This chapter should be cited as follows:
Okogbenin SA, Danny AA, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.417993
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 17
Maternal immunization
Volume Editors:
Professor Asma Khalil, The Royal College of Obstetricians and Gynaecologists, London, UK; Fetal Medicine Unit, Department of Obstetrics and Gynaecology, St George’s University Hospitals NHS Foundation Trust, London, UK
Professor Flor M Munoz, Baylor College of Medicine, TX, USA
Professor Ajoke Sobanjo-ter Meulen, University of Washington, Seattle, WA, USA

Chapter
Lassa Fever
First published: May 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Lassa fever is an acute viral hemorrhagic illness caused by the Lassa virus, a single-stranded RNA virus in the Arenavirus family.1 The illness was discovered in Nigeria in 1969 following the death of two missionaries.2,3,4 Since then, numerous outbreaks have occurred in several communities in Nigeria with the highest prevalence in two Southern States (Edo and Ondo) and the South Eastern State of Ebonyi.5,6 The highest incidence is between January and April but more recently, it has become endemic in many communities with cases seen all year round.5,7,8
The infection has spread to virtually all other West African countries9,10 especially those of the Mano River basin union, Sierra Leone,11 Guinea, Benin Republic,9 and Liberia.12 Cases have also been recorded in other countries such as Ghana, Senegal, and Mali.13,14 Lassa fever is the most exported viral hemorrhagic fever with reported cases in Europe, Canada, Israel, and the United States of America.15,16,17,18,19,20,21 The virus has become one of the WHO’s priority highly infectious pathogens in need of urgent research and countermeasures, including vaccine development, and it is classified as a category A bioterrorism agent capable of serving as a biological weapon.22,23
The virus is estimated to infect between 300,000 and 500,000 people with 5,000 people dying each year.24,25 This is believed to be largely under-estimated and there is currently a large multisite study across several countries in West Africa to estimate the true burden of the disease. There are also estimates of disease burden in some West Africa countries based on prevalence of antibodies in Nigeria (21%),26 Guinea (4–55%),27 and Sierra Leone (8–52%).28
TRANSMISSION
Lassa virus is a single-stranded RNA virus,29,30,31 spherical and enveloped. In cross-section, the ribosomes give it a sandy appearance and hence it is grouped as an Arenavirus.31,32,33,34 Different strains of the virus have been identified with clustering of particular stains in geographic locations. Clades I to III are found predominantly in Nigeria while clade IV is seen in Sierra Leone, Guinea, and Liberia. Clades V and VI have been identified in Mali and Togo, respectively.35,36,37 In Nigeria, the three clades are seen in three different locations. These clades have implications for development of diagnostic assays and vaccine.38,39,40,41
The recognized vector of viral transmission is the rodent, mastomys natalensis. This is a common rat in many West African countries.42,43,44 They are recognized by the multiple breasts on their ventral surface and their relatively hairless tail. There is also evidence that the African wood mouse Hylomyscus pamfi and the Guinea multimammate mouse Mastomys erythroleucus can act as reservoir host species for the Lassa virus.45,46,47,48 Mastomys natalensis are peri-domestic rodents. They often live in field crops and stored food and are efficient transmitter of the disease. They produce multiple offspring in a single birth and infected rats transmit the virus to their offspring and remain infected for life. The rodents are unharmed by the virus.9,28
The Lassa virus is transmitted from rats to humans when there is ingestion of food contaminated with the urine, feces or blood of infected mastomys. Humans also get infected when they handle, kill, or ingest infected rats as is common in some West African communities.43,49,50
Inhalation of aerosolized virus may also spread the infection through droplet transmission. This may occur during cleaning of surfaces or sweeping of floors containing infected rat droppings.51 But true airborne transmission is unlikely.
Human-to-human transmission is mainly in health-care settings when there is direct contact with blood, body fluid of infected patients, or unsafe injection practices. Contact with contaminated bed railings, bed linens, and medical equipment may also spread the infection.9,24 Skin cuts, abrasion, or wounds facilitate transmission. More recently, sequencing data show that the majority of transmissions are from rat-to-human and human-to-human transmission even in the same household is uncommon.52 This is in contrast with Ebola where the majority of transmission is human to human.
A report from a recent study suggests that sexual transmission is possible. The virus is reported to persist in semen for up to 12 months after discharge from hospital. The infectivity of such a long persisting virus was recently demonstrated by researchers from the Irrua Specialist Teaching Hospital and the Bernard Notch Institute of Tropical Medicine.53 There is also congenital transmission from mother to fetus or newborn. Transmission via breast milk is also believed to occur due to the high viral load seen in breast milk.54,55
CLINICAL COURSE
The infection is asymptomatic in 80% of people while in 20%, symptoms vary from mild to severe disease with death complicating about 1% of infected persons.24 However, during epidemics, mortality rate for hospitalized patients can be as high as 15–40%.24,56 The case fatality rate for hospitalized patients in Nigeria is currently about 20%.24
In pregnant patients, Lassa fever presents special challenges in diagnosis, management, and outcome. In non-pregnant women, mortality from Lassa fever is about 13% but in pregnancy, mortality rises to 30% and above.57,58,59 Perinatal mortality can be as high as 85–95% during epidemics. In endemic communities, Lassa fever disease contributes as much as 13.1–25% to the overall maternal mortality figures.59 Addressing the challenges of pregnant women or women in the puerperium should be a priority of any intervention.
The incubation period is from 2 to 21 days and illness usually last for 3 weeks resulting in recovery or death.1,51 The disease has an insidious onset and initial symptoms are mild and varied. At this stage, it is often taken lightly and may be presumed to be one of the common causes of febrile illnesses, such as malaria, typhoid fever, or gastroenteritis.60 The advice in endemic communities is to screen patients for Lassa fever if they fail to respond to antimalarials or antibiotics after 48 hours of treatment.54
The initial symptoms are fever (temperature greater than 38oC) malaise, headache, weakness, and body pains. More specific symptoms appear a few days later with pharyngitis, cough, retrosternal chest pain, muscle pain, abdominal pain, nausea, vomiting, and diarrhea. Further symptoms are usually due to complications. Disseminated Intravascular Coagulation (DIC) can present as petechial and conjunctival hemorrhage, ecchymosis, hematuria, hemoglobinuria, hemoptysis, etc. However, despite the fact that Lassa fever is a viral hemorrhagic fever, less the 30% actually have hemorrhagic symptoms.3,56,60 Acute kidney injury can present as oliguria, facial, pedal edema, and generalized edema. Acute respiratory distress syndrome can present as dyspnoea and reduced SPO2. Delirium, seizure, and coma may be signs of encephalopathy or central nervous system involvement. Jaundice with tender hepatomegaly may indicate hepatic involvement. In many settings patients often present late when complications have already set in. Unilateral or bilateral deafness of varying decree occurs in about one third of patients. This could be temporary or permanent (Table 1).61,62
1
Clinical features of Lassa fever.
Clinical features | ||
Initial features | Fever, headache, malaise, body aches, and pains | |
More specific features | Sore-throat, retrosternal chest pain, abdominal pain, cough, nausea, vomiting, conjunctival hemorrhage, diarrhea, proteinuria, microscopic hematuria. | |
Features of complications | Acute kidney injury | Oliguria, pedal and facial edema, confusion and seizure. |
Acute respiratory distress syndrome | Dyspnoea, tachycardia, reduced SPO2. | |
Disseminated intravascular coagulation | Petechial hemorrhage, ecchymosis, hematuria, hemoptysis, vaginal bleeding, bleeding from the gum, and oral mucosa. | |
Encephalopathy | Delirium, restlessness, seizure, coma. | |
Shock | Low blood pressure, increased pulse rate. | |
Liver involvement | Jaundice, elevated AST, elevated ALT, tender hepatomegaly. | |
Symptoms specific to pregnancy | Hyperemesis, abortion, prematurity, intrauterine fetal death, vaginal bleeding. Breast sign: enlarged, turgid, and tender. | |
Death usually results from acute kidney injury, encephalopathy and seizures, acute respiratory distress symptoms, sepsis and shock. Spontaneous recovery can occur in cases that do not progress to organ damage.
CLINICAL COURSE IN PREGNANCY
In pregnant women, it is not clear if there are as many asymptomatic cases as there are in non-pregnant patients. However, in symptomatic patients, the disease starts insidiously after 2–21 days of incubation and symptoms are similar to those seen in non-pregnant individuals. There are however symptoms specific to pregnancy.
In the first trimester, patients could present with hyperemesis associated with other Lassa fever symptoms. Abortion is common59,63,64 and may be the only presenting complaint. At presentation, the fetus is usually not viable and spontaneous abortive process may even be complete. An ultrasound scan done at this stage will confirm the state of the uterus. In endemic communities, Lassa fever must be suspected in patients with unexplained fetal loss that has been associated with a preceding febrile illness. Early screening should therefore be done for pregnant women with fever or ill health who are unresponsive to antimalaria and antibiotics in such communities.
In the late second or third trimester, there are two broad categories of presentation.59,63 In the first category, the fetus is viable and the patient presents with mild symptoms such as fever, malaise, headache, and body pain (Figure 1). At this stage, there are no signs of complication, thus suggesting an early presentation or a mild disease. The second broad category is defined by the presentation with a non-viable fetus. This is usually associated with other signs of severe disease such as extra vaginal bleeding, coma, seizure, sepsis, oliguria, etc. (see Figure 2).59 The disease in this category is usually fulminant and can progress rapidly to maternal death. The presence of a dead fetus in the late second or third trimester is therefore an emergency, which requires urgent evacuation of the contents of the uterus preferably via the vagina route. The stress of surgery in this category of patients may result in poor outcome.
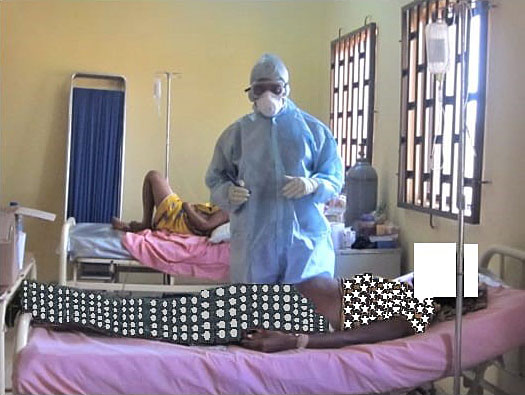
1
Lassa fever in pregnancy. Category 1: fetus is viable and symptoms are mild.

2
Lassa fever in pregnancy. Category 2: fetus is dead, Patient has severe symptoms. Blood in urine as seen in the urine bag, foot of bed is raised because patient is in shock. Oxygen is being delivered with a nasal prong.
Another specific feature seen in pregnancy is the breast sign (Figure 3).59,63 In women presenting with a viable fetus, one or both breasts may become enlarged, turgid, and painful without secretion of milk or colostrum. The cause of this breast sign, which is pathognomonic of Lassa fever in pregnancy is unknown. It is known that breast milk may contain the virus during puerperium and the breast may be a sanctuary site for persistent virus even after clearance from the blood. Recently, scientists from the Irrua Specialist Teaching Hospital and the Benard Notch Institute of Tropical Medicine (BNITM) reported persistent virus in some body fluids many months after survivors have been discharged following a negative serum PCR.53 Vaginal bleeding may also present in Lassa fever in pregnancy in the third trimester or post delivery period and may be misdiagnosed as antepartum or postpartum hemorrhage from obstetrics causes.59
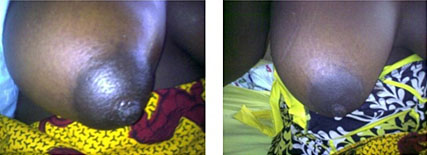
3
Breast sign in Lassa fever in Pregnancy: before and after treatment.
The worse prognosis seen in pregnancy has been attributed to various factors. The insidious, non-specific, and varied nature of symptoms in the early stages of Lassa fever disease may mimic early/constitutional pregnancy symptom or the general lassitude of pregnancy. This may result in delay in presentation to the hospital as well as inaccurate diagnosis. The Lassa fever virus has also been shown to have a higher affinity for the vascular tissues of the fetus and placenta. The maternal immunity is significantly altered in pregnancy while the fetal immunity is still maturing. The result is that pregnant women harbor significantly higher viral load than in the non-pregnant state and this has implication for overall outcome.65,66 In pregnancy, severe cases of Lassa fever disease may present with seizures, hemorrhage, and sepsis. These three presentations mimic eclampsia, obstetric hemorrhagic, and pregnancy-related sepsis, respectively; the three most common obstetric causes of maternal mortality. Hence the diagnosis of Lassa fever can be easily missed and thus Lassa fever may be a significant yet undiagnosed cause of many maternal mortality in endemic areas of West Africa.59 It is not uncommon to see obstetricians infected with Lassa fever after a cesarean section for a pre-operative diagnosis of eclampsia.
INVESTIGATIONS
A confirmatory test is necessary to establish the diagnosis. This is often done with a real-time RT-PCR. Lassa fever can also be diagnosed using enzyme-linked immunosorbent serologic assays that detect IgM and IgG antibodies, as well as the Lassa antigen. Viral culture is usually for research purposes and immune-histochemistry can be done on post-mortem tissues.
MANAGEMENT
Following diagnosis, patients should be admitted into a Lassa fever isolation facility and managed under standard and additional precautions to prevent contact transmission. A multidisciplinary team approach is best to provide optimum care. An infectious disease physician or a physician with interest in infectious disease is required. Other specialists that may be needed in the course of treatment include a nephrologist, a hematologist, and a critical care physician.
The base-line investigations for all patients include a full blood count, platelet count, blood sugar, clotting profile, serum electrolyte, urea, creatinine estimation, liver function test with AST and ALT. A low hematocrit could be due to the disease or the treatment with ribavirin. leucopenia, leucocytosis, thrombocytopenia, elevated AST and ALT are common as the disease progresses. AST is often more elevated compared to ALT and has been associated with poorer outcome. Urinalysis could also show proteinuria and microscopic hematuria. These investigations should be repeated on day 5 and day 10 for patients with mild disease who are responding to treatment. It is important to rule out other common causes of fever in the communities involved and blood test for malaria parasite and typhoid fever should be done. Urine microscopy culture and sensitivity is done to rule out urinary tract infection. These infections can co-exist with Lassa fever disease.
Severely ill patients and those with worsening disease will need more frequent repeat investigations and these could be done every 48 hours. More investigations are also necessary for severely ill patients. These could include blood gases, blood culture, serum calcium, chest X-ray or CT scan, EEG, and ECG.
Treatment is often commenced immediately with intravenous ribavirin using the McCormick multiple daily doses or the Irrua regimen which is a single daily dose. Supportive care is given with intravenous fluid, prophylactic broad-spectrum antibiotics, such as intravenous ceftriaxone or Augmentin. Oxygen therapy and blood transfusion may also be required. When complications threaten or set in, the necessary specialist should be consulted early. Dialysis is often required for acute kidney injury. Aminoglycosides and other nephrotoxic drugs are best avoided. Intravenous crystalloids and vasopressors are needed in shock. Low-dose haloperidol is useful to treat delirium67 and anticonvulsants may be given when there is seizure. Respiratory distress could require ventilatory support.
MANAGEMENT IN PREGNANCY
In pregnancy, an ultrasound scan is important to determine the state of the fetus and help determine the course of management to be adopted. This should be done on the day of admission as soon as possible. The ribavirin regime used in the Irrua Specialist Teaching Hospital for Pregnant women is the modified McCormick regime.
In the first trimester, abortion is the rule and many patients present with an ongoing abortive process. An ultrasound scan done will usually confirm an inevitable, incomplete or missed abortion. The patient is commenced on intravenous ribavirin, supportive care with intravenous infusion and prophylactic broad-spectrum antibiotics. An immediate evacuation of the contents of the uterus is done whenever there is a diagnosis of inevitable, incomplete or missed abortion. It is important to ensure that the uterus is completely emptied of the products of conception as any retained product could prolong the duration of the fever with poor outcome. An ultrasound scan 48 hours after the evacuation is recommended to ensure the uterus is empty if in doubt.
In the late second or third trimester, the two broad categories described earlier become relevant. In the first category in which the symptoms are mild and importantly the fetus is viable, conservative management with intravenous ribavirin and supportive care give better outcome for the mother and the fetus. In such circumstances the patient is also given prophylactic antibiotics, analgesics, and hematinics. Dexamethasone injection is often given for fetal lung maturity because of the high risk of preterm birth. However, tocolytics are to be avoided. Maternal and fetal monitoring is vital when on admission for conservative management. Fetal well-being is monitored with a baseline and weekly biophysical profile or a non-stress test and at least twice daily fetal heart rate check with a hand-held Doppler. This ensures immediately recognition of fetal compromise or intrauterine fetal demise. Maternal vital signs are monitored by temperature checks, blood pressure, and pulse-rate measurements. Real-time PCR checks are also done on day 5 and day 10 of treatment to determine the CT values and estimate the viral load. In the vast majority of patients, symptoms gradually abate and at about day 10, many patients have a negative PCR and are then moved from the isolation ward to a regular ward. Those who are still positive are treated for a few more days with the daily ribavirin dose until the PCR is negative otherwise a decision for delivery could be taken at this point depending on the gestational age, fetal well-being, and the severity of maternal symptoms or illness. When the PCR is negative and maternal symptoms have abated, patients are transferred to regular wards for continuous fetal monitoring until delivery. This is because intrauterine fetal death could still occur in about 30–50% of surviving fetuses. Fetal monitoring is done with twice daily fetal heart rate monitoring with the hand-held Doppler and also twice weekly biophysical profile or non-stress test. Delivery is initiated at 37 completed weeks or if fetal well-being test are non-reassuring. Vaginal delivery is to be preferred except there are obstetrics reason for a cesarean section.
In the second broad category of presentation in the late second or third trimester, there is intrauterine fetal death. This is often associated with symptoms of severe disease or organ involvement. Patients could therefore also present with vaginal or extra vaginal bleeding, oliguria from acute kidney injury and dyspnoea from acute respiratory distress syndrome. Seizure and coma are ominous signs. In this circumstance, the disease is often fulminant and can quickly progress to death. There is an urgent need to evacuate the dead fetus, placenta, and membranes. This gives the best chance of survival. Unfortunately, evacuation may not be achieved and many such patients die undelivered. However, should a vaginal delivery occur with complete delivery of the placenta and the membranes, the patient can then be treated as necessary with dialysis, blood and blood product transfusion, intravenous ribavirin, intravenous antibiotics, vasopressors, ventilation, and other critical care measures. Amazing recovery has been achieved in some patients, which is very well worth the effort.
It is important to note that during conservative management of pregnant women with mild disease, intrauterine fetal death could occur. In such circumstances, evacuation should be conducted immediately, however the patients are usually not in fulminant disease and the delivery could be conducted safely with good maternal outcome.
In all circumstances, delivery is conducted with standard and additional precautions to avoid contact transmission. The cord blood is taken for PCR and when positive, the newborn is handed over to the pediatricians for specialist care. When negative, neonates can be re-tested 2–3 weeks later or if illness ensues. Neonates born to mothers who have had some days of treatment often do better than neonates of mothers who are delivered before treatment could be initiated. At delivery, the breast milk should be tested for Lassa virus by PCR and if negative, the newborn can be breastfed otherwise artificial milk is given until the breast milk has a negative PCR result.
Multiple pregnancy is common in west Africa and it is not unusual to see Lassa fever infection complicating twin pregnancies. The presentation and management are essentially as in the singleton pregnancy. However, if during conservative management one twin dies, then delivery should be initiated. Outcome is essentially as in singleton pregnancy.
PREVENTION
At present there is no vaccine for the prevention of Lassa fever infection or disease. Their development should be prioritized and emphasis should be placed on the vulnerable population, such as pregnant women and their neonates. Currently efforts are directed at both passive and active surveillance, identifying cases and contacts and notifying public health authorities.
Environmental control efforts are focused on preventing rat and human contacts. Good quality housing helps to prevent rat entry into homes. This can be achieved by building houses with strong foundation using concrete or solid mud. It is also important to avoid or plug holes in windows, nets, roof, and doors from which rats could enter the house. Maintaining good hygiene of the immediate surroundings of houses is vital. Ensure proper waste disposal, cover waste bin around houses and avoid debris littering the area. Avoiding excessive vegetation, crop and animal farming in the immediate vicinity of houses also keeps the rodents away.
Within the house, both cooked and raw grains or food must be kept in rodent-proof containers and left-over food should be re-heated properly before consumption. Kitchen surfaces and utensils should be cleaned and food particles properly disposed after cooking. utensils should be stored in enclosed areas and re-washed before each use.
If any member of a house-hold is ill with fever in an endemic community or has a history of travel to an endemic country or has recently been in contact with someone who traveled recently, Lassa fever should be a consideration and avoidance of contact with the person or body fluids is advised.
To prevent nosocomial infection in the hospital setting, there should be a suspect or holding bay for suspect cases and confirmed cases should be managed in an isolation facility. There should be good supplies of personal protective equipment and the maintenance of infection prevention control measures.
CONCLUSION
Lassa fever is a significant health hazard and causes suffering and death in many endemic communities. It has the potential of spreading to many other countries and causing major epidemics. Prioritizing it for research and the development of new therapeutics and vaccine is the right thing to do. The concerns of special population, such as pregnant women and the newborn should be emphasized in any intervention effort.
PRACTICE RECOMMENDATIONS
Investigations
- Confirm diagnosis with real-time PCR.
- Base-line investigations include the following.
- Full blood count, platelet count (low hematocrit could be due to the disease or the ribavirin therapy. leucopenia or leucocytosis and thrombocytopaenia).
- Blood sugar.
- Clotting profile.
- Serum electrolyte, urea, and creatinine estimation.
- Liver function test with AST and ALT (increased levels of AST has been associated with poor outcome).
- Urinalysis for proteinuria and microscopic hematuria.
- Repeat real-time PCR and above investigations on day 5 and day 10 for patients with mild disease who are responding to treatment.
- For more severe disease repeat investigations every 48 h or as necessary.
- Rule out other common causes of fever in the communities such as blood test for malaria parasite and typhoid fever. Urine microscopy culture and sensitivity is done to rule out urinary tract infection.
- More investigations are also necessary for severely ill patients. These could include blood gasses, blood culture, serum calcium, chest X-ray or CT scan, EEG and ECG.
Management
- Admit into Lassa fever isolation facility.
- Manage under standard and additional precautions.
- A multidisciplinary team approach is best to provide optimum care.
- Commence intravenous ribavirin using the McCormick multiple daily doses or the Irrua regimen, which is a single daily dose.
- Supportive care is given with intravenous fluid.
- Prophylactic broad spectrum antibiotics with intravenous ceftriaxone or augmentin.
- Oxygen therapy and blood transfusion may also be required.
- Treat for malaria if present.
- When complications threaten or set in, the necessary specialist should be consulted early.
- Dialysis is often required for acute kidney injury.
- Aminoglycosides and other nephrotoxic drugs are best avoided.
- Intravenous crystalloids and vasopressors are needed in shock.
- Low-dose haloperidol is useful to treat delirium.
- Anticonvulsants may be given when there is seizure.
- Respiratory distress could require ventilatory support.
Management in Pregnancy (First Trimester)
- Do an ultrasound scan to determine the state of the fetus.
- In the first trimester an ultrasound scan done will usually confirm an inevitable, incomplete, or missed abortion.
- The patient is commenced on intravenous ribavirin.
- Supportive care with intravenous infusion.
- Prophylactic broad-spectrum antibiotics.
- An immediate evacuation of the contents of the uterus.
- It is important to ensure that the uterus is completely emptied of the products of conception as any retained product could prolong the duration of the fever and results in poor prognosis.
- An ultrasound scan done 48 h after the evacuation is recommended to ensure the uterus is emptied if in doubt.
Management in Pregnancy (Late Second or Third Trimester)
In the first category in which the symptoms are mild and importantly the fetus is viable, then the following is recommended.
- Conservative management with intravenous ribavirin and supportive care is adopted.
- Intravenous ribavirin using the modified McCormick regime.
- The patient is also given prophylactic antibiotics, analgesics, and hematinics.
- Dexamethasone injection is often given for fetal lung maturity because of the high risk of preterm birth.
- Avoid tocolytics even if there is preterm contraction.
- Monitor fetal well-being with a baseline biophysical profile or a non-stress test and at least twice daily fetal heart rate check with a hand-held Doppler.
- Monitor maternal vital signs by temperature checks, blood pressure, and pulse-rate measurements.
- Repeat real-time PCR on day 5 and day 10 of treatment to determine the CT values and estimate the viral load.
- In the vast majority of patients, symptoms gradually abate and at about day 10, many patients have a negative PCR and are then moved from the isolation ward to a regular ward.
- Those who are still positive are treated for a few more days with the daily ribavirin dose until the PCR is negative otherwise a decision for delivery could be taken at this point depending on the gestational age and the severity of maternal symptoms or illness.
- When the PCR is negative and maternal symptoms have abated, patients are transferred to regular wards for continuous fetal monitoring until delivery.
- Fetal monitoring in the regular ward is done with twice daily fetal heart rate monitoring with the hand-held doppler and also twice weekly biophysical profile or non-stress test.
- Delivery is initiated at 37 completed weeks or if fetal well-being test are non-reassuring.
- Vaginal delivery is to be preferred except when there is an obstetrics reason for a cesarean section.
In the second broad category of presentation in the late second or in the third trimester, there is intrauterine fetal death.
- Evacuate the dead fetus, placenta, and membranes from the uterus, this gives the best chance of survival.
- Treat as necessary with intravenous ribavirin, intravenous antibiotics, dialysis, blood and blood product transfusion, vasopressors, ventilation, and other critical care measures.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
WHO. Lassa fever 2017. Available at: https://apps.who.int/mediacentre/factsheets/fs179/en/index.html [Internet]. | |
Monath TP, Lassa Fever 1973. Tropical Doctor 1973;4:155–61. | |
Frame JD, Baldwin JM Jr, Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa I. Clinical description and pathological findings. Am J Trop Med Hyg 1970;19:670–6. | |
Troup JM, White HA, Fom AL, et al. An outbreak of Lassa fever on the Jos plateau, Nigeria, in January—February 1970. A preliminary report. Am J Trop Med Hyg 1970;19(4):695–6. | |
Nigeria Centre for Disease Control. Lassa Fever Situation report. Epi-week 2020;52:21–7. | |
Akpede GO, Asogun DA, Okogbenin SA, et al. Lassa fever outbreaks in Nigeria. Expert Rev Anti Infect Ther 2018;16(9):663–6. | |
Akpede G, Asogun D, Okokhere P, et al. Spatial and temporal trends of the Lassa fever epidemic in Nigeria 2001–2009, with particular reference to the Edo State experience. Intern J Infect Dis 2010;14:e476–7. | |
Asogun DA, Adomeh DI, Ehimuan J, et al. Molecular diagnostics for Lassa fever at Irrua specialist teaching hospital, Nigeria: lessons learnt from two years of laboratory operation. PLoSNegl Trop Dis 2012;6:e1839. doi: 10.1371/journal.pntd.0001839. | |
Ogbu O, Ajulijukwu E, Uneke CJ. Lassa fever in West African sub-region: an overview. J Vector Borne Dis 2007;44:1–11. | |
European Centre for Disease Prevention and Control. Lassa fever in Nigeria, Benin, Togo, Germany and USA, 23 March 2016. | |
McCormick JB, King IJ, Webb PA, et al. A case-control study of the clinical diagnosis and course of Lassa fever. J Infect Dis 1987;155:445–55. doi: 10.1093/infdis/155.3.445. | |
Frame JD. Clinical features of Lassa fever in Liberia. Rev Infect Dis 1989;11(Suppl 4):S783–9. doi: 10.1093/clinids/11.Supplement_4.S783 | |
WHO 2019. Lassa fever. https://www.who.int/emergencies/diseases/lassa-fever/en/. | |
Mylne AQ, Pigott DM, Longbottom J. Mapping the zoonotic niche of Lassa fever in Africa. Trans R Soc Trop Med Hyg 2015;109(8):483–92 | |
Holmes GP, McCormick JB, Trock SC, et al. Lassa fever in the United States. Investigation of a case and new guidelines for management. New Engl J Med 1990;323:1120–3. | |
Mahdy MS, Chiang W, McLaughlin B, et al. Lassa fever: the first confirmed case imported into Canada. Can Dis Wkly Rep 1989;15:193–8. | |
Zweighaft RM, Fraser DW, Hattwick MA, et al. Lassa fever: response to an imported case. New Engl J Med 1977;297:803–7. | |
Johnson KM, Monath TP. Imported Lassa fever: reexamining the algorithms. New Engl J Med 1990;323:1139–41. | |
Haas WH, Breuer T, Pfaff G, et al. Imported Lassa fever in Germany: surveillance and management of contacts. Clin Infect Dis 2003;36:1254–6. doi: 10.1086/374853. | |
Kofman A, Choi MJ, Rollin PE. Lassa fever in travellers from West Africa, 1969–2016. Emerg Infect Dis 2019;25:236–9. doi: 10.3201/eid2502.180836. | |
Choi MT, Worku S, Knust B, et al. A case of Lassa fever diagnosed at a community hospital-Minnesota 2014. Open Forum Infect Dis 2018;5:ofy131. doi: 10.1093/ofid/ofy131. | |
Mehand MS, Al-Shorbaji F, Millett P, et al. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Res 2018;159:63–7. | |
Kenmoe S, Tchatchouang S, Ebogo-Belobo JT, et al. ‘Systematic review and meta-analysis of the epidemiology of lassa virus in humans, rodents and other mammals in Sub-Saharan Africa’. PLoS Neglected Tropical Diseases 2020;14(8):1–29. | |
Center for Disease Control (CDC). Lassa Fever 2019. Available at: https://www.cdc.gov/vhf/lassa. | |
lassa Fever WHO newsletter Geneva, 2005. | |
Tomori O, Fabiyi A, Sorungbe A, et al. Viral hemorrhagic fever antibodies in Nigerian populations. Am J Trop Med Hyg 1988;38:407–10. | |
Lukashevich LS, Clegg JC, Sidibe K. Lassa virus activity in Guinea: distribution of human antiviral antibody defined using enzyme-linked immunosorbent assay with recombinant antigen. J Med Virol 1993;40:210–7. | |
McCormick JB, Webb PA, Krebs JW, et al. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis 1987;155:437–44. 4. | |
Auperin DD, McCormick JB. Nucleotide sequence of the Lassa virus (Josiah strain) S genome RNA and amino acid sequence comparison of the N and GPC proteins to other arenaviruses. Virology 1989;168:421–5. | |
Auperin DD, Sasso DR, McCormick JB. Nucleotide sequence of the glycoprotein gene and intergenic region of the Lassa virus S genome RNA. Virology 1986;154:155–67. 26. | |
McCormick JB. Arenaviruses. In: Fields BN, Knipe DM. (eds.) Fields virology. New York: Raven Press, 1990:1245–67. | |
Buckley SM, Casals J. Lassa fever, a new virus disease of man from West Africa.III. Isolation and Characterization of the virus. Amer J Trop Med Hyg 1970;19:670–6. | |
Radoshitzky SR, Bào Y, Buchmeier MJ, et al. Past, present, and future of arenavirus taxonomy. Arch Virol 2015;160(7):1851–74. | |
Andersen KG, Shapiro J, Matranga CB, et al. Clinical sequence uncovers origins and evolution of Lassa virus. Cell 2015;162:736–50. | |
Whitmer SLM, Strecker T, Cadar D, et al. New Lineage of Lassa Virus, Togo, 2016. Emerg Infect Dis 2018;24(3):599–602. | |
Kafetzopoulou LE, Pullan ST, Lemey P, et al. Metagenomic sequencing at the epicenter of the Nigeria 2018 Lassa fever outbreak. Science 2019;363(6422):74–7. 15. | |
Lukashevich IS. The search for animal models for Lassa fever vaccine development. Expert Rev Vaccines 2013;12(1):71–86. | |
Bowen MD, Rollin PE, Ksiazek TG, et al. Genetic diversity among Lassa virus strains. J Virol 2000;74(15):6992–7004. | |
Ehichioya DU, Hass M, Becker-Ziaja B, et al. Current molecular epidemiology of Lassa virus in Nigeria. J Clin Microbiol 2011;49(3):1157–61. | |
Hallam HJ, Hallam S, Rodriguez SE, et al. Baseline mapping of Lassa fever virology, epidemiology and vaccine research and development. NPJ Vaccines 2018;3(11):1–8. | |
Siddle KJ, Eromon P, Barnes KG, et al. Genomic Analysis of Lassa Virus during an Increase in Cases in Nigeria in 2018. N Engl J Med 2018;379(18):1745–53. | |
Lecompte, E. et al. Mastomys natalensis and Lassa fever, West Africa. Emerg Infect Dis 2006. doi: 10.3201/eid1212.060812. | |
Monath TP, Newhouse VF, Kemp GE, et al. Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone. Science 1974;185(4147):263–5. 16. | |
Olayemi A, Obadare A, Oyeyiola A, et al. Arenavirus Diversity and Phylogeography of Mastomys natalensis Rodents, Nigeria. Emerg Infect Dis 2016;22(4):694–7. | |
Coulibaly-N’Golo D, Allali B, Kouassi SK, et al. Novel Arenavirus Sequences in Hylomyscus sp. and Mus (Nannomys) setulosus from Co ˆte d’Ivoire: Implications for Evolution of Arenaviruses in Africa. PLoS ONE 2011;6(6):e20893. doi:10.1371/journal.pone.0020893. | |
Olayemi A, Cadar D, Magassouba N, et al. New Hosts of The Lassa Virus. Sci Rep 2016;6:25280. | |
Ehichioya DU, Dellicour S, Pahlmann M, et al. Phylogeography of Lassa Virus in Nigeria. J Virol 93:e00929–19. https:/doi.org/10.1128/jvi.00929-19. | |
Agbonlahor DE, Erah A, Agba IM, et al. Prevalence of Lassa virus among rodents trapped in three |south-south states of Nigeria. J Vector Borne Dis 2017;54:146–50 | |
McCormick JB. Epidemiology and control of Lassa fever. Curr Top Microbiol Immunol 1987;134:69–78. | |
Keenlyside RA, McCormick JB, Webb PA, et al. Case-control study of Mastomys natalensis and humans in Lassa virus-infected households in Sierra Leone. Am J Trop Med Hyg 1983;32:829–37. | |
CDC, Lassa Fever available at: cdc.gov/vhf/Lassa/transmission/index.html. | |
Kafetzopoulou LE, Pullan SI, Lemey P, et al. Metagenomic sequencing at the epicentre of Nigeria 2018 Lassa Fever Outbreak. Science 2019;363(6422):74–7. | |
Thielebein A, Ighodalo Y, Taju A, et al. Virus persistence after recovery from acute Lassa fever in Nigeria: a 2-year interim analysis of a prospective longitudinal cohort study. Lancet Microbe 2021. www.thelancet.com/microbe. Published online November 8, 20221 https://doi.org/10.1016/s2666-5247(21)00178-6. | |
Nigerian Centre for Disease Control. NCDC, National Guidelines for Lassa fever case Management 2018. Available at ncdc.gov.ng/themes/common/does.protocol/92_1547068532.pdf. | |
Salu OB, Amoo OS, Shaibu JO, et al. Monitoring of Lassa virus infection in suspected and confirmed cases in Ondo state, Nigeria. PAMJ Reseach 2020;36(253). doi: 10.11604/pamj.2020.36.253.22104. | |
Salu OB, Amoo OS, Shaibu JO, et al. Monitoring of Lassa virus infection in suspected and confirmed cases in Ondo state, Nigeria. PAMJ Reseach 2020;36(253). doi: 10.11604/pamj.2020.36.253.22104. | |
Akpede GO, Asogun DA, Okogbenin SA, et al. Caseload and Case Fatality of Lassa Fever in Nigeria, 2001–2018: A Specialist Center's Experience and Its Implications. Front. Public Health 2019. https://doi.org/10.3389/fpubh.2019.00170. | |
Price ME, Fisher-Hoch SP, Craven RB, et al. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. Br Med J 1988;297:584–7. | |
Harper TK. Lassa Fever. TKH Virology Notes 2004. http://www.tarakharper.com/v_lassa.htm. | |
Okogbenin S, Okoeguale J, Akpede G, et al. Retrospective Cohort Study of Lassa Fever in Pregnancy, Southern Nigeria. Emerg infect Dis 2019;25(8):1494–500. doi: 10.3201/eid2508.181299. | |
Okokhere P, Colubri A, Azubike C, et al. Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: a retrospective, observational cohort study. Lancet Infect Dis 2018;18(6):684–95. | |
Liao BS, Byl FM, Adour KK. Audiometric comparison of Lassa fever hearing loss and idiopathic sudden hearing loss: evidence for viral cause. Otolaryngol Head Neck Surg 1992;106(3):226–9. | |
Cummins D, McCormick JB, Bennett D, et al. Acute sensorineural deafness in Lassa fever. J Am Med Assoc 1990;264(16):2093–6. | |
Okogbenin SA, Asogun D, Akpede G, et al. New lessons from a case series review of Lassa fever in pregnancy. Int J inf Ds 2010;14:E380. | |
Kayem ND, Benson C, Aye CYL, et al. Lassa fever in pregnancy: a systemic review and meta-analysis. Trans R Soc Trop Med Hyg 2020;114:385–96. doi: 10.1093/trstmh/traa011 Advance Access publication 5 March 2020. | |
Walker DH, McCormick JB, Johnson KM, et al. Pathologic and virologic study of fatal Lassa fever in man. Am J Pathol 1982;107:349–56. [PMC free article] [PubMed] [Google Scholar]. | |
Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 2010;63:425–33. doi: 10.1111/j.1600-0897.2010.00836.x [PMC free article] [PubMed] [CrossRef] [Google Scholar]. | |
Okogbenin EO, Obagaye MO, Aweh BE, et al. One year retrospective review of psychiatric consultations in Lassa fever, Southern Nigeria. Emerging Infectious Disease 26(12):3091–3. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
