This chapter should be cited as follows:
Regoli D, De Curtis M, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.417963
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 16
The prevention and management of Rh disease
Volume Editors:
Professor Gerard HA Visser, Department of Obstetrics and Gynaecology, University Hospital of Utrecht, Heidelberglaan 100, Utrecht 3584EA, The Netherlands
Professor Gian Carlo Di Renzo, University of Perugia, Italy

Chapter
Management of the Neonate with Neonatal Hemolytic Disease
First published: January 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
SUMMARY
Delivery of an alloimmunized woman should take place in a level-3 center and if it is by cesarean section, it should be scheduled as far as possible.
In the case of preterm birth, the combination of anemia, hyperbilirubinemia and respiratory distress may require neonatal resuscitation and intensive care. It is not always possible to plan an alloimmune pregnancy delivery. Sometimes it may be necessary to intervene with a preterm emergency cesarean section, due to the presence of fetal distress or another obstetric indication. Also, the presence of severe anemia with heart failure may be incompatible with a natural birth and make it necessary to perform a cesarean section.
In less dramatic situations, a fetus with mild to moderate anemia can tolerate spontaneous delivery. Delayed cord clamping at birth is promoted since it results in a significantly higher hemoglobin at birth and decreases the number of exchange transfusions and delays the timing of the first transfusion.1,2
Given the great variability of clinical manifestations at birth, it is essential that the team of neonatologists know the detailed history of pregnancy, the diagnostic investigations carried out (type of antibody and antibody titer, ultrasound scans, genetic determination of the fetal group and Rh, evaluation of the middle cerebral artery peak systolic velocity, etc.) and the possible treatment to which the woman has been subjected (intravenous immunoglobulin, apheresis, intrauterine transfusion, etc.). It is also important that the birth process takes place in coordination between obstetricians and neonatologists in collaboration with the transfusion service and the laboratory.
INTRODUCTION
The estimated worldwide prevalence of hemolytic disease of the fetus and newborn (HDFN) is 276/100,000 live births in the year.3 A 2015 review reports about 2.6 cases per 100,000 live births in high-resource countries, while the prevalence is higher in low-to-middle income countries; for example, 529/100,000 in Eastern Europe/Central Asia and 386/100,000 in sub-Saharan Africa.4 After the introduction of anti-D immunoprophylaxis, the cases due to this antibody are drastically reduced, while those due to antibodies against so-called minor antigens of the Rh system (anti-c, anti-E) and other antigens of different blood group systems such as anti-Kell have relatively increased. A prevalence of 1 in 500 is estimated to be in alloimmunized pregnancy for antibodies other than D.5
CLINICAL MANIFESTATIONS
The severity of the disease depends on the extent of the hemolysis and the effectiveness of the erythropoietic response.6 At birth, the neonatologist may be confronted with a vital infant in good condition, without jaundice, with normal hemoglobin levels or only mild anemia. However, this situation, knowing the history of pregnancy and the titer of the antibodies, requires close monitoring of hemoglobin and total bilirubin levels, intervening with phototherapy and/or exchange transfusion or transfusion therapy or infusion of IGEV if needed. On the other hand, if, at the time of birth, there is already severe anemia with signs of cardiocirculatory overload, it is necessary to intervene quickly with blood transfusion, performed with concentrated red blood cells (generally group 0, Rh negative, CMV negative, irradiated, filtered). The procedure must be carried out very slowly to correct the anemia, with subsequent monitoring of total bilirubin and Hb to intervene as appropriate.7
However, the most serious situation that may occur is the presence of hydrops, which may require intensive neonatal resuscitation with the support of inotropic drugs, paracentesis, thoracentesis and adequate transfusion therapy.
In any case, a blood sample, taken from the umbilical cord, should always be sent for the determination of the blood group, Rh, direct antiglobulin test, blood count, reticulocytes and bilirubin dosage.
A particular and rare situation that may occur at birth, after a pregnancy with severe alloimmunization and the fetus subjected to one or more intrauterine transfusions, is that of a newborn born in good condition without anemia or jaundice. The direct antiglobulin test at birth could be negative and group 0, for red blood cells transfused into the fetus prenatally. These infants may show mild or moderate jaundice in the first week of life and may not need special treatment, but often show late hyporegenerative anemia.
These different situations are summarized in Figure 1.
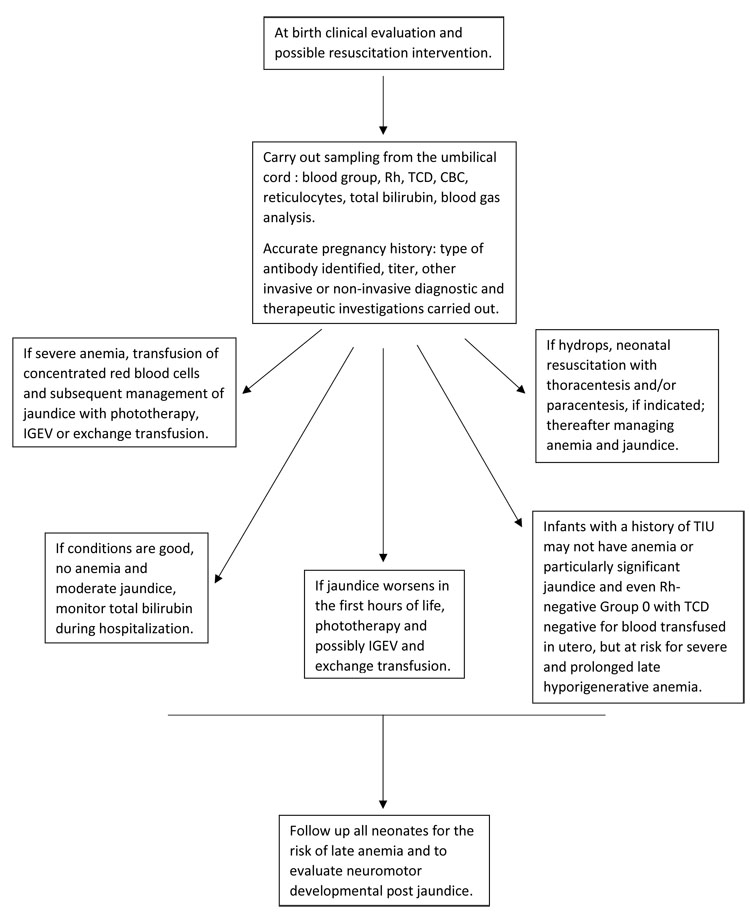
1
Management of the newborn with Rh hemolytic disease. TCD, direct Coombs test; CDC, complete blood count; TIU, intrauterine transfusion; IGEV, intravenous immunoglobulins.
PATHOGENESIS
The pathogenetic mechanism underlying fetal-neonatal hemolytic disease is known. The first moment is the sensitization, that is after the first contact of the blood of the woman with red blood cells that have antigen inherited from the father (first pregnancy and following maternal fetal hemorrhage at delivery for example, abortion, etc.) antibodies are formed, initially IgM that do not cross the placenta, and, after subsequent exposure, IgG. The IgG antibodies cross the placenta and attack the red blood cells of the fetus, with the antigen involved, which are then destroyed by the mononuclear phagocytic system. The destruction of red blood cells results in anemia on the one hand and an increase in bilirubin on the other, due to the heme degradation mechanism. In utero, bilirubin is eliminated by placental exchange to the mother, but after birth the "immature" liver of the newborn, has to eliminate bilirubin. For this reason, progressive jaundice is a symptom of the hemolytic disease of the newborn.8
Fetal anemia involves an increased erythropoietic stimulus involving not only the medulla, but also the liver and significant release into the circulation of erythroblasts and reticulocytes, hence the term for this disease: "fetal erythroblastosis", as which it was also known.
Hydrops fetalis is a consequence of hypervolemia, hypoalbuminemia, high vascular permeability and heart failure.
Improvements in diagnostics and therapies have made it possible to significantly improve the outcome of these newborns. Since the 1980s, after the introduction of intrauterine transfusions, there has been a reduction in the births of Rh sensitized babies with hydrops from 55% to 13% and a reduction in births before 32 weeks of gestational age from 10% to 2%.9 Infants born after intrauterine transfusion (IUT) treatment are less anemic at birth and require fewer blood transfusions. Therefore, a good management of high-risk pregnancies allows to obtain a better neonatal condition.
JAUNDICE
The destruction of red blood cells involves the degradation of heme by the enzyme heme oxygenase and hence the production of biliverdin, carbon monoxide (CO) and iron molecule. Biliverdin in turn is transformed into bilirubin by biliverdin reductase. The circulating bilirubin is taken up by the liver and transformed into conjugated bilirubin, which is then eliminated with the bile. The bacteria present in the intestine make it possible to transform the conjugated bilirubin into urobilinogen and from there into urobilin eliminated in the urine and in stercobilin eliminated in the feces.
In the newborn, liver uptake and bilirubin conjugation are less effective. Beta glucuronidase causes bilirubin deconjugation with indirect bilirubin return to the liver (enterohepatic circulation). Furthermore, the neonatal intestine is a sterile intestine with a low amount of bacteria, therefore the transformation into urobilinogen and urobilin is also poor.
The result is a greater accumulation of bilirubin, which if not bound to albumin can overcome the blood–brain barrier and cause brain damage.10
Neurological damage from hyperbilirubinemia in the newborn may also lead to death or acute multisystemic manifestations and long-term damage such as athetoid cerebral palsy and impaired speech, hearing and visual abilities.
Alterations occur when bilirubin levels exceed the neuroprotective abilities of the newborn and cause damage to the basal ganglia, central and peripheral auditory nerve pathways, hippocampus, diencephalon, subthalamic nuclei, pons, brainstem nuclei and cerebellum. The characteristic lesion of the MRI in the case of kernicterus is the increased signal in T2 symmetrical and bilateral in the pale globe and in the nucleus subthalamic.11
Among acute symptoms there may be changes in behavior, muscle tone, drowsiness, poor sucking, hypotonia and muscle tone that veers towards an increase in hypertonia, especially of the extensor muscles, cervical dystonia with retrocollis and opisthotonus, at first intermittent and then constant. There are two types of bilirubin encephalopathy: one is acute (acute bilirubin encephalopathy ABE) and one is chronic (chronic bilirubin encephalopathy CBE).12
A definition has also been given for a milder form of neuropathy, called BIND (bilirubin induced neurological dysfunction), which manifests itself with fine and gross motor incoordination, gait abnormalities, fine tremors and learning and behavioral problems. Being rather ’vague’ symptoms, they are sometimes difficult to diagnose and to refer to hyperbilirubinemia.11,13
1
Clinical management.
Treatment | Mechanism of action | Observations |
Phototherapy | Exposure to a light source with a particular wavelength causes isomerization of bilirubin not conjugated into water-soluble compounds (lumirubin and photobilirubin), which are excreted with bile. In this way the levels of bilirubinemia are reduced. | |
Exchange transfusion | Serum bilirubin is reduced. Circulating antibodies are reduced. Anemia is corrected. | This procedure is used increasingly rarely. It remains a life-saving practice in the case of worsening jaundice that does not respond to intensive phototherapy or to the administration of intravenous immunoglobulins. |
Intravenous immunoglobulins (IGEV) | The likely mechanism is to compete with the antigen-antibody complex by blocking the Fc receptor for IgG on macrophages and thus limit and slow down hemolysis. | The use of IGEV in prophylaxis of neonatal Rh hemolytic disease did not result a significant reduction in the number of exchange transfusions. Further studies are needed to better define the category of newborns, that would benefit most from IGEV as a therapy and at what bilirubin levels it should be carried out. |
Metalloporphyrins | They inhibit heme oxygenase hence the production of bilirubin. | |
Phenobarbital | Increases hepatic uridine diphosphate (UDP)-glucuronosyl transferase (UGT) activity and conjugation of bilirubin. | Its use was proposed a few decades ago, but is no longer recommended. |
PHOTOTHERAPY
The introduction of phototherapy as part of the treatment of neonatal jaundice dates back to the late sixties and originates, as often happens, from a casual discovery, namely that bilirubin levels decreased with exposure to the sun.14 The most effective light band to prevent hyperbilirubinemia appeared to be the wavelength between 400 and 500 nm with an absorption peak at 460 ± 10 nm.
Phototherapy is known to cause three reactions: structural and configurational isomerization and photo-oxidation. This transforms bilirubin into disposable derivatives without hepatic conjugation with urine and feces.
If we compare phototherapy to a drug, it is easier for us to understand how its effectiveness is dose dependent and derives not only from the correct wavelength used, but also from the radial spectrum of the lamp, the width of the treated skin and the distance of the lamp from the skin.
Over the years, thanks to advances in technology, better and more efficient types of equipment have been used: fluorescent lamps ("special blue", cold white lamps, daylight lamps), halogen lamps (they heat and must be positioned at suitable distance to prevent burns), lamps with an optical fiber system (used in blankets in contact with the skin placed under the newborn or that envelop the chest (very useful in intensive treatment in association with other types of phototherapy) and, finally, long-lasting and very effective LED lamps.
Phototherapy is generally used continuously with short breaks for meals, diaper changes and the time needed for cleaning. The infant is naked with only a minimal diaper, and eye protection. Continuous control with a pulse oximeter allows the condition of the child to be monitored, who may have a cyanotic crisis that is not recognized due to the blue light.
Initially, total bilirubin checks are carried out more closely (every 4–6 hours) and then the frequency is reduced. This depends on the initial value and the rate of increase of bilirubin.
There are several nomograms published by the international scientific community show the bilirubin levels beyond which it is necessary to start the phototherapy treatment, take into account both the gestational age and the postnatal age and any risk factors (hemolysis, sepsis, acidosis, asphyxia, G6PD deficiency).
Side-effects of phototherapy are rare. "Bronze baby syndrome" has been reported in children with cholestatic jaundice undergoing phototherapy for porphyrin accumulation in the skin and serum. Diarrhea, dehydration and fleeting erythema may also occur.15
EXCHANGE TRANSFUSION
In 1946 Wallerstein and Wiener introduced the technique of exchange transfusion (ET). At that time this consisted of a rather bloody method with infusion of blood into the saphenous vein and sampling from the radial artery. It was Diamond who introduced the use of umbilical vein catheterization. The first randomized and controlled study, published in 1952, confirmed the effectiveness of the technique in controlling bilirubin levels.16 This treatment consists of removing the baby's blood and replacing it with donor blood. It is performed using small exchange rates in a relatively short time. The exchange transfusion is performed using the push-pull technique with a special three-way stopcock. Donor red blood cells are used, devoid of the antigen involved in the disease, reconstituted with 50–55% plasma. Red blood cells should be fresh, therefore less than 5 days old, to limit possible storage damage for such large volumes. The final product must be irradiated.
Since the introduction of Rh immunoprophylaxis, the prenatal treatment, the increasingly effective phototherapy and the use of intravenous immunoglobulins, exchange transfusion is performed rarely, but it remains a very important "life-saving" technique in the event that bilirubin levels continue to rise despite intensive phototherapy.
ET aims to correct anemia, reduce bilirubin and circulating antibodies and reduce hemolysis by eliminating sensitized red blood cells. Immediate ET blood transfusion should also be performed when signs of acute encephalopathy appear.
There is no clear benefit with the use of single volume exchange transfusion over double volume exchange transfusion, which exchanges approximately 85% of neonatal blood. Therefore, the current practice is the use of double volume exchange transfusion. This practice allows the total bilirubin level in the blood to be reduced by half or three-quarters. The risk of death following this procedure is calculated to be between 0.5 and 2%. The technique also presents risks such as thrombocytopenia, coagulopathy, necrotizing enterocolitis, thrombosis of the portal vein, electrolyte alterations (hypocalcemia, hyperkalemia) and cardiac arrhythmias.
There are no data suggesting impaired outcome following ETs. ET are rare, expensive and time-consuming, require expertise and clinical experience, and should therefore preferably be performed in centers with sufficient expertise.
While in high-income countries, ET is carried out more and more rarely, an excessive rate of ET, with its associated risks, is being performed in low/middle income countries. A study carried out in the Middle East showed that more than 60% of newborns who are hospitalized for jaundice receive ET.17 In Latin America this figure is 21.5%.18
There are nomograms of total bilirubin values, for which ET is recommended.19,20
INTRAVENOUS IMMUNOGLOBULINS
While phototherapy and exchange transfusion are the most accredited therapies in the treatment of neonatal Rh hemolytic disease to avoid neurological damage, intravenous immunoglobulins (IVIg) have been proposed as an alternative therapy in order to avoid transfusion with its associated risks. The first use of IVIg in HDFN was published in 1987.21
The exact mechanism of IVIg's action to reduce hemolysis is unclear. Scientists suggest that IVIg most likely work by blocking antibody receptors located on the surface of red blood cells. Blocking these receptors may prevent the interaction between the antigens present on red blood cells and maternal antibodies, preventing the recognition of red blood cells by circulating macrophages and consequent decrease in hemolysis. Another mechanism is the increase in the clearance of cells linked to antibodies or downregulation of B cells to suppress the activation of the immune response.22
Although the overall results show a significant reduction in the need for exchange transfusion in infants treated with IVIg, the applicability of the results is limited due to poor quality of evidence. Studies with lower risk of bias have shown no benefit of IVIg. Further studies are therefore needed before IVIg might be used.23 For the time being, the use of IGEVs is limited to those situations in which total bilirubin reaches levels close to those recommended for ET to avoid its execution.19
METALLOPORPHYRINS
Metalloporphyrins are inhibitors of heme oxygenase (HO), the enzyme that oxidizes heme to biliverdin as an initial step in the production of bilirubin.24 Cases have been reported where their use was reserved for situations of major hemolysis, which did not respond to phototherapy and required exchange transfusion. They have also been used in situations where the parents oppose to transfusions for religious reasons.25
Long-term adverse effects have not been described and despite their use being proposed for several years, they are not yet authorized for use in neonatology.
OTHER THERAPIES
For the treatment of neonatal jaundice, other therapies have been proposed such as phenobarbital, which increases hepatic uridine diphosphate (UDP)-glucuronosyl transferase (UGT) activity and conjugation of bilirubin, or more recently clofibrate which induces glucuronosyl transferase.26,27 None of these therapies are currently accepted in common practice and need more in-depth studies.
LATE ANEMIA
Already at the end of the last century, some authors observed that 64% of newborns who needed an exchange transfusion experienced late anemia and also that 92% of those who did not need ET, most of whom had been treated in utero with intrauterine transfusions, developed late anemia.28 Late anemia most frequently occurs between the second and sixth week after birth and has several known causes, such as prolonged hemolysis, not treated with exchange transfusion, which is generally accompanied by elevated reticulocytosis. Late hyporegenerative anemia, instead, is caused by erythropoiesis depression and low production of reticulocytes. The mechanisms underlying this situation could be the destruction of erythroid precursors in the bone marrow and the suppression of the marrow due to intrauterine and postnatal transfusions.29 In this case, treatment with transfusions of concentrated and irradiated red blood cells is necessary, sometimes repeated several times in the first months of life; additionally recombinant human erythropoietin (HuEPO) may be used. Late anemia usually resolves by third month of life.29
CONCLUSIONS
Rh hemolytic fetal-neonatal disease still occurs, despite a drastic decrease due to the worldwide use of immunoprophylaxis, so it is very important that neonatologists know both the pathogenesis and diagnosis of the disease, as well as the therapeutics tools currently available. It represents an important cause of hyperbilirubinemia and kernicterus. Guidelines for the treatment of hyperbilirubinemia in this group of newborns must be followed closely. Follow-up of these children over time, to identify any early neurological and/or sensorineural deficit is essential.
More accurate studies on the use of pharmacological agents, such as metalloporphyrins or the use of albumin before transfusion, or phenobarbital, should be carried out in the future.
PRACTICE RECOMMENDATIONS
- Despite the spread of anti-D immunoprophylaxis, fetal-neonatal hemolytic disease remains an important hematological emergency, especially in low-resource countries, where antenatal care and prophylaxis are insufficient.
- The delivery of an alloimmunized woman should take place in a level-3 center, with coordinated care by obstetrician and neonatologist and with the support of the immunohematology and transfusion unit.
- The neonatologist must know the hemoglobin and bilirubin levels at which to intervene (nomograms) and the appropriate therapies.
- Young neonatologists should be trained to perform an exchange transfusion, eventually with simulation. In some cases, this intervention is still a life-saving practice.
- Follow-up of newborns with hemolytic disease is important to monitor and treat any late anemia or jaundice.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Garabedian C, Rakza T, Drumez E, et al. Benefits of delayed cord clamping in red blood cell alloimmunization. Pediatrics 2016;137:e20153236. | |
Sahoo T, Thukral A, Sankar MJ, et al. Delayed cord clamping in Rh-alloimmunised infants: a randomised controlled trial. Eur J Pediatr. doi.org/10.1007/s00431-020-03578-8. | |
Bhutani VK, Zipursky A, Blencowe H, et al. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res 2013;74(Suppl 1):86–100. doi: 10.1038/pr.2013.208. | |
Zipursky A, Bhutani VK. Impact of Rhesus disease on the global problem of bilirubin-induced neurologic dysfunction. Seminars in Fetal & Neonatal Medicine 2015;20:2–5. | |
Agrawal A, Hussain KS, Kumar A. Minor blood group incompatibility due to blood groups other than Rh (D) leading to hemolytic disease of fetus and newborn: a need for routine antibody screening during pregnancy. Intractable & Rare Diseases Research 2020;9(1):43–7. | |
Liley HG, Gardener G, Lopriore E, et al. Immune Hemolytic Disease in Nathan and Oski’s Hematology and Oncology of Infancy and Childhood, 8th edn. In: Orkin SH, Fisher DE, et al. (ed.) Elsevier Philadelphia, 2015:76–100. | |
Ross ME, Waldron PE, et al. Hemolytic disease of the fetus and newborn. in Neonatal Hematology, Pathogenesis, Diagnosis and Management of Hematologic Problems, 2nd edn. In: de Alarcòn PA, EJ, Werner EJ, Christensen R, (eds.) Cambridge University press, 2013:65–90. | |
de Haas M, Thurik FF, Koelewijn JM, et al. Haemolytic disease of the fetus and newborn. Vox Sanguinis 2015;109:99–113. | |
Zwiers C, Oepkes D, Lopriore E, et al. The near disappearance of fetal hydrops in relation to current state‐of‐the‐art management of red cell alloimmunization. Prenatal Diagnosis 2018;38:943–50. | |
Anderson NB, Calkins KL. Neonatal Indirect Hyperbilirubinemia. Neoreviews 2020;21(11):e749–60. | |
Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol 2014;38(7):422–8. | |
Volpe JJ. Bilirubin and Brain Injury in Neurology of the Newborn, 4th edn. WB Saunders Company: Philadelphia, 2001:521–46. | |
Bhutani VK, Wong RJ. Bilirubin neurotoxicity in preterm infants: risk and prevention. J Clin Neonatol 2013;2(2):61–9. | |
Maisels MJ Ward SJ. Phototherapy, and jaundice: a unique human and photochemical interaction. Journal of Perinatology 2015;35:671–5. doi:10.1038/jp.2015.56. | |
MacDonald MG, Ramasethu J, Rais-Bahrami K. Atlas of Procedures in Neonatology, 5th edn. Lippincott Williams and Wilkins (eds.) 2013:357–62. | |
Stockman III J A. Overview of the State of the Art of Rh Disease: History, Current Clinical Management, and Recent Progress. Journal of Pediatric Hematology/Oncology, 2001;23:8:554–62. | |
Hameed NN, Na 'Ma AM, Vilms R, et al. Severe neonatal hyperbilirubinaemia and adverse short-term consequences in Baghdad, Iraq. Neonatology 2011;100:57–63. | |
Salas AA, Mazzi E. Exchange transfusion in infants with extreme hyperbilirubinemia: an experience from a developing country. Acta Paediatr 2008;97:754–8. | |
American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Clinical practice guideline: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297–316. | |
Rennie J, Burman-Roy S, Murphy MS. Neonatal jaundice: summary of NICE guidance. BMJ 2010;340:C2409. | |
Sato K, Hara T, Kondo T, et al. High-Dose Intravenous Gammaglobulin Therapy for Neonatal Immune Haemolytic Jaundice due to Blood Group Incompatibility. Acta Pediatrica 1991;80(2):143–268. | |
Alsaleem M. Intravenous Immune Globulin Uses in the Fetus and Neonate: A Review Antibodies (Basel) 2020;9(4):60. | |
Zwiers C, Scheer-Rath MEA, Lopriore E, et al. Immunoglobulin for alloimmune hemolytic disease in neonates Cochrane Database Syst Rev 2018;3(3):CD003313. | |
Bhutani VK, Poland R, Meloy LD, et al. Clinical trial of tin mesoporphyrin to prevent neonatal hyperbilirubinemia. Perinatol 2016;36(7):533–9. doi: 10.1038/jp.2016.22. Epub 2016 Mar 3. | |
Kappas A, Drummond G S, Munson D P, et al. Sn-Mesoporphyrin interdiction of severe hyperbilirubinemia in Jehovah's Witness newborns as an alternative to exchange transfusion. Pediatrics 2001;108(6):1374–7. doi: 10.1542/peds.108.6.1374. | |
Kaabneh MA, Salama GS, Shakkoury AG, et al. Phenobarbital and phototherapy combination enhances decline of total serum bilirubin and may decrease the need for blood exchange transfusion in newborns with isoimmune hemolytic disease. Clin Med Insights Pediatr 2015;9:67–72. | |
Habibi M, Mahyar A, Ayazi P, et al. The effect of clofibrate on hyperbilirubinemia of term neonates. Acta Med Iran 2012;50:21–5. | |
al-Alaiyan S, al-Omran A. Late hyporegenerative anemia in neonates with Rhesus hemolytic disease. J Perinatol Med 1999;27:112–5. | |
Ree IMC, Smits-Wintjens V, van der Bom JG, et al. Neonatal management and outcome in alloimmune hemolytic disease, Expert Review of Hematology 10(7):607–16. DOI: 10.1080/17474086.2017.1331124. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)