This chapter should be cited as follows:
Brillo E, Tosto V, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.416613
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 6
Pregnancy complaints and complications: clinical presentations
Volume Editor: Professor Gian Carlo Di Renzo, University of Perugia, Italy

Chapter
Viral Infections in Pregnancy (except HIV)
First published: August 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Viral infectious diseases in pregnancy are major causes of maternal and fetal/neonatal morbidity and mortality. Most viruses can infect healthy human hosts through droplet infection, while some can also spread through sexual transmission, and/or transfusion of infected blood. For some viral infections, increased susceptibility of pregnant women has been recognized, while for other viruses a greater severity of infection has been identified in pregnant women compared to general population. Not all pregnant women appear to be symptomatic following a viral infection; however, the virus can have consequences on the fetuses even when mothers are asymptomatic.
Some viruses possess the ability to remain latent after a primary infection and be reactivated. Reactivation of these viruses can be symptomatic or asymptomatic for a pregnant woman, and may give rise to problems for the fetus. Viral infections can be transmitted to the fetus/newborn during the intrauterine (crossing the placenta), perinatal (peripartum i.e., during labor or delivery) or postnatal (postpartum) period.
For most viruses, the clinical manifestations of fetal-neonatal infections vary depending on the viral agent and gestational age at exposure.
Some viruses can cause miscarriages, stillbirths, fetal growth restrictions, preterm births, congenital malformations, or neonatal infections.
Multisystem congenital complications may ensue, encompassing the cardiovascular, neuromuscular, and gastrointestinal systems, and visual as well as auditory pathways.
It is important for the medical personnel who deal with pregnant women to understand better the underlying etiology, epidemiology, clinical manifestations, and key points in the management and prevention of these viral pathologies. The most important step in the management is to conduct a stringent screening protocol among those pregnant females who are at a relatively higher risk for these infectious diseases. The women who are predisposed to such conditions must be offered reliable tools for prophylaxis such as preconception vaccination. In addition, it is equally essential to carry out a full surveillance of the developing fetus so as to diagnose any life-threatening complications beforehand. The most efficient management plan lies in the multimodality approach which must also involve the will of the pregnant woman and her close relatives.
This chapter discusses the viral infections caused by the following pathogens: viruses belonging to the Herpesviridae family (i.e., cytomegalovirus, herpes simplex and varicella-zoster viruses), the Paramyxoviridae family (i.e., measles and mumps viruses), the Togaviridae family (i.e., rubella virus), the hepatitis viruses and newer agents such as zika virus, ebola virus, and new coronavirus (2019-nCoV or SARS-CoV-2). This chapter aims to develop a clinical understanding about the course of viral infectious disease management among pregnant women, while offering a special focus on the prophylactic measures which can be implemented among women to prevent them from viral infections during gestation.
MEASLES, MUMPS, RUBELLA, VARICELLA-ZOSTER AND PREGNANCY
The outcome of viral infections during pregnancy can range from no impact to spontaneous miscarriage. Paramyxoviruses including measles and mumps, and Togaviruses including rubella, are RNA viruses that have the potential to infect non-immune pregnant women as well as their fetuses or neonates. Varicella-zoster virus (VZV), commonly known as chickenpox, is a DNA virus which belongs to the Herpes family, and can also lead to drastic complications during the course of pregnancy. Congenital complications arising from these infections are well recognized.
In this section, all of these viral diseases are considered in detail, while their modes of transmission, clinical manifestations, diagnosis, treatment, and prophylactic measures among pregnant women are elaborated.
Measles and pregnancy
Measles is a highly contagious respiratory disease caused by a single-stranded, enveloped measles virus belonging to the genus Morbillivirus in the Paramyxoviridae family. Many countries have made significant progress to achieve the target of measles eradication.1 Nevertheless, the virus remains endemic in developing countries and outbreaks continue to occur in several regions worldwide.
Maternal transmission
Measles can be readily spread by means of inhalation of infectious droplets or aerosol from an infected person. The virus can remain infectious on various surfaces and even in the air for a period of 1–2 hours. Persons with measles are contagious from 4 days prior to and 4 days after the onset of morbilliform rash, and should be isolated during this period. Following exposure, approximately 90% of the susceptible population can develop disease manifestations.2 Once the virus enters the body via respiratory mucosa or conjunctivae, it replicates locally, spreads to regional lymph nodes, and is then thought to disseminate further via the bloodstream (first viremia).
Fetal transmission
Vertical transmission of measles to a fetus (termed as classic measles infection) has been found to occur among neonates born to the women who have experienced a measles infection 1–2 weeks before fetal delivery.3
Signs and symptoms in pregnant women
In general population, measles can produce a spectrum of clinical syndromes: (1) classic measles infection which affects immunocompetent individuals; (2) modified measles infection which infects subjects with pre-existing but incompletely protective anti-measles antibodies and results in an attenuated infection with milder symptoms and less clinical severity.
Classic measles infection can be subdivided into the following clinical stages: (1) incubation phase (6–21 days) where a patient usually remains asymptomatic (during which first and second viremia occur); (2) prodromal phase (2–4 days) characterized by anticipatory symptoms of the exanthematous phase (i.e., fever, fatigue, loss of appetite, and the “three Cs”: conjunctivitis, coryza, and cough); (3) enanthem (1–3 days) characterized by mucosal Koplik spots; (4) exanthem, that typically takes place 7–21 days following initial exposure; and (5) convalescence.4,5
The exanthem of measles arises approximately 2–4 days after the onset of fever; it comprises an erythematous, maculopapular rash that classically begins on the face and then spreads further to involve the neck, upper trunk, lower trunk, and extremities. The cranial-to-caudal progression of the rash is characteristic of measles but is not pathognomonic. After 3–4 days, the rash gradually darkens and then fades away, followed by fine desquamation in the more severely involved areas. During exanthem, clinical features such as generalized lymphadenopathy, pharyngitis, and conjunctivitis can appear. Clinical improvement typically ensues within 48 hours of the appearance of the rash. The occurrence of fever beyond the third or fourth day of rash implies a measles-associated complication.
Measles can be typically related to multiple organ-specific complications which can lead to inpatient hospitalization and even death, including gastrointestinal, neurological, respiratory, hematologic, renal, and skin-related complications,6 but the most prevalent complications include diarrhea, otitis media, and pneumonia. Historical data suggest that measles-related complications follow a more severe course among pregnant women where measles is often associated with an increased risk of life-threatening diarrhea, pneumonia, subacute sclerosing panencephalitis (SSPE), hospitalization, and mortality.7,8 The course of subacute sclerosing panencephalitis appears to be fulminant and the consequent fetal outcome is often unfavorable.
Women measles infected during the postpartum period could be also at high risk.9 Further study is needed to clarify this point.
Signs and symptoms in fetuses/newborns
Vertical transmission of measles can be associated with two clinical conditions depending upon the timing of its transmission: (1) congenital measles, defined as the appearance of rash at birth or within the first 10 days of life in a neonate whose mother was infected during late pregnancy (this early timing excludes transmission from mother to newborn post-delivery); (2) postnatal measles which is defined as appearance of measles rash within 14–30 days of birth.3,10
There is no proof of an increased risk of congenital abnormality among the infants of women who encounter measles during early pregnancy. Where defects have been reported, no conclusive evidence suggests that it is not a teratogenic agent.11,12
If a non-immune pregnant patient is exposed to measles just before delivery, intrauterine and intrapartum viral transmission is likely to cause a potentially serious infection in the newborn. Clinical data suggest an increased risk of fetal death, low birth weight (LBW), intrauterine growth retardation (IUGR), preterm birth and neonatal death following maternal measles, and neurological complications such as SSPE which can be associated with a high mortality risk.7,12,13,14,15,16
Diagnosis
The diagnosis of measles should be considered in a patient presenting with typical clinical manifestations, and can be confirmed with results obtained from serologic reports, culture, and viral polymerase chain reaction (PCR). Serologic testing plays a role in detecting anti-measles IgM by means of enzyme immunoassay (EIA) method. An acute measles infection can be confirmed with a four-fold or greater rise in anti-measles IgM levels between acute and convalescent sera.
Measles can also be diagnosed by identification of measles virus present within respiratory mucosal secretions, extracted from nasopharyngeal swabs. Detection of measles RNA by PCR amplification of RNA extracted from clinical samples can be done with primers targeted against highly conserved regions of measles genome. Primers that span a variable region combined with nucleotide sequencing allow the identification and characterization of measles virus genotypes for molecular epidemiological studies and can distinguish wild-type and vaccine measles virus strains.17
Treatment
The treatment of measles is largely conservative; there is no specific antiviral therapy available against the viral illness. Supportive therapy includes use of antipyretics, parenteral fluid resuscitation, and treatment of superinfections such as concurrent pneumonia and otitis media. Treatment of other complications, such as respiratory failure, may also be necessary. Vitamin A has been found to be useful for childhood measles, but there is no definite evidence for its administration in pregnant women.
Mumps and pregnancy
Mumps is a highly contagious viral infection. The virus is grouped along with measles virus in the Paramyxovirus family.
Transmission
It is transmitted through respiratory droplet, interpersonal contact, or infected fomites. The incubation period is usually around 16–18 days from the time of exposure to the point of onset of symptoms.
Signs and symptoms
Mumps typically begins with a few days of fever, headache, myalgia, fatigue, and anorexia; these manifestations are followed by the development of salivary gland swelling within a period of 48 hours. Mumps is a self-limiting disease where most of the individuals recover completely within a few weeks, but in some cases, mumps develops complications which involve meningoencephalitis, sensorineural hearing loss, myocardial involvement, pancreatitis, orchitis, and arthralgia. An asymptomatic infection occurs in 15–20% of population where individuals present with a non-specific clinical picture.
Mumps infection tends to be mild, even in pregnant women.10 In addition, mumps at any stage during pregnancy does not lead to an increased risk of fetal malformations.10,18 While some studies have shown that acquiring mumps during early pregnancy increases the risk of miscarriage, there remains no convincing evidence to suggest an association between mumps infection and pregnancy-related complications.19,20,21,22
Treatment
There is no treatment for mumps, but symptoms can be managed with over-the-counter pain medication such as acetaminophen (paracetamol). The best way to treat mumps is to prevent it in the first place specifically, with a vaccine.
Rubella and pregnancy
Rubella, also known as German measles, belongs to the genus Rubivirus of the Togavirus family, and humans are the only reservoir for rubella infection. Rubella causes a self-limited infection in most hosts, but can be transmitted to the fetus (i.e., congenital rubella infection or CRI) which may in turn lead to potentially deleterious consequences such as miscarriage, stillbirth, or congenital malformations (i.e., congenital rubella syndrome, CRS).
Maternal transmission
The virus is transmitted by direct droplet infection from nasopharyngeal secretions, and then replicates in the lymphoid tissue of the upper respiratory tract from where it spreads hematogenously.
Fetal transmission
Developing countries where rubella vaccination is not routinely performed possess a higher proportion of rubella infection and subsequent CRS cases.23 Even in the developed world, where rubella vaccination programs are available, the extent of vaccination is not always optimal due to which some areas have poor vaccination coverage. This results in limited cases of rubella which serve as a potential reservoir for fetal transmission.
The risk of fetal complications varies depending upon the timing of maternal infection, with the highest risk during the first 10 weeks of gestation. In the first trimester, fetal infection rates as high as 80% have been determined, which drop down to 25% in the late second trimester, while the rate of infection rises steeply to 100% for fetuses exposed beyond 36 weeks of gestation.24
Signs and symptom in pregnant women
The clinical manifestations vary depending upon the time of maternal infection. Rubella is generally a mild, self-limiting infectious disease associated with a characteristic exanthem. Symptoms tend to appear 14–21 days after initial exposure to the virus. Though asymptomatic in the majority, affected individuals may experience mild prodromal symptoms consisting of fever, conjunctivitis, pharyngitis, cough, and occasionally headache and malaise. These symptoms continue for 1–5 days. Rubella may also be associated with painful lymphadenopathy, which usually involves suboccipital, postauricular, and cervical lymph nodes. Just prior to the onset of the rash, approximately 20% of those infected will develop discrete rose spots on the soft palate (Forchheimer spots) that may later expand and coalesce.
Rubella typically causes an erythematous maculopapular rash which may be pruritic, and eventually evolves into pinpoint papules. The rash first appears on the face and then spreads to the trunk and body extremities within hours. It may last for as many as 3 days. Polyarthritis and polyarthralgia are potential sequelae. Other rare complications include post-infectious encephalitis, myocarditis, pericarditis, thrombocytopenia, hemolytic anemia, and hemolytic uremic syndrome.
Signs and symptoms in fetuses/newborns
The risk of clinical manifestations of CRI is dependent upon the timing of maternal infection, and is largely limited to maternal infection acquired during the first 16–17 weeks’ gestation.25 In the first trimester, the overall prevalence of fetal complications also increases.24,26 Cardiac and eye anomalies typically result when maternal infection occurs prior to 8 weeks, whereas hearing loss may be observed in maternal infections arising up to 18 weeks' gestation.24 Little, if any, risk of congenital defects is associated with infection after 18–20 weeks’ gestation, and intrauterine growth retardation may be the only sequelae of third trimester infection.24,27
In general, maternal immunity is protective against in-utero rubella infection. Although there have been CRS cases resulting from maternal reinfection,28,29 none of these have occurred among women infected after 12 weeks’ gestation.30 Intrauterine rubella infection leads to CRI, a chronic infection that possesses a broad spectrum of clinical manifestations: (1) asymptomatic infection; (2) effects on the developing fetus, resulting in miscarriage or stillbirth, intrauterine growth restriction, and very low birth weight; (3) CRS refers to a variable constellation of birth defects (e.g., deafness, congenital cataracts, and cardiac anomalies).31
The majority of infants with CRI have a subclinical infection during the neonatal period and, thus, remain asymptomatic at birth but they may develop manifestations over time (i.e., late manifestations).32 However, severe symptomatic neonatal infection can also occur (i.e., early manifestations). Early manifestations may include: (1) fetal growth restriction (FGR); (2) sensorineural hearing loss; (3) meningoencephalitis, microcephaly, sensorimotor dysfunction, and behavioral issues; (4) congenital heart disease; (5) eye disease, findings may include cataract, infantile glaucoma, and/or retinopathy; (6) petechiae and purpura ("blueberry muffin rash"), thrombocytopenia, hemolytic anemia, and hepatosplenomegaly.33 The risk of mortality is substantially increased in neonates with severe defects.
Late manifestations may include: (1) hearing loss, that is the most common late manifestation of CRI; it ranges in severity from mild to profound and may progress over time. Rarely, sudden onset of hearing loss may occur after years of normal hearing function; (2) endocrine disorders such as diabetes, thyroid disease, and growth hormone deficiency; (3) eye manifestations (e.g., retinopathy, cataracts, glaucoma, microphthalmos, strabismus, and keratoconus); (4) vascular disease (e.g., arteriosclerosis, systemic hypertension secondary to renal disease, and retinal neovascularization); (5) panencephalitis; (6) immunosuppression.34,35,36,37
Diagnosis
Immunity to rubella in pregnant women should be documented as part of initial prenatal care. Obstetric care includes routine testing for rubella as well as seromonitoring of susceptible women to ensure rapid diagnosis of primary rubella.
In susceptible pregnant women with suggestive clinical features, acute rubella syndrome can be diagnosed by one of the following: (1) a four-fold rise in IgM titers between acute (within 7–10 days of rash onset) and convalescent (2–3 weeks later) serum specimens; (2) a positive rubella culture (from nasal, blood, throat, urine, or cerebrospinal fluid samples); the virus is generally isolated from the pharynx 1 week before to 2 weeks after the onset of rash.
Rubella-specific IgM antibodies can be detected as a primary diagnostic approach to confirm acute infection. However, IgM testing may lead to a misinterpretation of the serological results. Therefore, it is crucial to distinguish IgM reactivity elicited by a recent primary infection from that resulting due to IgM persistence due to a previous infection, MMR vaccination,38 reinfection,39,40 polyclonal B-cell stimulation, or infections with other pathogens (e.g., EBV, parvovirus B19).41
If rubella IgM is incidentally detected in a pregnant woman without any history of rubella-like illness, further investigations are warranted. Nonetheless, due to a high rate of seroprevalence (~90%) in most of the developed countries,42 a majority of the anti-rubella virus IgM positive results are not associated with a primary rubella infection, but instead are mainly due to long-persisting IgM or false positivity, resulting in a decrease in the positive predictive value of IgM serology.43 In addition, widespread MMR vaccination programs have led to a decrease in rubella incidence while also resulting in a decline in the positive predictive value of IgM testing.43 In the light of these observations, the Center for Disease Control and Prevention (CDC) has downplayed the use of serum IgM for rubella screening in pregnancy.
A reliable diagnosis of rubella requires supplemental seroanalysis, such as follow-up serology for an evidence of seroconversion or a significant rise in the titers of rubella-specific IgG. Moreover, the determination of IgG avidity and the immunoblot detection of antibodies against E1 and E2 glycoproteins are considerably valuable in narrowing down the diagnosis.38,44 Therefore, avidity tests are potentially useful for distinguishing IgM due to primary rubella from long-persisting IgM antibodies. Likewise, anti-E1 IgG usually appears within 4–6 days following contact, whereas E2-specific antibodies are not identifiable until a few months following the infection, and therefore, rule out an acute bout of infection.45
Among the women who have a confirmed rubella diagnosis during pregnancy, fetal infection can be assessed by means of chorionic villous sampling and amniotic fluid samples through rubella-specific PCR assay. Ultrasonography can be helpful for achieving a prenatal diagnosis of CRS since findings associated with IUGR should prompt an evaluation for intrauterine viral infections such as rubella.
Treatment
Symptomatic management of pregnant women with acute rubella may include acetaminophen. Steroid therapy, platelet transfusion, and other supportive measures are reserved for subjects with viral complications such as thrombocytopenia or encephalopathy. The prognosis for pregnant women with rubella is generally excellent. However, owing to the potentially devastating effects of rubella on fetus, women must be counseled about the risk of fetal transmission, CRI or CRS, and they should be given a possible option regarding pregnancy termination. The latter is usually offered if there is positive IgM during the first trimester. After 20 weeks’ gestation, management should be individualized, and parents should be counseled about the potential for delayed consequences of rubella infection.
The use of immunoglobulins for pregnant women with an acute infection or following viral exposure remains controversial as it has not been shown to prevent disease or lessen its severity in any case.2 There are no data to indicate that immunoglobulins have a beneficial effect on the fetal response to disease. Therefore, the CDC recommends use of intravenous immunoglobulins among women who have a verified rubella exposure and those who adamantly decline abortion.46
Varicella-zoster virus and pregnancy
Varicella-zoster virus or VZV is a double-stranded DNA virus belonging to the Herpes family.47 VZV is the main causative factor behind two very common skin infections known as the chickenpox (primary infection) and herpes zoster also known as shingles (secondary infection).48 The incidence of chickenpox is low among adults as a majority of them are immune to infection. Although the incidence of chickenpox is not any higher among pregnant women as compared to non-pregnant females, the disease severity is relatively higher during pregnancy. In addition, chickenpox during pregnancy can expose the fetus and the newborn towards potentially disastrous complications.
Maternal transmission
The virus typically transmits through direct interpersonal contact, i.e., through contact with the skin lesions of chickenpox or via droplet infection from an infected individual. The patients can remain infectious from days prior to this rash until the lesions are crusted over.
Patients with herpes zoster have vesicles full of infectious virions that can become airborne and subsequently infect a nearby susceptible person, although herpes zoster is only about half as contagious as chickenpox and it usually requires close exposure to open cutaneous lesions.
Fetal transmission
Fetal transmission may occur in-utero, or in the perinatal period.49 Intrauterine or prenatal infection of the fetus is facilitated through transplacental transmission, while postnatally VZV is transmitted through respiratory droplets or direct contact with an infected person.49 The exact mechanism of in utero VZV infection is obscure. However, it is widely accepted that maternal viremia leads to transplacental transmission with a subsequent fetal infection. Therefore, prevention of maternal infection is of high priority.
VZV DNA may be detected in multiple fetal organs; histologic examination of the placenta demonstrates granulomas and acute inflammation. The sites of VZV replication in the fetus are currently unclear. It is believed that the fetus develops primary infection in utero which is followed by resolution and subsequent infection of the dorsal root ganglia. This results in destruction of the nervous tissue, which may account for limb denervation changes.
Signs and symptoms and pregnant women
The most prominent clinical feature of the primary infection caused by VZV (chickenpox) is a vesicular pruritic rash that occurs mainly on the trunk, head, and neck region. Infection begins with viral replication in the regional tonsils and lymph nodes, and possibly within the salivary glandular ducts. This is the first viremia that lasts about 4–6 days. The second viremia occurs during 10–14 days post-exposure where VZV is again released into the blood, and invades skin tissues thereby resulting in the exanthemous phase in about 14–21 days.
The skin lesions appearing due to chickenpox are replete with infectious, well-formed viruses which are aerosolized and are then transmitted to others who have not had the disease previously. The skin lesions commonly occur in crops and progress from papules to vesicles to crusts over a few days. There may be anywhere from a few to many hundreds of skin vesicles, with an average of about 500. More severe cases manifest more severe rashes and take a longer time for healing. Concomitant symptoms include malaise, fever, and fatigue, and the illness usually lasts about a week. Complications include bacterial superinfection of the skin, meningoencephalitis, and pneumonia. Adults and immunocompromised patients are more prone to severe infections as compared to healthy and immunocompetent children.50
Following the primary infection, VZV then becomes latent, primarily in the neurons of the peripheral autonomic ganglia as well as the dorsal root ganglia and cranial (trigeminal) nerve ganglia.50 VZV has the capability to stay dormant at these sites, and can initiate a secondary infection after a several years long dormancy.48
Up to decades later, latent VZV may undergo reactivation, either spontaneously or following one or more of a variety of triggering factors to cause secondary infection as herpes zoster.50 VZV reactivation becomes more frequent with the increasing age of the human host mediated by a diminishing cell-mediated immunity in such subjects. Herpes zoster usually appears as a painful and itchy cutaneous vesicular eruption that occurs in a characteristic dermatomal distribution. While the main and most important complication of herpes zoster is postherpetic neuralgia (PHN), it has now become increasingly recognized that VZV reactivation causes a variety of acute, subacute, and chronic neurological syndromes.
Subjects who have received live attenuated chickenpox vaccine may still develop infection after an exposure to the virus (either a person with chickenpox or one with herpes zoster). This situation is termed “breakthrough varicella” and is less contagious than primary varicella. When a person develops chickenpox despite receiving two doses of vaccine, the disease is often very minor and may be difficult to diagnose as chickenpox.50 Although the incidence of chickenpox is not much higher in pregnant women as compared to non-pregnant adults, the disease course can become drastically severe during pregnancy. The clinical features in pregnant women correspond to those illustrated for the general adult population. The incidence of chickenpox pneumonia does not appear to be substantially raised in pregnancy, but if it does occur, the morbidity and mortality from this infection is many times higher when compared to non-pregnant women, particularly if it occurs in the third trimester.51
Signs and symptoms in fetuses/newborns
Chickenpox in pregnant women can cause congenital varicella syndrome (CVS) or neonatal chickenpox (or neonatal varicella) which can be associated with a plethora of health-related complications as well as neonatal mortality.52 Maternal chickenpox acquired during the first two trimesters results in an intrauterine infection in up to 25% of cases,53 and congenital anomalies can be expected in approximately 12% of these infected fetuses.54 Maternal chickenpox within the first 20 weeks of pregnancy is associated with an overall incidence of CVS of 0.91–2%.55,56 No cases of CVS have been encountered due to maternal chickenpox following 28 weeks' gestation.56 However, if a mother acquires chickenpox near term or immediately after delivery, the newborn is at an increased risk for neonatal chickenpox.
The clinical features of CVS are widespread, but some tissues and organs are selectively implicated. It can cause many developmental defects in newborns, such as skin lesions and defects of the eyes (e.g., microphthalmos, uveitis, and congenital cataracts), musculoskeletal and neurological abnormalities (e.g., microcephaly and mental retardation), developmental delay, and abnormalities of the gastrointestinal and genitourinary tracts as well as the cardiovascular system.52 Most of the infants with CVS face an imminent mortality.57 They often have to experience recurrent reactivation of VZV and may have multiple cases of clinical herpes zoster during infancy.
In neonatal chickenpox, infection may occur by transplacental transmission, ascending infection from the birth canal or through direct contact with infectious lesions during and after delivery. If maternal infection takes place 1–4 weeks prior to delivery, up to 50% of babies may be infected.58 When the time between VZV infection and delivery is too short, the antibodies are seldom produced by the mother and transmitted to the fetus, while when this time is more than 7 days before delivery, antibodies against VZV can move into the fetal circulation and protect him.59,60
Maternal herpes zoster does not seem to determine CVS or neonatal VZV infection,61,62 although rare cases have been reported.63
Diagnosis
The diagnosis of VZV infection is generally made clinically by inspecting the appearance of the skin rash. In confusing or unusually appearing cases, the diagnosis may be confirmed through detection of viral DNA by PCR testing of skin scrapings from the base of the vesicle or through the detection of VZV antigen by immunofluorescence studies. Culture of VZV from vesicular fluid may also be used, but it is more expensive, takes more time (due to slower viral replication), and is less sensitive than the direct detection techniques (e.g., PCR).64 Serologic testing is usually not necessary for diagnosis of maternal varicella, and may be potentially confusing since the assays vary in sensitivity and specificity.
The diagnosis of varicella pneumonia should be considered when a pregnant woman has characteristic skin lesions, contact with varicella, and clinical symptoms suggesting chest infection. In women with suspected meningoencephalitis due to VZV, the viral DNA may be demonstrable in cerebrospinal fluid.64
After maternal infection, the risk of fetal infection can be estimated using PCR testing of the fetal blood or amniotic fluid samples for VZV DNA. PCR testing for VZV DNA is a sensitive test, usually obtained between 17 and 21 weeks of gestation. PCR and serological analysis carried out on samples of the fetus only confirm the presence of infection, but does not reflect whether the fetus is affected. In most cases, wherein the serological results are positive, the fetus is often found to have normal morphology at birth.
Ultrasonography should be added to PCR for detection of fetal abnormalities. A detailed ultrasound evaluation should be carried out at 5 weeks after maternal infection to assess fetal abnormalities consistent with CVS.49 Normal results of imaging and laboratory testing suggest a low risk of CVS. A normal ultrasound with detectable VZV DNA suggests potential risk; thus, a repeat ultrasound at 22–24 weeks should be performed. If the repeat ultrasound is normal, the risk of CVS is remote. In case the ultrasound shows evidence of CVS, the woman should be counseled regarding the imminent fetal complications.65 Enders and Miller have described prognostic value of prenatal diagnosis using ultrasound and PCR that can be very useful in cases of CVS suspicion.66
Intrauterine VZV infection can be demonstrated through the detection of anti-VZV IgM in the umbilical cord blood. Other criteria for intrauterine VZV infection are detection of VZV DNA in the newborn and persistence of VZV IgG beyond seven months of age; appearance of clinical zoster infection during early infancy.
Treatment
Pregnant women with a confirmed chickenpox could be treated with oral acyclovir therapy (800 mg 5 times per day for 7 days),67 but the therapy should be ideally started within 24 hours of symptomatic onset.68 If the diagnosis is uncertain, it is necessary to assess whether it is more appropriate to wait for confirmation of the diagnosis or to immediately begin therapy albeit a doubtful diagnosis. This therapy seems to reduce the duration of the signs and symptoms in chickenpox infected adults.52
Pregnant women with chickenpox that is complicated by pneumonia must be treated with prompt antiviral therapy as there is evidence of its efficacy in the prevention of maternal mortality.
It was suggested to administer acyclovir within 24–72 h of the onset of rash: 10–15 mg/kg every 8 hours for 5–10 days.67 The evidence available shows that acyclovir does not have a teratogenic effect69 and does not increase the risk of adverse events at any stage in pregnancy. The risk-benefit of treatment of maternal varicella infection outweighs any theoretical concerns regarding fetal toxicity.70 In case of maternal chickenpox at term, delivery can be delayed until 5–7 days after the onset of maternal rash so as to allow the transmission of maternal antibodies to the fetus.
Prevention of MMR and VZV infection
Three vaccine formulations are available in several countries: (1) a combined measles-mumps-rubella (MMR) vaccine that contains live attenuated measles, mumps and rubella viruses; (2) a monovalent varicella vaccine that contains live attenuated VZV (vaccine against chickenpox); (3) a combined measles-mumps-rubella-varicella (MMRV) vaccine that includes live attenuated measles, mumps, rubella viruses and VZV (vaccine against chickenpox). Single-antigen (i.e., monovalent) formulations of measles, mumps, and rubella vaccines are not available in all countries.
For all women in preconception period, formal documentation of immunity to rubella and measles should be established. The test of choice for determining rubella immunity is an immunoglobulin G (IgG) antibody titer, as an IgM antibody titer is only a marker of recent/acute infection. Once rubella immunity has been documented, repeat serology is unnecessary in subsequent pregnancies. Documentation of immunity to measles has become a component of preconception care in many countries due to the rising incidence of the infection in recent years.
For women in the childbearing age group who are at high risk of measles exposure (e.g., healthcare workers, travelers to endemic countries), the evidence of immunity includes documentation of age-appropriate completion of MMR vaccination (at least one dose of live measles-containing vaccine), laboratory evidence of immunity, or laboratory confirmation of measles.2 Any of the standard serologic assays for measles-specific IgG may be used for laboratory documentation of measles immunity.
In the absence of any evidence for immunity to rubella or measles, ensuring immunity against MMR in women of childbearing age is quite important since these immunizations are contraindicated during pregnancy and infection in non-immune pregnant women can adversely affect pregnancy outcomes. For this purpose, two doses of the MMR vaccine should be administered at least 28 days apart because preconception vaccination can prevent MMR infection in pregnancy.71 The MMR vaccine is an effective tool for preventing serious illness due to these viral infections.72,73 It is rare for immunocompetent individuals to not be fully immune after two doses of MMR.
If a woman has documented receipt of one or two doses of rubella-containing vaccine, but has rubella serology that is not clearly positive, she should receive one additional MMR vaccine dose (as there can be a maximum of three doses).2 If measles vaccination history cannot be ascertained, or the measles immunoglobulin G (IgG) is negative, non-pregnant women should receive one or two doses of the MMR vaccine based on their risk category.
Women vaccinated against MMR should be counseled to avoid pregnancy for 28 days straight after receiving MMR because of the theoretical risk of CRS. The risk of adverse events among women who became pregnant soon after receiving these vaccines has not been well established.74 Because a theoretical risk of transplacental fetal infection cannot be definitively excluded, the use of live vaccines such as MMR and chickenpox vaccines is discouraged during pregnancy. Nevertheless, harmful effects have not been reported in newborns of mothers who received live vaccines during pregnancy: (1) no adverse outcomes clearly attributable to measles following vaccination; (2) no congenital rubella syndrome-like defects (e.g., hearing loss, cataracts, congenital heart disease, bone lesions, IUGR, and mental retardation) have been observed in the offspring of women inadvertently vaccinated just before or during pregnancy.74,75,76 However, there have been reports of a subclinical infection. Termination of pregnancy after inadvertent vaccination is not warranted for this indication, given the absence of any serious documented congenital deformities.74
Before administering MMR or chickenpox vaccines to a woman of childbearing age, reasonable practices should include asking the woman if she is pregnant or could become pregnant in the next 4 weeks, and then counseling her about the potential fetal risks of vaccination during pregnancy or just prior to conception. Pregnancy testing of women of childbearing age is not necessary as long as pregnancy can reasonably be excluded by history.
If not done in the preconception period, rubella immunity should be documented as part of initial prenatal care among the pregnant women. It is important to counsel the susceptible or high-risk pregnant women regarding the risks for intrauterine rubella, and recommend that they restrict their contact with persons who have a confirmed, probable, or suspected rubella for >6 weeks (two incubation periods) after symptomatic onset in the affected individuals. Pregnant women who do not have evidence of immunity should receive a dose of MMR and/or chickenpox vaccine upon completion or termination of pregnancy and before discharge from the healthcare facility. Postpartum vaccination programs have been shown to significantly reduce rubella susceptibility in pregnant seronegative women.77 Repeat testing for serologic evidence of immunity thereafter is not required.
Postpartum women should receive all the recommended vaccines that could not be or were not administered during pregnancy (e.g., tetanus toxoid, reduced diphtheria toxoid, acellular pertussis [TDaP], and human papillomavirus). The vaccine can be given safely to postpartum women who are breastfeeding. Although rubella virus is excreted into breast milk, only seroconversion without serious infection has been reported in breastfeeding infants.78
For RhD-negative women who receive anti-D immunoglobulins in the postpartum period, MMR and/or chickenpox vaccination is still performed, if indicated following the delivery. A theoretical concern is the possibility that anti-rubella antibodies may be present in sufficient concentration in anti-D immunoglobulin to inhibit the woman’s immune response to the live rubella vaccine. However, there is evidence that anti-D immunoglobulins do not reduce a patient’s response to the rubella vaccination79,80,81 and there are no published reports of rubella vaccine failure after administration of anti-D immunoglobulin. Nevertheless, the CDC suggests that women who have received both anti-D immunoglobulins and rubella vaccine be serologically tested (~3 months) after vaccination, so as to ensure that seroconversion has occurred. The second vaccine dose should be administered at about 6 weeks later, although many countries are not yet using a two-dose schedule resulting in reduced protective effect (e.g., one chickenpox vaccine dose offers about 85% protection, while two doses offer up to 98% protection rate)82.
MMR may also be administered to household contacts of pregnant women (e.g., children) whenever indicated. Chickenpox vaccine can also be given to close contacts of a pregnant woman, regardless of her susceptibility to VZV. The risk of transmission of VZV from a healthy individual who received the varicella vaccine to a healthy but susceptible household contact is extremely low.61 Although there are rare cases of susceptible pregnant women being infected with the vaccine strain virus transmitted from their children who developed chickenpox following the live varicella vaccination,83 the actual risk of transmission of vaccine virus is considered much smaller than the risk of an unimmunized child developing chickenpox, and then transmitting it to a susceptible mother.
Post-exposure prophylaxis (PEP) for MMR and VZV infection
Measles
Within 72 hours of a confirmed measles exposure, post-exposure prophylaxis (PEP) for susceptible non-pregnant women consists of MMR vaccination.84 Although administration of vaccine is preferable to immune globulin therapy (since vaccination provides active, long-lasting immunity against measles), pregnant women, who cannot be vaccinated with MMR, should receive a passive immunization through immune globulin therapy.85 Immune globulin therapy can prevent or diminish the clinical severity of measles if administered to non-immune individuals within 6 days of exposure.77 Administration of immune globulin is appropriate for those exposed individuals who are at an increased risk of measles-related complications with relative contraindication for MMR vaccination.
Pregnant women are at increased risk for severe measles and complications. Therefore, administration of immune globulin may be appropriate for the pregnant women without any documented evidence of measles immunity who have been exposed to measles. Non-immune women with measles exposure who received immune globulin in pregnancy, should also receive MMR vaccine in postpartum, but no earlier than 8 months after immune globulin. With regard to the dose and the route of administration, the national advisory committee on immunization (NACI) measles-mumps-rubella-varicella working group (MMRVWG)85 has proposed an intramuscular immune globulin (IMIg) dose of 0.5 mL/kg to provide an immediate protection to pregnant women, but IMIg can be provided up to a maximum volume of 15 mL. Therefore, anyone weighing 30 kg or more will not receive an optimal dose of IMIg at 0.5 mL/kg. Large volumes, greater than 3–5 mL for adults, should be divided and injected at two or more sites; therefore, anyone receiving 15 mL of IMIg would be subject to multiple injections. Multiple injections may not be acceptable to all subjects, and intravenous immune globulin (IVIg) may be preferred (providing it at a dose of 400 mg/kg).85
Mumps and rubella
Following mumps or rubella exposure, neither post-exposure MMR nor immune globulin has been shown to prevent disease or lessen disease severity.
VZV
Susceptible women who have had a significant exposure to chickenpox (e.g., household contact, face-to-face contact for at least 5 minutes with a contagious person) or herpes zoster infection (i.e., close contact or exposure to open cutaneous lesions of a contagious patient), should receive immunoprophylaxis as PEP intervention.
Passive immunization with an immune globulin product containing high titers of anti-VZV antibodies should be guaranteed to VZV seronegative pregnant woman who have had a significant exposure to chickenpox or herpes zoster infection. Ideally, a VZV serologic test should be conducted prior to administration of immunoprophylaxis (for reasons of economy) among those women who report a negative or uncertain history of chickenpox.86 However, rapid screening is necessary since prophylaxis should be offered within 10 days of exposure. If results of serologic testing are not available within this time frame, then immune globulin should be offered.
In some countries, the oral anti-VZV immune globulin preparation currently available is VariZIG. It is a purified immune globulin preparation made from human plasma containing high levels of anti-VZV antibodies. VariZIG is supplied in 125 IU vials and should be given intramuscularly. The recommended dose is 125 IU/10 kg where a maximum of 625 IU (five vials) can be used simultaneously. VariZIG should be ideally administered within 96 hours (4 days) post-exposure to obtain maximal efficacy.87 It was suggested but not proven that immunoprophylaxis with anti-VZV antibodies would only be effective when given prior to the primary viremia, and that perhaps antiviral therapy (i.e., acyclovir) should be considered against secondary viremia.56,88 In some countries, intravenous anti-VZV immune globulin is currently available and can be useful for pregnant women who cannot receive VariZIG within 10 days of exposure. Intravenous anti-VZV immune globulin is administered a single dose at 400 mg/kg.
Anti-VZV immune globulin is offered to all the exposed pregnant women in order to reduce the risk of chickenpox and also attenuates the severity of infection in those who have seroconverted. Anti-VZV immune globulin could decrease the risk of vertical transmission of VZV due to a lower viremia. Current evidence does not clearly support on whether immunoprophylaxis is beneficial in reducing the risk of CVS. Any patient who receives VZV immune globulin should be observed closely for pathognomonic clinical features of chickenpox for up to 28 days after exposure.46 Antiviral therapy should be instituted immediately if signs or symptoms of varicella occur despite PEP.
Women who do not develop chickenpox after PEP should be administered live chickenpox vaccine after delivery and at least 5 months following administration of VZV-specific immune globulin administration.
There is no evidence that antiviral therapy (e.g., acyclovir) could be useful as a PEP intervention among VZV susceptible pregnant woman who have had a significant exposure to chickenpox or herpes zoster infection.
CYTOMEGALOVIRUS AND PREGNANCY
Cytomegalovirus (CMV) is a major contributing pathogen implicated in TORCH infections89 encountered during the course of pregnancy. CMV has been categorized as an enveloped DNA virus, belonging to the herpes virus family. CMV can be passed from person-to-person, usually via close contact CMV because infected saliva is a major factor responsible for interpersonal dissemination of the viral agent.90 Vertical transmission of the CMV has been significantly correlated with a broad range of developmental complications, including loss of hearing or vision, mental retardation, and cerebral palsy.91
Thus, special attention is warranted to understand the transmission, clinical manifestations, and management of this condition in details.
Maternal transmission
A woman may acquire CMV infection prior to conception, or during early pregnancy. Several routes of transmission have been established for maternal CMV infection. These include the following:92
- Non-sexual dissemination: exposure to saliva, urine, cervix secretions, semen, breast milk and in other bodily fluids CMV (with transmission to hands then to mucosal surfaces or directly to the mucous membranes);93
- Transfusion of blood or blood-based products, and organ transplantation;
- Transmission via droplet and/or aerosol (that, as for other herpes viruses, has been downplayed in case of CMV);
- Sexual contact.
The incubation period is on average of 40 days (28–60 days) and viremia lasts 2–3 weeks after primary infection. After this period, IgM followed by IgG antibody begin to be produced. During primary maternal CMV infection, CMV is shed from multiple bodily fluids.
An estimation of asymptomatic CMV infection among the women of childbearing potential can be carried out by means of serological assays (IgG antibody titers). It has been found that the seroprevalence of CMV ranges from 45% to as high as 99.9% throughout the world. The highest seroprevalence rates have been documented for women belonging to the Mediterranean, African, and Asian regions, whereas other Western countries report the lowest infection rates.94,95
A common trait of all herpes viruses is that they could enter into a latent stage in host cells following a primary infection. This is also true for CMV, and the dormant virus can become reactivated during later events of immunosuppression, infection or trauma experienced during pregnancy, thereby leading to secondary infection. In-vitro studies have shown that some common latency sites could include myeloid cells (monocytes) and vascular endothelial cells.96,97 Secondary infection can occur after reactivation of the latent endogenous CMV strain or by reinfection with a different exogenous viral strain.
In summary, pregnancy-associated CMV transmission can be strongly correlated to the following epidemiological factors: (1) poor socioeconomic status; (2) geographical and ethical/religious factors; (3) advancing maternal age; (4) multiparous women; (5) contact with infected family members; (6) having a child who attends daycare94,98,99,100 because CMV occurs more frequently in children under 2 years.101
Fetal or neonatal transmission
Maternal infection when associated with seroconversion during pregnancy, poses the highest threat for fetal CMV transmission because the virus in maternal blood can cross through the placenta and infect the developing fetus. Therefore, vertical transmission can occur when a primary CMV infection develops during or just before pregnancy.
The overall risk for fetal infection is approximately 30–40% during primary maternal infection, but it depends upon the gestational period where it becomes the highest during the third trimesters (40–72%) as compared to the second and first trimester (34–38% and 30%, respectively), and periconception period.102,103,104,105
Despite a considerably higher rate of fetal infection during the later trimesters of pregnancy, studies have shown that this is seldom accompanied by an increased probability of debilitating CMV complications. Following routes of maternal-fetal (vertical) transmission have been confirmed: (1) transplacental infection; (2) intrapartum infection, via exposure to infected maternal genital secretions during delivery; (3) postnatal infection, mainly through breastfeeding.98
In line with the maternal CMV transmission, congenital CMV infection has been found to be profoundly associated with demographic factors. Statistics reveal that more than 1% of pregnancies may become associated with congenital CMV infection in the Asian and African continents, while far lower percentage is seen in the developed world.
Signs and symptoms in pregnant women
Although primary CMV infection almost always produces no symptoms or a few mild symptoms in healthy subjects, including children and pregnant women,106 it can sometimes cause the following major clinical features:107,108,109,110,111
- Mild fever, malaise, headache, and sweats;
- Flu-like symptoms associated with rhinitis, pharyngitis, sore throat and lymphadenopathy;
- Generalized fatigue, arthralgias, and body aches;
- Skin manifestations such as maculopapular rash which may resemble that seen in rubella, measles, and group A beta-hemolytic streptococcal infections;
- Colitis, uveitis, retinitis, arteriovenous thrombosis, myocardial disease, pneumonia or others).
Surprisingly, this list is by no means exhaustive (see Table 1).
Initial symptoms of primary CMV infection can sometimes be indistinguishable from mononucleosis due to Epstein-Barr virus.112
During primary CMV infection, viremia is present for the first 2–3 weeks and a series of deranged biochemical findings have been reported among CMV infected individuals.107,113 A few of them are reported as follows: (1) lymphocytosis, (2) thrombocytopenia; (3) hemolytic anemia; (4) abnormally elevated hepatic transaminases (ALT, AST) and bilirubin.
Generally, nonprimary maternal infections (reactivation and/or reinfection) are not associated with any severe signs or symptoms in the immune population.
Signs and symptoms in fetuses/newborns
As mentioned above, the rate of CMV vertical transmission increases as gestation progresses, but the severity of congenital CMV disease is greatest if it develops early in pregnancy.
Based on the data obtained from analytical studies, as many as 11–13% of the seropositive neonates subsequently develop symptoms which can be specifically attributed to CMV.114,115
However, not all of these infected neonates will go on to develop crippling complications such as permanent hearing loss. Despite a low prevalence of CMV-related complications, neonatal CMV infection can quickly develop into a multisystem disorder where it can manifest itself through the following signs and symptoms:90,116,117 (1) fetal growth restriction (FGR) leading to small-for-gestational age (SGA) neonates; (2) jaundice accompanied by hepatosplenomegaly; (3) dermatologic manifestations (e.g., blueberry muffin rash); (4) microcephaly; (5) mental retardation; (6) hydrocephalus; (7) meningoencephalitis and seizures; (8) decreased neuromuscular tone; (9) delayed childhood milestones; (10) sensorineural deafness; (11) visual disturbance due to CMV uveitis, retinitis and optic disc atrophy; (12) thrombocytopenic purpura (Table 1).
Some evidence has shown that maternal reinfection can lead to vertical transmission, fetal damage, and long-term sequelae.118,119,120
CMV disease due to postnatal infection is uncommon in full-term infants probably because of two reasons: (1) the protective effect conferred by maternal antibodies that transfer to the fetus predominantly during the last weeks of gestation, and (2) the more efficient immune system of the babies who are born at full term compared with those who are born early.121,122
Instead, very low birth weight (VLBW) and premature infants are at risk for developing symptomatic postnatal CMV disease (characterized by any of the following: neutropenia, thrombocytopenia, hyperbilirubinemia, elevated liver enzymes, hepatopathy, jaundice, petechiae or CMV pneumonia), including CMV-related sepsis-like syndrome (defined as sepsis-like symptoms, such as bradycardia, apnea, or respiratory deterioration with CMV viruria and without bacterial infection).122,123
1
Summary of CMV-related maternal-fetal manifestations.
Constitutional features |
|
Gastrointestinal system |
|
Skin and subcutaneous tissues |
|
Central nervous system |
|
Eyes |
|
Cardiovascular system |
|
Hematology |
|
Pregnancy-related complications
Viral infections including CMV, per se, have been implicated in a plethora of serious issues throughout pregnancy. There exists a growing consensus that CMV and other viral infections can lead to placental pathology, FGR, fetal loss (miscarriage or stillbirth), preterm labor, and/or preterm premature rupture of the membranes (PPROM)124,125,126,127,128,129 as well as congenital and postnatal CMV disease with or without consequent disability (described above).
In addition, CMV also leads to an adverse impact over maternal health, with a higher risk for maternal morbidity and mortality.130
Diagnosis
During pregnancy, diagnostic evaluation of CMV infection can be carried out for both the mother and fetus. A timely management can certainly help boost the maternal/fetal survival rate, while averting the onset of many life-threatening complications.
Maternal CMV status can be checked at the time of first booking visit. This can be accompanied by laboratory-based assessment of other infectious diseases (TORCH) as well. However, CDC and American College of Obstetricians and Gynecologists (ACOG) do not recommend routinely prenatal serologic screening for CMV infection because test cannot predict if the baby will be infected or if he will have health problems.131,132
If a woman has clinical findings or symptoms suggestive of CMV infection, a careful physical examination could help point to the need of a confirmatory test for CMV.
An ELISA-based estimation of anti-CMV immunoglobulins (IgG) in the maternal blood is the best method of identifying the infection. In this regard, detecting a positive seroconversion (anti-CMV IgG-negative to IgG-positive) or a four-fold rise in IgG titers through two serum samples taken 3–4 weeks apart, is enough for establishing a confirmed diagnosis of infection.133
Although the former condition is not always possible, serological testing still represents the best tool for validating a maternal CMV infection.134,135 However, the sensitivity of antibody testing to distinguish between primary and nonprimary CMV infections is very poor. The anti-CMV IgM, which is usually a marker of an acute infectious process, can remain elevated in the serum even 12 months after the primary infection. This undermines its role for differentiating between an acute CMV infection, a latent or secondary infection, and reinfection with another viral strain. This drawback can be overcome through a careful estimation of antibody avidity, a technique that relies on measuring the overall binding strength between the antibody and its corresponding CMV antigen.134
This binding affinity of an antibody is known to be low during recent onset of CMV infection in pregnant women (the first 2–4 months), but increases in the later stages of infection.136 The simultaneous presence of anti-CMV IgM and low-avidity anti-CMV IgG is consistent with primary infection.135
Upon correctly identifying a maternal CMV infection, it remains to be seen whether transplacental transmission has occurred or not. In addition, the fetus needs to be meticulously assessed for any imminent complications. For this purpose, the following methods maybe utilized ultrasonography, amniocentesis and other less common tests.
Ultrasound offers a reliable and noninvasive approach towards diagnosis of fetal CMV. It can adequately assess fetal risk, and help modulate the course of case management.137 Some notable fetal ultrasound findings specific for CMV include:134 (1) reduced head circumference and/or biparietal diameter (microcephaly); (2) ventricular dilatation; (3) calcifications in the periventricular area; (4) cerebellar hypoplasia; (5) retarded growth parameters (e.g., subnormal crown-rump length, reduced abdominal circumference, and decreased femur length); (6) abnormal amniotic fluid index (could be oligohydramnios or polyhydramnios); (7) hepatosplenomegaly; (8) fetal edema. Even if ultrasound fails to reveal diagnostic findings in the earlier stages, the slightest clinical or laboratory-based suspicion of CMV should be followed with a series of ultrasonographic examinations throughout the course of pregnancy.
In case of positive ultrasound findings or a seropositive diagnosis of a maternal blood sample, it is advisable to carry out invasive fetal testing in the form of amniocentesis.138 This can help in charting the future course of pregnancy as some individuals might support the idea of terminating the pregnancy before any permanent sequelae appear. Amniocentesis can be used to withdraw a sample of amniotic fluid from within the gestational sac; the procedure is usually carried after 15 weeks of pregnancy. CMV DNA can then be detected by amniotic fluid PCR (polymerase chain reaction) technique with a fairly high sensitivity. However, when carried out prior to 21 weeks’ gestation, amniocentesis might possibly fail to detect CMV. This is mainly due to an insufficient renal excretion of the viral particles into the amniotic space.
Other less commonly used tests include fetal blood sampling by means of cordocentesis where the umbilical artery can be directly approached for extracting a blood sample,139 in which thrombocytopenia, abnormal liver biochemistry, or other biomarkers can be assessed.
In any case, CMV DNA especially after 21 weeks, is the most sensitive and specific test for fetal infection. However, a positive result does not mean that the fetus will be affected by permanent disability as a consequence of CMV infection. Ultrasound detection of fetal or placental abnormalities is more predictive of CMV disease and long-term consequences.
In addition, imaging modalities other than ultrasound (e.g., magnetic resonance imaging, MRI) can also be used for detecting fetal anomalies.
Treatment
Currently, there is no effective vaccine or therapy to prevent maternal CMV infection or fetal infection.102,140 The only effective strategy is to reduce CMV exposure in women especially during pre-pregnancy and pregnancy period.
All pregnant women and women trying to conceive should routinely receive information about CMV prevention.
If during pregnancy the diagnostic signs of congenital anomalies become grossly evident, the couple must be carefully counseled. The couple must then be offered a plan of conservative management that involves the following strategies:134 (1) symptomatic treatment (e.g., antipyretics); (2) antiviral drugs against CMV; (3) hyperimmune globulin (HIG).
Following are a few antiviral drugs utilized against CMV:
- Valacyclovir, which has been found to be potentially useful against CMV infection during pregnancy. There are a few limited trials that indicate only a partial reduction in fetal as well as maternal viral concentrations.140 However, a recent randomized controlled trial (N = 90) has profoundly supported the role of valacyclovir in the prevention of vertical transmission of CMV.141
- Ganciclovir/valganciclovir, drugs that are mostly utilized in neonates with a confirmed, symptomatic CMV disease. Experts have shown an efficacy of a combination of these two agents in infantile CMV where an initial intravenous ganciclovir therapy can be followed by the oral administration of valganciclovir. This drug combination can significantly improve sensorineural hearing deficit.142 In contrast, due to a possible risk of teratogenicity, their use during early pregnancy is questionable.134
The hyperimmune globulin (HIG) can be administered to the pregnant women who possess a potentially high risk for transplacental infection in the developing fetus. HIG mostly carries out its function by downregulating the inflammatory mediators at the maternal-fetal interface, while it has also been linked to a reduction in T-cell mediated immunity.143,102 Experimental trials have indicated that administration of HIG before 17 weeks' gestation can drastically improve the fetal outcome.144 Other studies have found that using HIG during pregnancy does not significantly impact the rate of fetal infection, but it is a plausible option in preventing long-term complications.145 A few trials have even undermined the role of HIG in cutting down the maternal/fetal levels of viral DNA, while stating that it does not offer any better fetal outcome for the CMV-complicated pregnancies.102
There is currently no indication about the timing and route of delivery for pregnant women with CMV infection, not even in the case of recovery of CMV from the cervix or urine. The mode of delivery is determined by standard maternal and fetal indications.
Prevention
In the absence of any guaranteed treatment or vaccination protocol, prevention of maternal CMV infection is the best strategy against congenital CMV infection. All pregnant women and women trying to conceive, should be adequately educated regarding sources of maternal CMV infection, its permanent sequelae in the children, and methods of hygiene to prevent exposure to CMV, as part of routine pre-pregnancy and pregnancy care. Pregnant women should be advised to avoid close contact with the immunocompromised individuals or other subjects who could possibly be a carrier of CMV, such as young children. Women should be warned to: (1) wash hands often especially after changing diapers, feeding a young child, wiping a young child’s nose or drool; (2) avoid to kiss younger children directly on the mouth or sharing utensils or any other items of personal use with younger children. Moreover, a proper body hygiene must be maintained at all times.146,147
As breastfed VLBW and premature infants are at risk to develop postnatal CMV disease, seropositive mothers should be informed about the possibility of CMV infant infection through breast milk.
The American Academy of Pediatrics stated that the benefits of breastfeeding outweigh the potential risks of a postnatal CMV infection, based on the absence of evidence to support the possibility of long-term neurodevelopmental abnormalities.148
Although the heating of the milk is able to eliminate CMV viral load (i.e., holder pasteurization or high-temperature short pasteurization), American Academy of Pediatrics recommends fresh mother’s own milk because heating affects bioactive factors and nutrients.148 On the other hand, freezing of milk reduces CMV viral load, but is not able to eliminate CMV viral load.149
Active immunization in prepregnancy (e.g., vaccines against measles, rubella, varicella) or gestation period (e.g., vaccines against influenza) offers a sustainable protection against a number of viral illnesses, but a vaccine against CMV is not yet available. Nevertheless, a few potential candidates have been put forward in this respect. A vaccine which targets a CMV envelop protein is being evaluated in advanced clinical trials.134
HERPES SIMPLEX VIRUS 1–2 AND PREGNANCY
The Herpes simplex virus (HSV) is a ubiquitous, enveloped, and double-stranded DNA virus belonging to Alphaherpesvirinae, a subfamily of the Herpesviridae family that also includes VZV.
Two viral types of HSV exist, type 1 (HSV-1) and type 2 (HSV-2), and both of which are known to cause widespread infection in humans, thus representing a serious global burden.150,151,152 The global seroprevalence of HSV-1 infection in 2012 was estimated of about 67% and HSV-2 of about 11%.150,151
A pre-existing HSV-1 or HSV-2 infection is common among pregnant women.153,154 The major concern of HSV during pregnancy infection is transmission to the fetus or newborn sometimes with devastating consequences.
The age of antiviral therapy has contributed to better clinical outcomes in infected neonates, but infants with invasive HSV disease continue to suffer substantial morbidity and mortality. This analysis provides a summary of the epidemiology and clinical characteristics associated with the transmission of HSV from mother to infant. As well as emerging methods for preventing the vertical transmission of HSV infections, treatment recommendations are briefly addressed.
Maternal transmission
HSV-1 is primarily transmitted by oral–oral transmission, whereas HSV-2 is primarily sexually transmitted. Sexually transmission of HSV may occur quickly in new sexual relationships155 and can also occur during periods of subclinical viral shedding.
In each trimester there is the same probability of HSV infection.156
Fetal or neonatal transmission
Maternal HSV infection can be transmitted to the fetus or newborn. HSV transmission to fetus/newborn can occur during intrauterine, perinatal (peripartum) or postnatal (postpartum) period, but usually occurs during labor and delivery (perinatal transmission) as a result of direct contact with virus shed from infected external or internal genitals.157
Intrauterine transmission is extremely rare, especially when HSV genital infection arises early in pregnancy allowing the development of type-specific HSV antibodies before the delivery.156 This transmission has been documented only in case of primary HSV genital infection and has been associated with miscarriage, preterm delivery, congenital defects, growth restriction, and/or low birth weight.156,158,159,160
Some factors that can increase the risk of perinatal transmission include:
- Newly acquired HSV infection near the time of delivery (particularly within 6 weeks of delivery) either symptomatic or asymptomatic161 because: (a) the duration, quantity, and concentration of viral shedding and the time to total healing are greater in newly acquired genital HSV infections, (b) the mother has no type-specific anti-HSV antibodies in newly acquired genital HSV infections (they develop within the first 12 weeks after infection162 resulting in loss of their protective effect on the fetus/newborn;163
- Presence of maternal fever;164,165
- Prolonged ruptured of membranes (>6 hours), probably as a result of virus ascending;
- Disruption of cutaneous barrier through application of fetal scalp monitors or others practices that can provide a site of inoculation of the virus;
- Preterm birth;161
- HSV serotype, because genital infections due to HSV-1 expose the fetus to greater risk than infections by HSV-2.
It is important to remember that viral shedding can occur in the absence of maternal signs and/or symptoms166,167 (30–50% of HSV-2 positive women shed HSV in their genitals during delivery),169,170 and identify whether a pregnant woman has a primary, nonprimary first-episode or recurrent HSV genital infection, because in a newly acquired HSV infection (primary or nonprimary first-episode) near the time of delivery, the risk of vertical transmission is higher than in a recurrent infection).161,162 The probability of perinatal transmission, among neonates delivered vaginally when newly acquired genital HSV infections occur in pregnant women at the time of delivery, has been calculated about 40–80%.156,161 The probability of perinatal transmission, among neonates delivered vaginally when recurrent genital HSV infections occur in pregnant women at the time of delivery, is lower than that associated with newly acquired genital HSV infections. The risk of perinatal transmission appears to decrease with higher titers of anti-HSV antibodies.171
However, most perinatal transmission occurs during unrecognized HSV genital lesions or asymptomatic HSV shedding.
Postnatal HSV transmission is more likely than intrauterine and less than perinatal transmission. It occurs as a result of direct contact with subjects infected with HSV (active HSV infection), typically from a cutaneous or mucosal source.
Signs and symptoms in women
HSV can cause orolabial lesions (oral herpes) or genital lesions (genital herpes). In orolabial lesions, HSV-1 predominates and is usually present in the trigeminal ganglia, while HSV-2 is found most frequently in the lumbosacral ganglia. However, both the orofacial regions and the genital tract may be affected by these viruses.172 Even, according to the CDC, anogenital infections due to HSV-1 infection are increased especially among young women.
Like all Herpesviridae viruses, HSV migrates to nerve tissues, where it persists in a latent state. It can reactivate and causes recurrent infections in the host.
The symptoms of primary oral herpes consist in ulcerative lesions involving the hard and soft palate, tongue, buccal mucosa, and others facial areas. There are some oral sequelae caused by HSV: (1) recrudescent herpes labialis; (2) asymptomatic shedding of HSV-1 in saliva; (3) recrudescent localized intraoral; (4) recrudescent intraoral herpes infection mimicking primary herpetic gingivostomatitis.173 By definition, it is named recurrence when an episode of asymptomatic viral reactivation occurs, and recrudescence when a viral reactivation with herpetic signs occurs,174 but usually the term recurrent infection is indifferently used to identify both conditions. Prodromal symptoms (i.e., pain, itching or burning at the site of subsequent eruption) can precede oral HSV sequelae.
HSV genital infection have three different clinical manifestations:
- Primary infection that occurs when a susceptible individual (deficiency of pre-existing HSV-1 and HSV-2 antibodies) is exposed to HSV;
- Nonprimary first-episode that occurs when a person with preexisting HSV antibodies (against HSV-1 or HSV-2) encounters a first episode with the opposite type of HSV;
- Recurrent infections that can occur in case of viral reactivations in a person with preexisting antibodies against the same form of HSV.
Primary and nonprimary first-episode HSV genital infections can be asymptomatic or symptomatic with lesions of the vulva, labia, vaginal introitus, or cervix. Asymptomatic or subclinical HSV genital infections seem to be more common than symptomatic ones in pregnant women as well as in nonpregnant women156 and that is one of the reasons why most genital HSV infection are underdiagnosed.
Cutaneous lesions occur after 2–20 days of incubation. They are usually present as painful erythematous papules which progress rapidly to clear fluid-filled characteristic vesicular lesions, often appearing in a cluster. Usually, these fragile vesicles explode, but an excess of inflammatory cells can cause the lesions to grow into pustules. Each lesion will appear as a shallow ulcer on an erythematous base after the rupture. Typically, mucosal lesions do not have vesicles and progress directly to ulcerations. Lesions can last as little as 8–10 days or as long as 21 days for the complete process. In addition to pain, these can be accompanied by pruritus, dysuria, vaginal discharge, and/or local lymphadenopathy. Skin lesions can be localized in the perineal area, buttocks and/or inner thigh.
Sometimes primary genital infection can result in a more serious disease where systemic involvement may also occur with fever, inguinal lymphadenopathy, myalgia and headache. Autonomic neuropathy is also an exceptional but possible consequence.
HSV-1 and HSV-2 involve a similar symptomatology during primary genital infection.
Most of the time, symptomatic nonprimary first-episode genital infection results in a mild symptomatology compared with symptomatic primary infection. Indeed, the severity of symptomatology and reactivation with HSV-2 infection are lower in women with prior HSV-1 infection.175
Recurrent HSV genital infections tend to be milder than in primary HSV genital infections. There may be symptomatic or asymptomatic viral reactivation from latency and prodromal symptoms (e.g., burning, pruritus, or pain) can occur before genital lesions become apparent. In recurrent infections, symptoms are usually mild, short-lived, localized, with sometimes atypical lesions (e.g., vulvar irritation). Clinical or subclinical recurrent infections are almost certain for all women HSV-2-infected during their lifetime.176
During pregnancy, about three-quarters of women with recurrent genital HSV experience at least one HSV (recurrent) episode during pregnancy177 and about one in seven women experiences a clinical HSV recurrence or prodromal symptoms at delivery.177,178
Viral shedding can occur during each of the clinical and subclinical HSV genital recurrent infections.
Generally, the severity and frequency of recurrent infections are greater with HSV-2 than HSV-1.
Infection by HSV-2 increase the risk of HSV-1 and HIV infection179 and it is frequently present in subject with a history of sexually transmitted infections.180
Signs and symptoms in newborns
Neonatal HSV-1 and HSV-2 infections, and consequent diseases, acquired during perinatal or postnatal period can be further classified into three main categories, that are predictive of morbidity and mortality:157,181,182
- Localized to the skin, eyes, and/or mouth disease (SEM disease) that accounts for approximately 45% of neonatal HSV cases and that may progress to the other two clinical forms if not treated early;
- Central nervous system disease (CNS disease), with or without SEM disease, that accounts for about 30% of neonatal HSV cases;
- Disseminated disease involving multiple organs, including lungs, liver, adrenal glands, CNS and/or SEM, that accounts for about 25% of neonatal HSV cases.
Neonatal HSV infection acquired during postnatal period can be as severe and lethal as that acquired during perinatal period.
Diagnosis
A clinical diagnosis of genital HSV infection should always be confirmed by laboratory testing, also because typical genital HSV lesions may be absent in many infected women making clinical diagnosis difficult. A diagnosis of HSV infections based on clinical findings is insensitive.183,184
When a genital HSV infection is suspected, a laboratory confirmation is needed. Laboratory tests includes techniques for viral detection (i.e., viral culture and HSV antigen detection by PCR) and techniques for antibody detection.
Virus isolation in cell culture has many limitations: (1) has a low sensitivity, since it is conditioned by virus, quality of specimen, primary or recurrent infection; (2) requires a slow process and a complex samples management; (3) is conditioned by the virus recovery that changes depending on the stage of skin lesions (i.e., greater in vesicles and less in crusted lesions); (4) needs a sample collected from the lesions the first 3 days after their appearance.
PCR has a greater sensitivity than viral culture especially in the case of a recurrent episode and it allows detection of HSV specific DNA even beyond 3 days from the moment of the appearance of lesions. In addition, real-time PCR systems has increased the specificity of the classic PCR.
Viral culture or PCR can produce negative results, but this does not exclude the possibility of an HSV infection also because viral shedding may be intermittent.
A type-specific serologic assay should be performed routinely in association with viral culture to confirm the diagnosis of HSV185 because knowing the serotype has prognostic implications and it consequently requires specific counseling and management of women and their babies.
Serologic testing can be useful when: (1) viral culture of specimen is negative because a positive serology result suggests a primary infection; (2) an HSV infection is clinically suspected, but virus and antibody detection tests have resulted negative. In this case serologic testing should be repeated 3–4 weeks later and (1) if no seroconversion is found, HSV infection can be excluded as cause of the genital lesion; (2) if seroconversion of one or both type-specific antibodies is demonstrated, diagnosis of primary infection (i.e., primary seroconversion with no type-specific antibodies at baseline) or nonprimary first-episode (when the other type-specific antibody was negative at baseline) can be made. In addition, HSV serotyping in pregnant women with a history of genital HSV lesions and an episode during the third trimester of pregnancy seems to be effective and cost-effective as it improves outcomes and decrease costs.186
Serological assays that are not type-specific (HSV-1/HSV-2) have limited clinical utility.
In summary: (1) if a woman has a history of laboratory confirmed genital HSV lesions, further tests are not required; (2) if a woman has a history of genital lesions but without a previous laboratory diagnosis, and she manifests a genital lesion during pregnancy, a viral test should be performed on the ulcer to detect HSV and a type-specific HSV serology should be made to classify the HSV infection as primary, nonprimary, or recurrent; (3) if a woman has no history of genital lesions but one of these appears during pregnancy, type-specific serologic and virologic assays should be performed.
Prevention and treatment
Identify women and fetuses/newborns at risk of HSV infection and disease
Before or during pregnancy, a detailed anamnesis should be gained to identify susceptible women at greater risk of HSV infection having a seropositive sexual partner. These couples should be counseled because women could acquire HSV genital infection during pregnancy with consequent risk of neonatal infection.
A history of undiagnosed atypical genital lesion should be identified and eventually investigated. Women should be properly informed about genital HSV manifestations and associated neonatal risks.
Providing health information to women
All women should be made aware that: (1) both HSV-1 and HSV-2 can be sexually transmitted; (2) the use of condoms does not completely eliminate the possibility of an HSV infection; (3) HSV-1 infection does not protect against HSV-2 and vice versa; (4) HSV infection can be transmitted to fetus/newborn causing morbidity and mortality; (5) reporting to healthcare providers the onset of prodromal symptoms is important for the HSV management during pregnancy; (6) postnatal transmission can be prevented by taking care to avoid contact of the child with the HSV lesions (oropharyngeal, cutaneous or genital) or contaminated hands (i.e., caretakers with active lesions should cover them and pay attention to hand washing before touching the baby).
Procedures to avoid in HSV-infected pregnant women and their fetuses
Pregnant women with HSV genital lesions should not undergo transcervical procedures to avoid the risk of transferring the infection to placenta or membranes, whereas transabdominal procedures are not contraindicated in these women. Both procedures are not contraindicated for asymptomatic pregnant women for HSV.187
Application of fetal scalp electrode and use of vacuum or forceps are not recommended because they could increase the risk for HSV infection.
Antiviral treatment
Acyclovir, valacyclovir, and famciclovir are commonly used as antiviral agents to treat HSV infections. Acyclovir is the most well-studied in pregnancy and its safety has been recognized at all trimesters, while there are no published data on the use of famciclovir in pregnancy. Valacyclovir has been studied enough in pregnancy and is considered an alternative to acyclovir for treatment of pregnant women.
Antiviral treatment can be necessary, especially in newly acquired genital HSV infections (primary or nonprimary first-episode).188 If a newly acquired genital HSV infection arises anytime during pregnancy, antiviral therapy can be immediately administered189 in order to decrease the duration and severity of symptoms as well as to reduce the duration of viral shedding.40 Treatment with acyclovir 400 mg orally three times a day, for 7–10 days or, alternatively, valacyclovir 1 g orally, twice a day, for 7–10 days is indicated in the case described above.188 In case of severe disease and lesions not yet healed, both treatments can be extended for more than 10 days.162 In the case of severe genital HSV infection or disseminated herpetic infections during pregnancy, acyclovir may be administered intravenously: 5–10 mg/kg every 8 hours for 2–7 days and the remaining 8–3 days of oral administration.
Empiric antiviral therapy can be started in women with suspicion of newly acquired genital HSV infections (i.e., genital ulcers suggestive of HSV infection in women without a history of genital HSV), while waiting for diagnosis. This approach has the advantage of speeding up the treatment, but the disadvantage of administering a potentially inappropriate treatment.
When newly acquired genital HSV infections occurs in the third trimester, antiviral therapy could be continued until delivery for the same reasons as above.188
A suppressive antiviral therapy from the 36 weeks of gestation to the onset of labor could be administered in women with a genital HSV lesion anytime during pregnancy, regardless of the clinical classification of the infection (primary, nonprimary first-episode, or recurrent), as well as in women with a history of genital HSV lesions in order to reduce symptomatic recurrent HSV infections and asymptomatic viral shedding at the time of delivery/labor.188 Treatment with acyclovir 400 mg orally three times a day or, alternatively, valacyclovir 500 g orally, twice a day is indicated in the case described above.188
However, current suppressive therapies cannot guarantee to eliminate viral shedding or neonatal HSV acquisition.168
If a recurrent HSV outbreak occurs during pregnancy, antiviral therapy should be administered for the same reasons as above.188 Treatment with acyclovir 400 mg orally three times a day, for 5 days (or 800 mg orally twice a day, for 5 days) or, alternatively, valacyclovir 500 mg orally, twice a day, for 3 days (or 1 g daily for 5 days) is indicated in the case described above.
Topical treatments
Topical treatments are not recommended for genital HSV lesions, since their use can cause resistance and are less effective than oral antiviral treatments.188
Mode of delivery
Mother-to-child HSV transmission is more likely when a primary or nonprimary first-episode genital HSV infection is acquired near the time of delivery because the fetus could be exposed to virus shed from the genital tract. Therefore, cesarean delivery may be an effective option and it may be offered to women with primary or nonprimary first-episode HSV genital infection during the third trimester of pregnancy, owing to the possibility of prolonged viral shedding. Cesarean delivery is especially recommended in case of active genital lesions, prodromal symptoms in women with a history of genital HSV39 or a newly acquired HSV genital infection develops within the last 4–6 weeks’ gestation (because seroconversion may not be complete and maternal antibodies may not be available to protect the fetus). For these reasons, all women with a history of genital HSV infection should be examined in order to identify any genital lesions before labor and delivery.
If not done earlier, a viral swab from the lesions should be taken, as the result can help clinicians in the management of the neonate.190
Conversely from the American College of Obstetricians and Gynecologists (ACOG) guidelines described above, those of the Royal College of Obstetricians and Gynaecologists (RCOG) recommend cesarean delivery only with primary genital HSV infections when the lesions appear within 6 weeks of estimated delivery.190 However, cesarean delivery does not completely prevent vertical transmission to the neonate.
In absence of an active genital HSV lesion at the time of childbirth or of a positive result to tests (e.g., viral culture repeated twice) the risk of vertical transmission is low and vaginal delivery can be considered. In other words, women with a history of genital HSV infection, but no active genital lesions or prodromal symptoms during labor, can give birth vaginally. Even women with active non-genital lesions (e.g., lesions on thigh or buttock) can give birth vaginally.
In cases of uncomplicated preterm premature rupture of the membranes, the common practice is expectant management, but if the woman presents active genital HSV lesions, the risk of morbidity and mortality related to prematurity should be weighed against the risk of neonatal HSV infection. Antiviral therapy is recommended in case of expectant management.
Screening for genital HSV
Routine screening for genital HSV during pregnancy or near delivery is not recommended in asymptomatic women with a history of recurrent genital HSV.188
Breastfeeding
Breastfeeding is not contraindicated. It is contraindicated only when a HSV lesion is located on the breast.
Acyclovir was found in the breast milk, but in concentrations considered safe for newborns. Valacyclovir has a good safety profile for lactating women and their babies.
HSV vaccines and microbicides
A prophylactic vaccine to prevent genital HSV-1 and HSV-2 infection would have a significant impact on the population as HSV infection of the genital tract is one of the most common transmitted infection and a significant health problem. A therapeutic HSV vaccine could also be very useful, as it could significantly reduce recurrent infections. Novel HSV vaccines have appeared promising in preclinical studies, but clinical studies are still ongoing.
Several topical microbicides are currently being studied.191
HEPATITIS VIRUSES AND PREGNANCY
Hepatitis could be a possible condition in pregnancy. The management of viral hepatitis in the setting of pregnancy requires special consideration. There are five liver-specific viruses (hepatitis virus A, B, C, D and E), each with unique epidemiology, tendency to chronicity, risk of liver complications, risk of obstetrics outcomes, immunization features, preventive strategies and treatments, breastfeeding considerations.
Acute viral hepatitis in pregnancy could be asymptomatic or have mild clinical disease. Patients may present with nonspecific symptoms such as jaundice, nausea, anorexia, abdominal pain or discomfort, fatigue, malaise, myalgia, and dark urine. Clinical symptoms are unable to differentiate the various viral hepatitis. Serological exams need to confirm or exclude hepatitis and which type of virus is eventually involved. Generally, maternal serological status on hepatitis virus B (HBV) and hepatitis virus C (HCV) is also checked during pregnancy for prophylaxis strategy (for HBV) and for define the best birth management and breastfeeding options. Other hepatitis viruses are usually not routinely screened. In presence of suspects signs and symptoms, the liver function test is assessed as the initial biomarker elevated in acute viral hepatitis. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) are elevated during the acute episode. ALT is elevated 2-to-100 fold depending on the severity of the acute viral hepatitis. International normalized ratio (INR), prothrombin time (PT), albumin, and ammonia are evaluated for acute and chronic viral hepatitis infections. The prevention of mother-to-child transmission is key to reducing the global burden of chronic viral hepatitis infection in pregnancy. Prevention strategies should be a priority worldwide.
Hepatitis A
Epidemiology and transmission
Hepatitis A virus is a positive-sense, single-stranded RNA virus belonging to the family Picornaviridae.192 There is a single serotype worldwide. The virus is mainly transmitted through the fecal–oral route. This is most prevalent in developing countries with poor hygiene and sanitation, which results in food and water contamination. The virus can be also transmitted through sexual contact and also rarely through blood exposure in injecting drug users and during a blood transfusion.
The average incubation period for hepatitis A is 30 days. Hepatitis A virus has the highest concentration in the feces, serum, and saliva, respectively. There has been no report of chronic sequelae among persons infected with hepatitis A.
Obstetrics outcomes
Intrauterine and perinatal transmission of hepatitis A is a rare occurrence.193,194 It is estimated that 1 : 1000 pregnant women are infected with acute hepatitis A virus. The disease is mostly self-limited with mortality of 0.3–0.6%.195 Hepatitis A has been associated with gestational complications, including premature contractions, placental abruptio, premature rupture of membranes, and vaginal bleeding, and these have been reported in a study evaluating the impact of acute hepatitis A on pregnancy.196 Fetal ascites and meconium peritonitis, which is a rare occurrence, have been reported.193
Diagnosis
Pregnant women who had contact with persons with acute hepatitis A should be screened for acute hepatitis A virus infection. Hepatitis A viral infection is diagnosed by detecting the immunoglobulin M antibody to the hepatitis A (anti-HAV IgM) virus in pregnant women and also in the fetus/newborn.197
Prevention and treatment
Is recommended to administer hepatitis A immunoglobulin to pregnant women who had contact with persons with acute hepatitis A viral infection and newborns infected during the third trimester. Newborns are to receive hepatitis A immunoglobulin within 48 hours of birth, as recommended by the CDC and the American College of Pediatric Association.6 There is a minimal risk of transmission of hepatitis A virus via breastmilk. Nevertheless, the benefit of breastfeeding greatly outweighs stopping breastfeeding. There is no contraindication to breastfeeding for mothers infected with hepatitis A.
Hepatitis B
Epidemiology and transmission
Hepatitis B virus (HBV) is an enveloped virus, partially double-stranded virus, circular DNA genome, and belonging to the family Hepadnavirus. Chronic hepatitis B infection is associated with cirrhosis and hepatocellular carcinoma. Hepatitis B virus does not cross the placenta and cannot infect the fetus unless there have been breaks in the maternal–fetal barrier. Perinatal transmission accounts for more than 50% of cases worldwide. Pregnant women with chronic hepatitis B and positive hepatitis B virus E antigen (HBeAg) have a 90% likelihood of their newborns being infected with the hepatitis B virus.198 Other modes of transmission include sexual intercourse, body fluids, and blood transfusion. Hepatitis B affects more than 250 million individuals worldwide and is the most common cause of chronic hepatitis worldwide. Of women childbearing age, 65 million are infected with chronic hepatitis B virus.
Obstetrics outcomes
The adverse effect of the hepatitis B virus on pregnancy is rare in patients with acute or chronic HBV infection. There is increased maternal and perinatal death associated with the hepatitis B virus infection during pregnancy. Complications such as placenta abruption, preterm birth, gestational hypertension, and fetal growth restriction have been associated with chronic HBV during pregnancy. Moreover, chronic HBV in pregnancy increases the risk of progression to cirrhosis.
Diagnosis
Vertical transmission of hepatitis B virus from infected mothers to their fetuses or newborns results in a 90% likelihood of the newborn being infected if the pregnant woman has chronic hepatitis B and is positive for hepatitis B virus E antigen (HbeAg).198 Universal screening for hepatitis B virus infection during pregnancy at the first prenatal visit has been recommended by the U.S. Preventive Services and Task Force (USPSTF) and the American Congress of Obstetricians and Gynecologists (ACOG). Thus, every pregnant woman should be screened for the hepatitis B surface antigen at the initial visit. This is to decrease the mother-to-child transmission of the hepatitis B virus. It is also recommended to reassess the serological status for HBV during the third trimester.
Prevention, treatment and management
Several parameters are key to planning for an approach to prevent mother-to child transmission of HBV in different countries and settings. These include the epidemiology of HBV infection (prevalence of chronic HBV infection and of high HBV DNA levels or HBeAg positivity), service coverage of immunization, including the birth dose of hepatitis B vaccine, availability of health services, experience with testing and peripartum prophylaxis with antivirals (still geographical heterogeneity). According to recent World Health Organization recommendations, hepatitis B immunoglobulin and hepatitis B vaccine should be administered within 12–24 hours of birth to all babies of hepatitis surface antigen positive (HBsAg) mothers or those with unknown/undocumented HBsAg status. This is regardless of whether maternal antiviral therapy was administered during the pregnancy. The implementation of this approach has reduced the risk of exposure from 90% to 5–10% in exposed neonates. Decreasing vertical hepatitis B virus transmission through cesarean section is not recommended as the sole indication.199 Also, the administration of both hepatitis B immunoglobulin and hepatitis B vaccine HBV vaccine within 12–24 hours of birth, to reduce in utero infection has been recommended by both the European Association for the Study of Liver Disease and Society for Maternal-Fetal Medicine (SMFM).200 Fetal exposure risk has not been increased with the use of antiviral medications during pregnancy.201 In the WHO 2015 Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection, the Guidelines Development Group had concluded that a formal recommendation could not be made on the routine use of antiviral therapy to prevent mother-to-child transmission. However, since the indications for treatment in HBV-infected pregnant women are the same as that for other adults, all pregnant women should first be assessed for eligibility for long-term treatment based on their own health needs before initiation of prophylaxis.202 While prophylaxis with antivirals during the third trimester of pregnancy was known to be effective in reducing mother-to-child transmission of HBV, at the time there was insufficient information available on the programmatic implications. Using antiviral therapy such as tenofovir, telbivudine or lamivudine after 28–32 weeks of gestation in Hepatitis B virus-infected pregnant women with high viral load (>6–8 log 10 copies/mL) has been associated with less than 3% risk of transmission. Recently, WHO recommends that pregnant women testing positive for HBV infection (HBsAg positive) with an HBV DNA ≥5.3 log10 IU/mL (≥200,000 IU/mL) receive tenofovir prophylaxis from the 28th week of pregnancy until at least birth, to prevent mother-to-child transmission of HBV. This is in addition to three-dose hepatitis B vaccination in all infants, including timely birth dose (conditional recommendation, moderate quality of evidence).203 Moreover, another new WHO recommendation suggests that in settings in which antenatal HBV DNA testing is not available, HBeAg testing can be used as an alternative to HBV DNA testing to determine eligibility for tenofovir prophylaxis to prevent mother-to-child transmission of HBV.203 Breastfeeding is encouraged after newborns receive the appropriate immunoprophylaxis by the American College of Pediatrics, Centers for Disease Control and Prevention (CDC), ACOG, and Society for Materno-Fetal Medicine (SMFM). Six months following the completion of the prophylaxis and active immunization series, a negative anti-hepatitis B core antibody test indicates that the prophylaxis was effective. Pre-pregnancy and antenatal prophylaxis include avoidance of high-risk behavior, unprotected intercourse with multiple partners, use of unclean injection needles, avoidance of blood contact in occupations that involve contact with human blood.
Hepatitis virus D
Epidemiology and transmission
Hepatitis D is caused by the hepatitis delta virus (HDV), which is a single-stranded, circular RNA and a defective virus with an incomplete RNA requiring the assistance of the hepatitis B virus, specifically hepatitis B surface antigen (HBsAg) to be infectious.204
It is a highly pathogenic virus and may cause a rapid disease progression from fulminant hepatitis to development of hepatocellular carcinoma in patients infected with HBV. Hepatitis D is mostly transmitted through the same route as the hepatitis B virus. The parenteral mode is the major mode of transmission, and vertical transmission during pregnancy is rare. Coinfection of hepatitis D virus and hepatitis B virus leads to severe acute infection. Superinfection of hepatitis D virus on chronic HBV leads to higher progression to chronic hepatitis D. Hepatitis D virus affects 15–20 million people worldwide with hepatitis B virus carriers. New studies estimate the prevalence of hepatitis D to be closer to 62–72 million. The prevalence of HDV in the US is estimated to range from 2% to 50%, depending on the patient population.205 The prevalence of HDV in a study in Pakistan revealed an estimated 20.63% in pregnant women with chronic hepatitis B virus infection.
Obstetrics outcome
Chronic hepatitis D is associated with a high risk of severe chronic liver disease in pregnant women.
Diagnosis
WHO recommends screening of pregnant women infected with hepatitis B virus for hepatitis D virus. Serum immunoglobulin M anti-HDAg is detected during active infection.
Prevention and treatment
Hepatitis D virus infection is treated with long-term α-interferon and PEGylated interferon. These are both contraindicated during pregnancy. Transmission of the hepatitis D virus has largely decreased due to perinatal prevention and treatment of hepatitis B virus infection. Nevertheless, a high level of attention is still needed, especially in geographical areas in which HBV infection is more common and HDV is endemic. HBV immunization programs and serological screening for HBV are crucial to prevent hepatitis virus D infection.
Hepatitis virus C
Epidemiology and transmission
Hepatitis C virus (HCV) is a partially double-stranded, plus-sense RNA virus with 11 major genotypes and 15 different subtypes.206
Acute hepatitis C infection occurs during the first 6 months after exposure. Failure to clear the hepatitis C virus after 6 months would progress to chronic hepatitis C. Hepatitis C is a major cause of cirrhosis and hepatocellular carcinoma worldwide. The major mode of transmission of HCV is mostly through parental transmission, which includes infected blood transmission, intravenous drug users sharing needles, sexual contact, and mother-to-child transmission.207 Vertical (mother-to-child) transmission is the leading cause of HCV infection in children. Perinatal transmission of HCV is mostly during the last month of pregnancy or delivery. Invasive procedures such as amniocentesis and chronic villus sampling break the maternal–fetal barrier: this increases the risk of vertical transmission of the hepatitis C virus during pregnancy and delivery. Hepatitis C virus affects more than 170 million people worldwide. About 8% of pregnant women are infected with HCV. The estimated prevalence of antenatal HCV infection in the United States is 1–2.5%.208
Obstetrics outcome
HCV infection during pregnancy is not usually associated with severe adverse maternal–fetal–neonatal outcomes. Infection is not an absolute contraindication to vaginal birth. Nevertheless, some adverse negative outcomes, such as fetal growth restriction, brachial plexus injury, fetal distress, cephalohematoma, neonatal seizures, and intraventricular hemorrhage are observed in HCV-infected pregnant women.209
Diagnosis
Transmission is associated with pregnant women with higher levels of HCV RNA. ACOG and CDC recommend risk-based screening for HCV in pregnant women. The anti-HCV antibody is tested as the screening tool during pregnancy.
Prevention and treatment
Prevention of HCV infection is crucial in every clinical setting; identification of populations at higher risk is mandatory. Education programs, should be encouraged. The safety profile in pregnancy for women taking direct antiviral agents has not yet been established. Direct antiviral agents treatment is mostly deferred until postpartum.210 Currently, there is no immunization for hepatitis C virus-infected mothers and infants. There is no contraindication for breastfeeding in hepatitis C virus-infected mothers and infants. It is crucial to understand hepatitis C in pregnancy due to the potential for increased prevalence, vertical transmission, risk of adverse maternal and neonatal outcomes, and subsequent sequelae such as chronic hepatitis C, cirrhosis, and hepatocellular carcinoma in the infant. The availability of new, potentially curative therapies will also likely shape the approach to treating peripartum HCV infection in both pregnant women and infants in the coming years, although additional prospective studies will be required.211,212,213,214
Hepatitis E
Epidemiology and transmission
There are about 20.1 million new hepatitis E virus (HEV) infections every year worldwide. HEV infection is prevalent in developing countries. Hepatitis E viral infection accounts for 70,000 deaths and 3000 stillbirths each year.215
Pregnant women in the second and third trimester are mostly affected during epidemics. The mortality rate as high as 5–25%. There is a higher mortality rate in pregnant women who rapidly progress to fulminant hepatitis. Hepatitis E virus (HEV) is an icosahedral, non-enveloped virus with a single-stranded, positive RNA virus classified into the family Hepeviridae and the genus Orthohepevirus. There about seven genotypes of the hepatitis E virus and genotypes 1–4 are known to affect humans.216 The main transmission route for HEV infection is the fecal–oral route and is most prevalent in developing countries with poor sanitation. Vertical transmission of the hepatitis E virus varies between 23.3% and 50%. Sporadic cases not associated with travel have been reported in developed countries, and these are mostly caused by genotype 3, and mainly attributed to the immunocompromised state.217 It has been rarely reported to be transmitted through sexual intercourse.218
Obstetrics outcome
Pregnant women are more susceptible to infection by HEV and progression to fulminant hepatic failure with high mortality rates (15–25%) and preterm deliveries.214
The cause of elevated maternal mortality of pregnant women infected with HEV1 living in developing countries has been the subject of many studies. HEV genotype could explain the poorer outcome in pregnant women, at least in part, since HEV3 is not particularly deadly for pregnant women. HEV1 is associated with increased apoptosis and necrosis at the maternal–fetal interface with alterations in the architecture of the placental barrier.219,220,221 HEV1 also produces more infectious virions and triggers the production of a panel of pro-inflammatory cytokines like IL-6 and chemokines. These changes in the cytokine microenvironment correlate with viral load and contribute to tissue damage. Other host factors, such as nutritional status, including micronutrient or folate deficiencies, or differences in major histocompatibility complex, may also influence the immune response of pregnant women to HEV.222
Diagnosis
Liver injury coincides with an elevation of transaminases and the appearance of anti-HEV immunoglobulin M (IgM). Anti-HEV IgM is tested during pregnancy for suspected cases. The mechanisms behind its aggressive course during pregnancy are still not clearly understood.
Clinical presentation varies from asymptomatic infection to anicteric, icteric, and fulminant hepatitis. Common presenting symptoms include yellowing of the eye and urine, fever, chills, anorexia, nausea, and abdominal pain. Aminotransferases are markedly elevated and may precede the onset of symptoms.214
Prevention and treatment
Since the virus has a fecal–oral route of transmission, the disease can be better prevented by improved sanitation, provision of clean drinking water, and avoiding raw pork and venison.223 Hospitalization should be considered for pregnant women. Ribavirin or pegylated interferon α, or both combined, are effective but contraindicated in pregnancy due to the risk of teratogenicity.224,225 An urgent liver transplant can be a successful option in acute liver failure. It is necessary to inform pregnant women of the potential effects of HEV on the fetus; they should be advised to avoid eating and drinking contaminated food and water to prevent possible HEV exposure. In addition, pregnant women in developed countries should avoid traveling to the hepatitis E virus endemic regions.
A HEV vaccine may hold great promise for reducing HEV-associated mortality in pregnant women. Finally, there is also a substantial need for novel therapies to treat HEV in pregnancy.
Conclusions
Viral hepatitis in pregnancy is variably associated with significant gestational and fetal complications. Adhering to screening guidelines is key to reduce mother-to-child transmission in these cases. A multidisciplinary team including obstetric care providers, internists, gastroenterologists, nurses, midwives, and pediatricians is important in providing a holistic and integrated approach to pregnant women with exposure or infected with viral hepatitis to achieve the best possible outcomes for the couple mother–baby. Universal screening of mothers for hepatitis B and C infection during antenatal care is essential. It is important to consider additional serological tests for hepatitis A, D and E when needed (HBV infection, signs and symptoms suggestive for HAV or HEV infections). Moreover, remember to consider other differential diagnoses, such as Epstein Barr virus infection (EBV) and non-infectious conditions as possible diagnostic hypothesis.
EBOLA AND PREGNANCY
Although Ebola virus (EBOV) was first discovered more than 40 years ago, it remained a relatively obscure pathogen until 2014, when it became widely known, including within the obstetrics and gynecology community. Prior to 2014, EBOV was associated with small, relatively limited disease outbreaks in rural Africa. However, in 2014–2016, West Africa experienced the largest outbreak of Ebola virus disease (EVD) in history, with more than 28,000 people infected and 11,000 deaths.226 Although the vast majority of cases occurred in three West African countries (Guinea, Sierra Leone, and Liberia), seven further countries, including the United States, reported local transmission. In the United States, four people were diagnosed with EBOV infection with one of them dying from EVD.226,227
Transmission
EBOV, first discovered in 1976 in Yambuku, Democratic Republic of Congo, along the Ebola River, is a zoonotic RNA virus and a member of the Filoviridae family. The virus is easily transmitted from person-to-person through direct contact with blood or body fluids, including through unsafe burial practices and through breast milk and sexual contact with semen. It is unknown whether sexual transmission from women occurs. In addition, transmission can also occur from contact with contaminated objects such as needles or syringes and contact with infected animals including ingestion of infected meat.228 As well as the terrible morbidity and mortality, overall gaps in reproductive health services in affected countries became apparent. It highlighted the importance of screening for travel history to avoid importation of disease. Because EBOV is highly transmissible, it also brought into focus the importance of infection control strategies, particularly in labor and delivery settings where blood and body fluid contamination are common. EBOV can cross the placenta, and pregnant woman infected with the virus will likely transmit it to the fetus. Placental tissues from patients with EVD have demonstrated EBOV antigen throughout numerous different types of placental cells on pathological exams. EBOV RNA has also been detected in amniotic fluid, fetal meconium, vaginal secretions, umbilical cord, and buccal swab samples from neonates.
Signs and symptoms
The clinical course of EVD has been well described in the past few years, even if many of the cases show a heterogeneous and aspecific clinical presentation. Signs and symptoms of EVD include fever, severe headache, muscle pain, abdominal pain, diarrhea, vomiting, and unexplained hemorrhage. While classically individuals present with a febrile gastrointestinal illness, up to 18% of confirmed cases may present without fever.229 Symptoms during the initial course of EVD may be non-specific and mirror the symptoms from infections endemic to the same region. The incubation period from exposure to the development of symptoms is estimated in 2–21 days with an average of about 7 days.230 Healthcare services should be prepared to screen patients for EVD and have a plan in place for how to triage, identify and manage these patients. They should:
- Know the signs and symptoms of EBOV;
- Ask patients about recent travel to a country with widespread EBOV transmission or contact with a person with EBOV;
- Assess patients for fever and other signs and symptoms of EBOV if they have recent travel to a country with widespread EBOV transmission or contact with a person with EBOV.
Obstetrics outcomes
The clinical presentation and disease course of EVD appears to be similar among pregnant and non-pregnant women, however, data are limited. An increased risk for severe disease and death among pregnant women compared to non-pregnant women has been reported in previous outbreaks with maternal mortality estimated at about 90%. Although there is some evidence that mortality rates in pregnant women in the recent outbreak were lower than reported in prior outbreaks, the strength of reported data is still limited.231 After a pregnant woman recovers from EVD, she should be offered counseling on the increased risk of spontaneous abortion, preterm labor, stillbirth, or neonatal death associated with EVD during pregnancy. She should be informed that there is a risk of transmission if others are exposed to pregnancy-related fluids or tissues, such as amniotic fluid, placental or fetal tissue. Data reported that pregnant women with EVD appear to be at an increased risk for obstetrics complications reported below:
- Postpartum hemorrhage (PPH);
- Retained placenta;
- Prolonged labor.
Obstetric procedures (such as cesarean section, vacuum extraction, artificial rupture of membranes, episiotomy) among pregnant women with acute EVD should be limited and reserved for the purpose of reducing maternal morbidity (i.e., not done for fetal indications), given the risk of infection via pregnancy-related fluids and tissues. No data exist to suggest the best method of delivery for pregnant women with EVD, with respect to maternal or neonatal outcomes or the safety of healthcare workers.
Diagnosis
A diagnosis of EVD is initially based on clinical presentation in the context of potential exposure history, either recent travel or potential EBOV exposure in endemic areas. Laboratory testing via reverse transcriptase polymerase chain reaction (RT-PCR) of blood or oral swabs to detect EBOV RNA may be positive within the first 24 hours after symptom onset; however, such positivity can be delayed for up to 72 hours after symptom onset.232 Enzyme linked immunosorbent assay (ELISA) can also be performed to detect EBOV specific IgM or IgG, with antibodies developing as early as 2 days and 6 days after symptom development and persisting for up to 6 months and 10 years for IgM and IgG, respectively.227,232,233,234,235,236 Prolonged detection of EBOV has been demonstrated in certain body fluids including semen and vaginal secretions. There may be considerable overlap in symptoms between early EVD and pregnancy-related conditions in pregnant women. As such, evaluation of pregnant women by obstetric and EBOV-response trained healthcare providers is essential, and clinical management should be informed by risk of exposure to EVD, symptom history, and an obstetric evaluation.
Treatment
There is limited information regarding specific care and treatment recommendations for pregnant women. Thus, supportive care recommendations should be similar to those for the non-pregnant women with primarily supportive care focusing on hydration to replace expected losses from mainly gastrointestinal sources of diarrhea and vomiting. Avoidance of secondary infections is critical, particularly during pregnancy when alterations in immunity may occur. In 2018, a multidisciplinary panel of international experts formulated evidence-based recommendations for delivering optimized supportive care to individuals with acute EVD. Recommendations were formulated based on the quality of evidence as well as benefits, harms, values, and preferences. The majority of recommendations, including rehydration therapy, systematic vital sign assessment, and biochemistry assessment were strongly recommended to all patients with suspected, probable or confirmed EVD as steps to reduce mortality and optimize care.237 Breastfeeding should be stopped if acute EVD is suspected or confirmed in lactating women or in a breastfeeding child. The child should be separated from the breastfeeding woman and provided a breastmilk substitute as needed. If a breastfeeding woman and her child are both diagnosed with EVD, breastfeeding should be discontinued, the pair should be separated, and appropriate breastmilk substitutes provided.238 A woman who has recovered from EVD, cleared viremia and wants to continue breastfeeding should wait until after two consecutive negative EBOV breastmilk tests by RT-PCR, separated by 24 hours. During this time, the child should be given a breastmilk substitute.238
Prevention
Current recommendations to limit EVD infection in pregnant women are based on control measures that include:238
- Invasive procedures should not be performed for fetal indications in pregnant women with acute EVD;
- All pregnant women with acute EVD should be managed using both standard precautions and EBOV-specific infection prevention control (IPC) measures;
- All pregnant women who have recovered from EVD (with conception prior to EVD) should be enabled and encouraged to attend frequent antenatal care (ANC). If there is no risk of exposure to pregnancy-related fluids during the ANC visit, only standard precautions are required. Complications associated with childbirth and pregnancy should be managed at ETCs and EBOV IPC measures should be used in addition to standard precautions;
- Among women who become pregnant after EVD (with conception after acute EVD), standard IPC precautions should be used.
Pregnant and breastfeeding women should be offered vaccination with the prequalified Ervebo live-replicating rVSV-ZEBOV vaccine during an active Zaire EBOV outbreak in affected areas, in the context of rigorous research or in accordance with a compassionate use protocol. Vaccination should occur with informed consent and in compliance with good clinical practice.238
Conclusions
EBOV caused a shift in worldwide global perspective of disease. A paucity of scientific evidence exists on how to best treat pregnant or breastfeeding women with suspected or confirmed EVD. Historical reports suggest that, among women who acquire EVD during pregnancy, there is increased mortality and morbidity, and a high rate of adverse pregnancy outcomes. To mitigate these outcomes and limit the spread of disease, it is critical to develop recommendations on the prevention, treatment, and surveillance of women who are exposed to and/or acquire EBOV during pregnancy or breastfeeding, or survive EVD with ongoing pregnancies.
ZIKA VIRUS AND PREGNANCY
Epidemiology and transmission
Zika virus (ZIKV) is a mosquito-borne virus that was first isolated from Zika forest, Uganda, in 1947. Since its inception, major and minor outbreaks have been documented from several parts of world. Aedes spp. mosquitoes are the primary vectors of ZIKV, but the virus can also be transmitted through sexual practices, maternal–fetal transmission, and blood transfusion. The recent Indian ZIKV outbreak suggests that the virus is circulating in the South East Asian region and may cause new outbreaks in future. The global epidemic of ZIKV has been a major public health problem affecting pregnant women and their infants.
Signs and symptoms
The clinical presentations of symptomatic ZIKV infections are similar to others infections, such as dengue and chikungunya.239 ZIKV in acute stage is believed to be asymptomatic in up to 80% of the infected people and it is classically characterized by low fever, arthralgia, maculopapular rash accompanied by pruritis, and conjunctivitis. After a 12 days incubation period, symptoms usually last only a few days. Apart from the consequences in pregnancy, infections in healthy adults were associated with Guillain–Barre syndrome as well.240 However, infection during pregnancy can cause intrauterine growth restriction, birth defects, vision and hearing loss, resulting in cognitive and speaking problems accompanied by social and motor development problems in children. ZIKV disease is very often misdiagnosed because the symptoms are similar to the ones of dengue fever and chikungunya.
Diagnosis
The diagnostic value of ZIKV disease differs between geographical regions and specific population groups. Travelers in high-risk areas or people in contact with an infected person must be screened with a serological blood test. Furthermore, pregnant women with possible ZIKV exposure, with or without symptoms and women who were diagnosed with fetal microcephaly must be tested for ZIKV infection. Exposed neonates should be evaluated with special Zika Outcomes and Development in Infants and Children (ZODIAC) tools.241,242 Current diagnostic tools to identify ZIKV infections are serological blood test. Fetal ultrasound, especially an accurate fetal neurosonography evaluation, is an important tool to suspect ZIKV and for subsequent pregnancy surveillance. A recent systematic review examined the role of prenatal ultrasonography and amniocentesis in the diagnosis of congenital Zika syndrome (CZS). As with other congenital infections, prenatal detection may vary with timing of infection, timing of ultrasound examination, technical expertise, and severity of abnormalities. The detection of ZIKV RNA in amniotic fluid in the included studies did not predict the risk for congenital ZIKV syndrome abnormalities in these cases, and clearance of ZIKV RNA from amniotic fluid appears possible after maternal infection. Diagnostic testing for ZIKV infection remains a shared decision between patients and clinicians, and more data are needed to define clinical predictors that will inform these decisions.
Obstetrics outcomes
Congenital microcephaly and autoimmune disorders like Guillain–Barré syndrome may occur in pregnant women with ZIKV infection. The fetal neurological damage caused by ZIKV is well-described in literature. Recently, studies and case reports were incorporated in a review; most studies have linked maternal infection during pregnancy to the development of neonatal microcephaly. The period considered most dangerous is the first trimester and the beginning or the whole of the second trimester.243 According to Ellington et al. in 2016, the probability of microcephaly in infants was about 1–13%, with limited data for the second and third trimesters.244 Microcephaly is defined as a head circumference measurement that is smaller than for infants of the same age and sex. The measurement value for microcephaly is more than 2 standard deviations below the average. During routine ultrasounds in pregnancy, microcephaly can be diagnosed in the second or early third trimester.
Clinical data derived from a research conducted in Colombia, reported that in pregnant women with laboratory-confirmed ZVD, brain or eye defects in infants or fetuses were more common during the ZIKV outbreak than during the periods immediately before and after the outbreak. The frequency of such defects was increased among women with a symptom onset early in pregnancy.245 Additionally, other abnormalities described include craniosynostosis, fetal growth restriction, craniofacial malformations, pulmonary hypoplasia, and arthrogryposis.246 Eye injuries were also detected in CZS newborns; most cases occurred in babies with a small cephalic diameter at birth and in mothers who reported symptoms in the first trimester of pregnancy.247 In different regions where the viral outbreaks occurred, similar lesions were found, such as macular lesions, optical nerve abnormalities, chorioretinal atrophy/scarring, focal pigment mottling of retina, microphthalmia, glaucoma, cataract, iris coloboma, and subluxation. Understanding how ZIKV could evade the innate immune defenses of the mother, placenta, and fetus has become central to determining how the virus can travel into the fetal brain. ZIKV, like other flaviviruses, evades host innate immune responses by leveraging viral proteins and other processes that occur during viral replication to allow spread to the placenta. Within the placenta, there are diverse cell types with coreceptors for ZIKV entry, creating an opportunity for the virus to establish a reservoir for replication and infect the fetus. The fetal brain is vulnerable to ZIKV, particularly during the first trimester, when it is beginning a dynamic process, to form highly complex and specialized regions orchestrated by neuroprogenitor cells.248 Figures 1 and 2 explain the principles of ZIKV vertical transmission and adverse effects of congenital and postnatal ZIKV infections, respectively.249
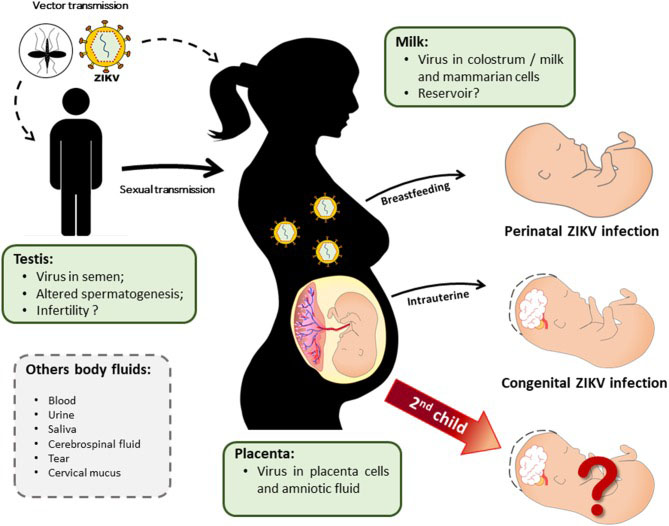
1
ZIKV vertical transmission and a possible maternal reservoir. ZIKV infection in pregnant women may occur by mosquito bite or sexual contact with an infected partner. Mother-to-child transmission can either occur in utero (infection in the first trimester of pregnancy is related to congenital ZIKV syndrome) or in the perinatal period via breastfeeding. ZIKV presents tropism for multiple tissues and is present in several body fluids, which contribute to its transmission by different routes. However, after gestational infection with the congenital involvement of the child, it is still unknown whether the ZIKV establishes a reservoir in the mother that may influence the course of a second pregnancy.249
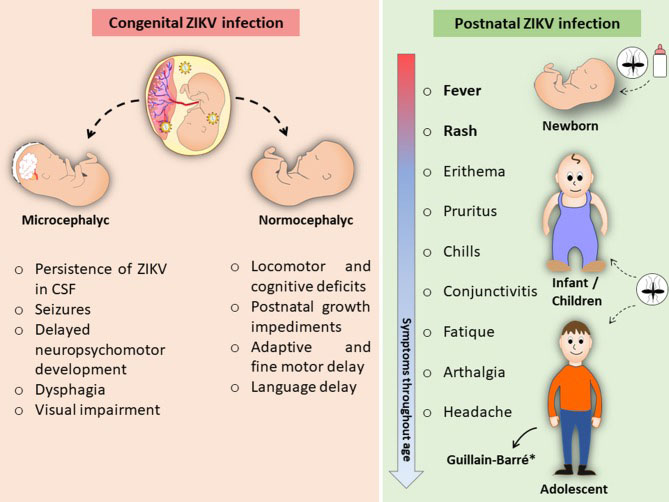
2
Adverse effects of congenital and postnatal ZIKV infections. Intrauterine exposure to ZIKV may lead to congenital infection causing fetal microcephaly, among other CZS-related effects. Even in infants who have not had microcephaly, congenital ZIKV infection can cause delays in locomotor and cognitive development. Pediatric ZIKV infection is self-limiting and typically causes mild and even asymptomatic disease similar to adult infection.*Rare cases of ZIKV-associated Guillain-Barré.249
Prevention and treatment
A higher prevalence of the ZIKV infection was observed in poor communities, where there is a deficit in prevention and supporting services.
At present, no specific vaccines or antivirals are available to treat ZIKV, so management and control of ZIKV infections rely mostly on preventive measures. The current protocol of ZIKV infection management involves only symptomatic care. Due to the serious problems faced by children exposed during pregnancy and their adverse effects on the economy and society, the early diagnosis of exposure is very important.
Interestingly, in response to the ZIKV outbreak in Puerto Rico (2015–2016) a campaign was launched to promote contraception services and awareness in order to prevent pregnancy during the ZIKV outbreak: the Zika Contraception Access Network (Z-CAN) was established to provide same-day access to the full range of reversible contraception at no cost to women.250
Despite the reduction in the number of new ZIKV infections, the development of a safe protective vaccine is a public health priority. In the face of the recent ZIKV infection epidemic, several groups have developed different vaccine strategies, that are capable of inducing high levels of neutralizing antibodies (nAbs), as well as generating protection against the challenge of infection in non-pregnant mouse and non-human primate models. However, in these studies, it has not been demonstrated whether this protection prevents congenital changes or has a long-term protective effect. Although several vaccine strategies have progressed into phase I clinical trials in humans, gestational protection vaccine studies are still limited.251,252,253,254 Future studies of vaccine strategies that generate an optimal neutralizing antibody response are essential, considering that passive mother-to-child immunity against ZIKV during pregnancy could prevent congenital infection.
Further investigations required regarding ZIKV infection in pregnancy include:
- Define well-established screening and management programs worldwide, especially in endemic zones. Remembering the high risk immigrant communities of the non-endemic areas.
- There is an urgent need to understand the complex relationship between cross-reactive anti-viral immunity, disease susceptibility, and severity in the face of differential exposure to related circulating Flaviviruses.
- Another relevant field of interest is that the ultrasound screening during pregnancy should be systematized and expanded.
- The importance of offering protection to women of childbearing age and their children by developing prophylactic maternal vaccines.
SARS-COV-2 AND PREGNANCY
Epidemiology and transmission
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2, has been declared a pandemic by WHO. As the pandemic evolves rapidly, there are data emerging to suggest that pregnant women diagnosed as having COVID-19 can have morbidities (up to 9%), even if the majority of data showed good maternal and neonatal outcomes. Person-to-person transmission is now known to occur via fomites, droplets through close proximity aerosols, and prolonged close contact within a 2 m perimeter. A study revealed that patients can continue to shed the virus as evidenced by positive RT-PCR results for up to 13 days after disease resolution. It is probable that COVID-19 infection during pregnancy cannot lead to transplacental vertical transmission. Available clinical data on COVID-19 infection in pregnancy are still limited. There is, therefore, a need to improve understanding of the transmission of the disease throughout pregnancy.
Signs and symptoms
COVID-19 can present with a spectrum of clinical manifestations ranging from mild signs and symptoms,255 such as fever, cough, sore throat, myalgia, and malaise, to severe illness, such as pneumonia with or without acute respiratory distress syndrome (ARDS)256 renal failure, and multiorgan dysfunction that may require immediate advanced critical care support. Clinical presentations in pregnant patients with COVID-19 could be atypical with normal temperature (56%) and leukocytosis.257 Clinical manifestations of COVID-19 also include features of acute respiratory illnesses. Typical radiologic findings consists of patchy infiltrates on chest radiograph and ground glass opacities on computed tomography scan of the chest. Patients who are pregnant may present with atypical features such as the absence of fever as well as leukocytosis. Confirmation of COVID-19 is by RT-PCR from upper airway swabs. When the RT-PCR test result is negative in suspect cases, chest imaging should be considered. A pregnant woman with COVID-19 is at the greatest risk when she is in labor, especially if she is acutely ill. Key decisions are made based on the presence of maternal and/or fetal compromise, adequacy of maternal oxygenation (SpO2 >93%) and stability of maternal blood pressure. Although vertical transmission is unlikely, there must be measures in place to prevent neonatal infections. Routine birth processes such as delayed cord clamping and skin-to-skin bonding between mother and newborn need to be revised.
Obstetrics outcome
Due to the physiological changes in their immune and cardiopulmonary systems, pregnant women are more likely to develop severe illness after infection with respiratory viruses. Interestingly, the impact of COVID-19 infection on pregnant women appears to be less severe. Currently, there is no evidence that pregnant women are more susceptible to COVID-19 infection and that those with COVID-19 infection are more prone to developing severe pneumonia. The clinical characteristics of pregnant women with laboratory-confirmed COVID-19 in the third trimester comprise mainly fever and cough. Other symptoms included myalgia, malaise, sore throat, diarrhea and shortness of breath. Data from laboratory tests often show that the majority of patients have lymphopenia and increased C-reactive protein, and chest CT scans reveal multiple patchy ground-glass shadows in the lungs. Pregnancy complications that appear after the onset of COVID-19 infection mainly included fetal distress and premature rupture of the membranes. A recent systematic review of 104 cases reported obstetrical, perinatal and neonatal outcomes occurred during the previous months of COVID-19 pandemic. Cesarean section was the mode of delivery for half of the women (50.0%), although no information was available for 28.8% of the cases. Regarding obstetrical and neonatal outcomes, fetal distress (13.5%), pre-labor rupture of membranes (9.6%), prematurity (8.7%), fetal death (4.8%), and abortion (2.9%) were reported. There were no positive results of neonatal infection by RT-PCR.263 Given that healthy pregnant women have evidence of increased generation of thrombin and a prothrombotic state, as well as increased intravascular inflammation that is exaggerated in the context of infection, such patients may be at an increased risk for thrombosis when affected by COVID-19. The International Society of Thrombosis and Haemostasis has generated a simple algorithm for the management of COVID-19 coagulopathy. The recommendation has been made that low-molecular-weight heparin should be considered in all such patients.264 Recently, Mendoza et al. published a paper which investigated the incidence of clinical, ultrasonographic and biochemical findings related to pre-eclampsia (PE) in pregnancies with COVID-19, and assessed their accuracy to differentiate between PE and the PE-like features associated with COVID-19. This study showed that pregnant women with severe COVID-19 can develop a PE-like syndrome that might be distinguished from PE by angiogenic factors (soluble fms-like tyrosine kinase-1/placental growth factor [sFlt-1/PlGF]), LDH and uterine artery pulsatility index (UtAPI) assessment. Thus, healthcare providers should be aware of its existence.265 The number of COVID-19 pregnancy outcomes is not large enough to draw absolute conclusions and long-term outcomes as the pandemic is still ongoing at the time of publication. Active and intensive follow-up is needed in order to provide robust data for the future.
Diagnosis
Confirmation of the disease is done using nucleic acid amplification tests (NAATs), such as real-time RT-PCR. Average RT-PCR testing takes up to 2 hours, but it takes between 6 and 10 hours for completion, or even longer when batch testing is done by laboratories. Imaging of the lungs is important in assessing the extent of COVID-19 pneumonia and in follow-up. Evidence on ultrasonographic imaging of the lung in patients with COVID-19 is evolving. In up to 85% of patients, abnormalities are found on imaging during the acute phase. Radiologic features of COVID-19 include patchy infiltrates on chest x-ray (CXR) and ground glass opacities (GGOs) on chest computed tomography (CT) scan.258 CXRs can be rapidly performed at bedside, but may have reduced sensitivity in the early stages of infection. Chest CT scan is more sensitive than CXR, but its widespread use is limited by availability, the practical but no less important consideration of the need for terminal cleaning to prevent nosocomial transmission, and acceptance by pregnant women. On chest CT, multilobar GGOs are most commonly seen, whereas lower lobe consolidation is more frequently observed in patients with severe and prolonged disease.259 Given its relatively untested specificity, its use as a first-line diagnostic tool has been discouraged by the American College of Radiology.260 In a case series of 15 pregnant patients with COVID-19 who were exposed to ionizing radiation between 2.3 and 5.8 mGy, all were found to have CT findings of mild disease, which did not worsen with pregnancy.260 In some circumstances when an earlier diagnosis of COVID-19 would alter the management of an obstetric patient, particularly if the patient is in respiratory distress raising concerns about significant pneumonia or concomitant pathology (e.g., pulmonary embolism), CXR, and thereafter chest CT if needed, could be considered. Clinicians should consider differential diagnosis. COVID-19 is primarily a respiratory illness. As understanding of the diagnostic imaging features of COVID-19 evolves, significant overlap with other viral and atypical pneumonias has been increasingly reported. On CXR, COVID-19 pneumonia often presents with multifocal, bilateral airspace opacification. This distinguishes it from the more common unifocal involvement noted in SARS, but not in MERS. When imaged by CT, the distribution seen in COVID-19 is similar to that noted in other viral and coronaviral pneumonias, such as influenza, parainfluenza, respiratory syncytial virus, and adenovirus. Even the multifocal GGOs, described in more than 80% of COVID-19 pneumonias, are common features of atypical (e.g,, Mycoplasma pneumoniae) and opportunistic (e.g., Pneumocystis jirovecii) pneumonias.261 As with other viral pneumonias, lymphadenopathy and pleural effusions are uncommon associated findings.262 In the later stages of COVID-19, confluent consolidation and interstitial thickening become more pronounced, with up to 20% patients developing features of ARDS. Given the significant overlap of imaging findings with other acute viral respiratory infections, imaging alone is unlikely to supplant the role of RT-PCR for the primary diagnosis of COVID-19.
Prevention and treatment
The spread of infection has been reported from asymptomatic patients, thereby rendering early detection and disease containment difficult.266 There is a possibility of viral dissemination when a patient is forcefully exhaling when in pain during active labor.267 Hence, it is essential to prepare for an adequate assistance at birth through a reorganization of the obstetrics units.268 All healthcare staff attending to women in active labor need to dress full personal protective equipment (PPE). Moreover, it is prudent to consider early epidural analgesia for optimal pain control.
General treatment of pregnant women with COVID-19 infection includes maintain fluid and electrolyte balance; symptomatic treatment consists in antipyretic, antibiotics and antidiarrheal medicines. Maternal surveillance is based on close and vigilant monitoring of vital signs and oxygen saturation level to minimize maternal hypoxia; conduct arterial blood-gas analysis; repeat chest imaging (when indicated); regular evaluation of complete blood count, renal and liver function testing, and coagulation testing.
Fetal surveillance consists in cardiotocography (CTG) for fetal heart rate (FHR) monitoring when gestational age is beyond the limit of viability based on local practice. The pregnancy should be managed according to the clinical findings, regardless of the timing of infection during pregnancy. All visits for obstetric emergencies should be offered in agreement with current local guidelines. All routine follow-up appointments should be postponed by 14 days or until positive test results (or two consecutive negative test results) are available.269
About breastfeeding, general recommendations suggest that if a mother previously identified as COVID-19 positive or under investigation for COVID-19 is asymptomatic or paucisymptomatic at delivery, rooming-in is feasible, and direct breastfeeding is advisable, under strict measures of infection control. On the contrary, when a mother with COVID-19 is too sick to care for the newborn, the neonate will be managed separately. Evidence and knowledge about COVID-19 and breastfeeding are still evolving.270
In conclusion, management of COVID-19 pregnant women should be shaped according to each clinical case. Ongoing collection of clinical data and research will answer questions in relation to the risk of congenital infection and the optimal antenatal and intrapartum management, timing and mode of delivery. Table 2 resumes key points on the current state of knowledges/recommendations on COVID-19 in pregnancy.
2
COVID-19 and pregnancy: key concepts on prevention, diagnosis and management.
DIAGNOSIS OF INFECTION (history, signs and symptoms, RT-PCR and instrumental examination)
CLINICAL CLASSIFICATION FOR STRATIFICATION OF RISK AND MANAGEMENT CHEST RADIOGRAPHY DURING PREGNANCY
MANAGEMENT
RE-ORGANIZATION OF OBSTETRIC UNITS
|
LYMPHOCYTIC CHORIOMENINGITIS VIRUS AND PREGNANCY
Lymphocytic choriomeningitis virus (LCMV) is an Arenavirus, discovered by Armstrong and Lillie in 1933.271 From 1955 to 2017, a total of 58 cases of congenital LCMV infections have been reported worldwide; all were diagnosed postnatally. An influenza-like illness was described in 50% of pregnant women, and exposure to rodents was reported by 33%.
Transmission
Although mice are the most common reservoir for LCMV, humans can acquire it by direct contact with fomites, through breathing in aerosolized virus or through organ transplantation.272 Infection with LCMV as an adult or child is similar to symptoms of meningitis and will lead to a full recovery. However, if contacted during pregnancy, this single-stranded RNA virus can cause transplacental human fetal infections with serious clinical consequences. Lymphocytic choriomeningitis virus can cause intrauterine infection from vertical transmission across the placenta or exposure to maternal vaginal secretions or blood during maternal viremia. Recent data suggest that innate immune response to LCMV infection of the human placenta is more vigorous in the third trimester than in the first trimester. The absence of viral replication in term placental explants may be attributable to the robust innate antiviral response in this tissue. Such findings parallel the clinical observation of decreased transplacental transmission and less severe fetal phenotypes of viral pathogens acquired in later gestation. Continued research into LCMV as a model of human congenital infection and immunity is warranted.273
Signs and symptoms
In immunocompetent adults, LCMV infection leads to an influenza-like illness or aseptic meningitis that usually resolves spontaneously; infection can also be asymptomatic.274 Most frequent and aspecific symptoms are weakness, myalgia, retroorbital headache, and photophobia.
Obstetrics outcomes
Like many congenital pathogens, LCMV has a tropism for fetal neural and retinal tissue, causing issues with brain development including microencephaly, periventricular calcification, cerebellar hypoplasia, and hydrocephalus.275,276 Meta-analysis demonstrated that children with congenital LCMV infection have a 35% mortality rate by approximately 2 years of age; those who survive have long-term neurological impairment and/or vision disability.276 The incidence of congenital LCMV is unknown, and infants with suspected congenital infection are not commonly tested for this viral pathogen. However, 9% of mice carry LCMV and 5% of humans are seropositive for the virus,277,278 indicating that it may be an underdiagnosed etiology of fetal/neonatal pathology. Congenital viral infections generally manifest with more severe fetal disease following the first-trimester maternal infection, when compared to infection later in gestation. For example, fetuses infected with Zika virus during the first trimester are known to be at increased risk for structural abnormalities.279,280 In a rat model of LCMV, pups introduced to the virus early in gestation (days 1–10) had more frequent and severe neuropathologies compared to pups exposed later in gestation.281 In part, this effect has been attributed to the teratogenic impact of infection during early fetal development – most evident after early transplacental rubella and varicella infections.282,283 However, maternal–fetal immune interactions evolve throughout pregnancy,284 possibly changing the placental response to viral pathogens as pregnancy progresses. Although few cases are described in the literature, the majority of published reports suggest that the virus is selectively neurotropic when transmitted in utero, targeting the brain and retina in 87.5% of cases.285,286,287,288,289,290,291 In animal models, Bonthius et al. demonstrated several mechanisms that explain the effect of the virus on the fetal brain: (a) the virus exhibits a strong tropism for neuroblasts; (b) disturbs the migration of neurons; and (c) triggers an inflammatory response, driven by cytotoxic T lymphocytes. The gestational age of the fetus also significantly affects the “patterns of infection and pathology within the brain”.285 The estimated mortality rate for infants with prenatal LCMV infection is 30–35% at the age of 21 months. Almost all survivors have neurologic sequelae of which 67% are severe.285
Diagnosis
Congenital lymphocytic choriomeningitis virus infection is often misdiagnosed as other infectious, neurologic, ophthalmologic, or chromosomal syndromes, or is lethal in utero.286,292,293 It has therefore been previously suggested that lymphocytic choriomeningitis virus be added to the list of congenital infections currently included in the TORCH acronym (Toxoplasmosis, Rubella, Cytomegalovirus, Herpes, and Syphilis).285,286,287,294 Due to the diverse clinical presentations for congenital lymphocytic choriomeningitis virus infection and the small number of cases described in the literature, further epidemiologic studies are needed to elucidate the natural history of congenital lymphocytic choriomeningitis virus infection, and to develop guidelines for inclusion of lymphocytic choriomeningitis virus in prenatal testing. These are the necessary steps to take to substantially reduce the risk of LCMV infection, since there is no treatment or vaccine to prevent the infection in pregnant women or their offspring.295 Fetal ultrasound examination could be really useful to insinuate the suspect for LCMV infection due to signs of fetopathy, such as hepatosplenomegaly, thymic hypertrophy, ascites, and pericardial and pleural effusion. Examination of the brain could show severe ventriculomegaly, microcephaly with polymicrogyria, a thin cortex, and diffuse periventricular calcifications. A bilateral chorioretinitis could be detected.
When ultrasonographic signs suggestive of infection are identified, complete ultrasonography can be performed to identify associated abnormalities and conventional congenital infections. If results of this initial assessment are negative, testing for LCMV is indicated for fetal samples and maternal serum samples. A definitive diagnosis relies on virus identification by serologic analysis or direct evidence, such as virus isolation or detection of LCMV RNA in fetal or maternal samples.
Treatment
No vaccine or effective treatment is available for infection with LCMV. Current recommendations are based on prevention strategies of transmission. Obstetricians should educate their pregnant patients about the risks of exposure to laboratory, pet, and wild rodents, and they should consider this infection in the differential diagnosis in suspected cases.
Conclusions
LCMV infection in pregnancy is rare and often underdiagnosed. There are many prenatal ultrasonographic signs of LCMV infection, involving mostly the central nervous system. Of these signs, ventriculomegaly is the most common. This infection should be considered a possible etiology requiring laboratory investigations for cases of evocative neurologic malformations or nonimmunologic anasarca not caused by common TORCH infections or genetic or chromosomal abnormalities. Further research, to define the prevalence of this infection in human and rodent populations, and prospective studies, to delineate the complete clinical spectrum of congenital infection, are needed. Healthcarers should be made aware of the hazard that wild, pet, and laboratory rodents pose to pregnant women.
PRACTICE RECOMMENDATIONS
Measles, mumps, rubella, varicella-zoster and pregnancy
- Routine screening for viral infections such as VZV and MMR, is substantially useful for women during their early pregnancy.
- Healthcare providers who deal with women of childbearing age should routinely determine MMR immunity by means of laboratory confirmation (clinical diagnosis without laboratory confirmation is not a sufficient evidence of immunity) and vaccinate all susceptible non-pregnant women.
- Serologic evidence of past VZV infection should be obtained at least in women of childbearing age who report a negative or uncertain history of chickenpox. All susceptible non-pregnant women should be vaccinated against chickenpox.
- Healthcare providers should at least consider administration of antiviral therapy to pregnant women with uncomplicated chickenpox. Pregnant women with complicated chickenpox pneumonia must receive antiviral therapy as soon as possible.
- Since many women possess a serologic evidence of past MMR or VZV infection, it is wise to serologically screen them prior to prophylaxis (PEP).
- PEP consisting of high titers of measles-specific immune globulins, should be offered to susceptible pregnant women who have had a significant exposure to a person with measles.
- PEP consisting of high titers of VZV-specific immune globulins should be offered to susceptible pregnant women who have had a significant exposure to a person with chickenpox or herpes zoster.
- After maternal chickenpox, the risk of CVS can be estimated using PCR testing of fetal blood or amniotic fluid for VZV DNA, in conjunction with ultrasonography for detection of congenital abnormalities. Still however, the women who acquire VZV infection during pregnancy, the risk of CVS is significantly low.
- Whenever feasible, close household contacts of mothers can also be immunized so as to ensure minimal viral transmission to a woman during pregnancy.
Cytomegalovirus and pregnancy
- CMV infection of women during pregnancy or periconceptional period can be transmitted to the fetuses or newborns.
- Only a minority of babies with congenital CMV develop permanent disability, but each year, more than 5000 babies develop a disability due to congenital CMV.
- Vaccines for prevention of maternal and congenital CMV infection are being developed but not yet available.
- Passive immunization and antiviral therapies are being investigated, but it is not yet clear whether these are effective in reducing the risk of vertical transmission.
- The best strategy against congenital CMV and its sequelae is to educate women on how to avoid exposure to CMV by following a few prevention guidelines.
- All pregnant women and women trying to conceive should routinely receive information about CMV prevention as avoiding contact with urine or saliva especially from babies and young children, and washing hands often with soap.
Herpes simplex virus 1–2 and pregnancy
- HSV infection during pregnancy can cause neonatal infection and consequent serious morbidity and mortality.
- Women and their babies have a higher risk when a newly acquired genital HSV infection (primary or nonprimary first-episode genital infections) occurs during the third trimester.
- In case of suspicious of genital HSV infection in pregnancy, a direct viral test (viral culture or PCR) and type-specific serologic assay should be performed.
- Mother-to-child HSV transmission occurs mainly during peripartum period through direct contact with virus shed from genital HSV lesions.
- Infected women can shed HSV both in the presence and in the absence of genital lesions.
- It is not recommended to test asymptomatic women with a history of genital HSV infection.
- Women with active lesions at delivery, a primary or nonprimary first-episode genital HSV infection during the third trimester of pregnancy, or prodromal symptoms in case of a history of genital HSV, should be offered cesarean delivery to avoid perinatal transmission.
Hepatitis viruses and pregnancy
- Routine screening for hepatitis A virus (HAV) is not recommended (but consider geographical areas).
- HAV prevention includes adherence to sanitary practices, active and passive immunoprophylaxis.
- Universal HAV vaccination for pregnant mothers is not recommended. Vaccination is recommended for high-risk patients and mothers.
- Immune globulin: available for postexposure HAV prophylaxis.
- Universal maternal screening and passive-active immunoprophylaxis of newborns have reduced transmission of HBV, but the addition of antiviral therapy is necessary to further decrease immunoprophylaxis failure.
- HBV-DNA follow-up levels as reference to guide pharmacotherapy (tenofovir, telbivudine, and lamivudine can be used safely in pregnancy without apparent teratogenicity or other harmful effects on mother or baby).
- HBV DNA ≥5.3 log10 IU/mL (≥200,000 IU/mL): receive tenofovir prophylaxis from the 28th week of pregnancy until at least birth, to prevent mother-to-child transmission of HBV.
- Multidisciplinary management: plan of care between patient, obstetrician, specialist in infectious diseases and hepatologist.
- Screening pregnant women infected with HBV for HDV.
- HBV screening and immunization programs are at the basis to prevent HDV infection.
- Serum immunoglobulin M anti-HDAg is detected during active HDV infection.
- 3–5% transmit HCV to their child at the time of birth.
- All pregnant women especially at risk, should be screened at the first prenatal visit for HCV.
- HCV infection should not influence the mode of delivery, and breastfeeding is not contraindicated.
- New, potentially curative therapies will be available to manage peripartum HCV infection in both pregnant women and infants in the coming years, but additional prospective studies will be required
- Keep in mind the possibility of HEV infection in pregnant women (tested Anti-HEV IgM in suspected cases).
- HEV has an aggressive course and high-mortality risk (15–25%) in pregnant population.
- Fecal–oral route transmission of HEV: prevention based on improving sanitation measures, provision of clean drinking water and avoiding raw pork and venison.
Ebola and pregnancy
- EVD is a relatively recent infectious disease and mainly linked to rural outbreaks.
- High maternal morbidity and mortality was reported.
- Increased risk of spontaneous abortion, preterm labor, stillbirth, or neonatal death. Others obstetrics complications described were postpartum hemorrhage, retained placenta, prolonged labor.
- Breastfeeding should be discouraged in acute EVD and mothers critically ill.
- Paucity of scientific evidence: to limit the spread of disease consider epidemiological data, prevention strategies, early detection, supportive therapies.
Zika virus and pregnancy
- The global epidemic of ZIKV is a major public health problem affecting pregnant women and their infants
- ZIKV infection in acute stage is asymptomatic in up to 80% of the infected people
- Vertical transmission could cause a severe congenital ZIKV syndrome: anamnestic data, fetal ultrasound examination, maternal serological sample, amniotic fluid examination for the diagnosis
- At present, no specific vaccines or antivirals are available, so management and control of ZIKV infections are based mostly on preventive and supportive measures.
SARS-CoV-2 and pregnancy
- Covid-19 infection is a current global health emergency: clinical data, also for pregnancies and obstetrics outcomes, are in continuous evolution.
- Covid-19 vaccination in pregnancy: a topic of debate.
- Need to prevent viral infection, observe cases, collect data, investigate pathological pathways.
Lymphocytic choriomeningitis virus and pregnancy
- LCMV is an emerging teratogen pathogen
- Congenital LCMV disease: wide range spectrum of disease; main trophism for fetal brain and retina.
- Neuropathology severity differs among cases.
- Diagnosis: (1) laboratory (serological confirmation); (2) fetal ultrasonographic features (structural brain anomalies, chorioretinitis, microencephaly, periventricular calcifications, ventriculomegaly, pachygyria, cerebellar hypoplasia, porencephalic cysts, periventricular cysts, and hydrocephalus); (3) differential diagnosis with other TORCH infections, genetics or chromosomal aberrations.
- Only preventive strategies based on patients education.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Perry RT, Gacic-Dobo M, Dabbagh A, et al. Centers for Disease Control and Prevention (CDC). Global control and regional elimination of measles, 2000–2012. MMWR Morb Mortal Wkly Rep 2014;63(5):103–7. | |
McLean HQ, Fiebelkorn AP, Temte JL, et al. Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013;62(RR-04):1–34. Erratum in: MMWR Recomm Rep 2015;64(9):259. | |
Charlier C, Hourrier S, Leruez-Ville M, et al. Polyvalent immunoglobulins in neonates after perinatal exposure to measles: Benefits and long-term tolerance of immunoglobulins. J Infect 2015;71(1):131–4. doi: 10.1016/j.jinf.2015.01.010. | |
Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004;189(1):S4–16. doi: 10.1086/377712. | |
McLean HQ, Fiebelkorn AP, Temte JL, et al. Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013;62(RR-04):1–34. Erratum in: MMWR Recomm Rep 2015;64(9):259. | |
Chovatiya R, Silverberg JI. Inpatient morbidity and mortality of measles in the United States. PLoS One 2020;15(4):e0231329. doi: 10.1371/journal.pone.0231329. | |
Ogbuanu IU, Zeko S, Chu SY, et al. Maternal, fetal, and neonatal outcomes associated with measles during pregnancy: Namibia, 2009–2010. Clin Infect Dis 2014;58(8):1086–92. doi: 10.1093/cid/ciu037. | |
Eberhart-Phillips JE, Frederick PD, Baron RC, et al. Measles in pregnancy: a descriptive study of 58 cases. Obstet Gynecol 1993;82(5):797–801. | |
Rasmussen SA, Jamieson DJ. What Obstetric Health Care Providers Need to Know About Measles and Pregnancy. Obstet Gynecol 2015;126(1):163–70. doi: 10.1097/AOG.0000000000000903. | |
Gershon AA. Chickenpox, measles, and mumps. In: Remington JS, Klein JO, Wilson CB, et al. (eds.) Infectious Diseases of the Fetus and Newborn Infant, 7th edn. Philadelphia: Elsevier, 2011. | |
Hardy JB. Fetal consequences of maternal viral infections in pregnancy. Archives of Otolaryngology (Chicago, Ill: 1960) 1973;98(4):218–27. | |
Ali ME, Albar HM. Measles in pregnancy: maternal morbidity and perinatal outcome. International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics 1997;59(2):109–13. | |
Dao B, Koalaga AP, Ki Zerbo G, et al. [Measles and pregnancy. Apropos of 16 cases in Burkina Faso]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1997;26(6):606–9. | |
Siegel M, Fuerst HT. Low birth weight and maternal virus diseases. A prospective study of rubella, measles, mumps, chickenpox, and hepatitis. JAMA 1966;197(9):680–4. | |
Dasopoulou M, Covanis A. Subacute sclerosing panencephalitis after intrauterine infection. Acta Paediatrica (Oslo, Norway: 1992) 2004;93(9):1251–3. | |
Cruzado D, Masserey-Spicher V, Roux L, et al. Early onset and rapidly progressive subacute sclerosing panencephalitis after congenital measles infection. European Journal of Pediatrics 2002;161(8):438–41. | |
Moss WJ, Griffin DE. Measles. Lancet 2012;379(9811):153–64. doi: 10.1016/S0140-6736(10)62352-5. | |
Center for Disease Control and Prevention (CDC) Mumps. In: Atkinson W, Wolfe S, Hamborsky J. (eds.) Epidemiology and Prevention of Vaccine-Preventable Diseases, 12th edn. Washington, DC: Public Health Foundation, 2011. | |
Hviid A, Rubin S, Mühlemann K. Mumps. Lancet 2008;371(9616):932–44. doi: 10.1016/S0140-6736(08)60419-5. | |
Siegel M, Fuerst HT, Peress NS. Comparative fetal mortality in maternal virus diseases. A prospective study on rubella, measles, mumps, chicken pox and hepatitis. N Engl J Med 1966;274(14):768–71. doi: 10.1056/NEJM196604072741404. | |
Bowers D. Mumps during pregnancy. West J Surg Obstet Gynecol 1953;61(2):72–3. | |
Siegel M. Congenital malformations following chickenpox, measles, mumps, and hepatitis. Results of a cohort study. JAMA 1973;226(13):1521–4. | |
Grant GB, Desai S, Dumolard L, et al. Progress Toward Rubella and Congenital Rubella Syndrome Control and Elimination – Worldwide, 2000–2018. MMWR Morb Mortal Wkly Rep 2019;68(39):855–9. doi: 10.15585/mmwr.mm6839a5. | |
Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet 1982;2(8302):781–4. doi: 10.1016/s0140-6736(82)92677-0. | |
Morgan-Capner P, Miller E, Vurdien JE, et al. Outcome of pregnancy after maternal reinfection with rubella. CDR (Lond Engl Rev) 1991;1(6):R57–9. | |
Peckham CS. Clinical and laboratory study of children exposed in utero to maternal rubella. Arch Dis Child 1972;47(254):571–7. doi: 10.1136/adc.47.254.571. | |
Grillner L, Forsgren M, Barr B, et al. Outcome of rubella during pregnancy with special reference to the 17–24th weeks of gestation. Scand J Infect Dis 1983;15(4):321–5. doi: 10.3109/inf.1983.15.issue-4.01. | |
Bullens D, Smets K, Vanhaesebrouck P. Congenital rubella syndrome after maternal reinfection. Clin Pediatr (Phila) 2000;39(2):113–6. doi: 10.1177/000992280003900207. | |
Paludetto R, van den Heuvel J, Stagni A, et al. Rubella embryopathy after maternal reinfection. Biol Neonate 1994;65(5):340–1. doi: 10.1159/000244081. | |
Robinson J, Lemay M, Vaudry WL. Congenital rubella after anticipated maternal immunity: two cases and a review of the literature. Pediatr Infect Dis J 1994;13(9):812–5. doi: 10.1097/00006454-199409000-00013. | |
Reef SE, Plotkin S, Cordero JF, et al. Preparing for elimination of congenital Rubella syndrome (CRS): summary of a workshop on CRS elimination in the United States. Clin Infect Dis 2000;31(1):85–95. doi: 10.1086/313928. | |
Forrest JM, Turnbull FM, Sholler GF, et al. Gregg's congenital rubella patients 60 years later. Med J Aust 2002;177(11–12):664–7. doi: 10.5694/j.1326-5377.2002.tb05003.x. | |
Oster ME, Riehle-Colarusso T, Correa A. An update on cardiovascular malformations in congenital rubella syndrome. Birth Defects Res A Clin Mol Teratol 2010;88(1):1–8. doi: 10.1002/bdra.20621. | |
Sever JL, South MA, Shaver KA. Delayed manifestations of congenital rubella. Rev Infect Dis 1985;7(1):S164–9. doi: 10.1093/clinids/7.supplement_1.s164. | |
Preece MA, Kearney PJ, Marshall WC. Growth-hormone deficiency in congenital rubella. Lancet 1977;2(8043):842–4. doi: 10.1016/s0140-6736(77)90781-4. | |
Duszak RS. Congenital rubella syndrome–major review. Optometry 2009;80(1):36–43. doi: 10.1016/j.optm.2008.03.006. | |
Ameratunga R, Woon ST, Koopmans W, et al. Cellular and molecular characterisation of the hyper immunoglobulin M syndrome associated with congenital rubella infection. J Clin Immunol 2009;29(1):99–106. doi: 10.1007/s10875-008-9219-y. | |
Best JM, Enders G. Chapter 3 Laboratory Diagnosis of Rubella and Congenital Rubella. In: Banatvala J, Peckham C. (eds.) Perspectives in Medical Virology 2007;15:39–77. London: Elsevier, 2007. | |
Aboudy Y, Barnea B, Yosef L, et al. Clinical rubella reinfection during pregnancy in a previously vaccinated woman. J Infect 2000;41(2):187–9. doi: 10.1053/jinf.2000.0716. | |
Best JM, Banatvala JE, Morgan-Capner P, et al. Fetal infection after maternal reinfection with rubella: criteria for defining reinfection. BMJ 1989;299(6702):773–5. doi: 10.1136/bmj.299.6702.773. | |
Tipples GA, Hamkar R, Mohktari-Azad T, et al. Evaluation of rubella IgM enzyme immunoassays. J Clin Virol 2004;30(3):233–8. doi: 10.1016/j.jcv.2003.11.006. | |
Nardone A, Tischer A, Andrews N, et al. Comparison of rubella seroepidemiology in 17 countries: progress towards international disease control targets. Bull World Health Organ 2008;86(2):118–25. doi: 10.2471/blt.07.042010. | |
Best JM, O'Shea S, Tipples G, et al. Interpretation of rubella serology in pregnancy–pitfalls and problems. BMJ 2002;325(7356):147–8. doi: 10.1136/bmj.325.7356.147. | |
Böttiger B, Jensen IP. Maturation of rubella IgG avidity over time after acute rubella infection. Clin Diagn Virol 1997;8(2):105–11. doi: 10.1016/s0928-0197(97)00018-4. | |
Pustowoit B, Liebert UG. Predictive value of serological tests in rubella virus infection during pregnancy. Intervirology 1998;41(4–5):170–7. doi: 10.1159/000024932. | |
Marin M, Güris D, Chaves SS, et al. Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR-4):1–40. | |
Sehrawat S, Kumar D, Rouse BT. Herpesviruses: Harmonious Pathogens but Relevant Cofactors in Other Diseases? Front Cell Infect Microbiol 2018;8:177. doi: 10.3389/fcimb.2018.00177. | |
Hamborsky J, Kroger A. Epidemiology and prevention of vaccine-preventable diseases, E-Book: The Pink Book: Public Health Foundation, 2015. | |
Enright AM, Prober CG. Herpesviridae infections in newborns: varicella zoster virus, herpes simplex virus, and cytomegalovirus. Pediatr Clin North Am 2004;51(4):889–908, viii. doi: 10.1016/j.pcl.2004.03.005. | |
Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers 2015;1:15016. doi: 10.1038/nrdp.2015.16. | |
Harger JH, Ernest JM, Thurnau GR, et al. National Institute of Child Health and Human Development, Network of Maternal-Fetal Medicine Units. Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J Infect Dis 2002;185(4):422–7. doi: 10.1086/338832. | |
Ahn KH, Park YJ, Hong SC, et al. Congenital varicella syndrome: A systematic review. J Obstet Gynaecol 2016;36(5):563–6. doi: 10.3109/01443615.2015.1127905. | |
Sauerbrei A, Wutzler P. Varicella-zoster virus infections during pregnancy: epidemiology, clinical symptoms, diagnosis, prevention and therapy. Current Pediatric Reviews 2005;1(3):205–15. https://doi.org/10.2174/157339605774574962 | |
Prober CG, Gershon AA, Grose C, et al. Consensus: varicella-zoster infections in pregnancy and the perinatal period. Pediatr Infect Dis J 1990;9(12):865–9. | |
Kennedy PGE, Gershon AA. Clinical Features of Varicella-Zoster Virus Infection. Viruses 2018;10(11):609. doi: 10.3390/v10110609. | |
Tan MP, Koren G. Chickenpox in pregnancy: revisited. Reprod Toxicol 2006;21(4):410–20. doi: 10.1016/j.reprotox.2005.04.011. | |
Schulze A, Dietzsch HJ. The natural history of varicella embryopathy: a 25-year follow-up. J Pediatr 2000;137(6):871–4. doi: 10.1067/mpd.2000.109005. | |
Miller E, Cradock-Watson JE, Ridehalgh MK. Outcome in newborn babies given anti-varicella-zoster immunoglobulin after perinatal maternal infection with varicella-zoster virus. Lancet 1989;2(8659):371–3. doi: 10.1016/s0140-6736(89)90547-3. | |
Ehrlich RM, Turner JA, Clarke M. Neonatal varicella; a case report with isolation of the virus. The Journal of Pediatrics 1958;53(2):139–47. doi: 10.1016/s0022-3476(58)80165-1. | |
Schutte TJ, Rogers LC, Copas PR. Varicella pneumonia complicating pregnancy: a report of seven cases. Infect Dis Obstet Gynecol 1996;4(6):338–46. doi: 10.1155/S1064744996000683. | |
Enders G, Miller E, Cradock-Watson J, et al. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet 1994;343(8912):1548–51. doi: 10.1016/s0140-6736(94)92943-2. | |
Paryani SG, Arvin AM. Intrauterine infection with varicella-zoster virus after maternal varicella. N Engl J Med 1986;314(24):1542–6. doi: 10.1056/NEJM198606123142403. | |
Higa K, Dan K, Manabe H. Varicella-zoster virus infections during pregnancy: hypothesis concerning the mechanisms of congenital malformations. Obstet Gynecol 1987;69(2):214–22. | |
Marin M, Watson TL, Chaves SS, et al. Varicella among adults: data from an active surveillance project, 1995–2005. J Infect Dis 2008;197(2):S94–S100. doi: 10.1086/522155. | |
Essex-Cater A, Heggarty H. Fatal congenital varicella syndrome. J Infect 1983;7(1):77–8. doi: 10.1016/s0163-4453(83)91173-8. | |
Enders G, Miller E. Varicella and herpes zoster in pregnancy and the newborn. Varicella-zoster virus Virology and clinical management. University Press Cambridge (UK), 2000. p. 317–47. | |
Tunbridge AJ, Breuer J, Jeffery KJ, British Infection Society. Chickenpox in adults – clinical management. J Infect 2008;57(2):95–102. doi: 10.1016/j.jinf.2008.03.004. | |
Wallace MR, Bowler WA, Murray NB, et al. Treatment of adult varicella with oral acyclovir. A randomized, placebo-controlled trial. Ann Intern Med 1992;117(5):358–63. doi: 10.7326/0003-4819-117-5-358. | |
Stone KM, Reiff-Eldridge R, White AD, et al. Pregnancy outcomes following systemic prenatal acyclovir exposure: Conclusions from the international acyclovir pregnancy registry, 1984–1999. Birth Defects Res A Clin Mol Teratol 2004;70(4):201–7. doi: 10.1002/bdra.20013. | |
Eldridge RR, Ephross SA, Heffner CR, et al. Monitoring pregnancy outcomes following prenatal drug exposure through prospective pregnancy registries and passive surveillance: a pharmaceutical company commitment. Prim Care Update Ob Gyns 1998;5(4):190–91. doi: 10.1016/s1068-607x(98)00115-2. | |
Coonrod DV, Jack BW, Boggess KA, et al. The clinical content of preconception care: immunizations as part of preconception care. Am J Obstet Gynecol 2008;199(6 Suppl 2):S290–5. doi: 10.1016/j.ajog.2008.08.061. | |
Bester JC. Measles and Measles Vaccination: A Review. JAMA Pediatr 2016;170(12):1209–15. doi: 10.1001/jamapediatrics.2016.1787. | |
Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev 2012;2012(2):CD004407. doi: 10.1002/14651858.CD004407.pub3. Update in: Cochrane Database Syst Rev 2020;4:CD004407. | |
Keller-Stanislawski B, Englund JA, Kang G, et al. Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 2014;32(52):7057–64. doi: 10.1016/j.vaccine.2014.09.052. | |
Bar-Oz B, Levichek Z, Moretti ME, et al. Pregnancy outcome following rubella vaccination: a prospective controlled study. Am J Med Genet A 2004;130A(1):52–4. doi: 10.1002/ajmg.a.30225. | |
Badilla X, Morice A, Avila-Aguero ML, et al. Fetal risk associated with rubella vaccination during pregnancy. Pediatr Infect Dis J 2007;26(9):830–5. doi: 10.1097/INF.0b013e318124a9f4. | |
Watson JC, Hadler SC, Dykewicz CA, et al. Measles, mumps, and rubella–vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1998;47(RR-8):1–57. | |
Landes RD, Bass JW, Millunchick EW, et al. Neonatal rubella following postpartum maternal immunization. J Pediatr 1980;97(3):465–7. doi: 10.1016/s0022-3476(80)80207-1. | |
Black NA, Parsons A, Kurtz JB, et al. Post-partum rubella immunisation: a controlled trial of two vaccines. Lancet 1983;2(8357):990–2. doi: 10.1016/s0140-6736(83)90979-0. | |
Pavelka R, Salzer H, Reinold E. Zur Rötelnimpfung und Anti-D-Prophylaxe post partum. Post partum rubella vaccination and anti-D prevention. Zentralbl Gynakol 1978;100(16):1025–9. German. | |
Edgar WM, Hambling MH. Rubella vaccination and anti-D immunoglobulin administration in the puerperium. Br J Obstet Gynaecol 1977;84(10):754–7. doi: 10.1111/j.1471-0528.1977.tb12487.x. | |
Shaw J, Gershon AA. Varicella Virus Vaccination in the United States. Viral Immunol 2018;31(2):96–103. doi: 10.1089/vim.2017.0136. | |
Salzman MB, Sharrar RG, Steinberg S, et al. Transmission of varicella-vaccine virus from a healthy 12-month-old child to his pregnant mother. J Pediatr 1997;131(1 Pt 1):151–4. doi: 10.1016/s0022-3476(97)70140-9. | |
Marin M, Marlow M, Moore KL, et al. Recommendation of the Advisory Committee on Immunization Practices for Use of a Third Dose of Mumps Virus-Containing Vaccine in Persons at Increased Risk for Mumps During an Outbreak. MMWR Morb Mortal Wkly Rep 2018;67(1):33–38. doi: 10.15585/mmwr.mm6701a7. | |
Tunis MC, Salvadori MI, Dubey V, et al. National Advisory Committee on Immunization (NACI)*. Updated NACI recommendations for measles post-exposure prophylaxis. Can Commun Dis Rep 2018;44(9):226–30. doi: 10.14745/ccdr.v44i09a07. | |
Koren G, Money D, Boucher M, et al. Serum concentrations, efficacy, and safety of a new, intravenously administered varicella zoster immune globulin in pregnant women. J Clin Pharmacol 2002;42(3):267–74. doi: 10.1177/00912700222011283. | |
Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG–United States, 2013. MMWR Morb Mortal Wkly Rep 2013;62(28):574–6. | |
Alonso AM, Perrotin F, Harchaoui Y, et al. Pneumopathie varicelleuse en cours de grossesse après double contage au 2e trimestre. Valeur de la séroprophylaxie. Varicella pneumonia during pregnancy after double exposure in the 2nd trimester. Value of seroprophylaxis. J Gynecol Obstet Biol Reprod (Paris) 1999;28(8):838–41. | |
Neu N, Duchon J, Zachariah P. TORCH infections. Clin Perinatol 2015;42(1):77–103, viii. doi: 10.1016/j.clp.2014.11.001. | |
Plosa EJ, Esbenshade JC, Fuller MP, et al. Cytomegalovirus infection. Pediatr Rev 2012;33(4):156–63; quiz 163. doi: 10.1542/pir.33-4-156. | |
Fowler KB, Boppana SB. Congenital cytomegalovirus infection. Semin Perinatol 2018;42(3):149–54. doi: 10.1053/j.semperi.2018.02.002. | |
Numazaki K, Chiba S, Asanuma H. Transmission of cytomegalovirus. Lancet 2001;357(9270):1799–800. doi: 10.1016/S0140-6736(00)04913-8. | |
Stowell JD, Forlin-Passoni D, Radford K, et al. Cytomegalovirus survival and transferability and the effectiveness of common hand-washing agents against cytomegalovirus on live human hands. Appl Environ Microbiol 2014;80(2):455–61. doi: 10.1128/AEM.03262-13. | |
Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010;20(4):202–13. doi: 10.1002/rmv.655. | |
Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol 2019;29(3):e2034. doi: 10.1002/rmv.2034. | |
Dupont L, Reeves MB. Cytomegalovirus latency and reactivation: recent insights into an age old problem. Rev Med Virol 2016;26(2):75–89. doi: 10.1002/rmv.1862. | |
Wills MR, Poole E, Lau B, et al. The immunology of human cytomegalovirus latency: could latent infection be cleared by novel immunotherapeutic strategies? Cell Mol Immunol 2015;12(2):128–38. doi: 10.1038/cmi.2014.75. | |
Pass RF, Anderson B. Mother-to-Child Transmission of Cytomegalovirus and Prevention of Congenital Infection. J Pediatric Infect Dis Soc 2014;3(Suppl 1):S2–6. doi: 10.1093/jpids/piu069. | |
Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 2011;21(4):240–55. doi: 10.1002/rmv.695. | |
Hui L, Wood G. Perinatal outcome after maternal primary cytomegalovirus infection in the first trimester: a practical update and counseling aid. Prenat Diagn 2015;35(1):1–7. doi: 10.1002/pd.4497. | |
Zheng QY, Huynh KT, van Zuylen WJ, et al. Cytomegalovirus infection in day care centres: A systematic review and meta-analysis of prevalence of infection in children. Rev Med Virol 2019;29(1):e2011. doi: 10.1002/rmv.2011. | |
Revello MG, Lazzarotto T, Guerra B, et al. CHIP Study Group. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 2014;370(14):1316–26. doi: 10.1056/NEJMoa1310214. | |
Nigro G, Adler SP, La Torre R, et al. Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 2005;353(13):1350–62. doi: 10.1056/NEJMoa043337. | |
Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 2003;289(8):1008–11. doi: 10.1001/jama.289.8.1008. | |
Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 1986;256(14):1904–8. | |
Stagno S, Britt W. Cytomegalovirus Infections. In: Remington JS, Klein JO, Wilson CB, et al. (eds.) Infectious Diseases of the Fetus and Newborn Infant. Philadelphia, PA: Elsevier Saunders, 2006. pp. 739–81. | |
Nigro G, Anceschi MM, Cosmi EV, Congenital Cytomegalic Disease Collaborating Group. Clinical manifestations and abnormal laboratory findings in pregnant women with primary cytomegalovirus infection. BJOG 2003;110(6):572–7. | |
Lancini D, Faddy HM, Flower R, et al. Cytomegalovirus disease in immunocompetent adults. Med J Aust 2014;201(10):578–80. doi: 10.5694/mja14.00183. | |
Abgueguen P, Delbos V, Ducancelle A, et al. Venous thrombosis in immunocompetent patients with acute cytomegalovirus infection: a complication that may be underestimated. Clin Microbiol Infect 2010;16(7):851–4. doi: 10.1111/j.1469-0691.2009.03022.x. | |
van Spronsen DJ, Breed WP. Cytomegalovirus-induced thrombocytopenia and haemolysis in an immunocompetent adult. Br J Haematol 1996;92(1):218–20. doi: 10.1046/j.1365-2141.1996.00288.x. | |
Just-Nübling G, Korn S, Ludwig B, et al. Primary cytomegalovirus infection in an outpatient setting–laboratory markers and clinical aspects. Infection 2003;31(5):318–23. doi: 10.1007/s15010-003-3129-y. | |
Mocarski ESJ, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM. (eds.) Fields Virology Philadelphia: Lippincott, Williams & Wilkins, 2007. pp. 2701–72 | |
Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis 2003;37(12):1603–6. doi: 10.1086/379711. | |
Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr 2006;148(3):332–6. doi: 10.1016/j.jpeds.2005.09.003. | |
Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007;17(5):355–63. doi: 10.1002/rmv.544. | |
Ostrander B, Bale JF. Congenital and perinatal infections. Handb Clin Neurol 2019;162:133–53. doi: 10.1016/B978-0-444-64029-1.00006-0. | |
Ross SA, Boppana SB. Congenital cytomegalovirus infection: outcome and diagnosis. Semin Pediatr Infect Dis 2005;16(1):44–9. doi: 10.1053/j.spid.2004.09.011. | |
Boppana SB, Fowler KB, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 1999;104(1 Pt 1):55–60. doi: 10.1542/peds.104.1.55. | |
Boppana SB, Rivera LB, Fowler KB, et al. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 2001;344(18):1366–71. doi: 10.1056/NEJM200105033441804. | |
Yamamoto AY, Mussi-Pinhata MM, Boppana SB, et al. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 2010;202(3):297.e1–8. doi: 10.1016/j.ajog.2009.11.018. | |
Mussi-Pinhata MM, Pinto PC, Yamamoto AY, et al. Placental transfer of naturally acquired, maternal cytomegalovirus antibodies in term and preterm neonates. J Med Virol 2003;69(2):232–9. doi: 10.1002/jmv.10271. | |
Dworsky M, Yow M, Stagno S, et al. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics 1983;72(3):295–9.) | |
Lanzieri TM, Dollard SC, Josephson CD, et al. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics 2013;131(6):e1937–45. doi: 10.1542/peds.2013-0076. Epub 2013 May 27. Erratum in: Pediatrics 2014;133(4):755. | |
Fisher S, Genbacev O, Maidji E, et al. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol 2000;74(15):6808–20. doi: 10.1128/jvi.74.15.6808-6820.2000. | |
Chan G, Guilbert LJ. Ultraviolet-inactivated human cytomegalovirus induces placental syncytiotrophoblast apoptosis in a Toll-like receptor-2 and tumour necrosis factor-alpha dependent manner. J Pathol 2006;210(1):111–20. doi: 10.1002/path.2025. | |
Chaudhuri S, Lowen B, Chan G, et al. Human cytomegalovirus interacts with toll-like receptor 2 and CD14 on syncytiotrophoblasts to stimulate expression of TNFalpha mRNA and apoptosis. Placenta 2009;30(11):994–1001. doi: 10.1016/j.placenta.2009.09.001. | |
Lopez H, Benard M, Saint-Aubert E, et al. Novel model of placental tissue explants infected by cytomegalovirus reveals different permissiveness in early and term placentae and inhibition of indoleamine 2,3-dioxygenase activity. Placenta 2011;32(7):522–30. doi: 10.1016/j.placenta.2011.04.016. | |
Njue A, Coyne C, Margulis AV, et al. The Role of Congenital Cytomegalovirus Infection in Adverse Birth Outcomes: A Review of the Potential Mechanisms. Viruses 2020;13(1):E20. doi: 10.3390/v13010020. | |
Tanaka K, Yamada H, Minami M, et al. Screening for vaginal shedding of cytomegalovirus in healthy pregnant women using real-time PCR: correlation of CMV in the vagina and adverse outcome of pregnancy. J Med Virol 2006;78(6):757–9. doi: 10.1002/jmv.20619. | |
Slyker JA, Lohman-Payne BL, Rowland-Jones SL, et al. The detection of cytomegalovirus DNA in maternal plasma is associated with mortality in HIV-1-infected women and their infants. AIDS 2009;23(1):117–24. doi: 10.1097/QAD.0b013e32831c8abd. | |
Cytomegalovirus (CMV) and Congenital CMV Infection. [Internet]. USA: Centers for Disease Control and Prevention (CDC) [cited 2021 Jan 22]; [about 2 screens]. Available from: https://www.cdc.gov/cmv/fact-sheets/parents-pregnant-women.html. | |
Practice bulletin no. 151: Cytomegalovirus, parvovirus B19, varicella zoster, and toxoplasmosis in pregnancy. Obstet Gynecol 2015;125(6):1510–25. doi: 10.1097/01.AOG.0000466430.19823.53. Erratum in: Obstet Gynecol 2016;127(2):405. | |
Practice bulletin no. 151: Cytomegalovirus, parvovirus B19, varicella zoster, and toxoplasmosis in pregnancy. Obstet Gynecol 2015;125(6):1510–1525. doi: 10.1097/01.AOG.0000466430.19823.53. Erratum in: Obstet Gynecol 2016;127(2):405. | |
Carlson A, Norwitz ER, Stiller RJ. Cytomegalovirus infection in pregnancy: should all women be screened? Rev Obstet Gynecol 2010;3(4):172–9. | |
Lazzarotto T, Guerra B, Lanari M, et al. New advances in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 2008;41(3):192–7. doi: 10.1016/j.jcv.2007.10.015. | |
Lazzarotto T, Varani S, Guerra B, et al. Prenatal indicators of congenital cytomegalovirus infection. J Pediatr 2000;137(1):90–5. doi: 10.1067/mpd.2000.107110. | |
Benoist G, Salomon LJ, Jacquemard F, et al. The prognostic value of ultrasound abnormalities and biological parameters in blood of fetuses infected with cytomegalovirus. BJOG 2008;115(7):823–9. doi: 10.1111/j.1471-0528.2008.01714.x. | |
Society for Maternal-Fetal Medicine (SMFM), Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol 2016;214(6):B5–B11. doi: 10.1016/j.ajog.2016.02.042. | |
Pass RF, Arav-Boger R. Maternal and fetal cytomegalovirus infection: diagnosis, management, and prevention. F1000Res 2018;7:255. doi: 10.12688/f1000research.12517.1. | |
Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol 2016;215(4):462.e1–462.e10. doi: 10.1016/j.ajog.2016.04.003. | |
Shahar-Nissan K, Pardo J, Peled O, et al. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: a randomised, double-blind, placebo-controlled trial. Lancet 2020;396(10253):779–85. doi: 10.1016/S0140-6736(20)31868-7. Erratum in: Lancet 2020;396(10257):1070. | |
Amir J, Wolf DG, Levy I. Treatment of symptomatic congenital cytomegalovirus infection with intravenous ganciclovir followed by long-term oral valganciclovir. Eur J Pediatr 2010;169(9):1061–7. doi: 10.1007/s00431-010-1176-9. | |
Adler SP, Nigro G. Findings and conclusions from CMV hyperimmune globulin treatment trials. J Clin Virol 2009;46(4):S54–7. doi: 10.1016/j.jcv.2009.08.017. | |
Visentin S, Manara R, Milanese L, et al. Early primary cytomegalovirus infection in pregnancy: maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clin Infect Dis 2012;55(4):497–503. doi: 10.1093/cid/cis423. | |
Blázquez-Gamero D, Galindo Izquierdo A, Del Rosal T, et al. Prevention and treatment of fetal cytomegalovirus infection with cytomegalovirus hyperimmune globulin: a multicenter study in Madrid. J Matern Fetal Neonatal Med 2019;32(4):617–25. doi: 10.1080/14767058.2017.1387890. | |
Centers for Disease Control and Prevention (CDC). Knowledge and practices of obstetricians and gynecologists regarding cytomegalovirus infection during pregnancy–United States, 2007. MMWR Morb Mortal Wkly Rep 2008;57(3):65–8. | |
ACOG practice bulletin. Perinatal viral and parasitic infections. Number 20, September 2000. (Replaces educational bulletin number 177, February 1993). American College of Obstetrics and Gynecologists. Int J Gynaecol Obstet 2002;76(1):95–107. PMID: 11905409. | |
American Academy of Pediatrics. Breastfeeding and the Use of Human Milk. Pediatrics 2012;129(3):e827–41. doi: https://doi.org/10.1542/peds.2011-3552. | |
Maschmann J, Hamprecht K, Weissbrich B, et al. Freeze-thawing of breast milk does not prevent cytomegalovirus transmission to a preterm infant. Arch Dis Child Fetal Neonatal Ed 2006;91(4):F288–90. doi: 10.1136/adc.2004.050625. | |
Looker KJ, Magaret AS, May MT, et al. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One 2015;10(10):e0140765. doi: 10.1371/journal.pone.0140765. | |
Looker KJ, Magaret AS, Turner KM, et al. Correction: Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015;10(5):e0128615. doi: 10.1371/journal.pone.0128615. Erratum for: PLoS One 2015;10(1):e114989. | |
Jansen MA, van den Heuvel D, Bouthoorn SH, et al. Determinants of Ethnic Differences in Cytomegalovirus, Epstein-Barr Virus, and Herpes Simplex Virus Type 1 Seroprevalence in Childhood. J Pediatr 2016;170:126–34.e1–6. doi: 10.1016/j.jpeds.2015.11.014. | |
Bernstein DI, Bellamy AR, Hook EW 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis 2013;56(3):344–51. doi: 10.1093/cid/cis891. | |
Patton ME, Bernstein K, Liu G, et al. Seroprevalence of Herpes Simplex Virus Types 1 and 2 Among Pregnant Women and Sexually Active, Nonpregnant Women in the United States. Clin Infect Dis 2018;67(10):1535–42. doi: 10.1093/cid/ciy318. | |
Wald A, Krantz E, Selke S, et al. Knowledge of partners' genital herpes protects against herpes simplex virus type 2 acquisition. J Infect Dis 2006;194(1):42–52. doi: 10.1086/504717. | |
Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med 1997;337(8):509–15. doi: 10.1056/NEJM199708213370801. | |
Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med 2009;361(14):1376–85. doi: 10.1056/NEJMra0807633. Erratum in: N Engl J Med 2009;361(27):2681. | |
Fa F, Laup L, Mandelbrot L, et al. Fetal and neonatal abnormalities due to congenital herpes simplex virus infection: a literature review. Prenat Diagn 2020;40(4):408–14. doi: 10.1002/pd.5587. | |
Gibson CS, Goldwater PN, MacLennan AH, et al. South Australian Cerebral Palsy Research Group. Fetal exposure to herpesviruses may be associated with pregnancy-induced hypertensive disorders and preterm birth in a Caucasian population. BJOG 2008;115(4):492–500. doi: 10.1111/j.1471-0528.2007.01653.x. | |
Kalu EI, Ojide CK, Chuku A, et al. Obstetric outcomes of human herpes virus-2 infection among pregnant women in Benin, Nigeria. Niger J Clin Pract 2015;18(4):453–61. doi: 10.4103/1119-3077.154210 | |
Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 2003;289(2):203–9. doi: 10.1001/jama.289.2.203. | |
Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64(RR-03):1–137. Erratum in: MMWR Recomm Rep 2015;64(33):924. | |
Johnston C, Magaret A, Selke S, et al. Herpes simplex virus viremia during primary genital infection. J Infect Dis 2008;198(1):31–4. doi: 10.1086/588676. | |
Caviness AC, Demmler GJ, Selwyn BJ. Clinical and laboratory features of neonatal herpes simplex virus infection: a case-control study. Pediatr Infect Dis J 2008;27(5):425–30. doi: 10.1097/INF.0b013e3181646d95. | |
Knezevic A, Martic J, Stanojevic M, et al. Disseminated neonatal herpes caused by herpes simplex virus types 1 and 2. Emerg Infect Dis 2007;13(2):302–4. doi: 10.3201/eid1302.060907. | |
Tronstein E, Johnston C, Huang ML, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 2011;305(14):1441–9. doi: 10.1001/jama.2011.420. | |
Corey L, Wald A, Patel R, et al. Valacyclovir HSV Transmission Study Group. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004;350(1):11–20. doi: 10.1056/NEJMoa035144. | |
Watts DH, Brown ZA, Money D, et al. A double-blind, randomized, placebo-controlled trial of acyclovir in late pregnancy for the reduction of herpes simplex virus shedding and cesarean delivery. Am J Obstet Gynecol 2003;188(3):836–43. doi: 10.1067/mob.2003. | |
Mostad SB, Kreiss JK, Ryncarz AJ, et al. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. J Infect Dis 2000;181(1):58–63. doi: 10.1086/315188. | |
Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med 1991;324(18):1247–52. doi: 10.1056/NEJM199105023241804. | |
Prober CG, Sullender WM, Yasukawa LL, et al. Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. N Engl J Med 1987;316(5):240–4. doi: 10.1056/NEJM198701293160503. | |
Kriebs JM. Understanding herpes simplex virus: transmission, diagnosis, and considerations in pregnancy management. J Midwifery Womens Health 2008;53(3):202–8. doi: 10.1016/j.jmwh.2008.01.010. | |
Christie SN, McCaughey C, Marley JJ, et al. Recrudescent herpes simplex infection mimicking primary herpetic gingivostomatitis. J Oral Pathol Med 1998;27(1):8–10. doi: 10.1111/j.1600-0714.1998.tb02083.x. | |
Norval M. Herpes simplex virus, sunlight and immunosuppression. Rev Med Microbiol 1992;3:227–34. | |
Corey L. The current trend in genital herpes. Progress in prevention. Sex Transm Dis 1994;21(2):S38–44. | |
Wald A, Zeh J, Selke S, et al. Virologic characteristics of subclinical and symptomatic genital herpes infections. Int J Gynaecol Obstet 2020;333:770–5. | |
Sheffield JS, Hill JB, Hollier LM, et al. Valacyclovir prophylaxis to prevent recurrent herpes at delivery: a randomized clinical trial. Obstet Gynecol 2006;108(1):141–7. doi: 10.1097/01.AOG.0000219749.96274.15. Erratum in: Obstet Gynecol 2006;108(3 Pt 1):695. | |
Patel R, Kennedy OJ, Clarke E, et al. 2017 European guidelines for the management of genital herpes. Int J STD AIDS 2017;28(14):1366–79. doi: 10.1177/0956462417727194. | |
Corey L, Wald A, Celum CL, et al. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr 2004;35(5):435–45. doi: 10.1097/00126334-200404150-00001. | |
Magdaleno-Tapial J, Hernández-Bel P, Valenzuela-Oñate C, et al. Genital Infection With Herpes Simplex Virus Type 1 and Type 2 in Valencia, Spain: A Retrospective Observational Study. Actas Dermosifiliogr 2020;111(1):53–8. doi: 10.1016/j.ad.2019.06.002. | |
Kimberlin DW, Lin CY, Jacobs RF, et al. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics 2001;108(2):223–9. doi: 10.1542/peds.108.2.223. | |
Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev 2004;17(1):1–13. doi: 10.1128/cmr.17.1.1-13.2004. | |
Mullan HM, Munday PE. The acceptability of the introduction of a type specific herpes antibody screening test into a genitourinary medicine clinic in the United Kingdom. Sex Transm Infect 2003;79(2):129–33. doi: 10.1136/sti.79.2.129. | |
Cowan FM, Johnson AM, Ashley R, et al. Antibody to herpes simplex virus type 2 as serological marker of sexual lifestyle in populations. BMJ 1994;309(6965):1325–9. doi: 10.1136/bmj.309.6965.1325. | |
Hensleigh PA, Andrews WW, Brown Z, et al. Genital herpes during pregnancy: inability to distinguish primary and recurrent infections clinically. Obstet Gynecol 1997;89(6):891–5. doi: 10.1016/s0029-7844(97)00121-x. | |
Chatroux IC, Hersh AR, Caughey AB. Herpes Simplex Virus Serotyping in Pregnant Women With a History of Genital Herpes and an Outbreak in the Third Trimester of Pregnancy: A Cost-Effectiveness Analysis. Obstet Gynecol 2021;137(1):63–71. doi: 10.1097/AOG.0000000000004181. | |
Westhoff GL, Little SE, Caughey AB. Herpes simplex virus and pregnancy: a review of the management of antenatal and peripartum herpes infections. Obstet Gynecol Surv 2011;66(10):629–38. doi: 10.1097/OGX.0b013e31823983ec. | |
Management of Genital Herpes in Pregnancy: ACOG Practice Bulletinacog Practice Bulletin, Number 220. Obstet Gynecol 2020;135(5):e193–202. doi: 10.1097/AOG.0000000000003840. | |
Lebrun-Vignes B, Bouzamondo A, Dupuy A, et al. A meta-analysis to assess the efficacy of oral antiviral treatment to prevent genital herpes outbreaks. J Am Acad Dermatol 2007;57(2):238–46. doi: 10.1016/j.jaad.2007.02.008. Epub 2007 Apr 9. PMID: 17416440. | |
Foley E, Clarke E, Beckett VA, et al. Management of Genital Herpes in Pregnancy. Royal College of Obstetricians and Gynecologists; 2014 [cited 2021 Jan 2] 26 p. Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/management-genital-herpes.pdf. | |
Farr Zuend C, Nomellini JF, Smit J, et al. Generation of a Dual-Target, Safe, Inexpensive Microbicide that Protects Against HIV-1 and HSV-2 Disease. Sci Rep 2018;8(1):2786. doi: 10.1038/s41598-018-21134-1. | |
Lemon SM, Ott JJ, Van Damme P, et al. Type A viral hepatitis: A summary and update on the molecular virology, epidemiology, pathogenesis and prevention. J Hepatol 2017. | |
Leikin E, Lysikiewicz A, Garry D, et al. Intrauterine transmission of hepatitis A virus. Obstet Gynecol 1996;88(4 Pt 2):690–1. | |
Radivojevic K, Rosenkranz M, Weninger M. [Intrauterine transmission of hepatitis A: a case report]. Gynakol Rundsch 1989;29(3):186–8. | |
Chaudhry SA, Koren G. Hepatitis A infection during pregnancy.Can Fam Physician.2015;61(11):963–4 | |
Elinav E, Ben-Dov IZ, Shapira Y, et al. Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology 2006;130(4):1129–34. | |
Advisory Committee on Immunization Practices (ACIP). Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-7):1–23. | |
Joshi SS, Coffin CS. Hepatitis B and Pregnancy: Virologic and Immunologic Characteristics. Hepatol Commun 2020;4(2):157–71. | |
Chang MS, Gavini S, Andrade PC, et al. Caesarean section to prevent transmission of hepatitis B: a meta-analysis. Can J Gastroenterol Hepatol 2014;28(8):439–44. | |
Pan CQ, Han GR, Jiang HX, et al. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clin Gastroenterol Hepatol 2012;10(5):520–6. | |
Ehrhardt S, Xie C, Guo N, et al. Breastfeeding while taking lamivudine or tenofovir disoproxil fumarate: a review of the evidence. Clin Infect Dis 2015;60(2):275–8. | |
Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: World Health Organization, 2015 (http://apps.who.int/iris/bitstream/10665/154590/1/9789241549059_eng.pdf?ua=1&ua=1, accessed 20 March 2020). | |
Prevention of mother-to-child transmission of hepatitis B virus: guidelines on antiviral prophylaxis in pregnancy. World Health organization, 2020. | |
Taylor JM. Structure and replication of hepatitis delta virus RNA. Curr Top Microbiol Immunol 2006;307:1–23. | |
Chen HY, Shen DT, Ji DZ, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut 2019;68(3):512–21. | |
Simmonds P. Viral heterogeneity of the hepatitis C virus. J Hepatol 1999;31(1):54–60./p> | |
Mok J, Pembrey L, Tovo PA, et al., European Paediatric Hepatitis C Virus Network. When does mother to child transmission of hepatitis C virus occur? Arch Dis Child Fetal Neonatal Ed 2005;90(2):F156–60. | |
Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5(9):558–67. | |
Salemi JL, Whiteman VE, August EM, et al. Maternal hepatitis B and hepatitis C infection and neonatal neurological outcomes. J Viral Hepat 2014;21(11):e144–53. | |
Society for Maternal-Fetal Medicine (SMFM). Electronic address: pubs@smfm.org. Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol 2017;217(5):B2–12. | |
Yeung C-Y, Lee H-C, Chan W-T, et al. Vertical transmission of hepatitis C virus: current knowledge and perspectives. World J. Hepatol 2014;6:643–51. | |
Chehreh MEG, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV− mothers: A meta-analysis. Arch Gynecol Obstet 2011;283:255–60. | |
Dibba P, Cholankeril R, Li AA, et al. Hepatitis C in pregnancy. Diseases 2018;6(2) pii: E31. | |
Di Renzo GC, Tosto V, Giardina I, et al. Perinatal Infections. Good and Malpractice in Labour and Delivery 2019;24:129. | |
Chaudhry SA, Verma N, Koren G. Hepatitis E infection during pregnancy. Can Fam Physician 2015;61(7):607–8. | |
Pérez-Gracia MT, Suay-García B, Mateos-Lindemann ML. Hepatitis E and pregnancy: current state. Rev Med Virol 2017;27(3):e1929. | |
Charre C, Ramière C, Dumortier J, et al. Chronic Genotype 3 Hepatitis E in Pregnant Woman Receiving Infliximab and Azathioprine. Emerg Infect Dis 2018;24(5):941–3. | |
Anty R, Ollier L, Peron JM, et al. First case report of an acute genotype 3 hepatitis E infected pregnant woman living in South-Eastern France. J Clin Virol 2012;54:76–8. doi: 10.1016/j.jcv.2012.01.016. | |
Tabatabai J, Wenzel JJ, Soboletzki M, et al. First case report of an acute hepatitis E subgenotype 3c infection during pregnancy in Germany. J Clin Virol 2014;61:170–2. doi: 10.1016/j.jcv.2014.06.008. | |
Bouthry E, Benachi A, Vivanti AJ, et al. Autochthonous Hepatitis E during Pregnancy, France. Emerg Infect Dis 2018;24:1586–7. doi: 10.3201/eid2408.180105. | |
Gouilly J, Chen Q, Siewiera J, et al. Genotype specific pathogenicity of hepatitis E virus at the human maternal-fetal interface. Nat Commun 2018;9:4748. doi: 10.1038/s41467-018-07200-2. | |
Kmush BL, Labrique A, Li W, et al. The Association of Cytokines and Micronutrients with Hepatitis E Virus Infection During Pregnancy and the Postpartum Period in Rural Bangladesh. Am J Trop Med Hyg 2016;94:203–11. doi: 10.4269/ajtmh.15-0238. | |
Kumar N, Das V, Agarwal A, et al. Fetomaternal outcomes in pregnant women with hepatitis E infection; still an important fetomaternal killer with an unresolved mystery of increased virulence in pregnancy. Turk J Obstet Gynecol 2017;14(2):106–13. doi: 10.4274/tjod.15045. | |
Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. J Viral Hepat 2015;22:965–73. | |
Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E Lancet 2012:379:2477–88 | |
CDC. Ebola Outbreak in West Africa – Case Counts. 2014;2016. https://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Accessed August 25, 2017. | |
Haddad LB, Horton J, Ribner BS, et al. Ebola Infection in Pregnancy: A Global Perspective and Lessons Learned. Clin Obstet Gynecol 2018;61(1):186–96. doi: 10.1097/GRF.0000000000000332 | |
World Healh Organization (WHO) Ebola virus disease Fact Sheet. 2017. http://www.who.int/mediacentre/factsheets/fs103/en/. Accessed August 25, 2017. | |
Jamieson DJ, Uyeki TM, Callaghan WM, et al. What obstetrician-gynecologists should know about Ebola: a perspective from the Centers for Disease Control and Prevention. Obstet Gynecol 2014;124(5):1005–10. | |
WHO. Ebola Response Team. Aylward B, Barboza P, Bawo L, et al. Ebola virus disease in West Africa–the first 9 months of the epidemic and forward projections. N Engl J Med 2014;371(16):1481–95. | |
Kamali A, Jamieson DJ, Kpaduwa J, et al. Pregnancy, Labor, and Delivery after Ebola Virus Disease and Implications for Infection Control in Obstetric Services, United States. Emerg Infect Dis 2016;22(7). | |
Chertow DS, Kleine C, Edwards JK, et al. Ebola virus disease in West Africa–clinical manifestations and management. N Engl J Med 2014;371(22):2054–7. | |
Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis 1999;179(1):S28–35. | |
Ksiazek TG, Rollin PE, Williams AJ, et al. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 1999;179(1):S177–87. | |
Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun 2014;55:1–9. | |
Martinez VP, Bellomo CM, Iglesias AA. Laboratory preparation for the diagnosis of Ebola virus disease in Argentina. Medicina (B Aires) 2014;74(6):506–7. | |
Lamontagne F, Fowler RA, Adhikari NK, et al. Public health evidence-based guidelines for supportive care of patients with Ebola virus disease. Lancet 2018;391(10121):700–8. | |
WHO. Guidelines for the management of pregnant and breastfeeding women in the context of Ebola virus disease. February 2020. | |
Sharma V, Sharma M, Dhull D, et al. Zika virus: an emerging challenge to public health worldwide. Can J Microbiol 2020;66(2):87–98. | |
Petersen LR, Jamieson DJ, Powers AH, et al. N Engl J Med 2016;374:1552–63. doi: 10.1056/NEJMra1602113. | |
CDC Zika Outcomes Investigation (ZODIAC). CDC. [(accessed on 13 February 2020)]; Available online: https://www.cdc.gov/pregnancy/zika/research/zodiac.html. | |
Zika Virus. [(accessed on 5 February 2020)]; Available online: https://www.cdc.gov/zika/fs-posters/index.html. | |
Antoniou E, Orovou E, Sarella A, et al. Zika Virus and the Risk of Developing Microcephaly in Infants: A Systematic Review. Int J Environ Res Public Health 2020;17(11):3086. | |
Ellington SR, Devine O, Bertolli J, et al. Estimating the Number of Pregnant Women Infected With Zika Virus and Expected Infants With Microcephaly Following the Zika Virus Outbreak in Puerto Rico, 2016. JAMA Pediatr 2016;170:940–5. doi: 10.1001/jamapediatrics.2016.2974. | |
Ospina ML, Tong VT, Gonzalez M, et al. Zika Virus Disease and Pregnancy Outcomes in Colombia. N Engl J Med 2020;383(6):537–45. doi: 10.1056/NEJMoa1911023. | |
Marques VM, Santos CS, Santiago IG, et al. Neurological complications of congenital zika virus infection. Pediatr Neurol 2019;91:3–10. doi: 10.1016/j.pediatrneurol.2018.11.003. | |
Oliveira Melo AS, Malinger G, Ximenes R, et al. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016;47:6–7. doi: 10.1002/uog.15831 | |
Nelson BR, Roby JA, Dobyns WB, et al. Immune Evasion Strategies Used by Zika Virus to Infect the Fetal Eye and Brain. Viral Immunol 2020;33(1):22–37. doi: 10.1089/vim.2019.0082. | |
Teixeira FME, Pietrobon AJ, de Mendonca Oliveira L, et al. Maternal-Fetal Interplay in Zika Virus Infection and Adverse Perinatal Outcomes. Front Immunol 2020;11:175. | |
August EM, Rosental J, Torrez R, et al. Community Understanding of Contraception During the Zika Virus Outbreak in Puerto Rico. Health Promot Pract 2020;21(1):133–41. doi: 10.1177/1524839919850764. | |
Larocca RA, Abbink P, Peron JP, et al. Vaccine protection against Zika virus from Brazil. Nature 2016;536:474–8. doi: 10.1038/nature18952. | |
Abbink P, Larocca RA, De La Barrera RA, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016;353:1129–32. doi: 10.1126/science.aah6157. | |
Pardi N, Hogan MJ, Pelc RS, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017;543:248–51. doi:10.1038/nature21428. | |
Poland GA, Kennedy RB, Ovsyannikova IG, et al. Development of vaccines against Zika virus. Lancet Infect Dis 2018;18:211–9. doi: 10.1016/S1473-3099(18)30063-X. | |
Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. | |
Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. | |
Liu H, Liu F, Li J, et al. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect 2020. | |
Shi H, Han X, Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425–34. | |
Hosseiny M, Kooraki S, Gholamrezanezhad A, et al. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. AJR Am J Roentgenol 2020. | |
Liu D, Li L, Wu X. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol 2020. | |
Salehi S, Abedi A, Balakrishnan S, et al. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 2020. | |
Ong SWX, Tan YK, Chia PY. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA 2020. | |
Ghayda RA, Li H, Lee KH, et al. COVID-19 and Adverse Pregnancy Outcome: A Systematic Review of 104 Cases. J Clin Med 2020;9(11):E3441. | |
Di Renzo GC, Giardina I. Coronavirus disease 2019 in pregnancy: consider thromboembolic disorders and thromboprophylaxis. Am J Obstet Gynecol 2020;223(1):135. doi: 10.1016/j.ajog.2020.04.017 | |
Mendoza M, Garcia-Ruiz I, Maiz N, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG 2020;127(11):1374–80. doi: 10.1111/1471-0528.16339. | |
Ng K, Poon BH, Kiat Puar TH. COVID-19 and the risk to health care workers: a case report. Ann Intern Med 2020. | |
Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol 2020;55:435–7. | |
Capanna F, Haydar A, McCarey C, et al. Preparing an obstetric unit in the heart of the epidemic strike of COVID-19: quick reorganization tips. J Matern Fetal Neonatal Med 2020;12:1–7. doi: 10.1080/14767058.2020.1749258 | |
Poon LC, Yang H, Kapur A, et al. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: Information for healthcare professionals. Int J Gynaecol Obstet 2020;149(3):273–86. doi: 270.1002/ijgo.13156 | |
Mei Ng YP, Fen Low Y, Lei Goh X, et al. Breastfeeding in COVID-19: A Pragmatic Approach. Am J Perinatol 2020;37(13):1377–84. doi: 10.1055/s-0040-1716506. | |
Armstrong C, Lillie RD. Experimental lymphocytic choriomeningitis of monkeys and mice produced by a virus encountered in studies of the 1933 St. Louis encephalitis epidemic. Public Health Rep 1934;49:1019–27. doi: 10.2307/4581290. | |
Fischer SA, Graham MB, Kuehnert MJ, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. The New England Journal of Medicine 2006;354(21):2235–49. doi: 10.1056/NEJMoa053240. | |
Enninga EAL, Theiler NR. Lymphocytic Choriomeningitis Virus Infection Demonstrates Higher Replicative Capacity and Decreased Antiviral Response in the First-Trimester Placenta. J Immunol Res 2019;2019:7375217. | |
Barton LL, Mets MB. Congenital lymphocytic choriomeningitis virus infection: decade of rediscovery. Clin Infect Dis 2001;33:370–4. doi: 10.1086/321897 | |
Jamieson DJ, Kourtis AP, Bell M, et al. Lymphocytic choriomeningitis virus: an emerging obstetric pathogen? American Journal of Obstetrics & Gynecology 2006;194(6):1532–6. doi: 10.1016/j.ajog.2005.11.040. | |
Wright R, Johnson D, Neumann M, et al. Congenital lymphocytic choriomeningitis virus syndrome: a disease that mimics congenital toxoplasmosis or cytomegalovirus infection. Pediatrics 1997;100(1):E9. doi: 10.1542/peds.100.1.e9. | |
Ksiazek TG, Childs JE, Korch GW, et al. Lymphocytic choriomeningitis virus infection and house mouse (Mus musculus) distribution in urban Baltimore. The American Journal of Tropical Medicine and Hygiene 1992;47(1):27–34. doi: 10.4269/ajtmh.1992.47.27. | |
Stephensen CB, Blount SR, Lanford RE, et al. Prevalence of serum antibodies against lymphocytic choriomeningitis virus in selected populations from two U.S. cities. Journal of Medical Virology 1992;38(1):27–31. doi: 10.1002/jmv.1890380107. | |
Pacheco O, Beltran M, Nelson CA, et al. Zika virus disease in Colombia – preliminary report. The New England Journal of Medicine 2016. doi: 10.1056/NEJMoa1604037. | |
Reynolds MR, Jones AM, Petersen EE, et al. Vital signs: update on Zika virus–associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure – U.S. Zika Pregnancy Registry, 2016. Morbidity and Mortality Weekly Report 2017;66(13):366–73. doi: 10.15585/mmwr.mm6613e1. | |
Bonthius DJ, Nichols B, Harb H, et al. Lymphocytic choriomeningitis virus infection of the developing brain: critical role of host age. Annals of Neurology 2007;62(4):356–74. doi: 10.1002/ana.21193. | |
Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerging Infectious Diseases 2006;12(11):1638–43. doi: 10.3201/eid1211.060152. | |
Tanimura K, Kojima N, Yamazaki T, et al. Second trimester fetal death caused by varicella-zoster virus infection. Journal of Medical Virology 2013;85(5):935–8. doi: 10.1002/jmv.23531. | |
Enninga EAL, Nevala WK, Creedon DJ, et al. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. American Journal of Reproductive Immunology 2015;73(3):251–62. doi: 10.1111/aji.12303. | |
Bonthius DJ. Lymphocytic Choriomeningitis Virus: An Underrecognized Cause of Neurologic Disease in the Fetus, Child, and Adult. Semin Pediatr Neurol 2012;19:89–95. | |
Barton LL, Mets MB. Congenital lymphocytic choriomeningitis virus infection: Decade of rediscovery. Clin Infect Dis 2001;33:370–4. | |
Bonthius DJ, Wright R, Tseng B, et al. Congenital lymphocytic choriomeningitis virus infection: Spectrum of disease. Ann Neurol 2007;62:347–55. | |
Bonthius DJ, Nichols B, Harb H, et al. Lymphocytic choriomeningitis virus infection in brain: Critical role of host age. Ann Neurol 2007;62:356–74. | |
Wright R, Johnson D, Neumann M, et al. Congenital lymphocytic choriomeningitis virus syndrome: A disease that mimics congenital toxoplasmosis or cytomegalovirus infection. Pediatrics 1997;100:E9. | |
Sheinbergas MM. Hydrocephalus due to prenatal infection with the lymphocytic choriomeningitis virus. Infection 1976;4:185–91. | |
Bonthius DJ, Mahoney J, Buchmeier MJ, et al. Critical role for glial cells in the propagation and spread of lymphocytic choriomeningitis virus in the developing rat brain. J Virol 2002;76:6618–35. | |
Bonthius DJ, Karacay B. Meningitis and encephalitis in children: an update. Neurol Clin 2002;4:1013–38. | |
Yu JT, Culican SM, Tychsen L. Aicardi-like chorioretinitis and maldevelopment of the corpus callosum in congenital lymphocytic choriomeningitis virus. J AAPOS 2005;10:58–60. | |
Meritet JF, Krivine A, Lewin F, et al. A case of congenital lymphocytic choriomeningitis virus (LCMV) infection revealed by hydrops fetalis. Prenatal Diagnosis 2009;29:626–7. | |
Anderson JL, Levy PT, Leonard KB, et al. Congenital lymphocytic choriomeningitis virus: when to consider the diagnosis. J Child Neurol 2014;29(6):837–42. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)
