This chapter should be cited as follows:
Couck I, Lewi L, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.415003
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 4
Fetal development and maternal adaptation
Volume Editor: Professor Asma Khalil, The Royal College of Obstetricians and Gynaecologists, London, UK; Fetal Medicine Unit, Department of Obstetrics and Gynaecology, St George’s University Hospitals NHS Foundation Trust, London, UK

Chapter
The Fetoplacental Circulation in Twin Pregnancies and Clinical Correlates
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Twin pregnancies, in general, have higher loss rates and morbidity compared to singleton pregnancies because of their increased risk of preterm birth, growth restriction, and pre-eclampsia. Monochorionic twins face the highest risks as their unique placental vascular anastomoses may lead to transfusion imbalances and make their well-being interrelated. In this chapter, the typical placental findings in twin pregnancies are discussed. We describe how to distinguish the monochorionic from the dichorionic placenta, the typical pathology findings in common twin complications, and the anomalies more frequently encountered in twins. Further, we describe how to document the vascular anastomoses in monochorionic placentas and their relationship to the typical monochorionic complications.
DISTINGUISHING MONOCHORIONIC FROM DICHORIONIC PLACENTAS BY EXAMINING THE INTERTWIN SEPTUM
Monochorionic twins are monozygotic and share a single placenta. About one in five twin pregnancies has a monochorionic placenta,1 and in the overall majority, placental vascular anastomoses connect the circulation of both twins.2 In about 15%, an imbalance in intertwin blood exchange occurs, leading to complications, such as the twin–twin transfusion syndrome or twin anemia polycythemia sequence.3 Monochorionic twin pregnancies are thus high-risk pregnancies that merit 2-weekly sonographic follow-up to ensure timely detection of any transfusion imbalances.4,5,6
We identify monochorionic diamniotic twin pregnancies at the first-trimester ultrasound scan between 12 and 14 weeks by assessing the membranes that separate the twins. In monochorionic twin pregnancies, only two thin amniotic membranes (amnion-amnion) separate the twins, and the yolk sacs are in a common exocoelomic cavity. The intertwin septum inserts on the placental disk, forming an empty lambda (early first trimester) or T-sign (late first trimester) (Figure 1). After birth, we also confirm the chorionicity by assessment of the intertwin septum. In the monochorionic placenta, the intertwin septum is translucent and consists of two amniotic membranes that we can easily separate and remove from the placental disk (Figure 2). In about 5% of monochorionic twin pregnancies, the twins also share a common amniotic cavity (Figure 3). In these monoamniotic twin pregnancies, there is no intertwin septum.
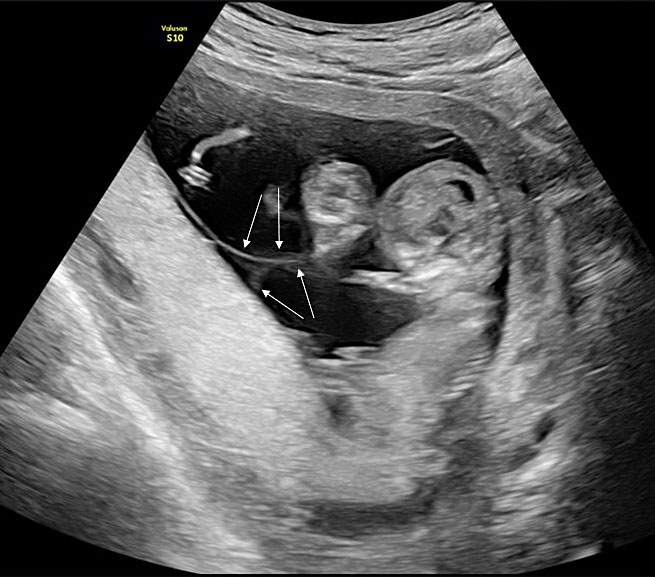
1
Ultrasound image of a monochorionic diamniotic twin pregnancy at 12 weeks. The intertwin membrane inserts on the placental disk as an empty lambda sign formed by the apposition of the two thin amniotic membranes (thin arrows) that separate the twins. The twins are, thus, separated by two layers (amnion-amnion).
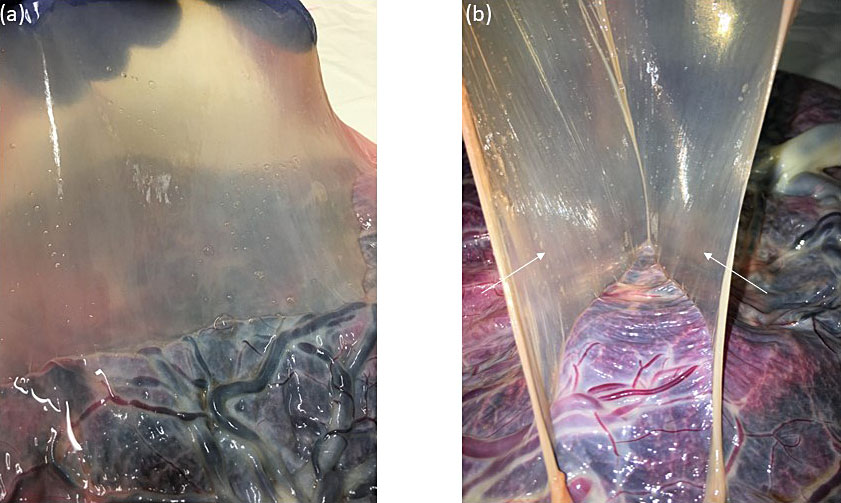
2
Macroscopic image of the intertwin septum of a monochorionic diamniotic twin pregnancy. (a) The intertwin septum is translucent; (b) The intertwin septum consists of two amniotic membranes that can be easily separated and removed from the placental disk (thin arrows)
3
Ultrasound image of a monochorionic monoamniotic twin pregnancy at 11 weeks. (a) Both twins move freely within the same amniotic cavity, and a single amniotic membrane (thin arrows) encircles both twins. (b) Typically, the cords are entangled already in the first trimester.
In dichorionic twin pregnancies, each twin has its own placenta, and vascular anastomoses are absent. Dizygotic twins always have a dichorionic placenta, whereas one-third of monozygotic twins have a dichorionic placenta, and two-thirds have a monochorionic placenta. Dichorionic twins are not connected to one another, and so they are not at risk of intertwin transfusion imbalances. Dichorionic twins always have separate amniotic sacs.
At the first-trimester scan, the intertwin septum consists of a thick chorionic membrane that is flanked on each side by two thin amniotic layers (amnion-chorion-amnion). The yolk sacs are in separate amniotic cavities and the intertwin septum inserts on the placental disk, forming a full lambda sign or twin peak sign formed by the triangular extension of chorion frondosum into the base of the intertwin membrane (Figure 4). After birth, the intertwin septum of the dichorionic twin placenta is thick and opaque with linear streaks running through it, representing atrophied chorionic vessels. We can separate the two thin amniotic layers on each side of the chorionic layer and remove them. However, we cannot remove the chorionic layer, which is firmly attached to the placental disk (Figure 5). So, it is the intertwin septum that determines the chorionicity before as well as after birth.
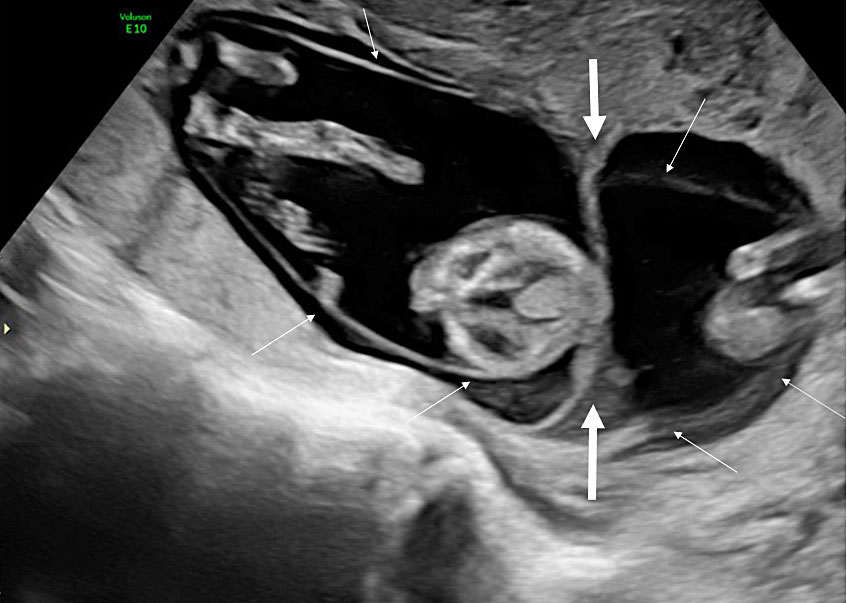
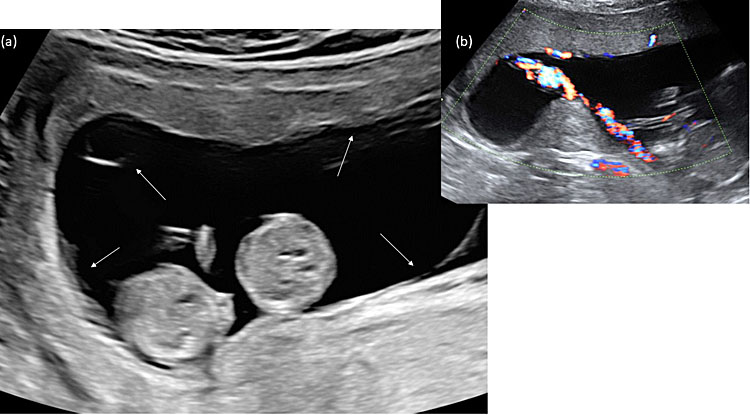
4
Ultrasound image of a dichorionic twin pregnancy at 13 weeks. The chorionic layer of the intertwin septum inserts on the placental disk forming a full lambda or twin peak sign (thick arrows). The two thin amniotic membranes (thin arrows) can be visualized separately on each side of the septum. The twins are thus separated by three layers (amnion-chorion-amnion).
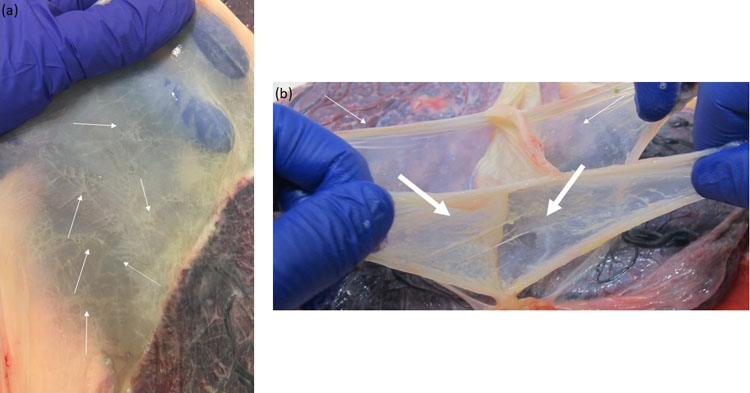
5
Macroscopic image of the intertwin septum of a dichorionic twin pregnancy. (a) The intertwin septum is opaque with linear streaks representing atrophied chorionic vessels (thin arrows); (b) The intertwin septum consists of two amniotic membranes (thin arrows) that we can easily remove. In contrast, the two chorionic layers (thick arrows) in the middle cannot be removed and are firmly attached to the placental disk.
Histology of the intertwin membrane further confirms the amnion-amnion and amnion-chorion-amnion configuration of the monochorionic diamniotic and dichorionic placentation (Figure 6). Looking at the number of placental disks is an unreliable way to determine the chorionicity because dichorionic placentas commonly fuse, and monochorionic placentas rarely consist of two separate lobes (Figure 7)7 with velamentous anastomoses running between these lobes.
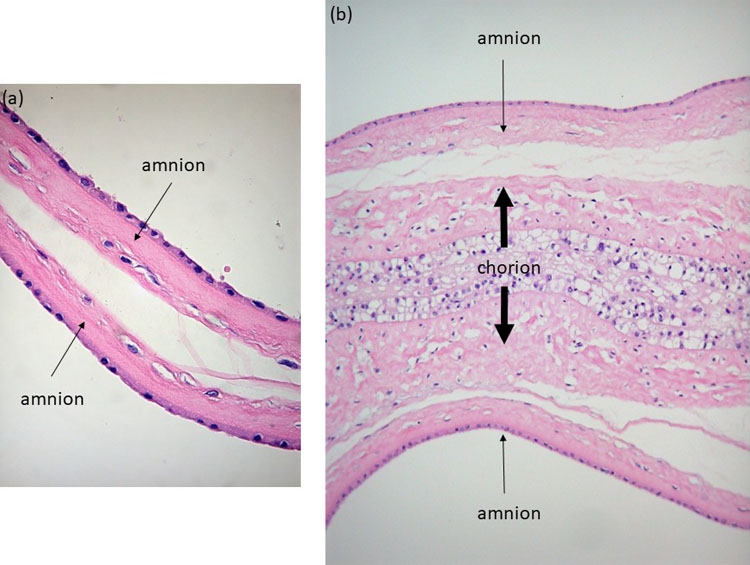
6
(a) Histologic image of a monochorionic diamniotic intertwin septum: only amnion-amnion is present (thin arrows); (b) Histologic image of a dichorionic intertwin septum: the two amniotic layers (thin arrows) are separated by a thick chorionic layer (thick arrow).
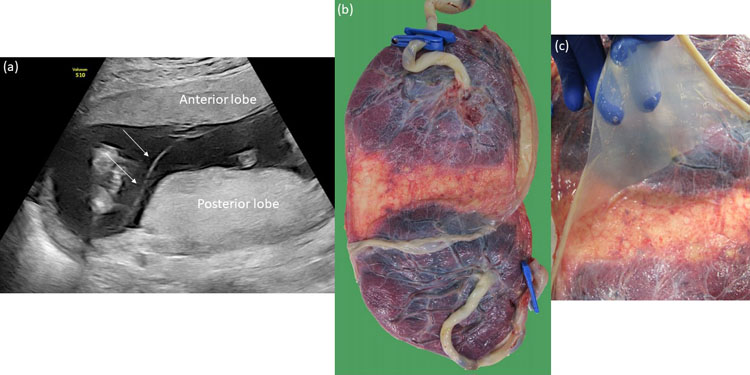
7
(a) Ultrasound image of a bilobar monochorionic diamniotic placenta with an anterior and posterior lobe at 16 weeks. The pregnancy was initially thought to be dichorionic because of the two separate placental masses. (b) and (c) After birth, the placenta was confirmed to be a bilobar monochorionic placenta. There were no anastomoses between the two parts.
It is important to note that mixed placentation may occur where part of the septum is diamniotic, and part is dichorionic (partial monochorionic twins) or where only a part of the septum is formed (partial monoamniotic twins).8 Partial monochorionic twin placentas may have vascular anastomoses, and in partial monoamniotic twins, the umbilical cords may entangle, so antenatal surveillance should be similar to that for monochorionic and monoamniotic twin pregnancies, respectively. Also, rarely dizygotic twins may have a monochorionic placentation, and thus, boy–girl pairs with a monochorionic placenta have been reported.9
THE GENERAL PATHOLOGIC EXAMINATION OF TWIN PLACENTAS
Twin pregnancies have higher risks of pregnancy complications than singleton pregnancies. Preterm birth, intrauterine growth restriction and pre-eclampsia occur more frequently in twins compared to singletons4 and the placentas are therefore more commonly sent for pathology. The pathology exam of a twin placenta is somewhat similar to the examination of two singleton placentas. As such, the individual part of each twin is assessed separately using a standardized approach.10
Ideally, the cord of the first-born twin will be labeled by a single clamp. If the cords are not marked, inspection of the gestational sacs usually allows for the correct identification of the cords, as the cord insertion of the first twin is accessible from the defect in the gestational sac, whereas the insertion of the second-born twin will be accessible from the hole in the intertwin membrane.
In dichorionic twin pregnancies, the placental part of each twin is easily identified by the dichorionic septum that cannot be removed from the placental disk. In monochorionic twin pregnancies, the placental territory of each twin is delineated along the vascular equator where the anastomoses meet, although this slightly overestimates the degree of discordance11 (Figure 8).
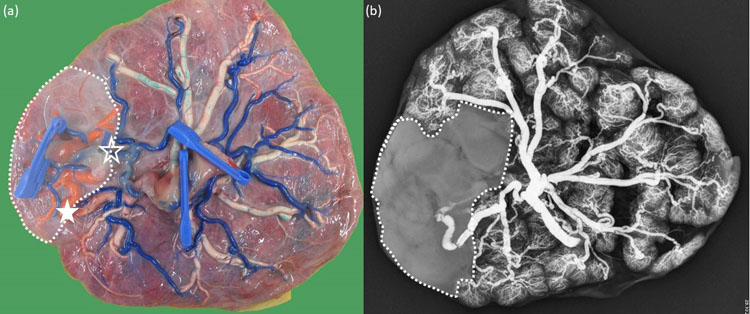
8
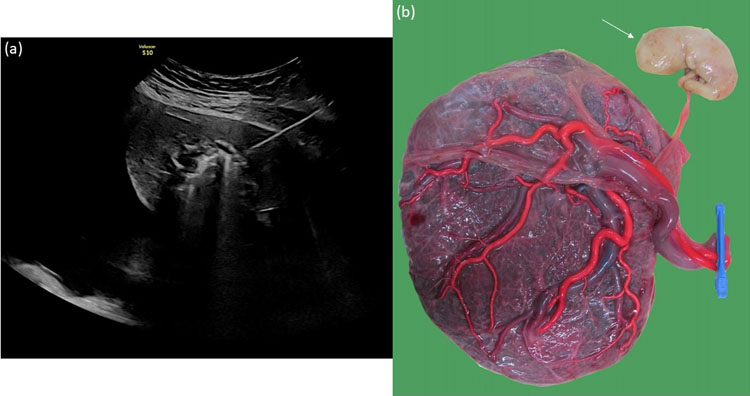
(a) Macroscopic image of a monochorionic diamniotic twin placenta with type III discordant growth. The chorionic vessels are injected with dye, and the amniotic membranes are removed. The babies were born at 32 weeks. Twin 1 (1 clamp) was the smaller twin with the smaller share and little individual territory, as nearly all vessels are part of an anastomosis. There is large arterio-arterial anastomosis (empty star) and venovenous anastomosis (full star) present. The arteries are blue, the veins of twin 1 are red, and of twin 2 are white. The territory of twin 1 is indicated with a dotted line. (b) X-ray image of the same placenta after injection of the veins of twin 1 with barium sulfate. Twin 1 has a larger venous territory than is suggested on the macroscopic image, which slightly overestimates the degree of discordance.
Preterm birth is the most important contributor to mortality and morbidity in twins. The incidence of birth before 28 weeks, before 32 weeks and before 37 weeks is about ten times higher in twins than in singletons: 4% versus 0.4%; 10% versus 1%, and 60% versus 6%, respectively.12 While the placenta may not show any specific findings and preterm birth in twins may be related to the increased mass, a detailed placental examination is indicated to exclude chorioamnionitis, which may affect each twin differently.13 Due to the ascending nature of the infection, the placenta and membranes of the presenting twin may show a more pronounced inflammatory response. This differential impact appears to be more common in dichorionic compared to monochorionic twins. In advanced chorioamnionitis (involving the umbilical cord as well), non-presenting twins from dichorionic pairs with separate placentas have a lower risk of being infected, whereas monochorionic twins are more likely to be affected equally.
Growth restriction is also more common in twin pregnancies as the human uterus is ill-equipped to nurture two babies. As such, one in four twins has a birth weight below the 10th centile using singletons growth charts.14 Typically, one twin becomes growth restricted, whereas the other continues to grow normally, leading to discordant growth. A difference in birth weight of more than 25% occurs in about 10% of mono- and dichorionic twin pregnancies.15
As most dichorionic twins are dizygotic, discordant growth may be due to differences in their genetic make-up, without any underlying pathology. However, impaired function of the placenta of one twin can also explain why growth restriction affects only one twin. A large prospective study showed that placental histological lesions are more common in smaller twins of a discordant dichorionic pair, compared to the larger twins and normal controls.16 Rarely, discordance for chromosomal anomalies and genetic syndromes may cause differences in growth. Congenital infections, like cytomegalovirus, can also affect only one twin of a dichorionic pair, resulting in growth restriction of the affected fetus.17
In monochorionic placentas with discordant growth, the number of histologic lesions does not differ between the placental parts of the smaller and larger twin16 as differences in growth are mostly related to an unequal distribution of the placenta or imbalances in blood exchange, as discussed below.
The incidence of pre-eclampsia before 37 weeks is also ten times higher in twin than in singleton pregnancies (6% versus 0.6%).18 However, maternal and fetal vascular malperfusion lesions are less frequently seen in twins than in singletons, suggesting that other mechanisms play a role in twin pre-eclampsia.19
PATHOLOGIES THAT ARE MORE COMMON IN TWIN PREGNANCIES
Velamentous cord insertion
Twins are three times more likely to have a velamentous cord insertion than singletons. A velamentous cord is especially more prevalent in monochorionic (35%) as compared to dichorionic (8%) twins.20 Also, subfertility treatment increases the risk of a velamentous insertion in dizygotic twins.21
In dichorionic twins, a velamentous insertion inserts either directly onto the uterine wall or on the intertwin septum. In contrast, a velamentous insertion in monochorionic twins always inserts on the uterine wall and never on the intertwin septum, which lacks the chorionic layer. Therefore, in a dichorionic twin pregnancy, a velamentous insertion on the intertwin septum must always be ruled out. If the cord of the second twin inserts on the septum, then artificial rupture of the septum can cause acute exsanguination during birth. About one in 25 velamentous insertions is accompanied by vasa previa,22 which are also more common in twin pregnancies and must be ruled out at the time of the second-trimester scan. Postnatal placental evaluation should include careful inspection of any velamentous vessels for evidence of disruption and their relationship to the site of membrane rupture.
In monochorionic twins, a velamentous insertion also increases the risk of growth restriction and intrauterine demise, whereas no such association exists in dichorionic twins.16,20,23 Sato and Benirschke observed an increased rate of fetal vessel thrombosis in monochorionic twin placentas, especially in the presence of a velamentous cord insertion. Together with unequal sharing, fetal vessel thromboses may contribute to growth restriction and intrauterine demise.24 In monochorionic twins, a velamentous insertion also increases the risk of twin–twin transfusion syndrome. In the overall majority, it is the growth-restricted twin or donor twin who has a velamentous insertion23 (Figure 9).
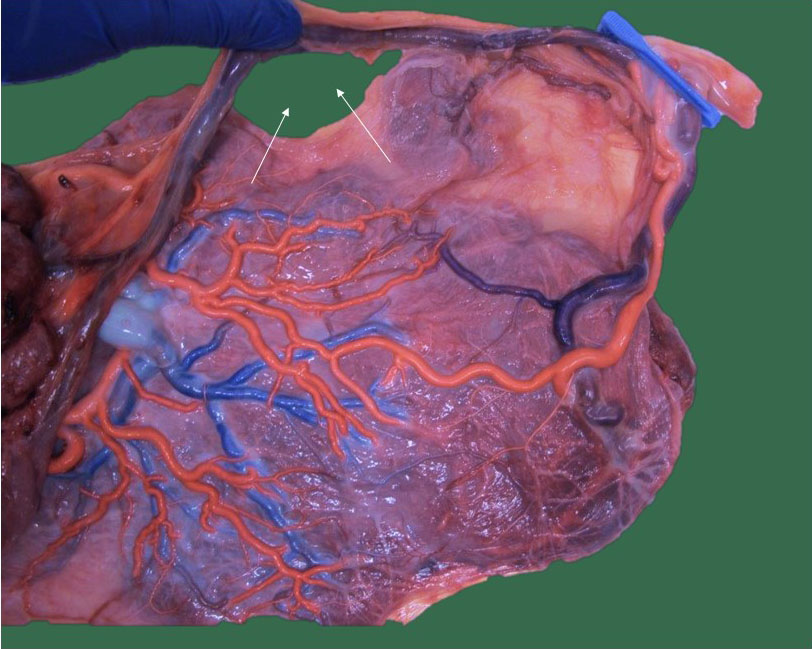
9
Macroscopic image of a monochorionic diamniotic twin placenta with type I growth restriction. The chorionic vessels are injected with dye and the amniotic membranes are removed. Twin 1 (1 clamp) was the smaller twin with a velamentous cord insertion with vasa previa. An elective cesarean section was performed with the incision (small arrows) just underneath a large velamentous vein.
Single umbilical artery
A single umbilical artery is more common in twins than in singletons (2–5% versus 1%),25,26 with similar incidences in mono- and dichorionic pairs. Usually, the smaller twin has a single umbilical artery. Interestingly, the single umbilical artery in twins does not seem to show the same compensatory dilatation as in singletons, which may account for its association with poor growth in twin pregnancies.25
Discordance for molar pregnancy and placental mesenchymal dysplasia
Unique to a dizygotic twin setting is the combination of a complete hydatidiform mole (diploid, 46 chromosomes, all from paternal origin) with a normal pregnancy. These pregnancies pose difficult management decisions and have a high risk of developing early pre-eclampsia and malignant trophoblastic disease.27,28 A few case studies report also on a partial mole (69 chromosomes, triploid, extra set of paternal chromosomes) in monochorionic and dichorionic pairs, which can be managed by a selective reduction of the triploid twin.29
Placental mesenchymal dysplasia (mosaicism confined to the chorionic mesoderm with 46 chromosomes, diploid, all from paternal origin) can mimic a partial mole on prenatal ultrasound scan (Figure 10). It can exist in a dichorionic,30 but also in a monochorionic setting, affecting the entire placenta31 or only one part of the placenta.32 In contrast to partial moles, this condition is limited to the placenta and may result in good outcomes, albeit with increased risks of growth restriction, genetic syndromes like Beckwith-Wiedemann syndrome, and fetal demise.33 Placental mesenchymal dysplasia can be differentiated from a partial mole by amniocentesis, which will demonstrate a normal karyotype in mesenchymal dysplasia and triploidy in partial moles.
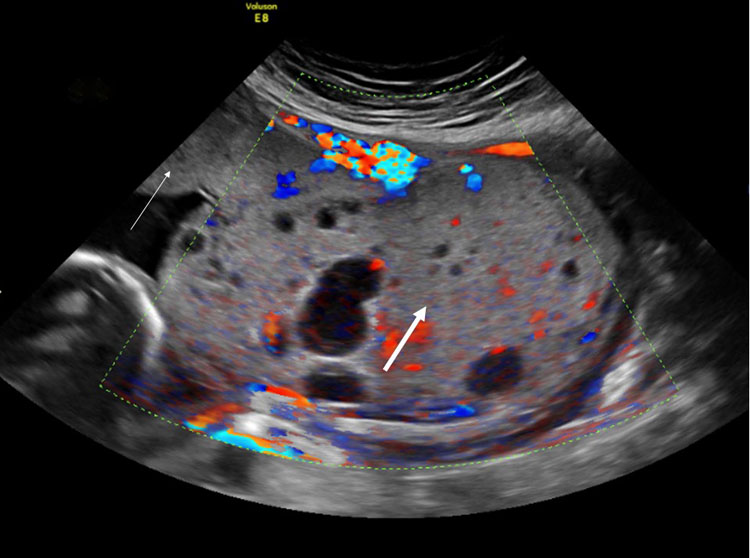
10
Ultrasound image of a monochorionic diamniotic twin placenta at 20 weeks with placental mesenchymal dysplasia of the placental part of the growth-restricted twin (thick arrow). The placental part of the appropriately growing twin had a normal appearance (thin arrow).
Placenta previa
Placenta previa is defined as placenta that inserts within 2 cm from the internal os. A low-lying placenta is more common in dichorionic than in monochorionic and singleton pregnancies34 due to the implantation of two placental masses, as well as to the increased use of subfertility treatment, a known risk factor for placenta previa.35 We diagnose placenta previa on the second-trimester ultrasound scan. However, it is essential to counsel patients that the overall majority of low-lying placentas resolve by the time of birth.34
THE VASCULAR ANASTOMOSES IN MONOCHORIONIC TWIN COMPLICATIONS
The different types of anastomoses
About 95% of monochorionic twins have placental vascular communications that connect the fetal circulations.2 Anastomoses can be of two types. Arterio-arterial and venovenous anastomoses are called “bidirectional” and “superficial” connections because they allow flow in both directions, and they directly connect the arteries and veins of both twins on the placental surface. When injecting the cord vessels with dye, the color will pass from one cord to the other in the presence of an arterio-arterial or venovenous anastomosis. Arteriovenous anastomoses are labeled as “unidirectional” and “deep” as they allow flow in one direction only and the anastomosis of the artery of one twin and the vein of the other twin occurs in the depth of the placenta. When injecting the cord vessels with dye, the color will not pass from one cord to the other if only deep unidirectional arteriovenous anastomoses are present. Because of the arteriovenous anastomoses, the monochorionic placenta consists of three parts: two parts are perfused by each twin individually, whereas a third part that is shared and perfused by arteriovenous anastomoses. On the placental surface, arteries can be distinguished from veins because the arteries almost always lie on top of the veins.
The typical monochorionic placenta has one arterio-arterial anastomosis and several arteriovenous anastomoses.2 The bidirectional character of the arterio-arterial anastomosis protects against any transfusion imbalance across the unidirectional arteriovenous anastomoses. The arterio-arterial anastomosis acts as a flexible arteriovenous anastomosis (Figure 11). Venovenous anastomoses are only present in about one in four monochorionic placentas.36 They also allow flow in both directions, but they may increase the risk of a transfusion imbalance37 and fetal loss.36
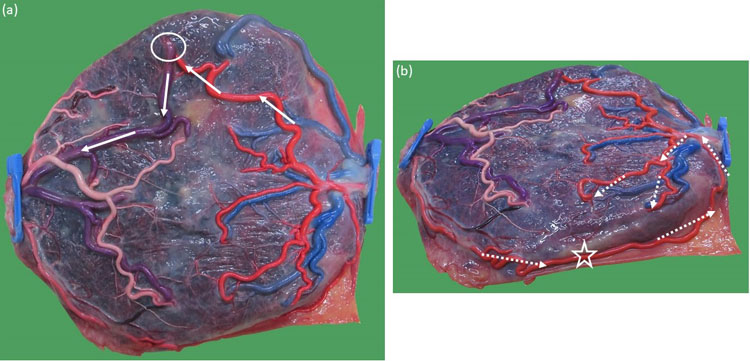
11
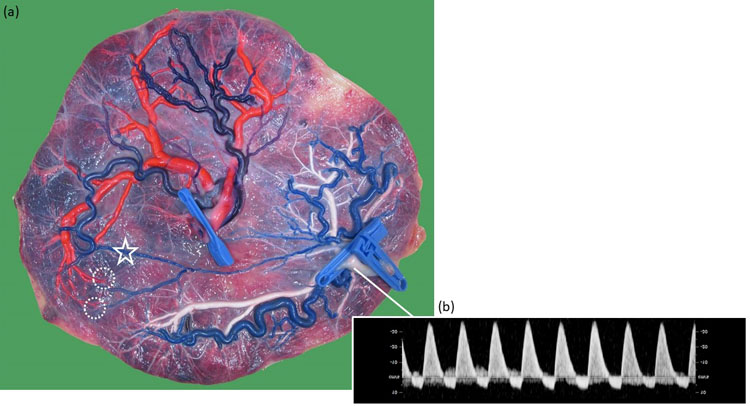
Macroscopic image of a monochorionic diamniotic twin placenta of an uncomplicated twin pregnancy delivered at 36 weeks. The chorionic vessels are injected with dye, and the amniotic membranes are removed. (a) There is one large arteriovenous anastomosis (empty circle) from twin 1 to twin 2. (b) This large unidirectional anastomosis would have caused an immediate acute exsanguination (full arrows) if there had not been a large bidirectional arterio-arterial anastomosis hidden underneath the placental edge (open star) that functions as an oppositely directed arteriovenous anastomosis from twin 2 to twin 1 (dashed arrows).
The anastomoses in twin–twin transfusion syndrome
Twin–twin transfusion syndrome (TTTS) is characterized by a severe discordance in amniotic fluid with one oliguric twin (donor) who has little or no fluid (deepest pocket <2 cm on ultrasound scan) and one polyuric twin (recipient) who has too much fluid (>6 cm before 16 weeks, >8 cm between 16 and 20 weeks and >10 cm after 20 weeks).38 TTTS occurs in about one in ten monochorionic diamniotic twin pregnancies, usually between 16 and 26 weeks.3
The typical TTTS placenta has several arterio-venous anastomoses and lacks a bidirectional arterio-arterial anastomosis (Figure 12). As such, an arterio-arterial anastomosis is found in 84% of non-TTTS placenta in contrast to 24% of TTTS placentas.36 On the other hand, venovenous anastomoses may be more common in TTTS placentas. Since the venous circulation has a low resistance, external factors (such as fetal position) may trigger a intertwin transfusion imbalance.37
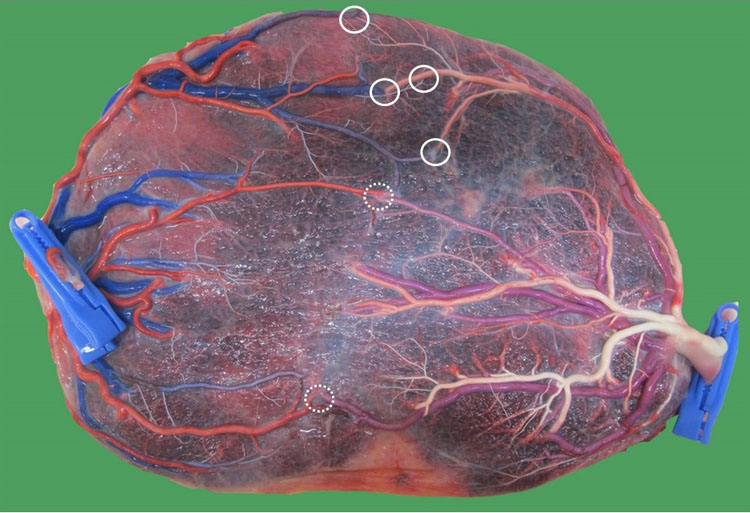
12
Macroscopic image of a monochorionic diamniotic twin placenta of a pregnancy complicated by twin-to-twin transfusion syndrome with hydrops of the recipient twin at 31 weeks. The chorionic vessels are injected with dye, and the amniotic membranes are removed. Twin 2 was the donor (2 clamps; 1140 g) and twin 1 the recipient (1600 g). Only unidirectional arteriovenous anastomoses are present (full circles from twin 1 to twin 2; dashed circles from twin 2 to twin 1).
Fetoscopic laser coagulation of the vascular anastomoses is the best treatment of TTTS and results in the resolution of the amniotic fluid discordance.38 If all anastomoses are coagulated, then the diuresis in the donor resumes resulting in increasing amniotic fluid, whereas the polyuria in the recipient stops leading to a disappearance of the polyhydramnios.
The anastomoses in twin anemia polycythemia sequence (TAPS)
Twin anemia polycythemia sequence (TAPS) is characterized by a severe discordance in red blood cells with anemia in one pale twin (donor) and polycythemia in one plethoric twin (recipient) without the severe amniotic fluid discordance required for the diagnosis of TTTS. TAPS is diagnosed if there is a significant difference (1 multiple of the median (MoM)) in the peak systolic velocity in the middle cerebral artery between the twins. Alternatively, the anemic donor must have increased blood velocities in the brain (1.5 MoM), whereas the polycythemic recipient has decreased velocities (0.8 MoM).39 The placental part of the anemic donor is often white and thick, whereas the recipient’s part is dark and thin.40 TAPS occurs in about one in 20 previously uncomplicated twin pregnancies3 and may present as early as the first trimester,41 but often also occurs after 26 weeks in the viable period.42,43
The TTTS and TAPS placenta is very similar as regards the type of anastomoses. TAPS placentas typically have arteriovenous anastomoses and miss the arterio-arterial anastomosis, but the anastomoses in TAPS are minuscule and fewer in number44 (Figure 13). TAPS seems to be a chronic net transfusion of red blood cells through tiny anastomoses. TTTS probably also starts as a transfusion imbalance in red blood cells, but because of the larger anastomoses and more elaborate blood exchange, the donor masks the anemia through volume contraction and hemoconcentration, resulting in oliguria and anhydramnios. In contrast, the recipient masks its polycythemia through volume expansion and hemodilution, resulting in polyuria and polyhydramnios.45 If, during fetoscopy, we only coagulate the large anastomoses but miss any minuscule connections, then TTTS will disappear, but TAPS may develop several weeks later46 (Figure 14). To prevent postoperative TAPS, we now draw a coagulation line across the entire placenta along the vascular equator to ascertain whether these tiny anastomoses are also coagulated.47
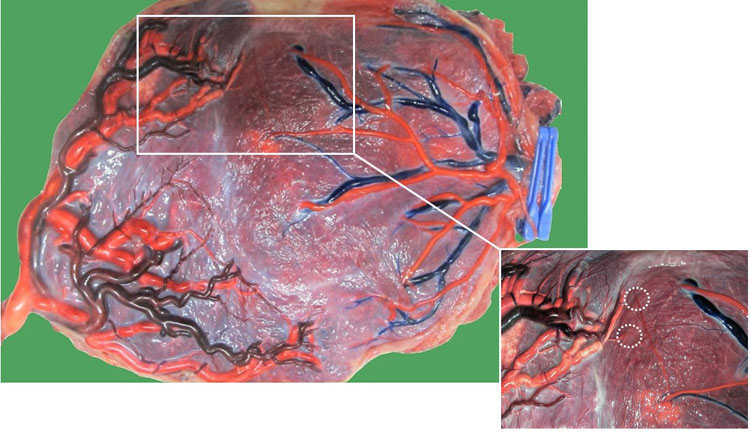
13
Macroscopic image of a monochorionic diamniotic twin placenta of a pregnancy complicated by spontaneous twin anemia polycythemia sequence at 31 weeks. The chorionic vessels are injected with dye, and the amniotic membranes are removed. A week earlier, the donor received an intrauterine transfusion for a hemoglobin of 7 g/dL. The chorionic vessels are injected with dye and the amniotic membranes are removed. Twin 1 was the recipient (1900 g; hemoglobin: 25 g/dL) and twin 2 the donor (1520 g; hemoglobin: 8 g/dL). There were only minuscule unidirectional arteriovenous anastomoses present from twin 2 to twin 1 (dashed circles).
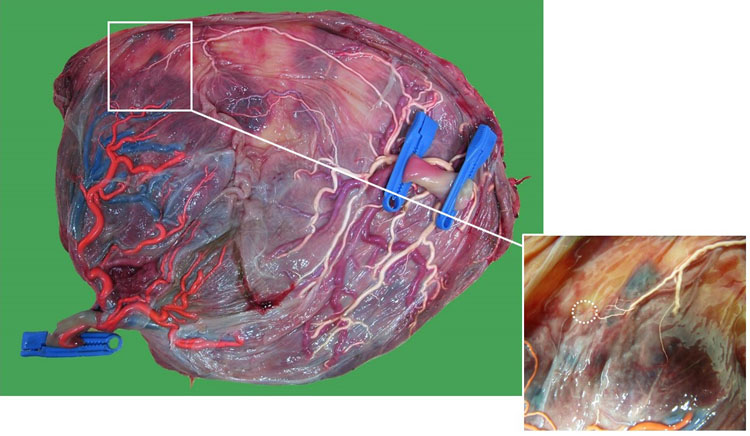
14
Macroscopic image of a monochorionic diamniotic twin placenta of a pregnancy complicated by post-laser spontaneous twin anemia polycythemia sequence at 29 weeks. The chorionic vessels are injected with dye, and the amniotic membranes are removed. Fetoscopic laser surgery for twin–twin transfusion syndrome was performed at 20 weeks. The poly- and oligohydramnion disappeared, but at 23 weeks twin anemia polycythemia sequence developed, which was treated with three intrauterine transfusions of the donor and one exchange transfusion of the recipient. There was one tiny missed arteriovenous anastomosis from donor (twin 2; 1276 g) to recipient (twin 1; 1050 g) (dashed circle) at the placental edge.
The anastomoses in acute intrapartum transfusion
TAPS must be distinguished from an acute intertwin transfusion during labor. The exact incidence of acute intrapartum transfusion is not known, but it is thought to be rather rare. Both TAPS and intrapartum transfusion result in the birth of a pale and plethoric baby. However, TAPS results from a very slow transfusion over tiny anastomoses, whereas an acute intertwin transfusion is caused by rapid exsanguination across large, bidirectional anastomoses. In TAPS, the anemic baby will not show signs of hypovolemic shock, and the reticulocyte count is raised due to chronic anemia. Also, in TAPS, the maternal side of the placenta will typically show a thickened, pale part and thin, dark part (Figure 15), whereas, in intrapartum transfusion, both parts have the same color.48
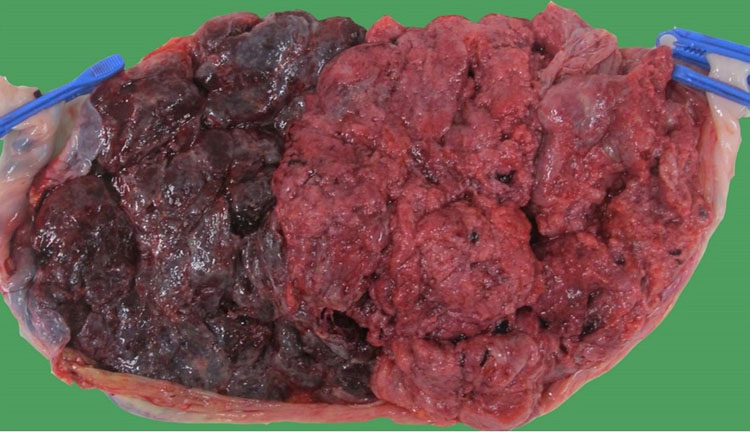
15
Maternal side of the placenta in Figure 12. The pale part belongs to the donor (2 clamps), whereas the dark part belongs to the recipient (1 clamp).
The anastomoses in twin reversed arterial perfusion sequence (TRAP)
TRAP is a unique anomaly in monochorionic pregnancies. It occurs in about 2.5% of monochorionic pregnancies49 and may arise when one of the twins dies in early pregnancy. On ultrasound scan, there is one structurally normal twin who pumps blood (hence the name pump twin) towards the demised co-twin that does not usually have any cardiac activity (hence the name acardiac twin), and looks grossly abnormal. The arterial blood flow in the acardiac’s cord is reversed and towards rather than away from the acardiac twin (hence the name twin reversed arterial perfusion). A prerequisite for TRAP to develop is the presence of a large arterio-arterial and venovenous anastomosis. The acardiac twin does not have any placental territory of its own and is entirely perfused by the pump twin. Usually, the acardiac twin has a single umbilical artery, and its cord originates close to the pump’s cord or sometimes even bifurcates from it.50 Because TRAP may result in high-output cardiac failure in the pump twin, we can arrest the reversed flow by intrafetal coagulation51 (Figure 16).
16
(a) Ultrasound image of the intrafetal coagulation of an acardiac mass at 21 weeks to arrest the reversed flow. (b) Macroscopic image of the placenta after intrafetal coagulation with maceration of the acardiac mass (thin arrow).
The anastomoses in isolated discordant growth
Monochorionic twins are monozygotic, so differences in genetic make-up cannot explain a difference in growth. Transfusion imbalances like TTTS and TAPS commonly lead to growth restriction in the donor twin. However, in the absence of a transfusion imbalance, an unequal division of the shared placenta is the most common cause of discordant growth.
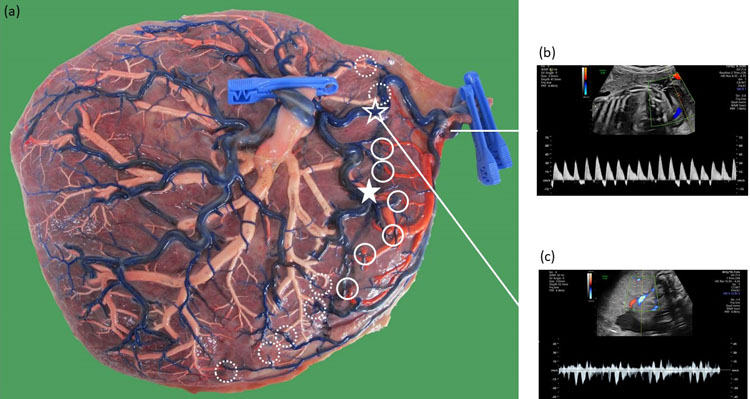
Discordant growth with a birth weight difference of 25% or more occurs in about 10–15% of monochorionic twins.15,52 We classify isolated discordant growth according to the Doppler pattern in the umbilical artery of the smaller twin as measured at the placental cord insertion. In most cases, the umbilical artery blood flow is normal (type I), and these cases have an excellent outcome, with a 95% survival rate. Usually, the placenta is moderately unequally shared, and the anastomoses are similar to those of uncomplicated monochorionic pregnancies. Rarely, there is a continuous absent end-diastolic flow in the umbilical artery of the smaller twin (type II), and these cases have the worst outcome with only 55% survival. Type II placentas are also only moderately unequally shared with few and rather small anastomoses and probably an impaired placental dysfunction of the share of the smaller twin (Figure 17). Finally, type III cases have a cyclical pattern in the umbilical artery of the smaller twin varying from normal to absent or reversed in one Doppler sweep. Type III placentas are very unequally divided with almost no individual territory for the smaller twin, and all its placental vessels are involved in huge anastomoses. The cyclical variation indicates the presence of a large arterio-arterial anastomosis. In type III, the smaller twin entirely survives thanks to the extensive blood exchange with its co-twin on the larger share (Figure 18). Type III cases usually have good outcomes, with an 85% survival rate. However, they are at risk of unpredictable and often double intrauterine demise.52,53
17
(a) Macroscopic image of a monochorionic diamniotic twin placenta of a pregnancy complicated by type II discordant growth at 30 weeks. The chorionic vessels are injected with dye, and the amniotic membranes are removed. Twin 1 weighed 1300 g, and twin 2 weighed 680 g and had continuous reversed flow in the umbilical artery. Both twins seem to have a sufficient part of the placenta. There is a small arterio-arterial anastomosis (open star) and two arteriovenous anastomoses from twin 2 to 1 (dashed circles); (b) Ultrasound image demonstrating the continuous reversed end-diastolic flow pattern in the umbilical cord of the smaller twin.
18
(a) Macroscopic image of a monochorionic diamniotic twin placenta of a pregnancy complicated by type III discordant growth at 32 weeks. The chorionic vessels are injected with dye, and the amniotic membranes are removed. Twin 1 weighed 2160 g and twin 2 weighed 1300 g and had an intermittent absent and reversed flow in the umbilical artery. Twin 2 had a very small part of the placenta. Nearly all of its vessels are involved in anastomoses. Fetoscopic laser coagulation of these large vessels would be very difficult and leave almost no placenta for the smaller twin. There is a large arterio-arterial anastomosis (open star), venovenous anastomosis (full star) and several arteriovenous anastomoses from twin 1 to twin 2 (full circles) and from twin 2 to twin 1 (dashed circles); (b) Ultrasound image demonstrating the intermittent absent and reversed end-diastolic flow pattern in the umbilical cord of the smaller twin; (c) Ultrasound image demonstrating the bidirectional wave-form in the large arterio-arterial anastomosis.
The anastomoses in single demise
In dichorionic twin pregnancies, a single demise will lead to atrophy of the placenta of the demised twin (Figure 19). In monochorionic twin pregnancies, the surviving twin can recruit the placental part of its demised co-twin through an arterio-arterial and venovenous anastomosis and expand its placental surface. At birth, the entire placenta is perfused, and the atrophied part is missing.54
19
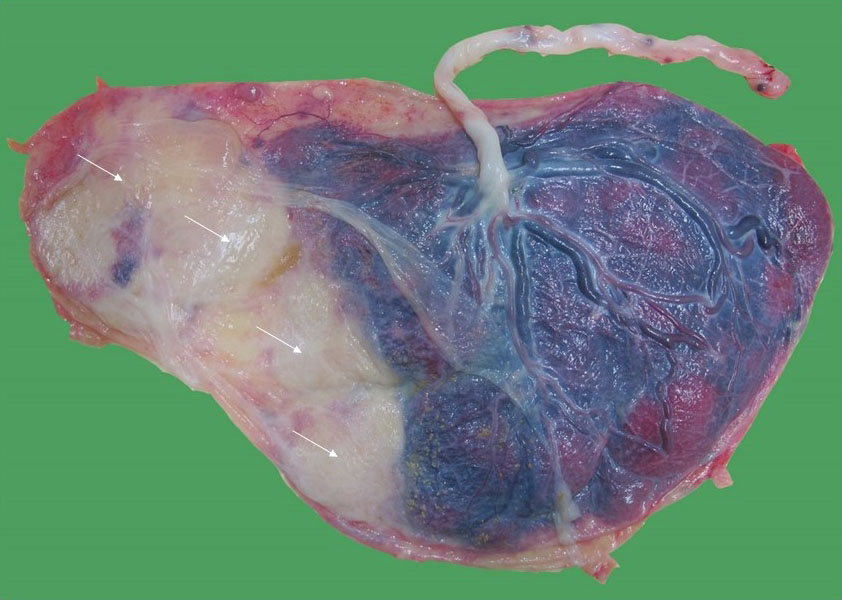
Macroscopic image of a monochorionic diamniotic twin placenta at 36 weeks of a pregnancy complicated by early twin-anemia polycythemia sequence, which led to the demise of the anemic donor twin at 18 weeks. Because of the tiny anastomoses, the surviving twin did not recruit placenta from its demised co-twin and its placental part became atrophic (thin arrows).
The anastomoses in monoamniotic twin pregnancies
Monoamniotic twins constitute 5% of monochorionic twin pregnancies.1 They share not only the same placenta, but also the same amniotic sac. In about 50% of monoamniotic placentas, the cords insert close to one another with large bidirectional arterio-arterial anastomoses linking the two fetal circulations55,56,57 (Figure 20). Usually, the cords are already entangled from the first trimester onward. Because of the large bidirectional connections, monoamniotic pregnancies are less likely to develop TTTS or TAPS, but they are at increased risk of an acute intertwin transfusion causing unexpected and usually double fetal demise.55,58,59,60 If only one of a monoamniotic pair dies, then in 30% of cases, the surviving twin dies several weeks later due to cord entanglement.60
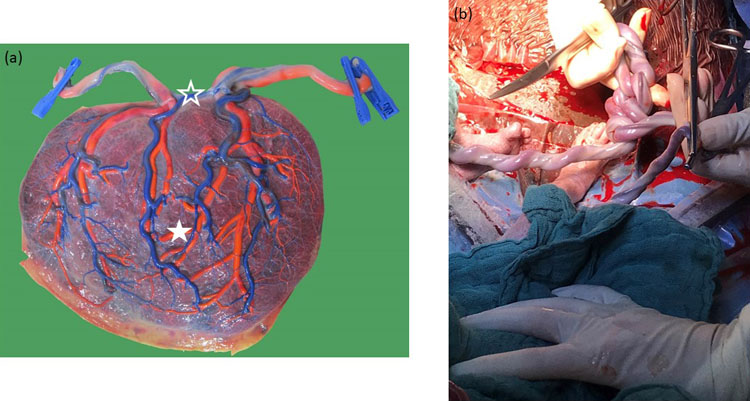
20
(a) Macroscopic image of a monochorionic monoamniotic twin placenta of a pregnancy at 33 weeks. The chorionic vessels are injected with dye, and the amniotic membrane is removed. The cords insert close to one another, and a large arterio-arterial (open star) and venovenous (full star) is present. (b) Illustration of the cord entanglement at the time of the cesarean section.
Dye injection to demonstrate the anastomoses in a monochorionic placenta
Because the vascular anastomoses account for the excessive risk in monochorionic pregnancies, documentation of the anastomoses and the division of the placenta is extremely informative. Also, in fetal medicine centers practicing fetoscopic laser surgery, checking whether any anastomoses were missed serves as a means of quality control. For color injection, the placenta should be stored fresh in a regular refrigerator at 4°C. Placentas that are stored in formalin or in the freezer can no longer be injected. It is best to examine the placenta within a week after birth. We catheterize each cord vessel and use barium sulfate mixed with colors (eosin and ink) for injection, but poster paint can be used as well.46,61 The solution should not be too watery as it will otherwise leak out from the maternal surface. Barium sulfate and color paint are both sufficiently viscous to clog up the capillaries and thereby prevent leakage. Water and air injections are not good methods as they fail to document any tiny anastomoses.
For catheterization, one can use intravenous catheters or umbilical cord catheters. The injection should be slow while massaging the dye into the peripheral placental vessels. Usually, one can inject about 20 mL in the arteries of each part and about 30–40 mL of dye in the vein. After all peripheral vessels are filled with dye, we clamp the cord and rinse the placenta with cold tap water to remove any blood or stains. In the end, we peel off the amniotic membrane as tiny vessels then become more visible and take a picture of the chorionic surface.
CONCLUSION
The macroscopic and pathologic examination of twin placentas is not only essential to confirm the chorionicity, but also helps to explain any adverse outcomes such as preterm birth and growth restriction. In monochorionic twin pregnancies, the documentation of the vascular anastomoses and placental sharing by color injection is especially useful to understand any acute and chronic transfusion imbalances or growth differences. Dye injection also serves as quality control to detect any missed anastomoses after fetoscopic laser surgery. A better understanding of the pathophysiology of twin complications may ultimately improve the management of these high-risk pregnancies.
PRACTICE RECOMMENDATIONS
- Macroscopic examination of the intertwin septum after birth accurately confirms the chorionicity.
- Twin pregnancies are at increased risk of preterm birth, growth restriction and pre-eclampsia, and pathologic examination of the twin placenta helps to document the presence of chorioamnionitis and placental lesions.
- A velamentous cord insertion, vasa previa, single umbilical artery and placenta previa are more common in twin than in singleton pregnancies.
- Dye injection of the monochorionic placenta and the documentation of the vascular anastomoses and sharing are indispensable to explain any transfusion imbalances or discordant growth.
CONFLICTS OF INTEREST
Author(s) statement awaited.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Litwinska E, Syngelaki A, Cimpoca B, et al. Outcome of twin pregnancy with two live fetuses at 11–13 weeks' gestation. Ultrasound Obstet Gynecol 2020;55(1):32–8. | |
Lewi L, Cannie M, Blickstein I, et al. Placental sharing, birthweight discordance, and vascular anastomoses in monochorionic diamniotic twin placentas. Am J Obstet Gynecol 2007;197(6):587 e1–8. | |
Lewi L, Jani J, Blickstein I, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol 2008;199(5):514 e1–8. | |
NICE guideline [NG137]: Twin and triplet pregnancy 2019 [Available from: https://www.nice.org.uk/guidance/ng137. | |
Khalil A, Rodgers M, Baschat A, et al. ISUOG Practice Guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol 2016;47(2):247–63. | |
Society for Maternal-Fetal M, Simpson LL. Twin-twin transfusion syndrome. Am J Obstet Gynecol 2013;208(1):3–18. | |
Lopriore E, Sueters M, Middeldorp JM, et al. Twin pregnancies with two separate placental masses can still be monochorionic and have vascular anastomoses. Am J Obstet Gynecol 2006;194(3):804–8. | |
Galjaard S, Moerman P, Corveleyn A, et al. Partial monochorionic and monoamniotic twin pregnancies: a report of two cases. Ultrasound Obstet Gynecol 2014;44(6):722–4. | |
Peters HE, Konig TE, Verhoeven MO, et al. Unusual Twinning Resulting in Chimerism: A Systematic Review on Monochorionic Dizygotic Twins. Twin Res Hum Genet 2017;20(2):161–8. | |
Khong TY, Mooney EE, Ariel I, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med 2016;140(7):698–713. | |
Couck I, Aertsen M, Jespers A, et al. The assessment of placental sharing using X-ray angiogram versus digital photograph: A prospective study. Placenta 2019;83:1–4. | |
Perinatale activiteiten Vlaanderen 2018 2018 [ | |
Phung DT, Blickstein I, Goldman RD, et al. The Northwestern Twin Chorionicity Study: I. Discordant inflammatory findings that are related to chorionicity in presenting versus nonpresenting twins. Am J Obstet Gynecol 2002;186(5):1041–5. | |
Ananth CV, Vintzileos AM, Shen-Schwarz S, et al. Standards of birth weight in twin gestations stratified by placental chorionicity. Obstet Gynecol 1998;91(6):917–24. | |
Litwinska E, Syngelaki A, Cimpoca B, et al. Intertwin discordance in fetal size at 11–13 weeks' gestation and pregnancy outcome. Ultrasound Obstet Gynecol 2020;55(2):189–97. | |
Kent EM, Breathnach FM, Gillan JE, et al. Placental pathology, birthweight discordance, and growth restriction in twin pregnancy: results of the ESPRiT Study. Am J Obstet Gynecol 2012;207(3):220 e1–5. | |
Samedi VM, Skappak C, Jantzie L, et al. Comparison of Presentation, Course, and Outcome of Congenital and Acquired Cytomegalovirus Infection in Twins. AJP Rep 2016;6(1):e1–5. | |
Francisco C, Wright D, Benko Z, et al. Hidden high rate of pre-eclampsia in twin compared with singleton pregnancy. Ultrasound Obstet Gynecol 2017;50(1):88–92. | |
Aviram A, Giltvedt MK, Sherman C, et al. The role of placental malperfusion in the pathogenesis of preeclampsia in dichorionic twin and singleton pregnancies. Placenta 2018;70:41–9. | |
Costa-Castro T, Zhao DP, Lipa M, et al. Velamentous cord insertion in dichorionic and monochorionic twin pregnancies – Does it make a difference? Placenta 2016;42:87–92. | |
Delbaere I, Goetgeluk S, Derom C, et al. Umbilical cord anomalies are more frequent in twins after assisted reproduction. Hum Reprod 2007;22(10):2763–7. | |
Vintzileos AM, Ananth CV, Smulian JC. Using ultrasound in the clinical management of placental implantation abnormalities. Am J Obstet Gynecol 2015;213(4 Suppl):S70–7. | |
Couck I, Mourad Tawfic N, Deprest J, De Catte L, Devlieger R, Lewi L. Does site of cord insertion increase risk of adverse outcome, twin-to-twin transfusion syndrome and discordant growth in monochorionic twin pregnancy? Ultrasound Obstet Gynecol 2018;52(3):385–9. | |
Sato Y, Benirschke K. Increased prevalence of fetal thrombi in monochorionic-twin placentas. Pediatrics 2006;117(1):e113–7. | |
Klatt J, Kuhn A, Baumann M, et al. Single umbilical artery in twin pregnancies. Ultrasound Obstet Gynecol 2012;39(5):505–9. | |
Stout MJ, Odibo AO, Longman R, et al. The incidence of isolated single umbilical artery in twins and adverse pregnancy outcomes. Prenat Diagn 2013;33(3):269–72. | |
Lin LH, Maesta I, Braga A, et al. Multiple pregnancies with complete mole and coexisting normal fetus in North and South America: A retrospective multicenter cohort and literature review. Gynecol Oncol 2017;145(1):88–95. | |
Wee L, Jauniaux E. Prenatal diagnosis and management of twin pregnancies complicated by a co-existing molar pregnancy. Prenat Diagn 2005;25(9):772–6. | |
Nugent CE, Punch MR, Barr M, Jr, et al. Persistence of partial molar placenta and severe preeclampsia after selective termination in a twin pregnancy. Obstet Gynecol 1996;87(5 Pt 2):829–31. | |
Surti U, Hill LM, Dunn J, et al. Twin pregnancy with a chimeric androgenetic and biparental placenta in one twin displaying placental mesenchymal dysplasia phenotype. Prenat Diagn 2005;25(11):1048–56. | |
Robertson M, Geerts LT, de Jong G, Wainwright H. Mesenchymal dysplasia in a monochorionic diamniotic twin pregnancy with review of the differential diagnosis of cystic changes in the placenta. J Ultrasound Med 2007;26(5):689–93. | |
Gheysen W, Strybol D, Moerman P, et al. Discordance for placental mesenchymal dysplasia in a monochorionic diamniotic twin pregnancy: A case report. Clin Case Rep 2018;6(8):1557–60. | |
Nayeri UA, West AB, Grossetta Nardini HK, Copel JA, Sfakianaki AK. Systematic review of sonographic findings of placental mesenchymal dysplasia and subsequent pregnancy outcome. Ultrasound Obstet Gynecol 2013;41(4):366–74. | |
Weis MA, Harper LM, Roehl KA, et al. Natural history of placenta previa in twins. Obstet Gynecol 2012;120(4):753–8. | |
Qin JB, Wang H, Sheng X, et al. Assisted reproductive technology and risk of adverse obstetric outcomes in dichorionic twin pregnancies: a systematic review and meta-analysis. Fertil Steril 2016;105(5):1180–92. | |
Denbow ML, Cox P, Taylor M, et al. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am J Obstet Gynecol 2000;182(2):417–26. | |
Zhao DP, Cohen D, Middeldorp JM, et al. The role of veno-venous anastomoses in twin-twin transfusion syndrome. Placenta 2014;35(5):334–6. | |
Senat MV, Deprest J, Boulvain M, et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med 2004;351(2):136–44. | |
Khalil A, Gordijn S, Ganzevoort W, et al. Consensus diagnostic criteria and monitoring of twin anemia polycythemia sequence: a Delphi procedure. Ultrasound Obstet Gynecol 2019. | |
Tollenaar LSA, Lopriore E, Middeldorp JM, et al. Prevalence of placental dichotomy, fetal cardiomegaly and starry-sky liver in twin anemia polycythemia sequence. Ultrasound Obstet Gynecol 2019. | |
Couck I, Valenzuela I, Russo F, et al. Spontaneous regression of twin anemia-polycythemia sequence presenting in the first trimester. Ultrasound Obstet Gynecol 2019. | |
Lewi L, Gucciardo L, Huber A, et al. Clinical outcome and placental characteristics of monochorionic diamniotic twin pairs with early- and late-onset discordant growth. Am J Obstet Gynecol 2008;199(5):511 e1–7. | |
Tollenaar LSA, Slaghekke F, Lewi L, et al. Treatment and outcome in 370 cases with spontaneous or post-laser twin anemia polycythemia sequence managed in 17 different fetal therapy centers. Ultrasound Obstet Gynecol 2020. | |
Lopriore E, Deprest J, Slaghekke F, et al. Placental characteristics in monochorionic twins with and without twin anemia-polycythemia sequence. Obstet Gynecol 2008;112(4):753–8. | |
Couck I, Lewi L. The Placenta in Twin-to-Twin Transfusion Syndrome and Twin Anemia Polycythemia Sequence. Twin Res Hum Genet 2016;19(3):184–90. | |
Lewi L, Jani J, Cannie M, et al. Intertwin anastomoses in monochorionic placentas after fetoscopic laser coagulation for twin-to-twin transfusion syndrome: is there more than meets the eye? Am J Obstet Gynecol 2006;194(3):790–5. | |
Slaghekke F, Lopriore E, Lewi L, et al. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: an open-label randomised controlled trial. Lancet 2014;383(9935):2144–51. | |
Tollenaar LSA, Zhao DP, Middeldorp JM, et al. Can color difference on the maternal side of the placenta distinguish between acute peripartum twin-twin transfusion syndrome and twin anemia-polycythemia sequence? Placenta 2017;57:189–93. | |
van Gemert MJ, van den Wijngaard JP, Vandenbussche FP. Twin reversed arterial perfusion sequence is more common than generally accepted. Birth Defects Res A Clin Mol Teratol 2015;103(7):641–3. | |
Van Allen MI, Smith DW, Shepard TH. Twin reversed arterial perfusion (TRAP) sequence: a study of 14 twin pregnancies with acardius. Semin Perinatol 1983;7(4):285–93. | |
Lewi L, Valencia C, Gonzalez E, et al. The outcome of twin reversed arterial perfusion sequence diagnosed in the first trimester. Am J Obstet Gynecol 2010;203(3):213 e1–4. | |
Couck I, Ponnet S, Deprest J, et al. Outcome of selective intrauterine growth restriction in monochorionic twin pregnancies at 16, 20 or 30 weeks according to the new consensus definition. Ultrasound Obstet Gynecol 2020. | |
Gratacos E, Lewi L, Munoz B, et al. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery Doppler flow in the smaller twin. Ultrasound Obstet Gynecol 2007;30(1):28–34. | |
Deneckere S, Couck I, Devlieger R, et al. Placental vascular recruitment after single intrauterine demise: A newly diagnosed phenomenon unique to monochorionic pregnancies. Prenat Diagn 2019;39(5):409–12. | |
Hack KE, van Gemert MJ, Lopriore E, Schaap AH, Eggink AJ, Elias SG, et al. Placental characteristics of monoamniotic twin pregnancies in relation to perinatal outcome. Placenta 2009;30(1):62–5. | |
Umur A, van Gemert MJ, Nikkels PG. Monoamniotic-versus diamniotic-monochorionic twin placentas: anastomoses and twin-twin transfusion syndrome. Am J Obstet Gynecol 2003;189(5):1325–9. | |
Zhao DP, Peeters SH, Middeldorp JM, et al. Monochorionic placentas with proximate umbilical cord insertions: definition, prevalence and angio-architecture. Placenta 2015;36(2):221–5. | |
Van Mieghem T, De Heus R, Lewi L, Klaritsch P, Kollmann M, Baud D, et al. Prenatal management of monoamniotic twin pregnancies. Obstet Gynecol 2014;124(3):498–506. | |
Diehl W, Glosemeyer P, Tavares De Sousa M, Hollwitz B, Ortmeyer G, Hecher K. Twin anemia-polycythemia sequence in a case of monoamniotic twins. Ultrasound Obstet Gynecol 2013;42(1):108–11. | |
Hack KE, Derks JB, Schaap AH, et al. Perinatal outcome of monoamniotic twin pregnancies. Obstet Gynecol 2009;113(2 Pt 1):353–60. | |
Lopriore E, Slaghekke F, Middeldorp JM, et al. Accurate and simple evaluation of vascular anastomoses in monochorionic placenta using colored dye. J Vis Exp 2011;(55):e3208. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)