This chapter should be cited as follows:
Lam AYR, Lim W, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.416423
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 8
Maternal medical health and disorders in pregnancy
Volume Editor:
Dr Kenneth K Chen, Alpert Medical School of Brown University, USA
Originating Editor: Professor Sandra Lowe

Chapter
Clinical Management of Diabetes in Pregnancy
First published: August 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Diabetes mellitus is one of the most common metabolic conditions affecting pregnancy.1,2 Diabetes in pregnancy can be categorized into pre-gestational diabetes mellitus (type 1 or type 2), and gestational diabetes mellitus (GDM). It is estimated that diabetes mellitus affects 1 in 6 (16.8%) pregnancies, with 13.6% from pre-gestational diabetes and 86.4% from GDM.3
Diabetes mellitus in pregnancy increases the risk of adverse maternal and neonatal outcomes. With comprehensive multidisciplinary care, as well as preconception counseling for pre-gestational diabetes mellitus, the risk of these complications can be mitigated.
PHYSIOLOGY: GLUCOSE HOMEOSTASIS IN PREGNANCY
During pregnancy, several metabolic adaptations occur to ensure adequate fetal growth and development, and to provide the mother with adequate energy stores for the increased demands of pregnancy.4
Glucose is the main energy substrate for the fetus and placenta. Fetal gluconeogenesis is minimal; thus, the fetus is highly reliant on placental supply of glucose from the maternal circulation. Glucose crosses the placenta by facilitated carrier-mediated diffusion through glucose transporter proteins (GLUTs). Maternal glucose concentrations exceeding that of the fetus will drive net glucose transport toward the fetus.5
Insulin sensitivity in the mother changes over the course of pregnancy (Figure 1). Early pregnancy is an anabolic state, involving increases in maternal fat stores and insulin sensitivity.6 This enables nutrient storage in early pregnancy to meet fetoplacental and maternal requirements during late gestation and lactation. Insulin sensitivity peaks between gestational weeks 9 and 16, resulting in reduced insulin requirements and a higher likelihood of hypoglycemia in insulin-treated women.7 Increasing insulin resistance and insulin requirements occur during the remainder of pregnancy, most pronounced in the third trimester.8 This is attributed to the effects of placental hormones, including human placental lactogen, progesterone, prolactin, placental growth hormone, and cortisol.9 In non-diabetic pregnancy, the increasing insulin resistance is accompanied by compensatory hyperinsulinemia. This compensatory rise in endogenous insulin production does not occur in women with pre-existing diabetes, resulting in greater maternal hyperglycemia, particularly postprandially.
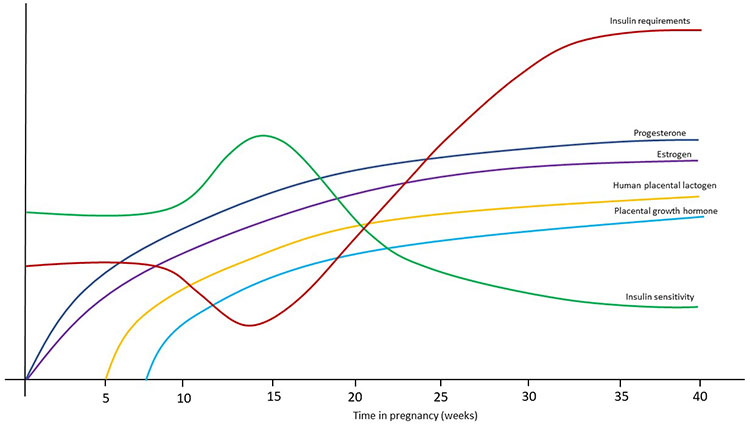
1
Insulin requirements and sensitivity throughout pregnancy.
Pregnancy is also a state of “accelerated starvation”, or an exaggerated response to overnight fasting compared to the non-pregnant state. This involves a greater fall in plasma glucose and amino acids, and a more pronounced rise in free fatty acids with enhanced ketogenesis during periods of fasting.6
POTENTIAL FETAL, NEONATAL, AND MATERNAL RISKS OF DIABETES IN PREGNANCY
Pre-gestational diabetes is associated with increased risks of adverse fetal, neonatal, and maternal outcomes during pregnancy (Table 1). The risk of adverse fetal and neonatal outcomes correlates largely with the degree of maternal glycemic control.10,11
1
Adverse pregnancy outcomes associated with pre-gestational diabetes mellitus.
Fetal | Neonatal | Maternal |
|
|
|
The adverse effects of GDM on pregnancy are largely similar, except for the risk of congenital malformations, which is mostly associated with pre-gestational diabetes mellitus. A meta-analysis by Balsells et al. suggested an increased risk of congenital malformations in gestational diabetes, albeit less than pre-gestational diabetes mellitus.12 A limitation of this study was that potential contributing factors such as maternal age, obesity, and possible pre-conception hyperglycemia could not be ascertained.
These are summarized in Table 2.
2
Adverse pregnancy outcomes associated with gestational diabetes mellitus.
Fetal | Neonatal | Maternal |
|
|
|
PRECONCEPTION CARE
Structured care by a multidisciplinary team should start from the preconception period. Preconception care programs aim to empower women with pre-existing diabetes with knowledge on how to achieve a healthy and successful pregnancy. These programs have been shown to improve pregnancy preparation and reduce adverse pregnancy outcomes in women with T1DM and T2DM.13,14 They also result in significant healthcare cost savings.15,16,17
Preconception counseling
The American Diabetes Association recommends that preconception counseling should be part of routine diabetes care for women who have reproductive potential, starting at puberty.18
Preconception counseling is the responsibility of both primary care physicians and specialists who provide diabetes care. The content of preconception counseling may vary, depending on the patient’s current and future childbearing goals. However, crucial points to address include:
- Potential fetal, neonatal, and maternal risks of diabetes in pregnancy.
- The importance of planning a pregnancy, and the increased risks associated with an unplanned pregnancy.
- How to access preconception care.
- Contraception and family planning.
- What to do should an unexpected pregnancy occur.
Preconception care of women who are actively planning pregnancy
Women who are actively planning a pregnancy should do so in conjunction with a multidisciplinary team comprised of endocrinologists, diabetes nurse educators, and dieticians.
Components of preconception care in women who are actively planning pregnancy
- Glycemic control
- Medication optimization
- Optimization of glucose-lowering medications
- Cessation/substitution of potentially teratogenic medications
- Folic acid supplementation
- Screening and management of diabetes-related complications
- Diabetic retinopathy
- Diabetic nephropathy
- Cardiovascular disease
- Assessment for and management of co-existing medical conditions
- Hypertension
- Hyperlipidemia
- Thyroid disorders
- Weight management and nutrition
Glycemic control
Stringent glycemic control is crucial before withdrawing reliable contraception, as periconception maternal hyperglycemia is associated with a higher risk of spontaneous abortion and congenital malformations.19,20 Periconception glycemic control has been reported as an independent predictor of congenital malformation (adjusted odds ratio [aOR] 1.3 [95% CI 1.2, 1.4] per 1% [11 mmol/mol] linear increase in HbA1c above 6.3% [45 mmol/mol]).19 The risk of spontaneous abortion in women with diabetes mellitus can be up to double that of non-diabetic women.10,21 This risk appears to be reversed with optimal glycemic control.22
Therefore, the goal is to minimize fetal exposure to maternal hyperglycemia during the crucial period of fetal organogenesis, which occurs prior to 7 weeks' gestation and often before a woman first discovers that she is pregnant.23 Most International bodies (see Table 3) recommend a preconception HbA1c target of <48 mmol/mol or <6.5%, as this is associated with risk of congenital anomalies equivalent to the non-diabetic population.18,19,24 There is little evidence regarding capillary blood glucose (CBG) or interstitial fluid glucose derived from continuous glucose monitoring (CGM) targets in women who are planning pregnancy. Despite the absence of such evidence, various International bodies have published their recommendations for such targets. The 2015 NICE guidelines recommend that women with T1DM and T2DM who are planning pregnancy aim for the same CBG targets as recommended for all people with T1DM.25 These are CBG levels of 5–7 mmol/l upon waking, 4–7 mmol/l before meals at other times of the day, and 5–9 mmol/l at least 90 min post-meal.26 The use of real-time CGM during preconception period did not yield significant pregnancy outcome benefits.27 Currently, CGM has not been recommended in routine preconception care.
3
Summary of preconception glycemic targets for pregnancy from international guidelines.
Guideline | HbA1c (mmol/mol, %) | Fasting/pre-meal glucose (mmol/l) | Postprandial glucose (mmol/l) |
American Diabetes Association (2020)18 | <48, 6.5% | NIL | NIL |
Diabetes Canada Clinical Practice Guidelines Expert Committee (2018)28 | ≤48, 6.5% | NIL | NIL |
The National Institute for Health and Care Excellence (NICE) (2015)25 | <48, 6.5% | 5–7 mmol/l on waking 4–7 mmol/l before meals at other times of the day | 5–9 mmol/l at least 90 min postprandially |
The Endocrine Society (2013)29 | Aim for HbA1c levels as close to normal as possible when they can be safely achieved without undue hypoglycemia | ≤5.3 mmol/l | 1 h after the start of a meal: ≤7.8 mmol/l 2 h after the start of a meal: ≤6.7 mmol/l |
Medication optimization
Optimization of glucose-lowering medications
Women with T2DM on treatment with oral glucose-lowering agents who are planning to become pregnant should be counseled on the safety of these medications in pregnancy. The two main oral glucose-lowering agents used in pregnancy are metformin and glibenclamide (glyburide). ADA recommends converting oral agents to insulin therapy as a preferred management strategy.18 NICE recommends that metformin may continue to be used as an adjunct or alternative to insulin, when the potential benefits of improved glycemia outweighs the risk of harm. NICE also recommends ceasing all other blood glucose-lowering agents.30 Diabetes Canada recommends continuing metformin and/or glibenclamide if glycemic control is optimal until pregnancy is achieved, while those on other agents should be switched to insulin therapy.28
Insulin-treated women with diabetes seeking to conceive should be treated with multiple daily insulin injections (MDIIs) or continuous subcutaneous insulin infusion (CSII), as these regimens are more likely than premixed insulin therapy to achieve target blood glucose levels during the preconception period and during pregnancy. MDII therapy involves the use of intermediate- or long-acting basal insulin to inhibit hepatic glucose production and lipolysis throughout the day, and mealtime short- or rapid-acting insulin to cover dietary carbohydrate intake. CSII therapy involves the continuous infusion of rapid-acting insulin by an insulin pump. Changes to insulin regimens should be made in advance of withdrawing contraceptive measures.29
Insulin
In view of transfer of glucose and gluconeogenic substrates to the fetus, women with T1DM have a two–threefold higher propensity for hypoglycemia, and a reduction in insulin requirements by approximately 10–20% in the first trimester.31,32 From the second trimester, insulin requirements increase, plateauing at 36–37 weeks' gestation.32 Peak insulin requirements may be up to twice that pre-pregnancy.32 Immediately postpartum, diabetogenic pregnancy hormones are cleared rapidly, with swift recovery of insulin sensitivity. The dosage of glucose lowering medications must therefore be reduced postpartum. In the initial few postpartum days, insulin requirements are approximately half of that needed at the time of delivery.18
Table 4 describes the time-action profiles of various insulin preparations in non-pregnant populations. There may be large inter- and intra-individual variability in the onset, peak, and duration of action of the various types of insulin.
Insulin type | Insulin | Onset of action | Time to peak | Duration of action | FDA pregnancy category |
Short-acting | Regular insulin | 30–60 min | 90–120 min | 5–12 h | B |
Rapid-acting | Insulin lispro | 10–15 min | 30–90 min | 3–5 h | B |
Insulin aspart | 10–15 min | 40–50 min | 3–5 h | B | |
Insulin glulisine | 10–15 min | 55 min | 3–5 h | C | |
Intermediate-acting | Neutral protamine Hagedorn (NPH) | 1–2 h | 4–10 h | 16–18 h | B |
Long-acting | Insulin detemir | 1–2 h | None | Up to 24 h | B |
Insulin glargine | 1–2 h | None | Up to 24 h | No human pregnancy data (previously C*) | |
Insulin glargine | None | No human pregnancy data | |||
Insulin degludec | 1–2 h | None | 42 h | No human pregnancy data |
*In 2015, the US FDA has amended the pregnancy labeling rule for prescription drug products to require labeling that includes a summary of risk, a discussion of the data supporting that summary, and relevant information to help health-care providers make prescribing decisions and counseling women about the use of drugs during pregnancy. Pregnancy categories A, B, C, D, and X are being phased out.
Human insulins
Prior to the development of analog insulins, human insulins were the only formulations available for use in pregnancy. Soluble (regular) insulin is a quick-acting human insulin formulation, which has a slower onset and a longer duration of action than endogenous insulin. This confers an increased risk of undesirable postprandial hypoglycemia.34 Neutral protamine Hagedorn (NPH) insulin is an intermediate-acting human insulin formulation, which has a duration of action of 16–18 h and must be administered twice a day to provide adequate 24-h basal insulin cover. NPH insulin also has a peak at 4–10 h post-injection, which may confer higher risks of hypoglycemia in the fasted state.33
Insulin analogs
Rapid-acting insulin analogs include lispro, aspart, and glulisine. These can be used as mealtime insulin in MDII therapy, or in an insulin pump. Rapid-acting insulin analogs have faster onset of action, earlier time to peak, and shorter duration of action than regular insulin, mimicking endogenous insulin physiology more closely. This allows rapid-acting insulin analogs to provide superior control of postprandial hyperglycemia and reduce risks of hypoglycemia in the late post-meal period. Superior control of postprandial hyperglycemia is crucial, as the postprandial glucose level after the first trimester is the most important contributor to the risk of macrosomia.37 Compared to regular insulin, lispro and aspart use in women with pre-gestational DM was associated with similar obstetric, fetal, and neonatal outcomes.38,39,40,41,42 Lispro and aspart have been approved by the European Medicines Agency for use in pregnancy, and are FDA category B. There has been no published data on the use of glulisine in pregnancy, and it is not FDA approved for use in pregnancy.
Long-acting insulin analogs include detemir and glargine. They are “peakless” and have a longer duration of action compared to NPH insulin. The efficacy and safety of detemir has been established in a multinational randomized controlled trial involving pregnant women with T1DM.43,44 Detemir has demonstrated lower fasting glucose levels, similar hypoglycemia rates and perinatal outcomes, and noninferior glycemic control compared to NPH.41,43 Detemir has been approved for use by the European Medicines Agency for use in pregnancy, and is FDA category B. Some women may require twice-daily dosing of detemir, due to inter-individual variability in the duration of action.
Glargine has a higher receptor affinity for the type 1 insulin-like growth factor receptor. This has led to theoretical concerns about undesirable effects of glargine on mitogenicity, fetal growth, and diabetic retinopathy.33,45 Limited data on the glargine use in women with pre-gestational DM suggest comparable maternal, fetal, and neonatal outcomes compared with other basal insulins.46,47,48 Insulin glargine does not carry an FDA classification consistent with current labeling.
Newer insulin analogs
Glargine U-300 is a concentrated form of glargine, containing 300 units/ml instead of 100 units/ml. Insulin degludec is an ultra-long-acting insulin with a duration of action exceeding 42 h. They are both not currently approved for use in pregnancy.49,50,51
Fiasp is a rapid-acting insulin analog containing insulin aspart with the addition of vitamin B3 and L-Arginine to hasten absorption. There are no available data with Fiasp in pregnant women to inform a drug-associated risk for major birth defects and miscarriage.52
Metformin
The recommendation by various international groups for the use of oral glucose lowering agents in pregnancy vary considerably.53 Some international groups have expressed extreme reluctance regarding the use of metformin, such as ADA and ACOG,18,54 whilst others include metformin as an option particularly for gestational diabetes, such as CDA, NICE, SMFM, HKCOG.55,28,30,56
Metformin alone is not considered adequate treatment for women with pre-existing T2DM in pregnancy as they are unlikely to achieve adequate glycemic control. Up to 43% of women primarily treated with metformin during pregnancy required supplementary insulin therapy to achieve optimal glycemic control.57,58,59
NICE recommends that women with T2DM may use metformin as an adjunct or alternative to insulin in the preconception period and during pregnancy, when the likely benefits from improved blood glucose outweigh the potential for harm.25
Metformin crosses the placenta readily and metformin levels in the fetal circulation have been reported to be half or the same as the maternal metformin levels.60,61 Despite metformin crossing the placenta, there is reassuring data regarding the short-term safety of metformin use in both early and late pregnancy. Several meta-analyzes showed no evidence of an increased risk for major malformations and adverse neonatal outcomes with first-trimester metformin use.62,63,64
However, there is limited long-term follow-up information, especially concerning metformin use in pregnancies complicated by pre-existing diabetes although good data on pediatric follow up has been published from its use for gestational diabetes.65 At age 7, 290 children from the Australian cohort were assessed and there were no differences in offspring measures. Two years later, 99 children all from the New Zealand cohort were reviewed. Of note, their mothers had a higher BMI at enrolment than those randomized to insulin (35.4 versus 32.0, p = 0.08)). These children were larger by measures of weight, arm, and waist circumferences, waist: height (p <0.05), body mass index, triceps skin fold (p: 0.05), DEXA fat mass and lean mass (p: 0.07).56 Body fat percentage was similar by DEXA and bioimpedence. Visceral adipose tissue and liver fat were similar by magnetic resonance imaging (MRI). Metabolic markers including HbA1c, fasting glucose, fasting lipids, adiponectin, and leptin were all similar.
Further data regarding the long-term impact of these small but statistical differences in body composition is awaited along with the results of newer trials including the offspring from the EMPOWaR and MOP trials may shed more light as these studies compared metformin with placebo rather than insulin. The MiTy Kids' trial will assess the children of mothers with type 2 diabetes receiving metformin in addition to insulin.66,67,68
Cessation/substitution of potentially teratogenic medications
Women should be reviewed with a plan to cease potentially teratogenic or fetotoxic medications either in advance of conception to allow substitution with more appropriate medications or as early as possible after confirmation of pregnancy. These include ACE inhibitors (ACE-I), angiotensin receptor blockers (ARB), and statins (see below).69,70
Folic acid supplementation
Folate requirements increase to 600 ug/day during pregnancy. Women with diabetes who are planning a pregnancy should receive folic acid supplements to reduce the risk of neural tube defects.71,72 Supplementation should be started at least 1 month prior to conception, as neural-tube development begins as early as the third week after conception.73 Although not evidence based, a higher dose (5 mg/day) is sometimes recommended for women with diabetes and should be given until week 12 of gestation, after which the dose may be reduced to 0.4 to 1.0 mg/day.25,29
Screening and management of diabetes-related complications
Management of diabetic retinopathy: preconception, antenatal, and postpartum care
preconception
Women should undergo retinal examination prior to conception to accurately determine the presence and severity of diabetic retinopathy (DR).18 Ophthalmologist referral should be made if severe non-proliferative diabetic retinopathy (NPDR), proliferative diabetic retinopathy (PDR), or diabetic macular edema is detected at screening. Women should be counseled about the risks of worsening retinopathy during pregnancy and up to 1 year postpartum, especially if retinopathy is severe.74,75,76,77 Overall, 10 to 26% of women without retinopathy may develop background retinopathy changes during pregnancy. However, these are generally mild, do not require intervention, and usually regress postpartum.74,77,78,79 If the degree of pre-existing retinopathy warrants therapy, The Endocrine Society recommends deferring conception until the retinopathy has been treated and stabilized.29
Antenatal and postpartum
Dilated eye examinations should occur ideally before pregnancy or in the first trimester. Patients should be monitored every trimester and for 1 year postpartum as indicated by the degree of retinopathy.18 As hypertension and poor glycemic control are modifiable risk factors for DR progression, these should be optimized. Rapid improvement of hyperglycemia is associated with potential worsening of pre-existing diabetic retinopathy. The postulated mechanisms include (i) reduced blood flow or increased ischemia, and (ii) elevated insulin-like growth factor 1 (IGF-1) secondary to improved glycemia. IGF-1 levels are also elevated due to the pregnancy state.80 Despite this, retinopathy should not delay blood glucose optimization in women with a high HbA1c in early pregnancy.81
Pan-retinal laser photocoagulation may be used safely and effectively for treatment of PDR during pregnancy.82 Anti-VEGF medications (Ranibizumab, Aflibercept and Bevacizumab) are used for treatment of DME in non-pregnant patients. These agents have been assigned FDA pregnancy category C, as animal studies have shown evidence of embryo-fetal toxicity, but there are no controlled data in human pregnancy and a risk–benefit approach should be taken regarding their use.83
Management of diabetic nephropathy: preconception, antenatal, and postpartum care
A diagnosis of diabetic nephropathy (DN) is made based on the presence of albuminuria and/or reduced estimated glomerular filtration ratio (eGFR) in the absence of other primary causes of kidney damage.84 It is important to note that during pregnancy, renal hyperfiltration results in underestimation of eGFR, hence one should use creatinine level to monitor progression of nephropathy.85,86
Preconception
Women planning a pregnancy should undergo screening for DN with measurement of serum creatinine and evaluation of albuminuria with a spot urine albumin-to-creatinine ratio or a 24-h urinary protein measurement. Women who are found to have DN preconception should be counseled that pregnancies complicated by DN are associated with increased rates of cesarean delivery, pre-eclampsia, preterm delivery, IUGR and perinatal mortality. However, with improved antenatal care, women in developed countries with diabetic nephropathy largely achieve successful pregnancy outcomes. In one retrospective cohort study, the perinatal mortality rate was 3%, comparable to patients with DM but no renal involvement.87
Women with moderate-to-severe renal insufficiency with serum creatinine ≥124 umol/l (1.4 mg/dl) have significant risks of accelerated DN progression in pregnancy, which may not be reversible.88 Women with a serum creatinine ≥120 umol/l, urine ACR >30 mg/mmol or eGFR <45 ml/min should see a nephrologist before discontinuation of contraception. In women with more advanced renal dysfunction, pregnancy may lead to permanent worsening of renal function and accelerate progression to end stage renal failure. These women need specialist counseling as dialysis or transplant may be required prior to, or even during, pregnancy.
Blockade of the renin-angiotensin-aldosterone system (RAAS) with either an ACE-i or ARB is recommended in non-pregnant patients to decrease proteinuria and slow the progression of diabetic nephropathy.89,90 Use of these agents during pregnancy is not recommended.
Evidence of ACE-I and ARB-associated teratogenicity for first-trimester exposures is conflicting. In contrast, there is unequivocal associated fetal renin angiotensin aldosterone blockade for second- and third-trimester exposures, that may result in renal failure oligohydramnios, intrauterine growth retardation (IUGR), and death.69,91
In women with significant proteinuria who are planning to conceive, stopping RAAS blockade may cause worsening proteinuria and more rapid decline in renal function, especially as successfully conceiving may take an indeterminate period.
Therefore, the risks and benefits of conceiving while taking these agents must be carefully deliberated with the patient, preferably by a nephrologist.70,92,93,94,95 Enalapril and captopril are considered compatible with breastfeeding by the American Academy of Pediatrics, and may be restarted after delivery.96
Antenatal and postpartum
Blood pressure control using agents which are safe in pregnancy is crucial for the management of DN during pregnancy. Blood pressure-lowering therapy should be started if blood pressure consistently exceeds 135/85 mmHg. Blood pressure targets should not range lower than 120/80 mmHg, as lower targets may impair fetal growth.18 Prospective studies in pregnant women with T1DM treated to blood pressure <135/85 mmHg and urinary albumin excretion <300 mg/24 h showed a reduction in pre-eclampsia and preterm delivery.97,98 Safe and effective antihypertensive agents, which can be substituted for ACE inhibitors and angiotensin receptor blockers while actively planning or during pregnancy, include methyldopa, nifedipine, labetalol, diltiazem, prazosin, and clonidine.18,29
There is no guidance on the schedule of care for women with CKD, however, their renal function and obstetrics progress should be monitored regularly antenatally and in the postpartum period. One suggested approach is to monitor serum creatinine and urine albumin/creatinine ratio at least 4 weekly, and at least 2 weekly beginning at 32 weeks' gestation.99
Cardiovascular disease
Although ischemic heart disease is rarely encountered in pregnancy, it is a major cause of maternal morbidity and mortality.100 Women who have symptoms of, or documented ischemic heart disease should be referred to a cardiologist prior to withdrawal of contraception. Pregnancy is contraindicated in severe cardiac disease.101
Assessment for and management of co-existing medical conditions
Hypertension
Due to vasodilation in normal pregnancy, blood pressure decreases early in pregnancy, nadirs mid-gestation, and continues to rise until delivery. Previously, the early drop in diastolic blood pressure was reported to range between 10 and 15 mmHg. A recent study seemed to suggest a much less profound drop of between 0 and 2 mmHg from 12 weeks' gestation to nadir at around 19 weeks' gestation, with a subsequent increase of between 8 and 10 mmHg to delivery.102
Hypertensive disorders in pregnancy are common in women with diabetes, with an overall incidence of 45% in T1DM and 40% in T2DM. T1DM is more often associated with pre-eclampsia, while T2DM is more often associated with chronic hypertension.103
Most guidelines recommend a blood pressure target of <135/85 mmHg for women with pre-existing diabetes.21,30,54,104,105 Blood pressures lower than 120/80 mmHg should be avoided.
Hyperlipidemia
Women with pre-existing diabetes may have concomitant metabolic syndrome and dyslipidemia. Several hormonally mediated changes in lipid metabolism occur in pregnancy: very low-density lipoprotein cholesterol (VLDL) cholesterol, triglycerides and low-density lipoprotein cholesterol (LDL-C) increase, with peak levels occurring at term. High-density lipoprotein cholesterol (HDL-C) rises by mid-gestation and declines at term.106
Statins are not recommended for use during pregnancy. It is unlikely that withdrawing statins for the limited duration of conception, pregnancy, and breastfeeding will have significant long-term adverse health outcomes.18,29
Women who have significant hypertriglyceridemia should receive dietary counseling to achieve a low-fat diet with less than 20% of calories from fat. Omega-3 fatty acids (3–4 g per day) lower TG levels and prevent essential fatty acid deficiency, which may otherwise result from a fat-restricted diet.106 Routine use of fibrates and niacin during pregnancy is not recommended.29
Thyroid disorders
Autoimmune thyroid disease such as Hashimoto thyroiditis and Graves’ disease occur more frequently in women with T1DM. Women with T1DM desiring pregnancy should have their serum TSH measured before withdrawing contraception. Thyroid peroxidase antibodies (TPOAb) should be measured if not done previously. Women with T1DM are also more prone to postpartum thyroid dysfunction, with an incidence of 25%.107 They should be screened for postpartum thyroiditis with serum TSH measurements at 3 and 6 months postpartum.29
Weight management and nutrition
Obesity is common in patients with T2DM, and increasingly prevalent in patients with T1DM.108Being overweight and obese are independent risk factors for poor perinatal outcomes. Women with a body mass index (BMI) ≥27 kg/m2 desiring pregnancy should be given advice on weight loss prior to conception.81
ANTENATAL CARE
Antenatal care of pre-existing diabetes in pregnancy
Components of antenatal care for women with pre-existing diabetes in pregnancy
- Glycemic targets and monitoring
- Pharmacotherapy of diabetes in pregnancy
- Glycemic control
- Blood pressure control
- Aspirin for pre-eclampsia prevention
- Folic acid
- Lifestyle management and medical nutrition therapy
- Medical nutrition therapy
- Weight management
- Risk assessment/antenatal screening
- Timing and mode of delivery
- Management of diabetes-related complications in pregnancy
- Chronic complications – microvascular and macrovascular
- Acute complications – hypoglycemia, DKA
Glycemic targets and monitoring
Women are more insulin sensitive in the first trimester, but become increasingly insulin resistant in the second and third trimesters.109 Summarized data from 11 studies evaluating third-trimester glucose levels of non-diabetic pregnant women suggests that the average fasting glucose is 3.9 ± 0.4 mmol/l, 1-h postprandial glucose is 5.3–6.8 mmol/l and 2-h postprandial glucose is 4.9–6.1 mmol/l.110
Currently, there are no adequately powered randomized controlled trials evaluating the impact of different glycemic targets for diabetes in pregnancy. Targets recommended by various guidelines are summarized in Table 5. Most of these targets are extrapolated from studies evaluating normoglycemia in pregnancy, and pre-gestational and gestational DM.111,112,113,114
5
Summary of target glucose and HbA1c levels for pre-existing diabetes in pregnancy from international guidelines.
Guideline | Fasting/pre-prandial (mmol/l) | 1-h postprandial (mmol/l) | 2-h postprandial (mmol/l) | HbA1c |
The American College of Obstetricians and Gynecologists (ACOG) 201854 | <5.3 | <7.8 | <6.7 | <42, 6.0 |
American Diabetes Association (ADA) 202018 | <5.3 | <7.8 | <6.7 | <42, 6.0 |
The National Institute for Health and Care Excellence (NICE) 201525 | <5.3 | <7.8 | <6.4 | <48, 6.5 |
Australasian Diabetes in Pregnancy Society (ADIPS) (2005)115 | ≤5.5 | ≤8.0 | ≤7.0 | <42, 6.0 |
Diabetes Canada Clinical Practice Guidelines Expert Committee (2018)28 | <5.3 | <7.8 | <6.7 | <48, 6.5 (first and second trimester) <43, 6.1 (third trimester) |
Glycated hemoglobin (HbA1c)
In the non-pregnant state, glycated hemoglobin (HbA1c) reflects average blood glucose concentrations during the preceding 12 weeks.116 In pregnancy, HbA1c values tend to be lower due to increased red cell turnover.117 HbA1c may therefore be monitored more frequently during pregnancy, for example once every 4 weeks.118 HbA1c does not reveal the degree of glycemic variability, or postprandial hyperglycemia, which drives macrosomia.119 Therefore, self-monitoring of blood glucose is the primary method of glucose monitoring in pregnancy.18
Elevated first-trimester HbA1c is associated with poor outcomes such as stillbirth, spontaneous abortion, and congenital abnormalities (see Figure 2).24,120,121 Elevated third-trimester HbA1c is associated with macrosomia, preterm delivery, and pre-eclampsia.122,123 Most guidelines suggest a HbA1c target of <6.0–6.5%.21,30,54,104,105 While striving for these tight targets, avoidance of hypoglycemia remains important due to associated risks of low birth weight, in addition to the usual risks of hypoglycemia.119
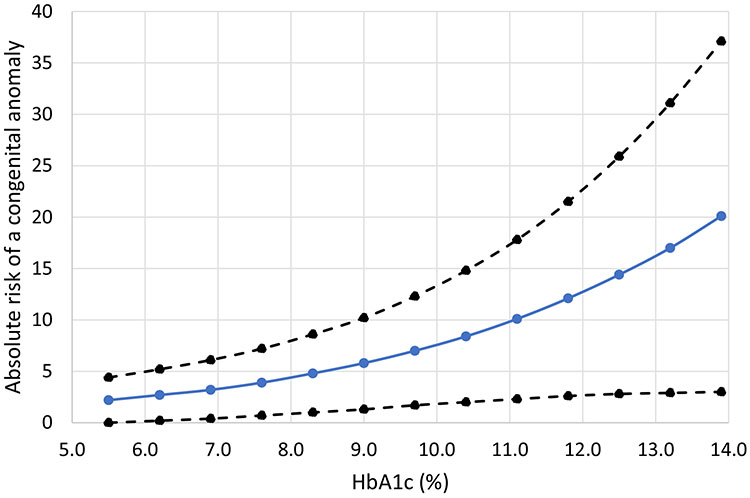
2
Absolute risk of a major or minor congenital anomaly according to periconceptional HbA1c. Data presented as absolute risk (solid line) and ±95% CIs (dashed lines). Adapted from Guerin A, Nisenbaum R, Ray JG. Use of maternal GHb concentration to estimate the risk of congenital anomalies in the offspring of women with pre-existing diabetes in pregnancy. Diabetes Care 2007;30:1920–1925.121
Capillary blood glucose monitoring
Frequent self-monitoring of blood glucose (SMBG) is required for intensification of DM treatment in pregnancy. Postprandial glucose is superior to preprandial glucose as a predictor of fetal macrosomia.112,123,124
SMBG should be performed preprandial, postprandial (1 or 2 h after the start of a meal), and at bedtime.21,30,54,104,115 The timing of postprandial glucose checks should ideally capture peak postprandial glucose levels. A study utilizing continuous glucose monitoring demonstrated that the average peak postprandial glucose level occurs 90 min after a meal, with significant variation between individuals.125 Multiple factors influence this, such as variable gastric emptying, meal timings, glycemic index, or macronutrient content of meals.126,127,128
Newer methods of blood glucose monitoring
The need for frequent fingerpricks for capillary blood glucose (CBG) monitoring is cumbersome and painful. Newer methods of glucose monitoring involve measurement of the interstitial fluid glucose by continuous blood glucose monitoring (CGM) and flash glucose monitoring systems.129 However, these technologies are more costly and not all have been validated for use in pregnancy.
CGM devices measure interstitial glucose at 5–10 s intervals, providing about 288 values per day.129 There are two types of CGM: real-time and retrospective CGM. Glucose sensors for CGM devices typically last for a week. At least two CBG readings per day are required for sensor calibration. CGM may identify glucose excursions, which go undetected by CBG testing.125,130,131
CONCEPTT (Continuous Glucose Monitoring in Pregnant Women with Type 1 Diabetes Trial) was a randomized controlled trial of real-time CGM in addition to CBG monitoring, versus CBG monitoring alone for pregnant women with T1DM. CGM use resulted in small improvements in HbA1c, greater time-in-target, fewer hypoglycemic episodes, and better neonatal outcomes.27 Patients benefit from CGM when it is used continuously rather than intermittently.132,133,134 In women with type 1 diabetes, higher mean glucose levels, higher glucose variability and less time in target range were associated with an increased risk of LGA and neonatal composite outcomes.135
The Abbott Freestyle Libre flash glucose monitoring system (flash-GMS) similarly measures interstitial fluid glucose. A reader or smartphone is used to scan a glucose sensor worn on the back of the arm. There is no need for calibration with CBG readings, and the sensor lasts for up to 14 days.136,137 Flash-GMS is not approved for use in pregnancy in most countries.138,139 One study compared the accuracy of flash-GMS to CBG monitoring and found the mean absolute difference to be acceptable at 11.8%.21,140 A more recent study revealed concerns regarding accuracy of flash-GMS in pregnancy, with readings tending to be lower than CBG readings, which may result in divergent treatment decisions.21 There are no available randomized controlled trials to validate the use of flash-GMS in pregnancy.
The ADA recommended that CGM should not be used as a replacement for CBG monitoring but rather as a supplementary modality of glycemic monitoring.18 The use of flash-GMS in pregnancy is not currently advocated by any guideline.
Adjuvant therapies for women with diabetes in pregnancy
Aspirin for pre-eclampsia prevention
Pre-eclampsia is a multisystem disorder of pregnancy, usually defined as hypertension and proteinuria diagnosed after 20 weeks' gestation. Women with pre-existing DM have an increased risk of pre-eclampsia, further increased in those with longer histories of DM and vascular complications.141,142
Low-dose aspirin (80–150 mg) reduces the risk of pre-eclampsia when initiated before 16 weeks' gestation and taken throughout pregnancy.143 Efficacy likely increases with increasing aspirin dosages, and when aspirin is taken at night.144,145,146 However, the optimal aspirin dose is unclear.
Lifestyle management and medical nutrition therapy
Medical nutrition therapy
Nutrition therapy is the cornerstone of DM management. All individuals with DM in pregnancy should be referred to a registered dietitian for individualized medical nutritional therapy. The goals are to provide enough nutrients for mother and fetus, achieve optimal weight gain, and maintain ideal glycemic control.
There is no optimal diet for diabetes in pregnancy. The Institute of Medicine (IOM) has suggested a rough macronutrient distribution for all populations: 40–60% carbohydrates, 30% protein, and 10% fats. Carbohydrate is the primary nutrient affecting maternal glucose levels. The minimum carbohydrate requirements increase in pregnancy from 130 g/day to 175 g/day.147
Carbohydrate requirements should be individualized based on weight-gain targets, insulin doses, glucose readings, and patient preference. Individuals should choose carbohydrates that have low glycemic index to improve postprandial glycemic control.148
Dietary calcium and iodine should be increased due to increased pregnancy requirements.
Weight management
Overweight or obese women who experience pregnancy weight gain greater than IOM recommendations have more adverse outcomes, including increased macrosomia, LGA, and higher rates of cesarean deliveries.149,150
Although not specific to women with pre-gestational diabetes, IOM guidelines recommend weight gain in pregnancy based on a woman’s pre-pregnancy weight or BMI (Table 6).
Weight loss is not recommended during pregnancy because of potential adverse fetal outcomes. All weight-loss efforts in women who are overweight or obese should take place prior to conception.
6
2009 Institute of Medicine Recommendations for weight gain during pregnancy.151
Pre-pregnancy BMI | Total weight gain | Rates of weight gain in second and third trimester | ||
Range (kg) | Range (lb) | Mean weight gain (range), kg/week | Mean weight gain (range), lb/week | |
Underweight (<18.5 kg/m2) | 12.5–18 | 28–40 | 0.51 (0.44–0.58) | 1 (1–1.3) |
Normal weight (18.5–24.9 kg/m2) | 11.5–16 | 25–35 | 0.42 (0.35–0.50) | 1 (0.8–1) |
Overweight (25.0–29.9 kg/m2) | 7–11.5 | 15–25 | 0.28 (0.23–0.33) | 0.6 (0.5–0.7) |
Obese (≥30.0 kg/m2) | 5–9 | 11–20 | 0.22 (0.17–0.27) | 0.5 (0.4–0.6) |
Diabetic ketoacidosis in pregnancy
Diabetic ketoacidosis (DKA) in pregnancy is associated with 4–15% maternal and 10–35% neonatal mortality.21,152,153 Due to constant transplacental transfer of glucose and nutrients, DKA in pregnancy may present atypically with euglycemic DKA.154
Physiological changes in pregnancy makes one more susceptible to DKA. Firstly, in pregnancy, fat breakdown, ketosis, and protein catabolism occur quicker. Secondly, there is reduced buffering capacity in view of increased alveolar minute ventilation, with resultant compensatory increase in renal excretion of bicarbonate. Lastly, the presence of diabetogenic hormones such as human placental lactogen, free cortisol, prolactin, and progesterone, results in increased insulin resistance.155 The usual precipitants for DKA are intercurrent illness, hyperemesis, non-compliance, and medications such as β2-agonists and steroids, which may be given for tocolysis and for fetal lung maturation.
Management of DKA in pregnancy is like that in non-pregnant individuals, involving rapid stabilization of maternal biochemistry with aggressive intravenous hydration, intravenous insulin infusion and close monitoring for fetal distress. All maternity units caring for women with diabetes should have protocols available for management of this rare but life-threatening complication. Admission to a high-dependency setting capable of monitoring both mother and fetus is essential. Non-reassuring fetal heart rate tracings are common, but resolve quickly with treatment of DKA.156 Therefore, emergency delivery should rarely be required.
There are no long-term studies on the outcomes of offspring born to mothers with higher levels of serum ketones. However, there are some concerns about neurocognitive and behavioral detrimental effects. One study analyzed children who were born to women who had high blood ketones and fatty acids during the third trimester and found that they had lower scores of behavioral and intellectual developments at age 2 and 5 years.157,158
Gestational diabetes
See chapter on screening and pathophysiology
Management of gestational diabetes (GDM) begins with glucose monitoring and modifications to diet and exercise. Pharmacologic treatment should be initiated when target glucose levels cannot be achieved through diet modifications and exercise. Recommended glucose targets for patients with gestational diabetes remain controversial and a range of targets have been recommended but never tested prospectively. These are summarized in Table 7.
7
Summary of target glucose and HbA1c levels for gestational diabetes from international guidelines.
Guideline | Fasting/Pre-prandial (mmol/l) | 1-h postprandial (mmol/l) | 2-h postprandial (mmol/l) | HbA1c |
The American College of Obstetricians and Gynecologists (ACOG) 201854 | <5.3 | <7.8 | <6.7 | NA |
American Diabetes Association (ADA) 202018 | <5.3 | <7.8 | <6.7 | NA |
The National Institute for Health and Care Excellence (NICE) 201525 | <5.3 | <7.8 | <6.4 | <48, 6.5 |
Australasian Diabetes in Pregnancy Society (ADIPS) (2014)159 | ≤5.0 | ≤7.4 | ≤6.7 | NA |
The various international groups vary considerably regarding recommended pharmacotherapy in gestational diabetes mellitus.53 Some international guidelines suggested insulin as first line pharmacotherapy should lifestyle modifications fail to achieve optimal glycemic control.18,104,160 Others recommended a trial of oral glucose lowering agents (e.g., metformin, glibenclamide), with subsequent addition of insulin therapy if oral glucose-lowering agents are insufficient to achieve satisfactory glycemia.29,30 These oral agents are known to cross the placenta, and fail to provide adequate glycemic control in a substantial proportion of women with GDM.161,162,163 In the MIG trial, up to 50% of women on metformin therapy required supplementary insulin later in pregnancy.164 Similarly, glibenclamide appeared to have a failure rate of 20%, with these women requiring insulin therapy.165
Compared to women with normoglycemic pregnancies, women with GDM have a higher risk of developing T2DM. There is heterogeneity in the relative risks quoted by various studies, which could be attributed by variables such as ethnicity, BMI, family history of DM, and age. A systemic analysis and meta-analysis by Bellamy et al. suggested an at least sevenfold increased risk of T2DM for women with GDM (RR 7.43, 95% CI 4.79–11.51).166 In view of this increased risk, all women with GDM should undergo a 75-g, 2-hour OGTT at 4–12 weeks' postpartum, and repeat testing every 1–3 years thereafter if this is normal.18
Risk assessment/antenatal screening
Pregnancies complicated by pre-existing or GDM should be managed as high-risk pregnancies. These women do not represent a homogenous group. The following categories are at particular risk:
- Long duration of pre-existing T1DM or T2DM
- Advanced maternal age
- Very young mothers and adolescents
- Obesity
- Women with DM complications
- Co-existing medical conditions (e.g., hypertension)
- T1DM with frequent hypoglycemia
- Women with a poor obstetric history
- Smokers
- Poor compliance and frequent failure to attend
All women with pre-existing DM should be offered a first-trimester scan for accurate dating. Diabetes adversely affects the screening performance of second-trimester maternal serum screening test for fetal chromosomal anomalies. Therefore, mothers with DM should commence antenatal care early, and undergo the first trimester combined screening test using fetal nuchal translucency measurements and maternal biochemistry.
Symptoms and signs of pre-eclampsia should be sought at every visit after 20 weeks given the higher risk of those with both GDM and pre-existing DM (Table 8). Those with pre-existing hypertension, nephropathy, and a previous history of pre-eclampsia are at particular risk.
8
Risk of pre-eclampsia.
Category | % of pregnancies affected by pre-eclampsia |
2–7% | |
10–30% | |
15–20% | |
10–14% |
For women with pre-existing DM, a detailed fetal anomaly scan at 18–20 weeks and a detailed fetal echocardiography at 20 weeks should be performed.30 Serial growth scans are performed thereafter in the third trimester. For both GDM and pre-existing DM, routine antenatal growth scans are performed at 28, 32, and 36 weeks to detect excessive fetal growth.25
Subsequent serial ultrasonography is targeted towards surveillance of fetal growth, specifically macrosomia and polyhydramnios. Biometry should be charted as abdominal circumference correlates with birth weight. In a study evaluating T1DM women, fetal abdominal circumference measurements exceeding the 90th percentile at 30–33 weeks' gestation has a 88% sensitivity and 83% specificity for the prediction of birth weight greater than the 90th centile.176 Ultrasound-guided management has been suggested with commencement of insulin in women with GDM if the fetal AC is >70th centile.165,177 A meta-analysis showed an increased need for insulin (ten women with GDM needing insulin to prevent one newborn with an abnormal birthweight).178 Conversely, another study had shown that a higher maternal glucose threshold to start insulin therapy may be permissible if the fetal abdominal circumference was <70th percentile, allowing more women to avoid insulin.179
Shoulder dystocia/perinatal trauma
Birth injuries and brachial plexus injuries are rarely associated with GDM, even if it is untreated. The results are discordant and the risk seems to be mainly associated with macrosomia.180
The NICE diabetes' guideline recommends that pregnant women with diabetes who have a normally grown fetus should be offered elective birth through induction of labor, or by elective cesarean section if indicated, after 38 completed weeks.25
Elective cesarean section should be considered to reduce the potential morbidity for pregnancies complicated by pre-existing or gestational diabetes, regardless of treatment, with an estimated fetal weight of greater than 4.5 kg.181
Studies involving the largest number of vaginal deliveries (34,800 to 267,228) report incidences of shoulder dystocia between 0.58% and 0.70%. Infants of diabetic mothers have a two- to fourfold increased risk of shoulder dystocia compared with infants of the same birth weight born to non-diabetic mothers.182
The incidence of brachial plexus injury is 1–2/1000 births. Most recover spontaneously or with physiotherapy.182 All birth units looking after diabetic pregnancies should be trained in the recognition and prompt team management of shoulder dystocia.
Timing and mode of delivery
The timing and mode of delivery of mothers with DM on insulin should be individualized. Where possible this should jointly involve the multidisciplinary team and the mother, taking into account maternal factors including glycemic control, the presence of other pregnancy complications, past obstetric history and fetal factors including size. Following the well-publicized Montgomery versus Lanarkshire case, which involved a severe case of shoulder dystocia in a diabetic mother resulting in significant permanent neonatal injury, the mother’s views and concerns, which are of material importance to her, should be taken on board in the decision making.183 Women should be advised about the risks of macrosomia with potential risks of difficult labor requiring emergency cesarean section, and risks of shoulder dystocia with risks of birth injuries.
Two arguments have been advanced for the delivery of an infant of a diabetic mother 1 or 2 weeks before term. One is the avoidance of unexpected fetal death. The second is the avoidance of fetal trauma associated with vaginal delivery of a large infant of a diabetic mother.
The timing of 38 completed weeks' gestation in the presence of good glycemic control is therefore a compromise between the competing risks of respiratory distress syndrome in the newborn delivered earlier against the risk of fetal demise if the pregnancy continues. Older studies suggested an increased still birth rate in women with GDM, but newer studies using contemporary screening and management approaches have not confirmed this.184 The absolute risk of stillbirth and infant death in GDM was found to be very equal or even lower than non-diabetic controls, possibly because of closer antenatal monitoring.185 NICE recommends delivery after 38 completed weeks in diabetes in pregnancy.25
However, if DM is poorly controlled or affects the fetus, it is not recommended that the pregnancy exceed 38 completed weeks' gestation, and consideration be given to earlier delivery on a case-by-case basis. One study randomized women with a suspected macrosomic fetus to induction of labor between 37 and 39 weeks, or expectant management up to 41 weeks. It demonstrated that IOL was associated with better rates of spontaneous vaginal births (RR 1.14, 95% Cl 1.01–1.29), reduced rates of shoulder dystocia (RR 0.32, 95% Cl 0.12–0.85).186
Generally, cesarean sections (CSs) should be recommended on the basis of obstetric indications. Where the timing has been decided, and in the absence of any indication for cesarean section, induction of labor would be performed with an aim towards achieving vaginal delivery.
Estimating the birth weight at which CSs should be recommended to prevent shoulder dystocia and perinatal trauma is debatable. The RCOG suggests that elective CSs should be considered to reduce the potential morbidity for pregnancies complicated by both pre-existing and gestational diabetes, regardless of treatment, when the estimated fetal weight exceeds 4.5 kg.182
MANAGEMENT DURING LABOR AND DELIVERY
Management of labor
Several considerations are necessary for a woman with diabetes prior to admission for delivery. Delivery is best performed in a facility which has a 24-h anesthesia service, a special care baby unit/Neonatal Intensive Care Unit, and staff who are trained in the care of women with DM and their infants.
Latent phase
The woman would be allowed a normal diet and encouraged to ambulate.
Active phase
For both the mother and the fetus, labor is a stress test: the mother has lower insulin requirements because of increased calorie consumption and reduced intake. The fetus must cope with the intermittent hypoxia produced by contractions and unpredictable glucose levels.
Fetal monitoring during labor
NICE suggests continuous electronic fetal monitoring in pregnancies with diabetes.25
Second/third stage of labor
The combination of fetal macrosomia, prolonged labor, and mid-cavity delivery carries a high risk of shoulder dystocia, especially if vacuum-assisted delivery is carried out.187 Any decision for instrumental delivery performed for a prolonged second stage needs careful consideration, and perhaps as a trial in theater, especially if the labor has been induced and the first stage prolonged.
Analgesia and anesthesia
The obstetric anesthetist is a vital member of the multidisciplinary team.188 Timely communication is important and the relevant considerations would include:
- If the diabetes is pre-existing or gestational
- The presence of co-existing diabetes' complications
- The degree of glycemic control
- The presence of obstetric complications (e.g., pre-eclampsia)
- Drugs administered
NICE suggests that women with diabetes and comorbidities such as obesity or autonomic neuropathy should be offered an anesthetic assessment in the third trimester.25 If general anesthesia is used, blood glucose should be monitored regularly (every 30 min) from induction of general anesthesia until after the baby is born and the woman is fully conscious. For intravenous pre-hydration before operative anesthesia, a balanced electrolyte solution (0.9% saline) is used rather than a dextrose solution to avoid a large glucose load, which reduces umbilical cord pH and can cause neonatal hypoglycemia.189,190
Management of preterm labor
Preterm delivery
Two studies have specifically analyzed the risk of spontaneous premature birth in gestational diabetic mothers.191,192 In a cohort study of more than 46,000 pregnancies (after exclusion of women with pre-gestational diabetes), the risk of spontaneous preterm delivery increased with the level of maternal blood glucose, independently of any perinatal complications associated with prematurity. The RR was 1.42 (1.15–1.77) for GDM pregnancies.193 In a study by Ostlund et al., premature delivery before 37 weeks was more common in women with GDM (11.4% versus 5.4%, p = 0.005) and was one of the main reasons for NICU admission. After adjustment for other prematurity-related risk factors (hypertension, pre-eclampsia, maternal BMI), hyperglycemia was a risk factor for premature birth <37 weeks (OR = 2.0 [1.0–3.9]).194
Tocolytics
The oxytocin receptor antagonist (Atosiban) and calcium channel blockers (Nifedipine) can be used without any specific precautions. However, beta2-agonists have an unfavorable risk: benefit ratio as they cause hyperglycemia.
Corticosteroids
Prenatal corticosteroids, when clearly indicated, should not be avoided solely because of their potential to cause hyperglycemia. Women in whom antenatal corticosteroids are indicated may require hospitalization for closer monitoring of their glucose profile in anticipation of increased insulin requirements. In one study of steroids given to mothers with T1DM, it was noted that the insulin requirement increased by 20% on day 1 and 4, and by 40% on day 2 and 3, with a return to pre-existing requirements by day 6 and 7.195
There is no benefit in the late administration of corticosteroids (i.e., delivery close to 37 weeks) to mothers with DM, in view of the risks of hyperglycemia.196
Peripartum glycemic control
The main goal of good peripartum glycemic control is to minimize the incidence of neonatal hypoglycemia, which is thought to be due to fetal hyperinsulinism induced by maternal hyperglycemia. The target peripartum blood glucose level is between 4 and 7 mmol/l.18,25,104 This can be achieved by means of intravenous regular insulin infusion and dextrose drip, with hourly capillary blood glucose monitoring and infusion rate adjustments.197,198
Women receiving insulin pump therapy can continue to use their insulin pump in labor, if they are physically capable of doing so. They usually require suspension of the basal insulin during active labor, and may resume their pre-pregnancy pump settings postpartum.18
POSTPARTUM CARE
Lactation
Women with plans to breastfeed should be educated about the benefits of breastfeeding for the baby, and for their own metabolic health.199 Medications that are safe for breastfeeding should be prescribed.
Pharmacotherapy
Breastfeeding lowers blood glucose levels. Women may require further insulin dose reductions, or a carbohydrate snack prior to breastfeeding to avoid hypoglycemia.
Women with T1DM may generally resume their pre-pregnancy insulin doses. Women with T2DM may continue metformin therapy, as it is excreted only minimally in breast milk. Glyburide and glipizide are undetectable in breast milk, and may also be used during breastfeeding.200 Limited information is available for other sulphonylureas, other oral glucose-lowering medications and GLP-1 receptor analogs, and their use is not recommended during lactation. If additional glucose-lowering is required, insulin may be added.
Contraception
Contraception is an often overlooked yet extremely crucial element of management. It should be discussed with the patient before she is discharged postnatally, particularly if the woman is at risk of non-attendance at the postnatal clinic, in which case postpartum contraception is desirable. Important considerations include the presence of diabetes' complications, breastfeeding, smoking, age, and plans for future fertility. Contraception is often necessary for effective pre-pregnancy care and optimization of glycemic control before a planned pregnancy. The WHO has issued excellent guidelines regarding contraceptive use.201
SUMMARY
While several challenges are present in the management of pregnancies with diabetes, the combined efforts of the multidisciplinary team and the woman living with diabetes can make the experience of pregnancy and motherhood a successful and joyous one.
PRACTICE RECOMMENDATIONS
- Periconception glycemic control is an independent predictor of congenital malformations and most international guidelines recommend a preconception HbA1c target of <48 mmol/l or <6.5%.
- Folic acid supplementation should be initiated at least 1 month prior to conception to reduce the risk of fetal neural-tube defects.
- Maternal insulin sensitivity changes over the course of pregnancy, peaking between 9 and 16 weeks' gestation, resulting in reduced insulin requirements and higher risk of hypoglycemia in insulin-treated women. Insulin resistance increases thereafter and plateaus by around 36 weeks' gestation.
- Oral glucose-lowering agents (OGLAs) such as glibenclamide and metformin can be considered for use during pregnancy, while other OGLAs should be stopped due to lack of evidence of their safety in pregnancy.
- Human insulins (NPH insulin, regular insulin) and insulin analogs (detemir, lispro, aspart) have safety and efficacy studies supporting their use in pregnancy.
- Self-monitoring of blood glucose is the primary method of glucose monitoring in pregnancy. HbA1c may not be reflective of glycemic variability and postprandial hyperglycemia that drives macrosomia.
- Low-dose aspirin (80–150 mg) should be started for pre-eclampsia prophylaxis in women with pre-gestational diabetes mellitus, prior to 16 weeks' gestation and taken throughout the pregnancy.
- Excessive weight gain during pregnancy should be avoided due to increased risk of adverse outcomes such as macrosomia, large for gestational age, and higher rates of cesarean deliveries.
- Detailed fetal anomaly and echocardiography scans at 18–20 weeks, and serial growth scans at 28, 32, and 36 weeks should be performed for women with pre-gestational diabetes mellitus.
- The timing and mode of delivery of women with diabetes mellitus in pregnancy should be individualized, taking into consideration presence of other pregnancy complications, glycemic control, past obstetrics history, and fetal factors such as size.
- A peripartum blood glucose target of between 4 and 7 mmol/l is recommended. This may be achieved by means of intravenous regular insulin infusion and dextrose drip, with hourly capillary blood glucose monitoring and infusion rate adjustments.
- In the postpartum period, insulin sensitivity quickly reverts to pre-pregnancy state, necessitating a significant reduction of insulin doses to minimize risk of hypoglycemia.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and Changes in Preexisting Diabetes and Gestational Diabetes Among Women Who Had a Live Birth – United States, 2012–2016. MMWR Morb Mortal Wkly Rep 2018;67(43):1201–7. | |
Coton SJ, Nazareth I, Petersen I. A cohort study of trends in the prevalence of pregestational diabetes in pregnancy recorded in UK general practice between 1995 and 2012. BMJ Open 2016;6(1):e009494. | |
Lapolla A, Metzger BE. (eds.) Gestational Diabetes. A Decade after the HAPO Study. Front Diabetes Basel, Karger, 2020;28:1–10 (DOI: 10.1159/000480161). doi: 10.1159/000480161. | |
Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Semin Fetal Neonatal Med 2009;14(2):66–71. | |
Lager S, Powell TL. Regulation of Nutrient Transport across the Placenta. J Pregnancy 2012;2012:1–14. | |
Freinkel N. Banting Lecture 1980: of Pregnancy and Progeny. Diabetes 1980;29(12):1023–35. | |
García-Patterson A, Gich I, Amini SB, et al. Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: three changes of direction. Diabetologia 2010;53(3):446–51. | |
Ryan EA. Hormones and insulin resistance during pregnancy. Lancet Lond Engl 2003;362(9398):1777–8. | |
Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol 2007;50(4):938–48. | |
Miodovnik M, Skillman C, Holroyde JC, et al. Elevated maternal glycohemoglobin in early pregnancy and spontaneous abortion among insulin-dependent diabetic women. Am J Obstet Gynecol 1985;153(4):439–42. | |
Temple R, Aldridge V, Greenwood R, et al. Association between outcome of pregnancy and glycemic control in early pregnancy in type 1 diabetes: population based study. BMJ 2002;325(7375):1275–6. | |
Balsells M, García-Patterson A, Gich I, et al. Major congenital malformations in women with gestational diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev 2012;28(3):252–7. | |
Yamamoto JM, Hughes DJF, Evans ML, et al. Community-based pre-pregnancy care programme improves pregnancy preparation in women with pregestational diabetes. Diabetologia 2018;61(7):1528–37. | |
Murphy HR, Roland JM, Skinner TC, et al. Effectiveness of a regional prepregnancy care program in women with type 1 and type 2 diabetes: benefits beyond glycemic control. Diabetes Care 2010;33(12):2514–20. | |
Elixhauser A, Weschler JM, Kitzmiller JL, et al. Cost-benefit analysis of preconception care for women with established diabetes mellitus. Diabetes Care 1993;16(8):1146–57. | |
Herman WH, Janz NK, Becker MP, et al. Diabetes and pregnancy. Preconception care, pregnancy outcomes, resource utilization and costs. J Reprod Med 1999;44(1):33–8. | |
Egan AM, Danyliv A, Carmody L, et al. A Prepregnancy Care Program for Women With Diabetes: Effective and Cost Saving. J Clin Endocrinol Metab 2016;101(4):1807–15. | |
Association AD. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes – 2020. Diabetes Care 2020;43(1):S183–92. | |
Bell R, Glinianaia SV, Tennant PWG, et al. Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: a population-based cohort study. Diabetologia 2012; | |
Jensen DM, Korsholm L, Ovesen P, et al. Peri-conceptional A1C and risk of serious adverse pregnancy outcome in 933 women with type 1 diabetes. Diabetes Care 2009;32(6):1046–8. | |
Gabbe SG, Mestman JH, Hibbard LT. Maternal mortality in diabetes mellitus: an 18-year survey. Obstet Gynecol 1976;(48):549–51. | |
Rosenn B, Miodovnik M, Combs CA, et al. Pre-conception management of insulin-dependent diabetes: improvement of pregnancy outcome. Obstet Gynecol 1991;77(6):846–9. | |
Mills JL, Baker L, Goldman AS. Malformations in infants of diabetic mothers occur before the seventh gestational week. Implications for treatment. Diabetes 1979;28(4):292–3. | |
Nielsen GL, Møller M, Sørensen HT. HbA1c in early diabetic pregnancy and pregnancy outcomes: a Danish population-based cohort study of 573 pregnancies in women with type 1 diabetes. Diabetes Care 2006;29(12):2612–6. | |
National Collaborating Centre for Women’s and Children’s Health. Diabetes in Pregnancy: Management of Diabetes and its Complications from Preconception to the Postnatal Period. February 2015. Available from: https://www.nice.org.uk/guidance/ng3. | |
National Clinical Guideline Centre (UK). Type 1 Diabetes in Adults: Diagnosis and Management [Internet]. London: National Institute for Health and Care Excellence (UK), 2015 [cited 2019 Jul 7]. (National Institute for Health and Care Excellence: Clinical Guidelines). Available from: http://www.ncbi.nlm.nih.gov/books/NBK315808/. | |
Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet Lond Engl 2017;390(10110):2347–59. | |
Diabetes Canada Clinical Practice Guidelines Expert Committee, Feig DS, Berger H, et al. Diabetes and Pregnancy. Can J Diabetes 2018;42 Suppl 1:S255–82. | |
Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2013;98(11):4227–49. | |
1 Recommendations | Diabetes in pregnancy: management from preconception to the postnatal period | Guidance | NICE [Internet]. [cited 2020 Jan 10]. Available from: https://www.nice.org.uk/guidance/ng3/chapter/1-Recommendations#antenatal-care-for-women-with-diabetes-2. | |
Evers IM, Braak EWMT ter, Valk HW de, et al. Risk Indicators Predictive for Severe Hypoglycemia During the First Trimester of Type 1 Diabetic Pregnancy. Diabetes Care 2002;25(3):554–9. | |
García-Patterson A, Gich I, Amini SB, et al. Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: three changes of direction. Diabetologia 2010;53(3):446–51. | |
Lambert K, Holt RIG. The use of insulin analogues in pregnancy. Diabetes Obes Metab 2013;15(10):888–900. | |
Toledano Y, Hadar E, Hod M. Pharmacotherapy for hyperglycemia in pregnancy – The new insulins. Diabetes Res Clin Pract 2018;145:59–66. | |
Blum AK. Insulin Use in Pregnancy: An Update. Diabetes Spectr 2016;29(2):92–7. | |
Hirsch IB. Insulin analogues. N Engl J Med 2005;352(2):174–83. | |
Combs CA, Gunderson E, Kitzmiller JL, et al. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care 1992;15(10):1251–7. | |
Durnwald CP, Landon MB. A comparison of lispro and regular insulin for the management of type 1 and type 2 diabetes in pregnancy. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet 2008;21(5):309–13. | |
Di Cianni G, Volpe L, Ghio A, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes mellitus treated with lispro or aspart insulin: comparison with regular insulin. Diabetes Care 2007;30(4):e11. | |
Heller S, Damm P, Mersebach H, et al. Hypoglycemia in type 1 diabetic pregnancy: role of preconception insulin aspart treatment in a randomized study. Diabetes Care 2010;33(3):473–7. | |
Hod M, Damm P, Kaaja R, et al. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol 2008;198(2):186.e1–7. | |
Mathiesen ER, Kinsley B, Amiel SA, et al. Maternal glycemic control and hypoglycemia in type 1 diabetic pregnancy: a randomized trial of insulin aspart versus human insulin in 322 pregnant women. Diabetes Care 2007;30(4):771–6. | |
Mathiesen ER, Hod M, Ivanisevic M, et al. Maternal efficacy and safety outcomes in a randomized, controlled trial comparing insulin detemir with NPH insulin in 310 pregnant women with type 1 diabetes. Diabetes Care 2012;35(10):2012–7. | |
Mathiesen ER, Damm P, Jovanovic L, et al. Basal insulin analogues in diabetic pregnancy: a literature review and baseline results of a randomised, controlled trial in type 1 diabetes. Diabetes Metab Res Rev 2011;27(6):543–51. | |
Kurtzhals P, Schäffer L, Sørensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000;49(6):999–1005. | |
Pöyhönen-Alho M, Rönnemaa T, Saltevo J, et al. Use of insulin glargine during pregnancy. Acta Obstet Gynecol Scand 2007;86(10):1171–4. | |
Pantalone KM, Faiman C, Olansky L. Insulin glargine use during pregnancy. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol 2011;17(3):448–55. | |
Lepercq J, Lin J, Hall GC, et al. Meta-Analysis of Maternal and Neonatal Outcomes Associated with the Use of Insulin Glargine versus NPH Insulin during Pregnancy. Obstet Gynecol Int 2012;2012:649070. | |
TOUJEO (insulin glargine injection) U-300 [prescribing information]. Bridgewater, NJ: Sanofi-Aventis, 02/2015. | |
Keller MF, Vestgaard M, Damm P, et al. Treatment with the long-acting insulin analog degludec during pregnancy in women with type 1 diabetes: An observational study of 22 cases. Diabetes Res Clin Pract 2019;152:58–64. | |
Bonora BM, Avogaro A, Fadini GP. Exposure to insulin degludec during pregnancy: report of a small series and review of the literature. J Endocrinol Invest 2019;42(3):345–9. | |
FIASP® (insulin aspart injection) [prescribing information]. Plainsboro, NJ: Novo Nordisk Inc., 09/2017. | |
Zhang M, Zhou Y, Zhong J, et al. Current guidelines on the management of gestational diabetes mellitus: a content analysis and appraisal. BMC Pregnancy Childbirth 2019;19(1):200. | |
ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol 2018;131(2):e49. | |
Guidelines_on_GDM_updated.pdf [Internet]. [cited 2020 Oct 10]. Available from: http://www.hkcog.org.hk/hkcog/Download/Guidelines_on_GDM_updated.pdf. | |
SMFM Statement: Pharmacological treatment of gestational diabetes. Am J Obstet Gynecol 2018;218(5):B2–4. | |
Hickman MA, McBride R, Boggess KA, et al. Metformin Compared with Insulin in the Treatment of Pregnant Women with Overt Diabetes: A Randomized Controlled Trial. Am J Perinatol 2013;30(06):483–90. | |
Ibrahim MI, Hamdy A, Shafik A, et al. The role of adding metformin in insulin-resistant diabetic pregnant women: a randomized controlled trial. Arch Gynecol Obstet 2014;289(5):959–65. | |
Priya G, Kalra S. Metformin in the management of diabetes during pregnancy and lactation. Drugs Context [Internet]. 2018 Jun 15 [cited 2020 Oct 10];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6012930/. | |
Pharmacokinetics of metformin during pregnancy – PubMed [Internet]. [cited 2020 Oct 10]. Available from: https://pubmed-ncbi-nlm-nih-gov.libproxy1.nus.edu.sg/20118196/. | |
Charles B, Norris R, Xiao X, et al. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006;28(1):67–72. | |
Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metformin: a meta-analysis. Fertil Steril 2006;86(3):658–63. | |
Cassina M, Donà M, Di Gianantonio E, et al. First-trimester exposure to metformin and risk of birth defects: a systematic review and meta-analysis. Hum Reprod Update 2014;20(5):656–69. | |
Elmaraezy A, Abushouk AI, Emara A, et al. Effect of metformin on maternal and neonatal outcomes in pregnant obese non-diabetic women: A meta-analysis. Int J Reprod Biomed Yazd Iran 2017;15(8):461–70. | |
Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diabetes Res Care 2018;6(1):e000456. | |
MiTy Kids (Metformin in Women With Type 2 Diabetes in Pregnancy Kids Trial) – Full Text View – ClinicalTrials.gov [Internet]. [cited 2020 Oct 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT01832181. | |
Chiswick C, Reynolds RM, Denison F, et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2015;3(10):778–86. | |
Syngelaki A, Nicolaides KH, Balani J, et al. Metformin versus Placebo in Obese Pregnant Women without Diabetes Mellitus. N Engl J Med 2016;374(5):434–43. | |
Bullo M, Tschumi S, Bucher BS, et al. Pregnancy Outcome Following Exposure to Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Antagonists: A Systematic Review. Hypertension 2012;60(2):444–50. | |
Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major Congenital Malformations after First-Trimester Exposure to ACE Inhibitors. N Engl J Med 2006;354(23):2443–51. | |
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA 2017;317(2):183. | |
Wilson RD, GENETICS COMMITTEE, MOTHERISK. Pre-conceptional vitamin/folic acid supplementation 2007: the use of folic acid in combination with a multivitamin supplement for the prevention of neural tube defects and other congenital anomalies. J Obstet Gynaecol Can JOGC J Obstet Gynecol Can JOGC 2007;29(12):1003–13. | |
Cavalli P. Prevention of Neural Tube Defects and proper folate periconceptional supplementation. J Prenat Med 2008;2(4):40–1. | |
Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy. The Diabetes in Early Pregnancy Study. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care 1995;18(5):631–7. | |
Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care 2000;23(8):1084–91. | |
Vestgaard M, Ringholm L, Laugesen CS, et al. Pregnancy-induced sight-threatening diabetic retinopathy in women with Type 1 diabetes. Diabet Med J Br Diabet Assoc 2010;27(4):431–5. | |
Rasmussen KL, Laugesen CS, Ringholm L, et al. Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia 2010;53(6):1076–83. | |
Moloney JB, Drury MI. The effect of pregnancy on the natural course of diabetic retinopathy. Am J Ophthalmol 1982;93(6):745–56. | |
Axer-Siegel R, Hod M, Fink-Cohen S, et al. Diabetic retinopathy during pregnancy. Ophthalmology 1996;103(11):1815–9. | |
Chantelau E, Kohner EM. Why some cases of retinopathy worsen when diabetic control improves. BMJ 1997;315(7116):1105–6. | |
National Collaborating Centre for Women’s and Children’s Health (UK). Diabetes in Pregnancy: Management of Diabetes and Its Complications from Preconception to the Postnatal Period [Internet]. London: National Institute for Health and Care Excellence (UK), 2015 [cited 2019 Jul 7]. (National Institute for Health and Care Excellence: Clinical Guidelines). Available from: http://www.ncbi.nlm.nih.gov/books/NBK293625/. | |
Morrison JL, Hodgson LA, Lim LL, et al. Diabetic retinopathy in pregnancy: a review. Clin Experiment Ophthalmol 2016;44(4):321–34. | |
Polizzi S, Mahajan VB. Intravitreal Anti-VEGF Injections in Pregnancy: Case Series and Review of Literature. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther 2015;31(10):605–10. | |
American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes – 2019. Diabetes Care 2019;42(1):S124–38. | |
Smith MC, Moran P, Ward MK, et al. Assessment of glomerular filtration rate during pregnancy using the MDRD formula. BJOG Int J Obstet Gynaecol 2008;115(1):109–12. | |
Koetje PM, Spaan JJ, Kooman JP, et al. Pregnancy reduces the accuracy of the estimated glomerular filtration rate based on Cockroft-Gault and MDRD formulas. Reprod Sci 2011;18:456–62. | |
Klemetti MM, Laivuori H, Tikkanen M, et al. Obstetric and perinatal outcome in type 1 diabetes patients with diabetic nephropathy during 1988–2011. Diabetologia 2015;58(4):678–86. | |
Purdy LP, Hantsch CE, Molitch ME, et al. Effect of pregnancy on renal function in patients with moderate-to-severe diabetic renal insufficiency. Diabetes Care 1996;19(10):1067–74. | |
Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993;329(20):1456–62. | |
Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345(12):861–9. | |
Podymow T, Joseph G. Preconception and pregnancy management of women with diabetic nephropathy on angiotensin converting enzyme inhibitors. Clin Nephrol 2015;83(2):73–9. | |
Walfisch A, Al-maawali A, Moretti ME, et al. Teratogenicity of angiotensin converting enzyme inhibitors or receptor blockers. J Obstet Gynaecol J Inst Obstet Gynaecol 2011;31(6):465–72. | |
Wiles K, Chappell L, Clark K, et al. Clinical practice guideline on pregnancy and renal disease. BMC Nephrol 2019;20(1):401. | |
Porta M, Hainer JW, Jansson S-O, et al. Exposure to candesartan during the first trimester of pregnancy in type 1 diabetes: experience from the placebo-controlled DIabetic REtinopathy Candesartan Trials. Diabetologia 2011;54(6):1298–303. | |
Li D-K, Yang C, Andrade S, et al. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ 2011;343:d5931. | |
American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics 2001;108(3):776–89. | |
Nielsen LR, Damm P, Mathiesen ER. Improved pregnancy outcome in type 1 diabetic women with microalbuminuria or diabetic nephropathy: effect of intensified antihypertensive therapy? Diabetes Care 2009;32(1):38–44. | |
Nielsen LR, Müller C, Damm P, Mathiesen ER. Reduced prevalence of early preterm delivery in women with Type 1 diabetes and microalbuminuria–possible effect of early antihypertensive treatment during pregnancy. Diabet Med J Br Diabet Assoc 2006;23(4):426–31. | |
Bramham K. Diabetic Nephropathy and Pregnancy. Semin Nephrol 2017;37(4):362–9. | |
Pollard K. MBRRACE-UK Report launch December 2016. Pract Midwife 2017;20(2):30–2. | |
Elkayam U, Goland S, Pieper PG, et al. High-Risk Cardiac Disease in Pregnancy: Part I. J Am Coll Cardiol 2016;68(4):396–410. | |
Green LJ, Mackillop LH, Salvi D, et al. Gestation-Specific Vital Sign Reference Ranges in Pregnancy. Obstet Gynecol 2020;135(3):653–664. | |
Cundy T, Slee F, Gamble G, et al. Hypertensive disorders of pregnancy in women with Type 1 and Type 2 diabetes. Diabet Med J Br Diabet Assoc 2002;19(6):482–9. | |
Diabetes Canada Clinical Practice Guidelines Expert Committee, Feig DS, Berger H, et al. Diabetes and Pregnancy. Can J Diabetes 2018;42 Suppl 1:S255–82. | |
Overview | Hypertension in pregnancy: diagnosis and management | Guidance | NICE [Internet]. [cited 2020 Jan 10]. Available from: https://www.nice.org.uk/guidance/ng133. | |
Goldberg AS, Hegele RA. Severe hypertriglyceridemia in pregnancy. J Clin Endocrinol Metab 2012;97(8):2589–96. | |
Alvarez-Marfany M, Roman SH, Drexler AJ, et al. Long-term prospective study of postpartum thyroid dysfunction in women with insulin dependent diabetes mellitus. J Clin Endocrinol Metab 1994;79(1):10–6. | |
Corbin KD, Driscoll KA, Pratley RE, et al. Obesity in Type 1 Diabetes: Pathophysiology, Clinical Impact, and Mechanisms. Endocr Rev 2018;39(5):629–63. | |
Pregnancy and Insulin Resistance | Metabolic Syndrome and Related Disorders [Internet]. [cited 2020 Jan 10]. Available from: https://www.liebertpub.com/doi/10.1089/met.2006.4.149. | |
Hernandez TL, Friedman JE, Van Pelt RE, et al. Patterns of Glycemia in Normal Pregnancy. Diabetes Care 2011;34(7):1660–8. | |
Willman SP, Leveno KJ, Guzick DS, et al. Glucose threshold for macrosomia in pregnancy complicated by diabetes. Am J Obstet Gynecol 1986;154(2):470–5. | |
Veciana MD, Toohey JS. Postprandial versus Preprandial Blood Glucose Monitoring in Women with Gestational Diabetes Mellitus Requiring Insulin Therapy. N Engl J Med 1995;333(19):5. | |
Crowther CA, McPhee AJ. Effect of Treatment of Gestational Diabetes Mellitus on Pregnancy Outcomes. N Engl J Med 2005;10. | |
Landon MB, Carpenter MW, Wapner RJ, et al. A Multicenter, Randomized Trial of Treatment for Mild Gestational Diabetes. N Engl J Med 2009;10. | |
adips_pregdm_guidelines.pdf [Internet]. [cited 2020 Jan 10]. Available from: http://www.adips.org/downloads/adips_pregdm_guidelines.pdf. | |
Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes 2009;1(1):9–17. | |
Nielsen LR, Ekbom P, Damm P, et al. HbA1c Levels Are Significantly Lower in Early and Late Pregnancy. Diabetes Care 2004;27(5):1200–1. | |
Jovanovi? L, Savas H, Mehta M, et al. Frequent Monitoring of A1C During Pregnancy as a Treatment Tool to Guide Therapy. Diabetes Care 2011;34(1):53–4. | |
Combs CA, Gunderson E, Kitzmiller JL, et al. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care 1992;15(10):1251–7. | |
Suhonen L, Hiilesmaa V, Teramo K. Glycaemic control during early pregnancy and fetal malformations in women with type I diabetes mellitus. Diabetologia 2000;43(1):79–82. | |
Guerin A, Nisenbaum R, Ray JG. Use of Maternal GHb Concentration to Estimate the Risk of Congenital Anomalies in the Offspring of Women with Prepregnancy Diabetes. Diabetes Care 2007;30(7):1920–5. | |
Maresh MJA, Holmes VA, Patterson CC, et al. Glycemic Targets in the Second and Third Trimester of Pregnancy for Women With Type 1 Diabetes. Diabetes Care 2015;38(1):34–42. | |
Jovanovic-Peterson L, Peterson CM, Reed GF, et al. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development–Diabetes in Early Pregnancy Study. Am J Obstet Gynecol 1991;164(1 Pt 1):103–11. | |
Manderson JG, Patterson CC, Hadden DR, et al. Preprandial versus postprandial blood glucose monitoring in type 1 diabetic pregnancy: a randomized controlled clinical trial. Am J Obstet Gynecol 2003;189(2):507–12. | |
Ben-Haroush A, Yogev Y, Chen R, et al. The postprandial glucose profile in the diabetic pregnancy. Am J Obstet Gynecol 2004;191(2):576–81. | |
Kerssen A, de Valk HW, Visser GHA. Day-to-day glucose variability during pregnancy in women with Type 1 diabetes mellitus: glucose profiles measured with the Continuous Glucose Monitoring System. BJOG Int J Obstet Gynaecol 2004;111(9):919–24. | |
Sivan E, Weisz B, Homko CJ, et al. One or two hours postprandial glucose measurements: are they the same? Am J Obstet Gynecol 2001;185(3):604–7. | |
Stanley K, Magides A, Arnot M, et al. Delayed gastric emptying as a factor in delayed postprandial glycaemic response in pregnancy. Br J Obstet Gynaecol 1995;102(4):288–91. | |
Hewapathirana NM, O’Sullivan E, Murphy HR. Role of continuous glucose monitoring in the management of diabetic pregnancy. Curr Diab Rep 2013;13(1):34–42. | |
Kerssen A, de Valk HW, Visser GHA. The Continuous Glucose Monitoring System during pregnancy of women with type 1 diabetes mellitus: accuracy assessment. Diabetes Technol Ther 2004;6(5):645–51. | |
Yogev Y, Ben-Haroush A, Chen R, et al. Continuous glucose monitoring for treatment adjustment in diabetic pregnancies–a pilot study. Diabet Med J Br Diabet Assoc 2003;20(7):558–62. | |
Secher AL, Ringholm L, Andersen HU, et al. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care 2013;36(7):1877–83. | |
Polsky S, Garcetti R. CGM, Pregnancy, and Remote Monitoring. Diabetes Technol Ther 2017;19(S3):S49–59. | |
Voormolen DN, DeVries JH, Sanson RME, et al. Continuous glucose monitoring during diabetic pregnancy (GlucoMOMS): A multicentre randomized controlled trial. Diabetes Obes Metab 2018;20(8):1894–902. | |
Kristensen K, Ögge LE, Sengpiel V, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia 2019;62(7):1143–53. | |
Bidonde J, Fagerlund BC, Frønsdal KB, et al. FreeStyle Libre Flash Glucose Self-Monitoring System: A Single-Technology Assessment [Internet]. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH), 2017 [cited 2020 Jan 13]. (NIPH Systematic Reviews). Available from: http://www.ncbi.nlm.nih.gov/books/NBK482068/. | |
Leelarathna L, Wilmot EG. Flash forward: a review of flash glucose monitoring. Diabet Med J Br Diabet Assoc 2018;35(4):472–82. | |
Snapshot [Internet]. [cited 2020 Jan 13]. Available from: https://www.freestylelibre.co.uk/libre/discover/diabetes-management-pregnancy.html. | |
Health C for D and R. Freestyle Libre 14 Day Flash Glucose Monitoring System – P160030/S017. FDA [Internet]. 2019 Mar 29 [cited 2020 Jan 13]; Available from: http://www.fda.gov/medical-devices/recently-approved-devices/freestyle-libre-14-day-flash-glucose-monitoring-system-p160030s017. | |
Gatti M, Amato AML, Bruttomesso D. FreeStyle Libre flash glucose monitoring system in pregnant woman with type 1 diabetes: a focus on accuracy. Acta Diabetol 2019;56(8):969–70. | |
Bennett SN, Tita A, Owen J, et al. Assessing White’s classification of pregestational diabetes in a contemporary diabetic population. Obstet Gynecol 2015;125(5):1217–23. | |
Sibai BM, Caritis S, Hauth J, et al. Risks of preeclampsia and adverse neonatal outcomes among women with pregestational diabetes mellitus. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol 2000;182(2):364–9. | |
Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol 2018;218(3):287–293.e1. | |
Roberge S, Nicolaides K, Demers S, et al. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol 2017;216(2):110–120.e6. | |
Atallah A, Lecarpentier E, Goffinet F, et al. Aspirin for Prevention of Preeclampsia. Drugs 2017;77(17):1819–31. | |
Tong S, Mol BW, Walker SP. Preventing preeclampsia with aspirin: does dose or timing matter? Am J Obstet Gynecol 2017;216(2):95–7. | |
Dietary Reference Intakes Tables and Application?: Health and Medicine Division [Internet]. [cited 2020 Jan 14]. Available from: http://nationalacademies.org/hmd/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx. | |
Louie JCY, Brand-Miller JC, Moses RG. Carbohydrates, Glycemic Index, and Pregnancy Outcomes in Gestational Diabetes. Curr Diab Rep 2013;13(1):6–11. | |
Yee LM, Cheng YW, Inturrisi M, et al. Effect of gestational weight gain on perinatal outcomes in women with type 2 diabetes mellitus using the 2009 Institute of Medicine guidelines. Am J Obstet Gynecol 2011;205(3):257.e1–6. | |
Harper LM, Shanks AL, Odibo AO, et al. Gestational weight gain in insulin-resistant pregnancies. J Perinatol Off J Calif Perinat Assoc 2013;33(12):929–33. | |
Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines [Internet]. Rasmussen KM, Yaktine AL. (eds.) Washington (DC): National Academies Press (US), 2009 [cited 2019 Jul 2]. (The National Academies Collection: Reports funded by National Institutes of Health). Available from: http://www.ncbi.nlm.nih.gov/books/NBK32813/. | |
Montoro MN, Myers VP, Mestman JH, et al. Outcome of pregnancy in diabetic ketoacidosis. Am J Perinatol 1993;10(1):17–20. | |
Rougerie M, Czuzoj-Shulman N, Abenhaim HA. Diabetic ketoacidosis among pregnant and non-pregnant women: a comparison of morbidity and mortality. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet 2019;32(16):2649–52. | |
Jaber JF, Standley M, Reddy R. Euglycemic Diabetic Ketoacidosis in Pregnancy: A Case Report and Review of Current Literature. Case Rep Crit Care 2019;2019:8769714. | |
Kamalakannan D, Baskar V, Barton DM, et al. M. Diabetic ketoacidosis in pregnancy. Postgrad Med J 2003;79(934):454–7. | |
Ng YHG, Ee TX, Kanagalingam D, et al. Resolution of severe fetal distress following treatment of maternal diabetic ketoacidosis. BMJ Case Rep 2018;2018. | |
Rizzo T, Metzger BE, Burns WJ, et al. Correlations between antepartum maternal metabolism and intelligence of offspring. N Engl J Med 1991 26;325(13):911–6. | |
Bronisz A, Ozorowski M, Hagner-Derengowska M. Pregnancy Ketonemia and Development of the Fetal Central Nervous System [Internet]. International Journal of Endocrinology 2018 [cited 2020 Jan 14]. Available from: https://www.hindawi.com/journals/ije/2018/1242901/. | |
2014ADIPSGDMGuidelinesV18.11.2014_000.pdf [Internet]. [cited 2020 Jan 10]. Available from: https://www.adips.org/downloads/2014ADIPSGDMGuidelinesV18.11.2014_000.pdf. | |
Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynecol Obstet 2015;131:S173–211. | |
Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009;85(6):607–14. | |
Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol 2016;12(6):691–9. | |
Gante I, Melo L, Dores J, et al. Metformin in gestational diabetes mellitus: predictors of poor response. Eur J Endocrinol 2018;178(1):129–35. | |
Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 2008;358(19):2003–15. | |
Kjos SL, Schaefer-Graf UM. Modified Therapy for Gestational Diabetes Using High-Risk and Low-Risk Fetal Abdominal Circumference Growth to Select Strict Versus Relaxed Maternal Glycemic Targets. Diabetes Care 2007;30(2):S200–5. | |
Bellamy L, Casas J-P, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet Lond Engl 2009;373(9677):1773–9. | |
Knuist M, Bonsel GJ, Zondervan HA, et al. Intensification of fetal and maternal surveillance in pregnant women with hypertensive disorders. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 1998;61(2):127–33. | |
Hauth JC, Ewell MG, Levine RJ, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol 2000;95(1):24–8. | |
Weissgerber TL, Mudd LM. Preeclampsia and Diabetes. Curr Diab Rep 2015;15(3):9. | |
Yogev Y, Xenakis EMJ, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol 2004;191(5):1655–60. | |
Montoro MN, Kjos SL, Chandler M, et al. Insulin Resistance and Preeclampsia in Gestational Diabetes Mellitus. Diabetes Care 2005;28(8):1995–2000. | |
Jensen DM, Damm P, Moelsted-Pedersen L, et al. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care 2004;27(12):2819–23. | |
Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care 2009;32(11):2005–9. | |
Knight KM, Thornburg LL, Pressman EK. Pregnancy outcomes in type 2 diabetic patients as compared with type 1 diabetic patients and nondiabetic controls. J Reprod Med 2012;57(9–10):397–404. | |
Groen B, Links TP, van den Berg PP, et al. Similar adverse pregnancy outcome in native and nonnative dutch women with pregestational type 2 diabetes: a multicentre retrospective study. ISRN Obstet Gynecol 2013;2013:361435. | |
Leinonen PJ, Hiilesmaa VK, Kaaja RJ, et al. Maternal mortality in type 1 diabetes. Diabetes Care 2001;24(8):1501–2. | |
Buchanan TA, Kjos SL, Montoro MN, et al. Use of Fetal Ultrasound to Select Metabolic Therapy for Pregnancies Complicated by Mild Gestational Diabetes. Diabetes Care 1994;17(4):275–83. | |
Balsells M, García-Patterson A, Gich I, et al. Ultrasound-guided compared to conventional treatment in gestational diabetes leads to improved birthweight but more insulin treatment: systematic review and meta-analysis. Acta Obstet Gynecol Scand 2014;93(2):144–51. | |
Kjos SL, Schaefer-Graf U, Sardesi S, et al. A Randomized Controlled Trial Using Glycemic Plus Fetal Ultrasound Parameters Versus Glycemic Parameters to Determine Insulin Therapy in Gestational Diabetes With Fasting Hyperglycemia. Diabetes Care 2001;24(11):1904–10. | |
Mitanchez D. Foetal and neonatal complications in gestational diabetes: perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab 2010;36(6 Pt 2):617–27. | |
Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102(4):850–6. | |
RCOG Greentop Guideline No. 42. | |
Montgomery v Lanarkshire Health Board: SC 11; [2015] UKSC 11, 2015 GWD 10–179, [2015] Med LR 149, 2015 SCLR 315, (2015) 143 BMLR 47, 2015 SLT 189, [2015] 2 WLR 768, [2015] 1 AC 1430, [2015] 2 All ER 1031, [2015] WLR(D) 123, [2015] PIQR P13, UKSC 2013/0136, 2015 SC (UKSC) 63. | |
Rosenstein MG, Cheng YW, Snowden JM, et al. The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes. Am J Obstet Gynecol 2012;206(4):309.e1–309.e7. | |
Feig DS, Hwee J, Shah BR, et al. Trends in Incidence of Diabetes in Pregnancy and Serious Perinatal Outcomes: A Large, Population-Based Study in Ontario, Canada, 1996–2010. Diabetes Care 2014;37(6):1590–6. | |
Boulvain M, Senat M-V, Perrotin F, et al. Induction of labour versus expectant management for large-for-date fetuses: a randomised controlled trial. Lancet Lond Engl 2015;385(9987):2600–5. | |
Mollberg M, Hagberg H, Bager B, et al. Risk factors for obstetric brachial plexus palsy among neonates delivered by vacuum extraction. Obstet Gynecol 2005;106(5 Pt 1):913–8. | |
Robertshaw HJ, McAnulty GR, Hall GH. Strategies for managing the diabetic patient. Best Pract Res Clin Anaesthesiol 2004;18(4):631–43. | |
Grylack LJ, Chu SS, Scanlon JW. Use of intravenous fluids before cesarean section: effects on perinatal glucose, insulin, and sodium homeostasis. Obstet Gynecol 1984;63(5):654–8. | |
Kenepp NB, Kumar S, Shelley WC, et al. Fetal and neonatal hazards of maternal hydration with 5% dextrose before caesarean section. Lancet Lond Engl 1982;1(8282):1150–2. | |
Farrell T, Neale L, Cundy T. Congenital anomalies in the offspring of women with type 1, type 2 and gestational diabetes. Diabet Med J Br Diabet Assoc 2002;19(4):322–6. | |
Donnelly V, Foran A, Murphy J, et al. Neonatal brachial plexus palsy: an unpredictable injury. Am J Obstet Gynecol 2002;187(5):1209–12. | |
Yogev Y, Langer O. Spontaneous preterm delivery and gestational diabetes: the impact of glycemic control. Arch Gynecol Obstet 2007;276(4):361–5. | |
Ostlund I, Hanson U, Björklund A, et al. Maternal and fetal outcomes if gestational impaired glucose tolerance is not treated. Diabetes Care 2003;26(7):2107–11. | |
Mathiesen ER, Christensen A-BL, Hellmuth E, et al. Insulin dose during glucocorticoid treatment for fetal lung maturation in diabetic pregnancy: test of an algorithm [correction of analgoritm]. Acta Obstet Gynecol Scand 2002;81(9):835–9. | |
Thiebaugeorges O, Guyard-Boileau B. Obstetrical care in gestational diabetes and management of preterm labour. Diabetes Metab 2010;36(6 Pt 2):672–81. | |
Jovanovic L, Peterson CM. Insulin and glucose requirements during the first stage of labor in insulin-dependent diabetic women. Am J Med 1983;75(4):607–12. | |
Golde SH, Good-Anderson B, Montoro M, et al. Insulin requirements during labor: a reappraisal. Am J Obstet Gynecol 1982;144(5):556–9. | |
Schwarz EB, Nothnagle M. The Maternal Health Benefits of Breastfeeding. Am Fam Physician 2015;91(9):602–4. | |
Anderson PO. Treating Diabetes During Breastfeeding. Breastfeed Med 2018;13(4):237–9. | |
WHO, Department of Reproductive Health and Research. Medical Eligibility Criteria for Contraceptive Use, 5th edn. (2015). |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)
