This chapter should be cited as follows:
Dangor Z, Madhi SA, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.420433
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 17
Maternal immunization
Volume Editors:
Professor Asma Khalil, The Royal College of Obstetricians and Gynaecologists, London, UK; Fetal Medicine Unit, Department of Obstetrics and Gynaecology, St George’s University Hospitals NHS Foundation Trust, London, UK
Professor Flor M Munoz, Baylor College of Medicine, TX, USA
Professor Ajoke Sobanjo-ter Meulen, University of Washington, Seattle, WA, USA

Chapter
Respiratory Syncytial Virus
First published: May 2023
Updated: October 2024
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
BACKGROUND
Lower respiratory tract infections (LRTIs) are the commonest cause of hospitalization and death in children 1–59 months of age, particularly in low- and middle-income countries (LMICs).1 Respiratory syncytial virus (RSV) is the commonest cause (30–80%) of LRTI hospitalization in children.2,3 Approximately two-thirds of children will be infected at least once by RSV in the first 2 years of life, a third of whom will develop LRTI.4
There has been no change in the burden of RSV over the past 2 decades, except when there was disruption of RSV circulation in 2020 and 2021 (in some settings) during the Covid-19 pandemic. Similar to estimates for 2005, there were an estimated 33 million cases of RSV-LRTI in children less than 5 years of age, including 3.6 million who were hospitalized in 2019. The proportion of RSV cases as a fraction of LRTI was similar between high-income (29%) and LMICs (23–26%).5 Furthermore, in 2019, it was estimated that there were 101,400 (uncertainty range: 84,500–125,200) RSV attributable deaths, 99% of which occurred in LMICs and 50% of which were in children less than 6 months of age.5 Although preterm birth is a risk factor for severe RSV-LRTI, the majority (~80%) of hospitalization from RSV-LRTI occurs in children born at term.3,6 Death from RSV is likely to be underestimated in low-income settings. Investigation of unexplained community deaths in Argentina, noted that the peak in those deaths were temporally associated with the RSV epidemic and RSV attributable deaths in the health facility.7 Furthermore, RSV-LRTI during infancy could predispose to adverse respiratory sequelae, including obstructive and restrictive lung disease, which could persist into adulthood.8 There is currently no antiviral treatment for children with RSV infection and management of RSV-LRTI is symptom based.2 Globally, it is estimated that RSV infection in children has a direct medical cost of 3.1 billion US dollars per annum.2 Vaccines to prevent RSV-LRTI are considered to be a priority by the World Health Organization.9
RSV was first identified in 1955 in chimpanzees with coryza,10 and later in infants.11 RSV is a single-stranded RNA virus that belongs to the Pneumoviridae family. There are two antigenic types, RSV A and RSV B, both occur variably between seasons and geographic regions. The RSV genome codes for 11 proteins (two non-structural and nine structural), each of which have distinct virulence properties.12 Of these are two important surface proteins that are targets for neutralizing antibodies; the attachment protein (G) attaches the virus to the host cell, and the fusion (F) protein for membrane fusion. The highly conserved F protein is a better vaccine target and occurs in two stable forms: the pre-fusion (pre-F) and post-fusion (post-F) structure. Six antigenic (Ø, I-V) neutralization sites have been identified on the pre-F and post-F forms with varying neutralizing potency. The site Ø and V epitopes induce the most potent neutralizing body and are only visible in the pre-fusion configuration of the F protein.12
Innate and adaptive immune responses are ill-equipped for RSV infection in early infancy. Neutralizing antibodies administered to infants or transferred from the mother to fetus in-utero may protect against RSV disease.12,13 Nevertheless, there are established correlates of protection against RSV LRTI, including in relation to maternal acquired neutralizing antibody.14
This may in part relate to maternal exposure to RSV before or during pregnancy and its impact on maternal titers, the titer level required to protect against severe LRTI compared with milder illness, and the quality of maternal antibody transferred to the fetus.15,16,17
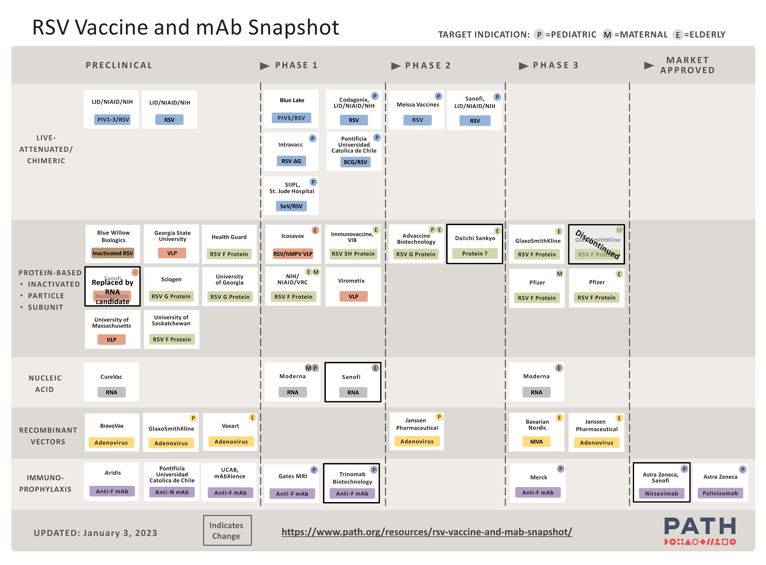
RSV vaccine development was hindered following studies of a formalin inactivated RSV vaccine in the 1960s, where vaccine-associated enhanced disease was observed among the vaccinated children in their first RSV season. The heightened susceptibility to severe RSV illness among vaccinated children who were sero-negative at time of vaccination, was attributed to vaccination inducing a Th2 dominant immune response.12 Following a subsequent hiatus of developing RSV prophylaxis, a monoclonal antibody (palivizumab) was developed in 1998. More recently, there are several additional preventative strategies in development, including longer-acting monoclonal antibodies and vaccines targeted at the elderly, children and pregnant women with the aim of protecting their infants in the first few months of life (Figure 1).
1
Snapshot of the respiratory syncytial virus vaccine and monoclonal antibody. Reproduced with permission from PATH (2023) (for latest updated version see https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/).18,19
As of December 2022, the only licensed effective strategy to reduce the risk of RSV-LRTI hospitalization has been the use of palivizumab, a monoclonal antibody targeting the RSV F protein site II.2 The effectiveness of palivizumab in reducing the risk of RSV hospitalization was 51% (RR 0.49; 95% CI: 0.37 to 0.64) in a meta-analysis.18,20 Nevertheless, the high cost of palivizumab with an estimated average seasonal cost ranging from $3221 to $12,568 in the USA in 2016–2017,21 resulted in the American Academy of Pediatrics recommending its use be restricted for high-risk infants less than 12 months of age at the onset of the RSV season; limited to infants born at <29 weeks' gestation, those with congenital heart disease with hemodynamically significant lesions, and in children with chronic lung disease of prematurity.3 Due to a half-life of 19–27 days for palivizumab, monthly dosing is required throughout the course of the RSV season. Furthermore, the high costs and logistical challenges related to monthly dosing of palivizumab has limited its availability and use in LMICs.
Recent advances in extending the half-life (63–73 days) of monoclonal antibodies through YTE mutations of the Fc component of IgG, and targeting of sites of the RSV F pre-fusion protein associated with heightened neutralizing antibody activity, offers further opportunities for expanding the use of monoclonal antibody beyond only infants at high risk of severe RSV. Nirsevimab, is one such long-acting monoclonal antibody, which is targeted against site Ø of the F-protein. The efficacy of Nirsevimab was 78.4% (95% CI: 51.9–90.3) against RSV LRTI hospitalization in infants born at 29 to <35 weeks, and 76.8% (95% CI: 49.4–89.4) in near term or term infants.22,23,24 Nirsevimab has the advantage over palivizumab in only requiring a single dose, which can be timed with the predicted onset of the RSV season, and predictability in antibody kinetics during the course of the RSV season.25,26 Nevertheless, its accessibility and cost, which is yet to be determined may make it unaffordable in LMICs, where the need is the greatest. Also, being targeted against a single epitope, ongoing surveillance would be required to determine whether mutations evolve in the selected epitope with its widespread use, which was not the case for palivizumab.
Since Nirsevimab was approved for use in Europe and North America in 2023, multiple studies have reported effectiveness of Nirsevimab against RSV infection, hospitalization, and ICU admission. In a population-based study from Spain, Nirsevimab was administered to 1083 infants within 7 days after birth – effectiveness for preventing hospitalization for RSV LRTI was 88.7% (95% CI: 69.6–95.8), and it had similar effectiveness for preventing emergency room consultation (87.9%; 95% CI: 70.3–995.1) and ICU admissions (85.9%; 95% CI: 13.2–97.7).27 Compared to the pre-Nirsevimab period, PICU admissions for RSV bronchiolitis declined from 9.0 (95% CI: 8.6–9.5) per 1000 admissions to 4.3 (CI: 95% 33.2–6.1).28 In a matched case–control study from France, 60 of 690 hospitalized infants with RSV bronchiolitis compared to 97 of 345 non-RSV hospital visits received Nirsevimab, the adjusted effectiveness of Nirsevimab was 83.0% (95% CI: 73.4–89.2).29 Furthermore, in a meta-analysis of studies conducted in the US and three European countries over the 2023/2024 RSV season, the real-world vaccine effectiveness was 90.5% (95% CI: 87.1–92.9) against RSV-associated LRTI.30 In a 2023/2024 immunization campaign in Spain wherein 9408 infants received Nirsevimab, the effectiveness was 82.0% (95% CI: 65.6–90.2) against RSV-related LRTI, and 69.2% (95% CI: 55.9–78.0) against all-cause LRTI hospitalizations, and 66.2% (95% CI: 56.0–73.7) against all-cause hospitalizations.31
Suptavumab was another experimental RSV monoclonal antibody, which targeted site V of the F protein, but failed to demonstrate efficacy against RSV-LRTI due to already circulating RSV-B strains that had acquired 2-amino acid substitutions in the suptavumab epitope.32
Furthermore, preliminary phase 2b/3 results of the long-acting RSV monoclonal antibody Clesrovimab (MK-1654) showed significantly reduced medically attended LRTI caused by RSV through day 150, meeting its primary safety and efficacy endpoints in infants born preterm and at term, as well as in those with underling congenital heart or chronic lung disease (NCT04767373).
In addition to the long-acting monoclonal antibodies, active vaccination against RSV is being explored in multiple phase I–III studies that are currently underway (Figure 1). Already, pre-fusion F protein-based vaccines have been shown to be efficacious in reducing the risk of RSV-LRTI in people older than 65 years.33 In healthy adults 18 to 50 years of age, vaccine efficacy (VE) was 86.7% (95% CI: 53.8–96.5) for symptomatic RSV after a single intramuscular injection of the bivalent pre-fusion F vaccine.34 Similarly, in an ongoing phase-III trial amongst adults ≥60 years of age, a single intramuscular injection of a pre-fusion F vaccine had a VE of 62.1% (95% CI: 37.1–77.9) against RSV-associated respiratory illness compared to placebo.35
Adaptations of the pre-fusion F-protein vaccines used in the elderly are also being explored as a strategy to vaccinate pregnant women to enhance transplacental transfer of RSV neutralizing antibodies to the fetus that could confer protection during the first 3–6 months of life when risk of severe RSV-LRTI is greatest and protection by direct vaccination of the infant is unlikely to be effective.6 In addition, breastmilk secretory IgA may be an additional source of protection from women vaccinated during pregnancy.36
The maternal RSV immunization strategy will, however, likely need to be coupled with further active RSV vaccination of the infant to provide protection beyond the period when the transplacental acquired antibody has waned below the threshold associated with efficacy. A number of RSV vaccine candidates are being investigated in phase I–III studies in children older than 3–6 months of age (Figure 1).
The use of maternal vaccination as a strategy to protect pregnant women and their young infants from disease has already been established for tetanus, pertussis, influenza virus, and more recently COVID-19; for which routine vaccination of pregnant women is recommended.36 Immunization of pregnant women against influenza has also been associated with lower rates of preterm labor (8–13%) and stillbirths (27–31%).37,38 Similarly, vaccinating pregnant women against COVID-19 was associated 10% and 15% lower risk of preterm labor and stillbirth, respectively.39 In addition, there are vaccines that are safe during pregnancy that are not routine but may be required in ad hoc situations or outbreaks such as the meningococcal, cholera, rabies and typhoid vaccines.36 Other vaccines being considered for pregnant women include vaccines against group B streptococcus, cytomegalovirus, Ebola virus, Zika virus, and malaria.36 The benefits of maternal vaccination are highlighted in Figure 2.
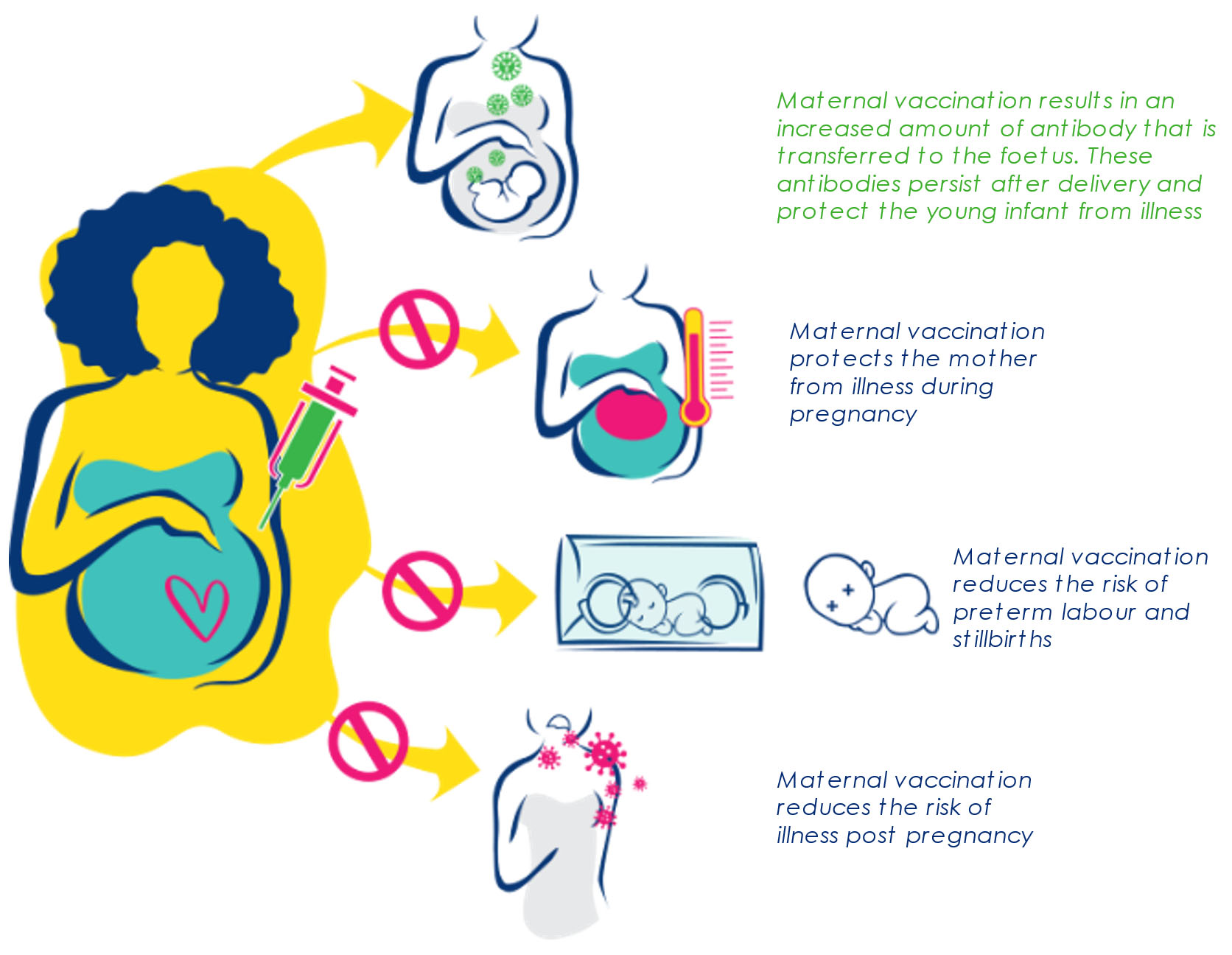
2
Infographic demonstrating the benefits of maternal vaccination.
MATERNAL RSV VACCINATION
How does RSV maternal vaccination work?
The pre-requisites for an effective maternal RSV vaccine are highlighted in Box 1. Vaccination of pregnant women is being targeted during the second or third trimester of pregnancy, and aimed to increase neutralizing antibody levels in the pregnant women that are then transplacentally transferred to the fetus. Transplacental antibody transfer is an active process involving the attachment of maternal IgG to the Fc receptor (FcRn), which is expressed by syncytiotrophoblasts, followed by transcytosis into the fetal circulation.36 IgG1 is most actively transferred, followed by IgG4, IgG3, IgG2. Protein-based vaccines such as that for RSV predominantly induce IgG1 and IgG3 responses. These antibodies are then cumulatively transferred to the fetus over the next weeks until delivery. The estimated amount of antibody transferred to the fetus is about half of the mother’s levels early in the third trimester and matching or even exceeding maternal levels at full-term pregnancy. The rate of IgG transfer can, however, be affected by IgG subclass, antigen specificity; and negatively by maternal characteristics such as chronic infections, advanced age, high parity, high body mass index, HIV infection, and hypergammaglobulinemia.
Box 1 Pre-requisites for an effective maternal RSV vaccine
Pre-requisites for an effective maternal RSV vaccine include the following:
- safety established in the pregnant women, including risk of adverse pregnancy outcomes, such as preterm labor or stillbirth, and congenital abnormalities;
- immunogenicity of the vaccine in the women, and particularly functional antibody – including neutralizing antibody activity;
- subclass of the IgG antibodies induced by the vaccine;
- efficiency of transplacental antibody transfer to fetus;
- adequate gestational time to allow for optimal transfer;
- timing of vaccination in relation to birth of child and onset of RSV season/exposure;
- gestational age at time of birth, which could affect efficiency of transplacental antibody acquisition;
- antibody kinetics in the women and infant, including half-life that could determine the duration of protection;
- non-inference with other vaccines recommended for pregnant women, particularly if given concurrently;
- non-interference with other vaccines children would receive.
Current vaccine landscape and regulatory approval
The first phase-III maternal RSV vaccine trial that demonstrated proof of concept was the PREPARE RSV F nanoparticle vaccine (Novovax) that aimed to determine the efficacy of maternal immunization with the RSV F vaccine against medically significant symptomatic RSV-LRTI through 90, 120, 150, and 180 days of life in infants.40 This randomized, observer-blind, placebo-controlled trial was performed in pregnant women 18 to 40 years at 87 sites in 11 countries. There were 4636 pregnant women that underwent randomization to receive one intramuscular injection of the RSV F vaccine (120 μg of RSV F vaccine adsorbed to 0.4 mg of aluminum) or placebo at 28–36 weeks' gestational age, and the infant followed up to 1 year of age. The vaccine was safe, although expectantly women that received the vaccine were more likely to have mild injection-site reactions than placebo recipients (40.7% vs. 9.9%). In infants, serious adverse events were coded as “pneumonia” and occurred in 2.2% of infants whose mothers were vaccinated as compared to 4.5% in the placebo group. The vaccine was immunogenic and induced an 18.6-fold increase in geometric mean concentration of anti-F IgG 2 weeks after vaccination with high levels at delivery and a decline at 180 days postpartum.
This study failed to reach the primary endpoint, but several important findings were noted. The VE through to 90 days was 39.4% (95% CI: 5.3–61.2) against medically significant RSV-LRTI, and 48.3% (95% CI: −8.2–75.3) against RSV-LRTI with severe hypoxemia.40 The cord-maternal anti-F IgG antibody ratio was 1.17 (95% CI, 1.14 to 1.19). This study also showed a 46% reduction in all-cause severe LRTI in infants of vaccinated mothers. Notably only 28 and 32 women need to be vaccinated to prevent a single episode of all-cause LRTI hospitalization and LRTI with severe hypoxemia, respectively.41 In South Africa, the VE was higher against medically significant RSV-LRTI (56%; 95% CI: 33–74) and RSV-LRTI with severe hypoxemia (74%; 95% CI: 50–98) compared with VE estimates for high-income countries (48% against medically significant RSV-LRTI and 28% RSV-LRTI with severe hypoxemia).
Enrolment into the GSK phase-III double-blind, placebo controlled randomized trial was terminated early on the recommendation of the Data and Safety Monitoring Board, because of safety concerns in vaccinated recipients.42 This single-dose unadjuvanted RSV prefusion F protein maternal vaccine (60 μg and 120 μg doses) was administered intramuscularly to pregnant women 18–49 years of age and the primary endpoint was a VE against medically assessed RSV-LRTI up to 6 months of age. The final results of this safety and efficacy study are yet to be published.
The phase-III double-blind, placebo-controlled MATISSE Pfizer study was conducted in 18 countries.43,44 This study aimed to determine the VE against medically attended RSV-associated LRTI in infants within 90, 120, 150, and 180 days after birth. Pregnant women (24–36 weeks) were randomly assigned to receive a single intramuscular injection of 120 μg of a bivalent RSV prefusion F protein-based vaccine or placebo. Adverse events within 1 month after vaccination in pregnant women and within 1 month after birth in infants were similar in the vaccine and placebo group. The VE for severe medically attended RSV-LRTI was 81.8% (95% CI: 40.6–96.3) at 90 days after birth, and 69.4% (95% CI: 44.3–84.1) at 180 days after birth.
In 2023, the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved the GSK (Arexvy) and the Pfizer (Abrysvo) pre-fusion F protein-based vaccine to prevent RSV in adults, and the Pfizer (Abrysvo) bivalent pre-fusion F protein-based maternal RSV vaccine to reduce the burden of RSV in infants. The US FDA, however, approved licensure for pregnant women 32 through 36 weeks rather than from 24 weeks. This decision was based on a non-statistically significant higher proportion of preterm birth in the vaccine recipients (5.6%) compared to placebo (4.7%, RR 1.20; 95% CI: 0.98–1.46) in the MATISSE Pfizer phase-III trial. Preterm births were higher in upper-middle-income countries among vaccine recipients (7.4%) compared to placebo (4.0%; RR 1.85, p <0.05), and coincided with the Omicron and Delta SARS-CoV2 waves. Furthermore, no links between the delivery and timing of the vaccine was observed, and preterm births occurred >30 days after the vaccination was administered.
In the US, vaccine safety monitoring post implementation undertaken by the Vaccine Safety Data link has shown no change in preterm delivery incidence (4.1%) compared to pre-vaccine introduction (3.1–6.1%). Additionally, a systematic review of six randomized control trials (four with the pre-F protein vaccine and two with the RSV protein nanoparticle vaccine) showed no significant association between maternal RSV vaccination and preterm birth (RR 1.16; 95% CI: 0.99–1.36), stillbirth (RR 0.81; 95% CI: 0.38–1.72), intrauterine growth restriction (RR 1.32; 95% CI: 0.75–2.33), congenital abnormalities (RR 0.96; 95% CI: 0.88–1.04), infant deaths (RR 0.81; 95% CI: 0.36–1.81), or maternal deaths (RR 3.00; 95% CI: 0.12–73).45
Considerations of RSV vaccine in specific populations
Women living with HIV (WLWH) have a 2.7-fold (95% CI: 1.5–4.6) and 3.0-fold (95% CI: 1.1–7.6) higher rate of RSV infection than HIV-uninfected women during pregnancy and the postpartum period, respectively.46 Similarly, HIV-exposed infants (i.e., infants born to HIV-infected pregnant women) have a 1.4-fold (95% CI: 1.3–1.5) higher incidence and 2.1-fold (95% CI: 1.1–3.8) increased case-fatality rate from RSV infection compared to unexposed infants.47 Notably, HIV-exposed infants have lower antibody levels at birth compared to HIV-unexposed infants;48 this may be from lower levels of baseline antibody in the mother or the inefficient transplacental transfer of antibody. Maternal hypergammaglobulinemia due to HIV infection, or other causes, saturates the FcRn diminishing the binding of placental antibody and consequently resulting in lower transplacental transfer of antibody. Cord-maternal anti-RSV antibody ratios were 22% lower in mothers that had a total IgG >15 g/L.49 HIV-infected-exposed pregnant–newborn dyads had lower cord–maternal transfer ratios in South Africa (0.67; 95% CI: 0.61–0.72 vs. 0.82; 95% CI: 0.75–0.91) and Gaborone (1.02; 95% CI: 0.93–1.12 vs. 1.15; 95% CI: 1.03–1.27) than HIV-uninfected-unexposed dyads.49,50 These findings are not specific to RSV, and similar findings have been demonstrated for other pathogens.51 Importantly, IgG1 was the most affected by maternal HIV infection and this is the predominant IgG subclass for protein vaccines such as RSV.51
As WLWH may have reduced immunogenicity to vaccines,52 alternative doses or dosing schedules may need to be considered to heighten the antibody responses in the women, and enrich the transplacental antibody transfer to their fetuses.
Pre- and post-licensure maternal vaccination planning
For a RSV maternal vaccine to be successfully incorporated into public health antenatal programmes, particularly in LMICs, certain key initiatives need to be considered. Caregiver awareness and education programmes, and public engagement with religious and cultural groups are necessary to overcome vaccine hesitancy. This includes addressing misinformation through personal and online engagement. Sharing testimonials of pregnant women or mothers of children that demised or have significant sequelae from RSV may also be beneficial in this digital era. Maternal and paternal awareness and acceptability requires stakeholder campaigns – this builds confidence and changes attitudes to vaccination and health-seeking behavior. In a study exploring parental perspectives for use of nirsevimab in infants and/or the maternal RSV preF vaccine, many factors impacted the decision by caregivers to adopt either of these strategies. Individual and contextual influences such as healthcare access and cost, climate and geographical factors, acceptability of the vaccines, and cultural and religious influences were key when deciding which option to choose. Furthermore, the recommendations from health care providers, safety and efficacy, and perceptions of the disease itself played a significant role in these decisions.53
Policymakers need to prioritize pregnant women in fiscal health decisions. There needs to be a concerted effort by pharma to reduce cost, including for countries that are not Gavi-subsidized. Antenatal programs need to be aligned so vaccination (including cold chain) during pregnancy can be considered the standard-of-care during the second and third trimester.
Safety and vaccine coverage need to be monitored. Fetal (miscarriage and stillbirth) and birth outcomes (death, disability, and dysmorphism) using pregnancy registries are crucial to measuring population-specific adverse outcomes. Health-care worker education and engagement with professional obstetrics/midwives and neonatology societies is required to frame a united public-health stance. Lastly, surveillance systems to measure the effectiveness of maternal vaccination on RSV (incidence, seasonality, mortality, and long-term sequelae), and all-cause LRTI hospitalization and death need to be identified prior to vaccine roll-out in order to measure the full impact of the vaccine, while vaccine probe studies may be necessary in less resourced areas. Lastly, a strategy needs to be in place for women presenting in preterm labor that were unvaccinated or had limited gestational time for adequate antibody transfer.
Another important consideration is vaccine co-administration. The CDC has recommended the co-administration of the Pfizer (Abrysvo) bivalent pre-fusion F protein-based maternal RSV vaccine with COVID-19 and influenza vaccines. Reduced immunogenicity to pertussis and influenza antigens have been reported with co-administration with the Pfizer (Abrysvo) bivalent pre-fusion F protein-based.54
CONCLUSION
Few vaccines have been recommended for use in pregnant women and the Pfizer (Abrysvo) bivalent pre-fusion F-protein maternal RSV vaccine has been licensed in Europe and North America. An expanded “antenatal” program on immunization, endorsed by WHO, and accepted by major stakeholders is urgently required. Key markers of success are vaccine safety, awareness, availability, acceptability, and confidence.
The broad aims of maternal RSV vaccination would be to provide immediate protection to the infant in the first 6 months of life and thereby reducing under-5 mortality. In addition, prevention of hospitalization will relieve pressure on health-care facilities during the RSV season, particularly in low-income settings with high bed occupancy, smaller intensive care facilities, and limited access to invasive or non-invasive ventilation. Importantly, reducing RSV infection also reduces the risk of secondary bacterial or co-infection.40 Furthermore, vaccination can reduce RSV infection in the mother during pregnancy or in the postpartum period.46 It is however unknown whether RSV maternal infection increases the risk of stillbirth or premature labor, as shown for infections like pertussis and influenza.55 Therefore, maternal vaccination is an attractive and implementable option, but may not necessarily be the only choice in high-income settings with access to newer monoclonal antibodies or emerging anti-viral therapy. The major limitation of maternal vaccination is that it will not protect infants born prematurely who have not had enough time for placental transfer.
PRACTICE RECOMMENDATIONS
- Respiratory syncytial virus (RSV) is the commonest cause of lower respiratory tract infection (LRTI) hospitalization in children and there is currently no antiviral treatment for children with RSV infection.
- RSV infection may be prevented by the administration of monoclonal antibodies and vaccines targeted at the young infant, or by vaccinating pregnant women with the aim of protecting their infants in the first few months of life.
- The use of maternal vaccination as a strategy to protect pregnant women and their young infants from disease has already been established for tetanus, pertussis, influenza virus, and COVID-19; for which routine vaccination of pregnant women is recommended.
- Vaccination of pregnant women is being targeted during the second or third trimester of pregnancy to increase neutralizing antibody levels in the pregnant women that are then transplacentally transferred to the fetus.
- The transplacental transfer of antibody can be negatively affected by maternal chronic infections, advanced age, high parity, high body-mass index, HIV infection, and hypergammaglobulinemia.
- The Pfizer bivalent RSV prefusion F protein has completed phase-III evaluation – the vaccine is safe and immunogenic, and the vaccine efficacy against severe medically attended LRTI was reported at 81.8% (CI: 40.6–96.3%) through the first 90 days.
- Caregiver awareness, education programmes, and public engagement are necessary to overcome vaccine hesitancy.
CONFLICTS OF INTEREST
Shabir Madhi has conflicts – funding of clinical trials, money to institution, in relation to Pfizer, GSK, Novavax, AstraZenca (Medimmue) and MSD. Grant funding to institution from BMGF. Ziyaad Dangor and Shabir A Madhi are clinical trial investigators on RSV maternal vaccination and monoclonal antibody trials.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Perin J, Mulick A, Yeung D, et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the Sustainable Development Goals. The Lancet Child & Adolescent Health 2022;6(2):106–15. | |
Mazur NI, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. The Lancet Infectious Diseases 2022. | |
Meissner HC. Viral Bronchiolitis in Children. The New England Journal of Medicine 2016;374(18):1793–4. | |
Glezen WP, Taber LH, Frank AL, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986;140(6):543–6. | |
Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet (London, England). 2022;399(10340):2047–64. | |
Rha B, Curns AT, Lively JY, et al. Respiratory Syncytial Virus-Associated Hospitalizations Among Young Children: 2015–2016. Pediatrics 2020;146(1). | |
Geoghegan S, Erviti A, Caballero MT, et al. Mortality due to Respiratory Syncytial Virus. Burden and Risk Factors. American Journal of Respiratory and Critical Care Medicine 2017;195(1):96–103. | |
Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010;65(12):1045–52. | |
WHO Preferred Product Characteristics for Respiratory Syncytial Virus (RSV). Vaccines. Geneva: World Health Organization, 2017. Licence: CC BY-NC-SA 3.0 IGO. | |
Blount RE Jr, Morris JA, Savage RE. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med 1956;92(3):544–9. | |
Chanock R, Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). II. Epidemiologic aspects of infection in infants and young children. Am J Hyg 1957;66(3):291–300. | |
Taleb SA, Al Thani AA, Al Ansari K, et al. Human respiratory syncytial virus: pathogenesis, immune responses, and current vaccine approaches. Eur J Clin Microbiol Infect Dis 2018;37(10):1817–27. | |
Chu HY, Tielsch J, Katz J, et al. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology 2017;95:90–5. | |
Fong Y, Huang Y, Borate B, et al. Antibody Correlates of Protection From Severe Respiratory Syncytial Virus Disease in a Vaccine Efficacy Trial. Open Forum Infect Dis 2023;10(1):ofac693. doi: 10.1093/ofid/ofac693. PMID: 36655191; PMCID: PMC9835761. | |
Ogilvie MM, Vathenen AS, Radford M, et al. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol 1981;7(4):263–71. | |
Piedra PA, Jewell AM, Cron SG, et al. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 2003;21(24):3479–82. | |
Nyiro JU, Sande CJ, Mutunga M, et al. Absence of Association between Cord Specific Antibody Levels and Severe Respiratory Syncytial Virus (RSV) Disease in Early Infants: A Case Control Study from Coastal Kenya. PloS One 2016;11(11):e0166706. | |
Andabaka T, Nickerson JW, Rojas-Reyes MX, et al. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 2013(4):Cd006602. | |
PATH, 2022. https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/. | |
Gonzales T, Bergamasco A, Cristarella T, et al. Effectiveness and Safety of Palivizumab for the Prevention of Serious Lower Respiratory Tract Infection Caused by Respiratory Syncytial Virus: A Systematic Review. Am J Perinatol 2022. | |
Shahabi A, Peneva D, Incerti D, et al. Assessing Variation in the Cost of Palivizumab for Respiratory Syncytial Virus Prevention in Preterm Infants. Pharmacoecon Open 2018;2(1):53–61. | |
Griffin MP, Yuan Y, Takas T, et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. The New England Journal of Medicine 2020;383(5):415–25. | |
Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. The New England Journal of Medicine 2022;386(9):837–46. | |
Muller WJ, Madhi SA, Seoane Nuñez B, et al. Nirsevimab for Prevention of RSV in Term and Late-Preterm Infants. N Engl J Med 2023;388(16):1533–4. doi: 10.1056/NEJMc2214773. Epub 2023 Apr 5. PMID: 37018470. | |
Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 2019;7(8):e1031–e45. | |
Li Y, Hodgson D, Wang X, et al. Respiratory syncytial virus seasonality and prevention strategy planning for passive immunisation of infants in low-income and middle-income countries: a modelling study. The Lancet Infectious Diseases 2021;21(9):1303–12. | |
Ezpeleta G, Navascués A, Viguria N, Herranz-Aguirre M, Juan Belloc SE, Gimeno Ballester J, Muruzábal JC, García-Cenoz M, Trobajo-Sanmartín C, Echeverria A, Martínez-Baz I, Vera-Punzano N, Casado I, López-Mendoza H, Ezpeleta C, Castilla J. Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study. Vaccines (Basel). 2024 Apr 4;12(4):383. doi: 10.3390/vaccines12040383. PMID: 38675765; PMCID: PMC11054679. | |
Alejandre C, Penela-Sánchez D, Alsina J, Agüera M, Soler A, Moussalam S, Muñoz-Almagro C, Brotons P, Cambra FJ, Forner OR, Balaguer M, Launes C, Jordan I. Impact of universal immunization program with monoclonal antibody nirsevimab on reducing the burden of serious bronchiolitis that need pediatric intensive care. Eur J Pediatr. 2024 Jun 23. doi: 10.1007/s00431-024-05634-z. Epub ahead of print. PMID: 38910199. | |
Assad Z, Romain AS, Aupiais C, Shum M, Schrimpf C, Lorrot M, Corvol H, Prevost B, Ferrandiz C, Giolito A, Valtuille Z, Bendavid M, Cohen JF, Toubiana J, de Pontual L, Delande CF, Levy M, See P, Cohen R, Levy C, Angoulvant F, Lenglart L, Gits-Muselli M, Biran V, Diallo K, Alemede O, El Hebil MM, Durrmeyer X, Labouret G, Casanovas N, Hallak B, Maréchal O, Jung C, Bréhin C, Ouldali N. Nirsevimab and Hospitalization for RSV Bronchiolitis. N Engl J Med. 2024 Jul 11;391(2):144-154. doi: 10.1056/NEJMoa2314885. PMID: 38986058. | |
Riccò M, Cascio A, Corrado S, Bottazzoli M, Marchesi F, Gili R, Giuri PG, Gori D, Manzoni P. Impact of Nirsevimab Immunization on Pediatric Hospitalization Rates: A Systematic Review and Meta-Analysis (2024). Vaccines (Basel). 2024 Jun 8;12(6):640. doi: 10.3390/vaccines12060640. PMID: 38932369; PMCID: PMC11209424. | |
Ares-Gómez S, Mallah N, Santiago-Pérez MI, Pardo-Seco J, Pérez-Martínez O, Otero-Barrós MT, Suárez-Gaiche N, Kramer R, Jin J, Platero-Alonso L, Alvárez-Gil RM, Ces-Ozores OM, Nartallo-Penas V, Mirás-Carballal S, Piñeiro-Sotelo M, Malvar-Pintos A, González-Pérez JM, Rodríguez-Tenreiro-Sánchez C, Rivero-Calle I, Salas A, Durán-Parrondo C, Martinón-Torres F; NIRSE-GAL study group. Effectiveness and impact of universal prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia, Spain: initial results of a population-based longitudinal study. Lancet Infect Dis. 2024 Aug;24(8):817-828. doi: 10.1016/S1473-3099(24)00215-9. Epub 2024 Apr 30. Erratum in: Lancet Infect Dis. 2024 Jul;24(7):e419. doi: 10.1016/S1473-3099(24)00355-4. PMID: 38701823 | |
Simões EAF, Forleo-Neto E, Geba GP, et al. Suptavumab for the Prevention of Medically Attended Respiratory Syncytial Virus Infection in Preterm Infants. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America 2021;73(11):e4400–e8. | |
Falsey AR, Walsh EE, Scott DA, et al. Phase 1/2 Randomized Study of the Immunogenicity, Safety, and Tolerability of a Respiratory Syncytial Virus Prefusion F Vaccine in Adults With Concomitant Inactivated Influenza Vaccine. J Infect Dis 2022;225(12):2056–66. | |
Schmoele-Thoma B, Zareba AM, Jiang Q, et al. Vaccine Efficacy in Adults in a Respiratory Syncytial Virus Challenge Study. The New England Journal of Medicine 2022;386(25):2377–86. | |
Walsh EE, Pérez Marc G, Zareba AM, et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N Engl J Med 2023;388(16):1465–77. doi: 10.1056/NEJMoa2213836. Epub 2023 Apr 5. PMID: 37018468. | |
Etti M, Calvert A, Galiza E, et al. Maternal vaccination: a review of current evidence and recommendations. Am J Obstet Gynecol 2022;226(4):459–74. | |
Nunes MC, Aqil AR, Omer SB, et al. The Effects of Influenza Vaccination during Pregnancy on Birth Outcomes: A Systematic Review and Meta-Analysis. Am J Perinatol 2016;33(11):1104–14. | |
Bratton KN, Wardle MT, Orenstein WA, et al. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: a systematic review and meta-analysis. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America 2015;60(5):e11–9. | |
Prasad S, Kalafat E, Blakeway H, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun 2022;13(1):2414. | |
Madhi SA, Polack FP, Piedra PA, et al. Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. The New England Journal of Medicine 2020;383(5):426–39. | |
Madhi SA, Cotton M, Ahmed K, et al. Efficacy of nano-particle respiratory syncytial virus (RSV) f-protein vaccine immunization of pregnant women against RSV lower respiratory tract infection (LRTI) in South African infants: a randomised placebo controlled trial. European Society of Paediatric Infectious Diseases 2020. | |
Dieussaert I, Hyung Kim J, Luik S, Seidl C, Pu W, Stegmann JU, Swamy GK, Webster P, Dormitzer PR. RSV Prefusion F Protein-Based Maternal Vaccine - Preterm Birth and Other Outcomes. N Engl J Med. 2024 Mar 14;390(11):1009-1021. doi: 10.1056/NEJMoa2305478. PMID: 38477988. | |
Simões EAF, Center KJ, Tita ATN, et al. Prefusion F Protein-Based Respiratory Syncytial Virus Immunization in Pregnancy. The New England Journal of Medicine 2022;386(17):1615–26. | |
Kampmann B, Madhi SA, Munjal I, et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med 2023;388(16):1451–64. doi: 10.1056/NEJMoa2216480. Epub 2023 Apr 5. PMID: 37018474. | |
Phijffer EW, de Bruin O, Ahmadizar F, et al. Respiratory syncytial virus vaccination during pregnancy for improving infant outcomes. Cochrane Database Syst Rev. 2024;5(5):CD015134. doi:10.1002/14651858.CD015134.pub2 | |
Nyawanda BO, Otieno NA, Otieno MO, et al. The Impact of Maternal Human Immunodeficiency Virus Infection on the Burden of Respiratory Syncytial Virus Among Pregnant Women and Their Infants, Western Kenya. J Infect Dis 2022;225(12):2097–105. | |
Cohen C, Moyes J, Tempia S, et al. Epidemiology of Acute Lower Respiratory Tract Infection in HIV-Exposed Uninfected Infants. Pediatrics 2016;137(4). | |
Bengtson AM, Sanfilippo AM, Hughes BL, et al. Maternal immunisation to improve the health of HIV-exposed infants. The Lancet Infectious Diseases 2019;19(4):e120–e31. | |
Jallow S, Agosti Y, Kgagudi P, et al. Impaired Transplacental Transfer of Respiratory Syncytial Virus-neutralizing Antibodies in Human Immunodeficiency Virus-infected Versus -uninfected Pregnant Women. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America 2019;69(1):151–4. | |
Patel SM, Jallow S, Boiditswe S, et al. Placental Transfer of Respiratory Syncytial Virus Antibody Among HIV-Exposed, Uninfected Infants. J Pediatric Infect Dis Soc 2020;9(3):349–56. | |
Alonso S, Vidal M, Ruiz-Olalla G, et al. Reduced Placental Transfer of Antibodies Against a Wide Range of Microbial and Vaccine Antigens in HIV-Infected Women in Mozambique. Front Immunol 2021;12:614246. | |
Nunes MC, Weinberg A, Cutland CL, et al. Neutralization and hemagglutination-inhibition antibodies following influenza vaccination of HIV-infected and HIV-uninfected pregnant women. PloS One 2018;13(12):e0210124. | |
Treston B, Geoghegan S. Exploring parental perspectives: Maternal RSV vaccination versus infant RSV monoclonal antibody. Hum Vaccin Immunother. 2024 Dec 31;20(1):2341505. doi: 10.1080/21645515.2024.2341505. Epub 2024 May 9. PMID: 38723786; PMCID: PMC11085959. | |
Peterson JT, Zareba AM, Fitz-Patrick D, Essink BJ, Scott DA, Swanson KA, Chelani D, Radley D, Cooper D, Jansen KU, Dormitzer PR, Gruber WC, Gurtman A. Safety and Immunogenicity of a Respiratory Syncytial Virus Prefusion F Vaccine When Coadministered With a Tetanus, Diphtheria, and Acellular Pertussis Vaccine. J Infect Dis. 2022 Jun 15;225(12):2077-2086. doi: 10.1093/infdis/jiab505. PMID: 34637519; PMCID: PMC9200146. | |
Giles ML, Davey MA, Wallace EM. Associations Between Maternal Immunisation and Reduced Rates of Preterm Birth and Stillbirth: A Population Based Retrospective Cohort Study. Front Immunol 2021;12:704254. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)