This chapter should be cited as follows:
Castleman JS, Kilby MD, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.418123
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 16
The prevention and management of Rh disease
Volume Editors:
Professor Gerard HA Visser, Department of Obstetrics and Gynaecology, University Hospital of Utrecht, Heidelberglaan 100, Utrecht 3584EA, The Netherlands
Professor Gian Carlo Di Renzo, University of Perugia, Italy

Chapter
Medical Management of Rhesus Disease
First published: November 2022
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
ABSTRACT
Fetal red cell destruction by maternal antibodies remains an important cause of fetal and neonatal morbidity and mortality. Intrauterine fetal blood transfusion is the therapy of choice for severe hemolytic disease of the fetus. Invasive fetal therapy carries risk of miscarriage, preterm birth, fetal demise, and further sensitization. Severe, early onset fetal anemia is more hazardous because fetal intravascular needle insertion is more technically challenging due to small vessel size. Non-invasive, medical treatments are important to minimize or avoid procedure-related risks. Intravenous immunoglobin (IVIg) and novel monoclonal antibody (M281, nipocalimab) treatments may attenuate the transplacental passage and fetal effects of IgG antibodies. By delaying the onset of fetal anemia, immunological therapies can defer the need for first transfusion. These medical therapies in early onset hemolytic disease of the fetus and newborn (HDFN) improve fetal survival. Their wider impact on perinatal management, and the longer-term outcomes for survivors of HDFN are important to elucidate.
INTRODUCTION
Prompt recognition and in utero treatment of alloimmune fetal anemia is vital to avoid fetal death and adverse neonatal outcomes.1,2 In maternal red blood cell (RBC) alloimmunization, IgG class antibodies cross the placenta to destroy fetal erythroid cells expressing the involved antigen. The antigens D, Kell, and c are considered the most high risk3,4 and alloimmunized pregnancies should be identified and managed according to international evidence-based guidelines as previously described.5,6,7,8 Women with a previous pregnancy affected by HDFN or a critical level of high-risk alloantibody, require review by a fetal medicine specialist.8
In order to understand the mechanism of action of contemporary medical treatments, it is important to appreciate the underlying pathophysiology of HDFN. The fetal/neonatal Fc receptor (FcRn) is a heterodimer, first identified in the neonatal rodent intestine transporting IgG in breast milk.9 FcRn is now recognized as an important transmembrane protein at the maternal–fetal interface, involved in IgG and albumin homeostasis10 (Figure 1). FcRn in vascular endothelial cells recycles the circulating serum IgG pool, which is a key role in immunoglobulin homeostasis. FcRn in the placental syncytiotrophoblast transports maternal IgG subtypes across the placenta, by transcytosis, into the fetal circulation.10 There are also several subtypes of IgG antibody that have a differing pathogenicity and rate of transplacental passage.11
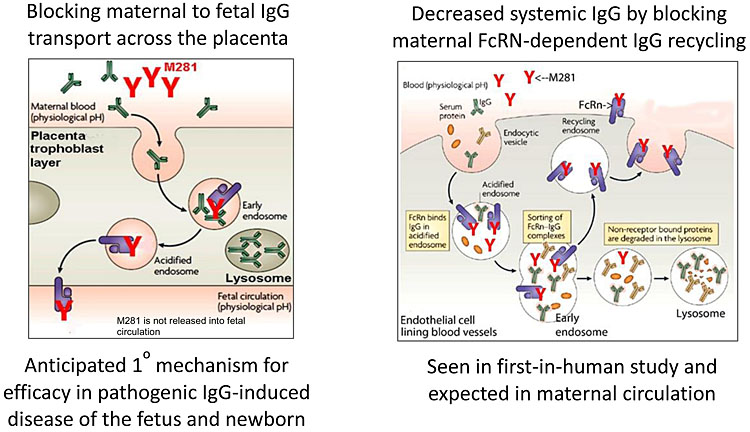
1
Schematic diagram of FcRn function and M281 blockade. Adapted from Roopenian & Akilesh Nat Rev Immunol 2007;7(9):715–25. doi:10.1038/nri2155, © Momenta Pharmaceuticals Inc. All rights reserved.
The movement of IgG across the placenta by FcRn is dependent on several factors, including the amount of antibody in the maternal circulation, the gestational age and placental "integrity". Features of the antibody and antigen are also important, for example the subclass and degree of glycosylation of IgG, and the expression of antigen (those from the thymus being more intense). The physiological role of FcRn is to provide passive immunity to the fetus by transferring IgG across the placenta, and protecting IgG from degradation. In human disease, this mechanism is potentially harmful, such as in HDFN when alloantibodies enter the fetal circulation to target RBCs for destruction. Passage of the IgG1 subclass is most efficient and most readily causes HDFN.12
INVASIVE FETAL THERAPY WITH INTRAUTERINE BLOOD TRANSFUSION
Fetal blood transfusion is described elsewhere in this volume. The risks and benefits of invasive fetal therapy must be borne in mind to appreciate the role of medical therapies in attenuating the disease process and deferring the need for intervention. Needle procedures are associated with pregnancy loss, ruptured membranes, and increased alloantibody formation.13,14 The largest reported retrospective study of 1678 IUTs in 589 fetuses over three decades from Leiden University Medical Center in the Netherlands, reported a procedure-related complication rate of 3.3% with perinatal loss rate of 1.8%.15 Intravascular transfusion before 22 weeks is associated with higher overall fetal mortality because the small anatomical dimensions of the fetal vasculature make the procedure more technically challenging.16,17 A series of 30 intrauterine, intravascular transfusions performed before 22 weeks (range 16–22 weeks) in Toronto reported a procedure-related complication rate of 6.7% and an overall perinatal loss rate of 20%.16 The Leiden group reported a similar number of cases performed prior to 20 weeks (n = 29, range 16–19+6 weeks) with a procedure-related complication rate of 5% and a non-procedure-related complication risk of 11% (per procedure).17 The perinatal loss rate overall (per fetus) was 24%, with 85% of losses occurring before 20 weeks. Fetal intraperitoneal transfusion is an option prior to 20 weeks, to defer the time of first fetal intravascular transfusion.18
Pregnancies with early-onset fetal anemia (prior to 22 weeks) are the main beneficiaries of medical treatments, which dampen the alloimmune antibody-mediated red cell destruction, as they have the highest rates of procedure-related fetal loss. For women with a previous pregnancy affected by HDFN, previous early-onset disease or hydropic stillbirth are risk factors for being susceptible to early-onset disease again. The onset of fetal anemia is usually 4–6 weeks earlier in the subsequent affected pregnancy.13
There are four potential mechanisms of action for medical management of red cell alloimmunization. Firstly, a drug might reduce the amount of circulating maternal IgG antibody by preventing its production. Secondly, a molecule might eliminate IgG from the maternal or fetal circulation. A third mechanism would be to prevent IgG against red cell antigen from crossing the placenta (via FcRn) into the fetal circulation. Finally, a therapy could target the antibody–antigen complex and alter the interaction between the antibody and target fetal antigen. Apart from mechanism of action, it is important to consider unwanted effects and cost. Maternal (or fetal) immunosuppression can lead to life-threatening infectious morbidity. When considering any fetal therapy the protection of the woman from complications is a key tenet.19,20 Maternal immune therapies may be expensive, time consuming, and demanding on healthcare resources.
Maternal immunomodulation therapy
Evidence exists from small cohort series for the use of azathioprine, promethazine, and prednisolone to prevent red cell alloimmunization.21,22 Such immunomodulation is rarely used, if at all, in contemporary practice. Mouse models of “peptide immune therapy” to alter the recognition of the red cell antigens by T-helper cells to induce active tolerance have yielded promising results but there have been no subsequent reports of this potential therapy.23 Ongoing research is evaluating the prevention of RBC alloantibody formation following antigen exposure, including the humoral immune processes distinguishing responders from non-responders.24 Antigen recognition, clearance and their interaction with alloantibodies are potential future therapeutic targets.24
Plasma exchange
Maternal plasma exchange has historically been used to clear alloantibodies (non-selectively) from the maternal circulation. Plasmapheresis leads to loss of electrolytes and important proteins like coagulation factors and other immunoglobulins as well as alloantibodies. Other concerns with this technique relate to altered maternal hemodynamics and reduced placental perfusion as well as the impact on resources as it requires specialist skills for vascular access and delivery. A rebound increase in antibody levels after therapeutic plasma exchange occurs due to immune activation as autoregulatory factors are removed.25,26 Serial plasmapheresis can be combined with IVIg infusion to reduce the chance of further antibody formation.27 Immunoadsorption and intravenous IVIg has been used with good outcomes.28 This technique is rarely now practiced in the management of severe maternal red cell alloimmunization.
Intravenous immunoglobulin (IVIg)
High dose immunoglobulins were first used as a treatment for autoimmune disease in the context of pediatric immune thrombocytopenia in the early 1980s.29 This-therapeutic strategy is a "paradox", as IVIg was originally used as an antitoxin to treat infectious disease (before antibiotics were available) and in immunodeficiency as replacement therapy.30
Delaying the natural history of HDFN can defer the need for transfusion until a later, safer gestation.18,31,32,33 In women with severe alloimmunization in previous pregnancies, the use of maternal IVIg can postpone invasive intrauterine transfusions.18,34 The main mechanism of action of IVIg is competitive inhibition, blocking the transplacental transfer of alloimmune IgG. It also dilutes alloantibody, increases antibody “turnover”, reduces the woman’s own antibody production and blocks fetal secondary macrophage function.35,36 IVIg can cause systemic adverse reactions, such as fever, urticarial (type I hypersensitivity) reaction, hemolytic anemia, aseptic meningitis, and thrombosis. Patients receiving IVIG should be advised that headaches are the commonest side effect, occurring in up to 15%.37 As a derived blood product, there is the theoretical risk of transmitting blood borne infection. IVIg is an expensive, scarce resource ($6000/£4800/€5500 per week) requiring careful health economic consideration.38
The "Postponing Early intrauterine Transfusion with Intravenous immunoglobulin Treatment" (PETIT) study was an international collaboration, pooling multicenter, retrospective observational cohort study data. Cases were enrolled with a qualifying pregnancy, which was affected by HDFN prior to 24 weeks. A subsequent pregnancy with an antigen-positive fetus was treated with or without IVIg.39 The “treatment group” comprised 24 women who received IVIg (at a dose of 1 g per kilogram maternal weight per week). A “control” group of 28 women were not treated with IVIg. Each of the 52 women also served as their own control as outcomes in each pregnancy were compared. IVIg therapy delayed the onset of clinically significant anemia compared to the previous pregnancy by 15 days as compared to the group not treated with IVIg where the delay was 9 days. IVIg also appeared to reduce the overall incidence of fetal hydrops (4% vs. 24%, odds ratio (OR) 0.03; 95% confidence interval, 0–0.5; P = 0.011) and the need for neonatal exchange transfusion (9% vs. 37%; OR: 0.1; 95% confidence interval, 00.5; P = 0.009). Overall survival was 88%, with no difference between IVIg or control groups.39 A subgroup analysis indicated that if the IVIg was initiated before 13 weeks of gestation, fetal anemia was delayed by 25 days as compared to the previous pregnancy. Anemia prior to 20 weeks' gestation occurred less often (23%) as compared to the untreated previous pregnancy (54%). A treatment that could make a more clinically useful reduction in the need for invasive transfusion would revolutionize care.
Experimental monoclonal antibody therapy: M281 (nipocalimab)
M281 is a next-generation Fc receptor (FcRn) blocking agent, which binds with high affinity and specificity to the IgG binding site of FcRn. It may be an effective non-surgical intervention for those women with HDFN likely to require IUT during early gestation when the procedure-related risks are relatively high.40,41 Also known as nipocalimab, M281 is a fully human, recombinant, aglycosylated IgG1 monoclonal antibody formulated for intravenous infusion. M281 has the potential to inhibit IgG transport across the placenta via FcRn from mother to fetus, including the transfer of anti-red cell alloantibodies. FcRn also prevents the degradation of IgG, instead recycling it during normal internalization of circulating proteins into endothelial cells and maintaining its long half life.10 The blockade of FcRn-IgG binding is expected to decrease IgG half-life and serum concentrations including those of pathogenic IgG.42,43 Similar mechanisms have also been postulated for IVIg where high concentrations of administered IgG work by competitive inhibition of endogenous IgG for placental transport and recycling as previously discussed. M281 has greater than 1000-fold higher affinity binding to FcRn than IgG and is therefore likely to be more efficient in inhibiting these processes.
In preclinical studies, M281 demonstrated inhibition of placental IgG transfer from maternal to fetal circulation in a human placental perfusion model within 4 to 6 h, suggesting rapid saturation of FcRn and a fast onset of action.44 The risk of fetal and neonatal M281 exposure is considered low because transfer from the maternal to fetal circulation is insignificant. In a murine model, anti-FcRn antibody-protected rodent fetuses from thrombocytopenia due to maternal antiplatelet antibodies45 and from miscarriage due to maternal antibodies inducing placental damage.46
There were no serious or severe adverse events in the first in-human study of M281.40 The drug was well tolerated with only mild reactions reported in similar frequency to placebo. As predicted by its mechanism of action and preclinical studies, M281 induced rapid dose-dependent lowering of serum IgG upon administration of single and multiple doses. FcRn has a role in albumin homeostasis42 and M281 was shown to lower serum albumin, but this effect was to a lesser extent than IgG. The human volunteers in the study did not have any signs or symptoms of hypoalbuminemia and the effect was not severe. Studies of other anti-FcRn agents in rheumatology patients have also not reported any serious adverse events.42 Early concerns regarding increased risk of infection related to the inhibition of FcRn-mediated lowering of IgG serum concentrations have not been confirmed by preliminary data with M281 or other anti-FcRn agents.42 Antagonists of FcRn do not affect other antibody classes. There should be no impact on the ability to fight infection as the IgM response to new antigen is preserved.47 Clearly maternal, fetal, and neonatal safety of this novel therapy are of paramount importance to the trial teams and clinicians in research and clinical practice. FcRn antagonism is a promising mechanism of action, with translational research confirming proof of concept, tolerability, and clinical utility.
The UNITY (NCT03755128) study is a multicenter, open-label, proof-of-principle (phase II) clinical study sponsored by Momenta Pharmaceuticals Inc. (Cambridge, Massachusetts, USA) (https://clinicaltrials.gov/ct2/show/NCT03842189?term=M281&draw=2&rank=2). UNITY is designed to evaluate the potential of weekly intravenous M281 to delay or prevent the need for IUT in pregnant mothers at risk of early onset HDFN prior to 24 weeks, the same population analyzed in the PETIT study. M281 is initiated upon confirmation of positive fetal antigen status at approximately 14 weeks of gestation. Weekly dosing until 35 weeks aims to block placental IgG transfer of pathogenic alloantibodies for as long as possible. The study will enroll 15 pregnant women affected by early onset HDFN. Primary endpoints in the study are maternal and infant safety and efficacy as determined by the frequency of live births at ≥32 weeks gestational age without the requirement for an IUT throughout pregnancy. The inclusion criteria make the eligibility quite rare, therefore collaboration between international centers is required. Robust safety of data for this experimental therapy, including detailed pharmacodynamic and pharmacokinetic data in pathologic pregnancies, as well as short- and long-term follow up of surviving children is mandatory.
CONCLUSION
HDFN is an alloimmune pathology associated with an increased risk of perinatal mortality and neonatal morbidity, both from prematurity and the direct effects of anemia and hyperbilirubinemia. A small but significant cohort of women with red cell alloimmunization are at risk of developing early-onset fetal anemia and although IUT is possible (prior to 22 weeks), it carries increased risk of procedure-related complications including miscarriage. In these patients, IVIG administered late in the first trimester may postpone the gestational age when an IUT is required to a technically safer time. A novel strategy to inhibit transplacental alloantibody transfer with maternal M281 monoclonal antibody therapy is under evaluation with potential for greatest impact in these alloimmunized pregnancies at risk of early-onset fetal anemia.
PRACTICE RECOMMENDATIONS
- Maternal red cell alloimmunization carries increased fetal risk of perinatal morbidity and mortality and requires antenatal monitoring.
- The most sensitive and specific, non-invasive screening for fetal anemia is by serial middle cerebral artery Doppler peak systolic velocity.
- In utero transfusion of the fetus is the "gold standard" therapy but carries increased risks at early gestation (<20 weeks).
- Maternal therapy, such as serial intravenous immunoglobulin (IVIG) infusions may prolong the gestation of first in utero transfusion in this high-risk group.
- The use of IVIG or more contemporary immunological therapies may attenuate transplacental IgG transfer, reducing the need for in utero transfusion and requires more research assessment before introduction into clinical practice.
CONFLICTS OF INTEREST
Professor Kilby receives no direct funding from Momenta Pharmaceuticals. Birmingham Women’s and Children’s Research Development/University of Birmingham, UK receives funding from Momenta for the ongoing phase II clinical trial.
Data availability statement
There is no original data presented in this review article. Data from published studies are referenced.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Castleman JS, Gurney LRI, Kilby MD, et al. Identification and management of fetal anaemia: a practical guide. The Obstetrician and Gynaecologist 2021;23:196–205. | |
Daniels G, Finning K, Martin P, et al. Noninvasive prenatal diagnosis of fetal blood group phenotypes: current practice and future prospects. Prenatal Diagnosis 2009;29:101–7. | |
Karafin MS, Westlake M, Hauser RG, et al. Risk factors for red blood cell alloimmunization in the Recipient Epidemiology and Donor Evaluation Study (REDS-III) database. British Journal of Haematology 2018;181:672–81. | |
Delaney M, Wikman A, van de Watering L, et al. Blood Group Antigen Matching Influence on Gestational Outcomes (AMIGO) study. Transfusion 2017;57:525–32. | |
Committee on Practice B-O. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstetrics & Gynecology 2017;130:e57–70. | |
Mari G, Norton ME, Stone J, et al. Guideline for blood grouping and red cell antibody testing in pregnancy. Transfusion Medicine 2016;26:246–63. | |
Mari G, Norton ME, Stone J, et al. Society for Maternal-Fetal Medicine (SMFM) Clinical Guideline #8: the fetus at risk for anemia–diagnosis and management. American Journal of Obstetrics and Gynecology 2015;212:697–710. | |
Surendran SK AS, Regan F. Royal College of Obstetricians and Gynaecologists. The Management of Women with Red Cell Antibodies during Pregnancy. Green-top Guideline No. 65, 2014. | |
Jones EA, Waldmann TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. Journal of Clinical Investigation 1972;51:2916–27. | |
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nature Reviews Immunology 2007;7:715–25. | |
Lambin P, Debbia M, Puillandre P, et al. IgG1 and IgG3 anti-D in maternal serum and on the RBCs of infants suffering from HDN: relationship with the severity of the disease. Transfusion 2002;42:1537–46. | |
Lynen R, Krone O, Legler TJ, et al. A newly developed gel centrifugation test for quantification of RBC-bound IgG antibodies and their subclasses IgG1 and IgG3: comparison with flow cytometry. Transfusion 2002;42:612–8. | |
Zwiers C, van Kamp IL, Oepkes D. Management of red cell alloimmunization in Fetal Therapy: Scientific basis and critical appraisal of clinical benefits, 2nd edn. Cambridge University Press, 2020. | |
Doyle B, Quigley J, Lambert M, et al. Red cell alloimmunisation following intrauterine transfusion and the feasibility of providing extended phenotype-matched red cell units. Transfusion Medicine 2014;24:311–5. | |
Zwiers C, Lindenburg ITM, Klumper FJ, et al. Complications of intrauterine intravascular blood transfusion: lessons learned after 1678 procedures. Ultrasound in Obstetrics & Gynecology 2017;50:180–6. | |
Yinon Y, Visser J, Kelly EN, et al. Early intrauterine transfusion in severe red blood cell alloimmunization. Ultrasound in Obstetrics & Gynecology. 2010;36:601–6. | |
Lindenburg IT, van Kamp IL, van Zwet EW, et al. Increased perinatal loss after intrauterine transfusion for alloimmune anaemia before 20 weeks of gestation. BJOG 2013;120:847–52. | |
Fox C, Martin W, Somerset DA, et al. Early intraperitoneal transfusion and adjuvant maternal immunoglobulin therapy in the treatment of severe red cell alloimmunization prior to fetal intravascular transfusion. Fetal Diagnosis and Therapy 2008;23:159–63. | |
Charles AG, Blumenthal LS. Promethazine hydrochloride therapy in severely Rh-sensitized pregnancies. Obstetrics & Gynecology 1982;60:627–30. | |
Moise KJ. The rationale of fetal therapy. In: Kilby M, Johnson A, Oepkes D (eds.) Fetal Therapy – Scientific basis and critical appraisal of clinical benefits, 1st edn. Cambridge University Press, 2012. | |
Moise KJ, Jr. Changing trends in the management of red blood cell alloimmunization in pregnancy. Archives of Pathology & Laboratory Medicine 1994;118:421–8. | |
Wong KS, Connan K, Rowlands S, et al. Antenatal immunoglobulin for fetal red blood cell alloimmunization. Cochrane Database of Systematic Reviews 2013:CD008267. | |
Hall AM, Cairns LS, Altmann DM, et al. Immune responses and tolerance to the RhD blood group protein in HLA-transgenic mice. Blood 2005;105:2175–9. | |
Hendrickson JE, Delaney M. Hemolytic Disease of the Fetus and Newborn: Modern Practice and Future Investigations. Transfusion Medicine Reviews 2016;30:159–64. | |
Dau PC. Increased antibody production in peripheral blood mononuclear cells after plasma exchange therapy in multiple sclerosis. Journal of Neuroimmunology 1995;62:197–200. | |
Reeves HM, Winters JL. The mechanisms of action of plasma exchange. British Journal of Haematology 2014;164:342–51. | |
Ruma MS, Moise KJ, Jr, Kim E, et al. Combined plasmapheresis and intravenous immune globulin for the treatment of severe maternal red cell alloimmunization. American Journal of Obstetrics and Gynecology 2007;196:138 e1–6. | |
Colpo A, Tison T, Gervasi MT, et al. Personalized treatment with immunoadsorption and intravenous immunoglobulin in a case of severe Rh alloimmunization during pregnancy unresponsive to plasma – exchange. Transfusion and Apheresis Science 2017;56:480–3. | |
Imbach P, Barandun S, d'Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. The Lancet 1981;1:1228–31. | |
Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. Journal of Experimental Medicine 2007;204:11–5. | |
Connan K, Kornman L, Savoia H, et al. IVIG – is it the answer? Maternal administration of immunoglobulin for severe fetal red blood cell alloimmunisation during pregnancy: a case series. Australian and New Zealand Journal of Obstetrics and Gynaecology 2009;49:612–8. | |
Kriplani A, Malhotra Singh B, Mandal K. Fetal intravenous immunoglobulin therapy in rhesus hemolytic disease. Gynecologic and Obstetric Investigation Journal 2007;63:176–80. | |
Voto LS, Mathet ER, Zapaterio JL, et al. High-dose gammaglobulin (IVIG) followed by intrauterine transfusions (IUTs): a new alternative for the treatment of severe fetal hemolytic disease. Journal of Perinatal Medicine 1997;25:85–8. | |
Deka D, Buckshee K, Kinra G. Intravenous immunoglobulin as primary therapy or adjuvant therapy to intrauterine fetal blood transfusion: a new approach in the management of severe Rh-immunization. Journal of Obstetrics and Gynaecology Research 1996;22:561–7. | |
van den Akker ES, Oepkes D. Fetal and neonatal alloimmune thrombocytopenia. Best Practice & Research Clinical Obstetrics & Gynaecology 2008;22:3–14. | |
Gelfand EW, Ochs HD, Shearer WT. Controversies in IgG replacement therapy in patients with antibody deficiency diseases. The Journal of Allergy and Clinical Immunology 2013;131:1001–5. | |
Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfusion Medicine Reviews 2013;27:171–8. | |
Thung SF, Grobman WA. The cost effectiveness of empiric intravenous immunoglobulin for the antepartum treatment of fetal and neonatal alloimmune thrombocytopenia. American Journal of Obstetrics and Gynecology 2005;193:1094–9. | |
Zwiers C, van der Bom JG, van Kamp IL, et al. Postponing Early intrauterine Transfusion with Intravenous immunoglobulin Treatment; the PETIT study on severe hemolytic disease of the fetus and newborn. American Journal of Obstetrics and Gynecology 2018;219:291 e1–291 e9. | |
Ling LE, Hillson JL, Tiessen RG, et al. M281, an Anti-FcRn Antibody: Pharmacodynamics, Pharmacokinetics, and Safety Across the Full Range of IgG Reduction in a First-in-Human Study. Clinical Pharmacology & Therapeutics 2019;105:1031–9. | |
Zuercher AW, Spirig R, Baz Morelli A, et al. Next-generation Fc receptor-targeting biologics for autoimmune diseases. Autoimmunity Reviews 2019;18:102366. | |
Gable KL, Guptill JT. Antagonism of the Neonatal Fc Receptor as an Emerging Treatment for Myasthenia Gravis. Frontiers in Immunology 2019;10:3052. | |
Sockolosky JT, Szoka FC. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Advanced Drug Delivery Reviews 2015;91:109–24. | |
Roy S, Nanovskaya T, Patrikeeva S, et al. M281, an anti-FcRn antibody, inhibits IgG transfer in a human ex vivo placental perfusion model. American Journal of Obstetrics and Gynecology 2019;220:498 e1–498 e9. | |
Chen P, Li C, Lang S, et al. Animal model of fetal and neonatal immune thrombocytopenia: role of neonatal Fc receptor in the pathogenesis and therapy. Blood 2010;116:3660–8. | |
Li C, Piran S, Chen P, et al. The maternal immune response to fetal platelet GPIbalpha causes frequent miscarriage in mice that can be prevented by intravenous IgG and anti-FcRn therapies. Journal of Clinical Investigation 2011;121:4537–47. | |
Nixon AE, Chen J, Sexton DJ, et al. Fully human monoclonal antibody inhibitors of the neonatal fc receptor reduce circulating IgG in non-human primates. Frontiers in Immunology 2015;6:176. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
