This chapter should be cited as follows:
Martínez Egea J, Pumarola Brussosa C, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.419133
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 18
Ultrasound in obstetrics
Volume Editors:
Professor Caterina M (Katia) Bilardo, Amsterdam UMC, Amsterdam and University of Groningen, Groningen, The Netherlands
Dr Valentina Tsibizova, PREIS International School, Florence, Italy

Chapter
Doppler in the Management of the Growth-Restricted Fetus
First published: January 2024
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Fetal growth restriction (FGR) is defined as a failure to achieve the endorsed growth potential because of a uteroplacental insufficiency or dysfunction. This pathology represents one of the phenotypes that constitute the heterogeneous population of small-for-gestational-age (SGA) fetuses and should be differentiated from the other SGA phenotypes: constitutional origin and infectious and genetic causes.1
FGR is associated with adverse perinatal outcome (emergency cesarean section, neonatal acidosis, and ICU admission), severe neonatal morbidity and mortality (death and cerebral palsy), poorer neurodevelopment (cognitive and language) and higher incidence of metabolic syndrome.2
Prenatal identification of FGR is clinically relevant because it has proven to be an effective strategy to reduce adverse perinatal outcomes and stillbirth.3,4 Prenatal detection of FGR allows for a more intensive monitoring and planning of the optimal timing of delivery, especially in early gestational age cases where the risk of prematurity must be weighed against the risk of intrauterine death or high neonatal morbidity.
The Doppler study allows assessment of the uteroplacental function and the fetal hemodynamic status. The fetal chronic hypoxia leads to a gradual worsening of the fetus generating a sequence of hemodynamic changes in distinct locations of the fetus, from the initial adaptation to this hostile environment to a progressive fetal surrender. Therefore, as we will see in this chapter, Doppler study is key to identify and classify FGR and to determine the severity and the optimal time of delivery.
DOPPLER EVALUATION OF UTERINE ARTERY (UTA)
Placental insufficiency is a consequence of inadequate trophoblastic invasion of the spiral arteries, leaving a high-resistance circulation and alterations in the villous vascular tree. This high-resistance circulation could be reflected by a lack of physiological transformation of the uterine arteries from high- to low-resistance vessels.5 Therefore, the persistence of high UtA mean pulsatility index (PI) (above the 95th percentile) is associated with placental insufficiency and maternal vascular malperfusion of the placenta.6
Moreover, uterine Doppler has the advantage of reflecting placental insufficiency from the maternal side, so could also capture placental insufficiency secondary to pathophysiological mechanisms other than early defective trophoblastic invasion.7 Abnormal UtA Doppler has been associated with an increased risk of intrapartum fetal distress, emergency cesarean delivery, and admission to intensive care unit.8,9,10
In clinical practice, UtA can be analyzed both quantitatively (PI) and qualitatively (presence/absence of notching). Notching consists in a reduced early diastolic velocity before diastolic velocity increases to its maximum. However, only median PI between both uterine arteries improves the clinical performance and should be analyzed according to gestational-age-related reference ranges.
Technical Aspects of Uterine Artery Doppler Measurement
This measurement can be performed both transvaginally and transabdominally. The transvaginal approach is preferred until the 12th week of gestation and the transabdominal is preferred after the 20th week of gestation, and it is then the approach used for managing purposes.
Transabdominally, the probe should be located longitudinally in the lower lateral quadrant of the abdomen, in parallel with the iliac crest and the uterine wall. A false image of the uterine artery crossing the external iliac artery is visualized with a little medial movement of the probe. The uterine artery should be ideally studied 1–2 cm distally to this point.
The following technical aspects should be taken into consideration:
- The vessel must be first visualized with Doppler color, using high velocity scales (>60 cm/s) for a selective identification of the vessel.
- Zoom should be set appropriately such that the region of concern takes up more than 50% of the screen.
- Insonation angle must be lower than 30°, being optimal when aligned with the direction of blood flow.
- The sample volume of the Doppler should be equal to the vessel diameter and should be located at the center of the vessel.
- Three or more similar waves should be visualized, in an optimal scale of pulse-wave Doppler (PRF). The waveforms should fill at least 75% of the Doppler screen with the basal line in the lower quarter of the axis.
- Doppler horizontal sweep speed should be fast enough to show five to ten waves on the screen.
Figure 1 shows the correct insonation and obtention of the flow velocity wave of uterine artery.
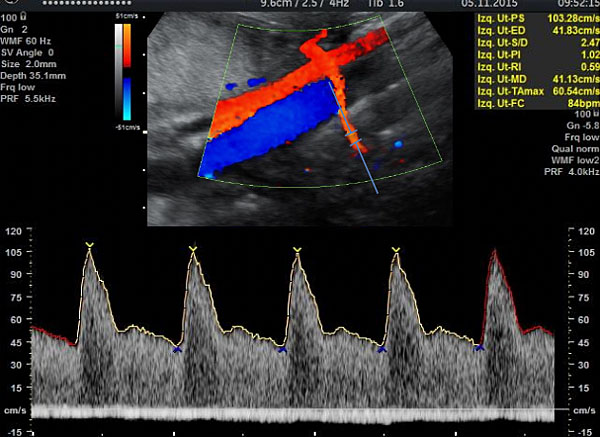
1
Insonation and obtention of the flow velocity wave of uterine artery. Observe the insonation site at 1–2 cm distally to the crossing.
DOPPLER EVALUATION OF UMBILICAL ARTERY (UA)
The progressive reduction of placental surface available for gas and nutrition exchange and the increase in fetal resistance to afterload results in a progressive increase of PI of umbilical artery (UA). As placental insufficiency worsens and reported 1 week before to the acute fetal deterioration,11 end-diastolic flow can become absent and, in more severe cases, reversed.12
Abnormal PI is defined as higher than the 95th percentile according to gestational age. To consider absent or reverse diastolic flow, at least diastolic flow should be absent or reverse, respectively, at some point of the diastole, constantly (more than 50% of the cycles), and persistently (at two different measurements separated by at least 12 h) in both arteries.
Technical aspects of Doppler measurement
The UA can be studied at the fetal insertion, in a free loop or at the placental insertion of the umbilical cord. The less distance to the placenta, the less pulsatility the UA will present. It is recommended to perform the Doppler study in a free cord loop for different reasons: it is an easier technique; most reference curves have been constructed in this site; and all the randomized studies that have validated its utility have been performed at this level.
Technical recommendations are as follows:
- The vessel must be first visualized with Doppler color, using median velocity scales (between 20 to 40 cm/s) for a selective identification of the vessel.
- Zoom should be set appropriately such that the region of concern takes up more than 50% of the screen.
- Insonation angle must be lower than 30°.
- The sample volume of the Doppler should be equal to the vessel diameter and should be located at the center of the vessel.
- The measurement should be recorded in the absence of fetal breathing and body movements since they can simulate an absence of the diastolic flow.
- Three or more similar waves should be visualized, in an optimal scale of pulse-wave Doppler (PRF). The waveforms should fill at least 75% of the Doppler screen with the basal line in the lower quarter of the axis.
- Doppler horizontal sweep speed should be fast enough to show five to ten waves on the screen.
- At the beginning of the second trimester, 30% of fetuses can present more than 20% differences in the PI of both umbilical arteries; becoming lower as the pregnancy progresses. Both arteries should be assessed in case of abnormal pulsatility, and the best measurement should be used.
- The study should be performed three times and the better measurement (less pulsatile) should be chosen.
Figure 2 shows the correct insonation and obtention of the flow velocity wave of UA and Figure 3 shows the progressive changes of the flow velocity wave of UA when placental insufficiency occurs.
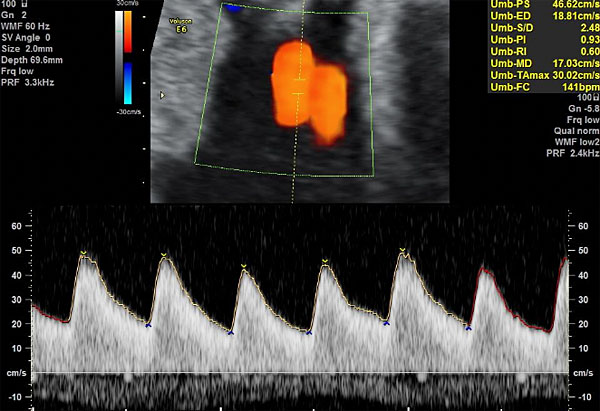
2
Insonation and obtention of the flow velocity wave of umbilical artery. Observe the insonation angle closer to 0°, the optimization of the color scale to avoid aliasing and the adjustment of the velocity scale aiming that the wave fills 75% of the Doppler screen.
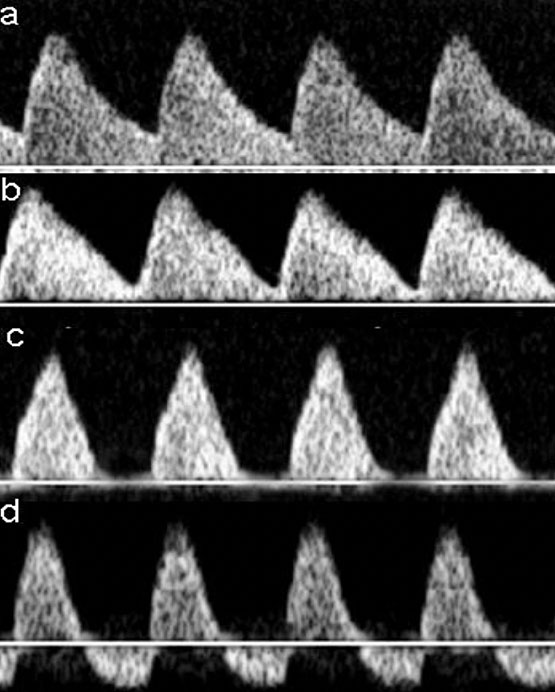
3
Progressive changes of the flow velocity wave of umbilical artery when placental insufficiency occurs. (a) Normal diastolic flow; (b) decreased but positive diastolic flow; (c) absent diastolic flow; (d) reverse diastolic flow.
DOPPLER EVALUATION OF MIDDLE CEREBRAL ARTERY (MCA)
The middle cerebral artery (MCA) informs about the existence of cerebral vasodilation, a surrogate marker of hypoxia. The fetus responses physiologically to hypoxia with a redistribution of cardiac output preferentially to the coronary arteries, brain, and adrenal glands.13 Decreased MCA-PI is associated with adverse perinatal and neurological outcomes.14,15
To evaluate cerebral vasodilation, PI should be assessed. Cerebral vasodilation is considered when the MCA-PI is persistently lower than the fifth percentile according to the gestational age (in two determinations 12 h apart).
The cerebro-placental ratio (CPR), which is calculated as MCA-PI/UA-PI, is essentially a diagnostic index. Arterial redistribution of the brain perfusion should be considered when CPR is persistently lower than the fifth percentile according to the gestational age (in two determinations 12 h apart). This index provides greater sensitivity for detecting fetuses at risk of poor outcomes than either UA or MCA Doppler alone. This is because placental impedance (represented by UA) is often combined with reduced cerebral resistance (MCA). Therefore, CPR decreases when one of its components undergoes small changes within normality.16 Recently, the longitudinal changes of CPR between 37 and 40 weeks have also been associated with the need for cesarean section for non-reassuring fetal status during labor in low-risk pregnancies.17
Technical aspects of Doppler measurement
The MCA should be identified in an axial section of the brain at the level of the circle of Willis, viewing its trajectory from its origin in the internal carotid artery to its end next to the parietal bone. The more distal from the circle, the more pulsatility the vessel will present. In addition, the vessel is divided distally in two to four branches. Therefore, the assessment should be performed at the proximal portion near to the division from internal carotid artery.
The following technical aspects should be taken into consideration:
- The vessel must be first visualized with Doppler color, using median velocity scales (between 20 to 40 cm/s) for a selective identification of the vessel.
- Zoom should be set appropriately such that the region of concern takes up more than 50% of the screen.
- Insonation angle must be lower than 15°, and as closer to 0°.
- The sample volume of the Doppler should be equal to the vessel diameter and should be located at the center of the vessel.
- The measurement should be recorded in the absence of fetal breathing and body movements. Interference in the waveform may occur in bradycardia or tachycardia and the IP would be poorly measurable.
- Excessive pression to the fetal head must be avoided since it can increase the pulsatility index (PI) and decrease the peak systolic value (PSV).
- Three or more similar waves should be visualized, in an optimal scale of pulse-wave Doppler (PRF). The waveforms should fill at least 75% of the Doppler screen with the basal line in the lower quarter of the axis.
- Doppler horizontal sweep speed should be fast enough to show five to ten waves on the screen.
- The study should be performed three times and the better measurement (more pulsatile) should be chosen.
Figure 4 shows the correct insonation and obtention of the flow velocity wave of MCA and Figure 5 shows the progressive changes of the flow velocity wave of MCA when hypoxia occurs.
4
Insonation and obtention of the flow velocity wave of the middle cerebral artery. Observe the zoom, the insonation angle closer to 0° in the most proximal site next to the circle of Willis and the adjustment of the velocity scale aiming that the wave fills 75% of the Doppler screen.
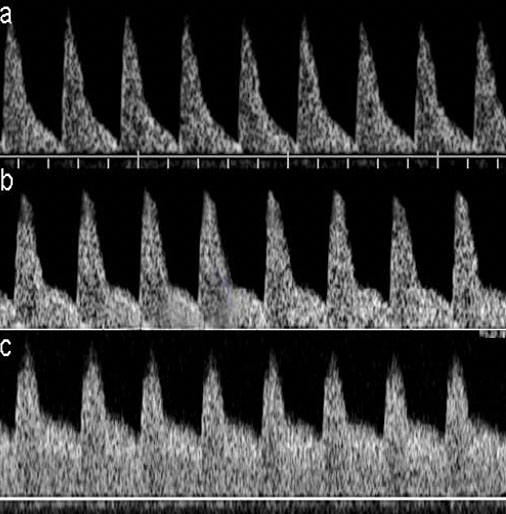
5
Progressive changes in the flow velocity wave of the middle cerebral artery when hypoxia. (a) Normal flow velocity wave with high resistance. (b) Moderate increase of diastolic velocities with a moderate decrease of pulsatility. (c) Marked increase of diastolic velocities with a marked decrease of pulsatility.
DOPPLER EVALUATION OF DUCTUS VENOSUS (DV)
The ductus venosus (DV) is the strongest single Doppler parameter to predict the short-term risk of fetal death in early-onset FGR.
The DV is evaluated using the pulsatility index (PI) and the presence or absence of flow during the atrial contraction (a-wave). The DV is defined as pulsatile when the PI is higher than the 95th percentile according to the gestational age. The finding of absent-reversed a-wave must be constant (more than 50% of the cycles) and persistent (in two occasions separated by more than 12 h).
To increase the blood flow toward the heart and compensate an extreme hypoxia, a progressive dilatation of the DV occurs and it is reflected as alteration in the DV flow velocity waveform, especially with absent or reversed a-wave.18 Absent or reversed a-wave in the ductus venosus could also be a consequence of increased intra-atrial pressure due to high cardiac afterload due to an increased vascular placental resistance and/or a direct effect of fetal acidemia on myocardial cell function.19
Technical aspects of Doppler measurement
The study of DV can be performed at a midsagittal view or in an oblique-transverse of an abdominal fetal section.
The ductus venosus starts from the umbilical vein and it is easily identified by Doppler color because it presents high velocities. It is essential to differentiate DV from the hepatic veins because the waveform of these veins can simulate a pathologic DV. For the differentiation of these two vessels, it is important to have a good knowledge of the fetal anatomy and consider that hepatic veins present lower velocities than DV.
The technical recommendations are as follows:
- The vessel must be first visualized with Doppler color using high-velocity scales (40–60 cm/s).
- Zoom should be set appropriately such that the region of concern takes up more than 50% of the screen.
- Insonation angle should be lower than 30°, which is easier to achieve with transverse view.
- The sample volume should be reduced to avoid the possible contamination produced by adjacent vessels. The sample volume must be in the most proximal point of the exit of the umbilical vein, which is the point that presents the highest velocities.
- The measurement should be recorded in the absence of fetal breathing and maternal movements.
- Three or more similar waves should be visualized, in an optimal scale of pulse-wave Doppler (PRF). The waveforms should fill at least 75% of the Doppler screen with the basal line in the lower quarter of the axis.
- Doppler horizontal sweep speed should be fast enough to show five to ten waves on the screen.
- The study should be performed three times (ideally in different planes) and the better measurement (less pulsatile) should be chosen.
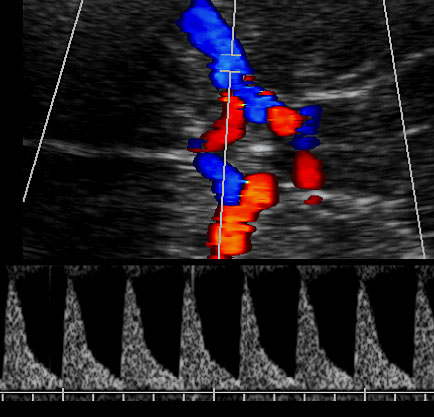
Figures 6 and 7 show the color Doppler imaging of the ductus venosus in transverse view and the correct insonation and obtention of the flow velocity wave of DV, respectively. Figure 8 shows the progressive changes of the flow velocity wave of DV when acidosis occurs.
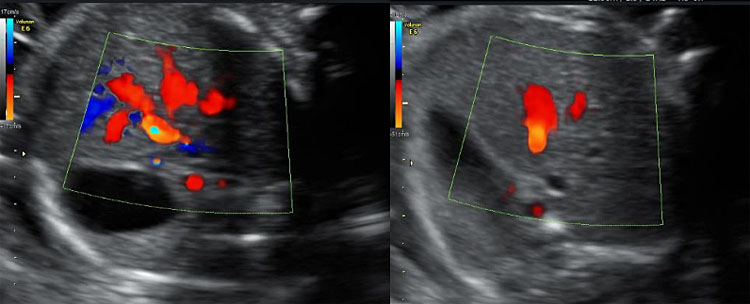
6
Color Doppler imaging of the ductus venosus in transverse view. Observe that adjusting velocities at a high range selectively marks the ductus and allows identification of the optimal point of insonation, which corresponds with the highest velocity zone.
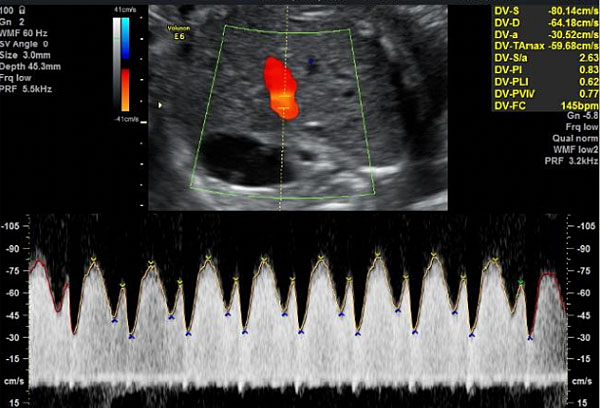
7
Insonation and obtention of the flow velocity wave of ductus venosus. Observe the insonation site, which corresponds with the highest velocity identified by the aliasing.
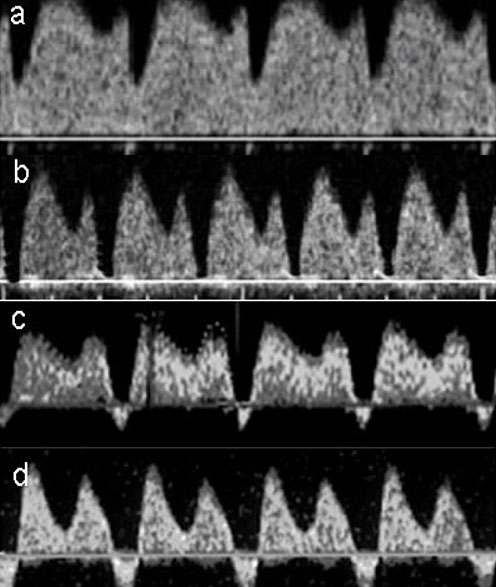
8
Progressive changes in the flow velocity wave of ductus venosus in a condition of acidosis. (a) Normal flow velocity wave. (b) Absent flow during atrial contraction. (c) Moderate reverse flow during atrial contraction. (d) Marked reverse flow during atrial contraction.
USE OF DOPPLER FOR THE CLASSIFICATION OF FGR
Distinction between fetal growth restriction (FGR) and small for gestational age (SGA)
SGA is considered when estimated fetal weight (EFW) or abdominal circumference (AC) is below the 10th percentile in the reference growth curves.20
Within this definition there are several phenotypes:
The smallest group are pathological fetuses, which includes those fetuses with congenital malformations (including chromosomopathies) or infections. These should be suspected in early and severe diagnosis and when other markers or malformations are present. Infection (cytomegalovirus)21 and genetic studies are recommended in these cases. A genetic array (based either on CGH or SNPs) has a 3–10% of incremental yield over conventional karyotyping.22
Without suspicion of structural anomaly, two other groups of small fetuses should be differentiated: the true cases of FGR, that do not achieve their endowed growth potential mainly due to placental insufficiency; and the SGA fetuses with a constitutional origin, the largest group.
Because FGR fetuses are at increased risk of intrauterine fetal impairment, fetal death, and overall worse perinatal outcome compared to normally growing fetuses and SGA, it is important to identify them.
In general, FGR is associated with Doppler signs suggestive of hemodynamic redistribution reflecting the fetal adaptation to increased placental resistance and hypoxia. However, in many cases, especially those with late presentation (defined as beyond 32 weeks), these differences may be more subtle.
There is extensive evidence that the UA Doppler accurately reflects placental insufficiency that appears early in gestation. With a 29% decrease in perinatal deaths, there is compelling evidence that using UA Doppler in high-risk pregnancies improves perinatal outcomes.23 Reversed end-diastolic flow in the UA is associated to adverse perinatal outcome with a sensitivity and specificity of about 60%, independently of prematurity.24 After 30 weeks, the risks of preterm outweigh the risk of stillbirth in a fetus with isolated reversed end-diastolic velocities in the UA Doppler.25,26,27
According to the 2016 Delphi expert consensus,27 before 32 weeks' gestation, UA with absent or reversed diastolic flow is by itself diagnostic of FGR as is pulsatility (PI-UA above the 95th percentile) associated with fetal growth defect (EFW or AC below the 10th percentile). These criteria have been reaffirmed in recent guidelines.5
In late onset FGR the assessment of UA is less useful, as this parameter is not altered in most cases. Less than 3% of late FGR fetuses had an altered UA Doppler in a longitudinal study of 171 fetuses.30 In a recent observational study with more than 1,000 late-onset small fetuses, altered UA Doppler was found in 6.7% of cases at diagnosis and of those fetuses that reached 37 weeks, only 5.3% had pathological UA.31
MCA is particularly valuable for the identification and prediction of adverse outcomes among late-onset FGR.14,29,32 MCA vasodilatation occurs in 15–20% of small fetuses at term with normal UA Doppler. This sign is associated with a worse perinatal outcome and poorer neurodevelopment in the short and medium term.8,32 Fetuses with abnormal MCA-PI had a six-fold risk of emergency cesarean section for fetal distress when compared with SGA fetuses with normal MCA-PI,34 which is particularly relevant because labor induction at term is the current standard of care of late-onset FGR.15,35 Late-FGR with abnormal MCA-PI have poorer neurobehavioral competence at birth and at two years of age.14,15 MCA is considered a rather late manifestation, with acceptable specificity but low sensitivity, which is improved using CPR.
CPR is more sensitive to hypoxia than its individual components.36 In late-SGA fetuses, abnormal CPR is present before delivery in 20–25% of the cases,37 and it is associated with a higher risk of adverse outcome at induction.34 Recently, Meler et al.31 observed impaired CPR in 22.3% of fetuses at high risk of adverse perinatal outcomes compared to 8.3% in the case of impaired MCA. The literature reports sensitivity and specificity of up to 93 and 76%, respectively, in predicting perinatal death in small fetuses38 and compared to alteration of other Doppler parameters, CPR is the one most associated with emergency cesarean section due to an altered cardiotocographic recording.39 A significant deterioration of this parameter has also been reported between 37 weeks and the last determination before delivery,30 with 1.5–2 times more CPR and MCA values altered before delivery. These findings suggest that these Doppler parameters may better capture placental deterioration and it is for this reason that it is used to identify the FGR fetus when we find a low EFW above 32 weeks.
The role of UtA for the diagnosis of FGR is controversial. The most recent guidelines consider uterine Doppler as one of the diagnostic criteria for early FGR but not for late FGR.5,28 However, observational studies have shown an association between late-onset FGR and a higher frequency of placental signs such as maternal malperfusion,7,29 placental aging,40 and an increased risk of perinatal adverse events independent of fetal weight and cerebral Doppler.33,41,42 Increased risk of hemodynamic redistribution and adverse perinatal events has also been demonstrated in late FGR fetuses with impaired Doppler of the UtA at diagnosis compared to those with normal UtA at diagnosis.33 Compared with CPR, a similar prediction of adverse perinatal events (neonatal ICU admission, emergency cesarean section, neonatal acidosis and perinatal death) has also recently been shown.43 Therefore, more studies are needed to assess more objectively the role of uterine artery Doppler in the low weight fetus.
Finally, regardless of Doppler, an isolated EFW or AC below the third percentile is also established as a diagnostic criterion for FGR at any gestational age.5,28 EFW below the third percentile has a much higher risk of adverse perinatal outcomes (including intrauterine death), independent of Doppler parameters.44,45 Furthermore, after 2 years of follow-up, the DIGITAT study concluded that severe growth restriction (defined by an EFW below the 2.3 percentile) was, together with neonatal admission, the most important predictor of neurodevelopmental impairment at 2 years of age.46
Distinction between early-onset FGR and late-onset FGR
Two clinical patterns of intrauterine growth restriction are well-described, which are differentiated primarily by the gestational age of onset: early-onset FGR and late-onset FGR. Although they share as common pathogenesis the existence of placental insufficiency, both clinical forms present differences in several aspects: the pattern of alteration of the Doppler study, the speed of worsening, the degree of severity and perinatal results, and the association with hypertensive states, among others.5,47,48 Table 1 summarizes the main differences between early-onset and late-onset FGR.
1
Summary of the main differences between early and late-onset FGR.
Early-onset FGR | Late-onset FGR |
Prevalence: ~1% | Prevalence: 3–5% |
Severe placental disease: UA Doppler abnormal, high association with PE | Mild placental disease: UA Doppler normal, low association with PE |
Severe hypoxia ++: systemic CV adaptation | Mild hypoxia: central CV adaptation |
High mortality and morbidity. | Lower mortality (but common cause of late stillbirth). |
FGR, fetal growth restriction; PE, pre-eclampsia; CV, cardiovascular.
Although there is still no clear consensus on the optimal cut-off point, gestational age of onset is the variable most used to differentiate the two clinical forms. In the 2016 Delphi expert consensus,28 89% of the participants agreed to define the cut-off point at week 32. Savchev et al.49 also observed that the differences between the two patterns were magnified at 32 weeks, both in terms of perinatal mortality, adverse perinatal outcomes and association with pre-eclampsia. 32 weeks is also the cut-off point recommended by the ISUOG guideline.5
However, in clinical practice, there is significant overlap between the two forms of FGR with a continuous spectrum in the transition from one clinical form to the other. Savchev et al.,49 in their evaluation of the cut-off point between early and late onset of FGR, showed that Doppler, specifically the UA Doppler discriminated better than gestational age two groups with different timing, natural history, and rate of adverse outcome.
In cases of early-onset FGR, a large proportion of the placenta is affected, and the fetus suffers from chronic hypoxia frequently reflected by an alteration of the UA doppler.50 In early stages the MCA and the Doppler of the UtA (in more than 60% of cases) may be affected. An increased PI-UA represents typically the first of the Doppler changes in the cascade of fetal impairment that occurs as the hypoxic condition worsens. These alterations may be present weeks before severe cardiovascular and metabolic deterioration occurs. As placental function progressively deteriorates, the diastolic flow of the UA becomes absent. The severe placental insufficiency is reflected by reverse diastolic flow in the UA and fetal cardiovascular and metabolic critical impairment by alterations in the ductus venosus (absent or reversed a-wave).51,52
DV pulsatility index and short-term variation (STV) of fetal heart rate become abnormal only in advanced stages of fetal compromise.11,24,47,51 Absent or reversed a-wave is associated with perinatal mortality independently of the gestational age at delivery53 with a risk ranging from 40 to 100% in early-onset FGR.25,51 In about 50% of cases abnormal DV precedes the loss of STV in computerized CTG,47 and in about 90% of cases it is abnormal 48–72 h before the biophysical profile.51 Thus, this sign is normally considered sufficient to recommend delivery at any gestational age, after completion of steroids. Hence, it is considered to provide a better window of opportunity for delivering fetuses in critical conditions at very early gestational ages.
This pattern of alterations allows us to monitor the fetal deterioration and to decide electively the best time for delivery. Regardless, this sequence is relatively constant, the time to deterioration is affected by the severity of placental involvement and severe pre-eclampsia can distort the natural history.
Figure 9 represents in a schematic and simplified way the pathophysiological progression with the main adaptation/consequence on placental-fetal physiology, and the cascade of changes in Doppler parameters.
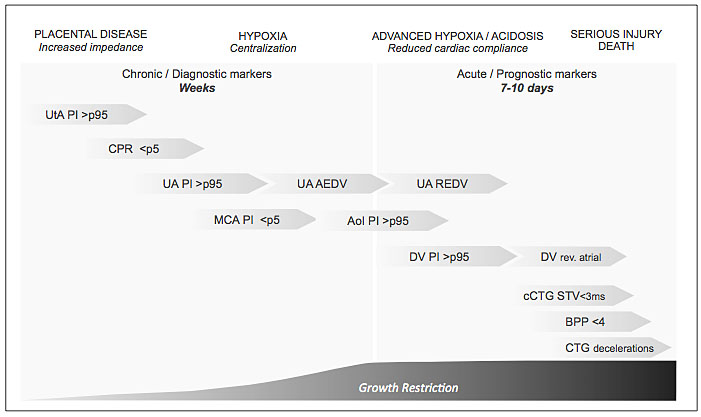
9
Fetal impairment and early FGR monitoring. UtA, uterine artery; PI, pulsatility index; CPR, cerebroplacental ratio; AU, umbilical artery; AEDV, absent end-diastolic velocity; REDF, reverse end-diastolic flow; MCA, middle cerebral artery; AoI, aortic isthmus; DV, ductus venosus; cCTG, computed cardiotocography; STV, short-term variability; BPP, biophysical profile; CTG, cardiotocography.
In the case of late FGR, the alteration of UA Doppler above the 95th percentile is infrequent because placental involvement is milder. So, it is not a good prognostic marker. In contrast, in 15–20% of late FGR, cerebral vasodilatation (MCA-PI <5th percentile) is observed, suggesting chronic hypoxia.30,54 However, the first hemodynamic parameter that is usually affected is CPR, which can identify subtle changes between placental and cerebral blood-flow perfusion that may not be appreciated by evaluation of UA Doppler or MCA separately.30
Although most cases persist with altered and constant CPR over time and not progress to severe hypoxia, both abnormal MCA and CPR correlate with adverse outcomes in late FGR, increasing the risk of loss of fetal well-being and emergency cesarean section.54,55 A higher risk of rapid fetal deterioration has been observed above 37 weeks, possibly due to a combination of increased susceptibility to hypoxia of the mature fetuses at term and the more frequent presence of uterine contractions in late gestation.
Figure 10 shows schematically the pathophysiological progression of late-onset FGR fetuses.
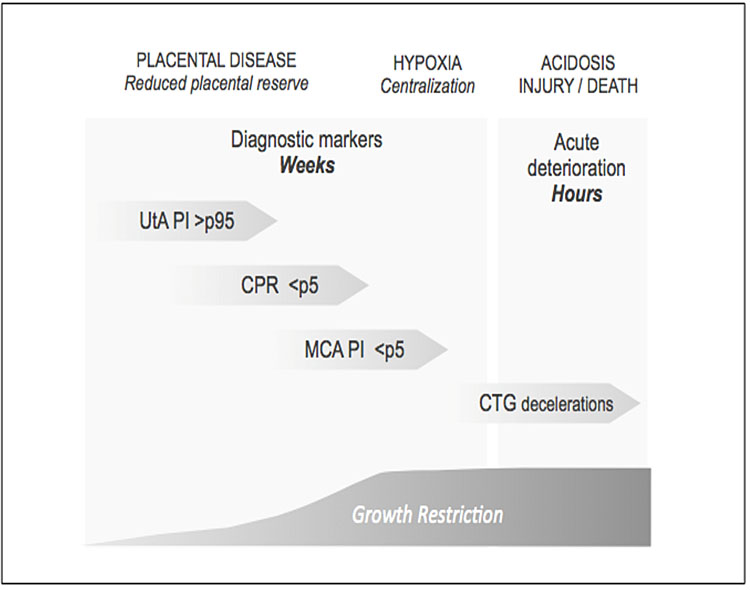
10
Fetal impairment and late FGR monitoring. UtA, uterine artery; PI, pulsatility index; CPR, cerebroplacental ratio; MCA, middle cerebral artery; DV, ductus venosus; CTG, cardiotocography.
Based on the evidence, the Delphi consensus28 established the differences described in Table 2 to classify early and late onset FGR. This classification is also supported by the most recent guidelines.5
2
Consensus-based definitions for early and late FGR.
Early-onset FGR | Late-onset FGR |
EFW/AC <third percentile or absent/reverse diastolic flow on UA, or:
| EFW/AC <third percentile, or two of the following three:
|
FGR, fetal growth restriction; EFW, estimated fetal weight; AC, abdominal circumference; UA, umbilical artery; mUtA, mean uterine artery; CPR, cerebro-placental ratio; PI, pulsatility index.
USE OF DOPPLER FOR THE MANAGEMENT OF FGR
As described below, there are no differences in the clinical management between early and late-onset FGR.
Evidence has shown that classifying exclusively based on UA is not sufficient to select those fetuses with adverse outcomes. If we include UtA Doppler, CPR and EFW below the third percentile, we can identify up to 40% of fetuses at risk of poor outcome if any of them are altered, compared to 11% if all of them are normal, and only 8% in those fetuses with normal weight.
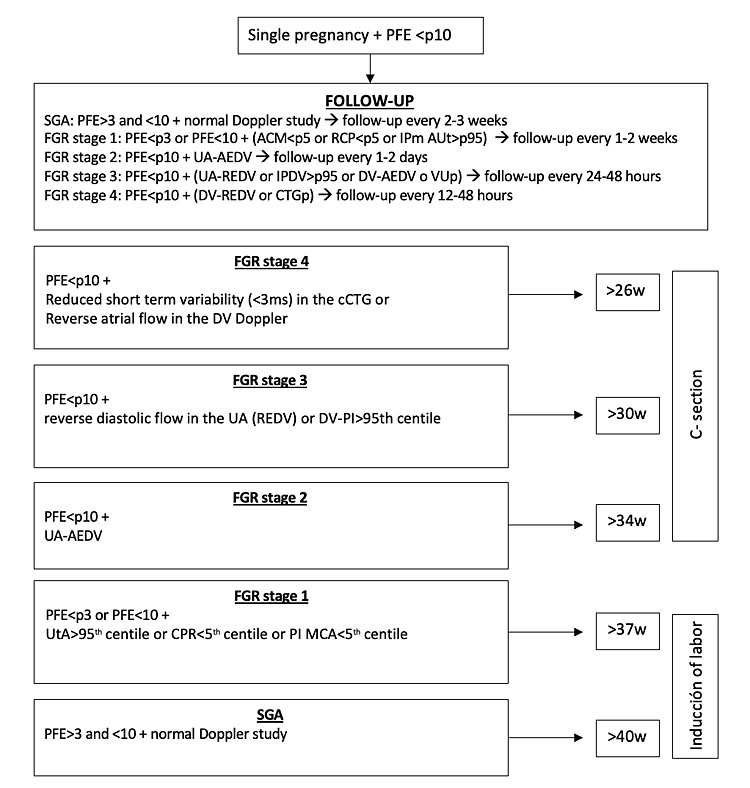
In a first step, when a small fetus is identified (EFW <percentile 10), a Doppler should be assessed (mUtA-PI, UA-PI, MCA-PI and CPR) to classify the fetus as SGA or FGR. In case of FGR fetuses, changes in UA Doppler, DV and CTG are used to define the stage of deterioration.
SGA: Defined as EFW in the third percentile or above and under tenth percentile, with normal Doppler. Fortnightly Doppler and growth assessment is safe and constitutes standard practice. Labor induction should be recommended at 40 weeks.56
Stage I FGR [severe smallness or mild placental insufficiency]: Defined as EFW below the third percentile or between third and tenth percentile with at least one of the following: UtA-PI >95th percentile or CPR or MCA–PI <fifth percentile. Available evidence suggests a low risk of fetal deterioration before term. Labor induction beyond 37 weeks is acceptable, but the risk of intrapartum fetal distress is increased.34 If MCA – PI <fifth percentile emergency cesarean section rate is 50%. Monitoring in these cases can be weekly.
Stage II FGR [severe placental insufficiency]: This stage is defined by absent end-diastolic flow in UA. Delivery should be recommended after 34 weeks, prior lung maturation with corticosteroids. The risk of emergent cesarean section at labor induction exceeds 50%, and, therefore, elective cesarean section is a reasonable option. Monitoring twice a week is recommended.
Stage III FGR [advanced fetal deterioration, low-suspicion signs of fetal acidosis]: The stage is defined by reverse diastolic flow in the UA or DV-PI>95th percentile. There is an association with a higher risk of stillbirth and poorer neurological outcome. However, since signs suggesting a very high risk of stillbirth within days are not present yet, it seems reasonable to delay elective delivery to reduce as much as possible the effects of severe prematurity. We suggest delivery should be recommended by cesarean section after 30 weeks, prior lung maturation with corticosteroids and under prophylaxis of cerebral palsy with magnesium sulphate. Monitoring every 24–48 h is recommended.
Stage IV FGR [high suspicion of fetal acidosis and high risk of fetal death]: There are spontaneous fetal heart rate decelerations, reduced short-term variability (<3 ms) in the computerized CTG, or reverse atrial flow in the DV Doppler. Delivery is recommended after 26 weeks by cesarean section at a tertiary-care center under steroid treatment for lung maturation and prophylaxis of cerebral palsy with magnesium sulphate. Intact survival exceeds 50% only after 26–28 weeks, and before this threshold parents should be counseled by multidisciplinary teams. Monitoring every 12–24 h is recommended.
Particularly at early gestational ages and at any stage in which severe pre-eclampsia coexists, the natural history of fetal deterioration may be precipitated, so stricter fetal monitoring is warranted (increasing follow-up to the next stage of severity).
Stage-based decision algorithm for the management of FGR and the recommendation of ending the gestation is illustrated in Figure 11.
11
Stage-based decision algorithm for the management and the determination of the time of delivery in FGR.
PRACTICE RECOMMENDATIONS
- To correctly assess the fetal-placental Doppler it is essential to take into account the technical aspects: identify the vessel in the correct view, using color Doppler with a correct velocity scale according to the vessel; adjust the zoom so that the region of concern takes up more than 50% of the screen; apply an insonation angle as close to zero as possible; adjust the sample volume to the size of the vessel; adjust the PRF so that the waves take up 75% of the screen and the sweep speed to show five to ten waves.
- In the assessment of UA, MCA and DV, three measurements should be made (in the case of DV in different views, if possible) and the best value should be taken.
- Although early-onset FGR and late-onset FGR differ in severity, speed of worsening, perinatal results and Doppler alteration pattern, the clinical management should be the same for both forms.
- FGR fetuses are at increased risk of worse perinatal outcome and the Doppler assessment of UtA, UA, MCA, and CPR is recommended when EFW/AC is below the tenth percentile to classify the fetus as SGA or FGR.
- Doppler changes in UA Doppler, DV, and CTG should be used to define the stage of fetal deterioration and to decide electively the best time for delivery, when the risk of prematurity is lower than the risk of intrauterine death or high neonatal morbidity.
- In stage I delivery at term (>37 weeks) is acceptable. If MCA-PI is below the fifth percentile, the 50% risk of emergency cesarean section should be considered. Stage II is defined by absent end-diastolic flow in UA, indicator of impaired placental function and elective cesarean section is recommended at 34 weeks of pregnancy. In stage III either the end-diastolic flow of UA is reversed, or DV-PI is above 95th percentile, reflecting that placental function is severely impaired and the first signs of fetal acidosis. Elective cesarean section is recommended at 30 weeks of pregnancy. In stage IV a-wave of DV can be absent or reversed or pathologic CTG can occur. A high risk of fetal death exists since delivery is recommended after 26 weeks by cesarean section.
- If delivery is needed, lung maturation with steroids is recommended below 35 weeks of pregnancy and prophylaxis of cerebral palsy with magnesium sulphate is recommended below 33 weeks of pregnancy.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Darendeliler F. IUGR: Genetic influences, metabolic problems, environmental associations/triggers, current and future management. Best Pract Res Clin Endocrinol Metab 2019;33(3):101260. doi: 10.1016/j.beem.2019.01.001. Epub 2019 Jan 22. PMID: 30709755. | |
Sacchi C, Marino C, Nosarti C, et al. Association of Intrauterine Growth Restriction and Small for Gestational Age Status With Childhood Cognitive Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatr 2020;174(8):772–81. doi: 10.1001/jamapediatrics.2020.1097. PMID: 32453414; PMCID: PMC7251506. | |
Lindqvist PG, Molin J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol 2005;25(3):258–64. doi: 10.1002/uog.1806. PMID: 15717289. | |
Gardosi J, Madurasinghe V, Williams M, et al. Maternal and fetal risk factors for stillbirth: population based study. BMJ 2013;346:f108. doi: 10.1136/bmj.f108. PMID: 23349424; PMCID: PMC3554866. | |
Lees CC, Stampalija T, Baschat A, et al. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol 2020;56(2):298–312. doi: 10.1002/uog.22134. PMID: 32738107. | |
Levytska K, Higgins M, Keating S, et al. Placental Pathology in Relation to Uterine Artery Doppler Findings in Pregnancies with Severe Intrauterine Growth Restriction and Abnormal Umbilical Artery Doppler Changes. Am J Perinatol 2017;34(5):451–7. doi: 10.1055/s-0036-1592347. Epub 2016 Sep 20. PMID: 27649292. | |
Llurba E, Turan O, Kasdaglis T, et al. Emergence of late-onset placental dysfunction: relationship to the change in uterine artery blood flow resistance between the first and third trimesters. Am J Perinatol 2013;30(6):505–12. doi: 10.1055/s-0032-1329181. Epub 2012 Dec 19. PMID: 23254384. | |
Severi FM, Bocchi C, Visentin A, et al. Uterine and fetal cerebral Doppler predict the outcome of third-trimester small-for-gestational age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 2002;19(3):225–8. doi: 10.1046/j.1469-0705.2002.00652.x. PMID: 11896941. | |
Ghosh GS, Gudmundsson S. Uterine and umbilical artery Doppler are comparable in predicting perinatal outcome of growth-restricted fetuses. BJOG 2009;116(3):424–30. doi: 10.1111/j.1471-0528.2008.02057.x. PMID: 19187375. | |
Vergani P, Roncaglia N, Andreotti C, et al. Prognostic value of uterine artery Doppler velocimetry in growth-restricted fetuses delivered near term. Am J Obstet Gynecol 2002;187(4):932–6. doi: 10.1067/mob.2002.127137. PMID: 12388980. | |
Ferrazzi E, Bozzo M, Rigano S, et al. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet Gynecol 2002;19(2):140–6. doi: 10.1046/j.0960-7692.2002.00627.x. PMID: 11876805. | |
Burton GJ, Woods AW, Jauniaux E, et al. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009;30(6):473–82. doi: 10.1016/j.placenta.2009.02.009. Epub 2009 Apr 17. PMID: 19375795; PMCID: PMC2697319. | |
Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol A Mol Integr Physiol 1998;119(3):717–23. doi: 10.1016/s1095-6433(98)01010-1. PMID: 9683411. | |
Eixarch E, Meler E, Iraola A, et al. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol 2008;32(7):894–9. doi: 10.1002/uog.6249. PMID: 19035538. | |
Oros D, Figueras F, Cruz-Martinez R, et al. Middle versus anterior cerebral artery Doppler for the prediction of perinatal outcome and neonatal neurobehavior in term small-for-gestational-age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 2010;35(4):456–61. doi: 10.1002/uog.7588. PMID: 20178115. | |
Gramellini D, Folli MC, Raboni S, et al. Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol 1992;79(3):416–20. doi: 10.1097/00006250-199203000-00018. PMID: 1738525. | |
Cancemi A, Rial-Crestelo M, Martinez J, et al. Longitudinal change in cerebro-placental ratio (CPR) between 37 and 40 weeks of pregnancy is associated with non-reassuring fetal status and increased risk of cesarean section. J Matern Fetal Neonatal Med 2023;36(1):2191776. doi: 10.1080/14767058.2023.2191776. PMID: 36948221. | |
Kiserud T, Kessler J, Ebbing C, et al. Ductus venosus shunting in growth-restricted fetuses and the effect of umbilical circulatory compromise. Ultrasound Obstet Gynecol 2006;28:143–9. | |
Ferrazzi E, Lees C, Acharya G. The controversial role of the ductus venosus in hypoxic human fetuses. Acta Obstet Gynecol Scand 2019;98:823–9. | |
Lees CC, Stampalija T, Baschat A, et al. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol 2020;56(2):298–312. doi: 10.1002/uog.22134. PMID: 32738107. | |
Yamamoto R, Ishii K, Shimada M, et al. Significance of maternal screening for toxoplasmosis, rubella, cytomegalovirus and herpes simplex virus infection in cases of fetal growth restriction. J Obstet Gynaecol Res 2013;39(3):653–7. doi: 10.1111/j.1447-0756.2012.02012.x. Epub 2012 Oct 29. PMID: 23107457. | |
Borrell A, Grande M, Pauta M, et al. Chromosomal Microarray Analysis in Fetuses with Growth Restriction and Normal Karyotype: A Systematic Review and Meta-Analysis. Fetal Diagn Ther 2018;44(1):1–9. doi: 10.1159/000479506. Epub 2017 Sep 9. PMID: 28889126. | |
Alfirevic Z, Stampalija T, Dowswell T. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev 2017;6(6):CD007529. doi: 10.1002/14651858.CD007529.pub4. PMID: 28613398; PMCID: PMC6481396. | |
Cosmi E, Ambrosini G, D'Antona D, et al. Doppler, cardiotocography, and biophysical profile changes in growth-restricted fetuses. Obstet Gynecol 2005;106(6):1240–5. doi: 10.1097/01.AOG.0000187540.37795.3a. PMID: 16319247. | |
Cruz-Lemini M, Crispi F, Van Mieghem T, et al. Risk of perinatal death in early-onset intrauterine growth restriction according to gestational age and cardiovascular Doppler indices: a multicenter study. Fetal Diagn Ther 2012;32(1–2):116–22. doi: 10.1159/000333001. Epub 2012 Jul 6. PMID: 22777088. | |
GRIT Study Group. A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG 2003;110(1):27–32. doi: 10.1016/s1470-0328(02)02514-4. PMID: 12504932. | |
Thornton JG, Hornbuckle J, Vail A, et al. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 2004;364(9433):513–20. doi: 10.1016/S0140-6736(04)16809-8. PMID: 15302194. | |
Gordijn SJ, Beune IM, Thilaganathan B, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 2016;48:333–9. | |
Parra-Saavedra M, Crovetto F, Triunfo S, et al. Association of Doppler parameters with placental signs of underperfusion in late-onset small-for-gestational-age pregnancies. Ultrasound Obstet Gynecol 2014;44(3):330–7. doi: 10.1002/uog.13358. Epub 2014 Aug 7. PMID: 24615982. | |
Oros D, Figueras F, Cruz-Martínez R, et al. Longitudinal changes in uterine, umbilical and fetal cerebral Doppler indices in late-onset small-for-gestational age fetuses. Ultrasound Obstet Gynecol 2011;37(2):191–5. doi: 10.1002/uog.7738. Epub 2010 Jul 8. PMID: 20617509. | |
Meler E, Mazarico E, Eixarch E, et al. A 10-year experience of protocol-based management of fetal growth restriction: perinatal outcomes in late pregnancy cases diagnosed after 32 weeks. Ultrasound Obstet Gynecol 2020. doi: 10.1002/uog.23537. Epub ahead of print. PMID: 33159370. | |
Hershkovitz R, Kingdom JC, Geary M, et al. Fetal cerebral blood flow redistribution in late gestation: identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 2000;15(3):209–12. doi: 10.1046/j.1469-0705.2000.00079.x. PMID: 10846776. | |
Cruz-Martínez R, Savchev S, Cruz-Lemini M, et al. Clinical utility of third-trimester uterine artery Doppler in the prediction of brain hemodynamic deterioration and adverse perinatal outcome in small-for-gestational-age fetuses. Ultrasound Obstet Gynecol 2015;45(3):273–8. doi: 10.1002/uog.14706. Epub 2015 Jan 27. PMID: 25346413. | |
Cruz-Martínez R, Figueras F, Hernandez-Andrade E, et al. Fetal brain Doppler to predict cesarean delivery for nonreassuring fetal status in term small-for-gestational-age fetuses. Obstet Gynecol 2011;117(3):618–26. doi: 10.1097/AOG.0b013e31820b0884. PMID: 21343765. | |
Boers KE, Vijgen SM, Bijlenga D, et al. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT). BMJ 2010;341:c7087. doi: 10.1136/bmj.c7087. PMID: 21177352; PMCID: PMC3005565. | |
Arbeille P, Maulik D, Fignon A, et al. Assessment of the fetal Po2 changes by cerebral and umbilical Doppler on lamb fetuses during acute hypoxia. Ultrasound Med Biol 1995;21(7):861–70. | |
Cruz-Martinez R, Figueras F, Hernandez-Andrade E, et al. Longitudinal brain perfusion changes in near-term small-for-gestational-age fetuses as measured by spectral Doppler indices or by fractional moving blood volume. Am J Obstet Gynecol 2010;203(1):42.e1–6. doi: 10.1016/j.ajog.2010.02.049. Epub 2010 May 1. PMID: 20435282. | |
Conde-Agudelo A, Villar J, Kennedy SH, et al. Predictive accuracy of cerebroplacental ratio for adverse perinatal and neurodevelopmental outcomes in suspected fetal growth restriction: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;52(4):430–41. doi: 10.1002/uog.19117. Epub 2018 Sep 5. PMID: 29920817. | |
Villalaín C, Herraiz I, Quezada MS, et al. Fetal Biometry and Doppler Study for the Assessment of Perinatal Outcome in Stage I Late-Onset Fetal Growth Restriction. Fetal Diagn Ther 2018;44(4):264–70. doi: 10.1159/000485124. Epub 2018 May 4. PMID: 29730664. | |
Paules C, Dantas AP, Miranda J, et al. Premature placental aging in term small-for-gestational-age and growth-restricted fetuses. Ultrasound Obstet Gynecol 2019;53(5):615–22. doi: 10.1002/uog.20103. Epub 2019 Apr 12. PMID: 30125412. | |
Monaghan C, Binder J, Thilaganathan B, et al. Perinatal loss at term: role of uteroplacental and fetal Doppler assessment. Ultrasound Obstet Gynecol 2018;52(1):72–7. doi: 10.1002/uog.17500. PMID: 28436166. | |
Vergani P, Roncaglia N, Andreotti C, et al. Prognostic value of uterine artery Doppler velocimetry in growth-restricted fetuses delivered near term. Am J Obstet Gynecol 2002;187(4):932–6. doi: 10.1067/mob.2002.127137. PMID: 12388980. | |
Martínez-Portilla RJ, Caradeux J, Meler E, et al. Third-trimester uterine artery Doppler for prediction of adverse outcome in late small-for-gestational-age fetuses: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2020;55(5):575–85. doi: 10.1002/uog.21940. PMID: 31785172. | |
Savchev S, Figueras F, Cruz-Martínez R, et al. Estimated weight centile as a predictor of perinatal outcome in small-for-gestational-age pregnancies with normal fetal and maternal Doppler indices. Ultrasound Obstet Gynecol 2012;39(3):299–303. doi: 10.1002/uog.10150. Epub 2012 Feb 7. PMID: 22102177. | |
Pilliod RA, Cheng YW, Snowden JM, et al. The risk of intrauterine fetal death in the small-for-gestational-age fetus. Am J Obstet Gynecol 2012;207(4):318.e1–6. doi: 10.1016/j.ajog.2012.06.039. PMID: 23021697; PMCID: PMC3724359. | |
Van Wyk L, et al. Effects on (neuro) developmental and behavioral outcome at 2 years of age of induced labor compared with expectant management in intrauterine growth-restricted infants: long-term outcomes of the DIGITAT trial. Am J Obstet Gynecol 2012;206(5):406.e1–7. | |
Hecher K, et al. Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound Obstet Gynecol 2001;18(6):564–70. | |
Figueras F, Gratacós E. Stage-based approach to the management of fetal growth restriction. Prenat DIagn 2014;34(7):655–9. | |
Savchev S, Figueras F, Sanz-Cortés M, et al. Evaluation of an optimal gestational age cut-off for the definition of early-and late-onset fetal growth restriction. Fetal Diagn Ther 2014;36(2):99–105. DOI: 10.1159/000355525. | |
Turan OM, Turan S, Gungor S, et al. Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet Gynecol 2008;32(2):160–7. DOI: 10.1002/uog.5386. | |
Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol 2001;18(6):571–7. doi: 10.1046/j.0960-7692.2001.00591.x. PMID: 11844191. | |
Crimmins S, Desai A, Block-Abraham D, et al. A comparison of Doppler and biophysical findings between liveborn and stillborn growth-restricted fetuses. Am J Obs Gynecol 2014;669(e1):10. doi: 10.1016/j.ajog.2014.06.022. | |
Schwarze A, Gembruch U, Krapp M, et al. Qualitative venous Doppler flow waveform analysis in preterm intrauterine growth-restricted fetuses with ARED flow in the umbilical artery–correlation with short-term outcome. Ultrasound Obstet Gynecol 2005;25(6):573–9. doi: 10.1002/uog.1914. PMID: 15912468. | |
Figueras F, Gratacós E. An integrated approach to fetal growth restriction. Best Pract Res Clin Obstet Gynaecol [Internet] 2017;38:48–58. | |
Vollgraff Heidweiller-Schreurs CA, De Boer MA, Heymans MW, et al. Prognostic accuracy of cerebroplacental ratio and middle cerebral artery Doppler for adverse perinatal outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51(3):313–22. doi: 10.1002/uog.18809. | |
McCowan LME, Harding JE, Roberts AB, et al. A pilot randomized controlled trial of two regimens of fetal surveillance for small-for-gestational-age fetuses with normal results of umbilical artery Doppler velocimetry. Am J Obstet Gynecol 2000;182(1 I):81–6. doi: 10.1016/s0002-9378(00)70494-7. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
