This chapter should be cited as follows:
Escobar MF, Zambrano MA, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.413083
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 13
Obstetric emergencies
Volume Editor: Dr María Fernanda Escobar Vidarte, Fundación Valle del Lili, Cali, Colombia

Chapter
Non-surgical Approaches to Refractory PPH
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
NON-SURGICAL APPROACHES TO REFRACTORY PPH
In the management of PPH refractory to first-line treatment with uterotonics and tranexamic acid (TXA), mechanical methods are a useful and effective option for hemostasis.1 In low-resource settings, these devices, which may be the sole therapeutic options, can contribute to improved outcomes, including survival prior to definite management.2 This chapter reviews the available mechanical methods, indications for use, instructions of use, and recommendations.
The non-surgical life-saving, mainly temporizing, measures include:
- Temporizing measures prior to definitive management:
- Bimanual uterine compression;
- Aortic compression;
- Non-pneumatic antishock garment (NASG).
- Temporizing measure, that can also be definitive treatment:
- Intrauterine balloon tamponade.
The choice of method will depend on the etiology and severity of the hemorrhage; the condition of the woman; what human resources are available and their training; and what supplies and equipment are available. As with all cases of PPH, the first step is evaluating the source/etiology of the hemorrhage.
In addition to these specific measures, there are best practices for management of hemorrhage that are required of healthcare facilities and individual providers as outlined in the other chapters and in various WHO guidelines and manuals on PPH.3 As with all procedures, it is important to communicate calmly and clearly with the woman and her family about what is happening and what you, the provider, are doing to manage the situation and provide best care.
BIMANUAL UTERINE COMPRESSION
Bimanual uterine compression involves one hand internally putting upward pressure on the uterus, while the external hand puts pressure on the fundus through the abdomen. After ensuring proper antiseptic preparation, the operator should introduce her gloved hand into the vagina, form a fist, and press upward on the lower uterus whilst pressing the uterine fundus with the external hand in the direction of the symphysis pubis. The operator exerts pressure with both hands in order to compress the uterus between the two hands and stop the hemorrhage (Figure 1). The uterus should be kept compressed until medical support arrives or during transport to the operating theater or to a higher-level facility (WHO 2012 PPH Recommendations classify this as a “weak recommendation, very low-quality evidence”).4,5 Occasionally bimanual compression alone will stop the hemorrhage and no other hemostatic measures will be necessary. If the woman has gone into shock from the blood loss, other measures, such as blood transfusions may be required.
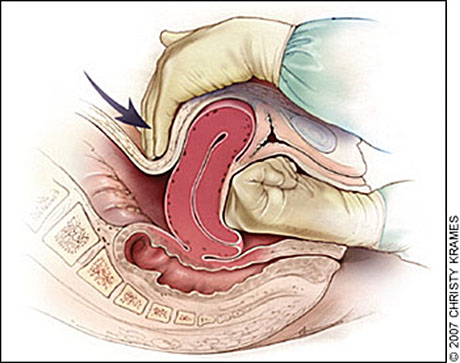
1
Bimanual uterine compression. Redrawn with permission from Anderson J, Etches D, Smith D. Postpartum hemorrhage. In: Baxley E. Advanced Life Support in Obstetrics course syllabus. 4th edn. Leawood, KS: American Academy of Family Physicians, 2001.6
AORTIC COMPRESSION
Aortic compression can be performed by trained, qualified health professionals. The concept is to maintain blood flow in the superior portion of the body, while preventing blood flow in the inferior portion; thus keeping the vital organs perfused.4
The operator stands to the right of the patient and places the left fist on the superior left side of the patient’s umbilicus, while simultaneously palpating for femoral pulses with the right hand. During this process the operator must feel the pulse before applying pressure to the abdominal aorta. Once the femoral pulse has been found, downward pressure must be applied by leaning on the patient, using body weight to increase pressure on the aorta (Figure 2). Absence of femoral pulse confirms adequate compression.7
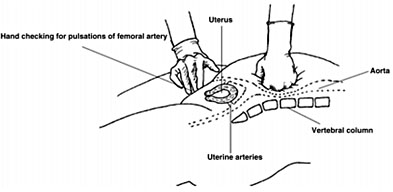
2
Aortic compression. From FIGO Safe Motherhood and Newborn Health (SMNH) Committee. 2018.8
Both aortic and bimanual compression are older procedures9 that can be performed by anyone who has been trained and knows how to palpate the uterus for location and tone, for aortic compression, and how to palpate for femoral pulses. The only supplies necessary are sterile gloves for bimanual compression. Both procedures have been recommended by the WHO in 2012 (“weak recommendation, very low quality evidence”).5 One limitation to these procedures is that they require the operators to involve both their hands, which requires another operator to perform any other procedure(s); in low-resource or low-volume settings there may only be one provider. Also, these compressive techniques are uncomfortable for the patient and tiring for the provider. Further, it may be difficult to sustain adequate pressure over long periods of time (such as delays in transport). An observational study in Benin, Rwanda, Jamaica, and Ecuador10 found that only 22% of skilled birth attendants were able to perform bimanual uterine compression correctly. One study, conducted in Ghana with a uterine simulator that measured strength of compression, found that a team of two people, one putting pressure on the uterus through the vagina and the other using both hands to compress the uterus through the abdomen were able to maintain adequate compression for up to 5 minutes, while those conducting bimanual compression as a single provider could maintain adequate pressure for only 2 minutes.11
NON-PNEUMATIC ANTISHOCK GARMENT (NASG)
The non-pneumatic antishock garment (NASG) is considered a low-technology first-aid measure. Its purpose is to increase the survival in women with hypovolemic shock secondary to postpartum hemorrhage (PPH), extending the available time frame in order to perform definitive surgical interventions, blood transfusion, or transfer to more specialized centers.12
Non-pneumatic antishock garment history
The use of the pneumatic anti-shock garment began in 1903 when Dr George Crile developed the first compression garment for use during surgery to increase blood pressure. This device increased systemic vascular resistance and arterial pressure, while lowering blood loss.13
Around 1942, the United States Army Air Corps introduced the G-suit, an anti-gravity suit that helped to avoid syncope while pilots performed rapid ascent. During the Vietnam war, it was modified from a full-body suit to a half-suit, called the Military/Medical Anti-Shock Trousers® (MAST) or pneumatic antishock garment (PASG), and was used for resuscitation and stabilization of soldiers injured in combat.2 This pneumatic device achieved compression by inflating a series of air bladders, this resulted in over-inflation, leaking and loss of air, which led to underinflation; the device was also complicated to use.2
Later, between 1970 and 1980 the Pneumatic Antishock Garment was introduced into emergency medical practice for the pre-hospital care of trauma patients in shock. However, its effectiveness and safety standards were questioned. From 1990 onwards several side-effects such as diminished urinary output, hypoxia, respiratory distress, and hyperinflation-related complications, such as ischemia and compartment syndrome, were reported.14,15,16,17 Further, several of the case series and clinical studies conducted demonstrated that there were no statistically significant differences regarding morbidity and mortality between use and non-use of PASG.18,19,20,21 Around the early 2000s, the Cochrane Collaboration published a systematic review, which reported that there was insufficient evidence to justify the use of PASG for pre-hospital emergency trauma care, given the side-effects, and a possible increased rate of mortality.22
In 1971, due to the negative or lack of improved outcomes related to hyperinflation, the National Aeronautics and Space Administration (NASA), under the supervision of Ralph Pelligra, worked to develop a Non Pneumatic Antishock Garment (NASG). In 1991 the NASG was marketed as the Non-Inflatable Anti-Shock Garment™ by the Zoex Corporation. The device received FDA approval for medical use.
The NASG has several advantages over the PASG such as:2
- Lower risk of ischemia, decreased urine output, hypoxia, respiratory distress and other outcomes related to hyperinflation;
- Lower production costs and market prices;
- No valve or pressure systems are required, therefore, it is much easier to train and use.
Non-pneumatic antishock garment design
NASG is a lightweight and relatively inexpensive suit, made from neoprene and Velcro™, composed of articulated segments which fit in the lower body: three segments for each lower extremity (ankles, calves, and thighs), one pelvic segment and two abdominal segments. The latter includes a compression ball that is placed over the uterus (Figure 3). The NASG applies approximately 20–40 mmHg of circumferential pressure.2 This compression over the abdomen diminishes blood flow to inferior portions of the body, including the pelvis, which results in decreased uterine bleeding. At the same time, the NASG increases venous return, increasing cardiac output, which results in improved upper body tissue perfusion. All these mechanisms contribute to faster recovery from shock.23
Additional aspects of the NASG are easy access to vagina and perineum, which facilitates genital tract medical and surgical procedures. The garment is adjustable to women of different heights and abdominal girths. It is reusable, the NASG can be disinfected and washed over 100 times without losing its compressive effects.12
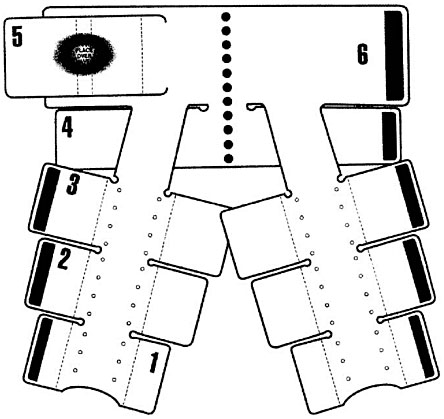
3
Non-pneumatic antishock garment (NASG) design. From Miller et al., 2008, with permission.24
Safety studies
Studies performed in humans have demonstrated that NASG can be used safely. No abdominal wall lesions or other secondary effects due to pressure have been reported. These results are independent of the patient's BMI or the force with which the NASG is applied.25
Doppler studies have demonstrated that flow rate (volume/time) of the distal aorta is diminished by 33% when the NASG is applied, additionally the resistive index (inversely proportional to flow rate) of the internal iliac arteries is increased when the garment’s pelvic and abdominal segments are applied. When resistive index (RI) of uterine arteries was evaluated by transvaginal echography in healthy non-pregnant women there was a confirmed increase in uterine RI.26
Indications
Any woman presenting with postpartum hemorrhage and clinical signs of shock or hemodynamic instability is eligible for NASG application. It is a first-aid device that should be quickly applied to reverse shock and stabilize her vital signs. Once the woman is hemodynamically stable, it is crucial that she receive definitive treatment to control the source of bleeding.
The following guidelines serve as reference for indicators for NASG application:12
1
Indications for NASG use. Adapted from FIGO Safe Motherhood and Newborn Health Committee, 2015.12
Estimated blood loss | Systolic blood pressure | Heart rate | |
Low complexity centers (transfer to a higher center required) | 500 ml | <100 mmHg | >100 bpm |
High complexity centers | 1,000 ml | <90 mmHg | >110 bpm |
Instructions for use
The folded NASG should be stored in an easily accessible area (Figure 4). To apply, the NASG must be unfolded, and placed under the woman's body with the superior edge of the top segment at the level of the lowest rib (not above this rib). Each segment must be stretched with maximum force around the woman and the Velcro closed tightly, starting with ankle segment #1 and continuing successively.2 To guarantee joint mobility, it is better to leave both knees free between the calf and thigh segments. Patient height is a factor that must be taken into account. In case of shorter women, segment #1 should be folded inside segment #2 prior to placement. If two healthcare providers are available, by working in tandem, each on one side of the body, the three leg segments can be placed simultaneously. If there is only one provider, then she can close segment pairs on both sides of the body.
Pelvic segment #4 is placed approximately on the pubic symphysis, the abdominal segments #5 and 6 over the umbilicus (Figure 5). The abdominal and pelvic segment should be closed by only one person using as much force as possible. Two people can work together, where one presents the opposite segment to the one who will close the two segments together. The abdominal segment should be closed tightly, but not so tightly that the woman is unable to take a deep breath.
If the woman begins to present signs of respiratory distress, the abdominal segment can be gently released, but not removed. It is important to perform continuous monitoring of the patient’s vital signs. If there is no initial improvement following the placement of the NASG, blood pressure must be verified and fluid reanimation therapy optimized.2 In case surgery is necessary, only the abdominal and pelvic segments need to be opened, they must be closed again at the end of the procedure.

4
Folded NASG. Source: Miller S. University of California, San Francisco, Safe Motherhood Program.
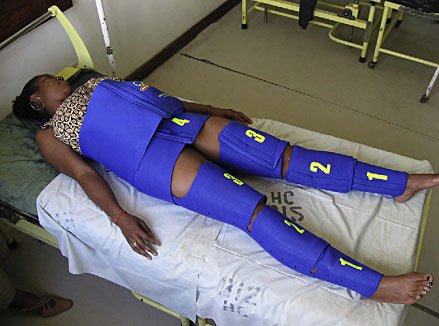
5
Patient with non-pneumatic antishock garment (NASG). From Miller et al., 2008, with permission.2
NASG monitoring and removal
The NASG can be safely used for up to 48 hours until the cause of hemorrhage is resolved and the woman is hemodynamically stable; however, there are anecdotal reports of use for longer than 72 hours. NASG removal must be done only under strict medical supervision with continuous monitoring of vital signs, with intravenous fluids still running, and in an institution with the capacity for definite treatment in case of recurrent hemorrhage.2,12 It should be noted that replacement of the NASG during removal is very rare.
To safely remove the NASG, blood loss must be confirmed to be less than 50 mL/h, heart rate less than 100 bpm, and SBP equal or greater than 100 mmHg (“Rule of 100s”) for at least 2 hours.2 Once these parameters have been verified, the removal of the segments can be safely initiated. This is done in the same order as they were initially placed, starting with segment #1 at the ankles and continuing upwards. After opening the first segment, allow 15 minutes to pass, take another set of vital signs, if the vital signs remain stable, continue with each segment pair, pausing for 15 minutes and taking vital signs before proceeding. However, if there is a drop in SBP ≥20 mmHg or an increase in heart rate ≥20 bpm (“Rule of 20s”), the segments must be closed once again. If this occurs, fluid therapy must be optimized. This change in vital signs indicates that the source of bleeding was not fully controlled. Care must be taken to follow these instructions, as not adhering to these steps or removal of the NASG’s segments in an incorrect order (especially opening the abdominal segment first), could result in hemodynamic instability and recurrence of shock.2
If there are no signs of hemodynamic instability, the whole suit can be removed in 1 hour and 15 minutes. Following removal, hemoglobin and hematocrit levels must be monitored. Values over 7.5 mg/dL of hemoglobin and a hematocrit of 23% are recommended; however, there is no consensus about these values and further evidence of exact values dependent on women’s size and context is needed.
Side-effects
Previous side-effects attributed to the older PASG have been substantially diminished with the introduction of the NASG. To date, no side-effects have been identified with the correct usage and implementation of the NASG,2 and no increase in the types of adverse events, such as kidney, respiratory, cardiac, or cerebral dysfunctions that women with severe hypovolemic shock might experience.27
Contraindications
There are no absolute medical contraindications to the placing of NASG.12 Conditions such as heart failure, severe mitral stenosis, and pulmonary hypertension have been reported as relative contraindications in non-pregnant patients;2 however, in severe hemorrhage the benefits of the NASG are generally considered to outweigh any potential problems.
Effectiveness and advantages
Observational quasi-experimental studies carried out in higher-level referral facilities in Egypt, Nigeria, India, Zambia, and Zimbabwe involving 2,330 women, showed a 48% reduction in maternal mortality by hypovolemic shock secondary to PPH, after the introduction of NASG. Additionally, in the patient group who received NASG, there were fewer women that had to be transferred to higher complexity centers along with reduced numbers of abdominal hysterectomy as definite treatment (RR 0.44, CI 95% 0.23–0.86).27
A randomized cluster clinical trial carried out in Zambia and Zimbabwe by Miller et al., which aimed to evaluate whether placing the NASG before patient referral from primary healthcare centers (PHCs) to higher complexity centers diminished adverse outcomes (mortality and severe morbidities) compared to only placing it on women at the higher complexity centers. This randomized cluster clinical trial found that the earlier placement of the NASG at the PHC was associated with a statistically significant reduction in shock recovery time (defined as shock index <0.9), with a median time to recovery of 170 minutes for the NASG group and 209 minutes in the control group with a Hazard Ratio (HR) of 1.25 (95% CI 1.02–1.52, p = 0.03). The 54% decrease in mortality and severe morbidities found is a clinically highly significant finding. This 54% decrease was not statistically significant, perhaps because the study was underpowered, with a total sample of only 880 women instead of the 2,400 required for adequate power.28
In a 2015 systematic review, Pileggi-Castro et al. analyzed five quasi-experimental studies and the one randomized cluster clinical trial, all of which compared standard of care management for PPH (uterotonics, fluids, uterine massage) vs. standard care plus NASG. The researchers found that the latter course of treatment was associated with a statistically significant 48% reduction in maternal mortality, with a relative risk (RR) of 0.52 (95% CI 0.36–0.77). Additionally, it demonstrated a statistically significant 68% lower combined adverse outcomes, mortality and severe morbidities (RR 0.31; 95% CI 0.17–0.59). No additional benefit was found for need for blood transfusions (RR 0.99; 95% CI 0.97–1.02). There was no difference in adverse events, demonstrating safety as well as efficacy of the NASG.23
Finding decreased maternal mortality and severe morbidity and no safety issues, the NASG was included in the World Health Organization (WHO) PPH management guidelines as a temporizing measure until definitive care was obtained (“weak recommendation, low quality evidence”).5 Specific recommendations for inclusion of the NASG in curricula for medical personnel education, recommendations for procurement, and for inclusion in national PPH protocols and guidelines were published in 2014.29
The FIGO Safe Motherhood and Newborn Health Committee guidelines recommend the use of NASG for clinical stabilization and later patient transfer to higher complexity centers. The NASG is in use in over 40 countries globally, and recommended by FIGO, GLOWM, WHO, and other national and international guidelines,3,30,31,32 NASGs should be present in health service centers everywhere, as it has demonstrated efficacy, effectiveness, and significant reductions in PPH-related mortality.4,5,12
HYDROSTATIC INTRAUTERINE BALLOON TAMPONADE
Hydrostatic intrauterine balloon tamponade (UBT) is considered one of the best non-surgical interventions available for the management of atonic postpartum hemorrhage refractory to uterotonics (WHO evidence classified as “weak recommendation, very-low-quality evidence”).5,33 This technique has replaced uterine packing with gauze and compresses, which was associated with risks of trauma and infection, and was frequently difficult to apply.34 The Bakri balloon was first described in 195135 and UBTs have been diffused globally at many levels of the healthcare system. The Bakri’s design has had several modifications to overcome certain disadvantages, including cost, which directly affect its availability in hospitals of low-income countries. The UBTs in this chapter are classified as specific devices (those designed for uterine atony: Bakri, EBB, and BT-Cath), non-specific (those that have other uses: Foley catheter, Sengstaken-Blakemore gastroesophageal tube, and Rusch balloon), and improvized devices (such as condoms attached to catheters).
Mechanism of action of all intrauterine balloon tamponade
All UBTs, whether specific or non-specific, including condom catheter types, work on the same principles: externally compressing the site of hemorrhage, increasing intrauterine pressure, thus exerting enough hydrostatic pressure upon the uterine vessels to control bleeding.36 This is important during uncontrollable hemorrhage cases when uterine muscles have lost their ability to contract impeding their constriction of bleeding vessels.37
It is presumed that in addition to compression of the bleeding area that there are other mechanisms involved in the homeostasis.38 One of the additional proposed mechanisms of UBTs is the induction of uterine contractions. This was evaluated in a study comparing the estimated firmness of the uterine corpus and cervix before and after the UBT insertion, using radiation force impulse elastography. The study showed an immediate increase of the uterine firmness after UBT placement, suggesting a correlation with the induction of uterine contractions.38 This property may be associated with the production of prostaglandins generated after the device is placed; nevertheless, more studies are needed to support this association.39,40
Specific intrauterine balloon devices
Among the specific intrauterine devices available, the Bakri balloon is the one that has been most often used. The Bakri balloon is an elastic silicone balloon-shaped device that connects with a catheter that allows the flow of saline in to the device. The rapid instillation set include polymer tubing with an IV bag spike and three-way valve. This balloon has a maximum recommended volume of 500 mL and can be inserted transvaginal or transabdominal depending on the delivery route. Placement procedure will be further explained in “How to insert Bakri balloon” section below. Once inside the uterine cavity, the Bakri balloon is filled with saline which causes distention of the device walls, compressing the bleeding area, stopping the hemorrhage (Figure 6).4,41
There are two other specific devices that differ somewhat from the Bakri, the Ebb® and the BT-Cath. The Ebb has an additional balloon that is placed in the vaginal tract, which decreases the risk of the device slipping out of the uterus. This balloon is composed of a thin layer of polyurethane that holds up to 750 mL of fluid (Figure 7).42,43 The most important structural difference of the BT-Cath balloon is that of a “more pronounced umbilicated drainage channel (Figure 8) and a thinner silicone balloon layer” compared with the Bakri balloon.42 Table 2 briefly summarizes the most important aspects of these three specific intrauterine balloons.
2
Specifications of uterine-specific balloon tamponade devices. Adapted from Georgiou, 2014;42 and Antony et al., 2017.44
Bakri | BT-Cath | Ebb | |
Manufacturer/Distributor | Cook Medical | UTAH Medical Products Inc. | Glenveigh |
Balloon material | Silicone | Silicone | Polyurethane |
Recommended volume | 500 mL | 250 mL (gastric balloon) 150 mL (esophagus balloon) | 750 mL (uterine balloon 300 mL (vaginal balloon) |
Drainage of uterine cavity | Yes | Yes | Yes |
Balloon number(s) in device | 1 | 1 | 2 |
Rupture volume | 2,850 mL | > 5,000 mL | > 5,000 mL |
Rupture pressure | 64 mmHg | 17 mmHg | 9 mmHg |
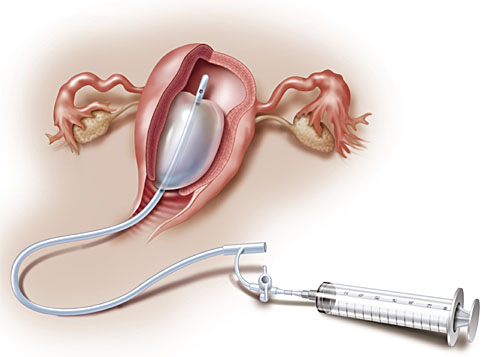
6
Bakri balloon. From Posner GD, Jessica DY, Black A, Jones, GD. Human Labour & Birth, 6th edn.45 www.obgyn.mhmedical.com. Copyright © McGraw-Hill Education. All rights reserved.
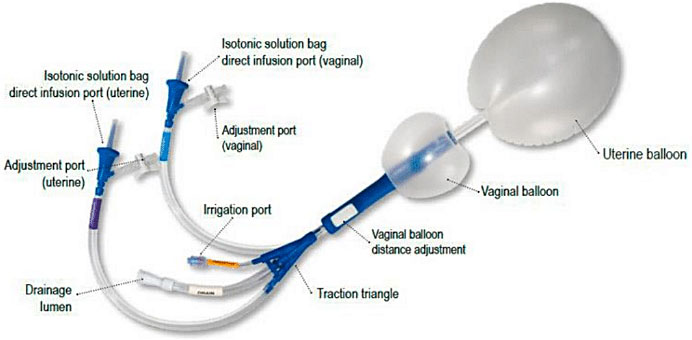
7
Ebb system balloon. From Ebb Quick Reference guide [Internet]. Clinical Innovations. 2014. http://www.kentecmedical.com/wp-content/uploads/2016/05/ebb-Quick-Reference-Guide1.pdf.
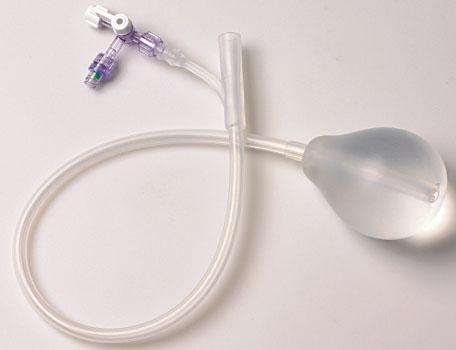
8
BT-Cath balloon. From BT-Cath Uterine Balloon Tamponade Catheter for Postpartum Hemorrhage [Internet]. Utah Medical Products Inc. http://www.utahmed.com/pdf/58281.pdf.
Indications
UBTs are indicated in refractory PPH, with an estimated blood loss less than 1,500 mL, after uterotonics have not stopped the bleeding, and the origin of any source of hemorrhage, besides uterine atony, has been corrected, this includes repairing lacerations, and ruling out retained placenta and uterine inversion.46 In cases with an estimated blood loss greater than 1,500 mL, a surgical approach should be considered first.
Prophylactic UBT use has been reported in placenta previa cases after cesarean delivery and to avoid recurrence of uterine inversion; however, there is insufficient evidence to recommend its application.42
Although the UBT may not always give total control of the hemorrhage, it can still be used as a temporary method by allowing a marked bleeding reduction and extending initial stabilization time to get definite treatment. This will also be useful in centers where hemocomponents may not be available or transfer to a higher-level complexity institution is required.47
Contraindications
The use of the UBT must be avoided in women who have an allergic history to silicone, latex, polyurethane, rubber, or any other of the components of the chosen balloon. Additionally, its use is not recommended in women who have anatomic alterations of the uterine cavity, suspected uterine, cervical, or vaginal infection, or that have uterine rupture.34
How to insert a Bakri balloon
The Bakri balloon is an UBT which can be inserted transvaginally (anterogradely) usually after delivery or retrogradely after cesarean delivery.42 As previously stated, it is recommended that the insertion of the balloon is only performed after a negative response to uterotonics, and when trauma and tissue retention as a cause of bleeding have been ruled out.48
Prior to the insertion of the sterile Bakri device, the patient must have an empty bladder. The device is introduced manually or with the help of Rampley forceps via the transvaginal route and the use of a speculum may facilitate clamping the anterior lip of the cervix. Clamp the superior pole of the Bakri balloon with a Rochester forceps and slide it slightly through the cervix until uterine fundus resistance is felt, release the Rochester forceps and carefully remove it trying not to displace the device from the uterus cavity. Transabdominal placement requires the uninflated balloon to be passed, inflation port first, through the cesarean incision and then slipped through the uterus and cervix. The physician must have an assistant to pull the shaft of the balloon through the vaginal canal until the deflated balloon base comes in contact with the internal cervical os. Inflation of the balloon should occur after closing the incision to avoid balloon puncture (Figure 9).
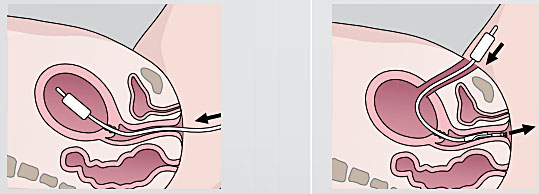
9
Anterograde and retrograde Bakri insertion. (Source: Bakri postpartum balloon with rapid instillation components. Cook Medical. 2017. https://www.cookmedical.com/data/resources/RH-D35085-ES-F_M3_2017–06–30_113153_1499282491477.pdf.)
After insertion and correct placement of the device, a tamponade test must be carried out. The balloon should be filled with 200 mL of saline solution, and the drainage port connected to a fluid collection bag to monitor blood loss. Any blood loss through the cervical walls must also be evaluated. In case the bleeding persists, an additional 50 mL should be added to the initial 200 mL and the bleeding re-evaluated. The tamponade test is considered to be negative if 500 mL of saline (maximum recommended volume) is reached and the bleeding has not subsided. If the tamponade test is negative, the woman should be considered for surgical management.49
Uterine compliance must be taken into account in the management of PPH secondary to uterine atony. Compliance is an inherent variable in determining the volume and pressure that must be applied inside the balloon and the uterine walls to achieve homeostasis. However, compliance is a difficult variable to measure, so monitoring based on the individual clinical evaluation is required to determine the precise balloon volume necessary to achieve adequate control of the hemorrhage.42
When a positive tamponade test is achieved, the inlet catheter should be clamped in order to maintain volume and vaginal tamponade performed preferably with sterile cotton compresses, avoiding the displacement of the balloon.
Handling and withdrawal time
The balloon can be left inside the uterine cavity for up to 24 hours.4 However, Einerson et al., compared the clinical outcomes of a group of women with PPH managed with tamponade and intrauterine balloon for more than 12 hours (n = 206, 75%) versus another group with 2–12 hours of management (n = 68, 25%). With respect to estimated blood loss after balloon placement (190 vs. 143 mL, p = 0.116), transfusion of blood components (62.1% vs. 51.5%, p = 0.120), uterine artery embolization (15.1% vs. 16.2%, p = 0.823), hysterectomy (0.0% vs. 1.5%, p = 0.248), admission to the ICU (8.7% vs. 7.4%, p = 0.721), in this study, no statistically significant differences were found between the groups.51
Methods of UBT removal have been debated. Some authors recommend that the device be gradually deflated at 2–3 intervals over 2 hours by removing 50–100 mL each time,4 so in case bleeding resumes during the process, the balloon can be rapidly inflated again. This differs from the findings of Wong et al., who showed less bleeding after the balloon was deflated, in one time period versus bleeding after removal in two time periods (2.1 vs. 31 mL, p = 0.005).52
Monitoring and adjunct medication
Use of continued oxytocin infusion is recommended for 24 hours after uterine tamponade.4 The use of antibiotics, mainly single dose cephalosporin, has been documented during the use of the balloon as prophylaxis; however, there is no clear evidence that has determined the risk of infection secondary to the use of balloon tamponade nor the antibiotic of choice, additional research is needed.34 The use of either analgesia or anesthesia during insertion has not been determined; therefore, its requirement and applicability should be determined under medical judgment.
Women with uterine tamponade require continuous monitoring for vital signs, uterine tone, estimation of vaginal blood loss, and urinary output. In addition, after the woman has stabilized, it is essential to guarantee adequate hydration. Intravenous fluids should be administered at 1 L every 4–6 hours, or, if intravenous access is not available, give oral rehydration sales, 200–500 mL per hour, until the absence of dehydration signs.4
Bakri balloon – effectiveness and advantages
Uterine tamponade with the Bakri balloon has proven to be a safe measure for PPH management. Its reported effectiveness rate ranges from 80 to 90%.53,54,55 The use of the balloon does not preclude the use of other measures. In scenarios where no definite management can be given, it provides a temporary control of bleeding that allows the transport of the patient to a center of higher complexity for more advanced interventions.
Notwithstanding the 80% success rates reported for the Bakri Balloon, the rate of effectiveness of balloon tamponade in PPH is controversial; there are retrospective cohort studies that report only 65% effectiveness. However, it is recognized that the maternal outcomes of successful cases of tamponade are much better when compared to other interventions (interventional radiology or hysterectomy), mostly in the length of hospital stay and ICU admission..56,57
Some retrospective cohort studies report that use of balloon tamponade resulted in a decrease in the frequency of surgical interventions (vascular ligation, uterine compression sutures, hysterectomy, or uterine artery embolization) in women with PPH refractory to uterotonics, from 28.4% (n = 74) to 6.6% (n = 91) (p = 0.0003).58 This was later confirmed by Revert et al. who reported, in a retrospective cohort study, a lower risk of surgical intervention among women with vaginal delivery managed with balloon tamponade (OR 0.14, 95% CI 0.08–0.27) (n = 26,299 with tamponade balloon vs. n = 29,024 without tamponade balloon).59
According to Revert et. al., several factors were associated with failure of balloon tamponade including cesarean delivery (OR 3.5, 95% CI 1.6–7.6), blood loss >1500 mL before balloon tamponade (OR 3.2, 95% CI 1.5–6.8) and coagulopathy (OR 5.6, 95% CI 2.5–13.0).55 The absence of placenta accreta correlates with a successful balloon tamponade in cases of placenta previa and concomitant PPH (OR 0.001, CI 0.000–0.974).60 It has also been reported that the presence of a positive tamponade test defined as ≤50 mL of blood drained from the uterus within the first 30 minutes after insertion of intrauterine balloon tamponade is a good predictor for success.61
One of the limitations and the greatest cause of failure is the balloon prolapsing into the vagina, this occurs mainly in cases of PPH after cesarean section despite of vaginal packing with gauze.62 As a means to solve this limitation, techniques such as "holding the cervix" have been designed, which consists of closing the cervix with forceps; this dual technique (packing and holding the cervix) was correlated with a 93% hemostatic success rate compared to the previous stated evidence without the technique.63
Non-specific and improvized devices
Other available alternatives to intrauterine balloon tamponade are the Foley catheter, Sengstaken-Blakemore gastroesophageal tube and the Rusch balloon, which have been shown to be effective in hemorrhage control in studies conducted to date.40 Nevertheless, these balloons have a smaller size and a lower capacity volume, which involves the need for introducing several balloons in order to achieve an effective tamponade. This represents a significant disadvantage when compared to specific balloons, which possess a greater volume capacity, enough to make a successful tamponade with only one balloon. Additionally, specific balloons have a drainage catheter that helps drain the residual blood from the area between the balloon and the uterus walls.42 Table 3 shows the most critical aspects of some of the non-specific intrauterine balloons.
3
Characteristics of non-specific uterine balloon tamponade devices. Adapted from Antony et al.44
Device | Foley catheter | Sengstaken Blakemore | Condom + urethral catheter | Rusch balloon |
Balloon material | Latex + silicone coating | Rubber | Latex/silicone | Latex/silicone |
Usual use | Urethral catheter | Gastric balloon | Contraceptive device | Urethral catheter |
Recommended maximum filling volume per instructions for use | 35 mL | 250 mL | Not available | 30–100 mL |
Rupture volume | 120 mL | 3,350 mL | 4,750 mL | Not available |
Rupture pressure | 377 mmHg | 61 mmHg | 3 mmHg | Not available |

10
Specific, non-specific, and improvized intrauterine tamponade balloons. From Antony et al.44 with permission.
In low-income countries, where maternal morbidity and mortality rates are the highest, the use of specific balloons would be ideal, but given the economic circumstances and difficult access to higher level facilities, specific uterine balloon tamponade devices may not be readily available. To reduce the costs, cheaper catheters and balloons have been adapted in order to simulate the mechanism of previous uterine specific balloons. These non-specific balloons have achieved close effectiveness rates to the Bakri balloon’s rate (Figure 10).
One of the most accepted, studied and used devices for uterine tamponade in low-resource settings is the condom catheter. This device usually has good outcomes and the supplies needed to assemble it are inexpensive and easy to access: a condom, a Foley catheter, syringe, gauze and sterile suture (silk, catgut or cotton) or any kind of string. The fluid used to insufflate the balloon can be any type of sterile solution.
To assemble the condom catheter, introduce the catheter inside the condom and secure the open end of the condom around the catheter with a sterile suture or string.40 Once positioned inside the uterus, fill the condom with the syringe through the catheter. Frequently, the condom is filled with 250–500 mL of saline or water, although case reports show that it can be filled up to 760 mL.64 This device takes around 4–15 minutes to control the hemorrhage and is removed at 6–72 hours.40
In a 2012 systematic review, Tindell et al. found that previous observational studies with condom catheters reported low failure and complications rates with an additional reduction in surgical procedures. However, in those studies the catheter was placed exclusively by medical personnel.40 Based on this premise, in 2010, Nelson et al. in collaboration with the ministry of South Sudan, a country with one of the highest rates of maternal deaths (789 per 100,000 live births) and with more than 90% of births attended by non-medical personnel;65 designed a program whose goal was to train at least 800 community-based health workers to manage postpartum hemorrhage.66 One year of follow-up later, the study concluded that after adequate training, the non-medical personnel successfully placed condom balloons without the need to call physicians with low risk of complications during the procedure.66
Based on these findings, the same research group, using the Every Second Matters for Mothers and Babies (ESM-UBT) device, replicated the study with community-based health workers and health personnel in Kenya, Sierra Leone, Senegal and Nepal, concluding that condom catheters are cheap, acceptable, and easy to implement devices after proper education and training. Also, maternal survival rates were reported up to 95% when these devices were included in the management; however, in these reports there were no comparison groups.67,68,69,70
In 2018, Darwish et al. compared the effectiveness of condom balloon and Bakri balloon, through a randomized clinical trial, participants included 66 women with PPH secondary to uterine atony after a vaginal delivery in an Egyptian hospital. The reported effectiveness was 91% for Bakri balloon and 85% for condom balloon, with no significant differences between groups, these results must be considered in light of the current literature that reports effectiveness of both balloons from 80 to 95%.71 These findings suggest that the condom balloon could be an alternative device to Bakri balloon in low-resource settings, given its low cost, similar effectiveness and no increase of surgical procedures associated with its use.
There is some controversy about the use of UBT to prevent adverse outcomes in refractory uterine atony. A randomized control trial of UBT vs. second line uterotonics for refractory PPH conducted by Dumont et al.72 in Mali and Benin, found that the proportion of primary composite outcome (death or uterine surgery) did not differ significantly between the tamponade arm (16%; 9/57) and the second dose of uterotonic arm (7%; 4/59), relative risk 2.33 (95% CI: 0.76–7.14, p = 0.238). The case fatality rate was higher in the tamponade group (10%; 6/57) than in the control group (2%; 1/59) (p = 0.059). Unpublished results, reported at FIGO, 2018 by Gynuity Health Projects73 of a stepped wedge randomized controlled trial of UBT vs. second-line uterotonics among 2388 women with PPH in Uganda, Senegal and Egypt, demonstrated that the use of UBT did not reduce PPH-related deaths or invasive procedures during the study period. Both of these studies have been found to be flawed by some investigators,74,75 but the results must be taken into consideration.
COMBINING METHODS: INTRAUTERINE BALLOON TAMPONADE AND NON-PNEUMATIC ANTISHOCK GARMENT
Reports have been published on successful use of UBTs and NASGs used together.76,77,78 The theory behind this dual use is to “sandwich” the uterus between an external device (NASG) and an internal device (UBT) in order to have a double effect on the hemorrhage and to reverse shock simultaneously. In addition, if a woman has stopped bleeding due to the action of a UBT, but has already lost so much blood that she is in hypovolemic shock, she should also receive an NASG to reverse the shock until a blood transfusion can be obtained.
CONCLUSION
The use of these interventions designed to control refractory postpartum hemorrhage, is supported and recommended by WHO, mostly in cases related to uterine atony for the balloon tamponade; and as a temporizing measure for bimanual uterine compression, external aortic compression, and NASG. The NASG and UBT, used individually and together, have demonstrated a lower need of surgical interventions and a reduction in maternal mortality and severe morbidity associated with PPH. The implementation of these interventions in a wide variety of contexts and communities has shown good results after proper education and training. When using these devices, health care providers must be alert to clinical evolution and changes in the bleeding volume, in order to identify non-responsive signs and the need for surgical interventions and blood transfusions. Health care providers should be trained in their use during pre-service education and should continue to practice their use once they are in-service. Training videos and illustrative materials are available on the GLOWM website. (http://www.glowm.com/resources/glowm_www/mobile/mother.app/mother.welcome.html)
PRACTICE RECOMMENDATIONS
- The non-surgical, life-saving interventions indicated in postpartum hemorrhage refractory to uterotonics and tranexamic acid include bimanual uterine compression, aortic compression, non-pneumatic antishock garment (NASG), and intrauterine balloon tamponade (UBT).
- The choice of these methods will depend on the etiology of the hemorrhage, severity of hemorrhage, condition of the woman, what human resources are available and their training, and what supplies and equipment are available.
- NASGs and UBTs can be used simultaneously.
- The non-pneumatic antishock garment (NASG) is a first-aid temporizing measure that extends the time for definite surgical interventions and/or blood transfusions, or transfer to more specialized centers.
- NASG should be considered in any woman with PPH and clinical signs of shock or hemodynamic instability.
- Intrauterine balloon tamponade is recommended for the treatment of refractory PPH due to uterine atony.
- A tamponade test must be carried out to ensure the correct placement and filling volume of the UBT, and to determine if the intervention will be successful.
- A negative tamponade test may indicate that the etiology of the hemorrhage is not uterine atony, and the next steps might be not only finding the source of the hemorrhage, but also applying one of the other procedures/devices in this chapter (compressive measures or NASG).
- After the administration of compressive measures or any of the PPH devices, women will require strict medical supervision, continuous monitoring for vital signs, uterine tone, estimation of vaginal blood loss and urinary output, as well as ensuring proper hydration.
- Use of intravenous infusion of oxytocin is recommended during 24 hours after uterine tamponade placement.
- The condom balloon could be an alternative device to Bakri balloon in low-resource settings, given its low cost, similar effectiveness, and potential for decreasing surgical procedures.
CONFLICTS OF INTEREST
Professor Suellen Miller declares that her university, the University of California, San Francisco, licenses the trade mark 'LifeWrap', to one brand of NASG. The manufacturer of the LifeWrap NASG pays a royalty for the use of the trademark.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: clinical management guidelines for obstetrician gynecologists, no. 76, October 2006: postpartum hemorrhage. Obstet Gynecol 2006;108:1039–47. | |
Miller S, Martin HB, Morris JL. Anti-shock garment in postpartum haemorrhage. Best Pract Res Clin Obstet Gynaecol 2008;22(6):1057–74 | |
Managing complications in pregnancy and childbirth: a guide for midwives and doctors – 2nd edn. Geneva: World Health Organization, 2017. Licence: CC BY-NC-SA 3.0 IGO | |
FIGO Safe Motherhood and Newborn Health (SMNH) Committee. Prevention and treatment of postpartum hemorrhage in low-resource settings. International Journal of Gynecology and Obstetrics 2012;117:108–18. | |
World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva 1st edn. Published 2012. | |
Anderson J, Etches D, Smith D. Postpartum hemorrhage. In: Baxley E. Advanced Life Support in Obstetrics course syllabus. 4th edn. Leawood, Kan.: American Academy of Family Physicians, 2001. | |
World Health Organization (WHO). Managing Eclampsia: Education Material for Teachers of Midwifery. http://whqlibdoc.who.int/publications/2006/9241546662_5_eng.pdf. Published 2006. | |
FIGO Safe Motherhood and Newborn Health (SMNH) Committee. Prevention and treatment of postpartum hemorrhage in low-resource settings. International Journal of Gynecology and Obstetrics 2012;117:108–18. | |
Keogh J, Tsokos N. Aortic compression in massive postpartum haemorrhage an old but lifesaving technique. Aust N Z J Obstet Gynaecol 1997;37(2):237–8. | |
Harvey, SA, Ayabaca P, Bucagu M, et al. Skilled birth attendant competence: an initial assessment in four countries, and implications for the Safe Motherhood movement. Int J Gynaecol Obstet 2004;87(2):203–10. | |
Andreatta P, Perosky J, Johnson TR. Two-provider technique for bimanual uterine compression to control postpartum hemorrhage. J Midwifery Womens Health 2012;57(4):371–5. | |
FIGO Safe Motherhood and Newborn Health Committee. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet 2015;128(3):194–5. | |
Sternbach G. George W. Crile: the pneumatic rubber suit. J Emerg Med 1984;1(5):439–42. | |
Vahedi M, Ayuyao A, Parsa M, et al. Pneumatic antishock garment-associated compartment syndrome in uninjured lower extremities. J Trauma 1995;384(4):616–8. | |
Aprahamian C, Gessert G, Bandyk DF, et al. MAST-associated compartment syndrome (MACS): a review. J Trauma 1989;29(5):549–55. | |
Brotman S, Browner BD, Cox EF. MAS trousers improperly applied causing a compartment syndrome in lower-extremity trauma. J Trauma 1982;22(7):598–9. | |
Maull KI, Capehart JE, Cardea JA, et al. Limb loss following Military Anti-Schock Trousers (MAST) application. J Trauma 1981;21(1):60–2. | |
Bickell WH, Pepe PE, Bailey ML, et al. Randomized trial of pneumatic antishock garments in the prehospital management of penetrating abdominal injuries. Ann Emerg Med 1987;16(6):653–8. | |
Mattox KL, Bickell W, Pepe PE, et al. Prospective MAST study in 911 patients. J Trauma 1989;29(8):1104–11. discussion: 1111–2. | |
Pepe PE, Bass RR, Mattox KL. Clinical trials of the pneumatic antishock garment in the urban prehospital setting. Ann Emerg Med 1986;15(12):1407–10. | |
Chang FC, Harrison PB, Beech RR, et al. PASG: does it help in the management of traumatic shock? J Trauma 1995;39(3):453–6. | |
Dickinson K, Roberts I. Medical anti-shock trousers (pneumatic anti-shock garments) for circulatory support in patients with trauma. Cochrane Database Syst Rev 2000;(2):CD001856 | |
Pileggi-Castro C, Nogueira-Pileggi V, Tunçalp Ö, et al. Non-pneumatic anti-shock garment for improving maternal survival following severe postpartum haemorrhage: a systematic review. Reprod Health 2015;12:1–13. | |
Miller S, Martin HB, Morris JL. Anti-shock garment in postpartum hemorrhage. Best Pract Res Clin Obstet Gynaecol 2008;22(6):1057–74 | |
Stenson AL, Miller S, Lester F. The Mechanisms of Action of the Non-Pneumatic Anti-Shock Garment. In: Arulkumaran SS, Karosh M, Keith LG, Lalonde AB, B-Lynch C (eds.) A comprehensive textbook of postpartum hemorrhage an essential clinical reference for effective management 2nd edn. London: Sapiens Publishing, 2012;331–40. | |
Lester F, Stenson A, Meyer C, et al. Impact of the Non-pneumatic Antishock Garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol 2011;204(5):409.e1–5. | |
Mourad-Youssif M, Ojengbede OA, Meyer CD, et al. Can the Non-Pneumatic Anti-Shock Garment (NASG) reduce adverse maternal outcomes from postpartum hemorrhage? Evidence from Egypt and Nigeria. Reprod Health 2010;7:1–8. | |
Miller S, Bergel EF, El Ayadi AM, et al. Non-Pneumatic Anti-Shock Garment (NASG), a First-Aid Device to Decrease Maternal Mortality from Obstetric Hemorrhage: A Cluster Randomized Trial. Send to. PLoS One 2013;8(10):1–12 | |
World Health Organization. WHO recommendations on prevention and treatment of postpartum haemorrhage. Highlights and Key Messages from New 2012 Global Recommendations. Geneva 1st edn. Published 2014. | |
Camacho-Castro FA, Rubio-Romero JA. Recomendaciones internacionales para el tratamiento médico de la hemorragia posparto. [International recommendations on medical treatment in postpartum hemorrhage]. Rev Fac Med 2016;64(1):87–92. | |
Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go next? BJOG 2015;122(2):202–10. | |
Hofmeyr GJ, Quereshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol 2016;36:68–82. | |
Management of postpartum haemorrhage Royal Australian and New Zealand College of Obstetricians and Gynaecologists; College statements, C-Obs 43, Melbourne: RANZCOG, 2014. | |
Likis FE, Sathe NA, Morgans AK, et al. Management of Postpartum Hemorrhage. Agency for Healthcare Research and Quality. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015 Apr. Report No.: 15-EHC013-EF. | |
Holtz RS. The control of postpartum hemorrhage by the intrauterine balloon. Am J Obstet Gynecol 1951;62(02):450–1. | |
Cho Y, Rizvi C, Uppal T, Condous G. Ultrasonographic visualization of balloon placement for uterine tamponade in massive primary postpartum hemorrhage. Ultrasound Obstet Gynecol 2008;32:711–3. | |
Nelson SH, Suresh MS. Lack of reactivity of uterine arteries from patients with obstetric hemorrhage. Am J Obstet Gynecol 1992;166:1436–43. | |
Yorifuji T, Tanaka T, Makino S, et al. Balloon tamponade in atonic bleeding induces uterine contraction: attempt to quantify uterine stiffness using acoustic radiation force impulse elastography before and after balloon tamponade. Acta Obstet Gynecol Scand. | |
Marasinghe JP, Du Plessis J, Epitawela D, et al. Management of postpartum haemorrhage with uterine balloon tamponade: The way forward. Aust N Z J Obstet Gynaecol 2015;55(4):315–7. | |
Tindell K, Garfinkel R, Abu-Haydar E, et al. Uterine balloon tamponade for the treatment of postpartum haemorrhage in resource-poor settings: a systematic review. BJOG 2013;120(1):5–14. | |
Lalonde A, Daviss BA, Acosta A, et al. Postpartum hemorrhage today: ICM/FIGO initiative 2004–2006. Int J Gynecol Obstet 2006;94(3):243–53. | |
Georgiou, C. A review of current practice in using Balloon Tamponade Technology in the management of postpartum haemorrhage. Hypertens Res Pregnancy 2014;2:1–10. | |
Uygur D, Altun Ensari T, Ozgu-Erdinc AS. Successful use of BT-Cath1 balloon tamponade in the management of postpartum haemorrhage due to placenta previa. Eur J Obstet Gynecol Reprod Biol 2014;181:223–8. | |
Antony KM, Racusin DA, Belfort MA, et al. Under pressure: intraluminal filling pressures of postpartum hemorrhage tamponade balloons. Ajp Rep 2017;7(2):e86–e92. | |
Posner GD, Jessica DY, Black A, et al. Human Labour & Birth, 6th edn. www.obgyn.mhmedical.com. | |
Aibar L, Aguilar MT, Puertas A, et al. Bakri balloon for the management of postpartum hemorrhage. Acta Obstet Gynecol Scand 2013;92(4):465–7. | |
Georgiou C, Suharjono H, Ruey S, Lim C. Using balloon tamponade technology to minimize blood loss during transfer of patients in the management of postpartum haemorrhage. BJOG 2012;119:2–25 (FC1.49). | |
Vintejoux E, Ulrich D, Mousty E, et al. Success factors for Bakri™ balloon usage secondary to uterine atony: a retrospective, multicentre study. Aust N Z J Obstet Gynaecol 2015;55(6):572–7. | |
Georgiou C. Using the uterine-specific Bakri balloon in the management of postpartum haemorrhage: Case series and conceptual/practical guidelines. Chapter 48. In: Arulkumaran S, B-Lynch C, Keith LG, Lalonde AB, Karoshi M (eds.) A Comprehensive Textbook of Postpartum Hemorrhage, 2nd edn. London: Sapiens Publishing, 2012;387–97. | |
Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet 2001;74(2):139–42 | |
Einerson BD, Son M, Schneider P. The association between intrauterine balloon tamponade duration and postpartum hemorrhage outcomes. Am J Obstet Gynecol 2017;216(3):300.e1–5. | |
Wong M, Greene N, Gregory K. Removal of Bakri Intrauterine Balloon by Rapid or Stepwise Deflation: Which Technique is Better? Obstetrics & Gynecology 2018;131:10S. | |
Grönvall M, Tikkanen M, Tallberg E, et al. Use of Bakri balloon tamponade in the treatment of postpartum hemorrhage: a series of 50 cases from a tertiary teaching hospital. Acta Obstet Gynecol Scand 2013;92(4):433–8. | |
Aibar L, Aguilar MT, Puertas A, et al. Bakri balloon for the management of postpartum hemorrhage. Acta Obstet Gynecol Scand 2013;92(4):465–7. | |
Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum haemorrhage in a perinatal network: a prospective cohort study. BJOG 2017;124(8):1255–62. | |
Olsen R, Reisner DP, Benedetti TJ, et al. Bakri balloon effectiveness for postpartum hemorrhage: a "real world experience". J Matern Fetal Neonatal Med 2013;26(17):1720–3. | |
Martin E, Legendre G, Bouet PE, et al. Maternal outcomes after uterine balloon tamponade for postpartum hemorrhage. Acta Obstet Gynecol Scand 2015;94(4):399–404. | |
Gauchotte E, De La Torre M, Perdriolle-Galet E, et al. Impact of uterine balloon tamponade on the use of invasive procedures in severe postpartum hemorrhage. Acta Obstet Gynecol Scand 2017;96:877–82. | |
Revert M, Rozenberg P, Cottenet J. Intrauterine Balloon Tamponade for Severe Postpartum Hemorrhage. Obstet Gynecol 2018;131(1):143–9. | |
Maher MA, Abdelaziz A. Comparison between two management protocols for postpartum hemorrhage during cesarean section in placenta previa: Balloon protocol versus non-balloon protocol. J Obstet Gynaecol Res 2017;43(3):447–55. | |
Kong CW, To WW. Prognostic factors for the use of intrauterine balloon tamponade in the management of severe postpartum hemorrhage. Int J Gynaecol Obstet 2018;142(1):48–53 | |
Soyama H, Miyamoto M, Ishibashi H, et al. Analysis of prophylactic Bakri balloon tamponade failure in patients with placenta previa. Taiwan J Obstet Gynecol 2019;58(1):159–63. | |
Ogoyama M, Takahashi H, Usui R, et al. Hemostatic effect of intrauterine balloon for postpartum hemorrhage with special reference to concomitant use of "holding the cervix" procedure (Matsubara). Eur J Obstet Gynecol Reprod Biol 2017;210:281–5. | |
Manaktala U, Dubey C, Takkar A, et al. Condom catheter balloon in management of massive nontraumatic postpartum haemorrhage during cesarean section. J Gynecol Surg 2011;27:115–7. | |
World Health Organization. Country Cooperation Strategy at a glance South Sudan. Published 2018. | |
Nelson BD, Stoklosa H, Ahn R, et al. Use of uterine balloon tamponade for control of postpartum hemorrhage by community-based health providers in South Sudan. Int J Gynaecol Obstet 2013;122(1):27–32. | |
Natarajan A, Kamara J, Ahn R, et al. Provider experience of uterine balloon tamponade for the management 3 of postpartum hemorrhage in Sierra Leone. Int J Gynaecol Obstet 2016;134(1):83–6. | |
Natarajan A, Chavez J, Ahn R, et al. Provider experiences with uterine balloon tamponade for uncontrolled postpartum hemorrhage in health facilities in Kenya. Int J Gynaecol Obstet 2015;131(2):201–4. | |
Natarajan A, Alaska Pendleton A, Nelson BD, et al. Provider experiences with improvised uterine balloon tamponade for the management of uncontrolled postpartum hemorrhage in Kenya. Int J Gynaecol Obstet 2016;135(2):210–3. | |
Burke TF, Ahn R, Nelson BD, et al. A postpartum haemorrhage package with condom uterine balloon tamponade: a prospective multi-centre case series in Kenya, Sierra Leone, Senegal, and Nepal. BJOG 2016;123(9):1532–40. | |
Darwish AM, Abdallah MM, Shaaban OM, et al. Bakri balloon versus condom-loaded Foley’s catheter for treatment of atonic postpartum hemorrhage secondary to vaginal delivery: a randomized controlled trial. J Matern Fetal Neonatal Med 2018;31(6):747–53 | |
Dumont A, Bodin C, Hounkpatin B, et al. Uterine balloon tamponade as an adjunct to misoprostol for the treatment of uncontrolled postpartum haemorrhage: a randomised controlled trial in Benin and Mali. BMJ Open 2017;7:1–9. | |
Gynuity Health Projects and FIGO. New Thinking and Innovations in the Management of Postpartum Haemorrhage. Paper presented at: XXII FIGO World Congress of Gynecology and Obstetrics; 2018 October 14–19; Rio de Janeiro, Brasil. | |
Burke T. Critical analysis of the Dumont, et al. Uterine balloon randomized controlled trial in Benin and Mali. 2017;7:1. | |
Burke T. Saving Mothers from PPH deaths – how can the new PPH bundles accelerate progress. Paper presented at: Advances in PPH Control. XXII FIGO World Congress of Gynecology and Obstetrics; 2018 October 14–19; Rio de Janeiro, Brasil. | |
Escobar MF, Füchtner CE, Carvajal JA, et al. Experience in the use of non-pneumatic anti-shock garment (NASG) in the management of postpartum haemorrhage with hypovolemic shock in the Fundación Valle Del Lili, Cali, Colombia. Reprod Health 2017;14(1):58. | |
Escobar MF, Fernández Pérez PA, Carvajal JA, et al. Impact of nonpneumatic antishock garment in the management of patients with hypoperfusion due to massive postpartum hemorrhage. J Matern Fetal Neonatal Med 2019:1–5. | |
Jelks A, Berletti M, Hamlett L, et al. Nonpneumatic Antishock Garment Combined with Bakri Balloon as a Nonoperative “Uterine Sandwich” for Temporization of Massive Postpartum Hemorrhage from Disseminated Intravascular Coagulation. 2015:1–3. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
