This chapter should be cited as follows:
Kumpel B, Hazell M, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.418853
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 16
The prevention and management of Rh disease
Volume Editors:
Professor Gerard HA Visser, Department of Obstetrics and Gynaecology, University Hospital of Utrecht, Heidelberglaan 100, Utrecht 3584EA, The Netherlands
Professor Gian Carlo Di Renzo, University of Perugia, Italy

Chapter
Fetomaternal Hemorrhage and Laboratory Methods for its Determination
First published: January 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
DISCOVERY OF FETOMATERNAL HEMORRHAGE
In 1948, Wiener1 concluded that two cases of severe anemia of newborn infants were due to occult (unseen) placental hemorrhage during pregnancy or parturition. The bleeding was from the fetal surface of the placenta into the maternal circulation (he termed this fetomaternal transfusion) and the loss of blood had led to post-hemorrhagic shock in the babies.1 At birth, the infants’ hemoglobin (Hb) levels were 8.7 and 6.5 g per 100 mL. Chown2 later described a similar case of an infant with severe anemia (Hb 7.8 g per 100 mL), proven by differential red-cell (RBC) agglutination with anti-D, antibody absorption and biochemical analysis of fetal and maternal Hb to be caused by fetomaternal transfusion. Because 5–10% of the RBC in the mother’s circulation were fetal RBC and she rapidly developed anti-D 3 weeks after delivery, he concluded that the fetomaternal transfusion may have been ongoing for weeks.2
CAUSES OF FETOMATERNAL HEMORRHAGE
Fetal blood circulates via the umbilical cord through the placenta to gain oxygen, nutrients and IgG from maternal blood. The large placental blood vessels branch repeatedly, through finger-like villi, forming capillaries near the surface of terminal villi. These are bathed in maternal blood from uterine arteries. Syncytiotrophoblasts form the entire outer surface of the villi and are metabolically very active, mediating the transport of many substances from maternal blood to the fetal capillaries. They are continuously replaced by underlying cytotrophoblasts; this is normally well controlled but occasionally there may be small breaks in the epithelial cover.3,4 Because the blood pressure in the fetal circulation is higher than that in the surrounding maternal blood, any breaks in the epithelium means blood will flow from the fetus to the mother, i.e., fetomaternal hemorrhage (FMH). This was originally known as fetomaternal transfusion or transplacental hemorrhage (TPH) but these are less specific than FMH. Passage of blood in the opposite direction, from the mother to the fetus (maternal–fetal hemorrhage), seldom occurs, then in tiny amounts and is of no known significance.5
In some cases, massive FMH can be caused by intraplacental choriocarcinoma (IC), when a nodule approximately 2–3 cm wide develops in placental tissue adjacent to maternal blood, destroying the syncytiotrophoblast barrier at that site. The presentation of massive FMH with severe neonatal anemia and IC was first reported in 1962.6 Trophoblast cells in a nodule were positive for β-human chorionic gonadotropin (HCG).7 Another case had high maternal serum levels of hCG at 37 weeks.8 Because IC in pregnancy is rare and develops without obvious symptoms, fatal antepartum FMH may remain unpredictable. However, surveillance of β-HCG levels might reduce mortality.9
Of 134 babies with FMH >50 dL, a cause was not known in 82%, although presenting symptoms (which were too late) were decreased or absent fetal body movements (27%), neonatal anemia (35%), and unexpected stillbirth (12.5%).10 Trauma, such as a fall, may cause FMH but in reality this is unpredictable; only two women of six had FMH after trauma at 17–35 weeks' gestational age (WGA), the case at 37 WGA being an intrauterine death (IUD).11 Various obstetric interventions may cause FMH,12 although no significant differences were found in the incidence of large FMH between “high-risk” cesarean section and normal “low-risk” vaginal deliveries.13,14
Most FMHs occur without symptoms, so the cause of FMH is rarely found.
INCIDENCE, VOLUME, AND CONSEQUENCES OF FMH FOR THE FETUS
Massive FMH is relatively uncommon; the incidence was estimated at approximately 1 in 1000 births (0.1%) for FMH ≥50 mL,15 1 in 1146 (0.09%) for ≥80 mL, and 1 in 2813 (0.035%) for ≥150 mL fetal blood.16 Since the fetal blood volume at term is approximately 250 mL and the placental blood volume is about 150 mL, totaling 400 mL,17 the loss of 150 mL comprises nearly 40% of the feto-placental blood, explaining the severity of fetal and neonatal anemia. With FMHs ≥150 mL, 15 of 41 infants died.18 Of eight cases of massive FMH (over 200 mL), five babies died (four IUDs and one neonatal death) with average FMH of 238 mL blood, and three survived (with severe anemia (Hb 30–38 g/L) requiring transfusion) with average FMH of 340 mL blood.11 IUDs caused by massive FMH occurred at 36 WGA on average.11,15,19 Fortunately, after the perinatal period, surviving infants generally make a good recovery.15,16,20 A large study found no reduction in rates of massive FMH between 1987 and 2012.19
Many women have little or no detectable fetal blood in their circulation during pregnancy, and then only in small volumes (<0.1 mL) that may increase slightly in the third trimester.21,22 The incidence of antenatal FMH in Kenya and Thailand was reported to be higher than in Caucasians.23,24 After delivery, the incidence and volume of FMH is usually greater.21,22 This explains why more women are D-immunized after delivery than during pregnancy.
Mollison25 plotted the available data from three large studies of the estimates of the magnitude of FMH on a logarithmic scale against the cumulative frequency of FMH following normal delivery, also on a logarithmic scale. This can be easily memorized as follows:
- Approximately 0.1% of women have FMH ≥30 mL RBC
- Approximately 0.3% of women have FMH ≥10 mL RBC
- Approximately 1% of women have FMH ≥3 mL RBC
- Approximately 3% of women have FMH ≥1 mL RBC
- Approximately 10% of women have FMH ≥0.3 mL RBC
- Approximately 30% of women have FMH ≥0.1 mL RBC
Slightly different figures for FMH were quoted by Sebring and Polesky,18 given in volumes of whole blood (approximately twice that of RBC):
- Less than 0.5 mL fetal blood in 93% of women (~93% of women have <0.25 mL RBC)
- Less than 1 mL fetal blood in 96% of women (~96% of women have <0.5 mL RBC)
- Less than 2 mL fetal blood in 98% of women (~98% of women have <1 mL RBC)
- More than 30 mL fetal blood in 0.3% of women (~0.3% of women have >15 mL RBC)
CONSEQUENCES OF FMH FOR THE D-NEGATIVE MOTHER
Pregnant and more especially post-partum D-negative women are at risk of becoming D-immunized in response to FMH of D-positive blood. They need to receive an adequate dose of anti-D immunoglobulin (anti-D Ig) to destroy all the fetal RBC in their circulation in order to prevent D-immunization. D-immunization risks HDFN in future D-positive pregnancies.
Countries have adopted different practices to prevent D-immunization. In the UK, standard doses of prophylactic anti-D Ig are 500 iu (100 μg), which is considered sufficient to clear up to 4 mL fetal RBC, 1250 iu (250 μg) to destroy up to 10 mL RBC and 1500 iu (300 μg) to remove up to 12 mL RBC.12 In the USA, the dose is 300 μg (1500 iu), which is considered to suppress immunization by 30 mL blood (or 15 mL RBC).26 In Australia and New Zealand, doses are 625 IU (125 μg), which will protect against 6 mL RBC and 250 IU (50 μg) for additional dosing for larger FMH and protection in the first trimester of pregnancy.27 Confusingly, these figures and the notations used are slightly different. None of these doses will prevent D-immunization of the few women who have a large FMH.
Therefore, there will be some women who will not be protected by a “standard dose” of anti-D Ig. The minimum volume of fetal RBC that will immunize pregnant and post-partum women is thought to be approximately 0.1 mL.25 This occurs in about 30% of these women and without anti-D Ig, about 15% are immunized by their pregnancy. Therefore, it is best practice for all D-negative women to be screened after delivery for FMH and for those with a positive result to have the volume of FMH measured.
FMH quantitation is also performed to aid diagnosis of obstetric complications, often in women with no D incompatibility.11 As shown above, large volumes of FMH can lead to fetal death or severe neonatal anemia requiring prompt transfusion.
Determination and quantitation of FMH
Methods to detect and quantitate FMH determine either the percentage of RBCs containing fetal Hb or the percentage of RBCs expressing the D antigen in maternal blood. The first can be used for patients with any blood group and both are applicable for calculating the correct doses of prophylactic anti-D Ig. Two detailed reviews describing the methods available at the time were published in 201026 and 2011.28
RBC with adult and fetal Hb
Hemoglobin (Hb) is a tetramer comprising two alpha chains and either two beta chains with adult Hb (HbA) or two gamma chains with fetal Hb (HbF). HbF can bind oxygen at lower oxygen levels (as the situation in the fetus) than HbA. The fetus has exclusively HbF in RBCs until approximately 30 WGA when the gene for adult Hb is switched on and HbA begins to be synthesized, in addition to HbF. At term, 40 WGA, HbF predominates, comprising approximately 50–70% in fetal RBCs.29 Later in infancy the HbF gene is not entirely switched off; although most adult RBCs have only HbA, some RBCs (usually less than 5%) contain some HbF.30 These are known as F cells. Hereditary persistence of fetal hemoglobin (HPFH) occurs when the numbers of F cells are greatly increased. In some hematological conditions (delta-beta-thalassemia, sickle-cell disease treated with hydroxyurea and some leukemias) F cells can predominate.31
KLEIHAUER-BETKE ACID-ELUTION TEST
A short report in 1957 showed that HbA was eluted from RBCs on glass slides by acidified citrate-phosphate buffer, whereas HbF was not. After counterstaining with May–Grünwald–Giemsa, RBCs with HbF were easily seen among the majority of “ghost” RBCs.32 The first FMH assay was done by Zipursky and colleagues by adapting this acid-elution (AE) test. They made mixtures of fetal RBCs added to adult blood to determine the quantifiable ranges and the best method of counting, then they used this test to investigate blood from 42 postnatal women. Nine women were found to have FMH, all except one of less than 1 ml blood.33 Subsequently, Kleihauer and Betke published their technical procedure, which is known as the Kleihauer–Betke test or the acid-elution assay,34 here abbreviated to the KB/AE test.
Standardized reagents and kits for performing the KB/AE assay are readily available from many companies, with protocols for performing the test. The BCSH Guidelines 200912 describe the procedure below in detail.
Counting fetal and adult RBCs fixed on slides
The equipment required includes a benchtop microscope with two 10× eyepieces, one 10× objective, one 40× objective, and a graticule (Miller square or Indexed square grid, Figure 1). Glass rectangular slides, staining troughs and pipettes are also necessary. The graticule fits in one eyepiece and enables accurate counting. A Miller square has a large square and a small square, 1/9th of the area, inside it. An Indexed square grid has a large square with 100 smaller squares inside it. These enable the adult RBC “ghosts” to be counted in one or more of the smaller squares and the far less numerous stained fetal RBC to be counted in the large square areas. A “clicker” hand-held counter is helpful.
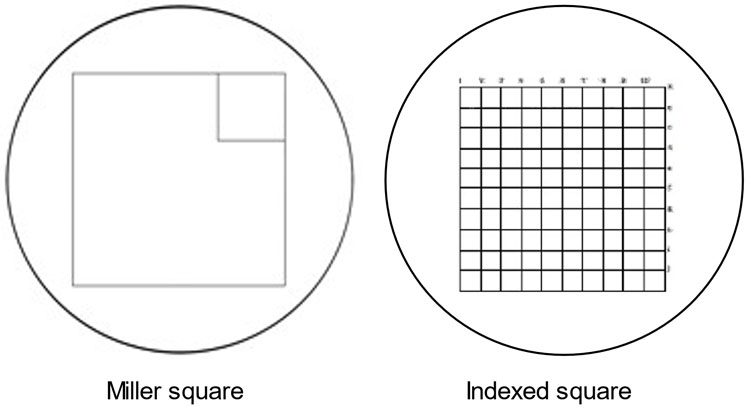
1
Miller square and Indexed square graticules. Miller square and Indexed square graticules for use in microscopic counting of RBC in the KB/AE assay.
Blood collected in EDTA is diluted 1 in 2 or 1 in 3 with normal saline and mixed well. Controls are normal adult blood (negative control) and normal adult blood containing 1% (1 : 100) ABO matched umbilical cord blood (positive control), diluted as above in saline. Blood smears are made on clean, dry slides, as follows. Label the slides. Add a drop of diluted blood near one end of a slide then using another slide, place it at an angle on the drop until the blood is spread sideways across the width of the slide and then swiftly push the blood along the slide. This should be done in a continuous motion because it is essential to have an even spread of cells throughout the slide. The RBCs should be close or touching each other but not overlapping. (Practise first!) Air dry the blood films by standing the slides upright. Fix the cells in fixative (20% ethanol) for 5 minutes, air dry vertically for 10 minutes, place in the acidified staining solution (containing hematoxylin) for the required time, rinse in water, stain with contrast stain (eosin or erythrosine) then rinse and air dry.
First, examine the controls to check the staining of fetal cells is dark and the elution of HbA from adult cells is satisfactory; the RBCs on the films must be evenly spread, close together but not overlapping. Variability of the density of RBCs on blood films results in large errors. Leucocytes should be stained blue/purple. Second, do a quick screen. Count all the fetal RBCs in 25 fields of view under low power (10× objective). These fields are the circle of cells visible; the slide must be moved so that each field is not overlapping the next. (Most microscopes have a stage on which the slide is fixed; this can be moved horizontally in two directions so that adjacent fields can be systematically viewed.) If there are fewer than 10 fetal RBCs in the 25 fields, then the FMH is <2 mL RBC or <4 mL blood and there is no need to count any more. If there are more than 10 fetal RBCs in the 25 low-power fields, a detailed count must be made.
Using the Miller square and the 40× objective (high power), count all the adult “ghost” RBCs in the small inner square. Then multiply by 9 to estimate the total in the large square (= A1). Still using the 40× objective, count all the dark red/pink stained RBCs in the large square (high power) (= F1). Repeat these counts in different but adjacent high-power fields (A2, F2; A3, F3 etc) until a total of at least 10,000 adult cells (Atotal) have been surveyed. Add up the fetal RBCs from each field of view (Ftotal). (When using an Indexed square instead of a Miller square, the adult “ghost” RBCs in 10 of the 100 squares are counted and multiplied by 10 to estimate the total.)
The calculation of FMH assumes that the maternal RBC volume is 1800 mL, that fetal RBCs are 22% larger than maternal RBCs and that only 92% of fetal RBCs stain darkly. The Mollison formula35 is:
| FMH = | Number of fetal cells per high-power field | × | 1800 | × | 122 | × | 100 | |
| Number of adult cells per high-power field | 100 | 92 |
or simplified to:
| FMH = | Ftotal | × | 2400 | |
| Atotal |
or FMH (mL packed RBC) = % fetal cells × 24
or FMH (mL fetal blood) = % fetal cells × 48
The accuracy of results depends on the number of RBCs surveyed (more is best; 2,000 cells were insufficient, compared to 10,000)36 and the correctness of the assumptions above taken for the several variables listed above. Other formulas are used, listed by Kim and Makar.28 Poorly visible adult “ghost” cells may be missed, giving counting errors.28 The presence of maternal F cells that are stained to differing extents has also been recognized as a problem for counting, usually leading to overestimation of FMH by the KB/AE test.37,38,39 (See Figure 2 for examples.)
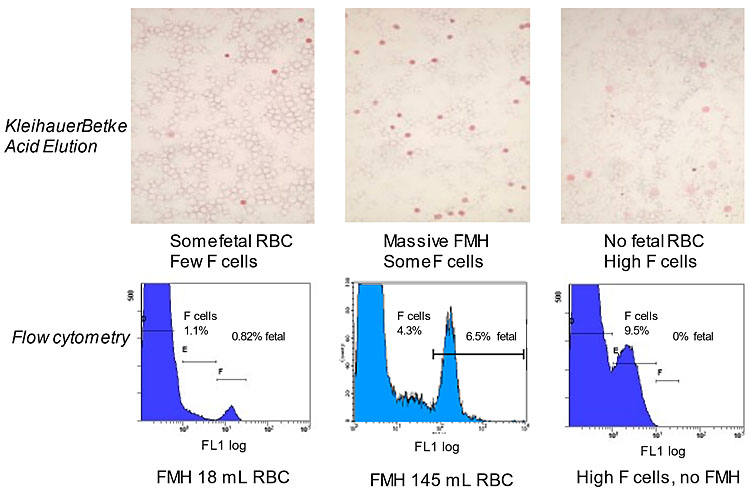
2
Assays of HbF-positive RBC. Top row: photomicrographs of cells in the KB/AE assay. Fetal cells stain dark pink/red. Maternal F cells are stained variably, from very pale to dark, but rarely as dark as fetal RBC. They also appear slightly larger. Maternal cells without HbF are not stained, hence appearing as “ghosts”. Bottom row: the same samples tested by flow cytometry with anti-HbF-FITC. The maternal cells with no or low HbF merge with F cells because the flow cytometric assay is sensitive to very small amounts of HbF in the RBC. The fetal RBC may also merge with F cells if they are at high concentration. Placing the two markers over the populations enables much more accurate quantitation than is possible with the KB/AE assay. FMH is recorded as packed RBCs.
Most (85%) normal adults have less than 0.6% of total Hb as HbF, which is restricted to F cells that comprise up to 4.4% of the total RBC. However, HbF and F cells can be tenfold higher, under genetic control.40 Thus the presence of increased F cells in normal maternal blood samples cannot be predicted. Of 12 patients misdiagnosed with FMH by KB/AE tests and given high doses of prophylactic anti-D, all were found (by flow cytometry) to have no FMH but high (mean 13%) F cells.11 Furthermore, 30% of maternal samples with estimated FMH of >2 mL by KB/AE actually had elevated F-cells (over 4%) but no FMH.41 Two recent studies in Ireland found 3% of pregnant and post-partum women had >16% F cells38 and 22% of maternal and gynecological patients had >10% F cells.39
Therefore, if the intensity of staining ranges from very pale to dark in different RBC, this strongly indicates they are F cells, which should not be counted.
ROSETTE SCREEN
Developed by Sebring and Polesky in 1982,18 this is one of the earliest FMH tests. It is a microscopic test for the presence of fetal D-positive RBCs in maternal D-negative blood.
First, a drop of IgG anti-D is added to a drop of a 3% suspension of maternal blood and incubated at 37°C for 15 minutes, followed by washing the cells 4 times in saline. Then a drop of indicator RBCs are added; this is a 0.5% suspension of ficin-treated group OR2R2 cells. The cells are mixed then centrifuged and the sedimented RBCs suspended in saline. A drop is placed on a microscope slide and examined for the presence of clumps of RBCs at low power (100×). These aggregated (agglutinated) RBC are the indicator cells captured by the anti-D molecules that were adhering to a fetal D-positive cell. Photographs are shown.26,28 A positive result is if 3 or more aggregates (rosettes) are observed in nine low-power fields. This indicates the FMH was approximately 10 mL blood (4 mL RBC) and it should be quantified by a more sensitive test, such as the KB/AE or a flow cytometry test. The rosette assay cannot assess D-negative FMH or those in D-positive women. It is performed predominantly in the USA.
Other assays testing for D-positive RBC in D-negative maternal blood were developed, enzyme-linked antiglobulin tests42,43 and gel agglutination card tests.44,45 Unfortunately, none of these convenient bench-top assays were sensitive enough to detect and quantitate 0.2% (4.4 mL) D-positive RBC.
FLOW CYTOMETRY
Flow cytometers are instruments that detect individual cells passing through a very narrow tube. They have two or more lasers that can detect molecules, which emit fluorescence on being excited by light of certain wavelengths. There are many fluorochromes available, which can be bound to monoclonal antibodies (mAbs) with specificity for cell-surface antigens. The lasers independently measure the quantity and type of fluorescence emitted by each cell at a particular wavelength as they pass at high speed though the machine, allowing the collection of huge amounts of data. Powerful in-built computers enable storage and analysis of the data. There are now probably thousands of different mAb-fluorochrome combinations, which have enormously expanded the knowledge in some areas of medicine by their use in the analysis of mixtures of cells. For determination of FMH, mAbs to the D antigen and to HbF conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE) are commonly used.
Only small samples of cells are needed for flow cytometry. Mix the blood sample well, add 50 μL of blood to 2 mL phosphate buffered saline (PBS) in a tube and centrifuge at 1000 × g in a semi-fixed rotor centrifuge for 1 minute, aspirate the fluid and resuspend the cells in 500 μL PBS. This is approximately a 5% suspension of RBC. Controls comprising 1% and 0.2% fetal D-positive RBC in adult D-negative can be used.
FLOW CYTOMETRY TO DETECT D-POSITIVE FETAL RBC
Label the RBC: Add 5 μL of anti-D (BRAD3-FITC)11 to 45 μL of PBS, mix, add 20 μL of the 5% RBC suspension, mix, incubate at 37°C for 30 minutes, wash once in PBS. Add 1 mL PBS for analysis.
Analysis: Gate the cells on log forward scatter (FS) versus log side scatter (SS) dot plots to exclude debris and agglutinated RBC, then (optionally) gate on SS versus FL2 (PE) dot plots. Set up a histogram of FL1 (FITC) fluorescence of the twice-gated RBCs with the Y-axis expanded to approximately 0–500 counts in order to show the peaks of fetal cells, although the top of the large D-negative RBC peak will be cut off. The X-axis shows the level of fluorescence of each cell. Place markers over the low fluorescent adult cells (D-negative RBC) and the cells with high fluorescence (D-positive RBC). Analyze 100,000–500,000 events. The computer will determine the percentages and mean fluorescence of the cells under the two markers.
The calculation of FMH assumes that the average maternal RBC volume is 1800 mL and that fetal RBCs are on average 22% larger than adult RBCs.46 The volume of FMH (mL packed fetal RBCs) is calculated as:
| % events under the marker for D-positive cells | × | 1800 | × | 122 | × | 100 | = | % positive events | × | 21.96 |
| 100 | 100 |
or FMH (mL packed RBCs) = % D-positive cells × 22
or FMH (mL fetal blood) = % D-positive cells × 44
Comments: There may be many cells with fluorescence between the D-negative and D-positive populations. These are granulocytes (mainly neutrophils) from maternal blood. They have Fc receptors and bind IgG through the Fc portion, therefore also capturing anti-D-FITC. Granulocytes are dense and sediment with RBC on centrifugation. The stress of labor induces an inflammatory response with outpouring of leucocytes.47 When measuring the neutrophil counts of 67 FMH patients and 40 female blood donors it was found the patients had markedly increased numbers of neutrophils (Figure 3) compared to the donors.41 A negative control, AEVZ5.3-FITC, had been introduced as an isotope control earlier but without a clinical trial and was later found to give erroneous results; of 36 postpartum samples with no FMH, this method gave 50% of samples a negative FMH of minus 0.3 to minus 4.5 mL and only 36% had a correct (zero) result.41 However, the granulocytes can be easily excluded from analysis by adding 5 μL of anti-CD66b/granulocyte (BIRMA17C-PE) to the RBCs together with BRAD3-FITC. The PE-labeled granulocytes have high fluorescence in the FL2 region outside the gate on the SS versus FL2 plot (Figure 4). Conjugated mAbs to leucocytes (CD45-PE) may be used11,48 but have lower fluorescence than BIRMA17C-PE.41
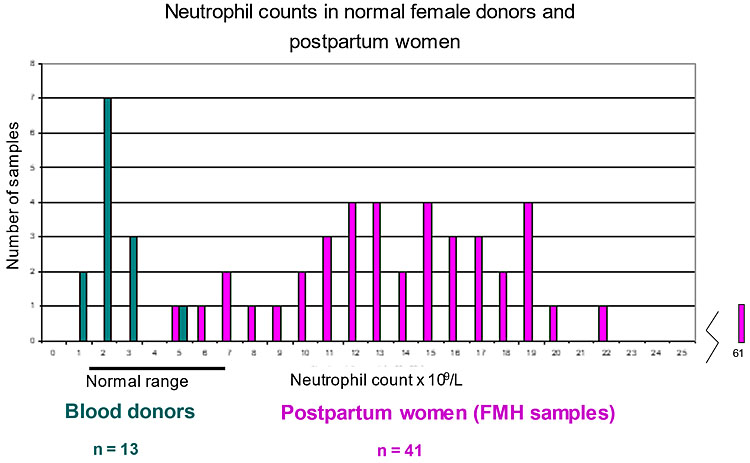
3
Neutrophil counts in blood of women. Comparison of the neutrophil counts in blood of female donors and FMH samples (postpartum maternal blood) obtained with a hematology blood analyzer. There is almost no overlap between the two populations. The median counts for donors was 3.97 × 109 cells/L and for postpartum patients was 10.28 × 109 cells/L. Neutrophils are the most numerous granulocytes in blood.
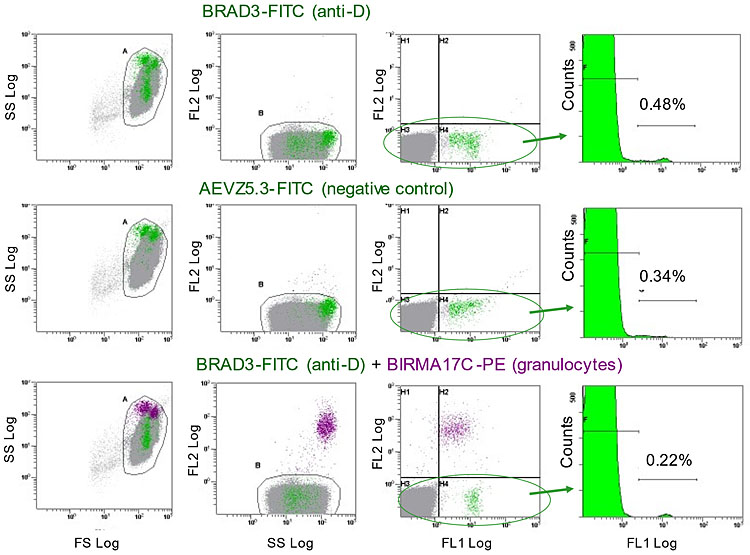
4
Flow cytometry analysis of D-positive RBC in FMH. Method of analysis of FMH by flow cytometry in a representative patient. Three dot plots and one histogram are set up on the flow cytometer. The dot plots are forward scatter logarithmic (FS Log) versus side scatter (SS Log), SS Log versus fluorescence channel 2 (FL2 Log), FL1 Log versus FL2 Log and the histogram is of FL1 Log versus Counts (events). A gate (A) is placed around the dense area of cells in the FS versus SS plot and these cells are then displayed on the SS versus FL2 plot. The PE labeled granulocytes are separated on this plot and are eliminated from analysis by placing a second gate, B, over the main group of cells. The FL1 versus FL2 plot shows how the FITC-labeled cells are separated from the unlabeled majority. Finally, the cells in the gate B, or another gate in FL1 versus FL2 encompassing only the FITC-labeled cells, are displayed in the histogram. Markers are placed over the labeled cells and the computer gives their percentage of the total. The FMH = % positive events × 22 (mL RBC).
For this patient, BRAD3-FITC labeling resulted in a FMH estimate of 10.6 mL RBC. AEVZ5.3-FITC labeling resulted in a FMH estimate of 7.5 mL RBC; these are labeled granulocytes. Using this negative control gives the FMH as 10.6 minus 7.5 = 3.1 mL RBC. This is below the level of 4 mL, which is the maximum the routine dose of 500 iu anti-D Ig will protect against D-immunization – the patient would not need an extra dose. However, after eliminating the granulocytes with BIRMA17C-PE, the FMH is correctly found to be 4.8 mL, requiring an additional dose. The assay with BRAD3-FITC + BIRMA17C-PE is quicker, needs no subtraction calculation and is much more accurate.
If a patient has been given anti-D Ig before a blood sample is taken for FMH testing, the FMH test can be performed as usual because the concentration of prophylactic anti-D is too low to block the D-antigen sites.49
FLOW CYTOMETRY TO DETECT RBC WITH HBF
The first reports of the use of FITC-conjugated anti-HbF for FMH quantitation appeared in 1998.50,51 Because the HbF molecules are inside RBC, the cells have to be fixed and then permeabilized before incubating with the antibody.
Fixing the RBC: Add 20 μL glutaraldehyde to 1 mL of PBS (0.05%), mix, add 20 μL of the 5% suspension of RBC, retain at room temperature (RT) for 10 minutes, then wash in PBS 3 times.
Permeabilize the RBC: Add 0.5 mL PBS containing 0.1% Triton X-100 plus 0.1% bovine serum albumin to the fixed RBC pellet, resuspend the RBCs, retain at RT for 5 minutes, wash in PBS.
Label the RBC: Add 10 μL of anti-HbF-FITC conjugate, retain at RT for 15 minutes in the dark, wash in PBS. Add 1 mL PBS, keep in the dark before analysis.
Analysis: Gate the cells on log forward scatter (FS) versus log side scatter (SS) dot plots to exclude debris and agglutinated RBC, then gate on SS versus FL2 dot plots to exclude autofluorescent (FL2-positive) granulocytes (analogous to the BIRMA17C-PE exclusion). Set up a histogram of FL1 fluorescence of the twice-gated RBC with the Y-axis expanded to approximately 0–500 counts in order to show the peaks of fetal cells, cutting off the top of the peak of adult cells. The X-axis shows the level of fluorescence of each cell. Place markers over the cells with medium fluorescence (F cells) and cells with the highest fluorescence (fetal cells). Analyze 100,000–500,000 events. The computer will determine the percentages and mean fluorescence of the cells under the two markers (Figures 2 and 4).
The calculation of FMH assumes that the maternal RBC volume is 1800 mL and that fetal RBC are 22% larger than maternal RBC.46
FMH (mL packed RBC) = % positive events under the marker for fetal cells × 22
FMH (mL fetal blood) = % positive events under the marker for fetal cells × 44
Comments: The blood samples should be tested as soon as possible because as the blood ages the RBCs become more agglutinated after the fixing, permeabilizing and staining procedures. This shows as particles with high FS and SS falling outside the gated area, leading to erroneous quantitation of FMH. The amount of anti-HbF-FITC added should be accurate; if more (50 μL), the percentage of F cells may increase, if less (1 in 10 dilution) the FMH will be lower.11 This error in dilution occurred in one study, which then underestimated the FMH.52 It is important to read the small print in publications.
FLOW CYTOMETRY USING ANTIBODIES TO HBF AND ADULT RBC SIMULTANEOUSLY
A Fetal Cell Count Kit (FCC kit) containing anti-HbF-FITC and anti-CA-PE (carbonic anhydrase, present in adult RBC) can distinguish between fetal RBCs (HbF+, CA−), F cells (HbF+, CA+) and adult RBCs (HbF−, CA+). This enabled fetal cells to be evaluated when maternal F-cells were high.53 Another study compared the FCC kit with single-color HbF flow cytometry for quantitation of fetal cells in pregnant patients with hemoglobinopathies. The dual-color staining consistently underestimated the quantity of fetal cells, showing the difficulties with high F-cells in these patients.54 In two cases of massive FMH (>100 mL RBC), fetal cells were clearly separated from F cells (very high, 11.5%, in one case) with the FCC kit, but again the quantitation of fetal cells was lower than in the standard single-color HbF assay, considered to be due to some hemolysis in the assay.11 Another study found the KB/AE, the anti-HbF flow cytometry, the anti-D flow cytometry and the FCC kit all had advantages and disadvantages in different clinical situations.36
COMPARISON OF FMH ASSAYS
Nance and colleagues in 198955 compared the KB/AE, Rosette, and anti-D flow cytometry assays, finding flow cytometry was more accurate, reproducible, and sensitive than the earlier tests and KB/AE overestimated the FMHs. Workers in India56 and Ethiopia57 found the FMHs estimated by KB/AE and anti-D flow cytometry correlated well. Others recorded the KB/AE overestimated the FMH when compared to anti-HbF flow cytometry11,37,38,39,58 and also compared to anti-D flow cytometry.11,37,55
High-performance liquid chromatography (HPLC) is used for hemoglobinopathy screening of pregnant women in some areas. HPLC was not found to be able to detect the raised levels of HbF in FMH samples (determined by KB/AE and flow cytometry), even in cases with high F cells or massive FMH.37
FMH DETERMINATION BY HEMATOLOGY ANALYZERS
Three studies have been found. The first used an Abbott Cell-Dyn CD4000 hematology analyzer in Scotland to measure FMH of cells labeled with FITC-anti-D (probably Quant-Rho-FITC) using artificial standards and clinical samples. There was an excellent agreement between observed and expected FMH. The limit of detection was <1 mL and 1.6 mL FMH could be quantified. The analyzer performance was similar to that for anti-D flow cytometry. Parallel KB/AE assessments gave higher values.59
A CELL-DYN Sapphire hematology analyser (Abbott Diagnostics) was used to analyze 236 blood samples from pregnant women after labeling with anti-HbF-FITC plus propidium iodide nuclear stain (FMH QuikQuant kit) and comparing with HbF flow cytometry as reference. Although the maximum FMH was 11 mL RBC, there were no results from the analyzer.60 A photograph showed a screen shot of the analyzer with a very high simulated FMH of 1.75% (38.5 mL RBC) that show a small peak of fetal cells, but this represents a rare massive FMH.25 Another study used a similar procedure with artificial FMH preparations and post-partum samples to study the hemoglobin assessed by oximetry and spectrophotometry in an automatic blood analyzer (ABA ABL800flex, Radiometer Medical) with HbF flow cytometry. There was a low correlation between HbF flow cytometry FMH and the analyzer, the fetal and F cells were not distinguished by the analyzer and it overestimated the FMH.61
Analysis of fetal Hb by blood analyzers encounters the same difficulty as HPLC – which is because a FMH may be large, the fetal Hb is still a small proportion in adult blood that has F cells. However, it is surprising that hematology analyzers have not been used to quantitate FMH labeled with anti-D. The use of BRAD3-FITC appears to be superior to QuantRho-FITC because it gave >3 times greater fluorescence with three different D-positive cells.11
1
Comparison of equipment, reagents, and assays for methods described in the text.
Assays for HbF-positive RBC | Assays for D-positive RBC |
KB/AE test (Kleihauer) Equipment: Reagents: Assay: Results: | Rosette test Equipment: Reagents: Assay: Results: |
Anti-HbF flow cytometry Equipment: Reagents: Assay time: Results: | Anti-D flow cytometry Equipment: Reagents: Assay time: Results: |
PRACTICE RECOMMENDATIONS
- The current routine doses of anti-D Ig will not protect all D-negative women pregnant with a D-positive fetus against D-immunization because a few women will have FMH, which exceed the doses.
- Women should be screened approximately one hour after delivery or potential antenatal sensitizing events to discover those with high FMH. Anti-coagulated peripheral blood samples should be analyzed in laboratories as rapidly as possible.
- The method of analysis chosen will depend on several variables including the costs to run the assays, the precision of the results required, the technical expertise available, and the ability to afford the prophylaxis medication.
- The accuracy of the assays depends greatly on the number of RBC assessed because the fetal cells are rare and will be randomly distributed among the maternal cells. It is advised to count 10,000 maternal cells in the KB/AE tests and 500,000 cells in the flow cytometry assays.
- One inaccuracy is the variability of maternal blood volume that is rarely known.
- Therefore, it is advisable to always err on the side of caution and give more, rather than less, anti-D Ig prophylaxis.
REAGENTS AND SUPPLIERS
BRAD 3 FITC (Anti-D): IBGRL Research Products; IQ Products
AEVZ 5.3 FITC: IBGRL Research Products; IQ Products
BIRMA 17C PE (Anti-Granulocyte): IBGRL Research Products
Quant-Rho FITC (Anti-D): Alpha Laboratories; Quotient Biodiagnostics
Anti-Com-DF (anti-D PE, anti-HbF FITC): Millipore; Sigma
HbF FITC (anti-HbF): Chemicon; Caltag Medsystems; BD Biosciences; ThermoFisher; Miltenyi Biotech
FMH QuikQuant (anti-HbF FITC, propidium iodide (PI): IQ Products; Caltag Medsystems
Fetal Cell Count Kit (anti-HbF FITC, anti-CA PE): IQ Products; Caltag Medsystems
FETALtrol (stabilized fetal D-positive RBC in D-negative RBC; low, medium, high): IQ Products; Caltag Medsystems; ThermoFisher; other companies
CONFLICTS OF INTEREST
Author(s) statement awaited.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Wiener AS. Diagnosis and treatment of anemia of the newborn caused by occult placental hemorrhage. Am J Obstet Gynecol 1948;56(4):717–22. | |
Chown B. Anaemia from bleeding of the fetus into the mother’s circulation. Lancet 1954;266(6824):1213–5. | |
Kumpel BM, Sibley K, Jackson DJ, et al. Ultrastructural localization of glycoprotein IIIa (GPIIIa, β3 integrin) on placental syncytiotrophoblast microvilli: implications for platelet alloimmunization during pregnancy. Transfusion 2008;48(10):2077–86. | |
Kumpel BM, Manoussaka MS. Placental immunology and maternal alloimmune responses. Vox Sang 2012;102(1):2–12. | |
Brossard Y, Pons JC, Jrad I, et al. Maternal-fetal hemorrhage: a reappraisal. Vox Sang 1996;71(2):103–7. | |
Benson PF, Goldsmith KLG, Rankin GLS. Massive foetal haemorrhage into fetal circulation as a complication of choriocarcinoma. BMJ 1962;1(5281):841–2. | |
Takai N, Miyazaki, T, Yoshimatsu J, et al. Intraplacental choriocarcinoma with fetomaternal transfusion. Pathol Int 2000;50(3):258–61. | |
Aso K, Tsukimori K, Yumoto Y, et al. Prenatal findings in a case of massive fetomaternal hemorrhage associated with intraplacental choriocarcinoma. Fetal Diagn Ther 2009;25(1):158–62. | |
Hookins B, Vatsayan A. Intraplacental choriocarcinoma and fetomaternal haemorrhage and maternal disseminated intravascular coagulopathy in a term pregnancy: A case report. Case Reports in Women’s Health 2020;27:e00216. | |
Kumpel BM, MacDonald AP, Bishop DR, et al. Quantitation of fetomaternal haemorrhage and F cells in unusual maternal blood samples by flow cytometry using anti-D and anti-HbF. Transfus Med 2013;23(3):175–86. | |
Giacoia GP. Severe fetomaternal hemorrhage: a review. Obstet Gynecol Surv 1997;52(6):372–80. | |
Austin E, Bates S, de Silva M, et al. Guidelines for the estimation of fetomaternal haemorrhage. Working party of the British Committee for Standards in Haematology, Transfusion Taskforce, 2009. | |
Ness PM, Baldwin ML, Niebyl JR. Clinical high-risk designation does not predict excess fetal-maternal hemorrhage. Am J Obstet Gynecol 1987;156(1):154–8. | |
Lubusky M, Simetka O, Studnikova M, et al. Fetomaternal hemorrhage in normal vaginal delivery and in delivery by cesarian section. Transfusion 2012;52(9):1977–82. | |
Rubod C, Deruelle P, Le Goueff F, et al. Long-term prognosis for infants after massive fetomaternal hemorrhage. Obstet Gynecol 2007;119(2 Pt 1):256–60. | |
De Almeida V, Bowman JM. Massive fetomaternal hemorrhage: Manitoba experience. Obstet Gynecol 1994;83(3):323–8. | |
Morris JA, Hustead RF, Robinson RG, et al. Measurement of fetoplacental blood volume in the human previable fetus. Am J Obstet Gynecol 1974;118(7):927–34. | |
Sebring ES, Polesky HF. Fetomaternal hemorrhage: incidence, risk factors, time of occurrence, and clinical effects. Transfusion 1990;39(4):344–57. | |
O’Leary BD, Walsh CA, Fitzgerald JM, et al. The contribution of massive fetomaternal hemorrhage to antepartum stillbirth: a 25-year cross-sectional study. Acta Obstet Gynecol Scand 2015;94(12):1354–8. | |
Larsen R, Berkowicz A, Lousen T, et al. Massive fetomaternal hemorrhage: clearance of fetal red blood cells after intravenous anti-D prophylaxis monitored by flow cytometry. Transfusion 2008;48(8):1707–12. | |
Bowman JM, Pollock JM, Penston LE. Fetomaternal transplacental hemorrhage during pregnancy and after delivery. Vox Sang 1986;51(2):117–21. | |
Solomonia N, Playforth K, Reynolds EW. Fetal-maternal hemorrhage: a case and literature review. Am J Perinatol Rep 2012;2(1):7–14. | |
Kizza AP, Rogo KO. Feto-maternal haemorrhage in Kenya. East Afr Med J 1990;67(11):801–7. | |
Choavaratana R, Uer-Areewong S, Makanantakosol S. Feto-maternal transfusion in normal pregnancy and during delivery. J Med Assoc Thai 1997;89(2):96–100. | |
Mollison PL. Incidence of transplacental haemorrhage. In: Blood Transfusion and Clinical Medicine 6th edn. Oxford UK: Blackwell Scientific Publications, 1979:330. | |
Sandler S, Sathiayamoorthy S. Laboratory methods for Rh immunoprophylaxis: a review. Immunohematology 2010;26(3):92–103. [This can be freely accessed through ResearchGate.net, via Google.] | |
Scientific Subcommittee of the Australian and New Zealand Society of Blood Transfusion Inc. Guidelines for laboratory assessment of fetomaternal haemorrhage, 2002. | |
Kim YA, Makar RS. Detection of fetomaternal hemorrhage. Am J Hematol 2012;87(4):417–23. | |
Bard H. The postnatal decline of hemoglobin synthesis in normal full-term infants. J Clin Invest 1975;55(2):395–8. | |
Boyer SH, Belding TK, Margolte L, et al. Variations in the frequency of fetal hemoglobin-bearing erythrocytes (F-cells) in well adults, pregnant women, and adult leukemics. Johns Hopkins Med J 1975;137(3):105–15. | |
Wood WG, Stamatoyannopoulos G, Lim G, et al. Blood 1975;46(5):671–82. | |
Kleihauer E, Braun H, Betke K. [Demonstration von fetalem hämoglobin in den erythrocyten eines blutausstrichs.] Klin Wochenschr 1957;35(12):637–8 [In German]. | |
Zipursky A, Hull A, White FD, et al. Foetal erythrocytes in the maternal circulation. Lancet 1959;i(7070):451–2. | |
Kleihauer E, Betke K. [Practical use of the demonstration of cells containing hemoglobin F in fixed blood smears. Der Internist [or Izv Mikrobiol Inst (Sofiia)] 1960;1:292–5 [In German]. | |
Mollison PL. Quantitation of transplacental haemorrhage. Br Med J 1972;3(5817):31–4. | |
Gielezynska A, Stachurska A, Fabijanska-Mitek J, et al. Quantitative fetomaternal hemorrhage assessment with the use of five laboratory tests. Int J Lab Hematol 2016;38(4):419–25. | |
Chambers E, Davies L, Evans S, et al. Comparison of haemoglobin F detection by the acid elution test, flow cytometry and high-performance liquid chromatography in maternal blood samples analysed for fetomaternal haemorrhage. Transfus Med 2012;22(3):199–204. | |
Corcoran D, Murphy D, Donnelly JC, et al. The prevalence of maternal F cells in a pregnant population and potential overestimation of foeto-maternal haemorrhage as a consequence. Blood Transfus 2014;12(4):570–4. | |
Cormack OM, Guilfoyle F, Flynn CM. The prevalence of an elevated F cell population in a maternal and gynaecology cohort. Transfus Med 2019;29(5):369–73. | |
Rochette J, Craig JE, Thein SL. Fetal hemoglobin levels in adults. Blood Rev 1994;8(4):213–24. | |
Kumpel B, Hazell M, Guest A, et al. Accurate quantitation of D+ fetomaternal hemorrhage by flow cytometry using a novel reagent to eliminate granulocytes from analysis. Transfusion 2014;54(5):1305–16. | |
Ness PM. The assessment of fetal-maternal hemorrhage by an enzyme-linked antiglobulin test for Rh immune globulin recipients. Am J Obstet Gynecol 1982;143(7):788–92. | |
Greenwalt TJ, Dumaswala UJ, Domino MM. The quantification of fetomaternal hemorrhage by an enzyme-linked antibody test with glutaraldehyde fixation. Vox Sang 1992;63(4):268–71. | |
Salama A, David M, Wittman G, et al. Use of the gel agglutination technique for determination of fetomaternal haemorrhage. Transfusion 1998;38(2):177–80. | |
Agaylan A, Meyer O, Ahrens N, et al. A rapid gel agglutination test for the determination of fetomaternal haemorrhage. Transfus Med 2007;17(5):395–8. | |
Kumpel BM. Analysis of factors affecting quantification of fetomaternal hemorrhage by flow cytometry. Transfusion 2000;40(11):1376–83. | |
Molloy EJ, O’Neill AJ, Grantham JJ, et al. Labor induces a maternal inflammatory response syndrome. Am J Obstet Gynecol 2004;190(2):448–55. | |
Spychalska J, Uhrynowska M, Pyl H, et al. [Standardisation of the quantitative flow cytometric test with anti-D antibodies for fetomaternal hemorrhage in RhD negative women.] Ginekol Pol 2015;86(7):486–93 [In Polish]. | |
Kumpel BM. Labeling D+ RBCs for flow cytometric quantification of fetomaternal hemorrhage after the RBCs have been coated with anti-D. Transfusion 2001;41(8):1059–63. | |
Davis BH, Olsen S, Bigelow NC, et al. Detection of fetal red cells in fetomaternal hemorrhage using a fetal hemoglobin monoclonal antibody by flow cytometry. Transfusion 1998;38(8):749–56. | |
Nelson M, Zarkos K, Popp H, et al. A flow cytometric equivalent of the Kleihauer test. Vox sang 1998;75(3):234–41. | |
Kennedy GA, Shaw R, Just S, et al. Quantification of feto-maternal haemorrhage (FMH) by flow cytometry: anti-fetal hemoglobin labeling potentially underestimates massive FMH in comparison to labelling with anti-D. Transfus Med 2003;13(1):25–33. | |
Porra V, Bernaud J, Gueret P, et al. Identification and quantification of fetal red blood cells in maternal blood by a dual-color flow cytometric method: evaluation of the Fetal Cell Count kit. Transfusion 2007;47(7):1281–9. | |
Othman J, Orellana D, Chen LS, et al. The presence of F cells with a fetal phenotype in adults with hemoglobinopathies limits the utility of flow cytometry for quantitation of fetomaternal hemorrhage. Cytometry B Clin Cytom 2018;94(4):695–8. | |
Nance SJ, Nelson JM, Arndt PA, et al. Quantitation of fetal-maternal hemorrhage by flow cytometry. A simple and accurate method. Am J Clin Pathol 1989;91(3C):288–92. | |
Savithrisowmya S, Singh M, Kriplani A, et al. Assessment of fetomaternal hemorrhage by flow cytometry and Kleihauer-Betke test in Rh-negative pregnancies. Gynecol Obstet Invest 2008;65(2):84–8. | |
Urgessa F, Tsegaye A, Gebrehiwot Y, et al. Assessment of feto-maternal hemorrhage among rhesus D negative pregnant mothers using the kleihauer-betke test (KBT) and flow cytometry (FCM) in Addis Ababa, Ethiopia. BMC Pregnancy Childbirth 2014;14:358. | |
Pastoret C, Le Priol J, Fest T, et al. Evaluation of FMH QuikQuant for the detection and quantification of fetomaternal hemorrhage. Cytometry B Clin Cytom 2013;84(1):37–43. | |
Little BH, Robson R, Roemer B, et al. Immunocytometric quantitation of foeto-maternal haemorrhage with the Abbott Cell-Dyn CD4000 haematology analyser. Clin Lab Haematol 2005;27(1):21–31. | |
De Wit H, Nabbe KCAM, Kooren JA, et al. Reference values of fetal erythrocytes in maternal blood during pregnancy established using flow cytometry. Am J Clin Pathol 2011;136(4):631–6. | |
Cardoso MR, de Souza-Araujo CN, Talarico MCR, et al. Evaluation of automatic blood analyser as screening method in fetomaternal hemorrhage. Biomed Res Inst 2019;26:6481654. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
