This chapter should be cited as follows:
Borges da Fonseca E, Moreira de Sá RA, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.419073
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 19
Pregnancy shortening: etiology, prediction and prevention
Volume Editors:
Professor Arri Coomarasamy, University of Birmingham, UK
Professor Gian Carlo Di Renzo, University of Perugia, Perugia, Italy
Professor Eduardo Fonseca, Federal University of Paraiba, Brazil

Chapter
Prevention of Preterm Birth
First published: February 2024
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Preterm is defined as babies born alive before 37 weeks of pregnancy are completed; however, the low-gestational age cutoff, or that used to distinguish preterm birth from spontaneous abortion, varies by location.1,2,3 There are subcategories of preterm birth (PTB), based on gestational age:1,2,3 (a) extremely preterm (less than 28 weeks), (b) very preterm (28–32 weeks), and (c) moderate to late preterm (32–37 weeks).
The incidence of PTB varies from region to region, averaging around 10% of pregnancies. In almost all countries with reliable data, PTB rates are increasing, much of this increase is explained by rising numbers of indicated preterm births.1,2 The population characteristics may impact on incidence of PTB. For instance, nutritional status during pregnancy, described by indicators such as body mass index (BMI), is associated with spontaneous preterm delivery. Low pre-pregnancy BMI is associated with a higher risk of spontaneous preterm birth. Obesity might be protective for spontaneous preterm labor; however, it increases the rate of medically indicated preterm delivery.1,3 Contributing factors include the increasing frequency of births to women older than 35 years, and the use of infertility treatments, with their significant enhancement of multiple birth rates.1,2,3
An estimated 15 million babies are born prematurely every year. This is more than 1 in 10 babies. The morbidity and mortality in this group is high with serious illness or death during the neonatal period, especially for those born before 32 weeks. Without appropriate treatment, those who survive are at increased risk of lifelong disability and poor quality of life.1
PTB accounts for 75% of perinatal mortality and more than half the long-term morbidity. Approximately 1 million children die each year due to complications of PTB. Although most preterm babies survive, they are at increased risk of neurodevelopmental impairments and respiratory and gastrointestinal complications.2,3 Hence, PTB is the main cause of neonatal mortality and the second highest cause of mortality in children aged up to 5 years, decreasing the survivors’ quality of life because of short- and long-term morbidities.2 PTB not only increases costs for the health care system, but also for families who also undergo considerable psychological difficulties afterwards.1 Spontaneous PTB represents two-thirds of all PTB and is often multifactorial,3 therefore the use of prediction tools and prevention strategies is sometimes difficult. Additionally, most women who undergo preterm delivery are low risk,4 making the effectiveness of strategies based on risk factors alone weak. Therefore, preterm delivery predictors and preventive strategies evaluated in recent years have not reduced overall prematurity.
As the etiology of spontaneous PTB is multifactorial, the prevalence of PTB is affected by several characteristics in the studied population (e.g., single or multiple pregnancies), influencing the results of prediction and preventive measures. The most commonly used prediction/prediction strategies involve recognizing risk factors for prematurity, such as prior history of PTB, multiple pregnancies and short cervical length by midtrimester transvaginal ultrasound.
The use of progesterone, cerclage, and pessary as preventive strategies has shown promising results; however, only in certain groups of patients.
This chapter reviews prediction and prevention strategies for a group of asymptomatic women, divided into singleton or multiple pregnancies.
PREDICTION OF SPONTANEOUS PTB IN ASYMPTOMATIC WOMEN
Although several risk factors are associated with PTB, the majority of PTB has no identifiable risk factors, and the presence of these factors does not increase the predictive value. The most important risk factors for preterm birth are prior history of PTB, multiple pregnancies (representing 15–20% of all PTB, and 40–50% of spontaneous PTB), short cervical length at transvaginal ultrasound at mid-trimester, an interval between deliveries of <6 months, socioeconomic difficulties and low educational levels, lack of prenatal care, low BMI, maternal infections, uterine malformations, cervical excisional procedures, second trimester maternal surgery, depression, smoking, drug use, black race, periodontal disease, and advanced age.3,4,5
To identify those at high risk for PTB, these factors may be combined to create risk scores. Unfortunately, such scores have low positive predictive values (38%) with high false-positive rates (17%).6,7
Despite these limitations, the recognition of risk factors during the care of asymptomatic women should be encouraged as it is easy, free of charge, and gives rise to the possibility of using corrective measures directed to certain groups, leading to a possible reduction of the individual influence of each factor on the risk of prematurity. The various combinations of clinical characteristics allow for the stratification from population groups in which the validation of more advanced prediction and prevention strategies have been tested, such as prematurity history, twin pregnancies, and a short cervix.
Prediction of PTB in singleton pregnancy
The traditional view that has governed the study of parturition is that preterm and term labor are fundamentally the same process, albeit occurring at different gestational ages.3,4,5 Indeed, preterm and term labor share a common terminal pathway, which it has defined as the anatomic, biochemical, endocrinologic, and clinical events that occur in term and preterm parturition. For example, the uterine components of the common pathway include: (a) increased uterine contractility; (b) cervical ripening; and (c) decidual membrane activation.
Screening tests are available to detect the activation of each of the components of the common pathway. Increased myometrial uterine contractility is often detected by patients and documented using external tocodynamometers. Short cervical length, that could be a sign of effacement in progress, can be detected by transvaginal ultrasound at midtrimester. Membrane/decidual activation can be detected subclinically by a positive fetal fibronectin, insulin-like growth factor binding protein-1, placental α-microglobulin-1, or other analytes.4,5,6,7,8,9,10
Biochemical methods
Fetal fibronectin (fFN) is a glycoprotein produced in the extracellular matrix. fFN is present in the vaginal fluid until 18 weeks; thereafter, levels begin to decrease before increasing again closer to delivery. However, in the case of mechanical or inflammatory damage to this matrix, fFN levels rise and subsequently increase the risk of premature birth.8 The PTB prediction by fFN has a high negative predictive value, while the positive predictive value is not as good.8 A meta-analysis of nine randomized controlled trials (RCTs) (1236 women) on fFN accuracy reported that in asymptomatic women without risk factors, this method presents with a sensitivity, specificity, positive- and negative-probability ratio of 0.48 (95% CI 0.20–0.77), 0.96 (95% CI 0.86–0.99), 12.01 (95% CI 4.70–30.68), and 0.54 (95% CI 0.30–0.97), respectively.9 The values in asymptomatic women with risk factors conceptualized heterogeneously between the studies, with sensitivity, specificity, positive, and negative-probability ratios of 0.34 (95% CI 0.24–0.43), 0.91 (95% CI 0.88–0.93), 3.47 (95% CI 2.84–4.24), and 0.75 (95% CI 0.68–0.82), respectively.9 Despite the high capacity to predict outcomes in women without risk factors, it seems unlikely that the use of fFN alone as a screening tool is acceptable or feasible in this population. Furthermore, its use in asymptomatic women with risk factors has not changed clinical management thus far.9
A recent prospective study, the Evaluation of a Quantitative Instrument for the Prediction of Preterm Birth (EQUIPP), reported that fibronectin concentration in the vagina is directly proportional to the risk of prematurity, offering a better prediction value than the qualitative test alone.8,9,10 This study demonstrated that values <10 ng/mL (present in 70% of asymptomatic women) represent a similar risk of premature birth to the general population of about 3.0%, with high sensitivity and negative predictive value. Values above 200 ng/mL represent a positive predictive value of only 38%, but higher than the value obtained with qualitative analysis.10 Because of these characteristics, fFN can be associated with cervical length measurements to assist in the clinical management of women with a short cervix.10
Owing to the likely inflammatory origin of premature labor, several biochemical markers of inflammatory response, such as insulin-like growth factor binding protein-1, placental α-microglobulin-1, interleukin (IL)-6, IL-1α, IL-8, tumor necrosis factor α (TNFα), and C-reactive protein, are collected from the blood, cervicovaginal fluids, or amniotic fluid. However, these markers still need to be validated and better understood to determine their clinical usefulness.10,11,12
A new line of research for the prediction of premature births is that of genomic, transcriptomic, proteomic, and metabolomic technologies based on the study of genes and particles that are substrates of chemical reactions that may be associated with the onset of premature delivery.13 Although promising, the results of their use, both isolated and associated with other prediction methods, are still conflicting and there are no prevention measures related to these findings, limiting their use in clinical practice.11
Biophysical methods
To assess the risk of PTB in the index pregnancy, transvaginal ultrasonographic cervical length (US-CxL) is the single most powerful predictor for PTB. The presence of a short cervix in low-risk women at the mid-trimester has been consistently associated with prematurity, resulting in a relative risk of 4 if <30 mm, 6 if <26 mm, 9 if <22 mm, and 14 if <13 mm, with a positive predictive value range from 6% to 44% and a sensitivity of 47−47%.14 Thus, the identification of a short cervix, is associated with a higher risk for PTB, and in such groups the use of progesterone, which can reduce preterm birth by about 40%.15,16,17 Considering these results, several studies have demonstrated that universal sonographic measurement of the cervix at mid-trimester and use of vaginal progesterone is cost-effective and yields the greatest reduction in PTB at 34 weeks’ gestation.18,19,20
Although such a strategy appears to be cost-effective, the recommendation for universal screening is not so clear in most guidelines.21,22 The FIGO working group on Best Practice in Maternal-Fetal Medicine recommend that transvaginal sonographic cervical length measurement should be performed in all pregnant patients at 19–236/7 weeks. This can be done at the same time as the ultrasound performed for the anatomical survey.23
There is a consensus on the use of transvaginal ultrasound for cervical length measurement in women at high risk for PTB,21 which includes women with a prior history of PTB, uterine anomalies, and cervical excisional treatments (loop electrosurgical excision procedure or cone biopsy).14 Serial cervical length measurements from 16 to 24 weeks seem to offer advantages over their isolated use in women with a history of prematurity <34 weeks.22 The examination should be repeated every 2 weeks if cervical length remains ≥30 mm or weekly if it is between 25 and 29 mm23 according Figure 1. This screening, associated with cerclage in cases with a cervix <25 mm, reduces births <24 weeks (RR 0.44, 95% CI 0.21–0.92), which in turn reduces perinatal death (RR 0.54, 95% CI 0.29–0.99), and when cervical length is <15 mm, the realization of cerclage to reduce birth <35 weeks (RR 0.23, 95% CI 0.08–0.66).23,24,25
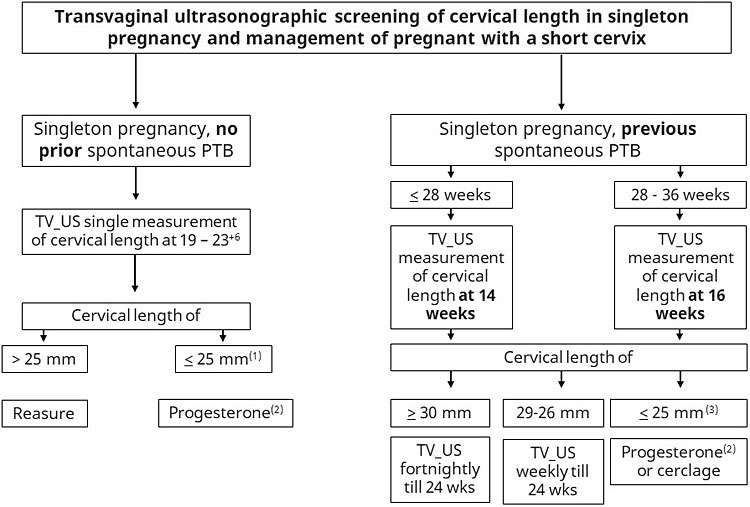
1
Approach to transvaginal ultrasonographic (TV_US) screening of cervical length in singleton pregnancy and management of pregnant people with a short cervix. (1) If cervical length is equal or less than 10 mm, cerclage might be considered; (2) vaginal micronized progesterone, 200 mg every night; (3) progesterone and cerclage are equally efficient, the decision for one of these treatments should consider safety, cost-benefit and patient/physician preference.
Association of methods – prediction models
The proposal of associating cervical length measurements and fibronectin dosage to improve the accuracy of the methods used individually in asymptomatic high-risk women was validated in 2016. This resulted in a prediction model that includes history of prematurity or premature rupture of membranes (PROM), cervical length measurement, and fibronectin dosage with an accuracy ranging from 77% to 99%. The model also has the ability to predict PTB within 2–4 weeks, with a sensitivity of 75.0% (19.4–99.4%) and 63.6% (30.8–89.1%), specificity of 97.7% (96.2–98.8%) and 95.8% (93.9–97.2%), positive predictive value of 17.6% (3.8–43.4%) and 21.2% (9.0–38.9%), negative predictive value of 99.8% (99.1–100%) and 99.3% (98.3–99.8%), positive probability ratio of 33.3 (15.5–71.6) and 15.0 (8.4–27.0), and negative probability ratio of 0.3 (0.1–1.4) and 0.4 (0.2–0.8) for 2- and 4-week predictions, respectively.26 The authors have also created an application called QUIPP to facilitate its use by physicians from all over the world.26 However, initial research suggests that although the use of this application would double the use of preventive measures in the studied population, benefiting women who would progress to a premature delivery, it would also increase the inappropriate use of preventive measures in other subpopulations. Therefore, further research is necessary before its use in clinical practice.27
Prediction of PTB in multiple pregnancy
Biochemical methods
The use of fFN in multiple pregnancies was evaluated in a meta-analysis of 15 studies including 1211 asymptomatic twin pregnancies, most of which (82%) underwent serial dosages between 22 and 34 weeks.28 Most studies analyzed birth prediction before 34 weeks with a sensitivity of 35–45%, specificity of 81–94%, and positive and negative probability ratios of approximately 2.5 and 0.7, respectively, concluding that the accuracy of this test in this population is limited.28 A more recent meta-analysis also stated that the accuracy of fFN in asymptomatic twin pregnancies is inconclusive.9
Biophysical methods
A short cervix (<25 mm) at 22–24 weeks is more prevalent in women with twin pregnancies (18%) than in women with single pregnancies (9%) and the same cervical measurement seems to offer more risk of prematurity in twin than in single pregnancies.4 The strong association between short cervix in asymptomatic women with twin pregnancies and the risk of prematurity was reported in a meta-analysis of 16 studies.29 Findings indicated that cervical length measurement is a good predictor for prematurity and the most accurate cutoff point for births <32 and <34 weeks is <20 mm between 20 and 24 weeks. The sensitivity, specificity, and positive and negative probability ratios for births <34 weeks was 39%, 96%, 10.1, and 0.64, respectively.29
The use of cervical length measurements in asymptomatic women with twin pregnancies has helped to identify women at higher risk of prematurity and inspired studies to analyze the best prevention strategy. Other publications have shown the possible benefits of prevention strategies in the subgroup of asymptomatic women with twin pregnancies and a short cervix,30,31,32 encouraging the use of cervical length measurement in women with twin pregnancies between 19 and 24 weeks.21,33 However, further studies are still needed to conclusively prove the real benefits of prevention strategies and to show the cost-effectiveness of universal cervical screening to modify the current recommendations.4,34
PREVENTION MEASURES
Primary prevention measures are directed to all women, including recommendations to stop smoking, implantation of few embryos in in vitro fertilization, correction of maternal pathologies, treatment of infectious diseases, reduction of stress, and better socioeconomic conditions with access to health services. Secondary prevention measures are directed to certain groups of women exposed to certain risk factors, including the use of cerclage, pessary, and progesterone, with different interpretations and outcomes in populations of asymptomatic women with single or twin pregnancies.2
Prevention of PTB in asymptomatic single pregnancy women
Cerclage
Cerclage consists of applying suture in the cervical region of the uterus to reinforce the structure of the cervix in women with cervical isthmus insufficiency. The suture is normally removed at 37 weeks of pregnancy. Cerclage can be performed transvaginally using the McDonald’s or Shirodkar’s technique or abdominally by laparotomy or laparoscopy.35,36 Cerclage is generally indicated in the following three situations: (1) based on history (history-indicated cerclage); (2) ultrasound findings (ultrasound-indicated cerclage); or (3) physical examination finding (emergency cerclage).35,36
History-indicated cerclage
History indication is based on reports of late second trimester losses or extreme premature deliveries and is considered a classic indication for cerclage. However, controversy exists as to the number of losses required as well as the effectiveness of this indication.37 The Royal College of Obstetricians and Gynaecologists (RCOG) recommends cerclage after three gestational losses,36 while the American College of Obstetricians and Gynecologists (ACOG) recommends prophylactic cerclage between 13 and 14 weeks for all women with a history of ≥1 second trimester losses, typically <24 weeks and without labor or placental detachment, or if there is a history of painless cervical dilatation cerclage in a previous pregnancy.35 Neither of the guidelines recommend the use of resistance tests or radiographic examinations to diagnose cervical isthmus insufficiency.35,36 The Cochrane systematic review (2017) failed to demonstrate the effectiveness of history-indicated cerclage.38 Approximately 40–70% of women with a history of second trimester loss or extreme premature births have a normal cervix up to 24 weeks of pregnancy, and of these, about 90% will have their deliveries after 34 weeks.36 These data have supported the use of ultrasound-indicated cerclage, avoiding unnecessary procedures. Cervical length measurement from 16 to 23 weeks among patients with second trimester loss or prior extreme preterm birth might be considered as a good clinical practice.35
Ultrasound-indicated cerclage
Ultrasound indication is based on serial transvaginal ultrasound screening from 16 to 24 weeks to diagnose cervical shortening in women with a prior history of preterm birth before 34 weeks.25,37,39 In this population, when the cervix is shorter than 25 mm, cerclage should be performed to significantly reduce the risk of preterm birth (RR 0.70, 95% CI 0.55–0.89) and perinatal morbidity and mortality (RR 0.64, 95% CI 0.45–0.91).25 Nevertheless, cerclage should not be indicated for those with cervix longer than 25 mm.36
On the other hand, short cervical length without prior history of PTB before 34 weeks is not an indication for cerclage.35,36,40 A systematic review and meta-analysis published by Berghella et al. in 2017 has demonstrated no benefit of cerclage in 419 women with single pregnancies, short cervix and no history of PTB.41 However, these authors reported a reduction of births less than 35 weeks in women with a cervical length <10 mm (39.5% vs. 58.0%, RR 0.68, 95% CI 0.47–0.98), even without a prior history of PTB. Further studies are required for a definitive conclusion because this study does not corroborate a previous RCT on 253 women to evaluate the efficacy of Shirodkar cerclage in women with cervix <15 mm in a population in which <20% of women had a history of PTB from 16 to 32 weeks. The study concluded that it was not possible to demonstrate the benefits of cerclage.40
Emergency cerclage
The presence of cervical dilatation during physical examination, without active labor or placental detachment, has also been used as an indicator for emergency cerclage. However, there are limited data regarding such indication.35
Ultrasound examination is recommended before any type of cerclage to evaluate fetal condition and confirm gestational age.42 There is no evidence to suggest that the use of antibiotics and tocolytics associated with emergency cerclage is beneficial.35 Cerclage should preferably be performed under spinal anesthesia42 and McDonald or Shirodkar cerclage sutures present similar outcomes regarding PTB prevention.43 Mersilene™ or Ethibond™ sutures are commonly used and seem to provide similar results, although there are records detailing the successful use of absorbable rescue sutures. Meta-analysis of results from six studies comparing the use of one or two sutures suggests that the use of two sutures reduce births <34 (OR 0.59, 95% CI 0.40–0.86) and <28 weeks (OR 0.43, 95% CI 0.26–0.73) without differences in neonatal morbidity and mortality.44 Furthermore, patients were discharged on the day of the procedure or the following day if performed after 20 weeks.42
Progesterone
Progesterone seems to act both on the myometrium, reducing the contractile response and frequency of contractions, and on the uterine cervix, reducing cervical remodeling. Remodeling occurs due to progesterone’s local anti-inflammatory properties, especially when administered vaginally.17,45
Some studies have used vaginal progesterone in asymptomatic women with single gestation and short cervix at doses of 90, 100 and 200 mg/day, the latter being the most studied.15,16,46 Progesterone has reduced the risk of preterm delivery for those with a prior history of PTB and a short cervical length regardless of the dose used.17 A systematic review and meta-analysis published in 2012 showed that vaginal progesterone in women with short cervix in the second trimester reduces the rate of birth before 28, 33, and 35 weeks, and such reducing is translated to a better neonatal outcome.33 These findings were reinforced by a Cochrane systematic review involving 36 RCTs (8523 women and 12,515 newborns) that reported the benefit of progesterone versus placebo in asymptomatic women with a short cervix, reducing birth <28 (RR 0.59, 95% CI 0.37–0.93) and 34 weeks (RR 0.64, 95% CI 0.45–0.90).47
In 2016, the OPPTIMUM trial did not find positive results, although it has demonstrated the micronized progesterone was safe.48 In 2018, a new meta-analysis was released with the largest number of individuals to date and including data from the OPPTIMUM trial. This meta-analysis included five studies on individual asymptomatic women with single pregnancies and cervix <25 mm, and analysis confirmed the efficacy of progesterone.17 These authors demonstrated the important effect of vaginal progesterone on reducing births <33 weeks (14% vs. 22%, RR 0.62, 95% CI 0.47–0.81, P = 0.0006, numbers needed to treat [NNT] 12, 95% CI 8–23). The study also confirmed the reduction in births before 36, 35, 34, 32, 30, and 28 weeks regardless of prematurity history, thus being an alternative to cerclage in women with prematurity history and cervical shortening of <25 mm.17,33 The authors also demonstrated reduced RDS, neonatal composite morbidity, weight <1500 g, and <2500 g at NICU admission and a tendency to reduce mortality (RR 0.44, 95% CI 0.18–1.07, P = 0.07).17 Despite the tendency to reduce neonatal mortality by 40–50%, intraventricular hemorrhage, and sepsis,17 these and other neonatal benefits still need to be better confirmed in women with this profile. Additionally, long-term follow-ups confirm that there is no evidence of malformations or development changes in children aged up to two years due to the use of progesterone.17,48
Consistent evidence regarding the benefits of progesterone for women with a history of PTB and single pregnancies is shown in the systematic Cochrane review with reduced births <34 weeks (RR 0.31, 95% CI 0.14–0.69), <37 weeks, and a significant extension of pregnancy, in addition to several neonatal benefits such as reduced perinatal mortality (RR 0.50, 95% CI 0.33–0.75), birth <2500 g (RR 0.58, 95% CI 0.42–0.79), use of assisted ventilation, necrotizing enterocolitis, neonatal mortality, and NICU admission.47 Although most RCTs demonstrate the advantage of using vaginal progesterone, the benefit of this therapy has been questioned by some studies, such as that by O’Brien et al. in 2007 and the PROGRESS study of 2017.49 The first study was a RCT with 611 women and analyzed the use of progesterone gel in women with a history of prematurity; their findings did not indicate reduced in neonatal prematurity or morbidity49 The second study was also a multicenter RCT involving 39 centers and a progesterone dose of 100 mg in 787 women in which the primary finding was the occurrence of RDS. The study also reported no benefits with regards to the use of progesterone neither for reduction of RDS or for reduction of PTB. One of the questioning points in this study was the fact that about 2% of the sample was composed of twin pregnancies.46 Divergent results lead to the need of meta-analysis with individual data to better understand the size of the vaginal progesterone effect in women with a history of prematurity.46
Pessary
The pessary is a flexible ring-shaped silicone device used to try to prevent premature birth. Most studies published to date used the Arabin pessary (CE0482, MED/CERT ISO 9003/EN 46003; Dr Arabin GmbH and Co., KG).50,51 The most important advantages of a pessary are easy outpatient placement and removal that does not require anesthesia and is not invasive. It acts by keeping the cervix closed and angulated, changing the direction of the cervical canal posteriorly, and focusing the weight on the anterior segment of the uterus.52,53,54
The use of a pessary in women with single pregnancies and short cervix has presented controversial results. The cervical pessary in pregnant women with a short cervix (PECEP) study was published by Goya et al. in 2012. It was one of the first RCTs on the use of pessary for prematurity prevention and involved five Spanish hospitals.52 A total of 380 women with single gestation and cervix <25 mm between 18 and 22 weeks were selected and randomly compared for pessary and placebo use, with an important reduction in births <34 weeks (6% vs. 27%, OR 0.18, 95% CI 0.08–0.37; P <0.0001) in the pessary group.52
In contrast, Hui et al. in 2013, analysis of 108 women indicated no advantages following the use of a pessary for birth prevention <34 weeks.55 However, they did not reach the number of women intended for adequate power of the study (1120 women) due to interruptions early in the research process.55
In 2016, a third multicenter RCT was published by Nicolaides et al. including women between 20 and 24 weeks + 6 days with cervix <25 mm. Women with cervix <15 mm received vaginal progesterone. The authors did not identify differences in births <34 weeks and neonatal changes.56
Some methodological differences between studies may explain the differences in results. A study by Goya et al. showed that 16% of the patients had their pessary repositioned, which did not occur in the other studies. Differences in the studied populations may also have occurred, with fewer women with a history of PTB in the study by Goya et al. than in the study by Nicolaides et al. (10.8% vs. 16.8%, respectively).52,57 The incidence of PTB for the control group in the study by Goya et al. (26.8%) was much higher than that of other studies (5.5% in the study by Hui et al., and 10.8% in the study by Nicolaides et al. ). Also, in the study by Goya et al. 20% of women were smokers and 90% were Latin American, raising doubt about the fact that the women included in this study had different characteristics from the women included in other studies. The study by Nicolaides et al. presented a high rate of pessary removal (24.5%), mainly due to the occurrence of premature rupture of membrane (PROM) or at the patient’s request. After pessary removal most patients progressed to labor.57 The pessary was also removed in cases of PROM in the study by Hui et al., in 14.5% of the women in the study group.55 The study by Goya et al. maintained the device despite the occurrence of PROM and reported that the infection rate did not differ from the control group. Unlike the other studies, the findings of Nicolaides et al. indicated that 45% of women (with cervix <15 mm) made concomitant use of vaginal progesterone in both groups, which may have reduced the effect of the pessary alone.56 Likewise, the analysis of these RCTs does not allow definitive conclusions regarding the interpretations of the results by adequately stratifying the history of PTB or cervical length ranges, which were divergent among the studies and must be considered for future studies.57
Systematic reviews and meta-analyses published in 2017 by Saccone et al.57 and Jin et al.58 involving the three RCTs and totaling 1420 women concluded that the use of the Arabin pessary in asymptomatic women with cervix <25 mm between 20 and 24 weeks + 6 days does not reduce the rate of premature delivery or improve perinatal outcomes. However, it is worth noting that more than half of the meta-analysis sample came from the study by Nicolaides et al. (2016) and included women with and without a history of prematurity.57,58
Kabarsian et al. (2016) tested the association of pessary use with the use of 400 mg vaginal progesterone in 144 women with single pregnancies and cervix <25 mm between 18 and 22 weeks. The pessary was removed in the presence of PROM and in women undergoing periodic cervical length measurements.59 This study mixed women with and without a history of prematurity and 14.5% had PROM. The prematurity rate in the control group was 19.7% even with the use of progesterone, and did not differ from the study group, thus showing no benefits from associating the pessary with the isolated use of progesterone.59
To reduce population heterogeneity, Saccone et al. (2017) published a RCT on women with cervix <25 mm between 18 and 23 weeks + 6 days and with no history of prematurity conducted in a single center and providing vaginal progesterone for women with cervix <20 mm in both groups.53 In addition to training the team to place the pessary, it was maintained in case of PROM and proper positioning was confirmed monthly through digital examination. Different from the meta-analysis published by the same author, this RCT showed a protective effect of the pessary for birth <34 weeks (7.3% vs. 15.3%, RR 0.48, 95% CI 0.24–0.95, P = 0.04) even with a mean cervical length lower than that in the study by Nicolaides et al. More than 80% of the sample was composed of cervix <20 mm and used progesterone. However, the authors recognized the limitation of external generalization of their results, since it was not a multicenter study, and also noted a high incidence of prematurity in the control group (32.7% <37 weeks and 15.3% <34 weeks).
A meta-analysis published by Corrêa et al. in 2019 involving all five RCTs concluded that although studies point to a lack of pessary efficacy, study heterogeneity allows no definitive conclusions and better evaluation of its association with progesterone is still required.60
Cruz-Melguizo et al. (2018) published a RCT directly comparing the use of pessary and progesterone, which included 27 centers and 254 asymptomatic women with single pregnancies and cervix <25 mm between 19 and 22 weeks with a history of PTB and ultrasound verification of pessary positioning; it did not consider PROM as a criterion for pessary removal.61 The authors concluded that the use of a pessary is not inferior to progesterone even in cervical length or history of PTB subgroups, but increases vaginal discharge and discomfort.61
Finally, in 2020, Conde Agudelo brought the biggest systematic review and meta-analysis, involving 12 RCT (4687 women and 7167 fetuses/infants), being eight evaluated pessary vs. no pessary in women with a short cervix, two assessed pessary vs. no pessary in unselected multiple gestations, and two compared pessary vs. vaginal progesterone in women with a short cervix. The paper demonstrated that there were no significant differences between the pessary and no pessary groups in the risk of spontaneous PTB <34 weeks among singleton gestations with a cervical length ≤25 mm (relative risk, 0.80; 95% confidence interval, 0.43–1.49; six trials, 1982 women), unselected twin gestations (relative risk, 1.05; 95% confidence interval, 0.79–1.41; one trial, 1177 women), twin gestations with a cervical length <38 mm (relative risk, 0.75; 95% confidence interval, 0.41–1.36; three trials, 1128 women), and twin gestations with a cervical length ≤25 mm (relative risk; 0.72, 95% confidence interval, 0.25–2.06; two trials, 348 women). Overall, no significant differences were observed between the pessary and no pessary groups in preterm birth <37, <32, and <28 weeks of gestation, and most adverse pregnancy, maternal, and perinatal outcomes, there were no significant differences in the risk of spontaneous PTB <34 weeks between pessary and vaginal progesterone in singleton gestations with a cervical length ≤25 mm (relative risk, 0.99; 95% confidence interval, 0.54–1.83; one trial, 246 women) and twin gestations with a cervical length <38 mm (relative risk, 0.73; 95% confidence interval, 0.46–1.18; one trial, 297 women). In conclusion, the authors demonstrated that there is no current evidence to support the use of cervical pessary to prevent preterm birth or to improve perinatal outcomes in singleton or twin gestations with a short cervix and in unselected twin gestations.50
Comparison and association of prevention strategies
Some studies, directly or indirectly, have tried to compare the abovementioned methods and the superiority of these strategies in relation to a placebo. An example of these studies was published by Alfiveric et al. (2013) and compared the follow-up outcomes of women with a history of PTB and short cervix assigned to different cohorts according to the prematurity prevention strategy used (progesterone, vaginal, or cerclage). The authors concluded that all strategies have similar effectiveness for this patient profile.62 However, Jarde et al. in meta-analysis involving 40 RCTs with 11,311 pregnant women showed that progesterone (primarily when administered vaginally) is the best option to prevent prematurity and neonatal death in women with a history of prematurity or short cervix.63
Strategies to prevent prematurity in asymptomatic women with twin pregnancy
Cerclage
The use of cerclage in women with twin pregnancies is controversial as it is associated with increased gestational risks. Its placement is not routinely indicated as only some subgroups have been found to benefit from this method.24,35,36,64
Ultrasound-indicated cerclage
A systematic Cochrane review published in 2014 and a meta-analysis published in 2015 by Saccone et al.65 proved the risks of cerclage in women with twin pregnancies. However, they only included a few RCTs therefore have a limited quality to allow for definitive conclusions. The negative points identified include a possibly increased incidence of births about 4 weeks earlier than in the control group, and a higher number of newborns with a low birth weight and respiratory distress syndrome (RDS).65,66 The number of women with a history of prematurity in the meta-analysis by Saccone et al. was extremely scarce and does not allow for conclusions. This point is important because cerclage indicated due to ultrasound only demonstrates benefits in women with single pregnancies and history of prematurity, especially prior to 34 weeks. Therefore, it is important that studies on twin pregnancies analyze the influence of short cervix, history of prematurity, and the phase in which prematurity occurred.
Although cerclage indicated due to ultrasound is not routinely recommended, a recent systematic review and meta-analysis published by Li et al. (2019) including 16 studies and 1211 women suggested some positive points for cerclage in twin pregnancies.67 Of the total studies included, nine studies (three RCT and six cohort studies) including 471 women were analyzed. Although the RCTs confirmed the lack of benefits, the cohort studies included in the meta-analysis showed that women with cervix <15 mm between 16 and 24 weeks had a significant pregnancy prolongation with reduced birth rates <37, <34, and <32 weeks when undergoing cerclage.67
Emergency cerclage
Similar to that of single pregnancies, emergency cerclage seems to offer benefits when compared to expectant behavior, with scarce RCT literature on this topic. A meta-analysis of three cohort studies (244 women) evaluated the use of cerclage indicated due to cervical dilatation >10 mm at physical examination and reported a mean pregnancy prolongation of 6.8 weeks, reducing birth incidence <34, <28, <2, and improved perinatal outcomes.67 A large RCT called Emergency Cerclage in Twin Pregnancies at Imminent Risk of Preterm Birth: an Open-Label Randomized Controlled Trial (ENCIRCLE) is in progress (ClinicalTrials.gov Identifier: NCT03818867) and intends to evaluate the real effect of emergency cerclage in twin pregnancies and the effect of cerclage in women with short cervix and twin–twin transfusion syndrome.
History-indicated cerclage
A case-control study with 82 women, of whom 41 underwent cerclage in the first trimester due to their history, demonstrated that the women who underwent cerclage presented with a higher gestational age at birth, lower birth rates <24, <28, 32, and 34 weeks, higher birth weight, and lower neonatal morbidity, NICU admission, respiratory distress syndrome (RDS), intraventricular hemorrhage, necrotizing enterocolitis, and neonatal mortality compared with the control group.68 However, recent meta-analysis on the subject states that it is not yet possible to demonstrate the real effectiveness of cerclage indicated only due to history as long as women present cervix >15 mm ) and further studies are needed.67
Progesterone
Progesterone has not demonstrated beneficial effects in unselected twin pregnancies either by intramuscular or vaginal route.15,63,69,70,71 Increased doses of vaginal progesterone from 200 mg to 400 mg daily also showed no benefits,72 although they seem not to cause fetal or neurodevelopmental changes up to 4–5 years of life after being used during pregnancy.70,32 A recent Cochrane systematic review including 16 studies (4548 women) also concluded that the administration of vaginal or intramuscular progesterone does not seem to be associated with decreased prematurity or improved neonatal outcomes.73
Nevertheless, secondary meta-analysis data published in 2012 suggested that progesterone reduced perinatal composite morbidity and mortality (23.9% vs. 39.7%, RR 0.52, 95% CI 0.29–0.93) in the subgroup of women with twin pregnancies and short cervix.33 Rehal et al., in a RCT involving 1079 pregnant women, have demonstrated that early vaginal progesterone in twin pregnancies does not reduce that rate of PTB. However, post hoc time-to-event analysis suggested that progesterone may reduce the risk of spontaneous birth before 32 weeks in women with a cervical length of <30 mm, and it may increase the risk for those with a cervical length of ≥30 mm.74 The results need to be interpreted with caution, but progesterone does not be indicated for those with a longer cervix of 30 mm once such indication might be harmful. On the other hand, vaginal progesterone appears to be benefit for those with short cervix.
Finally, a systematic review and meta-analysis, published in 2023, involving 11 RCT (3401 women and 6802 fetus/infants) showed that vaginal progesterone does not reduce neither the risk of PTB or improve neonatal outcomes in an unselected group of twins. Nevertheless, in a subgroup analyses, the authors showed that in a select group of twins with a transvaginal sonographic short cervix less than 30 mm (6 studies; 306 women and 612 fetuses/infants), and less or equal than ≤25 mm (6 studies; 95 women and 190 fetuses/infants), vaginal progesterone is associated with a significant decrease in the risk of PTB.75
Therefore, current data considering the apparent absence of deleterious progesterone effects, the high risk of PTB for women with twin pregnancies and short cervix between 20 and 24 weeks and the apparent neonatal benefits show that the use of progesterone in such group seems acceptable. However, further studies on this population are required to clarify the real effectiveness of this prevention strategy, the appropriate dose, and the degree of benefit in different cervical length ranges.
Pessary
Since 2003, when Arabin published the results of his study showing reduced prematurity in women with short cervix in single or twin pregnancies, several studies have tried to prove the effectiveness of this strategy. In 2013, Liem et al. published the results of a multicenter RCT called ProTWIN and showed a reduced risk of prematurity at <28 and <32 weeks and poor perinatal outcomes in women with twin pregnancies and use of pessary when the cervical length <25th percentile, defined in the study as 38 mm.76 Long-term evaluation did not show any neurodevelopment changes up to 3 years of age.77
Furthermore, a large multicenter RCT published by Nicolaides et al. (2016) of 1180 women with unselected twin pregnancies concluded that the use of a pessary does not reduce the incidence of prematurity or perinatal morbidity and mortality in these women or even in the subgroup with cervical length <25 mm.78 In this study, the authors recognized that adequate placement of the pessary could not be guaranteed, and it was removed in 22.3% of women before 34 weeks (8% secondary to PROM).78 However, in the same year, Goya et al. published the PECEP-TWIN study, a multicenter RCT on the use of pessary in 134 women with twin pregnancies and cervix <25 mm to be removed at 37 weeks, but maintaining the device in cases of PROM, which occurred in only 1.5% of women in the pessary group versus 9.1% of women in the control group.31 The authors reported reduced birth <34 weeks (16.2% vs. 39.4%, RR 0.41, 95% CI 0.22–0.76), low birth weight, and neonatal composite morbidity. They reported no differences for births prior to 28 weeks, which according to the authors could be due to the inefficacy of the pessary in a very short cervix.31
A meta-analysis involving women with short cervix presented in the three RCTs described above showed no benefits for the use of a pessary to prevent prematurity or neonatal outcomes.79 According to the data available so far, new RCTs directed at women with twin pregnancies and short cervix are needed, including adequate training and control for pessary positioning, standardizing cutoff points for short cervix definition, and stratifying results by history of prematurity. The real role and risks of pessary maintenance in women with PROM still need to be better clarified and a focused study is required to achieve this.
Potential benefits of omega-3 fatty acid supplementation in pregnancy on prevention of PTB
The importance of good nutrition in pregnancy is well accepted. Over the last 25 years, evidence has accumulated that intake of omega-3 long-chain polyunsaturated fatty acids (n-3 PUFAs) can benefit pregnancy.80,81
The two major categories of polyunsaturated fatty acids (PUFAs) are the omega-3 (also called n-3) and omega-6 (n-6) fatty acids, based on the location of the first double bond in the fatty acid chain. The three major dietary omega-3s are: (a) eicosapentaenoic acid (EPA), (b) docosahexaenoic acid (DHA), and (c) alpha-linolenic acid (ALA). The first two (marine omega 3) are mostly present in fish and shellfish, being the major components of fish oil supplements.80,81
Marine omega-3 fatty acids have several biologic effects. DHA and EPA are components of the phospholipids that form the structures of cell membranes, being important in the development of retina and brain. In addition, high concentrations of EPA and DHA tip the eicosanoid balance toward less inflammatory activity. Therefore, as the fetus depends on transplacental transfer of DHA for optimal visual and cognitive development, an appropriate maternal marine omega-3 fatty acid intake during pregnancy might reduce inflammation-mediated disorders, such as PTB, pre-eclampsia, and allergic/atopic disease in offspring.82,83,84,85
The first evidence on reducing PTB came from a multicenter RCT published in 2000 that included 19 hospitals across Europe. Pregnant women with a prior history of PTB were provided fish oil capsules containing 2.7 g of omega-3 (including 900 mg/d DHA and 1200 mg/d EPA) from 20 weeks.86 Supplementation reduced recurrent PTB roughly 36% and also delayed delivery.18 Two later RCTs of high DHA supplementation (600 mg/d of DHA or 800 mg/d of DHA plus 100 mg EPA) were conducted in women at low risk for PTB. Both found a significant reduction in early PTB as a secondary outcome among women with low intakes or blood levels of omega-3.19,20 In 2018, Cochrane Review evaluated omega-3 supplementation during pregnancy. The systematic review found strong evidence that pregnant women assigned to consume fish, fish oil, DHA or DHA+EPA, or given dietary advice to consume foods with omega-3 had an 11% risk reduction of all cause PTB <37 weeks (risk ratio – 0.89, 95% confidence interval of 0.81–0.97), and a 42% risk reduction of all cause of early PTB <34 weeks (risk ratio 0.58, 95% confidence interval of 0.44–0.77).21 The doses of DHA plus EPA ranged from 200 mg to 2700 mg/d, however, the results were largely driven by trials that provided greater than 500 mg/d of DHA.87
Current recommendations for DHA or DHA+EPA intakes in pregnancy
The World Health Organization (WHO), European Food Safety Authority (EFSA), United States Environmental Protection Agency and Food and Drug Administration, and The International Federation of Gynecology and Obstetrics (FIGO) recommend that pregnant women consume fish and seafood for general nutritional support.80,88,89,90 The American College of Obstetricians and Gynecologists (ACOG) recommends that women who are pregnant, or planning to be, should consume 8–12 ounces (227–340 g) of seafood/week.91 However, none of them specifically mentions that fish provides DHA and EPA that may reduce the risk of PTB and early PTB. The Perinatal Lipid Intake Working Group recommends at least 200 mg/d of DHA for pregnant women and those of childbearing age but also does not tie the recommendation to PTB or early PTB.92 The intake of omega-3 in the general population and in pregnant women is safe and does not appear to be harmful for doses up to 1000 mg daily of DHA plus EPA or DHA alone.
Finally, for pregnant women a regular intake of at least 350–450 mg/day DHA is recommended by the European Food Safety Authority.89 Those pregnant with a low DHA intake and/or low DHA blood levels are at increased risk of PTB and should receive a regular supply of about 600–1000 mg/day of DHA plus EPA, or DHA alone. In addition, this supply should preferably begin in the second trimester of pregnancy and not later than 20 weeks.
PRACTICE RECOMMENDATIONS
- There are many maternal and fetal risk factors for preterm birth (PTB). The identification of these risk factors before conception or early in pregnancy might allow early interventions.
- The short cervical length in transvaginal ultrasound at mid-trimester is the single most powerful predictor for PTB in the index pregnancy.
- Micronized vaginal progesterone is the first line treatment for singleton pregnancy with a short cervical length ≤25 mm. Cerclage might be an option for those with a short cervix ≤10 mm.
- Micronized vaginal progesterone and cerclage are equally effective for preventing PTB in pregnant women with singleton pregnancy, prior history of PTB and mid-trimester transvaginal ultrasonographic short cervix. The choice of treatment should consider safety, cost-benefit and the patient/physician decision.
- Micronized vaginal progesterone might be useful in multiple pregnancy with a mid-trimester short cervix ≤25 mm. Nevertheless, such treatment should not be an option for those with a cervix >30 mm, once there is evidence from RCTs that the treatment could be harmful.
- There is scientific evidence that intake of omega-3 long-chain polyunsaturated fatty acids (n-3 PUFAs) can benefit pregnancy, reducing PTB in the general population, However, further studies are needed to confirm this finding and define the best dose to be used.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Heal 2019;7(1):e37–46. | |
WHO recommendations on interventions to improve preterm birth outcomes. Available from: www.who.int/reproductivehealth. | |
Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. | |
Son M, Miller ES. Predicting preterm birth: Cervical length and fetal fibronectin. Semin Perinatol 2017;41(8):445–51. | |
Vogel JP, Chawanpaiboon S, Moller AB, et al. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol [Internet] 2018;52:3–12. Available from: https://doi.org/10.1016/j.bpobgyn.2018.04.003. | |
Witkin SS, Moron AF, Ridenhour BJ, et al. Vaginal biomarkers that predict cervical length and dominant bacteria in the vaginal microbiomes of pregnant women. MBio 2019;10(5):1–13. | |
Honest H, Bachmann LM, Sundaram R, et al. The accuracy of risk scores in predicting preterm birth – A systematic review. J Obstet Gynaecol (Lahore) 2004;24(4):343–59. | |
Hezelgrave NL, Shennan AH. Quantitative fetal fibronectin to predict spontaneous preterm birth: A review. Women’s Heal 2016;12(1):121–8. | |
Dos Santos F, Daru J, Rogozińska E, et al. Accuracy of fetal fibronectin for assessing preterm birth risk in asymptomatic pregnant women: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2018;97(6):657–67. | |
Conde-Agudelo A, Papageorghiou AT, Kennedy SH, et al. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. Br J Obstet Gynaecol 2011;118:1042–54. | |
Souza RT, McKenzie EJ, Jones B, et al. Trace biomarkers associated with spontaneous preterm birth from the maternal serum metabolome of asymptomatic nulliparous women – parallel case-control studies from the SCOPE cohort. Sci Rep 2019;9(1):1–10. | |
Suff N, Story L, Shennan A. The prediction of preterm delivery: What is new? Semin Fetal Neonatal Med 2019;24(1):27–32. | |
Cecatti JG, Souza RT, Sulek K, et al. Use of metabolomics for the identification and validation of clinical biomarkers for preterm birth: Preterm SAMBA. BMC Pregnancy Childbirth [Internet] 2016;16(1):1–9. Available from: http://dx.doi.org/10.1186/s12884-016-1006-9. | |
Lim K, Butt K, Crane JM, et al. Ultrasonographic Cervical Length Assessment in Predicting Preterm Birth in Singleton Pregnancies. J Obstet Gynaecol Canada [Internet] 2011;33(5):486–99. Available from: http://dx.doi.org/10.1016/S1701-2163(16)34884-8. | |
Fonseca EB da, Celik E, Parra-Cordero M, et al. Progesterone and the Risk of Preterm Birth among Women with a Short Cervix. N Engl J Med 2007;357:462–9. | |
Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blinded, placebo-controlled trial 2012;38(1):18–31. | |
Romero R, Conde-Agudelo A, Fonseca E Da, et al. Vaginal Progesterone for Preventing Preterm Birth Gestations With a Short Cervix : a Meta-Analysis of. Am J Obs Gynecol 2018;218(2):161–80. | |
Cahill AG, Odibo AO, Caughey AB, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. Am J Obstet Gynecol [Internet] 2010;202(6):548.e1–548.e8. Available from: http://dx.doi.org/10.1016/j.ajog.2009.12.005. | |
Werner EF, Hamel MS, Orzechowski K, et al. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: An update. Am J Obstet Gynecol [Internet] 2015;213(4):554.e1–554.e6. Available from: http://dx.doi.org/10.1016/j.ajog.2015.06.020. | |
Pizzi LT, Seligman NS, Baxter JK, et al. Cost and cost effectiveness of vaginal progesterone gel in reducing preterm birth: An economic analysis of the PREGNANT trial. Pharmacoeconomics 2014;32(5):467–78. | |
Medley N, Poljak B, Mammarella S, et al. Clinical guidelines for prevention and management of preterm birth: a systematic review. BJOG: An International Journal of Obstetrics and Gynaecology 2018;125. | |
Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. J Am Med Assoc 2001;286(11):1340–8. | |
FIGO – Working Group on Best Practice In Maternal-Fetal Medicine. FIGO Committee Report. International Journal of Gynecol & Obstet 2015;128:80–2. | |
Berghella V, Odibo AO, To MS, et al. Cerclage for short cervix on ultrasonography: Meta-analysis of trials using individual patient-level data. Obstet Gynecol 2005;106(1):181–9. | |
Berghella V, Rafael TJ, Szychowski JM, et al. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol 2011;117(3):663–71. | |
Kuhrt K, Smout E, Hezelgrave N, et al. Development and validation of a tool incorporating cervical length and quantitative fetal fibronectin to predict spontaneous preterm birth in asymptomatic high-risk women. Ultrasound Obstet Gynecol 2016;47(1):104–9. | |
Goodfellow L, Care A, Sharp A, et al. Effect of QUiPP prediction algorithm on treatment decisions in women with a previous preterm birth: a prospective cohort study. BJOG An Int J Obstet Gynaecol 2019;126(13):1569–75. | |
Conde-Agudelo A, Romero R. Cervicovaginal fetal fibronectin for the precdiction of spontaneous preterm birth in multiple pregnancies: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2010;23(12):1365–76. | |
Conde-Agudelo A, Romero R, Hassan SS, et al. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and meta-analysis. Am J Obs Gynecol 2010;203(2):128.e1–128.12. | |
El-refaie W, Abdelhafez MS, Badawy A. Vaginal progesterone for prevention of preterm labor in asymptomatic twin pregnancies with sonographic short cervix: a randomized clinical trial of efficacy and safety. Arch Gynecol Obstet 2016;293(1):61–7. | |
Goya M, De La Calle M, Pratcorona L, et al. Cervical pessary to prevent preterm birth in women with twin gestation and sonographic short cervix: A multicenter randomized controlled trial (PECEP-Twins). Am J Obstet Gynecol 2016;214(2):145–52. | |
Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol 2017;49(3):303–14. | |
Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbity: a systematic review and meta-analysis of individual patient data Roberto. Am J Obs Gynecol 2012;206(2):124.e1–19. | |
McIntosh J, Feltovich H, Berghella V, et al. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol [Internet] 2016;215(3):B2–7. Available from: http://dx.doi.org/10.1016/j.ajog.2016.04.027. | |
Cerclage for the Management of Cervical Insufficiency. Pratice Bulletin No. 142. American College of Obstetricians and Gylecologists. Obstet Gynecol 2014;123(2):372–9. | |
Shennan A, To M. Green-top guideline No 60: Cervical cerclage. RCOG 2011. | |
Goddijn M, Christiansen OB, Elson J, et al. Recurrent pregnancy loss. Guideline of the European Society of Human Reproduction and Embryology. Eur Soc Hum Reprod Embryol 2017:0–153. | |
Alfirevic Z, Stampalija T, Medley N. Cochrane Database of Systematic Reviews Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy (Review) Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy (Review) 2017;(6). Available from: www.cochranelibrary.com. | |
Berghella V, Palacio M, Ness A, et al. Cervical length screening for prevention of preterm birth in singleton pregnancy with threatened preterm labor: systematic review and meta-analysis of randomized controlled trials using individual patient-level data. Ultrasound Obstet Gynecol 2017;49(3):322–9. | |
To MS, Alfirevic Z, Heath VCF, et al. Cervical cerclage for prevention of preterm delivery in women with short cervix: Randomised controlled trial. Lancet 2004;363(9424):1849–53. | |
Berghella V, Ciardulli A, Rust OA, et al. Cerclage for sonographic short cervix in singleton gestations without prior spontaneous preterm birth: systematic review and meta-analysis of randomized controlled trials using individual patient-level data. Ultrasound Obstet Gynecol 2017;50(5):569–77. | |
Berghella V, Ludmir J, Simonazzi G, et al. Transvaginal cervical cerclage: evidence for perioperative management strategies. Am J Obstet Gynecol 2013;209(3):181–92. | |
Odibo A, Berghella V, To M, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol 2007;24(1):55–60. | |
Pergialiotis V, Vlachos DG, Prodromidou A, et al. Double versus single cervical cerclage for the prevention of preterm births. J Matern Fetal Med 2015;28:379–85. | |
Furcron AE, Romero R, Plazyo O, et al. Vaginal progesterone, but not 17α-hydroxyprogesterone caproate, has antiinflammatory effects at the murine maternal-fetal interface. Am J Obstet Gynecol [Internet] 2015;213(6):846.e119. Available from: http://dx.doi.org/10.1016/j.ajog.2015.08.010. | |
Crowther CA, Ashwood P, McPhee AJ, et al. Vaginal progesterone pessaries for pregnant women with a previous preterm birth to prevent neonatal respiratory distress syndrome (the PROGRESS Study): A multicentre, randomised, placebo-controlled trial. PLoS Med 2017;14(9). | |
Dodd JM, Jones L, Flenady V, et al. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev 2013;2013(7). | |
Norman JE, Marlow N, Messow CM, et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): A multicentre, randomised, double-blind trial. Lancet [Internet] 2016;387(10033):2106–16. Available from: http://dx.doi.org/10.1016/S0140-6736(16)00350-0. | |
O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: Primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2007;30(5):687–96. | |
Conde-Agudelo A, Romero R, Nicolaides KN. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol 2020;223:42–65.e2. doi: 10.1016/j.ajog.2019.12.266. | |
ARABIN® Cerclage Pessary perforated [Internet] [cited 2019 Dec 21]. Available from: https://dr-arabin.de/produkt/arabin-cerclage-pessary-perforated/?lang=en. | |
Goya M, Pratcorona L, Merced C, et al. Cervical pessary in pregnant women with a short cervix (PECEP): An open-label randomised controlled trial. Lancet [Internet] 2012;379(9828):1800–6. Available from: http://dx.doi.org/10.1016/S0140-6736(12)60030-0. | |
Saccone G, Maruotti GM, Giudicepietro A, et al. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length a randomized clinical trial. JAMA – J Am Med Assoc 2017;318(23):2317–24. | |
Arabin B, Halbesma JR, Vork F, et al. Is treatment with vaginal pessaries an option in patients with a sonographically detected short cervix? J Perinat Med 2003;31(2):122–33. | |
Lao TT, Leung F. Hui S, et al. Cerclage Pessary for Preventing Preterm Birth in Women with a Singleton Pregnancy and a Short Cervix at 20 to 24 Weeks: A Randomized Controlled Trial. Am J Perinatol 2012;30(04):283–8, 2013. | |
Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med 2016;374(11):1044–52. | |
Saccone G, Ciardulli A, Xodo S, et al. Cervical Pessary for Preventing Preterm Birth in Singleton Pregnancies With Short Cervical Length: A Systematic Review and Meta-analysis. J Ultrasound Med 2017;36(8):1535–43. | |
Jin XH, Li D, Huang LL. Cervical Pessary for Prevention of Preterm Birth: A Meta-Analysis. Sci Rep [Internet] 2017;7:1–6. Available from: http://dx.doi.org/10.1038/srep42560. | |
Karbasian N, Sheikh M, Pirjani R, et al. Combined treatment with cervical pessary and vaginal progesterone for the prevention of preterm birth: A randomized clinical trial. J Obstet Gynaecol Res 2016;42(12):1673–9. | |
Corrêa TD, Amorim EG, Tomazelli JAG, et al. Use of the Pessary in the Prevention of Preterm Delivery. Revista Brasileira de Ginecologia e Obstetricia. Georg Thieme Verlag, 2019;41:53–8. | |
Cruz-Melguizo S, San-Frutos L, Martínez-Payo C, et al. Cervical pessary compared with vaginal progesterone for preventing early preterm birth a randomized controlled trial. Obstet Gynecol 2018;132(4):907–15. | |
Alfirevic Z, Owen J, Carreras Moratonas E, et al. Vaginal progesterone, cerclage or cervical pessary for preventing preterm birth in asymptomatic singleton pregnant women with a history of preterm birth and a sonographic short cervix. Ultrasound Obstet Gynecol 2013. | |
Jarde A, Lutsiv O, Beyene J, et al. Vaginal progesterone, oral progesterone, 17-OHPC, cerclage, and pessary for preventing preterm birth in at-risk singleton pregnancies: an updated systematic review and network meta-analysis. BJOG An Int J Obstet Gynaecol 2019;126(5):556–67. | |
Huang X, Saravelos SH, Li TC, et al. Cervical cerclage in twin pregnancy. Best Pract Res Clin Obstet Gynaecol [Internet] 2019;59(xxxx):89–97. Available from: https://doi.org/10.1016/j.bpobgyn.2019.06.001. | |
Saccone G, Rust O, Althuisius S, et al. Cerclage for short cervix in twin pregnancies: Systematic review and meta-analysis of randomized trials using individual patient-level data. Acta Obstet Gynecol Scand 2015;94(4):352–8. | |
Rafael TJ, Berghella V, Alfirevic Z. Cervical stitch (cerclage) for preventing preterm birth in multiple pregnancy. Cochrane Database Syst Rev 2014;10(9). | |
Li C, Shen J, Hua K. Cerclage for women with twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol [Internet] 2019;220(6):543–557.e1. Available from: https://doi.org/10.1016/j.ajog.2018.11.1105. | |
Rottenstreich A, Levin G, Kleinstern G, et al. History-indicated cervical cerclage in management of twin pregnancy. Ultrasound Obstet Gynecol 2019;54(4):517–23. | |
Rouse D, Caritis S, Peaceman A, et al. A Trial of 17 Alpha-Hydroxyprogesterone Caproate to Prevent Prematurity in Twins. N Engl J Med 2007;357:454–61. | |
Rode L, Klein K, Nicolaides KH, et al. Prevention of preterm delivery in twin gestations (PREDICT): A multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol 2011;38(3):272–80. | |
Brizot ML, Hernandez W, Liao AW, et al. Vaginal progesterone for the prevention of preterm birth in twin gestations: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol [Internet] 2015;213(1):82.e1–9. Available from: http://dx.doi.org/10.1016/j.ajog.2015.02.021. | |
Serra V, Perales A, Meseguer J, et al. Increased doses of vaginal progesterone for the prevention of preterm birth in twin pregnancies: A randomised controlled double-blind multicentre trial. BJOG An Int J Obstet Gynaecol 2013;120(1):50–7. | |
Dodd JM, Grivell RM, OBrien CM, et al. Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy. Cochrane database Syst Rev 2019;2019(11). | |
Rehal A, Benkő Z, Matallana CP, et al. Early vaginal progesterone versus placebo in twin pregnancies for the prevention of spontaneous preterm birth: a randomized, double-blind trial. Am J Obstet Gynecol 2021;224:86.e1–19. doi: 10.1016/j.ajog.2020.06.050. | |
Conde-Agudelo A, Romero R, Rehal A, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in twin gestations: a systematic review and meta-analysis. Am J Obstet Gynecol 2023;229:599–616. | |
Liem S, Schuit E, Hegeman M, et al. Cervical pessaries for prevention of preterm birth in women with a multiple pregnancy (ProTWIN): A multicentre, open-label randomised controlled trial. Lancet 2013;382(9901):1341–9. | |
van ‘t Hooft J, van der Lee JH, Opmeer BC, et al. Pessary for prevention of preterm birth in twin pregnancy with short cervix: 3-year follow-up study. Ultrasound Obstet Gynecol 2018;51(5):621–8. | |
Nicolaides KH, Syngelaki A, Poon LC, et al. Cervical pessary placement for prevention of preterm birth in unselected twin pregnancies: A randomized controlled trial. Am J Obstet Gynecol [Internet] 2016;214(1):3.e1–9. Available from: http://dx.doi.org/10.1016/j.ajog.2015.08.051. | |
Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in twin pregnancies with short cervical length: a systematic review and meta-analysis. J Matern Neonatal Med [Internet] 2017;30(24):2918–25. Available from: http://dx.doi.org/10.1080/14767058.2016.1268595. | |
Hanson MA, Bardsley A, De-Regil LM, et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: "Think Nutrition First". Int J Gynaecol Obstet 2015;131(Suppl 4):S213–53. | |
Almeida CAN, Pimentel C, Fonseca EB. Além da Nutrição – O impacto da nutrição matrena na saúde de futuras gerações. 1ª edição, São Paulo, 2019:134–43. Acess on https://abran.org.br/new/wp-content/uploads/2019/08/ALEM_DA_NUTRICAO.pdf. | |
Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr 1992;120:S129. | |
Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Micronutrients). Washington, DC: The National Academies Press, 2002/2005. | |
McCann JC, Ames BN. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr 2005;82:281. | |
Lewin GA, Schachter HM, Yuen D, et al. Effects of omega-3 fatty acids on child and maternal health. Evid Rep Technol Assess (Summ) 2005;1. | |
Olsen SF, Secher NJ, Tabor A, et al. Randomised clinical trials of fish oil supplementation in high risk pregnancies. Fish Oil Trials In Pregnancy (FOTIP) Team. BJOG 2000;107(3):382–95. | |
Middleton P, Gomersall JC, Gould JF, et al. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev 2018;11:CD003402. | |
World-Health-Organization-&-Food-and-AgricultureOrganization-of-the-United-Nations. Report of the joint FAO/WHO expert consultation on the risks and benefits of fish consumption, 25–29 January 2010, Rome, Italy. Rome: World-Health-Organization, 2011. | |
EFSA-Panel-on-Nutrition-Novel-Foods-and-Food-Allergens. Scientific Opinion on health benefits of seafood (fish and shellfish) consumption in relation to health risks associated with exposure to methylmercury. EFGSA Journal 2014;12(7):3761. | |
United-States-Einvironmental-Protection.-Agency-and_Food-and-Drug-Administration. EPA-FDA Advice about Eating Fish and Shellfish For Those Who Might Become Pregnant, Are Pregnant, Are Breastfeeding, and for Children. 2021. | |
American-College-of-Obstetrics-and-Gynecology. Nutrition During Pregnancy. Frequently Asked Questions. 2022. | |
Koletzko B, Cetin I, Brenna JT. Dietary fat intakes for pregnant and lactating women. Br J Nutr 2007;98:873–7. doi: 10.1017/S0007114507764747. Epub 2007 Aug 10. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
