This chapter should be cited as follows:
Cook S, Fiander A, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.416533
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 15
The puerperium
Volume Editors:
Dr Kate Lightly, University of Liverpool, UK
Professor Andrew Weeks, University of Liverpool, UK

Chapter
Postnatal Family Planning
First published: November 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Family planning is a highly effective public health intervention that saves lives. Globally 214 million women would like to prevent or delay pregnancy but have no access to contraception.1 Postnatal family planning is an important strategy for meeting unmet need for contraception, and reducing child and maternal morbidity and mortality. Short inter-pregnancy intervals increase the risk of preterm birth, low birth weight, stillbirth, and neonatal death.2,3 Meeting the global unmet need for contraception would prevent one third of maternal deaths from obstetric complications or unsafe abortion and prevent 1 in 10 child deaths if pregnancies are spread more than 2 years apart.4,5 Globally 40% of pregnancies are unplanned.
The World Health Organization recommends a 24 month inter-pregnancy interval after delivery.6 Worldwide more than 9 out of 10 women want to avoid pregnancy for 2 years after having had a baby, but 1 in 7 of them are not using contraception. This means that pregnancies soon after childbirth are not uncommon and many of these are unplanned. Provision of postnatal family planning is therefore vitally important but is often ignored and a number of biases and misconceptions limit its availability.
Childbirth presents an opportunity for providing contraception, as women have contact with healthcare providers with the skills to offer a full range of methods, and are often highly motivated to start using an effective method. There is a window of opportunity in the first 48 hours postpartum when most methods can be used effectively, followed by a “wait” period for some methods, until 4–6 weeks postpartum (see Figure 2).
RETURN OF FERTILITY IN NON-BREASTFEEDING WOMEN
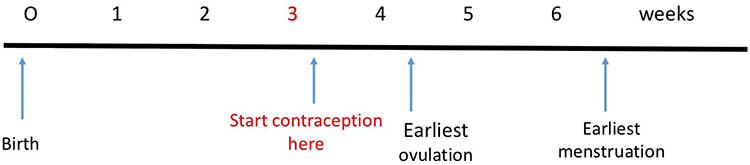
Following the delivery of the placenta, levels of estrogen and progesterone fall and follicle-stimulating hormone (FSH) and luteinising hormone (LH) gradually rise, allowing ovulation to recommence. Ovulation in a non-breastfeeding woman occurs around 4–7 weeks (average 45 days) after delivery. The woman’s first menses will then occur 2 weeks after this. Many women start to become sexually active following birth before this time; a UK study found that 32% of women had resumed sexual activity by 6 weeks postpartum.7 It is therefore possible to become pregnant again before menstruation occurs. In order to prevent this from happening, contraception needs to be started by 3 weeks after delivery (see Figure 1).
1
Return of ovulation after childbirth in non-breastfeeding women.
It is important to note that ovulation can occur as early as 8 days after first- or second-trimester miscarriage, abortion or ectopic pregnancy. Therefore, if these women wish to delay or prevent future pregnancies, contraception should be started within 5 days.
Exclusive breastfeeding suppresses LH secretion, meaning that the menstrual cycle may not resume until 6 months, or even later, after delivery. Some women may choose to rely on breastfeeding initially for contraception. They can be advised that this is around 98% effective contraception in the first 6 months after delivery, as long as conditions for the lactational amenorrhea method (LAM) are met (see below).
Lactational amenorrhea method (LAM) for breastfeeding women
Conditions required for effective contraceptive use of LAM:
- Fully or nearly fully breastfeeding day and night (no other liquid/food given, or only given as very small amounts infrequently); no long intervals (>4 h daytime, >6 h night time) between breastfeeds.
- Amenorrheic.
- Within 6 months of delivery.8
It is important to discuss and plan contraception options with the woman before this 6 month period is over (or earlier if any of the above criteria are no longer fulfilled.
Family planning can be integrated with other sexual reproductive health services, e.g. childbirth, infant and child immunization, HIV/STI testing and treatment, cervical screening, and assessment for gender-based violence.
TIMING OF CONTRACEPTION PROVISION
Immediate postpartum contraception is beneficial in terms of convenience for the woman and is particularly important in situations where women may have difficulties accessing healthcare providers and facilities. Immediate postpartum contraception ensures that the woman can start the method before her first ovulation. When contraception provision is delayed, women are less likely to access it9 and may then be at risk of unplanned pregnancy and short inter-pregnancy intervals.
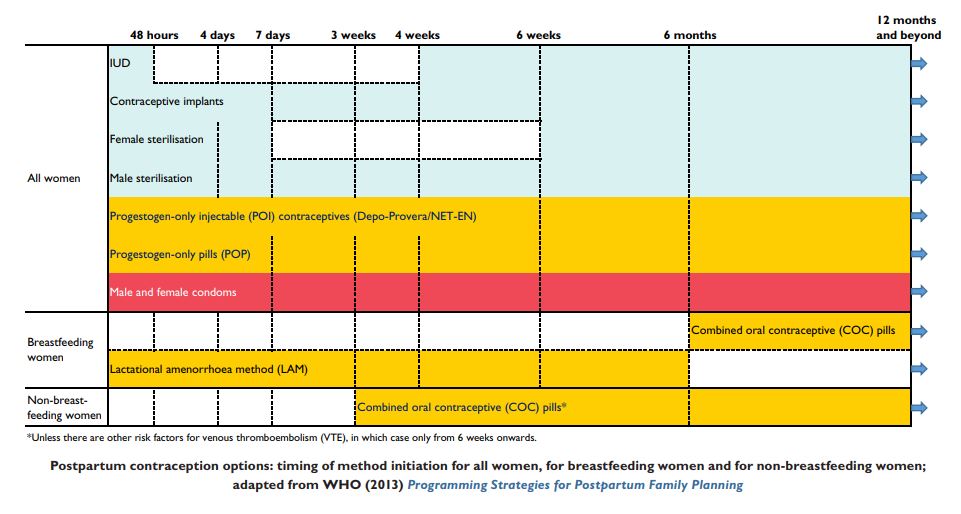
Whilst the majority of contraceptive methods can be started immediately postpartum, some can not and some women may decline immediate provision, but decide to commence contraception at a later date. Furthermore, advice varies for some methods according to whether the woman is breastfeeding or not. The chart below depicts the timings for initiating different methods.
If any method is started later than 3 weeks postpartum, the practitioner should be reasonably certain that the woman is not pregnant or at risk of pregnancy due to recent unprotected sex. If there is a recent risk of pregnancy, emergency contraception should be considered, in the form of either oral hormonal emergency contraception (levonorgestrel or ulipristal acetate) or the Copper intrauterine contraceptive device (Cu-IUC). However, ulipristal acetate is excreted in breastmilk and there is uncertainty about the safety for neonates. Women are therefore advised not to breastfeed for 1 week after use, or to express and discard milk for this time.8 In situations where women are currently breastfeeding it therefore may be more appropriate to avoid ulipristal acetate and rather offer levonorgestrel emergency contraception when an oral method is desired.
2
Timing of method initiation for postnatal family planning for all women, breastfeeding and non-breastfeeding women. Reproduced with permission from RCOG, 2015.10
COUNSELING A WOMAN ABOUT CONTRACEPTION
Discussions about postnatal family planning and contraceptive options should ideally start in the antenatal period. Firstly, because many methods can be provided at the time of delivery (particularly the intrauterine contraceptive methods) or in the immediate postpartum period while the women is still in the birthing facility. Secondly, prior to delivery, women may have more time to consider their options.
Contraceptive counseling should be non-judgmental and promote informed decision-making for the woman to decide on her preferred contraceptive options.
Information and postnatal family planning can be provided during one-to-one appointments, group counseling sessions, and through posters and leaflets.
Things to ask when counseling about postnatal family planning10
- Age.
- Methods used in the past and experience of them.
- Any methods the woman has in mind – and why.
- Any methods the woman would not like to use – and why.
- Desired family size (is family planning for spacing or for limiting?).
- Infant feeding intentions (and previous experience).
- Past medical history including HIV status.
- Current health.
- Views of partner or other family members.
How to ensure opportunities for postnatal family planning counseling are not missed
Postnatal family planning should be discussed at every opportunity and if possible the discussion should start while the woman is still pregnant so that she is able to start her chosen method as soon as possible after delivery.
In the antenatal clinic
- Women should be given verbal and written information about all contraceptive options and told about the particular benefits of postnatal family planning, particularly of intrauterine contraceptives (IUC) and implants, as the most effective methods.
- For women who are considering limiting their family size, it may be appropriate to discuss vasectomy or female sterilization with the woman and her partner at this time.
- For women who are considering limiting their family size and undergoing a planned cesarean section, the possibility of concurrent tubal ligation should be discussed.
- Women should be given the opportunity to ask questions about contraception every time they are seen in the antenatal clinic.
- The method that the woman has chosen should be documented in the appropriate case record so that it can be provided as soon as possible after childbirth.
- If hormonal pills or barrier methods are chosen, these could be provided during late pregnancy so that women have a supply at home to start at the appropriate time after childbirth.
In the labor ward
- Women should be asked whether they have received contraceptive advice antenatally and, if so, the method they have chosen should be confirmed and then provided unless complications during pregnancy or delivery indicate the need for review.
- If the chosen method is not available in the labor ward, the method should be provided before the woman leaves the hospital or she should be referred to the most convenient place where the contraceptive method can be provided.
- Contraception should not be discussed with a woman who is in active labor.
- In women having a cesarean section, intrauterine contraceptives can be fitted as soon as the placenta has been delivered. Insertion is simple and expulsion rates are low.
In the postnatal ward
- If a woman has not had the chance to discuss contraception before she arrives on the postnatal ward, it should be discussed with her before she leaves the hospital and her chosen method (including an implant, or an intrauterine contraceptive if within 48 h of delivery) should be provided.
In the postpartum care clinic
- Women attending for postpartum care should be asked whether they are using, or have a supply of, contraception.
- It should be confirmed with women who have chosen their method that they are happy with their choice, are knowledgeable about the method, have sufficient supplies and know where they can get more (if appropriate).
- If a woman has not chosen a contraceptive method, she should be told about all methods, particularly the most effective methods, and arrangements made to provide her with the method she has chosen.
In the baby immunization clinic
- Women bringing their babies for immunization should be asked whether they are using contraception.
- It should be confirmed with women who have chosen their method that they are happy with their choice, are knowledgeable about the method, have sufficient supplies and know where they can get more (if appropriate).
- If a woman is not using contraception, she should be told about all methods, particularly the most effective methods, and arrangements made to provide her with the method she has chosen.
WHAT DO WOMEN NEED TO KNOW ABOUT POSTNATAL CONTRACEPTION?
What a woman needs to know about each main method of family planning10
- The effectiveness of the method and how long it lasts.
- How to use the method.
- When the method can be started.
- The common side effects and risks.
- When she should attend for follow-up.
- When and how the method needs to be renewed or how to stop using it.
- When fertility returns after the method is stopped.
- How to protect against sexually transmitted infections (STIs).
In a little more detail:
Effectiveness and correct use |
|
Side effects |
|
Follow-up care and re-supply |
|
Stopping a method |
|
STI prevention |
|
Effectiveness and correct use
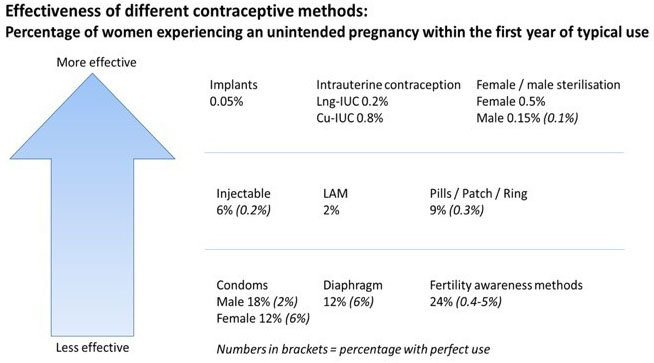
It is important that women are advised about the range of contraceptive options available to them. Provided the options are medically safe and suitable, the woman should be free to choose the method she wishes. Many factors influence a woman’s choice of contraceptive method; effectiveness is often one of the most important factors. The World Health Organization (WHO) therefore recommends providing information about different methods using a tiered effectiveness model, which gives information about methods in terms of the effectiveness of each method.11 With no method of contraception, approximately 85% of women who are regularly sexually active will become pregnant within 1 year. Figure 3 depicts the effectiveness of different methods, comparing the percentage of women who will become pregnant by the end of 1 year with typical and perfect use. Perfect use refers to what happens when the method is used correctly 100% of the time, whereas typical use refers to what happens in reality when the method may be used incorrectly or inconsistently by some women. The top line of Figure 3 shows that for these methods, the typical and perfect use is either the same or very similar; this is because these methods do not rely on the user to remember something, e.g. taking a pill each day or using a condom every time they have sex. WHO have a pictorial version of this chart, which can be useful for discussions with patients.11
Safety of different contraceptive methods (medical eligibility)
When thinking and talking about family planning methods, it is important to remember that you are advising someone to use a method in order to avoid pregnancy. While many people worry about the theoretical risks of contraception, it is always the case that the risks of pregnancy and childbirth are greater than those associated with contraception. So, for example, the most common serious risk among all the methods of family planning is the risk of blood clots [venous thromboembolism (VTE)] among women taking the combined oral contraceptive pill (COCP). It is true that women who take the COCP have double or triple the risk of getting a VTE compared with women who are not taking the pill, but the risk of VTE in pregnancy is five times higher and in the postpartum period it is 60 times higher than in a woman who is not pregnant. It is also important to remember that, while it sounds serious to say that the risk of something is doubled or tripled, if the risk is only 1 in 1,000 then doubling or tripling it only makes it 2 or 3 in 1,000, so 997 women are not at risk. Quite apart from the medical risks associated with unintended pregnancy, whether the pregnancy is continued or not, it also carries profound social, economic, and emotional consequences.
All methods of contraception are associated with side effects: most of them are simply a nuisance but they often result in the woman stopping the method unless you can reassure her that the side effects are not dangerous and putting up with them is better and safer than being pregnant when she does not want to be.
Very rarely, the theoretical or known risks of a method will outweigh the benefits of that method for a particular woman.
The World Health Organization’s Medical Eligibility Criteria for contraceptive use (MEC)12 provides guidance to practitioners regarding the use of various contraceptive methods in the context of a variety of health conditions and characteristics. It primarily considers whether the contraceptive method could worsen the health condition or create additional risks, and secondarily whether the health condition may make the contraceptive method less effective. The contraceptive method is then given a category according to that health condition (see below).
Medical Eligibility Criteria (MEC) categories for contraceptive eligibility12
1 | A condition for which there is no restriction on the use of the contraceptive method. |
2 | A condition where the advantages of using the method generally outweigh the theoretical or proven risks. |
3 | A condition where the theoretical or proven risks usually outweigh the advantages of using the method. |
4 | A condition that represents an unacceptable health risk if the contraceptive method is used. |
The WHO have produced MEC tables and wheels that can be used to work out the category for a particular contraceptive method for a woman but it should be remembered that, even if a woman has a condition that is categorized as 3 for a particular method, if there is no other method available or acceptable then she can still use it. For example, if a woman has breast cancer, she should not take the combined pill but actually pregnancy would be a far greater risk for her.
A medical history should be taken from all women and their medical eligibility checked. This will need to be checked again if labor or delivery is complicated (e.g. puerperal sepsis). There should be no restrictions on provision of any method based on age, parity, or the number of children a woman has had, unless there is a medical reason.
MOST EFFECTIVE CONTRACEPTIVE METHODS
Long-acting reversible methods
Progestogen-only subdermal implants and intrauterine contraceptives (IUC) are highly effective reversible methods of contraception and are referred to together as long-acting reversible contraceptives (LARCs). The provision of LARC immediately after childbirth has been shown to be associated with a reduced risk of unintended pregnancy.13
Implants
The progestogen-only subdermal implant works primarily by inhibiting ovulation and can be started any time following childbirth (Figure 4). Subdermal implants are effective for 3–5 years, depending on the implant. They are usually inserted in the upper arm. There are a range of different implants, two of the most commonly used worldwide are an implant containing etonorgestrel that lasts 3 years and comes as one rod (e.g. Nexplanon) and an implant containing levonorgestrel that lasts 5 years and comes as two rods (e.g. Jadelle). The insertion procedure for Nexplanon is summarized below. The most common side effect is an irregular bleeding pattern. Fertility returns immediately after removal.
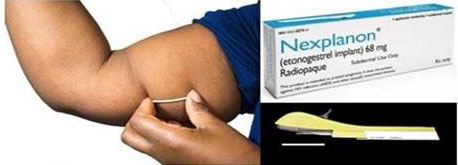
4
Progestogen-only subdermal implant. (Reproduced from RCOG LSC program.)
Checklist for implant insertion
Below is a checklist of the steps involved in inserting the implant Nexplanon according to the manufacturer’s guidance. Steps for insertion of other devices will differ. This checklist should only be used in conjunction with practical training.
Implant insertion checklist (Source: Summary of Product Characteristics for Nexplanon 2019.)
Steps for insertion of an IUC using placental forceps | ||
GETTING READY FOR INSERTION | ||
1. | Check the woman’s record to ensure that it is appropriate for her to have an implant and that she has given her consent. Rule out conditions that prevent insertion of an implant, as per the WHO Medical Eligibility Criteria. | |
2. | Confirm that supplies are available. Talk to the woman with kindness and respect. Confirm with the woman whether she still wants an implant. Answer any questions that she might have. | |
3. | Perform hand hygiene and put on sterile or high level disinfection gloves. | |
4. | Position the woman’s arm: have the woman lie on her back with her non-dominant arm flexed at the elbow and externally rotated so that her hand is positioned next to her head. | |
5. | Identify the insertion site: this should be the inner side of the upper arm about 8–10 cm above the medical epicondyle of the humerous. The sulcus (groove) between biceps and triceps should be AVOIDED. The implant should be inserted subdermally, just under the skin. | |
6. | Make two marks with a marker pen. First, where the implant will be inserted, and second a few centimeters above (proximal) to the first as a direction guide. | |
7. | Clean the insertion site with antiseptic and anesthetize the area with anesthetic spray or by injecting 2 ml 1% lidocaine. | |
INSERTING NEXPLANON | ||
8. | Remove the implant from its packaging and remove the transparent needle protection cap. | |
9. | Check that the white colored implant is visible within the needle. | |
10. | Hold the implant applicator with your dominant hand. With your free hand stretch the skin around the insertion site with your thumb and index finger. | |
11. | Puncture the skin with the tip of the needle at an angle of less than 30°. | |
12. | Lower the applicator to be horizontal to the skin and slide the needle to its full length. The needle should be seen just beneath the skin. You can best see movement of the needle and that it is inserted just beneath the skin if you are seated and looking at the applicator from the side. | |
13. | Keep the needle inserted to its full length. Move the purple slider fully back until it stops. The implant is now released from the applicator and the applicator can be disposed of in a sharps' container. | |
AFTER INSERTION | ||
16. | Check the presence of the implant in the woman’s arm immediately by palpating both ends of the implant. | |
17. | Apply a dressing to the site. A pressure bandage can be used in addition for 24 h to minimize bruising. | |
18. | Perform hand hygiene. All infection prevention steps should be followed as per standard infection prevention procedures and the facility protocol for waste management. | |
19. | Provide the woman with the post-insertion instructions. Provide an implant card showing the type of implant and the date of insertion. Inform her about possible side effects and normal postpartum symptoms. Emphasize that she should come back any time she has a concern. Reassure the woman that the implant will not affect breastfeeding and breast milk. | |
20. | Record information regarding the implant insertion in the woman’s clinical file. | |
Post-insertion instructions to the woman |
The most common side effect of the implant is irregular bleeding. |
Remove the pressure bandage after 24 h and the dressing after 3–5 days. |
Remember that an implant does not protect against STIs and HIV so if you are at risk of acquiring infection you should ask your partner to use a condom. |
Return for removal of the implant at any time you decide that you want to become pregnant and you will have almost immediate return of fertility. |
Give a card to the woman with the following information in writing:
- type of implant inserted;
- date of implant insertion;
- month and year when the implant will need to be removed or replaced;
- where to go or call if she has problems or questions about her implant.
Intrauterine contraceptives (IUC)
Intrauterine contraceptive methods, both the Copper-releasing IUC (Cu-IUC) and the levonorgestrel-releasing IUC (Lng-IUC) (e.g. Mirena®, Levosert®, Kyleena®, Jaydess® etc.), can be inserted immediately after delivery of the placenta (0–48 h) (Figure 5). This can either be at the time of cesarean section or following vaginal delivery. Due to a lack of evidence for the safety of insertion between 48 h and 4 weeks, it is recommended insertion is avoided during this time. The insertion of IUC within the first 48 h after childbirth is associated with an increased risk of expulsion of the device, but also higher continuation rates at 6–12 months postpartum.13,14 The insertion technique within 48 h of delivery differs from insertion in the non-postnatal period. See table below for a step-by-step checklist for insertion of IUC within the first 48 h after delivery. Insertion at the time of cesarean section can be performed by placing the device with the sterile gloved hand at the uterine fundus prior to closure. Insertion following vaginal delivery requires different equipment and training for insertion due to the enlarged uterus, compared to interval insertion (after 4–6 weeks). Insertion is most effective when performed using long placental forceps.14
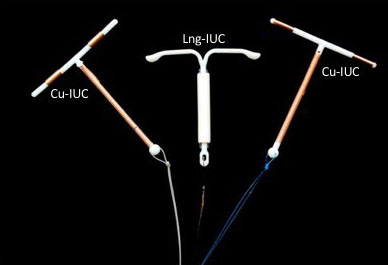
5
Intrauterine contraceptives. (Reproduced from RCOG LSC program.)
IUC work through a mixture of both pre- and post-fertilization effects. Cu-IUC are effective for 5–10 years, depending on the device. The most common side effect is heavier or more painful menstruation. Return of fertility is immediate following removal. Lng-IUC are effective for 3–5 years, depending on the device. The most common side effect is lightened, or no bleeding, occasionally the bleeding may be heavier or more frequent in the first few months following insertion.
Checklist for insertion of intrauterine contraception
Below is a checklist of the steps involved in inserting intrauterine contraception within the first 48 hours after delivery. This checklist should only be used in conjunction with practical training.
Immediate postpartum IUC insertion checklist (Source: Leading Safe Choices Logbook. RCOG 2015.)
Steps for insertion of an IUC using placental forceps | ||
GETTING READY FOR INSERTION | ||
1. | Check the woman’s record to ensure that it is appropriate for her to have a postpartum IUC and that she has given her consent. Rule out conditions that prevent insertion of an IUC, such as:
| |
2. | Confirm that sterile instruments, supplies, and a light source are available in the labor room for immediate post-placental insertion of the IUC. Talk to the woman with kindness and respect. Confirm with the woman whether she still wants an IUC. Explain that you will insert the IUC following delivery of the placenta. Answer any questions that she might have. | |
3. | Perform hand hygiene and put on sterile gloves. | |
4. | Arrange instruments and supplies on a sterile tray or draped area. | |
5. | Position the woman on the bed and inspect the perineum, labia, and vaginal walls for lacerations. If lacerations are not bleeding heavily, insert the IUC first and repair the lacerations afterwards if needed. | |
6. | Gently visualize the cervix by inserting a Sims speculum in the vagina and depressing the posterior wall of the vagina. | |
7. | Gently clean the cervix with antiseptic solution such as povidone-iodine or chlorhexidine. | |
INSERTING THE IUC | ||
8. | Gently grasp the anterior lip of the cervix with the ring forceps up to the first lock. (The same ring forceps that was used to clean the cervix can be used.) | |
9. | Grasp the IUC in the sterile package with the long placental forceps using a no-touch technique. It should be held just on the edge of the placental forceps so that it can be easily released from the instrument when opened. | |
10. | Apply gentle traction on the anterior lip of the cervix using the ring forceps and insert the IUC into the lower uterine cavity. Avoid touching the walls of the vagina. Pass the placental forceps with the IUC carefully into the lower uterine cavity. | |
11. | Once the placental forceps is in the lower uterine cavity, lower the ring forceps that is holding the anterior lip of the cervix. Move your left hand to the woman’s abdomen and push the entire uterus superiorly (upwards, towards the woman’s head). This is to straighten out the angle between the vagina and the uterus, so that the instrument can easily move upwards towards the uterine fundus. | |
12. | Gently move the placental forceps upwards towards the fundus following the curve of the uterine cavity. Take care not to apply excessive force. If the uterus is not pushed upwards, the angle between the cervix and the uterus may not allow the instrument to advance smoothly. Always keep the instrument closed so that the IUC is not dropped accidentally in the mid-portion of the uterine cavity. | |
13. | Confirm that the end of the placental forceps has reached the fundus and tilt the forceps slightly inwards. When it reaches the uterine fundus, you will feel resistance and you will also feel the thrust of the instrument at the fundus of the uterus with your left hand, which is placed on the abdomen. | |
14. | Open the placental forceps and release the IUC at the fundus. Sweep the placental forceps to the side wall of the uterus. Stabilize the uterus (using the base of your hand against the lower part of the body of the uterus). To help prevent the IUC being drawn downwards in the uterus, the instrument is swept to the right (i.e., to the woman’s left) to ensure that the instrument is away from the IUC. If the instrument closes and catches the strings of the IUC, it can accidentally pull the IUC down from its fundal position, increasing the risk of expulsion. Slowly remove the placental forceps from the uterine cavity, keeping it slightly open. Take particular care not to dislodge the IUC as the placental forceps is removed. Stabilize the uterus until the placental forceps is completely out of the uterus. | |
15. | Examine the cervix to ensure that there is no bleeding. If the IUC is seen protruding from the cervix, remove and reinsert it. It is important to check that the IUC is not visible at the cervical os. If it is visible, or if the strings appear to be very long, then the IUC has not been adequately placed at the fundus and the chance of spontaneous expulsion is higher. If it appears that the IUC is not placed high enough, you can use the same forceps to remove the IUC and repeat the steps of insertion using aseptic procedures. | |
AFTER IUC INSERTION | ||
16. | Remove all instruments used and place them in 0.5% chlorine solution for 10 min for decontamination. | |
17. | Allow the woman to rest for a few minutes. Support the initiation of routine postpartum care, including immediate breastfeeding. The woman should rest on the table for a few minutes following the insertion procedure. You should reassure her that the insertion was done smoothly and that she now has an effective, safe, and reliable long-term spacing method of contraception. | |
18. | Immerse both gloved hands in 0.5% chlorine solution. Remove the gloves by turning them inside out and disposing of them. Perform hand hygiene. All infection prevention steps should be followed as per standard infection prevention procedures and the facility protocol for waste management. | |
19. | Provide the woman with the post-insertion instructions. Provide an IUC card showing the type of IUC and the date of insertion. Inform her about the IUC side effects and normal postpartum symptoms. Tell the woman when to return for IUC follow-up/postnatal care/newborn check-up. Emphasize that she should come back any time she has a concern or experiences warning signs. Inform her about the warning signs regarding IUCs. Explain how to check for expulsion and what to do in case of expulsion. Reassure the woman that the IUC will not affect breastfeeding and breast milk. Ensure that the woman understands the post-insertion instructions. These instructions should be reinforced again by the staff of the postpartum unit and repeated to the woman, and if possible with her family. | |
20. | Record information regarding the IUC insertion in the woman’s clinical file. | |
Post-insertion instructions to the woman |
There may be vaginal bleeding or spotting or cramping for the initial few days or weeks after insertion. These symptoms are normally experienced by women in the postpartum period. Take ibuprofen, paracetamol, or other pain reliever as needed. Persistent severe pain may indicate perforation, although this is rare for IUC insertion. Report persistent severe pain to your HCP. |
Spontaneous expulsion can happen in some cases, and is most likely to occur during the first 3 months postpartum. Be observant whether the IUC comes out. If it does, come to the health facility immediately for reinsertion or another contraceptive. |
At 6 weeks postpartum, the IUC strings can be felt by some women and/or their partners. It is not necessary for you to check the strings. You may come to the health facility if you have any concern about the strings. |
Remember that an IUC does not protect against STIs and HIV so if you are at risk of acquiring infection you should ask your partner to use a condom. You may resume intercourse at any time that you feel ready. |
Return for removal of the IUC at any time you decide that you want to become pregnant and you will have almost immediate return of fertility. |
Warning signs for the woman |
Before discharge, the following warning signs should be highlighted and the woman should be encouraged to call or come to the facility immediately for assessment:
|
Give a card to the woman with the following information in writing:
- type of IUC inserted;
- date of IUC insertion;
- month and year when the IUC will need to be removed or replaced;
- date of postpartum follow-up visit;
- where to go or call if she has problems or questions about her IUC.
Permanent methods
Both female sterilization and vasectomy can be safely performed at any time postnatally. However, there is an increased risk of regret when sterilization is requested in association with pregnancy. This is particularly the case when female sterilization is performed at the time of cesarean section and the women has felt pressurized into it by their healthcare provider.15,16 If sterilization is considered during the antenatal period, newborn survival rates should be discussed.
EFFECTIVE CONTRACEPTIVE METHODS
The lactational amennorhea method is effective in the first six months postpartum if the woman is breastfeeding exclusively, see above.
Progestogen-only injectable contraceptives and pills
Progestogen-only injectable contraceptives are provided by either the subcutaneous or intramuscular route and work primarily by inhibiting ovulation. Depot (intramuscular) and subcutaneous medroxyprogesterone acetate both last 13 weeks and norethisterone enanthate lasts 8 weeks. The WHO recommends that the risks may outweigh the benefits of providing the progestogen-only injectable before 6 weeks postpartum in breastfeeding women due to a theoretical concern regarding exposure of the neonate to DPMA/NET-EN.12 However, other organizations, including the Faculty of Sexual and Reproductive Healthcare and the Royal College of Obstetricians and Gynaecologists disagree with this advice and advise provision any time following childbirth is acceptable.8,10 The typical failure rate of 6% with injectable contraceptives is usually due to failure to get a repeat injection within the correct timescale. Amenorrhea is common with long-term use, with around 70% of women amenorrheic after 1 year of use. There may be a delay in the return of fertility with the use of this method of up to 1 year.
Checklist: what a woman needs to know about the progestogen-only injectable.
- It is 94% effective.
- Lasts 13 weeks.
- Can be given by intramuscular or subcutaneous injection (can self-administer by the subcutaneous route).
- Can be started at any time postnatally, including immediately before discharge home from the health facility.
- Common side effects include irregular bleeding, particularly in the first few months, with around 70% of women experiencing amenorrhea after 1 year.
- She should attend either after 13 weeks for a further injection, or if she is self-injecting, after 1 year for a review, or at any time if she has concerns.
- There can be a delay in fertility of up to 1 year after stopping the injectable.
- The injectable does not protect against STIs and HIV so if she is at risk of acquiring infection she should use condoms.
The progestogen-only pill can be started any time following childbirth. Traditional progestogen only pills primarily work by thinning the uterine endometrium and thickening cervical mucus, whereas newer desogestrel-containing pills also inhibit ovulation in most users. Traditional progestogen-only pills should be taken within 3 hours of the same time every day, whereas the desogestrel progestogen only pill should be taken within 12 hours of the same time every day. This requirement to remember a pill every day is the reason that both progestogen-only and combined pills have a typical failure rate of 9%. Progestogen-only pills may cause irregular bleeding patterns or amenorrhea. Fertility returns immediately once the woman ceases to take them.
Checklist: what a woman needs to know about the progestogen-only pill.
- It is 91% effective.
- One pill needs to be taken every day (within 3 or 12 hours, depending on the pill).
- If a pill is missed (i.e., more than 3 or 12 hours late), the pill should be taken as soon as remembered and then the next pill taken at the usual time and additional precautions (e.g. condoms) used for the next 2 days.
- Can be started at any time postnatally, including immediately before discharge home from the health facility.
- Common side effects include irregular bleeding and amenorrhea.
- Should attend before she runs out of the pill for further supplies. It is sensible to issue 1 year’s supply at the time if possible.
- Fertility returns immediately after stopping the pill.
- The pill does not protect against STIs and HIV so if she is at risk of acquiring infection she should use condoms.
Combined hormonal contraception
Combined hormonal contraception (CHC) works primarily by suppressing ovulation, due to exerting feedback on the hypothalamo-pituitary-ovarian axis resulting in suppression of LH and FSH. CHC can be administered orally (pills), transdermally (patches) or vaginally (vaginal ring). Patches are replaced weekly, vaginal rings every 3 weeks and pills are required to be taken every day. Traditionally, combined methods were designed to be taken for 3 weeks, followed by a 1 week break to allow the woman to have a regular withdrawal bleed. However, there is now guidance that allows women to take them in an extended format, which allow women to reduce the frequency of, or eliminate entirely, their withdrawal bleeds.17 Fertility returns immediately once the woman ceases to take CHC.
CHC, including the combined oral hormonal contraceptive pill (COCP) carries a risk of venous thromboembolism (VTE) and therefore should not be started prior to 3 weeks postpartum. In non-breastfeeding women, it may be started at, or after, 3 weeks postpartum as long as the woman does not have any additional risk factors for VTE, if she has additional risk factors for VTE, initiation should be delayed to 6 weeks postpartum. In breastfeeding women, initiation prior to 6 weeks is MEC 4, and between 6 weeks and 6 months MEC 3. This is due to inconsistent evidence regarding effects on breastfeeding continuation or exclusivity and a lack of evidence regarding long-term effects of estrogen on the neonate.12
Checklist: what a woman needs to know about the combined hormonal contraceptive pill.
- It is 91% effective.
- One pill needs to be taken every day (within 24 hours).
- If a pill is missed (i.e., more than 24 h late), the pill should be taken as soon as remembered and then the next pill taken at the usual time. If two or more pills are missed, the following rules apply:
- Additional precautions (i.e., condoms)/abstainence for the next 7 days.
- If there are seven or more pills left in the pack after the last missed pill – finish the pack, and take 7-day pill-free break as normal.
- If there are less than seven pills left in the pack after the missed pill – finish the pack and start a new pack the next day, i.e., miss out the pill-free break.
- Emergency contraception should be offered if two or more pills are missed in the first week of a pack and the woman had unprotected sex in the previous 7 days.
- Can be started after 3 weeks in non-breastfeeding women, and after 6 months in breastfeeding women.
- Common side effects include nausea, breast tenderness, and headaches. More serious adverse effects include venous thromboembolism (clots in the legs or lungs), but these are not common. She should be encouraged to return if she has any concerns about side effects.
- Should attend before she runs out of the pill for further supplies or sooner if she has any concerns. It is sensible to issue 1 year's supply at the time if possible.
- Fertility returns immediately after stopping the pill.
- The pill does not protect against STIs and HIV so if she is at risk of acquiring infection she should use condoms.
LESS EFFECTIVE CONTRACEPTIVE METHODS
Barrier methods
Male and female condoms can be used at any time postnatally and have the benefit of protecting against sexually transmitted infections. However, they have a high typical failure rate for pregnancy prevention due to the requirement for them to be used correctly and consistently for every episode of sexual intercourse. Women may wish to use both condoms and another, more effective contraceptive method together to give dual protection against pregnancy and sexually transmitted infections. Diaphragms also have a high typical use failure rate and do not protect against sexually transmitted infections. Their use should be delayed until 6 weeks after delivery when uterine involution is complete.
Fertility awareness methods
Fertility awareness methods include a variety of different techniques used by women to identify when in their cycle they are most and least likely to be fertile. They have a relatively high failure rate with typical use. Furthermore, they are not appropriate for use in the immediate postnatal period as the woman needs to have completed at least three or four menstrual cycles first.
FOLLOW UP FOR WOMEN USING CONTRACEPTION
Women who receive contraception should be followed up to ask about satisfaction with the method and side-effects. This is predominantly led by the women for LARCs, and COCPs can be provided in up to 1 year’s supply at a time. Follow-up for progesterone-only injectables can be at the time of the next injection. In some countries, including the UK, some women have been taught to self administer the injectable Sayana Press every 13 weeks (subcutaneous medroxyprogesterone acetate) and then a year’s supply of injectables may be provided at a time. Specific training is needed for some methods, such as IUC string checks for women who attend with problems or concerns about their IUC.
PRACTICE RECOMMENDATIONS
- Provision of postnatal contraception is a highly effective public health intervention that saves lives and therefore every opportunity to counsel and provide postnatal family planning should be taken.
- Ideally women (and their partners) should receive counseling on postnatal family planning during the antenatal period, so they can choose a method before delivery.
- Long-acting reversible contraceptive methods (LARCs) are the most effective contraceptive methods and the most likely to achieve optimal birth spacing. Women and their partners should be aware of the benefits of LARCs.
- Safety of contraceptive methods can be determined using WHO Medical Eligibility Criteria (MEC) criteria but in the majority of cases family planning is considerably safer than pregnancy.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
KEY RESOURCES
World Health Organization medical eligibility criteria for contraceptive use (WHOMEC).
https://www.who.int/reproductivehealth/publications/family_planning/Ex-Summ-MEC-5/en/.
RCOG Best Practice in Postpartum Family Planning. Best Practice Paper No. 1: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/bpp1/.
FSRH Guideline: Contraception after pregnancy https://www.fsrh.org/documents/contraception-after-pregnancy-guideline-january-2017/.
REFERENCES
Guttmacher Institute factsheet. Adding it up: Investing in contraception and maternal and newborn health. New York: Guttmacher Institute, 2017. Available from: https://www.guttmacher.org/fact-sheet/adding-it-up-contraception-mnh-2017. | |
Bigelow CA, Bryant AS. Short interpregnancy intervals: an evidence-based guide for clinicians. Obstet Gynecol Surv 2015;70:458–64. | |
Smith GCS, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study. Br Med J 2003;327:313. | |
Darroch JE, Audam S, Biddlecom A, et al. Adding it up: The costs and benefits of investing in sexual and reproductive health. New York: Guttmacher Institute, 2017. Available from: https://www.guttmacher.org/fact-sheet/adding-it-up-contraception-mnh-2017. | |
Rutstein SO. Effects of preceding birth intervals on neonatal, infant and under-five years mortality and nutritional status in developing countries: evidence from the demographic and health surveys. Int J Gynaecol Obstet 2005;89(1):S7–24. | |
World Health Organization. Report of a WHO Technical Consultation on Birth Spacing. Geneva: WHO, 2005. Available from: http://www.who.int/maternal_child_adolescent/documents/birth_spacing05/en/. | |
Barrett G, Pendry E, Peacock J, et al. Women’s sexual health after childbirth. BJOG 2000;107(2):186–95. | |
FSRH Guideline: Contraception after pregnancy. London: FSRH, 2017. Available from: https://www.fsrh.org/documents/contraception-after-pregnancy-guideline-january-2017/. | |
Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol 2015;126:5–11. | |
RCOG Best practice in postpartum family planning. Best Practice Paper No. 1. London: RCOG, 2015. Available from: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/bpp1/. | |
World Health Organization. Family planning: a global handbook for providers. Geneva: WHO, 2018. | |
World Health Organization. Medical eligibility criteria for contraceptive use, 5th edn. Geneva: WHO, 2015. | |
Goldthwaite LM, Shaw KA. Immediate postpartum provision of long-acting reversible contraception. Curr Opin Obstet Gynecol 2015;27:460–4. | |
Lopez LM, Bernholc A, Hubacher D, et al. Immediate postpartum insertion of intrauterine device for contraception. Cochrane Database Syst Rev 2015;6:CD003036. | |
Chi IC, Petta CA, McPheeters M. A review of safety, efficacy, pros and cons, and issues of puerperal tubal sterilization – an update. Adv Contracept 1995;11:187–206. | |
Emens JM, Olive JE. Timing of female sterilisation. Br Med J 1978;2:1126. | |
FSRH Guideline: Combined hormonal contraception. London: FSRH, 2012. Available from: https://www.fsrh.org/standards-and-guidance/documents/combined-hormonal-contraception/. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)