This chapter should be cited as follows:
Vousden N, Benova L, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.415413
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 2
Health and risk in pregnancy and childbirth
Volume Editors:
Professor Claudia Hanson, Karolinska Institutet, Sweden
Dr Nicola Vousden, King’s College, London, UK

Chapter
A Critical Review of Biological Risk and Pregnancy Outcomes
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Risk in pregnancy is influenced by multiple, interrelated factors. These can be considered at a number of levels. Thaddeus and Maine established the three delays framework which describes determinants influence on deciding, reaching and receiving care.1 Gabrysch and Campbell identified four themes: (1) sociocultural factors; (2) perceived benefit/need of skilled attendance; (3) economic accessibility; and (4) physical accessibility.2 Similarly, McCarthy and Maine concluded that morbidity in pregnancy was a consequence of five intermediate determinants: (1) health status; (2) reproductive status; (3) access to health services; (4) health care behavior; and (5) unknown factors which are underpinned by the underlying socioeconomic and cultural background of the mother.3 For the purpose of this chapter we consider these factors at the level at which they influence a woman’s risk during pregnancy. Individual level factors include biological risks such as age, parity or birth spacing as well as sociodemographic factors, such as education and occupation. Individual factors sit within the wider sociocultural environment, which includes factors such as ethnicity, religion and wealth. This, in turn, is dependent on wider political and contextual factors such as the functioning health system and geographical location. A further two chapters in this volume cover each of these factors in turn and describes their impact on maternal and neonatal mortality and morbidity. This chapter includes the biological factors and behavioral factors such as substance misuse and a subsequent chapter describes the sociodemographic factors, sociocultural environment and wider health system factors.
WEIGHT AND HEIGHT
Prevalence
Obesity is one of the most commonly occurring risk factors in obstetric practice and the prevalence is increasing globally.4 According to the World Health Organization (WHO), body mass index (BMI) can be grouped into the categories (Table 1).5
1
World Health Organization classification of body mass index (BMI).
Classification | BMI (kg/m2) |
Underweight | <18.5 |
Normal range | 18.5–24.9 |
Overweight | ≥25.0 |
Pre-obese | 25.0–29.9 |
Obese class I | 30.0–34.9 |
Obese class II | 35.0–39.9 |
Obese class III | ≥40.0 |
In the United Kingdom, less than half of pregnant women (47.3%) have a BMI in the normal range and 21.3% of the antenatal population are obese.6 Robust country wide data for BMI in pregnancy from low- and middle-resource settings are lacking7 but the prevalence of obesity in pregnancy in Africa is reported to range from 6.5% in Democratic Republic of Congo and 44% in South Africa7 compared to 2% in Asia.8 Globally, the prevalence of overweight and obesity is rising in all regions as shown in Figure 1. The prevalence of low BMI has decreased in Africa and Asia, the regions where it was highest, but remains greater than 10%.8
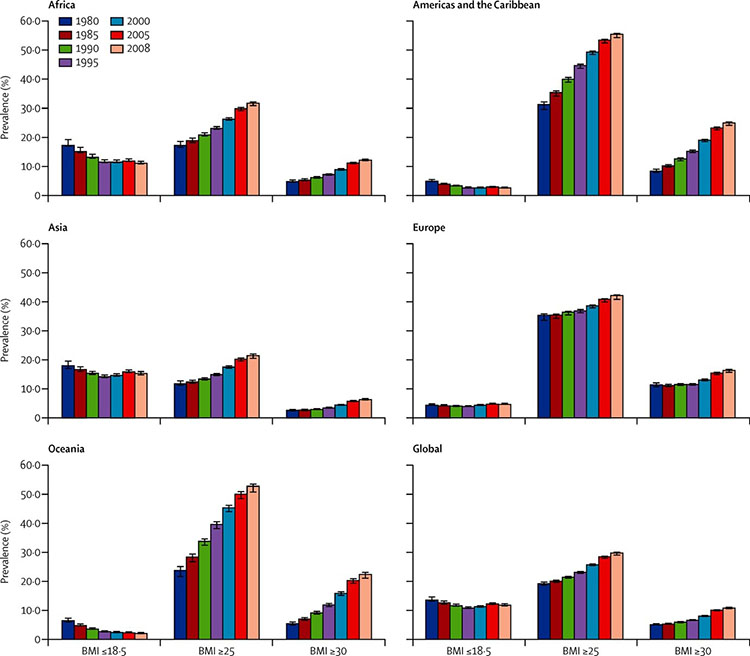
1
Trends in low BMI, overweight, and obesity, using population weighted average prevalences for women aged 20–49 years across UN regions and globally, 1980–2008.8
Pregnancy risk
The impact of a high BMI on pregnancy, delivery and the postnatal period is well established. Evidence from a high-quality systematic review, demonstrated that a low pre-pregnancy BMI was associated with a 25% reduction in the risk of developing gestational diabetes mellitus (GDM) compared to women with a normal BMI (OR 0.75; 95% confidence interval 0.69–0.82). However, for every 1 kg increase in BMI, the risk of GDM increases by 0.92% (0.73–1.10), so the risk in overweight women is double that of women with a normal BMI (overweight OR 1.97; 1.77–2.19) and in obese women the risk is increased nearly four-fold (OR 3.76; 2.34–3.87)).9 This means women with a BMI >30 kg/m2 are advised to have an oral glucose tolerance test between 24 and 28 weeks and should receive dietary advice.
Women that are overweight or obese prior to pregnancy are also at increased risk of pre-eclampsia in pregnancy. A high quality systematic review reported that the unadjusted risk for overweight, obese and severely obese women to develop pre-eclampsia were 1.58 (1.44–1.72), 2.68 (2.39–3.01) and 3.12 (2.24–4.36), respectively.10 Evidence for the impact of BMI on timing and severity of pre-eclampsia is less conclusive. Some studies suggest an association between high BMI and severe pre-eclampsia,11 and others a stronger association with mild or moderate pre-eclampsia.12,13 Similarly, other studies have reported that high BMI is a greater risk for late onset pre-eclampsia than early onset.12,14,15 It is therefore recommended that blood pressure and proteinuria are measured at every contact..16,17
Few studies have explored the association between height and pre-eclampsia. One study demonstrated that women who were short (<164 cm) had greater risk of pre-eclampsia compared to women of a normal height (163–171 cm) (mild to moderate pre-eclampsia aOR 1.07; 1.02–1.11; severe pre-eclampsia aOR 1.22; 1.15–0.30) and women that were tall (>172 cm) had reduced risk (mild to moderate pre-eclampsia OR 0.90; 0.86–0.94; severe pre-eclampsia aOR 0.85; 0.79–0.90).18 However, this and a further study that identified a positive association19 were both undertaken only in women born in Scandinavian countries, whereas a study in Latin America and the Caribbean found no association,20 therefore the generalizability of this finding is not clear.
Labor and delivery
There is good evidence, from three systematic reviews, that obese women have double the risk of cesarean section compared to women with a normal BMI21 (OR 2.01; 1.87–2.1522 up to OR 2.36; 2.15–2.59).23 The risk of instrumental delivery was also reported to be increased by 20%.22 Short-stature is also associated with greater risk of cesarean section24,25 with a study from 34 sub-Saharan African countries reporting that risk gradually increased as height decreased. Women who were short (145.0–149.9 cm) had more than double the risk of cesarean compared to women of average height (155.0–159.9 cm).26
Women who are obese or morbidly obese also have significantly increased risk of hemorrhage and infection from all sources compared to those with a normal BMI (OR 1.24; 1.20–1.28 and OR 3.34; 2.74–4.06, respectively).22 Surgical site infection is also reported to be more common.21,27,28 In comparison, women who were underweight have a reduced risk of cesarean section and hemorrhage (OR 0.81; 0.72–0.90 and 0.67; 0.55–0.82, respectively).22 These risks should therefore be taken into consideration when planning the place of birth, ensuring that facilities to provide assisted deliveries are available.
Obesity can also impact on the timing of delivery. It is important to consider spontaneous and induced preterm birth (PTB) separately due to the increased risk of pre-eclampsia and GDM previously described. Overweight and obesity is reported to significantly increase the risk of induced PTB.29 Whereas, there is no increased risk of spontaneous preterm birth at less than 37 weeks (aOR 0.93; 0.85–1.01)29 and some studies suggest a high BMI is protective against spontaneous PTB (aOR 0.83; 0.75–0.92) even with appropriate adjustment for confounders.30 This is potentially due to increased cervical length in this group compared to normal or underweight women. Some studies report that more severely elevated BMI may affect the severity of PTB, however, few studies have looked at spontaneous PTB alone, with appropriate adjustment for confounders.30 For women with a low BMI, there is high quality evidence that the risk of both spontaneous and induced PTB is increased (aRR1.29; 1.15–1.46, aRR 1.32; 1.10–1.57, respectively).31
Neonatal outcomes
There is also a clear association between infant birthweight and maternal BMI. Women who have a high BMI before pregnancy are less likely to deliver a low birth weight infant (below the 10th centile) (OR 0.81; 0.80–0.83). When this effect is examined in different regions, the protective effect of being overweight or obese was found to be greater in low- and middle-income countries (LMIC) compared to high-income countries (HIC) (OR 0.5; 0.47–0.71 versus 0.90; 0.79–1.01).29 However, women who are overweight or obese prior to pregnancy are twice as likely to have an infant that is large for gestational age (above the 90th centile) (OR 2.08; 1.95–2.23)32 and three times as likely to be macrosomic (>4500 g) (OR 3.23; 2.39–4.37)) compared to infants born to normal-weight women.22 In comparison, women that are underweight prior to pregnancy have an increased risk of having a small for gestational age (OR 1.81; 1.76–1.87) or low birth weight infant (OR 1.47; 1.27–1.71).32
There is strong evidence that obese mothers are at greater risk of having infants with congenital defects. Associated abnormalities include neural tube defects (OR, 1.87; 1.62–2.15), spina bifida (OR 2.24; 1.86–2.69), cardiovascular anomalies (OR 1.30; 1.12–1.51), cleft palate (OR 1.23; 1.03–1.47), anorectal atresia (OR 1.48; 1.12–1.97) and hydrocephaly (OR, 1.68; 1.19–2.36). However, the risk of gastroschisis is reduced in women who are obese compared to those of an normal BMI (OR 0.17; 0.10–0.30).33 All mothers should therefore receive an ultrasound before 24 weeks of gestation to check for fetal abnormalities, in addition to confirming gestational age and multiple pregnancies.16
Maternal obesity also increases the risk of stillbirth, with greater risk as maternal BMI increased (OR 1.24; 1.18–1.30 per 5 BMI units34 and overweight OR 1.47; 1.08–1.94, obese: OR 2.07; 1.59–2.74)35. This may be due to the increased risk of pregnancy comorbidities and congenital anomalies, or reduced ability to detect changes in fetal movements. The risk of miscarriage (OR 1.31; 1.18–1.46),36 neonatal and infant death is also reported to be increased (OR 1.15; 1.07–1.23 and OR 1.18; 1.09–1.28 per 5 BMI unit increase, respectively).34 All these outcomes highlight the importance of appropriate weight management prior to pregnancy in order to reduce subsequent risks to pregnancy and the infant. The Royal College of Obstetrics and Gynaecology has recently released guidance stating that all women of childbearing age with a BMI >30 kg/m2 should receive information about the risks to pregnancy and childbirth and should have the opportunity to optimize their weight before pregnancy.37
RCOG Green-top Guideline No. 72, 2018: Care of women with obesity in pregnancy
https://obgyn.onlinelibrary.wiley.com/doi/full/10.1111/1471-0528.15386#
WHO recommendations on antenatal care for a positive pregnancy experience, 2016
https://apps.who.int/iris/bitstream/handle/10665/250796/9789241549912-eng.pdf?sequence=1
Smaller studies suggest that short maternal height is also associated with increased risk of infant injuries such as such as shoulder dystocia or clavicle fracture.38 A study in sub-Saharan Africa also reported increased risk of neonatal death, especially on the day of delivery or the first postnatal day, compared to average height women (OR 2.36; 1.57–3.55 and 2.34; 1.19–2.60, respectively).26 This indicates that improved intrapartum care is required for short women, although the majority of neonatal deaths will occur in women of average height, since they are the largest population, therefore height is not recommended as a screening tool for referral.26
SMOKING, ALCOHOL AND SUBSTANCE MISUSE
Prevalence
It is estimated that globally 1.7% of women smoke during pregnancy (0.0–4.5). This varies in different geographical regions from 8.1% in Europe, 5.9% in the Americas, 1.2% in Southeast Asia, , and 0.8% in Africa (Figure 2). Globally, 52.9% (45.6–60.3) women who smoke daily before pregnancy continue to smoke daily during pregnancy, again this varied by region from 30.6% (25.6–36.4) in the Europe to 79.6% (44.2–100.0) in the Western Pacific.39
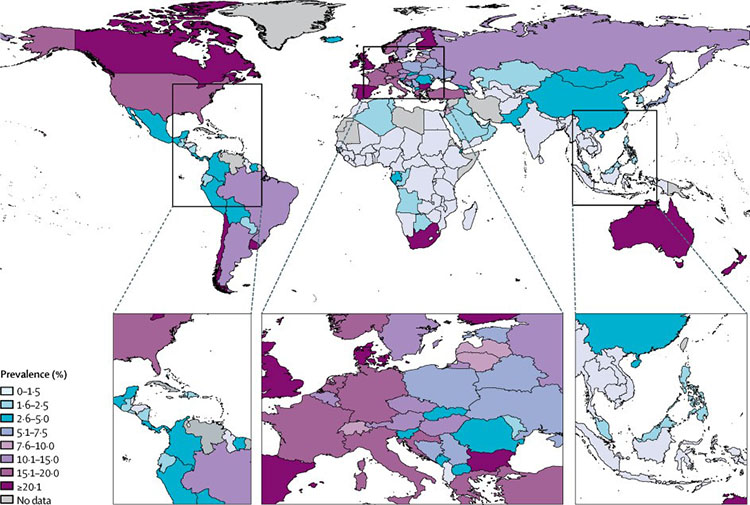
2
Global prevalence of smoking during pregnancy in 2015. Reproduced from Lange et al., The Lancet Global Health, 2018. 39
This study, which was based 295 empirical studies from 104 countries, found that globally the prevalence of smoking during pregnancy has significantly decreased over the past 30 years, but this was mainly driven by reductions in the USA. This suggests that globally health care professionals need greater awareness of the impact of smoking and to offer smoking cessation interventions, ideally prior to conception.39
It is estimated, from data from 50 countries, that globally 9.8% (8.9–11.1) of women drink alcohol during pregnancy and the estimated prevalence of fetal alcohol syndrome in the general population is 14.6/100,000 people (9.4–23.3).40 This equates to one woman in every 67 that drink alcohol during pregnancy, delivering a child with fetal alcohol syndrome. This prevalence varies worldwide. The highest prevalence of drinking alcohol during pregnancy, is in Europe (25.2%, 21.2–29.6), compared to 11.2% (9.4–12.6) in the Americas, 10% (8.5–11.8) in Africa and 1.8% (0.9–5.1) in South East Asia.40
There are less robust data on the prevalence of drug misuse during pregnancy globally. Estimates from the USA state that in 2015 2% of pregnant women reported illicit opioid use, including prescription opioids.41 Rates were highest in pregnant women aged 15–17 compared to older age groups. However, it is likely that a substantial population do not disclose drug use during pregnancy. This was demonstrated by a survey in South Africa, where 5231 pregnant women were screened for illicit drug use and 8.8% tested positive for at least one drug, compared to 3.6% that self-reported.42
Pregnancy outcome
Smoking is associated with a number of pregnancy complications. In early pregnancy, smoking increases the risk of miscarriage (RR 1.32; 1.21–1.44) by 1% per cigarette smoked per day. Second-hand smoke exposure during pregnancy also increases the risk of miscarriage by 11%.43 Meta-analysis of 1167 studies has shown that smoking also increases the risk of placental abruption by up to 80% (OR 1.80; 1.75–1.85).44 This is thought to be due to a chronic inflammatory process.45 Whilst less compelling, several studies also report that smoking is associated with a greater risk of placenta praevia, a low-lying placenta, possibly due to the increased weight and size of the placenta.45 Two systematic reviews concluded that smoking during pregnancy reduces the incidence of pre-eclampsia, and the more cigarettes smoked the lower the risk.46,47 Despite this, the adverse effects of cigarette smoking during pregnancy for both the mother and baby far outweigh this benefit.
The impact of alcohol intake in pregnancy is dependent on the volume and frequency of intake. Heavy or abusive intake of alcohol during pregnancy is associated with increased risk of preterm birth, even taking into account the increased likelihood of maternal smoking.48 Although a number of studies have explored the impact of low to moderate alcohol intake during pregnancy (less than 12 g (or 1.5 units) per day), the majority are small and have methodological flaws, such as failure to adjust for smoking. Therefore, a systematic review concluded that there is limited evidence of adverse pregnancy outcome such as miscarriage, preterm birth following low to moderate levels of alcohol consumption during pregnancy.49 However, as the evidence is inconclusive, safety to consume alcohol at any level has not been demonstrated.
There is a lack of well-designed studies regarding the effect of drug use during pregnancy and conclusions are often complicated with use of multiple substances at the same time. There is evidence that cocaine use during pregnancy increases the risk of adverse pregnancy outcome such as premature rupture of membranes and placental abruption.50 A meta-analysis of cannabis use during pregnancy concluded that there was increased risk of maternal anemia but not gestational diabetes, hypertensive disorder of pregnancy or postpartum hemorrhage.51 Some studies have reported that opioid use during pregnancy increases the risk of pregnancy complications including preterm birth, preterm premature rupture of membranes and chorioamnionitis but there are several inconsistency among studies.52 Despite the lack of evidence for the impact of individual drugs, polydrug use itself is associated with adverse pregnancy outcome.50 Therefore, WHO recommends that all women should have access to affordable prevention and treatment services.53
Neonatal outcome
Smoking is also associated with increased risk of a poor pregnancy outcome. Even taking into account the physical and socioeconomic status of the parents, it is associated with approximately double the risk of delivering an infant that is low birth weight (OR 2.0, 1.77–2.26)54, and 20% increased risk of having a preterm birth at less than 37 weeks of gestation (OR 1.21, 1.19–1.24).55,56 The likelihood of an adverse outcome such as low birth weight or preterm birth are even greater for mothers who smoke heavily (>20 per day).57 Accordingly, cessation of smoking during pregnancy reduces these risks. For example, the probability of a preterm birth in women aged 25–29 years who smoke 1–9 cigarettes throughout pregnancy is 9.8%, compared to 9% for those who stop smoking in the second trimester and 7.8% for those who stop smoking at the start of pregnancy.58 A further study demonstrated that women who only smoke in the first trimester have no increased risk of preterm birth <37 weeks but are at 20% increased risk of an extreme preterm birth at <28 weeks (OR 1.20; 1.02–1.40).55 Similarly, a meta-analysis of smoking cessation trials demonstrated that the risk of having a low birth weight infant was decreased in those who stopped smoking in pregnancy (0.65; 0.42–0.88).59 Smoking cessation should therefore be encouraged at the earliest opportunity.
There is some evidence that congenital defects such as cleft palate are more likely to occur in mothers who smoke (RR 1.22, 1.10–1.35).60 Gastroschisis and small intestinal atresia are also reported to be more likely, especially in mothers who smoke in combination with vasoconstrictive drugs such as cocaine, amphetamines and decongestants but this is based on a small number of cases.61 There is more convincing evidence that active (OR 1.25; 1.16–1.34) and passive smoking (OR 2.24, 1.81–2.77) increases the risk of congenital heart defects such as atrial septal defect and right ventricular outflow tract obstruction.62
There is also convincing evidence that smoking increases the risk of stillbirth by 47% (OR 1.47; 1.37–1.57) and that risk increases the more cigarettes smoked. For example, the risk in those who smoke 1–9 cigarettes a day is increased by 9% compared to 52% in those who smoke ≥10 cigarettes a day.63 A similar increased risk of stillbirth was also shown by Pineles et al. who additionally demonstrated an increased risk in neonatal death (RR 1.22; 1.14–1.30) and perinatal death (RR 1.33; 1.25–1.41) in active smokers. This risk was also increased with the amount the mother smoked. The risk of stillbirth and perinatal death was also increased in women exposed to passive smoke (OR 1.40; 1.06–1.85 and OR 1.42; 1.10–1.85, respectively).64 Therefore, health care professionals should be aware of this and promote smoking cessation within the home environment.
There is a large volume of literature demonstrating that heavy alcohol intake during pregnancy is associated with fetal alcohol spectrum disorders (FASD) and neonatal poor outcomes such as growth restriction.48 The most risky pattern of alcohol intake is binge drinking, with exposure during the first trimester reported to have the greatest impact. FASD is an umbrella term that describes a range of features related to fetal alcohol exposure, the most severe being fetal alcohol syndrome, which is associated with impaired growth, facial abnormalities and damage to the central nervous system resulting in sustained behavioral and cognitive dysfunction.65
Similar to pregnancy outcome, there is limited consistent evidence showing that low to moderate alcohol intake during pregnancy is associated with adverse neonatal outcome, including stillbirth, impaired growth or congenital malformations. There is some evidence that even light to moderate alcohol intake (less than daily) can cause sustained changes in behaviors.66 Therefore, again, the absence of evidence at this level of alcohol intake does not mean that safety has been demonstrated and abstinence is recommended by several national bodies.49
Studies have suggested that cocaine exposure during pregnancy increases the risk of the infant being low birth weight and small for gestational age, although this effect was also seen in polydrug use without cocaine.50 Cannabis use is also associated with a decrease in birth weight and increased risk of admission to the neonatal intensive care unit compared to mothers that did not use cannabis during pregnancy.51 Neonates exposed to opiates during pregnancy have a greater risk of major birth defects such as septal defects (2.0; 1.2–3.6), spina bifida (2.0; 1.3–3.2) and gastroschisis (1.8; 1.1–2.9).67 In addition, between 50% and 80% of infants born to opiate-dependent mothers have neonatal abstinence syndrome due to physical dependence on them, and therefore suffer hyperreflexia, jitteriness, hypertonia and convulsions.68
A large proportion of pregnancies are unplanned; indeed alcohol and drug use may increase the risk of unplanned pregnancy. Therefore, women may continue to smoke, drink alcohol or take illicit drugs at their pre-pregnancy level until pregnancy is confirmed. The gold standard is pre-conception advice to optimize healthy behaviors in all women of reproductive age and contraceptive counseling. However, screening in pregnancy and referral to smoking cessation or substance abuse programs is also important to avoid the negative consequences to the pregnancy and fetus.
Guideline for identification and management of substance use and substance use disorders in pregnancy; 2014, World Health Organization
https://www.who.int/substance_abuse/publications/pregnancy_guidelines/en/
AGE, PARITY AND BIRTH SPACING
Advanced maternal age
Advanced maternal age is defined as childbearing in a woman aged over 35 years. This group represents a significant proportion of pregnancies in higher income countries. In 2017, 22% of births in the UK were to women aged 35 and over69 and the prevalence in Norway is even higher at 33%.70 The trend towards delaying childbirth to later reproductive years is clear in this setting, although this group also includes multiparous women who continue childbearing. Advances in and availability of assisted reproductive technologies is likely to be an important factor. In lower-income countries, sociodemographic factors such as marital status and education differ significantly with age, but it is estimated that 14.0% of births were in women of advanced maternal age in 2015–2020,71 which has gradually declined over the last 25 years. The prevalence in individual countries is estimated to vary substantially, for example from 3% in Nepal to 22% in Afghanistan.72
Pregnancy outcome
As maternal age increases, fertility declines and the rate of spontaneous abortion increases (Table 2).
2
Risk of infertility and spontaneous miscarriage with age.73
Maternal age (years) | Fertility rate per 1000 married women | Spontaneous miscarriages (%) |
20–24 | 470 | 11 |
25–29 | 440 | 12 |
30–34 | 400 | 15 |
35–39 | 330 | 25 |
40–44 | 190 | 51 |
≥45 | 40 | 93 |
The prevalence of maternal death, maternal near miss and severe morbidity increases substantially with increasing maternal age. Evidence from a secondary analysis of the WHO multi-country study of 29 countries reported that the risk of maternal near miss (surviving a life-threatening pregnancy complication) was more than double in women aged 35–39 compared to 20–34 years of age and more than triple in women aged 40–44 (4/1000 live births in women aged 20–34; 9/1000 in women aged 35–39 and 14/1000 in women aged 40–44).
The majority of births, and therefore deaths, are in women aged 25–34.74 Therefore, even though women aged 35–39 had a 70% increased risk of maternal death compared to those aged 20–34, the overall numbers are small (OR 1.7; 1.2–2.6).72 Others have reported that the risk rapidly increases after age 30, becoming progressively greater as age advances. This risk is high irrespective of the underlying maternal mortality ratio (MMR) of the region (Figure 3), suggesting that the barriers to improved maternal health outcomes are more resistant to change at advanced maternal ages74 and risk is influenced by common biological and social factors. These are explored elsewhere in this volume.
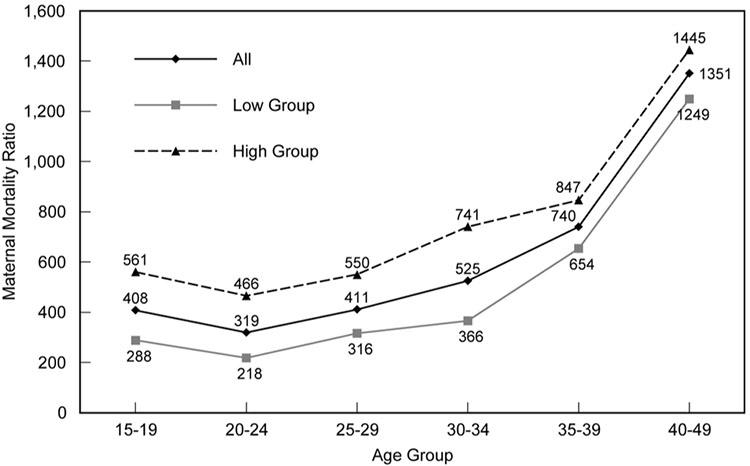
3
Maternal mortality ratios (MMR), all countries and low- and high-MMR groups for increasing age groups. High group, overall MMR >500; low group, overall MMR <500. Reproduced from Blanc et al., PLoS One, 2013. 74
Several studies have shown that compared to younger women, those aged over 35 are less likely to have skilled attendance at birth75 and postnatal care.76 However, these studies failed to take into account that parity increases with age and the negative influence that this has on care-seeking behaviors. When studies appropriately adjust for parity they find that either age has no effect on use of skilled care attendants, or older women are more likely to use skilled attendants than younger mothers.2 In addition to differences in care, women who conceive when older are more likely to have low education levels and greater poverty, which both contribute to increased parity.74 Women who deliver aged over 35 years are nearly twice as likely to develop pre-eclampsia (OR 1.99; 1.65–2.36) and nearly three times as likely to develop gestational diabetes than younger women (OR 2.85; 2.46–3.32).77 These likely contribute to the increased risk of preterm birth reported at <37 weeks of gestation (OR 1.10; 1.03–1.17)).78 The risk of cesarean section and operative delivery is also increased in advanced maternal age, irrespective of parity (RR varied from 1.39 to 2.76).79 It is hypothesized that this is due to poorer uterine contractility than in younger mothers.
Neonatal outcome
Advanced maternal age is associated with increased risk of a range of adverse pregnancy outcomes. It is estimated that 6.7% (uncertainty 6.3–7.3%) of all stillbirths are attributable to older maternal age.80 A review which included over 44 million births found that the risk of stillbirth was increased by 75% in women aged over 35 compared to younger women (OR 1.75; 1.62–1.89). The majority of the studies were undertaken in high-income countries but separate analysis by geographical region found similar increased risk in low and-middle income regions (East Asia (n = 5 studies), OR 1.99; 1.44–2.74), South America (n = 4), OR 1.80; 1.28–2.53), Africa (n = 2), OR 1.76; 1.63–1.90). The risk of fetal growth restriction is also reported to be increased by 23% (OR 1.23, 1.01–1.52) and other pregnancy outcomes are also more likely, such as the infant being small for gestational age (OR 1.16, 1.06–1.27), low birth weight (<2500 g) (OR 1.37, 1.26–1.50) and having a neonatal death (OR 1.48, 1.30–1.67)77 with similar increased risk in LMIC.72 In the UK, experts have therefore argued that women aged 40 years or older should be offered induction of labor at 39–40 weeks of gestation to reduce the risk of stillbirth.
RCOG Scientific Impact Paper 34, 2013: Induction of Labor at Term in Older Mothers
https://www.rcog.org.uk/globalassets/documents/guidelines/scientific-impact-papers/sip_34.pdf
However, it is important to note that only few of studies exploring the relationship between maternal age and pregnancy outcome evaluate the impact of parity. Those that have (nine studies) report that nulliparous women aged over 35 were not at increased risk of stillbirth (compared to nulliparous women aged less than 35 years) but that multiparous women were (OR 1.88; 1.54–2.28), so parity plays an important role in the increased risk in this age group and should be taken into account when making clinical decisions. Similarly, a study from low-income countries concluded that after adjusting for parity, advanced maternal age was no longer a significant risk factor for low birth weight.81 Evidence is less conclusive for preterm birth, which some studies suggest are increased in mothers aged over 35 years, regardless of parity,77 but others report no association after parity and past medical history are adjusted for.78 Pre-eclampsia and gestational diabetes are reported to be increased irrespective of parity in mothers aged over 35 years.77 Although, the impact of other factors such as body mass index, pre-existing medical problems and smoking are likely to be of greater importance.
It is therefore vital that family planning programs clearly communicate the greater risks associated with older age, notably subfertility, severe maternal morbidity or death, stillbirth, preterm birth, low birth weight, and neonatal death.
Low maternal age
In 2017, 3.0% of births in the UK were to mothers under 20 years of age, a figure which has steadily been declining over the past 20 years.69 In low-income countries, this proportion is estimated to be much higher at an average of 14.9% in 2015–2020.82 This represents an estimated 18.5 million adolescent girls (10–19 years old) that give birth every year,83 2.5 million of which are under 16 years old.84 However, this varies by geographical regions, from 115 per 1000 in west Africa to 45 per 1000 women in South-Eastern Asia and 7 per 1000 women in Eastern Asia.85 The number of live births per 1000 women aged between 15 and 19 years is steadily declining in every region of the world,82 but as the adolescent population continues to grow, projections indicate that adolescent pregnancies will increase globally by 2030.86
Maternal outcome
Adolescents pregnancy is associated with greater risk to both the mother and the baby.83 Historically, advocacy articles have reported the risk of maternal death to be doubled in adolescent mothers (aged under 20 years) compared to women in their twenties.87,88 However, there are limited data to support this. One compilation of 13 data sets concluded that the risk of mortality was either equivalent (six studies) or only slightly increased (less than 26%, six studies) in adolescents aged 15–19 compared to women aged 20–24.89 A subsequent analyses of 38 low-income countries' demographic health surveys reported the risk for those aged 15–19 to be increased by 28%, much less than the increased risk in women aged over 35 years.74 This pattern is seen in all regions of the world apart from southeast Asia, where maternal mortality ratio is lower in those aged 15–19 years than for 20–24 year olds (Figure 4).90
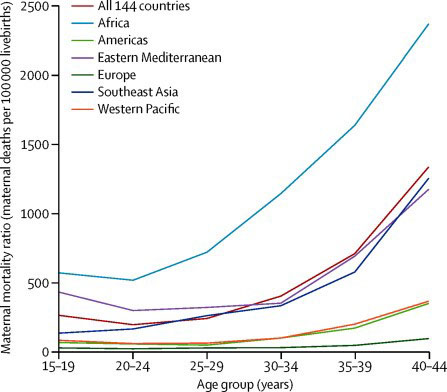
4
Age-specific maternal mortality ratios by region. Reproduced from Nove et al. The Lancet Global Health, 2014.90
Few studies separate outcomes for adolescent mothers by age, but all that do, demonstrate that decreasing age is associated with increased risk. One study of 854,377 Latin American women reported that, even taking into account other factors such poverty, parity and maternal health care, girls under 15 years of age were four times more likely to die in pregnancy and childbirth than those aged 20–24.91 In Chad, Guinea, Mali, Mozambique, Niger and Sierra Leone more than 10% of girls are mothers before the age of 16. These countries have some of the highest MMR in the world, therefore the risk of mortality in this group will be extremely high.84
The causes of maternal death in this group are comparable with other age groups: hypertensive disorders of pregnancy, hemorrhage, abortion and sepsis.92 As in older age groups, the increased risk in this group is likely to be a combination of factors such as poverty, low educational attainment and access to high quality healthcare.93,94 However, adolescents are also more likely to be primiparous, which is associated with increased risk of mortality at any age.90 The increased risks associated with pregnancy and childbirth in adolescence are therefore compounded by the risk of first pregnancy and delivery. There are insufficient data to adjust for this, therefore comparison of risk in this age group between countries may be unreliable where the proportion of births and parity in this age group differ.
Adolescents are less likely to use antenatal care, and more likely to book later in pregnancy and receive fewer care components.95 In some cultures, they are also less likely to have a skilled assistant at delivery.96 This is likely a result of socioeconomic factors but also stigma from health care providers. Physiologically, adolescents (<20 years) are at greater risk of severe anemia as well as indirect causes of morbidity and mortality such as malaria and dengue in those under 18 years.97 The prevalence of morbidity such as eclampsia, hemorrhage and puerperal endometritis is also significantly increased in those under 20 years compared to 20–24 years.91,97,98 There is also evidence to suggest that younger girls (<15) are at greater risk of obstructed labor (OR 1.32; 1.13–1.55), a risk not seen in older adolescents.99 This is suggested to be because the pelvic bones and birth canal are still growing. Few studies have quantified the risk of obstetric fistula in this age group, but in Ethiopia and Nigeria, more than 25% of fistula patients had become pregnant under 15 years of age and more than 50% were under 18 years. This results in life long disability and disadvantage.100 Surgical management of fistula is available that can minimize this impact, however, access across LMIC is limited.101 Pregnant adolescents are less likely to continue education and, therefore, have fewer opportunities for employment, which perpetuates cycles of poverty. The economic cost to countries is also significant due to the loss of lifetime annual income.102 Therefore, the risks that adolescents face in giving birth are still severe and have lifelong impact.
Neonatal outcome
Adolescent pregnancy is also associated with increased risk of poor neonatal outcomes, especially at younger ages. The risk of neonatal mortality is significantly increased in mothers aged under 16 years compared to those aged 20–29 years, even after adjusting for socioeconomic, demographic and health care factors. Whereas, only a minority of countries (e.g. Maldives and Malawi) report significantly increased risk in mothers aged 18–19 years.103 Similarly, a study undertaken in 29 countries by WHO found that adolescents under 20 years old were more likely to have a preterm delivery (<37 weeks) and that risk was increased as age decreased (prevalence 11.2% in girls aged 15 years and under, 8.6% aged 16–17 years, 7.7% aged 18–19 years and 7% aged 20–24 years, p <0.001).97 The risk for neonatal severe conditions (birth weight <1500 g, gestational age at delivery <32 weeks or 5-minute Apgar score of <7) shows a similar pattern, with the greatest risk in those under 15 years of age (3.6% aged 15 years, 2.7% aged 16–17, 2.3% aged 18–19 and 1.9% aged 20–24 years (p <0.001)). This study found similar, non-significant trends for low birth weight (p = 0.357),97 but others have found a significantly increased risk of low birth weight in all adolescent groups compared to women aged 20–24, with the greatest risk in those aged 15 years and under (12.8% of <15 years compared to 8.1% of 20–24 years OR 1.62; 1.54–1.71,91 13.9% compared to 6.5% respectively, RR 1.81; 1.40–2.34)104. Fewer studies have found a significant change in the risk of stillbirth.91,97,99,104 Again it is important to note the impact of parity on risk. In all three regions, the risk of neonatal mortality associated with adolescent birth was greater for second or subsequent deliveries than in the first birth when compared to women in their 20s of the same parity (Figure 5).103
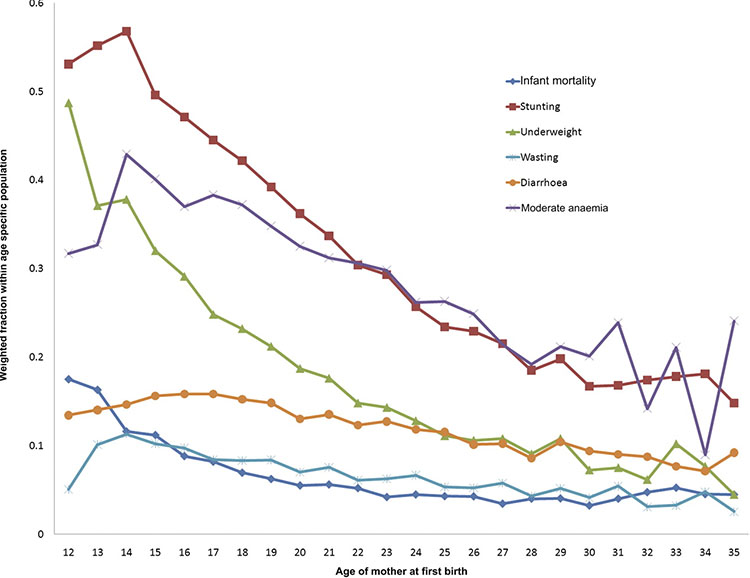
5
Child health indicator weighted prevalence by age of the mother at first birth. Reproduced from Finlay et al., BMJ Open, 2011, with permission.105
In addition to perinatal outcomes, the prevalence of poor child health outcomes such as infant mortality, stunting, underweight and anemia are also increased in younger mothers. Evidence based on data from 55 LMIC suggests the elevated risk in infant mortality is seen in all women who have their first-born child at ages below 27–29 years, although the effect is only significant in women below 18 years. Even when other socioeconomic, demographic and health care factors are taken into account, the risk associated with age remained similar, suggesting that it is an independent risk factor for poor infant outcomes.105 However, adolescents who have multiple births before the age of 20 years are likely to be among the most deprived. This section demonstrates an important link between adolescent sexual health and child health. Prevention of adolescent pregnancies in very young age groups should be prioritized to reduce maternal and neonatal mortality and poor infant outcomes. In addition, access to high quality health care for adolescents during pregnancy and labor is likely to reduce risks by ensuring early detection and management of underlying factors such as anemia and hypertension.
Parity and birth spacing
Recent evidence demonstrated that on average across 30 LMIC, 6.3% of all second-order and high-order births were in adolescents aged <20 years. This varied from 0.8% in Rwanda to 12.5% in Bangladesh and 12.1% in Chad.106 Whilst these percentages are relatively small, the numbers of adolescents affected can be large. USAID estimated that of the 22.5 million adolescents who gave birth in 2017, 4.1 million were a second or higher order birth.107 Whilst many adolescent pregnancies occur in marriage, it is likely that closely spaced pregnancies are unintended. Rapid repeat pregnancy is a concern, which presents further risks for both the mother and child. Systematic reviews have identified that short pregnancy intervals in women with a previous cesarean section are associated with increased risks of uterine rupture and uteroplacental disorders (placental abruption and previa).108 The risk of other adverse maternal outcomes such as anemia, hemorrhage and maternal death are less conclusive.108 In comparison, a longer interval between pregnancy is associated with an increased risk of pre-eclampsia, with increasing risk as the interpregnancy interval increases.108
There is also a clear relationship between a short duration between pregnancies (<18 months) and increased risk of adverse neonatal outcomes including a 61% increased risk of having a low birth weight infant (OR 1.61; 1.39–1.86), 40% increased risk of preterm birth (OR 1.40; 1.24–1.58) and 26% increased risk of small for gestational age infants (OR1.26; 1.18–1.33) compared to intervals of 18–23 months. Long intervals between pregnancies of over 59 months have similarly increased risks for neonates.109 There is limited evidence to conclude why there is increased risk with shorter interpregnancy intervals, but maternal nutritional depletion, cervical insufficiency, transmission of infections,110 socioeconomic status and inadequate healthcare usage have all been hypothesized to contribute.108
Nulliparity and grand multiparity are widely reported as risk factors for pregnancy and labor. Studies from many different countries demonstrate that the risk of maternal mortality is raised for primiparous women, then reduced for second and third order births, then risk increases as parity increases with the greatest risk seen at parities greater than six.111 Therefore, avoiding births above parity five has been proposed as strategy to substantially reduce maternal mortality worldwide.
Nulliparity is also associated with increased neonatal morbidity such as small for gestational age births (OR 1.51; 1.39–1.64) and neonatal mortality (OR 1.28; 1.11–1.51) compared to parous women also aged between 18 and 35. The same was also seen in nulliparous women aged <18 compared to parous women in this age group. This may be due to the increased risk of obstetric complications such as eclampsia, malaria and obstructed labor. Increased risk is also seen in women with parity ≥3, where greater risk of preterm birth is seen irrespective of age (age 18–<35: OR 1.20, 1.06–1.35, age ≥35: OR 1.43, 1.21–1.69).112 High parity (≥3) also increases the risk of neonatal mortality irrespective of age (age 18–<35: OR 1.30, 1.11–1.51, age ≥35: OR 1.66, 1.23–2.23).112 It therefore appears that parity is an independent risk factor for neonatal adverse outcome, however, other studies have concluded that in contexts where women had appropriate access to care there was no increased risk,113 therefore it is possible that residual confounding persists.
In clinical practice, it is the combination of these risk factors, age, parity and interpregnancy interval that confer the greatest risks of mortality. One study that attempted to quantify the contribution of these risks used data from 45 countries Demographic and Health Surveys, which demonstrated that combination of these categories, such as maternal age >40 and parity >4 that conferred the greatest risks for infant mortality.114 This suggests that meeting unmet contraceptive need in all age groups is an effective way of reducing pregnancy-related morbidity and mortality by avoiding high-risk births in these groups.115
PRACTICE RECOMMENDATIONS
- All women of childbearing age with a BMI >30 should receive information about the risks to pregnancy and childbirth, and should have the opportunity and support to optimize their weight before pregnancy.
- All pregnant women should have their weight and height measured, and their BMI calculated and recorded at the antenatal booking visit.
- Advice should be given about healthy diets in pregnancy rather than specific weight gain targets.
- All pregnant women with a booking BMI 30 or greater should be screened for gestational diabetes.
- Women who smoke should be informed of the risk and supported to stop, prior to pregnancy or at the booking appointment.
- Women should be advised that there is no known safe level of alcohol intake during pregnancy and therefore abstinence is advised.
- Women should be screened for substance misuse in pregnancy and have access to affordable prevention and treatment services.
- Women should be informed about the impact of age and parity on pregnancy and neonatal risk in order to make informed decision about family planning.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Thaddeus S, Maine D. Too far to walk: maternal mortality in context. Social Science and Medicine 1994;38(8):1091–110. | |
Gabrysch S, Campbell OM. Still too far to walk: literature review of the determinants of delivery service use. BMC Pregnancy Childbirth 2009;9:34. | |
McCarthy J, Maine D. A framework for analyzing the determinants of maternal mortality. Stud Fam Plann 1992;23(1):23–33. | |
World Health Organisation. Obesity and Overweight Factsheet. 2018 https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. [Accessed 15th January 2019] | |
World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: WHO, 2000. | |
National Maternity and Perinatal Audit Project Team. National Maternity and Perinatal Audit Clinical Report. London, 2017. | |
Onubi OJ, Marais D, Aucott L, et al. Maternal obesity in Africa: a systematic review and meta-analysis. Journal of Public Health (Oxford, England) 2016;38(3):e218-e31. | |
Goldstein RF, Abell SK, Ranasinha S, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Medicine 2018;16(1):153. | |
Torloni MR, Betran AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity 2009;10(2):194–203. | |
Wang Z, Wang P, Liu H, et al. Maternal adiposity as an independent risk factor for pre-eclampsia: a meta-analysis of prospective cohort studies. Obesity reviews: an official journal of the International Association for the Study of Obesity 2013;14(6):508–21. | |
Eskenazi B, Fenster L, Sidney S. A multivariate analysis of risk factors for preeclampsia. JAMA 1991;266(2):237–41. | |
Odegard RA, Vatten LJ, Nilsen ST, et al. Risk factors and clinical manifestations of pre-eclampsia. BJOG 2000;107(11):1410–6. | |
Sohlberg S, Stephansson O, Cnattingius S, et al. Maternal body mass index, height, and risks of preeclampsia. American Journal of Hypertension 2012;25(1):120–5. | |
Mbah AK, Kornosky JL, Kristensen S, et al. Super-obesity and risk for early and late pre-eclampsia. BJOG 2010;117(8):997–1004. | |
Catov JM, Ness RB, Kip KE, et al. Risk of early or severe pre-eclampsia related to pre-existing conditions. Int J Epidemiol 2007;36(2):412–9. | |
World Health Organisation. WHO recommendations on antenatal care for a positive pregnancy experience. 2016 www.who.int/reproductivehealth/publications/maternal_perinatal_health/anc-positive-pregnancy-experience/en/. [Accessed 22nd August 2018] | |
National Institute for Health and Care Excellence. Antenatal care for uncomplicated pregnancies CG62. London, UK: National Institute for Health and Care Excellence, 2019. | |
Sohlberg S, Stephansson O, Cnattingius S, et al. Maternal Body Mass Index, Height, and Risks of Preeclampsia. American Journal of Hypertension 2012;25(1):120–5. | |
Basso O, Wilcox AJ, Weinberg CR, et al. Height and risk of severe pre-eclampsia. A study within the Danish National Birth Cohort. Int J Epidemiol 2004;33(4):858–63. | |
Conde-Agudelo A, Belizan JM. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG 2000;107(1):75–83. | |
Marchi J, Berg M, Dencker A, et al. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obesity reviews: an official journal of the International Association for the Study of Obesity 2015;16(8):621–38. | |
Heslehurst N, Simpson H, Ells LJ, et al. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity 2008;9(6):635–83. | |
Poobalan AS, Aucott LS, Gurung T, et al. Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women–systematic review and meta-analysis of cohort studies. Obesity reviews: an official journal of the International Association for the Study of Obesity 2009;10(1):28–35. | |
McGuinness BJ, Trivedi AN. Maternal height as a risk factor for Caesarean section due to failure to progress in labour. The Australian & New Zealand Journal of Obstetrics & Gynaecology 1999;39(2):152–4. | |
Burke N, Burke G, Breathnach F, et al. Prediction of cesarean delivery in the term nulliparous woman: results from the prospective, multicenter Genesis study. Am J Obstet Gynecol 2017;216(6):598.e1-.e11. | |
Arendt E, Singh NS, Campbell OMR. Effect of maternal height on caesarean section and neonatal mortality rates in sub-Saharan Africa: An analysis of 34 national datasets. PLoS One 2018;13(2):e0192167. | |
Stamilio DM, Scifres CM. Extreme obesity and postcesarean maternal complications. Obstetrics and Gynecology 2014;124(2 Pt 1):227–32. | |
Corcoran S, Jackson V, Coulter-Smith S, et al. Surgical site infection after cesarean section: implementing 3 changes to improve the quality of patient care. American Journal of Infection Control 2013;41(12):1258–63. | |
McDonald SD, Han Z, Mulla S, et al. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 2010;341:c3428. | |
Torloni MR, Betrán AP, Daher S, et al. Maternal BMI and preterm birth: A systematic review of the literature with meta-analysis. The Journal of Maternal-Fetal & Neonatal Medicine 2009;22(11):957–70. | |
Han Z, Mulla S, Beyene J, et al. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol 2011;40(1):65–101. | |
Yu Z, Han S, Zhu J, et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One 2013;8(4):e61627. | |
Stothard KJ, Tennant PW, Bell R, et al. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 2009;301(6):636–50. | |
Aune D, Saugstad OD, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 2014;311(15):1536–46. | |
Chu SY, Kim SY, Lau J, et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol 2007;197(3):223–8. | |
Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Seminars in Reproductive Medicine 2011;29(6):507–13. | |
Denison FC, Aedla NR, Keag O, et al. Care of Women with Obesity in Pregnancy. BJOG: An International Journal of Obstetrics & Gynaecology 2019;126(3):e62-e106. | |
Gudmundsson S, Henningsson A-C, Lindqvist P. Correlation of birth injury with maternal height and birthweight. BJOG: An International Journal of Obstetrics & Gynaecology 2005;112(6):764–7. | |
Lange S, Probst C, Rehm J, et al. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. The Lancet Global health 2018;6(7):e769-e76. | |
Popova S, Lange S, Probst C, et al. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. The Lancet Global Health 2017;5(3):e290-e9. | |
Substance Abuse and Mental Health Services Administration: US Department of health and human services. Center for Behavioral Health Statistics and Quality Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health. (HHS Publication No. SMA 15–4927, NSDUH Series H-50). 2015. | |
Petersen Williams P, Jordaan E, Mathews C, et al. Alcohol and Other Drug Use during Pregnancy among Women Attending Midwife Obstetric Units in the Cape Metropole, South Africa. Advances in Preventive Medicine 2014;2014:871427. | |
Pineles BL, Park E, Samet JM. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. American Journal of Epidemiology 2014;179(7):807–23. | |
Shobeiri F, Masoumi SZ, Jenabi E. The association between maternal smoking and placenta abruption: a meta-analysis. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2017;30(16):1963–7. | |
Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Human Development 2007;83(11):713–20. | |
Conde-Agudelo A, Althabe F, Belizan JM, et al. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol 1999;181(4):1026–35. | |
Wei J, Liu CX, Gong TT, et al. Cigarette smoking during pregnancy and preeclampsia risk: a systematic review and meta-analysis of prospective studies. Oncotarget 2015;6(41):43667–78. | |
O’Leary CM, Nassar N, Kurinczuk JJ, et al. The effect of maternal alcohol consumption on fetal growth and preterm birth. BJOG: An International Journal of Obstetrics & Gynaecology 2009;116(3):390–400. | |
Henderson J, Gray R, Brocklehurst P. Systematic review of effects of low–moderate prenatal alcohol exposure on pregnancy outcome. BJOG: An International Journal of Obstetrics & Gynaecology 2007;114(3):243–52. | |
Addis A, Moretti ME, Ahmed Syed F, et al. Fetal effects of cocaine: an updated meta-analysis. Reproductive Toxicology (Elmsford, NY) 2001;15(4):341–69. | |
Gunn JKL, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 2016;6(4):e009986. | |
Roper V, Cox KJ. Opioid Use Disorder in Pregnancy. Journal of Midwifery & Women's Health 2017;62(3):329–40. | |
World Health Organisation. Guidelines for the identification and management of substance use and substance use disorders in pregnancy. Geneva, Switzerland, 2014. | |
Pereira PP, Da Mata FA, Figueiredo AC, et al. Maternal Active Smoking During Pregnancy and Low Birth Weight in the Americas: A Systematic Review and Meta-analysis. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco 2017;19(5):497–505. | |
Moore E, Blatt K, Chen A, et al. Relationship of trimester-specific smoking patterns and risk of preterm birth. Am J Obstet Gynecol 2016;215(1):109.e1–6. | |
Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. American Journal of Obstetrics and Gynecology 2000;182(2):465–72. | |
Ko TJ, Tsai LY, Chu LC, et al. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatrics and Neonatology 2014;55(1):20–7. | |
Soneji S, Beltran-Sanchez H. Association of Maternal Cigarette Smoking and Smoking Cessation With Preterm Birth. JAMA Network Open 2019;2(4):e192514. | |
Veisani Y, Jenabi E, Delpisheh A, et al. Effect of prenatal smoking cessation interventions on birth weight: meta-analysis. The Journal of Maternal-Fetal & Neonatal Medicine 2019;32(2):332–8. | |
Little J, Cardy A, Munger RG. Tobacco smoking and oral clefts: a meta-analysis. Bull World Health Organ 2004;82(3):213–8. | |
Werler MM, Sheehan JE, Mitchell AA. Association of vasoconstrictive exposures with risks of gastroschisis and small intestinal atresia. Epidemiology (Cambridge, Mass) 2003;14(3):349–54. | |
Zhao L, Chen L, Yang T, et al. Parental smoking and the risk of congenital heart defects in offspring: An updated meta-analysis of observational studies. European Journal of Preventive Cardiology 2019:2047487319831367. | |
Marufu TC, Ahankari A, Coleman T, et al. Maternal smoking and the risk of stillbirth: systematic review and meta-analysis. BMC Public Health 2015;15:239. | |
Pineles BL, Hsu S, Park E, et al. Systematic Review and Meta-Analyses of Perinatal Death and Maternal Exposure to Tobacco Smoke During Pregnancy. American Journal of Epidemiology 2016;184(2):87–97. | |
World Health Organisation. Prevention of harm caused by alcohol exposure in pregnancy: Rapid review and case studies from member states. 2016. | |
Flak AL, Su S, Bertrand J, et al. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta-analysis. Alcoholism, clinical and experimental research. 2014;38(1):214–26. | |
Broussard CS, Rasmussen SA, Reefhuis J, et al. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol 2011;204(4):314.e1–11. | |
Greenough AK, Z. Effects lf substance abuse during pregnancy. Current Topics and Opinions 2005;125(5):212–4. | |
Office National Statistics. Birth Summary Tables, England and Wales. 2017 https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthsummarytables. [Accessed 23rd March 2019] | |
Wang Y, Tanbo T, Abyholm T, et al. The impact of advanced maternal age and parity on obstetric and perinatal outcomes in singleton gestations. Archives of Gynecology and Obstetrics 2011;284(1):31–7. | |
United Nations. Population Division: World population prospects. 2017. https://population.un.org/wpp/DataQuery/. [Accessed 23rd March 2019] | |
Laopaiboon M, Lumbiganon P, Intarut N, et al. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG: An International Journal of Obstetrics & Gynaecology 2014;121(s1):49–56. | |
Heffner LJ. Advanced maternal age–how old is too old? The New England journal of medicine 2004;351(19):1927–9. | |
Blanc AK, Winfrey W, Ross J. New findings for maternal mortality age patterns: aggregated results for 38 countries. PLoS One 2013;8(4):e59864. | |
Stanton C, Blanc AK, Croft T, et al. Skilled care at birth in the developing world: progress to date and strategies for expanding coverage. Journal of Biosocial Science 2007;39(1):109–20. | |
Fort A KM, Abderrahim N. Postpartum care: levels and trends in the developing world. Calverton, Maryland: Macro International Inc., 2006. | |
Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PloS one 2017;12(10):e0186287-e. | |
Fuchs F, Monet B, Ducruet T, et al. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS One 2018;13(1):e0191002. | |
Bayrampour H, Heaman M. Advanced Maternal Age and the Risk of Cesarean Birth: A Systematic Review. Birth 2010;37(3):219–26. | |
Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016;387(10018):587–603. | |
Fall CH, Sachdev HS, Osmond C, et al. Association between maternal age at childbirth and child and adult outcomes in the offspring: a prospective study in five low-income and middle-income countries (COHORTS collaboration). The Lancet Global Health 2015;3(7):e366–77. | |
United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). Levels and Trends in Child Mortality: Report 2017, Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation, 2017 https://www.unicef.org/publications/files/Child_Mortality_Report_2017.pdf. [Accessed 04th December 2017] | |
UNFPA. Girlhood, not motherhood: Preventing adolescent pregnancy. 2015. https://www.unfpa.org/sites/default/files/pub-pdf/Girlhood_not_motherhood_final_web.pdf. [Accessed 23rd March 2019] | |
Neal S, Matthews Z, Frost M, et al. Childbearing in adolescents aged 12–15 years in low resource countries: a neglected issue. New estimates from demographic and household surveys in 42 countries. Acta Obstetricia et Gynecologica Scandinavica 2012;91(9):1114–8. | |
UN DESA. SDG Indicators: Global Database, 2017. https://unstats.un.org/sdgs/indicators/database/. [Accessed 23rd March 2019] | |
UNFPA. Adolescent pregnancy: A review of the evidence. 2013. https://www.unfpa.org/sites/default/files/pub-pdf/ADOLESCENT%20PREGNANCY_UNFPA.pdf. [Accessed 23rd March 2019] | |
World Health Organization. The second decade: improving adolescent health and development. Geneva: WHO, 2001. | |
UN Millennium Project. Who's got the power? Transforming health systems for women and children. 2005. | |
Stover J, Ross J. How increased contraceptive use has reduced maternal mortality. Maternal and Child Health Journal 2010;14(5):687–95. | |
Nove A, Matthews Z, Neal S, et al. Maternal mortality in adolescents compared with women of other ages: evidence from 144 countries. The Lancet Global Health 2014;2(3):e155-e64. | |
Conde-Agudelo A, Belizan JM, Lammers C. Maternal-perinatal morbidity and mortality associated with adolescent pregnancy in Latin America: Cross-sectional study. Am J Obstet Gynecol 2005;192(2):342–9. | |
Neal S, Mahendra S, Bose K, et al. The causes of maternal mortality in adolescents in low and middle income countries: a systematic review of the literature. BMC Pregnancy and Childbirth 2016;16(1):352-. | |
Loto OM, Ezechi OC, Kalu BK, et al. Poor obstetric performance of teenagers: is it age- or quality of care-related? Journal of obstetrics and gynaecology: the journal of the Institute of Obstetrics and Gynaecology 2004;24(4):395–8. | |
Raatikainen K, Heiskanen N, Verkasalo PK, et al. Good outcome of teenage pregnancies in high-quality maternity care. European Journal of Public Health 2006;16(2):157–61. | |
Owolabi OO, Wong KLM, Dennis ML, et al. Comparing the use and content of antenatal care in adolescent and older first-time mothers in 13 countries of west Africa: a cross-sectional analysis of Demographic and Health Surveys. The Lancet Child & Adolescent Health 2017;1(3):203–12. | |
Magadi MA, Agwanda AO, Obare FO. A comparative analysis of the use of maternal health services between teenagers and older mothers in sub-Saharan Africa: evidence from Demographic and Health Surveys (DHS). Soc Sci Med 2007;64(6):1311–25. | |
Ganchimeg T, Ota E, Morisaki N, et al. Pregnancy and childbirth outcomes among adolescent mothers: a World Health Organization multicountry study. BJOG 2014;121 Suppl 1:40–8. | |
Granja AC, Machungo F, Gomes A, et al. Adolescent maternal mortality in Mozambique. The Journal of adolescent health: official publication of the Society for Adolescent Medicine 2001;28(4):303–6. | |
Ganchimeg T, Mori R, Ota E, et al. Maternal and perinatal outcomes among nulliparous adolescents in low- and middle-income countries: a multi-country study. BJOG: An International Journal of Obstetrics & Gynaecology 2013;120(13):1622–30. | |
World Health Organisation. Adolescent pregnancy: a culturally complex issue. Bulletin of the World Health Organisation, 2009;87(6). [Accessed 23rd March 2019] | |
Adler AJ, Ronsmans C, Calvert C, et al. Estimating the prevalence of obstetric fistula: a systematic review and meta-analysis. BMC Pregnancy and Childbirth 2013;13(1):246. | |
World Health Organisation. Adolescent Pregnancy Factsheet. Geneva, World Health Organisation, 2018. | |
Neal S, Channon AA, Chintsanya J. The impact of young maternal age at birth on neonatal mortality: Evidence from 45 low and middle income countries. PloS One 2018;13(5):e0195731-e. | |
Althabe F, Moore JL, Gibbons L, et al. Adverse maternal and perinatal outcomes in adolescent pregnancies: The Global Network’s Maternal Newborn Health Registry study. Reproductive Health 2015;12(2):S8. | |
Finlay JE, Özaltin E, Canning D. The association of maternal age with infant mortality, child anthropometric failure, diarrhoea and anaemia for first births: evidence from 55 low- and middle-income countries. BMJ Open 2011;1(2):e000226. | |
Benova L, Neal S, Radovich EG, et al. Using three indicators to understand the parity-specific contribution of adolescent childbearing to all births. BMJ Glob Health 2018;3(6):e001059. | |
Norton M, Chandra-Mouli V, Lane C. Interventions for Preventing Unintended, Rapid Repeat Pregnancy Among Adolescents: A Review of the Evidence and Lessons From High-Quality Evaluations. Global health, Science and Practice. 2017;5(4):547–70. | |
Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Effects of birth spacing on maternal health: a systematic review. American Journal of Obstetrics and Gynecology 2007;196(4):297–308. | |
Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth Spacing and Risk of Adverse Perinatal OutcomesA Meta-analysis. JAMA 2006;295(15):1809–23. | |
Conde-Agudelo A, Rosas-Bermudez A, Castano F, et al. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann 2012;43(2):93–114. | |
Cleland J, Conde-Agudelo A, Peterson H, et al. Contraception and health. The Lancet 2012;380(9837):149–56. | |
Kozuki N, Lee AC, Silveira MF, et al. The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health 2013;13(3):S2. | |
Mor-Yosef S, Seidman DS, Samueloff A, et al. The effects of the socioeconomic status on the perinatal outcome of grand multipara. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1990;36(1–2):117–23. | |
Rutstein SOW, R. The effects of fertility behavior on child survivial and child nutritional status: evidence from the Demographic and Health Surveys. Rockville, USA: ICF International, 2014. | |
Brown W, Ahmed S, Roche N, et al. Impact of family planning programs in reducing high-risk births due to younger and older maternal age, short birth intervals, and high parity. Seminars in Perinatology 2015;39(5):338–44. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)
