10.1016/j.fertnstert.2008.04.009. Epub 2008 Jun 18. PMID: 18565515. | |
Vigoureux S, Fernandez H, Capmas P, Levaillant JM, Legendre G. Assessment of Abdominal Ultrasound Guidance in Hysteroscopic Metroplasty. J Minim Invasive Gynecol. 2016 Jan;23(1):78-83. doi: 10.1016/j.jmig.2015.08.882. Epub 2015 Aug 28. PMID: 26319796. | |
Ohl J, Bettahar-Lebugle K. Ultrasound-guided transcervical resection of uterine septa: 7 years' experience. Ultrasound Obstet Gynecol. 1996 May;7(5):328-34. doi: 10.1046/j.1469-0705.1996.07050328.x. PMID: 8774097. | |
Korkmazer E, Tekin B, Solak N. Ultrasound guidance during hysteroscopic myomectomy in G1 and G2 Submucous Myomas: for a safer one step surgery. Eur J Obstet Gynecol Reprod Biol. 2016 Aug;203:108-11. doi: 10.1016/j.ejogrb.2016.03.043. Epub 2016 May 17. PMID: 27267872. | |
Ludwin A, Ludwin I, Pityński K, Basta P, Basta A, Banas T, Jach R, Wiecheć M, Grabowska R, Stangel-Wójcikiewicz K, Milewicz T, Nocuń A. Transrectal ultrasound-guided hysteroscopic myomectomy of submucosal myomas with a varying degree of myometrial penetration. J Minim Invasive Gynecol. 2013 Sep-Oct;20(5):672-85. doi: 10.1016/j.jmig.2013.05.001. Epub 2013 Jul 11. PMID: 23850363. | |
Letterie GS, Catherino WH. A 7.5-MHz finger-grip ultrasound probe for real-time intraoperative guidance during complex reproductive surgical procedures. Am J Obstet Gynecol. 2002 Dec;187(6):1588-90. doi: 10.1067/mob.2002.128400. PMID: 12501068. | |
Morgan M, Aziz M, Mikhail M, Henein M, Atalla R. Ultrasound guided treatment of cornual ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009 Apr;143(2):126. doi: 10.1016/j.ejogrb.2008.11.004. Epub 2009 Jan 20. PMID: 19157677. | |
Thakur Y, Coker A, Morris J, Oliver R. Laparoscopic and ultrasound-guided transcervical evacuation of cornual ectopic pregnancy: an alternative approach. J Obstet Gynaecol. 2004 Oct;24(7):809-10. doi: 10.1080/01443610400009576. PMID: 15763795. | |
Faschingbauer F, Mueller A, Voigt F, Beckmann MW, Goecke TW. Treatment of heterotopic cervical pregnancies. Fertil Steril. 2011 Apr;95(5):1787.e9-13. doi: 10.1016/j.fertnstert.2010.10.043. Epub 2010 Dec 3. PMID: 21122844. | |
Buckett WM. A meta-analysis of ultrasound-guided versus clinical touch embryo transfer. Fertil Steril. 2003 Oct;80(4):1037-41. doi: 10.1016/s0015-0282(03)01015-x. PMID: 14556830. | |
Sallam HN, Sadek SS. Ultrasound-guided embryo transfer: a meta-analysis of randomized controlled trials. Fertil Steril. 2003 Oct;80(4):1042-6. doi: 10.1016/s0015-0282(03)01009-4. PMID: 14556831. | |
Acharya G, Morgan H, Paramanantham L, Fernando R. A randomized controlled trial comparing surgical termination of pregnancy with and without continuous ultrasound guidance. Eur J Obstet Gynecol Reprod Biol. 2004 May 10;114(1):69-74. doi: 10.1016/j.ejogrb.2003.09.042. PMID: 15099874. | |
Ma C, Wang Y, Li TC, Qiao J, Yang Y, Song X, Yang S. Trans-abdominal ultrasound guided transvaginal hydrolaparoscopy is associated with reduced complication rate. Eur J Obstet Gynecol Reprod Biol. 2012 Feb;160(2):166-9. doi: 10.1016/j.ejogrb.2011.11.008. PMID: 22289262. | |
Jones BP, Saso S, Farren J, El-Bahrawy M, Ghaem-Maghami S, Smith JR, Yazbek J. Ultrasound-Guided Laparoscopic Ovarian Wedge Resection in Recurrent Serous Borderline Ovarian Tumours. Int J Gynecol Cancer. 2017 Nov;27(9):1813-1818. doi: 10.1097/IGC.0000000000001096. PMID: 28763365. | |
Franchi D, Boveri S, Radice D, Portuesi R, Zanagnolo V, Colombo N, Testa AC. Ultrasonographic diagnosis and longitudinal follow-up of recurrences after conservative surgery for borderline ovarian tumors. Am J Obstet Gynecol. 2016 Dec;215(6):756.e1-756.e9. doi: 10.1016/j.ajog.2016.07.024. Epub 2016 Jul 18. PMID: 27443811. | |
Kasaven LS, Chawla M, Jones BP, Al-Memar M, Galazis N, Ahmed-Salim Y, El-Bahrawy M, Lavery S, Saso S, Yazbek J. Fertility Sparing Surgery and Borderline Ovarian Tumours. Cancers (Basel). 2022 Mar 14;14(6):1485. doi: 10.3390/cancers14061485. PMID: 35326636; PMCID: PMC8946233. | |
Mascilini F, Quagliozzi L, Bolomini G, Scambia G, Testa AC, Fagotti A. Intraoperative ultrasound through laparoscopic probe in fertility-sparing surgery for borderline ovarian tumor recurrence. Ultrasound Obstet Gynecol. 2019 Aug;54(2):280-282. doi: 10.1002/uog.20138. PMID: 30288807. | |
Moruzzi MC, Bolomini G, Albanese M, Catena U, Romito I, Fagotti A, Scambia G, Testa AC. Intraoperative Endoscopic Ultrasound Guided Surgical Treatment of Herlyn-Werner-Wunderlich Syndrome. Case Report and a Systematic Literature Review. Obstet Gynecol Res 2020;3(1):37–80. | |
Saso S, Chatterjee J, Yazbek J, Thum Y, Keefe KW, Abdallah Y, Naji O, Lindsay I, Savage PM, Seckl MJ, Smith JR. A case of pregnancy following a modified Strassman procedure applied to treat a placental site trophoblastic tumour. BJOG. 2012 Dec;119(13):1665-7. doi: 10.1111/j.1471-0528.2012.03501.x. Epub 2012 Oct 19. PMID: 23078379. | |
Moruzzi MC, Moro F, Bolomini G, Macchi C, Cavaliere AF, Fagotti A, Scambia G, Testa AC. Intraoperative ultrasound assistance during myomectomy in pregnant woman. Ultrasound Obstet Gynecol. 2020 Jun;55(6):840-841. doi: 10.1002/uog.21881. PMID: 31587408. | |
De Blasis I, Tortorella L, Macchi C, Arciuolo D, Scambia G, Testa AC. Intraoperative ultrasound diagnosis of metastatic lymph node in serous borderline ovarian tumor. Ultrasound Obstet Gynecol. 2019 Oct;54(4):562-563. doi: 10.1002/uog.20234. PMID: 30740802. | |
Moro F, Uccella S, Testa AC, Scambia G, Fagotti A. Intraoperative Ultrasound-Guided Excision of Cardiophrenic Lymph Nodes in an Advanced Ovarian Cancer Patient. Int J Gynecol Cancer. 2018 Nov;28(9):1672-1675. doi: 10.1097/IGC.0000000000001363. PMID: 30371564. | |
Nabeshima H, Murakami T, Nishimoto M, Sugawara N, Sato N. Successful total laparoscopic cystic adenomyomectomy after unsuccessful open surgery using transtrocar ultrasonographic guiding. J Minim Invasive Gynecol. 2008 Mar-Apr;15(2):227-30. doi: 10.1016/j.jmig.2007.10.007. PMID: 18312998. | |
Nabeshima H, Murakami T, Terada Y, Noda T, Yaegashi N, Okamura K. Total laparoscopic surgery of cystic adenomyoma under hydroultrasonographic monitoring. J Am Assoc Gynecol Laparosc. 2003 May;10(2):195-9. doi: 10.1016/s1074-3804(05)60298-8. PMID: 12732771. | |
Mascilini F, Moro F, De Leo R, Scambia G, Fagotti A, Testa AC. Intraoperative ultrasound assistance for surgical removal of lost intrauterine device. Ultrasound Obstet Gynecol. 2019 May;53(5):705-706. doi: 10.1002/uog.19167. PMID: 29947114. | |
Yang WT, Yuen PM, Ho SS, Leung TN, Metreweli C. Intraoperative laparoscopic sonography for improved preoperative sonographic pathologic characterization of adnexal masses. J Ultrasound Med. 1998 Jan;17(1):53-61. doi: 10.7863/jum.1998.17.1.53. PMID: 9440109. | |
Umranikar S, Clark TJ, Saridogan E, Miligkos D, Arambage K, Torbe E, Campo R, Di Spiezio Sardo A, Tanos V, Grimbizis G; British Society for Gynaecological Endoscopy /European Society for Gynaecological Endoscopy Guideline Development Group for Management of Fluid Distension Media in Operative Hysteroscopy. BSGE/ESGE guideline on management of fluid distension media in operative hysteroscopy. Gynecol Surg. 2016;13(4):289-303. doi: 10.1007/s10397-016-0983-z. Epub 2016 Oct 6. PMID: 28003797; PMCID: PMC5133285. | |
Aydeniz B, Gruber IV, Schauf B, Kurek R, Meyer A, Wallwiener D. A multicenter survey of complications associated with 21,676 operative hysteroscopies. Eur J Obstet Gynecol Reprod Biol. 2002 Sep 10;104(2):160-4. doi: 10.1016/s0301-2115(02)00106-9. PMID: 12206931. | |
AAGL Advancing Minimally Invasive Gynecology Worldwide; Munro MG, Storz K, Abbott JA, Falcone T, Jacobs VR, Muzii L, Tulandi T, Indman P, Istre O, Jacobs VR, Loffer FD, Nezhat CH, Tulandi T. AAGL Practice Report: Practice Guidelines for the Management of Hysteroscopic Distending Media: (Replaces Hysteroscopic Fluid Monitoring Guidelines. J Am Assoc Gynecol Laparosc. 2000;7:167-168.). J Minim Invasive Gynecol. 2013 Mar-Apr;20(2):137-48. doi: 10.1016/j.jmig.2012.12.002. PMID: 23465255. | |
Nahlawi S, Gari N. Sonography Transvaginal Assessment, Protocols, and Interpretation. 2022 Sep 16. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 34283450. | |
Silas AM, Kruskal JB, Kane RA. Intraoperative ultrasound. Radiol Clin North Am. 2001 May;39(3):429-48. doi: 10.1016/s0033-8389(05)70290-6. PMID: 11506086. | |
Kasaven LS, Jones BP, Ghaem-Maghami S, Verbakel JYJ, El-Bahrawy M, Saso S, Yazbek J. Study protocol for a randomised controlled trial on the use of intraoperative ultrasound-guided laparoscopic ovarian cystectomy (UGLOC) as a method of fertility preservation in the management of benign ovarian cysts. BMJ Open. 2022 Jul 14;12(7):e060409. doi: 10.1136/bmjopen-2021-060409. PMID: 35835531; PMCID: PMC9289018. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)