This chapter should be cited as follows:
Marchant A, Kollmann TR, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.419463
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 17
Maternal immunization
Volume Editors:
Professor Asma Khalil, The Royal College of Obstetricians and Gynaecologists, London, UK; Fetal Medicine Unit, Department of Obstetrics and Gynaecology, St George’s University Hospitals NHS Foundation Trust, London, UK
Professor Flor M Munoz, Baylor College of Medicine, TX, USA
Professor Ajoke Sobanjo-ter Meulen, University of Washington, Seattle, WA, USA

Chapter
Neonatal Immunization
First published: May 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Immunity in early life is dependent on passive immunity provided by the mother (maternal antibodies transferred through the placenta and breastmilk) and on the development of active immunity within the newborn and young infant. Maternal and newborn/infant immunization are therefore complementary strategies to prevent infectious diseases in early life. The aim of neonatal immunization is to provide early priming of immunity to pathogens that can be subsequently enhanced by booster vaccination. Vaccination at birth can also favor vaccine coverage when neonates are in contact with health systems.
Neonates have been vaccinated since the 19th century and perceptions of the immune competence of the newborn has evolved since then. Current knowledge of the development of the immune system indicates that neonates are uniquely able to adapt to rapidly changing environmental exposure to non-self-antigens and can respond efficiently to many vaccines. Antibody responses to vaccination in early life can be impacted by high levels of transferred maternal antibodies; this raises important questions for the integration of maternal and infant vaccination programs. Beyond protection against their target pathogens, vaccines protect neonates against unrelated pathogens through mechanisms that are not fully delineated.
In this chapter, we review current evidence regarding the use of vaccines in neonates and we discuss prospects for protection against a larger range of infectious disease affecting young children.
A BRIEF HISTORY OF NEONATAL IMMUNIZATION
The 19th and beginning of the 20th centuries
The history of neonatal immunization started with the first human vaccine and the first vaccination program. In 1853, the Parliament of the United Kingdom passed the Vaccination Act making vaccination against smallpox statutory for newborn children.1 Until the beginning of the 20th century, it was considered that newborn children respond efficiently to vaccination. Opinions changed in the 1920s and 1930s when it was reported that antibody responses to toxins and bacteria improved with age in young children and young animals.2 These observations led to the concept of serologic maturation, involving a progressive increase in the capacity of young infants to produce antibodies in response to vaccination.3 The concept seemed supported by data from serum protein electrophoresis showing that, after waning of maternal antibodies, infants acquire adult levels of gamma-globulins very slowly, contrasting with the rapid acquisition of the other serum globulins.4 Although the role of exposure to microbial antigens in the progressive acquisition of infant antibodies was recognized, it was concluded that young infants appear to have very limited capacity to produce antibodies in response to antigenic stimulation.3 Based on these data, it was proposed that the optimal age for immunization against, e.g., pertussis was the second half of the first year of life.5
The 1940s and the 1950s
In the 1940s, studies showed that alum-adjuvanted tetanus, diphtheria, and pertussis vaccines could induce high levels of antibodies already in the first weeks of life and provide protection to young infants against pertussis.6,7,8 In parallel, it was recognized that high levels of maternal antibodies transferred to the newborn were associated with decreased vaccine responses.9,10,11 Differences were observed between antigens, with higher responses observed after newborn immunization with tetanus and diphtheria toxoids than with pertussis antigens.8,11 Based on these data, it was concluded that antibody responses could be induced by vaccination soon after birth and that interference by high levels of maternal antibodies was the dominant factor limiting responses to some, but not all, vaccines in early life.11 In 1952, the American Academy of Pediatrics recommended three monthly doses of alum-containing diphtheria, tetanus, and pertussis (DTP) vaccine starting at the age of 3 months.
The risk of inducing immunological tolerance by vaccinating newborn children has been a concern for decades. This risk was experimentally supported by the work of Billingham, Brent and Medawar who described in 1953 the phenomenon of actively acquired tolerance to foreign cells in fetal animals.12 While no evidence of immunological tolerance has been observed with vaccines administered at birth, reduced response to booster vaccination following the administration of a first dose of pertussis vaccine at birth has been observed in some studies13,14 but not in others.15,16,17 However, rather than immunological tolerance, reduced responses were related to interference between certain vaccine formulations.18
Modern times
In the 1970s, the original guidelines for the expanded Program on Immunization (EPI) of the World Health Organization (WHO) were to initiate routine infant vaccination at 3 months of age. But many countries had already set up successful immunization programs beginning at 6 to 8 weeks of age; in 1983, the EPI guidelines stipulated that DTP and oral poliomyelitis (OPV) vaccines could be administered from 6 weeks after birth.19 As discussed below, the administration of a first dose of trivalent (t) OPV was changed to newborn administration soon after.19 Later, hepatitis B immunization of newborns was shown to be effective and was recommended to prevent vertical transmission of hepatitis B virus (HBV). The efficacy of bacillus Calmette-Guerin (BCG) immunization of newborns had been shown through prospective and retrospective clinical studies since the 1930s and this led the WHO to recommend the administration of the BCG vaccine as soon as possible after birth in 1980.20 Vaccination of newborns with OPV, HBV and BCG is currently part of the immunization program of many countries. Following many decades of investigations and debate, it has become clear that the neonate is not prone to immunological tolerance and can effectively respond to vaccination. This insight was based on the increasing understanding of the basic mechanisms driving the development of the human immune system.
DEVELOPMENT OF THE HUMAN IMMUNE SYSTEM
Research conducted over the last two decades demonstrated that the immune system of the newborn infant is not immature but follows distinct rules that are essential to face unique challenges. From life in utero to postnatal exposure to a large diversity of non-self-molecules, the immune system must adapt and maintain tolerance to non-inherited maternal antigens and to non-harmful microbes, while controlling the replication of invasive pathogens. A description of the immune effectors contributing to these unique tasks is beyond the scope of this chapter and has been reviewed elsewhere.21 Here, we will summarize the characteristics of the immune components that are directly involved in immune responses to vaccination. In contrast to some other species, the human immune system develops early during fetal life. By the end of the first trimester of pregnancy, most immune cells can be identified in blood or lymphoid organs.22,23
Humoral immunity
As discussed in sections 4 and 5, newborn children can develop potent antibody responses to some vaccines (HBV, tetanus, and diphtheria toxoids) whereas responses to other vaccines (pertussis and OPV) are lower than in older infants. The mechanisms underlying these differences remain incompletely understood and probably involve lower expression of specific activating receptors by neonatal B lymphocytes and reduced formation of germinal centers, the lymphoid structure required for antibody responses to protein antigens (Figure 1). The repertoire of antibodies expressed by B lymphocytes is established in utero and is as diverse in neonates as it is in adults, supporting the notion that newborn children can respond to very diverse antigens.24 In contrast to protein antigens, responses to polysaccharide antigens are only developed during the second year of life.25 The mechanisms underlying this delayed capacity remain unclear but probably involve the reduced expression of activating receptors by infant B cells and the development of spleen structures required for T-cell-independent antibody responses.26 The effectiveness of conjugate vaccines further emphasizes the capacity of young infants to develop T-cell-dependent antibody responses.26,27
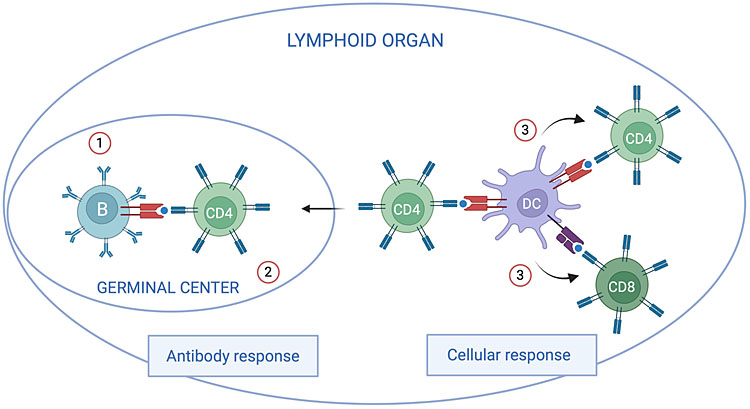
1
Schematic view of factors potentially limiting humoral and cellular responses to some vaccines in neonates. Antibody response: antibody responses to protein antigens require cognate interactions between antigen-specific B lymphocytes and CD4 T lymphocytes recognizing the same antigens. These interactions take place in specialized structures of secondary lymphoid organs called germinal centers (GC). The lower antibody responses to some vaccines in neonates as compared to older infants could be related to a lower expression of activating receptors on B lymphocytes and to lower levels of B-cell molecules stimulating CD4 T lymphocytes (1). These lower signals could be involved in reduced formation of GC (2).144,145 Vaccines stimulating potent antibody responses in neonates overcome these limitations through mechanisms that have not yet been identified. Cellular response: dendritic cells activate and induce the differentiation of CD4 and CD8 T lymphocytes producing effector cytokines and cytolytic molecules controlling intracellular pathogens. Neonatal dendritic cells have a different functional program than dendritic cells of older children and adults, involving the production of lower levels of cytokines promoting the differentiation of Th1 and cytotoxic CD8 T cells (3).146 This may limit cellular responses to some vaccines. The induction of potent cellular responses by some vaccines, like BCG, likely relates to their capacity to overcome this limitation and to induce potent activation of neonatal dendritic cells (created with BioRender.com).
Cellular immunity
The control of intracellular pathogens requires the differentiation of effector T lymphocytes producing anti-microbial cytokines and cytolytic molecules (Figure 1). Like antibody responses, effector T-cell responses can be induced by some vaccines (BCG, whole cell pertussis (wP)) in neonates and young infants, whereas cellular responses to other vaccines (OPV, HBV) are lower than in adults.28,29,30,31 These differences probably involve differential capacity of vaccines to stimulate the capacity of antigen-presenting cells to induce the differentiation of effector T lymphocytes (Figure 1). In parallel with B lymphocytes, the repertoire of T lymphocyte receptors is fully diversified at birth, allowing the response to very diverse antigens.24
Genes and environment
Twin studies have shown that genetic factors play an important role in regulating early response to vaccines in neonates.32,33,34 On the other hand, environmental factors appear to play a dominant role in the persistence and in the avidity maturation of vaccine-induced antibodies.35 Colonization with commensals provide important signals shaping the development of the immune system in early life.36 Some studies indicate an association between the composition of the microbiome and vaccine responses in young infants.37,38,39 However, a causal relationship remains to be established.40
In summary, immune components involved in vaccine responses develop early during human fetal life and allow efficient responses to several vaccines administered to neonates. The mechanisms underlying lower responses in early life to some but not all vaccines remain poorly understood.
VACCINES RECOMMENDED FOR HUMAN NEWBORNS
Neonates are vaccinated against tuberculosis and hepatitis B in countries where these diseases are endemic and neonatal OPV vaccination is an important part of ongoing polio eradication efforts.41,42,43 The efficacy of these interventions supports the notion that neonatal vaccination is a useful approach for the control of infectious diseases.
BCG
BCG is currently the only licensed vaccine against tuberculosis. It is a live-attenuated vaccine developed by in vitro subculturing of Mycobacterium bovis.44 It was first administered orally to a newborn child in 1921.44 Multiple strains were derived from the original BCG vaccine and are currently in use.45 In 2020, intradermal BCG vaccination of neonates was part of the vaccination program of 156 countries, with a coverage ranging from 68% in the Regions of the Americas to 95% in the West Pacific Region.46 BCG vaccination has a very good safety profile in immunocompetent neonates.41 Although no correlate of vaccine-induced protection against tuberculosis has been identified yet, it is considered that cellular immunity plays a predominant role in controlling mycobacteria.47 BCG vaccination induces comparable effector T-cell responses in neonates and in older children.30,31,48,49 The first clinical trials demonstrating the efficacy of intradermal BCG vaccination of neonates were conducted in the 1930s.50,51 More recent studies indicate that neonatal BCG vaccination provides 60% protection against tuberculosis in children below 5 years but is ineffective in protecting adolescents and adults.52 Recent estimates of incident pediatric tuberculosis cases in children under 4 years of age indicate about 500.000 cases per year, emphasizing the importance of administering BCG to newborns in countries where tuberculosis is endemic.52 As considerable research efforts are invested in the development of a vaccine providing higher protection against tuberculosis, it remains essential to maintain an adequate supply of the BCG vaccine.53,54
OPV
OPV is a live-attenuated vaccine against poliomyelitis. It replicates in the intestine and induces both systemic and mucosal immunity, thereby reducing viral shedding and transmission following subsequent wild-type viral exposure.55 Serum antibody response to OPV is lower in neonates as compared to older children, but IgA responses can be detected in stools of infants who shed the virus and have no detectable serum IgG response.56 Importantly, a first dose of OPV at birth accelerates the production of serum IgG induced by booster vaccine doses.57 Efficacy of neonatal vaccination with OPV has been demonstrated in many populations.56 However, the immunogenicity of OPV is lower in tropical countries as compared to industrialized countries, possibly because of interference by other enteroviruses.58 In populations with low levels of immunity, OPV can revert to virulent vaccine-derived polio virus (VDPV) and cause vaccine-associated paralytic polio (VAPP).59 To prevent this risk, industrialized countries have been using the inactivated polio vaccine (IPV) since the late 1990s to immunize infants from 6 weeks of age.43 IPV induces potent systemic but no mucosal immunity and therefore does not prevent transmission.55 As most VAPP cases were caused by OPV serotype 2 (OPV2) and as the last case of wild-type type 2 polio had occurred in 1999, the Global Polio Eradication Initiative coordinated in 2016 the global switch from tOPV to a bivalent (b) OPV containing the serotypes 1 and 3, supplemented with at least one dose of IPV.59 The administration of IPV to infants primed with bOPV at birth induces potent systemic immunity but no mucosal immunity to OPV2, and therefore no population immunity against poliovirus type 2 transmission.60 The development of OPV2 with higher genetic stability will be important for global eradication of poliovirus type 2.61
HBV
HBV is an adjuvanted sub-unit vaccine containing the hepatitis B surface antigen with aluminum salt. Neonatal vaccination against hepatitis B is implemented in 114 countries with a coverage ranging from 6% in the African Region to 85% in the Western Pacific Region and is recommended globally for neonates born to mothers with chronic hepatitis B infection.46,62 Antibody response to HBV in neonates is comparable to the response of older children or adults and is not affected by transferred maternal antibodies.28,63,64,65 Anti-viral T-cell responses to HBV appear lower in neonates as compared to adults.28 Vaccination initiated at birth is highly protective against vertical transmission.65 Protection is further increased by the co-administration of hyper immune globulins with no interference with the immunogenicity of the vaccine.66,67 Importantly, neonatal vaccination against hepatitis B provides long-term protection against carriage and liver cancer, underscoring the importance of further increasing vaccination coverage globally.62,68
VACCINES TESTED IN HUMAN NEWBORNS
Viral vaccines
As mentioned above, live smallpox vaccination of neonates was one of the first public health vaccine campaigns.1,69 Rotavirus represents a significant infectious threat causing nearly one third of diarrhea-associated deaths in childhood.70 The rationale for live-attenuated rotavirus vaccination in early infancy is based on the high incidence of severe rotavirus disease in infants below 6 months of age.70 The WHO recommends the first dose of rotavirus vaccine be administered as soon as possible after 6 weeks.71 Rotavirus infection of neonates is common but fortunately most often mild or asymptomatic.72,73,74 Importantly, even asymptomatic neonatal rotavirus infection can provide protection against severe, but not mild, rotavirus diarrhea later in life.72 Encouraged by this clinical observation of protection following neonatal infection, several rotavirus vaccines were tested in newborns.75,76 In addition to the monovalent human rotavirus vaccine (strain RV3-BB), Rotateq® (pentavalent) in a neonatal dosing schedule was found to be safe and immunogenic77,78,79,80,81 and likely clinically and cost-effective.82 Neonatal vaccination targeting human immunodeficiency virus (HIV) via subunit vaccines or live viral vectors has been assessed in several small studies and found to be safe and immunogenic;83,84,85,86 however, clinical efficacy of this approach remains to be determined.87,88
Conjugated polysaccharide vaccines
Pneumococcus (Streptococcus pneumoniae) is a leading cause of deaths in children under 5 years of age.89 The WHO recommends a three-dose schedule starting as early as 6 weeks of age.90 Given the significant burden of neonatal invasive pneumococcal disease,91,92 efforts to increase protection earlier has been attempted.93,94,95,96 Several trials of the 7-valent (PCV7) vaccine in newborns have been reported.97,98,99,100 Neonatal PCV7 vaccination was reported as safe but with waning of titers over time.93 Similarly, various Hemophilus influenza (Hi) vaccine formulations have been tested in newborns producing conflicting results (reviewed in101,102).
Diphtheria, tetanus and pertussis vaccines
Individual and combination DTP vaccines have been in use for over 100 years.103 The WHO recommends three doses of DTP-containing vaccines with the first dose administered from 6 weeks of age.104,105,106 Pertussis is a serious threat to newborns and infants prior to receipt of their first dose.107 Pertussis vaccination induces lower antibody responses in neonates as compared to older infants.5,6,7,8,108 And neonatal vaccination with wP or acellular pertussis (aP) has been associated with decreased response to booster vaccination in some but not in other studies.13,14,15,16,17,109,110 This phenomenon of vaccine interference following neonatal pertussis vaccination remains incompletely understood and may involve the diphtheria, rather than the pertussis, antigen component of the vaccines administered to newborns.18 Immunization against tetanus and diphtheria induces similar antibody levels in neonates and in older infants.7,8,108 The time required to induce antibodies against tetanus toxin does not allow protection against neonatal tetanus that is best prevented by maternal antibodies transferred following immunization during pregnancy.106 As neither diphtheria nor postnatally acquired tetanus (i.e., beyond neonatal tetanus) are common in the neonate,111,112,113 immunization against these two toxins is performed at the same time as pertussis immunization, using combined DTP vaccines.104,106
INTERACTIONS WITH MATERNAL ANTIBODIES
The reduced antibody response to infant vaccination in the presence of high levels of transferred maternal antibodies has been recognized since the 1940s.11 This reduction is variable between vaccines and vaccine antigens.113,114,115,116 Maternal antibodies have limited or no impact on antibody responses to tetanus toxoid or hepatitis B surface antigen.9,64,65 A different impact of maternal antibodies on the infant antibody response to individual antigens contained in the pertussis vaccine has also been observed.117,118,119 In contrast to antibody responses, maternal antibodies do not appear to interfere with infant T-cell responses to vaccines.120 The mechanisms underlying reduced antibody, but not T-cell, responses to some, but not all, vaccine antigens remain incompletely understood (Figure 2). Masking of antigenic epitopes by maternal antibodies probably plays a role.121 Studies in animals suggest that maternal IgG crosslink the inhibitory FcgRIIb at the surface of infant B cells and thereby reduce B-cell activation.122,123 Such a mechanism of B-cell regulation rather than masking of antigenic epitopes has important potential implications for the outcome of infant B-cell responses. Indeed, FcgRIIb is a key regulator of B-cell responses in germinal centers and participates in the selection of B cells producing high-affinity antibodies.124 Evidence that maternal antibodies regulate germinal center reaction has been obtained in infant mice.125 Whether similar processes are involved in the interaction between maternal antibodies and infant B cells in humans has not been established. Data from a study in Thailand suggests that the reduction of infant antibodies levels by maternal pertussis vaccination are not associated with a reduction of antibody-dependent B. pertussis killing.118 Also reassuring is current evidence from high-income countries indicating that pertussis immunization during pregnancy is not associated with an increased risk of whooping cough in toddlers.126 Continued surveillance for the potential impact of maternal pertussis immunization on whooping cough in children is required, particularly in LMIC as infant antibody response to wP vaccination may be more impacted by maternal antibodies than the response to aP vaccination.127
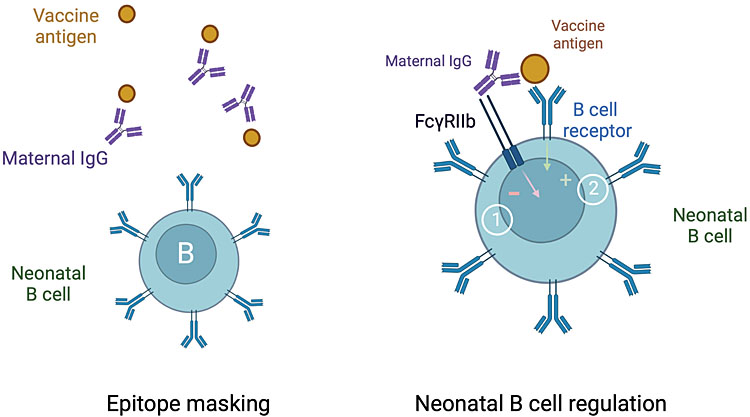
2
Interactions with maternal antibodies. High levels of transferred maternal antibodies are associated with reduced neonatal and infant antibody responses to some vaccines through mechanisms that remain poorly understood. Maternal antibodies (IgG) probably mask epitope on vaccine antigens and thereby prevent their recognition by neonatal B lymphocytes. Animal studies indicate that maternal IgG crosslink the inhibitory receptor FcgRIIb at the surface of newborn B cells (1). This negative signal reduces the activation of newborn B cells induced by the crosslinking of the surface B-cell receptor (2). The consequences of this regulation on the quality and repertoire of infant antibodies induced by vaccination (created with BioRender.com).
PATHOGEN-AGNOSTIC EFFECTS OF VACCINES IN NEWBORNS
Beyond targeting the pathogens, vaccines were initially designed to protect from, i.e., their pathogen-specific benefits, vaccination has been shown to provide another powerful benefit: reduction of newborn death beyond those attributable to the specific pathogen, i.e., pathogen-agnostic benefits (also known as non-specific, off-target, or secondary effects of vaccines).128 Specifically, safe and effective vaccines currently deployed in neonatal vaccine programs such as BCG and OPV can reduce newborn death by 30% independent of and in addition to protection from infection with the specific pathogen.128,129 Given that the list of potential pathogens threatening newborns is very long, pathogen-agnostic protective interventions have important public health potential.129,130,131,132,133 Importantly, these pathogen-agnostic protective effects are fast-acting because in high-mortality populations significant reduction in overall newborn mortality occurs within the 3 days of vaccination, thus precisely targeting the peak period of infectious neonatal deaths.129,131 Yet, while the potential pathogen-agnostic impact of neonatal HBV vaccination remains to be elucidated, the WHO confirmed that BCG provides significant pathogen-agnostic benefit to newborns.134 While neonatal vaccination with BCG and OPV is part of the vaccination program of most low- and middle-income countries, BCG vaccination coverage remains relatively low in many countries.46 In part, this relates to current policy focused on reducing BCG vaccine waste by opening BCG vials only when a minimum number of eligible neonates are present.135,136 Elucidating the mechanisms underlying the pathogen-agnostic effects of vaccines is essential to further build on this approach for the protection of neonates.130 It has been identified that BCG given to neonates induces granulocyte colony stimulating factor (GCSF), which leads to activation of emergency granulopoiesis (i.e., increased production of neutrophils) increasing neutrophils to provide BCG’s pathogen-agnostic protection137,138 (Figure 3). Acquired innate immunity, or trained immunity, may also contribute to pathogen-agnostic protection induced by BCG but this has not yet been explored in neonates.139 And while it remains unknown how OPV protects from neonatal infectious death, switching from OPV to IPV may unintentionally remove this benefit for neonates, especially in LMIC.
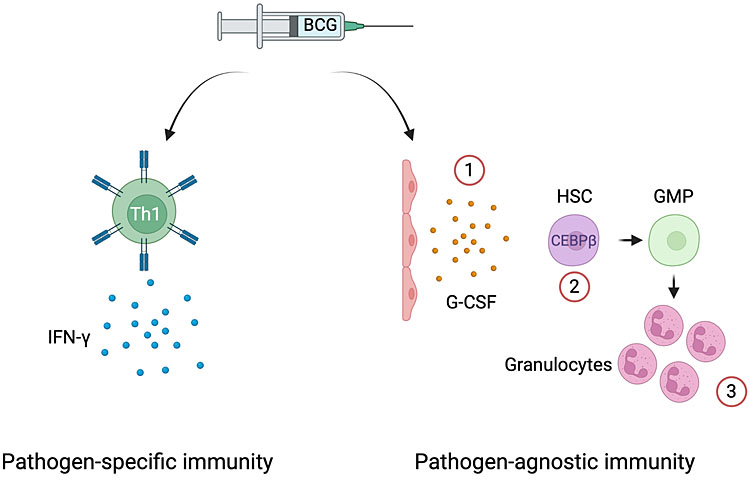
3
BCG’s pathogen-agnostic effects via emergency granulopoiesis. In addition to the induction of mycobacteria-specific T cells producing effector cytokines (IFN-g), BCG can trigger the expansion of hematopoietic stem cells and their differentiation to myeloid progenitors in the neonate. This effect involves the production of G-CSF by an unknown cell type, likely endothelial cell (1), and the upregulation of the canonical transcription factor of emergency granulopoiesis, namely CEBPβ (2). As a result, progenitors are biased towards enhanced granulopoiesis (3). HSC: hematopoietic stem cell; MPP: multipotent progenitor; GMP: granulocyte monocyte progenitor; G-CSF: granulocyte colony-stimulating factor (created with BioRender.com).
FUTURE OF NEONATAL VACCINATION
Infectious diseases remain a major cause of childhood morbidity and mortality worldwide, involving many pathogens that are not covered by existing vaccines.129,140 Vaccination of neonates will likely remain an important strategy to provide early protection against infectious diseases affecting infants and to increase vaccination coverage by further integrating vaccination in neonatal healthcare systems. However, the time required to induce pathogen-specific immunity limits the efficacy of neonatal vaccination against infectious diseases occurring during the first days and first weeks of life. Current knowledge clearly supports the notion that the newborn immune system can mount protective immune responses to live attenuated as well as inactivated and subunit formulations. The mechanisms underlying the lower responses to some vaccines in neonates as compared to older children remain poorly understood. Identifying determinants of efficient responses to neonatal vaccination will inform the development of future vaccines and adjuvants adapted to this age group.21,102,141
Vaccination during pregnancy is increasingly recognized as an effective and safe strategy to provide immunity to pathogens in the first months of life.142 Therefore, integrating maternal and infant immunization strategies and programs will be essential for optimal protection of young children against infectious pathogens. Blunting of infant antibody responses to vaccines by high levels of maternal antibodies remains a significant concern. Yet, current understanding of the mechanisms involved and of the impact of maternal antibodies on the quality of vaccine-induced antibodies is poor. Filling this knowledge gap could help optimize maternal and infant vaccination programs beyond delaying the age at infant vaccination.143 Finally, pathogen-agnostic immunity induced by neonatal vaccination may represent a powerful strategy to protect neonates from the large number of pathogens causing severe infectious diseases in the first weeks of life. Rapid exploration of this promising approach as an additional tool to protect newborns is needed to optimize this strategy and to better integrate it in vaccination programs across the world.129
PRACTICE RECOMMENDATIONS
- In countries or settings with a high incidence of tuberculosis and/or high leprosy burden, the WHO recommends a single dose of BCG vaccine to all healthy neonates at birth, for prevention of tuberculosis and leprosy.41 Studies indicate that neonatal BCG vaccination also provides pathogen-agnostic immunity, in addition to protection against tuberculosis in childhood.134 Recommendation: administer BCG at birth.
- All children worldwide should be fully vaccinated against polio. For countries using OPV in their national immunization program, the WHO recommends three doses of bOPV and two doses of IPV as the vaccination schedule.43 Studies suggest that neonatal OPV vaccination provides pathogen-agnostic immunity, in addition to protection against poliomyelitis. Recommendation: all children worldwide should be fully vaccinated against polio, including a dose at birth where bOPV is given.
- Hepatitis B vaccination is recommended for all children, the WHO recommends that all national programs include a monovalent hepatitis B vaccine birth dose.42 Neonates born to mothers with chronic hepatitis B should receive a hepatitis B vaccine and hepatitis B hyperimmune globulins. Recommendation: hepatitis B vaccination should be given to all children worldwide.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Williamson S. Anti-vaccination leagues. Arch Dis Child 1984;59(12):1195. | |
Baumgartner L. Age and Antibody-Production. J Immunol 1937;33(6):477–88. | |
McKhann CF, Kapnick I. Immunity and susceptibility to disease in early infancy. J Pediatrics 1938;13(6):907–18. | |
Moore DH, Pan RMD, Buxton CL. An electrophoretic study of maternal, fetal, and infant sera. Am J Obstet Gynecol 1949;57(2):312–22. | |
Sauer LW. The age factor in active immunization against whooping cough. Am J Pathology 1941;17(5):719–23. | |
Sako W, Treuting WL, Witt DD, et al. Early Immunization against Pertussis with Alum Precipitated Vaccine. J Amer Med Assoc 1945;127(7):379–84. | |
Sant’Agnese PA di. Simultaneous Immunization of New-Born Infants against Diphtheria, Tetanus, and Pertussis: Production of Antibodies and Duration of Antibody Levels in an Eastern Metropolitan Area. Am J Public Health N 1950;40(6):674–80. | |
Sant’Agnese Pad. Combined immunization against diphtheria, tetanus and pertussis in newborn infants; production of antibodies in early infancy. Pediatrics 1949;3(1):20–33. | |
Agnese Pads. Combined immunization against diphtheria, tetanus and pertussis in newborn infants, duration of antibody levels; antibody titers after booster dose; effect of passive immunity to diphtheria on active immunization with diphtheria toxoid. Pediatrics 1949;3(2):181–94. | |
Osborn JJ, Dancis J, Julia JF. Studies of the immunology of the newborn infant. II. Interference with active immunization by passive transplacental circulating antibody. Pediatrics 1952;10(3):328–34. | |
Evans DG, Smith JWG. Response of the Young Infant to Active Immunization. Brit Med Bull 1963;19(3):225–9. | |
Billingham RE, Brent L, Medawar PB. ‘Actively Acquired Tolerance’ of Foreign Cells. Nature 1953;172(4379):603–6. | |
Halasa NB, O’Shea A, Shi JR, et al. Poor Immune Responses to a Birth Dose of Diphtheria, Tetanus, and Acellular Pertussis Vaccine. The Journal of Pediatrics 2008;153(3):327–32.e1. | |
Provenzano RW, Wetterlow LH, Sullivan CL. Immunization and Antibody Response in the Newborn Infant – Pertussis Inoculation within Twenty-Four Hours of Birth. New Engl J Medicine 1965;273(18):959–65. | |
Wood N, McIntyre P, Marshall H, et al. Acellular Pertussis Vaccine at Birth and One Month Induces Antibody Responses By Two Months of Age. Pediatric Infect Dis J 2010;29(3):209–15. | |
Knuf M, Schmitt H-J, Wolter J, et al. Neonatal Vaccination with an Acellular Pertussis Vaccine Accelerates the Acquisition of Pertussis Antibodies in Infants. The Journal of Pediatrics 2008;152(5):655–60.e1. | |
Belloni C, Silvestri AD, Tinelli C, et al. Immunogenicity of a Three-Component Acellular Pertussis Vaccine Administered at Birth. Pediatrics 2003;111(5):1042–5. | |
Siegrist C-A. Blame Vaccine Interference, Not Neonatal Immunization, for Suboptimal Responses after Neonatal Diphtheria, Tetanus, and Acellular Pertussis Immunization. The Journal of Pediatrics 2008;153(3):305–7. | |
Halsey N, Galazka A. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bulletin of the World Health Organization 1985;63(6):1151–69. | |
BCG vaccination policies. Report of a WHO study group. World Heal Organization Technical Rep Ser 1980;652:1–17. | |
Kollmann TR, Kampmann B, Mazmanian SK, et al. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 2017;46(3):350–63. | |
McGovern N, Shin A, Low G, et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 2017;546(7660):662–6. | |
Li N, Unen V van, Höllt T, et al. Mass cytometry reveals innate lymphoid cell differentiation pathways in the human fetal intestine. J Exp Med 2018;215(5):1383–96. | |
Rechavi E, Lev A, Lee YN, et al. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Science Translational Medicine 2015;7(276):276ra25. | |
Adderson EE. Antibody repertoires in infants and adults: effects of T-independent and T-dependent immunizations. Springer Semin Immunopathol 2001;23(4):387–403. | |
Breukels MA, Zandvoort A, Rijkers GT, et al. Complement Dependency of Splenic Localization of Pneumococcal Polysaccharide and Conjugate Vaccines. Scand J Immunol 2005;61(4):322–8. | |
Rappuoli R, Gregorio ED, Costantino P. On the mechanisms of conjugate vaccines. Proceedings of the National Academy of Sciences 2019;116(1):14–6. | |
Ota MOC, Vekemans J, Schlegel-Haueter SE, et al. Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine 2004;22(3–4):511–9. | |
Vekemans J, Ota MOC, Wang ECY, et al. T cell responses to vaccines in infants: defective IFNγ production after oral polio vaccination. Clin Exp Immunol 2002;127(3):495–8. | |
Vekemans J, Amedei A, Ota MO, et al. Neonatal bacillus Calmette-Guérin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. European journal of immunology 2001;31(5):1531–5. | |
Marchant A, Goetghebuer T, Ota MO, et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guérin vaccination. Journal of immunology (Baltimore, Md : 1950). 1999;163(4):2249–55. | |
Newport MJ, Goetghebuer T, Weiss HA, et al. Genetic regulation of immune responses to vaccines in early life. Genes and Immunity 2004;5(2):122–9. | |
Yan K, Cai W, Cao F, et al. Genetic effects have a dominant role on poor responses to infant vaccination to hepatitis B virus. J Hum Genet 2013;58(5):293–7. | |
Lee YC, Newport MJ, Goetghebuer T, et al. Influence of genetic and environmental factors on the immunogenicity of Hib vaccine in Gambian twins. Vaccine 2006;24(25):5335–40. | |
Marchant A, Pihlgren M, Goetghebuer T, et al. Predominant influence of environmental determinants on the persistence and avidity maturation of antibody responses to vaccines in infants. The Journal of infectious diseases 2006;193(11):1598–605. | |
Brodin P. Immune-microbe interactions early in life: A determinant of health and disease long term. Science 2022;376(6596):945–50. | |
Koff EM de, Baarle D van, Houten MA van, et al. Mode of delivery modulates the intestinal microbiota and impacts the response to vaccination. Nat Commun 2022;13(1):6638. | |
Zhao T, Li J, Fu Y, et al. Influence of gut microbiota on mucosal IgA antibody response to the polio vaccine. Npj Vaccines 2020;5(1):47. | |
Jong SE de, Olin A, Pulendran B. The Impact of the Microbiome on Immunity to Vaccination in Humans. Cell Host Microbe 2020;28(2):169–79. | |
Church JA, Parker EP, Kirkpatrick BD, et al. Interventions to improve oral vaccine performance: a systematic review and meta-analysis. Lancet Infect Dis 2019;19(2):203–14. | |
Organization WH. BCG vaccines: WHO position paper – February 2018 [Internet]. Available from: https://apps.who.int/iris/rest/bitstreams/1095887/retrieve. | |
Organization WH. Hepatitis B vaccines: WHO position paper – July 2017 [Internet]. Available from: https://apps.who.int/iris/rest/bitstreams/1086442/retrieve. | |
Organization WH. Polio vaccines: WHO position paper – June 2022 [Internet]. Available from: https://apps.who.int/iris/rest/bitstreams/1438202/retrieve. | |
Calmette A. Preventive Vaccination against Tuberculosis with BCG. J Roy Soc Med 1931;24(11):1481–90. | |
Oettinger T, Jørgensen M, Ladefoged A, et al. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tubercle Lung Dis 1999;79(4):243–50. | |
Muhoza P, Danovaro-Holliday MC, Diallo MS, et al. Routine Vaccination Coverage – Worldwide, 2020. Morbidity Mortal Wkly Rep 2021;70(43):1495–500. | |
Plotkin SA. Recent updates on correlates of vaccine-induced protection. Front Immunol 2023;13:1081107. | |
Burl S, Adetifa UJ, Cox M, et al. Delaying Bacillus Calmette-Guerin Vaccination from Birth to 4 1/2 Months of Age Reduces Postvaccination Th1 and IL-17 Responses but Leads to Comparable Mycobacterial Responses at 9 Months of Age. The Journal of Immunology 2010;185(4):2620–8. | |
Ritz N, Casalaz D, Donath S, et al. Comparable CD4 and CD8 T cell responses and cytokine release after at-birth and delayed BCG immunisation in infants born in Australia. Vaccine 2016;34(35):4132–9. | |
Ferguson R, Simes A. BCG vaccination of Indian infants in Saskatchewan A study carried out with financial assistance from the National Research Council of Canada. Tubercle 1949;30(1):5–11. | |
Dam HG ten, Hitze KL. Does BCG vaccination protect the newborn and young infants? B World Health Organ 1980;58(1):37–41. | |
Martinez L, Cords O, Liu Q, et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: a systematic review and individual participant data meta-analysis. Lancet Global Heal 2022;10(9):e1307–16. | |
Dockrell HM, Butkeviciute E. Can what have we learnt about BCG vaccination in the last 20 years help us to design a better tuberculosis vaccine? Vaccine 2022;40(11):1525–33. | |
Preez K du, Seddon JA, Schaaf HS, et al. Global shortages of BCG vaccine and tuberculous meningitis in children. Lancet Global Heal 2019;7(1):e28–9. | |
Connor RI, Brickley EB, Wieland-Alter WF, et al. Mucosal immunity to poliovirus. Mucosal Immunol 2022;15(1):1–9. | |
Halsey N, Galazka A. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. B World Health Organ 2008;63(6):1151–69. | |
Dong DX, Hu XM, Liu WJ, et al. Immunization of neonates with trivalent oral poliomyelitis vaccine (Sabin). B World Health Organ 1986;64(6):853–60. | |
Grassly NC, Praharaj I, Babji S, et al. The effect of azithromycin on the immunogenicity of oral poliovirus vaccine: a double-blind randomised placebo-controlled trial in seronegative Indian infants. Lancet Infect Dis 2016;16(8):905–14. | |
Pallansch MA. Ending Use of Oral Poliovirus Vaccine – A Difficult Move in the Polio Endgame. New Engl J Med 2018;379(9):801–3. | |
Macklin GR, Grassly NC, Sutter RW, et al. Vaccine schedules and the effect on humoral and intestinal immunity against poliovirus: a systematic review and network meta-analysis. Lancet Infect Dis 2019;19(10):1121–8. | |
Damme PV, Coster ID, Bandyopadhyay AS, et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet 2019;394(10193):148–58. | |
Qu C, Chen T, Fan C, et al. Efficacy of Neonatal HBV Vaccination on Liver Cancer and Other Liver Diseases over 30-Year Follow-up of the Qidong Hepatitis B Intervention Study: A Cluster Randomized Controlled Trial. Singal A, editor. PLoS Medicine 2014;11(12):e1001774. | |
Barin F, Denis F, Chiron JP, et al. IMMUNE RESPONSE IN NEONATES TO HEPATITIS B VACCINE. Lancet 1982;319(8266):251–3. | |
Prozesky OW, Stevens CE, Szmuness W, et al. Immune response to hepatitis B vaccine in newborns. J Infection 1983;7:53–5. | |
Maupas P, Barin F, Chiron J-P, et al. Efficacy of Hepatitis B Vaccine in Prevention of Early HBsAg Carrier State in Children Controlled Trial in an Endemic Area (Senegal). Lancet 1981;317(8215):289–92. | |
Beasley RP, LEE GC-Y, Roan C-H, et al. Prevention of Perinatally Transmitted Hepatitis B Virus Infections with Hepatitis B Immune Globulin and Hepatitis B Vaccine. Lancet 1983;322(8359):1099–102. | |
Ko SC, Fan L, Smith EA, et al. Estimated Annual Perinatal Hepatitis B Virus Infections in the United States, 2000–2009. J Pediatric Infect Dis Soc 2016;5(2):114–21. | |
Sande MAB van der, Waight P, Mendy M, et al. Long-Term Protection against Carriage of Hepatitis B Virus after Infant Vaccination. J Infect Dis 2006;193(11):1528–35. | |
Eyler JM. Smallpox in history: the birth, death, and impact of a dread disease. J Lab Clin Med 2003;142(4):216–20. | |
Troeger C, Khalil IA, Rao PC, et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. Jama Pediatr 2018;172(10):958–65. | |
Organization WH. Rotavirus vaccines: WHO position paper – July 2021 [Internet]. Available from: https://apps.who.int/iris/rest/bitstreams/1357146/retrieve. | |
Bishop RF, Barnes GL, Cipriani E, et al. Clinical Immunity after Neonatal Rotavirus Infection – A Prospective Longitudinal Study in Young Children. New Engl J Medicine 1983;309(2):72–6. | |
Haffejee IE. Neonatal Rotavirus Infections. Clin Infect Dis 1991;13(5):957–62. | |
Kilich E, Sadarangani M. Use of rotavirus vaccines in preterm babies on the neonatal unit. Expert Rev Vaccines 2016;15(12):1463–5. | |
Vesikari T. Neonatal rotavirus vaccination making headway. Lancet Infect Dis 2015;15(12):1362–3. | |
Ruuska T, Vesikari T, Delem A, et al. Evaluation of RIT 4237 Bovine Rotavirus Vaccine in Newborn Infants: Correlation of Vaccine Efficacy to Season of Birth in Relation to Rotavirus Epidemic Period. Scand J Infect Dis 1990;22(3):269–78. | |
Bines JE, Thobari JA, Satria CD, et al. Human Neonatal Rotavirus Vaccine (RV3-BB) to Target Rotavirus from Birth. New Engl J Medicine 2018;378(8):719–30. | |
Chen M-Y, Kirkwood CD, Bines J, et al. Rotavirus specific maternal antibodies and immune response to RV3-BB neonatal rotavirus vaccine in New Zealand. Hum Vacc Immunother 2017;13(5):1126–35. | |
Saleh E, Eichner B, Clark DW, et al. Open-Label Pilot Study to Compare the Safety and Immunogenicity of Pentavalent Rotavirus Vaccine (RV5) Administered on an Early Alternative Dosing Schedule with Those of RV5 Administered on the Recommended Standard Schedule. J Pediatric Infect Dis Soc 2017;7(1):82–5. | |
Cowley D, Sari RM, Handley A, et al. Immunogenicity of four doses of oral poliovirus vaccine when co-administered with the human neonatal rotavirus vaccine (RV3-BB). Vaccine 2019;37(49):7233–9. | |
Witte D, Handley A, Jere KC, et al. Neonatal rotavirus vaccine (RV3-BB) immunogenicity and safety in a neonatal and infant administration schedule in Malawi: a randomised, double-blind, four-arm parallel group dose-ranging study. Lancet Infect Dis 2022;22(5):668–78. | |
Geard N, Bradhurst R, Tellioglu N, et al. Model-based estimation of the impact on rotavirus disease of RV3-BB vaccine administered in a neonatal or infant schedule. Hum Vacc Immunother 2022;18(6):2139097. | |
Borkowsky W, Wara D, Fenton T, et al. Lymphoproliferative Responses to Recombinant HIV-1 Envelope Antigens in Neonates and Infants Receiving gp120 Vaccines. J Infect Dis 2000;181(3):890–6. | |
Cunningham CK, Wara DW, Kang M, et al. Safety of 2 Recombinant Human Immunodeficiency Virus Type 1 (HIV-1) Envelope Vaccines in Neonates Born to HIV-1-Infected Women. Clin Infect Dis 2001;32(5):801–7. | |
Johnson DC, McFarland EJ, Muresan P, et al. Safety and Immunogenicity of an HIV-1 Recombinant Canarypox Vaccine in Newborns and Infants of HIV-1–Infected Women. J Infect Dis 2005;192(12):2129–33. | |
McFarland EJ, Johnson DC, Muresan P, et al. HIV-1 vaccine induced immune responses in newborns of HIV-1 infected mothers. Aids 2006;20(11):1481–9. | |
Fouda GG, Paris KD, Levy O, et al. Immunological mechanisms of inducing HIV immunity in infants. Vaccine 2020;38(3):411–5. | |
Goswami R, Berendam SJ, Li SH, et al. Harnessing early life immunity to develop a pediatric HIV vaccine that can protect through adolescence. Plos Pathog 2020;16(11):e1008983. | |
O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009;374(9693):893–902. | |
Organization WH. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019 [Internet]. Available from: https://www.who.int/publications/i/item/10665-310968. | |
Saha SK, Schrag SJ, Arifeen SE, et al. Causes and incidence of community-acquired serious infections among young children in south Asia (ANISA): an observational cohort study. Lancet 2018;392(10142):145–59. | |
Alsubaie SS. Early-Onset Neonatal Pneumococcal Infection. Infect Dis Clin Prac 2019;27(2):68–72. | |
Clarke E, Kampmann B, Goldblatt D. Maternal and neonatal pneumococcal vaccination – where are we now? Expert Rev Vaccines 2016;15(10):1305–17. | |
Shahid NS, Hoque SS, Begum T, et al. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet 1995;346(8985):1252–7. | |
Lehmann D, Pomat WS, Riley ID, et al. Studies of maternal immunisation with pneumococcal polysaccharide vaccine in Papua New Guinea. Vaccine 2003;21(24):3446–50. | |
Chang AB, Toombs M, Chatfield MD, et al. Study Protocol for Preventing Early-Onset Pneumonia in Young Children Through Maternal Immunisation: A Multi-Centre Randomised Controlled Trial (PneuMatters). Frontiers Pediatrics 2022;9:781168. | |
Biggelaar AHJ van den, Pomat W, Bosco A, et al. Pneumococcal conjugate vaccination at birth in a high-risk setting: No evidence for neonatal T-cell tolerance. Vaccine 2011;29(33):5414–20. | |
Scott JAG, Ojal J, Ashton L, et al. Pneumococcal Conjugate Vaccine Given Shortly After Birth Stimulates Effective Antibody Concentrations and Primes Immunological Memory for Sustained Infant Protection. Clin Infect Dis 2011;53(7):663–70. | |
Pomat WS, Biggelaar AHJ van den, Phuanukoonnon S, et al. Safety and Immunogenicity of Neonatal Pneumococcal Conjugate Vaccination in Papua New Guinean Children: A Randomised Controlled Trial. Plos One 2013;8(2):e56698. | |
Aho C, Michael A, Yoannes M, et al. Limited impact of neonatal or early infant schedules of 7-valent pneumococcal conjugate vaccination on nasopharyngeal carriage of Streptococcus pneumoniae in Papua New Guinean children: A randomized controlled trial. Vaccine Reports 2016;6:36–43. | |
Saso A, Kampmann B. Vaccine responses in newborns. Semin Immunopathol 2017;39(6):627–42. | |
Whittaker E, Goldblatt D, McIntyre P, et al. Neonatal Immunization: Rationale, Current State, and Future Prospects. Front Immunol 2018;9:532. | |
Plotkin S. History of vaccination. Proc National Acad Sci 2014;111(34):12283–12287. | |
Organization WH. Diphtheria vaccine: WHO position paper – August 2017 [Internet]. Available from: https://www.who.int/publications/i/item/who-wer9231. | |
Organization WH. Pertussis vaccines: WHO position paper – August 2015 [Internet]. Available from: https://www.who.int/publications/i/item/WHO-WER9035. | |
Organization WH. Tetanus vaccines: WHO position paper – February 2017 [Internet]. Available from: https://www.who.int/publications/i/item/WHO-WER9206. | |
Kilgore PE, Salim AM, Zervos MJ, et al. Pertussis: Microbiology, Disease, Treatment, and Prevention. Clin Microbiol Rev 2016;29(3):449–86. | |
Sauer LW, Tucker WH. Simultaneous Immunization of Young Children against Diphtheria, Tetanus, and Pertussis: Experience in a Northern Metropolitan Area. Am J Public Health N 1950;40(6):681–5. | |
MD MK, MD H-JS, PhD J-MJ, et al. Booster Vaccination After Neonatal Priming with Acellular Pertussis Vaccine. The Journal of Pediatrics 2010;156(4):675–8. | |
Wood N, Nolan T, Marshall H, et al. Immunogenicity and Safety of Monovalent Acellular Pertussis Vaccine at Birth. Jama Pediatr 2018;172(11):1045–52. | |
Sharma NC, Efstratiou A, Mokrousov I, et al. Diphtheria. Nat Rev Dis Primers 2019;5(1):81. | |
Thwaites CL, Beeching NJ, Newton CR. Maternal and neonatal tetanus. Lancet 2015;385(9965):362–70. | |
Yen LM, Thwaites CL. Tetanus. Lancet 2019;393(10181):1657–68. | |
Siegrist C. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 2003;21(24):3406–12. | |
Edwards KM. Maternal antibodies and infant immune responses to vaccines. Vaccine 2015;33(47):6469–72. | |
MD PAM, DPhil MS, PhD MG, et al. Maternal immunisation: collaborating with mother nature. The Lancet infectious diseases 2017:1–12. | |
Maertens K, Caboré RN, Huygen K, et al. Pertussis vaccination during pregnancy in Belgium: Follow-up of infants until 1 month after the fourth infant pertussis vaccination at 15 months of age. Vaccine. 2016. | |
Wanlapakorn N, Maertens K, Vongpunsawad S, et al. Quantity and Quality of Antibodies After Acellular Versus Whole-cell Pertussis Vaccines in Infants Born to Mothers Who Received Tetanus, Diphtheria, and Acellular Pertussis Vaccine During Pregnancy: A Randomized Trial. Clin Infect Dis 2019;71(1):72–80. | |
Abu-Raya B, Maertens K, Munoz FM, et al. The Effect of Tetanus-Diphtheria-Acellular-Pertussis Immunization During Pregnancy on Infant Antibody Responses: Individual-Participant Data Meta-Analysis. Front Immunol 2021;12:689394. | |
Orije MRP, García-Fogeda I, Dyck WV, et al. Impact of Maternal Pertussis Antibodies on the Infants’ Cellular Immune Responses. Clin Infect Dis 2021;75(3):442–52. | |
Siegrist C-A, Córdova M, Brandt C, et al. Determinants of infant responses to vaccines in presence of maternal antibodies. Vaccine 1998;16(14–15):1409–14. | |
Kim D, Huey D, Oglesbee M, et al. Insights into the regulatory mechanism controlling the inhibition of vaccine-induced seroconversion by maternal antibodies. Blood 2011;117(23):6143–51. | |
Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies, 2014:1–15. Available from: http://journal.frontiersin.org/Journal/10.3389/fimmu.2014.00446/abstract. | |
Pincetic A, Bournazos S, DiLillo DJ, et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nature Immunology 2014;15(8):707–16. | |
Vono M, Eberhardt CS, Auderset F, et al. Maternal Antibodies Inhibit Neonatal and Infant Responses to Vaccination by Shaping the Early-Life B Cell Repertoire within Germinal Centers. CellReports 2019;28(7):1773–84.e5. | |
Abu-Raya B, Forsyth K, Halperin SA, et al. Vaccination in Pregnancy against Pertussis: A Consensus Statement on Behalf of the Global Pertussis Initiative. Nato Adv Sci Inst Se 2022;10(12):1990. | |
Abu-Raya B, Edwards KM. Interference With Pertussis Vaccination in Infants After Maternal Pertussis Vaccination. Pediatrics 2020;146(3):e20193579. | |
Benn CS, Fisker AB, Rieckmann A, et al. Vaccinology: time to change the paradigm? Lancet Infect Dis 2020;20(10):e274–83. | |
Kollmann TR, Marchant A, Way SS. Vaccination strategies to enhance immunity in neonates. Science 2020;368(6491):612–5. | |
Goodridge HS, Ahmed SS, Curtis N, et al. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol 2016;16(6):392–400. | |
Biering-Sørensen S, Aaby P, Lund N, et al. Early BCG-Denmark and Neonatal Mortality Among Infants Weighing <2500 g: A Randomized Controlled Trial. Clin Infect Dis Official Publ Infect Dis Soc Am 2017;65(7):1183–90. | |
Prentice S, Nassanga B, Webb EL, et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomised controlled trial. Lancet Infect Dis 2021;21(7):993–1003. | |
Aaby P, Nielsen S, Fisker AB, et al. Stopping Oral Polio Vaccine (OPV) After Defeating Poliomyelitis in Low- and Middle-Income Countries: Harmful Unintended Consequences? Review of the Nonspecific Effects of OPV. Open Forum Infect Dis 2022;9(8):ofac340. | |
Organization WH. meeting of the Strategic advisory group of experts on immunization, November 2013 – conclusions and recommendations [Internet]. Available from: http://www.who.int/immunization/sage/meetings/2014/april/3_NSE_Epidemiology_review_Report_to_SAGE_14_Mar_FINAL.pdf. | |
Organization WH. Monitoring vaccine wastage at country level. [Internet]. Available from: https://apps.who.int/iris/bitstream/handle/10665/68463/WHO_VB_03.18.Rev.1_eng.pdf?sequence=1&;isAllowed=y. | |
Schaltz-Buchholzer F, Frankel HN, Benn CS. The real-life number of neonatal doses of Bacille Calmette-Guérin vaccine in a 20-dose vial. Global Health Action 2017;10(1):1267964. | |
Brook B, Harbeson DJ, Shannon CP, et al. BCG vaccination–induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci Transl Med 2020;12(542). | |
Brook B, Schaltz-Buchholzer F, Ben-Othman R, et al. A place for neutrophils in the beneficial pathogen-agnostic effects of the BCG vaccine. Vaccine 2022;40(11):1534–9. | |
Geckin B, Föhse FK, Domínguez-Andrés J, et al. Trained immunity: implications for vaccination. Curr Opin Immunol 2022;77:102190. | |
MacLennan CA, Saul A. Vaccines against poverty. Proceedings of the National Academy of Sciences 2014;111(34):12307–12. | |
Kollmann TR, Marchant A. Towards Predicting Protective Vaccine Responses in the Very Young. Trends Immunol 2016;37(8):523–34. | |
Sadarangani M, Kollmann T, Bjornson G, et al. The Fifth International Neonatal and Maternal Immunization Symposium (INMIS 2019): Securing Protection for the Next Generation. Msphere 2021;6(1):e00862–20. | |
Barug D, Pronk I, Houten MA van, et al. Maternal pertussis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: an open-label, parallel, randomised controlled trial. Lancet Infect Dis 2019;19(4):392–401. | |
Siegrist C-A, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009;9(3):185–94. | |
Mastelic-Gavillet B, Vono M, Gonzalez-Dias P, et al. Neonatal T Follicular Helper Cells Are Lodged in a Pre-T Follicular Helper Stage Favoring Innate Over Adaptive Germinal Center Responses. Front Immunol 2019;10:1845. | |
Kollmann TR, Levy O, Montgomery RR, et al. Innate Immune Function by Toll-like Receptors: Distinct Responses in Newborns and the Elderly. Immunity 2012;37(5):771–83. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)