This chapter should be cited as follows:
Bartin R, Salomon LJ, Glob. libr. women's med.,
ISSN: 1756-2228; DOI 10.3843/GLOWM.419343
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 18
Ultrasound in obstetrics
Volume Editors:
Professor Katia Bilardo, University of Groningen
Dr Valentina Tsibizova, PREIS International School, Firenze, Italy

Chapter
Fetal Biometry: From Pregnancy Dating to Assessment of Fetal Size and Growth
First published: August 2023
Study Assessment Option
By completing 4 multiple-choice questions (randomly selected) after studying this chapter readers can qualify for Continuing Professional Development awards from FIGO plus a Study Completion Certificate from GLOWM
See end of chapter for details
INTRODUCTION
Fetal biometry is the cornerstone of modern prenatal care. Since the introduction of the ultrasonic fetal measurements in the 1960s, biometry assessment has become common practice,1,2,3 and its use depends on the gestational age at ultrasound examination, with several objectives. First-trimester measurements are mainly used for pregnancy dating, which relies essentially on measurements of the crown-rump length (CRL). Accurate estimation of gestational age is the key to further interpretation of fetal biometry. Later on, the main fetal biometric parameters measured are biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femur diaphysis length (FL). These measurements may be used to assess fetal size (fetal biometry at a given timepoint compared to normal charts) and growth (dynamic evolution of fetal biometry across gestation), along with their respective disorders and can also be combined to estimate fetal weight (EFW). Growth disorders mainly consist of fetal growth restriction, often associated with small-for-gestational age (SGA), or large-for-gestational age (LGA), which may lead to fetal macrosomia at delivery. Growth and size disorders are associated with a variety of adverse maternal and perinatal outcomes. Hence, biometry is used to determine the optimal management of these pregnancies and the best timing for delivery. Nonetheless, as for all non-automatic measurements and estimates, fetal biometry is subject to both inter and interobserver variability that could be reduced by standardizing acquisitions and routine quality control of ultrasound images.
This chapter, mainly based on international guidelines, gives an overview of the technique used to obtain the most accurate measurements of fetal biometry and estimation of fetal weight, and provides the keys to the interpretation of fetal size and growth.
PREGNANCY DATING
Accurate assessment of gestational age is central information to modern obstetrical practice. It allows estimation of the expected date of delivery and is an essential prerequisite for further interpretation of fetal biometry and growth, which is key to an optimal management for all pregnancies. While the exact date of conception is certain in pregnancies arising from assisted reproductive technology, it cannot be determined in spontaneous pregnancies. The use of the last menstrual period carries too much uncertainty and is known to be unreliable.4 Hence, ultrasound assessment of gestational age based on first-trimester biometry has become the preferred method for pregnancy dating.5,6,7
Crown-rump length measurement
Crown-rump length (CRL) is currently the most reliable fetal measurement for pregnancy dating in the first trimester.7,8,9,10,11 Ideally, the measurement should be performed for a CRL between 45 and 84 mm, along with the assessment of fetal anatomy and screening for common aneuploidies.10,11 As even small errors can have a significant impact on fetal size and growth assessment later on,12,13,14 standardization of measurements is the key to obtain the most reliable assessment of gestational age. The CRL can be measured either through abdominal or vaginal ultrasound, using a set of various criteria and anatomical landmarks:10,15,16,17,18
- Mid-sagittal section: CRL should be measured on a mid-sagittal section of the entire fetus. Ideally, the midline facial profile (with nasal bones), spine, genital tubercle, and rump should all appear on a single image. Both crown and rump should be clearly visible for adequate placement of the calipers.
- Horizontal position: the fetus should be oriented horizontally on the image, perpendicular to the ultrasound beam.
- Neutral position of the head: amniotic fluid should be visible between the chin and the chest. Cervical hyperextension and hyperflexion are responsible for over and underestimation of the CRL measurement, respectively.
- Optimal magnification: the entire fetus should fill the largest part of the image.
- Caliper positioning: the calipers should be placed on the outer border of the skin covering the crown and rump.
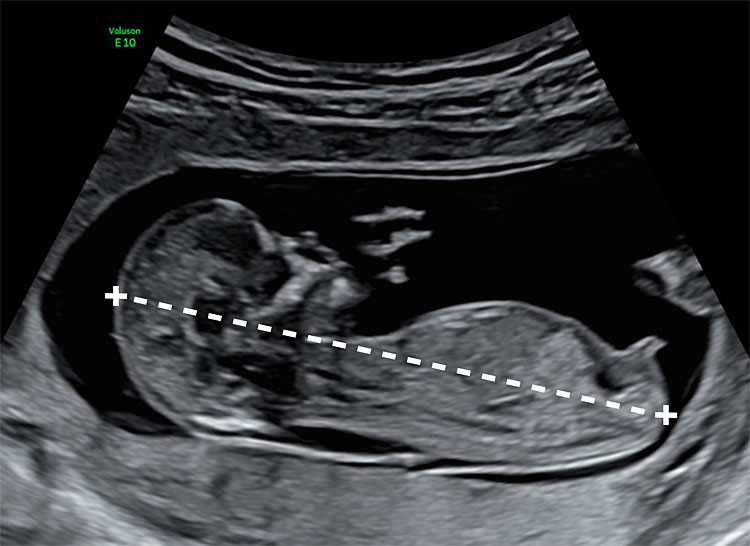
1
Transabdominal acquisition of the crown-rump length at 13 weeks’ gestation. On this mid-sagittal section, the fetus is in neutral position (with amniotic fluid between the head and the neck). Both crown and rump are clearly identified. The genital tubercle is visible above the bladder.
Biparietal diameter and head circumference
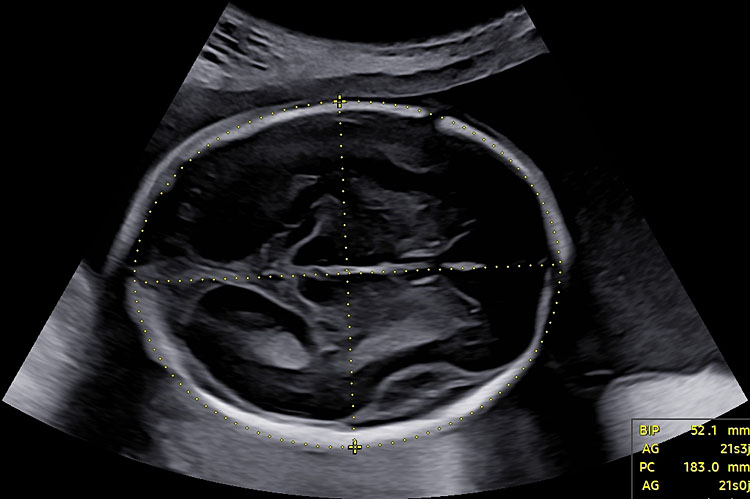
In addition to fetal crown-rump length, the main measurements used for pregnancy dating are biparietal diameter (BPD) and head circumference (HC). Both should be measured on a symmetrical axial section of the fetal head. Landmarks used to assess the quality of the measurements include the midline third ventricle, interhemispheric fissure and falx separating symmetrically both hemispheres and choroid plexuses. Whenever possible, cephalic measurements should be performed on the transthalamic plane (Figure 2). HC is commonly measured by an ellipse drawn around the outer border of the skull. For BPD, both outer-to-inner and outer-to-outer measurements are described and are acceptable, as long as the nomogram used is in adequation with the measurement technique.
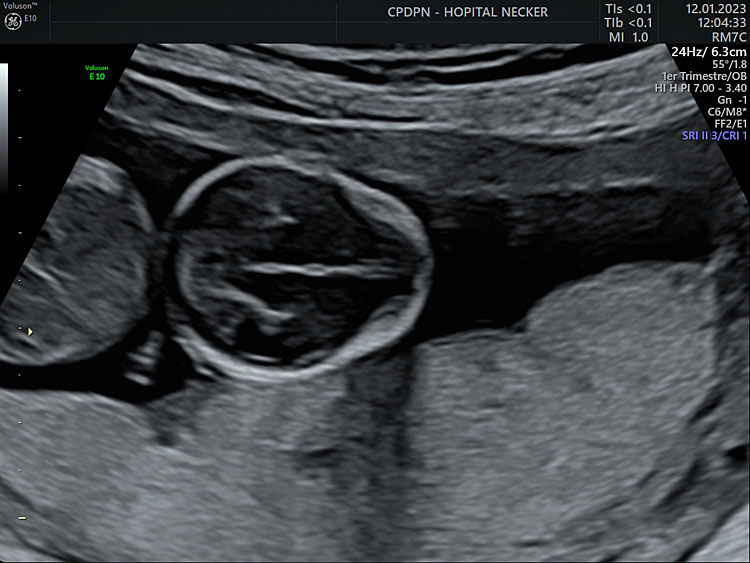
2
Axial section of the fetal head at 13+6 weeks’ gestation, at the level of the thalami, for biparietal diameter and head-circumference measurements. Interhemispheric fissure separates both hemispheres. The plane remains above the posterior fossa.
Overall, a CRL below 84 mm is more than or at least as accurate as other measurements for pregnancy dating in the first trimester of the pregnancy and also used routinely to adjust other first-trimester markers, such as nuchal translucency and/or maternal serum markers. It should therefore be used preferentially. Beyond this threshold of 84 mm, age estimates from head circumference should be used if a first-trimester scan is not available (ISUOG Guidelines).10,11,19
FETAL BIOMETRY AND ESTIMATED FETAL WEIGHT
Appropriate assessment of fetal biometry is a prerequisite for the estimation of fetal weight (EFW), size, and growth. The main objective is to screen for fetal size and growth disorders, including intrauterine growth restriction (IUGR) often associated with small-for-gestational age (SGA), and large-for-gestational age (LGA) fetuses. As both may generate adverse perinatal outcomes, an accurate classification of the pregnancy is essential to the subsequent management of the pregnancy. To this end, according to ISUOG guidelines, the biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femur length (FL) should be measured routinely for the assessment of fetal size and performed in a standardized manner, thus reducing the interobserver and intraobserver variabilities.19,20,21,22,23
Cephalic measurements
Anatomical landmarks
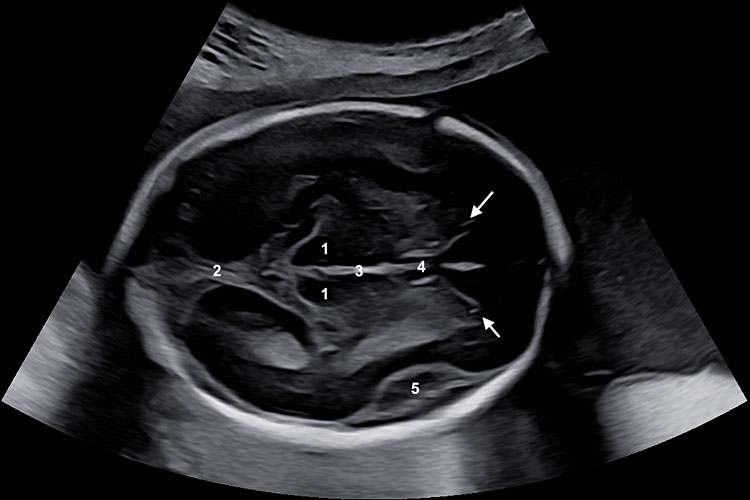
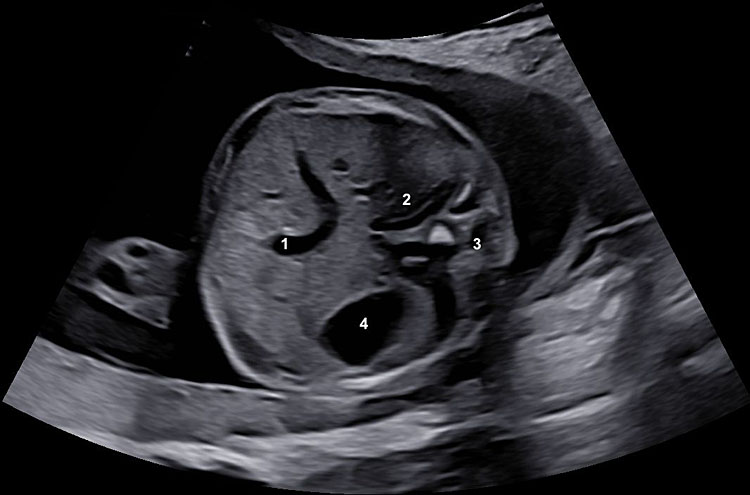
Both biparietal diameter and head circumference can be measured on a symmetrical axial section of the fetal head, with the following anatomical landmarks visible to ensure an optimal acquisition (Figure 3A):
- The axial section should pass through the thalami, separated by the third ventricle.
- Horizontal acquisition: the angle between the ultrasound beam and the midline echo should be kept as close to 90° as possible.
- Both hemispheres should appear symmetrically on both parts of the midline echo.
- The midline echo (falx cerebri) is interrupted anteriorly only by the cavum septi pellucidi.
- The plane should be kept above the posterior fossa.
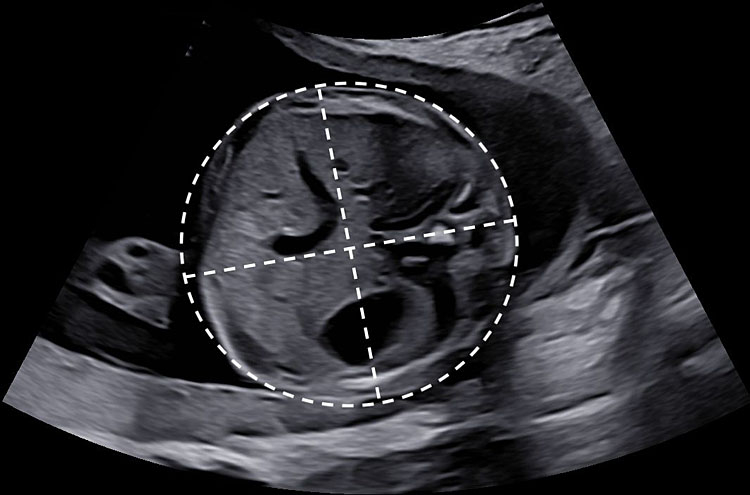
Biparietal diameter
The biparietal diameter should be measured at the widest part of the skull, perpendicular to the midline. Both outer-to-inner edge (i.e., "leading edge" technique) and outer-to-outer edge measurements have been described, and reference charts have been generated accordingly. Nonetheless, ISUOG guidelines recommend an outer-to-outer placement of when measuring BPD, as it eases reproducibility and quality control (Figure 3B).20,24
Head circumference
Two methods may be used to determine the head circumference. First, HC can be measured directly by placing an ellipse around the outer border of the fetal skull (Figure 3B). Alternatively, HC can be estimated from biparietal and occipitofrontal diameters: the BPD is measured using the inner-to-outer border technique and the OFD is obtained by placing the calipers in the middle of the bone echo of frontal and occipital skull bones, perpendicularly to the BPD. HC can therefore be calculated as HC = 1.62 × (BPD + OFD). Although outer-to-outer placement of calipers is preferable, both methods are acceptable.20
(A) |
(B) |
3
Symmetrical axial section of the fetal head for measurements of biparietal diameter and head circumference. (A) (1) Thalami, (2) falx, (3) third ventricle, (4) cavum septi pellucidi, (5) Sylvian fissure (arrows) anterior horns. (B) The biparietal diameter is measured at the widest part of the skull, perpendicular to the midline. HC can be measured by an ellipse drawn around the outer border of the skull or deduced from the biparietal and the occipital-frontal diameters.
Abdominal circumference
Abdominal circumference should be measured on a transverse section of the fetal abdomen, as circular as possible. The level of this section is given by the following anatomical landmarks (Figure 4):
- The intrahepatic portion of the umbilical vein should be visible at the level of the portal sinus.
- The stomach bubble should be visible on this section.
- The kidneys should not appear on this section and adrenal glands may be visible, next to the vertebra.
- Ideally, the vertebra should be placed in the 3- or 9-o’clock position to ensure the best visualization of all landmarks and boundaries.
(A) |
(B) |
4
Axial section of the abdomen, for estimation of the abdominal circumference. (A) (1) Intrahepatic portion of the umbilical vein, at the level of the portal sinus. (2) Adrenalin gland. (3) Vertebrae. (4) Stomach bubble. (B) AC can be measured by an ellipse drawn around the outer border of the skin or deduced from both transverse (TAD) and anteroposterior (APAD) abdominal diameters.
As for the HC, AC can be directly measured using an ellipse, where calipers should be placed at the outer surface of the skin line. Alternatively, AC can be calculated from linear measurements made perpendicular to each other, including the anteroposterior abdominal diameter (APAD) and transverse abdominal diameter (TAD). To measure the APAD, the calipers are placed on the outer borders of the body outline, from the skin covering the vertebra (posterior landmark) to the anterior abdominal wall. The TAD is obtained by placing the calipers on the outer borders of the body outline, across the abdomen at the widest point, perpendicular to the APAD. The AC is then calculated using the formula:
AC = π(APAD+TAD)/2=1.57 (APAD+TAD).20,25,26
Femur length
To obtain an optimal femur length measurement (FL), the femur closest to the probe should be imaged preferably. FL is imaged with both ends of the ossified diaphysis visible and the longest axis of the ossified diaphysis is measured. Ideally, the femur should be kept as horizontal as possible, but an angle of insonation between 45° and 90° is acceptable. The focal point should be placed at the same level as the diaphysis.
Each caliper is placed at the ends of the ossified diaphysis without including the distal femoral epiphysis if it is visible (Figure 5).
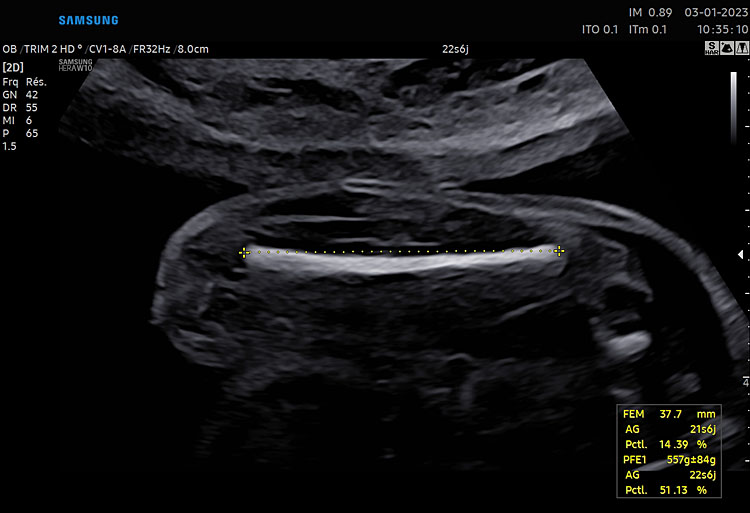
5
Femur length measurement. The closest femur is orientated horizontally on the image (i.e., with an angle between the ultrasound beam and the femur of 90°). Calipers are placed at both ends of the ossified diaphysis.
Estimated fetal weight
Apart from the independent study of each measurement to screen for specific anomalies, fetal biometric parameters may be combined to obtain an estimated fetal weight (EFW). Various combinations of either BPD, HC, AC, or FL have led to the publication of many formulas.27,28,29 While the accuracy of most of these equations are within the range of ± 15%, equations based on three or four biometric measurements appear to provide the most accurate estimation of fetal weight.30,31,32,33 Hence, the most used equation of estimated fetal weight worldwide is the three-parameter Hadlock equation based on HC, AC, and FL given by
Log10 (weight) = 1.326–0.00326 × AC × FL + 0.0107 × HC + 0.0438 × AC + 0.158 × FL.28
ASSESSMENT OF FETAL GROWTH
Relevant measurements and metrics to assess fetal growth
Fetal size and growth trajectories are pertinent indicating factors of fetal health. The use of EFW is interesting for practitioners, as it provides a clinical parameter that speaks to pediatricians, obstetricians, and parents. Another advantage of EFW is that growth can be monitored prenatally and postnatally with the same parameter. Moreover, this gives an overview of the expected birth weight, particularly relevant in the case of preterm delivery or suspected growth anomalies as it is predictive of perinatal adverse outcomes.
Nonetheless, the use of EFW also has disadvantages:19,34,35 errors in single-parameter measurements are multiplied; accuracy of EFW is compromised by large intra- and interobserver variability. Finally, very different fetal phenotypes can have the same EFW, as variations in cephalic, femur, and abdominal measurements will all affect differently the estimated weight. Among these parameters, the AC is the metric that influences the most the estimation of fetal weight. Hence, both EFW and AC should be evaluated together, as they are the most representative parameters of fetal growth and size, especially when screening for small fetuses.36
Assessment of fetal growth: concepts
Interpretation of fetal biometry and estimated fetal weight should always include the gestational age at which the measurement has been performed along with the expected weight in an assumed normal population, thus allowing care providers to properly assess the fetal development. It is also important to differentiate the concepts of fetal weight or size, describing the fetal biometric measurements at a given timepoint, and fetal growth, a dynamic process across gestation. Hence, biometric parameters must be expressed accordingly:
First, measurements may be reported as raw data: specific measurements are expressed in mm or cm, and EFW in grams, providing an idea of the fetal biometry at the moment of the estimation. Second, because measurements evolve across gestation, centiles or Z-scores are helpful to assess the fetal development, as they measure the deviation of biometric parameters from the mean of a population.
Therefore, fetuses can be classified as as follows:
- Appropriate for gestational age (AGA): when the fetal weight is within the normal range for its gestational age (i.e., individual biometric parameters and/or EFW between the 10th and 90th percentiles).
- Large for gestational age (LGA): when EFW and/or AC are above a predefined threshold, generally the 90th percentile. Macrosomia usually refers to a birthweight above 4000 g.
- Small for gestational age (SGA): when EFW and/or AC are below a predefined threshold, generally the 10th percentile, although the exact cut-off for SGA definition is debated. A fetus considered as SGA is not necessarily growth restricted but may simply be constitutionally small with excellent perinatal outcome, although the risk of fetal growth restriction is higher in this population.37
- Fetal growth restriction (FGR): the definition behind the concept of fetal (FGR) or intrauterine (IUGR) growth restriction is more complex. Basically, this refers to a fetus that could not achieve its predetermined growth potential, mainly because of a placental insufficiency. Fetuses with suspected FGR may fail to achieve their growth potential despite not being SGA at birth.
Assessing for fetal growth, a dynamic process across gestation, requires serial measurements throughout the pregnancy. After a second ultrasound performed around 18–22 weeks’ gestation to assess for fetal morphology and biometry, there is currently no consensus on the frequency of ultrasound examination in the third trimester, that should depend on local guidelines and the presence of risk factors for abnormal growth.19 Given the +/- 15% error in estimation of fetal weight, the time interval between scans should typically be at least 2–3 weeks, to minimize false-positive detection of fetal growth disorders.
Fetal growth references and standards
Many published fetal growth charts are available, and the choice of one of them requires careful consideration. The concepts of fetal growth “standards” and fetal growth “references”, and fetal growth charts based on estimated fetal weight vs those based on birth weight can be confusing. Moreover, fetal growth charts can be derived from prescriptive or descriptive, population-based, or customized. We intend to clarify these notions.
Population-based growth charts
Population-based charts are constructed based on a large population of uncomplicated pregnancies and include both descriptive references and prescriptive standards of growth. Descriptive references consist in population-based curves constructed retrospectively that describe the distribution of a measurement in a given population, considered as “normal” based on maternal characteristics and perinatal outcomes, particularly the distribution of birth weights. Considering the retrospective construction of such references, many confusing factors cannot be controlled, and the methodological quality behind the construction of such references is therefore often questionable.
Prescriptive standards describe theoretical growth under optimal conditions. These standards are constructed mainly from prospective data, with large and international populations at low risk of perinatal adverse outcomes, appropriate pregnancy dating, ultrasound protocols, and quality control. Thus, international standards for fetal biometry describing optimal growth in fetuses from pregnancies at low risk of FGR have been constructed. Two main charts are currently available, and include the INTERGROWTH-21st (IG) and the World Health Organization (WHO).38,39,40,41
Nonetheless, despite being all international population-based prospective charts, these standards may not have the same efficacy in all populations, especially in the detection of SGA or IUGR, as centiles estimation may differ for a same measurement. While prescriptive standards have been largely adopted and are recommended by the ISUOG, the choice between either one of these prescriptive standards is debated and should be adapted to local considerations.19,42,43
An alternative to the use of international population-based charts is to use local charts that have been developed on own population.
Some elements differentiating the IG from the WHO must, however, be underlined. IG provides a complete set of prescriptive tools developed from the same cohort: pregnancy dating, biometry, EFW, Doppler, dynamic growth assessment, etc. All measurements were blinded and subject to external quality control. Statistical modeling allows easy calculation of Z-score and quality control. The population size was larger despite the exclusion of all pathological conditions and post-natal follow-up beyond 2 years of all pregnancies was available, making the cohort very robust.
Birth weight charts vs. estimated fetal weight charts
Although there is theoretically a continuum in fetal and neonatal growth, birth weight charts significantly diverge from EFW charts, regardless of the methodology used to construct references. One limitation of the reference ranges of birth weights lies in an overrepresentation of pathological pregnancies (and among them, fetal growth restriction) particularly in pregnancies delivering before 37 weeks of gestation. Preterm infants tend to have lower BW distribution compared to the expected EFW for a same gestational age, and the difference tend to be greater in the earliest deliveries.44,45,46 Hence, EFW should not be plotted on newborn birth-weight charts.19
In a recent study, the Fetal Medicine Foundation proposed reference population for both EFW and BW charts.46 The development of these charts was based on the assumption of a common median but a difference in the deviation around the median (and thus, centile estimation). The population from which BW charts were constructed also included fetuses still in utero (and thus, ultrasound EFW) along with newborn children. While this population had a lower prevalence of pathological pregnancies, which supposedly reduces bias, including both accurate birth weight and estimated fetal weight may lead to measurement heterogeneity and variability.
Customized charts
Customized and conditional charts have been proposed as an alternative to population-based or reference charts. These reference charts consist in adjusting for single or multiple factors known to affect fetal weight and growth: maternal height and weight, ethnicity, parity, or fetal sex.47,48,49,50 Compared with population-based non-customized reference charts, a customized chart will assign a different proportion of fetuses as either appropriate or small for gestational age. The benefit of customized charts is, however, uncertain.
SCREENING FOR FETAL GROWTH RESTRICTION
When an abnormal biometry is found on a routine ultrasound scan, patients should be referred for detailed assessment of the fetus. The first step is to confirm the accuracy of pregnancy dating, as an approximation or error in the estimated date of conception may result in misclassification of the pregnancy. A detailed evaluation of fetal anatomy is also required along with the evaluation for uteroplacental insufficiency, including maternal (uterine artery) and fetal (umbilical artery, cerebroplacental ratio and if necessary, Ductus venosus) Doppler patterns. This objective placental morphology assessment (location of cord insertion, and size and appearance of the placenta). Depending on the sonographic findings, an amniocentesis may be considered depending on for karyotype or CGH array analysis.
Ultrasound diagnosis of fetal growth restriction
As seen above, intrauterine growth restriction (IUGR) is difficult to define and refers to a fetus that does not reach its biological growth potential, mainly because of impaired placental function. Fetuses with IUGR are at higher risk for perinatal morbidity and mortality,51 and poor long-term outcomes, such as neurodevelopmental impairment or cardiovascular and endocrine diseases.52,53,54 An international Delphi consensus held in 2016 proposed a set of criteria for the diagnosis of IUGR, using the following:36
- A differentiation was made between early and late-onset IUGR, with a cut-off at 32 weeks’ gestation.
- Biometric parameters: both fetal size and growth were considered in the diagnosis of IUGR.
- Doppler patterns: including maternal (uterine artery – UtA) or fetal (umbilical – UA or cerebral/umbilical ratio (CPR), as they partially describe placental function and fetal adaptation to placental impairment.
Early-onset IUGR refers to a IUGR occurring before 32 weeks’ gestation with the following criteria:
- AC/EFW <3rd centile or UA-AEDF (absent end diastolic flow in the umbilical artery)
Or the association of
- AC/EFW <10th centile with
- UtA-PI >95th centile and/or
- UA-PI> 95th centile
Late IUGR refers to a IUGR occurring before 32 weeks’ gestation with the following criteria:
- AC/EFW <3rd centile
Or at least two of the following criteria:
- AC/EFW <10th percentile
- AC/EFW crossing >2 quartiles on growth centiles (non-customized centiles)
- CPR <5th centile or UA-PI >95th centile
These definitions highlight that SGA and IUGR are two different concepts that are not necessarily entangled. Thus, an AC/EFW <10th centile is not sufficient for the diagnosis of IUGR. Conversely, a fetus with an AC/EFW >10th centile could be growth-restricted: a drop in AC/EFW centile along with abnormal Doppler patterns are signs of IUGR regardless of the exact value of AC/EFW.
Overall, assessment of fetal growth and diagnosis of IUGR requires serial evaluation of fetal biometry and Doppler patterns across gestation.
Overview of fetal growth restriction management
Pregnancies characterized by intrauterine growth restriction should be referred to an appropriate unit for individualized management. Evaluation of the pregnancy is twofold:
- Maternal evaluation: in the absence of fetal malformation, pregnancies affected by growth restriction should trigger a clinical and/or biological maternal evaluation to screen for pre-eclampsia, as the association is frequent due to a placental dysfunction. This includes maternal hypertension, proteinuria, and specific complications.
- Fetal assessment: early identification of fetuses that will require emergency and/or preterm delivery is the priority in this situation, along with finding the optimal timing for antenatal corticosteroid administration when necessary. Antenatal testing strategies include regular controls of cardiotocography, amniotic fluid volume assessment and fetal Doppler patterns of the umbilical artery, fetal middle cerebral artery and cerebroplacental ratio, and aortic isthmus or ductus venosus flow. The frequency of surveillance depends on local guidelines and the severity of IUGR.
CONCLUSION
Assessment of fetal biometry is one of the main components of modern prenatal care. Accurate pregnancy dating is essential for further interpretation of both fetal size and growth. Measuring biometrics parameters, including head circumference (HC), biparietal diameter (BPD), abdominal circumference (AC), and femur length (FL), and combining them into an estimated fetal weight should be part of the routine ultrasound exam. Serial measurements across gestation provide useful information about growth velocity and may improve the diagnosis of growth disorders. Nonetheless, biometrics parameters and EFW are subject to measurement errors and approximations. Hence, measurements should be performed in a standardized manner to reduce inter- and intraobserver variability.
PRACTICE RECOMMENDATIONS
- To ensure the most accurate fetal measurements, all biometric parameters should be acquired in a standardized manner based on international guidelines. Biometric images should undergo quality-control checks routinely.
- Appropriate pregnancy dating is the key to further interpretation of fetal biometry and growth. CRL measurement is the most accurate method for pregnancy dating when performed between 45 and 84 mm along with the assessment of fetal anatomy and screening for aneuploidies.
- Routine examination of second-trimester ultrasound should include measurements of head circumference (HC), biparietal diameter (BPD), abdominal circumference (AC), and femur length (FL).
- Both AC and estimated fetal weight (EFW) may be used to screen for fetal growth disorders and should be compared to existing nomograms.
- Prescriptive fetal biometry standards, obtained from prospective data with large and international populations at low risk of adverse outcomes, should be used preferentially to assess for fetal growth.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Willocks J, Donald I, Duggan TC, et al. Foetal Cephalometry by Ultrasound. BJOG Int J Obstet Gynaecol 1964;71(1):11–20. doi:10.1111/j.1471-0528.1964.tb04236.x. | |
Willocks J, Donald I, Campbell S, et al. Intrauterine Growth Assessed by Ultrasonic Foetal Cephalometry. BJOG Int J Obstet Gynaecol 1967;74(5):639–47. doi:10.1111/j.1471-0528.1967.tb03774.x. | |
Campbell S. An Improved Method of Fetal Cephalometry by Ultrasound. BJOG Int J Obstet Gynaecol 1968;75(5):568–76. doi:10.1111/j.1471-0528.1968.tb00161.x. | |
Waldenström U, Axelsson O, Nilsson S. A Comparison of the Ability of a Sonographically Measured Biparietal Diameter and the Last Menstrual Period to Predict the Spontaneous Onset of Labor. J Diagn Med Sonogr 1991;7(1):42–3. doi:10.1177/875647939100700133. | |
Robinson HP, Sweet EM, Adam AH. The Accuracy of Radiological Estimates of Gestational Age using Early Fetal Crown-Rump Length Measurements by Ultrasound as a Basis for Comparison. BJOG Int J Obstet Gynaecol 1979;86(7):525–8. doi:10.1111/j.1471-0528.1979.tb10804.x. | |
Thorsell M, Kaijser M, Almström H, et al. Expected day of delivery from ultrasound dating versus last menstrual period-obstetric outcome when dates mismatch. BJOG Int J Obstet Gynaecol 2008;115(5):585–9. doi:10.1111/j.1471-0528.2008.01678.x. | |
Salomon LJ, Pizzi C, Gasparrini A, et al. Prediction of the date of delivery based on first trimester ultrasound measurements: An independent method from estimated date of conception. J Matern Fetal Neonatal Med 2010;23(1):1–9. doi:10.3109/14767050903078672. | |
Salomon LJ, Bernard JP, Duyme M, et al. Revisiting first-trimester fetal biometry: First-trimester fetal biometry. Ultrasound Obstet Gynecol 2003;22(1):63–6. doi:10.1002/uog.162. | |
Chalouhi GE, Bernard JP, Benoist G, et al. A comparison of first trimester measurements for prediction of delivery date. J Matern Fetal Neonatal Med 2011;24(1):51–7. doi:10.3109/14767051003728229. | |
ISUOG Practice Guidelines: performance of first‐trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2013;41(1):102–13. doi:10.1002/uog.12342. | |
ISUOG Practice Guidelines (updated): performance of 11–14‐week ultrasound scan. Ultrasound Obstet Gynecol 2023;61(1):127–43. doi:10.1002/uog.26106. | |
Gadsbøll K, Wright A, Kristensen SE, et al. Crown–rump length measurement error: impact on assessment of growth. Ultrasound Obstet Gynecol 2021;58(3):354–9. doi:10.1002/uog.23690. | |
Kagan KO, Hoopmann M, Baker A, et al. Impact of bias in crown-rump length measurement at first-trimester screening for trisomy 21. Ultrasound Obstet Gynecol 2012;40(2):135–9. doi:10.1002/uog.11095. | |
Salomon LJ, Bernard M, Amarsy R, et al. The impact of crown-rump length measurement error on combined Down syndrome screening: a simulation study. Ultrasound Obstet Gynecol 2009;33(5):506–11. doi:10.1002/uog.6371. | |
Ioannou C, Sarris I, Hoch L, et al. for the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). Standardisation of crown-rump length measurement. BJOG Int J Obstet Gynaecol 2013;120:38–41. doi:10.1111/1471-0528.12056. | |
Wanyonyi SZ, Napolitano R, Ohuma EO, et al. Image-scoring system for crown-rump length measurement. Ultrasound Obstet Gynecol 2014;44(6):649–54. doi:10.1002/uog.13376. | |
Dhombres F, Khoshnood B, Bessis R, et al. Quality of first-trimester measurement of crown-rump length. Am J Obstet Gynecol 2014;211(6):672.e1–5. doi:10.1016/j.ajog.2014.06.012. | |
Liao Y, Wen H, Ouyang S, et al. Routine first-trimester ultrasound screening using a standardized anatomical protocol. Am J Obstet Gynecol 2021;224(4):396.e1–15. doi:10.1016/j.ajog.2020.10.037. | |
Salomon LJ, Alfirevic Z, Da Silva Costa F, et al. ISUOG Practice Guidelines: ultrasound assessment of fetal biometry and growth. Ultrasound Obstet Gynecol 2019;53(6):715–23. doi:10.1002/uog.20272. | |
Salomon LJ, Alfirevic Z, Berghella V, et al. ISUOG Practice Guidelines (updated): performance of the routine mid‐trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2022;59(6):840–56. doi:10.1002/uog.24888. | |
Salomon LJ, Bernard JP, Duyme M, et al. Feasibility and reproducibility of an image‐scoring method for quality control of fetal biometry in the second trimester. Ultrasound Obstet Gynecol 2006;27(1):34–40. doi:10.1002/uog.2665. | |
Sarris I, Ioannou C, Chamberlain P, et al. Intra- and interobserver variability in fetal ultrasound measurements. Ultrasound Obstet Gynecol 2012;39(3):266–73. doi:10.1002/uog.10082. | |
Perni SC, Chervenak FA, Kalish RB, et al. Intraobserver and interobserver reproducibility of fetal biometry. Ultrasound Obstet Gynecol 2004;24(6):654–8. doi:10.1002/uog.1717. | |
Napolitano R, Donadono V, Ohuma EO, et al. Scientific basis for standardization of fetal head measurements by ultrasound: a reproducibility study. Ultrasound Obstet Gynecol 2016;48(1):80–5. doi:10.1002/uog.15956. | |
Watson W, Chescheir N, Seeds J. Ultrasound Determination of Fetal Abdominal Circumference: A Comparison of Measurement Methods. Am J Perinatol 1990;7(02):182–3. doi:10.1055/s-2007-999476. | |
O’Gorman N, Salomon LJ. Fetal biometry to assess the size and growth of the fetus. Best Pract Res Clin Obstet Gynaecol 2018;49:3–15. doi:10.1016/j.bpobgyn.2018.02.005. | |
Warsof SL, Gohari P, Berkowitz RL, et al. The estimation of fetal weight by computer-assisted analysis. Am J Obstet Gynecol 1977;128(8):881–92. doi:10.1016/0002-9378(77)90058-8. | |
Hadlock FP, Harrist RB, Sharman RS, et al. Estimation of fetal weight with the use of head, body, and femur measurements – A prospective study. Am J Obstet Gynecol 1985;151(3):333–7. doi:10.1016/0002-9378(85)90298-4. | |
Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology 1991;181(1):129–33. doi:10.1148/radiology.181.1.1887021. | |
Dudley NJ. A systematic review of the ultrasound estimation of fetal weight: Ultrasound EFW review. Ultrasound Obstet Gynecol 2005;25(1):80–9. doi:10.1002/uog.1751. | |
Melamed N, Yogev Y, Meizner I, et al. Sonographic Fetal Weight Estimation: Which Model Should Be Used? J Ultrasound Med 2009;28(5):617–29. doi:10.7863/jum.2009.28.5.617. | |
Milner J, Arezina J. The accuracy of ultrasound estimation of fetal weight in comparison to birth weight: A systematic review. Ultrasound 2018;26(1):32–41. doi:10.1177/1742271X17732807. | |
Melamed N, Baschat A, Yinon Y, et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynecol Obstet 2021;152(S1):3–57. doi:10.1002/ijgo.13522. | |
Mayer C, Joseph KS. Fetal growth: a review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet Gynecol 2013;41(2):136–45. doi:10.1002/uog.11204. | |
Hiersch L, Melamed N. Fetal growth velocity and body proportion in the assessment of growth. Am J Obstet Gynecol 2018;218(2):S700&ndah;11.e1. doi:10.1016/j.ajog.2017.12.014. | |
Gordijn SJ, Beune IM, Thilaganathan B, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2016;48(3):333–9. doi:10.1002/uog.15884. | |
Monier I, Blondel B, Ego A, et al. Poor effectiveness of antenatal detection of fetal growth restriction and consequences for obstetric management and neonatal outcomes: a F rench national study. BJOG Int J Obstet Gynaecol 2015;122(4):518–27. doi:10.1111/1471-0528.13148. | |
Papageorghiou AT, Ohuma EO, Altman DG, et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. The Lancet 2014;384(9946):869–79. doi:10.1016/S0140-6736(14)61490-2. | |
Stirnemann J, Villar J, Salomon LJ, et al. International estimated fetal weight standards of the INTERGROWTH-21st Project: International estimated fetal weight standards. Ultrasound Obstet Gynecol 2017;49(4):478–86. doi:10.1002/uog.17347. | |
Kiserud T, Benachi A, Hecher K, et al. The World Health Organization fetal growth charts: concept, findings, interpretation, and application. Am J Obstet Gynecol 2018;218(2):S619–29. doi:10.1016/j.ajog.2017.12.010. | |
Papageorghiou AT, Kennedy SH, Salomon LJ, et al. The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am J Obstet Gynecol 2018;218(2):S630–40. doi:10.1016/j.ajog.2018.01.011. | |
Hua X, Shen M, Reddy UM, et al. Comparison of the INTERGROWTH-21st, National Institute of Child Health and Human Development, and WHO fetal growth standards. Int J Gynecol Obstet 2018;143(2):156–63. doi:10.1002/ijgo.12637. | |
Cheng YKY, Lu J, Leung TY, et al. Prospective assessment of INTERGROWTH-21st and World Health Organization estimated fetal weight reference curves: Assessment of INTERGROWTH 21st and WHO EFW reference curves. Ultrasound Obstet Gynecol 2018;51(6):792–8. doi:10.1002/uog.17514. | |
Villar J, Giuliani F, Fenton TR, et al. INTERGROWTH-21st very preterm size at birth reference charts. The Lancet 2016;387(10021):844–5. doi:10.1016/S0140-6736(16)00384-6. | |
Sotiriadis A, Eleftheriades M, Papadopoulos V, et al. Divergence of estimated fetal weight and birth weight in singleton fetuses. J Matern Fetal Neonatal Med 2018;31(6):761–9. doi:10.1080/14767058.2017.1297409. | |
Nicolaides KH, Wright D, Syngelaki A, et al. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obstet Gynecol 2018;52(1):44–51. doi:10.1002/uog.19073. | |
Gardosi J, Francis A. Adverse pregnancy outcome and association with small for gestational age birthweight by customized and population-based percentiles. Am J Obstet Gynecol 2009;201(1):28.e1–8. doi:10.1016/j.ajog.2009.04.034. | |
Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol 2015;213(4):449.e1–41. doi:10.1016/j.ajog.2015.08.032. | |
Grantz KL, Hediger ML, Liu D, et al. Fetal growth standards: the NICHD fetal growth study approach in context with INTERGROWTH-21st and the World Health Organization Multicentre Growth Reference Study. Am J Obstet Gynecol 2018;218(2):S641 55.e28. doi:10.1016/j.ajog.2017.11.593. | |
Fay E, Hugh O, Francis A, et al. Customized GROW vs. INTERGROWTH-21st birthweight standards to identify small for gestational age associated perinatal outcomes at term. Am J Obstet Gynecol MFM 2022;4(2):100545. doi:10.1016/j.ajogmf.2021.100545. | |
Lees C, Marlow N, Arabin B, et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: Cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet Gynecol 2013;42(4):400–8. doi:10.1002/uog.13190. | |
von Beckerath AK, Kollmann M, Rotky-Fast C, et al. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol 2013;208(2):130.e1–6. doi:10.1016/j.ajog.2012.11.014. | |
Lees CC, Marlow N, Van Wassenaer-Leemhuis A, et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): A randomised trial. The Lancet 2015;385(9983):2162–72. doi:10.1016/S0140-6736(14)62049-3. | |
Jaddoe VWV, de Jonge LL, Hofman A, et al. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ 2014;348:g14–g14. doi:10.1136/bmj.g14. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can now automatically receive 2 Continuing Professional Development credits from FIGO plus a Study Completion Certificate from GLOWM for successfully answering 4 multiple choice questions (randomly selected) based on the study of this chapter.
Medical students can receive the Study Completion Certificate only.
(To find out more about FIGO’s Continuing Professional Development awards programme CLICK HERE)


CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.