This chapter should be cited as follows:
Hull ML, Nguyen TT, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.417703
The Continuous Textbook of Women’s Medicine Series – Gynecology Module
Volume 3
Endometriosis
Volume Editors:
Professor Andrew Horne, University of Edinburgh, UK
Dr Lucy Whitaker, University of Edinburgh, UK

Chapter
Management of Endometriosis-associated Infertility
First published: November 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Endometriosis afflicts 1 in 9 (11%) people assigned female at birth,1 taking an average of 6.4 years from first symptoms to diagnosis. It is associated with period pain and on average eight additional comorbid symptoms.2 The incidence of infertility is 30–50% higher for people with endometriosis, although many people with endometriosis can conceive without fertility treatment. There is also a 25–50% higher incidence of endometriosis in infertility populations.3,4
The association between endometriosis and reduced fertility is well documented. Without treatment, the monthly fecundity rate (MFR; monthly chance of conception) in endometriosis cohorts ranges from 2–10%, compared to 15–20% in healthy women.5,6 This is reflected in both lower livebirth rates (LBR; the number of livebirths per year per 1000 couples, 5.6 versus 12), and cumulative livebirth rates (CLR; the number of livebirths in 36 months per 1000 couples, 16 versus 25), when compared to a general infertility cohort. Women classified with severe (rASRM stage III and IV) endometriosis were particularly impacted, with a LBR of 1 and CLR of 5.1. While older studies did not demonstrate a significant difference in MFR between women with and without mild/moderate endometriosis,7 a prospective study in 2004 reported lower pregnancy rates over 3 years in an untreated cohort of 75 women with mild/moderate endometriosis compared to a cohort of 117 women without endometriosis (36% versus 55% respectively; P <0.05).8 Similarly, when women undertook donor sperm insemination in natural cycles (controlling for confounding by suboptimal sperm quality)9 the MFR was 3.6% in women with untreated endometriosis versus 12% in those with a normal pelvis. Finally, women with endometriosis demonstrate lower MFRs in ovulation induction (OI) and intrauterine insemination (IUI) cycles.10
In our recent online survey, insensitive fertility advice was commonly reported. This included advocating pregnancy ‘as soon as possible’, often at an inappropriate time (during teenage years) or circumstance (no current partner).11 With improved understanding of the pathophysiology of endometriosis-associated infertility, better non-invasive diagnostics for detecting endometriosis, increasing access to fertility preservation through egg and embryo freezing, and evidence of benefit from tailored, endometriosis-specific fertility treatments, medical professionals are compelled to improve fertility dialogs with endometriosis patients.12
DOES DIAGNOSIS MAKE A DIFFERENCE FOR FERTILITY OUTCOMES?
Between 43 and 63% of women with a diagnosis of unexplained infertility are subsequently found to have endometriosis when a laparoscopy is undertaken.13,14,15,16,17 In an Australian Women’s Health cohort of 1322 couples, 34.7% of women had an endometriosis diagnosis, with 65.6% diagnosed before and 34.4% after their first IVF treatment. If endometriosis was diagnosed after their first IVF cycle, women were twice as likely to have used IUI, four times more likely to have had 11–36 IVF cycles and less likely to report a birth.18 Investigating endometriosis prior to using assisted reproductive technologies (ART) optimizes treatment outcomes.
Laparoscopy is costly, difficult to access and carries surgical risk.19 Prior to 2022, keyhole surgery was considered the ‘gold standard’ and only diagnostic test for endometriosis. The diagnostic test accuracy of transvaginal ultrasound scans (TVUS) and magnetic resonance imaging (MRI) for endometriosis has improved with advances in scan technology and improved skills in gynecological imaging.20 In February 2022, the European Society for Human Reproduction and Embryology (ESHRE) strongly recommended the use of specialist endometriosis TVUS and MRIs in the diagnostic workup for endometriosis.21 Subsequently the National Institute for Health and Care Excellence (NICE)22 and Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) guidelines23 were modified to be concordant, albeit without MRI imaging being recommended as a first line diagnostic test. A caveat of all guidelines is that when a positive finding is seen, the diagnosis is highly accurate (specificity 97% and 95% with TVUS and MRI, respectively), but if endometriosis is not detected, then sensitivity is only moderate (63% and 73%, respectively),12 and endometriosis cannot be excluded. When a patient presents for fertility investigation, a non-invasive diagnostic ultrasound scan may detect endometriosis but also permits assessment of both ovarian reserve (antral follicle count) and ovarian accessibility for oocyte retrieval.
LOW OVARIAN RESERVE AND FERTILITY PRESERVATION
Early natural menopause was associated with laparoscopically diagnosed endometriosis in two large prospective cohorts. Biopsies from the cortex of ovaries containing endometrioma contain reduced numbers of oocytes compared to contralateral endometriosis-free ovaries from the same person.24,25 The follicles were smaller and markers of activated follicular recruitment and increased apoptosis were evident, suggesting follicular ‘burnout’.26 Consistent with these biological findings, women with endometriosis have lower anti-mullerian hormone (AMH) levels,27 lower antral follicle counts28 and higher day 3 follicle stimulating hormone (FSH) levels29 which are biomarkers of ovarian reserve.30,31
Endometriosis alone confers risk for low ovarian reserve, which is likely due to oxidative stress and increased apoptosis of oocytes.32 Analysis of follicular fluid composition shows high levels of reactive oxygen species (ROS), inflammatory cytokines33,34 and low levels of antioxidant enzyme activity when endometriosis is present.35,36,37 Further, granulosa cells from endometriosis patients display markers indicating high oxidative DNA damage and apoptosis. Interestingly, bovine oocytes incubated with endometriotic follicular fluid have greater disruption of meiotic spindles compared to oocytes incubated with follicular fluid from people without endometriosis or media-only control fluid.38 An inflammatory intrafollicular environment appears to contribute to oocyte damage and degeneration.
Assessment of ovarian reserve is relevant for anyone with endometriosis. It provides both information about the length of their fertility window and opportunities to preserve fertility by egg and embryo freezing. In 2013, the American Society for Reproductive Medicine (ASRM) endorsed the clinical use of egg freezing for fertility preservation.39,40 Advances in vitrification improved oocyte survival and fertilization rates when compared to slow freezing39,41,42 and led to its rapid uptake. Although egg utilization is impacted by loss at thawing and lower rates of fertilization when compared to fresh eggs, embryos created from fresh and frozen eggs have similar pregnancy rates. There is no maximal number of frozen eggs that will guarantee pregnancy and most studies report a freeze-thaw oocyte to livebirth efficiency of approximately 6%.43 In those under 36 years, the cumulative livebirth increased with every additional oocyte until a plateau at 14 mature oocytes, with a cumulative livebirth rate of 85%.44 When fertility preservation was undertaken in the presence of endometrioma, less oocytes were retrieved (5.4 ± 3.8 versus 8.1 ± 4.8).45 Thus, more than one stimulated cycle may be required to store reasonable numbers of frozen oocytes. It may not be possible financially or emotionally, for people with endometriosis and a low ovarian reserve to freeze large numbers of eggs or it may entail multiple IVF cycles. It is reasonable to discuss fertility preservation with young people after a diagnosis of endometriosis as success rates from egg freezing are highest before 36 years of age.46,47
Cobo et al. assessed the outcomes of oocyte freezing for women with endometriosis in a large retrospective cohort of 485 patients.48 Inclusion criteria included having an endometrioma of at least 1 cm and an antral follicle count of at least 3. Oocyte survival rates after thawing were high at 83.2% and there was a cumulative LBR of 46.4%. People with endometriosis had a high oocyte utilization rate of 43% compared to other cohorts and a short interval between freezing and thawing oocytes (mean 1.5 years).43 This evidence of clinical benefit supports oocyte preservation as a suitable treatment option for endometriosis.
Endometriosis confers an increased risk of infection after transvaginal oocyte retrieval,49,50,51 although this complication is rare and difficult to quantify statistically. If the ovaries are adherent to the bowel or pelvic side wall, organs and blood vessels can be damaged, whereas accessing the aberrantly located ovaries through the myometrium is unlikely to cause complications. In these situations, prophylactic antibiotic cover in oocyte preservation cycles is a sensible precaution.
Endometriosis surgery is associated with a reduction in markers of ovarian reserve,52,53 especially when excising endometriomas, high stage disease, or when oophorectomy is contemplated. Surgery for bilateral endometrioma is associated with a lower age at onset of menopause54 and a small risk (2.4%) of immediate post-surgical premature ovarian insufficiency.55 Attempts at conception can also be delayed by surgery (myomectomy, removal of hydrosalpinges, colostomy and its revision, long waiting lists). Pre-operative embryo freezing permits a planned approach where an embryo can be transferred at an appropriate later date. This approach preempts a post-surgical reduction in oocytes and maintains the high pregnancy and low miscarriage rates of the age of the patient when the embryo was created and frozen, rather than of the more advanced age when the embryo is transferred. If an embryo is not transferred in a fertility preservation cycle, a progestogen containing intrauterine device or subcutaneous, slow-release implant can be left in situ, and progesterone primed ovarian stimulation (PPOS) regimens can be utilized, thus reducing proliferation of the endometrium and endometriotic lesions.
INFLAMMATORY MEDIATORS IN PERITONEAL FLUID ALTER GAMETE FUNCTION AND EMBRYO DEVELOPMENT IN ENDOMETRIOSIS
In women, endometriotic lesions display estrogen and progesterone receptors,56,57,58 but it is not ethically possible to observe cyclical or longitudinal, visual, or histological changes with repetitive laparoscopy. Our understanding of ectopic endometrial tissue responses to reproductive hormones have thus been delineated in animal models. Baboons with spontaneous and iatrogenic endometriosis have increased levels of inflammatory leucocytes, chemokines and cytokines in their peritoneal fluid and blood.59 A cycle of tissue breakdown and repair is seen in engrafted ectopic lesions in endometriosis mouse models of endometriosis60,61 (see Figure 1 below). The main difference in steroid response between eutopic and ectopic endometrium is that in endometriosis, dying tissues and cells in ectopic lesions are unable to leave the body. The immune system is thus required to launch an inflammatory, innate immune response to phagocytose dying endometrial cells in the pelvis. When the tissue is not completely cleared chronic inflammation, fibrosis, neovascularization and neural sensitization results.62 Endometriosis patients experience these events as period pain, bloating and heavy menstrual loss.
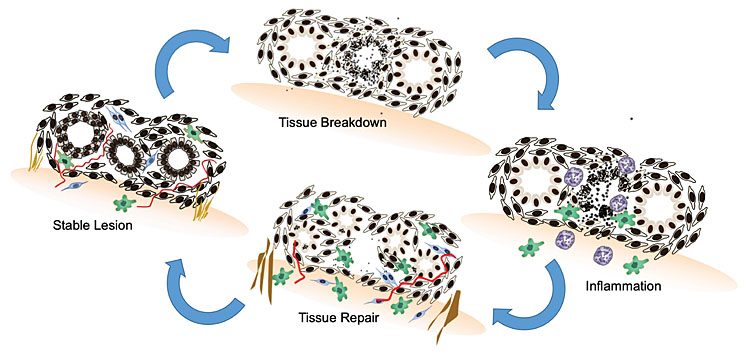
1
Endometriotic lesions undergo tissue breakdown in response to progesterone withdrawal at the end of the menstrual cycle. Neutrophils (purple) and macrophages (green) trigger an initial acute inflammatory response, phagocytosing dying endometriotic cells and tissue debris. In the estrogenic, proliferative phase of the menstrual cycle, neovascularization (red), fibroblast infiltration (blue), fibrosis (brown) and cellular proliferation characterize endometriotic tissue healing and repair.
Endometriosis-exposed peritoneal fluid has high numbers of peritoneal macrophages associated with an increased inflammatory response and activation of the NFκB pathway.63 They secrete proinflammatory chemokines and cytokines such as monocyte chemotactic protein 1 (MCP-1),64 prostaglandin E2 (PGE2),65 interleukin 1 (IL-1),66 IL-6,67 IL-10,67 tumor necrosis factor (TNF) and regulated on activation normal T cell expressed and secreted (RANTES).68,69,70,71 Markers of oxidative stress are dysregulated in the peritoneal fluid from people with endometriosis72 including glutathione peroxidase, superoxide dismutase,32 lipid peroxidase73, xanthine oxidase and catalase.74 Inflammation and oxidative stress associated with endometriotic peritoneal fluid, negatively impacts the function of gametes and embryos.
Reduced sperm motility75 and binding to fallopian tube epithelium76 were seen after exposure to peritoneal fluid from endometriotic women. In combination but not in isolation, IL-6 and IL-6R suppressed sperm motility and the number of rapidly moving sperm. Increased sperm DNA damage was also seen when sperm were incubated with endometriosis-associated peritoneal fluid for up to 3 hours.77
In a mouse model of endometriosis, the presence of ectopic endometrial lesions did not impact the number of mature MII oocytes per mouse but was associated with a lower proportion of normal oocytes (61 versus 83%) and incomplete extrusion or division of the first polar body and spindle abnormalities.78 When metaphase II mouse oocytes were incubated with peritoneal fluid from women with endometriosis, the proportion of oocytes with microtubule damage or chromosomal alterations was higher (68% and 64%, respectively) compared to control peritoneal fluid (24% and 15%) and tubal fluid groups (13% and 13%).79
Exposure to endometriosis tissue in vitro and endometriosis-like lesions in vivo also negatively impacts fertilization and embryo development. Fertilization was reduced in an in vitro co-culture system where mouse oocytes were exposed to human endometriotic stromal cells. A mouse model of endometriosis also demonstrated lower numbers of zygotes compared to control mice that were not exposed to endometriosis-like lesions.80,81
ENDOMETRIOSIS PRIMARILY INTERFERES WITH OOCYTE QUALITY
The comparative influence of endometriosis on oocyte quality and endometrial receptivity can be evaluated in human donor oocyte programs. In a case-control trial, when egg donors had rASRM III/IV endometriosis, lower pregnancy rates were observed in recipients independent of their endometriosis status. When oocytes from the same healthy egg donor were used to create embryos which were then transferred to recipients with or without endometriosis, similar implantation rates were seen.82,83,84 There is evidence that there is a reduced effect of progesterone on decidualization and endometrial receptivity85 in endometriosis. Any impact of this effect, however, appears to be secondary to the impact of endometriosis on oocyte quality.
ENDOMETRIOSIS AND INFERTILITY TREATMENTS
Apart from surgery, there are few fertility therapies that are specifically tailored for endometriosis patients. Treatment strategies have often overlapped with approaches to ‘unexplained infertility’, as people with endometriosis commonly commence fertility treatment without a diagnosis. From a biological perspective, strategies that enhance oocyte utilization and reduce an inflammatory pelvic environment are likely to be most effective.
Improving natural conception
Pursuing natural conception is an option for those with endometriosis. The inflammatory pelvic environment delays rather than prevents conception and many people with endometriosis conceive without using artificial reproductive technologies (ART). Optimizing lifestyle factors, such as reducing smoking, and marijuana use, making healthy dietary changes, increasing exercise, and limiting coffee and alcohol intake, decreases oxidative stress in cells and improves fertility outcomes. This is particularly relevant when endometriosis-derived inflammatory changes are difficult to modify. Suboptimal sperm concentration or quality will contribute to lower fertilization rates and poor embryo development and should be assessed and treated. When sperm counts are significantly low, intracytoplasmic sperm injection (ICSI) and assisted reproductive technologies (ART) should be considered as a first treatment option.
Recurrence of pain often occurs when people with endometriosis stop period-suppressing contraception and try to conceive naturally. Dysmenorrhea is the hallmark of endometriosis; however, if a complex pain syndrome develops, ovulation pain is a common sequela. Subsequent sexual dysfunction can be a barrier to successful natural conception. Women with endometriosis have a nine-fold increased risk of dyspareunia compared to the general female population.86 Pelvic floor physiotherapy (stretching of the pelvic floor muscles) or low doses of neural modulators such as amitriptyline can improve pain symptoms. Higher levels of estradiol produced by growing follicles in ovulatory or ART cycles promotes endometriosis tissue growth and can negatively impact pain symptoms over time. It is important to reassess pain throughout fertility therapy and continually reconsider surgically removing lesions or reducing time to pregnancy with in vitro fertilization (IVF) thus limiting exposure to repeated menstrual pain triggers.
Ovulation suppression is not recommended during fertility treatment
Gonadotropin-releasing hormone (GnRH) agonists, antagonists, danazol and the combined oral contraceptive (COC) pill suppress ovulation and inhibit estradiol-dependent proliferation of endometriosis. This explains why pain and disease recurrence are reduced when these medications are prescribed after excisional endometriosis surgery.87 A Cochrane library review evaluated 23 randomized controlled trials to determine whether ovulation suppression improved subsequent pregnancy rates upon cessation of these medications.88 No benefit for pregnancy was seen when ovulation suppression was used compared to placebo or no treatment (OR 0.97, 95% CI: 0.68 to 1.34, P = 0.8). Supressing ovulation did not improve natural fertility in endometriosis patients and may delay conception by reducing ovulatory cycles. Postoperative ovulation suppression is only recommended by ESHRE to improve pain management for people not desiring immediate pregnancy.21
Antioxidants to improve fertility
In endometriosis pain management, the use of antioxidants including vitamin C, vitamin E, melatonin,89 resveratrol,90 curcumin91 and co-enzyme Q10 (CoQ10) are associated with lower inflammatory markers in peritoneal fluid and animal models, and pain reduction in clinical endometriosis trials.92,93 There is evidence of improved fertility outcomes when antioxidants are used. For example, CoQ10 reduced endometriotic tissue volume and inflammatory and angiogenic markers in a rat model of endometriosis.94 Additionally, mouse oocytes that were exposed to follicular fluid from people with endometriosis in vivo, had a low maturation rate that trended to recovery when CoQ10 was used as a media supplement.95 However, there have been no randomized clinical trials assessing CoQ10’s benefit on fertility outcomes in women. Sixty-three randomized controlled trials were reviewed in a Cochrane study of antioxidant use in women attending fertility clinics, including people with endometriosis.96 The evidence was low quality and there were no studies that evaluated its effect on people with endometriosis as an independent group. Evidence that antioxidants may improve clinical pregnancy rates [odds ratio (OR) 1.65, 95% confidence interval (CI) 1.43–1.89; P <0.001, I2 = 63%; 35 RCTs, 5165 women] and with less certainty livebirth rates (OR 1.81, 95% CI: 1.36–2.43; P <0.001, I2 = 29%; 13 RCTs, 1227 women) was presented. Based on this evidence and biological plausibility, it seems reasonable to support the use of CoQ10 or other antioxidants to women with endometriosis during fertility treatment.
Tubal flushing with oil-based contrast medium (Lipiodol®)
If couples affected by endometriosis wish to conceive without IVF, fallopian tube patency should be assessed, as endometriosis-associated adhesions and anatomical distortion can disrupt tubal function and hydrosalpinges are more common in endometriosis. The brackish fluid in hydrosalpinges trickles back into the uterine cavity creating inflammation and compromising endometrial receptivity for embryos, which negatively impacts IVF conception rates.97,98 Tubal patency is assessed by flushing saline, contrast media or dye through the fallopian tubes and assessing its flow into the peritoneal cavity during a hysterosalpingogram (HSG, X-ray), hystero-salpingo contrast sonography (HyCosy, ultrasound) or laparoscopy and dye surgery. Lipiodol is an oil based, iodinated, contrast medium used for fallopian tube patency testing, which is safe in natural conception.99
Lipiodol (ethiodized oil) has a long half-life (~50 days)100 and after traveling through the fallopian tubes, may alter the pelvic environment in endometriosis. Other postulated mechanisms of action include flushing of debris from the fallopian tube, modification of peritoneal macrophage function and altered regulation of implantation-related factors in the endometrium.101 Further studies are warranted to assess the biological effect of lipiodol on the pelvic environment in people with and without endometriosis.
Oil-based contrast media (Lipiodol) was compared to water soluble media at hysterosalpingography in a large multi-centered randomized controlled trial of 2238 couples with unexplained fertility.101 In the oil-based contrast group, 220 of 554 (39.7%) women had an ongoing pregnancy compared to 161 of 554 (29.1%) women in the water group (rate ratio, 1.37; 95% CI: 1.16–1.61). Endometriosis was not evaluated in the enrolled couples, although the proportion with this condition in an unexplained fertility cohort is likely to be 40–60%.
The FLUSH [Flushing with Lipiodol for Unexplained (and endometriosis-related) Subfertility by Hysterosalpingography] study randomized 158 women with normal fallopian tubes to an HSG using lipiodol or no treatment. A subgroup analysis compared those with proven endometriosis (n = 62) to those without proven endometriosis (n = 96). In the endometriosis group, lipiodol flushing was associated with a higher 6-month pregnancy rate (48.0% vs. 10.8%, RR 4.44, 95% CI: 1.61–12.21) and live birth rate (40.0 vs. 10.8%, RR 3.70, 95% CI: 1.30–10.50) which was not seen in the group with unknown endometriosis status. A network meta-analysis that compared interventions for endometriosis related infertility showed that lipiodol led to higher odds of clinical pregnancy when compared with placebo/no intervention (OR = 7.56; 95% CI: 2.02–29.37).102 A Cochrane review concluded that flushing fallopian tubes with oil contrast media may improve the clinical pregnancy rate (OR 1.42, 95% CI: 1.10–1.85, 6 RCTs, 2598 women, I2 = 41%, low‐quality evidence), but with only three studies, there was not enough evidence to determine if the livebirth rate was affected.103
Lipiodol is generally well tolerated, although can cause cramping pain and bleeding. Extremely rare side-effects include allergy and embolism. Lipiodol contains a high dose of iodine which can lead to temporary subclinical hypothyroidism in some women. Assessment of thyroid function and appropriate thyroxine supplementation should be instituted for 6 months after use, particularly in early pregnancy.104 The evidence supports the use of lipiodol oil-based contrast in unexplained infertility and there is limited evidence of its benefit for people with endometriosis desiring fertility. It is thus reasonable to consider its use, particularly if tubal patency testing is required, for those with endometriosis who wish to conceive without using ART.
Intrauterine insemination and endometriosis
Intrauterine insemination (IUI) improves pregnancy rates by ensuring sperm are present in sufficient numbers around a recently ovulated oocyte when it is receptive to fertilization. When gonadotropins or ovulation induction agents are used, more than one follicle and higher estradiol levels may be seen. IUI can benefit couples with mild male factor defects, who struggle to have intercourse close to ovulation, who find it difficult to track ovulation or who live in different geographical locations. However, IUI does not alter the peritoneal environment in endometriosis and tubal patency is a requirement. The number of chances to fertilize an egg per month is capped at 1 or 2, due to risks of multiple pregnancy.
Randomized controlled trials that assessed IUI in endometriosis patients were conducted in the 1990s in small cohorts. In 1997, a 5.6-times higher livebirth rate was observed when 53 people with minimal to mild endometriosis were randomized to ovarian stimulation with follicle stimulating hormone (FSH) and IUI compared to 50 who received expectant management.105 FSH use significantly impacted the rate of human chorionic gonadotropin (hCG) positive pregnancies in a partially randomized study in 57 couples with minimal to mild endometriosis.106 Thirty-one couples were randomized to FSH and IUI and 15 of 127 cycles led to a positive pregnancy test (11.8%). This was 5.1 times higher than 26 couples not using FSH, where only 2 of 96 cycles resulted in pregnancy. Compared to expectant management and IUI alone, IUI with ovarian stimulation appears to improve pregnancy rate in minimal to mild endometriosis and underpins ESHRE’s recommendations that IUI may be offered to couples affected by rASRM stage I/II endometriosis.
When compared to couples with unexplained infertility, IUI and ovarian stimulation appeared less effective for people with endometriosis. The relative risk (RR) for pregnancy in unexplained fertility was 8.8 (95% CI: 1.1–71.3) compared to 5.1 (95% CI: 1.1–22.5) in the endometriosis group.106 Similarly, higher pregnancy rates after IUI and ovarian stimulation when 119 couples with unexplained infertility (33.6%) were compared to those who had endometriosis at laparoscopy (16.3%).107 The presence of lesions may influence IUI outcomes as there was no difference in pregnancy rates after FSH-IUI, when women who had surgical excision of rASRM stage I/II in the previous 6 months, were compared to those without endometriosis.
In a retrospective review of 65 patients having IUI with rASRM stage III/IV endometriosis, ovarian stimulation was associated with higher cumulative ongoing pregnancy rates (40% vs. 15.6%).108 Kim et al. compared ultralong and long GnRH agonist use before IUI in 80 women with endometriosis.109 The pregnancy rates were not influenced by GnRH agonist use with minimal/mild endometriosis, but they were increased in patients using an ultra-long downregulation GnRH protocol when higher stage endometriosis was present. The utility of IUI in rASRM III/IV endometriosis is less evident, however ESHRE supports its use, even though IVF provides a better chance of conception.109
Surgery
Excision of endometriosis removes tissue prone to breakdown and repair in response to cyclic reproductive hormones, limiting an inflammatory immune response to dying endometrial cells in the pelvic cavity. Surgical removal of endometriosis is associated with reduced concentrations of inflammatory cytokines in plasma from women with endometriosis,110 although its impact on the peritoneal environment requires exploration. In a cohort of women with endometriosis who were trying to conceive, 50% conceived naturally within 6 months after an operative laparoscopy.111
A 2020 Cochrane review assessed whether surgical treatment increased the chance of spontaneous pregnancy if endometriosis was present.112 Moderate quality evidence from three RCTs113,114,115 that included women with in rASRM stage I/II disease, showed surgical excision of endometriosis increased viable clinical pregnancy rates (OR 1.89; 95% CI: 1.25–2.86) when compared to diagnostic laparoscopy only. A similar conclusion was reached in a network meta-analysis which demonstrated an increased odds ratio for clinical pregnancy following surgical laparoscopy compared with placebo (OR 1.63; 95% CI: 1.13–2.35).102 Jin et al. 2014 reported that live birth rate was significantly increased after laparoscopic surgery for endometriosis (relative risk [RR] 1.52; 95% CI: 1.26–1.84, 4 studies; 741 patients).116 Based on this evidence the ESHRE guidelines recommend operative laparoscopy as a treatment option for infertility in rASRM stage I/II endometriosis as it improves the rate of ongoing pregnancy.
It is less clear whether surgery benefits fertility outcomes in the presence of endometriomas and deep infiltrating endometriosis, as no studies have compared conception after surgery for moderate to severe endometriosis with diagnostic laparoscopy or no treatment. There are also no comparisons between operative endometriosis surgery and IVF.112 However, expectant management of moderate to severe endometriosis was associated with very low pregnancy rates (MFR 3.2%) in a prospective cohort of 123 infertility patients with endometriosis. Conversely, pregnancy rates after surgical treatment of endometrioma were 43.8% (95% CI: 22.5–66.4) in a review of eight studies,117 whereas two reviews indicated postsurgical pregnancy rates of 37–50.5% when deep endometriosis was excised,118,119 although pregnancy rates were lower (21–28%) if the bowel was involved.118,120 With less compelling evidence, ESHRE guidelines have formulated weak recommendations that clinicians may consider operative laparoscopy for the treatment of endometrioma, and deep endometriosis associated infertility, as it may increase the chance of natural pregnancy and represents a treatment option.21,121 The ESHRE guideline development group recommended that the decision to perform surgery should be guided by the presence or absence of pain symptoms, patient age and preferences, history of previous surgery, presence of other infertility factors, ovarian reserve, and estimated endometriosis fertility index.
In vitro fertilization (IVF)
Stimulating multifollicular development with gonadotropins and retrieving oocytes preovulation for fertilization in the lab (IVF), moderates the impact of both lower oocyte numbers and the inflammatory pelvic environment for people with endometriosis. IVF also addresses fallopian tube dysfunction, sexual dysfunction and reduces time to conception compared to treatments where oocytes are left in situ. The disparity in pregnancy rates and birth outcomes seen in endometriosis when fertilization and embryo development occur in the reproductive tract, is ameliorated when ART is used.
Fertility outcomes for women with endometriosis have been compared to other infertility causes in cohorts and in large databases of reported ART outcomes. In 2016, 347,185 autologous fresh and frozen ART cycles recorded in the USA’s Society for Assisted Reproductive Technology’s (SART) national database from 2008 to 2010.122 Couples with an endometriosis diagnosis were more likely to have a canceled cycle (11.3%) than those with isolated endometriosis, tubal factor, or unexplained infertility (8.5%, 8.3%, 8.1% respectively, P <0.0001). When fresh cycles were analyzed, endometriosis was associated with a reduction in oocyte yield (RR 0.91 [0.91–0.92]), and the proportion of blastocysts transferred (RR 0.96 [0.93–0.99]).123 IVF cycles are more likely to be canceled, demonstrate a reduced response to FSH124 and have a lower number of oocytes retrieved and embryos created in endometriosis cohorts.125
Interestingly, most studies report that IVF livebirth rates for couples with rASRM stage I/II endometriosis are comparable to couples with non-endometriosis associated infertility, despite a lower number of oocytes retrieved. A large systematic review did not find a significant reduction in implantation rates (RR = 0.83, 95% CI: 0.68–1.01, P = 0.07), clinical pregnancy rates (RR = 0.94, 95% CI: 0.83–1.07, P = 0.35) or livebirth rates (RR = 0.92, 95% CI: 0.83–1.02, P = 0.10) in mild/moderate endometriosis, although fertilization rates were significantly lower (RR 0.93; 95% CI: 0.87–0.99; 7 studies; 2044 patients). A similar conclusion was reached when patients with endometriosis only were compared to other infertile groups in the SART analysis122 and in an analysis of 1749 patients from the Latin American Registry maintained by the Latin America Network of Assisted Reproduction (REDLARA).126 An explanation for this consistent finding is that, in mild/moderate endometriosis, even a smaller cohort of oocytes likely contains at least one of sufficient quality to support a term pregnancy.
In contrast, the literature reviews that compare rASRM stage III/IV endometriosis to other causes of infertility, indicate that pregnancy rates are significantly lower. Harb et al. 2016 found a lower implantation rate (RR = 0.79, 95% CI: 0.67–0.93, P = 0.006) and clinical pregnancy rate (RR = 0.79, 95% CI: 0.69–0.91, P = 0.0008) in rASRM stage III/IV disease, although the lower livebirth rate did not reach statistical significance (RR = 0.86, 95% CI: 0.68–1.08, P =0.19, 9 studies; 312 patients).127 Similarly, Hamdan and colleagues described a significantly lower clinical pregnancy rate (OR 0.60; 95% CI: 0.44–0.81; 15 studies; 9,471 patients) and live birth rate (OR 0.77; 95% CI: 0.64–0.92; 8 studies) when those with stage III/IV endometriosis were compared to those with no endometriosis.123 In a retrospective cohort analysis of 164 IVF-ET cycles in 148 women, a significantly lower pregnancy rate was seen when IVF cycles impacted by stage III/IV endometriosis were compared to stage I/II disease or tubal factor infertility (PR 9.7, 25 and 26.1%, respectively).128 There is good evidence that the stage of endometriosis is a factor to take into consideration when planning IVF with rASRM stage III/IV disease being associated with poorer IVF outcomes.
Concomitant fertility risk factors such as male factor infertility, tubal factor or diminished ovarian reserve also significantly increase the likelihood of poor IVF outcomes when endometriosis is present. In the SART database review, endometriosis in association with other reproductive pathology was associated with the lowest chance of livebirth. Live birth rates were reduced by 19–26% in fresh cycles and 12–18% reduction in frozen cycles in comparison to unexplained, tubal factor and all diagnostic groups combined. In contrast endometriosis in isolation was associated with a similar or slightly higher live birth rate compared to other infertility diagnoses.122
Surgical removal of endometriomas prior to IVF did not appear to impact pregnancy outcomes in two systematic reviews studies.123,129 Although cycle cancelation rates were higher and oocyte yield was lower in the group with endometrioma, comparable clinical pregnancy and livebirth rates were observed when compared to those without endometrioma. Surgical management of endometrioma prior to IVF did not alter pregnancy outcomes and the authors recommended individualizing care. ESHRE guidelines recommend antibiotic prophylaxis as a good practice point if endometriomas are present, although the risk of pelvic infection is low.49
Stimulation regimens
When embryos are transferred in fresh IVF cycles the luteinizing hormone (LH) surge is suppressed to prevent early ovulation, which occurs when multifollicular development induces supraphysiological estradiol levels. GnRH agonists can be used from the midluteal phase of the previous cycle to drain LH from the pituitary and prevent the LH surge. If GnRH agonist are used for up to 3 months, endometrial proliferation is suppressed, and endometriosis lesions become quiescent reducing pain symptoms when periods cease. In one small study, women with endometriosis were randomized to ultra-long 3-month downregulation with a GnRH agonist (n = 25) or a standard GnRH agonist IVF cycle (n = 26). The ultra-long downregulation demonstrated significantly higher ongoing pregnancy rates (80% vs. 53.85%) and a trend toward higher implantation rates (42.68% vs. 30.38%).130 However ultra-long downregulation with GnRH agonists may down regulate FSH receptors on granulosa cells and may suppress follicular recruitment and oocyte numbers.
GnRH antagonists block GnRH signaling to the pituitary preventing the release of LH and be used in shorter, patient friendly IVF cycles with less side effects. Benschop et al. reviewed four trials that assessed stimulation regimes on IVF outcomes in endometriosis.131 Standard GnRH agonist and GnRH antagonist treatment in women with endometriomas resulted in similar clinical pregnancy rates, but women using agonist cycles had slightly more mature oocytes retrieved (MD 1.6; 95% CI: 2.4–0.76).
A recent Cochrane review (8 studies, 640 participants) was not able to determine if ultra-long GnRH agonist cycles impacted livebirth (RR 0.48, 95% CI: 0.26–0.87) and clinical pregnancy rates (RR 0.48, 95% CI: 0.26–0.87) or mean number of oocytes (MD 0.72, 95% CI: 0.06–1.38) or embryos (MD -0.76, 95% CI: -1.33 to -0.19) as the quality of evidence was very low.132 It is important to weigh up each individual’s ovarian reserve, degree of endometriosis/adenomyosis and the possible side effects of treatment when choosing an IVF stimulation regimen.
Managing miscarriage
Several large population cohorts and linkage studies have reported an association between endometriosis and pregnancy loss;133,134 however, smaller prospective and retrospective studies have reported conflicting and uncertain results.135 A 2020 systematic review (39 studies, 697,984 women) identified a higher risk of miscarriage in women with endometriosis with a spontaneous conception (OR: 1.81, 95% CI: 1.44–2.28, I2 = 96%).136 Concordantly, a 2019 systematic review (104 studies) detected an increased risk of miscarriage (OR 1.30, 95% CI: 1.25–1.35) and other obstetric complications in endometriosis patients.125
There is some evidence that miscarriage risk is impacted by exposure of eggs and embryos to factors in the peritoneal environment when endometriosis is present. For women with endometriosis who undertook IVF (and the oocytes were taken out of the pelvic environment), the risk of miscarriage was comparable to those with tubal infertility (OR: 1.03, 95% CI: 0.92–1.14, I2 = 0%). Additionally, endometriosis, but not adenomyosis, conferred a risk of miscarriage when a small cohort of 214 of women with MRI identified endometriosis and adenomyosis were compared to those with adenomyosis alone (OR 3.2, 95% CI: 1.1–9.65). This risk was significantly higher with deep infiltrating endometriosis (OR 4.37, 95% CI: 1.32–14.53).125 It is important to continue to support women with endometriosis in the first trimester of pregnancy, and ensure their obstetrician is aware the increased risk endometriosis attributes to pregnancy.
Laboratory incubation in IVF may improve the conditions for fertilization and early embryo development. Pre-implantation screening can also be undertaken, which reduces miscarriage risk via the transfer of euploid embryos. Progesterone supplementation has been shown to reduce the risk of miscarriage risk in those with bleeding in the first trimester and a history of three previous miscarriages.137 Progesterone supplementation is commonly used in ART cycles and may especially benefit endometriosis patients, as it is theorized that the endometrium is ‘resistant’ to progesterone.85 IVF may prove to be beneficial in reducing miscarriage risk for women with endometriosis, but more research is required to establish this premise in prospective cohorts and trials.
SUMMARY (ALSO SEE FIGURE 2)
Endometriosis is associated with low ovarian reserve and reduced fertility. Specialist transvaginal ultrasound scans and MRIs improve detection of endometriosis before fertility treatment, which enhanced fertility outcomes in a large cohort of women. This information and accurate ovarian reserve testing, empowers young people with endometriosis with choices that include preserving fertility by freezing eggs.
Effective treatments for endometriosis-associated infertility optimize utilization of oocytes and reduce exposure to an inflammatory peritoneal environment. Minimizing pelvic pain and dyspareunia and reducing other causes of oxidative stress optimizes natural conception when surgery or ART is not desired. Limited research suggests flushing the fallopian tubes with oil-based contrast media (Lipiodol) may specifically benefit women with endometriosis, but the mechanism of effect needs to be elucidated.
Surgery improves spontaneous pregnancy rates in mild/moderate endometriosis, but has less impact in more severe disease. When lowering ovarian reserve is a risk, oocyte or embryo freezing can be undertaken beforehand and the embryos transferred after surgical recovery. Age, ovarian reserve, pain symptoms and patients’ preference are factors to take into consideration when contemplating surgery as a treatment option.
IVF extracts oocytes and embryos from an inflammatory peritoneal environment and utilizes oocytes efficiently. Pregnancy rates are comparable to other causes of infertility in mild and moderate stage endometriosis but decreased in late-stage disease. This is despite lower ovarian reserve, less oocytes retrieved, less mature oocytes and less embryos created in IVF cycles undertaken by women with endometriosis.
There is a paucity of evidence to support a particular preference between GnRH agonist and antagonist cycles. Progesterone primed ovarian stimulation (PPOS) has several theoretical advantages in endometriosis treatment when an embryo transfer is not contemplated, including suppressing lesion growth and minimizing menstrual loss and pain at the end of the cycle.
Finally, there is an increased risk of miscarriage in those with endometriosis and support in early pregnancy is recommended. Further research is required to determine if IVF or progesterone supplementation will reduce the risk of miscarriage.
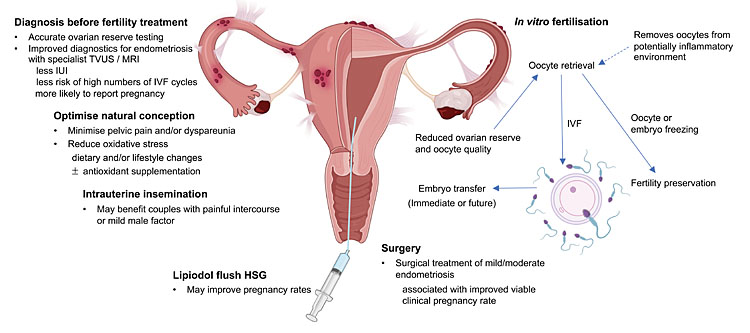
2
Summary of fertility management and current treatment options available for women with endometriosis. Created with BioRender.com.
PRACTICE RECOMMENDATIONS
- Although natural conceptions occur, endometriosis is associated with a lower monthly chance of conception.
- A diagnosis of endometriosis before fertility treatment is associated with less IUI use, a lower risk of high numbers of IVF cycles and a higher likelihood of reporting a livebirth.
- Specialist endometriosis transvaginal ultrasound and magnetic resonance imaging scans are the first line diagnostic tests, but negative scans cannot exclude endometriosis.
- Oocyte freezing is a suitable treatment for young people with endometriosis as they have a higher risk of low ovarian reserve.
- Limited evidence suggests flushing the fallopian tubes with oil-based contrast (Lipiodol) may improve natural conception rates and more research is required.
- Surgical removal of endometriosis lesions appears to improve pregnancy outcomes, but the fertility benefit is less apparent when endometriomas or severe endometriosis are present.
- In mild/moderate endometriosis, pregnancy rates from IVF are similar to other fertility patients, even though less oocytes are retrieved and less embryos created.
- Severe endometriosis is associated with lower pregnancy rates with IVF.
CONFLICTS OF INTEREST
M.L.H is founder of Adelaide-based fertility clinic, Embrace Fertility, and receives research funding for both Imagendoreg; (https://imagendo.org.au/) and EndoZone (https://www.endozone.com.au/) projects. All other contributing authors of this chapter declare that they have no interests that conflict with the contents of this chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Rowlands IJ, Abbott JA, Montgomery GW, et al. Prevalence and incidence of endometriosis in Australian women: a data linkage cohort study. BJOG 2021;128(4):657–65. https://doi.org/10.1111/1471-0528.16447. | |
Evans SF, Brooks TA, Esterman AJ, et al. The comorbidities of dysmenorrhea: a clinical survey comparing symptom profile in women with and without endometriosis. Journal of Pain Research 2018;11:3181–94. https://doi.org/10.2147/JPR.S179409. | |
Strathy JH, Molgaard CA, Coulam CB, et al. Endometriosis and infertility: A laparoscopic study of endometriosis among fertile and infertile women. Fertil Steril 1982;38:667–672. | |
Verkauf BS. Incidence, symptoms, and signs of endometriosis in fertile and infertile women. J Fla Med Assoc 1987;74:671–675. | |
Collins JA, Burrows EA, Willan AR. The prognosis for live birth among untreated infertile couples** Funded by the National Health Research and Development Program, National Department of Health and Welfare (project no. 6606-2628-44) and by contract no. 91-R-515 from the Royal Commission on New Reproductive Technology, Ottawa, Ontario, Canada. Fertility and Sterility 1995;64(1):22–8. https://doi.org/10.1016/S0015-0282(16)57650-X. | |
Schwartz D, Mayaux MJ. Female Fecundity as a Function of Age. New England Journal of Medicine 1982;306(7):404–6. https://doi.org/10.1056/nejm198202183060706. | |
Bérubé S, Marcoux S, Langevin M, et al. Fecundity of Infertile Women with Minimal or Mild Endometriosis And Women with Unexplained Infertility. Fertility and Sterility 1998;69(6):1034–41. https://doi.org/10.1016/S0015-0282(98)00081-8. | |
Akande VA, Hunt LP, Cahill DJ, et al. Differences in time to natural conception between women with unexplained infertility and infertile women with minor endometriosis. Human Reproduction 2004;19(1):96–103. https://doi.org/10.1093/humrep/deh045. | |
Jansen RPS. Minimal endometriosis and reduced fecundability: prospective evidence from an artificial insemination by donor program. Fertility and Sterility 1986;46(1):141–3. https://doi.org/10.1016/S0015-0282(16)49474-4. | |
De Hondt A, Peeraer K, Meuleman C, et al. Endometriosis and subfertility treatment: a review. Minerva Ginecol 2005;57(3):257–67. | |
Sirohi D, Freedman S, Freedman L, et al. Patient experiences of being advised by a healthcare professional to get pregnant to manage or treat endometriosis: A mixed method study. In Submission 2023. | |
Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;2(2):CD009591. https://doi.org/10.1002/14651858.CD009591.pub2. | |
KANDA Y, IKEDA M, ISHIKAWA M, et al. Laparoscopy for the treatment of unexplained infertility. Reproductive Medicine and Biology 2006;5(1):59–64. https://doi.org/10.1111/j.1447-0578.2006.00124.x. | |
Tsuji I, Ami K, Miyazaki A, et al. Benefit of Diagnostic Laparoscopy for Patients with Unexplained Infertility and Normal Hysterosalpingography Findings. The Tohoku Journal of Experimental Medicine 2009;219(1):39–42. https://doi.org/10.1620/tjem.219.39. | |
Bonneau C, Chanelles O, Sifer C, et al. Use of laparoscopy in unexplained infertility. European Journal of Obstetrics and Gynecology and Reproductive Biology 2012;163(1):57–61. https://doi.org/10.1016/j.ejogrb.2012.03.036. | |
Tanahatoe SJ, Lambalk CB, Hompes PGA. The role of laparoscopy in intrauterine insemination: a prospective randomized reallocation study. Human Reproduction 2005;20(11):3225–30. https://doi.org/10.1093/humrep/dei201. | |
Bhandari S, Singh A, Agrawal P, et al. Findings in diagnostic laparoscopy in patients with unexplained infertility [Original Article]. Fertility Science and Research 2015;2(1):29–33. https://doi.org/10.4103/2394-4285.180497. | |
Moss KM, Doust J, Homer H, et al. Delayed diagnosis of endometriosis disadvantages women in ART: a retrospective population linked data study. Human Reproduction 2021;36(12):3074–82. https://doi.org/10.1093/humrep/deab216. | |
Eisenberg VH, Decter DH, Chodick G, et al. Burden of Endometriosis: Infertility, Comorbidities, and Healthcare Resource Utilization. J Clin Med 2022;11(4). https://doi.org/10.3390/jcm11041133. | |
Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol 2016;48(3):318–32. https://doi.org/10.1002/uog.15955. | |
Becker CM, Bokor A, Heikinheimo O, et al. ESHRE guideline: endometriosis†. Human Reproduction Open 2022;2022(2). https://doi.org/10.1093/hropen/hoac009. | |
Kuznetsov L, Dworzynski K, Davies M, et al. Diagnosis and management of endometriosis: summary of NICE guidance. BMJ 2017;358:j3935. https://doi.org/10.1136/bmj.j3935. | |
RANZCOG. Australian clinical practice guideline for the diagnosis and management of endometriosis. Melbourne, Australia: WHO Press, 2021. | |
Thombre Kulkarni M, Shafrir A, Farland LV, et al. Association Between Laparoscopically Confirmed Endometriosis and Risk of Early Natural Menopause. JAMA Network Open 2022;5(1):e2144391-e2144391. https://doi.org/10.1001/jamanetworkopen.2021.44391. | |
Kitajima M, Defrère S, Dolmans M-M, et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertility and Sterility 2011;96(3):685–91. https://doi.org/10.1016/j.fertnstert.2011.06.064. | |
Kitajima M, Dolmans M-M, Donnez O, et al. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertility and Sterility 2014;101(4):1031–7. https://doi.org/10.1016/j.fertnstert.2013.12.049. | |
Romanski PA, Brady PC, Farland LV, et al. The effect of endometriosis on the antimüllerian hormone level in the infertile population. Journal of Assisted Reproduction and Genetics 2019;36(6):1179–84. https://doi.org/10.1007/s10815-019-01450-9. | |
Martyn F, O'Brien YM, Wingfield M. Review of clinical indicators, including serum anti-Müllerian hormone levels, for identification of women who should consider egg freezing. International Journal of Gynecology & Obstetrics 2017;138(1):37–41. https://doi.org/10.1002/ijgo.12167. | |
Hock DL, Sharafi K, Dagostino L, et al. Contribution of diminished ovarian reserve to hypofertility associated with endometriosis. J Reprod Med 2001;46(1):7–10. | |
Carrillo L, Seidman DS, Cittadini E, et al. The role of fertility preservation in patients with endometriosis. Journal of Assisted Reproduction and Genetics 2016;33(3):317–23. https://doi.org/10.1007/s10815-016-0646-z. | |
Feferkorn I, Suarthana E, Kigloo HN, et al. Combined effects of age and endometriosis on ovarian reserve in women with infertility. International Journal of Gynecology & Obstetrics 2023;161(1):129–36. https://doi.org/10.1002/ijgo.14519. | |
Szczepańska M, Koźlik J, Skrzypczak J, et al. Oxidative stress may be a piece in the endometriosis puzzle. Fertility and Sterility 2003;79(6):1288–93. https://doi.org/10.1016/S0015-0282(03)00266-8. | |
Nasiri N, Moini A, Eftekhari-Yazdi P, et al. Oxidative Stress Statues in Serum and Follicular Fluid of Women with Endometriosis. Cell J. Winter 2017;18(4):582–7. https://doi.org/10.22074/cellj.2016.4724. | |
Garrido N, Navarro J, Remohí J, et al. Follicular hormonal environment and embryo quality in women with endometriosis. Human Reproduction Update 2000;6(1):67–74. https://doi.org/10.1093/humupd/6.1.67. | |
Amreen S, Kumar P, Gupta P, et al. Evaluation of Oxidative Stress and Severity of Endometriosis. J Hum Reprod Sci. Jan-Mar 2019;12(1):40–6. https://doi.org/10.4103/jhrs.JHRS_27_17. | |
Prieto L, Quesada JF, Cambero O, et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertility and Sterility 2012;98(1):126–30. https://doi.org/10.1016/j.fertnstert.2012.03.052. | |
Singh AK, Chattopadhyay R, Chakravarty B, et al. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reproductive Toxicology 2013;42:116–24. https://doi.org/10.1016/j.reprotox.2013.08.005. | |
Da Broi MG, Malvezzi H, Paz CCP, et al. Follicular fluid from infertile women with mild endometriosis may compromise the meiotic spindles of bovine metaphase II oocytes. Human Reproduction 2013;29(2):315–23. https://doi.org/10.1093/humrep/det378. | |
Baldwin K, Culley L, Hudson N, et al. Reproductive technology and the life course: current debates and research in social egg freezing. Hum Fertil (Camb) 2014;17(3):170–9. https://doi.org/10.3109/14647273.2014.939723. | |
Practice Committees of the American Society for Reproductive M, the Society for Assisted Reproductive T. Mature oocyte cryopreservation: a guideline. Fertil Steril 2013;99(1):37–43. https://doi.org/10.1016/j.fertnstert.2012.09.028. | |
Rienzi L, Romano S, Albricci L, et al. Embryo development of fresh 'versus' vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod 2010;25(1):66–73. https://doi.org/10.1093/humrep/dep346. | |
Parmegiani L, Cognigni GE, Bernardi S, et al. Efficiency of aseptic open vitrification and hermetical cryostorage of human oocytes. Reprod Biomed Online 2011;23(4):505–12. https://doi.org/10.1016/j.rbmo.2011.07.003. | |
Doyle JO, Richter KS, Lim J, et al. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril 2016;105(2):459–66 e2. https://doi.org/10.1016/j.fertnstert.2015.10.026. | |
Cobo A, García-Velasco JA, Coello A, et al. Oocyte vitrification as an efficient option for elective fertility preservation. Fertility and Sterility 2016;105(3):755–64.e8. https://doi.org/10.1016/j.fertnstert.2015.11.027. | |
Kim SJ, Kim SK, Lee JR, et al. Oocyte cryopreservation for fertility preservation in women with ovarian endometriosis. Reprod Biomed Online 2020;40(6):827–34. https://doi.org/10.1016/j.rbmo.2020.01.028. | |
Gale J, Clancy AA, Claman P. Elective egg freezing for age-related fertility decline. CMAJ 2020;192(6):E142. https://doi.org/10.1503/cmaj.191191. | |
Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril 2013;100(2):492–9 e3. https://doi.org/10.1016/j.fertnstert.2013.04.023. | |
Cobo A, Giles J, Paolelli S, et al. Oocyte vitrification for fertility preservation in women with endometriosis: an observational study. Fertil Steril 2020;113(4):836–44. https://doi.org/10.1016/j.fertnstert.2019.11.017. | |
Benaglia L, Somigliana E, Iemmello R, et al. Endometrioma and oocyte retrieval-induced pelvic abscess: a clinical concern or an exceptional complication? Fertil Steril 2008;89(5):1263–6. https://doi.org/10.1016/j.fertnstert.2007.05.038. | |
Kasapoğlu I, Türk P, Dayan A, et al. Does the presence of endometriosis cause a challenge for transvaginal oocyte retrieval? A comparison between patients with and without endometriosis. J Turk Ger Gynecol Assoc 2018;19(3):151–7. https://doi.org/10.4274/jtgga.2017.0146. | |
Younis JS, Ezra Y, Laufer N, et al. Late manifestation of pelvic abscess following oocyte retrieval, for in vitro fertilization, in patients with severe endometriosis and ovarian endometriomata. J Assist Reprod Genet 1997;14(6):343–6. https://doi.org/10.1007/bf02765839. | |
Hwu Y-M, Wu FS-Y, Li S-H, et al. The impact of endometrioma and laparoscopic cystectomy on serum anti-Müllerian hormone levels. Reproductive Biology and Endocrinology 2011;9(1):80. https://doi.org/10.1186/1477-7827-9-80. | |
Streuli I, de Ziegler D, Gayet V, et al. In women with endometriosis anti-Müllerian hormone levels are decreased only in those with previous endometrioma surgery. Human Reproduction 2012;27(11):3294–303. https://doi.org/10.1093/humrep/des274. | |
Coccia ME, Rizzello F, Mariani G, et al. Ovarian surgery for bilateral endometriomas influences age at menopause. Human Reproduction 2011;26(11):3000–7. https://doi.org/10.1093/humrep/der286. | |
Busacca M, Riparini J, Somigliana E, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol 2006;195(2):421–5. https://doi.org/10.1016/j.ajog.2006.03.064. | |
Bergqvist A, Ferno M. Estrogen and progesterone receptors in endometriotic tissue and endometrium: comparison according to localization and recurrence. Fertil Steril 1993;60(1):63–8. | |
Regidor PA, Regidor M, Metz KA, et al. Immunohistochemical detection of estrogen and progesterone receptors in endometriotic tissue. A comparative study of paraffin embedded and fresh frozen tissues. Arch Gynecol Obstet 1994;255(4):181–7. https://doi.org/10.1007/BF02335083. | |
Jänne O, Kauppila A, Kokko E, et al. Estrogen and progestin receptors in endometriosis lesions: Comparison with endometrial tissue. American Journal of Obstetrics and Gynecology 1981;141(6):562–6. https://doi.org/10.1016/S0002-9378(15)33278-6. | |
D’Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Human Reproduction Update 2002;8(1):84–8. https://doi.org/10.1093/humupd/8.1.84. | |
Flores I, Rivera E, Ruiz LA, et al. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil Steril 2007;87(5):1180–99. https://doi.org/10.1016/j.fertnstert.2006.07.1550. | |
Hull ML, Escareno CR, Godsland JM, et al. Endometrial-peritoneal interactions during endometriotic lesion establishment. Am J Pathol 2008;173(3):700–15. https://doi.org/10.2353/ajpath.2008.071128. | |
Lousse JC, Van Langendonckt A, Defrere S, et al. Peritoneal endometriosis is an inflammatory disease. Front Biosci (Elite Ed) 2012;4(1):23–40. https://doi.org/10.2741/e358. | |
Ramírez-Pavez TN, Martínez-Esparza M, Ruiz-Alcaraz AJ, et al. The Role of Peritoneal Macrophages in Endometriosis. International Journal of Molecular Sciences 2021;22(19):10792. | |
Yih S, Katabuchi H, Araki M, et al. Expression of monocyte chemoattractant protein-1 in peritoneal endometriotic cells. Virchows Archiv 2001;438(1):70–7. https://doi.org/10.1007/s004280000263. | |
Sacco K, Portelli M, Pollacco J, et al. The role of prostaglandin E2 in endometriosis. Gynecological Endocrinology 2012;28(2):134–8. https://doi.org/10.3109/09513590.2011.588753. | |
Kondera-Anasz Z, Sikora J, Mielczarek-Palacz A, et al. Concentrations of interleukin (IL)-1α, IL-1 soluble receptor type II (IL-1 sRII) and IL-1 receptor antagonist (IL-1 Ra) in the peritoneal fluid and serum of infertile women with endometriosis. European Journal of Obstetrics and Gynecology and Reproductive Biology 2005;123(2):198–203. https://doi.org/10.1016/j.ejogrb.2005.04.019. | |
Punnonen J, Teisala K, Ranta H, et al. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol 1996;174(5):1522–6. https://doi.org/10.1016/s0002-9378(96)70600-2. | |
Braun DP, Gebel H, House R, et al. Spontaneous and induced synthesis of cytokines by peripheral blood monocytes in patients with endometriosis. Fertil Steril 1996;65(6):1125–9. | |
Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 01 Feb. 2002 2002;123(2):217–26. https://doi.org/10.1530/rep.0.1230217. | |
Halme J, Becker S, Hammond MG, et al. Increased activation of pelvic macrophages in infertile women with mild endometriosis. Am J Obstet Gynecol 1983;145(3):333–7. https://doi.org/10.1016/0002-9378(83)90720-2. | |
Gmyrek GB, Sieradzka U, Goluda M, et al. Flow cytometric evaluation of intracellular cytokine synthesis in peripheral mononuclear cells of women with endometriosis. Immunol Invest 2008;37(1):43–61. https://doi.org/10.1080/08820130701554962. | |
Van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril 2002;77(5):861–70. https://doi.org/10.1016/s0015-0282(02)02959-x. | |
Liu Y, Luo L, Zhao H. Levels of lipid peroxides and superoxide dismutase in peritoneal fluid of patients with endometriosis. J Tongji Med Univ 2001;21(2):166–7. https://doi.org/10.1007/BF02888087. | |
Ota H, Igarashi S, Tanaka T. Xanthine oxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil Steril 2001;75(4):785–90. https://doi.org/10.1016/s0015-0282(01)01670-3. | |
Oral E, Arici A, Olive DL, et al. Peritoneal fluid from women with moderate or severe endometriosis inhibits sperm motility: the role of seminal fluid components. Fertil Steril 1996;66(5):787–92. https://doi.org/10.1016/s0015-0282(16)58637-3. | |
Reeve L, Lashen H, Pacey AA. Endometriosis affects sperm–endosalpingeal interactions. Human Reproduction 2005;20(2):448–51. https://doi.org/10.1093/humrep/deh606. | |
Mansour G, Aziz N, Sharma R, et al. The impact of peritoneal fluid from healthy women and from women with endometriosis on sperm DNA and its relationship to the sperm deformity index. Fertil Steril 2009;92(1):61–7. https://doi.org/10.1016/j.fertnstert.2008.05.048. | |
Cohen J, Ziyyat A, Naoura I, et al. Effect of induced peritoneal endometriosis on oocyte and embryo quality in a mouse model. J Assist Reprod Genet 2015;32(2):263–70. https://doi.org/10.1007/s10815-014-0390-1. | |
Mansour G, Sharma RK, Agarwal A, et al. Endometriosis-induced alterations in mouse metaphase II oocyte microtubules and chromosomal alignment: a possible cause of infertility. Fertil Steril 2010;94(5):1894–9. https://doi.org/10.1016/j.fertnstert.2009.09.043. | |
Du YB, Gao MZ, Shi Y, et al. Endocrine and inflammatory factors and endometriosis-associated infertility in assisted reproduction techniques. Arch Gynecol Obstet 2013;287(1):123–30. https://doi.org/10.1007/s00404-012-2567-0. | |
Furukubo M, Fujino Y, Umesaki N, et al. Effects of endometrial stromal cells and peritoneal fluid on fertility associated with endometriosis. Osaka City Med J 1998;44(1):43–54. | |
Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril 2019;111(4):611–7. https://doi.org/10.1016/j.fertnstert.2019.02.009. | |
Diaz I, Navarro J, Blasco L, et al. Impact of stage III-IV endometriosis on recipients of sibling oocytes: matched case-control study. Fertil Steril 2000;74(1):31–4. https://doi.org/10.1016/s0015-0282(00)00570-7. | |
Simon C, Gutierrez A, Vidal A, et al. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum Reprod 1994;9(4):725–9. https://doi.org/10.1093/oxfordjournals.humrep.a138578. | |
Zhang P, Wang G. Progesterone Resistance in Endometriosis: Current Evidence and Putative Mechanisms. Int J Mol Sci 2023;24(8). https://doi.org/10.3390/ijms24086992. | |
Ballard KD, Seaman HE, de Vries CS, et al. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study–Part 1. BJOG 2008;115(11):1382–91. https://doi.org/10.1111/j.1471-0528.2008.01878.x. | |
Chen I, Veth VB, Choudhry AJ, et al. Pre- and postsurgical medical therapy for endometriosis surgery. Cochrane Database Syst Rev 2020;11(11):CD003678. https://doi.org/10.1002/14651858.CD003678.pub3. | |
Hughes E, Brown J, Collins JJ, et al. Ovulation suppression for endometriosis. Cochrane Database Syst Rev 2007;2007(3):CD000155. https://doi.org/10.1002/14651858.CD000155.pub2. | |
Schwertner A, Conceicao Dos Santos CC, Costa GD, et al. Efficacy of melatonin in the treatment of endometriosis: a phase II, randomized, double-blind, placebo-controlled trial. Pain 2013;154(6):874–81. https://doi.org/10.1016/j.pain.2013.02.025. | |
Dull AM, Moga MA, Dimienescu OG, et al. Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways. Molecules 2019;24(4). https://doi.org/10.3390/molecules24040667. | |
Vallee A, Lecarpentier Y. Curcumin and Endometriosis. Int J Mol Sci 2020;21(7). https://doi.org/10.3390/ijms21072440. | |
Amini L, Chekini R, Nateghi MR, et al. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res Manag 2021;2021:5529741. https://doi.org/10.1155/2021/5529741. | |
Santanam N, Kavtaradze N, Murphy A, et al. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl Res 2013;161(3):189–95. https://doi.org/10.1016/j.trsl.2012.05.001. | |
Akarca-Dizakar SO, Demirel MA, Coskun Akcay N, et al. The therapeutic effects of coenzyme Q10 on surgically induced endometriosis in Sprague Dawley rats. J Obstet Gynaecol 2022;42(7):3290–8. https://doi.org/10.1080/01443615.2022.2114322. | |
Romero S, Pella R, Zorrilla I, et al. Coenzyme Q10 improves the in vitro maturation of oocytes exposed to the intrafollicular environment of patients on fertility treatment. JBRA Assist Reprod 2020;24(3):283–8. https://doi.org/10.5935/1518-0557.20200003. | |
Showell MG, Mackenzie-Proctor R, Jordan V, et al. Antioxidants for female subfertility. Cochrane Database Syst Rev 2020;8(8):CD007807. https://doi.org/10.1002/14651858.CD007807.pub4. | |
Strandell A, Lindhard A, Waldenstrom U, et al. Hydrosalpinx and IVF outcome: a prospective, randomized multicentre trial in Scandinavia on salpingectomy prior to IVF. Hum Reprod 1999;14(11):2762–9. https://doi.org/10.1093/humrep/14.11.2762. | |
Blazar AS, Hogan JW, Seifer DB, et al. The impact of hydrosalpinx on successful pregnancy in tubal factor infertility treated by in vitro fertilization. Fertil Steril 1997;67(3):517–20. https://doi.org/10.1016/s0015-0282(97)80079-9. | |
Johnson NP, Farquhar CM, Hadden WE, et al. The FLUSH trial–flushing with lipiodol for unexplained (and endometriosis-related) subfertility by hysterosalpingography: a randomized trial. Hum Reprod 2004;19(9):2043–51. https://doi.org/10.1093/humrep/deh418. | |
Miyamoto Y, Tsujimoto T, Iwai K, et al. Safety and pharmacokinetics of iotrolan in hysterosalpingography. Retention and irritability compared with Lipiodol. Invest Radiol 1995;30(9):538–43. https://doi.org/10.1097/00004424-199509000-00005. | |
Dreyer K, van Rijswijk J, Mijatovic V, et al. Oil-Based or Water-Based Contrast for Hysterosalpingography in Infertile Women. N Engl J Med 2017;376(21):2043–52. https://doi.org/10.1056/NEJMoa1612337. | |
Hodgson RM, Lee HL, Wang R, et al. Interventions for endometriosis-related infertility: a systematic review and network meta-analysis. Fertil Steril 2020;113(2):374–82 e2. https://doi.org/10.1016/j.fertnstert.2019.09.031. | |
Wang R, Watson A, Johnson N, et al. Tubal flushing for subfertility. Cochrane Database Syst Rev 2020;10(10):CD003718. https://doi.org/10.1002/14651858.CD003718.pub5. | |
Glanville EJ, Venetis C, Boothroyd CA, et al. The use of oil-soluble contrast media for tubal flushing in infertility: A consensus statement from ACCEPT (Australasian CREI Consensus Expert Panel on Trial evidence). Aust N Z J Obstet Gynaecol 2020;60(5):667–70. https://doi.org/10.1111/ajo.13222. | |
Tummon IS, Asher LJ, Martin JS, et al. Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertil Steril 1997;68(1):8–12. https://doi.org/10.1016/s0015-0282(97)81467-7. | |
Nulsen JC, Walsh S, Dumez S, et al. A randomized and longitudinal study of human menopausal gonadotropin with intrauterine insemination in the treatment of infertility. Obstet Gynecol 1993;82(5):780–6. | |
Omland AK, Tanbo T, Dale PO, et al. Artificial insemination by husband in unexplained infertility compared with infertility associated with peritoneal endometriosis. Hum Reprod 1998;13(9):2602–5. https://doi.org/10.1093/humrep/13.9.2602. | |
van der Houwen LE, Schreurs AM, Schats R, et al. Efficacy and safety of intrauterine insemination in patients with moderate-to-severe endometriosis. Reprod Biomed Online 2014;28(5):590–8. https://doi.org/10.1016/j.rbmo.2014.01.005. | |
Kim CH, Cho YK, Mok JE. Simplified ultralong protocol of gonadotrophin-releasing hormone agonist for ovulation induction with intrauterine insemination in patients with endometriosis. Hum Reprod 1996;11(2):398–402. https://doi.org/10.1093/humrep/11.2.398. | |
Monsanto SP, Edwards AK, Zhou J, et al. Surgical removal of endometriotic lesions alters local and systemic proinflammatory cytokines in endometriosis patients. Fertil Steril 2016;105(4):968–77 e5. https://doi.org/10.1016/j.fertnstert.2015.11.047. | |
Abbott J, Hawe J, Hunter D, et al. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril 2004;82(4):878–84. https://doi.org/10.1016/j.fertnstert.2004.03.046. | |
Bafort C, Beebeejaun Y, Tomassetti C, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev 2020;10(10):CD011031. https://doi.org/10.1002/14651858.CD011031.pub3. | |
Moini A, Bahar L, Ashrafinia M, et al. Fertility Outcome after Operative Laparoscopy versus No Treatment in Infertile Women with Minimal or Mild Endometriosis. Int J Fertil Steril 2012;5(4):235–40. | |
Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med 1997;337(4):217–22. https://doi.org/10.1056/NEJM199707243370401. | |
Gad MS, Badroui MHH. Evidence-based therapy for infertility associated with early stage endometriosis [Journal article]. International journal of gynaecology and obstetrics 2012;119:S548. | |
Jin X, Ruiz Beguerie J. Laparoscopic surgery for subfertility related to endometriosis: a meta-analysis. Taiwan J Obstet Gynecol 2014;53(3):303–8. https://doi.org/10.1016/j.tjog.2013.02.004. | |
Alborzi S, Zahiri Sorouri Z, Askari E, et al. The success of various endometrioma treatments in infertility: A systematic review and meta-analysis of prospective studies. Reprod Med Biol 2019;18(4):312–22. https://doi.org/10.1002/rmb2.12286. | |
Iversen ML, Seyer-Hansen M, Forman A. Does surgery for deep infiltrating bowel endometriosis improve fertility? A systematic review. Acta Obstet Gynecol Scand 2017;96(6):688–93. https://doi.org/10.1111/aogs.13152. | |
Meuleman C, Tomassetti C, D'Hoore A, et al. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update. May-Jun 2011;17(3):311–26. https://doi.org/10.1093/humupd/dmq057. | |
Cohen J, Thomin A, Mathieu D'Argent E, et al. Fertility before and after surgery for deep infiltrating endometriosis with and without bowel involvement: a literature review. Minerva Ginecol 2014;66(6):575–87. | |
Olive DL, Stohs GF, Metzger DA, et al. Expectant management and hydrotubations in the treatment of endometriosis-associated infertility. Fertil Steril 1985;44(1):35–41. https://doi.org/10.1016/s0015-0282(16)48674-7. | |
Senapati S, Sammel MD, Morse C, et al. Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the Society for Assisted Reproductive Technologies Database. Fertil Steril 2016;106(1):164–71 e1. https://doi.org/10.1016/j.fertnstert.2016.03.037. | |
Hamdan M, Omar SZ, Dunselman G, et al. Influence of endometriosis on assisted reproductive technology outcomes: a systematic review and meta-analysis. Obstet Gynecol 2015;125(1):79–88. https://doi.org/10.1097/AOG.0000000000000592. | |
Al-Fadhli R, Kelly SM, Tulandi T, et al. Effects of different stages of endometriosis on the outcome of in vitro fertilization. J Obstet Gynaecol Can 2006;28(10):888–91. https://doi.org/10.1016/S1701-2163(16)32285-X. | |
Horton J, Sterrenburg M, Lane S, et al. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: a systematic review and meta-analysis. Hum Reprod Update 2019;25(5):592–632. https://doi.org/10.1093/humupd/dmz012. | |
Murta M, Machado RC, Zegers-Hochschild F, et al. Endometriosis does not affect live birth rates of patients submitted to assisted reproduction techniques: analysis of the Latin American Network Registry database from 1995 to 2011. J Assist Reprod Genet 2018;35(8):1395–9. https://doi.org/10.1007/s10815-018-1214-5. | |
Harb H, Gallos I, Chu J, et al. The effect of endometriosis on in vitro fertilisation outcome: a systematic review and meta-analysis. BJOG: An International Journal of Obstetrics & Gynaecology 2013;120(11):1308–20. https://doi.org/10.1111/1471-0528.12366. | |
Coccia ME, Rizzello F, Mariani G, et al. Impact of endometriosis on in vitro fertilization and embryo transfer cycles in young women: a stage-dependent interference. Acta Obstet Gynecol Scand 2011;90(11):1232–8. https://doi.org/10.1111/j.1600-0412.2011.01247.x. | |
Alshehre SM, Narice BF, Fenwick MA, et al. The impact of endometrioma on in vitro fertilisation/intra-cytoplasmic injection IVF/ICSI reproductive outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet 2021;303(1):3–16. https://doi.org/10.1007/s00404-020-05796-9. | |
Surrey ES, Silverberg KM, Surrey MW, et al. Effect of prolonged gonadotropin-releasing hormone agonist therapy on the outcome of in vitro fertilization-embryo transfer in patients with endometriosis. Fertil Steril 2002;78(4):699–704. https://doi.org/10.1016/s0015-0282(02)03373-3. | |
Benschop L, Farquhar C, van der Poel N, et al. Interventions for women with endometrioma prior to assisted reproductive technology. Cochrane Database Syst Rev 2010;(11):CD008571. https://doi.org/10.1002/14651858.CD008571.pub2. | |
Georgiou EX, Melo P, Baker PE, et al. Long‐term GnRH agonist therapy before in vitro fertilisation (IVF) for improving fertility outcomes in women with endometriosis. Cochrane Database of Systematic Reviews 2019;(11). https://doi.org/10.1002/14651858.CD013240.pub2. | |
Saraswat L, Ayansina DT, Cooper KG, et al. Pregnancy outcomes in women with endometriosis: a national record linkage study. BJOG 2017;124(3):444–52. https://doi.org/10.1111/1471-0528.13920. | |
Boje AD, Egerup P, Westergaard D, et al. Endometriosis is associated with pregnancy loss: a nationwide historical cohort study. Fertil Steril 2023;119(5):826–35. https://doi.org/10.1016/j.fertnstert.2022.12.042. | |
Leone Roberti Maggiore U, Ferrero S, Mangili G, et al. A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes. Hum Reprod Update 2016;22(1):70–103. https://doi.org/10.1093/humupd/dmv045. | |
Huang Y, Zhao X, Chen Y, et al. Miscarriage on Endometriosis and Adenomyosis in Women by Assisted Reproductive Technology or with Spontaneous Conception: A Systematic Review and Meta-Analysis. Biomed Res Int 2020;2020:4381346. https://doi.org/10.1155/2020/4381346. | |
Coomarasamy A, Harb HM, Devall AJ, et al. Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT. Health Technol Assess 2020;24(33):1–70. https://doi.org/10.3310/hta24330. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
