This chapter should be cited as follows:
Vash-Margita A, Rodriquez L, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.418443
The Continuous Textbook of Women’s Medicine Series – Gynecology Module
Volume 2
Adolescent gynecology
Volume Editor: Professor Judith Simms-Cendan, University of Miami, USA

Chapter
Dysmenorrhea, Pelvic Pain, Endometriosis and Functional Abdominal Pain
First published: November 2022
Updated: September 2025
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Written by a team of multidisciplinary experts, this chapter addresses differential diagnosis and management of pelvic pain in postmenarchal adolescent females with special focus on endometriosis and functional abdominal pain. Readers will enhance their ability to assess chronic pelvic pain and associated impairment including functional disability and mental health symptoms. Topics include etiology of pelvic pain and appropriate diagnostic evaluations, including imaging and mental health screening. The chapter describes medical and behavioral interventions for pain, functional disability, and mental health symptoms, as well as surgical interventions. This chapter is aimed at physicians, advanced practice providers, nurses and other providers caring for adolescent females with chronic pelvic pain.
Chronic pelvic pain is a persistent pain perceived in structures related to the pelvis and is often associated with negative cognitive, behavioral, sexual and emotional consequences and symptoms suggestive of lower urinary tract, sexual, bowel, myofascial, or gynecologic dysfunction.1 Usually, pain lasting 6 months or longer is defined as chronic unless central sensitization pain mechanisms (cognitive, behavioral, and emotional impairment) are documented.1 Dysmenorrhea is defined as cyclic pelvic or abdominal pain associated with menstruation. It is divided into primary dysmenorrhea, which refers to painful menstruation without underlying pelvic pathology, and secondary dysmenorrhea, which refers to menstrual pain caused by an identifiable condition such as endometriosis, adenomyosis, fibroids, or pelvic inflammatory disease. Chronic pelvic pain, in contrast, is non-cyclic pain lasting for at least 6 months and can be a debilitating condition, affecting up to 15% of postmenarchal females.2 The prevalence of primary dysmenorrhea is high, with one study reporting 41.7% among female university students.3 Pain usually presents after ovulatory cycles have been achieved (6–12 months after menarche).
The most common cause of secondary dysmenorrhea in adolescents is endometriosis.4 Endometriosis is defined as ectopic location of the endometrial-like tissue outside the uterus, which is often associated with pelvic pain. According to a recent systematic literature review, endometriosis has been diagnosed on average in 64% of adolescent females undergoing diagnostic laparoscopy due to pelvic pain.5 There is an indication that the previously reported high prevalence of pelvic pain may, in fact, be lower in a different population but nevertheless it remains a significant health issue.6 In spite of high prevalence of the disease in females with pelvic pain, diagnosis is often delayed by as much as 6.7 years between onset of symptoms and a surgical diagnosis of endometriosis in women aged 18–45 years..7 The notorious delay in diagnosing endometriosis in adolescents exacerbates stigma and distress.8,9 Other causes of pelvic pain include primarily genitourinary and gastrointestinal problems as well as musculoskeletal and psychological causes (see below).
ETIOLOGY AND RISK FACTORS OF ENDOMETRIOSIS
The etiology of endometriosis is unclear at this time. A number of risk factors have been identified such as early menarche, nulliparity, shorter menstrual cycle, abnormal uterine bleeding (AUB), obstructive Müllerian anomalies, being born prematurely, having a low birth weight, being formula-fed, and having been exposed to diethylstilbestrol (DES) in utero.10,11
Earlier studies indicated that sociodemographic factors such as ethnicity and race may influence prevalence of endometriosis. However, these findings have been challenged by more recent work indicating that access to healthcare and variations in sociocultural acceptance of surgical intervention may vary among women of different races/ethnicities, raising the possibility of biased conclusions.7,12,13,14,15,16,17 A history of sexual and/or physical abuse has been implicated as a risk factor for endometriosis.13,18
A genetic component of the disease has been established after observational studies confirmed a higher prevalence of endometriosis in first-degree relatives of patients with endometriosis compared to disease-free controls. An Australian study in twins demonstrated a relative risk of 2.34 for endometriosis in siblings and a relative risk of approximately 2.0 in monozygotic/dizygotic twins.19 Various genes responsible for different physiologic compartments have been implicated in the pathogenesis of endometriosis and research is ongoing (Table 1).20
1
Genes implicated in the pathogenesis of endometriosis.
Mechanism | Cell growth | Sex hormone steroidogenesis | DNA repair | Metabolism | Inflammation and immune response | Neoangiogenesis |
Genes | ARF CDKN2A/B KRAS ID4 MAP3K4 VEZT | ESR1/2 PROGINS WNT4 FN1 GREB1 | XRCC1–3 XPD XPG APE1 HOGG1 | GSTM1 GSTP1 GSTT1 CYP1A1 CYP17A1 CYP19A1 NAT1/2 | IL10 TGFβ1 TNFα HLA-A/B HLA-DRB1/DQB1 | VEGF EGFR MMP 2–3-7 TIMP3–4 |
Pathogenesis
Several theories have been suggested regarding the origin of endometriosis. One category proposes that implants originate from the uterine endometrium and are due to tissue migration. Sampson’s theory suggests that endometriosis occurs due to the retrograde menstruation via the tubal transport of endometrial cells.21,22 Metastatic spread of endometrial tissue through blood and lymphatic vasculature has also been proposed. The other broad category describes endometrial implants that arise from the tissue outside the uterus. This involves aberrant differentiation and migration of Müllerian ducts with misplaced endometrial cells during fetal organogenesis. This process is called Müllerianosis.23,24
Another pathologic process proposed as the origin of endometriosis is metaplasia, the transformation of one differentiated cell type to another differentiated cell type. In this case, metaplastic differentiation of ovarian celomic epithelium leads to its transformation into endometrial tissue. Interestingly, a history of neonatal uterine bleeding has been reported more commonly in patients with endometriosis, although the reason for the association is unclear.10,25,26,27,28,29,30
A more recent proposal suggests that extrauterine stem/progenitor cells originating from bone marrow may differentiate into endometriotic tissue.31,32 Other candidates for the origin of the endometriosis are endometrial epithelial progenitor cells and endometrial mesenchymal stromal–stem-like cells (eMSC).33 Overall, current evidence supports a multifactorial process in the pathogenesis of endometriosis involving genetic predisposition, estrogen milieu, progesterone resistance, altered immune cell population, and functionality and inflammation.34
EVALUATION OF THE PATIENT WITH PELVIC PAIN
History
The most common presenting symptom associated with endometriosis in adolescents that leads to an evaluation by a gynecologist is pelvic pain. In a study by Laufer et al., only 9.4% of adolescent subjects had the classic adult symptom of cyclic pain associated with menses and 28.1% had non-cyclic pain alone.35 Both cyclic and non-cyclic pain were documented in 62.5%, meaning that the majority (90.6%) of adolescents with endometriosis had non-cyclic pain. This is a major distinction in the clinical presentation of pelvic pain associated with endometriosis in adolescents compared to adult women, who classically have predominantly cyclic pain.
Obtaining a thorough pain history can be challenging in adolescents. The adolescent patient should be encouraged to keep a menstrual diary using a calendar or utilize a secure smartphone app to record symptoms. History should include all possible gynecologic, gastrointestinal, urologic, musculoskeletal, and psychosocial causes. Pelvic pain in adolescents has been associated with nausea, vomiting, diarrhea, headaches, tiredness, back pain, irritable mood, syncope, anorexia, dizziness, constipation, and bloating.6,36,37 It is helpful to differentiate pelvic pain from non-pelvic abdominal pain. The provider may also ask the patient to localize pain above or below the umbilicus. The differential diagnosis is broad and should exclude non-gynecologic causes of pain (Table 2). Direct questions regarding pain should include location, duration, intensity, type of pain, relieving or provoking factors, and type of medication used in the past if any.
2
Differential diagnosis of pelvic pain.
Non-gynecologic causes | Gynecologic causes | |||
Gastrointestinal | Genitourinary | Musculoskeletal | Psychological | |
Appendicitis Abdominal migraine/functional abdominal pain Cholecystitis/cholangitis Constipation Diverticular disease Gastric ulcer Gastritis Inflammatory bowel disease Meckel diverticulum Mesenteric adenitis | Calculi Cystitis Painful bladder syndrome Pyelonephritis Ureteral obstruction Ureteral diverticulum/polyp | Bone and joint inflammation Congenital anomalies Fibromyalgia Nerve entrapment Tumors Trauma | Abuse (physical, emotional, sexual) Anxiety Depression Somatization Substance abuse | Adenomyosis Adnexal torsion Bartholin cyst abscess Cysts – ovarian and paraovarian Ectopic pregnancy Endometriosis Hydrosalpinx/pyosalpinx Lichen sclerosus Leiomyoma Mittelschmerz Müllerian anomalies – obstructive and non-obstructive Pelvic inflammatory disease/tubo-ovarian abscess Vaginismus Vaginitis Vulvodynia |
A complete gynecologic history should be obtained with notation of age at menarche, cyclicity of menses, timing of dysmenorrhea, progressive nature of dysmenorrhea, length of menses and amount of blood flow. It is important to establish whether pain associated with menses is disruptive to daily activities (school attendance, sport participation, etc.). Discussion of sexual activity should take place as appropriate in a private setting, and the presence of dyspareunia noted. Family history should specifically elicit history of Müllerian anomalies and/or endometriosis. In addition to the aforementioned sexual history, the psychosocial evaluation should include possibility of physical, emotional, or sexual abuse, and any other stressors such as food or housing insecurity.
Physical examination
In addition to the basic assessments of general wellbeing, vital signs, and BMI, a detailed abdominal examination is critical in patients with abdominal and pelvic pain. Focal tenderness elicited on palpation may lead to the diagnosis of musculoskeletal pain, a diagnosis often missed when relying exclusively on imaging studies. Carnett’s sign is a specialized physical examination technique during which the examiner applies deep palpation to focal points on the anterior abdominal wall localizing the painful site while the patient is requested to contract the diaphragm by either performing a Valsalva maneuver, raising and holding the head from the pillow or raising straight leg (Figure 1). With the patient’s abdominal muscles tense, the examiner applies pressure with his/her fingertip. The Carnett’s maneuver is considered positive when palpation of the abdomen in the tense position elicits the same or more tenderness as that in the resting position. The patient then relaxes the abdominal musculature, and tenderness typically lessens. Carnett's sign is a specialized physical examination technique that can help support the fact that the abdominal pain originates from the abdominal wall rather than from the abdominal viscera.38,39 Reportedly, it has a 78% sensitivity and 88% specificity for pain arising from an abdominal wall source.40
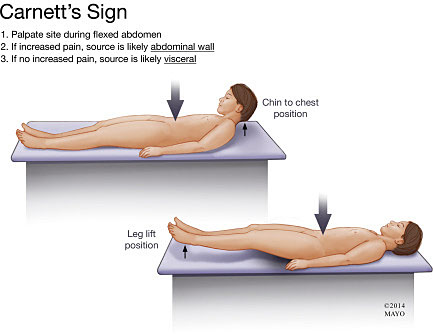
1
Patient positioning to elicit Carnett’s sign. (Reproduced from Kamboj AK, Hoversten P, Oxentenko AS. Chronic Abdominal Wall Pain: A Common Yet Overlooked Etiology of Chronic Abdominal Pain. Mayo Clin Proc. 2019; 94(1):139-144,38 used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
Pelvic examination in non-sexually active adolescent patients with pelvic pain is rarely utilized in the same way as it is with sexually active adolescents and adults. Visual inspection of the perineum will help with establishment of the Tanner stage for pubic hair, anatomy of the labia majora and minora, clitoris, external urethral meatus, configuration of the hymenal ring and position of the anus. Such an inspection may reveal hymenal variants such as microperforate hymen causing partial obstruction and hematocolpos. During inspection, a lubricated Q-tip may be used to probe patency and length of the vaginal canal. If the patient is menstruating, an imperforate hymen is unlikely but, in a case of uterus didelphys and oblique vaginal septum, partial obstruction may be diagnosed, although this is more likely to be diagnosed on imaging than examination. Rectal examination may be helpful if vaginal septum is suspected. With informed consent and proper coaching, rectal examination may be more acceptable in a non-sexually active teenage girl. Bimanual examination can identify trigger points of pain in the pelvic floor musculature but may not be tolerated in a non-sexually active adolescent.
Patients and their caregivers should be given an explicit explanation about the nature, reason for, and extent of any suggested examination. Shared decision-making as to which parts of the exam are to be performed and consent from parents as well as assent from the patient should be explicitly obtained. In general, palpation can yield information about neuromuscular sites of pain, where imaging is more useful for identifying masses or Müllerian anomalies.
Laboratory assessment
Blood work and urine testing has a limited place in evaluation of an adolescent patient with pelvic pain and is dictated by clinical presentation. A complete blood count and erythrocyte sedimentation rate may be obtained if anemia needs to be assessed and/or an inflammatory process is suspected. Urine pregnancy test is used as needed in sexually active adolescents to rule out intrauterine or ectopic pregnancy. Urine or cervical nucleic acid amplification testing (NAAT) should be performed for Chlamydia trachomatis and Neisseria gonorrhoeae infections, as indicated. If endometriosis is suspected, it is important to emphasize that, at this time, there are no blood tests available for clinical practice for diagnosis of endometriosis, but research on establishing non-invasive markers of endometriosis is ongoing.41,42
Imaging
Transabdominal and transvaginal ultrasound is the preferred imaging method in the evaluation of adolescent patients with pelvic or abdominal pain, especially if pelvic pathology is suspected (Figure 2). Normally, ultrasound does not rule out endometriosis in adolescent patients as lesions classically present as stage-I or -II small implants, which are not detected on an ultrasound.43 Ultrasound has its role in diagnosing adnexal pathology such as ovarian and paraovarian cysts or congenital uterine malformations (CUMs). The sensitivity for the diagnosis of deeply infiltrating endometriosis in the pelvis and the uterosacral ligaments, in particular, is lower (80% and 75%, respectively), but these data are available only for adult patients.44 In challenging cases or if a CUM is suspected, three-dimensional ultrasound or, if available, pelvic MRI with a protocol dedicated to the diagnosis of CUMs, are the imaging modalities of choice. Review of the clinical case and imaging findings in consultation with the radiologist can be particularly helpful.
a) | b) |
|
|
2
Right ovarian endometrioma in a 21-year-old patient with chronic pelvic pain: (a) transabdominal ultrasound, showing 4.74-cm right ovarian endometrioma (arrow); (b) intraoperative image of the same right adnexal endometrioma (arrow). Courtesy of Alla Vash-Margita, MD, with patient’s permission.
Differential diagnosis
There are multiple conditions of gynecologic and non-gynecologic origin that can present with pelvic and/or abdominal pain (Table 2). This list is extensive and thorough history and physical examination will lead the provider to correct diagnosis and appropriate and prompt referrals to other specialists. We want to draw special attention to functional abdominal pain disorders, given their high frequency and associated high costs to the medical system.45 These disorders have been considered a diagnosis of exclusion in the past, but current knowledge and the favorable prognosis associated with early diagnosis and appropriate therapy support their earlier consideration in the diagnostic process. Most disorders of the brain–gut interaction are diagnosed clinically, primarily using the Rome IV criteria (expert opinion-based clinical criteria). According to the Rome IV criteria, functional abdominal pain is defined as pain with a duration of at least 2 months and with a frequency of 4 days per week that does not meet criteria for other disorders of brain–gut interactions.46 The diagnosis of functional abdominal pain is clinical, with testing guided by the presence of red flags, including vomiting (bloody emesis may indicate inflammatory conditions and peptic disease; bilious emesis may point towards obstructive conditions), diarrhea that may suggest infections or inflammatory conditions of the gastrointestinal tract (particularly when bloody), and abdominal distention (may suggest malabsorption and obstructive conditions).
MANAGEMENT OF THE ADOLESCENT PATIENT WITH PELVIC PAIN
Medical treatment for pain related to menses
The optimal treatment for chronic pelvic pain is often multimodal and multidisciplinary. If evaluation of an adolescent patient points to primary dysmenorrhea, first-line treatment in a patient who does not need contraception is often a non-steroidal anti-inflammatory drug (NSAID) that can be started 1 to 2 days prior to onset of menses and continued as needed. Opioids are specifically not recommended due to risk of development of dependence.4 A few different types of NSAIDs, often ibuprofen or naproxen, can be tried prior to trying second-line treatments. Recommendation is made to increase fluid intake in the presence of headaches and ensure healthy sleep hygiene to improve tolerance to pain by increasing REM sleep. If NSAIDs are ineffective, or if a patient would prefer treatment that can be used for contraception as well, a variety of hormonal methods can be used. These are designed to suppress endometrial development and reduce prostaglandin production (Table 3). When prescribing combined oral contraceptives, it is important to assess the risk of venous thromboembolism (VTE) based on personal and/or family history of vascular thrombotic event, liver disease, and/or migraines with aura. In latter cases, estrogen containing preparations should be avoided, while progestin-only preparations, including depo-medroxyprogesterone acetate, the etonogestrel implant, and progestin-only pills remain excellent options. The levonorgestrel intrauterine system (IUS) has been recommended for treatment of primary dysmenorrhea. If the above measures do not bring relief, the provider must consider etiologies of secondary dysmenorrhea, especially endometriosis. If there is a positive finding on ultrasound (such as obstructive CUM) appropriate surgery will be recommended. If ultrasound and pelvic examination are not revealing, endometriosis should be suspected. Empirical treatment for endometriosis would be similar to treatment of primary dysmenorrhea with the most common choice being combined hormonal or progestin-only pill, usually administered in a continuous fashion (with skipping the placebo) for menstrual suppression. Due to significant side-effects, more potent modalities such as GnRH agonist or antagonists are ideally used in adolescents if there is surgical and pathologic diagnosis of endometriosis (Table 3). Note, that use of GnRH agonist or antagonists in adolescents younger than 18 years would be outside of Food and Drug Administration indications in the USA.
3
Hormonal preparations for treatment of primary dysmenorrhea or endometriosis.
Medication | Dose | Side effects |
Combination estrogen/progestin: pill, patch or ring | Pill: 20–35 mcg EE + various progestins | Risk of VTE |
Progestins | Norethindrone 0.35 mg* daily | Irregular bleeding |
GnRH agonists | Leuprolide: 11.25 mg IU every 3 months or 3.75 mg IU monthly | FDA approved >18-year-old |
Elagolix (GnRH antagonist) | 150 mg tablet daily or 200 mg twice per day | Not approved for <18-year-old, not approved as a contraceptive |
Danazol | 100 mg, 200 mg, 400 mg (daily) | Acne, hirsutism, weight gain, deepening of voice (permanent) |
*Formulation is approved for birth control.
EE, ethinyl estradiol; FDA, Food and Drug Administration; GnRH, gonadotropin releasing hormone; IM, intramuscular; VTE, venous thrombotic event.
Surgical treatment for dysmenorrhea
If the conservative treatment listed above does not alleviate the pain, diagnostic laparoscopy may be considered. Some patients and families desire to know whether there is an organic pathology, which can be discerned during laparoscopy. The American College of Obstetrics and Gynecologists Committee Opinion recommends the use of laparoscopy after 3–6 months if pain does not improve with patient diligently following medical regimen while amenorrhea is medically induced.4 During laparoscopy, special attention should be paid to subtle, clear vesicular lesions as endometriosis appears differently in adolescents (Figure 3). A technique called 'hydro-inspection' has been described when the operator 'dives in' with the laparoscope into the posterior cul-de-sac filled with saline and performs inspection of the peritoneal surfaces. Any suspicious lesion should be biopsied for histologic confirmation of the diagnosis. Any visible lesions should be destroyed, ablated, or excised by electrocautery, laser ablation, or excision. The American College of Obstetrics and Gynecologists recommends against the technique of 'peritoneal stripping' due to lack of short- and long-term outcome data in adolescents, and case reports of significant pelvic adhesions induced by this technique.4 Most adolescents with endometriosis will be diagnosed with stage-I or -II endometriosis. The most commonly used staging classification is that of the revised American Society of Reproductive Medicine.47
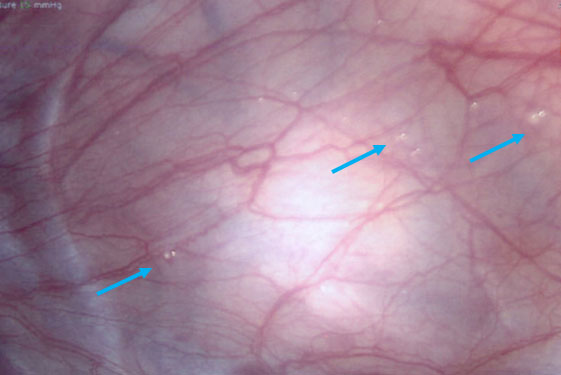
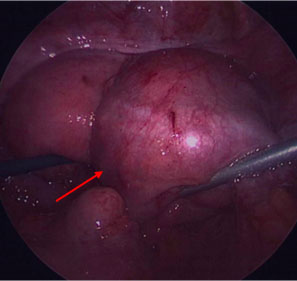
3
Endometriotic 1–2-mm vesicular lesions (arrows) in a 16-year-old patient with pelvic pain. Courtesy of Alla Vash-Margita, MD, with patient’s permission.
According to a recent Cochrane review, there is very low-quality evidence that laparoscopic excision and ablation are similarly effective in relieving pain (only in one study).48 Authors of the same review could not draw a conclusion with regard to the most effective laparoscopic surgical intervention for treatment of severe endometriosis and whether holistic or medical treatment modalities are more effective than laparoscopic surgery. More evidence is emerging that pelvic pain in adolescents should be treated conservatively with a recent study by Sachedina et al., commenting on the need for laparoscopy in only 8.1% of patients.6
Endometriosis is a disease of reproductive age and may recur if treatment is stopped. Following surgical identification of endometriosis, menstrual suppression is usually recommended until childbearing is desired.4
COMPLEMENTARY AND HOLISTIC THERAPY
Chronic abdominal pain can precipitate hypertonicity in the abdominal wall. Poor posture, poorly fitted footwear, heavy school bags and acute muscle strain all can exacerbate pain. Physical therapy has been a great adjunct to treatment if a myofascial pain component is present or significant physical deconditioning has occurred. Trigger point injections with local anesthetics and/or corticosteroids can be employed by pain specialists as well as neuropathic medications (amitriptyline, serotonin-noradrenaline re-uptake inhibitors, anticonvulsants). Cognitive behavioral therapy has been tailored to chronic pain conditions and shown to be beneficial. This is described in detail below.
Another complementary treatment that can alleviate the pain of dysmenorrhea in adult women is acupuncture. However, no studies have yet specifically addressed acupuncture in adolescents, other than a few case reports. A meta-analysis of six trials showed that acupuncture had a positive effect on the primary pain level in patients with dysmenorrhea compared with that of the control group.49
A recent randomized controlled trial by Manisha et al. studied the effect of transcutaneous electrical nerve stimulation (TENS) on primary dysmenorrhea in adolescent girls aged 14–19 years, with 140 subjects followed over 1 year. Lower abdominal and low back pain was measured by numerical pain rating scale and measurement of systolic and diastolic blood pressure. High-frequency TENS was applied at the low back region (root level L3–L5) with significant reduction of pain between intervention group and control.50
Therapeutic interventions for functional abdominal pain
The main goal in the treatment of functional abdominal pain is to improve symptoms. Treatment options include dietary and lifestyle changes, use of probiotics and complementary medicine, behavioral interventions, pain neuromodulators and some recent devices that have been developed to help regulate frequency and intensity of pain using electric neuromodulation. Despite the lack of evidence supporting the recommendation of dietary changes like fiber supplementation51 and lactose restriction52 to improve functional abdominal pain, they are used as first-line treatments given the lack of significant adverse effects and potential benefits for other associated symptoms (like constipation). A diet limiting monosaccharides, disaccharides and fermentable oligosaccharides has been reported as beneficial when compared to placebo to improve pain symptoms.53 Some have reported successful outcomes with the use of peppermint oil,54 which acts as an antispasmodic with minimal, if any, side-effects. The use of probiotics has increased over the last two decades due to low cost, availability, and lack of significant side-effects. Recent studies have shown their utility in functional abdominal pain, specifically preparations, which include Lactobacillus rhamnosus GG and VSL#3.55,56
Medications used to treat functional pain are grouped as those that help abort pain and those that prevent pain. Medications to abort pain include primarily anti-spasmodics, although there is limited evidence to recommend their use, and providers should be aware of the associated side-effects. A systematic review of 12 antispasmodics compared to placebo in adult patients found that only hyoscine and otilonium bromide demonstrated consistent benefit.57 The preventive medications used to decrease frequency and intensity of functional pain, also known as pain neuromodulators, include primarily cyproheptadine and antidepressants. Cyproheptadine is an antihistamine with important serotoninergic effects and has been reported as useful in the treatment of functional abdominal pain.58,59 The use of antidepressants for functional abdominal pain has been increasing over recent decades, including primarily selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs). Despite the increase in their use, there is no significant evidence to support their use in children and adolescents for functional abdominal pain.45 For this reason we recommend their use in selected cases and after attempting and failing all other medical interventions, as well as in cases in which psychiatric comorbidities are identified.
Percutaneous electric field neurostimulation has gained popularity in the last few years due to its efficacy and minimal adverse effects. This is achieved through nerve stimulation of cutaneous branches of cranial nerves V, VII, IX, and X as well as occipital nerves located in the ear lobe, resulting in decreased neuronal activity in the amygdala. Recent research has shown that after 3 weeks of treatment, patients report a significant decrease in functional abdominal pain that is sustained for a median of 6 months.60
Cognitive behavioral therapy
Cognitive behavioral therapy (CBT) is one of the most widely implemented and researched psychological interventions with documented efficacy on symptom reduction and improved quality of life.61,62,63,64 CBT has been adapted to several chronic medical conditions, including chronic pain, and is shown to be effective across a range of chronic pain conditions.65,66,67,68,69,70,71 The application of CBT to chronic pain coincides with the emergence of improved understanding of the brain’s active role in modulating pain signals and the impact of somatosensory, affective, and cognitive experiences on pain processing.72,73 CBT for chronic pain seeks to improve functional disability and modify the cognitive and affective interpretations of pain that contribute to maladaptive coping and disrupted functioning (e.g. avoidance/withdrawal from daily activities, fear of pain).74 CBT for chronic pain is demonstrated to decrease pain intensity and pain frequency, improve quality of life and functioning, and decrease symptoms of anxiety and depression – common sequelae of prolonged chronic pain.75,76,77 Although CBT is a psychological intervention, its application to chronic pain in no way suggests that chronic pain is a psychological condition. Rather, the experience of pain is multifaceted and is known to have detrimental effects on daily functioning, mood, and hypervigilance to pain, particularly as pain persists or worsens.78,79 Readers interested in learning more about the multidimensional experience of pain are encouraged to review additional literature on the neuromatrix model of chronic pain.80
Typical protocols for CBT for chronic pain range in duration from 6–10 visits, though in practice this varies widely. CBT interventions that include caregivers and are delivered by a trained behavioral health clinician may bolster treatment effects.81,82 CBT for chronic pain typically includes (1) biopsychosocial assessment, (2) education about the sensory and affective experience of pain, the role of the central nervous system in processing pain signals, and identification of factors that modify the experience of pain, (3) relaxation training, (4) behavioral activation and the development of a functional restoration behavior plan, (5) cognitive-based strategies including awareness and restructuring of unhelpful thoughts and beliefs about pain, and (6) lifestyle modifications to support improved coping including increased hydration, adherence to medical recommendations, and healthy sleep habits. As noted above, modifying family dynamics that may inadvertently exacerbate or maintain the experience of pain and associated functional disability (e.g. caregiver attention to pain, catastrophizing about pain, protective responding to pain symptoms) is an integral component of CBT for chronic pain in children and adolescents.83
Other psychological interventions
Other psychological interventions for chronic pain include clinical hypnosis (e.g. gut-directed hypnotherapy for chronic abdominal pain), mindfulness, and acceptance-based practices. Though evidence for these approaches is more robust in adults with chronic pain, growing literature is supporting the application of these modalities for children and adolescents as well as specific chronic pain conditions.
Clinical hypnosis is a therapeutic approach facilitated by a trained clinician and includes focused attention and concentration to guide patients in using their imagination to enhance self-awareness and regulation.84,85,86 Clinical hypnosis includes the use of elicitation by narrowing attention to a specific experience (such as a comforting place or the sensation of the breath) as well as intensification and exploration methods to support patients in feeling comfortable, relaxed, and open to suggestion. Clinical hypnosis strives to give patients a sense of agency over their experience and encourages the use of self-hypnosis outside of session. Gut-directed hypnotherapy typically utilizes imaginal experiences focused on modifying gut function or the sensation of pain (e.g. using the sensation of warmth after placing hands on belly to alleviate pain).86,87 A systematic review of gut-directed hypnotherapy for pediatric chronic abdominal pain demonstrates improvements in pain intensity that persist over time.88
Mindfulness-based strategies share some similarities with clinical hypnosis given that both approaches include focused attention; however, mindfulness differs in its emphasis on awareness to the present moment and the utility of recognizing and observing thoughts in a non-judgmental manner. Research on mindfulness meditation for chronic pain is fairly limited, though preliminary findings in adults suggest possible benefits for pain perception/intensity, symptoms of depression, and quality of life.89,90 Studies in pediatrics support mindfulness as a feasible and acceptable intervention, but also suggest limited impact on outcome measures as a standalone intervention, to date.91,92
Mindfulness practice is a critical component of acceptance-based interventions such as acceptance and commitment therapy.93 Acceptance-based therapies emphasize psychological flexibility and the importance of accepting the current moment while also engaging in behavior change and value directed action (i.e. behavior that aligns with one’s values).94 Applied to chronic pain, acceptance-based interventions encourage shifting away from the goal of pain relief and rather support patients in identifying how to lead a fulfilling and meaningful life even in the presence of pain.95 Acceptance-based interventions demonstrate positive effects on daily functioning, anxiety, and depression though effect sizes tend to be small to moderate.92
CONCLUSION
Chronic pelvic and abdominal pain among adolescent females represents a serious health condition worldwide. Endometriosis is one of the common causes of such pain but remains underdiagnosed. The Global Consortium for Endometriosis states that adolescent females represent an underserved group of the population with high morbidity and negative social impact. This Consortium recommended placing an emphasis on the development of non-invasive biomarkers specifically in young women, highlighting that more work on the role of microRNAs (miRNAs) is required, including using miRNAs as a biomarker and therapeutic tools for endometriosis among other approaches.96 Ongoing research has shown promising results in adult women with the use of specific miRNAs as non-invasive diagnostic markers with work ongoing in applying the same approach in adolescents.97 Functional abdominal pain is another common cause of chronic pelvic and abdominal pain in children and young adults. It is important to dedicate time to educate patients and families about this condition, minimize the use of diagnostic studies guided by the presence of red flags and to consider alternative therapies and medications along with behavioral interventions to manage this complicated patient population. Further work should be directed to the development of non-invasive diagnostic tools to support comprehensive assessment of the patient across developmental, biological, and psychological domains.
A timely and multidisciplinary approach resulting in comprehensive interventions targeting pain, mental health, and restored functioning is critical. A multidisciplinary team approach is recommended early in the diagnostic process. Psychological interventions are a necessary component of evidence-based pain management. CBT, in particular, demonstrates clinically significant improvements in pain intensity and pain-related disability as well as improved pain coping and emotional functioning. Despite the importance of behavioral-based interventions for chronic pain, effect sizes over time tend to be modest.98 Additional research is needed to match and tailor intervention approaches with specific patient characteristics, including psychological factors, to maximize treatment efficacy.99
PRACTICE RECOMMENDATIONS
- Consider endometriosis as a cause of cyclic or non-cyclic pelvic and/or abdominal pain in postmenarchal females.
- Empirical diagnosis of endometriosis may be achieved based on history, physical examination, and absence of other structural causes of pain on imaging modalities. Research devoted to the development of non-invasive biomarkers is ongoing. Laparoscopy in adolescent females should be reserved for challenging cases with poor or absent response to standard medical treatment.
- First-line medical treatments for primary or secondary dysmenorrhea are non-steroidal anti-inflammatory drugs (NSAIDs) and combined hormonal formulations that induce amenorrhea.
- Laparoscopy is the preferred surgical approach for diagnosis of endometriosis. Endometriotic lesions diagnosed during surgery can be destroyed with electrocautery, laser ablation, or excision. There is no preferred method of surgical removal of visible lesions. In addition, the American College of Obstetricians and Gynecologists recommends against the technique of 'peritoneal stripping' due to lack of short- and long-term outcome data in adolescents.
- Laparoscopy may be considered in patients after 3–6 months of medically induced amenorrhea if pain persists or the patient/family desire to proceed with surgery.
- Physical therapy has been a great adjunct to treatment if a myofascial pain component is present or significant physical deconditioning has occurred. Trigger point injections with local anesthetics and/or corticosteroids can be employed by pain specialists as well as neuropathic medications (amitriptyline, serotonin-noradrenaline re-uptake inhibitors, anticonvulsants).
- Acupuncture has been shown to alleviate dysmenorrhea in adult women with no studies specifically addressing acupuncture in adolescents other than a few case reports.
- Transcutaneous electrical nerve stimulation (TENS) may be beneficial for primary dysmenorrhea in adolescent girls. This modality may be considered as adjunct to the first-line treatment.
- Diagnosis of functional abdominal pain should be considered in adolescents with chronic abdominal pain in the absence of red flags. The use of antidepressants for functional abdominal pain has been increasing over recent decades, including primarily SSRIs and tricyclic antidepressants. Despite the increase in their use, there is no significant evidence to support their use in children and adolescents for functional abdominal pain. Dietary changes and alternative medicine as well as behavioral interventions should be considered first.
- Cognitive behavioral therapy has been adapted to several chronic medical conditions, including chronic pain, and is shown to be effective across a range of chronic pain conditions.
- A multidisciplinary approach resulting in comprehensive interventions targeting pain, mental health, and restored functioning remains a critical component during evaluation of an adolescent female patient with chronic abdominopelvic pain. A multidisciplinary team approach is recommended early in the diagnostic process.
ACKNOWLEDGMENT
The authors acknowledge the support of Alyssa Grimshaw, MSLIS, Harvey Cushing/John Hay Whitney Medical Library, Yale University.
CONFLICTS OF INTEREST
Dr Rodriquez serves as a Consultant on the NeurAxis Advisory Board.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Classification of Chronic Pain, 2nd edn. IASP Press, 2012. | |
Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician 2014;17(2):E141–7. PubMed PMID: 24658485. Epub 2014/03/25. | |
Hu Z, Tang L, Chen L, et al. Prevalence and Risk Factors Associated with Primary Dysmenorrhea among Chinese Female University Students: A Cross-sectional Study. J Pediatr Adolesc Gynecol 2020;33(1):15–22. PubMed PMID: 31539615. Epub 2019/09/21. | |
ACOG Committee Opinion No. 760: Dysmenorrhea and Endometriosis in the Adolescent. Obstet Gynecol 2018;132(6):e249–e58. PubMed PMID: 30461694. Epub 2018/11/22. | |
Hirsch M, Dhillon-Smith R, Cutner AS, et al. The Prevalence of Endometriosis in Adolescents with Pelvic Pain: A Systematic Review. J Pediatr Adolesc Gynecol 2020;33(6):623–30. PubMed PMID: 32736134. Epub 2020/08/01. | |
Sachedina A, Abu Bakar M, Dunford AM, et al. Dysmenorrhea in young people: Experiences from a tertiary center with a focus on conservative management. J Obstet Gynaecol Res 2021;47(1):352–8. PubMed PMID: 33084069. Epub 2020/10/22. | |
Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96(2):366–73.e8. PubMed PMID: 21718982. PMCID: PMC3679489. Epub 2011/07/02. | |
Rowlands IJ, Teede H, Lucke J, et al. Young women's psychological distress after a diagnosis of polycystic ovary syndrome or endometriosis. Hum Reprod 2016;31(9):2072–81. PubMed PMID: 27412249. Epub 2016/07/15. | |
DiVasta AD, Vitonis AF, Laufer MR, et al. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs. adulthood. Am J Obstet Gynecol 2018;218(3):324.e1–.e11. PubMed PMID: 29247637. Epub 2017/12/17. | |
Falcone T, Flyckt R. Clinical Management of Endometriosis. Obstet Gynecol 2018;131(3):557–71. PubMed PMID: 29420391. Epub 2018/02/09. | |
Ottolina J, Schimberni M, Makieva S, et al. Early-life factors, in-utero exposures and endometriosis risk: a meta-analysis. Reprod Biomed Online 2020;41(2):279–89. PubMed PMID: 32532666. Epub 2020/06/14. | |
Christ JP, Yu O, Schulze-Rath R, et al. Incidence, prevalence, and trends in endometriosis diagnosis: a United States population-based study from 2006 to 2015. Am J Obstet Gynecol 2021;225(5):500.e1–.e9. PubMed PMID: 34147493. Epub 2021/06/21. | |
Missmer SA, Hankinson SE, Spiegelman D, et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 2004;160(8):784–96. PubMed PMID: 15466501. Epub 2004/10/07. | |
Arumugam K, Templeton AA. Endometriosis and race. Aust N Z J Obstet Gynaecol 1992;32(2):164–5. PubMed PMID: 1520204. Epub 1992/05/01. | |
Bougie O, Yap MI, Sikora L, et al. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG 2019;126(9):1104–15. PubMed PMID: 30908874. Epub 2019/03/26. | |
Williams DR, Mohammed SA, Leavell J, et al. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci 2010;1186:69–101. PubMed PMID: 20201869. PMCID: PMC3442603. Epub 2010/03/06. | |
Bougie O, Healey J, Singh SS. Behind the times: revisiting endometriosis and race. Am J Obstet Gynecol 2019;221(1):35.e1–.e5. PubMed PMID: 30738028. Epub 2019/02/10. | |
Harris HR, Wieser F, Vitonis AF, et al. Early life abuse and risk of endometriosis. Hum Reprod 2018;33(9):1657–68. PubMed PMID: 30016439. PMCID: PMC6112577. Epub 2018/07/18. | |
Treloar SA, O'Connor DT, O'Connor VM, et al. Genetic influences on endometriosis in an Australian twin sample. sueT@qimr.edu.au. Fertil Steril 1999;71(4):701–10. PubMed PMID: 10202882. Epub 1999/04/15. | |
Deiana D, Gessa S, Anardu M, et al. Genetics of endometriosis: a comprehensive review. Gynecol Endocrinol 2019;35(7):553–8. PubMed PMID: 30909768. Epub 2019/03/27. | |
Sampson JA. Heterotopic or misplaced endometrial tissue. American Journal of Obstetrics and Gynecology 1925;10(5):649–64. | |
Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927;14:422–69. | |
Batt RE, Smith RA, Buck Louis GM, et al. Müllerianosis. Histol Histopathol 2007;22(10):1161–6. PubMed PMID: 17616942. Epub 2007/07/10. | |
Signorile PG, Baldi F, Bussani R, et al. Ectopic endometrium in human foetuses is a common event and sustains the theory of müllerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J Exp Clin Cancer Res 2009;28(1):49. PubMed PMID: 19358700. PMCID: PMC2671494. Epub 2009/04/11. | |
Brosens I, Benagiano G. Is neonatal uterine bleeding involved in the pathogenesis of endometriosis as a source of stem cells? Fertil Steril 2013;100(3):622–3. PubMed PMID: 23725803. Epub 2013/06/04. | |
Bianchi P, Benagiano G, Brosens I. Promoting awareness of neonatal menstruation. Gynecol Endocrinol 2017;33(3):173–8. PubMed PMID: 28079409. Epub 2017/01/13. | |
Puttemans P, Benagiano G, Gargett C, et al. Neonatal uterine bleeding as a biomarker for reproductive disorders during adolescence: a worldwide call for systematic registration by nurse midwife. J Matern Fetal Neonatal Med 2017;30(12):1434–6. PubMed PMID: 27454348. PMCID: PMC5505234. Epub 2016/07/28. | |
Brosens I, Gargett CE, Guo SW, et al. Origins and Progression of Adolescent Endometriosis. Reprod Sci 2016;23(10):1282–8. PubMed PMID: 27036950. Epub 2016/04/03. | |
Brosens I, Ćurčić A, Vejnović T, et al. The perinatal origins of major reproductive disorders in the adolescent: Research avenues. Placenta 2015;36(4):341–4. PubMed PMID: 25637411. Epub 2015/02/01. | |
Gargett CE, Schwab KE, Brosens JJ, et al. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol Hum Reprod 2014;20(7):591–8. PubMed PMID: 24674992. Epub 2014/03/29. | |
Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci 2008;1127:106–15. PubMed PMID: 18443337. PMCID: PMC3107843. Epub 2008/04/30. | |
Wang Y, Nicholes K, Shih IM. The Origin and Pathogenesis of Endometriosis. Annu Rev Pathol 2020;15:71–95. PubMed PMID: 31479615. PMCID: PMC7980953. Epub 2019/09/04. | |
Kong Y, Shao Y, Ren C, et al. Endometrial stem/progenitor cells and their roles in immunity, clinical application, and endometriosis. Stem Cell Res Ther 2021;12(1):474. PubMed PMID: 34425902. PMCID: PMC8383353. Epub 2021/08/25. | |
Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012;98(3):511–9. PubMed PMID: 22819144. PMCID: PMC3836682. Epub 2012/07/24. | |
Laufer MR, Goitein L, Bush M, Cramer DW, Emans SJ. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J Pediatr Adolesc Gynecol. 1997 Nov;10(4):199-202. doi: 10.1016/s1083-3188(97)70085-8. PMID: 9391902. | |
Dun EC, Kho KA, Morozov VV, et al. Endometriosis in adolescents. JSLS 2015;19(2). PubMed PMID: 26005317. PMCID: PMC4432718. Epub 2015/05/26. | |
Miller JA, Missmer SA, Vitonis AF, et al. Prevalence of migraines in adolescents with endometriosis. Fertil Steril 2018;109(4):685–90. PubMed PMID: 29605402. Epub 2018/04/02. | |
Kamboj AK, Hoversten P, Oxentenko AS. Chronic Abdominal Wall Pain: A Common Yet Overlooked Etiology of Chronic Abdominal Pain. Mayo Clin Proc 2019;94(1):139–44. PubMed PMID: 30611441. Epub 2019/01/07. | |
Carnett J. Intercostal neuralgia as a cause of abdominal pain and tenderness. Surg Gynecol Obstet 1926;42:625–32. | |
Srinivasan R, Greenbaum DS. Chronic abdominal wall pain: a frequently overlooked problem. Practical approach to diagnosis and management. Am J Gastroenterol 2002;97(4):824–30. PubMed PMID: 12003414. Epub 2002/05/11. | |
Nisenblat V, Bossuyt PM, Shaikh R, et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;2016(5):Cd012179. PubMed PMID: 27132058. PMCID: PMC7076288. Epub 2016/05/02. | |
Bendifallah S, Suisse S, Puchar A, Delbos L, Poilblanc M, Descamps P, Golfier F, Jornea L, Bouteiller D, Touboul C, Dabi Y, Daraï E. Salivary MicroRNA Signature for Diagnosis of Endometriosis. J Clin Med. 2022 Jan 26;11(3):612. doi: 10.3390/jcm11030612. PMID: 35160066; PMCID: PMC8836532 | |
Laufer MR, Sanfilippo J, Rose G. Adolescent endometriosis: diagnosis and treatment approaches. J Pediatr Adolesc Gynecol 2003;16(Suppl 3):S3–11. PubMed PMID: 12742180. Epub 2003/05/14. | |
Bazot M, Malzy P, Cortez A, et al. Accuracy of transvaginal sonography and rectal endoscopic sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2007;30(7):994–1001. PubMed PMID: 17992706. Epub 2007/11/10. | |
Park R, Mikami S, LeClair J, et al. Inpatient burden of childhood functional GI disorders in the USA: an analysis of national trends in the USA from 1997 to 2009. Neurogastroenterol Motil 2015;27(5):684–92. PubMed PMID: 25809794. PMCID: PMC5549670. Epub 20150322. | |
Koppen IJ, Nurko S, Saps M, et al. The pediatric Rome IV criteria: what's new? Expert Rev Gastroenterol Hepatol 2017;11(3):193–201. PubMed PMID: 28092724. Epub 20170124. | |
Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67(5):817–21. PubMed PMID: 9130884. Epub 1997/05/01. | |
Bafort C, Beebeejaun Y, Tomassetti C, Bosteels J, Duffy JM. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2020 Oct 23;10(10):CD011031. doi: 10.1002/14651858.CD011031.pub3. PMID: 33095458; PMCID: PMC8428328. | |
Xu Y, Zhao W, Li T, et al. Effects of acupuncture for the treatment of endometriosis-related pain: A systematic review and meta-analysis. PLoS One 2017;12(10):e0186616. PubMed PMID: 29077705. PMCID: PMC5659600. Epub 2017/10/28. | |
Manisha U, Anuradha L. Effect of high frequency transcutaneous electrical nerve stimulation at root level menstrual pain in primary dysmenorrhea. J Bodyw Mov Ther 2021;26:108–12. PubMed PMID: 33992229. Epub 2021/05/17. | |
Horvath A, Dziechciarz P, Szajewska H. Systematic review of randomized controlled trials: fiber supplements for abdominal pain-related functional gastrointestinal disorders in childhood. Ann Nutr Metab 2012;61(2):95–101. PubMed PMID: 22889919. | |
Huertas-Ceballos AA, Logan S, Bennett C, et al. Dietary interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database Syst Rev 2009;21(1):CD003019. PubMed PMID: 19160214. Epub 20090121. | |
Chumpitazi BP, Hollister EB, Oezguen N, et al. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes 2014;5(2):165–75. PubMed PMID: 24637601. PMCID: PMC4063841. Epub 20140127. | |
Kline RM, Kline JJ, Di Palma J, et al. GEnteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr 2001;138(1):125–8. PubMed PMID: 11148527. | |
Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther 2011;33(12):1302–10. PubMed PMID: 21507030. Epub 20110420. | |
Guandalini S, Magazzu G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr 2010;51(1):24–30. PubMed PMID: 20453678. | |
Ford AC, Talley NJ, Spiegel BM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ 2008;337:a2313. PubMed PMID: 19008265. PMCID: PMC2583392. Epub 20081113. | |
Sadeghian M, Farahmand F, Fallahi GH, et al. Cyproheptadine for the treatment of functional abdominal pain in childhood: a double-blinded randomized placebo-controlled trial. Minerva Pediatr 2008;60(6):1367–74. PubMed PMID: 18971897. | |
Rodriguez L, Diaz J, Nurko S. Safety and efficacy of cyproheptadine for treating dyspeptic symptoms in children. J Pediatr 2013;163(1):261–7. PubMed PMID: 23419589. PMCID: PMC3661691. Epub 20130216. | |
Kovacic K, Hainsworth K, Sood M, et al. Neurostimulation for abdominal pain-related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. Lancet Gastroenterol Hepatol 2017;2(10):727–37. PubMed PMID: 28826627. Epub 20170818. | |
Beck AT. Cognitive therapy and the emotional disorders. Penguin, 1976. | |
Beck JS. Cognitive behavior therapy: Basics and beyond. Guilford Publications, 1964. | |
Butler AC, Chapman JE, Forman EM, et al. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clinical Psychology Review 2006;26(1):17–31. | |
Hofmann SG, Asnaani A, Vonk IJ, et al. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive Therapy and Research 2012;36(5):427–40. | |
Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep and Biological Rhythms 2011;9(1):24–34. | |
Sadeghi K, Gharraee B, Fata L, et al. Effectiveness of cognitive-behavioral therapy in treating patients with obesity. Iranian Journal of Psychiatry and Clinical Psychology 2010;16(2):107–17. | |
Safren SA, Gonzalez JS, Wexler DJ, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes care 2014;37(3):625–33. | |
Bernardy K, Füber N, Köllner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome – a systematic review and metaanalysis of randomized controlled trials. The journal of Rheumatology 2010;37(10):1991–2005. | |
de C Williams AC, Fisher E, Hearn L, et al. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews 2020;(8). | |
Dixon KE, Keefe FJ, Scipio CD, et al. Psychological interventions for arthritis pain management in adults: a meta-analysis. Health Psychology 2007;26(3):241. | |
Hoffman BM, Papas RK, Chatkoff DK, et al. Meta-analysis of psychological interventions for chronic low back pain. Health Psychology 2007;26(1):1. | |
Melzack R. From the gate to the neuromatrix. Pain 1999;82:S121–S6. | |
Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150(3699):971–9. | |
Coakley R. When your child hurts: effective strategies to increase comfort, reduce stress and break the cycle of chronic pain. Yale University Press, 2016. | |
Kashikar-Zuck S, Sil S, Lynch-Jordan AM, et al. Changes in pain coping, catastrophizing, and coping efficacy after cognitive-behavioral therapy in children and adolescents with juvenile fibromyalgia. The Journal of Pain 2013;14(5):492–501. | |
Palermo TM, Eccleston C, Lewandowski AS, et al. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain® 2010;148(3):387–97. | |
Vinall J, Pavlova M, Asmundson GJ, et al. Mental health comorbidities in pediatric chronic pain: a narrative review of epidemiology, models, neurobiological mechanisms and treatment. Children 2016;3(4):40. | |
Gatchel RJ. Comorbidity of chronic pain and mental health disorders: the biopsychosocial perspective. Am Psychologist 2004;59(8):795. | |
Kashikar-Zuck S, Goldschneider KR, Powers SW, et al. Depression and functional disability in chronic pediatric pain.Clin J Pain 2001;17(4):341–9. | |
Moseley GL. A pain neuromatrix approach to patients with chronic pain. Manual Therapy 2003;8(3):130–40. | |
Law E, Fisher E, Eccleston C, et al. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev 2019;(3). | |
O’Connell C, Shafran R, Bennett S. A systematic review of randomised controlled trials using psychological interventions for children and adolescents with medically unexplained symptoms: A focus on mental health outcomes. Clin Child Psychol Psychiat 2020;25(1):273–90. | |
Palermo TM, Chambers CT. Parent and family factors in pediatric chronic pain and disability: An integrative approach. Pain 2005;119(1–3):1–4. | |
Kohen DP, Kaiser P. Clinical hypnosis with children and adolescents – What? Why? How?: Origins, applications, and efficacy. Children 2014;1(2):74–98. | |
Kohen DP, Olness K. Hypnosis and hypnotherapy with children. Routledge, 2012. | |
Vasant DH, Whorwell PJ. Gut‐focused hypnotherapy for Functional Gastrointestinal Disorders: Evidence‐base, practical aspects, and the Manchester Protocol. Neurogastroenterol Motil 2019;31(8):e13573. | |
Miller V, Whorwell PJ. Hypnotherapy for functional gastrointestinal disorders: a review. Int J Clin Exp Hypnosis 2009;57(3):279–92. | |
Rutten JM, Reitsma JB, Vlieger AM, et al. Gut-directed hypnotherapy for functional abdominal pain or irritable bowel syndrome in children: a systematic review. Arch Dis Childh 2013;98(4):252–7. | |
Chiesa A, Serretti A. Mindfulness-based interventions for chronic pain: a systematic review of the evidence. The Journal of Alternative and Complementary Medicine 2011;17(1):83–93. | |
Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Annals of Behavioral Medicine 2017;51(2):199–213. | |
Jastrowski Mano KE, Salamon KS, Hainsworth KR, et al. A randomized, controlled pilot study of mindfulness-based stress reduction for pediatric chronic pain. Alternative Therapies in Health & Medicine 2013;19(6). | |
Waelde LC, Feinstein AB, Bhandari R, et al. A pilot study of mindfulness meditation for pediatric chronic pain. Children 2017;4(5):32. | |
Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. Guilford press, 2011. | |
Hayes SC, Strosahl KD. A practical guide to acceptance and commitment therapy. Springer Science+ Business Media, 2005. | |
Hughes LS, Clark J, Colclough JA, et al. Acceptance and commitment therapy (ACT) for chronic pain. Clin J Pain 2017;33(6):552–68. | |
Rogers PA, Adamson GD, Al-Jefout M, et al. Research Priorities for Endometriosis. Reprod Sci 2017;24(2):202–26. PubMed PMID: 27368878. PMCID: PMC5933154. Epub 2016/07/03. | |
Moustafa S, Burn M, Mamillapalli R, et al. Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstet Gynecol 2020;223(4):557.e1–.e11. PubMed PMID: 32165186. Epub 2020/03/14. | |
Eccleston C, Morley S, Williams AdC. Psychological approaches to chronic pain management: evidence and challenges. British Journal of Anaesthesia 2013;111(1):59–63. | |
Simons LE, Basch MC. State of the art in biobehavioral approaches to the management of chronic pain in childhood. Pain Management 2016;6(1):49–61. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)