This chapter should be cited as follows:
Foreste V, Reppuccia S, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.420603
The Continuous Textbook of Women’s Medicine Series – Gynecology Module
Volume 8
Gynecological endoscopy
Volume Editors:
Professor Alberto Mattei, Director Maternal and Child Department, USL Toscana Centro, Florence, Italy
Dr Federica Perelli, Obstetrics and Gynecology Unit, Ospedale Santa Maria Annunziata, USL Toscana Centro, Florence, Italy

Chapter
Outpatient Hysteroscopy: Setting, Indication and Techniques
First published: January 2024
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Hysteroscopy is the gold standard procedure for the diagnosis and treatment of women with intrauterine pathology.1 Since 1869, the year of the first hysteroscopy performed by Pantaleoni,2 this procedure has undergone continuous improvement: the introduction of safe distending media, the development of bipolar energy and of various instruments, optics, and smaller scope sizes, have made hysteroscopy one of the most commonly performed procedures in contemporary gynecology.
However, despite its ubiquity, there has been a lack of consensus when describing hysteroscopic procedures, such that multiple terms being used across the international community without any clear definition as to what they mean. Indeed, hysteroscopic procedures are conducted in a variety of healthcare facilities with or without the use of anesthesia/analgesia or in a hospital operating room (theater) with an anesthetist in charge of pain management. The decision as to where and how to undertake hysteroscopic procedures depends upon a number of factors including the available infrastructure (staffing, equipment, facilities), preferences (both patient and clinician), the type of hysteroscopic procedure (i.e. feasibility, acceptability and effectiveness of diagnostic and operative procedures) and health economics (e.g. reimbursement, investment and cost-effectiveness). Thus, according to the recent consensus on standard nomenclature for hysteroscopic procedure,3 the hysteroscopy is considered as “outpatient” when the patient arrives and leaves the facility (outpatient clinic/department of a hospital, community clinic or a freestanding surgical center (public or private)) on the same calendar day (in the United States the term office and outpatient are used interchangeably as regards the model of care).
Hysteroscopy involves introducing a rigid or flexible hysteroscope through the cervical canal into the uterus and then using distending media to allow for complete visualization of the endometrial cavity. For simplicity, it is possible to divide the procedure in multiple stages:4
- Vaginoscopy;
- Examination of the uterine cervix;
- Examination of the uterine cavity.
Vaginoscopy is the time of inspection of the vaginal canal through a vaginoscopic approach. The vaginoscopic approach allows investigation of the morphology and size of the vaginal canal, along with identification of any septa. It is of crucial importance in cases of suspected congenital anomalies of the genital tract. Wherever there is no chance to obtain adequate distension of the vaginal cavity, is has proven useful to close the vaginal labia with the fingers to generate an increased pressure and an increased distension of the uterine walls, so as to facilitate identification of any longitudinal or transverse septa. The second stage of hysteroscopic examination includes a detailed assessment of the position, morphology and size of the uterine cervix. Proceeding in a stepwise fashion, the four vaginal fornices are identified by use of panoramic view of the cervix. Beyond the internal uterine ostium, in the uterine cavity, hysteroscopic evaluation is focused on morphology, size, and shape of the fundus, as well as on the number and characteristics of the tubal ostia.
SETTING
Equipment
An equipped ambulatory examination room, with sanitary facilities and a dressing room is necessary to carry out a diagnostic hysteroscopy. The nurse has to guarantee that all the necessary equipment and tools are readily available.
The patient must be well informed about the investigation to be submitted, and welcomed in a familiar and relaxing environment for a comfortable gynecological exam.
It has been observed that women who are more anxious before the procedure usually experience greater discomfort during hysteroscopy. Therefore, if the surgeon is able to reduce the patient’s anxiety, a more positive result and maximum satisfaction on the part of the woman can be expected. Indeed, it is recommended an emotional support to the patient (‘vocal anesthesia’) in order to reassure and involve her, inviting her to take a look at the additional monitor (if any), giving explanations of what is seen or any abnormality found to prevent her from feeling excluded and/or neglected.5,6
If the patient needs more advanced procedures, the hysteroscopic surgery may require the support of anesthesia, i.e., mild sedation, and of the contemporary use of sonographic techniques.7 This necessity, led surgeons to create a new concept of hysteroscopic setting, defined as digital hysteroscopic clinic (DHC), an innovative form of precision medicine which combines office and ambulatory surgical care with improved imaging technology for an efficient and accurate uterine diagnostic and therapeutic approach. Moreover, with this clinic, the patient who requires the post-surgery observation, does not require high-tech recovery infra-structure which enables the patient to leave the observation room after surgery.
Instrumentation
Instrumentation for diagnostic and operative office hysteroscopy4
- BETTOCCHI® Operating Hysteroscope 2.1 mm and 2.9 mm (KARL STORZ, Germany) : both hysteroscopes are available with two sheaths, one for irrigation and the other one for the suction, a 5-Fr operating channel and an oval cross-sectional profile. The operative channel of hysteroscopes permits the passage of mechanical instruments such as small hysteroscopic graspers, scissors, or a tenaculum, and of bipolar electrodes.
- Trophyscope® (KARL STORZ, Germany), with an outer diameter of 2.9 mm it allows an easier passage through the cervix. It can be employed as a single flow diagnostic hysteroscope or as a continuous flow operating hysteroscope by an adjunctive use of an outer sheath which increases the overall diameter to 4.4 mm.
- 15-Fr bipolar office resectoscope (KARL STORZ, Germany) is an effective alternative option which enables the gynecologists a standard resectoscopic operative procedure in an ambulatory setting.
- 15-Fr tissue removal devices (TRD): these systems allow the mechanical removal of endouterine lesions (polyps, small myomas and retained product of conception) reducing the time of the surgery with the system of simultaneous cutting and aspiration of tissue in office setting.
Instrumentation for and operative hysteroscopy in an operating room setting4
The instrumentation described for office hysteroscopy may also be used in the operating room, particularly in cases where owing to the type and/or dimensions of the lesion, it can be performed without cervical dilatation and the use of resectoscope. Bipolar resectoscope and TRD of large diameter that required cervical dilatation can be used.
INDICATIONS
Hysteroscopy is a useful diagnostic tool for the investigation of abnormal uterine bleeding and infertility.7
The need for hysteroscopy is often determined by the result of a previous ultrasound scan or in primary care when managing heavy menstrual bleeding or postmenopausal bleeding.8
Abnormal uterine bleeding
According to the International Federation of Obstetricians and Gynaecologists (FIGO), the causes of abnormal uterine bleeding (AUB) can be summarized using the acronym PALM-COEIN (polyps, adenomyosis, leiomyoma, malignancy or hyperplasia, coagulopathy, ovulatory dysfunction, endometrial disorders, iatrogenic, not yet classified).9
Structural causes of AUB affecting the uterine cavity, such as polyps, adenomyoma, leiomyoma, malignancy or hyperplasia can be detectable and treatable by hysteroscopy.
It is important to underline that, bleeding in women taking tamoxifen therapy, usually as adjuvant therapy for breast cancer, always needs investigating urgently, as the risk of endometrial hyperplasia and cancer is elevated in this population.10
Infertility
A significant proportion (40–50%) of women with infertility will have intrauterine pathology that could contribute to their condition.11
Hysteroscopy is indicated when the scan suggests an abnormality, to confirm the diagnosis and to offer treatment. However, hysteroscopy also has a role in patients with history of implantation failure or recurrent pregnancy loss to identify any missed pathologies. Indeed, uterine factors such as endometrial polyp, submucosal myoma, intrauterine adhesion, chronic endometritis or congenital uterine anomalies can be detected in these patients.12
In patients with repeated in vitro fertilization treatment failure, many efforts have been tried to improve the endometrial receptivity, and hysteroscopic endometrial scratch – intentional injury to the endometrium – is one of the procedures proposed. A Cochrane review found that endometrial scratch performed between day 7 of the previous cycle and day 7 of the embryo transfer cycle is associated with an improved live birth and clinical pregnancy rates versus those without scratch in women with >2 previous embryo transfers.13
TECHNIQUES
Basic operative hysteroscopy
Removal of IUDs
There are a variety of form, size and medicated/non medicated intrauterine devices (IUDs) and more than 150 million women worldwide use IUDs for contraception. To be effective, the IUD must be correctly positioned at the uterine fundus. The removal of the IUD is usually a straightforward procedure by gentle traction on the strings emerging from the external cervical ostium during gynecological examination. The procedure becomes problematic when the strings are not visible or when they break. In this case, blind removal of an IUD can be very difficult. Outpatient hysteroscopy provides a safe and effective method for removing IUDs under direct visual control in the ambulatory setting (Figure 1).14
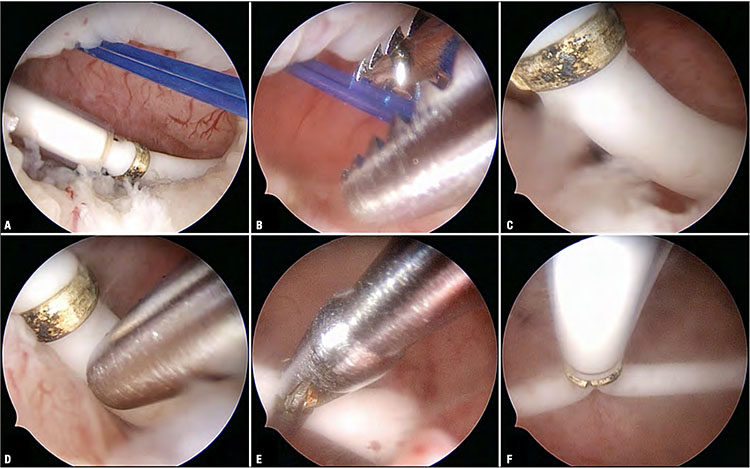
1
Hysteroscopic repositioning of a 52 mg levonorgestrel-medicated IUD (active for 6 years, Benilexa, Gedeon Richter Plc) transversely dislocated at the level of an isthmocoele (A). The technique mainly consists of repositioning the IUD until in-line with the longitudinal axis of the uterine body. This is accomplished by applying gentle traction on the string, causing the IUD to migrate toward the center of the cervical canal (B). Once the IUD has been realigned longitudinally passing across the cesarean scar niche (C), an alligator forceps is used to mobilize the arms of the device until it rests in the intended intrauterine position (D–F).
The procedure is very simple: once the device is identified, forceps with jaws wide open are used to grasp either the string or one of the IUD’s distal arms. With closed jaws, the entire hysteroscope is retracted from the uterine cavity, without withdrawing the tip of the forceps into the operating channel. Hysteroscopic examination should always exclude the incarceration of the IUD into the myometrial wall.
Occasionally, an intrauterine device is malpositioned in the isthmic region, most often resulting from anatomical difficulties such as cervical canal stenosis, or an isthmocele. Whatever gives rise to suspect an incorrectly positioned IUD or its dislocation, outpatient hysteroscopy has been shown to be effectively used to evaluate the state of the IUD and to identify the actual position of the device.14,15 In the case of IUD dislocation, the device can be repositioned by grasping the distal arm with a 5-Fr mechanical instrument, such as grasping forceps, or simply mobilize at the distal end or the vertical stem using the tip of the hysteroscope.
Endometrial polypectomy
Today the removal of the majority of endometrial polyps should be carried out in an outpatient setting. The experience of the surgeon, the size and the anatomical site of the polyp (in the fundal and/or cornual region) and the instruments available represent major factors limiting the feasibility of an ambulatory approach. Indeed, there are no guidelines stipulating the limits of the dimensions, location or the number of lesions beyond which polypectomy should be performed in the operating room. For this reason, the inpatient treatment of endometrial polyps should be reserved for those patients who can not tolerate an ambulatory operative procedure, or when the size and/or number of polyps would require too long.
5-Fr Instruments
Small polyps (<0.5 cm) should be removed using 5-Fr mechanical instruments such as scissor and/or grasping forceps. The endometrial polyp is approached by positioning the grasping, with opened jaws, at its implantation base. While gently closing the jaws, the grasping – with jaws in closed configuration – is finally pushed towards the uterine fundus (Figure 2).
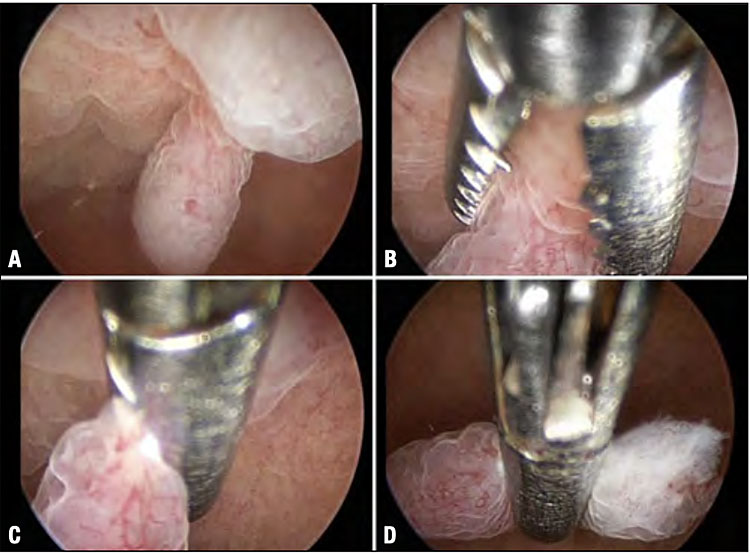
2
Endometrial polypectomy using an alligator forceps. A polypoid lesion is identified, <0.5 cm (A), and grasped at its base with the jaws of a forceps (B), which are closed (C) allowing the lesion to be gently mobilized toward the uterine fundus (D), resulting in its detachment from the base of implantation. This final step is particularly important, because the majority of surgeons tend erroneously, after closing the jaws, to withdraw the forceps towards the distal end of the hysteroscope. Such a maneuver fails to detach the polyp from its base.
Larger endometrial polyps (>0.5 cm) should be sectioned by scissors or bipolar electrode into small fragments that are subsequently extracted from the uterine cavity with grasping or tenaculum forceps. There is no universal rule as to which surgical approach is best suited to remove an endometrial polyp larger than the size of the internal uterine ostium (IUO). The fundamental rule, however, is to approach the base of the lesion only in the final stages of the procedure because this step is the most painful for the patient, on account of the proximity of subendometrial nerve endings.
Tissue removal device (TRD)
The TRD procedure is very simple, the learning curve is very fast, surgery time is significantly reduced, and it fulfills the requirements for an outpatient procedure. Once the hysteroscope is inserted into the uterine cavity using the vaginoscopic technique and a clear view is obtained, just placing the blade against the polyp, the device simultaneously aspirates and cuts the tissue, delivering it through the shaving system into a collecting pouch allowing for complete capture and histopathologic assessment of all fragments extracted from the uterine cavity.16,17
15-Fr Mini-resectoscope
The 15-Fr resectoscope represents an effective alternative to the available instrumentation, combining the advantages of traditional resectoscopy with the benefits of outpatient surgery. As always with the vaginoscopic approach, without cervical dilatation, the instrument is introduced into the uterine cavity and the slicing is done with the resectoscope by cutting from the free edge to the base. The polyp is sliced into multiple fragments, offering the possibility to insert the instrument just once throughout the internal ostium until the end of surgery also to remove big polyps, larger than the internal cervical ostium (Figure 3).18
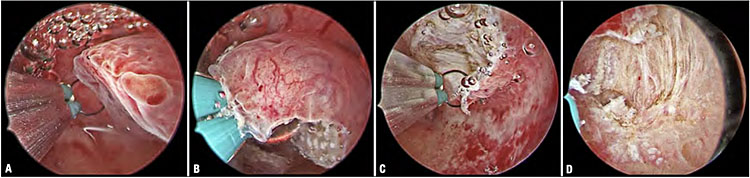
3
Technique of endometrial polypectomy using the 15-Fr bipolar resectoscope (KARL STORZ, Germany). Once the sessile polypoid lesion with a small base has been identified on the left lateral wall (A), the loop electrode is guided towards the implantation base with repetitive ‘slicing’ movements, starting from the free edge of the polyp (B–C) and progressing towards the base where the pedicle attaches to the endometrium (D).
Resectoscope
The technique of resectoscopic slicing involves a method of serial resection (slicing) of the polyp, starting with its free end and advancing towards the base of implantation.16 As a rule, polypectomy is performed with the aid of an angled cutting loop. There are, however, locations of endocavitary polyps (fundus or cornual area) that make it difficult to apply an angled loop, necessitating the use of more specific loops such as a straight cutting loop.
Cervical polypectomy
Outpatient treatment of polyps smaller than 0.5 cm usually involves the use of mechanical instruments: scissors should always be preferred over grasping forceps, considering that the fibrous nature of a polyp makes it difficult to completely remove using traction only. Lesions larger than 0.5 cm should always be treated by resecting the base of the polyp with a 5-Fr bipolar electrode. The use of mini-resectoscope and hysteroscopic morcellator has further reduced resectoscopic treatment of endocervical polypoid lesions, reserving it only for cases where ambulatory removal is not feasible (e.g., large size, impaired endoscopic vision). In this case, the technique is the same as described for endometrial polyps (Figure 4).
4
Polypectomy of a voluminous endocervical polyp with a straight 5-Fr bipolar electrode (KARL STORZ, Germany). The use of a bipolar electrode allows accurate dissection of the polypoid lesion at its sessile base (B–C), while providing for a good hemostasis (D).
Synechiolysis
Cervical synechiae
Cervical synechiae are the principal cause of the failure of office hysteroscopy. The main objective of treatment is to restore normal patency of the cervical canal.19
In case of mild synechiae, hysteroscopic lysis may be performed employing blunt division with the distal tip of a hysteroscope or, as an alternative option, by rotating the oval profile of the hysteroscope through 90° until its larger diameter is in line with the transverse axis of the cervical canal.
In cases of moderate synechiae, as previously described, a straight approach may be attempted. Alternatively, lysis may be accomplished with miniature mechanical instruments, such as grasping forceps or scissors. Grasping forceps should be introduced in the closed configuration and then, at the site where the synechia is attached, you open that.
Intrauterine synechiae (mild and moderate)
Hysteroscopic lysis of adhesions is the gold standard for treatment of intrauterine adhesions. Intrauterine synechiae are adhesive processes frequently detected in patients with a prior history of procedures involving the use of gynecological instruments both for diagnostic and therapeutic purposes.
Synechiae of a thin or filmy consistency can be lysed by gently tearing them down with the distal tip of the hysteroscope moved in a lateral direction. Lysis of moderate synechiae requires the use of 5-Fr hysteroscopic scissors or forceps to gradually transect fibrous bridges (Figure 5).
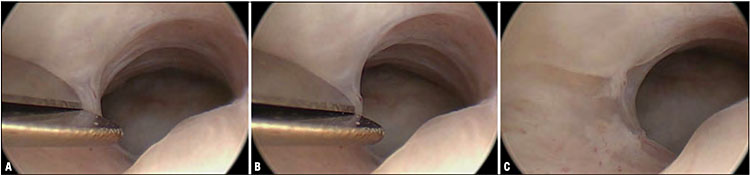
5
Synechiolysis with blunt 5-Fr scissors. Note, the synechia is located on the right lateral wall of the cervical canal (A) and resected with a clean cut through its central portion (B), allowing visualization at the end of the cut to see the separation between the anterior and the posterior wall (C).
The treatment of severe synechiae is described in paragraph Adhesive pathology
G0-G1 Myomectomy
According to the classification developed by Wamsteker et al.20 and the European Society for Gynecological Endoscopy (ESGE), which considers only the degree of myometrial penetration of the submucous myoma, a fibroid G0 is completely within the uterine cavity; a fibroid G1 has its larger part (>50%) in the uterine cavity; a fibroid G2 has its larger part (>50%) in the myometrium.21
The gold standard treatment for submucosal myomas (G0–G2) is hysteroscopic myomectomy. Currently, size and type of myoma, surgeon experience and available equipment will inform the choice of the setting and the technique performed. Among the basic procedures, outpatient myomectomy should only be considered for small, less than 2 cm, intracavitary myomas (G0–G1).
Resectoscope
The classical resectoscopic excision of the intracavitary fibroid is carried out with the technique of slicing. It consists of repeated and progressive passes of the cutting loop starting from the free margin of the lesion, carried out with the standard technique. During the resection of the fibroid the fragments sectioned and then accumulated into the cavity may interfere with a clear view. They must be removed from the uterine cavity by taking out the resectoscope after grasping the loose tissue elements with the loop electrode.
Tissue removal device
TRD is a new modality for mechanical resection of intrauterine tissues; myomectomy with TRD comes with the specific benefit of avoiding the risk of iatrogenic thermal injury which can be associated with the use of a standard resectoscope. Moreover, it overcomes several other limitations, such as the formation of gas bubbles, which can impede intracavitary vision. Because of the TRD’s unique property of simultaneous cutting and aspiration, no tissue chips are accumulated, which need to be retrieved repeatedly by inserting the instrument into the uterine cavity. In the case of small G0 myomas, the procedure can be performed on conscious patients with the advantage of reduced overall operative time, increased safety as well as patient compliance and satisfaction (Figure 6).22
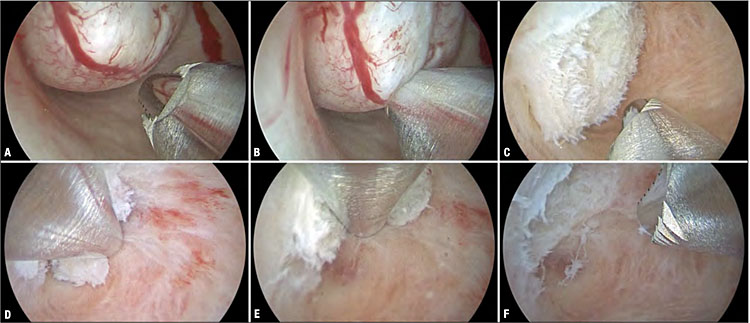
6
Technique of ambulatory myomectomy of a type 0 myoma with the 6.3 mm Intrauterine Bigatti Shaver (IBS) (KARL STORZ, Germany). Hysteroscopic view of the type 0 myoma (approx. diameter 1.5 cm) which is shown to have formed in the right cornual recess (A). Once the cutting window is brought in close contact with the myoma (B), the shaver system is activated causing the fibroid tissue to be cut away and aspirated synchronously. This is continued until the fibroid is removed completely (C–E). Final aspect of the uterine cavity upon complete removal of the myoma (F).
Advanced operative hysteroscopy
Endometrial biopsy
Endometrial biopsy on atrophic endometrium
In cases of thick endometrium or focal vegetating/exophytic lesions, the biopsy samples are easily obtained using the so-called grasping technique. Although, when the area is atrophic, the biopsy samples are obtained using 5-Fr scissors or bipolar electrodes, which are inserted through the operating channel of the hysteroscope. Making precise cuts with these instruments, parallel to the endometrial surface in order to avoid cutting deep into the myometrium, allows tissue samples to be collected and removed with grasping forceps.23 Alternatively, biopsy can be made with the cutting loop of the miniresectoscope.
Endomyometrial biopsy
The Campo Trophyscope® provides the possibility of using the outer sheet as a guide to insert an endometrial sampler or an endometrial–myometrial biopsy probe, called Spirotome. The Spirotome offers the possibility of performing a direct forwarded biopsy and obtaining a representative sample of tissue for further examination, including, when indicated, the different layers of endometrium, inner myometrium and outer myometrium, with no or minimal risk of cell spreading or bleeding (Figure 7).24
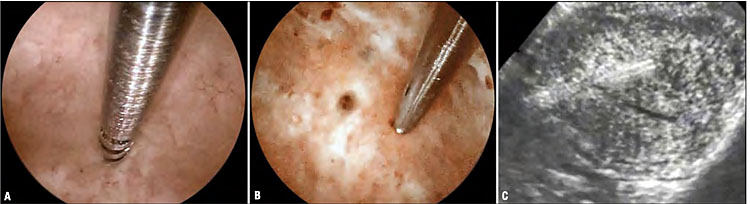
7
Biopsy sampling of subendometrial myometrium performed hysteroscopically using the Spirotome (BionciseNV,Belgium)(A–B) under sonographic guidance (C).
In this way, a correct anatomopathological examination from endometrium and junctional zone myometrium becomes possible. The hysteroscopic exploration of the subendometrial myometrium is also possible using the 5 Fr instruments, and highly suggestive images for adenomyosis such as the neovascularization, endometrial defects or chocolate dye-filled cysts with endometrial implants on the pseudocystic wall can be explored and resected for histological examination.
Adhesive pathology
Severe cervical stenosis
Severe uterine cervical synechiae are the main cause of failure in diagnostic hysteroscopy, causing uterine perforation, cervical laceration, false tracts and subsequent cervical scarring due to the trauma. The miniaturized instruments such as scissors and a bipolar electrode are the best for this purpose. Scissors are used to gradually trim the fibrous webs bridging the cervical walls. In cases of complete obliteration of the cervical canal and/or a keyhole-shaped external uterine ostium, it is highly recommended to make a ‘star-shaped’ incision in the stenotic tract and/or the external uterine ostium using a bipolar electrode to create an adequately sized entry, giving access to the uterine cavity.5,19,25
Asherman’s syndrome
The presence of a combination of pain, menstrual disturbance and subfertility due to severe adhesions in the endometrium is defined as Asherman’s syndrome.26 Severe intrauterine synechiae generally involves more than three-quarters of the uterine cavity, with thick bands and occlusion of one or both tubal ostia. The aim of surgery is to reestablish the uterine cavity with exposure of all residual micro-endometrial cavities and both intrauterine tubal ostia.
In the most of cases, the adhesiolysis can be performed in an outpatient procedure provided that both ultrasound instruments and hysteroscopes are available.27 Occasionally Asherman’s syndrome treatment requires conventional operating room. The surgical procedure begins with a transvaginal ultrasound to identify whether any endometrial area remains, which can be used as an ultrasound-guided target to unify residual endometrial micro-cavities. The classical approach, which is increasingly becoming out-dated, is to perform the lysis of adhesions with the classical resectoscope. During the adhesiolysis, constant ultrasound surveillance is important. However, it is important to note that, in cases of severe synechiae, cervical dilatation can be especially difficult due to the presence of fibrosis at the internal or external ostium.
Today, surgery for Asherman’s syndrome uses instruments of a total diameter of around 15 Fr. The mini resectoscope is therefore a marked improvement and an important aid in the minimal invasive approach when treating Asherman’s syndrome.28 Contrary to the conventional bipolar resectoscope, the small loop provides more precise, fine movements and greater accuracy in cutting capacities. Predominantly, the resectoscope is used to remove the cicatricial areas visible after liberation or creation of a cavity with mechanical instruments and balloon dilatation.
Myomectomy
The office treatment of <1.5 cm G0–G1 myomas, the inpatient treatment of G0–G1 myomas >2 cm and the treatment of all myomas with prevalent intramural development (G2) represent advanced hysteroscopic procedures.
5-Fr Instruments
In an ambulatory setting, depending on the compliance of the patient and instruments available, we limit the size of myoma G0 to maximal 1.5 cm.29 For small size G0 myoma, a technique is similar to that described for polypectomy is applied with the difference that, due to their higher tissue density, the myomas are first divided into two hemispheres with a 5-Fr bipolar electrode, then each of the two halves is cut into 3–4 fragments of such a size that they can be removed from the uterine cavity using a 5-Fr grasping or tenaculum forceps. For a small size G1 myoma with an intramural proportion, the myoma is separated from the capsule using mechanical instrument scissors, grasping forceps and bipolar needle for the coagulation of the vessels. This will prevent any myometrial stimulation or damage of the surrounding healthy myometrium and coagulate the important efferent and afferent vessels prior to the shaping procedure.
15-Fr Mini resectoscope
The use of a mini resectoscope represents an effective alternative procedure for an office myomectomy that has made it possible to treat these types of myomas while reducing operating time and simplifying the technique. The instrument is introduced into the uterine cavity always with a vaginoscopic approach, and the slicing is done with the resectoscope by cutting from the free edge of the myoma to the base (Figure 8).30
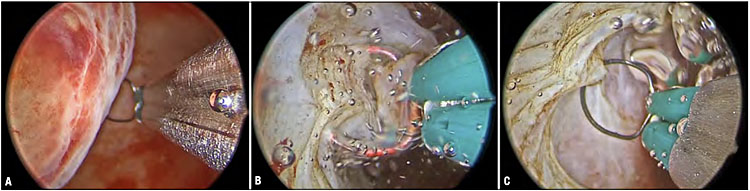
8
Resection of a type 1 myoma using a 15-Fr bipolar office resectoscope (KARL STORZ, Germany). Hysteroscopic view of the type 1 myoma which attaches to the right lateral wall (A). Myomectomy is performed using a slicing technique with the angled cutting loop (B–C). Intramyometrial slicing requires perfect identification of the proper surgical plane to make sure that resection does not go beyond the pseudocapsule.
Tissue removal device
As described for outpatient polypectomy, TRD can also be used to perform myomectomy. The technique is similar to that described for endometrial polyps. Once the hysteroscope is inserted into the uterine cavity using the vaginoscopic approach and a clear view is obtained, just placing the shaver against the myoma the device will simultaneously aspirate and cut tissue, delivering it through the shaving system.31
Resectoscope
The slicing technique represents the gold standard in the resectoscopic treatment of myomas. It involves repeated passes of the cutting loop in accordance with the following steps: the cutting loop is first placed behind the lesion and the current is activated only during retraction of the loop until the rest position is reached. Resection is normally initiated at the free margin of the myoma, then proceeding in a uniform manner towards its base of implantation. During myoma resection, fragments are serially resected, accumulate in the cavity and tend to impede vision. Therefore, tissue fragments need to be removed repeatedly during the operation.22 For G1–G2 myomas, the technique involves a first-stage procedure in which only the intracavitary portion of the myoma is removed, by means of the slicing technique. Then the intramural portion is approached.
Adenomyosis
Today, the combined ultrasound–hysteroscopic approach is becoming increasingly popular and has high accuracy for the surgical diagnostic and therapeutic approach of focal adenomyosis.32
During hysteroscopy the presence of focal hypervascularization, endometrial defects and cystic or firm elevations suggest adenomyosis. The technique of adenomyomectomy has yet to be exhaustively defined. The surgical procedure involves that the tissue protruding into the uterine cavity, is incised, evacuated and resected (by slicing) using a resectoscope with a cutting loop. Superficial adenomyotic nodules are usually treated with ease employing the technique above. In cases of deeply implanted lesions, the nodule may first be mobilized using various techniques that cause it to migrate into the uterine cavity.
The goal of surgery is to remove adenomyotic tissue without causing damage to surrounding healthy myometrial fibers. However, the lack of a distinct cleavage plane indicating the normal myometrial tissue can make the procedure quite challenging. This kind of surgery should be performed under ultrasound vision. Superficial diffuse adenomyosis may be treated with a variable rate of success by means of endo-myometrial ablation (endo-myomectomy) in the inpatient setting.33
The technique differs from the classical method of endometrial ablation in that resection is not limited to the endometrium and the first 2–3 mm of myometrium. Upon resection of the endometrial and superficial myometrial layer, the operator proceeds with continued slicing of the myometrial layer below until healthy myometrium is visualized and concludes the procedure by coagulation of endometrial residues. Endo-myomectomy is accomplished using 3 mm or 5 mm straight loops for ablation of the fundus and cornual recesses, as well as classic cutting loops for ablation of uterine walls.
Congenital uterine malformations
The gold standard in the treatment of Mullerian anomalies amenable to surgical correction is the operative hysteroscopy that offers numerous benefits regarding the intraoperative and postoperative aspects and the better reproductive outcomes.34
Dysmorphic uterus (class U1)
Different methods and instruments have been used for the metroplasty of a dysmorphic uterus (T-shaped, Y-shaped, tubular uterus), including scissors and resectoscope with a monopolar hook or bipolar energy.35,36,37
Di Spiezio et al. proposed in 2015 the HOME-DU technique, an outpatient technique performed under conscious sedation which increases the volume of the uterine cavity improving the final uterine morphology. Longitudinal lateral incisions 3–4 mm in depth are made with a 5-Fr bipolar electrode along the lateral uterine walls in the isthmic region and also on the anterior and posterior walls of the fundal region up to the isthmus.36 At the end of the procedure, an anti-adhesive gel is applied into the uterine cavity through the inflow channel of the hysteroscope, to prevent intrauterine adhesions (Figure 9).
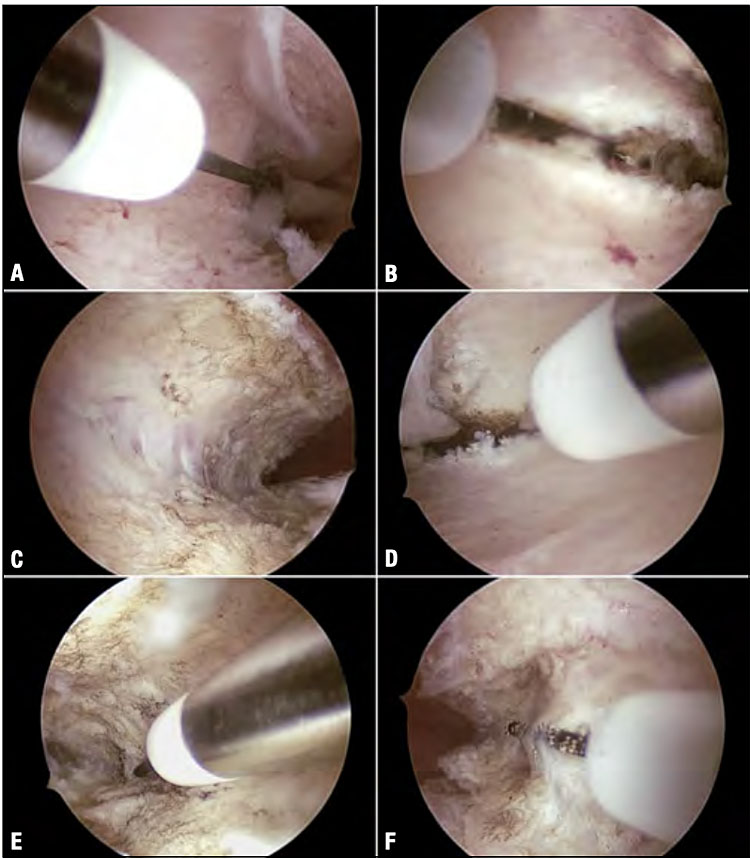
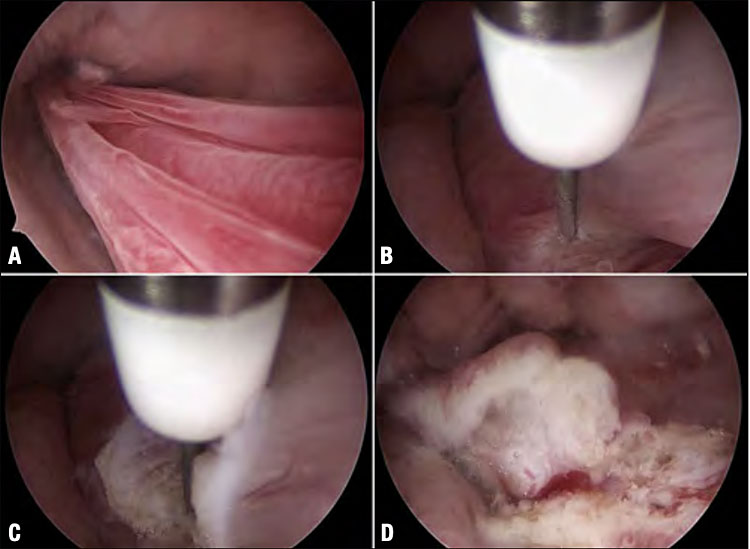
9
The HOME-DU technique (Hysteroscopic Outpatient Metroplasty To Expand Dysmorphic Uteri). An incision of 3–4 mm in depth is made in redundant fibromuscular tissue using a straight bipolar electrode (KARL STORZ, Germany) at the isthmic area of the right lateral wall (A–C). Incision in the isthmic area of the contralateral lateral wall (D–F). Additional incisions are then made on the anterior and posterior walls, extending from the fundal region up to the isthmus.
Septate uterus (class U2)
The septate uterus is the most frequent Mullerian anomaly, and it is associated with the highest rate of pregnancy complication. Additionally, the indication range for surgical treatment is the subject of a lively debate in literature; for this reason, most authors believe that a repeated/recurrent pregnancy loss is the main indication for a metroplasty while, our point of view is that considering the simplicity of the operation and the significant improvement in reproductive outcomes, the “prophylactic metroplasty” should be considered for these women.
Surgical technique is based on the incision of the septum along the median plane, starting from the apex and proceeding towards the fundus. The most delicate part of the procedure in when stop the incision of the septum in order to avoid any complications (such us perforation, synechiae). Normally, the metroplasty should be stop once both tubal ostia are simultaneously visible with a panoramic view or when during the incision of the myometrium, there is bleeding from small fundal myometrial vessels.
The resectoscopic treatment of the uterine septum involves the use of straight cutting loops or a pointed electrode. The septum is removed to the medial portion of the septum from the apex to the base with the cutting loop oriented in an anterograde direction (in any other resectoscopic operative procedure the cutting loop is moved in a retrograde direction).
In case of complete uterocervical septum the resection starts from the isthmic portion of the septum. The cervix of the larger uterine hemi cavity is gradually dilated in order to introduce a resectoscope with the classical straight loop; in the contralateral hemi cavity, a Hegar dilator is inserted and used as a guide for the incision. Cutting starts above the internal uterine ostium using an angular cutting loop. Gradual resection then follows toward the fundus using the classic technique.
The mini-invasive hysteroscopic technique with miniature instruments is based on the same rules of the resectoscopic approach. The hysteroscopic instruments are straight and hooked bipolar electrodes and miniaturized scissors. Also in this case, the resection starts from the apex of the septum, using bipolar electrode operating in pulsed mode, proceeding from one side of the to the other. Following removal of about ¾ of the septum, the procedure continues using the miniature scissors to remove the thickness of the remaining fundal tissue.
The advent of the 15-Fr bipolar office resectoscope has made a septate uterus amenable to surgical treatment on the same operative scheme as applicable in a traditional resectoscopic approach, eliminating the need for cervical dilatation. In this way the metroplasty can be performed in an outpatient setting. As mentioned above, the hysteroscopist adopts the same technique used with a classic resectoscope of a larger caliber. Also in this case the scissors facilitate trimming of the thickness of the remaining fundal tissue. In final stages of metroplasty, the use of palpatory instrument improve accuracy of the procedure (Figure 10).
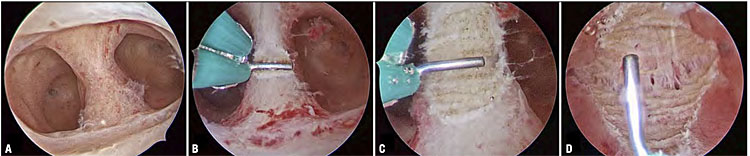
10
Hysteroscopic metroplasty for removal of an uterine septum with a 15-Fr bipolar office resectoscope (KARL STORZ, Germany). The septum is gradually resected with a pointed electrode beginning at its apex and proceeding towards the fundus (B–C) until both tubal ostia are visualized (D).
After metroplasty, it is suggested to apply an anti-adhesive gel to prevent intrauterine adhesions through the inflow channel of the hysteroscope.38,39
Removal of retained products of conception
Retained products of conception (RPOC) may occur after medical and surgical pregnancy termination, miscarriage, and vaginal or cesarean delivery. In 1997, Goldenberg et al. reported on the use of hysteroscopy for treatment of RPOC. This surgical approach has the advantage of reduced trauma to the endometrium, possibly reduced rates of intrauterine adhesions and improved future reproductive outcomes.40,41
5-Fr Instruments
The hysteroscopic outpatient approach commands the use of miniaturized mechanical instruments (e.g., alligator forceps) for appropriate removal of the retained products. The technique is to separate the RPOC from its myometrial bed by repeatedly opening and closing the jaws of the forceps; if the material is more adherent to the uterine wall, scissors may be used or, proceeding with great caution, a bipolar electrode, making sure to prevent iatrogenic damage to the myometrium.
Tissue removal device
An alternative surgical treatment option for women with RPOC is the TRD. A miniature shaver also be used in the ambulatory setting. The removal of RPOCs is a major indication for using a TRD and – given the instrument’s purely mechanical cutting action – should be considered particularly in cases where the remnant is found to be poorly vascularized. The procedure can be performed in a rapid and straightforward manner by bringing the cutting window in close contact with the placental remnant to be removed. Another benefit lies in the fact, that the procedure can be performed in an outpatient setting. While small-caliber TRDs up to 6 mm in diameter can be applied without prior cervical dilatation in an outpatient setting, the use of larger models (>6 mm) is limited to inpatient conditions in the operating room and necessitates the use of anesthesia (Figure 11).
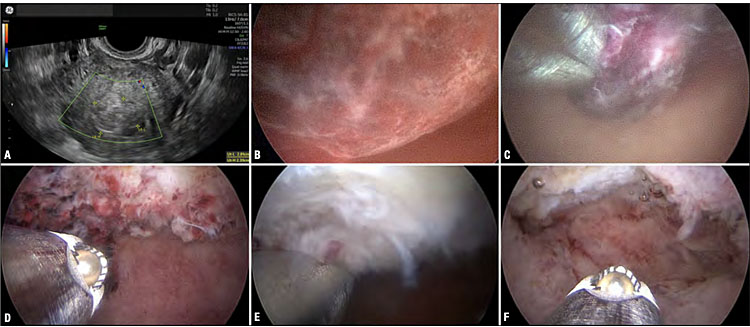
11
Treatment of a retained trophoblast with the 19-Fr Intrauterine Bigatti Shaver (IBS®) (KARL STORZ, Germany). Ultrasonographic appearance (A) and hysteroscopic image (B) of an avascular retained trophoblast. The cutting window of the TRD is brought in contact with the free margin of the lesion (C). Owing to the simultaneous removal and aspiration of tissue fragments, a clear visual field is maintained throughout the procedure (D–F).
15-Fr Resectoscope
An operative office mini resectoscope can be used for office treatment of RPOC, reducing the number of procedures performed in the operating room. These new tools are safe instruments that combine the advantages of traditional resectoscopy with the benefits of outpatient surgery. The instrument is introduced into the uterine cavity and the slicing is done using the electrosurgical bipolar loop as a curette in order to avoid thermal damage of the endometrium. In cases of large or adherent residual, the loop may be used with activated current.
Resectoscope
This type of treatment involves the use of a resectoscope with an angled loop used in a ‘cold way’ (i.e., not activated): it is generally not suitable to activate the loop in order to completely eliminate the risk of thermal myometrial damage. The loop is advanced until a cleavage plane is identified between the myometrium and trophoblastic tissue, followed by detachment of the material, adopting a technique similar to that used to guide a curette under visual control and taking care to preserve the integrity of the healthy myometrium. Occasionally, the cleavage plane is indiscernible, or the material to be removed seems to be tenaciously adherent. In this case, the loop may be used with activated current. Due caution is required, because there is still the possibility of abnormal placental growth (e.g., placenta accreta).
PRACTICE RECOMMENDATIONS
- Hysteroscopy is a useful diagnostic tool for the investigation of abnormal uterine bleeding and when there are concerns about fertility.
- It has a useful therapeutic role when combined with diagnosis as a see-and-treat service.
- The need for hysteroscopy is often determined by the result of a previous ultrasound scan, whether arranged in primary care when managing heavy menstrual bleeding or in secondary care for women with postmenopausal bleeding.
- Outpatient hysteroscopy for diagnosis and treatment is well established, but for a significant minority of women, an inpatient procedure is required.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Gkrozou F, Dimakopoulos G, Vrekoussis T, et al. Hysteroscopy in women with abnormal uterine bleeding: a meta-analysis on four major endometrial pathologies. Arch Gynecol Obstet 2015;291(6):1347–54. | |
Marlow JL. Media and delivery systems. Obstet Gynecol Clin North Am 1995;22(3):409–22. | |
Carugno J, Grimbizis G, Franchini M, et al. International Consensus Statement for recommended terminology describing hysteroscopic procedures. Facts Views Vis Obgyn 2021;13(4):287–94. | |
Di Spiezio Sardo A, Campo R. State-of-the-Art Hysteroscopic Approaches to Pathologies of the Genital Tract. EndoPress.Version: 2.0 04-2022. | |
Campo R, Molinas CR, Rombauts L, et al. Prospective multicentre randomized controlled trial to evaluate factors influencing the success rate of office diagnostic hysteroscopy. Hum Reprod 2005;20(1):258–63. | |
Di Spiezio Sardo A, Giampaolino P, Manzi A, et al. The Invisible External Cervical Os. Tips and Tricks to Overcome this Challenge during In-Office Hysteroscopy. J Minim Invasive Gynecol 2021;28(2):172–3. | |
Campo R, Meier R, Dhont N, et al. Implementation of hysteroscopy in an infertility clinic: The one-stop uterine diagnosis and treatment. Facts Views Vis Obgyn 2014;6(4):235–9. | |
The Use of Hysteroscopy for the Diagnosis and Treatment of Intrauterine Pathology: ACOG Committee Opinion, Number 800. Obstet Gynecol 2020;135(3):e138–48. | |
Munro MG, Critchley HO, Fraser IS. The FIGO systems for nomenclature and classification of causes of abnormal uterine bleeding in the reproductive years: who needs them? Am J Obstet Gynecol 2012;207(4):259–65. | |
Royal College of Obstetricians and Gynaecologists. Management of Endometrial Hyperplasia. Green-top Guideline No. 67. London: RCOG, 2016. www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg67 (accessed November 2019). | |
Taylor E, Gomel V. The uterus and fertility. Fertil Steril 2008;89(1):1–16. | |
Stamenov GS, Vitale SG, Della Corte L, et al. Hysteroscopy and female infertility: a fresh look to a busy corner. Hum Fertil (Camb) 2022;25(3):430–46. | |
Nastri CO, Lensen SF, Gibreel A, et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database Syst Rev 2015;22(3):CD009517, 1002/14651858.CD009517.pub3. | |
Siegler AM, Kemmann E. Location and removal of misplaced or embedded intrauterine devices by hysteroscopy. J Reprod Med 1976;16(3):139–44. | |
Salazar CA, Isaacson KB. Office Operative Hysteroscopy: An Update. J Minim Invasive Gynecol 2018;25(2):199–208. | |
Smith PP, Middleton LJ, Connor M, et al. Hysteroscopic morcellation compared with electrical resection of endometrial polyps: a randomized controlled trial. Obstet Gynecol 2014;123(4):745–51. | |
Noventa M, Ancona E, Quaranta M, et al. Intrauterine Morcellator Devices: The Icon of Hysteroscopic Future or Merely a Marketing Image? A Systematic Review Regarding Safety, Efficacy, Advantages, and Contraindications. Reprod Sci 2015;22(10):1289–96. | |
Dealberti D, Riboni F, Cosma S, et al. Feasibility and Acceptability of Office-Based Polypectomy With a 16F Mini-Resectoscope: A Multicenter Clinical Study. J Minim Invasive Gynecol 2016;23(3):418–24. | |
Guida M, Di Spiezio Sardo A, Acunzo G, et al. Vaginoscopic versus traditional office hysteroscopy: a randomized controlled study. Hum Reprod 2006;21(12):3253–7. | |
Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol 1993;82(5):736–40. | |
Salim R, Lee C, Davies A, et al. A comparative study of three-dimensional saline infusion sonohysterography and diagnostic hysteroscopy for the classification of submucous fibroids. Hum Reprod 2005;20(1):253–7. | |
Di Spiezio Sardo A, Mazzon I, Bramante S, et al. Hysteroscopic myomectomy: a comprehensive review of surgical techniques. Hum Reprod Update 2008;14(2):101–19. | |
Perez-Medina T, Lopez-Mora P, Rojo J. Comparison of the hysteroscopy-biopsy with the D & C in the diagnosis of abnormal uterine bleeding. Progresos de Obstetricia y Ginecologia 1994;37:479–86. | |
Janssens JP, Rotenberg L, Sentis M, et al. Caution with microbiopsies of the breast: displaced cancer cells and ballistics. Eur J Cancer Prev 2006;15(6):471–3. | |
Bettocchi S, Bramante S, Bifulco G, et al. Challenging the cervix: strategies to overcome the anatomic impediments to hysteroscopy: analysis of 31,052 office hysteroscopies. Fertil Steril 2016;105(5):e16–7. | |
AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: practice guidelines for management of intrauterine synechiae. J Minim Invasive Gynecol 2010;17(1):1–7. | |
Campo R, Van Belle Y, Rombauts L, et al. Office mini-hysteroscopy. Hum Reprod Update 1999;5(1):73–81. | |
Roy KK, Lingampally A, Kansal Y, et al. A Pilot Study Comparing Hysteroscopic Adhesiolysis by Conventional Resectoscope Versus Mini-resectoscope. Oman Med J 2017;32(6):492–8. | |
Bettocchi S, Ceci O, Di Venere R, et al. Advanced operative office hysteroscopy without anaesthesia: analysis of 501 cases treated with a 5 Fr. bipolar electrode. Hum Reprod 2002;17(9):2435–8. | |
Papalampros P, Gambadauro P, Papadopoulos N, et al. The mini-resectoscope: a new instrument for office hysteroscopic surgery. Acta Obstet Gynecol Scand 2009;88(2):227–30. | |
Noventa M, Ancona E, Quaranta M, et al. Intrauterine Morcellator Devices: The Icon of Hysteroscopic Future or Merely a Marketing Image? A Systematic Review Regarding Safety, Efficacy, Advantages, and Contraindications. Reprod Sci 2015;22(10):1289–96. | |
Gordts S, Grimbizis G, Campo R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Steril 2018;109(3):380–8.e1. | |
Molinas CR, Campo R. Office hysteroscopy and adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006;20(4):557–67. | |
Grimbizis GF, Gordts S, Di Spiezio Sardo A, et al. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum Reprod 2013;28(8):2032–44. | |
Meier R, Campo R. T-shaped uterus in Female Genital Tract Congenital Malformations: Classification, Diagnosis and Management. London: Springer, 2015. | |
Di Spiezio Sardo A, Florio P, Nazzaro G, et al. Hysteroscopic outpatient metroplasty to expand dysmorphic uteri (HOME-DU technique): a pilot study. Reprod Biomed Online 2015;30(2):166–74. | |
Alonso L, Haimovich S, Di Spiezio Sardo A, et al. Dysmorphic Uterus: Do We Need a T-Y-I Subclassification? J Minim Invasive Gynecol 2020;27(1):4–6. | |
Homer HA, Li TC, Cooke ID. The septate uterus: a review of management and reproductive outcome. Fertil Steril 2000;73(1):1–14. | |
Di Spiezio Sardo A, Zizolfi B, Bettocchi S, et al. Accuracy of Hysteroscopic Metroplasty With the Combination of Presurgical 3-Dimensional Ultrasonography and a Novel Graduated Intrauterine Palpator: A Randomized Controlled Trial. J Minim Invasive Gynecol 2016;23(4):557–66. | |
Capmas P, Lobersztajn A, Duminil L, et al. Operative hysteroscopy for retained products of conception: Efficacy and subsequent fertility. J Gynecol Obstet Hum Reprod 2019;48(3):151–4. | |
Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med 1997;42(1):26–8. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
