This chapter should be cited as follows:
Cutland CL, Makan N, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.419403
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 17
Maternal immunization
Volume Editors:
Professor Asma Khalil, The Royal College of Obstetricians and Gynaecologists, London, UK; Fetal Medicine Unit, Department of Obstetrics and Gynaecology, St George’s University Hospitals NHS Foundation Trust, London, UK
Professor Flor M Munoz, Baylor College of Medicine, TX, USA
Professor Ajoke Sobanjo-ter Meulen, University of Washington, Seattle, WA, USA

Chapter
Tetanus
First published: May 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Clostridium tetani (C. tetani) are rod-shaped, obligate anaerobic, Gram-positive bacilli, which are ubiquitous to the environment.1 On exposure to oxygen, C. tetani form spores, which are resilient to dehydration and extremes of temperature and are metabolically inert. C. tetani produce an exotoxin, or tetanospasmin, a potent neurotoxin, when the spores germinate after entering an anaerobic environment, typically through breaks in the skin.2 Deep penetrating puncture wounds, wounds with a significant amount of tissue injury and unsterile procedures – including the use of contaminated instruments during childbirth – promote spore germination.3
Tetanospasmin disseminates via lymphatic and vascular systems and enters nervous tissues where it disrupts inhibitory neurons or interneurons from releasing inhibitory neurotransmitters, gamma-aminobutyric acid (GABA) and glycine from Renshaw cells. This loss of inhibition (disinhibition) results in increased muscle tone and painful spasms due to the unopposed muscle contraction by acetylcholine. Tetanospasmin has an affinity for ganglioside-containing receptors at the nerve termini found throughout the peripheral and central nervous systems, including peripheral neuromuscular junctions, autonomic nervous system, spinal cord and brain.4 Once bound, tetanospasmin cannot be neutralized with tetanus antitoxin. The clinical manifestation of tetanus usually begins approximately 8 days after the injury but ranges from 3 to 21 days.
There are four recognized forms of tetanus classified according to the toxin distribution in the body, symptoms of the disease, and the age of those afflicted.
In generalized tetanus, muscle spasms usually start in the face, causing trismus or “lock-jaw” and a typical facial expression (risus sardonicus). Powerful, painful muscle contractions spread throughout the body, leading to impaired respiratory function, opisthotonis, and may be strong enough to cause muscle tears and broken bones.
Tetanus may be localized to an area around a wound. Cephalic tetanus affects the muscles supplied by the 12 cranial nerves, and is caused by injuries to the head, or infections like otitis media.
Neonatal tetanus occurs in newborn infants during the neonatal period, or first month of life. Tetanus spores usually enter the newborn’s body through the umbilical stump, either from a non-sterile instrument used to cut the cord after birth, or from unclean cord care practices. Poultices or herbal mixtures, which include mud, ash, or animal excrement, are believed, by some traditional healers, to aid in healing of the cord and protection of the newborn, but in some cases result in the source of tetanus infection.5 In infants, symptoms may take from 3 days to 2 weeks to develop and diagnosis is typically clinical in nature. Neonatal tetanus cases often present as an infant who had a normal suck and cry in the first few days of life, but lost this ability between 3 and 28 days of life, became stiff and/or had spasms.
Tetanus is the only vaccine-preventable disease that is infectious but not contagious and cannot be spread from person to person. Complete eradication, however, is not possible due to the ubiquitous nature of the spores in the environment and intestine of many animals. In addition, recovery from naturally acquired tetanus does not usually result in immunity and the majority of individuals remain susceptible to infection if exposed subsequently.6 Case-fatality rates vary from 10% to 70% depending on the age, general health of the patient,7 and treatment available. At the extremes of age, mortality rates approach 100% without the appropriate intensive care therapy,8 therefore, early treatment, proper wound management, and effective vaccination strategies are the cornerstones to tetanus control and elimination.
Diagnosis of tetanus
The diagnosis of tetanus is often clinical, by recognition of a triad of trismus, ridus sardonicus, and opisthotonus. In developed countries, where healthcare workers rarely observe cases due to the low disease incidence,9 tetanus cases may be misdiagnosed. A history of injury or wound is typically reported but absence of history of injury should not rule out the diagnosis, as symptoms have been known to present years following an injury and thus patients are unable to recall such events. The “spatula test”, may assist in the diagnosis. The posterior wall of the patient’s pharynx is touched with a soft instrument, and a reflex spasm of the jaw elicits a positive result with a high sensitivity (94%) and specificity (100%).10
Clinical diagnoses are often supported by epidemiological data and rarely depend on laboratory confirmation. C. tetani is recovered from the wound in only 30% of cases, however, it may also be isolated from patients who do not have tetanus. Isolating C. tetani is very difficult due to anaerobic slow growth in culture media. Laboratory identification of the organism depends on the demonstration of toxin production in mice. Tetanus toxin can be isolated from serum using a bioassay.
Clusters of tetanus cases have been identified as a complication of disasters, including earthquakes and tsunamis,11 and tetanus should be included as a differential diagnosis for people falling ill after disasters.
Treatment of tetanus
The treatment of tetanus should be in an intensive care setting. Treatment goals include three main aspects: (i) neutralization of circulating tetanus toxin; (ii) removal of the source of tetanospasmin; and (iii) supportive care to control muscle spasms and manage the airway and associated dysautonomia instability.9
Neutralization
Active and passive immunization should occur as soon as possible (at separate sites). In order to neutralize circulating tetanus toxin, human tetanus immunoglobulin (TIG) is indicated. Approximately 3000–6000 units should be administered IMI.12 It is important to provide TIG prior to debridement, as toxin can be released into circulation when the wound is debrided. If TIG is not available, equine antitoxin, which has a worse safety profile and greater risk of anaphylaxis than TIG, can be administered. A test dose of 0.1 mL in a 1 : 10 dilution should be administered to assess for hypersensitivity.
Clinical tetanus is one of the few bacterial diseases that does not confer immunity and thus both tetanus toxoid and TIG should be given to those with high-risk wounds.
Removal of source of toxin release
Removing the source of toxin production includes wide wound debridement to eliminate spores and necrotic tissue that promulgates C. tetani growth. Although regarded as having a secondary role (to wound debridement) in reducing organism load, antibiotics are universally recommended. Metronidazole is the treatment of choice (500 mg IVI every 6–8 hours). Penicillin G (2–4 million units 4–6 hourly) was previously used as an alternative to metronidazole, but is now avoided due to the associated GABA antagonism and potentiation of tetanospamin effects. The duration of therapy should be between 7 and 10 days. Other alternatives include erythromycin, tetracycline, chloramphenicol, and clindamycin.3
Supportive care
Once tetanospasmin has been taken up into the nervous tissue, it cannot be reversed, and symptoms will eventually subside when nerve fibers are replaced or heal (over a period of several months). For this reason, supportive therapies are the mainstay of treatment.
Endotracheal intubation is the initial measure to establish and protect the airway while allowing for ventilation. Early tracheostomy is recommended due to the likelihood of prolonged ventilation. Generalized muscle spasms are life threatening due to associated respiratory failure, fractures, and associated metabolic acidosis. Agents such as benzodiazepines (e.g., Diazepam 10–30 mg IVI), skeletal muscle relaxants (100 mcg intrathecally) and anticholinergics (e.g., Benztropine 1–2 mg IVI) should be used to control muscle rigidity and spasms. Serial doses are frequently required and, since agents are frequently used for several weeks, should be tapered to prevent withdrawal reactions. When sedation alone is inadequate, long-acting non-depolarizing muscle relaxants such as vecuronium should be considered.
Several drugs are available for use in order to manage the dysautonomia associated with tetanus and should be tailored according to patient profile and clinical presentation. Magnesium sulphate has been studied in a randomized control trial, and found to reduce receptor responsiveness to catecholamines and act as a presynaptic neuromuscular blocker. Other agents to consider include labetolol, propranolol, dobutamine, and dopamine.
PREVENTION OF TETANUS
There is usually no naturally acquired immunity to tetanus following infection. The amount of toxin that causes disease is insufficient to stimulate an immune response for the production of antibody. Therefore, reliance on active or passive immunization strategies is critical for the prevention of infection. Since disease is as a result of toxin effects, an appropriate and specific antibody-mediated immune response is needed to neutralize tetanus toxin. This is achieved through the use of tetanus vaccines to confer immunity within the individual as well as providing a means of passive immunization from mother to child in order to prevent cases of NT.
Tetanus is prevented through the administration of tetanus toxoid containing vaccines (TTCV), which are included in routine childhood immunization programs globally and used in many countries for the vaccination of pregnant women during antenatal care visits.7 TTCVs are available as single antigen vaccines (TT), or in combination with other vaccines. These include diphtheria vaccine (DT in children <5 years or Td low-dose diphtheria toxoid licenced for use after 5 years of age), or further combined with at least one of pertussis, Hepatitis B, Haemophilus influenza type B, and polio vaccine.13
The World Health Organization recommends the administration of a six-dose child and adolescent tetanus toxoid schedule, starting with a three-dose primary series of TTCV during infancy (starting at 6 weeks with 4 week intervals between doses; doses 2 and 3 given at 10 and 14 weeks, respectively). This is followed by three-dose booster series prior to adulthood.7 Booster doses are first administered in the second year of life (12–23 months), thereafter spaced approximately 4 years apart in childhood (4–7 years) and adolescence (9–15 years).13
Some national immunization schedules are variations of the WHO-recommended schedule and include the administration of the infant three-dose primary series doses at 2, 3 and 4 months; 3, 4 and 5 months; or 2, 4 and 6 months.7 A two-dose primary schedule, mainly used in Nordic countries, includes vaccination at 2 and 4 months or 3 and 5 months.14 Recommendations for booster doses also vary but are usually administered every 10 years. Such variations in schedules may be a result of differences in disease epidemiology, cost implications, and available resources and capacity of local health systems.
Historically, immunization strategies have focused on prevention of disease in infants and young children, however there has been a recent strong shift towards life course vaccination among adult population groups. Among adults who have not received prior TTCV, three doses in the primary series is recommended with the second dose administered 1–2 months after the first dose and the third dose ideally administered 6–8 months after the second dose (but a minimal interval of one month is permitted). Booster doses of TTCV should be given to adults who have completed a primary series and if the last dose was received 10 or more years previously.
Vaccination of adult groups, specifically pregnant women, provide an invaluable source of protection against cases of NT. Vaccination during pregnancy protects both mother and infant through the production of tetanus-specific IgG antibodies, which are transferred to the fetus through the placenta and provide protection through the neonatal and early infancy period when the infant is too young to receive TTCV and mount an appropriate immune response.
Pregnant women and their newborn infants are considered protected from tetanus if the mother received six TTCV doses during childhood, or five doses if a catch-up vaccination schedule was initiated after 1 year of age.
Additionally, at least two doses of TTCV are recommended during pregnancy if a pregnant woman is not considered fully protected or documentation of prior TTCV is not available, with the first two doses administered 4 weeks apart.13 At each subsequent pregnancy, one TTCV dose should be given to achieve long-term protection until the full series is completed as shown in Table 1.
1
TTCV vaccination schedule for women of reproductive age and pregnant women with unknown vaccination status or without previous exposure to TTCV.
Dose of TTCV | When to give | Expected duration of protection |
TTCV1 | At first contact/antenatal visit, or as early as possible in pregnancy | None |
TTCV2 | At least 4 weeks after TTCV1 (at the latest 2 weeks prior to birth) | 1–3 years |
TTCV3 | At least 6 months after TTCV2, or during subsequent pregnancy | At least 5 years |
TTCV4 | At least 1 year after TTCV3, or during subsequent pregnancy | At least 10 years |
TTCV5 | At least 1 year after TTCV4, or during subsequent pregnancy | For all childbearing age and much of adulthood |
This is an adaptation of an original work “Protecting all against tetanus: guide to sustaining maternal and neonatal tetanus elimination (MNTE) and broadening tetanus protection for all populations. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO”. This adaptation was not created by WHO. WHO is not responsible for the content or accuracy of this adaptation. The original edition shall be the binding and authentic edition.13
HIGH-RISK PRACTICES THAT CAN CAUSE TETANUS
A freshly cut umbilical stump is major port of entry into the neonate for invasive pathogens such as C. tetani.21 Perhaps the best known sociocultural practice with significant causal association with tetanus disease in neonates is the application of oils, herbs, bodily fluids, heat, and animal feces5 to the umbilical stump within the first few hours after birth. Contrary to health beliefs that inform these practices, applying substances to the stump worsens physiological function at the site of the umbilicus and reduces local immune mechanisms. Improved umbilical stump care in neonates together with clean birthing practices (i.e., active non-intervention) reduces the incidence of NT by 55–99%.22
Nearly 30% of births in lower and middle income countries occur outside of healthcare facilities, approaching 80% in some African countries.20 Tetanus exposure in pregnant women most commonly occurs during termination of pregnancy (medical or surgical), early pregnancy loss or unhygienic practices around delivery. Unsafe terminations of pregnancy and cultures that marginalize and discriminate against women’s reproductive rights is a leading cause of maternal morbidity and mortality, including maternal tetanus. Nearly 50% of termination of unplanned pregnancies (TOP) are unsafe, and 97% of these occur in developing countries.23 While unsafe surgical TOP is a direct cause of maternal tetanus, medical TOPs frequently result in incomplete miscarriages exposing the genitourinary tract to ascending infections such as tetanus, further followed by systemic insult and immune dysregulation.
Traditional treatment by scarification, the process of inflicting cuts (incisions) to the body, is another cultural practice that increases the risk of exposure to tetanus in both mothers and newborn infants. Traditional practitioners use scarification for various reasons including esthetics, marital ceremonies, and tribal affiliations, to "therapeutic" practices to let out "bad blood" or evil spirits, including for treatment of conditions such as ascites, dermatoses, hepatosplenomegaly, psychiatric conditions, or for pain relief.24,25 Despite widespread belief in the therapeutic benefits, scarification leads to significant morbidity and mortality from tetanus.26 Compounding the adverse sequelae of scarification is that these traditional practices most often occur in districts where health services (and hence access to vaccination, disease surveillance, and clean birth practices) are limited and strong sociocultural norms vests significant trust in traditional practices. Joint learning and partnering with traditional healers in an ethical and culturally sensitive nature is critical to empowering traditional healers to be champions of MNTE by maternal and neonatal safe practices in a culturally acceptable manner.27
HOW ARE TETANUS VACCINES MADE?
The earliest clinical descriptions of tetanus can be traced back to the fifth century BC where ancient civilisations recognized the relationship between infected wounds and fatal muscle contractions. However, it was only in 1884 that scientists Rattone and Carle produced tetanus in animals by injecting them with the pus from a fatal case of human tetanus.28 This experiment was replicated by injecting animals with soil samples. Five years later, following his successful isolation of the organism from soil samples, Shibasaburo Kitasato demonstrated C. tetani as the causal agent of tetanus and reported its neutralization by specific antibodies.29 In 1897, Nocard demonstrated passive immunity through injection of antitoxin, paving the way for prophylaxis and treatment used in World War I. In 1924, Descombey developed the first TT vaccine, which was used in World War II. In 1948, TT was rolled out internationally in the form of the DTP vaccine.
During manufacture of tetanus vaccine, toxigenic strains of C. tetani are grown in liquid media, and the resulting tetanus neurotoxin (TeNT) is harvested from the culture supernatant of C. tetani seed lots. Purified TeNT is inactivated by the addition of formaldehyde followed by several steps of purification and sterilization. The resulting tetanus toxoid maintains its antigenic properties but is not toxigenic. To enhance immunogenicity, the toxoid is adsorbed to an adjuvant (usually aluminum or calcium salts) prior to packaging and stringent batch release testing.15
The standard dose of TTCV is 0.5 ml and has a minimum potency of 40 IU per dose in the infant schedule.16 The vaccine is administered intra-muscularly (IM) in the anterolateral thigh of infants and in the deltoid muscle in older age groups.
MATERNAL AND NEONATAL TETANUS
The inability to eradicate C. tetani across the globe underscores the importance of encouraging behavioral changes in communities to reduce the risk of transmission. While near-elimination of cases has occurred in developed countries, where comprehensive vaccination programs have been rolled out and safe birthing practices are enforced, the risk of contracting C. tetani remains high in developing countries.17 Reducing maternal and neonatal tetanus (MNT) cases is a critical progress indicator for the Sustainable Development Goals and every case of MNT represents a failure of multiple levels of the healthcare system.18
Maternal tetanus is defined as tetanus occurring during pregnancy or within 6 weeks after pregnancy ends (with birth, miscarriage, or abortion), and has the same risk factors and means of prevention as neonatal tetanus. For this reason, NT elimination is considered a proxy for maternal tetanus elimination and is defined as less than 1 case of NT per 1000 live births in every district or similar administrative unit in the country each year.13,19 Maternal tetanus is assumed to be eliminated once NT elimination has been achieved.
The neonatal tetanus elimination (NTE) goal was formally launched during the 42nd World Health Assembly in 1989. The initial elimination target focused on eliminating NT from 59 priority countries by 1995, however, this target was revised in 1991 at the 44th World Health Assembly, just 2 years after it was set, due to slow implementation of elimination strategies. The revised target date for elimination of NT in all countries was 2000. In 1999, the NT global elimination goals were reviewed, and a new initiative was launched, which included the addition of maternal tetanus elimination to the NT elimination target. The Maternal and Neonatal Tetanus Elimination Initiative saw multiple local stakeholders and global partnerships commit to meeting their MNTE targets. The global elimination target date was set at 2005, then moved to 2015 and again to 2020. It has still not been met in several countries distributed in three of the six WHO regions.
MNTE strategy is a multi-faceted approach, which includes (i) strengthening of antenatal care immunization of pregnant women, (ii) supplementary TTCV immunization activities targeting women of reproductive age, (iii) promotion of clean birth and clean cord care, (iv) reliable NT surveillance.
HISTORY OF TETANUS VACCINATION IN PREGNANCY
Early work around TT in pregnancy looked to reduce the incidence of NT. A 1961 field trial in New Guinea was the first to document a significant reduction in NT following antenatal TT immunization.30 They noted NT cases in 10% of infants born to mothers who received either no doses or one dose of TT compared to 0.57% of infants whose mothers had received three doses. A double-blind controlled trial, concluded in 1966, validated these findings and further showed the elimination of NT in cases where two or more doses of TT were administered.31 A systematic review found that immunization of pregnant women or women of childbearing age with at least two doses of tetanus toxoid (TTCV2+) was estimated to reduce mortality from neonatal tetanus by 94% [95% confidence interval (CI) 80–98%].32
Immunization of pregnant women with TTCV is one of the most effective interventions against NT, independent of other interventions33 and forms a key pillar towards achieving MNTE globally.
TETANUS TOXOID VACCINATION COVERAGE IN WOMEN
Pregnant women and their newborn infants are considered protected from birth-associated tetanus if the mother has received at least six doses of TTCV (documented by card, immunization registry, and/or history) before the time of reproductive age, or five doses if a catch-up vaccination schedule was initiated after 1 year of age.
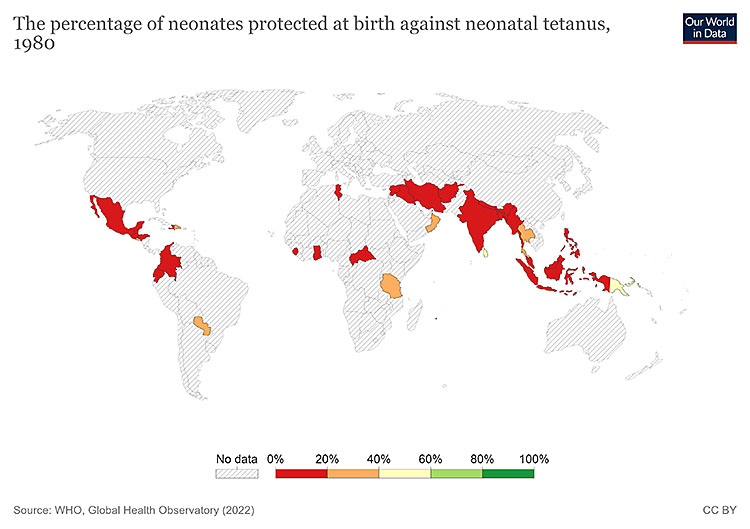
1
Map: neonates protected at birth against neonatal tetanus 1980. https://ourworldindata.org/tetanus#elimination-of-maternal-and-neonatal-tetanus.
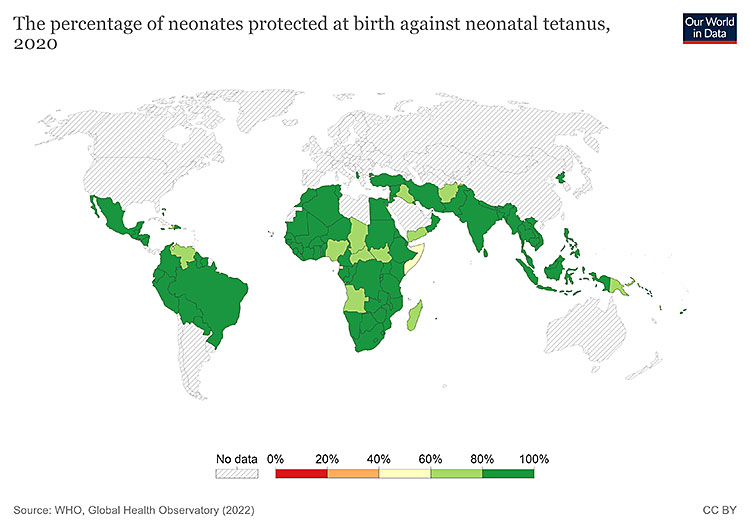
2
Map: neonates protected at birth against NT 2020. https://ourworldindata.org/tetanus#elimination-of-maternal-and-neonatal-tetanus. Hannah Behrens, Sophie Ochmann, Bernadeta Dadonaite and Max Roser (2019) – "Tetanus". Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.org/tetanus' [Online Resource].
PROGRESS TOWARDS GLOBAL MNTE
MNTE follows a three-staged approach: achieving, validating, and sustaining. Once elimination has been achieved, the country applies for a validation status. It is certified to have achieved validation for MNTE if the elimination status of NT (<1 case per 1000 live births) has been verified by district level data using several surveillance indicators. Following validation, continued measures are required to sustain the MNTE status.
Establishment of guidelines to achieve MNTE
The global MNTE initiative focused on key areas, including immunization and delivery practices, in order to achieve the target of <1 case of NT per 1000 live births in every district globally.
The recommended strategies for achieving MNTE include the following:
- Strengthening routine immunization of pregnant women, achieving ≥80% coverage with ≥2 doses of TTCV (TTCV2+);
- Targeting ≥80% of women of reproductive age 15–49 years in selected high-risk areas, with appropriately spaced doses of TTCV through supplementary immunization activities (SIAs);
- Promoting clean birth deliveries and cord care practices through the provision of care by a skilled birth attendant (SBA) present at ≥70% of deliveries;
- Enhancing reliable NT surveillance, including case investigation and response.
Routine Immunization of pregnant women
Antenatal clinic (ANC) visits are used as opportunities to immunize pregnant women against tetanus in many countries, and have led to significant reduction in MNT, especially where TTCV vaccination coverage in children is low. ANC visits provide a platform for administering booster TTCV doses as well as catch-up vaccination for those who have not previously been vaccinated. In women who have never been vaccinated with TTCV or those with no documented proof a total of five properly spaced doses is recommended: two doses given one month apart in the first pregnancy, the third dose given at least 6 months later, then one dose in each subsequent pregnancy (or intervals of at least 1 year), for a total of five doses.
The "high-risk approach"
Where routine vaccination and reproductive health services fail to reach a substantial proportion of pregnant women, several rounds of TTCV SIAs are required for MNTE. This strategy targets all women of reproductive age living in high-risk districts with limited or no access to routine vaccination. It is known as the "high-risk approach" and consists of three rounds of TTCV dose administration with an interval of at least 4 weeks between doses 1 and 2, and at least 6 months between doses 2 and 3, irrespective of prior vaccination status.
Clean deliveries
Clean birth practices is paramount in reducing maternal and neonatal infection, including tetanus. The principles are largely founded on infection prevention and control guidelines. They include clean hands of the birth attendant, a sterile delivery surface, clean umbilical cord cut and ties and clean stump practices. Where feasible, all deliveries should be carried out in health facilities by skilled birth attendants. For home or community-based deliveries, pregnant women, birth attendants, and their community should be educated about safe delivery and post-delivery practices and where to seek immediate help should complications arise.
Surveillance
Optimal surveillance for MNT is the key to assessing program deficits, setting up targets, planning and executing elimination strategies, and evaluating the success of the program. The areas where NT cases continue to pose a significant public health threat tend to be those in poorly developed marginalized communities with inadequate health infrastructure. Many neonatal deaths occur at home, and are often not reported. Thus MNT surveillance remains suboptimal in many parts of the world and require local and national partnerships to strengthen these surveillance systems. Several countries have integrated NT surveillance into pre-existing platforms such as local acute flaccid paralysis (AFP) and measles surveillance, as a cost-effective and integrative strategy for notifiable vaccine preventable diseases.
Recommendations by the WHO include case-based surveillance for NT cases, including zero-case reporting (submission of reports even in the absence of NT cases) to ensure surveillance systems remain sensitive and ongoing.19 In addition active surveillance strategies are recommended through regular site visits particularly in high-risk areas.19
Guidelines to maintain MNTE
Since complete eradication of tetanus is not possible due to the ubiquitous presence of tetanus spores in the soil and gut of many animal species globally, continued efforts are required to maintain MNTE in countries that have successfully achieved MNTE.
These efforts include the following:
- Ensuring adequate TTCV coverage among the majority of pregnant women (≥80%);
Ensuring high coverage with TTCV in infancy and childhood, including booster doses through routine immunization services or school-based programs; - Providing access to skilled health personnel and clean delivery and cord care practices;
- Continued monitoring of MNT cases through sensitive and reliable surveillance systems; identifying high-risk areas and targeting effective interventions when necessary; evaluating and improving upon established programs.
Validation of MNTE
When a country believes it has achieved MNTE, it applies for validation status through the WHO. This process has been standardized across the globe and consists of a complete district level review of the raw data for the previous several years. Representatives from government and non-governmental agencies, including those from the WHO and UNICEF, comprise the validation team and systematically review core and supplemental indicators, including reported NT cases per 1000 live births, to confirm elimination.34 A thorough review of existing surveillance systems is conducted to further ensure high-risk and remote areas are not overlooked.19 Upon completion of the review, one of three outcomes may occur:17
(a) MNT likely eliminated – determines if additional information or surveys are required;
(b) MNT not eliminated – corrective actions are recommended, including strengthening of surveillance systems, conducting additional SIA’s etc.;
(c) MNT likely eliminated – “lot quality assurance” cluster sampling field surveys are recommended in the high-risk districts.
MNTE sustainability
Following validation of a country’s MNTE status, continued efforts are required to strengthen existing strategies and sustain elimination. Program oversight, leading to decreased TTCV coverage or unsafe birthing practices, may result in a resurgence of MNT cases and loss of a country’s MNTE status. Validated countries are encouraged to apply the following four key strategies as recommended by the WHO in order to sustain elimination:13,35
(1) TTCV immunization: strengthening of routine immunization strategies by providing three primary doses of TTCV during infancy and three booster doses at 12–23 months, 4–7 years, and 9–15 years, respectively. This strategy focuses on immunizing the entire population before adolescence instead of only women of reproductive age to ensure long-term protection.
(2) Antenatal screening: verifying and documenting TTCV status of pregnant women during antenatal care and providing adequate doses of TTCV if needed to ensure tetanus protection at birth (PAB). Providing further information and education around safe birthing and umbilical cord care practices.
(3) Clean birthing practices: promoting clean delivery and umbilical cord care practices at a healthcare facility or at home, through increased access to skilled birth attendants (doctors, nurses, and midwives) at all deliveries.
(4) Improved surveillance: maintaining a sensitive NT surveillance, which allows for rapid identification of high-risk areas and implementation of corrective actions.
In addition, WHO recommends that validated countries conduct standalone post-validation assessments every 5 years to assess their MNTE sustainability status.13
Despite achieving sustainability, countries may lose their elimination status is control measures remain sub-optimal. Sub-optimal routine vaccination services, a lack of adequate antenatal care facilities and unclean birthing practices all threaten a countries MNTE status and therefore continued and improved surveillance systems with appropriate and responsive remedial action are needed.
Update on MNTE
The MNTE initiative, launched by the WHO and its partners in 1999, has made significant progress in eliminating maternal and neonatal tetanus globally. At its inception, 59 countries were prioritized for NTE with 47 (80%) of those countries having been validated for achieved MNTE as of December 2020. MNT continues to remain a public health problem in 12 countries across three of the six WHO regions (six in AFR, five in EMR, and one in WPR). The figure below depicts the distribution of these 12 countries.36
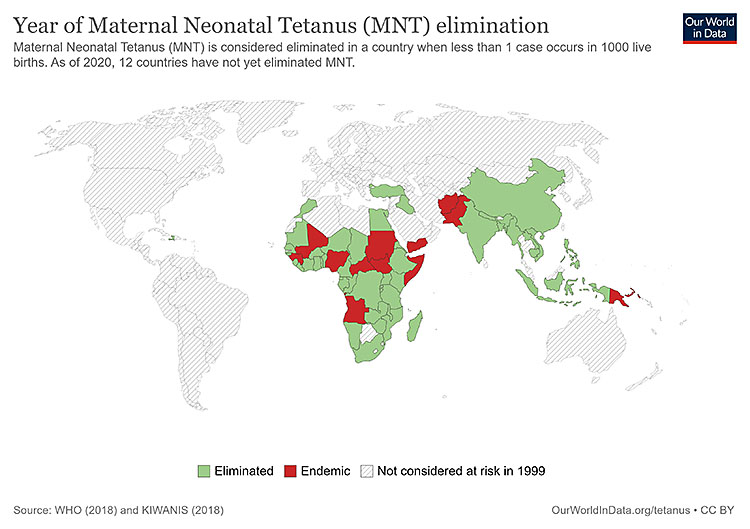
3
Map depicting elimination status of neonatal tetanus (MNT), 2020. The 12 countries where MNT is still a public health problem: Afghanistan, Angola, Central African Republic, Guinea, Mali, Nigeria, Pakistan, Papua New Guinea, Somalia, Sudan, South, Sudan, and Yemen. Our World in Data Maps downloaded from https://ourworldindata.org/tetanus#elimination-of-maternal-and-neonatal-tetanus. Hannah Behrens, Sophie Ochmann, Bernadeta Dadonaite and Max Roser (2019) – "Tetanus". Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.org/tetanus' [Online Resource].
Three of the countries have partially eliminated MNT:
- South East zone of Nigeria (Abia, Anambra, Ebonyi, Enugu, and Imo states) and Southwest zone of Nigeria (Ekiti, Lagos, Ogun, Ondo Osun, and Oyo states) and South-south zone (Akwa Ibom, Bayelsa, Cross River, Edo, and Rivers states);
- Pakistan (Punjab province);
- Mali (Southern Mali comprising of Sikasso, Kayes, Koulikoro, Ségou, Mopti, and Bamako regions).
Successful elimination of MNT in many priority countries can be attributed to a combination of elimination strategies and continued efforts employed by local and national stakeholders. Country-level data through administrative records and vaccination coverage surveys are reported annually to WHO and UNICEF. In 2020, less than one third of the priority countries (16/59) achieved TTCV2+ coverage ≥80%. Despite the majority of countries increasing routine vaccination coverage since 2000, nine countries have failed to maintain previous coverage levels two decades prior.
Reliance on TTCV SIA’s by 52 countries have assisted in increasing the number of children protected at birth from 74% (2000) to 86% (2020) with the majority of countries46 having achieved ≥80% of infants protected at birth in 2020. However, 59 million women targeted for protection by TTCV SIAs remained unreached and a further 16 million had services disrupted due to the Covid-19 pandemic.
The decades following the MNTE initiative also saw a 30% increase in deliveries assisted by a skilled birth attendant from 64% (2000–2006) to 84% (2015–2021).37 The elimination target of ≥70% of SBA-assisted deliveries was achieved by 28 countries (only 50 countries reported data) in 2020.
Through case-based and active surveillance methods, global NT cases decreased by 88% from 17,935 (2000) to 2229 (2020). In 2020, ten (17%) countries reported zero cases. Seven countries, namely Angola, Central African Republic, Chad, Congo, Ethiopia, Madagascar, and Mozambique reported more cases in 2020 than in 2000 but this may be as a result of improved surveillance systems over the years.
The WHO estimated that there were 14,230 neonatal tetanus (NT) deaths worldwide in 2019.38 This represents a 92% reduction in NT-related deaths from 170,829 cases in 2000, displaying significant progress towards global MNTE.38 Further, only 0.4% of all neonatal deaths were attributed to tetanus in 2019 a decrease from 7% in 2000.38 These figures represent a tremendous feat in global collaboration to eliminate MNT. Despite this progress, many countries continue to face challenges in MNTE, particularly amongst the poorest and most marginalized communities, where neonatal mortality remains high. MNT highlights global health inequities and socioeconomic challenges faced by many developing nations. Control measures for MNTE rely on low cost and highly effective measures, which require ongoing efforts to maintain elimination globally. Increased support and innovation is also needed to target the remaining 12 countries.
PRACTICE RECOMMENDATIONS
- Maintain high tetanus-toxoid-containing vaccine (TTCV) coverage (>80%) in childhood (six doses)
- Three primary doses of TTCV in infancy, usually at 6, 10, and 14 weeks of age
- Three booster doses of TTCV during childhood and adolescence at (i) 12–23 months of age, (ii) 4–7 years, and (iii) 9–15 years of age
- Implement and maintain TTCV vaccination coverage of women of child-bearing age and pregnant women
- Two doses given 1 month apart in the first pregnancy
- Third dose given at-least 6 months after second dose
- Then one dose in each subsequent pregnancy (or intervals of at least 1 year), for a total of five doses
- Encourage clean birth practices, cord care, community delivery packs
- Educate healthcare providers, traditional birth attendants, and women of reproductive age about practices that are high risk for tetanus
CONFLICTS OF INTEREST
Author(s) statement awaited.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Duerden BI, Brazier JS. Tetanus and other clostridial diseases. Medicine (Baltimore) 2009;37(12):638–40. | |
George EK, De Jesus O, Vivekanandan R. Clostridium Tetani. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022 [cited 2022 May 31]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK482484/. | |
Hsu SS, Groleau G. Tetanus in the emergency department: A current review. J Emerg Med 2001;20(4):357–65. | |
Chen C, Fu Z, Kim JJP, et al. Gangliosides as High Affinity Receptors for Tetanus Neurotoxin. J Biol Chem 2009;284(39):26569–77. | |
Coffey PS, Brown SC. Umbilical cord-care practices in low- and middle-income countries: a systematic review. BMC Pregnancy Childbirth 2017;17(1):68. | |
Matzkin H, Regev S. Naturally acquired immunity to tetanus toxin in an isolated community. Infect Immun 1985;48(1):267–8. | |
Tetanus vaccines: WHO position paper – February 2017 [Internet] [cited 2022 May 30]. Available from: https://www.who.int/publications-detail-redirect/WER9206. | |
Raza SA, Avan BI. Eliminating Maternal and Neonatal Tetanus and Promoting Clean Delivery Practices Through Disposable Clean Birth Kits. Front Public Health [Internet] 2019 [cited 2022 May 31];7. Available from: https://www.frontiersin.org/article/10.3389/fpubh.2019.00339. | |
Van Driessche A, Janssens B, Coppens Y, et al. Tetanus: A Diagnostic Challenge in the Western World. Acta Clin Belg 2013;68(6):416–20. | |
Apte NM, Karnad DR. Short report: the spatula test: a simple bedside test to diagnose tetanus. Am J Trop Med Hyg 1995;53(4):386–7. | |
Afshar M, Raju M, Ansell D, et al. Narrative review: tetanus-a health threat after natural disasters in developing countries. Ann Intern Med 2011;154(5):329–35. | |
Tetanus Treatment & Management: Approach Considerations, Initial Supportive Therapy and Wound Care, Pharmacologic Therapy, 2021 [cited 2022 May 31]. Available from: https://emedicine.medscape.com/article/229594-treatment#d9. | |
World Health Organization. Protecting all against tetanus: guide to sustaining maternal and neonatal tetanus elimination (MNTE) and broadening tetanus protection for all populations [Internet]. Geneva: World Health Organization, 2019 [cited 2022 May 17]. Available from: https://apps.who.int/iris/handle/10665/329882. | |
Salisbury DM, Martin RM, Van Damme P, et al. 68 – Immunization in Europe. In: Plotkin SA, Orenstein WA, Offit PA. (eds.) Vaccines (Sixth Edition) [Internet]. London: W.B. Saunders, 2013;1334–52 [cited 2022 May 31]. Available from: https://www.sciencedirect.com/science/article/pii/B9781455700905000689. | |
Pennings JLA, Abachin E, Esson R, et al. Regulation of Clostridium tetani Neurotoxin Expression by Culture Conditions. Toxins 2022;14(1):31. | |
World Health Organization. Manual for quality control of diphtheria, tetanus and pertussis vaccines [Internet]. World Health Organization, 2013 [cited 2022 May 30]. Report No.: WHO/IVB/11.11. Available from: https://apps.who.int/iris/handle/10665/80681. | |
Dhir SK, Dewan P, Gupta P. Maternal and Neonatal Tetanus Elimination: Where are We Now? Res Rep Trop Med 2021;12:247–61. | |
The Sustainable Development Goals and Maternal Mortality [Internet]. Maternal Health Task Force, 2017 [cited 2022 May 31]. Available from: https://www.mhtf.org/topics/the-sustainable-development-goals-and-maternal-mortality/. | |
World Health Organization. Neonatal Tetanus: Vaccine Preventable Diseases Surveillance Standards: Updated September 5 2018. [Internet] [cited 2022 May 28]. Available from: https://www.who.int/publications/m/item/vaccine-preventable-diseases-surveillance-standards-neonatal-tetanus. | |
Hernández-Vásquez A, Chacón-Torrico H, Bendezu-Quispe G. Prevalence of home birth among 880,345 women in 67 low- and middle-income countries: A meta-analysis of Demographic and Health Surveys. SSM – Popul Health 2021;16:100955. | |
Imdad A, Bautista RMM, Senen KAA, et al. Umbilical cord antiseptics for preventing sepsis and death among newborns. Cochrane Database Syst Rev [Internet] 2013;(5) [cited 2022 May 28]. Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008635.pub2/full. | |
Blencowe H, Cousens S, Mullany LC, et al. Clean birth and postnatal care practices to reduce neonatal deaths from sepsis and tetanus: a systematic review and Delphi estimation of mortality effect. BMC Public Health 2011;11(Suppl 3):S11. | |
Abortion [Internet] [cited 2022 May 30]. Available from: https://www.who.int/news-room/fact-sheets/detail/abortion. | |
Salami T, Eshiobo I, Emorinken A, et al. The Allure of Abdominal Scarification (`UDE`) among the Esan Tribe in South-South Nigeria – A Prospective Analysis. Int J Trop Dis Health 2020;47:9–19. | |
Emordi VC, Aisien E, Osagie OT, et al. Evisceration following Abdominal Scarification in Neonates. J Trop Pediatr 2018;64(3):237–40. | |
Tchuenkam LW, Ndame EK, Guifo ML, et al. An unusual complication of the traditional treatment of a closed fracture – generalized tetanus: a case report. J Med Case Reports 2017;11:298. | |
Street RA, Smith M, Moshabela M, et al. Traditional health practitioners and sustainable development: a case study in South Africa. Public Health 2018;165:1–5. | |
Plotkin’s Vaccines – 7th Edition [Internet] [cited 2022 May 30]. Available from: https://www.elsevier.com/books/T/A/9780323357616. | |
Behring E von. Ueber das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. 2013;. | |
Schofield FD, Tucker VM, Westbrook GR. Neonatal tetanus in New Guinea. Effect of active immunization in pregnancy. Br Med J 1961;2(5255):785–9. | |
Newell KW, Lehmann AD, Leblanc DR, et al. The use of toxoid for the prevention of tetanus neonatorum. Bull World Health Organ 1966;35(6):863–71. | |
Blencowe H, Lawn J, Vandelaer J, et al. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J Epidemiol 2010;39 Suppl 1:i102–109. | |
Gupta SD, Keyl PM. Effectiveness of prenatal tetanus toxoid immunization against neonatal tetanus in a rural area in India. Pediatr Infect Dis J 1998;17(4):316–21. | |
Chmielewski E. Report of the SAGE Working Group on Maternal and Neonatal Tetanus elimination and broader tetanus prevention. :27. | |
Njuguna HN. Progress Toward Maternal and Neonatal Tetanus Elimination – Worldwide, 2000–2018. MMWR Morb Mortal Wkly Rep [Internet] 2020;69 [cited 2022 May 28]. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6917a2.htm. | |
Progress towards global MNT elimination [Internet] [cited 2022 May 31]. Available from: https://www.who.int/initiatives/maternal-and-neonatal-tetanus-elimination-(mnte)/progress-towards-global-mnt-elimination. | |
Delivery care [Internet]. UNICEF DATA [cited 2022 May 31]. Available from: https://data.unicef.org/topic/maternal-health/delivery-care/. | |
Kanu FA, Yusuf N, Kassogue M, et al. Progress Toward Achieving and Sustaining Maternal and Neonatal Tetanus Elimination – Worldwide, 2000–2020. MMWR Morb Mortal Wkly Rep 2022;71(11):406–11. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
