This chapter should be cited as follows:
Tosto V, Tsibizova V, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.419063
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 19
Pregnancy shortening: etiology, prediction and prevention
Volume Editors:
Professor Arri Coomarasamy, University of Birmingham, UK
Professor Gian Carlo Di Renzo, University of Perugia, Perugia, Italy
Professor Eduardo Fonseca, Federal University of Paraiba, Brazil

Chapter
Preterm Birth Prediction
First published: November 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Preterm birth (PTB) occurs in approximately 10%1 of pregnancies worldwide and about 28% of all neonatal deaths are the result of PTB.2,3 PTB rates remain significant in both high- and low-resource countries, ranging from 5% to 18%, with the highest burden in low-income areas.4 Moreover, rates appear to increase in countries as data systems improve.5 Premature birth may be iatrogenic or spontaneous. Iatrogenic premature birth is the result of a medical intervention due to a fetal and/or maternal condition (e.g., fetal growth restriction, pre-eclampsia, other obstetric complications) necessitating early delivery. By contrast, spontaneous premature birth often occurs despite the best efforts to prolong the pregnancy. It is estimated that up to 80% of premature births fall into this category.
A great effort is being given by experts to reduce this adverse obstetric event through the use of prediction tools/strategies/models. The preterm parturition mechanism may be considered an example of predictive medicine application in obstetrics. Predictive medicine is a relatively new subspecialty in healthcare systems, yet the concept itself is not novel. In the most basic terms, predictive medicine utilizes specific tools (that may include instrumental examinations, laboratory, and genetic tests) to determine an individual's probability of developing disease or high-risk medical conditions.6
There is still not an ideal single or combined model screening for preterm birth. Interesting and promising investigations on metabolomics, proteomics, and microRNA profiling have brought a new aspect on this topic, as well as studies on ultrasound markers apart from cervical length measurements, that is the current most cost-effective method largely used in clinical practice.
Authoritative international and national associations provided useful guidelines and recommendations on PTB in recent years. Researchers are actively involved in the investigations of this complex syndrome in order to optimize its early detection and prevention.
The success of the reliable prediction/identification of women at risk of preterm birth translates into effective efforts to prolong pregnancy length and to optimize the outcome for the fetus, allowing for the transfer of the pregnant woman to a healthcare center with appropriate neonatal facilities, to administer corticosteroids to enhance fetal lung maturation, to administrate antibiotics when indicated, and to give magnesium for fetal neuroprotection.
PRETERM BIRTH SYNDROME
Preterm parturition is a complex and heterogeneous syndrome7 that can be induced by several factors able to trigger myometrial activation, premature rupture of membranes, and/or cervical maturation, thus resulting in threatened preterm labor, active preterm labor, and finally preterm delivery.
Most cases are of unknown cause. Although the mechanisms triggering PTB remain in part unclear, it seems reasonable from the scientific evidences, that an imbalanced inflammatory response and/or infectious conditions are the main contributors of this complication.8,9
Focused on prediction actions, which may help to identify and correctly manage the patients with true risk of PTB well, we can distinguish between predictors divided into the following categories:
- maternal characteristics (risk factor assessment),
- ultrasound markers,
- biomarkers and molecular techniques.
Maternal characteristics
Obstetric history is the first keystone to investigate during the first obstetric visit. A history of prior preterm birth in a previous pregnancy has an increased risk for preterm birth in a subsequent pregnancy. In a study by Iams et al. risk of recurrent PTB (<35 weeks) was 14–15% while women with a previous history of uncomplicated term delivery had 3% risk for spontaneous term delivery.10 By taking the increased risk for PTB into consideration in women with preterm birth history, precautions like cervical length screening or progesterone administration and intervention for maternal risk factors, like smoking cessation, treatment of underlying maternal comorbidities, and achieving ideal body mass index (BMI) should be considered in this population. All conditions that may led to imbalanced inflammatory response should be accurately investigated, for example, prepregnancy BMI, pregnancy weight gain, maternal infectious status, maternal vitamin deficiencies (i.e., vitamin D), even if there are controversial reports in the scientific literature about the real influence of these factors on preterm delivery risk.11,12,13,14,15
Ultrasound markers
The most important ultrasound marker in PTB prediction is the transvaginal cervical length (CL) measurement (Table 1). Screening of CL by transvaginal ultrasound is a good predictor of PBT risk in singleton pregnancies (Figure 1). Threshold of cervical length in 24 weeks of gestation for PBT risk was defined as 25 mm, with 37.3% sensitivity and 92.2% specificity.16 A meta-analysis showed that the knowledge of cervical length had a reduced risk for PTB before 37 weeks.17 A recent study in an extensive population reported that universal cervical screening program during mid-trimester sonogram in women without a history of preterm birth was associated with reduction in the PTB.18
A cervical length ≤15 mm was reported as the most optimal cut-off with 81% specificity and 83% positive predictive value for predicting the true preterm labor.19
The role of CL in predicting PTB risk in multiple pregnancies is more controversial. However, cervical length assessed in mid-trimester asymptomatic twin pregnancies was a poor predictor of PB <32 weeks' gestation.20 Within 3-week period, a shortening in cervical length >10% was found associated with increased risk of PB.21 There has been a conflicting evidence on screening of cervical length in the first trimester (11–13 weeks).22,23
1
Ultrasound cervical length measurement.
Ultrasound criteria |
|
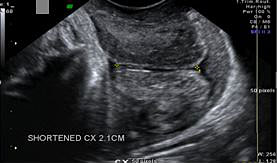
1
Transvaginal cervical length correctly measured.
Figure 2 briefly resumes some main technical aspects of the cervical length measurement with a transvaginal approach, and also shows possible ultrasound changes of CL in patients with threatened PTB (T, Y, V, and U shape funneling, reflecting a cervix at increased PTB risk).
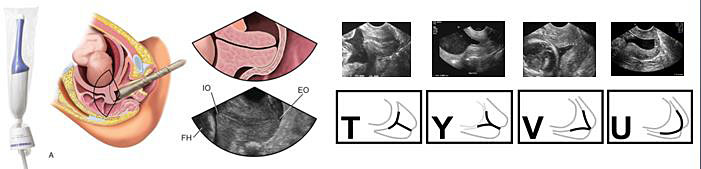
2
Cervical length measurement technical aspects.
In recent years, investigators studied others cervical parameters that may be helpful in PTB prediction: CL is only a morphologic analysis and cervix has consistence and structural typical changes during threatened PTB. Two methods have been better investigated for assessment of cervical elastography: strain elastography and shear wave elastography.24 These methods are promising but with obvious limitations in technical implementations and routine clinical use. Gesthuysen et al. observed that higher elastography index and strain pattern scores were correlated with an elevated risk of PTB and are superior to CL measurement as a predictive marker.25
Nazzaro et al. reported that women who delivered preterm had significantly lower hardness ratio (HR) compared to those who did not deliver preterm. Incidences of HR <50% and <35% were statistically significantly higher in women who delivered preterm compared to those who did not.26
Furthermore, the cervical consistency index (CCI), formulated as (AP1/AP) × 100, measuring anteroposterior cervical diameter before (AP) and after (AP1), is reported to be possibly more effective than cervical length in the prediction of PB.27,28 The uterocervical angle (UCA) is an adjunctive biophysical ultrasound (US) marker investigated for PTB prediction. The rationale behind this association is based on the mechanical properties of the UCA. An acute UCA seems act as a preventive mechanical barrier against preterm birth.29
A recent research showed that the UCA proved to be a novel ultrasound parameter that can be useful as a better predictor of preterm births in comparison to CL. In particular, a strong correlation exists between obtuse UCA and a risk of spontaneous preterm birth.30 UCA in the range of 95–105 degrees was found to be significantly associated with spontaneous PTBs at 34 to 37 weeks with a sensitivity of 78.1%, specificity of 93.7%, and p-value <0.001.30 This is in concordance with a previous study by Dziadosz et al.31 Interestingly, the UCA was studied to predict cerclage failure: it was observed in patients with transvaginal cerclage, an increasingly obtuse anterior uterocervical angle reflects an increased risk of cerclage failure in the mid-trimester.32
There are limited data about this issue for twin pregnancies in the literature. A recent systematic review investigated the relationship between uterocervical angle and preterm labor in twin pregnancies: a total of three studies were included in the analysis. It was observed that uterocervical angle was associated with a higher risk of preterm delivery in the overall effect.33
Figure 3 compares acute and obtuse UCA at transvaginal ultrasound.
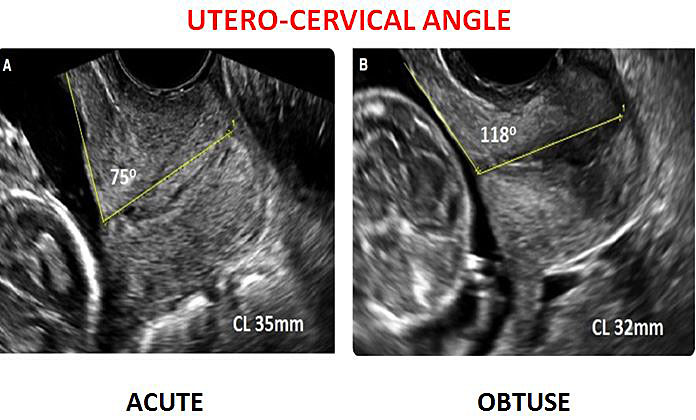
3
Transvaginal utero-cervical angle measurement.
Cervical sliding sign (CSS) is another ultrasound cervical marker analyzed for predicting spontaneous preterm birth. Debring et al. observed that positive CSS occurred more frequently in the preterm patients compared with the term pregnancy group.34
Currently, in the major part of clinical settings the traditional method of transvaginal CL measurement is the first ultrasound method of PTB detection. A combination of the parameters described could be considered for improving the PTB prediction performance (Table 2).
2
Ultrasound cervical markers in PTB prediction.
Ultrasound markers | Main findings |
EVOLVING CERVICAL IMAGING TECHNOLOGIES | |
Cervical length |
|
Cervical elastography |
|
Uterocervical cervical angle |
|
Fetal adrenal gland biometry was investigated as a potential PTB ultrasound marker, with contrasting results. Lemos et al. reported that the biometry for the central zone of the fetal adrenal gland predicted delivery within 7 days in pregnant women with spontaneous PB and had a predictive accuracy similar to that of CL measurement.35 On the other hand, Hoffman et al. observed that fetal adrenal size, as measured by ultrasonography between 22 and 30 weeks of gestation, is not predictive of spontaneous preterm birth in cohort of nulliparous women.36
Studies on prediction value of combining both fetal adrenal gland biometry, ultrasound cervix study, and/or biomarkers were conducted. Agarwal et al. combined fetal adrenal gland biometry and cervical elastography as predictors of preterm birth: fetal adrenal zone enlargement was shown to be a reliable marker of preterm birth. Furthermore, shear wave speed estimation of the antenatal cervix showed the highest sensitivity and specificity (96.7% and 87%, respectively) in the prediction of preterm birth and also showed a strong correlation with fetal adrenal gland enlargement.37 A study based on three-dimensional (3D) ultrasound measurement of fetal adrenal gland volume enlargement and cervicovaginal placental alpha microglobulin-1 (PAMG-1) test was conducted to predict the timing of delivery within 7 days in singleton pregnant women with threatened preterm labor. The combination of both markers increased sensitivity for the prediction of the timing of delivery.38
Biomarkers for prematurity prediction
Several studies assessed biomarkers predicting the pregnancy length and pregnancy duration after preterm premature rupture of membranes (p-PROM) in recent years.
Accessible biological fluids, including whole maternal blood/serum/plasma, urine, saliva, amniotic fluid, and cervicovaginal fluid (CVF), were studied to test which biomarkers were potentially useful for prediction purposes: these body fluids provide rich sources of proteins and metabolites that vary in concentration in response to pregnancy and pathological pregnancy states.
Biomarkers from different pathways of spontaneous preterm birth (cervical membrane degradation – fetal fibronectin, fFN), cervical remodeling (soluble E-cadherin), and inflammation (elafin, surfactant protein-D, interleukin-6 [IL-6]) were investigated. In particular, detection of some biomarkers in cervicovaginal fluid, such as fetal fibronection (fFN), placental alpha macroglobulin-1 (PAMG-1), and phosphorylated insulin-like growth factor binding protein 1 (phIGFBP-1) represent the most useful available tests for the prediction of PTB.
Fetal fibronectin (fFN) is a widely used biomarker for predicting premature delivery in women with symptomatic threatened PTB within 7–14 days. However, it shows the limited role in predicting PTB in asymptomatic women.39 Overall, fFN is used clinically for its negative predictive value, which exceeds 95% in some studies.
phIGFBP1 is secreted by decidual cells and leaks into cervical secretions when fetal membranes detach from decidua. It has been used to clinically assess cervical maturation. Clinical diagnostic trials indicate that, like fFN, phIGFBP1 is a good negative predictor of preterm birth (92% specificity).
Placental alpha microglobulin-1 is a relative novel biomarker detected in cervicovaginal discharge in patients with threatened preterm birth. More recently, Cnota et al. reported that PAMG-1 is a more accurate predictor of PTB when compared to CL.40 Nikolova et al. compared the PAMG-1 and the phIGFBP-1 alone and in combination with CL measurement for the prediction of imminent risk of PTB in symptomatic women. PAMG-1 resulted the best predictor: it seems significantly more specific than phIGFBP-1 for PTB prediction within 7 days, whereas both tests had comparable sensitivity.41,42 From cervical samples, it has been determined that the other proteins expressed at the highest levels in women with PTB are extracellular matrix protein 1, laminin, and calsintenin.43 Other studies found proteins such as desmoplakin 1, which participates in intercellular junctions and cell–cell communication, localized in human fetal membranes.
Maternal serum biomarkers were studied as a potential additional biomarker in PTB detection. Chiu et al. demonstrated that low levels of maternal serum PAPP-A and PlGF in the first trimester were associated with increased risks of spontaneous PTB and p-PROM at <37 weeks, providing an early option for preterm labor and birth detection.44 A recent systematic review analyzed if a maternal blood biomarker able to predict PTB exists: from 77 studies it has emerged that there is currently no known predictive biomarker for spontaneous PTB. Inflammatory and immune biomarkers show promise, but positive reporting bias limits the utility of results. The biomarkers identified may be more predictive in multi-marker models instead of as single predictors.45
Regarding the p-PROM, overall few studies investigated the role of biomarkers, including IL-6, IL-8, C-reactive protein (CRP), IL1RA, s-endoglin, human chorionic gonadotropin (hCG), alpha fetoprotein (AFP), urea, creatinine, oxygen radical absorbance capacity, lipocalin-2, endotoxin activity, metalloproteinases (MMP-8, MMP-9) and S100 A8/A9, were found to have a positive predictive value for delivery timing prediction. Furthermore, proinflammatory biomarkers, such as IL-6 or CRP, proved to be best correlated with delivery timing, independent of the occurrence of intrauterine infection.46
Recent studies investigated the role of neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) in the prediction of spontaneous preterm birth, showing high sensitivity and specificity.47,48
Endocrine biomarkers were also the object of investigation in preterm birth parturition mechanisms.
In this regard, a recent research proposed a nomogram for predicting the risk of premature delivery based on the thyroid function in pregnant women. The study identified the pregnancy-specific cut-off values for TPOAb positivity and TSH concentration associated: the pregnancy-specific cut-off values associated with the risk of premature delivery during the first trimester were 5.14 IU/mL for TPOAb positivity and 1.33 mU/L for TSH concentration.49
Another interesting study focused on the stress hormone axis: corticotropin-releasing hormone (CRH) has been proposed as part of a placental clock, with early activation of placental production resulting in preterm birth. CRH is a peptide hormone that increases exponentially in pregnancy and has been implicated in preterm birth because of its endocrine, autocrine, and paracrine roles. CRH has actions that increase placental production of estriol and of the transcription factor nuclear factor-κB, that likely play a key role in activating the myometrium.50
There is a relative paucity of strong evidence regarding the real effectiveness of urine and salivary (i.e., salivary progesterone) biomarkers.
New frontiers of investigations include cell-free fetal DNA (cfDNA). Studies evaluated the fetal fraction of cell-free DNA in maternal plasma at 11–13 weeks' gestation and observed different results: Farina et al. reported that high concentrations of fetal cell-free DNA in maternal serum were associated with an increased risk of spontaneous preterm delivery.51 Other studies did not find a significant correlation.52 Furthermore, cervical microRNAs were analyzed in association with spontaneous preterm birth and the length of gestation, but the extent to which microRNAs serve as predictive biomarkers remains unknown. Burris et al. conducted a prospective nested case-control study of cervical microRNA expression and spontaneous preterm birth and identified a global increase in microRNA expression and up-regulation of 95 distinct microRNAs in association with subsequent spontaneous preterm birth.53 cfRNA provides a molecular window on the maternal-fetal dyad. The approach to molecular diagnostics could also help guide patient management and preventive and therapeutic interventions by triggering treatments tailored to the observed molecular pathophysiology.54
Genetic contribution to PTB
Preterm birth likely depends on a number of interacting factors, including genetic, epigenetic, and environmental risk factors. Genetic studies may identify markers, which more accurately predict preterm birth than currently known risk factors. Relatively few studies were conducted, emphasizing the complexity of genetic influences on birth timing. While several candidate genes have been reported as associated with the pathological condition, inconsistency across studies exists.55,56
A recent published article provided an overview research on genetic factors that influence preterm birth in the context of neonatal phenotypic assessment: investigation of the human genome, epigenome, and transcriptome helps to identify molecular mechanisms linked with preterm delivery and premature newborn clinical appearance in early and late neonatal life and even predict developmental outcomes.57
Further researches and unbiased genetic approaches are needed for future studies to examine the genetic etiology of PTB.
CONCLUSIONS
Investigation on PTB prediction is active with interesting advances in genomic, proteomic, and metabolomic technologies. A significative number of predictive-risk markers/tools were investigated during the recent years, often showing a good predictive value, but with limits in the routinely clinical application (Table 3). Thus, a well codified screening for PTB prediction is not still available. Open questions remain regarding how to use and/or combine the predictors in a clinically reasonable manner and to cost-effective advantages.
Currently, ultrasound cervical length and biomarkers, such as cervical fetal fibronectin, PAMG1, alpha fetoprotein, C-reactive protein, and interleukin 6, may have an overall good diagnostic accuracy in identifying pregnancies at risk of spontaneous PTB. Further large prospective studies in a different sub-set of women are needed to ascertain which and whether the combination of different body fluids and additional imaging markers can improve antenatal prediction of this condition.
3
Keystones on current PTB detection.
Personal medical history |
|
Clinical presentation |
|
Ultrasound cervix evaluation (cervical length, cervical consistency, anterior utero-cervical angle, etc?) |
|
Maternal cervico-vaginal, serum (and amniotic fluid, saliva, urine) biomarkers |
|
PRACTICE RECOMMENDATIONS
- Previous preterm birth as a stronger risk factor to predict PTB
- Asymptomatic and symptomatic pregnant women should be distinguished
- Ultrasound cervical evaluation should include:
- CL measurement: widespread use, easy to perform, reproducible, cost-effectiveness, easy learning curve. Additional cervix parameters not largely used; need healthcare sonographer experience.
- Maternal cervico-vaginal, serum (and amniotic fluid, saliva, urine) biomarkers – few (fFn, PAMG1, phIGFB1, IL6) have concrete application in some clinical settings. Most do not (still) find clinical application, even if promising.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. Howson CP, Kinney MV, Lawn JE. (eds.) Geneva: WHO, 2012. | |
ACOG Practice Bulletin. Management of preterm labour. Number 42, May 2003. Int J Gynaecol Obstet 2003;82:127–35. | |
ACOG Practice Bulletin. Assessment of risk factors for preterm birth. Clinical Management guidelines for obstetrician-gynecologists. Number 31, October 2001. Obstet Gynecol 2001;98:709–16. | |
Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013;10(Suppl 1):S2. doi: 10.1186/1742-4755-10-S1-S2. | |
Ferrero DM, Larson J, Jacobsson B, et al. Cross-country individual participant analysis of 4.1 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PloS One 2016;11(9):e0162506. doi: 10.1371/journal.pone.0162506. | |
Jen MY, Shahrokhi M, Varacallo M. Predictive Medicine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. | |
Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345(6198):760–5. doi: 10.1126/science.1251816. | |
Di Renzo GC, Tosto V, Giardina I. The biological basis and prevention on preterm birth. Best Pract Res Clin Obstet Gynaecol 2018;52:13–22. doi: 10.1016/j.bpobgyn.2018.01.022. | |
Samuel TM, Sakwinska O, Makinen K, et al. Preterm birth: a narrative review of the current evidence on nutritional and bioactive solutions for risk reduction. Nutrients 2019;11(8):E1811. doi: 10.3390/nu11081811. | |
Iams JD, Goldenberg RL, Mercer BM, et al. The Preterm Prediction Study: Recurrence risk of spontaneous preterm birth. American Journal of Obstetrics & Gynecology 1998;178(5):1035–40. doi: 10.1016/S0002-9378(98)70544-7. | |
Ren H, Du M. Role of Maternal Periodontitis in Preterm Birth. Frontiers in Immunology 2017;8. doi: 10.3389/fimmu.2017.00139. | |
Macones GA, Parry S, Nelson DB, et al. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS). American Journal of Obstetrics & Gynecology 2010;202(2):147.e1–8. doi: 10.1016/j.ajog.2009.10.892. | |
Wagner CL, Baggerly C, McDonnell SL, et al. Post-hoc comparison of vitamin D status at three timepoints during pregnancy demonstrates lower risk of preterm birth with higher vitamin D closer to delivery. The Journal of Steroid Biochemistry and Molecular Biology 2015;148:256–60. doi: 10.1016/j.jsbmb.2014.11.013. | |
Zhou J, Su L, Liu M, et al. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: A prospective observational study in southern China. European Journal of Clinical Nutrition 2014;68(8):925–30. doi: 10.1038/ejcn.2014.99. | |
Qin L, Lu F, Yang S, et al. Does Maternal Vitamin D Deficiency Increase the Risk of Preterm Birth: A Meta-Analysis of Observational Studies. Nutrients 2016;8(5):301. doi: 10.3390/nu8050301. | |
Iams JD, Goldenberg RL, Meis PJ, et al. The Length of the Cervix and the Risk of Spontaneous Premature Delivery. The New England Journal of Medicine 1996;334(9):567–73. doi: 10.1056/NEJM199602293340904. | |
Berghella V, Palacio M, Ness A, et al. Cervical length screening for prevention of preterm birth in singleton pregnancy with threatened preterm labor: systematic review and meta-analysis of randomized controlled trials using individual patient-level data. Ultrasound in Obstetrics & Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology 2017;49(3):322–9. | |
Son M, Grobman WA, Ayala NK, et al. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. American Journal of Obstetrics & Gynecology 2016;214(3):365.e1–5. doi: 10.1016/j.ajog.2015.12.020. | |
Kunzier NB, Kinzler W L, Chavez MR, et al. The use of cervical sonography to differentiate true from false labor in term patients presenting for labor check. American Journal of Obstetrics & Gynecology 2016;215(3):372–.e5. doi: 10.1016/j.ajog.2016.03.031. | |
Pagani G, Stagnati V, Fichera A, et al. Cervical length at mid-gestation in screening for preterm birth in twin pregnancy. Ultrasound in Obstetrics & Gynecology 2016;48(1):56–60. doi: 10.1002/uog.15668. | |
Blanc J, Bretelle F. Outils prédictifs de l’accouchement prématuré dans une population asymptomatique à haut risque. Journal de Gynécologie Obstétrique et Biologie de la Reproduction 2016;45(10):1261–79. doi: 10.1016/j.jgyn.2016.09.009. | |
Parra-Cordero M, Sepulveda-Martinez A, Rencoret G, et al. Is there a role for cervical assessment and uterine artery Doppler in the first trimester of pregnancy as a screening test for spontaneous preterm delivery? Ultrasound in Obstetrics & Gynecology: The Official Journal of The International Society of Ultrasound in Obstetrics and Gynecology 2014;43(3):291–6. | |
Greco E, Gupta R, Syngelaki A, et al. First-trimester screening for spontaneous preterm delivery with maternal characteristics and cervical length. Fetal Diagnosis and Therapy 2012;31(3):154–61. doi: 10.1159/000335686. | |
Fruscalzo A, Mazza E, Feltovich H, et al. Cervical elastography during pregnancy: a critical review of current approaches with a focus on controversies and limitations. Journal of Medical Ultrasonics 2016;43(4):493–504. doi: 10.1007/s10396-016-0723-z. | |
Gesthuysen A, Hammer K, Möllers M, et al. Evaluation of Cervical Elastography Strain Pattern to Predict Preterm Birth. Ultraschall Med 2020;41(4):397–403. doi: 10.1055/a-0865-1711. | |
Nazzaro G, Saccone G, Miranda M, et al. Cervical elastography using E-cervix for prediction of preterm birth in singleton pregnancies with threatened preterm labor. J Matern Fetal Neonatal Med 2022;35(2):330–5. doi: 10.1080/14767058.2020.1716721. | |
Parra-Saavedra M, Gomez L, Barrero A, et al. Prediction of preterm birth using the cervical consistency index. Ultrasound in Obstetrics & Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology 2011;38(1):44–51. | |
Banos N, Murillo-Bravo C, Julia C, et al. Mid-trimester sonographic Cervical Consistency Index to predict spontaneous preterm birth in a low-risk population. Ultrasound Obstet Gynecol 2018;51(5):629–36. doi: 10.1002/uog.17482. | |
Daskalakis G. Assessment of uterocervical angle width as a predictor of preterm birth: a systematic review of literature. BioMed Res Int 2018;18:374–8. | |
Pramod KS, Resham S, Ishan K, et al. Evaluation of Uterocervical Angle and Cervical Length as Predictors of Spontaneous Preterm Birth. Indian J Radiol Imaging 2022;28:32(1):10–5. doi: 10.1055/s-0041-1741411. | |
Dziadosz M, Bennett T A, Dolin C. Uterocervical angle: a novel ultrasound screening tool to predict spontaneous preterm birth. Am J Obstet Gynecol 2016;215(03):3760–3.76E9. | |
Knight JC, Tenbrink E, Sheng J, et al. Anterior uterocervical angle measurement improves prediction of cerclage failure. J Perinatol 2017;37(4):375–9. doi: 10.1038/jp.2016.241. | |
Ercan I, Dincgez B, Uzunoglu A, et al. Evaluation of the predictive role of anterior uterocervical angle in preterm labor in twin gestation through meta-analysis: anterior uterocervical angle and preterm labor in twin gestation. J Gynecol Obstet Hum Reprod 2022;51(6):102397. doi: 10.1016/j.jogoh.2022.102397. | |
Debring B, Mollers M, Koster HA, et al. Cervical strain elastography: pattern analysis and cervical sliding sign in preterm and control pregnancies. J Perinat Med 2022;51(3):328–36. doi: 10.1515/jpm-2022-0166. | |
Lemos AP, Feitosa FE, Araujo Junior E, Feitosa HN, et al. Delivery prediction in pregnant women with spontaneous preterm birth using fetal adrenal gland biometry. J Matern Fetal Neonatal Med 2016;29(23):3756–61. doi: 10.3109/14767058.2016.1147556. | |
Hoffman MK, Turan OM, Parker CB, et al. Ultrasound Measurement of the Fetal Adrenal Gland as a Predictor of Spontaneous Preterm Birth. Obstet Gynecol 2016;127(4):726–34. | |
Agarwal S, Agarwal A, Joon P, et al. Fetal adrenal gland biometry and cervical elastography as predictors of preterm birth: A comparative study. Ultrasound 2018;26(1):54–62. doi: 10.1177/1742271X17748515. | |
Santipap M, Phupong V. Combination of three-dimensional ultrasound measurement of foetal adrenal gland enlargement and placental alpha microglobulin-1 for the prediction of the timing of delivery within seven days in women with threatened preterm labour and preterm labour. J Obstet Gynaecol 2018;38(8):1054–9. doi: 10.1080/01443615.2018.1446422. | |
Conde-Agudelo A, et al. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG: An International Journal of Obstetrics and Gynaecology 2011;118(9):1042–54. | |
Cnota W, Jagielska A, Janowska E, et al. Prediction of preterm birth using PAMG-1 test: a single centre experience – preliminary report. Ginekol Pol 2022;93(7):574–7. doi: 10.5603/GP.a2021.0171. | |
FIGO Working Group on Good Clinical Practice in Maternal-Fetal Medicine. Good clinical practice advice: prediction of preterm labor and preterm rupture of membranes. Int J Gynaecol Obstet 2019;144(3):340–6. doi: 10.1002/ijgo.12744. | |
Nikolova T, Uotila J, Nikolova N, et al. Prediction of spontaneous preterm delivery in women presenting with premature labor: a comparison of placenta alpha microglobulin-1, phosphorylated insulin-like growth factor binding protein-1, and cervical length. Am J Obstet Gynecol 2018;219(6):610.e1–9. doi: 10.1016/j. Ajog.2018.09.016. | |
Pereira L, Reddy AP, Jacob T, et al. Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J Proteome Res 2007;6:1269–76. 10.1021/pr0605421. | |
Chiu CPH, Feng Q, Chaemsaithong P, et al. Prediction of spontaneous preterm birth and preterm prelabor rupture of membranes using maternal factors, obstetric history and biomarkers of placental function at 11–13 weeks. Ultrasound Obstet Gynecol 2022;60(2):192–9. doi: 10.1002/uog.24917. | |
Hornaday KK, Wood EM, Slater DM. Is there a maternal blood biomarker that can predict spontaneous preterm birth prior to labour onset? A systematic review. PLoS One 2022;17(4):e0265853. | |
Feduniw S, Pruc M, Ciebiera M, et al. Biomarkers for Pregnancy Latency Prediction after Preterm Premature Rupture of Membranes-A Systematic Review. Int J Mol Sci 2023;24(9):8027. doi: 10.3390/ijms24098027. | |
Yuce E. Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) Can Predict Spontaneous Preterm Birth? J Inflamm Res 2023;16:2423–9. doi: 10.2147/JIR.S414305. | |
Ozel A, Alici Davutoglu E, Yurtkal A, et al. How do platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio change in women with preterm premature rupture of membranes, and threaten preterm labour? J Obstet Gynaecol 2020;40(2):195–9. doi: 10.1080/01443615.2019.1621807. | |
Meng Y, Lin J, Fan J. A Novel Nomogram for Predicting the Risk of Premature Delivery Based on the Thyroid Function in Pregnant Women. Front Endocrinol (Lausanne) 2022;12:793650. doi: 10.3389/fendo.2021.793650. | |
Herrera CL, Maiti K, Smith R. Preterm Birth and Corticotrophin-Releasing Hormone as a Placental Clock. Endocrinology 2022;164(2):bqac206. doi: 10.1210/endocr/bqac206. | |
Farina A, LeShane ES, Romero R, et al. High levels of fetal cell-free DNA in maternal serum: a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2005;193(2):421–5. doi: 10.1016/j.ajog.2004.12.023. | |
Quezada MS, Francisco C, Dumitrascu-Biris D, et al. Fetal fraction of cell-free DNA in maternal plasma in the prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol 2015;45(1):101–5. doi: 10.1002/uog.14666. | |
Burris HH, Gerson KD, Woodward A, et al. Cervical microRNA expression and spontaneous preterm birth. Am J Obstet Gynecol MFM 2023;5(1):100783. doi: 10.1016/j.ajogmf.2022.100783. | |
Camunas-Soler J, Gee EPS, Reddy M, et al. Predictive RNA profiles for early and very early spontaneous preterm birth. Am J Obstet Gynecol 2022;227(1):72.e1–16. doi: 10.1016/j.ajog.2022.04.002. | |
Plunkett J, Muglia LJ. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Ann Med 2008;40(3):167–95. doi: 10.1080/07853890701806181. | |
Esplin MS. Preterm birth: a review of genetic factors and future directions for genetic study. Obstet Gynecol Surv 2006;61(12):800–6. doi: 10.1097/01.ogx.0000248747.52343.5f. | |
Dauengauer-Kirlienė S, Domarkienė I, Pilypienė I, et al. Causes of preterm birth: Genetic factors in preterm birth and preterm infant phenotypes. J Obstet Gynaecol Res 2023;49(3):781–93. doi: 10.1111/jog.15516. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)