This chapter should be cited as follows:
Vijayaraghavan SB, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.420453
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 18
Ultrasound in obstetrics
Volume Editors:
Professor Caterina M (Katia) Bilardo, Amsterdam UMC, Amsterdam and University of Groningen, Groningen, The Netherlands
Dr Valentina Tsibizova, PREIS International School, Florence, Italy

Chapter
Ultrasound of the Fetal Abdominal Wall
First published: January 2024
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Abdominal wall defects (AWDs) consist of a spectrum of anomalies. The overall prevalence is about 1 per 2000 births.1 Gastroschisis and omphalocele are common among AWDs,2 while other complex defects like bladder exstrophy, cloacal exstrophy, body stalk anomaly, pentalogy of Cantrell, and abdominoschisis due to amniotic bands are uncommon. Many AWDs may be associated with other congenital abnormalities which influence prognosis and management. Approximately 60–100% of abdominal wall defects are diagnosed prenatally depending on the expertise. An increased understanding of the spectrum of fetal ventral wall defects will improve the prenatal diagnosis.
Embryology
By the end of 2nd week of embryonic life, a bilaminar disc is formed. This disc has an epiblast facing the amniotic cavity and a hypoblast facing the yolk sac. Gastrulation occurs by the end of 3rd week, when this bilaminar disc becomes a trilaminar disc. This disc comprises endoderm formed by the hypoblast, ectoderm formed by the epiblast and the mesoderm sandwiched in between. Further development of the embryo leads to craniocaudal folding and lateral folding resulting in the formation of ventral wall. The establishment of the primary body wall is mainly driven by lateral folding of the embryo resulting in an outer tube (abdominal wall) surrounding an inner tube (gastrointestinal tract) and is completed around the 5th week. During the 8th menstrual week, the midgut begins to elongate out of proportion to the increase in body length. To accommodate such a growth, the midgut herniates into the base of the umbilicus. Here the midgut undergoes a 90° counterclockwise rotation. This physiologic herniation may be detected sonographically and should not be mistaken for an anomaly. As the bowel returns into the abdominal cavity, it undergoes a further 180° counterclockwise rotation and the return is complete by 11th week. The return of the intestinal loops is the starting signal for (secondary) closure of the ventral body wall. The prerequisite for the second body wall closure is ventral migration of the somatic cells. There is no clear consensus explaining the precise embryological mechanisms leading to the pathogenesis of both omphalocele and gastroschisis.
Development of cloaca
The cloaca develops at the terminal portion of the hindgut. During the 6th and 7th weeks of development, the urorectal septum begins to grow caudal towards the cloacal membrane and divides the cloaca into urogenital sinus anteriorly and the rectum posteriorly. Once the urorectal septum reaches the cloacal membrane, the membrane ruptures and two orifices are created. The urogenital sinus will undergo further differentiation to become the urinary bladder, urethra, and in the females, a portion of the vagina.
Physiological herniation of bowel in the fetus
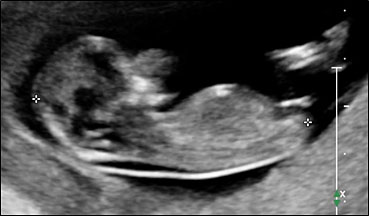
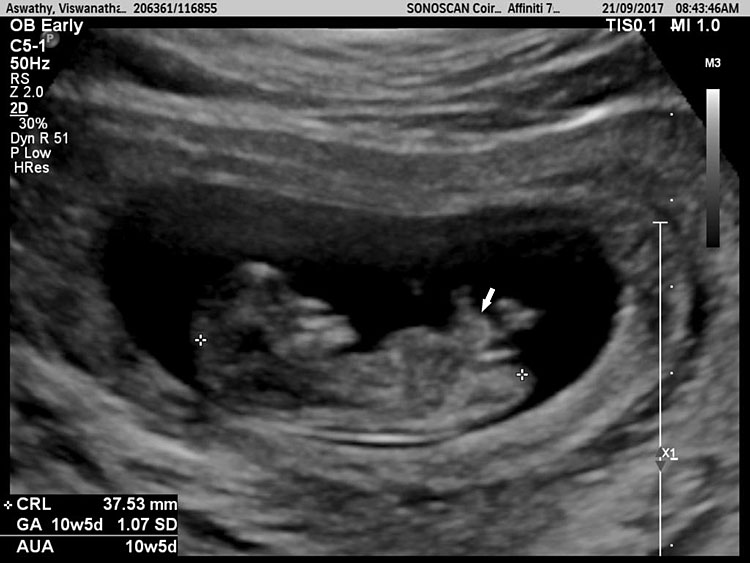
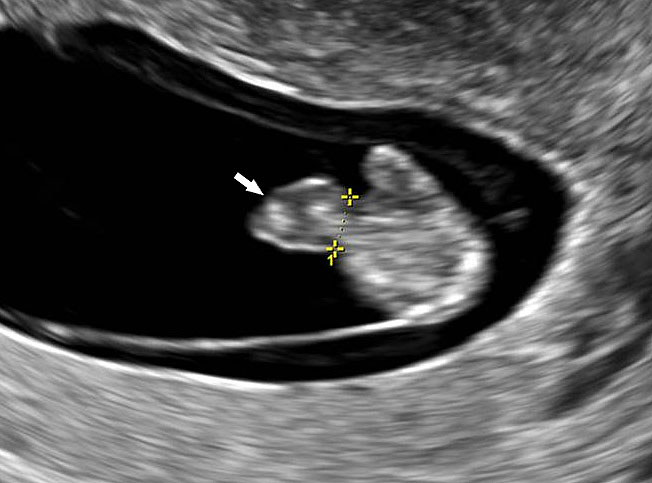
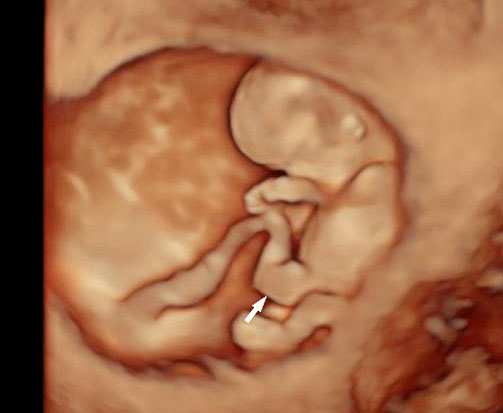
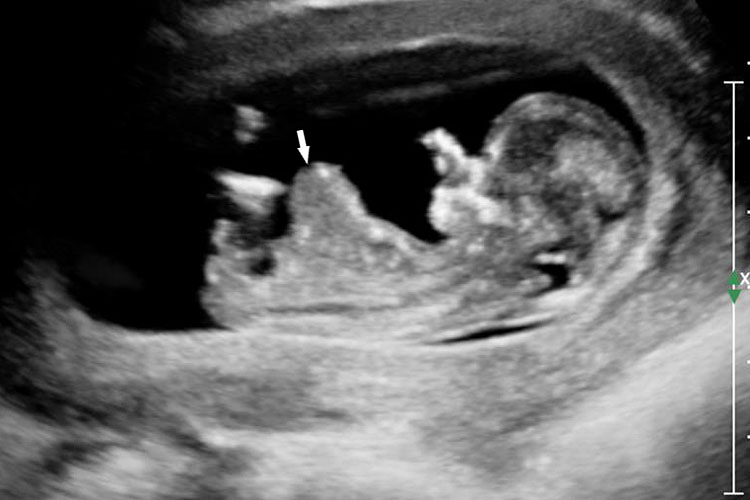
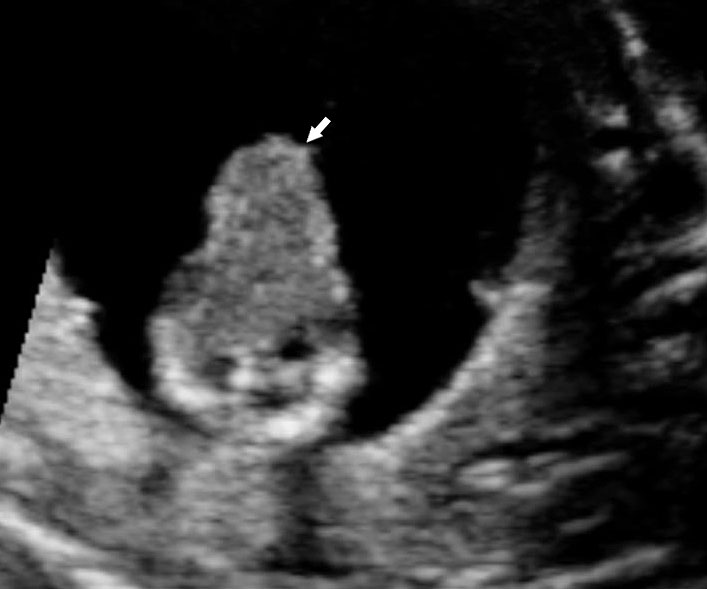
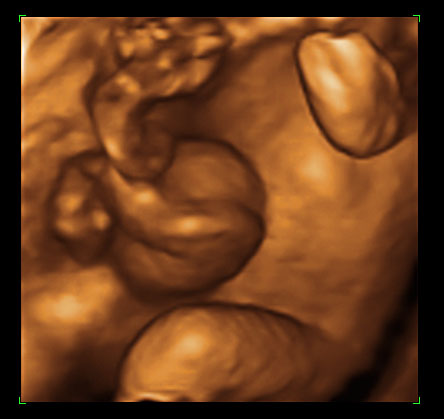
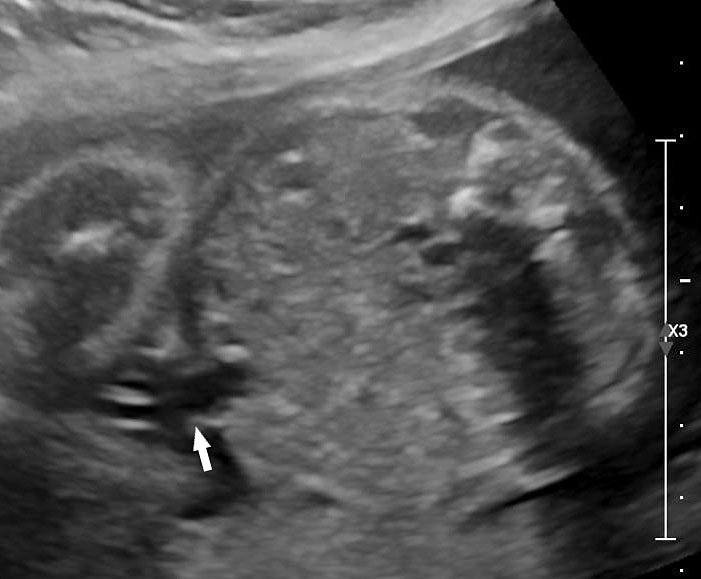
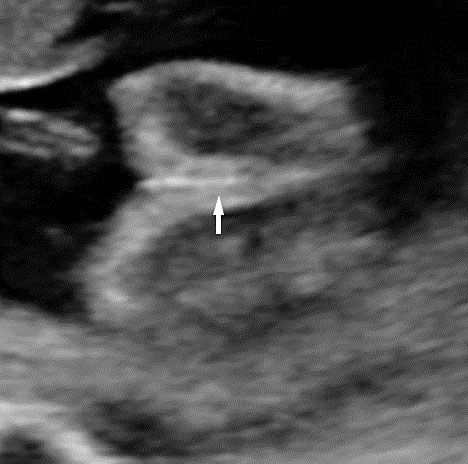
Physiological hernia of bowel in the fetus usually resolves by 11 weeks (CRL 45 mm). It should not be more than 1 cm into the cord and should never include the liver (Figure 1). If there is any doubt during the first trimester, then a follow-up scan at 14 weeks should be done to document the return of bowel into the abdominal cavity.3
(A) |
(B) |
(C) |
(D) |
1
Physiological herniation of bowel: (A) longitudinal scan of fetus of 10 weeks' gestation showing normal anterior abdominal wall, (B) longitudinal and (C) axial section of a fetus with physiological herniation of bowel (arrow). (D) Three-dimensional images of the same fetus.
OMPHALOCELE
Omphalocele is a defect in the closure of the abdominal wall involving the cord insertion site. This defect results in herniation of abdominal contents into the base of the umbilical cord through a wide umbilical opening. Possible causes include failure of the bowel to return to the abdominal cavity after normal physiologic herniation and failure of the abdominal wall to close.4 The defect is covered by a membrane composed of amnion on the outside and peritoneum on the inside.5 Omphalocele occurs in two to three cases per 10,000 live births.6 The incidence of omphalocele is higher in the first trimester due to a high spontaneous fetal loss or termination of pregnancy because of early pickup of associated chromosomal or other structural abnormalities. The organs frequently seen in the omphalocele sac are small bowel loops, liver, rarely colon, spleen and kidneys. The herniated organs are wrapped in a two-layered sac of peritoneum and the amnion.
Ultrasound features
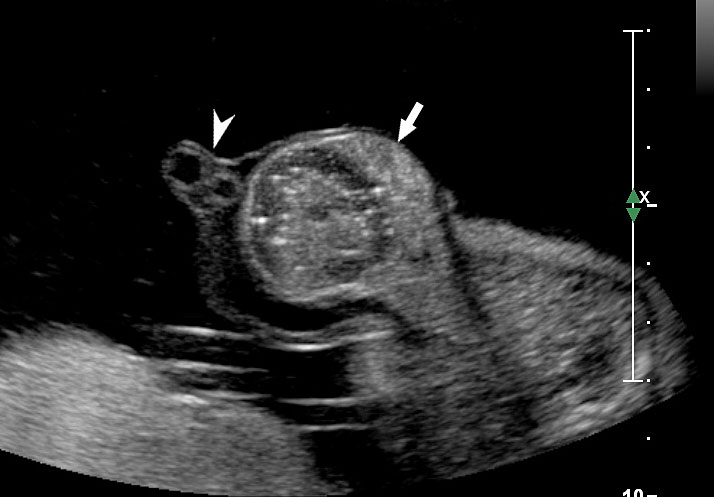
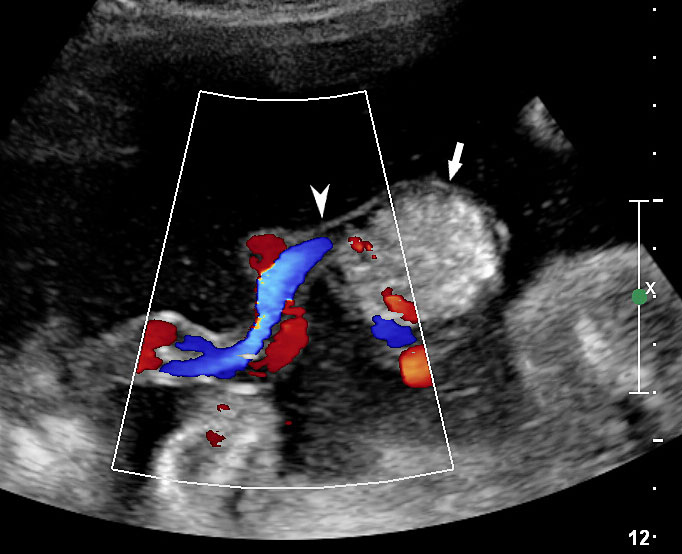
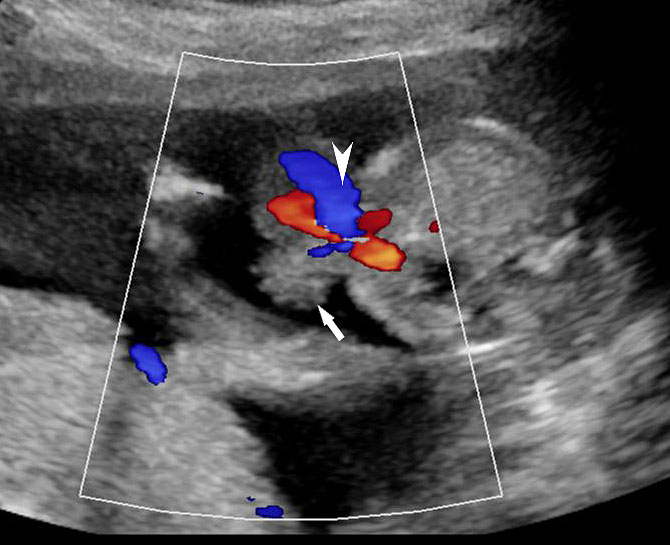
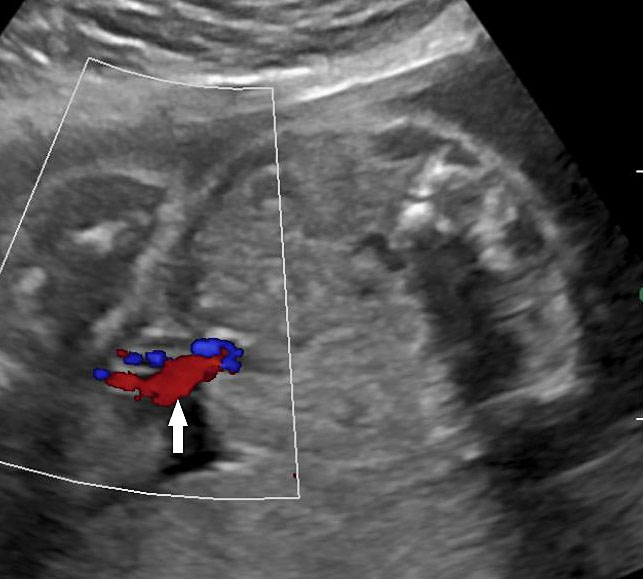
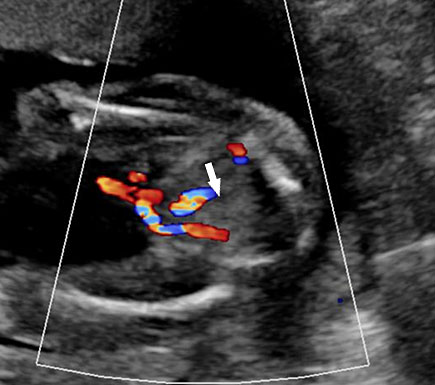
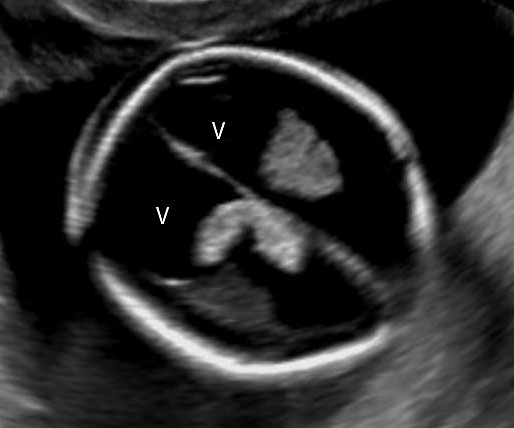
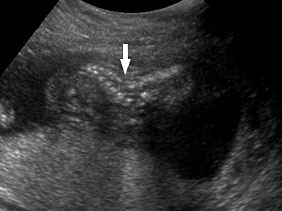
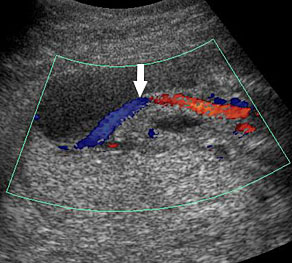
An omphalocele is sonographically seen as a bulging mass arising from the anterior abdominal wall, containing some abdominal viscera (liver and/or bowel) and the cord insertion is noted on its convexity. Color Doppler is useful to show the cord insertion on convexity (Figures 2 and 3).
(A) |
(B) |
2
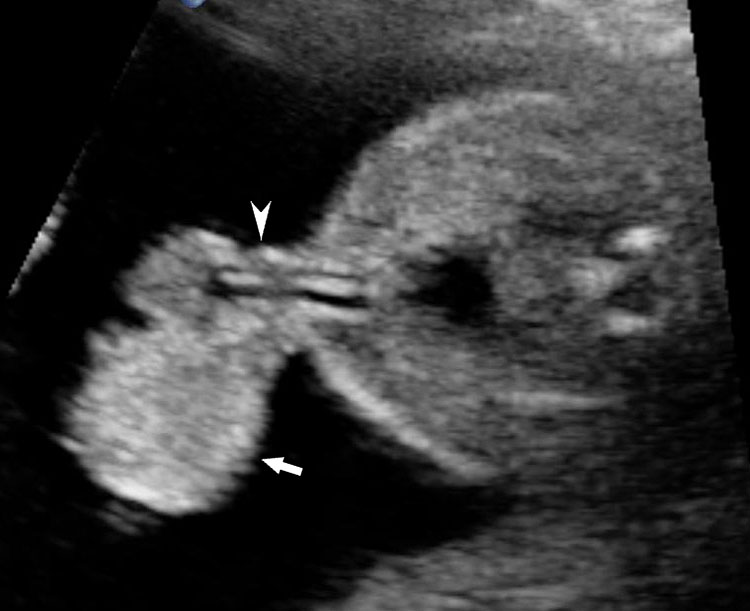
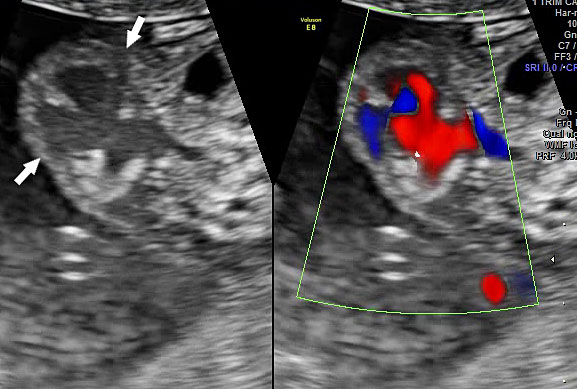
Omphalocele in 15 weeks fetus: (A) longitudinal and (B) axial scan showing the omphalocele (arrow).
(A) |
(B) |
(C) |
(D) |
3
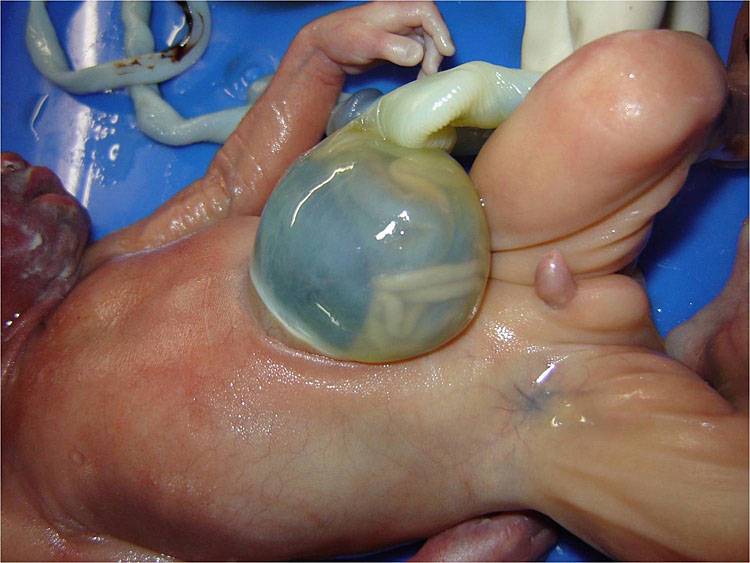
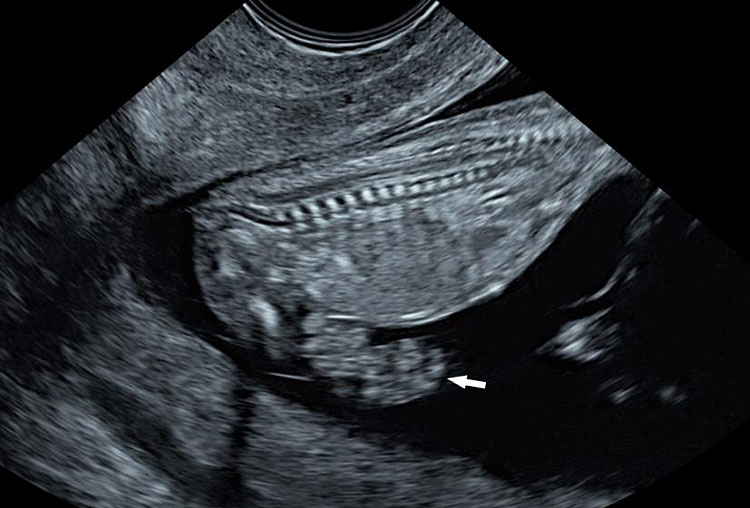
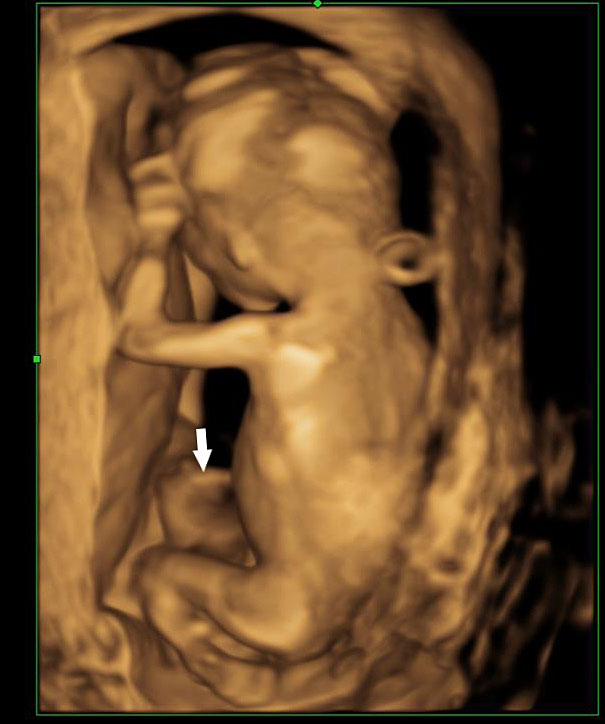
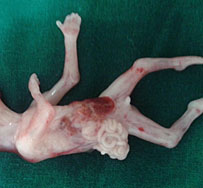
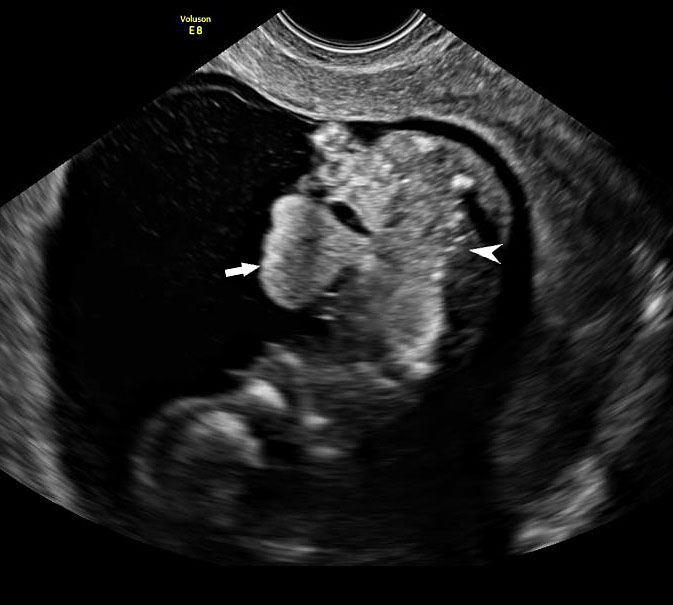
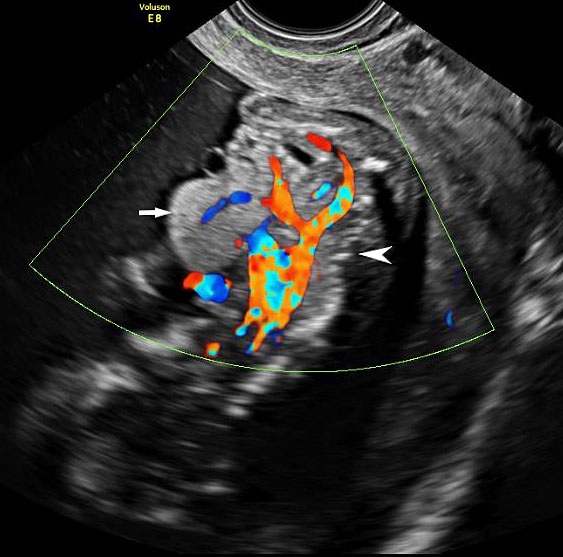
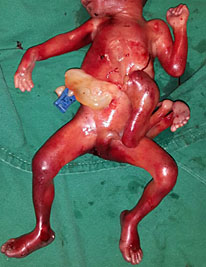
Omphalocele in a 20 weeks fetus: (A) axial scan and (B) color Doppler Scan and (C) 3D image showing the omphalocele (arrow) and the insertion of the umbilical cord on the convexity (arrowhead). (D) Photograph of the fetus.
Associated genetic and structural abnormalities
Up to 75% of cases of omphalocele have associated chromosomal and nonchromosomal congenital abnormalities.7 A study by Barisic et al.8 showed that 25% of cases were associated with chromosomal abnormalities (most commonly trisomy 18), 10% were part of a syndrome and 21% were associated with other major malformations. Cardiac, central nervous system, and urogenital abnormalities are the most common associated malformations. Omphalocele is also a component of the more complex AWDs, cloacal exstrophy and pentalogy of Cantrell. The association with other structural anomalies or increased nuchal translucency in the first trimester increase the possibility of genetic abnormalities.
Differential diagnosis
Omphalocele should be differentiated from other abdominal wall defects and physiological herniation of gut in the cord that disappears by 11 weeks' gestation. The herniated contents are contained in a sac in omphalocele, whereas in gastroschisis the bowel loops float freely in the amniotic fluid. The cord insertion site is normal in gastroschisis with a paramedian defect, while in an omphalocele, it inserts on the convexity of the sac. If a large omphalocele contains the whole of the liver, then this should be differentiated from the limb body wall complex (LBWC), which is characterized by evisceration of abdominal viscera outside the amniotic sac, major distortion of the body anatomy, with severe kyphoscoliosis and limb abnormalities. Cloacal exstrophy is another abnormality in which omphalocele extends caudally and is associated with bladder exstrophy and anomalies of the external genitalia. In the case of omphalocele, the recognition of a normal bladder in the pelvis rules out this very rare possibility of cloacal exstrophy.
Prognosis
The prognosis for prenatally diagnosed omphalocele is guarded. Amniocentesis/chorion villi sampling with genetic testing should be offered to rule out aneuploidy.
GASTROSCHISIS
In this AWD, the bowel herniates through a paramedian AWD, that is usually to the right of the cord insertion site and the herniated contents are not limited by a membrane. Incidence is 4.4 cases per 10,000 live births.9 Young maternal age is associated with increased incidence. The cause of gastroschisis is not completely understood; at one time, a vascular insult to the abdominal wall was hypothesized. A significant breakthrough in the study of gastroschisis was achieved in 2013 by Rittler and colleagues. They described gastroschisis as a defect of the umbilical ring: the umbilical cord was “only attached to the left side of the umbilical ring, while the right side remained uncovered allowing evisceration”.10
Ultrasound features
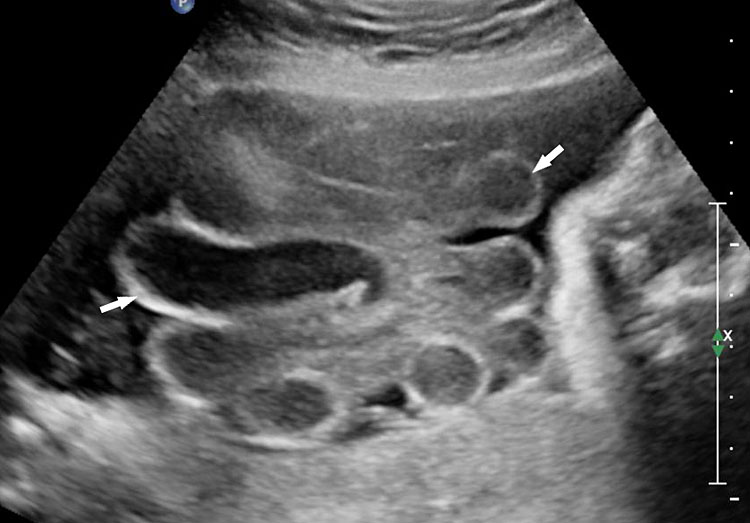
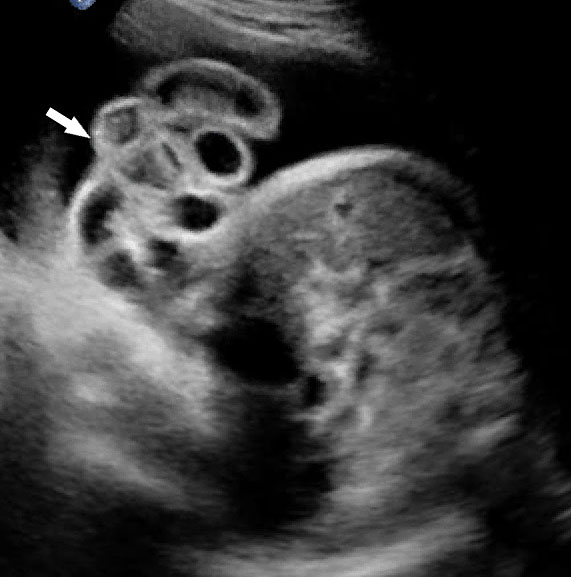
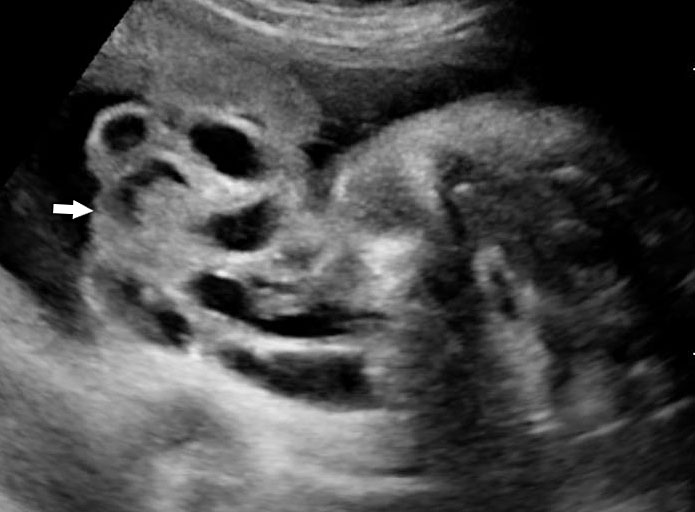
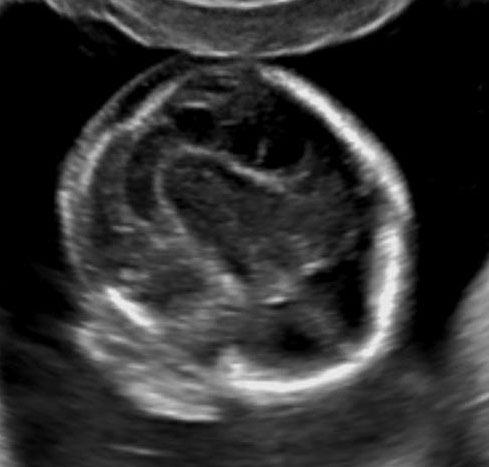
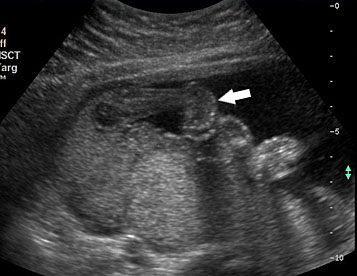
Prenatal diagnosis by ultrasound is based on the demonstration of the normally situated umbilical cord insertion and the herniated free-floating loops of intestine through a paramedian AWD that is usually to the right of the cord insertion site, without any membranous covering. The stomach may be positioned abnormally with the gastric fundus pulled toward the defect. The free-floating bowel loops can appear thickened and edematous or as an echogenic mass due to inflammation (Figures 4 and 5). Inflammation can progress to intestinal atresia. This may lead to the appearance of dilated bowel loops floating in the amniotic fluid and within the abdomen (Figure 6). The defect of gastroschisis is present without herniation of bowels in early gestation, preventing the diagnosis and may herniate at a later stage of gestation (Figure 7 and Video 1). Gastroschisis is usually an isolated anomaly and has good prognosis. Barisic et al.8 showed that 14% of fetuses with gastroschisis had additional anomalies, among which central nervous system and cardiac malformations were the most common.
(A) |
(B) |
(C) |
(D) |
(E) | |
4
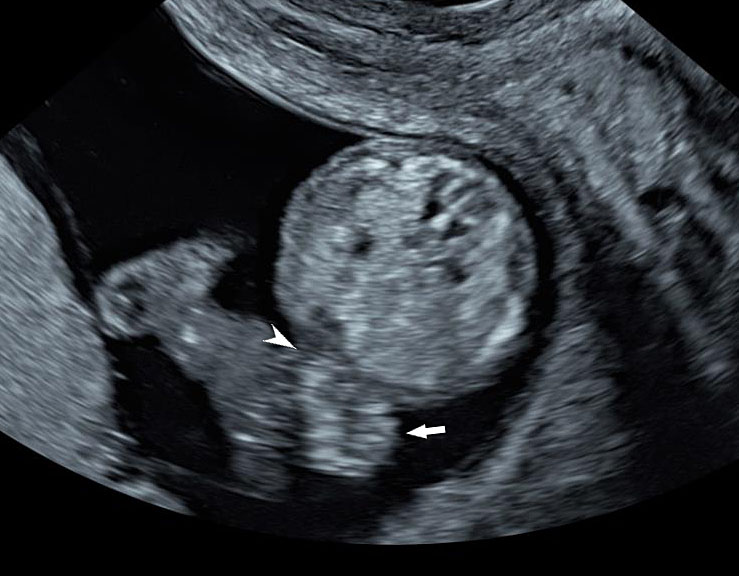
Gastroschisis in a 13-week fetus: (A) gray scale and (B) color Doppler axial scan of the fetal abdomen showing the free floating bowel loops (arrow) herniated through a defect to right of cord insertion (arrowhead). (C) Longitudinal scan showing the free floating bowels (arrow) in amniotic fluid. (D) 3D of the same fetus. (E) fetus showing the gastroschisis.
(A) |
(B) |
5
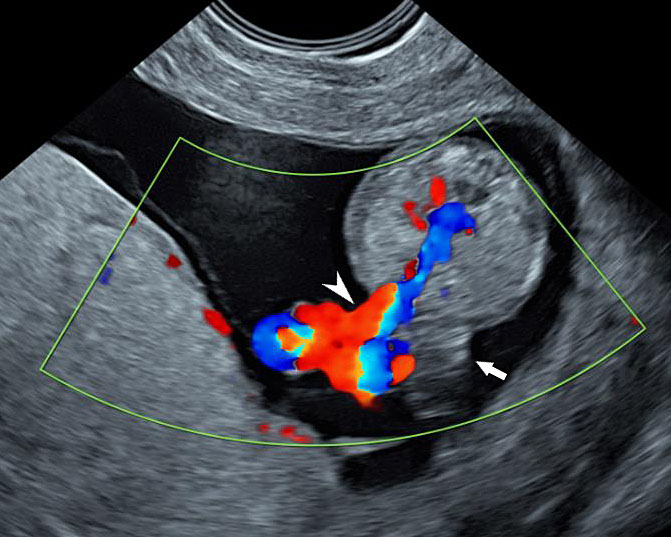
Gastroschisis in a 20-week fetus: (A) axial scan and (B) color Doppler study of the fetal abdomen showing the matted bowels seen as an echogenic mass (arrow) to right of cord insertion (arrowhead).
(A) |
(B) |
(C) | |
6
Gastroschisis with small bowel atresia: (A) axial scan and (B) color Doppler scan showing the dilated small bowel loops (arrow) floating in amniotic fluid herniated through a defect to right of cord insertion (arrowhead). (C) Longitudinal scan showing dilated loops (arrows) floating in the amniotic fluid.
(A) |
(B) |
(C) |
(D) |
(E) | |
7
Gastroschisis manifesting in late gestation: (A) axial scan and (B) color Doppler scan at 28 weeks showing normal insertion cord (arrow). There is no herniation of bowel loops (Video 1). (C) Axial scan of fetal abdomen at 37 weeks showing dilated loops (arrows) floating in the amniotic fluid (arrow). (D) Axial scan and (E) color Doppler scan showing the dilated small bowel loops (arrow) floating in amniotic fluid herniated through a defect to right of cord insertion (arrowhead).
1
Axial scan sweep of the normal cord insertion at 28 weeks showing absence of herniated bowel loops.
UMBILICAL CORD HERNIA
Congenital hernia of the umbilical cord is a different type of ventral AWD, in which the bowel usually herniates into the base of normally inserted umbilical cord through the umbilical ring. Unlike an omphalocele, which is covered only by amniotic membranes, a congenital umbilical hernia is always covered by intact skin and subcutaneous tissue.11,12 Umbilical hernia is associated with a better prognosis than is omphalocele and is usually an isolated finding. Its incidence is estimated to be 1 in 5000 and unlike omphalocele, it is not linked with chromosomal anomalies.
Ultrasound features
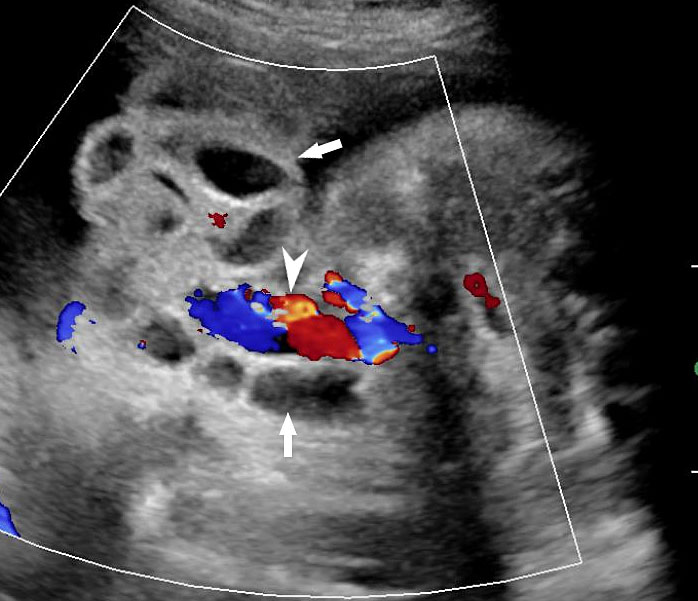
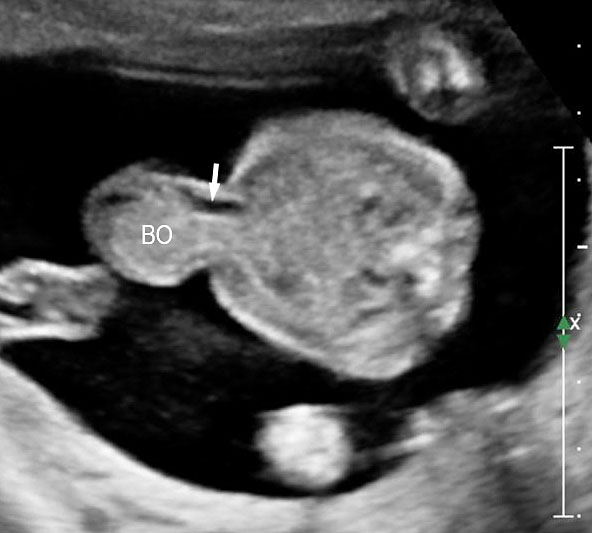
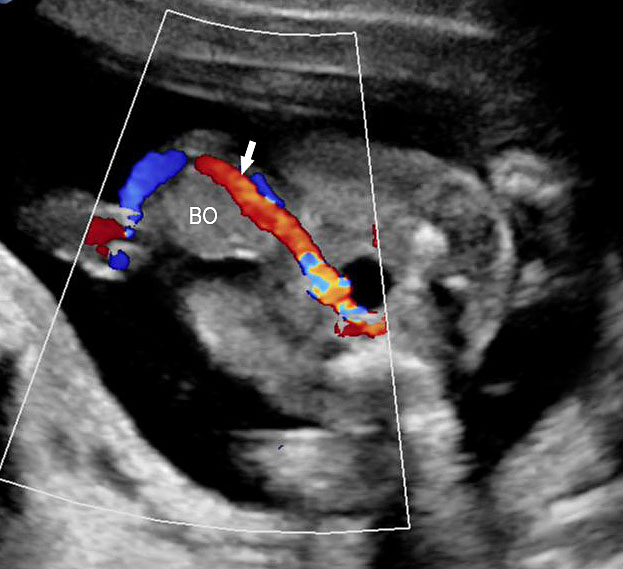
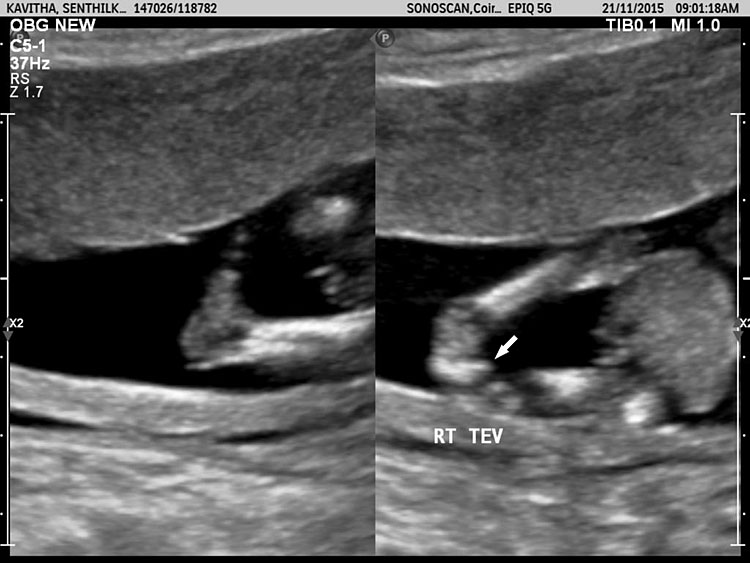
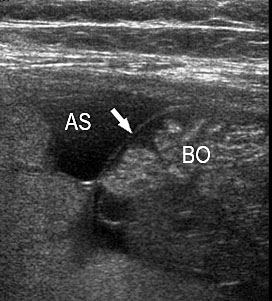
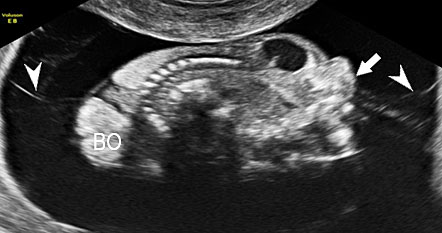
A loop of bowel is seen herniating into the base of normally inserted umbilical cord through the umbilical ring and is seen in between the umbilical vessels (Figure 8).
(A) |
(B) |
8
Umbilical cord hernia: (A) Axial scan and (B) color Doppler scan of fetal abdomen showing herniation of the loop of bowel (BO) into the base of umbilical cord by the side of cord vessels (arrow).
Outcome
Umbilical cord hernia is usually benign with a favorable outcome. However, prenatal diagnosis is useful to prevent inadvertent injury to the bowel during clamping of the cord at the time of delivery.
BLADDER AND CLOACAL EXSTROPHY
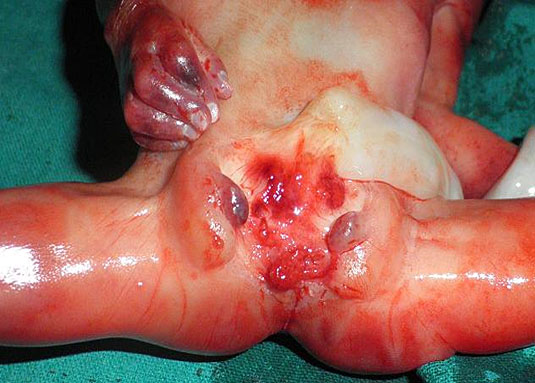
Bladder exstrophy and epispadias complex (BEEC) is an anterior midline defect with a variable expression involving the infraumbilical abdominal wall including the bony pelvis, urinary tract, and external genitalia. Genital abnormalities occur in association with bladder exstrophy. In males, epispadias, a short or split penis, and maldescended testes are common, whereas bifid clitoris and uterine and vaginal anomalies can be seen in females.13 Bladder exstrophy has been reported in one in 30,000 births and is more common in males.6 Bladder and cloacal exstrophy are two different expressions of a primary developmental defect. Exstrophy of the cloaca or OEIS complex (omphalocele-extrophy-imperforate anus-spinal defects) is due to failure of fusion of the genital tubercles and pubic rami, incomplete development of the lumbosacral vertebrae with spinal dysraphism, imperforate anus, cryptorchidism and epispadias in males, anomalies of the Mullerian duct derivatives in females and a wide range of urinary tract anomalies. Omphalocele is common and most patients have a single umbilical artery. It is essential to differentiate bladder exstrophy from cloacal exstrophy, which is a more severe AWD with a different prognosis and management implications. Whether these two malformations are distinct entities or a spectrum of the same underlying pathologic condition is controversial.14 Unlike in cloacal exstrophy, bowel loops are never extruded and the rectum is normally formed in bladder exstrophy.15
Etiopathogenesis
At around 6 weeks, the urorectal septum divides the cloaca into urogenital sinus anteriorly and the hindgut posteriorly. Simultaneously, the mesoderm extends medially to form an infraumbilical abdominal wall. If this is deficient, the cloacal membrane can rupture anteriorly. Rupture after the urorectal septum has reached the urogenital membrane results in bladder exstrophy and rupture before the complete descent of the urorectal septum results in cloacal exstrophy.
Prenatal ultrasound findings
Bladder Exstrophy
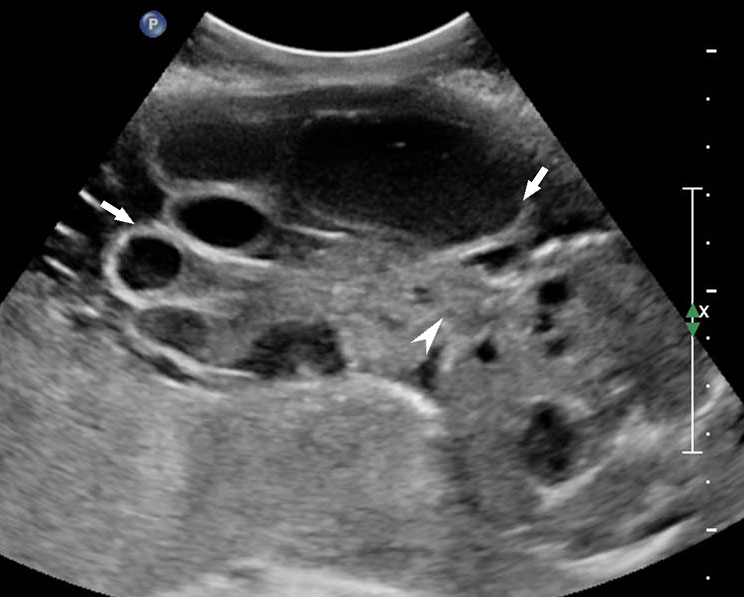
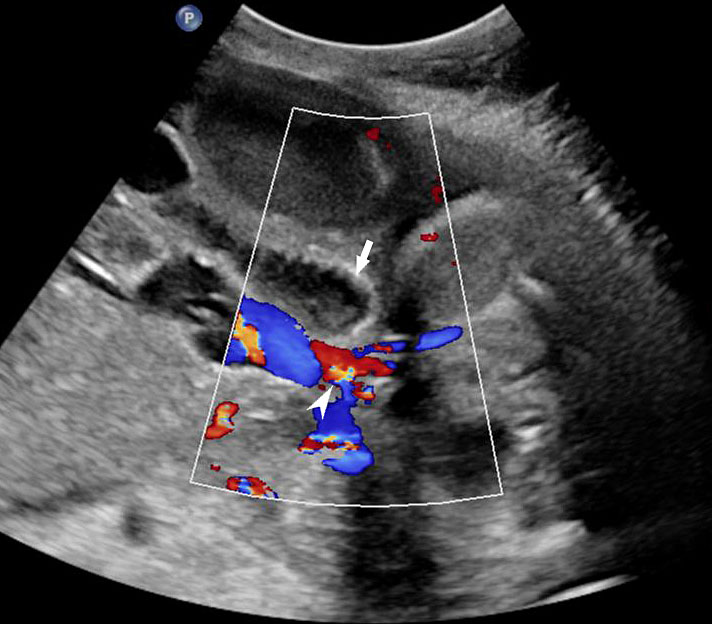
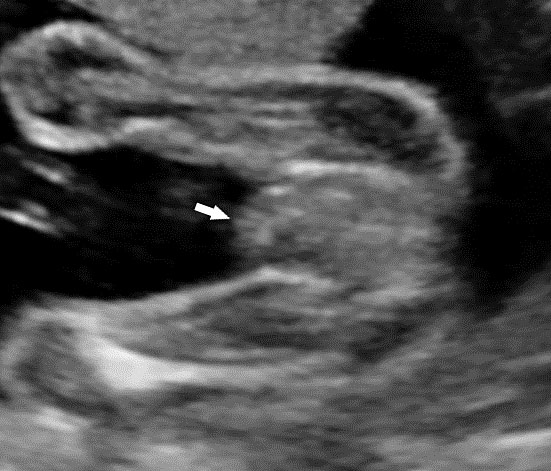
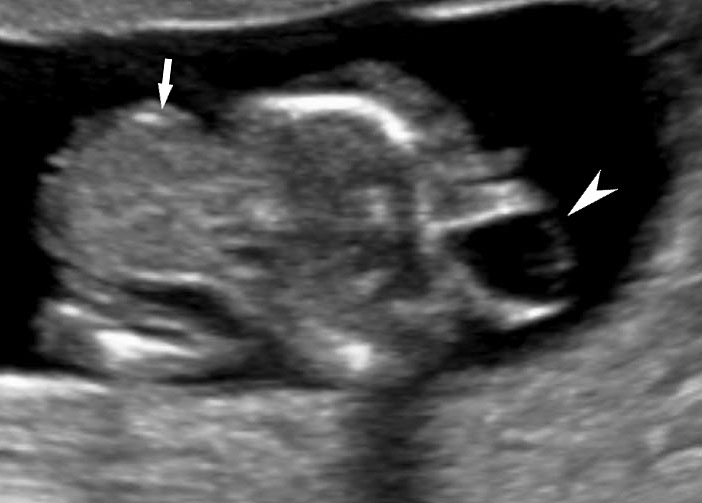
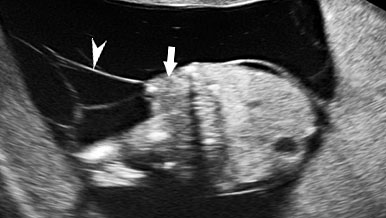
Persistently non-visualized urinary bladder with normal amniotic fluid should raise to the suspicion of bladder exstrophy. According to the 2013 American Institute of Ultrasound in Medicine guidelines, demonstration of a normal bladder is required at ultrasound.7 The bladder is a midline fluid-filled structure flanked by the umbilical arteries. It should fill and empty during the course of an examination. The hallmark of bladder exstrophy is a persistently “absent” urinary bladder. The bladder is open to the abdominal wall. Thus, urine is released immediately into the amniotic cavity and the bladder does not distend and, hence, is never visible as a fluid-filled structure. The bladder halves are exposed and everted. Inflammation of the exposed mucosa may cause irregular thickening of the posterior bladder wall which is seen as a soft tissue mass at the lower abdominal wall inferior to cord insertion (Figure 9). The cord inserts on the abdominal wall but may be lower than normal, with the AWD inferior to the cord insertion site.
(A) |
(B) |
(C) |
(D) |
(E) | |
9
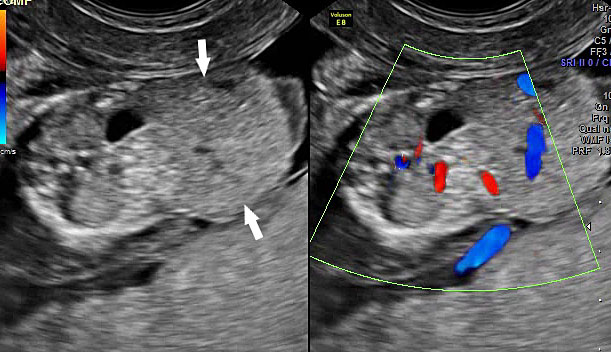
Bladder exstrophy: (A) axial scan of fetal abdomen revealing bilateral mild hydronephrosis. (B) Color Doppler scan of pelvis showing non-visualized urinary bladder between the umbilical arteries (arrow). (C) Image shows the redundant bladder mucosa seen as a soft tissue mass (arrow) at the lower abdominal wall inferior to cord insertion. (D) Scan of perineum revealing non-visualized anus (arrow). (E) Photo of fetus confirming the features.
Cloacal exstrophy or OEIS Complex
Cloacal exstrophy is a rare, but serious, congenital abnormality that has an estimated prevalence of one case per 200,000–400,000 live births, with higher rates among females.16 It is diagnosed when a persistently non-visualized bladder is associated with ventral wall defect, omphalocele, spinal defect, urinary tract anomaly along with ambiguous genitalia. The fetal anus is not seen. Bowel herniation between the bladder halves creates an “elephant trunk” appearance. Spinal defects include abnormalities of the vertebral column or spinal cord, tethered cord, lipomeningocele, and lipomyelocystocele.17 The most common genitourinary abnormalities are hydronephrosis, renal developmental variants, and abnormal genitalia. Urinary system abnormalities may be seen in up to 60% of cases and may result in oligohydramnios when renal obstruction is substantial. In the first trimester, a cystic mass is seen in the lower abdomen prior to the rupture of the cloacal membrane (Figures 10 and 11, also see Figure 2.54 in Ultrasound of the Fetal Gastrointestinal Tract).
(A) |
(B) |
(C) |
(D) |
10
Cloacal exstrophy: axial scan of fetal head showing (A) bilateral lateral ventriculomegaly and (B) Arnold Chiari malformation. (C) Axial scan of abdomen shows omphalocele (arrow) and meningocele (arrowhead). (D) Scan of lower limb shows talipesequinovarus deformity of one foot (arrow).
(A) |
(B) |
11
Cloacal exstrophy in first trimester: (A) longitudinal scan and (B) color Doppler study, of a 13-week fetus showing the omphalocele (arrow), exstrophy of bladder and kyphoscoliosis of spine (arrowhead).
Karyotyping along with microarray should be done to detect genetic abnormalities and to detect fetal sex which may be difficult to determine by ultrasound imaging.
Outcome of bladder and cloacal exstrophy
Outcome of bladder and cloacal exstrophy is guarded as it involves multiple surgical procedures with problems of urinary incontinence and sexual function.
ECTOPIA CORDIS AND PENTALOGY OF CANTRELL
Ectopia cordis is defined as an anomaly in which the fetal heart lies outside the thoracic cavity either completely or partially. It is a rare congenital abnormality. It is associated with omphalocele in pentalogy of Cantrell with an incidence of about 5.5 cases per 1 million live births and a male predominance of 2.7 : 1.15 The pentalogy of Cantrell is a rare anomaly characterized by a midline supraumbilical thoracoabdominal defect, a defect of the anterior diaphragm, a defect of the diaphragmatic pericardium and congenital cardiac anomalies. This constellation of defects is thought to result from abnormal formation and migration of the ventral mesoderm during days 14–18 of embryonic life and failure of fusion of the transverse septum of the diaphragm and lateral folds of the thorax.18
Ultrasound features
Diagnosis can be made as early as 10–12 weeks. The fetus shows a midline supraumbilical abdominal defect including herniated liver and ectopia cordis with a large omphalocele containing the intestines. Doppler and transvaginal ultrasound improves the precision in diagnosis (Figures 12 and 13, Videos 2 and 3).
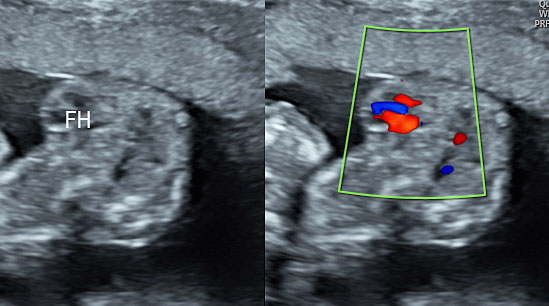
12
Ectopia cordis in a 13 weeks fetus: (A) Gray scale and (B) color Doppler study of axial scan of chest showing the fetal heart (arrows) lying outside the chest (Video 2).
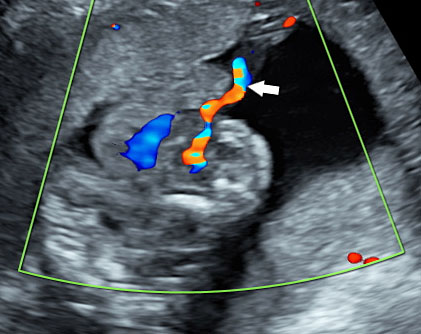
(A) |
(B) |
13
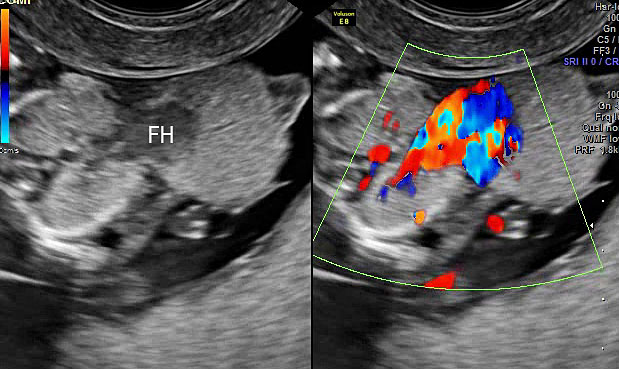
Pentalogy of Cantrell: (A) gray scale and color Doppler study of axial scan of fetal chest showing ectopia cordis (FH). (B) Gray scale and color Doppler scan of axial scan of fetal abdomen showing the large omphalocele (arrows) (Video 3).
2
Ectopia cordis: Axial section of fetal chest showing the heart lying outside the chest.
3
Pentalogy of Cantrell: Axial sweep of fetal chest and abdomen showing ectopia cordis and omphalocele.
Associated anomalies
Most cases are sporadic and without increased risk for recurrence. However, familial cases have been reported, and because of the reported association with trisomies 13 and 18 and Turner syndrome, amniocentesis should be performed. A detailed search for the associated anomalies is mandatory since the prognosis mostly depends on the severity of these associated findings. Cardiac defects are common and include septal defects, tetralogy of Fallot, left ventricular diverticulum, and Ebstein’s malformation.19 Other associated findings that may be seen in utero include facial defects (e.g., cleft lip or palate) and central nervous system (e.g., exencephaly, encephalocele, spina bifida, hydrocephalus) and musculoskeletal (e.g., clubfoot, absent radius) anomalies.14
LIMB BODY WALL COMPLEX/BODY STALK ANOMALY
Limb body wall complex (LBWC)/body stalk anomaly (BSA) is the rarest, most severe and invariably lethal abdominal wall defect with reported prevalence of 0.12 cases per 10,000 births (including both live and still births).20 It is a severe defect where the abdominal wall does not develop and thus there is evisceration of abdominal structures into the extraembryonic coelom and as a result it is characterized by attachment of visceral organs to the placenta and a short or absent umbilical cord. Various hypotheses exist regarding the pathogenesis of body stalk anomaly; the most likely one is abnormal lateral body folding resulting in failure of the coelom to form. The abdominal wall does not develop and thus the peritoneal cavity is open to the extraembryonic coelom and the fetus is attached to the placenta. Associated failed body stalk fusion results in a short or absent umbilical cord.15
Ultrasound features
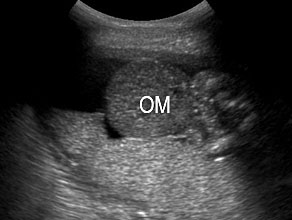
In the first trimester, fetal abdominal contents are seen eviscerated into the extra-embryonic coelom with a short or no cord. The fetal spine shows severe kyphoscoliosis (Figures 14 and 15, Videos 4 and 5). In second trimester the fetus is seen in fixed position with the eviscerated abdominal contents adherent to placental surface. Cord insertion site cannot be identified. There are no free-floating bowel loops. The rest of the fetus shows movements (Figure 16, Videos 6 and 7). A wide spectrum of limb anomalies, including clubfoot and absent limbs, has been described. In later gestation, oligoamnios is a prominent feature and it may be difficult to differentiate from omphalocele. High frequency scan will help to delineate the amniotic membrane and see the evisceration outside the amniotic membrane (Figure 17).
(A) |
(B) |
14
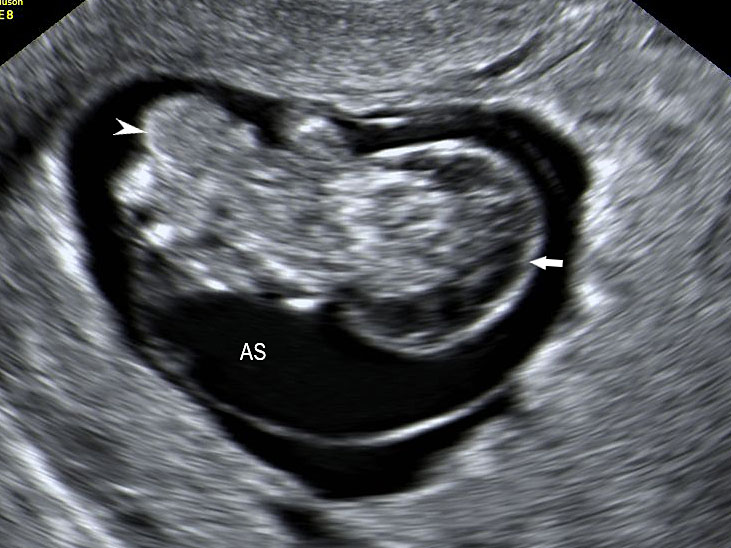
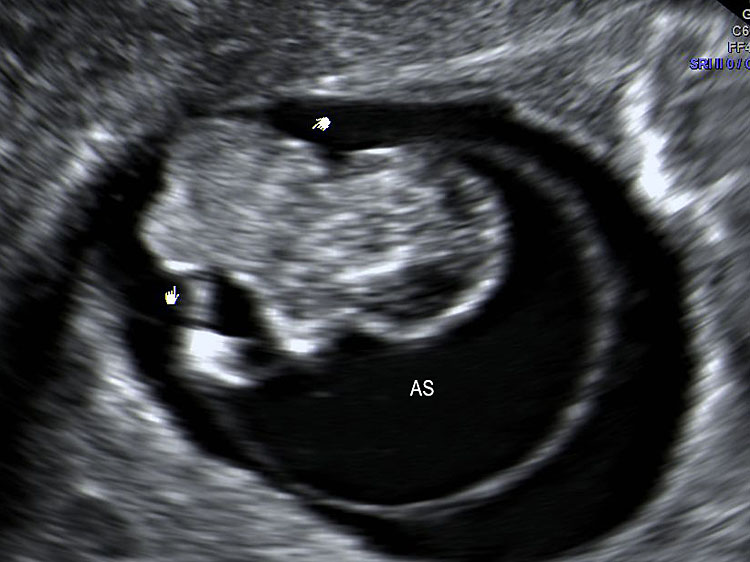
Limb body wall complex at 10 weeks: (A) Coronal scan of the fetus shows fetus in amniotic sac with scalp edema (arrow) and evisceration of abdominal contents (arrowhead) outside the amniotic sac (AS). (B) Axial scan of the fetus clearly shows the eviscerated abdominal contents outside the amniotic sac (AS).
(A) |
(B) |
(C) | |
15
Limb body wall complex anomaly at 13 weeks (A) Longitudinal and (B) axial scan of fetus shows evisceration of the viscera (arrow) into the extraamniotic coelom (arrowhead). (C) Photograph of the fetus showing the anomaly. AS, amniotic sac Videos 4 and 5.
4
Limb body wall complex in first trimester – Axial (Video 4) and Sagittal (Video 5) sweep of fetus shows evisceration of abnormal contents outside to amniotic sac.
5
Limb body wall complex in first trimester – Axial (Video 4) and Sagittal (Video 5) sweep of fetus shows evisceration of abnormal contents outside to amniotic sac.
(A) |
(B) |
(C) | |
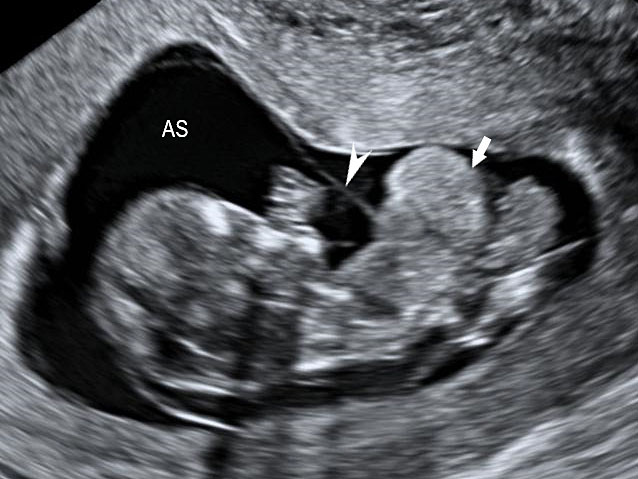
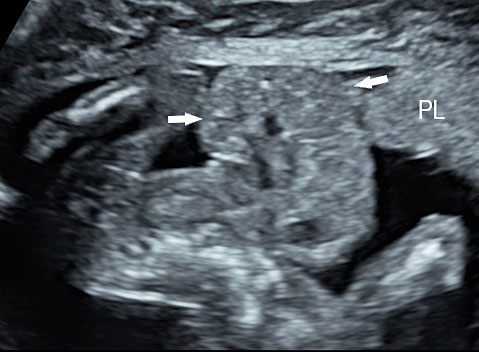
16
Limb body wall complex in early second trimester: (A) Oblique scan of fetus showing the evisceration of abdominal viscera (arrows) into extraembryonic coelom close to the placenta (PL). (B) Shows that the fetal heart (FH) is also eviscerated (video). (C) Color Doppler image showing the short umbilical cord (arrow). Video showing that the eviscerated parts are fixed to the placenta while rest of the fetus shows movements.
3.6
Limb body wall complex in early second trimester – Oblique section shows evisceration of heart and abdominal contents outside the amniotic sac.
3.7
Limb body wall complex – showing that the eviscerated bowels are fixed to placenta while rest of the fetus moves.
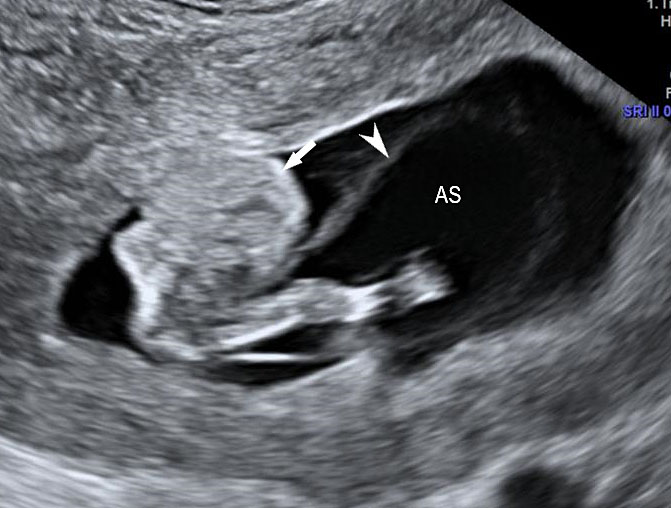
(A) |
(B) |
(C) |
(D) |
(E) | |
17
Limb body wall complex in late second trimester: (A) Longitudinal scan of fetus shows oligohydramnios which becomes a feature by this time of gestation and omphalocele (OM). (B) Shows talipesequinovarus deformity of a foot (arrow). (C) Shows kyphoscoliosis of spine (arrow). (D) High frequency scan shows the amniotic membrane (arrow) and that the bowels (BO) are outside the amniotic sac (AS) differentiating it from omphalocele. (E) Color Doppler scan shows short umbilical cord (arrow).
Associated anomalies
LBWC is a heterogeneous disease and is associated with varied internal anomalies. The central nervous system anomalies observed are anencephaly, encephalocele and alobar holoprosencephaly. Cardiovascular anomalies include primitive ventricle, common atrium atrial septal defects, common arterial trunk and membranous ventricular septal defect. The renal anomalies observed are unilateral or bilateral aplasia/hypoplasia of kidney. Genital abnormalities seen are abnormal external genitalia, absent gonad and exstrophy of bladder. Skeletal anomalies are most common and include club foot, oligodactyly, arthrogryposis, absent limb, single forearm bone, single lower leg bone, pesudosyndactyly, radial/ulnar hypoplasia, rotational defects and polydactyly. Karyotyping is usually normal.
Prognosis
LBWC is a lethal anomaly. Hence, it has to be differentiated from treatable causes like omphalocele or gastroschisis. But sometimes oligohydramnios may mask the underlying anomalies. High frequency probe is useful to demonstrate the amniotic membrane and evisceration of bowel. Early diagnosis followed by medical termination is the preferred treatment for this anomaly.
AMNIOTIC BAND SYNDROME
Amniotic band syndrome (ABS) is very rare and occurs in 0.5–1.0 of 10,000 births. Only a minority of cases involve the abdominal wall called abdominoschisis.
Etiopathogenesis
The cause of amniotic band syndrome is unclear. The exogenous and endogenous theories are proposed to explain ABS. The exogenous theory by Torpin suggests rupture of the amnion without rupture of the chorion as the initial event. Subsequent oligohydramnios and attachment of the fetus to the “sticky” chorionic surface lead to entanglement of fetal parts and disruption defects. The endogenous theory suggests that vascular endothelial injury is the underlying pathogenesis of constriction bands and secondary limb amputation.
Ultrasound features
The ultrasound features are highly variable depending upon the fetal body parts involved in amniotic bands. Earlier the amniotic rupture, more severe is the defect.21 The defects in amniotic band syndrome occur in a non-anatomic distribution and are described as “slash defects.” Recognition of such appearance should lead to a careful search for bands, which may be difficult to see. Amniotic bands are seen as fine linear echoes extending from the defect to the uterine wall. In abdominoschisis, the abdominal contents float in the amniotic fluid without a clear encasing membrane and bands may also trap the umbilical cord. Depending on the location of the band, it may be difficult to see the cord insertion site (Figure 18). Demonstration of a normal umbilical cord is useful to differentiate LBWC from abdominoschisis.
(A) |
(B) |
18
Abdominoschisis in amniotic band syndrome: (A) Endovaginal Sonography showing deformed cephalic pole (arrow) with amniotic bands (arrowheads) attached to it (B) shows eviscerated abdominal contents (arrow) with amniotic band (arrowhead) attached to it.
Prognosis in ABS
The prognosis is poor in abdominoschisis.
PARASITIC TWIN
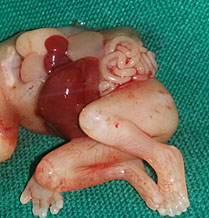
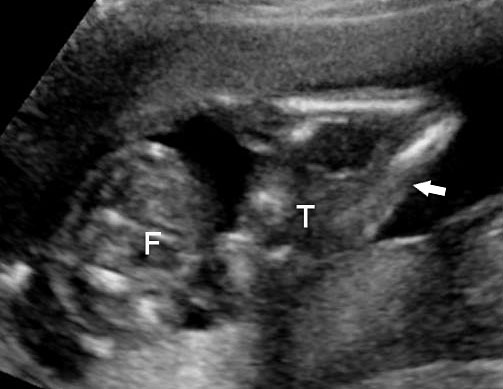
This is a very rare condition where in incomplete twin fetal part is attached to anterior abdominal wall. On ultrasound axial section fetal abdomen shows part of trunk and lower limbs of the parasitic twin attached to anterior abdominal wall of an otherwise normal fetus (Figure 19, Video 8).
(A) |
(B) |
19
Parasitic twin: (A) Axial scan of fetal abdomen showing trunk of parasitic twin (T) with lower limbs (arrow) attached to the anterior abdominal wall of the fetus (F). (B) Photograph of the fetus.
8
Parasitic twin: Axial sweep of fetal abdomen shows the parasitic twin with lower limbs attached to anterior abdominal wall of the fetus.
CONCLUSION
Abdominal wall defects are a complex group of congenital anomalies with a broad spectrum of manifestations and outcomes. The first step is evaluation of the cord insertion site in relation to the defect. The defects involving cord insertion are omphalocele, cloacal exstrophy, pentalogy of Cantrell, abdominoschisis and body stalk anomaly. Omphalocele as an isolated defect has good prognosis. In cloacal exstrophy, the associated defect is inferior and in pentalogy of Cantrell, the defect is superior. The defects with intact cord insertion are gastroschisis and bladder exstrophy. Absent urinary bladder is a finding in both bladder and cloacal exstrophy. Fixed fetal position and undefinable anatomic parts are hallmarks of abdominoschisis due to amniotic band syndrome and body stalk anomaly. Recognition of more complex AWDs is vital in pregnancy counseling and management.
PRACTICE RECOMMENDATIONS
- An omphalocele is sonographically seen as a bulging mass arising from the anterior abdominal wall, containing some abdominal viscera (liver and/or bowel) and the cord insertion is noted on its convexity. Color Doppler is useful to show the cord insertion on convexity.
- Diagnosis gastroschisis based on the demonstration of the normally situated umbilical cord insertion and the herniated free-floating loops of intestine without any membranous covering.
- Persistently non-visualized urinary bladder with normal amniotic fluid should raise to the suspicion of bladder exstrophy.
- OEIS complex is diagnosed when a persistently non-visualized bladder is associated with ventral wall defect, omphalocele, spinal defect, urinary tract anomaly along with ambiguous genitalia. The fetal anus is not seen.
- Ectopia cordis with a large omphalocele is diagnostic of pentalogy of Cantrell.
- In limb body wall complex (LBWC) the fetal abdominal contents are seen eviscerated into the extra-embryonic coelom with a short cord.
- In abdominoschisis, the abdominal contents float in the amniotic fluid without a clear encasing membrane with amniotic bands.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Gibbin C, Touch S, Broth RE, et al. Abdominal wall defects and congenital heart disease. Ultrasound Obstet Gynecol 2003;21(4):334–7. doi: 10.1002/uog.93. PMID: 12704739. | |
Glasser J. Omphalocele and Gastroschisis 2001. eMedicine http://www.emedicine.com/. | |
Cyr DR, Mack LA, Schoenecker SA, et al. Bowel migration in the normal fetus: US detection. Radiology 1986;161(1):119–21. doi: 10.1148/radiology.161.1.2945223. PMID: 2945223. | |
Nichol PF, Corliss RF, Yamada S, et al. Muscle patterning in mouse and human abdominal wall development and omphalocele specimens of humans. Anat Rec (Hoboken) 2012;295(12):2129–40. doi: 10.1002/ar.22556. Epub 2012 Sep 14. PMID: 22976993; PMCID: PMC3976953. | |
Roberts C. Intrauterine diagnosis of omphalocele. Radiology 1978;127(3):762. doi: 10.1148/127.3.762. PMID: 663174. | |
Boyd PA, Haeusler M, Barisic I. EUROCAT Report 9: Surveillance of congenital anomalies in Europe 1980–2008. Birth Defects Res A Clin Mol Teratol 2011;91(Suppl 1):S1. doi: 10.1002/bdra.20790. Epub 2011 Mar 7. PMID: 21384536. | |
Stoll C, Alembik Y, Dott B, et al. Omphalocele and gastroschisis and associated malformations. Am J Med Genet A 2008;146A(10):1280–5. doi: 10.1002/ajmg.a.32297. PMID: 18386803. | |
Barisic I, Clementi M, Häusler M, et al. Evaluation of prenatal ultrasound diagnosis of fetal abdominal wall defects by 19 European registries. Ultrasound Obstet Gynecol 2001;18(4):309–16. doi: 10.1046/j.0960-7692.2001.00534.x. PMID: 11778988. | |
Kirby RS, Marshall J, Tanner JP, et al. Prevalence and correlates of gastroschisis in 15 states, 1995 to 2005. Obstet Gynecol 2013;122(2 Pt 1):275–81. doi: 10.1097/AOG.0b013e31829cbbb4. PMID: 23969795; PMCID: PMC4605404. | |
Rittler M, Vauthay L, Mazzitelli N. Gastroschisis is a defect of the umbilical ring: evidence from morphological evaluation of stillborn fetuses. Birth Defects Res A Clin Mol Teratol 2013;97(4):198–209. doi: 10.1002/bdra.23130. Epub 2013 Apr 3. PMID: 23554304. | |
Muschaweck U. Umbilical and epigastric hernia repair. Surg Clin North Am 2003;83(5):1207–21. doi: 10.1016/S0039-6109(03)00119-1. PMID: 14533911. | |
Haas J, Achiron R, Barzilay E, et al. Umbilical cord hernias: prenatal diagnosis and natural history. J Ultrasound Med 2011;30(12):1629–32. doi: 10.7863/jum.2011.30.12.1629. PMID: 22123997. | |
Ludwig M, Ching B, Reutter H, et al. Bladder exstrophy-epispadias complex. Birth Defects Res A Clin Mol Teratol 2009;85(6):509–22. doi: 10.1002/bdra.20557. PMID: 19161161. | |
Martínez-Frías ML, Bermejo E, Rodríguez-Pinilla E, et al. Exstrophy of the cloaca and exstrophy of the bladder: two different expressions of a primary developmental field defect. Am J Med Genet 2001;99(4):261–9. doi: 10.1002/ajmg.1210. PMID: 11251990. | |
Pakdaman R, Woodward PJ, Kennedy A. Complex abdominal wall defects: appearances at prenatal imaging. Radiographics 2015;35(2):636–49. doi: 10.1148/rg.352140104. PMID: 25763744. | |
Feldkamp ML, Botto LD, Amar E, et al. Cloacal exstrophy: an epidemiologic study from the International Clearinghouse for Birth Defects Surveillance and Research. Am J Med Genet C Semin Med Genet 2011;157C(4):333–43. doi: 10.1002/ajmg.c.30317. Epub 2011 Oct 14. PMID: 22002951. | |
McLaughlin KP, Rink RC, Kalsbeck JE, et al. Cloacal exstrophy: the neurological implications. J Urol 1995;154(2 Pt 2):782–4. PMID: 7609179. | |
Cantrell JR, Haller JA, Ravitch MM. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium and heart. Surg Gynecol Obstet 1958;107(5):602–14. PMID: 13592660. | |
Chandran S, Ari D. Pentalogy of cantrell: an extremely rare congenital anomaly. J Clin Neonatol 2013;2(2):95–7. doi: 10.4103/2249-4847.116410. PMID: 24049753; PMCID: PMC3775145. | |
Bugge M. Body stalk anomaly in Denmark during 20 years (1970–1989). Am J Med Genet A 2012;158A(7):1702–8. doi: 10.1002/ajmg.a.35394. Epub 2012 May 31. PMID: 22653710. | |
Pumberger W, Schaller A, Bernaschek G. Limb-body wall complex: a compound anomaly pattern in body-wall defects. Pediatr Surg Int 2001;17(5–6):486–90. doi: 10.1007/s003830000497. PMID: 11527200. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)