This chapter should be cited as follows:
Osanan GC, Charry RC, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.413063
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 13
Obstetric emergencies
Volume Editor: Dr María Fernanda Escobar Vidarte, Fundación Valle del Lili, Cali, Colombia

Chapter
Non-conservative and Conservative Surgical Management of PPH
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Postpartum hemorrhage (PPH) represents one of the main causes of maternal mortality worldwide, representing approximately 25% of these deaths.1 Maternal mortality rates range from 15 to 443 for every 100,000 live births, most of which occur in low- and middle-income countries (LMIC).2 Thus, public policies and national strategies focused on reducing maternal deaths, including related to PPH, should be encouraged in these regions.3,4
Multidisciplinary teams in charge of treating patients with PPH must perform systematic and coordinated strategies. The procedures will increase in complexity as the golden hour for PPH treatment goes by. The golden hour aims to reduce morbidity and mortality related to the delayed management of PPH. It is well known that there is a direct relationship between the time taken to control the bleeding and a poor maternal outcome. Delayed control of bleeding may culminate in the lethal triad of hypovolemic shock, which consists of coagulopathy, hypothermia and acidosis. At this point, any strategy to avoid collapse or death can be unsuccessful.5
It is worthy noticing that the percentage of lethality from PPH is directly proportional to the elapsed time since its diagnosis. The golden hour can be divided into three periods of 20 minutes. The first 20 minutes mainly represents medical treatment, fluid replacement and identification and management of the main cause bleeding, taking into account the context of the 4 Ts: Tone, Trauma, Tissue, Thrombin.6 The following 20 minutes involves performing mechanical maneuvers, including the use of intrauterine tamponade balloon, uterine compressive sutures and pelvic vascular ligatures.7,8,9 Simultaneously, the non-pneumatic anti-shock garment (NASG) should be considered.10 Finally, the remaining 20 minutes are for non-conservative surgical techniques, which include hysterectomy and damage control surgery as final weapons to avoid maternal death.11
The following objectives are covered in this chapter:
- Review of the practical anatomical landmarks of vascular structures and anatomical references that are necessary for the appropriate surgical management of the pelvic cavity;
- Description of uterine-conservative hemostatic maneuvers and techniques;
- Detail of the non-conservative surgical techniques steps;
- Revision of the current evidence on the effectivity and rationale that sustain each procedure.
CONSERVATIVE SURGICAL MANAGEMENT
Conservative surgical management in PPH includes surgical procedures that can avoid hysterectomy and its morbidity, as well as can preserve patient’s fertility. The most used procedures are the hemostatic sutures, which refer to the ligation of pelvic vessels (LPV) and uterine compressive sutures (UCS). The success rate will depend on the cause, intensity and location of the bleeding as well as on the type of procedure chosen and the surgeon’s ability to perform the procedure.11,12,13,14
In general, there are three cornerstones for conservative surgical procedures: simplicity, safety and efficacy.15 Efficacy is defined as the ability to stop the bleeding after the hemostatic technique has been performed. Doumouchtsis et al. (2007) showed that conservative surgical procedures (pelvic vascular ligatures and uterine compressive sutures) seem to have similar success rates in controlling PPH compared to intrauterine tamponade balloon and uterine artery embolization.12 However, the rates of conservative surgical procedures may be deeply influenced by the topographic area of the bleeding and its cause (Table 1).11
1
Efficacy of conservative surgical techniques to stop uterine bleeding by topographic area and cause of PPH.11
Procedure | Topographic area and cause of bleeding | ||
Bleeding from the uterine body (S1) | Bleeding from the uterine cervix and upper vagina (S2) | ||
(Uterine atony) | (Placenta acreta) | Any cause | |
Bilateral uterine artery ligature | Excellent | Good | Poor or ineffective |
B-Lynch | Excellent | Good | Poor or ineffective |
Cho | Good | Excellent | Excellent |
Hayman | Excellent | Good | Poor or ineffective |
Pereira | Excellent | Good | Poor or ineffective |
Selective lower vascular ligature | Not applicable | Not applicable | Excellent |
Understanding and recognizing the origin, distribution and anastomosis of the genital arterial pedicles are essential skills to choose the most appropriate technique. The uterus can be didactically, divided into two vascular sectors: (a) Sector 1 (S1), which comprises the uterine body and it is supplied mainly by the uterine arteries and, to a lesser extent, by branches of the ovarian arteries and collaterals of the upper vesical artery; and (b) Sector 2 (S2), which includes the lower segment, uterine cervix and upper part of the vagina that is supplied by a group of pelvic subperitoneal collaterals, originating from the internal pudendal artery, and by accessory collateral vessels from the internal iliac arteries, uterine artery and lower vesical arteries (Figure 1).11
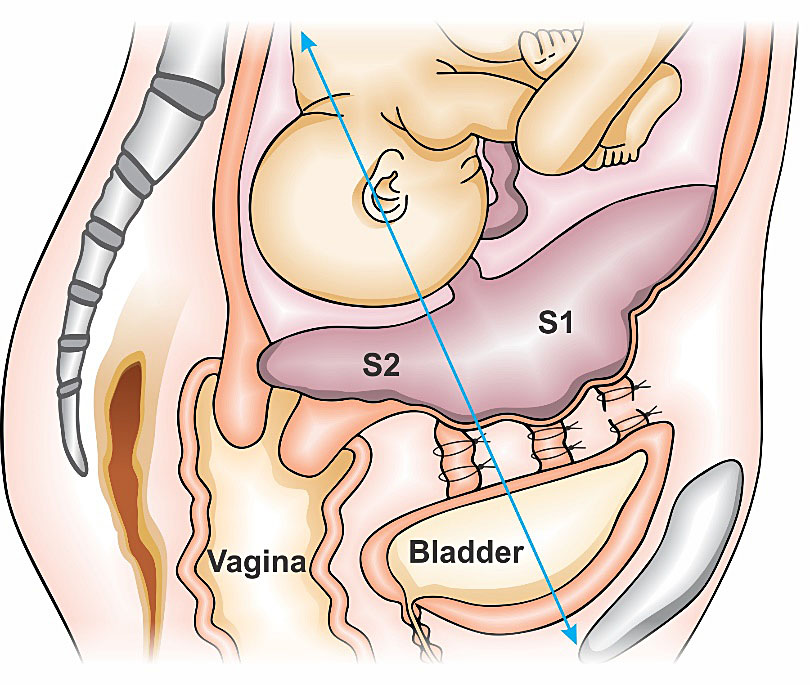
1
Saggital scheme of genital vascular regions S1 and S2. S1 refers to uterine fundus and body area and S2 compromises lower uterine segment, cervix and upper part of the vagina.
Simplicity as well as professional familiarity with the technique will influence the choice and the efficacy of the procedure. Obstetric skills training that involves practice on how to perform the conservative surgical procedures, especially when associated with detailed knowledge of pelvic anatomy, is likely to enhance the surgeon’s performance and to improve patient outcome.11
Finally, all techniques have related complications and so it is important to know them as well as the procedure indications and limitations. In clinical practice, professionals may try to associate conservative surgical methods, such as compresive sutures and some vessels ligations in an attempt to improve efficacy;16 however, some procedures may enhance complications, such as uterine ischemic necrosis17 and may prolong the time for deciding for hysterectomy
Theoretically, UCS should be able to control bleeding more quickly and in a safer manner when compared to hysterectomy. However, when it fails to stop PPH, radical surgery is indicated, since the patient's prognosis may worsen (as the time elapsed from diagnosis until the control of the bleeding will be prolonged) and the mortality rates may increase. For these reasons the conservative surgical management should be included into a PPH protocol, so the provider is able to decide, properly, how and when to use them.
UTERINE COMPRESSIVE SUTURES
Uterine compressive sutures (UCS) are an excellent surgical strategy to stop PPH. They can offer an easy, quick, safe, and effective control of bleeding at a laparotomy, especially in locations with low technical resources. It can avoid hysterectomy and its morbidity can also preserve patient fertility in most cases. The most known and used type UCS is the one described by B-Lynch et al. (1997) and its variations.7 Other techniques, such as Cho et al. (2000), Hayman et al. (2002) and Pereira et al. (2005) sutures have also been well studied.18,19,20 The main mechanisms of action of UCS are to promote a mechanical compression on specific uterine areas as well as a reduction of blood flow in the uterine arteries.7,20
Indications
The primary indication for UCS is a hemorrhage resulting from uterine atony that: (a) does not respond to uterotonics after a cesarean section, or (b) does not respond to pharmacological treatment and other nonsurgical management (such as intrauterine tamponade balloon and non-pneumatic anti-shock garment) in vaginal delivery.6,7
However, UCS can be used in any situation that compression of the uterus is considered an important step to control uterine bleeding. This situation may occur in cases of placenta previa or acreta, or even bleedings related to some types of uterine lacerations. More recently, UCS have been indicated as a prophylaxis for acute recurrence of uterine inversion.21,22
Most authors describe the importance of performing an efficacy test before the procedure (through manual uterine compression and observation of the blood loss), in order to see if uterine compression is sufficient to control bleeding. If this maneuver does not control hemorrhage, UCS is not indicated, as it will probably fail to control PPH and increase the duration of the bleeding. In these situations, the health professional team should move to the next step of PPH management.7,22
Efficacy
When it is indicated, UCS’s efficacy for controlling the bleeding and avoiding hysterectomy ranges from 60% to almost 100%,7,23,24, but the overall success rate is usually superior to 80% in most series.8,12 Until now, there is no consistent evidence to indicate “the best and unique” UCS technique. The studies usually do not find significant differences in the success rate among different sutures.12
Palacios-Jaraquemada (2011), however, showed interesting data, in which UCS efficacy varies according to the area and the cause of uterine bleeding.11 In this study, the sutures of B-Lynch, Hayman, Cho and Pereira’s achieved a >94% success rate for controlling bleeding due to uterine atony; but, for bleeding from the cervix or upper vagina only Cho suture was effective (>92% success rate), and the others were considered to have low efficacy or even to be ineffective for treating PPH in these areas.11
Some other conditions interfere with UCS’s efficacy. Kayem et al. (2011) showed that delays of 2–6 hours, between delivery and uterine compression suture, were associated with a fourfold increase in the risk of failure.13 Also, the author pointed out that women were more likely to have radical surgery if they were aged 35 years or older, multiparous or had a vaginal delivery.13
Complications
UCS have short- and long-term complications of which all surgeons should be aware. Its incidence is still unclear, as the UCS are relative new procedures. The most frequently reported complications are partial uterine ischemic necrosis, septic pyometra, hematometra, uterine synechiae and, less commonly, uterine rupture in subsequent gestation or intestinal entrapment. No cases of maternal death related to UCS have been published to date.17,22 In recent years, many UCS techniques have been created with the aim of reducing some adverse effects related to the more traditional compressive sutures.
UTERINE COMPRESSIVE SUTURES TECHNIQUES
UCS can be classified in different ways.15,25 All classifications were created to help improve understanding about the suture's mechanism of action, application, selection, complication and validity of these procedures.15 Here, is presented one classification that considers six categories for UCS (Table 2).15
2
Classification of uterine compressive surgery, based on six criteria.15
Categories/criteria | Subcategories | ||||||
According to the mode of UCS | a) Approximating both walls of uterus together, e.g. B-Lynch and Hayman sutures | b) Stapling both walls together, e.g. Cho, Hwu and ‘‘U’’ sutures | c) Sandwich sutures, e.g. Nelson suture | d) Location area sutures in single wall (either anterior or posterior walls), e.g. figure-of-eight suture | |||
According to the direction of suturing | a) Longitudinal sutures, e.g. B-Lynch and Hayman sutures | b) Transversal sutures, e.g. Ying suture | c) Crisscrossing sutures, e.g. M-Y sutures | d) Sutures around the uterus, e.g. Pereira suture | |||
According to the anatomic region | a) Lower uterine segment, e.g. Hwu, Dedes sutures, etc. | b) Upper uterine segment, e.g. B-Lynch suture and Hayman suture | c) Location area sutures, viz., focus region suture, e.g. Cho suture | d) Sutures around the uterus, e.g. Pereira suture sutures applied longitudinally and transversally around the uterus | |||
According to the causation of PPH | a) Uterine atony, e.g. B-Lynch suture and Hayman suture | b) Placental abnormalities, e.g. Cho suture and Li suture | c) Laceration of the endometrium, e.g. Cho suture or figure-of-eight suture, and so on | ||||
According to whether the sutures transfix uterine cavity | a) Transfixing uterine cavity, e.g. Hayman suture | b) No transfixing uterine cavity, e.g. Zheng suture | |||||
According to whether the sutures are removable | a) Irremovable suture, e.g. figure-of-eight suture, Cho suture, and so on | b) Removable uterine compression sutures, e.g. Aboulfalah suture | |||||
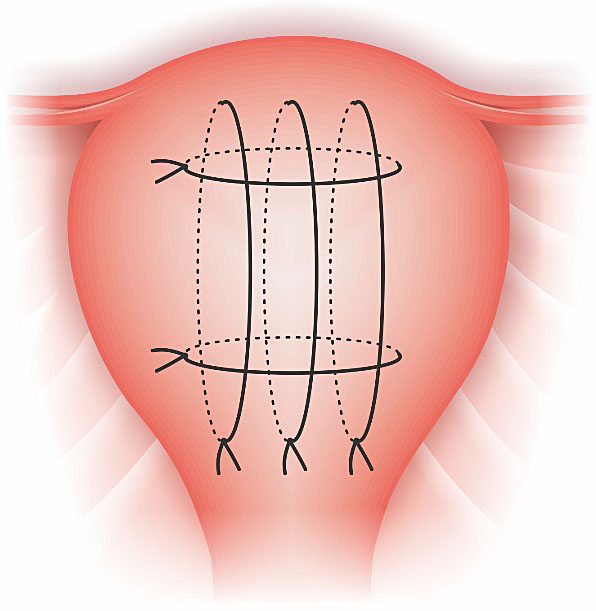
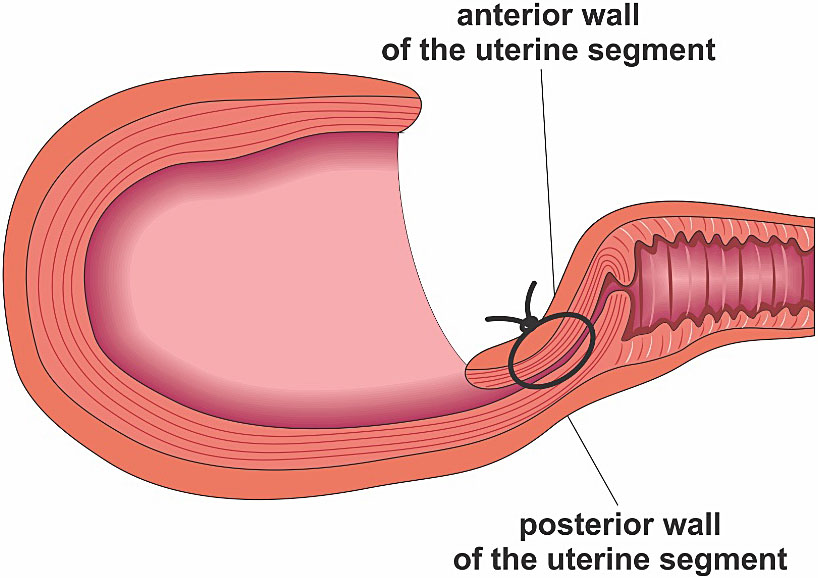
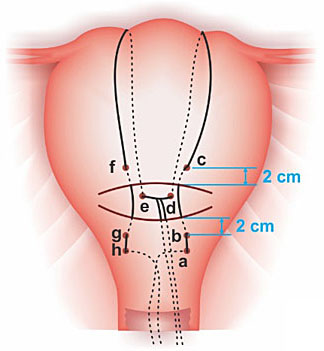
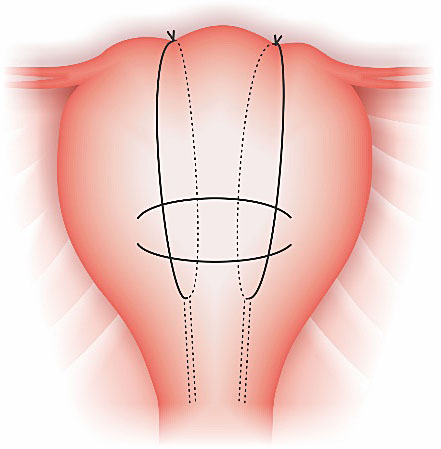
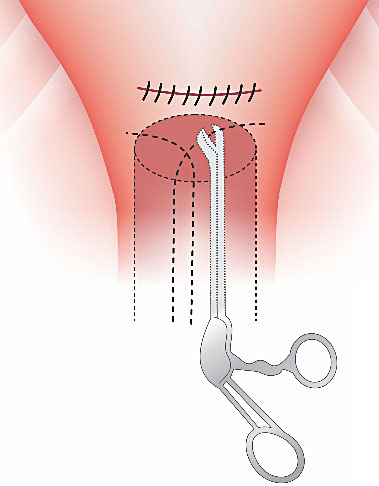
B-Lynch compressive suture
The B-Lynch compressive suture is the most known and probably the most widely used compression suture, it is an easy and very efficient procedure to control hemorrhages in the uterine body (S1).7,11 This suture has success rates above 90% due to atony, and controls bleeding by compressing the uterine body’s anterior wall against its posterior wall (Figure 2). The original technique requires hysterotomy, but some variations of B-Lynch technique may not need it.7
A test for the potential efficacy of the B-Lynch suture before performing the procedure is essential. The uterus is exteriorized and bimanual compression is performed. An assistant stands between the patient’s legs and evaluates the presence and extent of the bleeding. If the bleeding stops on applying such compression, there is a good chance that application of the B-Lynch suture will work and stop the bleeding.7
|
|
|
|
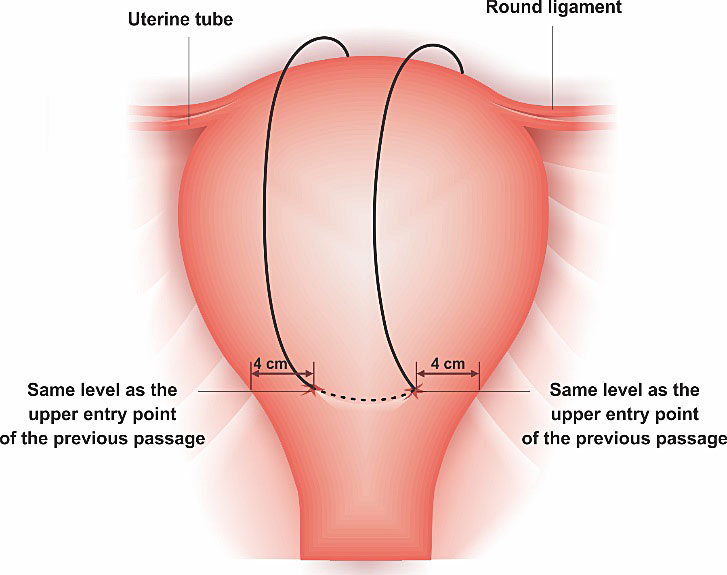
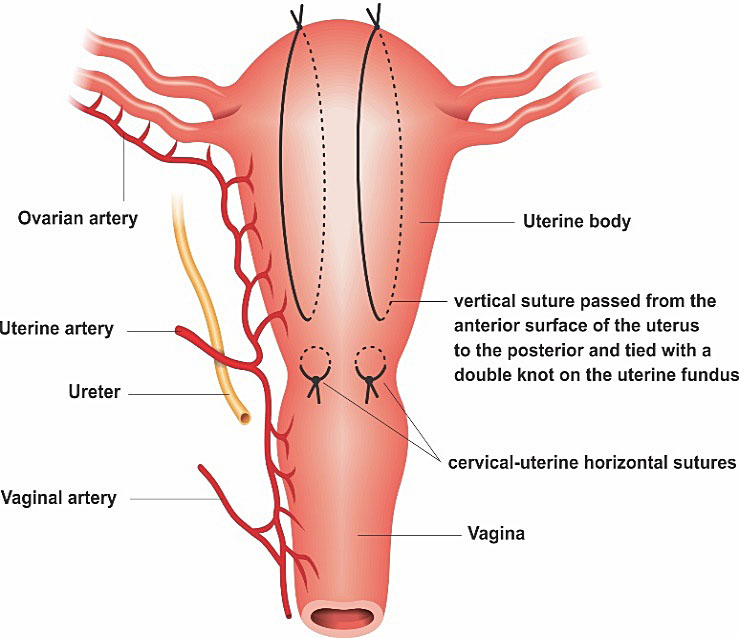
2
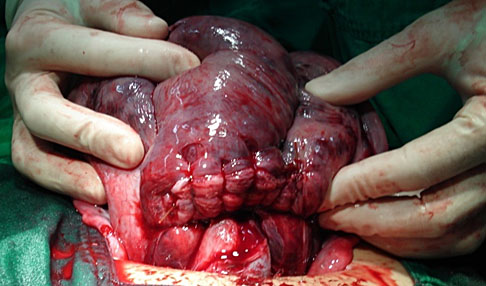
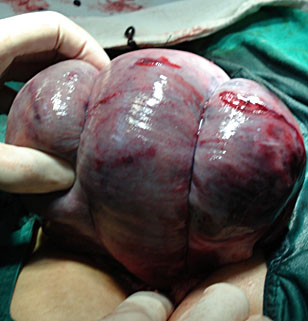
B-Lynch anterior and posterior view. B-Lynch suture technique: a stitch is placed 3 cm below the hysterotomy incision and threaded through the uterine cavity to emerge 3 cm above the upper incision margin. The suture is then carried vertically over the top of the uterus and to the posterior side of it. The stich now enters into the cavity at the level of the hysterotomy incision and the uterosacral ligament. With the suture inside the cavity, it is carried horizontally to the corresponding position on the other side of the uterus. The needle now transfixes the uterus from inside to the outer surface of the uterus. The suture, which is now outside of the uterine cavity posteriorly, will do the same suture initial steps, but in inverse sequence. Finally, the needle picks up the anterior wall at the same level as the initial entry point, and the thread is tied. Below, the anterior and superior views of the B-Lynch suture applied in Couvelaire's uterus.7
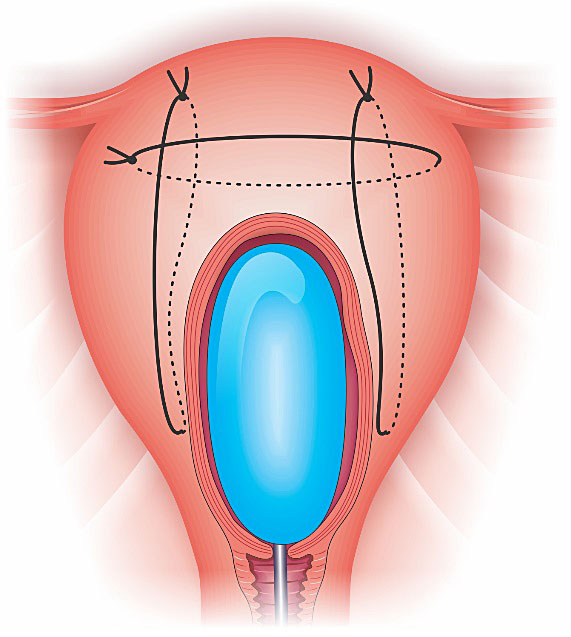
Hayman compressive suture
Hayman compressive suture resembles the B-Lynch technique in terms of success rate and indications. The main difference is that the two arm sutures are inserted into the anterior wall to the posterior, without hysterotomy. Hayman’s suture can also be performed using four longitudinal threads (instead of two) and, if a bleeding from lower uterine segment co-exists, it recommends the application of two isthmic-cervical compression sutures (Figure 3).19,22
|
|
A | B |
3
(A) Hayman suture anterior view. The needle transfixes the whole thickness of both uterine walls at the lower uterine segment level, with the thread being passed over to compress the fundus, and the thread tied at the fundus. The same is done on the opposite side. If bleeding from the lower uterine segment co-exists, two transverse cervicoisthmic sutures should be made. It does not need hysterotomy. (B) the superior view of Hayman's uterus suture after uterine atony.
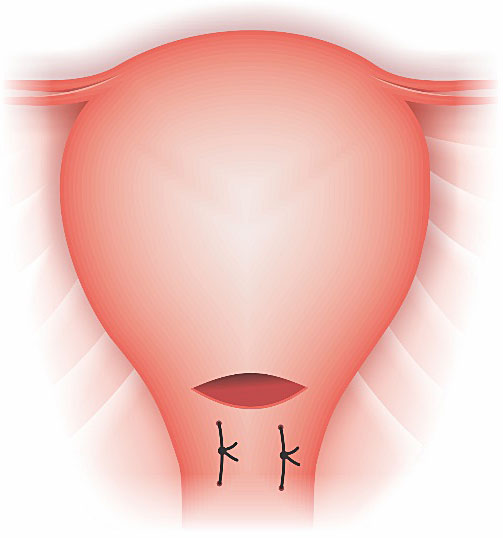
Cho compressive suture
Cho procedure is the “square” suture technique. It eliminates the spaces inside the uterine cavity, joining the anterior and posterior walls. It is useful for bleeding sites located in the body and lower segments of the uterus (Figure 4).18 Some authors, when applying Cho’s sutures in S2 area dilate the cervix so as to avoid hematometra.11 In an atonic uterus, four to five square sutures should be made (from the fundus to the uterine segment). In cases of placenta acreta the squares are applied in the bloodiest areas. In the placenta previa, the squares approach the walls of the uterine segment after bladder is displaced inferiorly.18
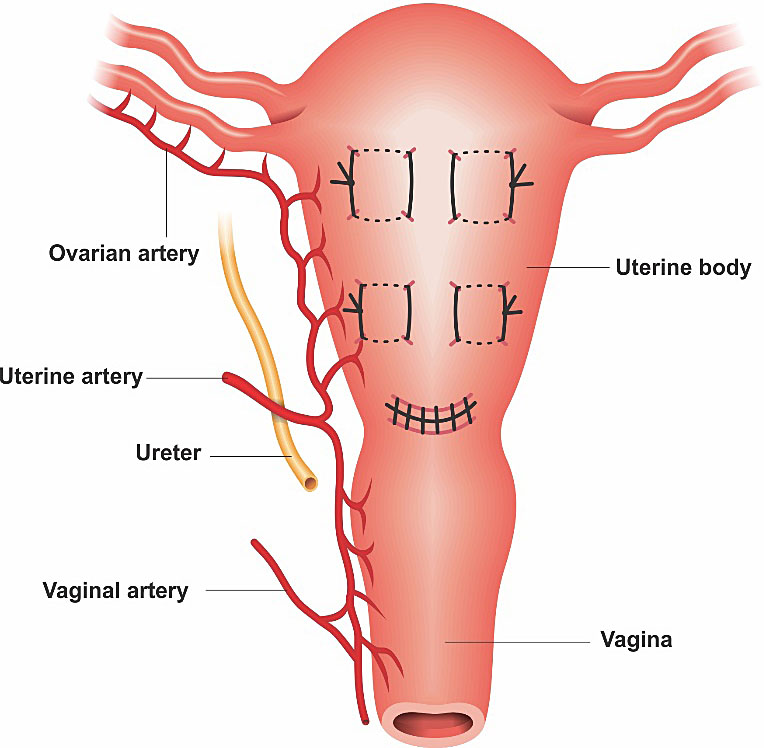
4
Cho suture anterior view. Technique: a needle transfixes the uterus from anterior to posterior wall and then from posterior to anterior. The same is done to approximate the anterior and posterior uterine walls in a “square” manner.18
Pereira compressive suture
The Pereira technique is a non-penetrating multiple transverse and longitudinal suture. The uterus is involved by several continuous sutures without penetrating the uterine cavity (Figure 5). This technique may reduce the risks of infection, uterine synechiae and also intestinal entrapment.20
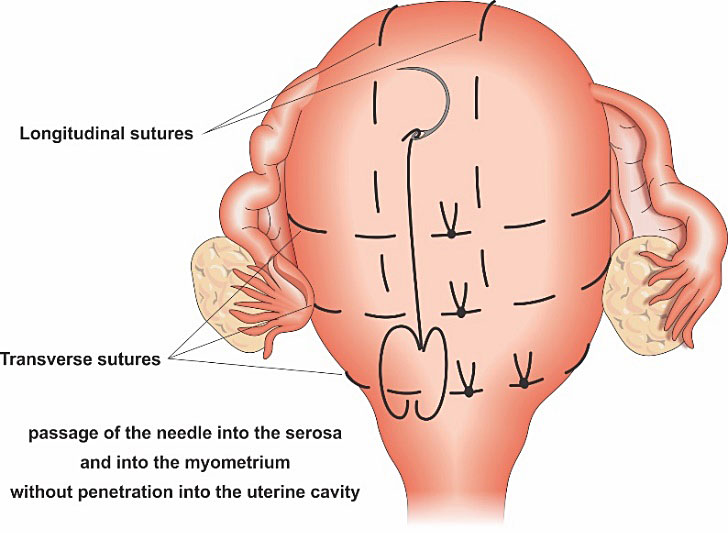
5
Pereira suture anterior view. Technique: placement of the sutures involved a series of bites inserted superficially, taking only the serous membrane and the subserous myometrium without penetrating the uterine cavity. When the suture crossed the broad ligament, it is important to select an avascular area and to be sure that the fallopian tube, the utero-ovarian ligament, and the round ligament are not inside the suture.20
OTHER UTERINE COMPRESSIVE SUTURES TECHNIQUES
Many other techniques have been described that can be useful. Most of them were developed to overcome the disadvantage or the complications of the more traditional UCS. Some techniques may also associate compressive sutures to other hemostatic procedures, such as uterine artery ligature or intrauterine tamponade balloon.
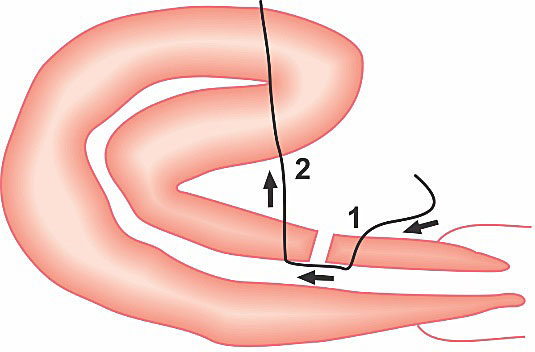
Matsubara-Yano compressive suture
The Matsubara-Yano (M-Y) suture was developed to overcome some B-Lynch’s suture drawbacks, such as the need to perform hysterotomy and the possibility of B-Lynch braces “slides off”.26,27
The M-Y suture transfixes uterine lower segment from anterior to posterior and then transfixes the uterine fundal edge from posterior to anterior, with the thread tied (longitudinal suture). After it, two transversal sutures are performed (crisscrossing suture). Hysterotomy is not necessary.26
A modified M-Y technique can also be associated to the use of intrauterine tamponade balloon in cases of placenta previa (Figure 6). This association is also known as M-Y sandwich technique. As can be observed, there are only two transversal sutures (instead of the three proposed by the original technique). M-Y sutures may be useful to avoid recurrence of uterine inversion.27
|
|
A | B |
6
(A) anterior view of the original Matsubara-Yano (M-Y) suture and (B) M-Y sandwich technique (modified suture associated to the insertion of an intrauterine tamponade balloon).
Dedes compressive suture
Dedes procedure is an effective circular isthmic-cervical compressive suture developed to treat cases of PPH due to placenta previa/acreta during a cesarean section.28 This technique interrupts blood circulation from the cervical artery and the branches of the vaginal artery; and keeps the cervix contracted. To avoid ureter and bladder injury, the bladder should be reflected downwards. A silastic drain (e.g. Foley catheter) is inserted into the internal and through the external os, so as to drain the uterine cavity and to keep the cervical canal open (Figure 7).
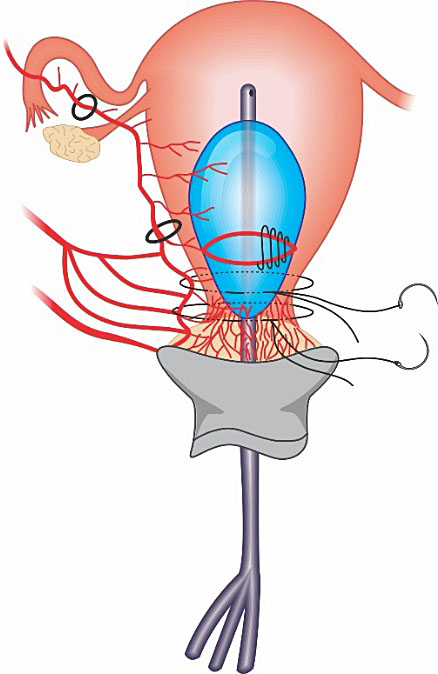
7
Dede compressive suture anterior vision: a circular isthmic-cervical compressive suture associated to a Foley catheter inserted into the cervical orifice to maintain drainage of the uterus cavity.
Multiple 8 compressive suture
The multiple compression suturing was described by Shazly (2012) as an additional procedure to control hemorrhage and preserve fertility in patients with placenta acreta (Figure 8). This procedure was performed after the bilateral uterine ligature and failed to stop the hemorrhage from anterior wall of the lower uterine segment. This technique may also be useful in cases of placenta previa.29
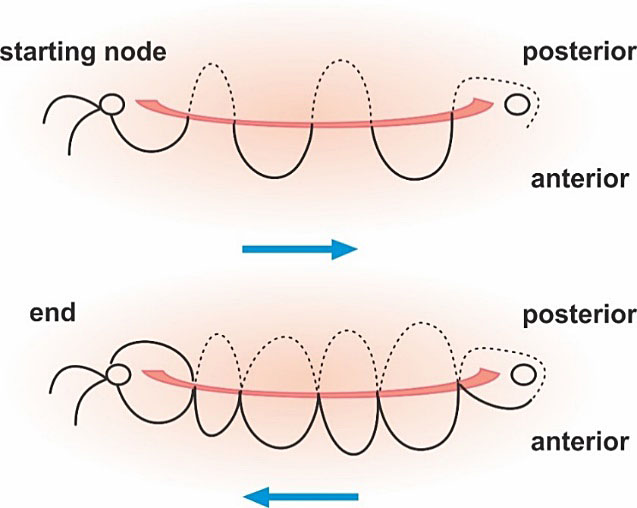
8
Technique of transverse lower uterine segment compression suture.
Hwu suture
Hwu compressive suture was designed to treat bleeding in cases of placenta previa or acreta during cesarean section.30 Two vertical compression sutures are placed in the lower segment to compress anterior to posterior wall. The bladder has to be reflected downward so as to adequately expose the underlying lower uterine segment (Figure 9).
|
|
A | B |
9
(A) anterior view of Hwu suture. (B) The needle is placed through the anterior wall of the lower segment. From inside the uterine cavity, the stitch is placed in the middle layer of the posterior wall of the lower segment. The suture is pulled from back to front through the uterine cavity and anterior wall of the lower segment. The knots are tied to compress the anterior and posterior walls of the lower segment.30
Li compressive suture
Li technique is a "belt-like suture", in which the uterine body and fundus bends over the anterior wall of the uterus, in order to obtain hemostasis by compression.31 It was first used in patients with uterine atony associated to abnormally adherent placenta (Figure 10). Li suture controlled PPH in 10 out of 11 patients when it was performed in the first series.31
|
|
A | B |
10
Li compressive suture: (A) lateral and (B) anterior views. The arrows indicate the direction and line of the suture; the numbers represent the puncture point and the pierce sequence.
Zheng compressive sutures
Zheng technique is a suture that it is performed without hysterotomy, without entering the uterine cavity and without suturing the anterior and posterior walls of the uterus (Figure 11). So its advantages involve reduced risk of complications (such as infection, uterine synechiae) and minimum damage to the atonic uterus (as it does not need hysterotomy).32
Zheng technique is non-penetrating two longitudinal sutures and is performed as follows: (a) the needle is inserted into the inner layer of the anterior wall of the lower segment and does not enter into the myometrium, (b) the needle is inserted into the middle layer of the fundus, (c) the needle is inserted into the inner layer of the posterior wall of the lower segment and does not enter into the myometrium, (d) the two ends of the thread are tied on the fundus of the uterus, and (e) The procedure is repeated on the other side.
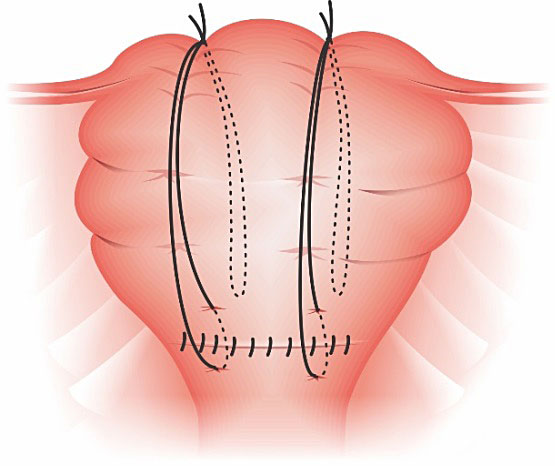
11
Zheng Compressive suture: anterior view.
REMOVABLE UTERINE COMPRESSION SUTURES
Removable uterine compression sutures (RUCS) were first described by Aboufalah et al. (2014).33 Later Zhang et al. (2015) described B-Lynch and Hayman removable sutures.34 All of these techniques have the aim of avoiding uterine synechiae, one of most common UCS complications.
Aboulfalah removable technique
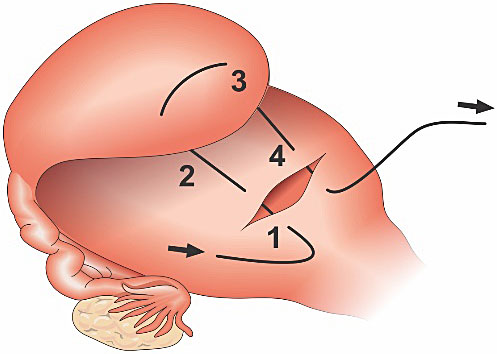
The Aboulfalah removable technique requires laparotomy, but not hysterotomy. It can be used in association to bilateral uterine artery ligature (BUAL). The procedure is performed bilaterally. Initially, an efficacy test should precede the UCS. The bladder should be reflected inferiorly. Using a number 2 sliding nonresorbable suture wire with 70 mm round-bodied hand needle or with wire guide, the first stitch is applied from outside, transfixing the full thickness of anterior abdominal wall above the pubis immediately and 2 cm laterally from the median line. Now the needle is inside the abdominal cavity, so it passes through the inferior uterine segment from the anterior to posterior wall as low as possible, under sutured hysterotomy, and 2 cm inside from uterine artery cross. The wire is then passed over as a brace to compress the uterine fundus by approximately 3 or 4 cm inside the corneal border. The last stitch is applied from inside to outside through the abdominal wall 2 cm above the first parietal stitch but 4 cm laterally from the median line. The right and the left lower suture extremities are tied anteriorly. The throws are visible to the skin (Figure 12). Blood reduction is immediately observed. The suture should be removed 24–48 hours later (maximum), without any anesthesia.33
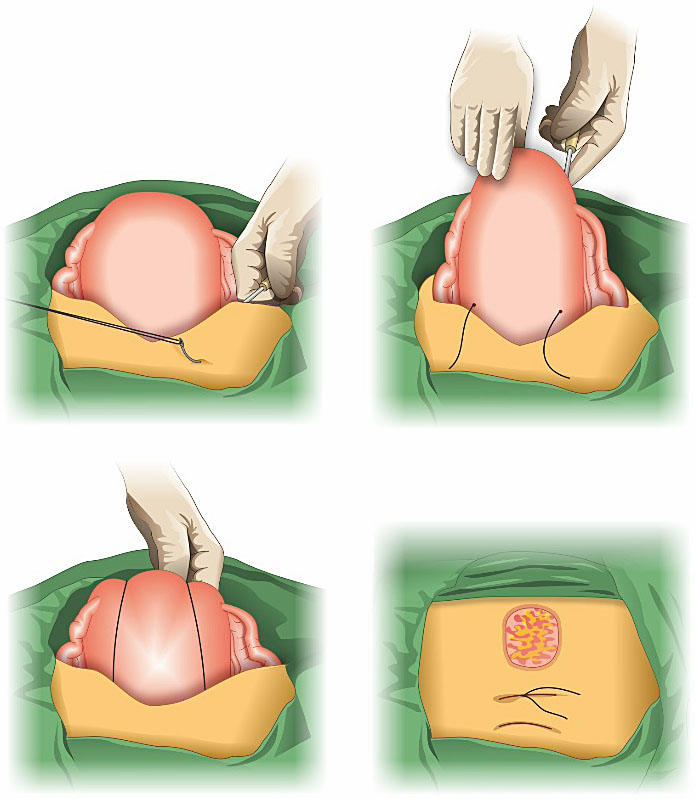
12
Steps of Aboulfalah removable technique.
Aboulfalah RUCS technique may reduce bleeding by two mechanisms: (1) compression of placental site by tight compression of uterine walls and (2) by obstruction of blood flow through uterine arteries by extreme forward flection of the uterus.
Zhang removal techniques
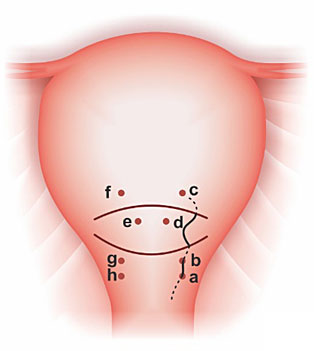
Zhang et al. (2015) described two RUCS techniques, a removable version of B-Lynch and Hayman sutures (Figure 13 and 14). They were applied in association with BUAL. A highlight of Zhang techniques is that its RUCS are removed vaginally (between 18 and 26 hours). The patients received antibiotics for 3–5 days. There were no complications related to RUCS.34
|
|
A | B |
13
Removable B-Lynch suture. (A) The letters a–h indicate the point at which the needle penetrates the uterine wall. The needle passes through the uterus in order from a to h. (B) Completed removable B-Lynch suture.34
|
|
A | B |
14
(A) Complete removable Hayman suture. (B) Demonstration on how to take out one of the stitches (a suction cannula is moved forward along the thread until the knot has been drawn into it), a pair of scissors is moved towards the knot along the inside wall of cannula and is used to cut it.34
PELVIC VASCULAR SUTURES
Pelvic vascular sutures (PVS) consist of selective ligatures of major pelvic vessels that nourish the pelvis, in order to reduce blood supply in a bleeding area. It usually does not treat the cause of PPH, instead of this, it reduces the perfusion pressure while definitive treatment is being performed. In some situations, it may be sufficient to control hemorrhage. PVS as well as UCS should be easily performed to quickly and safely control the bleeding since patient’s conditions may be aggravated by the delay in definitive treatment. To perform PVS, it is essential to know the origin, distribution and anastomosis of the genital arterial pedicles that are being ligated. The vessels most commonly addressed are the uterine arteries, the ovarian arteries, the round ligaments arteries and the internal iliac arteries.8,13,35 To guarantee the ligature efficacy, it is essential to monitor the persistence of bleeding during surgery. If bleeding persists, the healthcare provider should move to next step of PPH treatment (frequently hysterectomy).8,35
Efficacy
Ligature of major pelvic vessels seems to have similar efficacy to stop PPH when compared to UCS procedures and arterial embolization.12 In some series, the uterine artery ligation has showed a success rate of 80–96% for controlling uterine bleeding. The Internal iliac arteries efficacy, usually, varies from 42% to 93%.8,35 As occurs with UCS, all PVS procedures are highly dependent on the cause and the area of bleeding.11
Indications
The main indication for PVS are uterine bleeding, in which preservation of the uterus is desired; genital tract trauma when local measures fail to achieve hemostasis or are not feasible to control the bleeding; placental adhesive disorders, in which reducing blood loss is essential; and selected cases of coagulopathy, as an adjuvant procedure to reduce hemorrhage.8,12,13,35
Actually, any situation that results in devascularization of a bleeding area is essential to help to treat PPH, and it does not cause great harm or does not take too long to be performed, would indicate PVS. Uterine atony, after a vaginal delivery, should be treated initially with uterotonics and tamponade intrauterine balloon before laparotomy to perform PVS and/or compressive sutures. In cesarean section, PVS can be performed in earlier stage.
Complications
PVS complications are not very frequent. They usually are related to devascularization of non-target areas (that may complicate with necrosis, infertility and so on) and lesions of surrounding structures, such as nerves, vessels, ureters and bladder.8,35
Bilateral uterine artery ligature
The first cases of bilateral uterine artery ligature (BUAL) were published by Waters in 1952 and O’Leary in 1966.36 It is an easy, fast and safe procedure to stop bleeding located in sector 1 during a laparotomy. Uterine arteries are responsible for nearly 90% of irrigation of uterine body (S1), and so it may be a successful procedure for controlling bleeding in this area. However, it is not effective in bleeding from sector 2 (lower uterine segment, cervix and paracolpos). The main complication is ureteral injury, besides being rare, it is related to a technical mistake by placing the sutures too low. BUAL does not seem to interfere with menstrual function and the future reproductivity of the patient.36,37
A commonly technique consists of a suture 2–3 cm below and lateral to the segmental region of the hysterotomy (Figure 15). It is important to guarantee that bladder is away from the placement of the suture to avoid ureteric or bladder injury. The suture should encompass both the uterine artery and vein. It can be performed in association with compressive sutures or with other major pelvic vessels (such as ovarian artery).13,14
The ligature’s hemostatic effect will be observed right after the procedure. The uterus will become pale, with a pinkish hue, and bleeding will subside. The uterus may not become firm, but only less softened than before. The uterus will sometimes contract.36,37 Uterotonics (e.g. oxytocin) should be maintained in cases of uterine atony.
The BUAL optionally may be performed vaginally, through a 2-cm horizontal incision that is made in the anterior cervix, 1 cm beneath the vaginal cervical fold and the bladder reflected in the natural plane. After it, the uterine arteries are bilaterally accessed and ligated.38 The vaginal uterine artery ligation however may have more complications.
|
|
15
Distal uterine artery ligation.
Ligations of the ovarian arteries
The ovarian artery is usually performed when BUAL has failed to control the bleeding or in association with it. They are also useful for the treatment of bleeding from the uterine fundus and/or the upper portions of the uterine body. The ovarian artery has an anastomosis with the ascending branch of uterine artery, and corresponds to the other 10% of vascular irrigation of the uterine body. Its use for uterine bleeding from uterine sector 2 is not adequate. The ovarian arteries ligatures should be performed close to the level of the uterine-ovarian ligament (below or above it) (Figure 16). Its complications are usually related to accidental ureter ligature, lesions of surrounding structures or even ovarian failure.8,39,40
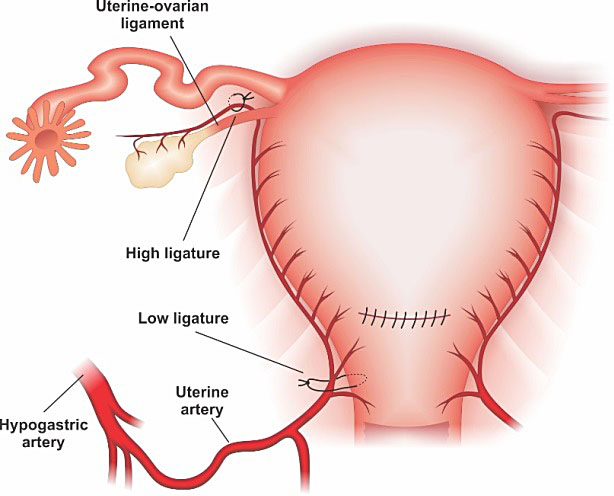
16
Utero-ovarian arteries ligatures above the ligament.
Tsirulnikov's triple ligation
Tsirulnikov's triple ligation includes bilateral ligatures of the ascending branches of the uterine arteries, the utero-ovarian connections and the arteries of the round ligament (which are branches of the utero-ovarian connections) (Figure 17).41
After ligation-division of the round ligament with its pedicle artery, and opening of the vesico-uterine peritoneum; the ascending branch of the uterine artery is ligated using the technique described by O’Leary. The utero-ovarian ligament is then ligated. The procedure is performed bilaterally.41
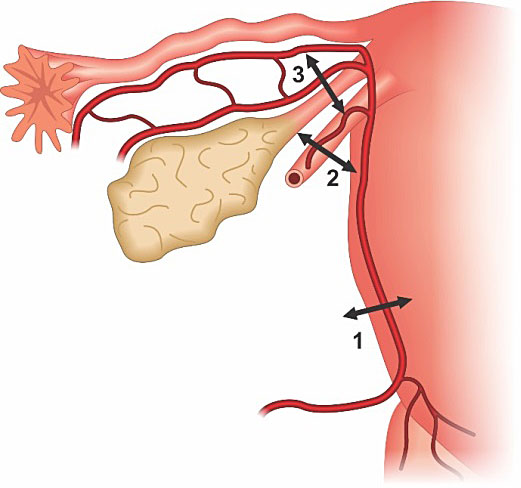
17
Tsirulnikov's triple ligation: 1. Uterine artery ligation, 2. Round ligament ligation, 3. and utero-ovarian ligation.
Stepwise sequential ligation
AbdRabbo's ligation advocates occlusion of the uterine arteries (including the cervico-vaginal pedicles) and ligation of the infundibulo-pelvic ligament (ovarian pedicle) (Figure 18). The procedure is performed in progressive steps and the sequence is defined by the persistence of bleeding after 10 minutes of the previous ligation.42 However, in severe PPH cases, it is not possible to wait such time, as patient may develop hypovolemic shock.
The initial step of stepwise ligature is bilateral distal ligation of uterine arteries. If bleeding persists, the second stage is a proximal ligation of the uterine arteries including the cervico-vaginal pedicles (it is essential to identify and protect the ureters). The final step described is bilateral ligation of the ovarian pedicle in the infundibulopelvic ligament.42
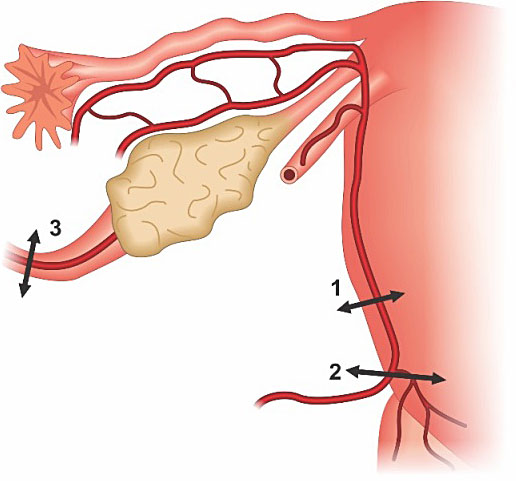
18
Stepwise sequential ligation: 1. Distal uterine artery ligation, 2. Proximal uterine artery ligation, 3. ligation of the ovarian pedicle in the infundibulopelvic ligament.
Morel’s step-by-step ligature
In the step-by-step technique described by Morel et al. consist of progressive sequence of vascular ligatures. The first step is to perform distal uterine artery ligation; the second step is round ligament ligation (rather than the infundibulopelvic ligaments, because of risks to future fertility); the third step is ligation of the utero-ovarian ligaments (rather than the infundibulopelvic ligaments, because of risks to future fertility), the fourth step is for controlling persistent bleeding is proximal uterine artery ligation, and finally the fifth and ultimate step is hypogastric artery ligation (Figure 19).8
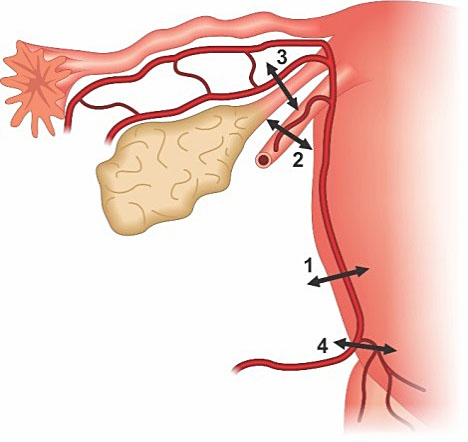
19
On the left: 1. Distal uterine artery ligation, 2. Round ligament ligation 3. and utero-ovarian ligation, 4. Proximal uterine artery ligation. On the right: internal iliac artery (hypogastric artery) ligation.
Ligations of the internal iliac arteries (hypogastric artery ligation)
Internal iliac artery ligation (IIAL) is indicated when there is an intractable hemorrhage, in an effort to reduce it. IIAL is more complex and so requires more surgical skills when compared to the other ligations. Due to a high level of anastomosis to the uterus and pelvis, some professionals do not use this technique frequently. Also IIAL needs more time to be performed, prevents further use of endovascular therapy (by closing the main access to pelvic branches) and is not more effective when compared to other conservative procedures.12,35
IIAL requires an abdominal approach. The uterus must be externalized and the broad ligament should be opened under the infundibulopelvic ligament. The bifurcation of the iliac trunk is identified and the hypogastric artery (internal iliac) is dissected over a distance of 3 cm, widely opening the vascular sheath to limit the risk of venous injury. After systematic identification of the ureter, a ligature is placed using a ligature passer about 2–3 cm below the bifurcation, taking care not to injure the vein (Figure 20). At the end of the procedure, we check the pulsations of the external iliac artery.8,14,43
The complications related to it can be infertility, buttock and thigh claudication, damage to the ureter, ischemic limb from damage to common or external iliac artery, damage to other pelvic vessels, damage to pelvic nerves, including the hypogastric plexus. The rate of complications may depend on the experience of the surgeon.8,43
|
|
20
Ligation of the right internal iliac artery (hypogastric ligation).
TECHNIQUES FOR TRANSITORY BLEEDING CONTROL DURING SURGERY
Internal aortic manual compression
Internal aortic manual compression can be used as a transitory vascular control during a laparotomy. It is simple, fast and efficient procedure to control bleeding. To perform aortic manual compression at laparotomy, it is necessary to move the uterus out of the pelvis, identify the sacral promontory and perform a manual compression on a laparotomy pads. It causes immediate and marked reduction in blood loss. Compression of infra-renal aorta reduce iliac internal and external flow and it can be safely maintained for 90 minutes, while a specialized team arrives or/and the anesthetist initiates adequate hemostatic resuscitation.44,45,46,47
Uterine tourniquet
Uterine tourniquet is traditionally used to control bleeding at myomectomy. But, in some critical situations after delivery it can be an option for transitory control of PPH. The technique may involve the placement of a Penrose drain or urinary catheter as low as possible around the lower uterine segment without incorporating the urinary bladder. It should be held tightly with a clamp. In settings lacking the facilities for definitive treatment, uterine tourniquets have been left in place for some hours during transfer to a higher-level facility. However, uterine tourniquet can cause uterine necrosis if placed for long periods.45
External uterine compression by elastic bandage (Esmarch bandage technique)
Esmarch Bandage is an elastic bandage that is usually used in orthopedic procedures. The use in obstetric bleeding was first described by Palacios-Jaraquemada e Fiorillo (2010), as an additional procedure to control hemorrhage, in an attempt to avoid hysterectomy in patients with PPH related coagulopathy.44
The Esmarch bandage should be positioned externally to the uterus (two wraps) from the fundus towards the cervix, so it can redirect blood flow out of the uterus (Figure 21). It determines such a relevant compression, that the uterine volume reduces by half. Cessation of bleeding is immediate and so hemostatic resuscitation can be successfully performed. The Esmarch bandage should not be used for longer than 6 hours, due to the risk of uterine necrosis.44
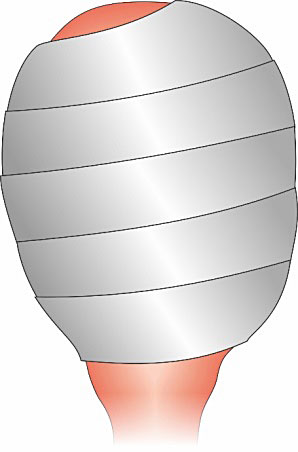
21
Esmarch Bandage uterine compression technique.
NON-CONSERVATIVE SURGICAL MANAGEMENT
Vasculature, anatomical landmarks of the pelvic cavity and modifications during pregnancy
Hormonal and hemodynamic changes, as well as the change in size and shape of the uterus during pregnancy will determine an increase in the caliber of arterial and venous vessels, which at the same time become more compliant. Other changes also take place in regards of distension, hypomotility and a discreet reorientation of hollow viscera, such as the ureter or appendix. These usually serve as anatomical landmarks for pelvic surgery.48 In certain circumstances, neovascularization or revascularization of pelvic territories may be present and can be a cause of bleeding.49
During emergencies, such as severe PPH scenario, surgical evaluation of the abdominal cavity may require a wide incision. This will allow initial recognition of the situation, quick identification of specific lesions, and fast approach to vascular trunks. This technique also will provide a rapid view of structures and anatomical landmarks that may accidentally be injured during emergency approach.49
Often, the uterus will need exteriorization. This maneuver allows the visualization of key structures such as the vesico-uterine pouch, round ligament, fallopian tubes, ovaries and uterosacral ligaments. Sectioning the round ligament and opening the leaflets of the broad ligament allow the approach and visualization of important anatomical landmarks, such as the hypogastric arteries and the point of origin of the uterine arteries from the hypogastric trunk, among others (Figure 22). In cases in which the anatomy of the inferior segment is distorted (e.g. cases of placenta acreta in any degree of invasion), this can add difficulties to the dissection (Figure 23).
Traction and displacement of the uterine fundus to the pubis can be performed. This maneuver allows identification of utero-sacral and pelvic infundibulum ligaments, and the access (through palpation or visualization) to anatomical structures such as the infra-mesenteric aorta and its bifurcation to common iliac arteries. Following their course up to their divisions into a branch that follows an anterior and external course, the external iliac artery, and a branch that follows a posterior and medial course, the hypogastric artery. One anatomical landmark of great importance is the visualization of the ureter crossing over the common iliac artery, 1–2 centimeters above the common iliac artery bifurcation. It is more dilated, crosses and then turns following a medial direction to the pelvic excavation. It is to be noted that to achieve direct visualization of these structures, we have to make the approach by sectioning the parietal peritoneum by the external margin of the pelvic infundibulum, which also allows visualization of the psoas muscle and homonymous veins (Figure 24).
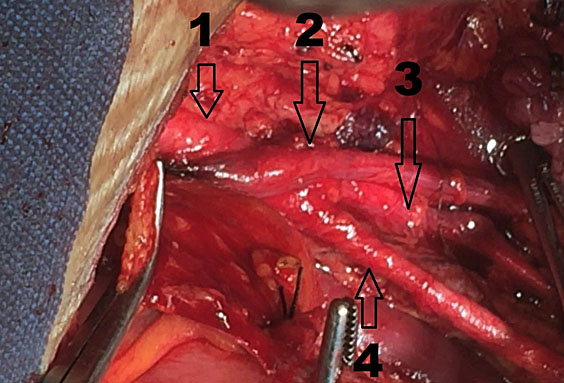
22
Dissection of pelvic vessels – Arrow 1. common iliac artery. Arrow 2. ureter crossing over the common iliac artery. Arrow 3. Internal iliac artery. (Surgeon Jorge Hoghel MD, photograph by Rafael Cortes Charry MD. Oncologic Hospital Padre Machado).
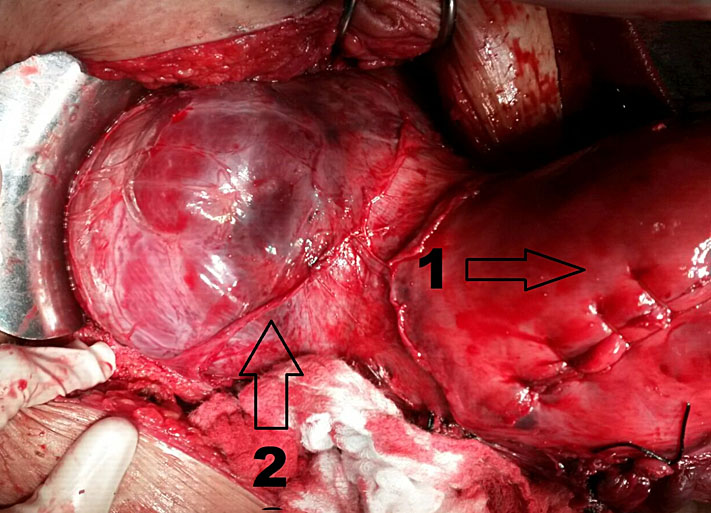
23
Cesarean hysterectomy due to placenta accreta. Arrow 1. uterine corporal incision (hysterorraphy). Arrow 2. Placenta accreta: abnormal insertion of placenta compromising uterine lower segment and vesico-uterine space (FIGO grade IIB).
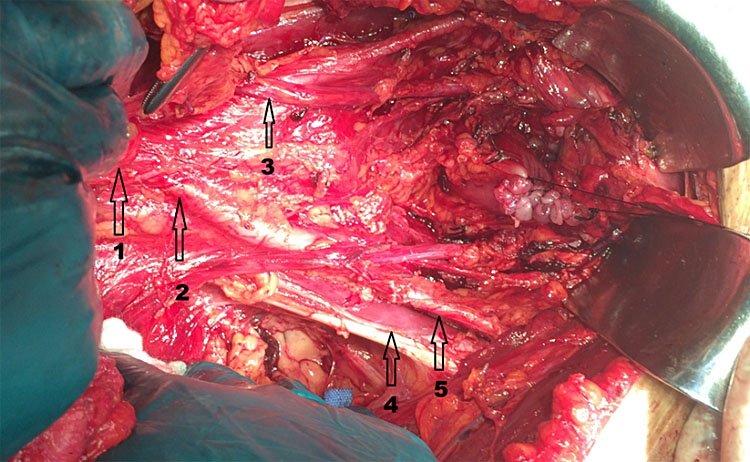
24
Anatomic landmarks of pelvic vasculature. Arrow 1. supra-iliac aorta. Arrow 2. common Iliac artery. Arrow 3. ureter crossing above Iliac artery. Arrow 4. psoas muscle. Arrow 5. external iliac artery. Surgeon: Jorge Hoghel MD. Photography by Rafael Cortes Charry MD. Oncologic Hospital Padre Machado.
Hysterectomy
Postpartum hysterectomy constitutes the final step to control PPH, as it can result in an additional blood loss of more than 2 liters.50 However, it can be a lifesaving procedure when it is indicated and so it should not be postponed.12,51
Hysterectomy will take place when all conservative measures (pharmacological, mechanical or other more conservative surgical strategies) have failed (or are not indicated) to achieve bleeding control in the setting of life threatening hemorrhage.9,51
Emergency postpartum hysterectomy has an incidence between 0.24 and 0.78 per 1000 deliveries52,53 and its most common indications are placenta accreta, uterine atony and uterine rupture.51,54
The effectiveness of hysterectomy on controlling PPH can be determined by a number of factors including PPH cause and intensity; previous use of blood products; presence of coagulopathy, and surgeon’s ability to perform it; moment of execution (if it is an elective or emergency procedure); and the place it is being performed (e.g. at a referral center or not).12,14,51,55
Technique
Wide lincision is essential to allow abdominal cavity evaluation and access during postpartum hysterectomy, Midline abdominal incisions can be accomplished or an already performed transversal incision can be enlarged. Once inside the abdominal cavity, the technique for hysterectomy can present variants related to findings, such as dissecting hematomas, previous selective or supra-selective vascular ligatures, hemostatic sutures, or placenta acreta spectrum disorders. If these variants are not present, it is recommended to follow, systematically, the usual steps of a non-obstetric hysterectomy.
The surgeon should be familiar with postpartum hysterectomy as well as with anatomic landmarks. First, the round ligament is clamped, sectioned and ligated on each side. During this step, the anterior and posterior leaflets of the broad ligament should be incised: the first one following an arciform trajectory, above and parallel to the vesico-uterine peritoneal pouch; and the second leaflet parallel and externally to the axis of the tubo-ovarian pedicle and pelvic infundibulum. We can also take advantage of this step and make a delicate digital disclosure of the soft tissue towards pelvic excavation, under the sectioned pedicle of the round ligament. The aim of this maneuver is to have direct visualization of the ureter, common iliac artery and its bifurcation, psoas muscle and some important anatomical landmarks.
Oophorectomy must be avoided, considering the fact that a hysterectomy already has a great impact on patient’s emotional sphere. The removal of the ovaries also determines a hormonal impact with all of its associated morbidities.
We then clamp, section and cut the pedicle that include the fallopian tube and the utero-ovarian ligament at once (as a block) in order to reduce surgical time. This represents a difference to an elective hysterectomy, in which bilateral salpingectomy is performed to prevent epithelial ovarian cancer.56 The following steps on this surgery are typically the ones described for a non-obstetric hysterectomy.
The advantages of performing a sub-total versus total hysterectomy are not clear. Those who defend the subtotal technique sustain the arguments that: surgical time is shortened; this procedure has less probability of ureteric or vesical lesions; it requires less surgical abilities; and it is better to preserve the sustaining elements of the pelvic floor. The authors who support total hysterectomy manifest that there may be some remaining vessels or tissue on the cervical stump which are responsible for bleeding; and that by performing a total hysterectomy, in countries with high prevalence of cervical cancer, it would be a measure to prevent these types of neoplasia.35,57
Variants on the surgical technique exist in cases of placenta acreta spectrum (PAS). FIGO’s 2018 consensus suggests that ideally the diagnosis of this condition should be made during pregnancy. If a patient has an antenatal diagnosis of PAS, she should be referred to a center of excellence, with an interdisciplinary team that has enough experience on the management of PAS.58
Depending on the suspected PAS invasion, hysterectomy can be preceded by ureteric endoscopy for catheterization of the ureter with double J stent; radiological selective arterial catheterization (whether of uterine, hypogastric, iliac arteries or aorta), for placement of intra-arterial occlusion balloons. Alternatively, there should be a thorough and selective vascular dissection of the aforementioned vascular beds and ureters before hysterectomy. All these measures tend to minimize incidental lesions of neighboring structures and the bleeding generated by placental infiltration (which also determines uterine and nearby organ hyper or neovascularization).49,58
In cases in which there is intraoperative bleeding during the course of the hysterectomy, mechanical compression techniques have been suggested, such as internal aortic manual compression, aortic loop, and selective use of tourniquets, among others.45,59
Team coordination must exist during surgery among anesthesiologists, hemodynamic specialists, hematotherapist. This will allow the team to make assertive surgical decisions, which can decrease maternal morbidity and mortality. The immediate postsurgical period must be strictly monitored, ideally in an intensive care unit. Likewise, it is important to maintain efficacious communication with family members throughout all stages of treatment.
Damage control surgery
To speak about damage control surgery (DCS) is to think back on the history of World War II. Damage control is a Navy term defined as “the capacity of a ship to absorb damage and maintain mission integrity”. In these situations, some specific repairs were made in order to absorb the damage, preserving a level of basic functioning that allowed them to complete their mission.60
Regarding DCS, the fundament is to perform a rapid and effective surgery that minimizes the patient's risk of imminent death, so that in the post-surgical time, medical interventions are performed to improve well-being and compensating the impact on organs and systems that were affected by shock. Once this objective is achieved, the patient can be carried on to a second surgical time performed in better conditions to a definitive surgical organ repair.60,61,62
During the 80s and 90s decades, there was a tendency of performing surgical correction of the cause of bleeding and immediate repair of damage caused on organs due to multiple trauma. Attempting to provide a single definitive procedure in these patients led them to ongoing bleeding from coagulopathy, an unresuscitatable shock state, or multiple organ failure. In the end, the objective of reducing patient’s mortality and morbidity would not be accomplished.63 Favorable results observed on patients with hepatic trauma, using packing for local compression, and total abdominal packing before a surgery for definitive correction.64,65 This management was adopted in cases of patients with severe PPH who did not respond to any medical or surgical measure.61,62
In some patients with severe bleeding surgical control cannot be achieved. So temporizing measures aimed at slowing the bleeding are better than continuing futile attempts in the operating room that further exacerbate the reasons for the refractory bleeding.61 This surgery is performed along with other supporting measures with the aim to avoid or to treat the lethal triad of hypovolemic shock, that consists of hypothermia, coagulopathy and acidosis.61
A DCS can be indicated during a surgery for controlling PPH when there is a: (a) venous bleeding not amenable to surgical control; (b) persistent bleeding despite transfusion of large amounts of blood products (greater than 10 units packed red cells); (c) persistent and escalating fluid requirements in the setting of active non arterial bleeding; (d) Hemodynamic instability or development of ventricular arrhythmias; (e) coagulopathy resulting from a combination of hypothermia (temperature less than 35˚C), acidosis (pH less than 7.3), and loss of clotting factors.61
Deffieux et al. (2017) found that DCS was performed in 1 of every 14 patients with obstetric hysterectomies, with a 24% mortality rate.66
Damage control surgery is not an isolated procedure but forms one of the links in a chain of actions that tend to counter the impact of shock, and which is divided into four stages (Table 3).62
3
Stages of damage control surgery.
STAGE 0 (Reanimation) | Initial reanimation: the objective at this stage is to optimize hemodynamic parameters. Into this stage all measures of vital support must be established in a coordinated way while DCS is performed. Initial reanimation; blood transfusion; correction of acidosis, hypothermia, and coagulopathy must have started. |
STAGE 1 (Initial management) | Abdominal packing: this stage is represented by the damage control surgery itself, where it begins with the hysterectomy, if not previously performed, followed by packing of the abdominal cavity and finally temporal closure of the abdominal cavity. |
STAGE 2 (Resuscitation) | Intensive unit care: at this moment the patient must be sent to an intensive care unit in order to enable rapid correction of metabolic and specific organ dysfunction or failure. |
STAGE 3 (Definitive care) | Definitive surgery: packing removal is performed, and then definitive surgical repair and final closure of the abdominal cavity are accomplished. |
Surgical technique of DCS
There are many techniques to perform packing in a way that is effective. One technique is described that may be applied in every setting and with a lesser learning curve.
First, a wide abdominal midline incision (xifo-pubic) is made, regardless of any previous incision. If it was not previously done, we perform hysterectomy. Normally, we would expect to find a layer of bleeding on all tissues, sites of venepuncture and insertion of vascular catheters (all factors that relate to consumption coagulopathy). Then, patient must be placed in Trendelemburg position to facilitate manual displacement of viscera towards the superior abdomen, so isolating them from the rest of the cavity. The viscera will “be packed” with predesigned perforated plastic bags that allow its total containment. If this is not available, we can create an artisanal perforated plastic field which accomplishes the same function. The objective is to avoid adherence of compresses to visceral serosa, and the perforations allow for fluid and blood drainage toward the inferior abdomen.
Another perforated plastic field is then placed over the abdominopelvic surface that is to be packed. This will isolate all exposed and bleeding portions of the parietal peritoneum, avoid the adherence of compresses and allow fluid and blood drainage, without losing the compressive function. Compresses must be folded in half twice to make a square, and then be placed in way it covers and compresses every abdominal and pelvic bleeding surface. To achieve the compressive effect, as many compresses may be used as required, and then complemented by the great pressure exerted by one plane closure of the abdominal cavity. This will remain partially open due to the separation caused by the compresses that fill it and do not allow for full union of the edges.
Excessive pressure or complete closure of the abdominal wall will increase the risk of developing abdominal compartment syndrome. It is mandatory to leave in place, draining systems with perforated intra-abdominal catheters connected to low negative pressure devices that allow the evaluation of how effective the surgery was, depending on hourly drainage. Then, the patient in this condition will be sent to the intensive care unit. After 48–72 hours have passed, we then proceed with damage repair in specific organs, and if it is the case, the final closure of the abdominal cavity.61 Finally, providers must follow up the patient closely, not only to determine any long-term sequelae, but also to provide emotional support.61.66
PRACTICE RECOMMENDATIONS
- Surgical approaches should be directed towards the cause of PPH, as early control of the bleeding site is the most effective measure to avoid hypovolemic shock.
- Conservative surgical management in PPH is used to avoid hysterectomy and its morbidity as well as to preserve patient’s fertility. The most used conservative surgical procedures are uterine compressive sutures (UCS) and ligation of pelvic vessels (LPV).
- UCS and LPV can offer an easy, quick, safe, and effective control of bleeding at a laparotomy, especially in locations with few technical resources. UCS also can be performed in association to LPV or intrauterine tamponade balloon.
- Understanding and recognizing the origin, distribution and anastomosis of the genital arterial pedicles are essential skills to choose the most appropriate hemostatic suture. Also, simplicity, as well as professional familiarity with the technique will influence the choice and the efficacy of the procedure.
- The primary indication for UCS is a hemorrhage resulting from uterine atony that does not respond to uterotonics after a cesarean section; or does not respond to pharmacological treatment and other nonsurgical management (such as intrauterine tamponade balloon and non-pneumatic anti-shock garment) in vaginal delivery.
- Manual compression of the infra-renal aorta during a laparotomy can be performed in cases of postpartum intractable hemorrhage. It can be safely maintained for 90 minutes, while a specialized team arrives.
- Postpartum hysterectomy is usually indicated when all conservative measures have failed (or are not indicated) to achieve bleeding control. However, when it is indicated, it should be performed as early as possible, to avoid the installation of coagulopathy.
- If a patient has an antenatal diagnosis of PAS, she should be referred to a center of excellence, with an interdisciplinary team that have sufficient experience on the management of PAS. When performing hysterectomy due to placenta accreta, the placenta should be left in situ, as the manipulation of it considerably increases patient blood loss.
- If there is a suspect of coagulopathy after hysterectomy, damage control surgery should be considered in order to reduce surgical time and to allow volume restoration, correction of coagulation disorders and treatment of organ dysfunctions.
- Training obstetric skills that involves practice on how to perform surgical procedures should be encouraged as it can enhance the surgeon’s performance and improve patient's outcome and safety.
ACKNOWLEDGMENTS
All the illustrations in this chapter are original but some of them have been developed by reference to illustrative material from the pioneering work of Professor B-Lynch whose surgical initiatives have made such an important contribution to this field.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: WHO; 2012. Access: https://apps.who.int/iris/bitstream/handle/10665/75411/9789241548502_eng.pdf;jsessionid=4464904EB17B692D2D2F46B0E2BA82E3?sequence=1. | |
GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1775–812. Doi: 10.1016/S0140–6736(16)31460-X. | |
Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: A systematic review. Lancet 2006;367(9516):1066–74. | |
Osanan GC, Padilla H, Reis MI, et al. Strategy for Zero Maternal Deaths by Hemorrhage in Brazil: A Multidisciplinary Initiative to Combat Maternal Morbimortality. Rev Bras Ginecol Obstet 2018;40(3):103–5. | |
Claroni C, Aversano M, Todde C, et al. Postpartum hemorrhage management: the importance of timing. CMI 2018;12(1):11–5. https://doi.org/10.7175/cmi.v12i1.1326. | |
Mavrides E, Allard S, Chandraharan E, et al., on behalf of The Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum haemorrhage. BJOG 2016;124:e106 e149. | |
B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol 1997;104:372–5. | |
Morel O, Malartic CJ, Muhlstein JE, et al. Pelvic arterial ligations for severe post-partum hemorrhage. Indications and techniques. J Visc Surg 2011;148:95–102. | |
Cetin BA, Mathyk BA, Aydin AA, et al. Comparing success rates of the Hayman compression suture and the Bakri balloon tamponade. J Matern Fetal Neonatal Med 2019;32(18):3034–8. DOI: 10.1080/14767058.2018.1455184. | |
Escobar MF, Suso JP, Hincapie MA, et al. Experience of combined use of a Bakri uterine balloon and non-pneumatic antishock garment in a university hospital in Colombia. Int J Gynaecol Obstet 2019. Doi: 10.1002/ijgo.12872 [Epub ahead of print]. | |
Palacios-Jaraquemada JM. Efficacy of surgical techniques to control obstetric hemorrhage: analysis of 539 cases. Acta Obstet Gynecol Scan 2011;90:1036–42. | |
Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv 2007;62(8):540–7. | |
Kayem G, Kurinczuk JJ, Alfirevic Z, et al. Uterine compression sutures for the management of severe postpartum hemorrhage. Gynecol 2011;117:14–20 | |
Porreco PR, Stettler RW. Surgical Remedies for Postpartum Hemorrhage. Clin Obstet Gynecol 53(1):182–95. Doi: 10.1097/GRF.0b013e3181cc4139. | |
Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety and efficacy. Arch Obstet 2015;292:949–52. | |
Kaya B, Tuten A, Daglar K, et al. B-Lynch uterine compression sutures in the conservative surgical management of uterine atony. Gynecol Obstet 2015;291:1005–14. | |
Fotopoulou C, Dudennhausen JW. Uterine compression sutures for preserving fertility in severe postpartum haemorrhage: an overview 13 years after the first description. Obstet Gynaecol 2010;30(4):339–49. | |
Cho JH, Jun HS, Lee CN. Hemostatic suturing technique for uterine bleeding during cesarean delivery. Obstet Gynecol 2000;96(1):129–31. | |
Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol 2002;99(3):502–6. | |
Pereira A, Nunes F, Pedroso S, et al. Compressive uterine sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol 2005;106:569–72. | |
Matsubara S. Uterine compression suture may be useful not only for hemostasis in postpartum hemorrhage but also for prophylaxis of acute recurrence of uterine inversion. Arch Gynecol Obstet 2010;281:1081–2. | |
Matsubara S, Yano H, Ohkuchi A, et al. Uterine compression sutures for postpartum hemorrhage: an overview. Acta Obstet Gynecol Scand 2013;92:378–85. | |
Keriakos R, Chaudhuri S. Operative interventions in the management of major postpartum haemorrhage. Obstet Gynaecol 2012;32:14–25. | |
Sathe NA, Likis FE, Young JL, et al. Procedures and Uterine-Sparing Surgeries for Managing Postpartum Hemorrhage: A Systematic Review. Obstet Gynecol Surv 2016;71(2):99–113. Doi: 10.1097/OGX.000000000000027 | |
Kim TH, Lee HH, Kim JM, et al. Uterine compression sutures theory. Gynecol Obstet 2015;292:947–8. | |
Matsubara S. A new compression suture to prevent ‘uterine sandwich’ from sliding off. Acta Obstet Gynecol Scand 2012;91:638–9. | |
Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. N Z J Obstet Gynaecol 2014;54(3):283–6. | |
Dedes A, Ziogas V. Circular isthmic-cervical sutures can be an alternative method to control peripartum haemorraghe during caesarean section for placenta praevia accreta. Ginecol Obstet 2008;278:555–7. | |
Shazly SA, Badee AY, Ali MK. The use of multiple 8 compression suturing as a novel procedure to preserve fertility in patients with placenta accreta: case series. N Z J Obstet Gynaecol 2012;52(4):395–9. | |
Hwu YM, et al. Parallel vertical compression sutures: a technique to control bleeding from placenta praevia or accreta during caesarean section. J Obstet Gynaecol 2005;112:1420–3. | |
Li GT, et al. Reflexed compression suture for the management of atonic postpartum hemorrhage with an abnormally adherent placenta. Obstet Invest 2015;80(4):228–33. | |
Zheng J, et al. A new uterine compression suture for postpartum haemorrhage with atony. J Obstet Gynaecol 2011;118:370–4. | |
Aboulfalah A, et al. A new removable uterine compression by a brace suture in the management of severe postpartum hemorrhage. Surg 2014;1(43):1–4. | |
Zhang ZW, et al. Removable uterine compression sutures for postpartum haemorrhage. BJOG 2015;122:429–33. | |
Rath W, Hackethal A, Bohlmann MK. Second-line treatment of postpartum haemorrhage (PPH). Arch Gynecol Obstet 2012;286:549–61. DOI 10.1007/s00404–012–2329-z | |
O’Leary JL, O’Leary JA. Uterine artery ligation in the control of intractable postpartum hemorrhage. J Obstet Gynecol 1966;94(7):920–4. | |
O’Leary JL, O’Leary JA. Uterine artery ligation for control of post cesarean section hemorrhage. Gynecol 1974;43(6):849–53. | |
Hebisch G, Huch A. Vaginal uterine artery ligation avoids high blood loss and puerperal hysterectomy in postpartum hemorrhage. Gynecol 2002;100:574–8. | |
Moise Jr KJ, Belfort MA. Damage control for the obstetric patient. Clin North Am 1997;77(4):835–52. | |
Roman H, Sentilhes L, Cingotti M, et al. Uterine devascularization and subsequent major intrauterine synechiae and ovarian failure. Fertil Steril 2005;83(3):755–7. | |
Tsirulnikov MS. Ligation of the uterine vessels during obstetrical hemorrhages. Immediate and long-term results. Gynecol Obstet Biol Reprod 1979;8:751–3. | |
Abdrabbo SA. Stepwise uterine devascularization: a novel technique for management of uncontrolled postpartum hemorrhage with preservation of the uterus. J Obstet Gynecol 1994;171:694–700. | |
Sziller I, Hupuczi P, Papp Z. Hypogastric artery ligation for severe hemorrhage in obstetric patients. J Perinat Med 2007;35(3):187–92. | |
Palacios-Jaraquemada JM, Fiorillo A. Conservative approach in heavy postpartum hemorrhage associated with coagulopathy. Acta Obstet Gynecol Scand 2010;89(9):1222–5. | |
Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Practice & Research Clinical Obstetrics and Gynaecology 2016;36:68–82. | |
Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage – an old but lifesaving technique. Aust NZ Gynaecol 1997;37:237–8. | |
Harris LJ. A New Instrument for Control of Hemorrhage by Aortic Compression: A Preliminary Report. Med Ass 1964;91:128–30. | |
Langdon P, Howard U. An atlas of pelvic operations. Editors by W.B. Saunders Company. Philadelphia USA. Edit by ELICIEN 1970 | |
Palácios-Jaraquemada JM, Mónaco RG, Barbosa N, et al. Lower uterine blood supply: extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstetricia et Gynecologica 2007;86:228234 | |
Henrich W, Surbeck D, Kainer F, et al. Diagnosis and treatment of peripartum bleeding. J Perinat Med 2008;36:467–78. | |
Zhang Y, Yan J, Han Q, et al. Emergency obstetric hysterectomy for life threatening postpartum hemorrhage: A 12-year review. Medicine (Baltimore) 2017;96(45):e8443. Doi: 10.1097/MD.0000000000008443. | |
Imudia AN, Awonuga AO, Dbouk T, et al. Incidence, trends, risk factors, indications for, and complications associated with cesarean hysterectomy: a 17-year experience from a single institution. Arch Gynecol Obstet 2009;280:619–23. | |
Demirci O, Tugrul AS, Yilmaz E, et al. Emergency peripartum hysterectomy in a tertiary obstetric center: nine years’ evaluation. J Obstet Gynaecol Res 2011;37:1054–60. | |
Machado LSM. Emergency peripartum hysterectomy: Incidence, indications, risk factors and outcome. N Am J Med Sci 2011;3(8):358–61. Doi: 10.4297/najms.2011.358 | |
Huls CK, Belfort MA, Wright JD. Center of excellence for placenta acreta. Am J Obstet Gynecol 2015;212(5):561–8. | |
Kim M, Kim YH, Kim YB, et al. Bilateral salpingectomy to reduce the risk of ovarian/fallopian/peritoneal cancer in women at average risk: a position statement of the Korean Society of Obstetrics and Gynecology (KSOG). Obstet Gynecol Sci 2018;6(5):542–52. | |
Vanessa L. Jacoby MD, MAS. Hysterectomy Controversies: Ovarian and Cervical Preservation. Clin Obstet Gynecol 2014;57(1):95–105. | |
Janiaux E, Bhide A, Kennedy A, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int J Gynecol Obstet 2018;140:274–80. | |
Long M, Cheng C, Xia A, et al. Temporary loop ligation of the abdominal aorta during cesarean hysterectomy for reducing blood loss in placenta accrete. Taiwan J Obstet Gynecol 2015;54:323e325. | |
Ball CG. Damage control resuscitation: history, theory and technique. Can J Surg 2014;57(1):55–60. | |
Pacheco DL, Lozada MJ, Saade GR, et al. Damage-Control Surgery for Obstetric Hemorrhage. Obstet Gynecol 2018;132:423–7. | |
Escobar MF, Carvajal JA, Burgos JM, et al. Damage Control surgery for the Management of Major Obstetric Hemorrhage: Experience from the Fundacion Valle del Lili, Cali, Colombia. Pan-American Journal of Trauma, Critical Care and Emergency Surgery 2017;6(1):1–7. | |
Waibel BH, Rotondo MM. Damage control surgery: it's evolution over the last 20 years. Rev Col Bras Cir 2012;39(4):314–21. | |
Pringle JH. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg 1908;48(4):541–9. | |
Calne RY, Mcmaster P, Pentlow BD. The treatment of major liver trauma by primary packing with transfer of the patient for definitive treatment. Br J Surg 1979;6(5):338–9. | |
Deffieux X, Vinchant M, Wigniolle I, et al. Maternal outcome after abdominal packing for uncontrolled postpartum hemorrhage despite peripartum hysterectomy. Plos One 2017;12(6). |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)