This chapter should be cited as follows:
Huynh V, Gomez-Lobo V, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.418473
The Continuous Textbook of Women’s Medicine Series – Gynecology Module
Volume 2
Adolescent gynecology
Volume Editor: Professor Judith Simms-Cendan, University of Miami, USA

Chapter
Addressing the Reproductive Health of Adolescent Girls with Cancer and Cancer Survivors
First published: November 2022
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
More than 400,000 children (aged 0–19 years) in the world are diagnosed with cancer each year.1,2 The most common types being leukemia, central nervous system tumors, neuroblastoma, lymphoma, and kidney tumors and bone tumors. After many advancements throughout the years, most childhood cancers can be cured with oncologic therapies, such as chemotherapy, radiotherapy, and surgery. In developed countries, the 5-year survival rate has increased to more than 80%.1 Globally, the 5-year survival rate is 37.4% with large variation by region.3
As childhood and adolescent cancer survival rates continue to increase, there is an increased emphasis on managing the late effects related to the oncologic therapies. In particular, the gonadotoxic effect of radiation therapy and chemotherapy, reproductive outcomes, and psychosocial impact.
THE IMPACT OF ONCOLOGY THERAPY ON FEMALE REPRODUCTIVE HEALTH
Although cancer treatments such as chemotherapy and radiation are designed to cure cancer, it is also associated with long-term sequelae, including pituitary dysfunction, hypothyroidism, adrenal complications, low bone mineral density, cardiovascular disease, and infertility.4 More specifically, females are born with an ovarian reserve that consists of a finite number of primordial follicles. In addition to the continued and gradually accelerated decline of these follicles, oncologic therapies can be gonadotoxic and significantly reduce this reserve. Depending on the degree of loss of the ovarian reserve, this could manifest as retaining ovarian function, amenorrhea after treatment completion, or experiencing menopause before the age of 40 years. Alkylating agents, radiation directed to the abdominopelvic region or cranium, and hematopoietic stem-cell transplant are all gonadotoxic and can significantly increase the risk of premature ovarian insufficiency and associated infertility.
Chemotherapy Effects on Fertility
At birth, the ovary contains approximately 1–2 million oocytes formed as primordial follicles, which decline with age. The number of primordial follicles is a direct indicator of fertility reserve in which there is an ongoing decline with age or ovarian disruption.5 Chemotherapy induces toxicity on the ovaries directly by targeting actively dividing cells, thus inducing apoptosis in granulosa cells, which are supporting cells around the oocyte, and destroying mature ovarian follicles. Chemotherapeutic treatments, such as alkylating agents, platinum-based compounds, and anthracycline antibiotics, are gonadotoxic by causing DNA strand breaks and oxidative stress leading to accelerated activation, apoptosis, and inflammation of primordial follicles.6 Their mechanisms are also implicated causing damage to blood vessels and increased fibrosis of the ovarian cortex.4,7,8 This mode of injury results in local ischemia leading to dysfunctional primordial follicle growth and survival.9,10
There are short-term and long-term effects of chemotherapy drugs on the ovaries. During treatment, the drugs may acutely destroy growing ovarian follicles causing temporary amenorrhea. The long-term effect of chemotherapy may be a reduced ovarian reserve leading to infertility and/or premature ovarian insufficiency (POI). Several factors may affect the extent of ovarian damage, including age, type of drug, and dosage. Alkylating drugs (cyclophosphamide, procarbazine, busulfan, chlorambucil) are considered the most damaging chemotherapies for fertility due to their non-specific cell-cycle function.11
Radiation Effects on Fertility
Radiotherapy represents a cornerstone in cancer treatment, in which several types of cancer may require radiation therapy. Radiation is an effective oncologic therapy through directly killing cancer cells or causing direct DNA damage leading to cell death. Similarly, radiation of the ovary can damage oocytes, destroy granulosa cells causing ovarian follicle loss, cause vascular damage, and increase stromal fibrosis.10 Additionally, increased fibrosis and vascular damage has also been seen in the uterus and vagina causing increased poor pregnancy outcomes or dyspareunia, respectively.
The impact of radiation on fertility also depends on several factors, including age, irradiation field, type, dose, and duration of the treatment.12 Younger patients are more likely to maintain fertility compared to adult survivors, likely due to the larger ovarian reserve present at the time of treatment.11 Due to the radiation-induced ovarian follicular atrophy and the subsequent depletion of the ovarian reserve, patients undergoing abdominopelvic radiation are at risk of accelerated reproductive aging potentially leading to the development of POI.7,13 Ovarian dysfunction is often unpredictable with intermittent periods of function, but POI may also be permanent.14,15
Cranial irradiation may also induce infertility by disrupting the hypothalamic-pituitary-gonadal axis with consequent disturbance of the normal hormone secretion.16 Radiation-induced dysfunction to the hypothalamus and anterior pituitary gland is dose dependent and can lead to gonadotropin deficiency.10 The pulsatile secretion of gonadotropin-releasing hormone (GnRH) by the hypothalamus is responsible for the luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion by the pituitary.17,18 Therefore, a disruption in the gonadal axis and subsequent gonadotropin deficiencies can affect the menstrual cycle and overall fertility. The replacement of sex steroid hormones with estrogen and progesterone will be required.19 However, for women desiring pregnancy who have not had gonadotoxic therapy to the ovaries, ovulation can be induced with exogenous gonadotropins, since the egg supply was not affected.20,21
Hematopoietic Stem-Cell Transplantation
Hematopoietic stem-cell transplant (HSCT) is a curative therapy for certain types of cancers, including leukemia and lymphoma. Before the transplant, myeloablative conditioning is often required to ensure sufficient immunosuppression and prevent rejection of the transplant. Myeloablation can be executed with regimens involving radiation, chemotherapeutic drugs, or both. The most common preparative regimen is total body irradiation or high-dose alkylating agents, which can lead to POI and infertility.22,23 HSCT is increasingly becoming the standard-of-care for malignant and nonmalignant diseases, and with their cures comes the considerable burden of deleterious effects on the ovaries.24,25 In addition to the increased risk of POI, another potential late effect of HSCT is graft-versus-host-disease (GVHD) to the genitals.
Risk Calculation
Infertility rates range from 11% to 26% among female childhood cancer survivors.26 The toxicity that incurs from oncologic treatment is not uniform across all adolescent females and all treatment plans. In prepubescent females, radiation directed at the abdominopelvic at greater than 15 Gy incurs the greatest risk of gonadotoxicity that can lead to developing POI and even infertility. Conversely, older patients are more sensitive to gonadotoxic therapy and radiation levels greater than 10 Gy will incur the same level of risk for postpubertal patients.27 Cranial radiation doses greater than 30 Gy significantly increase the level of risk.28 Alkylating agent doses greater than 12 cyclophosphamide equivalent dose (CED) in prepubertal patients and greater than 8 (CED) in postpubertal patients incur the highest level of gonadotoxicity risk.29 All myeloablative regimens are also associated with high levels of increased risk.26,30
Providing an estimate of gonadotoxic risk should be included during chemoradiation treatment discussions and patients/families should be given the option of pursuing fertility preservation if desired. Interventions in preserving fertility may be costly and come with exposure to additional risks from surgery and anesthesia. Therefore, the risk of POI is important clinical knowledge to provide expectations on infertility and make decisions on the appropriate measure of fertility preservation, ultimately to provide comprehensive cancer care.31 It is important to note that there is very limited data on the risks associated with a number of oncologic therapies. For further information on calculating infertility risks, please refer to the gonadal insufficiency and infertility risk stratification system provided by the Pediatric Initiative Network.30
FERTILITY PRESERVATION FOR ADOLESCENTS
Oncologic therapies can compromise ovarian function and future fertility of cancer survivors. Thus, fertility preservation has become an important quality-of-life factor for many survivors of adolescent cancer, and health care providers should initiate discussions during counseling prior to treatment. Ideally, fertility preservation interventions would be executed before cancer treatment, but oocyte and embryo cryopreservation can also be offered between chemotherapy cycles, after surgery but before chemotherapy, or after treatment completion if the patient has not endured permanent POI. Cancer treatment takes top priority, which can delay or interfere with the efforts of preserving fertility. Embryo and oocyte cryopreservation are standard strategies for fertility preservation only for postmenarcheal patients but require a delay in treatment of 2–5 weeks. Ovarian tissue cryopreservation (OTC) and ovarian transposition are available methods for both pre- and postmenarcheal patients and does not require a delay in cancer treatment. OTC is no longer considered an experimental procedure, as of 2019, however, there have only been two live births from ovarian tissue transplantation of previously cryopreserved tissue obtained from prepubertal/premenarcheal girls..32,33,34 With all the available interventions and associated risks and benefits, it is important for every health care provider to consider each patient’s unique goals and concerns and come to a shared decision.
Embryo and Oocyte Cryopreservation
Embryo and oocyte cryopreservation are clinically well-established techniques and the standard of care for fertility preservation. Embryo cryopreservation requires sperm from a male partner or a donor, which opens the door to ethical, legal, and moral concerns of embryo storage if the patient dies or she and her partner separate.35,36 Oocyte cryopreservation is a modality that allows a woman to preserve her ability to procreate with a chosen partner in the future.37 It is also only available for postmenarcheal females due to their mature hypothalamic-pituitary axis and ability to respond to controlled ovarian stimulation (COS).38 Although embryo and oocyte cryopreservation are ideal prior to initiating cancer treatment, it could also be offered after treatment. However, previous studies have demonstrated diminished response to ovarian stimulation after one to two courses of chemotherapy.39,40
Embryo and oocyte cryopreservation are procedures that require COS, which take approximately 10–14 days, however, it can take up to 4–5 weeks for referral to a fertility center, coordination of medical care, or if waiting for menstrual cycle onset.41 Therefore, it is not a suitable option for fertility preservation in patients with time constraints on initiating oncologic treatment. The stimulation regimen is initiated with gonadotrophins injections to stimulate follicular growth and development. This is then followed by gonadotrophin-releasing hormone (GnRH) antagonists to prevent premature ovulation and luteinization. The conventional final step for COS was using human chorionic gonadotropin (hCG) to induce final follicular maturation. However, due to the risk hCG has shown for ovarian hyperstimulation syndrome, a rare but serious and potentially fatal complication, recent studies and clinical practice have shifted to an increased use of GnRH agonists to trigger the final follicular maturation step.42,43,44
Follicle development is monitored throughout COS with transvaginal or transabdominal ultrasounds and blood tests for hormone levels. At the end of the stimulation regimen, matured oocytes are collected with ultrasound-guided transvaginal needle aspiration under sedation. Transabdominal needle aspiration is also an available option for younger females. Collected oocytes are subsequently isolated by stripping away cumulus cells and only mature oocytes (metaphase II) proceed with cryopreservation by vitrification. For embryo cryopreservation, the oocytes then undergo fertilization. This may be facilitated by conventional in-vitro fertilization (IVF), a method of co-incubating oocytes with sperm, or by intracytoplasmic sperm injection (ICSI), a method shown to have higher fertilization rates when compared to the conventional method.45,46 Embryos are then incubated and grown for 5–6 days to the blastocyst stage and then cryopreserved.47
Historically, oocytes and embryos were cryopreserved by a slow-freezing technique that takes 1–2 hours and requires expensive instrumentation equipment.48,49 Current recommended practice involves rapid cooling, also known as vitrification, which is a procedure that takes only a few minutes, does not require specialized equipment and is more likely to avoid the formation of ice crystals in the intracellular and extracellular space.50 Embryos are more resistant to damage caused by cryopreservation than oocytes. While in metaphase II, mature oocytes are fragile and susceptible to ice-crystal formation due to their large size, high water content, low surface to volume ratio, and presence of the meiotic spindles.51,52 Additionally, meiotic spindle microtubules are highly sensitive to temperature deviation with transient exposures causing irreversible damage, which may result in aneuploidy after fertilization of thawed oocytes.53,54,55,56 In previous years, slow-freezing was prone to ice-crystal formation that led to poor success rates for oocyte cryopreservation. With the continued advancements in vitrification, this rapid supercooling method has been more likely to avoid ice-crystal formation and increase post-thaw survival, fertilization, and clinical pregnancies to similar rates as fresh embryos and oocytes.57,58,59 In the general population, oocyte cryopreservation demonstrates post-thaw survival rates that are greater than 90% and successful pregnancy rates have been reported to be as high as 50% in adult women with expectations of higher rates in adolescents.60,61 It is important to note that the success rates are related to the number of oocytes retrieved in which underlying cancer may cause ovarian suppression leading to lower response rates. Additionally, patients with time constraints may be only allotted one attempt of COS as compared to other patients that may pursue multiple cycles to retrieve more oocytes. In embryo cryopreservation, post-thaw survival rates are varied between 82–99%, clinical pregnancy rates are between 26–60%, and live birth rates are between 15–56%.62 There continues to be a gap in research on fertility preservation outcomes in patients that underwent intervention in adolescence.
Ovarian Tissue Cryopreservation
Ovarian tissue cryopreservation (OTC) has recently been accepted as a standard method for fertility preservation in patients receiving gonadotoxic treatment.32 As it does not require COS, it is one of the few methods available for premenarcheal females. It is also an option for patients that cannot delay oncologic therapy to complete COS. OTC is the only fertility preservation method that has shown to restore global reproductive endocrine hormone function. However, there are concerns of reseeding malignant cells in graft ovarian tissue and the risk of relapse, especially for patients with leukemia.63,64
In this process partial or whole ovary is obtained by minimally invasive laparoscopic surgery, ovarian cortical tissue (where the oocytes reside) is then sliced into thin cortical strips, and cryopreserved. Despite the growing popularity with vitrification of ovarian tissue, slow freezing ovarian tissue has been shown better survival results than vitrification.59 Transplantation of ovarian cortical fragments are typically placed orthotopically (in the pelvic or peritoneal cavity), however heterotopic sites have also been attempted (in the forearm or anterior abdominal wall).65 The most common transplantation sites are onto a remaining non-functioning ovary or within the peritoneal bursa. After auto-transplantation, ovarian endocrine function is restored in approximately 95% of cases and occurs approximately 4–6 months after reimplantation with a mean duration of 4–5 years.20,25,26 As a newer clinically accepted fertility preservation option, pregnancy and live birth rates continue to be reported. As of 2017, there have been over 130 live births from successful pregnancies using this method.38 However, there have been only two successful live births from tissue that was cryopreserved from prepubertal/premenarcheal patients.36,65
In-vitro maturation (IVM) of aspirated oocytes is an extension of both COS with oocyte cryopreservation as well as OTC, which may be an additional form of protecting fertility. This method cultures immature oocytes (germinal vesicle or metaphase I) to a mature state (metaphase II) required for fertilization. IVM is still in its infancy and recently is no longer considered experimental but may be a feasible option for cryopreserving mature oocytes in the premenarcheal patient population.66 In OTC, it is an option that can be done before the tissue is cryopreserved or after the tissue is thawed.67,68,69 IVM of aspirated oocytes also serves as a safer option for patients at risk of possible malignancy reseeding.70 OTC has also been performed after complete remission and has shown a decrease in risk of seeding.71 Previous studies have shown no significant difference between pre- and postmenarcheal patients in the number of aspirated oocytes. However, the overall IVM rate was significantly higher in the postmenarcheal group.72 Additionally, very young females (less than 6 years) have substantially decreased IVM rates making this an invaluable option for this age group.73 To our knowledge, there have been six live births following the use of OTC and IVM of immature oocytes from ovarian tissue collected from adult women.74,75,76,77 However, clinical data on OTC-IVM outcomes is very limited and scarce, thus this method continues to be experimental.
In postmenarcheal patients, combining OTC with COS and subsequent oocyte/embryo cryopreservation is an innovative technique that can be used to maximize efficiency of preserving fertility. In these cases, OTC could be performed prior to starting COS to avoid cryopreserving tissue from expanded ovarian cortex or bleeding corpora lutea, after as a backup method if the ovary does not respond well to COS with few oocytes retrieved, or after chemoradiation is complete and in remission if spontaneous menses resumes.78,79 Tissue harvesting has been shown to not affect the number or quality of the collected matured oocytes after COS.80
Ovarian Transposition
Ovarian transposition, also known as oophorepexy, is the first procedure in history proposed to preserve fertility in females with cancer.81 The method is indicated in patients requiring abdominopelvic radiation as part of their oncologic treatment. This surgical procedure includes repositioning one or both ovaries out of the direct field of radiation. It is generally performed after neoadjuvant chemotherapy. The procedure may be a minimally invasive surgery on its own or performed during the resection of an abdominal or pelvic tumor. The procedure is associated with adverse effects such as ovarian torsion, adhesion formation, and ovarian cysts.82,83 Additionally, OTC could be performed concomitantly with the ovarian transposition procedure to increase the opportunity for future fertility. Ovarian endocrine function has been preserved relatively efficiently with ovarian transposition in approximately 60% of patients. However, only 15% of patients treated with transposition and desire conception are able to achieve pregnancy, though this is based on relatively low numbers of reported patients.84 Additionally, the long-term effects of ovarian transposition on adolescents have not been well studied.
Gonadal Protection
GnRH agonists have been considered to provide pharmacologic protection of the ovary during chemotherapy. It is thought to induce hypogonadotropism ovarian quiescence as well as reduce ovarian blood flow.85 Recent studies in adult women with breast cancer and lymphoma have shown contradictory evidence regarding the effectiveness of GnRH agonists in preventing premature ovarian insufficiency. The use of GnRH agonists exclusively for fertility protection remains controversial with clinical evidence remaining uncertain of the effects on ovarian function and should not be the first-line option for fertility preservation over oocyte cryopreservation or OTC.86
REPRODUCTIVE HEALTH MANAGEMENT FOR ONCOLOGIC THERAPY
Menstrual Suppression
Patients undergoing oncologic therapy are at risk of heavy menstrual bleeding (HMB) from treatment-induced myelosuppression leading to thrombocytopenia.87 Menstrual blood loss can threaten a patient who may already be affected by anemic, thrombocytopenic, or both potentially leading interventions, such as blood and platelet transfusion. HMB can affect health-related quality of life as well as be troublesome in an already stressful situation. To avoid the risk of HMB, the menstrual cycle can be temporarily suppressed with various medical treatments. Options for menstrual management include combined hormonal contraceptives, progestin-only therapy, GnRH agonists, and progestin-containing intrauterine devices (IUDs). Each option has different mechanisms of action and side-effect profiles. Therefore, it is appropriate to take into consideration the patient’s current menstrual status, hemoglobin and platelet counts, planned treatment regimen, risk of venous thromboembolism (VTE), and contraception goals.
GnRH agonists can be used to interrupt the hypothalamic-pituitary-gonadal axis and suppress the menstrual cycle.88 It has been demonstrated to be the most effective method for menstrual suppression with rates as high as 96%.89,90 GnRH agonists typically induce a transient increase in gonadotropins and estrogen, potentially leading to increased bleeding in the first 2–4 weeks on therapy with the initial dose.91 To avoid the risk of HMB, menstrual suppression with GnRH agonists is initiated approximately ≥2 weeks before the anticipated hemoglobin and platelet nadir from oncologic treatment.92 Leuprolide acetate (LA) is a frequently used GnRH agonist that produces very high rates of amenorrhea in patients. The most preferred method of administration is an intramuscular (IM) dose of 11.5 mg LA every 3 months.86 It is also available as subcutaneous or intravascular formulations. It may also be given as IM doses of 3.75 mg every month or 45 mg IM every 6 months. It is recommended to continue throughout cancer treatment until 3 months after chemotherapy is complete. GnRH agonists are also a popular option due to the potential ability to confer some fertility preservation benefit, although this is controversial and there is no clear consensus on this. It is important to note that GnRH agonists are not considered contraception. Therefore, if GnRH agonists are used for menstrual suppression, health care providers should assess a patient’s need for a contraceptive method.93
With the suppression of the menstrual cycle, patient may begin to experience menopausal symptoms including mood changes and vasomotor symptoms. This may be uncomfortable or even intolerable especially for young patients. Additionally, suppression of estrogen may also cause decreased bone mineral density. Adolescence is a particularly critical period for bone accrual. Therefore, hormonal add-back therapy should be strongly considered to mitigate these effects. Norethindrone acetate 5 mg orally daily is commonly used for add-back therapy and has been shown to protect bone health and prevent menopausal symptoms.94,95 It has also been used alone as a method of menstrual suppression. It must be noted that norethindrone acetate does have estrogen effects and may be associated with an increased risk of VTE.96,97
Other options for menstrual suppression include progestin-only therapy, such as progestin-only pills, depot medroxyprogesterone acetate (DMPA) IM injections, etonogestrel implant, and levonorgestrel IUDs. Progestin-only oral therapy includes medroxyprogesterone acetate (10–20 mg/day), norethindrone acetate (5–10 mg/day), norethindrone (0.35 mg/day), or drospirenone (4 mg/day). Medroxyprogesterone acetate is recommended for chronic and acute bleeding control, while norethindrone acetate is effective for acute bleeding control.98,99 Norethindrone and drospirenone do not produce the same control as other oral progestins but have been shown to have contraceptive action and have fewer side effects.100,101 Depot medroxyprogesterone acetate, etonogestral implant, and levonorgestrel IUDs all provide adequate contraception but do not deliver the same degree of amenorrhea when compared to GnRH agonists.102,103,104
When used consistently, combined hormonal contraceptives can effectively achieve amenorrhea.105,106 However, combined hormonal contraceptives are contraindicated in patients with active malignancy (metastatic, receiving therapy, or within 6 months after clinical remission) due to increased risk of VTE with estrogen use.107 Copper IUDs are known to cause abnormal uterine bleeding and are not considered for menstrual suppression, thus are not recommended.
Conditioning regimens and HSCT can also cause thrombocytopenia. In this special patient population, the use of intrauterine foreign bodies during neutropenia or active immunosuppression may be associated with increased likelihood of infections. However, no studies have provided data regarding the safety of IUDs.108 If a patient is already amenorrheic with a progestin-containing IUD prior to the conditioning regimen, health care providers must counsel the patient on the risks associated with continued IUD use while undergoing HSCT and decide together whether to keep it in place or remove it prior to transplant. Due to unpredictable bleeding post-insertion of IUDs and implants, these methods are not recommended for initiation as part of the transplant care.109
Preventative Reproductive Health
Many patients are unaware of their fertility status while on oncologic therapies. This may lead to patients underestimating their risk of pregnancy and engaging in riskier behaviors.110 Cancer treatments are also known to pose teratogenic risks to a fetus.111 Therefore, health care providers should assess the patient’s risk of pregnancy and discuss contraceptives needs, expectations, and concerns. Selection of a contraception method should consider the contraindications associated with cancer, including the innate hypercoagulability of cancer, in avoidance of estrogen or combined oral contraceptives.112 Two absolute contraindications are the use of hormonal contraceptive methods in patients with hormonally active breast cancers and IUD insertion in patients with cervical or endometrial cancer with concerns of seeding.113 Considerations should also be made about oral contraceptives and the associated nausea and vomiting from cancer or treatments as well as poor absorption or tolerance from mucositis or diarrhea.114,115 Due to the variety of available contraceptive methods, reproductive goals, and personal preferences of patients, discussions must be flexible to address individual needs and preferences of each patient. Patients need counseling not only on the proper and consistent use of contraceptives but also on protection from sexually transmitted infections by using barrier contraceptives.116
Conversations about fertility preservation may lead patients to develop incorrect beliefs about their fertility after treatment is completed.117 Additionally, patients experiencing amenorrhea or POI may continue to ovulate and with a 5–10% chance of conceiving spontaneously, even if the diagnosis has been established.118,119 Therefore, contraception discussions should continue to occur post-treatment into the patient’s cancer continuum. A patient’s reproductive goals during survivorship may differ from their initial goals.
REPRODUCTIVE OUTCOMES IN FEMALE SURVIVORS OF ADOLESCENT CANCER
Premature Ovarian Insufficiency
Oncological therapies may cause an increase of risk of either temporary or permanent ovarian dysfunction leading to loss of hormonal endocrine function. Premature ovarian insufficiency (POI) can occur through ovarian follicle dysfunction or depletion, in which chemotherapy and radiation affect both.120,121 Therefore, POI in cancer patients and survivors may manifest in multiple ways including transient “acute ovarian failure” during or after the completion of cancer treatment or experience menopause before the age of 40 years.122 Survivors who menstruate may continue to have an increased lifetime risk of POI, and those who experience POI after treatment may have the possibility of intermittent resumption of ovarian function.123 Both subsets may lead to early and often unexpected infertility.119 Estrogen deprivation may also result in menopausal symptoms, such as vaginal dryness and vasomotor symptoms, as well as lead to adverse health outcomes including osteoporosis, increased risk of cardiovascular diseases, and psychosexual dysfunction.124,125,126,127,128
Survivors who are at risk for ovarian dysfunction will benefit from long-term surveillance and screening for POI.129 Early detection allows for timely management that may preserve health and quality of life.130 There are no clear guidelines on diagnosing POI. However, commonly used parameters include amenorrhea for greater than 4 months, loss of ovarian function at ages less than 40 years, and follicle stimulating hormone greater than 25 IU/I measured twice at least one month apart.131,132 For adolescents, POI symptoms can be particularly distressing during a time of significant developmental changes. Special considerations are required when developing an optimal treatment plan for these patients that factor in both the physical and emotional needs during their development.
Treatment for adolescents with POI involve medications to replace hormone levels. Beyond the benefits of supporting bone and cardiovascular health, hormone replacement can alleviate distressing symptoms, such as vaginal dryness and vasomotor symptoms.133,134 To mimic normal ovarian functioning levels of estradiol, treatment can include transdermal estradiol patch 0.1 mg/day, oral 17 beta estradiol 1–2 mg/day, or oral conjugated equine estrogen 0.625–1.25 mg/day. Transvaginal estradiol can also be used for local symptoms. In adolescents who are prepubertal or peripubertal, estrogen levels can be titrated up to this adult dose to parallel gradual pubertal changes.14 There is an increased risk of VTE when using oral estrogens in comparison to transdermal estrogen due to the first pass effect and transdermal is the recommended route of administration.135,136 To prevent endometrial hyperplasia and abnormal bleeding, progesterone can be administered continuously or cyclically for 10–12 days a month.137,138 It is important to note that for systemic benefits, patients with POI should be given an estrogen continuously even during progestin administration. Progesterone is available as combined oral contraceptive pills, medroxyprogesterone tablets, injectables, implants, and intrauterine devices. Some combined oral contraceptives (COC) may contain higher doses of estrogen than what is preferred for hormone replacement therapy and are not recommended as first-line treatment. However, oral contraceptives are the most commonly used method of contraception in the adolescent population.139 Therefore, COCs should be used on a continuous regimen to ensure adequate hormone replacement may be preferred by the adolescent.140 Ultimately, the best hormone replacement therapy is the one that the patient is compliant with and thus as with contraception counseling careful examination of the patient’s preferences is important. In addition to estrogen therapy patients will benefit from adequate intake of calcium and vitamin D to preserve bone density.141
Pregnancy Outcomes
Oncologic therapies are associated with adverse reproductive health, including pregnancy rates and pregnancy outcomes. When compared to siblings, cancer survivors took significantly longer to achieve pregnancy than their siblings.142 Of the female cancer survivors, those with leukemia, breast, or cervical cancer have the lowest success rate in achieving post cancer pregnancy.143 Oncologic therapies have also been shown to increase the risk of developing adverse pregnancy outcomes, including increased prevalence of preterm birth, low birth weight, and cesarean delivery.144 Radiation may induce damage to the elastic properties of the uterus resulting in increased fibrosis that can negatively affect the uterine vasculature and volume. This results in restricted growth and blood flow to a fetus leading to preterm birth, low birth weight, and cesarean delivery.145,146 Chemotherapy has also been associated with increased risk of preterm birth and low birth weight with unknown underlying pathophysiology.147 An increase in fetal loss has been reported among Wilms tumor survivors that underwent high-dose abdominal irradiation.148,149 However, there is no evidence of increased rates of fetal loss in patients with other types of cancers.150 There is also no evidence of increase in frequency of congenital anomalies in the offspring of cancer survivors.151 Other less commonly reported adverse pregnancy outcomes include fetal malposition, abnormal placentation, postpartum hemorrhage, and uterine rupture.152,153,154,155
Genital Graft Versus Host Disease
GVHD is an adverse outcome of HSCT mediated by donor T-cells reacting against proteins found in the recipient. GVHD can manifest as a syndrome involving clinical features of the skin, eyes, mouth, lungs, liver, or gastrointestinal tract that typically arise after immunosuppression is withdrawn.156 Genital GVHD is a common but underreported complication that typically manifests 7–10 months after HSCT but can also develop late, over a year after transplant.157,158 Prevalence is higher in recipients of mobilized peripheral blood transplants compared to bone marrow transplants.159 Most patients with genital GVHD have concurrent involvement in other organs, but the severity does not necessarily correlate. In females, genital GVHD typically manifests as vulvar erythema, tender vestibular gland openings, or mucosal erosions and fissures. However, diagnostic signs include lichen planus-like features, vaginal scarring or stenosis, and vaginal adhesions. The most severe complication includes complete vaginal stenosis resulting in blood accumulating after the obstruction.
Management of genital GVHD includes routine questions about sexual health and urinary symptoms to identify early signs. The genital area should be cleaned with warm water while avoiding mechanical and chemical irritants, such as soap or feminine hygiene products. Emollients may be used on the external genitalia to provide relief from itching or irritation. Vaginal lubricants or cream may be used in the vagina for comfort. Signs and symptoms of genital GVHD are primarily treated with topical immunosuppressives. High potency corticosteroids such as clobetasol or triamcinolone ointments may be applied on the vulva once daily for 4–6 weeks. Hydrocortisone acetate foam can be applied to the vaginal daily for 4–6 weeks, followed by tapering doses. To prevent further atrophy or superinfection with Candida, the steroids can be reduced to a maintenance dose of 2–3 times a week. Mechanical interventions with vaginal self-examinations, regular vaginal intercourse, or vaginal dilators can be used to prevent or treat scarring and stenosis. The addition or standalone treatment of topical calcineurin ointments has also been used with success.160,161 In those with low estrogen states, the physical exam findings may present similar to hypoestrogenic vaginal atrophy and can be treated with topical estrogen therapy with or without the use of a vaginal dilator.162 Severe vaginal stenosis or scarring, dense vaginal adhesions, or labial fusion can be treated by surgical efforts.163
UNIQUE CONSIDERATIONS FOR ADOLESCENT SURVIVORS
With advancements in cancer treatment, more survivors are facing the consequence of exposure to intensive multimodal therapies. Adolescence is a period of rapid physical and developmental change. It is also a challenging and dynamic phase of life marked by career plans, romantic relationships, and gaining independence.164 For many adolescent patients, the cancer diagnosis, treatment, and long-term effects of therapy can significantly affect the patient’s physical, psychological, and social well-being.165,166 Being diagnosed with cancer and undergoing treatment regimens may lead to delays in achieving important milestones, including interacting with potential romantic partners, and forming intimate and sexual relationships.167,168 These patients have an elevated risk of depression and anxiety when compared to adult cancer patients.169,170 After intensive treatment, female adolescent survivors are more likely to face low self-esteem, body image issues, and anxiety regarding sexual activity.150 Sexual desire and satisfaction have also been shown to be compromised in cancer survivors.171 For them, cancer extends into and compromises their adult life.
The long-term impact of gonadotoxic treatments may also impact sexuality. Some patients are at an increased risk of experiencing early and unexpected infertility, which has notable negative effects on marriages, sexual satisfaction, and overall psychosocial well-being.172,173,174 The physiological effects of chemotherapy may disrupt sexual function by affecting ovarian hormones, including estrogen.175 This may lead to early menopausal symptoms, including vaginal dryness, loss of elasticity, and decreasing sexual interest.176 Treatment with estrogen can be used to mitigate some symptoms. Radiation has also been associated with changes in sexual sensitivity in females.177
It is important for health care providers to initiate the discussion about sexual health problems during and after treatment. Providers should be comfortable and knowledgeable enough to address all sexual health concerns and confer expectations. This may include a multidisciplinary team of the oncologist and the survivorship providers, including adolescent medicine, pediatric and adolescent gynecology, reproductive endocrinology infertility, and urology.178 There is also a strong need to provide resources and guidance that caters specifically to this patient population in addition to interventions to support sexual health and psychosexual adjustment.179 Improved symptom control will also improve the quality of life for young cancer survivors.
Shared-Decision Making
Young females generally desire to participate in health care decisions and wish to be well-informed regardless of whether they are the primary decision-maker. These patients also tend to rely on their parents to interpret and process information regarding serious health issues.180 Accordingly, it is appropriate for providers to have discussions using developmentally appropriate language, address both the patient’s and parents’ concerns and expectations, and include the patient in the decision-making process. In some nations, there are protective laws guaranteeing confidentiality for adolescents seeking and receiving health care. This allows increased access to health care services without requiring parental consent for certain medical care. In the United States, adolescent confidentiality is supported by case law and federal statutes. However, confidentiality laws vary from nation to nation, and health care providers should be familiar with their laws regarding adolescent confidentiality. Ethics may be a concern in the event of a parent’s right for decision making, a child’s decisional capacity and right to assent are at odds.181 It is then important to understand the factors that may contribute to the patient's and parents' health care decisions, including morals, values, and overall understanding.
1
Risk stratification for gonadotoxicity in female patients (Table modified from Meacham et al.30 and Angarita et al.182).
Minimally Increased Risk | Significantly Increased Risk | Greatest Increased Risk | Unknown Risk | |||
Alkylating Agents CED* gm/m2 | Cyclophosphamide Ifosfamide Procarbazine Melphalan Bulsulfan Chlorambucil | Prepubertal | CED <8 | CED 8–12 | CED >12 | |
Postpubertal | CED <4 | CED 4–8 | CED >8 | |||
Heavy Metals | Cisplatin Carboplatin | |||||
Anthracycline Antibiotics | Doxorubicin | |||||
Alkylating Antibiotics | Bleomycin Dactinomycin | |||||
Antimetabolic Agents | Methotrexate Mercaptopurine 5-Fluorouracil | |||||
Antimicrotubule Agents | Vincristine Vinblastine | |||||
Taxanes | Paclitaxel Docetaxel | |||||
Monoclonal Antibodies | Imatinib Rituximab Bevacizumab Trastuzumab | |||||
Chimeric Antigen Receptor (CAR) T-cell Therapy | Abecma Breyanzi Kymriah Tacartus Yescarta | |||||
Radiation** Exposure to Ovary | Prepubertal | <15 Gy | >15 Gy | |||
Postpubertal | <10 Gy | >10 Gy | ||||
Hematopoietic Stem Cell Transplant | Myeloablative Conditioning Regimens with high-dose Alkylating Agents and/or Total Body Irradiation | |||||
*CED,183 cyclophosphamide equivalent dose
**Craniospinal radiation is not included because though it causes infertility by disrupting the hypothalamic-pituitary-ovary axis, it does not damage or deplete the oocytes. Uterine radiation also may cause infertility by causing impaired growth or endometrial function but is separate from gonadotoxicity to the oocytes.
2
Brief description of fertility preservation methods for girls and adolescents with cancer.
Preservation Methods | Strategy | Timing | Logistics | Specifics |
Embryo Cryopreservation |
| Before or after cancer treatment (preferably before) |
|
|
Oocyte Cryopreservation |
| Before or after cancer treatment (preferably before) |
|
|
Ovarian Tissue Cryopreservation |
| Before or after treatment (preferably before) |
|
|
Ovarian Transposition |
| Before treatment |
|
|
3
Options for inducing menstrual suppression.
Medication | Formulation | Dosing | Amenorrhea | Limitations | Notes |
GnRH agonists | Leuprolide acetate (intramuscular, subcutaneous) | Intramuscular
| High rates (up to 96%) |
|
|
Progestin-only pills | Medroxy- | 10–20 mg/day | Unknown efficacy |
|
|
Norethindrone acetate | 5–10 mg/day (87%) | ||||
Norethindrone | 0.35 mg/day | ||||
Drospirenone | 4 mg/day | ||||
Progesterone-containing system | Depot medroxyprogesterone acetate (DMPA) – (intramuscular or subcutaneous) | 150 mg every 3 months | 50–75% after 1 year Rates increase over time |
|
|
Levonorgestrel intrauterine device (Mirena, Liletta, Skyla, Kyleena) | Every 3–7 years | 20% after 1 year 30–50% after 2 years Rates increase over time | |||
Etonogestrel implant (arm) | Every 3 years | Rates as low as 20% | |||
Combined hormonal contraceptive | Multiple formulations (pills, patch, ring) | Formulation-specific dosages | Rates may increase up to 50% |
|
|
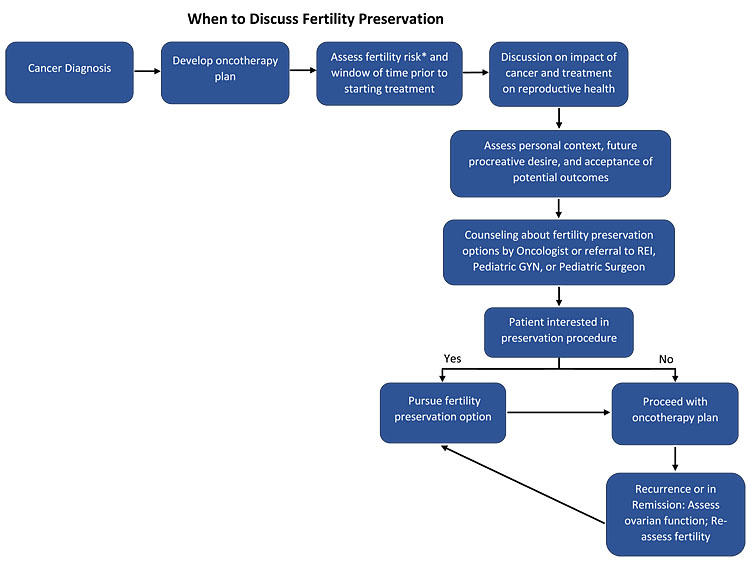
1
Illustrated representation of the process for discussing and assessing fertility preservation needs (*Refer to risk stratification in Table 1).
PRACTICE RECOMMENDATIONS
- Chemotherapy and radiation can be gonadotoxic, and these treatments may have immediate or delayed adverse effects. Risk for premature ovarian insufficiency can be calculated and fertility preservation options should be discussed.
- Ovarian tissue cryopreservation and ovarian transposition are the only methods of fertility preservation available for premenarchael patients.
- Fertility preservation methods for postmenarcheal patients include embryo cryopreservation, oocyte cryopreservation, ovarian tissue cryopreservation, and ovarian transposition.
- Cancer treatment is teratogenic, and contraception should be considered for adolescent patients at risk of pregnancy during oncologic therapies.
- Patients experiencing amenorrhea or diagnosed with premature ovarian insufficiency can spontaneously resume menstruation. Contraceptives should be discussed with patients who do not desire pregnancy.
- For patients at high risk of heavy menstrual bleeding as an effect of cancer treatment, reproductive health counseling should include the discussion of suppressing the menstrual cycle with medication.
- Hormone replacement therapy can alleviate menstrual symptoms in patients with premature ovarian insufficiency as well as preserve bone mineral health.
- Genital graft-versus-host-disease is a common adverse effect of hematopoietic stem-cell transplant with symptoms such as vulvar erythema, tender vestibular glands, or mucosal erosions and fissures. Health care providers should monitor female patients for signs and symptoms, especially if GVHD was diagnosed in other organ systems.
CONFLICTS OF INTEREST
Author(s) statement awaited.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Steliarova-Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001–10: a population-based registry study [published correction appears in Lancet Oncol 2017;18(6):e301]. Lancet Oncol 2017;18(6):719–31. doi:10.1016/S1470-2045(17)30186-9. | |
World Health Organization. CureAll framework: WHO global initiative for childhood cancer: increasing access, advancing quality, saving lives. World Health Organization 2021. | |
Ward ZJ, Yeh JM, Bhakta N, et al. Global childhood cancer survival estimates and priority-setting: a simulation-based analysis. Lancet Oncol 2019;20(7):972–83. doi:10.1016/S1470-2045(19)30273-6. | |
Gebauer J, Higham C, Langer T, et al. Long-Term Endocrine and Metabolic Consequences of Cancer Treatment: A Systematic Review. Endocr Rev 2019;40(3):711–67. doi:10.1210/er.2018-00092. | |
Meirow D, Dor J, Kaufman B, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod 2007;22(6):1626–33. doi:10.1093/humrep/dem027. | |
Luan Y, Edmonds ME, Woodruff TK, et al. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J Endocrinol 2019;240(2):243–56. doi:10.1530/JOE-18-0370. | |
Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol 2016;12(20):2333–44. doi:10.2217/fon-2016-0176. | |
Sonigo C, Beau I, Binart N, et al. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int J Mol Sci 2019;20(21):5342. Published 2019 Oct 27. doi:10.3390/ijms20215342. | |
Muñoz M, Santaballa A, Seguí MA, et al. SEOM Clinical Guideline of fertility preservation and reproduction in cancer patients (2016). Clin Transl Oncol 2016;18(12):1229–36. doi:10.1007/s12094-016-1587-9. | |
Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys 2009;73(5):1304–12. doi:10.1016/j.ijrobp.2008.12.016. | |
Weber GF. DNA Damaging Drugs. Molecular Therapies of Cancer 2014;9–112. Published 2014 Dec 8. doi:10.1007/978-3-319-13278-5_2. | |
Grigsby PW, Russell A, Bruner D, et al. Late injury of cancer therapy on the female reproductive tract. Int J Radiat Oncol Biol Phys 1995;31(5):1281–99. doi:10.1016/0360-3016(94)00426-L. | |
Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod 2003;18(1):117–21. doi:10.1093/humrep/deg016. | |
Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol 2014;124(1):193–7. doi:10.1097/01.AOG.0000451757.51964.98. | |
Waimey KE, Smith BM, Confino R, et al. Understanding Fertility in Young Female Cancer Patients. J Womens Health (Larchmt) 2015;24(10):812–8. doi:10.1089/jwh.2015.5194. | |
Bath LE, Anderson RA, Critchley HO, et al. Hypothalamic-pituitary-ovarian dysfunction after prepubertal chemotherapy and cranial irradiation for acute leukaemia. Hum Reprod 2001;16(9):1838–44. doi:10.1093/humrep/16.9.1838. | |
Marci R, Mallozzi M, Di Benedetto L, et al. Radiations and female fertility. Reprod Biol Endocrinol 2018;16(1):112. Published 2018 Dec 16. doi:10.1186/s12958-018-0432-0. | |
Crowne E, Gleeson H, Benghiat H, et al. Effect of cancer treatment on hypothalamic-pituitary function. Lancet Diabetes Endocrinol 2015;3(7):568–76. doi:10.1016/S2213-8587(15)00008-X. | |
Mikhael S, Punjala-Patel A, Gavrilova-Jordan L. Hypothalamic-Pituitary-Ovarian Axis Disorders Impacting Female Fertility. Biomedicines 2019;7(1):5. Published 2019 Jan 4. doi:10.3390/biomedicines7010005. | |
Fraietta R, Zylberstejn DS, Esteves SC. Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo) 2013;68 Suppl 1(Suppl 1):81–8. doi:10.6061/clinics/2013(sup01)09. | |
Zhu J, Chan YM. Fertility Issues for Patients with Hypogonadotropic Causes of Delayed Puberty. Endocrinol Metab Clin North Am 2015;44(4):821–34. doi:10.1016/j.ecl.2015.07.011. | |
Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009;15(12):1628–33. doi:10.1016/j.bbmt.2009.07.004. | |
Atilla E, Ataca Atilla P, Demirer T. A Review of Myeloablative vs. Reduced Intensity/Non-Myeloablative Regimens in Allogeneic Hematopoietic Stem Cell Transplantations. Balkan Med J 2017;34(1):1–9. doi:10.4274/balkanmedj.2017.0055. | |
Jadoul P, Anckaert E, Dewandeleer A, et al. Clinical and biologic evaluation of ovarian function in women treated by bone marrow transplantation for various indications during childhood or adolescence. Fertil Steril 2011;96(1):126–33.e3. doi:10.1016/j.fertnstert.2011.03.108. | |
Bresters D, Emons JA, Nuri N, et al. Ovarian insufficiency and pubertal development after hematopoietic stem cell transplantation in childhood. Pediatr Blood Cancer 2014;61(11):2048–53. doi:10.1002/pbc.25162. | |
Lehmann V, Chemaitilly W, Lu L, et al. Gonadal Functioning and Perceptions of Infertility Risk Among Adult Survivors of Childhood Cancer: A Report From the St Jude Lifetime Cohort Study. J Clin Oncol 2019;37(11):893–902. doi:10.1200/JCO.18.00965A. | |
Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab 2006;91(5):1723–8. doi:10.1210/jc.2006-0020. | |
Chemaitilly W, Li Z, Huang S, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the St Jude Lifetime Cohort study. J Clin Oncol 2015;33(5):492–500. doi:10.1200/JCO.2014.56.7933. | |
Green DM, Zhu L, Wang M, et al. Effect of cranial irradiation on sperm concentration of adult survivors of childhood acute lymphoblastic leukemia: a report from the St. Jude Lifetime Cohort Study†. Hum Reprod 2017;32(6):1192–201. doi:10.1093/humrep/dex082. | |
Meacham LR, Burns K, Orwig KE, et al. Standardizing Risk Assessment for Treatment-Related Gonadal Insufficiency and Infertility in Childhood Adolescent and Young Adult Cancer: The Pediatric Initiative Network Risk Stratification System. J Adolesc Young Adult Oncol 2020;9(6):662–6. doi:10.1089/jayao.2020.0012. | |
Lautz TB, Burns K, Rowell EE. Fertility Considerations in Pediatric and Adolescent Patients Undergoing Cancer Therapy. Surg Oncol Clin N Am 2021;30(2):401–15. doi:10.1016/j.soc.2020.11.009. | |
Demeestere I, Simon P, Dedeken L, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod 2015;30(9):2107–9. doi:10.1093/humrep/dev128. | |
Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 2019;112(6):1022–33. doi:10.1016/j.fertnstert.2019.09.013. | |
Matthews SJ, Picton H, Ernst E, et al. Successful pregnancy in a woman previously suffering from β-thalassemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecol 2018;70(4):432–5. doi:10.23736/S0026-4784.18.04240-5. | |
González C, Boada M, Devesa M, et al. Concise review: fertility preservation: an update. Stem Cells Transl Med 2012;1(9):668–72. doi:10.5966/sctm.2012-0076. | |
Donnez J, Dolmans MM. Fertility Preservation in Women. N Engl J Med 2017;377(17):1657–65. doi:10.1056/NEJMra1614676. | |
Noyes N, Knopman JM, Melzer K, et al. Oocyte cryopreservation as a fertility preservation measure for cancer patients. Reprod Biomed Online 2011;23(3):323–33. doi:10.1016/j.rbmo.2010.11.011. | |
Corkum KS, Rhee DS, Wafford QE, et al. Fertility and hormone preservation and restoration for female children and adolescents receiving gonadotoxic cancer treatments: A systematic review. J Pediatr Surg 2019;54(11):2200–9. doi:10.1016/j.jpedsurg.2018.12.021. | |
Ginsburg ES, Yanushpolsky EH, Jackson KV. In vitro fertilization for cancer patients and survivors. Fertil Steril 2001;75(4):705–10. doi:10.1016/s0015-0282(00)01802-1. | |
Dolmans MM, Demylle D, Martinez-Madrid B, et al. Efficacy of in vitro fertilization after chemotherapy. Fertil Steril 2005;83(4):897–901. doi:10.1016/j.fertnstert.2004.08.035. | |
Ajala T, Rafi J, Larsen-Disney P, et al. Fertility preservation for cancer patients: a review. Obstet Gynecol Int 2010;2010:160386. doi:10.1155/2010/160386. | |
Humaidan P, Quartarolo J, Papanikolaou EG. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril 2010;94(2):389–400. doi:10.1016/j.fertnstert.2010.03.028. | |
Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Hum Reprod Update 2003;9(3):275–89. doi:10.1093/humupd/dmg018. | |
Youssef MA, Van der Veen F, Al-Inany HG, et al. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst Rev 2014;(10):CD008046. Published 2014 Oct 31. doi:10.1002/14651858.CD008046.pub4. | |
Hu Y, Maxson WS, Hoffman DI, et al. A comparison of post-thaw results between cryopreserved embryos derived from intracytoplasmic sperm injection and those from conventional IVF. Fertil Steril 1999;72(6):1045–8. doi:10.1016/s0015-0282(99)00441-0. | |
Isikoglu M, Avci A, Kendirci Ceviren A, et al. Conventional IVF revisited: Is ICSI better for non-male factor infertility? Randomized controlled double blind study. J Gynecol Obstet Hum Reprod 2021;50(7):101990. doi:10.1016/j.jogoh.2020.101990. | |
Rienzi L, Gracia C, Maggiulli R, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017;23(2):139–55. doi:10.1093/humupd/dmw038. | |
Del-Pozo-Lérida S, Salvador C, Martínez-Soler F, et al. Preservation of fertility in patients with cancer (Review). Oncol Rep 2019;41(5):2607–14. doi:10.3892/or.2019.7063. | |
Practice Committees of the American Society for Reproductive Medicine and Society of Reproductive Biologists and Technologists. Electronic address: jgoldstein@asrm.org. A review of best practices of rapid-cooling vitrification for oocytes and embryos: a committee opinion. Fertil Steril 2021;115(2):305–10. doi:10.1016/j.fertnstert.2020.11.017. | |
Rivas Leonel EC, Lucci CM, Amorim CA. Cryopreservation of Human Ovarian Tissue: A Review. Transfus Med Hemother 2019;46(3):173–81. doi:10.1159/000499054. | |
Iussig B, Maggiulli R, Fabozzi G, et al. A brief history of oocyte cryopreservation: Arguments and facts. Acta Obstet Gynecol Scand 2019;98(5):550–8. doi:10.1111/aogs.13569. | |
Konc J, Kanyó K, Kriston R, et al. Cryopreservation of embryos and oocytes in human assisted reproduction. Biomed Res Int 2014;2014:307268. doi:10.1155/2014/307268. | |
Pickering SJ, Braude PR, Johnson MH, et al. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil Steril 1990;54(1):102–8. doi:10.1016/s0015-0282(16)53644-9. | |
Zenzes MT, Bielecki R, Casper RF, et al. Effects of chilling to 0 degrees C on the morphology of meiotic spindles in human metaphase II oocytes. Fertil Steril 2001;75(4):769–77. doi:10.1016/s0015-0282(00)01800-8. | |
Barritt J, Luna M, Duke M, et al. Report of four donor-recipient oocyte cryopreservation cycles resulting in high pregnancy and implantation rates. Fertil Steril 2007;87:e13–7. | |
Salazar FH, Loza EO, Lucas MT, et al. Successful pregnancies after oocyte and embryo vitrification. Ginecol Obstet Mex 2008;76:113–7. | |
Shi Y, Sun Y, Hao C, et al. Transfer of Fresh versus Frozen Embryos in Ovulatory Women [published correction appears in N Engl J Med. 2021;385(19):1824]. N Engl J Med 2018;378(2):126–36. doi:10.1056/NEJMoa1705334. | |
Mattawanon N, Spencer JB, Schirmer DA 3rd, et al. Fertility preservation options in transgender people: A review. Rev Endocr Metab Disord 2018;19(3):231–42. doi:10.1007/s11154-018-9462-3. | |
Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril 2011;96(2):277–85. doi:10.1016/j.fertnstert.2011.06.030. | |
Cobo A, García-Velasco JA, Coello A, et al. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril 2016;105(3):755–64.e8. doi:10.1016/j.fertnstert.2015.11.027. | |
Klipstein S, Fallat ME, Savelli S; COMMITTEE ON BIOETHICS; SECTION ON HEMATOLOGY/ONCOLOGY; SECTION ON SURGERY. Fertility Preservation for Pediatric and Adolescent Patients With Cancer: Medical and Ethical Considerations. Pediatrics 2020;145(3):e20193994. doi:10.1542/peds.2019-3994. | |
Hallamaa M, Seikkula J, Willman S, et al. Pregnancy potential and perinatal outcomes of embryos cryopreserved twice: a case-control study. Reprod Biomed Online 2021;43(4):607–13. doi:10.1016/j.rbmo.2021.06.028. | |
Dolmans MM, Marinescu C, Saussoy P, et al. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 2010;116(16):2908–14. doi:10.1182/blood-2010-01-265751. | |
Dolmans MM, Luyckx V, Donnez J, et al. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril 2013;99(6):1514–22. doi:10.1016/j.fertnstert.2013.03.027. | |
González C, Boada M, Devesa M, et al. Concise review: fertility preservation: an update. Stem Cells Transl Med 2012;1(9):668–72. doi:10.5966/sctm.2012-0076. | |
Practice Committees of the American Society for Reproductive Medicine, the Society of Reproductive Biologists and Technologists, and the Society for Assisted Reproductive Technology. Electronic address: jgoldstein@asrm.org. In vitro maturation: a committee opinion. Fertil Steril 2021;115(2):298–304. doi:10.1016/j.fertnstert.2020.11.018. | |
Son WY, Henderson S, Cohen Y, et al. Immature Oocyte for Fertility Preservation. Front Endocrinol (Lausanne) 2019;10:464. Published 2019 Jul 17. doi:10.3389/fendo.2019.00464. | |
Brouillet S, Ferrieres-Hoa A, Fournier A, et al. Cryopreservation of Oocytes Retrieved from Ovarian Tissue to Optimize Fertility Preservation in Prepubertal Girls and Women. J Vis Exp 2020;(164):10.3791/61777. Published 2020 Oct 23. doi:10.3791/61777. | |
Yang ZY, Chian RC. Development of in vitro maturation techniques for clinical applications. Fertil Steril 2017;108(4):577–84. doi:10.1016/j.fertnstert.2017.08.020. | |
Abir R, Ben-Aharon I, Garor R, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod 2016;31(4):750–62. doi:10.1093/humrep/dew007. | |
Shapira M, Raanani H, Barshack I, et al. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil Steril 2018;109(1):48–53. doi:10.1016/j.fertnstert.2017.09.001. | |
Karavani G, Schachter-Safrai N, Revel A, et al. In vitro maturation rates in young premenarche patients. Fertil Steril 2019;112(2):315–22. doi:10.1016/j.fertnstert.2019.03.026. | |
Karavani G, Wasserzug-Pash P, Mordechai-Daniel T, et al. Age-Dependent in vitro Maturation Efficacy of Human Oocytes – Is There an Optimal Age?. Front Cell Dev Biol 2021;9:667682. Published 2021 Jun 18. doi:10.3389/fcell.2021.667682. | |
Segers I, Bardhi E, Mateizel I, et al. Live births following fertility preservation using in-vitro maturation of ovarian tissue oocytes. Hum Reprod 2020;35(9):2026–36. doi:10.1093/humrep/deaa175. | |
Prasath EB, Chan ML, Wong WH, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod 2014;29(2):276–8. doi:10.1093/humrep/det420. | |
Uzelac PS, Delaney AA, Christensen GL, et al. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril 2015;104(5):1258–60. doi:10.1016/j.fertnstert.2015.07.1148. | |
Kedem A, Yerushalmi GM, Brengauz M, et al. Outcome of immature oocytes collection of 119 cancer patients during ovarian tissue harvesting for fertility preservation. J Assist Reprod Genet 2018;35(5):851–6. doi:10.1007/s10815-018-1153-1. | |
Dolmans MM, Hossay C, Nguyen TYT, et al. Fertility Preservation: How to Preserve Ovarian Function in Children, Adolescents and Adults. J Clin Med 2021;10(22):5247. Published 2021 Nov 11. doi:10.3390/jcm10225247. | |
Mahajan N. Fertility preservation in female cancer patients: An overview. J Hum Reprod Sci 2015;8(1):3–13. doi:10.4103/0974-1208.153119. | |
Dolmans MM, Marotta ML, Pirard C, et al. Ovarian tissue cryopreservation followed by controlled ovarian stimulation and pick-up of mature oocytes does not impair the number or quality of retrieved oocytes. J Ovarian Res 2014;7:80. Published 2014 Aug 26. doi:10.1186/s13048-014-0080-8. | |
Bieler EU, Schnabel T, Knobel J. Persisting cyclic ovarian activity in cervical cancer after surgical transposition of the ovaries and pelvic irradiation. Br J Radiol 1976;49(586):875–9. doi:10.1259/0007-1285-49-586-875. | |
Irtan S, Orbach D, Helfre S, et al. Ovarian transposition in prepubescent and adolescent girls with cancer. Lancet Oncol 2013;14(13):e601–8. doi:10.1016/S1470-2045(13)70288-2. | |
Barahmeh S, Al Masri M, Badran O, et al. Ovarian transposition before pelvic irradiation: indications and functional outcome. J Obstet Gynaecol Res 2013;39(11):1533–7. doi:10.1111/jog.12096. | |
Morice P, Thiam-Ba R, Castaigne D, et al. Fertility results after ovarian transposition for pelvic malignancies treated by external irradiation or brachytherapy. Hum Reprod 1998;13(3):660–3. doi:10.1093/humrep/13.3.660. | |
Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist 2007;12(9):1044–54. doi:10.1634/theoncologist.12-9-1044. | |
Falcone T, Moore H. GnRH agonist for gonadal protection during chemotherapy, Hum Reprod, 2015;30(12):2711–2. https://doi.org/10.1093/humrep/dev258. | |
Wang Y, Probin V, Zhou D. Cancer therapy-induced residual bone marrow injury-Mechanisms of induction and implication for therapy. Curr Cancer Ther Rev 2006;2(3):271–9. doi:10.2174/157339406777934717. | |
Ortmann O, Weiss JM, Diedrich K. Gonadotrophin-releasing hormone (GnRH) and GnRH agonists: mechanisms of action. Reprod Biomed Online 2002;5(Suppl 1):1–7. doi:10.1016/s1472-6483(11)60210-1. | |
Chiusolo P, Salutari P, Sica S, et al. Luteinizing hormone-releasing hormone analogue: leuprorelin acetate for the prevention of menstrual bleeding in premenopausal women undergoing stem cell transplantation. Bone Marrow Transplant 1998;21(8):821–3. doi:10.1038/sj.bmt.1701187. | |
Close AG, Jones KA, Landowski A, et al. Current practices in menstrual management in adolescents with cancer: A national survey of pediatric oncology providers. Pediatr Blood Cancer 2019;66(12):e27961. doi:10.1002/pbc.27961. | |
Perry CM, Brogden RN. Goserelin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in benign gynaecological disorders. Drugs 1996;51(2):319–46. doi:10.2165/00003495-199651020-00009. | |
American College of Obstetricians and Gynecologists’ Committee on Adolescent Health Care. Options for Prevention and Management of Menstrual Bleeding in Adolescent Patients Undergoing Cancer Treatment: ACOG Committee Opinion, Number 817. Obstet Gynecol 2021;137(1):e7–15. doi:10.1097/AOG.0000000000004209. | |
ACOG Committee Opinion No. 747 Summary: Gynecologic Issues in Children and Adolescent Cancer Patients and Survivors. Obstet Gynecol 2018;132(2):535–6. doi:10.1097/AOG.0000000000002764. | |
DiVasta AD, Feldman HA, Sadler Gallagher J, et al. Hormonal Add-Back Therapy for Females Treated With Gonadotropin-Releasing Hormone Agonist for Endometriosis: A Randomized Controlled Trial. Obstet Gynecol 2015;126(3):617–27. doi:10.1097/AOG.0000000000000964. | |
Sadler Gallagher J, Feldman HA, Stokes NA, et al. The Effects of Gonadotropin-Releasing Hormone Agonist Combined with Add-Back Therapy on Quality of Life for Adolescents with Endometriosis: A Randomized Controlled Trial. J Pediatr Adolesc Gynecol 2017;30(2):215–22. doi:10.1016/j.jpag.2016.02.008. | |
Vasilakis C, Jick H, del Mar Melero-Montes M. Risk of idiopathic venous thromboembolism in users of progestagens alone. Lancet 1999;354(9190):1610–1. doi:10.1016/S0140-6736(99)04394-9. | |
Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci 2012;19(6):563–71. doi:10.1177/1933719112438061. | |
James AH, Kouides PA, Abdul-Kadir R, et al. Evaluation and management of acute menorrhagia in women with and without underlying bleeding disorders: consensus from an international expert panel. Eur J Obstet Gynecol Reprod Biol 2011;158(2):124–34. doi:10.1016/j.ejogrb.2011.04.025. | |
Screening and Management of Bleeding Disorders in Adolescents With Heavy Menstrual Bleeding: ACOG COMMITTEE OPINION, Number 785. Obstet Gynecol 2019;134(3):e71–83. doi:10.1097/AOG.0000000000003411. | |
Palacios S, Colli E, Regidor PA. A multicenter, double-blind, randomized trial on the bleeding profile of a drospirenone-only pill 4 mg over nine cycles in comparison with desogestrel 0.075 mg [published correction appears in Arch Gynecol Obstet. 2020;301(6):1593]. Arch Gynecol Obstet 2019;300(6):1805–12. doi:10.1007/s00404-019-05340-4. | |
Board JA. Continuous norethindrone, 0.35 mg., as an oral contraceptive agent. Am J Obstet Gynecol 1971;109(4):531–5. doi:10.1016/0002-9378(71)90624-7. | |
Kaunitz AM. Injectable contraception. New and existing options. Obstet Gynecol Clin North Am 2000;27(4):741–80. doi:10.1016/s0889-8545(05)70171-6. | |
Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-Releasing Intrauterine Device Use in Female Adolescents with Heavy Menstrual Bleeding and Bleeding Disorders: Single Institution Review. J Pediatr Adolesc Gynecol 2017;30(4):479–83. doi:10.1016/j.jpag.2016.04.001. | |
Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril 2009;91(5):1646–53. doi:10.1016/j.fertnstert.2008.02.140. | |
Coutinho EM, O'Dwyer E, Barbosa IC, et al. Comparative study on intermittent versus continuous use of a contraceptive pill administered by vaginal route. Contraception 1995;51(6):355–8. doi:10.1016/0010-7824(95)00101-f. | |
Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol 2003;101(4):653–61. doi:10.1016/s0029-7844(03)00014-0. | |
Curtis KM, Tepper NK, Jatlaoui TC, et al. S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(No. RR-3):1–104. DOI: http://dx.doi.org/10.15585/mmwr.rr6503a1. | |
Brady PC, Soiffer RJ, Ginsburg ES. Continuation of a Levonorgestrel Intrauterine Device During Hematopoietic Stem Cell Transplant: A Case Report. Anticancer Res 2017;37(4):1985–7. doi:10.21873/anticanres.11541. | |
Chang K, Merideth MA, Stratton P. Hormone Use for Therapeutic Amenorrhea and Contraception During Hematopoietic Cell Transplantation. Obstet Gynecol 2015;126(4):779–84. doi:10.1097/AOG.0000000000001031. | |
Klosky JL, Howell CR, Li Z, et al. Risky health behavior among adolescents in the childhood cancer survivor study cohort. J Pediatr Psychol 2012;37(6):634–46. doi:10.1093/jpepsy/jss046. | |
Arnon J, Meirow D, Lewis-Roness H, et al. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum Reprod Update 2001;7(4):394–403. doi:10.1093/humupd/7.4.394. | |
Quinn SM, Louis-Jacques J. Menstrual management and reproductive concerns in adolescent and young adult women with underlying hematologic or oncologic disease. Curr Opin Pediatr 2016;28(4):421–7. doi:10.1097/MOP.0000000000000359. | |
Curtis KM, Tepper NK, Jatlaoui TC, et al. S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep 2016;65(No. RR-3):1–104. DOI: http://dx.doi.org/10.15585/mmwr.rr6503a1external. | |
Naidu MU, Ramana GV, Rani PU, et al. Chemotherapy-induced and/or radiation therapy-induced oral mucositis–complicating the treatment of cancer. Neoplasia 2004;6(5):423–31. doi:10.1593/neo.04169. | |
Davis MP, Hallerberg G. Palliative Medicine Study Group of the Multinational Association of Supportive Care in Cancer. A systematic review of the treatment of nausea and/or vomiting in cancer unrelated to chemotherapy or radiation. J Pain Symptom Manage 2010;39(4):756–67. doi:10.1016/j.jpainsymman.2009.08.010. | |
Murphy D, Klosky JL, Reed DR, et al. The importance of assessing priorities of reproductive health concerns among adolescent and young adult patients with cancer. Cancer 2015;121(15):2529–36. doi:10.1002/cncr.29466. | |
Abelman SH, Cron J. Contraception Counseling and Use Among Adolescent and Young Adult Female Patients Undergoing Cancer Treatment: A Retrospective Analysis. J Pediatr Adolesc Gynecol 2020;33(6):652–7. doi:10.1016/j.jpag.2020.09.004. | |
Nelson LM, Covington SN, Rebar RW. An update: spontaneous premature ovarian failure is not an early menopause. Fertil Steril 2005;83(5):1327–32. doi:10.1016/j.fertnstert.2004.11.059. | |
van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update 1999;5(5):483–92. doi:10.1093/humupd/5.5.483. | |
Mauri D, Gazouli I, Zarkavelis G, et al. Chemotherapy Associated Ovarian Failure. Front Endocrinol (Lausanne) 2020;11:572388. Published 2020 Dec 8. doi:10.3389/fendo.2020.572388. | |
Hao X, Anastácio A, Liu K, et al. Ovarian Follicle Depletion Induced by Chemotherapy and the Investigational Stages of Potential Fertility-Protective Treatments-A Review. Int J Mol Sci 2019;20(19):4720. Published 2019 Sep 23. doi:10.3390/ijms20194720. | |
Green DM, Sklar CA, Boice JD Jr, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27(14):2374–81. doi:10.1200/JCO.2008.21.1839. | |
Duffy C, Allen S. Medical and psychosocial aspects of fertility after cancer. Cancer J 2009;15(1):27–33. doi:10.1097/PPO.0b013e3181976602. | |
Zhou ES, Falk SJ, Bober SL. Managing premature menopause and sexual dysfunction. Curr Opin Support Palliat Care 2015;9(3):294–300. doi:10.1097/SPC.0000000000000156. | |
Pouillès JM, Trémollières F, Bonneu M, et al. Influence of early age at menopause on vertebral bone mass. J Bone Miner Res 1994;9(3):311–5. https://doi.org/10.1002/jbmr.5650090304. | |
Sullivan JM, Fowlkes LP. The clinical aspects of estrogen and the cardiovascular system. Obstet Gynecol 1996;87(2 Suppl):36S–43S. doi:10.1016/0029-7844(95)00432-7. | |
Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens 2011;20(2):133–8. doi:10.1097/MNH.0b013e3283431921. | |
Nappi RE, Cucinella L, Martini E, et al. Sexuality in premature ovarian insufficiency. Climacteric 2019;22(3):289–95. doi:10.1080/13697137.2019.1575356. | |
Gargus E, Deans R, Anazodo A, et al. Management of Primary Ovarian Insufficiency Symptoms in Survivors of Childhood and Adolescent Cancer. J Natl Compr Canc Netw 2018;16(9):1137–49. doi:10.6004/jnccn.2018.7023. | |
van Dorp W, Mulder RL, Kremer LC, et al. Recommendations for Premature Ovarian Insufficiency Surveillance for Female Survivors of Childhood, Adolescent, and Young Adult Cancer: A Report From the International Late Effects of Childhood Cancer Guideline Harmonization Group in Collaboration With the PanCareSurFup Consortium. J Clin Oncol 2016;34(28):3440–50. doi:10.1200/JCO.2015.64.3288. | |
Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med 2009;360(6):606–14. doi:10.1056/NEJMcp0808697. | |
European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016;31(5):926–37. doi:10.1093/humrep/dew027. | |
Popat VB, Calis KA, Kalantaridou SN, et al. Bone mineral density in young women with primary ovarian insufficiency: results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J Clin Endocrinol Metab 2014;99(9):3418–26. doi:10.1210/jc.2013-4145. | |
Langrish JP, Mills NL, Bath LE, et al. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension 2009;53(5):805–11. doi:10.1161/HYPERTENSIONAHA.108.126516. | |
Scarabin PY, Oger E, Plu-Bureau G; EStrogen and THromboEmbolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet 2003;362(9382):428–32. doi:10.1016/S0140-6736(03)14066-4. | |
Committee Opinion No. 698: Hormone Therapy in Primary Ovarian Insufficiency. Obstet Gynecol 2017;129(5):e134–41. doi: 10.1097/AOG.0000000000002044. PMID: 28426619. | |
Stuenkel CA, Davis SR, Gompel A, et al. Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015;100(11):3975–4011. doi:10.1210/jc.2015-2236. | |
Bjarnason K, Cerin A, Lindgren R, et al. Adverse endometrial effects during long cycle hormone replacement therapy. Scandinavian Long Cycle Study Group. Maturitas 1999;32(3):161–70. doi:10.1016/s0378-5122(99)00033-x. | |
Martinez GM, Abma JC. Sexual activity and contraceptive use among teenagers aged 15–19 in the United States, 2015–2017. NCHS Data Brief, no 366. Hyattsville, MD: National Center for Health Statistics. 2020. | |
Klein KO, Phillips SA. Review of Hormone Replacement Therapy in Girls and Adolescents with Hypogonadism. J Pediatr Adolesc Gynecol 2019;32(5):460–8. doi:10.1016/j.jpag.2019.04.010. | |
Rebar RW. Premature ovarian "failure" in the adolescent. Ann N Y Acad Sci 2008;1135:138–45. doi:10.1196/annals.1429.000. | |
Barton SE, Najita JS, Ginsburg ES, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2013;14(9):873–81. doi:10.1016/S1470-2045(13)70251-1. | |
Stensheim H, Cvancarova M, Møller B, et al. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer 2011;129(5):1225–36. doi:10.1002/ijc.26045. | |
Anderson C, Engel SM, Mersereau JE, et al. Birth Outcomes Among Adolescent and Young Adult Cancer Survivors. JAMA Oncol 2017;3(8):1078–84. doi:10.1001/jamaoncol.2017.0029. | |
Haggar FA, Pereira G, Preen D, et al. Adverse obstetric and perinatal outcomes following treatment of adolescent and young adult cancer: a population-based cohort study. PLoS One 2014;9(12):e113292. Published 2014 Dec 8. doi:10.1371/journal.pone.0113292. | |
Critchley HO, Wallace WH. Impact of cancer treatment on uterine function. J Natl Cancer Inst Monogr 2005;(34):64–8. doi:10.1093/jncimonographs/lgi022. | |
Madanat-Harjuoja LM, Malila N, Lähteenmäki PM, et al. Preterm delivery among female survivors of childhood, adolescent and young adulthood cancer. Int J Cancer 2010;127(7):1669–79. doi:10.1002/ijc.25157. | |
Li FP, Gimbrere K, Gelber RD, et al. Outcome of pregnancy in survivors of Wilms' tumor. JAMA 1987;257(2):216–9. | |
Hawkins MM, Smith RA. Pregnancy outcomes in childhood cancer survivors: probable effects of abdominal irradiation. Int J Cancer 1989;43(3):399–402. doi:10.1002/ijc.2910430309. | |
Mueller BA, Chow EJ, Kamineni A, et al. Pregnancy outcomes in female childhood and adolescent cancer survivors: a linked cancer-birth registry analysis. Arch Pediatr Adolesc Med 2009;163(10):879–86. doi:10.1001/archpediatrics.2009.112. | |
Green DM, Zevon MA, Lowrie G, et al. Congenital anomalies in children of patients who received chemotherapy for cancer in childhood and adolescence. N Engl J Med 1991;325(3):141–6. doi:10.1056/NEJM199107183250301. | |
Norwitz ER, Stern HM, Grier H, et al. Placenta percreta and uterine rupture associated with prior whole body radiation therapy. Obstet Gynecol 2001;98(5 Pt 2):929–31. doi:10.1016/s0029-7844(01)01435-1. | |
Huarte Ciganda M, Estaún Echavarren C, García Jiménez A, et al. Spontaneous Uterine Rupture in the Second Trimester in a Patient With Previous Pelvic Radiotherapy in Childhood: A Case Report. J Obstet Gynaecol Can 2020;42(1):84–7. doi:10.1016/j.jogc.2019.02.152. | |
van der Kooi ALF, Kelsey TW, van den Heuvel-Eibrink MM, et al. Perinatal complications in female survivors of cancer: a systematic review and meta-analysis. Eur J Cancer 2019;111:126–37. doi:10.1016/j.ejca.2019.01.104. | |
Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol 2010;116(5):1171–83. doi:10.1097/AOG.0b013e3181f87c4b. | |
Dignan FL, Clark A, Amrolia P, et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol 2012;158(1):30–45. doi:10.1111/j.1365-2141.2012.09129.x. | |
Spinelli S, Chiodi S, Costantini S, et al. Female genital tract graft-versus-host disease following allogeneic bone marrow transplantation. Haematologica 2003;88(10):1163–8. | |
Hamilton BK, Goje O, Savani BN, et al. Clinical management of genital chronic GvHD. Bone Marrow Transplant 2017;52(6):803–10. doi:10.1038/bmt.2016.315. | |
Flowers ME, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood 2002;100(2):415–9. doi:10.1182/blood-2002-01-0011. | |
Frey Tirri B, Häusermann P, Bertz H, et al. Clinical guidelines for gynecologic care after hematopoietic SCT. Report from the international consensus project on clinical practice in chronic GVHD. Bone Marrow Transplant 2015;50(1):3–9. doi:10.1038/bmt.2014.242. | |
Zantomio D, Grigg AP, MacGregor L, et al. Female genital tract graft-versus-host disease: incidence, risk factors and recommendations for management. Bone Marrow Transplant 2006;38(8):567–72. doi:10.1038/sj.bmt.1705487. | |
Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 2006;12(4):375–96. doi:10.1016/j.bbmt.2006.02.003. | |
Norian JM, Stratton P. Labial fusion: a rare complication of chronic graft-versus-host disease. Obstet Gynecol 2008;112(2 Pt 2):437–9. doi:10.1097/01.AOG.0000299876.18200.8 d. | |
Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol 2000;55(5):469–80. | |
Warner EL, Kent EE, Trevino KM, et al. Social well-being among adolescents and young adults with cancer: A systematic review. Cancer 2016;122(7):1029–37. doi:10.1002/cncr.29866. | |
Bellizzi KM, Smith A, Schmidt S, et al. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer 2012;118(20):5155–62. doi:10.1002/cncr.27512. | |
Abrams AN, Hazen EP, Penson RT. Psychosocial issues in adolescents with cancer. Cancer Treat Rev 2007;33(7):622–30. doi:10.1016/j.ctrv.2006.12.006. | |
Eiser C, Penn A, Katz E, Barr R. Psychosocial issues and quality of life. Semin Oncol 2009;36(3):275–80. doi:10.1053/j.seminoncol.2009.03.005. | |
Geue K, Brähler E, Faller H, et al. Prevalence of mental disorders and psychosocial distress in German adolescent and young adult cancer patients (AYA). Psychooncology 2018;27(7):1802–9. doi:10.1002/pon.4730. | |
Lang MJ, David V, Giese-Davis J. The Age Conundrum: A Scoping Review of Younger Age or Adolescent and Young Adult as a Risk Factor for Clinical Distress, Depression, or Anxiety in Cancer. J Adolesc Young Adult Oncol 2015;4(4):157–73. doi:10.1089/jayao.2015.0005. | |
Sanchez Varela V, Zhou ES, Bober SL. Management of sexual problems in cancer patients and survivors. Curr Probl Cancer 2013;37(6):319–52. doi:10.1016/j.currproblcancer.2013.10.009. | |
Cousineau TM, Domar AD. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol 2007;21(2):293–308. doi:10.1016/j.bpobgyn.2006.12.003. | |
Ramezanzadeh F, Aghssa MM, Jafarabadi M, et al. Alterations of sexual desire and satisfaction in male partners of infertile couples. Fertil Steril 2006;85(1):139–43. doi:10.1016/j.fertnstert.2005.07.1285. | |
Onat G, Kizilkaya Beji N. Effects of infertility on gender differences in marital relationship and quality of life: a case-control study of Turkish couples. Eur J Obstet Gynecol Reprod Biol 2012;165(2):243–8. doi:10.1016/j.ejogrb.2012.07.033. | |
Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst 2006;98(13):890–6. doi:10.1093/jnci/djj243. | |
Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas 2010;65(2):161–6. doi:10.1016/j.maturitas.2009.08.003. | |
Wettergren L, Kent EE, Mitchell SA, et al. Cancer negatively impacts on sexual function in adolescents and young adults: The AYA HOPE study. Psychooncology 2017;26(10):1632–9. doi:10.1002/pon.4181. | |
Cherven B, Sampson A, Bober SL, et al. Sexual health among adolescent and young adult cancer survivors: A scoping review from the Children's Oncology Group Adolescent and Young Adult Oncology Discipline Committee. CA Cancer J Clin 2021;71(3):250–63. doi:10.3322/caac.21655. | |
Stanton AM, Handy AB, Meston CM. Sexual function in adolescents and young adults diagnosed with cancer: A systematic review. J Cancer Surviv 2018;12(1):47–63. doi:10.1007/s11764-017-0643-y. | |
Quinn GP, Murphy D, Knapp C, et al. Who decides? Decision making and fertility preservation in teens with cancer: a review of the literature. J Adolesc Health 2011;49(4):337–46. doi:10.1016/j.jadohealth.2011.01.005. | |
Burns KC, Hoefgen H, Strine A, et al. Fertility preservation options in pediatric and adolescent patients with cancer. Cancer 2018;124(9):1867–76. doi:10.1002/cncr.31255. | |
Angarita AM, Johnson CA, Fader ANJ, et al. Fertility Preservation: A Key Survivorship Issue for Young Women with Cancer. Front Oncol 2016;6:102. doi: 10.3389/fonc.2016.00102. PMID: 27200291; PMCID: PMC4843761. | |
Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2014;61(1):53–67. doi:10.1002/pbc.24679 | |
Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril 2015;104(5):1097–8. doi:10.1016/j.fertnstert.2015.08.005. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
