This chapter should be cited as follows:
Zaima A, Chandraharan E, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.415163
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 11
Labor and delivery
Volume Editor: Dr Edwin Chandraharan, Director Global Academy of Medical Education and Training, London, UK

Chapter
Intrapartum Fetal Monitoring
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
The aim of intrapartum fetal monitoring is the timely identification of the onset of intrapartum fetal hypoxic stress so that appropriate action can be instituted immediately to avoid hypoxic ischemic encephalopathy and/or perinatal death without increasing unnecessary operative interventions for the mother. It is essential to ensure that assessment of the fetal heart rate (FHR) is carried out in the context of the wider clinical picture and taking into consideration the normal fetal physiological responses to ongoing hypoxic and mechanical stresses as well as the reserve of the individual fetus to mount an effective compensatory response. Although, several national and international guidelines have been produced to aid cardiotocograph (CTG) interpretation,1,2,3 it is vital to appreciate that the parameters stipulated by these guidelines and arbitrary time limits are designed for the population of human fetuses in general, and not for the individual fetus in question. In 2018, 34 CTG experts from 14 countries produced the first International Consensus Guidelines on Physiological CTG Interpretation4 to help individualize care.
In general, intermittent auscultation is recommended for a 'low risk' labor, whereas continuous electronic fetal heart rate monitoring (CEFM) using a CTG is recommended for high-risk labor.4 It is important to appreciate that the journey of labor which lasts for approximately 8–12 hours is fraught with the possibilities of the development of additional risk factors that may result in hypoxic and inflammatory damage to a fetus. Therefore, continuous and constant vigilance is essential during the intrapartum period, and an effective multidisciplinary team working with collective responsibility and individual leadership is vital to ensure excellent maternal and fetal outcomes. Interventions in response to abnormalities noted in the FHR should include immediate measures to improve the intrauterine environment (i.e. by reducing ongoing hypoxic stress by relaxation of the uterine muscle, correction of abnormal maternal environment such as hypotension and pyrexia that may cause abnormalities of the FHR), and/or an immediate operative delivery.
INTERMITTENT AUSCULTATION
Auscultation of the FHR at regular, predetermined intervals immediately following uterine contractions to determine the immediate fetal response to the stress of uterine contractions is recommended in low-risk pregnancies.2,3,4 Previous work has shown that CEFM in low-risk women is associated with an increased likelihood of intervention without any improvement in outcome.5 Therefore, women who are healthy and have had an uncomplicated pregnancy should be offered and recommended intermittent auscultation to monitor fetal wellbeing. This should be performed using Doppler ultrasound or a pinard stethoscope.2,4 All women must be fully informed of the risks and benefits of intermittent auscultation and CEFM.
Method
There is no robust evidence from trials to recommend any particular frequency or duration of intermittent auscultation. Therefore, it is more a ‘custom of practice’ than evidence-based approach.6 Auscultation is commonly carried out every 15 minutes in the first stage and every 5 minutes in the second stage – a practice adopted from the randomized controlled trials comparing intermittent auscultation and CEFM.
Initial assessment prior to commencing intermittent auscultation
- Record maternal perception of fetal movements over the preceding 24 hours.
- A full abdominal palpation to determine the lie, presentation and position of the baby.
- At the initial assessment use a pinard stethoscope on the mother’s abdomen in line with the fetal scapula to establish the real sounds of the fetal heart.7
- On first auscultation, one should listen for the FHR for at least one full minute in between contractions when the baby is at rest to establish a baseline FHR.5
- If in early labor, auscultation should be carried out during fetal movements or following stimulation of the baby. An acceleration should be noted, and, therefore, the presence of chronic hypoxia can be excluded.
- The maternal pulse should be palpated simultaneously while auscultating FHR to differentiate between the two, as it is possible to pick up inadvertently maternal heart rate from surrounding vessels. This should be done on the initiation of each auscultation and throughout if a FHR abnormality is detected.8
- During the first stage and passive (i.e. the cervix is fully dilated in the absence of active maternal expulsive efforts) second stage of labor, fetal heart should be auscultated immediately after a contraction for at least 1 minute every 15 minutes.
- During active (i.e. after the commencement of active maternal pushing) second stage of labor, the FHR should be auscultated every 5 minutes.9
- FHR should be documented as a single number and not as an average. If using Doppler, one should not rely on the range shown on the screen, as there have been instances in which the machine has miscalculated the FHR.8,10
- Record acceleration and decelerations, if heard.
- None of the literature suggests that variability can be determined by intermittent auscultation.7
- An observed rise in baseline rate, slow recovering decelerations or persistent accelerations (overshoot) after contractions should be confirmed by listening throughout the next three contractions to clarify the suspected pattern.8,9 Confirmation of an abnormality warrants a move to CEFM and transfer to obstetric-led care (Table 1).3,9
- Although a CTG machine utilizes the same technology as that of handheld Doppler, it should not be used in low-risk labor for intermittent auscultation, as this is inappropriate use of resources. Handheld Doppler has a narrow beam and is unlikely to pick up the maternal sound easily.10 It gives a swishing noise when tracked to a blood vessel compared with the electronic heartbeat sounds of the ultrasound transducer of a CTG machine, hence the handheld Doppler device is preferred.
1
Indications for converting from intermittent auscultation to cardiotocographic monitoring.
Maternal | Fetal |
Pulse over 120 bpm on two occasions 30 minutes apart | Undiagnosed breech presentation; transverse or oblique lie (review mode of delivery) |
A single reading of diastolic blood pressure ≥110 mmHg or systolic blood pressure ≥160 mmHg | Free-floating head in a nulliparous woman |
Diastolic blood pressure 90–109 mmHg or systolic blood pressure of 140–159 mmHg on two consecutive readings taken 30 minutes apart | Recurrent accelerations (immediately following a contraction i.e. overshoot) |
Maternal pyrexia (defined as ≥38.0°C once or ≥37.5°C on two occasions 1 hour apart) | Fetal heart rate below 110 or above 160 bpm, or perceived as inappropriate for gestational age. |
Any vaginal blood loss other than a show | Evidence of a rising baseline on the partogram |
The presence of meconium if birth is not imminent | 2 × decelerations in fetal heart rate heard on intermittent auscultation after two successive contractions |
Persistent pain in between contractions | |
Epidural analgesia |
2
Common indications for commencing cardiotocographic monitoring.
Maternal | Fetal |
Any condition that may impair utero-placental oxygenation | Suspected small-for-gestational age or macrosomia, gestation <37 or >42 weeks |
Induced labor | Known or suspected intrauterine growth restriction |
Administration of oxytocin | Oligohydramnios or polyhydramnios |
Ante/intrapartum hemorrhage | Abnormal fetal Dopplers |
Maternal illness (e.g. diabetes, cardiac, renal, hyperthyroidism) | Meconium-stained amniotic fluid |
Pre-eclampsia | Malpresentation |
Uterine scar (cesarean section or myomectomy) | Multiple pregnancy (all babies to be monitored) |
Contractions >5/10 minutes or lasting for more than 90 seconds | Reduced fetal movements in the previous 24 hours reported by the woman |
During/following insertion of an epidural block | Two-vessel cord |
Prolonged rupture of membranes >24 hours unless delivery is imminent | A rise in baseline, repeated decelerations or slow-to-recover decelerations or overshoots |
Maternal request | Fetal structural abnormalities diagnosed during the antenatal period and planned for CEFM |
CONTINUOUS ELECTRONIC FETAL MONITORING
CEFM is carried out using a CTG machine, which records features of the FHR (i.e. cardiograph) in relation to ongoing uterine contractions (tocograph). Although some argue that CTG could potentially reduce the mobility of the mother, CEFM is recommended in all cases in which there is an increased risk of intrapartum hypoxic injury. However, every effort should be made to facilitate the normal physiology of labor by encouraging the woman to adopt upright positions and mobilize. This can be facilitated by the use of wireless telemetry or the encouragement to move within the constraints of being connected to the monitor.
Deeper understanding of fetal physiology is essential to improve perinatal outcomes and to reduce unnecessary operative interventions. The 'population-based' CTG guidelines should not be blindly applied to every fetus in a labor ward because the arbitrary time limits that are stipulated by these guidelines (i.e. 30 minutes, 40 minutes, 90 minutes) would be meaningless in a fetus with intrauterine growth restriction or pre-existing hypoxia. Similarly, a fetus with chorioamnionitis will not be able to withstand the same intensity and duration of hypoxic stress as compared to a fetus not experiencing such an inflammatory response. Unfortunately, in many labor wards, the same CTG guideline with predetermined parameters is used in every fetus. Therefore, it is not surprising that a Cochrane systematic review (2017) concluded that the use of CTG is associated with an increase in the rate of cesarean section and instrumental delivery, without any significant benefit with regard to long-term outcome, except for a reduction in neonatal seizures.11
On the contrary, intense training in fetal physiology combined with mandatory competency testing on CTG interpretation and the use of fetal ECG as the additional central organ test has been shown to reduce the intrapartum emergency cesarean section rate as well as hypoxic ischemic encephalopathy rate.12 Therefore, it is important to interpret the CTG trace based on a deeper understanding of fetal behavioral states during labor, as well as the features on the CTG trace which differentiate fetal compensatory responses from the onset of decompensation, whilst incorporating the wider clinical context, so that care can be individualized.
Fetal Behavioral States
Fetal behavioral states13,14,15 refer to periods of:
- Quiescence – episodes of deep sleep associated with no eye movements. Deep sleep can last up to 50 minutes with very few fetal movements. This is demonstrated on the CTG as episodes of stable heart rate, rare accelerations and borderline heart rate variability.
- Active sleep – episodes of sleep associated with rapid eye movements and usually associated with moderate fetal activity. This is the most common behavioral state. It is demonstrated on the CTG by a moderate number of accelerations and normal heart rate variability.
- Wakefulness – active wakefulness is a rare state and is usually associated with a large number of fetal movements. This is demonstrated on the CTG by a large number of accelerations and normal variability. In this state accelerations may be so frequent as to pose a difficulty in determining the baseline heart rate (Figure 1).
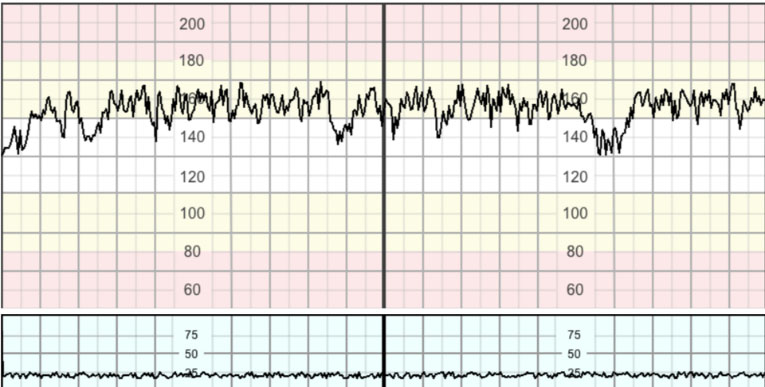
1
Confluence of accelerations.
The alternative epochs of active and quiet sleep cycles is referred to as cycling.15 This is a hallmark of fetal neurological responsiveness and absence of hypoxia and acidosis. Transitions between the different patterns become clearer after 32–34 weeks of gestation with the maturation of the fetal nervous system (Figure 2).
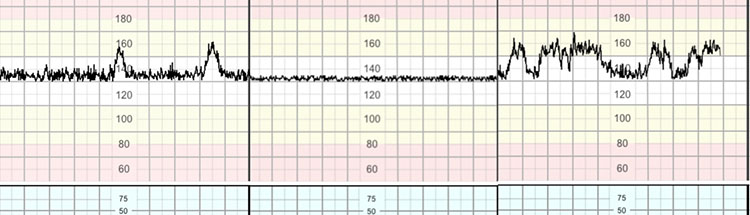
2
Fetal heart rate cycling.
Assessment of individual features of the cardiotocographic trace
The CTG is a graphical representation of the FHR and uterine activity over time. It is important to establish the standardized descriptive features of the trace as they will form the basis of all further discussions about fetal monitoring.
Baseline heart rate
The baseline heart rate is the mean FHR rounded to increments of five bpm. This is assessed over a minimum of 10-minute segment after the exclusion of episodes of accelerations, decelerations and periods of increased FHR variability (Figure 3). If the baseline cannot be established, that segment should be described as indeterminate.16 In tracings with unstable FHR signals, review of previous segments and evaluation of longer time periods may be necessary to determine the baseline.3 In myocardial hypoxia and acidosis, an unstable baseline FHR may be seen as a preterminal event.
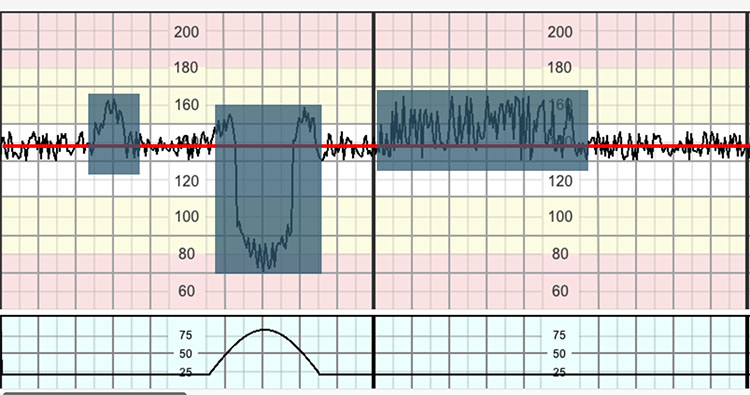
3
Assessment of the baseline FHR by excluding accelerations and decelerations.
Normal baseline heart rate lies between 110 and 160 bpm. A preterm fetus would tend to have a heart rate towards the upper end of this range, while a post-term fetus would have a heart rate towards the lower end. It has been shown that a baseline FHR >150 bpm after 40 weeks of gestation was associated a significantly higher incidence of meconium-stained amniotic fluid (OR 2.07), maternal intrapartum hyperpyrexia (OR 5.14) and urgent/emergency cesarean section (OR 2.65). Furthermore, a non-significantly greater occurrence of neonatal acidemia and admission to neonatal intensive care unit were also noted.17 When assessing a CTG it is important to note the normal baseline heart for each individual fetus, according to the gestational age of the fetus. The arbitrary range of 110–160 bpm which is applicable for population of human fetuses, should not be applied to every fetus irrespective of the gestational age. The baseline FHR should be compared with previously recorded CTG traces or documented FHR during antenatal visits.
Tachycardia refers to a baseline heart rate value above 160 bpm that lasts for over 10 minutes.
Bradycardia refers to a baseline value below 110 bpm lasting for more than 10 minutes.3 Although not very common, values between 90 and 110 bpm may occur in a normal fetus, especially in post-dates pregnancy. It is mandatory to confirm that this is not the maternal heart rate being picked up by the monitor, and that the trace demonstrates normal variability.21
Variability
Baseline variability refers to the oscillation in FHR. Evaluated as the average bandwidth of the signal in 1-minute segments and recorded in bpm.3 The fluctuation should be irregular in amplitude and frequency (Figure 4).16 Variability reflects integrity of the autonomic nervous system.
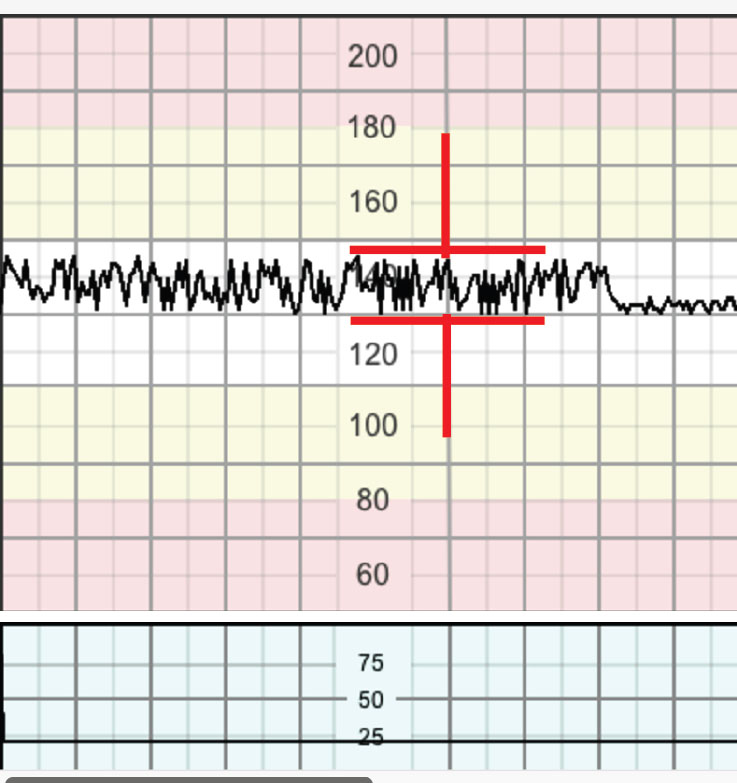
4
Assessment of baseline variability i.e. 'bandwidth'.
Normal variability is a bandwidth between 5 and 25 bpm.
Reduced variability refers to a bandwidth below 5 bpm, which is frequently associated with episodes of fetal quiescence, which can last up to 50 minutes.18 It is considered to be abnormal if it has persisted for over 50 minutes with a stable baseline.3 However, reduced variability is significant if it persists for >3 minutes within a deceleration3 indicating an acute brain hypoperfusion, which would warrant urgent intervention to improve fetal status.3,19
Increased variability (saltatory or the 'zigzag' pattern) is a bandwidth over 25 bpm. This pattern is presumed to result from fetal autonomic instability/hyperactivity. It may be seen linked to recurrent decelerations when hypoxia/acidosis evolves very rapidly. Saltatory or the 'zigzag' pattern persisting for over 30 minutes may indicate hypoxia even in the absence of decelerations (Figure 5). Interventions may be required sooner if this pattern is seen during the second stage or during decelerations.20 Recently it has been shown that the presence of the 'zigzag' pattern on the CTG trace is associated with 8.7–11.4 fold increase in the admission to the neonatal unit.21
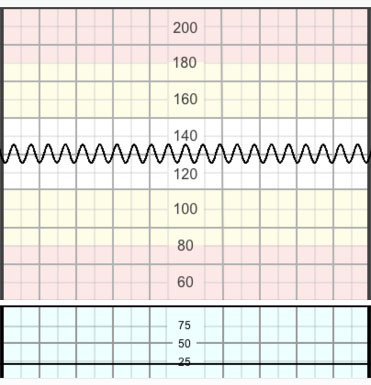
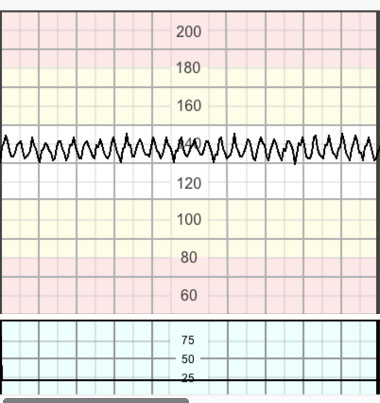
5
The 'zigzag' pattern due to autonomic instability as a result of a rapidly evolving hypoxia.
Typical sinusoidal pattern (Figure 6) is a regular smooth undulating signal resembling a sine wave, with an amplitude of 5–15 bpm and a frequency of 3–5 cycles/minute. It is usually associated with absence of accelerations and persists for over 30 minutes. it usually occurs in association with severe fetal anemia; e.g. anti-D alloimmunization. It has also been described in acute fetal hypoxia, infection, cardiac malformations, hydrocephalus and gastroschisis.
6
The 'typical' sinusoidal pattern due to chronic fetal anemia or fetal thumb sucking.
Atypical sinusoidal pattern (the 'Poole shark teeth') pattern resembles the 'typical' sinusoidal pattern, but with a more jagged ‘saw-tooth’ or 'shark teeth' appearance (Figure 7). This pattern seldomly persists for over 30 minutes and is usually preceded and followed by a normal pattern. The pathophysiology of this pattern is not completely understood. Some authors state that this is caused by acute fetal anemia caused by fetomaternal hemorrhage or ruptured vasa previa.22 This pattern has also been described after administration of analgesia, during periods of fetal sucking movements or other mouth movements.3
7
Atypical sinusoidal pattern or the 'Poole shark teeth' pattern due to acute fetal hypotension.
Pseudosinusoidal pattern is characterized by the presence of accelerations with a sinusoidal pattern. It can sometimes be difficult to distinguish this pattern from sinusoidal pattern, leaving the short duration of the pseudosinusoidal pattern to be the differentiating factor.
Accelerations
Accelerations are abrupt (onset to peak less than 30 seconds) increases in FHR above the baseline. An acceleration must start from and return to the baseline, have an amplitude of more than 15 beats and last for more than 15 seconds in duration; but less than 10 minutes. Before 32 weeks of gestation, amplitude and duration of accelerations may be lower (10 seconds and 10 bpm of amplitude).16 Although accelerations coinciding with uterine contractions may be caused by pressure sensation on the fetal skin, in late labor, there is a high chance of this being an erroneous recording of the maternal heart rate which is more likely to increase during contractions than FHR.
Decelerations
Decelerations are drops in FHR that last for over 15 seconds with an amplitude of over 15 beats. Although decelerations may be caused by parasympathetic stimulation due to head compression, they frequently occur as a protective reflex to hypoxic stress helping to maintain aerobic metabolism in the fetal myocardium. There have been many descriptive classifications that were adopted over the years. For the purpose of this text we will use the descriptive system published by FIGO in 2015.3
Early decelerations are gradual in onset (onset to nadir ≥30 s) and return to the baseline. They coincide with contractions16 and show normal variability within the deceleration. They are believed to be caused by vagal nerve stimulation secondary to head compression, hence they are likely to be seen in late first or second stage of labor. They do not indicate fetal hypoxia or acidosis.3
Variable decelerations (Figure 8) are V-shaped decelerations that exhibit a rapid drop (onset to nadir <30 s) followed by a rapid recovery to the baseline. These are believed to be caused by the stimulation of the baroreceptors secondary to cord compression. The rapid change in heart rate and the short duration of the deceleration can make it difficult to assess variability within the deceleration. Variable decelerations vary in size, shape and relationship to uterine contractions. They constitute the majority of decelerations during labor stimulation. They are seldom associated with fetal hypoxia/acidosis, unless they evolve to exhibit a U-shaped component ('sixties' criteria) with a reduced or an increased variability within the deceleration, and/or their individual duration exceeds 3 minutes.3,19
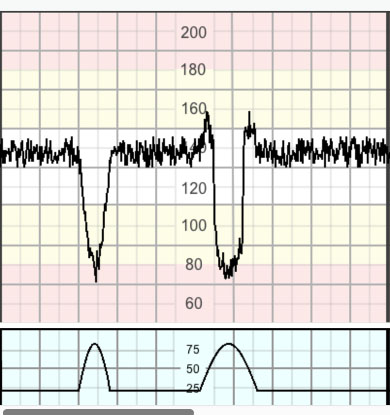
8
Variable decelerations.
Variable decelerations meet the 'sixties' criteria if two or more of the following are present: drops by 60 bpm or more, reaches 60 bpm or less, for the duration of 60 seconds or longer.19
Late decelerations (Figure 9) are decelerations with a gradual onset and/or a gradual return to the baseline and/or associated with reduced or increased variability within the deceleration. When contractions are adequately monitored, late decelerations frequently start over 20 seconds from the onset of the contraction, have a nadir after the acme of the contraction and start to return to the baseline after the end of the contraction.19 Late decelerations are indicative of a chemoreceptor mediated response to fetal hypoxemia.23
In a trace showing no accelerations and reduced variability, the definition of late decelerations also includes those with an amplitude of 5–15 bpm (shallow decelerations).
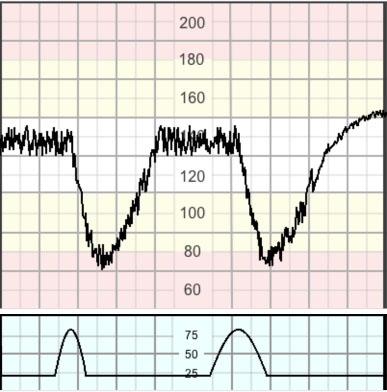
9
Late deceleration.
Prolonged decelerations (Figure 10) are decelerations that last for over 3 minutes. These are likely to include a chemoreceptor-mediated component even if initially triggered by a baroreceptor response, and thus to indicate hypoxemia. Decelerations exceeding 5 minutes with FHR maintained below 80 bpm and associated with reduced variability within the deceleration are frequently associated with acute fetal hypoxia/acidosis and require urgent intervention.19
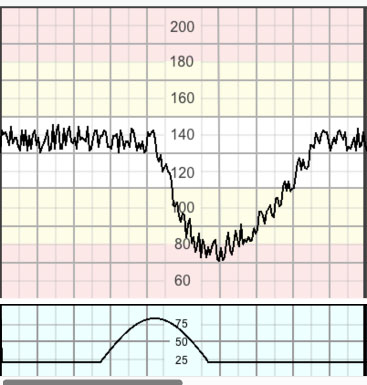
10
A 'single prolonged' deceleration.
Uterine contractions
Uterine contractions are recorded as bell-shaped gradual increases in the uterine activity signal followed by roughly symmetrical decreases. With the tocodynamometer, only the frequency of contractions can be reliably evaluated.3 The intensity and duration of contractions may be assessed by manual palpation. If the frequency of contractions cannot be assessed reliably by the tocodynamometer, manual palpation for 10 minutes every 30 minutes is required.
Tachysystole represents an excessive frequency of contractions and is defined as the occurrence of more than five contractions in 10 minutes in two successive 10-minute periods or averaged over a 30-minute period.23
Hyperstimulation refers to an exaggerated response to uterine stimulants, presenting as an increase in frequency of the contractions, strength of uterine contraction, increased uterine tone between contractions and/or prolonged contractions for over 2 minutes. These may lead to FHR changes. Therefore, any increased uterine activity; frequency, duration or strength, associated with CTG changes should be considered as uterine hyperstimulation. This picture may occasionally be seen in spontaneous labor without the use of stimulants. (To avoid over complication, the term hyperstimulation is used to include both iatrogenic and spontaneous increased uterine activity.)
TYPES OF FETAL HYPOXIA
It is important to appreciate the difference between hypoxemia i.e. an abnormally low concentration of oxygen in the blood, which can occur repeatedly without major implications, and hypoxia i.e. where the body or a region of the body is deprived of adequate oxygen supply at the tissue level, which if persistent for various durations of time may lead to actual tissue damage
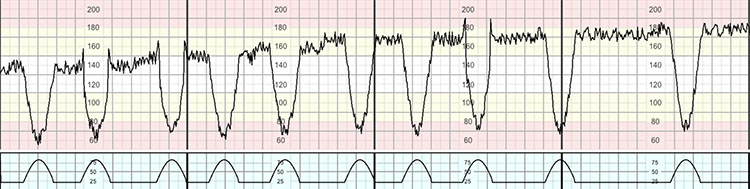
Acute hypoxia
Acute hypoxia24,25,26 is caused by sudden, almost complete interruption of oxygen supply to the fetus. It generally presents on the CTG as a prolonged deceleration lasting for more than 3 minutes (Figure 11). This causes rapid progression of hypoxia and acidosis within the tissues, with the pH dropping by approximately 0.01/minute.27,28
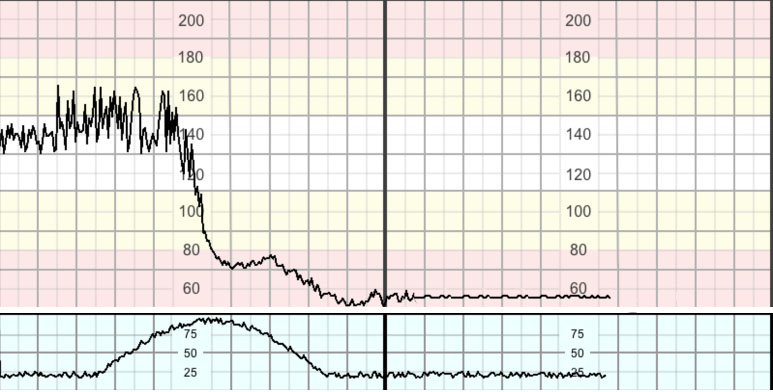
11
Acute hypoxia.
This sudden interruption of oxygen supply may be caused by an irreversible event namely placental abruption, uterine rupture or cord prolapse. More frequently, however, it is caused by a reversible situation such as prolonged uterine contraction, prolonged cord compression or maternal hypotension leading to uterine hypoperfusion.
Due to the rapid progress of hypoxia, the fetus has very little time to employ protective mechanisms apart from slowing down its heart to ensure adequate oxygen delivery to the heart muscle. The lack of protective mechanisms results in the rapid occurrence of tissue damage if this hypoxia is allowed to continue.
Management of acute hypoxia generally follows the 3-minute rule ('DEAR')
D – Diagnosis – when a deceleration exceeds 3 minutes with no signs suggestive of recovery, or with signs suggestive of worsening hypoxia; loss of variability within the deceleration prior to 3 minutes, the diagnosis of acute hypoxia should be made, and the emergency alarm must be triggered aiming at summoning the team.
E – Establishing the etiology (reversible or non-reversible causes) in the next 3 minutes (by 6 minutes), and measures to correct the reversible causes. Activating the 'crash delivery' for irreversible causes, ensuring delivery by the fastest and safest route.
A – Action (transfer to the operating theater, if the deceleration continues despite intrauterine resuscitation for reversible causes with no attempted signs of recovery) by 9 minutes.
R – Reassess the overall clinical picture in the operating theater and administer anesthesia if an emergency cesarean section is still required, by 12 minutes. Return to the labor ward if the deceleration has recovered or commence emergency cesarean section by 15 minutes.
Subacute hypoxia
Subacute hypoxia results from the fetus and placenta not being allowed adequate time to reverse hypoxemia.29 It presents on the CTG by the fetus spending more time responding to hypoxic events than at the baseline (Figure 12). This is frequently associated with episodes of increased variability which reflects rapid progression of hypoxia.21
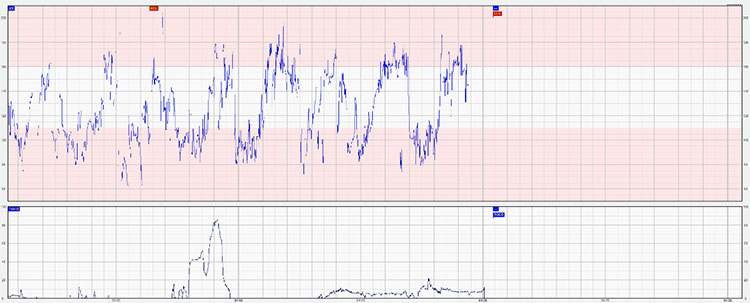
12
Subacute hypoxia.
It is important to note that, although partial cord compressions are seldom associated with significant hypoxemia, recurrent partial cord compression can significantly affect the delivery of oxygen from the placenta to the fetus. Subacute hypoxia is almost invariably caused by increased uterine activity; spontaneously or due to hyperstimulation, or by too frequent maternal pushing. The fetal pH may drop by 0.01, every 2–3 minutes.
Management of subacute hypoxia requires prompt attention and management to avoid significant/permanent damage.
- Reduce uterine activity, initially by reducing or stopping uterotonic; depending upon the duration of the hypoxia and signs of fetal reserve. If subacute hypoxia persists or if the increased activity is spontaneous, tocolytics may be necessary.
- Improve uterine and placental perfusion by avoiding supine hypotension.
- If hypoxia is caused by maternal pushing in the second stage advise the mother to stop pushing until the fetal status has recovered. Once recovered, pushing may be recommenced; occasionally it is advisable to push with every-other contraction if contractions remain too frequent.
- If hypoxia persists and is not corrected by the previous measures, delivery must be expedited.
Recently, the 'MOON' algorithm has been proposed to effectively manage subacute hypoxia during the second stage of labor.30 This includes incorporating the following whilst formulating the management plan: meconium, oxytocin infusion, oscillations (the 'zigzag' pattern) and 'nil visible' (i.e. spontaneous delivery is not imminent) to optimize outcomes.30
Gradually evolving hypoxia
Gradually evolving hypoxia,31 which develops over several hours, is the most common type of hypoxia during labor. The fetus has sufficient time to utilize protective mechanisms to compensate for such slowly evolving hypoxia for variable and frequently prolonged periods of time (Figure 13). This causes the changes to occur in a more subtle manner making it more likely to be missed, making this form of hypoxia the most dangerous. See Video 1 for a presentation that illustrates normal fetal circulation, to understand how the fetus can distribute blood in evolving hypoxia.
1
Illustration of normal fetal circulation.
13
A compensated evolving hypoxia with increased baseline FHR preceded by decelerations and with normal variability.
The changes during gradually evolving hypoxia tend to present in the following order (ABCDE):32
Evidence of hypoxic stress decelerations (to protect the myocardial workload)
A – Absence of accelerations
B – Baseline rise – catecholamine surge following decelerations becoming deeper and wider to redistribute blood and to perfuse vital organs
C – Compensated stress response – the fetus maintains a stable baseline and a reassuring variability despite ongoing deceleration and an increase in the baseline FHR
D – Decompensation characterized by loss of baseline variability and/or cycling, which may be followed by an unstable baseline FHR
E – End stage characterized by a progressive decline in the baseline FHR – the 'stepladder pattern to death' (Figure 14).
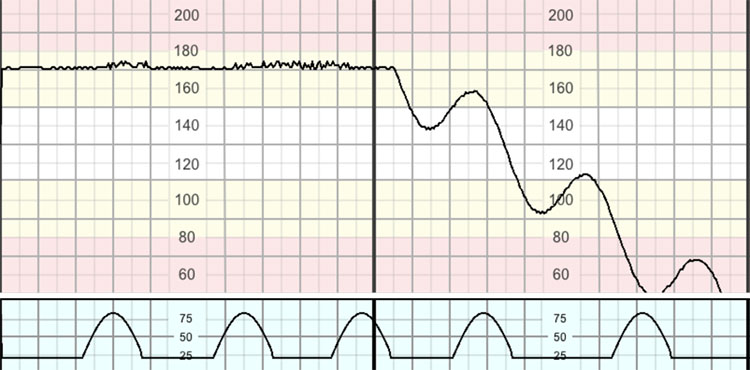
14
The 'end stage' of gradually evolving hypoxia: the 'stepladder pattern to death'.
Management of gradually evolving hypoxia should be aimed at improving fetal oxygenation by improving fetoplacental circulation so that decompensation and the onset of anaerobic metabolism in the fetal central organs are avoided. Urgent delivery is required if signs of decompensation cannot be reversed, despite the administration of a tocolytic drug to improve perfusion. A recent study on physiological interpretation of CTGs has confirmed that, even with ongoing catecholamine surge, if the baseline FHR variability is maintained, then, the likelihood of neonatal metabolic acidosis is 0%.33 However, if the baseline FHR variability is reduced, then the risk of neonatal metabolic acidosis is approximately 30%.33
Chronic hypoxia
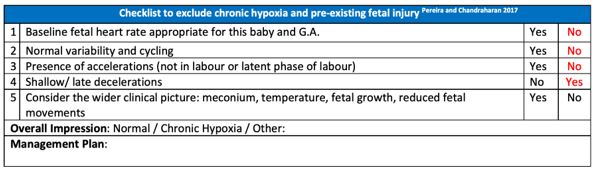
Chronic uteroplacental insufficiency occurs during the antenatal period, often over several days or weeks as a result of a gradual reduction of uteroplacental circulation.34,35 Video 2 shows the normal development of the placenta.
2
Normal development of the placenta.
Approximately 50–60% of the placental mass needs to be destroyed prior to observing changes in fetal Doppler blood flow suggestive of redistribution. Therefore, in a compensated state of chronic uteroplacental insufficiency in which <50% of placental mass has been destroyed, the CTG trace may be entirely normal with the presence of accelerations in the antenatal period or in very early labor. However, as uterine contractions become stronger, the fetus may show features of hypoxia within a short period of time, due to the reduced fetal reserve. If urgent action is taken to relieve hypoxic stress by administration of tocolytics and/or expediting delivery based on cervical dilatation, parity and the rate of progress of labor, then decompensation of the fetal central organs may occur rapidly.
Chronic hypoxia is a term used to describe the CTG changes in a fetus who has already exhausted all the compensatory mechanisms during the antenatal period and has evidence of depression in the fetal brain.35 A triad of CTG features (Figure 15) should be identified: a higher than expected baseline FHR for the given gestational age, reduced variability and/or absence of cycling and the presence of shallow or late decelerations.34 This is usually caused by poor placental function due to causes such as poor placentation, placental infarction or thrombosis, recurrent, but small abruptions or hyperplacentosis (gestational diabetes mellitus). Maternal history of reduced fetal movements and risk factors of chronic uteroplacental insufficiency such as pre-eclampsia and presence of thick meconium staining of amniotic fluid are often associated with chronic hypoxia.
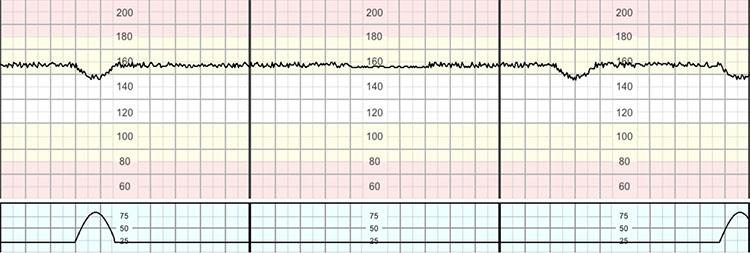
15
Chronic hypoxia. Note higher than expected baseline for 41 weeks, reduced variability/absence of cycling and presence of repetitive 'shallow' decelerations.
Pereira and Chandraharan proposed a 'Fetal Monitoring Checklist' to identify fetuses with chronic hypoxia at the onset of CTG recording.36 This checklist (Figure 16) is aimed at determining whether the fetus is fit to undertake the arduous and long journey of labor, so that immediate action can be taken to expedite delivery and to avoid further injury due to contractions-induced hypoxemia in an already compromised fetus.36
16
Chronic hypoxia checklist.
Oxygen administration to the mother who has a normal oxygen saturation does not alleviate fetal hypoxia and may actually be more harmful.37 Therefore, maternal oxygen administration should be carried out only to correct maternal hypoxemia and should not be administered to correct FHR abnormalities observed on the CTG trace. Similarly, administration of intravenous fluids in normotensive, well-hydrated women may lead to dilutional hyponatremia increasing the risk of maternal pulmonary edema as well as maternal and fetal convulsions secondary to electrolyte imbalance. Fluids should be administered only to correct CTG abnormalities when there is definitive evidence of maternal hypotension, dehydration or as part of treating a maternal medical condition (e.g. sepsis).
ADJUNCTIVE TECHNIQUES TO ASSESS FETAL WELLBEING
'Additional' or 'adjunctive' tests such as fetal scalp blood sampling (FBS) for estimating pH or lactate on the skin of the fetal scalp, fetal pulse oximetry, fetal scalp stimulation (FSS) and fetal ECG (ST-Analyzer or STAN) were introduced to reduce the very high false-positive rate of the CTG trace. These tests were necessitated as a result of the introduction of the CTG into clinical practice without conducting robust randomized controlled trials, and the resultant confusion amongst obstetricians and midwives with regard to the 'patterns'. Decelerations which are reflex fetal compensatory response to ongoing hypoxic stress were considered as 'non-reassuring' features and, based on their morphology and duration, CTG traces were 'suspicious' or 'pathological' by several guidelines. This resulted in an exponential increase in the rate of unnecessary operative interventions to mothers, without improving perinatal outcomes. Conversely, delay in accomplishing delivery by performing FBS, and false sense of security due to the contamination of the small sample of scalp blood with alkaline amniotic fluid may worsen perinatal outcomes.38,39
A deeper understanding of fetal physiological responses to ongoing hypoxic and mechanical stresses may alleviate the need for many of these anatomically and physiologically nonsensical, but historically important, 'adjunctive tests'.38
According to the current scientific evidence, if the CTG demonstrates a stable baseline and maintained variability, the risk of fetal acidosis is low.2,3,33 Therefore, one should not attempt to look for acidosis in the fetal scalp when there is a stable baseline and a reassuring variability in a 'pathological' CTG because the likelihood of hypoxia and acidosis is very low. Conversely, one should not attempt to check the pH or lactate in the skin of the scalp in the presence of an unstable baseline FHR or reduced variability because there is increased risk of acidosis (approximately 30%) in the fetal central organs. Therefore, attempting FBS in this case would delay delivery further and worsen perinatal outcomes.
Current scientific evidence and recommendations
Fetal scalp blood sampling
As quoted by NICE 2014 CTG Guideline,2 according to the current scientific evidence, the use of FBS increases the rate of cesarean section and operative vaginal birth for women. The Cochrane systematic reviews in 201340 and 201741 concluded that there is no available evidence of a correlation between fetal scalp pH and improvement in long-term outcomes. In addition, these reviews have also highlighted that contrary to the erroneous belief in the past, current evidence suggests that FBS may increase the number of cesarean sections and operative vaginal births. Performing more FBS (lactate) has been shown to double the rate of emergency cesarean section rate without improving the perinatal outcomes.42 The largest multicenter prospective study on FBS has concluded that contrary to what was believed by some senior obstetricians in the past, the use of FBS does not improve neonatal outcomes, and increases the rate of operative deliveries by 60%.43 Therefore, based on current scientific evidence and reported complications,38 FBS can no longer be recommended in clinical practice as the risks far outweigh the perceived benefits (none).
Fetal pulse oximetry
Transcervical, intrauterine application of a pulse oximeter on the accessible part of the fetal skin as an adjunctive test to reduce the false-positive rate of the CTG trace was attempted. However, the Cochrane systematic review in 201444concluded that fetal pulse oximetry did not reduce the cesarean section rate. Therefore, it can no longer be recommended in clinical practice.
Fetal scalp stimulation
Fetal scalp stimulation (FSS) attempts to elicit a fetal response by digitally stimulating the fetal scalp to 'wake up' a sleeping fetus, and observing an acceleration on the CTG trace has been considered as a reassuring sign that indicates the absence of fetal hypoxia and acidosis. A recent study has suggested that FSS was a reliable alternative to FBS, and even performed better than FBS.45 However, there is no consensus on the specific clinical situation in which FSS can be used.3,46 A deeper understanding of fetal physiology would obviate the need for FSS which is only useful in fetal sleep. In the presence of a stable baseline and reassuring variability, the likelihood of fetal hypoxia and acidosis is very unlikely, and therefore, FSS is not indicated. Conversely, in the presence of reduced variability at an abnormal baseline FHR and repetitive decelerations, the risk of fetal acidosis is very high, and therefore, precious time should not be wasted by performing FSS in this situation.
Fetal ECG – ST analyzer or STAN
STAN is a combined assessment of the standard CTG with an automated analysis of the fetal electrocardiogram (ECG). During episodes of ischemia, cardiac myocytes rely on anaerobic metabolism of glucose to meet their energy requirements. This metabolic pathway utilizes a larger amount of glucose for a much lower output of ATP. This deficit is compensated by further extraction of glucose from glycogen stores by means of glycogenolysis, a process releasing extra potassium as a biproduct. The extra potassium within cardiac tissues causes an increase in the height of the T-wave on the ECG.
The latest meta-analysis which included the largest US randomized trial on STAN reported a 36% statistically significant reduction in neonatal metabolic acidosis and 8% reduction in operative vaginal birth.47 Therefore, STAN is currently the only additional test of fetal wellbeing with robust scientific evidence from six randomized controlled trials (>26,000 babies) that showed a statistically significant reduction in neonatal metabolic acidosis.47 The current challenges to implement STAN in routine clinical practice are the lack of an in-depth understanding of fetal physiology and resistance to change by some obstetricians.48 Studies have shown that after instituting an intense training program for staff on fetal physiology, the use of STAN resulted in a significant reduction in emergency intrapartum cesarean section, neonatal metabolic acidosis and hypoxic ischemic encephalopathy.12,49 Therefore, based on the current scientific evidence, the use of STAN as an adjunctive test of fetal wellbeing is strongly recommended, after an intensive training program on fetal physiology for the users.
THE WIDER CLINICAL CONTEXT
The pneumonic 'MOTHERS' has been proposed to aid in the incorporation of the 'wider clinical picture' (Table 3), whilst interpreting and managing FHR changes during labor to optimize outcomes.32 It is beyond the scope of this chapter on intrapartum fetal monitoring to have a detailed discussion on the management of these individual risk factors during labor. However, it is important to appreciate that a preterm fetus has an underdeveloped autonomic nervous system, and therefore it may not show the 'typical' features described by some CTG guidelines which are designed to monitor term fetuses.50 Moreover, recent evidence has shown that chorioamnionitis is associated with specific features on the CTG trace, which may not be highlighted by some CTG guidelines which are designed to detect intrapartum fetal hypoxic stress and not fetal inflammation.51 Similarly, presence of any meconium (significant or non-significant) should be considered seriously as co-existing hypoxia or infection may increase the risk of meconium aspiration syndrome.52 Therefore, caution should be exercised whilst interpreting changes observed on the CTG in these situations.
3
The checklist 'MOTHERS' to incorporate the 'wider clinical picture'.
Meconium Oxytocin Temperature Hyperstimulation, hemorrhage Epidural, environment (maternal, fetal and organizational) Rate of progress of labor Scar, sepsis and size (both maternal and fetal) |
CONCLUSION
Intrapartum FHR monitoring is aimed at recognizing normal physiological responses of a human fetus to ongoing hypoxic and mechanical stresses during labor (Figure 17), so that timely action can be taken to avoid the onset of anaerobic metabolism and resultant perinatal brain injury or death. Using the 'fetal monitoring checklist' (Figure 16) at the beginning of labor to exclude pre-existing hypoxia, and developing a deeper understanding of different types of intrapartum hypoxia may help optimize outcomes. The wider clinical context (Table 3) should always be considered. Care should be individualized for the given fetus, and population-based CTG guidelines should not be blindly followed.
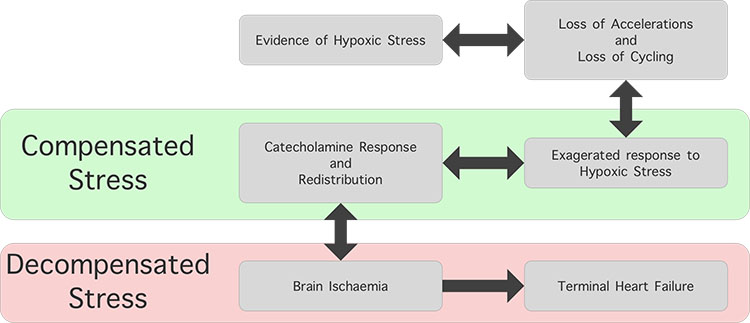
17
Fetal responses to evolving intrapartum hypoxic stress.
PRACTICE RECOMMENDATIONS
- Intermittent auscultation should be offered and recommended for low-risk women, whereas continuous electronic fetal heart rate monitoring using a CTG is indicated in high-risk labor.
- If the CTG continues to show a stable baseline FHR and reassuring variability, then the risk of fetal acidosis is very low.
- In acute hypoxia, the reversible causes should be corrected immediately, whereas in the case of irreversible causes, immediate delivery should be accomplished by the fastest and the safest route.
- The 'fetal monitoring checklist' is recommended at the beginning of labor to exclude pre-existing hypoxia.
- According to the current scientific evidence, fetal scalp blood sampling (FBS) and fetal pulse oximetry (FPO) are not recommended; however, the use of fetal ECG (ST analyzer or STAN) is associated with a 36% statistically significant reduction in neonatal metabolic acidosis and an 8% reduction in operative vaginal birth. Therefore, the authors recommend fetal ECG, after intensive training in fetal physiology to maximize its benefits.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol 2009;114(1):192–202. | |
Ayres-de-Campos D, Spong CY, Chandraharan E; FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int J Gynaecol Obstet 2015;131(1):13–24. | |
Maude RM, Skinner JP, Foureur MJ. Intelligent Structured Intermittent Auscultation (ISIA): evaluation of a decision-making framework for fetal heart monitoring of low-risk women. BMC Pregnancy Childbirth 2014;14:184. | |
Walsh D. CTG use in intrapartum care: assessing the evidence. British Journal of Midwifery 2008;16(6):367–9. | |
Munro J, Jokinen M. Evidence based guidelines for Midwifery-led Care in labour. Intermittent Auscultation (IA). The Royal College of Midwives Trust, 2012. | |
National Collaborating Centre for Women’s and Children’s Health Intrapartum care: care of healthy women and their babies during childbirth. RCOG Press: London, 2007. | |
Liston R, Sawchuck D, Young D, Society of Obstetrics and gynaecologists of Canada; British Columbia perinatal health program. Fetal health surveillance: Antepartum and Intrapartum consensus guideline. Journal of Obstetrics and gynaecology Canada 2007;29(9s4):s3–56. | |
Medicines and Healthcare products Regulatory Agency. Medical safety alert: Fetal monitor/cardiotocograph (CTG) – adverse outcomes still reported (All) adverse outcomes are still reported when CTG traces appear normal – this replaces alert SN 2002(23) issued August 2002. (MDA/2010/054) Gov.co.uk 28 June 2010. | |
Alfirevic Z, Gyte GML, Cuthbert A, et al. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No.: CD006066. DOI: 10.1002/14651858.CD006066.pub3. | |
Chandraharan E, Lowe V, Ugwumadu A, et al. Impact of fetal ECG (STAN) and competency based training on intrapartum interventions and perinatal outcomes at a Teaching Hospital in London: 5 year analysis. BJOG 2013;120:428–9. | |
Vindla S, James D. Fetal behaviour as a test of fetal wellbeing. BJOG: An International Journal of Obstetrics & Gynaecology 1995;102:597–9. | |
Pillai M, James D. Behavioural states in normal mature human fetuses. Arch Dis Child 1990;65(1 Spec No):39–43. | |
Preti M, Chandraharan E. Importance of fetal heart rate cycling during the interpretation of the cardiotocograph (CTG). Int J Gynecol and Reprod Sci 2018;1(1):10–2. | |
Macones GA1, Hankins GD, Spong CY, et al. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. | |
di Pasquo E, Fieni S, Chandraharan E, et al. Is FHR baseline >150 bpm associated with a higher risk of an adverse outcome? American Journal of Obstetrics & Gynecology 2020;222(1):S457–8. | |
Suwanrath C, Suntharasaj T. Sleep–wake cycles in normal fetuses. Arch Gynecol Obstet 2010;281(3):449–54. | |
Hamilton E, Warrick P, O’Keeffe D. Variable decelerations: do size and shape matter? J Matern Fetal Neonatal Med 2012;25(6):648–53. | |
Nunes I, Ayres-de-Campos D, Kwee A, et al. Prolonged saltatory fetal heart rate pattern leading to newborn metabolic acidosis. Clin Exp Obstet Gynecol 2014;41(5):507–11. | |
Gracia-Perez-Bonfils A, Vigneswaran K, Cuadras D, et al. Does the saltatory pattern on cardiotocograph (CTG) trace really exist? The ZigZag pattern as an alternative definition and its correlation with perinatal outcomes [published online ahead of print, 2019]. J Matern Fetal Neonatal Med 2019;1–9. doi:10.1080/14767058.2019.1686475 | |
Yanamandra N, Chandraharan E. Saltatory and sinusoidal fetal heart rate (FHR) patterns and significance of FHR ‘overshoots’. Curr Wom Health Rev 2014;9(3):175–82. | |
Peebles DM, Spencer JA, Edwards AD, et al. Relation between frequency of uterine contractions and human fetal cerebral oxygen saturation studied during labour by near infrared spectroscopy. Br J Obstet Gynaecol 1994;101(1):44–8. | |
Kamoshita E1, Amano K, Kanai Y, et al. Effect of the interval between onset of sustained fetal bradycardia and caesarean delivery on long-term neonatal neurologic prognosis. Int J Gynaecol Obstet 2010;111(1):23–7. doi: 10.1016/j.ijgo.2010.05.022. Epub 2010 Aug 4. | |
Leung TY1, Chung PW, Rogers MS, et al. Urgent caesarean delivery for fetal bradycardia. Obstet Gynecol 2009;114(5):1023–8. doi: 10.1097/AOG.0b013e3181bc6e15. | |
Cahill AG1, Caughey AB, Roehl KA, et al. Terminal fetal heart decelerations and neonatal outcomes. Obstet Gynecol 2013;122(5):1070–6. doi: 10.1097/AOG.0b013e3182a8d0b0. | |
Gull I, et al. Acid accumulation during end-stage bradycardia in term fetuses: how long is too long? Br J Obstet Gynaecol 1996. | |
Chandraharan E, Arulkumaran S. Prevention of birth asphyxia: responding appropriately to cardiotocograph (CTG) traces. Best Pract Res Clin Obstet Gynaecol 2007;21(4):609–. | |
Albertson A, Amer-Wahlin I, Loew V, et al. Incidence of acute hypoxia during active maternal pushing during labour. RCOG world congress, 2016. | |
Silvia Espuelas Malón, Edwin Chandraharan. Intrapartum Fetal Monitoring. In: Manual SOGIMIG de Assistência ao Parto e Puerpério. SOGIMIG, 2019. | |
Richardson BS1, Carmichael L, Homan J, et al. Fetal cerebral, circulatory, and metabolic responses during heart rate decelerations and cord compression. Am J Obstet Gynecol 1996;175(4 Pt 1):929–36. | |
Pinas A, Chandraharan E. Continuous cardiotocography during labour: Analysis, classification and management. Best Pract Res Clin Obstet Gynaecol 2016;30:33–47. doi:10.1016/j.bpobgyn.2015.03.022 | |
Jia YJ, Chen X, Cui HY, et al. Physiological CTG interpretation: the significance of baseline fetal heart rate changes after the onset of decelerations and associated perinatal outcomes [published online ahead of print, 2019]. J Matern Fetal Neonatal Med 2019:1–6. doi: 10.1080/14767058.2019.1666819 | |
Pulgar VM, Zhang J, Massmann GA, et al. Mild chronic hypoxia modifies the fetal sheep neural and cardiovascular responses to repeated umbilical cord occlusion. Brain Res 2007;1176:18–26. | |
McDonnell S, Chandraharan E. “The Pathophysiology of CTGs and Types of Intrapartum Hypoxia”. Current Women`s Health Reviews 2013;9:158. https://doi.org/10.2174/157340480903140131111654. | |
Pereira S, Chandraharan E. Recognition of chronic hypoxia and pre-existing foetal injury on the cardiotocograph (CTG): Urgent need to think beyond the guidelines. Porto Biomedical Journal 2017;2(4):124–9. doi: 10.1016/j.pbj.2017.01.004. | |
Fawole B, Hofmery GJ. Maternal oxygen administration for fetal distress. Cochrane Database Syst Rev 2012. | |
Chandraharan E. Fetal scalp blood sampling during labour: is it a useful diagnostic test or a historical test that no longer has a place in modern clinical obstetrics? BJOG 2014;121(9):1056–62. | |
Chandraharan E, Wiberg N. Fetal scalp blood sampling during labor: an appraisal of the physiological basis and scientific evidence. Acta Obstet Gynecol Scand 2014;93:544. | |
Alfirevic Z, Devane D, Gyte G. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev 2013;5:CD006066. | |
Alfirevic Z, Devane D, Gyte GML, et al. Continuous cardiotocography (CTG) as a form of electronic fetal moni-toring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev 2017;2:CD006066. | |
Holzmann M1, Wretler S2, Cnattingius S3, et al. Neonatal outcome and delivery mode in labors with repetitive fetal scalp blood sampling. Eur J Obstet Gynecol Reprod Biol 2015;184:97–102. | |
Al Wattar BH, Lakhiani A, Sacco A, et al. AB-FAB Study Group. Evaluating the value of intrapartum fetal scalp blood sampling to predict adverse neonatal outcomes: a UK multicentre observational study. Eur J Obstet Gynecol Reprod Biol 2019;240:62–7. | |
East CE, Begg L, Colditz PB, et al. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database of Systematic Reviews 2014;10. Art. No.: CD004075. DOI: 10.1002/14651858.CD004075.pub4 | |
Tahir Mahmood U, O'Gorman C, Marchocki Z, et al. Fetal scalp stimulation (FSS) versus fetal blood sampling (FBS) for women with abnormal fetal heart rate monitoring in labor: a prospective cohort study. J Matern Fetal Neonatal Med 2018;31(13):1742–7. doi:10.1080/14767058.2017.1326900 | |
Skupski DW, Rosenberg CR, Eglinton GS. Intrapartum fetal stimulation tests: a meta-analysis. Obstet Gynecol 2002;99(1):129–34. | |
Blix E, Brurberg KG, Reierth E, et al. ST waveform analysis versus cardiotocography alone for intrapartum fetal monitoring: a systematic review and meta-analysis of randomized trials. Acta Obstet Gynecol Scand 2016;95(1):16–27. | |
Chandraharan E. Foetal electrocardiograph (ST-analyser or STAN) for intrapartum foetal heart rate monitoring: a friend or a foe? J Matern Fetal Neonatal Med 2018;31(1):123–7. | |
Bhide A, Chandraharan E, Acharya G. Fetal monitoring in labor: Implications of evidence generated by new systematic review. Acta Obstet Gynecol Scand 2016;95(1):5–8. doi:10.1111/aogs.12830 | |
Afors K, Chandraharan E. Use of continuous electronic fetal monitoring in a preterm fetus: clinical dilemmas and recommendations for practice. J Pregnancy 2011;2011:848794. | |
Galli L, Dall'Asta A, Whelehan V, et al. Intrapartum cardiotocography patterns observed in suspected clinical and subclinical chorioamnionitis in term fetuses. J Obstet Gynaecol Res 2019;45(12):2343–50. doi:10.1111/jog.14133 | |
Bolten M, Chandraharan E. The Significance of ‘Non-Significant’ Meconium Stained Amniotic Fluid (MSAF): Colour versus Contents. Journal of Advances in Medicine and Medical Research 2019;30(5):1–7. https://doi.org/10.9734/jammr/2019/v30i530192. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)