This chapter should be cited as follows:
Arduini M, Caforio L, et al, Glob. libr. women's med.,
ISSN: 1756-2228; DOI 10.3843/GLOWM.419313
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 18
Ultrasound in obstetrics
Volume Editors:
Professor Katia Bilardo, University of Groningen
Dr Valentina Tsibizova, PREIS International School, Firenze, Italy

Chapter
Ultrasound Evaluation of Normal Second-Trimester Fetal Anatomy
First published: August 2023
Study Assessment Option
By completing 4 multiple-choice questions (randomly selected) after studying this chapter readers can qualify for Continuing Professional Development awards from FIGO plus a Study Completion Certificate from GLOWM
See end of chapter for details
INTRODUCTION
Congenital disorders can be defined as structural or functional anomalies that occur during intrauterine life. The World Health Organization estimated 6% of babies worldwide are born with a congenital disorder, resulting in hundreds of thousands of associated deaths. However, the true number of cases may be much higher because statistics do not often consider terminated pregnancies and stillbirths.
Around the world each year, an estimated 295,000 newborns die before reaching 4 weeks of age due to congenital disorders and associated complications.
Congenital anomalies develop prenatally and may be identified before birth.
The routine mid-trimester ultrasound is commonly made between the 18th and the 24th week of gestation. The second-trimester ultrasound is performed mainly for anatomical evaluation of the fetus.1
ULTRASOUND EVALUATION SECOND-TRIMESTER FETAL ANATOMY
The first step in the ultrasonographic evaluation of the fetus is the assessment of fetal visceral situs as some organs are arranged symmetrically and others, such as the abdominal and thoracic organs, are arranged asymmetrically.
Thorax
In the fetal thorax clavicles, the first ribs and the body of the first vertebra border the thoracic cavity above, the vertebral column posteriorly and the sternum anteriorly, the ribs laterally, and the diaphragm in the lower part. The right and left lungs occupy the majority of the thoracic cavity, with the heart occupying the central position.
The heart is positioned in the central part of the chest cavity. Two-thirds of the heart including the apex occupy the left side of the thorax while the remaining one-third including the base of the heart occupies the right side.
The normal cardiac axis lies at 45° to the left of the midline drawn from the spine to the anterior chest wall. The normal range is between 25 and 45 degrees.2
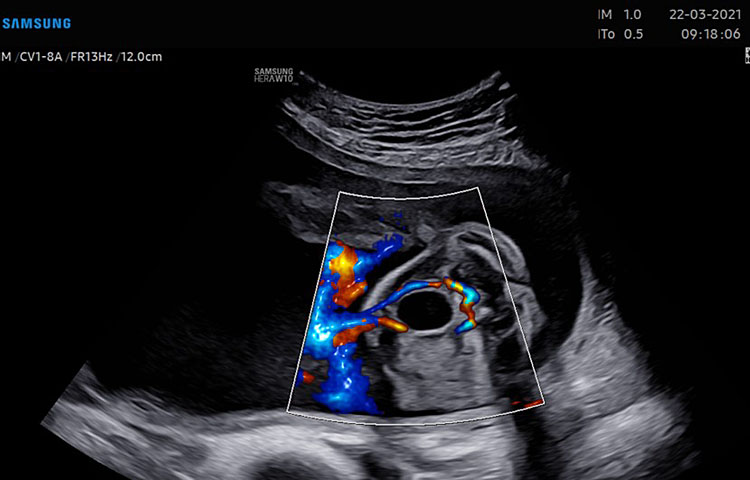
Normal heart position and axis in the fetal chest is defined levocardia. Dextrocardia defines a heart located in the right chest and mesocardia refers to a central position of the heart in the thorax (Video 1).
1
Four-chamber view.
Fetal heart
Congenital heart diseases (CHDs) are the most common severe congenital abnormalities. An incidence of 8–9/1000 live births has been reported in a large population study.3
The impact of fetal echocardiography on CHD should address two main issues: first, accuracy of the technique in the prenatal detection of CHD and secondly, the effectiveness of such detection in improving the perinatal outcome.
A recent meta-analysis of eight observational studies shows that prenatal diagnosis of critical congenital heart disease improves newborn preoperative survival.4
Unfortunately, in a recent meta-analysis van Velzen showed that the prenatal detection of CHD still fails in approximately half of the cases and the accuracy rate differs by type of heart defect.5
Surprisingly in half of the cases of undetected CHD revealed that the quality of the cardiac examination was inadequate, and the defect was not clearly visible due to lack of adaptational skills.
In a third of undetected cases, the defect was visible on the cardiac planes obtained, and it was therefore lack of recognition that led to it being missed.6
Ultrasound examination of the fetal heart is considered to be the most important and difficult part of a fetal anatomy analysis.
The study of fetal heart can be obtained by two types of exam: screening ultrasound and diagnostic echocardiography.
Screening examination of the fetal heart
The fetal cardiac screening examination includes the assessment of upper abdomen, four-chamber view, outflow tracts and three-vessel view.1
During the screening examination of the fetal heart, the use of color flow Doppler is not considered mandatory. The main scientific societies encourage the examiner to become familiar with its use.7
In this chapter all ultrasound planes used for the fetal cardiac screening examination are shown in grayscale and color Doppler.
A routine antenatal ultrasound performed between weeks 18 and 24 enables detection of most of these malformations.1
Four-chamber view (4CV)
The 4CV is the first plane indicated to the ultrasound assessment of the fetal heart throughout history.8
At present the correct four-chamber view is the first step toward a high-quality cardiac examination. In fact, many cardiac abnormalities are primarily suspected with a careful four-chamber view. However, other cardiac abnormalities, especially those affecting the great vessels, are associated with a normal four-chamber view.
The study of fetal heart starts with the assessment of the cardiac site.
From a transverse plane of the fetal upper abdomen, slide the transducer toward the fetal chest, maintaining the transverse plane, until the four-chamber view is imaged.
Stomach and heart are visualized from the same (left) side of the fetus.
It is recommended to run a correct symmetrical transverse plane section of the chest showing only one complete rib on each side of the fetal thorax (Video 1).
When multiple ribs are imaged along the two lateral thorax walls, an oblique rather than a transverse plane is obtained.
The four-chamber scan is defined apical when the apex of the heart is oriented towards the transducer, or transverse when the apex of the heart is directed laterally.
In the apical four-chamber view, ideally following an axis that connects the sternum anteriorly with the rachis posteriorly, it is possible to analyze the anatomical parts of the fetal heart.
The four-chamber view checklist includes the following.
- Right ventricle (lies directly behind the sternum).
- Inter-ventricular septum.
- Left ventricle.
- Two atrioventricular valves (tricuspid and mitral valve).
- Right atrium.
- Inter-atrial septum and foramen ovale.
- Left atrium and pulmonary veins (the most posterior cardiac chamber).
- Descending aorta (posteriorly, between the left atrium and the fetal spine).
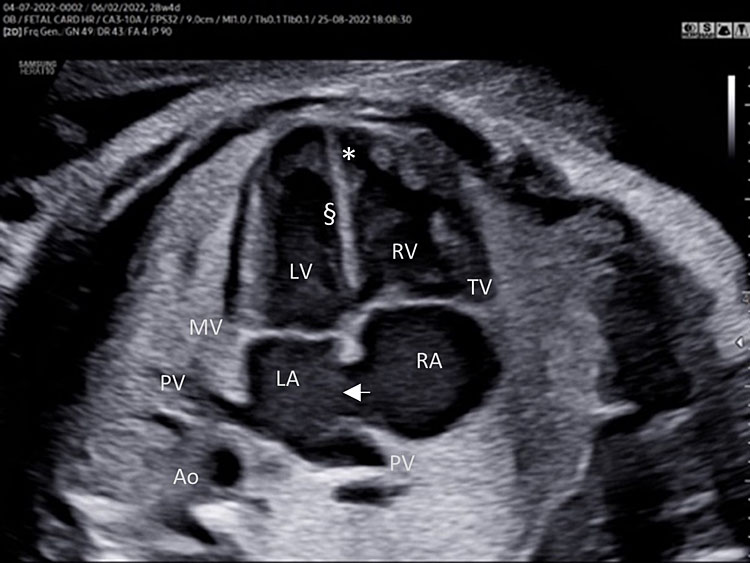
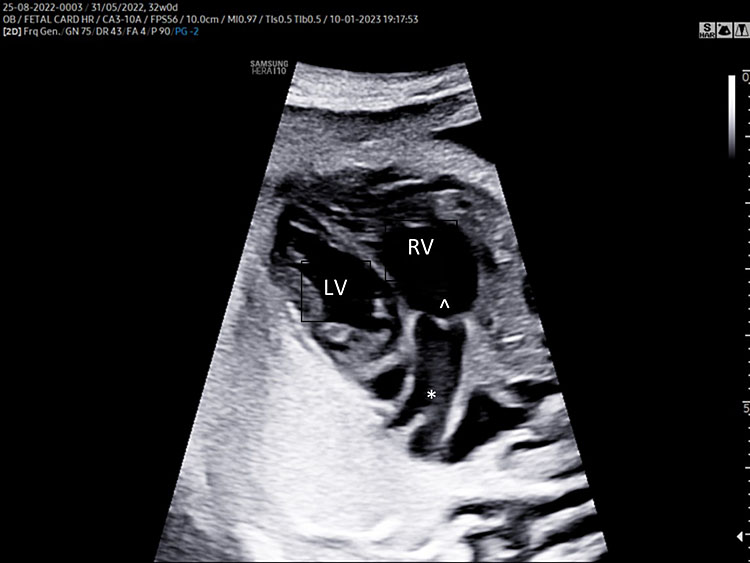
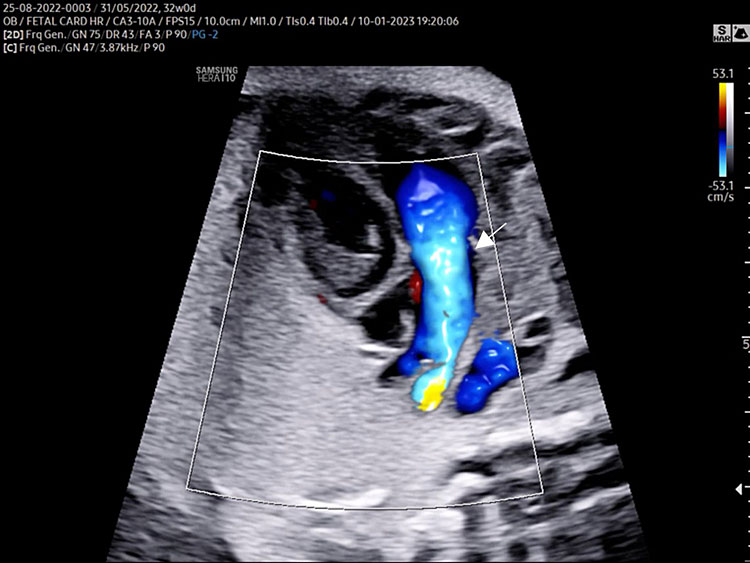
A detailed anatomy scan of the four-chamber view in the mid-trimester fetal ultrasound scan was resumed in Figure 1 and Video 1.
(A) |
(B) |
1
Apical four chamber view gray scale (A): Two ventricles, right and left, approximately the same size but different shape. Right ventricle (RV): the right ventricle is a round shape coarse and has the typical moderator band (*) in the apex. Left ventricle (LV): the left ventricle has a longer shape and forms the apex of the heart. Inter-ventricular septum (§): separates the right and left ventricles from the apex of the heart to the junction of the atrioventricular valve. Atrioventricular valve (AV) connects the atria with the ventricle chamber. The septal leaflet of the tricuspid valve (TV) inserts itself more apically on the inter-ventricular septum than the mitral valve (MV). The aspect of closed AV valves is defined “gull-wing shape”. Two atria (right and left) equal size: the left atrium (LA) represents the most posterior area of the fetal heart. The LA is located in front of the thorax spine and receives the two pulmonary veins (PV). The right atrium (RA) is situated anteriorly and on the right of the left atrium. It is connected to the left ventricle by the tricuspid valve. The LA and RA are separated by the inter-atrial septum formed by septum primum and sectum secundum. The LA and RA are connected by the foramen ovale (arrow). The leaflet of foramen ovale originates from the septum primum and opens into the left atrium with a right to left shunt. In the posterior area of the heart we can see the presence of two pulmonary veins (PV) connected to the left atrium, and transverse section of thoracic aorta (Ao) located in front and on the right the spine (Sp). Apical four-chamber view color Doppler (B): presence of normal atrio-ventricular flow (anterograde flow), ventricular same size, intact interventricular septum.
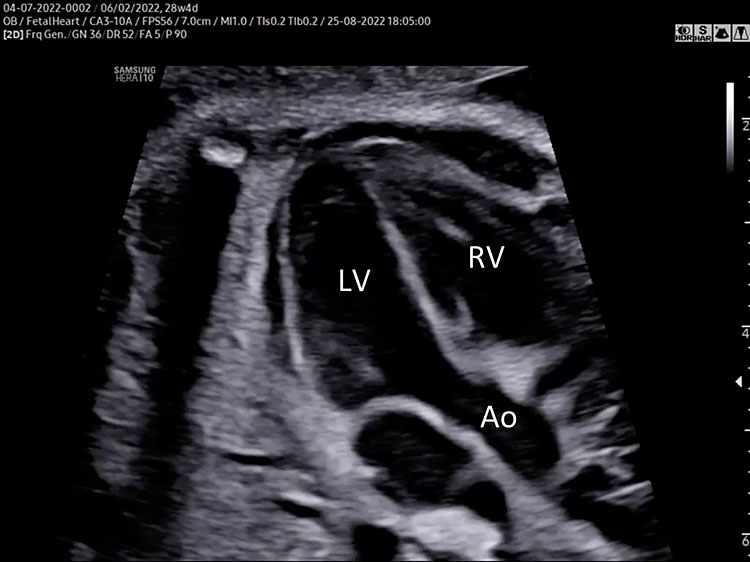
Great vessel (aorta and pulmonary artery)
The analysis of great vessels can be obtained by the five-chamber view and in the right ventricular outflow view.
Five-chamber view (5CV)
The five-chamber view is obtained from the four-chamber view with a slight angle of the transducer towards the fetal head and a slight rotation of the transducer towards the right shoulder.
The aortic outflow accounts for the fifth component of the five-chamber view. Tricuspid valve and right ventricular inflow are often not seen in the five-chamber view. The two superior pulmonary veins enter the posterior wall of the left atrium at the five-chamber view level.
The five-chamber view checklist includes the following.
- Presence of a large vessel with aortic characteristics (no bifurcation) originating from the morphologic left ventricle.
- Regular continuity of the anterior wall of the aorta with the inter-ventricular septum (membranous part).
- The leaflets of the aortic valve must be shown only in the diastolic phase (aortic valve closed).
- Normal size of ascending aorta (similar to pulmonary artery).
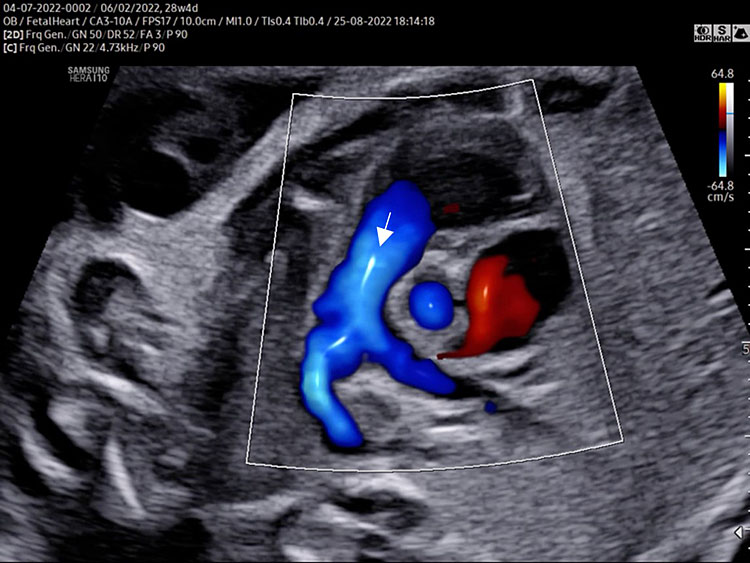
- A detailed anatomy scan of the five-chamber view in the mid-trimester fetal ultrasound scan was resumed in Figure 2 and Video 2.
(A) |
(B) |
2
The five-chamber view grayscale (A) assesses the presence of a great vessel with aortic characteristics “no bifurcation” (Ao) originating from the morphologic left ventricle. Continuity of the anterior wall of the aorta with the inter-ventricular septum (membranous part) should be documented. The aortic valve moves must happen freely. The leaflets should not be thickened and showed only in the diastolic phase (aortic valve closed). The size of the ascending aorta should be regular (similar to pulmonary artery). The two superior pulmonary veins enter the posterior wall of the left atrium at the five-chamber view level. Tricuspid valve and right ventricular inflow are often not seen in the five-chamber view. The five-chamber view color Doppler (B) assesses the laminar antegrade flow into the aortic artery and confirms the intact septum (arrow).
2
Five-chamber view.
Cardiac abnormalities commonly associated with an abnormal four-chamber view of the heart was reported in Table 1.
Right ventricular outflow view (long axis and short axis)
The right ventricular outflow view (RVOV) assesses the presence of a great vessel originating from the morphological right ventricle.
The right long axis view is obtained from the five-chamber view with a slight angulation of the transducer towards the fetal head (Video 3).
3
Right ventricular outflow view (short axis): scanning method.
The right short axis view is obtained from the four-chamber view with a slight angle of the transducer towards the fetal head and a slight rotation of the transducer towards the left shoulder (Video 3).
The right axis view (RVOT) checklist includes the following.
- Presence of a large vessel with pulmonary characteristics (bifurcation: the main pulmonary artery splits into left and right pulmonary artery) originating from the morphologic right ventricle.
- The pulmonary valve leaflets must be shown only in the diastolic phase (pulmonary valve closed).
- Normal size of the main pulmonary artery (usually slightly larger than the aortic artery).
- Regular great vessel (aorta-pulmonary) crossing.
- Normal course of ductus arteriosus (right short axis view) that connects the pulmonary artery to the descending aorta.
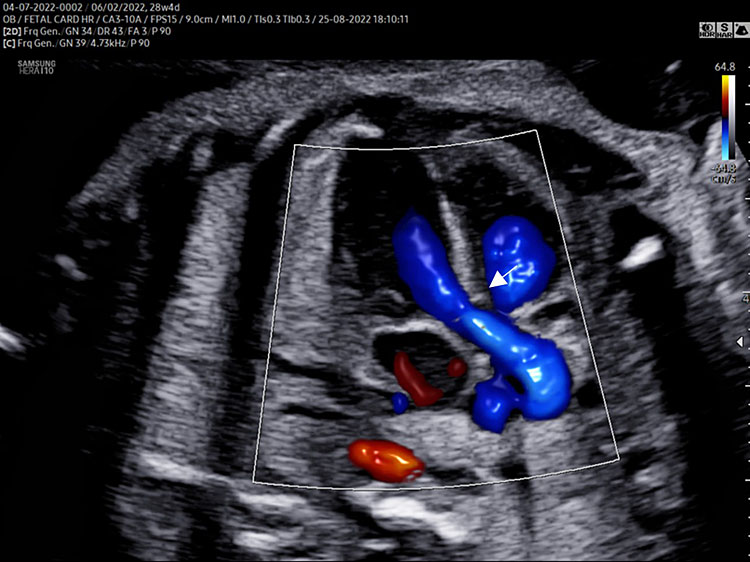
A detailed anatomy scan of the right ventricular outflow view in the mid-trimester fetal ultrasound scan was resumed in Figure 3 and Video 3.
(A) |
(B) |
(C) |
(D) |
3
The right outflow view [(A) long axis, (B) short axis] assesses the presence of a great vessel with pulmonary characteristics (*the main pulmonary artery splits into the left and right pulmonary artery) originating from the morphologic right ventricle (RV). The pulmonary valve moves must happen freely. The leaflets should not be thickened and showed only in the diastolic phase (pulmonary valve closed ^). The dimensions of pulmonary artery have to be regular (slightly larger than the aortic artery). Normal crossing of the great arteries can be assessed either directly in short axis view (B) or moving the transducer from five-chamber view to right long axis view and vice versa (video). Normal course of ductus arteriosus (DA arrow) [right short axis view (B)] that connects the pulmonary artery to the descending aorta (Ao). Long axis (C) and short axis (D) right outflow color Doppler view demonstrating laminar anterograde flow into the pulmonary artery (arrow).
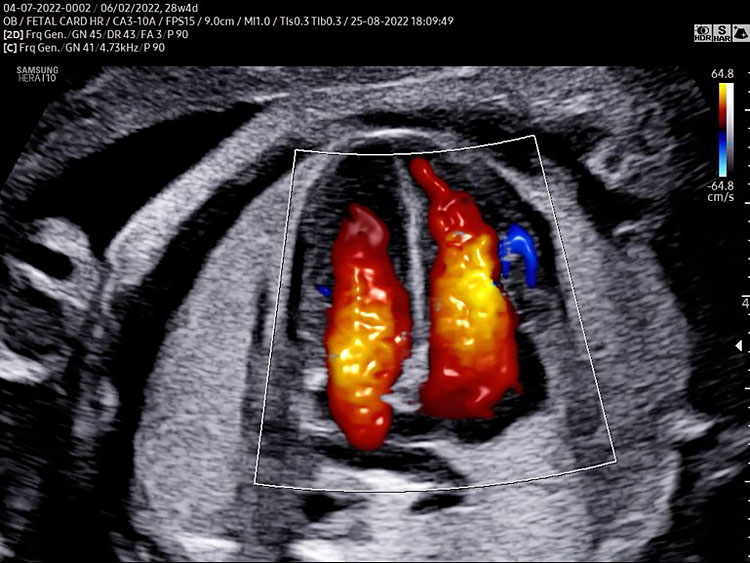
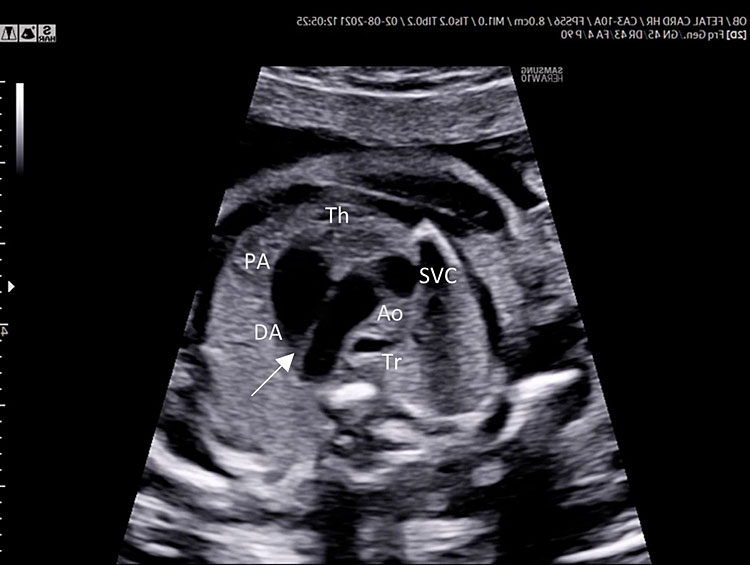
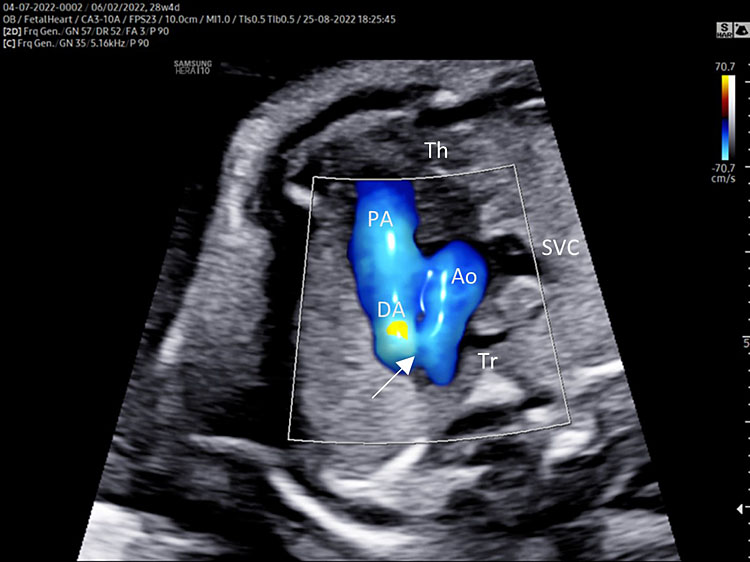
Three-vessel view and trachea (3VT)
Three-vessel view and trachea is obtained from the three-vessel view tilting the transducer towards the fetal head and a slight rotation of the transducer towards the left shoulder (Video 4).
4
Color Doppler.
This is the more important plane of the upper thorax evaluation.
In this plane several great vessels' congenital diseases can be detectable.
Three-vessel view and trachea checklist includes the following.
- Main pulmonary artery, ductus arteriosus (DA), transverse aortic arch (AoA).
- The DA converges into descending aorta “V shape signs”.
- Transverse section of superior vena cava.
- Transverse section of trachea.
- DA and AoA are directed to the left of the trachea (located anterior to the spine).
- Anterior the great vessels (PA and AoA), and behind the sternum can be shown the thymus.
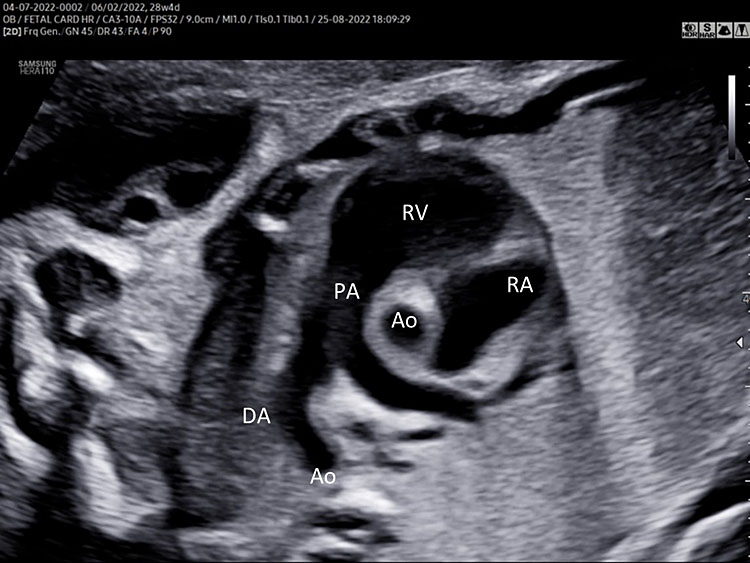
A detailed anatomy scan of the three-vessel view and trachea in the mid-trimester fetal ultrasound scan were resumed in Figure 4 and Video 4.
(A) |
(B) |
4
Three-vessel and trachea view (3VT): from left to right we can assess the pulmonary artery (PA) joining in the ductus arteriosus (DA), aortic arch (Ao), and superior vena cava (SVC). In this plane we can see the confluence (arrow) of the DU to the descending aorta in a "V shape" and their location to the left of the trachea (Tr). Ductus arteriosus and aortic arch have a similar size and blue color (ventral insonation) suggestive of anterograde flow (B). The anterior area behind the sternum and in front to the great arteries is occupied by the thymus gland (Th).
Fetal echocardiogram
Fetal echocardiogram is broadly defined as a detailed ultrasound evaluation that is used to identify and characterize heart anomalies before delivery. This specialized diagnostic procedure is an extension of fetal cardiac screening.
The echocardiography should be performed only for fetal–maternal valid medical reason indicated in recent guidelines of the American Institute of Ultrasound in Medicine (AIUM).9
Nevertheless, only 10% of fetal CHD cases occur in pregnancies with known risk factors, therefore the fetal-screening examination is the primary way to detect CHD before birth.4
The cardiac examination is performed optimally between 18 and 24 weeks’ menstrual age, although some cardiac structures may be better visualized before or after this period.
The fetal echocardiogram is a detailed evaluation of cardiac structure and function and includes the study of the following anatomical regions: four-chamber view, including pulmonary veins, left ventricular outflow tract, right ventricular outflow tract, branch pulmonary artery bifurcation, three-vessel view (including a view with pulmonary artery bifurcation and a more superior view with the ductal arch); short-axis views (“low” for ventricles and “high” for outflow tracts); long-axis view aortic arch, ductal arch, superior and inferior venae cavae.
Color and pulsed Doppler ultrasound should be used to evaluate heart rate and rhythm assessment, atrioventricular and semilunar valves' flow, ventricular and atrial septum, systemic and pulmonary veins, ductus venosus.
Cardiac biometry should be determined from two-dimensional images, including aortic and pulmonary valve anulus in systole and tricuspid and mitral valve anulus in diastole.9
Normal and main congenital diseases are resumed in Video 5.
5
Main congenital heart diseases commonly associated with abnormal four chamber view.
Abdomen
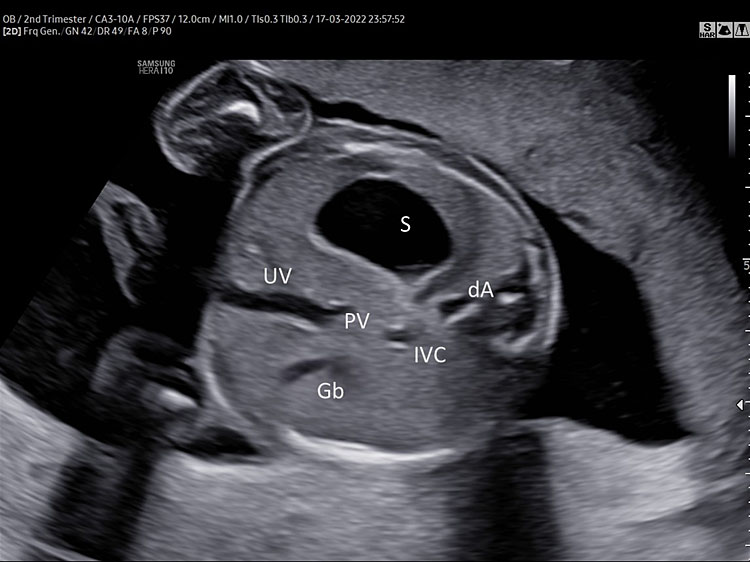
Abdominal circumference is obtained by a transverse section of the upper part of the fetal abdomen. This view is directly inferior to the four-chamber view. It can be obtained by placing the fetal spine horizontally across the screen and rotating the probe 90°.
The fetal spine has to be preferably in the 3- or 9-o’clock position, the stomach visible in the left side of the abdomen and the umbilical vein at the level of the portal sinus pointing right.
For the optimal measurement of AC, the transverse section of the fetal abdomen should be as circular as possible, one complete rib or no rib on each side of the fetal abdomen can be shown. Kidneys should not be visible (Figure 5).
(A) |
(B) |
(C) | |
5
(A) Normal transverse section of upper abdomen. Fetal spine three o’clock (fetus in breech position). Left side: stomach (S), and descending aorta (dA). Right side umbilical and portal vein (J shaped) inferior vena cava (IVC) and gallbladder (Gb). (B) Persistent non-visualization of the stomach. (C) 3D umbilical cord insertion.
Abdominal anatomy
The stomach (S) should be shown in the left side of the abdomen and should occupy about one-third of the left half of the transverse section of the fetal abdomen used for AC measurement.
A “J" shaped hypoechoic structure is seen in the midline; it should be one-third of the way across the abdomen and represents the internal portion of the umbilical vein (UV) branching to the right portal vein (PV).
The gallbladder (Gl) is visible as a tear shaped hypoechoic structure situated to the right anterior of the umbilical vein.
The large part of the fetal liver can be seen in the right side of the transverse section of the fetal abdomen.
Furthermore, the axial view of the fetal upper abdomen shows the anatomic location of the inferior vena cava (IVC) and its relationship to the descending aorta (DA). At this level the IVC is located anterior and to the right of the aorta.
The visualization of situs and shape of the stomach during upper abdomen section is recommended.
Persistent non-visualization or barely visible stomach, stomach expanding beyond the midline or presence of the “double bubble" should be reported to a reference center (Figure 5).
In a transverse section of the abdomen slightly inferior to the abdominal circumference it is possible to see the umbilical cord insertion of the fetal abdomen.
The best views are obtained with the cord insertion near the three or nine o’clock position. The skin line should be clearly visualized on both sides of the insertion. The base of the cord should enter cleanly into abdominal wall with no evidence of associated mass to suggest gastroschisis or omphalocele.
A detailed anatomy scan of fetal abdomen in mid-trimester was resumed in Figure 5.
The fetal kidney can be shown by a transverse section of the lower abdomen. Placing the spine uppermost we can obtain the best image. The kidneys can be seen on either side of the spine. Each hypoechoic renal pelvis can be measured in the anterior posterior direction. The normal renal pelvis measures ≤7 mm in the second trimester.1
In addition, a coronal section through the back just anterior to the fetal spine can be carried out.
A kidney should be seen on each side of the aorta. Each kidney is “C" shaped around a single renal pelvis (Figure 6).
(A) |
(B) |
(C) |
(D) |
6
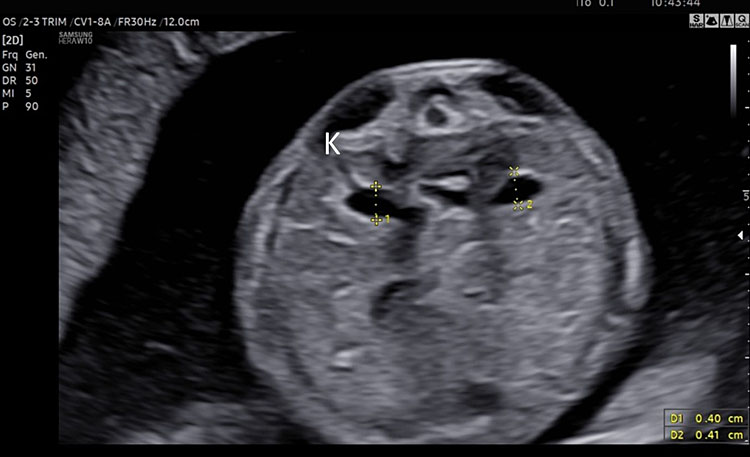
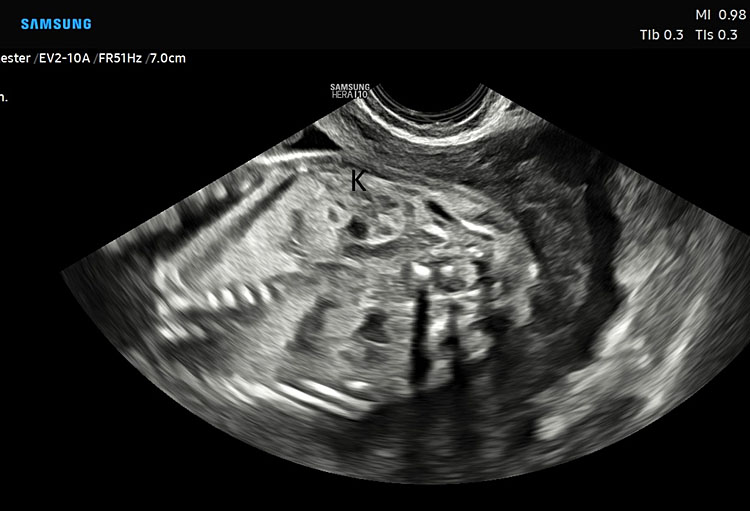
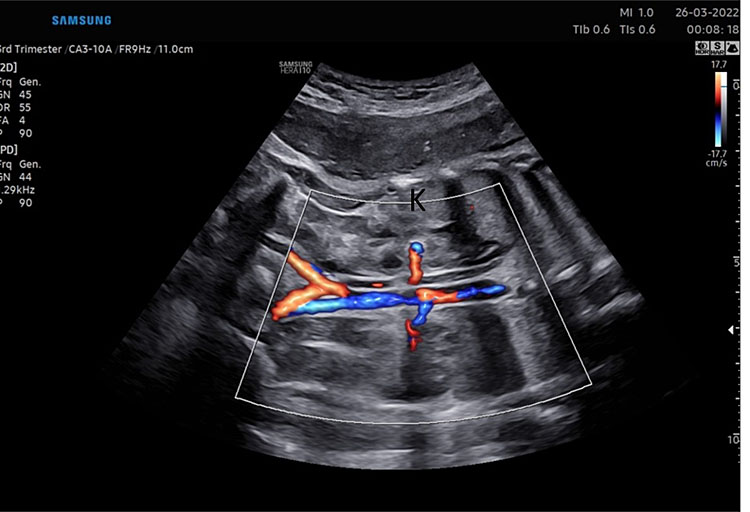
Fetal kidney (K) in transverse section of the lower abdomen (A) and coronal view (B). With the use of color Doppler, it is possible to identify the two renal arteries arising from the abdominal aorta (C) and the two umbilical artery around the bladder (D).
The axis of each kidney is roughly parallel to the aorta; if they angle towards each other at the caudal ends then this may indicate a horseshoe kidney. Each kidney should be similar sized (20–22 mm length in the second trimester) with no renal cysts visible. With the use of color Doppler, it is possible to identify the two renal arteries arising from the abdominal aorta.
Bladder and umbilical artery
This is a transverse section of the abdomen at the level of the bladder. Fluid should be seen within the bladder at some stage during the examination. The fetal bladder should not reach the level of the umbilical cord insertion. At 18 and 22 weeks, the 95th centile for the longitudinal bladder measurement is 14 and 23 mm, respectively.
A large bladder may be ”keyhole" shaped and may indicate urethral obstruction (partial or complete).
Conversely, persistent failure to visualize the bladder could be determined by a congenital malformation of the urinary tract.
Color or power Doppler is used to identify the two umbilical arteries that surround the bladder and then are directed towards the cord insertion (Figure 6).
These arteries need to be traced around the bladder towards the cord insertion to differentiate them from the iliac vessels that are seen more laterally in the pelvis directed towards the thighs. It is important to ensure that the Doppler settings are suitable for low flow to maximize the ability to detect both arteries.
Limbs and extremities
The presence of all four extremities (right and left arm, right and left leg) should be documented. The measurement of one femur is usually sufficient, unless there is suspicion of abnormality.
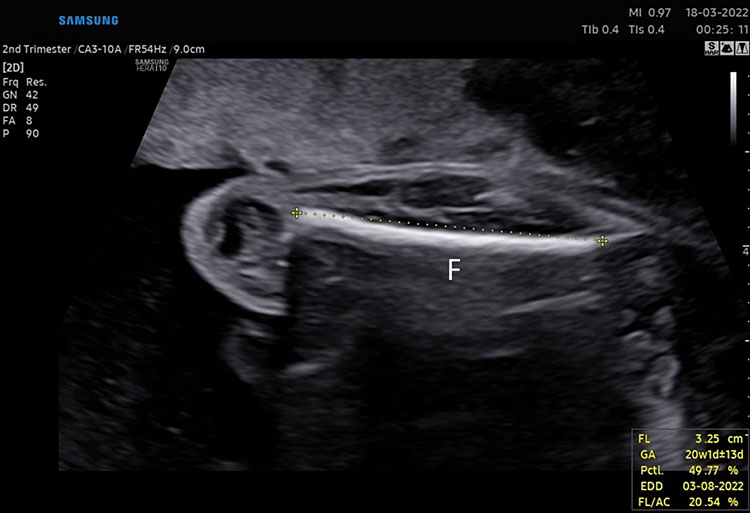
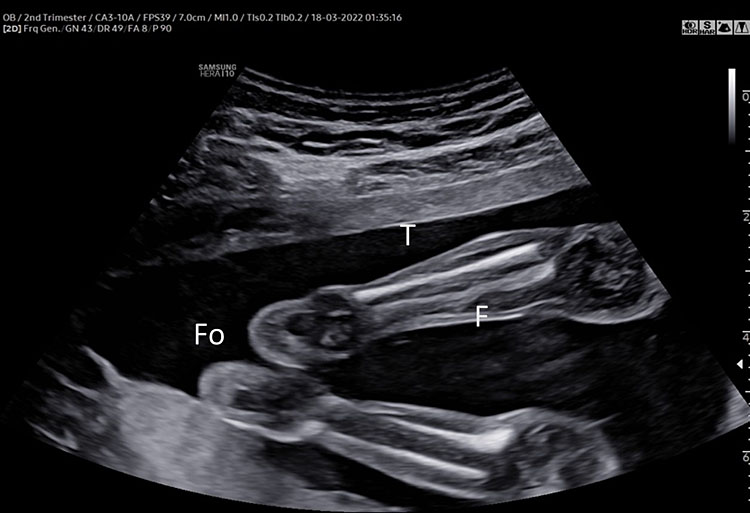
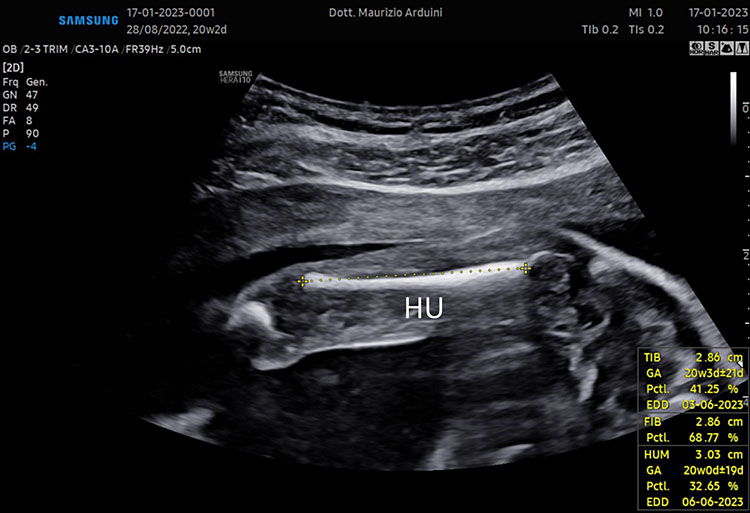
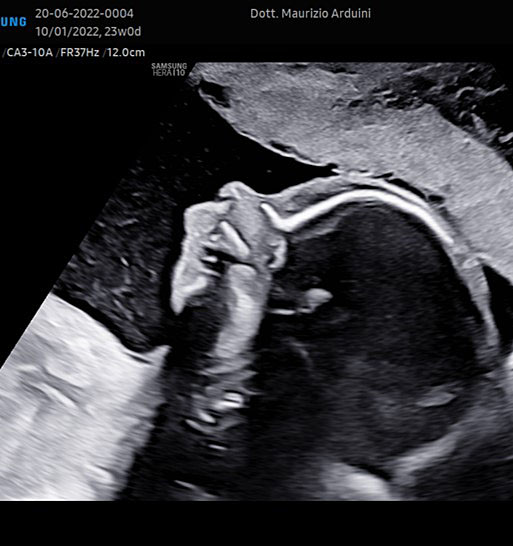
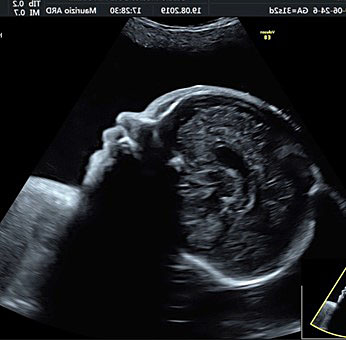
The femur should be measurement horizontally across the ossified diaphysis down the middle of the shaft of the bone avoiding any triangular echogenic extensions. An angle of insonation between 45° and 90° is typical (Figure 7).1
(A) |
(B) |
(C) |
(D) |
7
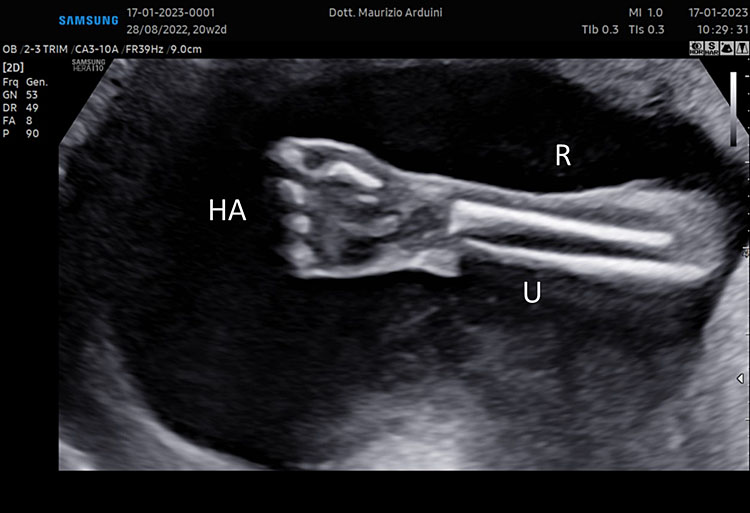
(A–B) lower limb, femur (F), tibia (T) fibula (F) and foot (Fo) in (C–D) upper limb humerus (HU), radius (R), ulna (U) and hand (HA).
The calliper is positioned at the ends of the ossified diaphysis without including the distal femoral epiphysis.
The anatomic study of the limb in mid-trimester ultrasound scan is recommended. All four limbs should be examined. The presence of all long bones, their symmetry, length, shape, alignment, position and movement should be surveyed. Counting fingers or toes is not required as part of the routine mid-trimester scan.
A detailed anatomy scan of fetal limb in mid-trimester fetal ultrasound scan was resumed in Figure 7.
Genitalia
The normal appearance of the external genitalia should be checked in the mid-trimester fetal ultrasound scan. The fetal genitalia are best assessed by taking two images. Firstly, a mid-sagittal image of the lower abdomen below the cord insertion; secondly a transverse image, just below the level of the bladder.1
Face
An examination of the face should be an essential part of any routine examination of fetal normality.
Three views are employed: axial, coronal and sagittal.
The basic examination of fetal face should include visualization of the upper lip, assessment of the presence and position of the orbits/eyes, and, if possible, evaluation of fetal profile.1
A detailed ultrasound face examination included other anatomical landmarks such as nose, nostrils, palate, maxilla, mandible, tongue, and position and size.
Ultrasound anatomy scan of upper lip, orbits and fetal profile in mid-trimester fetal ultrasound screening was resumed in Figure 8.
(A) |
(B) |
(C) |
(D) |
8
Bidimensional coronal view (A) upper and down lips, nose and nostrils. (B) Cleft lip. (C) and (D) 3D imaging in normal and clef lip.
(A) |
(B) |
(C) |
(D) |
9
Normal (A) and pathological fetal profile (B, C, D).
The fetal neck may be examined for its external contours and its interna structure. The fetal neck can be assessed in axial and sagittal views. The presence of obvious neck masses (cystic hygromas, goiter, or teratomas) should be documented (Figure 9).1
Central nervous system
The development of the central nervous system (CNS) is characterized by a complex interplay of growth and remodeling cycles of the various embryological structures that form it. This process begins during the first weeks of gestation. Around week 3 the neural plate originates from the superior portion of the thickened area of the ectoderm layer, dorsally to the column of mesenchymal cells, known as the notochord.
Three primary vesicles originate from the neural tube: the prosencephalon (forebrain), the mesencephalon (midbrain) and the rhomboncephalon (hindbrain).
The prosencephalon later gives rise to the telencephalon and the diencephalon, the rhomboncephalon to the metencephalon and myelencephalon, whereas the mesencephalon does not divide itself.
A genetic mutation or error that takes place during a specific step of CNS development is at the root of the different abnormalities that involve the CNS itself, which are among the most common congenital malformations.10 Their prevalence is of approximately 1–2 cases per 1000 births.11
Prognosis and postnatal neurocognitive development are variable and depend on the type of abnormality and the area of the nervous system that is involved.12,13
The CNS develops and matures throughout the whole period of gestation. As a result, neuroimaging assessment too changes throughout the pregnancy, depending on specific gestational age periods.
Ultrasonography (US) is the method of choice in the study of CNS anatomy and in the diagnosis and management of the congenital anomalies of this complex organ system.
To avoid diagnostic errors, it is crucial that the operator performing the US exam has a deep knowledge of neuroanatomy and the various stages of CNS development.
The second-trimester prenatal ultrasound screening test is used to diagnose these abnormalities, whereas neurosonography is employed for in-depth evaluation of the SNC and requires specific skills and appropriate technical facilities. Technological developments in ultrasonography have increased imaging resolution and diagnostic sensitivity of congenital CNS malformations (at present between 70 and 90%), also owing to the integration of 3D US, which allows neuroanatomy evaluation in the axial, sagittal, parasagittal, and coronal planes.14,15
In selected cases, fetal magnetic resonance imaging (MRI) may add useful information concerning cortical development and should preferably be performed at an appropriate gestational age (after 24 weeks of gestation), to optimize prenatal diagnostic accuracy..16,17
During the first trimester of pregnancy, it is possible to diagnose some serious malformations of the central nervous system with transvaginal and transabdominal ultrasound; in the second and third trimester, transabdominal US is employed to study the CNS and uses specific standardized planes. The evaluation of the CNS becomes more complex at later stages of gestation as skull ossification and fetal position may limit its visualization.18,19,20,21
Second-trimester ultrasonography has limitations mainly due to the fact that it uses axial planes, which may limit the visualization of the ipsilateral (with respect to the transducer) brain hemisphere, or of median structures such as the corpus callosum. Other limiting factors that may influence the quality of the exam are maternal tissue echogenicity and the type of machine and probes employed.
The optimal choice of probe and sound frequency depends on maternal habitus, fetal position, and the approach that is utilized.
Most CNS screening exams for low-risk pregnancies use 3.5 MHz convex transabdominal probes. If the fetus is in breech position the transabdominal probe should be positioned parallel to the maternal abdomen instead of perpendicularly.
If the fetus is in a cephalic presentation, it is possible to deepen CNS examination using a 5–10 MHz transvaginal probe, which allows evaluation of the coronal and sagittal planes, thereby increasing the accuracy of the exam for the diagnosis of congenital anomalies.
Neurosonography utilizes 3D volume acquisition imaging and color Doppler technique for cerebral vasculature identification.22,23,24,25,26,27
Basic CNS evaluation
A CNS screening test, using the transabdominal approach, includes a morphostructural and biometric study of the fetal head and spinal cord.28
To obtain high-quality images it is important to be familiar with the different sutures and fontanelles of the skull, firstly as they may be mistaken for anatomical defects and secondly as they can be employed as “acoustic windows” for the study of the fetal brain anatomy.
The cranial vault is formed by five bones: two frontal bones, two parietal bones, and one occipital bone (Figure 10).
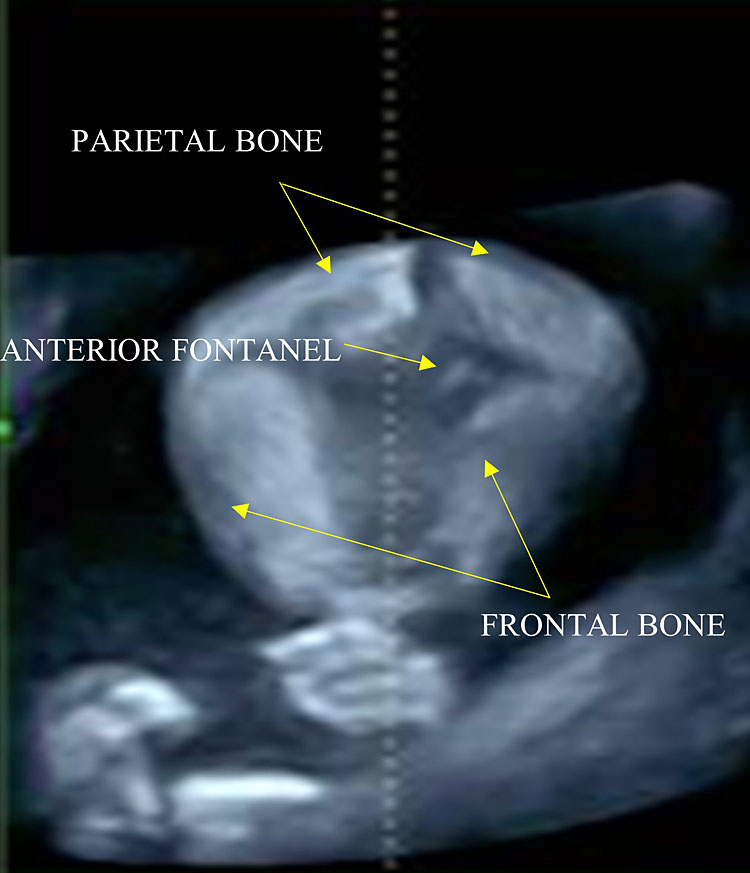
10
The anterior fontanel has a rhomboid shape and is located at the level of the bregma, where the two frontal and parietal bones converge. Scanning the fetal head in a cranial-caudal direction.
The junctional lines between these structures are called sutures and they meet in six points called fontanelles, two of which are located along the median line of the skull:
- The anterior (also known as bregmatic or quadrangular) fontanelle: it is the largest one, it has a rhomboid shape and is located at the level of the bregma, where the two frontal and parietal bones converge. It is visible using a median sagittal plane.
- The posterior (also known as lambdoid or triangular) fontanelle: it is located at the level of the lambda, at the point of convergence between the two parietal bones and the occipital bone.
The remaining four fontanelles are much smaller and are located on the lateral sides of the skull (two on each side):
- Two sphenoidal fontanelles, they are more anterior and are found at the level of the pterion, where the parietal, temporal, frontal, and sphenoidal bones meet.
- Two mastoid fontanelles, located more posteriorly at the level of the asterion, the point of convergence between the parietal, temporal, and occipital bones.
These fontanelles, along with the squamous suture, form an ideal acoustic window for the evaluation of the encephalic structures using transverse planes, whereas the mastoid fontanelle is used for the visualization of the cerebellum.29
Using the main axial/transverse planes it is possible to study the CNS, the interhemispheric falx (midline), the lateral ventricles, the choroid plexus, the cerebellum, the cisterna magna, the cavum septum pellucidum, and the bony structures of the skull; and to evaluate its integrity, bearing in mind that it is a dynamic, continuously developing, structure.
Severe abnormalities are often bilateral or associated with midline distortion or deviation, for this reason a basic CNS study should include evaluation of CNS symmetry.
There are three axial planes:
- Transventricular.
- Transthalamic (used for the evaluation of fetal head biometry).
- Transcerebellar.
These planes are obtained by scanning the fetal head in a cranial-caudal direction.28
The transventricular plane
This is the most superior plane and passes through the bodies of the lateral ventricles. This plane allows the visualization of the lateral ventricles, the cavum septum pellucidum, and the interhemispheric fissure (Figure 11).
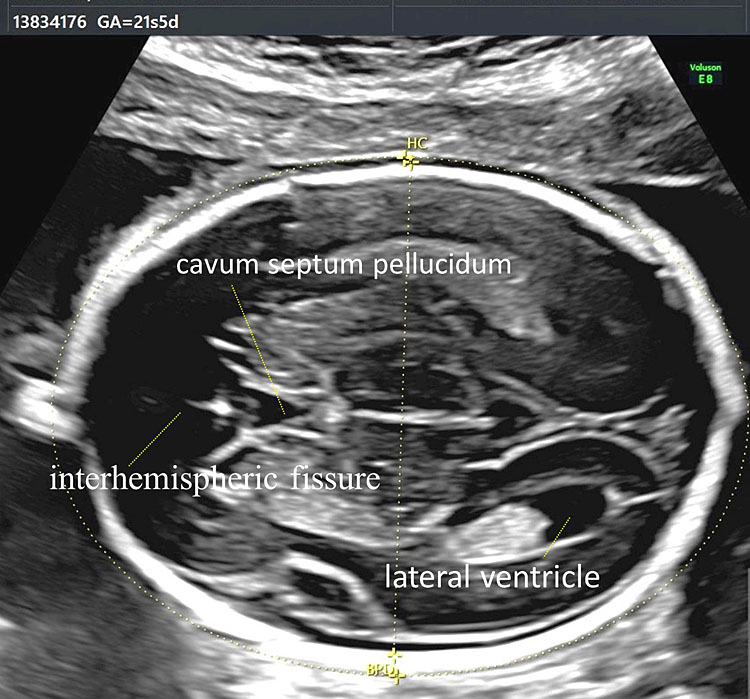
11
Transventricular plane allows the visualization of the lateral ventricles, the cavum septum pellucidum and the interhemispheric fissure.
Using this plane only the contralateral hemisphere, with respect to the transducer, can be correctly studied, as the ipsilateral hemisphere is more difficult to evaluate due to the presence of artifacts.
The lateral ventricles have both an anterior and posterior portion; beginning in the second trimester we may distinguish in each ventricle an anterior horn, body, atrium, and posterior horn, which is in part occupied by the choroid plexus, which has begun reabsorption. During the second trimester, the posterior portion of the lateral ventricle (posterior or occipital horn) is a structure formed by the atrium, which continues posteriorly in the posterior horn (Figure 11).
The atrium is nearly entirely filled by the glomus of the choroid plexus, which appears as hyperechoic, whereas the occipital horn is filled with fluid (Figure 12).
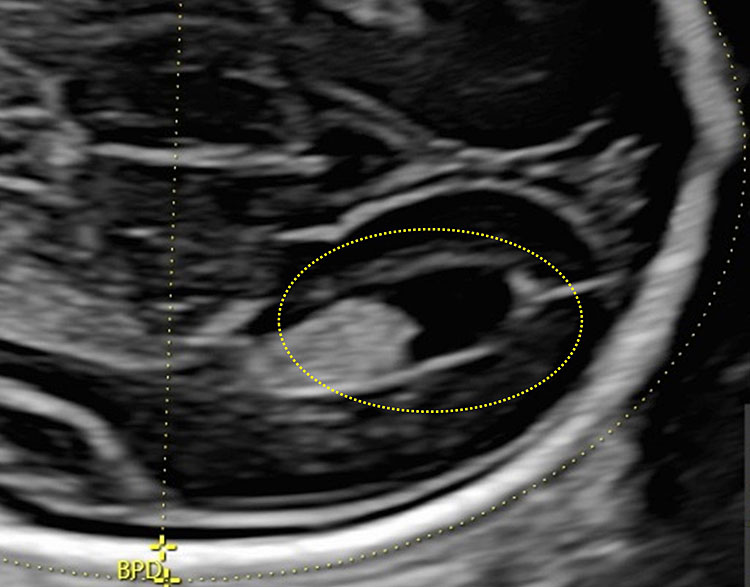
12
The posterior portion of the lateral ventricle (posterior or occipital horn) is a structure formed by the atrium, which continues posteriorly in the posterior horn.
Using this plane, it is possible to assess the size of the atrium by measuring the distance between the medial and lateral ventricular walls, which appear as hyperechoic lines that are parallel to the median line (Figure 13). Using a slightly more caudal plane, it is possible to visualize the anterior portion of the lateral ventricles (anterior horns) separated from each other by the cavum septum pellucidum (CSP). They appear as two fluid-filled, anechoic structures with a lateral hyperechoic wall.
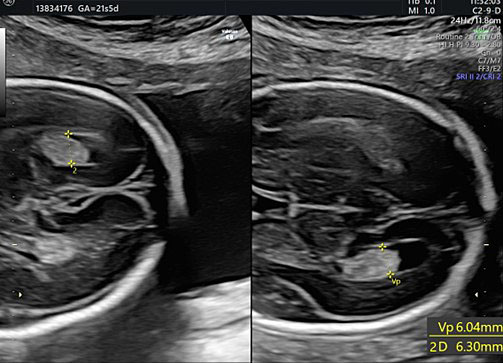
13
The glomus of the choroid plexus in distal and proximal ventricle. In this plane it is possible to assess the size of the atrium by measuring the distance between the medial and lateral ventricular walls, which appear as hyperechoic lines that are parallel to the median line.
The CSP is a fluid-filled cavity delimited by two thin membranes, which becomes visible around 16 weeks of gestation. This structure becomes obliterated late during gestation or during neonatal life due to fusion of these two membranes, which gives rise to the septum pellucidum. CSP should be identified during the second-trimester screening exam (Figure 14).30
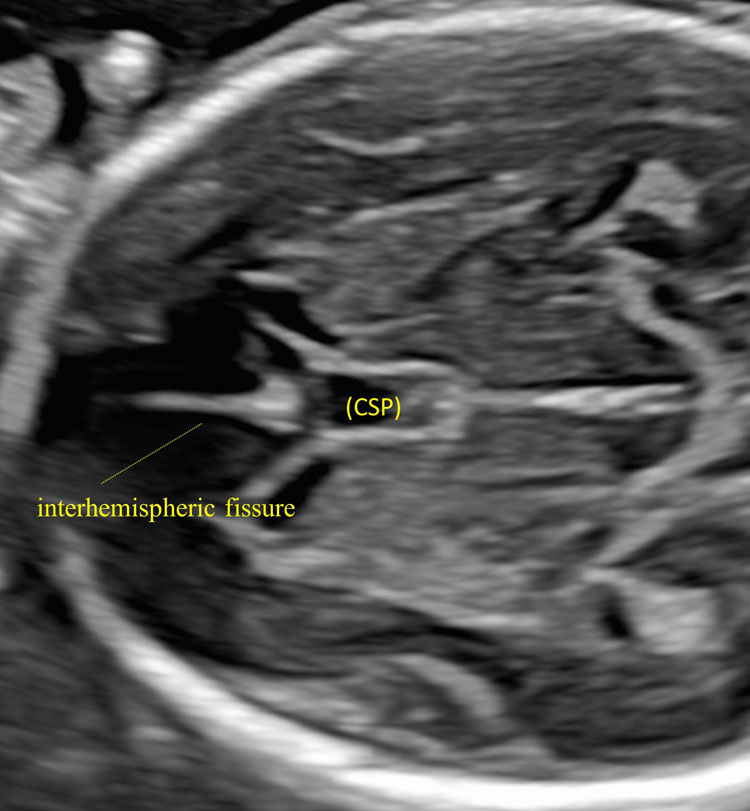
14
The anterior portion of the lateral ventricles (anterior horns) separated from each other by the cavum septum pellucidum (CSP).
Its absence or alteration has been associated with various cerebral abnormalities such as holoprosencephaly, corpus callosum agenesis or dysgenesis, severe hydrocephaly, or septo-optic dysplasia.30,31,32
Non-visualization of this structures before 16 weeks or after 37 weeks should not be considered pathological.
The transthalamic plane
This plane is used to evaluate fetal head biometry by measuring the biparietal (or transthalamic) diameter and the head circumference. It is located below the transventricular plane and includes the following anatomical landmarks: the frontal horns of the lateral ventricles, the cavum septum pellucidum, the thalami and the hippocampal gyri (Figure 15).29
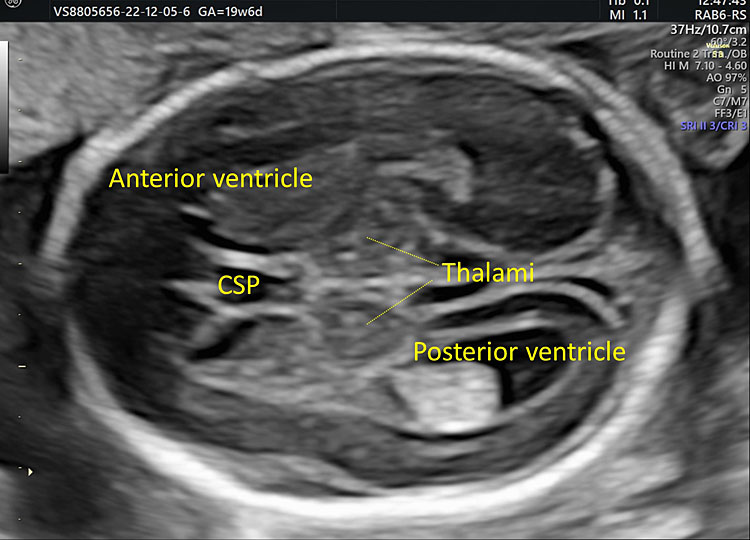
15
The transthalamic plane: it is located below the transventricular plane and includes the frontal horns of the lateral ventricles, the cavum septum pellucidum, the thalami, and the posterior horns of the lateral ventricles.
The lateral ventricles join in the third ventricle, a thin anechoic space located between the two hypoechoic thalami. This plane allows the evaluation of the subarachnoid spaces and the surface of the cerebral hemispheres. Laterally we can observe the external surface of the insular lobe, which is delimited by the frontal and occipital operculum, and will then form the Sylvian fissure. In the occipital area, it is possible to see the parieto-occipital fissure (Figure 16).
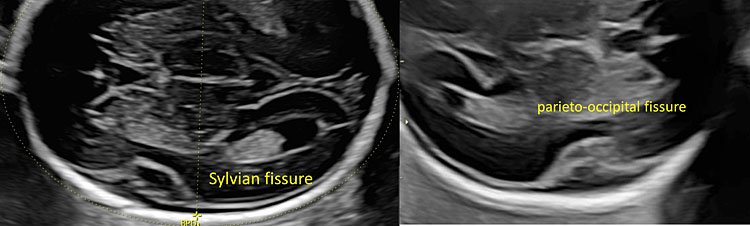
16
In the lateral part of the cerebral hemisphere you can observe the external surface of the insular lobe is delimited by the frontal and occipital operculum (Sylvian fissure). In the occipital area, it is possible to see the parieto-occipital fissure.
The transcerebellar plane
This plane is used to study the posterior cranial fossa (PF) and can be obtained by tilting the transducer posteriorly and by moving it slightly inferiorly compared to the transventricular plane (Figure 17).
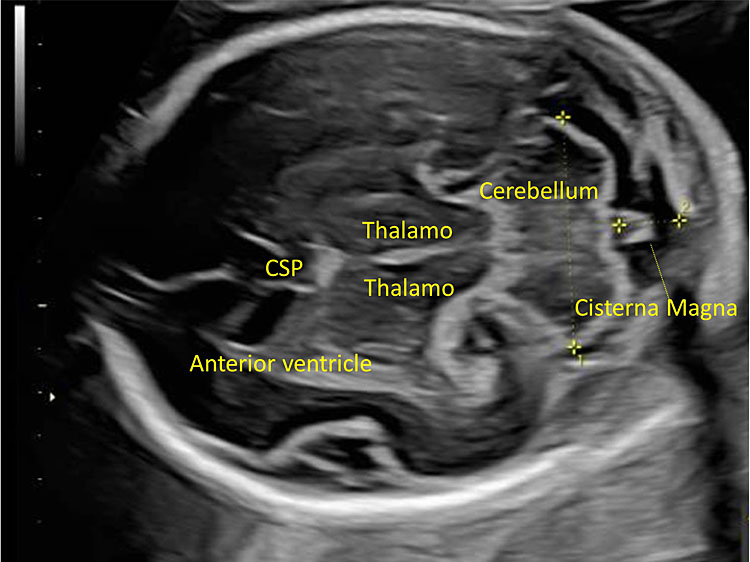
17
The transcerebellar plane allows the visualization of the frontal horns of the lateral ventricles, the CSP, the thalami, the cerebellar peduncles, the cerebellum and the cisterna magna.
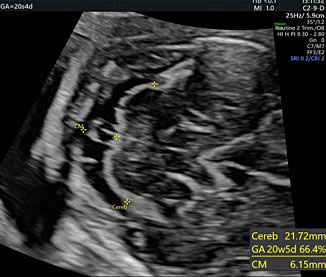
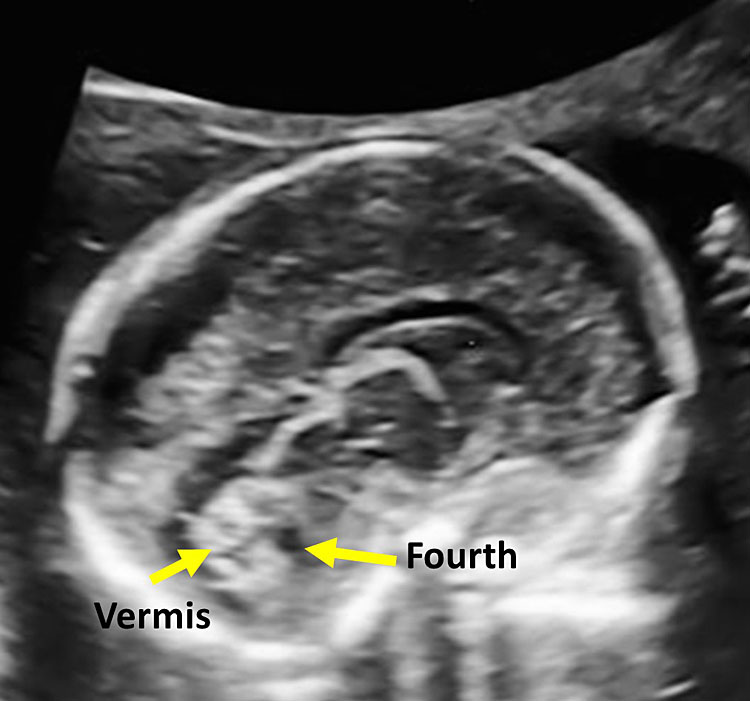
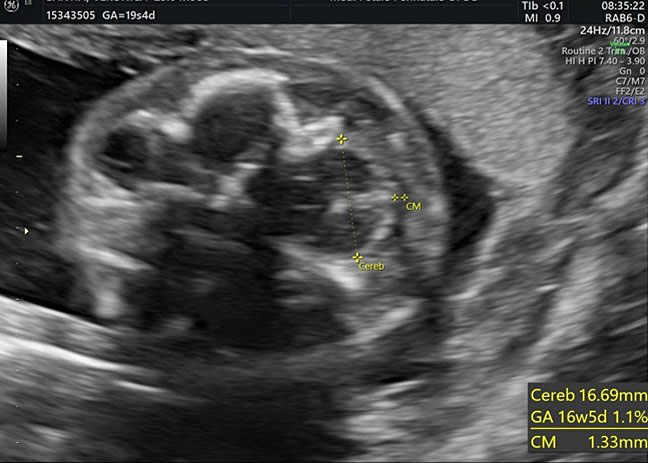
It allows the visualization of the frontal horns of the lateral ventricles, the CSP, the thalami, the cerebellar peduncles, the cerebellum, and the cisterna magna. The cerebellum is shaped like the “number-eight” and is formed by the cerebellar vermis medially, which appears slightly more hyperechoic with respect to the lateral cerebellar hemispheres. The transverse cerebellar diameter (TCD) should be measured using this plane. Anteriorly to the vermis and posteriorly to the cerebellar peduncles we find a small anechoic space known as the fourth ventricle. The cerebellum is surrounded posteriorly and laterally by another fluid-filled cavity, the cisterna magna (CM), or cisterna cerebello-medullaris. Thin septa can be found within this cavity and should not be confused with vascular or cystic structures. In some fetuses, it may be possible to observe remnants of Blake’s pouch. These are vestigial remnants that appear as two paramedian lines that may mimic a cystic structure (Figure 10).
Dimensions of the cisterna magna are measured using this plane (Figure 18).29,33
(A) |
(B) |
18
(A) Dimensions of the cerebellum and cisterna magna and retrocerebellar septa. (B) The medial sagittal plane: the vermis and the fourth ventricle.
By moving the transducer anteriorly, it is possible to see the orbits (Figure 19).
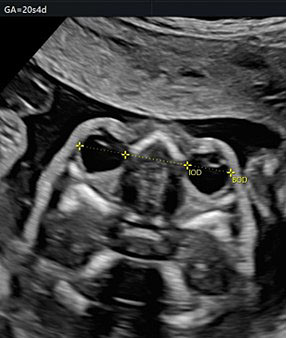
19
The orbital diameter and the measures of the binocular and interocular distance. BOD, binocular; IOD, interocular.
The sagittal and coronal planes
Fetal skull conformation and facial profile should also be evaluated.
Abnormal cranial conditions, such as craniostenosis, trigonocephaly, or turricephaly, can be a sign of bone abnormality or the result of a persistent abnormal fetal position, as in dolichocephaly, due to positional molding that affects breech fetuses. Visualization of the fetal profile allows the assessment of the fetal forehead, prefrontal thickness, nasal bone, nasal tip, fronto-nasal angle, and fetal chin (Figure 20).
Abnormal morphology of one of these structures could be a direct or indirect sign of fetal pathology.
These planes are more appropriate for the study of interhemispheric structures.
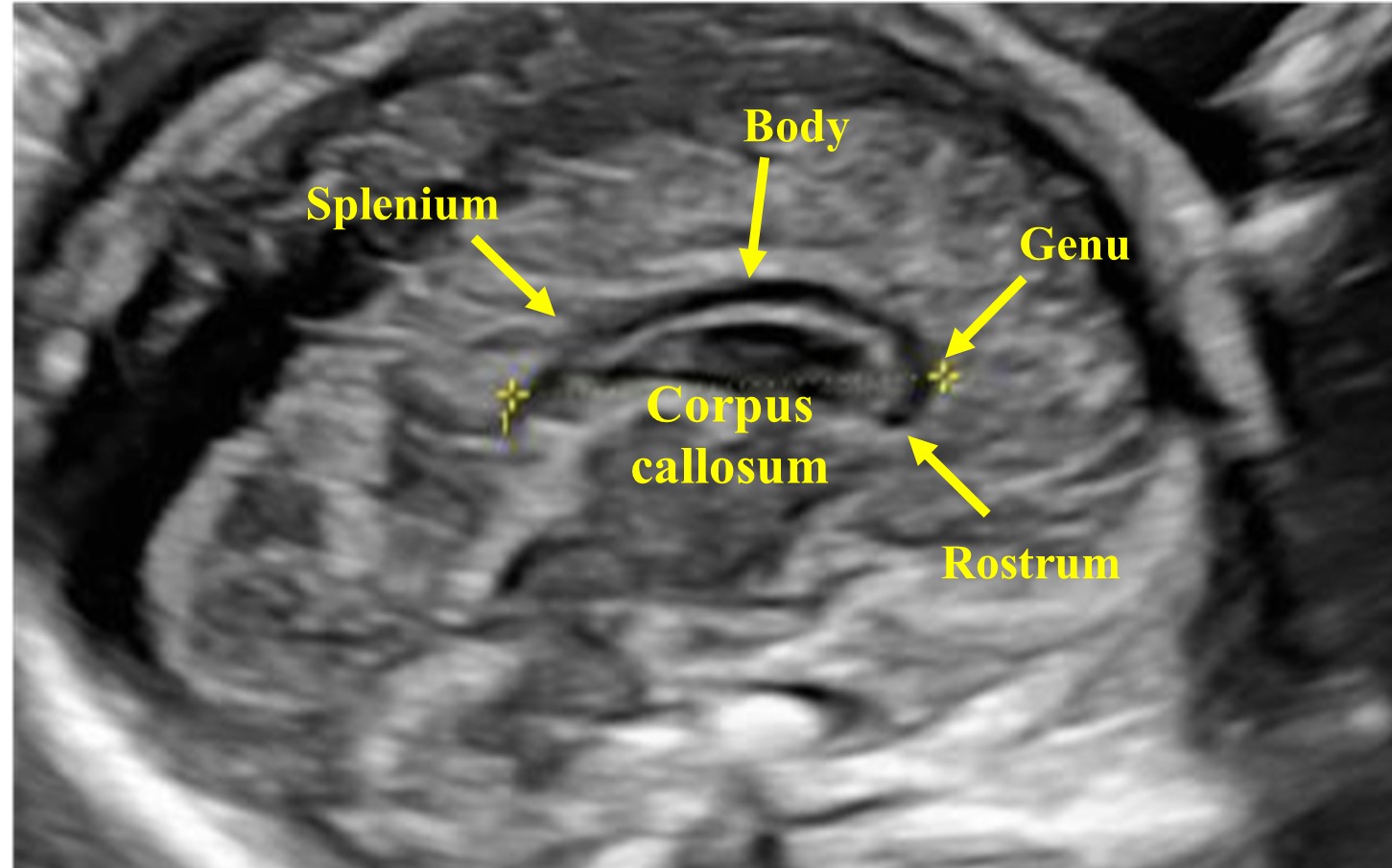
The sagittal plane can be obtained by placing the transducer above the anterior fontanelle. Using the medial sagittal plane, the corpus callosum (CC) can be evaluated, which is not possible using an axial plane. This structure can be observed from the second trimester onward, and it completes its morphogenesis around 18–20 weeks of gestation. It is a hypoechoic C-shaped structure, composed by the rostrum, the genu, the body, and the splenium (Figures 21 and 22).
Superiorly to the corpus callosum is the cingulate gyrus, located above the cavum septum pellucidum and the cavum vergae. Inferiorly is the third ventricle, postero-inferiorly the tentorium cerebelli and the posterior cranial fossa with the cerebellar vermis and the fourth ventricle.
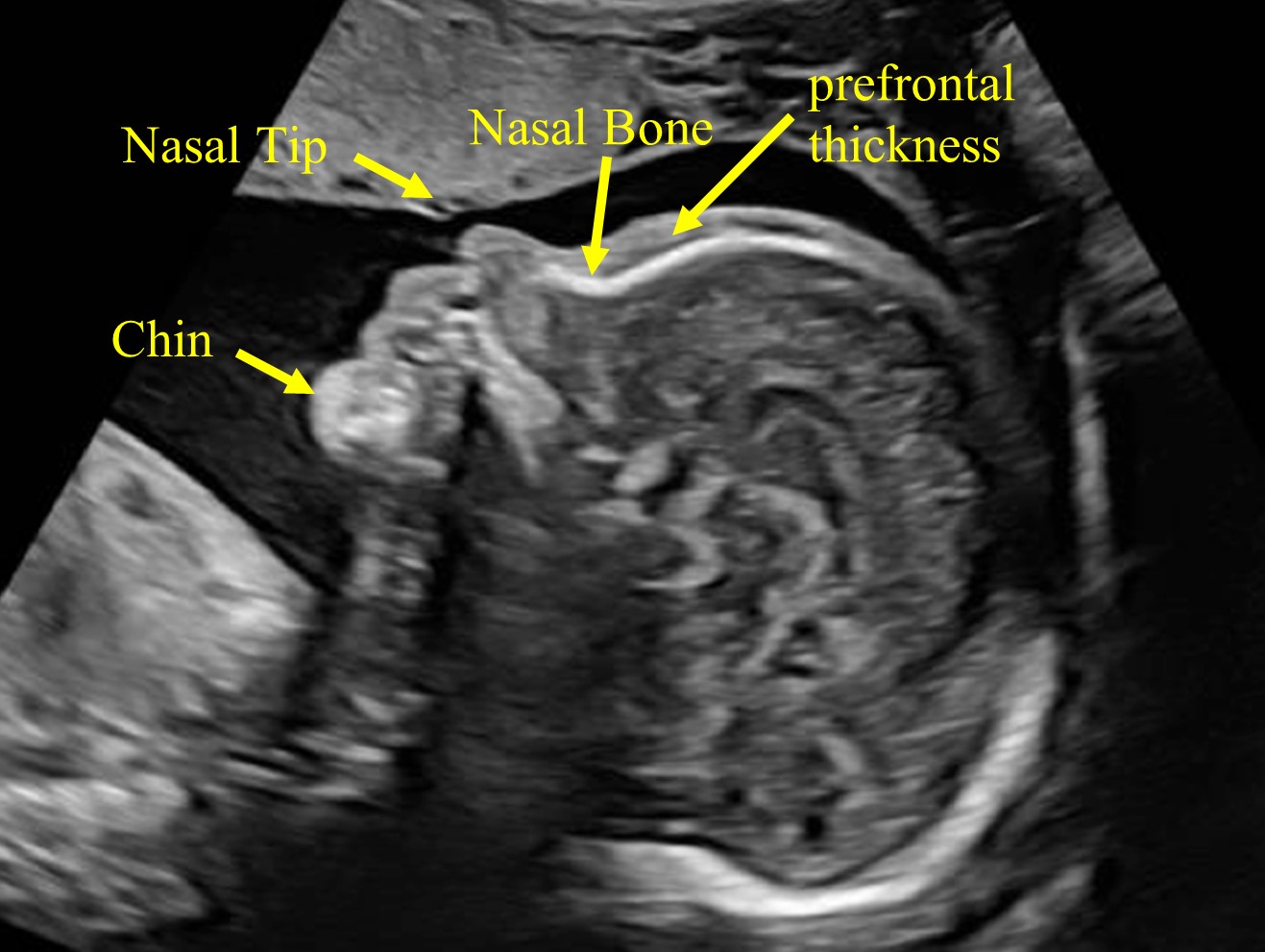
20
The fetal profile allows the assessment of the fetal forehead, prefrontal thickness, nasal bone, nasal tip, fronto-nasal angle, and fetal chin.
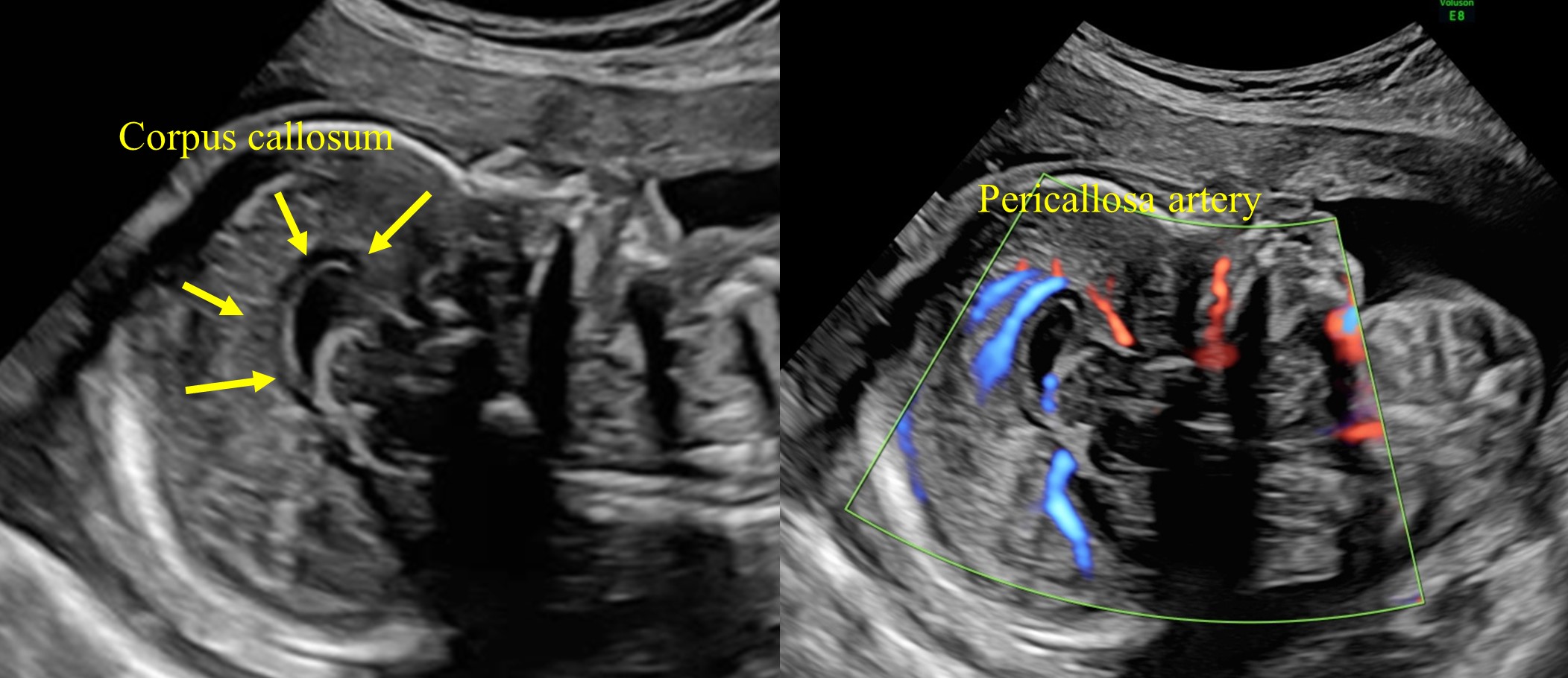
21
The medial sagittal plane, the corpus callosum, and pericallosa artery.
22
The corpus callosum (CC) is composed by the rostrum, the genu, the body, and the splenium.
The spinal cord
During the second-trimester screening test, the spinal cord should be examined using a longitudinal plane, while with neurosonography the following planes are used: axial, coronal, and sagittal.20,28,29
In the axial plane we can observe the three ossification centers of vertebrae, instead in sagittal plane we can also study the spinal cord and conus medullaris.
Skin integrity of the underlying vertebra should be examined using both an axial and longitudinal plane.
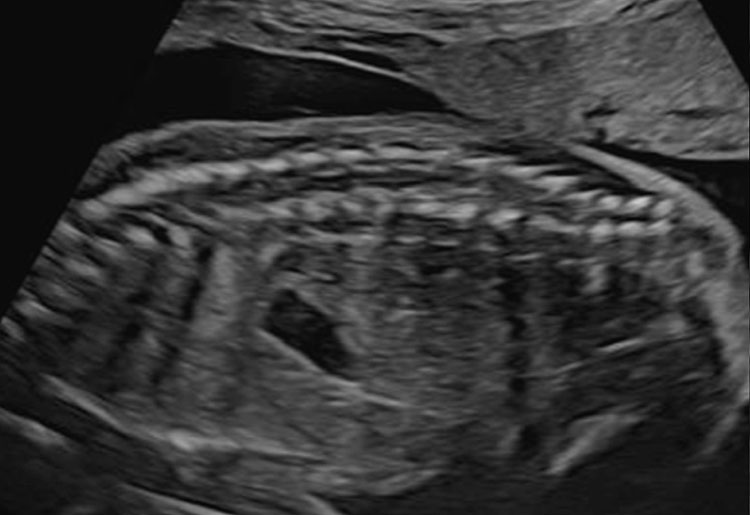
Depending on transducer orientation, the spinal cord appears as two or three parallel lines, that are formed by hyperechoic structures, which extend from the first cervical vertebra to the sacrum. These are the ossification centers of the vertebra (one at the center of the body of the vertebra and one between the lamina and the pedicle on each side of the vertebra) that surround the neural tube (Figures 23–25).
23
The spinal cord in sagittal plane with the ossification centers of vertebrae.
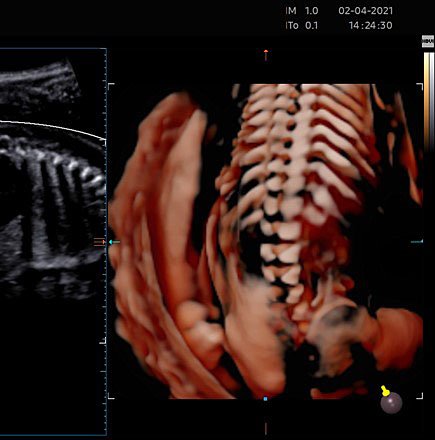
(A) |
(B) |
24
(A) The caudal portion of spinal cord and conus medullaris. (B) Spinal cord in axial plane and the ossification centers of vertebrae.
|
|
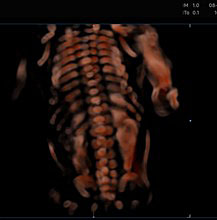
25
The spinal cord using 3D ultrasound.
Fetal position represents a limiting factor of the fetal cord exam. If the fetus is breech, a transvaginal approach may be useful. Spinal cord study should also exclude other spinal cord abnormalities such as morphofunctional abnormalities of the vertebra and sacral agenesis.29
SNC biometry
Biometry is a fundamental part of the second-trimester screening exam and includes measurement of the biparietal diameter (BPD), the head circumference (HC), the lateral ventricular diameter (AW), the transverse cerebellar diameter (TCD), and cisterna magna depth (CM).
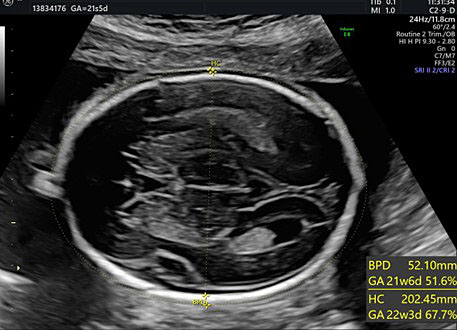
The BPD and the HC are used to assess fetal growth, to identify specific CNS abnormalities and, during the second trimester, to estimate effective gestational age. Both the transventricular and transthalamic planes can be used for these measurements. Different growth charts can be used where specific measures are associated with each gestational week.34,35,36
For BPD measurement the callipers must be placed at the outer edge of the near calvarial wall and at the inner edge of the far calvarial wall (external–internal measure) (Figure 26).1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,
32,33,34,35,36,37
26
The measurement of the biparietal diameter (BPD), the head circumference (HC).
External–external BPD is rarely used, and this measurement requests the use of different growth charts.28
Head circumference (HC) can be obtained using a mathematical formula that takes into consideration both the BPD and the occipitofrontal diameter. However, this formula has been overcome by modern ultrasonography devices that calculate the measure of an elliptical shape (the head circumference) by positioning the callipers of the area on the outer edges of the hyperechoic skull (Figure 26).
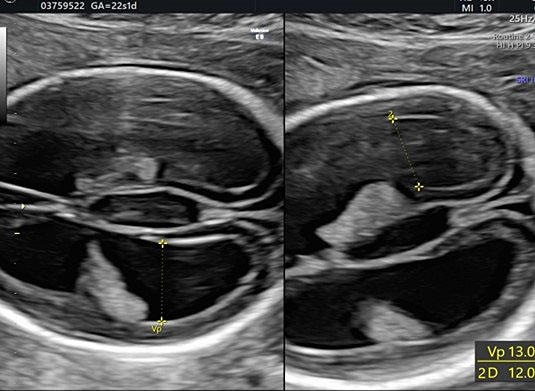
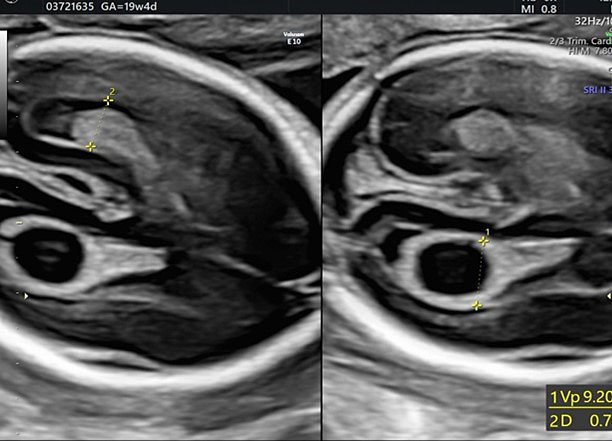
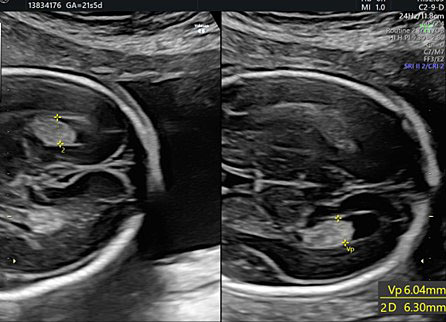
During second-trimester screening, measurement of the lateral ventricle should be performed at the level of the atrium, adjacent to the glomus of the choroid plexus, perpendicularly to the ventricular cavity (atrial width or AW). To avoid errors the lateral ventricle of the contralateral hemisphere, with respect to the transducer, should be measured, due to the presence of artifacts on the ipsilateral side. The callipers should be placed on the inner edges of the ventricular walls, using an internal–internal mode (Figure 27), to exclude ventriculomegaly. During the second trimester, its average length is 6–8 mm and it is considered normal when smaller than 10 mm.38,39,40 When this value is greater than 10 mm, we can define ventriculomegaly, which is a US finding possibly related to abnormal CNS development (Figure 27).40
27
The measurement of the lateral ventricular diameters.
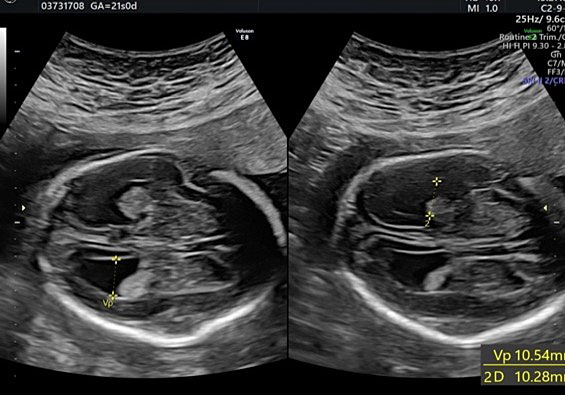
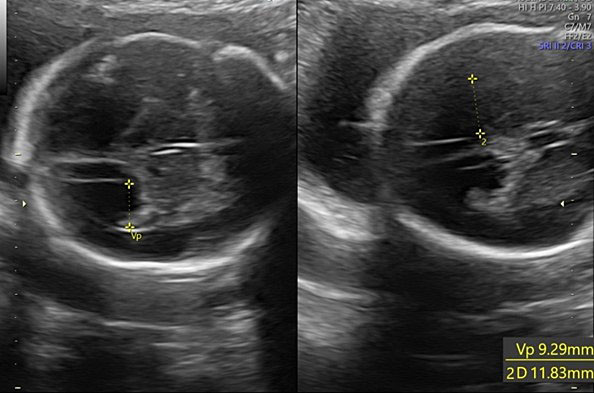
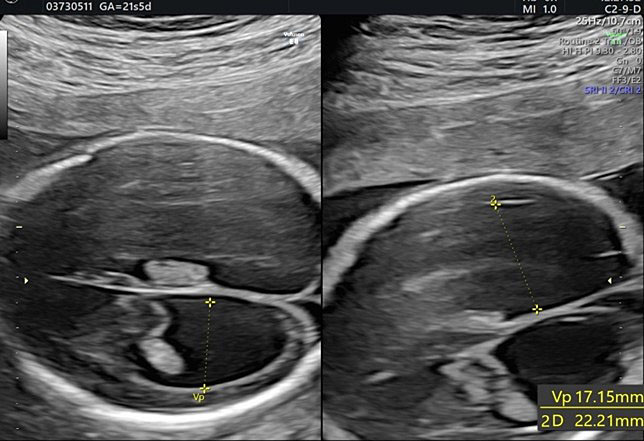
Transverse cerebellar diameter is measured using the transcerebellar plane, in which the cerebellum, vermis, fourth ventricle, and cisterna magna should be observed symmetrically (Figure 28).
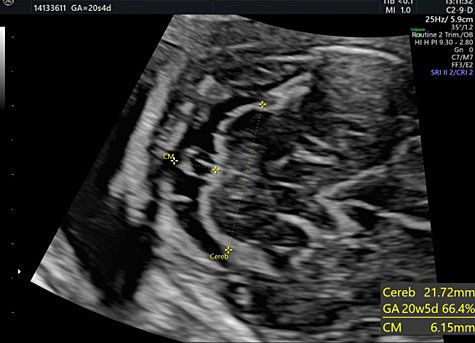
28
The transverse cerebellar diameter (TCD) and cisterna magna depth (CM).
The length of this diameter increases by approximately 1 cm per week from the beginning of the second trimester to approximately 21–22 weeks of gestation.41 This measure, along with the BPD and HC, is used to estimate gestational age during the second trimester.
The cisterna magna is measured by positioning the callipers between the posterior portion of the vermis and the internal edge of the occipital bone. During the second trimester, it should measure less than 10 mm (Figure 28).42
In the case of dolichocephaly, it is possible that this measure may be slightly increased.
Imaging fetal brain abnormalities
As previously mentioned, CNS abnormalities are among the most common congenital birth defects. The fetal abnormalities that can be diagnosed in the screening ultrasound of the second trimester of gestation are as follows: corpus callosum abnormalities (agenesis, hypoplasia/dysgenesis), absent cavum septi pellucidi, ventriculomegaly, posterior cranial fossa anomalies (vermian hypoplasia, cerebellum hypoplasia, Dandy–Walker malformation, Blake’s pouch cyst), intracranial cysts, encephalocele and myelomeningocele.
Below are some images of such diseases (Figures 29, 30 and 31).
Abnormalities of corpus callosum
The abnormalities of corpus callosum are as follows: agenesis, partial agenesis, hypoplasia/dysgenesis.
Agenesis of corpus callosum is characterized by the complete absence of all components of this median structure and by an anomalous development of the pericallous arteries.
Partial agenesis is characterized by the lack of a part of corpus callosum.
In the hypoplasia/dysgenesis the corpus callosum has reduced dimensions or appears thinned or dysmorphic.
In addition to the absence/hypoplasia/dysgenesis of corpus callosum, these abnormalities are characterized in the axial plane by non-visualization of cavum septi pellucidi, dilatation of occipital and frontal horns, ventriculomegaly, and colpocephaly. In coronal the third ventricle appears elevated (Figure 29).14,31,43,44,45,46
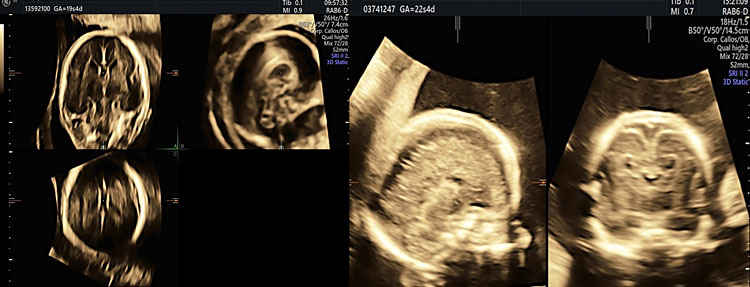
29
Corpus callosum abnormalities (hypoplasia agenesis).
(A) |
(B) |
(C) |
(D) |
(E) | |
30
Different conditions of ventriculomegaly related to an abnormality of the ventricular system. (A) Cerebral malformation and ventruculomegaly. (B) Isolated ventriculomegaly. (C) Chiari II malformation and ventruculomegaly. (D) Choroid plexus cysts. (E) Severe ventriculomegaly.
Absent cavum septi pellucidi
This is an anomaly characterized by the absence of the membrane of the septum pellucidum and is associated with several abnormalities of the CNS. In the transthalamic axial plane and in the coronal plane we can see a communication between anterior horns.14,45
Ventriculomegaly
Ventriculomegaly is a condition in which the size of the lateral ventricles is increased (>10 mm). It presents as monolateral or bilateral and may be related to an abnormality of the ventricular system and brain development with different etiopathogenesis (Figure 30).47
(A) |
(B) |
31
Vermian hypoplasia: the reduction of the vermian diameter can be assessed in the axial plane (A) and in the sagittal plane with increase of the pontus cerebellar angle and expansion of the fourth ventricle (B).
Posterior cranial fossa anomalies
In transcerebellar and in sagittal plane you can study morphology, position, biometry, and echogenicity of the cerebellum, the vermis, the fourth ventricle, the tentorium, and cisterna magna.
You can find a decreased transcerebellar diameter, <5th percentile for gestational age, in the case of cerebellar hypoplasia, but also the reduction of the vermian diameter with normal cerebellar hemispheres, that suggests vermian hypoplasia or dysplasia. An increase in the size of cisterna magna can be related to megacisterna magna, with normal anatomy, position, and biometry of cerebellum.
Other conditions that change and enlarge this fluid space are Blake’s pouch cyst or arachnoid cyst.
Blake’s pouch cyst is cystic, avascular anomaly, usually benign, characterized by an extroversion of the midline of the superior medullary veil inside the cisterna magna, which produces a slight rotation of the vermis and slight increase of the fourth ventricle.
Dandy–Walker malformation is a posterior cranial fossa anomaly characterized by anomalous implantation of the Tentorio, hypoplasia, or agenesis of the cerebellar vermis with increase of the pontus cerebellar angle and expansion of the fourth ventricle (Figure 31).48,49
Myelomengocele
The most common spinal cord abnormalities are due to abnormal closure of the neural tube, which, depending on the level at which they occur, can be classified as anencephaly, cephalocele/cranioschisis and spina bifida.
Spina bifida is caused by the abnormal closure of the vertebral arches. We can distinguish closed defects, in which the vertebral arches are not closed but the spinal cord and meninges do not occupy the lesion and there is overlying skin covering the defect and open defects, which involve both the vertebra and spinal cord. In this case, a posterior closure defect of the vertebra causes protrusion of the meninges and or the spinal cord, leading to meningocele (protrusion of the meninges) and myelomeningocele (protrusion of both the meninges and the spinal cord) (Figure 32).
(A) |
(B) |
(C) |
(D) |
32
Myelomeningocele is caused by the abnormal closure of the vertebral arches with protrusion of the meninges and/or the spinal cord. In 2D you can observe the defect in the sagittal and axial plane (A, B). Using 3D ultrasound you can study the defect in all three planes (C) and with the render you can assess also the skin (D).
These defects lead to alterations in liquor flow, which cause the cerebellum to slide inferiorly and occupy the foramen magnum, and to compress the spinal cord (Chiari II malformation). Open spinal defects are associated with characteristic ultrasonographic signs: ventriculomegaly, the “banana sign” due to the inferior displacement of the cerebellum and a shallow posterior fossa and the lemon sign, due to the typical frontal “pinching” of the calvarium (Figure 33).14,50
(A) |
(B) |
(C) | |
33
Chiari II malformation is characterized by inferior displacement of the cerebellum that obliterates the foramen magnum, and compresses the spinal cord. (A) In the medial sagittal plane we see the inferior displacement of the cerebellum. (B) This results in the “banana sign” seen at ultrasound. (C) In all three scan planes.
Encephalocele
This is an anomaly characterized by a bone defect that may affect the occipital, frontal, temporal and parietal part of the fetal skull, with erniation of the brain, meninges, or both. You can localize the bone defect in detail using a 3D ultrasound (Figure 34).14
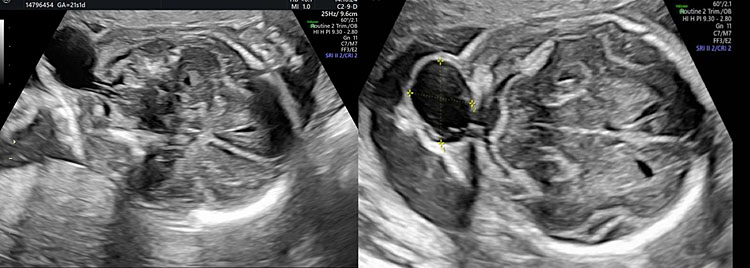
34
Encephalocele characterized by a defect of occipital bone with herniation of the meninges.
PRACTICE RECOMMENDATIONS
Before starting the exam, it is necessary:
- To evaluate gestational age
- To see habitus of the pregnant
- To choose the appropriate probe
- To know pregnancy history and, in particular,
- abnormalities at first-trimester screening ultrasound
- family history or previous pregnancy of abnormalities
- fetus with other ultrasound abnormalities
- suspected fetal infection
- chromosomal or genetic abnormalities
After the exam, if fetal anomalies are suspected at the mid-trimester ultrasound screen, a detailed second-trimester ultrasound examination and/or invasive procedure is mandatory for the diagnosis of fetal aneuploidies.
Fetal echocardiography is recommended in all cases of fetal malformation diagnosed by mid-trimester routine ultrasound.
Amniocentesis is indicated in cases of fetal malformation and/or in cases of suspected fetal infection.
The ultrasound follow-up must be performed at reference centers specialized in fetal medicine.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
ISUOG Practice Guidelines (updated): performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2022;59:840–56 | |
Comstock CH. Normal heart axis and position. Obstet Gynecol 1987;70:255–9 | |
Hoffman J, Kaplann S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39(12):1890–900. | |
van Velzen C, Clur S, Rijlaarsdam M, et al. Prenatal detection of congenital heart disease—results of a national screening programme. BJOG 2016;123(3):400–7 | |
van Velzen C, et al. l. Systematic review and meta-analysis of the performance of second-trimester screening for prenatal detection of congenital heart defects nt. J Gynaecol Obstet 2018;140(2):137–45 | |
Van Nisselrooij AEL, et al. l. Why are congenital heart defects being missed? Ultrasound Obstet Gynecol 2020;55:747–57 | |
ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol 2013;41:348–59. | |
Fermint L, De Getter B, et al. l. A Close Collaboration Between Obstetricians and Pediatric Cardiologists Allows Antenatal Detection of Severe Cardiac Malformations by Two-Dimensional Echocardiography. Pediatric Cardiology Proceedings of the Second World Congress 1986:34–7. | |
AIUM Practice Parameter for the Performance of Fetal Echocardiography. J Ultrasound Med 2020;39:E5–16 | |
Fotos J, Olson R, Kanekar S. Embryology of the brain and molecular genetics of central nervous system malformation. Semin Ultrasound CT MR 2011;32(3):159–66. | |
Morris JK, Wellesley DG, Barisic I, et al. l: Epidemiology of congenital cerebral anomalies in Europe: A multicentre, population-based EUROCAT study. Arch Dis Child 2019;104:1181–7 | |
Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Rare Diseases Epidemiology 2010:349–64. | |
Myrianthopoulos NC. Epidemiology of central nervous system malformations. In: Vinken PJ, Bruyn GW (eds.) Handbook of Clinical Neurology. Elsevier: Amsterdam, 1977:139–71. | |
Monteagudo A, Kuller JA, Craigo S, et al. SMFM fetal anomalies consult series# 3: intracranial anomalies. Am J Obstet Gynecol 2020;223(6):B2–46. | |
Anderson N, Boswell O, Duff G. Prenatal sonography for the detection of fetal anomalies: Results of a prospective study and comparison with prior series. AmJ Roentgenol 1995;165:943–50.3. | |
Malinger G, Ben-Sira L, Lev D, et al. Fetal brain imaging: a comparison between magnetic resonance imaging and dedicated neurosonography. Ultrasound Obstet Gynecol 2004;23:333–40.5. | |
Di Mascio D, et al. l Role of magnetic resonance imaging in fetuses with mild or moderate ventriculomegaly in the era of fetal neurosonography: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2019;54:164–71 | |
Ghi T, Pilu G, Savelli L, et al. Sonographic diagnosis of congenital anomalies during the first trimester. Placenta 2003;24(Suppl B):S84–7.7. | |
Monteagudo A, Timor-Tritsch IE. First trimester anatomy scan: pushing the limits. What can we see now? Curr Opin Obstet Gynecol 2003;15:131–41.8. | |
Salomon LJ, Alfirevic Z, Bilardo CM, et al. l ISUOG practice guidelines: performance of first-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2013;41(1):102–13. doi: 10.1002/uog.12342 | |
Volpe N, Sen C, Turan S, et al. l: First trimester examination of fetal anatomy: clinical practice guideline by the World Association of Perinatal Medicine (WAPM) and the Perinatal Medicine Foundation (PMF). J Perinat Med 2022;50(7):863–77. | |
Lecture presented by Prof Gustavo Malinger at ISUOG's Fetal anomalies course at the International Symposium in Athens, in 2018. | |
Lipa M, Pooh RK, Wielgoś M. Three-dimensional neurosonography – a novel field in fetal medicine. Ginekol Pol 2017;88(4):215–21. doi: 10.5603/GP.a2017.0041. | |
Pilu G, Segata M, Ghi T, et al. l: Diagnosis of midline anomalies of the fetal brain with the three-dimensional median view. Ultrasound Obstet Gynecol 2006;27:522–9.15. | |
Van den Wijngaard JA, Groenenberg IA, Wladimiroff JW, et al. Cerebral Doppler ultrasound of the human fetus. Br J Obstet Gynaecol 1989;96:845–9 | |
Pooh RK, Pooh K. Transvaginal 3D and Doppler ultrasonography of the fetal brain. Semin Perinatol 2001;25(1):38–43. doi: 10.1053/sper.2001.22895 | |
Monteagudo A, Timor-Tritsch IE, Mayberry P. Three-dimensional transvaginal neurosonography of the fetal brain: 'navigating' in the volume scan. Ultrasound Obstet Gynecol 2000;16(4):307–13. | |
Malinger G, Paladini D, Haratz K, et al. l: ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 1: performance of screening examination and indications for targeted neurosonography. Ultrasound Obstet Gynecol 2020;56(3):476–84. | |
Tutschek B, Blaas HK, Abramowicz J, et al. l: ISUOG 3D Special Interest Group. Three-dimensional ultrasound imaging of the fetal skull and face. Ultrasound Obstet Gynecol 2017;50(1):7–16. | |
Lerman-Sagie T, Pogledic I, Leibovitz Z, et al. A practical approach to prenatal diagnosis of malformations of cortical development. Eur J Paediatr Neurol 2021;34:50–61. | |
Malinger G, Lev D, Kidron D, et al. l. Differential diagnosis in fetuses with absent septum pellucidum. Ultrasound Obstet Gynecol 2005;25:42–9. | |
Sundarakumar DK, Farley SA, Smith CM, et al. l: Absent cavum septum pellucidum: a review with emphasis on associated commissural abnormalities. Pediatr Radiol 2015;45(7):950–64. | |
Malinger G, Lev D, Lerman-Sagie T. The fetal cerebellum. Pitfalls in diagnosis and management. Prenat Diagn 2009;29:372–80. | |
Hadlock FP, Harrist RB, Sharman RS, et al. Estimation of fetal weight with the use of head, body, and femur measurements – a prospective study. Am J Obstet Gynecol 1985;151(3):333–7. | |
Snijders RJ, Nicolaides KH. Fetal biometry at 14–40 weeks' gestation. Ultrasound Obstet Gynecol 1994;4(1):34–48. | |
Altman DG, Chitty LS. New charts for ultrasound dating of pregnancy. Ultrasound Obstet Gynecol 1997;10:174–91 | |
Salomon JC, Alfirevic Z, Berghella V, et al. l: Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan ISUOG Clinical Standards Committee. Ultrasound Obstet Gynecol 2011;37(1):116–26. | |
Salomon LJ, Chalouhi GE, Stirnemann J, et al. l: Cerebral ventricle width measurements vary in relation to gestational age, fetal gender and cephalometry. Ultrasound Obstet Gynecol 2011;37:369–72. | |
Salomon LJ, Bernard JP, Ville Y. Reference ranges for fetal ventricular width: a non-normal approach. Ultrasound Obstet Gynecol 2007;30:61–6.3. | |
Melchiorre K, Bhide A, Gika AD, et al. Counseling in isolated mild fetal ventriculomegaly. Ultrasound Obstet Gynecol 2009;34:212–24. | |
Garel C, Fallet-Bianco C, Guibaud L. The Fetal Cerebellum: Development and Common Malformations. Journal of Child Neurology 26(12):1483–92 | |
Gafner M, Yagel I, Fried S, et al. l: Fetal brain biometry in isolated mega cisterna magna: MRI and US study. pp:4199–207 | |
Leombroni M, Khalil A, Liberati M, et al. Fetal midline anomalies: Diagnosis and counselling Part 1: Corpus callosum anomalies. Eur J Paediatr Neurol 2018;22(6):951–62. | |
Paladini D, Pastore G, Cavallaro A, et al. l: Agenesis of the fetal corpus callosum: sonographic signs change with advancing gestational age. Ultrasound Obstet Gynecol 2013;42(6):687–90. | |
Borkowski-Tillman T, Garcia-Rodriguez R, et al. l: Agenesis of the septum pellucidum: Prenatal diagnosis and outcome. Prenat Diagn 2020;40(6):674–80. | |
Di Pasquo E, Kuleva M, Arthuis C, et al. l: Prenatal diagnosis and outcome of fetuses with isolated agenesis of septum pellucidum: cohort study and meta-analysis. Ultrasound Obstet Gynecol 2022;59(2):153–61. | |
Norton ME, Fox NS, Monteagudo A, et al. l: Fetal Ventriculomegaly. Society for Maternal-Fetal Medicine (SMFM). Review Am J Obstet Gynecol 2020;223(6):B30–3. | |
Klein O, Pierre-Kahn A, Boddaert N, et al. Dandy-Walker malformation: Pre-natal diagnosis and prognosis. Childs Nerv Syst 2003;19:484–9 | |
Ecker JL, Shipp TD, Bromley B, et al. The sonographic diagnosis of Dandy-Walker and Dandy Walker variant: associated findings and outcomes. Prenat Diagn 2000:328–32. | |
Copp AJ, Adzick NS, Chitty LS, et al. l: Spina Bifida. Review Nat Rev Dis Primers 2015;1:15007. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can now automatically receive 2 Continuing Professional Development credits from FIGO plus a Study Completion Certificate from GLOWM for successfully answering 4 multiple choice questions (randomly selected) based on the study of this chapter.
Medical students can receive the Study Completion Certificate only.
(To find out more about FIGO’s Continuing Professional Development awards programme CLICK HERE)