Benign, Proliferative Noninvasive (Borderline), and Invasive Epithelial Tumors of the Ovary
Authors
INTRODUCTION
The approximate distribution of ovarian epithelial tumors is shown in Table 1. Ovarian epithelial tumors comprise approximately half of all ovarian tumors, and account for approximately 40% of benign tumors and 86% of malignant tumors.
Table 1. Approximate distribution of ovarian epithelial tumors (% of all ovarian epithelial tumors).
Benign | Atypical proliferative (borderline) | Carcinoma | Total | |
Serous | 30.7 | 5.5 | 16.5 | 52.7 |
Mucinous | 23.7 | 3.8 | 3.6** | 31.1 |
Endometrioid | * | 0.4 | 5.7 | 6.1 |
Clear cell | * | 0.2 | 2.4 | 2.6 |
Transitional | 3.1 | 0.1 | 0† | 3.2 |
Undifferentiated | — | — | 2.1 | 2.1 |
Mixed | 0.5 | 0.1 | 1.8 | 2.4 |
Total‡ | 57.5 | 9.9 | 32.6 | 100 |
*Less than 0.5%.
** This is probably an overestimate since it is based on some literature prior to the recognition of the deceptive patterns of metastatic mucinous carcinoma in the ovaries.
†This percentage is inaccurate because older studies did not include this category.
‡The column totals are not exact sums because of rounding and lack of comparable data in some studies.
(Seidman JD, Russell P, Kurman RJ: Surface epithelial tumors of the ovary. In Kurman RJ [ed]: Blaustein's Pathology of the Female Genital Tract, pp 791–904, 6th ed. New York, Springer-Verlag, 2002.)
PATHOGENESIS AND PATTERNS OF SPREAD OF OVARIAN CARCINOMA
The pathogenesis of ovarian carcinoma is unknown. The traditional view that most carcinomas arise from the ovarian surface epithelium and the inclusions from which they are derived is not entirely supported by available data. The incessant ovulation hypothesis which holds that repeated ovulatory damage to the ovarian surface leads to formation of inclusions is belied by the prominence of inclusions in hypo-ovulatory women such as those with polycystic ovaries. Thus, the 'generally accepted' concept that 'surface epithelial inclusions' are derived from the ovarian surface epithelium is not without its detractors.
More important than the precise origin of ovarian carcinoma are its temporal and topographic modes of spread. Screening trials based on transvaginal ultrasound are grounded in the concept that typical ovarian carcinoma is confined to the ovaries at the outset and subsequently progresses to disseminated peritoneal involvement. However, this is an assumption and may not be correct. Comparisons of stage I and stage III carcinomas show that stage I tumors are larger, usually non-serous, and have a substantial noninvasive component in contrast to the typical stage III carcinoma that is serous, smaller and uncommonly displays a noninvasive component. These observations would suggest that with progression, ovarian carcinoma becomes smaller and changes from non-serous to serous; this would appear to be an unlikely sequence of events.1
Accumulating data support a recently proposed dualistic model of the pathogenesis of ovarian carcinoma.2 In this model, type I tumors are slow growing, usually confined to the ovaries for a prolonged period, and develop from well-described precursor lesions including atypical proliferative (borderline) tumors and endometriosis. Type II tumors are aggressive, rapidly growing, and do not have well-defined precursors. Molecular biological findings support this model, as type I tumors are characterized by genetic stability and mutations in specific genes including KRAS, BRAF, PTEN and β-catenin, while type II tumors are genetically unstable and harbor mutations of TP53.
SEROUS TUMORS
The classification of serous neoplasms of the ovary presented in this chapter, unlike the World Health Organization (WHO) classification, categorizes the proliferative noninvasive epithelial ovarian neoplasms as atypical proliferative tumors rather than borderline or of low malignant potential. The NCI-sponsored Borderline Ovarian Tumor Workshop considers both "atypical proliferative tumor" and "borderline tumor" acceptable and synonymous, but does not recommend "tumor of low malignant potential".3 The borderline category of ovarian epithelial tumors was introduced in the early 1970s to describe a group of tumors that did not display overtly malignant features but that occasionally appeared to behave in a malignant fashion.4, 5 Their behavior appeared to be intermediate between benign cystadenomas and frank serous carcinomas. The International Federation of Gynecology and Obstetrics (FIGO) committee charged with the development of the classification, which subsequently was adopted by the WHO, stated that this “intermediate” group may be composed of different types of tumors but that the differences could not be discerned.5 The classification was therefore viewed as provisional, but with its continued use over the past three decades, the borderline category has become firmly entrenched and is now regarded as a specific entity. Although it has been recognized for many years that the category includes a heterogeneous group of tumors, it is only recent studies that have clearly documented the biologic spectrum encompassed by the borderline category. Noninvasive serous tumors with a papillary architecture in which papillae display a hierarchical branching pattern are termed atypical proliferative serous tumors (APSTs), whereas those displaying a more complex papillary architecture characterized by delicate micropapillary and/or cribriform patterns are classified as noninvasive micropapillary serous carcinomas (MPSCs; or micropapillary serous borderline tumors). The intermediate behavior of tumors in the borderline group results from the inclusion of benign and malignant neoplasms, thereby creating the illusion of intermediate behavior. With the subdivision of the borderline group into benign and malignant tumors, in the opinion of some experts, the need for a borderline category disappears. Investigators who prefer to retain the borderline category cite recurrences and deaths in a minority of patients. They also maintain that, in at least some, peritoneal implants reflect spread from the ovarian tumors, that microinvasion indicates transformation to bona fide carcinoma, and that some associated lymph node lesions represent metastases.6
The literature shows that assessment of the prognosis of APST and MPSC requires evaluation of the peritoneal 'implants' that often accompany serous ovarian tumors. Forty percent of these tumors are associated with peritoneal implants. Studies over the past 20 years have shown that classifying implants as invasive or noninvasive plays a critical role in determining prognosis. Patients whose tumors are associated with noninvasive implants have a 10-year survival that is close to 100%. In contrast, patients whose tumors are associated with invasive implants have a mortality rate of 34% after more than 7 years of follow-up.7 The difference in survival for patients with invasive versus noninvasive implants is highly significant and supports the subclassification of implants for clinical management.
Benign Serous Tumors
Benign serous tumors include cystadenomas, adenofibromas, cystadenofibromas, and surface papillomas. These tumors are common, accounting for approximately 25% of all benign ovarian neoplasms and 58% of all ovarian serous tumors. The peak incidence is in the 4th and 5th decades, and the median age is 41 years. The symptoms and signs are nonspecific and most commonly include pelvic pain, discomfort, or an asymptomatic pelvic mass discovered on routine examination. Bilaterality rates are in the range of 12–23%.
Cystadenomas are composed of cysts filled with clear, watery (serous) fluid or thin mucoid material. Occasionally, they contain thick mucus-like material more typical of mucinous neoplasms. The external surfaces of the cysts are smooth and glistening, often with a prominent vascular pattern. Occasionally, small papillary excrescences are found on the external surface of the cyst. The tumors may be unilocular or multilocular and vary in size up to 30 cm, with a median of 9 cm. The lining of the cyst is either entirely flat or may have a varying number of coarse papillary projections. Such papillary excrescences rarely cover the entire inner surface of the cyst. Cystadenofibromas are solid neoplasms composed of tough, rubbery tissue with interspersed glandular spaces.
Normal-sized ovaries often have small papillary projections with a fibrotic stromal component resembling a microscopic adenofibroma or cystadenofibroma arising from the surface (surface papillomas); furthermore, simple germinal or cortical inclusions may become cystically dilated. Therefore, it has been suggested that serous neoplasms be diagnosed only if the lesion is greater than 1 cm in diameter. This is obviously arbitrary and it has been argued that this criterion is unlikely to distinguish true examples of neoplastic growth from simple serous cysts or nonneoplastic hyperplasias of the ovarian cortex.
There is a broad spectrum of epithelial proliferation in benign serous tumors that is manifested by variation in the prominence and complexity of the papillae, from a simple, single layer and blunt papillae to focal epithelial stratification and detachment of cell clusters approaching the degree of proliferation seen in APSTs. Identification of these features in 10% of the histologic material is the boundary between a cystadenoma and an APST.3 Cystadenomas are generally lined by a single layer of flattened-to-cuboidal cells with uniform basal nuclei. In addition, the epithelial cells can be pseudostratified and tubal in type, with the characteristic elongated (secretory cell) or rounded (ciliated cell) nuclei. In large cysts, the epithelium often becomes attenuated because of the pressure exerted by the cyst contents. Mitoses and atypia are generally absent. Psammoma bodies are present in the stroma in 15% of cystadenomas.
The stroma of benign serous tumors can resemble normal ovarian stroma but is generally more fibrous. When the tumor contains either thick fibrous papillae or large solid fibrous areas, the tumor can be designated as an adenofibroma.
A variety of benign cysts may occur in and around the ovary and broad ligament and may simulate serous cystadenomas, both grossly and microscopically. These include functional ovarian cysts, endometriotic cysts, hydatid cysts of Morgagni, mesonephric cysts, and mesothelial (peritoneal) cysts. Any of these cysts, if large, may adhere to or compress the ovary, thus suggesting an ovarian origin. Because serous cystadenomas are benign, unilateral salpingo-oophorectomy or ovarian cystectomy is adequate treatment. Recurrence is extremely rare and reflects either incomplete resection or a new primary tumor.
Atypical Proliferative (Borderline) Serous Tumors
Clinical Features and Operative Findings
Atypical proliferative serous tumors comprise approximately 10% of ovarian serous neoplasms and 56% of ovarian tumors classified as borderline. The clinical features of patients with APSTs are similar to those for serous cystadenomas, except that the mean patient age is slightly older for APSTs compared to cystadenomas.
Nearly 40% of atypical proliferative serous tumors are bilateral. Exophytic papillae reflecting ovarian surface involvement are common and are more often found in patients who also have peritoneal implants.8
APSTs are often associated with serous-type lesions involving the peritoneum. These include endosalpingiosis (benign glandular inclusions), found in 40% of patients, and noninvasive implants, found in approximately 30% of patients. The latter may appear as granular lesions or fibrous plaques.
Pathology
Atypical proliferative serous tumors have gross features similar to cystadenomas but tend to have finer, more friable, and more exuberant papillary projections. Papillae are nearly always present on internal surfaces of the cyst and are present on the external surfaces in up to 70% of cases. The adenofibromatous variant of APST is uncommon and grossly resembles its nonatypical counterpart.
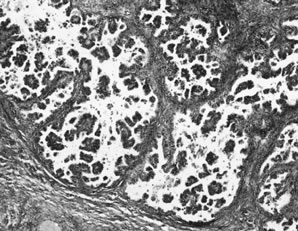
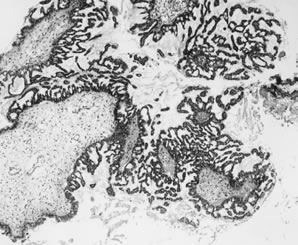
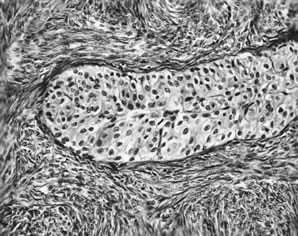
APSTs display extensive epithelial stratification, tufting and detachment of cell clusters, in addition to hierarchical branching with successively smaller papillae emanating from the larger, more centrally located papillae (Fig. 1). The two most easily quantifiable proliferative changes in these tumors are epithelial stratification and the extent of tufting or budding with detachment of cells from the surface. It has been recommended that a serous neoplasm should display stratification and budding in at least 10% of the available material to qualify as an APST.3
The cells in APSTs show features of epithelial and occasionally mesothelial differentiation. Ciliated cells resembling those in the fallopian tube are present in approximately one third of tumors. In addition, cells with abundant eosinophilic cytoplasm and rounded nuclei resembling mesothelial cells are also present, particularly on the tips of the papillae. The nuclei of APSTs resemble those in cystadenomas but tend to display slightly more atypia. Nuclei are basally located and tend to be ovoid or rounded. The chromatin is usually fine, but nucleoli are sometimes prominent. Mitoses are not common and rarely exceed four per ten high-power fields. Psammoma bodies are present in up to half of APSTs.
The most common type of microinvasion in APSTs is characterized by isolated cells with abundant eosinophilic cytoplasm that appear to be budding from the epithelium into the superficial stromal cores of the papillae.9 When carefully searched for, 10% of APSTs contain microinvasion, and recent data suggest 20% or more; however, in studies not specifically searching for microinvasion, it has been noted in only 1.3% of cases.7 The survival of 94 patients with microinvasion followed for a mean of 7.4 years was 100%.7 A recent large series suggests that microinvasion is an adverse prognostic indicator9 but in that series, most patients with an adverse outcome also had invasive peritoneal implants. It appears likely that microinvasion is frequently overlooked with no adverse affect on outcome. Although more data are needed, from the standpoint of prognosis and patient treatment, microinvasion at present appears to have no clinical relevance.
The peritoneum in patients with APSTs often contains serous epithelial proliferations displaying a range of proliferative changes: benign glands designated endosalpingiosis, papillary epithelial proliferations, sometimes with stromal desmoplasia, designated, noninvasive implants, and invasive carcinoma, also designated invasive implants. Some patients may have both invasive and noninvasive implants.
Endosalpingiosis, or benign glandular inclusions, may involve the peritoneal surfaces in patients with or without benign or malignant serous ovarian tumors. This is found in 40% of patients with APSTs. These glands typically are lined by simple columnar epithelium, often displaying tubal-type differentiation. The epithelium may display minor degrees of cytologic atypia and form simple papillary structures; psammoma bodies are sometimes present and may persist after degeneration of the associated epithelial structures. Mitotic figures are absent.
Among women with APSTs, about 30% have peritoneal epithelial lesions that display a degree of proliferation beyond that usually seen in endosalpingiosis, but that lack features of invasion. These have been designated noninvasive implants and have two morphologic forms: epithelial and desmoplastic. Mixtures of both types are not uncommon.
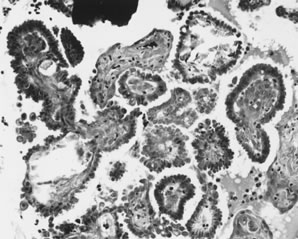
Epithelial implants are papillary and resemble the ovarian APST (Fig. 2).8 Mild atypia is often present, and occasionally atypia may be marked. Mitoses are usually absent. Calcification, usually in the form of psammoma bodies, is common and may be extensive.
Desmoplastic implants are densely fibrotic lesions that entrap glandlike structures. The characteristic architectural feature of the desmoplastic noninvasive implant is a plaquelike thickening overlying peritoneal surfaces that appears tacked on. They may extend into the septae that separate omental lobules and create a low-power appearance, which is suggestive of invasion. A chronic inflammatory response is usually present, and in 20% of cases, an acute inflammatory exudate overlies the implant. Mitotic figures are usually absent, and psammoma bodies are present in more than 90% of cases.8
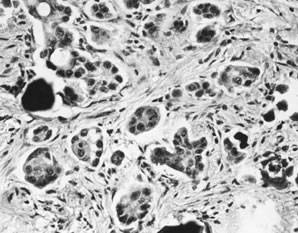
Invasive implants, also referred to as invasive serous carcinoma,3 have been reported in association with advanced stage APSTs in approximately 6% of cases. In contrast, they are found in association with nearly half of advanced stage MPSCs (see Micropapillary Serous Carcinoma, below). The characteristic architectural feature of an invasive implant is a haphazard infiltrative growth pattern (Fig. 3). A confluent or cribriform glandular pattern may be present. An exophytic micropapillary pattern, as described below (see Micropapillary Serous Carcinoma, below), in the opinion of some experts also qualifies for the designation of invasive implant (Fig. 4),3 but more often, micropapillae are nonbranched, embedded in fibrous stroma, and surrounded by a clear space or cleft. Sometimes, micropapillae are present within glands and may fuse with one another to create a weblike appearance. Invasive implants often display only mild cytologic atypia, but occasionally atypia is moderate and rarely marked. Mitotic figures are occasionally present. The distinction of desmoplastic implants from invasive implants (carcinoma) may be very difficult at times, but is important since it is this feature that is the best predictor of outcome for tumors with extraovarian disease.
|
Lymph node involvement has been reported in over 100 cases of APSTs.10 Excluded from consideration is endosalpingiosis involving lymph nodes, which are nonneoplastic glandular inclusions of müllerian type that occur in pelvic lymph nodes of 5–14% of unselected women and in 45–65% of women with borderline ovarian tumors. The most common type of lymph node lesion that has been associated with APSTs is characterized by individual cells and clusters of cells with abundant eosinophilic cytoplasm in the sinuses, predominantly subcapsular sinuses. The nature of these cells is unclear, but it has been suggested that they may be mesothelial in origin. Similar cells are nearly always present on the surface of primary APSTs and are often present in the stroma of noninvasive desmoplastic implants. It is plausible that these cells exfoliate and are filtered from the peritoneal fluid by regional lymph nodes—so-called deportation.
Identification of lymph node metastases of APSTs has been cited as evidence of malignant potential for APSTs (borderline tumors). In the largest series10 eight of 31 patients (26%) with lymph node involvement had invasive peritoneal implants. Among 22 patients with follow-up, four were alive with disease and there were two deaths, one in a patient with 'indeterminate' peritoneal implants. The presence of nodular aggregates of cells in the nodes was found to be an adverse prognostic factor. If patients with invasive implants (carcinoma) or indeterminate implants are excluded, then the overall survival for patients with lymph node involvement is about 98%.
Ovarian epithelial tumors are often heterogeneous, and carcinomas may have benign-appearing areas resembling a cystadenoma or an APST. It should therefore come as no surprise that approximately 20–30% of ovarian epithelial tumors diagnosed as atypical proliferative (borderline) at the time of frozen section examination prove to be carcinomas on further sampling. Because 15% of unilateral tumors are associated with extraovarian disease, it is important that the surgeon perform a thorough exploration when the frozen section is diagnosed as an APST, but formal staging is not necessary for a unilateral ovarian tumor unless suspicious peritoneal lesions are found. In contrast, 56% of bilateral tumors are associated with extraovarian disease, and therefore staging in this setting is advisable.
Behavior
The disease-specific survival rate of patients with APSTs confined to the ovaries after a mean of approximately 6.7 years, based on more than 2000 reported cases, exceeds 99.5%. As indicated above, survival of patients who had tumors that showed microinvasion is 100%, and survival of patients with lymph node involvement is 98%.7 In six prospective, randomized trials including approximately 373 patients with serous borderline tumors followed for a mean of 6.7 years, the survival was 100%.7 That the survival for stage I is virtually 100% was a general point of agreement at the 2003 Borderline Ovarian Tumor Conference.3 The behavior of APSTs with extraovarian disease is based on the type of implants that are present. The survival rate of patients with APSTs with noninvasive implants is 95–100% after a mean follow-up of 7.4 years.7 Recurrences and deaths reported in the literature are poorly documented in the majority of cases. When carefully documented, most deaths are either treatment related or are the result of complications from adhesions and bowel obstruction rather than carcinoma. Finally, in a literature review of more than 18,000 borderline tumors, we were unable to identify a single well-documented case of an APST with noninvasive peritoneal implants of which primary ovarian tumor had been adequately sampled to exclude invasion (one section per centimeter of maximum tumor diameter) that had progressed to documented invasive carcinoma.7 Among 27 cases that reportedly progressed to invasive carcinoma, none were documented to have been adequately sampled for pathologic examination.
In general, the noninvasive serous tumors associated with invasive implants are MPSCs, not APSTs. Based on a literature review of 467 noninvasive serous tumors, which included both invasive and noninvasive implants, the survival rate for patients with invasive implants was 66% after a mean follow-up of 7.4 years compared to 95% for patients with noninvasive implants. The difference was highly significant (p < 0.0001).7 The finding of invasive implants in association with an APST is very unusual and is probably due to inadequate sampling and reflects foci of occult invasion in the primary tumor.11
Malignant Serous Tumors
Micropapillary Serous Carcinoma
In 1978, Russell and Merkur described a noninvasive serous tumor, the morphology of which closely approached the degree of proliferation displayed by low-grade carcinomas but lacked invasion and, therefore, fulfilled the WHO criteria for serous borderline tumor. They designated these tumors as “grade IV proliferating serous tumors.” This pattern was also recognized by others around the same time. But despite numerous studies of ovarian borderline tumors or “tumors of low malignant potential” in the ensuing two decades, little data on this variant were published until 1996, when two series appeared12 followed by a third in 1999.13 At this time, some investigators observed that these tumors, despite no apparent evidence of invasion, behaved like low-grade carcinomas in contrast to the other tumors in this group, and therefore proposed that they be designated “micropapillary serous carcinoma”;12 other investigators preferred the term serous borderline tumor with a micropapillary pattern.13 The combined data of several additional recent series have confirmed these findings14, 15, 16, 17, 18 and also indicate that 49% of advanced stage MPSC have invasive peritoneal implants (carcinoma) as compared to 6% of advanced stage SBTs. MPSCs that do not display destructive infiltrative growth are considered noninvasive for purposes of this discussion, although it is conceivable that the complex exophytic micropapillary pattern is a form of invasion.
Clinical Features and Operative Findings
The mean age of patients with the noninvasive form of MPSC is 42 years. The most common presentation is an asymptomatic pelvic mass, but abdominal pain, fullness, and distention are common symptoms in advanced stage cases. Sixty-five per cent of tumors are bilateral. Forty-seven per cent of patients are stage I and the remainder are stages II and III. The mean tumor size is 8.2 cm. Surface involvement is present in 54% of cases.
Pathology
Because these tumors are very well differentiated, they tend to have a papillary and cystic gross appearance like APSTs and little, if any, necrosis in contrast to many typical serous carcinomas, which often have solid areas and extensive necrosis. Noninvasive MPSC is a proliferative serous neoplasm that displays a high degree of epithelial proliferation and complexity but is morphologically intraepithelial because diagnostic features of invasion are not present. Some investigators believe that the association of MPSC with invasive carcinoma and a high mortality rate warrants classification of this variant as a well-differentiated papillary serous carcinoma, thus the designation MPSC, while others prefer the term 'micropapillary serous borderline tumor'. In either case, this variant displays a characteristic pattern of papillary branching (Fig. 5). The distal papillary branches are thin and delicate with minimal fibrovascular support and emanate abruptly from thick, more centrally located papillae without intervening branches of successive intermediate sizes, unlike the hierarchical branching pattern of APSTs. The papillae often fuse to form a cribriform pattern. Because micropapillary and cribriform areas may be focally present in APSTs, an area of a confluent micropapillary pattern of 5 mm in diameter is required for the designation of MPSC; anything less than this, in the absence of other features of invasion, should be classified as an APST. Extensive sampling of the ovarian tumor may be necessary to resolve difficult cases. MPSC displays no apparent invasion of the stromal cores of the papillae. High-grade malignant nuclear features, even in the presence of the typical micropapillary patterns, warrant a diagnosis of ordinary papillary serous carcinoma. Invasion is recognized by a haphazard infiltrative growth composed of solid nests or complex glandlike structures displaying micropapillae or a confluent pattern. The nests, glands, and glandlike structures are often surrounded by a clear space or cleft. Psammoma bodies may be numerous in either the MPSC or invasive areas. Invasive MPSC is the typical form of invasive low grade serous carcinoma and represents about 9% of advanced stage serous carcinomas.19 Psammocarcinoma is a variant in which the bulk of the tumor is dominated by psammomatous calcification. The distinguishing morphologic features of serous neoplasms, including the invasive and noninvasive variants of MPSC, are shown in Table 2.
Table 2. Distinguishing morphologic features of serous ovarian neoplasms
Diagnosis | Atypia | Stratification and detachment | Micropapillary pattern | Stromal invasion |
Serous cystadenoma | Absent, or present in <10% | Absent, or present in 10% | Absent | Absent |
APST | Present in >10% | Present in >10% | May be present, <5 mm of confluence | Absent |
MPSC, noninvasive | Present | Usually present | Present, >5mm of confluence | Absent |
MPSC, invasive | Present | Usually present | Present | Present |
Serous carcinoma | Present | May be present | May be present | Present |
APST, atypical proliferative serous tumor; MPSC, micropapillary serous carcinomas.
(Seidman JD, Russell P, Kurman RJ: Surface epithelial tumors of the ovary. In Kurman RJ [ed]: Blaustein's Pathology of the Female Genital Tract, pp 791–904, 6th ed. New York, Springer-Verlag, 2002.)
The peritoneal implants associated with MPSC are frequently invasive (i.e. carcinoma). As noted above, among reported cases of advanced stage noninvasive MPSC, 49% of the implants were invasive in comparison to 6% of the implants associated with APSTs.
Behavior
Despite the apparent absence of destructive infiltrative growth in the noninvasive variant of MPSC, the limited available data indicate that it behaves as a low-grade serous carcinoma. Stage I MPSC appears to be cured by adnexectomy alone, although only a small number of stage I tumors have been reported. Based on relatively limited data, the five and 10-year survival rates for patients with advanced stage MPSC are approximately 80% and 50–60%, respectively.12, 13, 14, 15, 16, 17, 18 Documented recurrences of invasive carcinoma have been reported in one-third of advanced stage MPSCs. The invasive variant of MPSCs appear to have slightly worse survival rates (not statistically significant).18
Invasive Serous Carcinoma
Clinical and Operative Findings
Serous carcinoma is the most common type of ovarian cancer and accounts for approximately 50% of malignant ovarian neoplasms. The peak age group is 45–65 years, and the mean/median age depending on the source varies from 57–63 years. Inasmuch as over 80% of patients present in advanced stage (FIGO stage II or higher) with tumor disseminated throughout the abdominal and pelvic cavities, common presenting symptoms are abdominal pain and distention due to ascites or bulky abdominal tumor. Gastrointestinal symptoms are also common. Other symptoms may include urinary frequency, dysuria, and vaginal bleeding. Stage I tumors are rare and usually present as an asymptomatic mass on a routine pelvic examination.
Two thirds of cases involve both ovaries. For stage I cases, approximately 40% are bilateral. Nearly all advanced stage ovarian carcinomas spread along peritoneal surfaces, including the pelvic peritoneum (stage II), the surfaces of the bowel, and other abdominal organs (stage III). Both pelvic and abdominal spread can be by direct extension or metastasis. For example, direct extension to the rectosigmoid, broad ligament or uterus can occur by contiguous growth, or exfoliation of malignant cells can result in seeding of the peritoneal surfaces of the bowel or pelvic peritoneum. Tumor rupture before or during surgery may occur and warrants assignment of a stage I or II tumor to stage IC or IIC, respectively. Assessment of the character of the external surface is an important component of staging for tumors that are confined to the ovaries. This is best done by the surgeon but should also be evaluated by the pathologist. Grossly exophytic papillary tumor on the surface of an ovarian neoplasm that is confined to the ovaries warrants a stage of IC. Intracystic neoplasms without gross or microscopic tumor on the external surfaces do not qualify for surface involvement unless tumor cells invade through the full thickness of the cyst wall and are thus exposed to the peritoneal cavity. Dense adhesions to pelvic structures or bowel are generally reassigned to FIGO stage II or III, respectively, and although this appears to be a common but not universal practice, FIGO criteria are unclear as to whether this practice is permitted.
Patients with stage III disease usually have omental involvement which, in advanced cases, is manifested by a solid tumorous mass (omental cake). Pelvic and para-aortic lymph node metastases are found frequently, with a direct relationship to the extent of disease. Rarely, inguinal lymph nodes contain a metastatic tumor. Patients with tumors that otherwise appear to be stage I have lymph node metastases in 4–14% of cases; the corresponding figures for stages II, III, and IV are 36%, 41–68%, and 88%, respectively. Liver metastases usually manifest as studding of the peritoneal surface of the liver. Parenchymal liver metastases are rarely present, and splenic metastases are found on occasion, necessitating splenectomy.
In at least 10% of women with advanced stage papillary serous carcinoma, the ovaries are small and display predominantly surface involvement; occasionally, the ovaries are completely uninvolved by tumor. These findings warrant a diagnosis of primary peritoneal serous carcinoma. Recent data indicate that primary peritoneal serous carcinoma is more common than previously appreciated, comprising 18–28% of advanced stage serous carcinomas.
Pathology
Serous carcinomas range from microscopic (referred to as early de novo carcinoma) to approximately 20 cm in diameter. They are typically multilocular and cystic, with soft, friable papillae filling the cyst cavities and containing serous, turbid, or bloody fluid. The external surfaces may be smooth or bosselated and sometimes display surface papillae. Tumors are often solid, pink to gray with less-obvious papillae, and may be soft or firm, depending on the character of the tumor stroma. Hemorrhage and necrosis are often present. Omental metastases are characterized by firm nodules of variable size with white or gray cut surfaces, which may coalesce into an omental cake. If the omentum is grossly normal, microscopic omental metastases are found in 22% of cases.
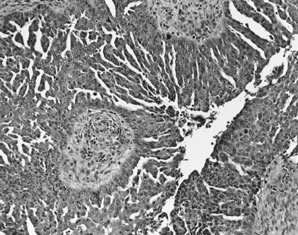
Well-differentiated serous carcinomas are discussed above (see micropapillary serous carcinomas above). Typical high grade serous carcinomas display complex papillary and solid patterns and marked nuclear atypia (Fig. 6). A frequently encountered and quite characteristic pattern is a lacelike or labyrinthine pattern. This pattern may be focal but often predominates and is characterized by extensive bridging and coalescence of papillae, resulting in slitlike spaces between the papillae. Areas of solid growth are common. A glandular pattern is not necessarily diagnostic of endometrioid differentiation, and the distinction of a high-grade endometrioid carcinoma from a serous carcinoma may be difficult. In fact, most high grade glandular (non-mucinous, non-clear cell) carcinomas are classified as serous, not endometrioid. The cells typically are large, pleomorphic, and display obviously malignant cytologic features.
|
Serous carcinomas that display extensive solid areas are usually composed of uniform sheets of cells with high-grade nuclear atypia and may display isolated bizarre mononuclear giant cells or syncytial-like aggregates. Mitoses, including abnormal mitoses, are usually numerous and necrosis is often pronounced. Nuclear grade is subjective and not very reproducible; we therefore designate these tumors as 'high grade'. A recently proposed binary grading system appears to be most clinically relevant.20 Usually there are focal areas with papillary architecture that permit the diagnosis of a serous carcinoma as opposed to an undifferentiated carcinoma. Psammoma bodies are present in 25% of cases. A completely solid carcinoma without any evidence of glands or papillae warrants a designation of undifferentiated carcinoma.
When faced with a neoplasm in the ovary, particularly when bilateral, the physician should always consider the possibility of metastatic tumor to the ovary. Although metastatic carcinomas to the ovary most often mimic mucinous or endometrioid ovarian carcinomas, metastases may display a variety of patterns.
Serous carcinomas may invade the fallopian tubes, and on occasion, the distinction of an ovarian from a primary tubal carcinoma may be difficult. Meticulous dissection of the fallopian tube, which is nearly always dilated and filled with tumor when primary, can be of value. Microscopically, the presence of carcinoma in situ in uninvolved tubal epithelium is also helpful. Recent data, particularly from prophylactic salpingo-oophorectomy specimens in high risk individuals, suggest that primary tubal carcinoma may be more frequent than previously acknowledged. The fimbriae in particular seems to be a common primary site.21
Grossly normal-sized ovaries with microscopic surface involvement or no involvement by serous carcinoma are classified as peritoneal carcinomas. From a practical standpoint, the distinction is not critical since the behavior and treatment of serous carcinomas of the ovary and peritoneum are similar.
Behavior
Stage I patients who have been comprehensively staged have a five-year survival rate that exceeds 90%. However, stage I serous carcinoma is rare; most stage I ovarian carcinomas are endometrioid, mucinous and clear cell carcinomas. Survival for stages III and IV is generally very poor. Some of the older literature overestimates the survival rate for stage III patients because of the inclusion of borderline tumors. Stage II tumors are uncommon (8% of serous carcinomas) and represent an intermediate group, which, depending on other factors including completeness of surgical removal and substage, can have widely varying survival and cure rates. Early de novo carcinoma, despite its small size, is associated with recurrence and death in approximately a third of cases, usually with widespread peritoneal disease.
ENDOMETRIOID TUMORS
The majority of endometrioid ovarian neoplasms are carcinomas. Endometrioid adenofibromas and atypical proliferative endometrioid tumors (APETs) are less common. Recent refinements in the criteria for these tumors suggest that many tumors diagnosed previously as well-differentiated endometrioid carcinomas would now be classified as APETs. Thus, APET may comprise up to 5% of ovarian epithelial neoplasms.
Benign Endometrioid Tumors
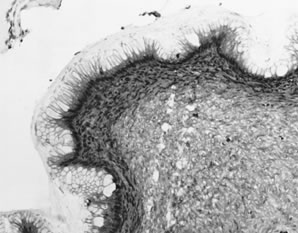
Endometrioid adenofibromas are usually unilateral. The median age of women with these tumors is 57 years. The mean diameter is approximately 10 cm. Seventeen per cent are bilateral. The external surface is smooth and the cut surface is densely fibrous, often with intermixed cystic areas creating a honeycomb appearance similar to a serous cystadenofibroma. The cysts contain clear or yellowish fluid. Microscopically, the dominant pattern is that of an adenofibroma or cystadenofibroma. The epithelial elements are arranged in branching tubular glands and cysts and usually resemble proliferative endometrium. The epithelium lining the glands is tall and columnar with oval nuclei containing coarse chromatin and small nucleoli; the cytoplasm is basophilic to amphophilic. Sometimes the nuclei resemble those of atrophic or inactive endometrium, with uniform, elongated dark nuclei and scanty cytoplasm. Mitoses are variable but usually rare. Secretory changes and focal squamous differentiation may be present. The stroma is usually densely fibrotic; focal areas may resemble ovarian cortex. Occasional endometrioid adenofibromas are associated with endometriosis. These tumors are benign, although rarely may recur.
Atypical Proliferative Endometrioid Tumors
There is a spectrum of epithelial proliferation, glandular crowding, and cytologic atypia in benign endometrioid neoplasms ranging from mild atypia, mild glandular crowding, and epithelial stratification slightly beyond that seen in typical endometrioid adenofibromas to confluent epithelial proliferation lacking stromal support in areas up to 5 mm in diameter resembling atypical hyperplasia and well-differentiated adenocarcinoma of the endometrium. A variety of terms have been used for these tumors, including proliferating or proliferative endometrioid tumor, atypical endometrioid adenofibroma, endometrioid tumor of borderline or low malignant potential, and atypical proliferative endometrioid tumor.22, 23, 24, 25 The criteria for these diagnoses have differed among these series. Because the behavior of these tumors has been benign, we prefer to combine these groups and refer to them as APET.
Among the three largest series, there were 96 patients with a mean patient age of 50 years. Five patients (5%) had bilateral tumors, and all but one were confined to the ovaries; one had a colonic 'implant'. The mean tumor size was approximately 8 cm. The characteristic gross appearance is solid and cystic; the cyst fluid is usually hemorrhagic, brown or green. A few patients have had endometriosis and some have also had endometrial hyperplasia.
The two characteristic microscopic architectural appearances of APET are adenofibromatous and glandular/papillary. The glandular/papillary proliferation can show varying degrees of glandular complexity and crowding. An underlying adenofibroma is often present. When the glandular proliferation becomes confluent, this is considered evidence of invasion by some experts but could also reasonably be considered examples of APET with intraepithelial carcinoma. A confluent epithelial proliferation that exceeds 5 mm in diameter warrants a diagnosis of carcinoma, according to one group.23 The glands show crowding and mild or moderate cytologic atypia and epithelial stratification; tufting and bridging may be present. Severe cytologic atypia warrants a diagnosis of intraepithelial carcinoma. Squamous metaplasia is present in up to half of cases. The stroma is usually cellular with periglandular cuffing. Necrosis is common and is often confined to gland lumens or cysts. The single case with a colonic implant most likely represents an independent lesion arising in endometriosis. Of 134 reported cases, all those with clinical follow-up have had a benign behavior after a mean of approximately 5 years.22, 23, 24, 25 In summary, microinvasion, intraepithelial carcinoma, and confluence of glandular growth, when limited in extent, are atypical morphologic features and appear to be biologically benign, but only a few cases have been reported and clinical follow-up data are limited.
Endometrioid Adenocarcinoma
Endometrioid carcinomas comprise 5.7% of ovarian surface epithelial neoplasms, 17.5% of ovarian carcinomas, and 93% of endometrioid ovarian neoplasms.
Clinical and Operative Findings
These tumors are most common in the 5th and 6th decades, and the mean patient age is 56 years. The most common symptoms are abdominal distention and pelvic or abdominal pain. Abnormal vaginal bleeding is also frequent. This is, in part, related to the association of endometrioid ovarian carcinoma with endometrial hyperplasia and carcinoma (see below). Most patients have an adnexal mass on pelvic examination.
Tumor size ranges from 12–20 cm with a mean of approximately 15 cm. The stage distribution differs significantly from serous carcinoma. A high proportion (43%) of endometrioid carcinomas are diagnosed in stage I. Approximately 13% of early stage (FIGO I–II) cases are bilateral. Endometriosis, which may be extraovarian, in the ipsilateral or contralateral ovary or within the tumor itself, is found in 15–20% of patients. However, recent studies have shown a stronger relationship witih endometriosis. It is likely that, if carefully sought, endometriosis can be identified in association with the majority of endometrioid carcinomas.
Pathology
Endometrioid carcinomas have a smooth outer surface. On cut section, they are solid and cystic, with the cysts containing friable soft masses and bloody fluid. Cysts occasionally contain mucus or greenish fluid. Less often, the tumor is solid with extensive hemorrhage and necrosis. Tumors arising in endometriosis may display gross findings of an endometriotic cyst containing chocolate-colored fluid, with a solid nodule in the wall reflecting the focus of malignant transformation.
Destructive infiltrative growth is definitional of ovarian carcinoma. Some authors also interpret a confluent glandular epithelial proliferation exceeding 5 mm as a pattern of invasion, which corresponds to a pattern of stromal invasion for tumors in the uterine corpus.23
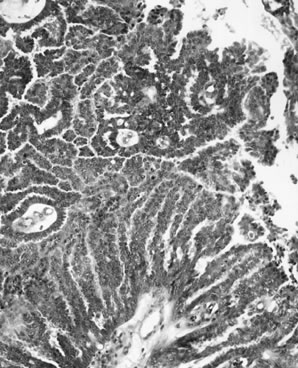
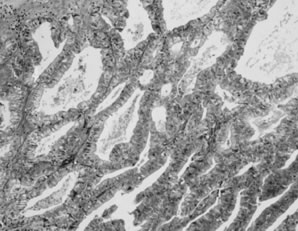
Well-differentiated endometrioid adenocarcinoma accounts for the majority of cases and is characterized by a confluent or cribriform proliferation of glands lined by tall, stratified columnar epithelium with sharp luminal margins (Fig. 7). A villoglandular growth pattern also occurs. Mitotic figures are often seen. Squamous differentiation is present in up to 50% of cases. Degeneration of squamous cells may induce a foreign body-type giant cell reaction in the stroma. Focal secretory changes are seen in up to a third of cases. This may be because of endogenous or exogenous progestin effect or may occur in the absence of hormonal stimulation. Areas of APET are commonly associated with endometrioid carcinoma.23 Moderately and poorly differentiated endometrioid carcinomas show solid growth and complex glandular and microglandular patterns, with significant overlap with poorly differentiated serous carcinomas. Nuclear pleomorphism and mitotic activity are marked and necrosis and hemorrhage are often prominent.
|
Approximately one third of ovarian carcinomas classified as endometrioid based on the predominant epithelial component are mixed with other epithelial types. A clear cell component is present in 20%, and a papillary serous component is seen in 10% of cases.
In addition to pure endometrioid carcinoma, which is the most common, several of the variants of endometrioid carcinoma of the uterine corpus also occur in the ovary. These include endometrioid carcinoma with squamous differentiation and secretory and ciliated variants. Other variant microscopic patterns of endometrioid ovarian carcinoma have been described. One variant has been designated 'sertoliform endometrioid carcinoma' or 'endometrioid carcinoma resembling sex cord-stromal tumor'.
The sertoliform or sex cordlike variant of endometrioid carcinoma creates the most problems in the differential diagnosis. This has important therapeutic and prognostic implications since sex cord-stromal tumors are usually low grade and require no therapy if confined to the ovaries, whereas patients with carcinomas usually require chemotherapy and have a worse prognosis. The age of the patient is helpful since the mean age of patients with Sertoli–Leydig cell tumors is 25 years, while women with endometrioid carcinoma are usually perimenopausal or postmenopausal. In addition, hormonal manifestations such as virilization are often associated with sex cord-stromal tumors and generally not with endometrioid carcinomas. In most cases of sertoliform endometrioid carcinoma, extensive sampling of the tumor discloses areas of typical endometrioid carcinoma.
Another common problem in the differential diagnosis of endometrioid carcinoma is metastatic adenocarcinoma, particularly metastatic adenocarcinoma from the colon. Colonic metastases to the ovaries are often cystic and grossly mimic primary ovarian neoplasms. Useful features in the distinction are a characteristic garland pattern characterized by cysts lined by cytologically malignant tall, columnar cells with bridging and cribriforming in addition to bilaterality and surface involvement.26, 27, 28 Although extensive dirty necrosis has also been cited as a feature of metastatic colonic carcinoma, this type of necrosis, characterized by abundant basophilic karyorrhectic nuclear debris intermixed with eosinophilic necrotic material, often in a geographic or zonal distribution, is often seen in primary ovarian carcinomas and therefore is of limited value.29 The immunoperoxidase panel of cytokeratin 7 and cytokeratin 20 can be of value in the distinction of primary ovarian versus metastatic colonic carcinoma.
Behavior
Overall, patients with endometrioid carcinomas of the ovary have a better prognosis than do those patients with typical serous carcinomas of the ovary. This is because of the high proportion of patients presenting in stage I. When stratified by stage, essentially all subtypes of ovarian carcinoma have a similar prognosis. The apparent favorable prognosis may also be due, in part, to a high proportion of grade I cases, although data on the influence of grade on prognosis are less clear. Treatment for endometrioid carcinoma is generally the same as that for other ovarian carcinomas. However, progestational agents, antiestrogens, tamoxifen, and other hormonal therapies have been used with limited success in previously treated endometrioid carcinomas; 10–15% response rates have been reported. There may be a correlation between the presence of steroid hormone receptors in tumor tissue and response rates, but data are limited. Hormonal therapy may be an option for treatment of recurrence in patients who did not respond or cannot tolerate chemotherapy or surgery.
Endometrioid Carcinoma Associated With Uterine Carcinoma
Approximately 15% of women with endometrioid ovarian carcinoma also have endometrial cancer of the uterine corpus. Endometrial hyperplasia is also commonly present. Because both tumors are often well-differentiated endometrioid adenocarcinomas, they usually resemble each other, and excluding the possibility that the ovarian tumor is metastatic can be a problem. Usually this can be determined based on a careful evaluation of the clinicopathologic features. If the uterine endometrial tumor is low grade with no or only inner half myometrial invasion, its metastatic potential is very low and the ovarian tumor can confidently be regarded as independent. If the endometrial tumor is high grade or deeply myoinvasive or both, the features of the ovarian tumors come into play. Bilaterality and a multinodular pattern, as well as other patterns characteristic of metastatic disease, indicate metastatic tumor. Close association of the ovarian tumors with either an underlying adenofibroma or endometriosis can provide evidence that the ovarian tumor is independent.
Follow-up of patients considered to have independent primary tumors also supports their independence, because most of these patients survive without recurrence, a finding compatible with stage I endometrial and ovarian carcinomas but not with a stage III endometrial cancer. Molecular studies can be of value in difficult cases.
Endometrioid Carcinoma Associated With Endometriosis
As noted above, it is likely that the majority of endometrioid carcinomas of the ovary are associated with endometriosis, which may occur within the tumor, the ipsilateral or contralateral ovary, or elsewhere. In only a small minority of cases, however, can direct continuity from endometriosis to atypical hyperplasia to carcinoma be shown. This is most commonly seen in the lining of an endometriotic cyst, which may contain a thickening of the cyst wall or a solid nodule protruding into the cyst. The mean age of women with endometrioid carcinoma associated with endometriosis is 5–10 years younger than when unassociated with endometriosis. Tumors associated with endometriosis, and particularly those arising in an endometriotic cyst, are usually well differentiated and stage I, and therefore the prognosis is excellent. On rare occasions, a limited atypical epithelial proliferation in an endometriotic cyst raises the differential diagnosis of atypical hyperplasia similar to the type seen in the uterine corpus versus well-differentiated endometrioid adenocarcinoma. In such cases, criteria for this distinction have been modified from those used in the uterine corpus.30
CLEAR CELL TUMORS
The majority of clear cell neoplasms of the ovaries are carcinomas. Clear cell adenofibromas and atypical proliferative clear cell neoplasms are rare.
Clear Cell Adenofibromas
Among approximately 12 reported cases of benign clear cell tumors, the mean age is 45 years. One case was bilateral. The median diameter was 12 cm. The tumors display a smooth, lobulated external surface, and the cut surfaces have a fine honeycomb appearance with minute cysts embedded in firm, rubbery stroma. The cyst fluid is clear. Microscopically, the tumor is characterized by tubular glands lined by one or two layers of peglike or hobnail cells that bulge into the lumen. The cytoplasm is either scanty, often in the hobnail cells, or abundant, clear, granular, or eosinophilic in the large polyhedral cells. Nuclear atypia and mitotic activity are minimal. The stroma is compact and fibrocollagenous. The cytoplasm usually contains glycogen. The clinical behavior is benign.
Atypical Proliferative Clear Cell Tumors
Among approximately 30 patients with APCCT (clear cell adenofibroma of borderline malignancy or low malignant potential) in the literature, the mean age is 60–70 years. The mean tumor diameter is approximately 15 cm. The gross appearance is similar to that of the clear cell adenofibroma, but in addition there are softer and fleshier areas. Microscopically, the architecture is similar to the clear cell adenofibroma. The tumor has greater epithelial proliferation and atypia than the adenofibroma and lacks stromal invasion. The cell types lining the glands and cystic spaces are similar to those in benign tumors but display significant nuclear atypia with coarse chromatin clumping, prominent nucleoli, and mitotic activity up to three per ten high-power field. The epithelium often displays stratification and budding; true papillary structures are uncommon. Small solid nests of clear cells may be present and can raise the possibility of stromal invasion.31
Peritoneal implants, as occur in the more common atypical proliferative serous tumor, have not been described with APCCTs. Among the limited reported cases, there is one alleged recurrence and no tumor deaths. Accordingly, we prefer the term atypical proliferative clear cell tumor for these neoplasms.
Clear Cell Carcinomas
Clinical and Operative Findings
Clear cell carcinomas comprise 2.4% of ovarian epithelial neoplasms and 7.4% of ovarian carcinomas. The mean age of patients with clear cell carcinoma is 57 years. Symptoms usually relate to a pelvic or abdominal mass. Interestingly, clear cell carcinoma is the most common epithelial ovarian neoplasm to be associated with paraneoplastic hypercalcemia, and there is also a higher frequency of thromboembolic events.
The relationship with endometriosis is strongest for clear cell carcinoma among all types of ovarian carcinoma, and accordingly, endometriotic implants are commonly present in close proximity to the tumor or elsewhere in the pelvis or abdomen. Approximately half of patients present in FIGO stage I and 15% in stage II.32 Four per cent of stage I cases are bilateral.
Pathology
Tumors range up to 30 cm in diameter with a mean of approximately 15 cm. Although they may be solid and fibrous with a honeycomb-cut surface resembling benign and atypical proliferative clear cell tumors, more commonly the cut surfaces show a thick-walled unilocular cyst with multiple yellow-beige fleshy nodules protruding into the lumen, or a multiloculated cystic mass with cysts containing watery or mucinous fluid. Tumors arising in endometriosis may display features of an endometriotic cyst, which typically contains chocolate-brown fluid and a thickened, polypoid or nodular area in the wall reflecting the area of malignant transformation.
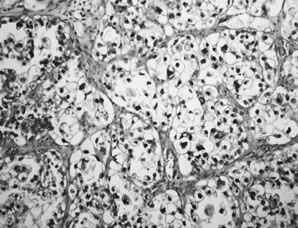
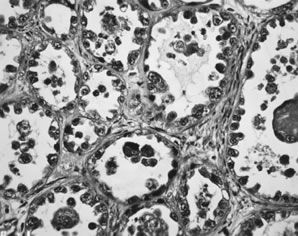
Clear cell carcinomas display several different patterns that often occur together.33 The most common patterns are solid and tubulopapillary. The solid pattern is characterized by sheets of polyhedral cells with abundant clear cytoplasm separated by delicate fibrovascular septae or dense hyalinized fibrotic stroma (Fig. 8). The tubulopapillary pattern is characterized by papillae with varying degrees of complexity. The fibrovascular cores of the papillae are often hyalinized. Complex tubules and cysts may be intermixed with the papillae, creating a tubulocystic pattern. The cell borders are usually prominent and the nuclei vary from small and rounded or angular to large and pleomorphic with large, prominent nucleoli. The cytoplasm is filled with glycogen. In the tubulopapillary pattern, the cells are often columnar with a hobnail appearance with the nucleus protruding from the papillae, gland or cyst, into the lumen (Fig. 9). Occasionally, the epithelium lining the glands and cysts is flattened. Mitotic activity is often prominent. Necrosis, hemorrhage, and stromal lymphocytic infiltrates are variable. Luteinized stromal cells and microcalcifications are occasionally present.
|
On average, 30–35% of ovarian clear cell carcinomas are associated with endometriosis, either in the involved ovary or elsewhere in the pelvis or abdomen. These figures vary widely and several studies have observed 50% or greater associated with endometriosis. In our experience, when carefully sought, nearly all ovarian clear cell carcinomas are associated with endometriosis and at least third of cases can be convincingly shown to have arisen within endometriosis, usually an endometriotic cyst.
Behavior
There are conflicting data on the behavior of clear cell carcinoma of the ovary. In some studies, the prognosis appears similar to that for other ovarian carcinomas, but in others, the prognosis is said to be worse. Several recent large series have not confirmed that clear cell carcinoma has a worse prognosis than the other cell types.34 It is possible that survival analyses are confounded by the fact that clear cell carcinomas are nearly always of high histologic grade, more often than other epithelial cell types. However, there are insufficient reproducible data correlating grade with prognosis of ovarian carcinomas at present to confirm this. The treatment for clear cell carcinoma is similar to that of other epithelial cell types of ovarian carcinoma.
MUCINOUS TUMORS
In the early 1970s, FIGO and WHO introduced a new classification in which tumors of surface epithelial origin were not only classified according to cell type but also were subdivided into benign, frankly malignant, or “of borderline malignancy” or “low malignant potential.”4, 5 This tripartite categorization, with the category of borderline malignancy at its core, was conceived to reconcile the disparate behavior and histologic appearance of a certain group of serous tumors, but the terminology was extended to include mucinous tumors as well as the other cell types.35 In the ensuing years, it was found that the long-term survival for mucinous borderline tumors (MBTs) confined to the ovary was close to 100%, whereas survival for advanced stage MBTs was 40–50%.36 Thus, the only MBTs that displayed malignant behavior were those that were advanced stage. A recent review showed that approximately 85% of all reported advanced stage MBTs was associated with pseudomyxoma peritonei (PMP).36 Furthermore, when analyzed carefully, virtually all of these can be shown to be secondary tumors from a mucinous cystadenoma of the appendix that ruptured and thus discharged mucinous epithelium into the peritoneal cavity.37, 38 This epithelium is capable of implanting on peritoneal surfaces and producing copious amounts of mucin. Bona fide primary ovarian MBTs that have ruptured before or at the time of surgery have never been reported to have recurred or resulted in the development of PMP. Thus, most investigators believe that PMP is a condition associated with certain gastrointestinal neoplasms, almost always mucinous cystadenomas of the appendix, but not with primary ovarian mucinous tumors.
Other studies have shown that metastatic carcinomas from the upper gastrointestinal tract, notably the pancreas and biliary tract, and occasionally the cervix can display a deceptive appearance in the ovary such that they are easily mistaken for ovarian MBTs. Furthermore, the ovarian tumors in these cases are typically much larger than the primary gastrointestinal tumor, thus heightening the resemblance to a primary ovarian tumor. Ovarian MBTs are a heterogeneous group of neoplasms. The majority are benign proliferative tumors confined to the ovary. The minority that are advanced stage are either secondary tumors from a ruptured mucinous adenoma of the appendix associated with PMP, or are metastatic carcinomas from a primary site in the upper gastrointestinal tract or cervix that masquerade as advanced stage MBTs. When diagnosed properly, ovarian MBTs are benign tumors that rarely, if ever, involve extraovarian sites. Thus, in our opinion the need for a category of borderline mucinous tumors no longer exists. We prefer the 'atypical proliferative' terminology, but 'borderline' is in widespread use and was accepted at the 2003 Borderline Ovarian Tumor Conference.3 Cystadenomas with increased proliferative activity and varying degrees of cytologic atypia are classified as atypical proliferative mucinous tumors (APMTs). Mucinous tumors with marked cytologic atypia represent 'APMT with intraepithelial carcinoma'. APMTs with clear-cut foci of invasion measuring less than 5 mm in diameter are designated 'APMT with microinvasion'.
Benign Mucinous Tumors
Mucinous cystadenomas and adenofibromas comprise 41% of all benign ovarian epithelial neoplasms, and 76% of ovarian mucinous neoplasms. Benign mucinous neoplasms occur most often in the 3rd to 6th decades with a mean age of approximately 50 years. Small tumors are uncommon and are often found incidentally, whereas typical examples are large tumors that present as a pelvic or abdominal mass.
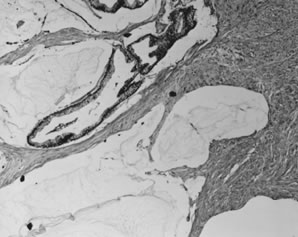
Bilaterality is uncommon, occurring in 2–5% of cases. Mucinous cystadenomas are typically large multiloculated cystic tumors that measure up to 50 cm in diameter. Locules are usually small and multiple but are variable in number; rarely, mucinous adenomas are large simple cysts with no loculations. These are the largest ovarian tumors on record; mucinous cystadenomas measuring 25–30 cm in diameter are not unusual, and tumors weighing more than 100 kg have been reported. The outer cyst wall is usually thick with a smooth opaque surface. The cysts contain thick tenacious gelatinous material that is usually pale yellow but may be turbid, brown, and more watery when associated with areas of infarction. Fibrous stroma is usually inconspicuous, but when it is abundant, these tumors are termed mucinous adenofibromas.
Microscopically, mucinous cystadenomas are lined by a single layer of uniform, tall columnar cells with clear or basophilic cytoplasm and small basal hyperchromatic nuclei (Fig. 10). Goblet cells are almost always present and indicate gastrointestinal-type differentiation. Rarely, endocervical-type mucinous epithelium predominates and is usually associated with a papillary architecture. Not infrequently, the cyst is lined by nondescript, tall columnar epithelium more typical of gastric antral-type epithelium. The intestinal-type tumors are usually glandular and cystic. Simple mucinous cysts have thick, collagenous, acellular outer walls, sometimes with dystrophic calcification. More complex cystadenomas have multiple locules that may be of similar or widely varying size, some of which display a peripheral arrangement of small acini, or daughter cysts, which may create a pseudoinvasive pattern. The complexity of glandular and papillary arrangements may be quite florid, and the presence or absence of invasion is often difficult to assess. Accumulation of mucin within the cysts leads to attenuation of the epithelium, which may lead to leakage of mucin into the stroma; the latter can also be caused by infarction. The mucin in the stroma may elicit no stromal reaction, an acute or chronic inflammatory response with muciphages, or a foreign body-type reaction. Extensive dissection of mucin through the tumor stroma is designated 'pseudomyxoma ovarii'. The stroma of mucinous cystadenomas is fibrocollagenous and the cellularity is variable but rarely approaches the high cellularity of the stroma of serous tumors. A minority of cases have a Brenner (transitional cell) tumor or component, usually within the outer wall. This frequency ranges from 1–4% in different series, although recent data suggest the frequency may be as high as 18%.39
|
Atypical Proliferative (Borderline) Mucinous Tumors
Clinical and Operative Findings
These tumors comprise about 38% of borderline tumors and 12% of mucinous ovarian neoplasms. The clinical features associated with APMTs are similar to those for mucinous cystadenomas. The age distribution of patients with these tumors peaks in the 5th and 6th decades.
APMTs are bilateral in up to 6% of cases. Like mucinous cystadenomas, APMTs are often very large neoplasms and may be adherent to other pelvic or abdominal organs. However, these tumors are noninvasive and therefore adherence to adjacent organs is not an ominous feature.
Pathology
The characteristic gross features of APMT are identical to those of mucinous cystadenomas. This is not surprising since APMTs arise in pre-existing mucinous cystadenomas (see below).
Atypical Proliferative Mucinous Tumors, Gastrointestinal Type
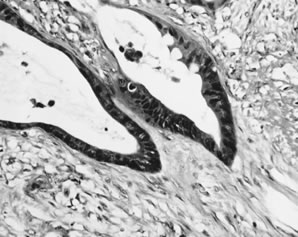
Atypical proliferative mucinous tumor of gastrointestinal type is characterized by cytologic atypia and epithelial stratification of mucinous epithelium that is otherwise similar to that of gastrointestinal-type mucinous cystadenomas (Fig. 11). At least 10% of the neoplasm should display these features to be included in the APMT category. Stromal invasion is absent. The epithelial stratification is often manifested by intraluminal papillary tufts of varying size that lack fibrovascular support. A villoglandular architecture is occasionally present. Although the older literature suggests that epithelial stratification greater than three cell layers conveys a worse prognosis,35 these data have not been substantiated, and furthermore, assessment of the degree and extent of epithelial stratification may not be reproducible. The glands are often crowded and may be back-to-back. Foci of a true cribriform pattern characterized by absence of fibrovascular support between adjacent glands may occur, but must be confined within cysts to qualify as an APMT. Nests or masses of epithelium displaying a cribriform pattern within the stroma reflect invasion and, depending on the size of these foci, qualify as microinvasive (less than 5 mm) or frank carcinoma (see below). Cytologic atypia and mitotic activity are variable. Marked cytologic atypia qualifies for the diagnosis of intraepithelial carcinoma (see below) and should prompt further sampling for areas of invasion.36, 37
|
The term atypical proliferative mucinous tumor with intraepithelial carcinoma is relatively new and has been used to describe a subset of atypical mucinous neoplasms displaying extensive epithelial stratification (greater than three cell layers) or marked cytologic atypia, which is a characteristic feature of invasive carcinoma in many sites; but in these cases, unequivocal stromal invasion cannot be shown.36 We reserve the diagnosis of APMT with intraepithelial carcinoma only for those tumors displaying areas of high-grade nuclear atypia. APMT with intraepithelial carcinoma comprises approximately one quarter of all mucinous neoplasms in the atypical proliferative and invasive carcinoma groups combined. The survival of patients with intraepithelial carcinoma exceeds 95% after salpingo-oophorectomy and for all practical purposes is the same as that of APMTs.36
To qualify as APMT with microinvasion, no single invasive area should exceed 5 mm in diameter. Multiple foci of invasion are permitted.3, 36 Some authors have proposed a 3 mm criterion as the maximum size of the invasive area to qualify for this diagnosis. In most cases, microinvasion measures less than 2 mm. Stromal invasion in mucinous neoplasms is described below (see mucinous carcinomas). Limited data on microinvasion in APMTs indicate a survival of 100% after a mean follow-up of six years.
The most important differential diagnostic consideration when evaluating a mucinous neoplasm in the ovary is whether the tumor is primary in the ovary or metastatic. If primary, an APMT must be distinguished from invasive carcinoma (see mucinous carcinoma below). Features suggestive of metastatic ovarian involvement include bilaterality, and if bilateral, size less than 10 cm; ovarian surface involvement; extensive pseudomyxoma ovarii; and certain patterns of haphazard, nodular infiltrative growth.
Atypical Proliferative Mucinous Tumors, Endocervical-Like Type
Fifteen per cent of ovarian APMTs are of the müllerian, endocervical-like type, also referred to as seromucinous to reflect the combination of serous and mucinous features.40 The mean patient age is 34 years. Seventy-seven per cent are stage I. Forty per cent are bilateral. In the largest reported series,40 all patients who had stage II and III disease (23% of all cases) did not have PMP, suggesting that the peritoneal 'implants' associated with this type of APMT are more akin to those seen with APSTs. This is also consistent with the papillary architecture of these tumors, a feature that closely resembles the architecture of serous tumors and is dissimilar to the architecture of intestinal-type mucinous neoplasms. The mean tumor size is 8 cm. The microscopic architectural features as noted above are similar to APSTs, with a hierarchical branching pattern, stratification, tufting, and detachment of cell clusters. A prominent acute inflammatory infiltrate is nearly always present, at least focally. The epithelium is tall, columnar, and mucinous, resembling endocervical epithelium. Cells with cilia can be found in a third of cases, reflecting serous-type differentiation. Atypia is usually minimal. Cells with slightly larger and rounded nuclei containing densely eosinophilic cytoplasm, particularly at the tips of the papillae, are present in nearly all cases. A small component (less than 10%) of other müllerian epithelial cell-type differentiation (e.g. endometrioid) is common. There is a strong association with endometriosis, which is present in at least 30% of patients; in two thirds of these patients, the neoplasm has been observed to arise directly from the atypical epithelial component of an endometriotic cyst.
Behavior
Atypical proliferative mucinous tumors are benign, with survival rates of 99% based on literature reviews, including approximately 900 patients.36 Rare reports of aggressive behavior most likely reflect inadequate sampling with failure to detect occult invasion, or the ovarian tumor may be a metastasis from an occult primary source elsewhere. Although the literature suggests that the survival of patients with advanced stage MBTs is only 54%, it is now clear that bona fide advanced stage MBT rarely, if ever, occurs. Eighty-five per cent of women who have been reported to have advanced stage MBTs present with the syndrome of pseudomyxoma peritonei, a condition that is not of ovarian origin36 (see below). For patients with APMT, endocervical-like type, after a short mean follow-up of 3.7 years, all reported patients were alive and well, but two who underwent unilateral salpingo-oophorectomy had contralateral APMTs develop, which most likely reflects new primary tumors.
Mucinous Tumors Associated With Pseudomyxoma Peritonei
Historically, the term pseudomyxoma peritonei (PMP) referred to the gross appearance of mucinous ascites associated with a mucinous tumor of the appendix, colon, or ovary. The literature on PMP before the 1990s is difficult to evaluate for several reasons. First, PMP has never been a specific histopathologic diagnosis but, rather, an operative/gross description. Second, much of the literature on this entity lacks descriptive data on the histology of the lesion. Third, PMP in women often involves the ovaries, and until recently, it had not been clear whether the primary site was in the appendix, ovary, or both in such women. Some investigators have also proposed that the primary site may in some cases be the colon. Another problem plaguing the diagnosis of PMP is the subclassification, by some authors, of cases into those with and without epithelial cells. These problems have recently been addressed. Some investigators have proposed that the clinical presentation and gross appearance of PMP encompass two distinct clinicopathologic entities, both of gastrointestinal origin: peritoneal mucinous carcinomatosis (PMCA), a highly aggressive tumor associated with a high early mortality rate; and disseminated peritoneal adenomucinosis (DPAM), an indolent condition nearly always derived from the appendix.41 In the latter condition, the mucinous epithelium released from the appendiceal neoplasm implants on peritoneal surfaces and is capable of producing copious amounts of mucin that persistently reaccumulates in the form of mucinous ascites. The mucinous epithelium has little capacity to invade tissue, but the mucin that accumulates in the peritoneal cavity is an irritant that induces fibrosis and dense adhesions. Patients with this entity usually have prolonged survival, but eventually many die of bowel obstruction. Although most cases of PMP can be reliably classified into DPAM or PMCA, approximately 10% display discordant or intermediate histologic features and, on average, have a prognosis intermediate between the two major groups.41
The development of bona fide invasive carcinoma in patients with DPAM is extremely rare. Conversely, mucinous neoplasms of the appendix that implant on the peritoneum as PMP are classified as carcinomas by some expert gastrointestinal pathologists, even if they display no significant cytologic atypia.42 It is not clear whether these tumors are true carcinomas. This is a very low-grade, clinically indolent neoplastic process that needs to be distinguished from the clinically aggressive PMCA rather than lumped together under the rubric PMP. The term PMP should be restricted to the description of the clinical entity and should not be used as a pathologic term.
Only 39% of patients with PMP in the largest series reported are women, and of these, 44% have ovarian involvement by a mucinous neoplasm.41 It is now generally accepted that ovarian involvement in women with PMP is secondary; PMP is nearly always of gastrointestinal origin, usually from a mucinous adenoma of the vermiform appendix.37, 38, 43 Mucinous ovarian tumors that are associated with PMP are typically classified as borderline because tumor is present throughout the peritoneal cavity. As noted above, these tumors are not ovarian in origin but are nearly always derived from appendiceal mucinous cystadenomas. Rarely, an ovarian mature cystic teratoma may have overgrowth of a mucinous component which can rupture and cause mucinous ascites, but recurrences in these cases have not been reported. Finally, it should be noted that the majority of the mucinous ovarian tumors associated with PMP show gross and microscopic features that are distinctive from those of primary ovarian MBTs (see below). We prefer to refer to the ovarian tumors in these cases descriptively as “ovarian involvement by DPAM from an appendiceal mucinous neoplasm”. They are identical to the appendiceal mucinous neoplasms from which they are derived.
Clinical and Operative Findings
The mean patient age is 44 years with two thirds of patients presenting in the 4th and 5th decades. Presenting clinical symptoms are often dominated by the effects of mucinous ascites, including abdominal distention and abdominal pain.
Mucinous ovarian tumors associated with PMP are bilateral in 80% of cases, with a mean diameter of 7 cm. When unilateral, there is a right-sided predominance. These findings contrast with primary ovarian APMTs, which are almost invariably unilateral, large (mean diameter greater than 15 cm), stage I, and equally distributed in the right and left ovaries. Mucinous ascites or mucoid nodules scattered throughout the peritoneal surfaces is characteristic.
Ovaries involved by DPAM are often cystic and usually display mucoid surfaces, surface nodules, or implants. Metastatic mucinous carcinomas (PMCA) in the ovaries appear similar but are more often solid. In 75% of patients with PMP, there is gross or microscopic evidence of rupture of the appendiceal tumor. Gastrointestinal perforations can be very small, can heal, and can be overlooked because of inadequate sampling by the pathologist; these are the best explanations for most of the remaining 25% of apparently unruptured appendices. It is also possible that there can be chronic leakage of mucus because of a small perforation that repeatedly heals and reruptures. In contrast, rupture of bona fide stage I primary ovarian MBTs is not associated with recurrence or development of PMP.
Pathology
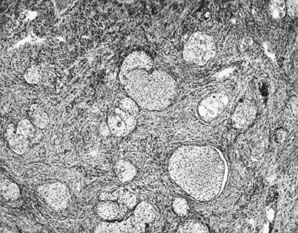
Microscopically, the majority of tumors associated with PMP display mucoid nodules or implants involving the ovarian surface; in 20% of cases, the involvement is confined to the surface. Surface and superficial or deep cortical involvement is seen in more than 50% of cases, and approximately 25% are confined to the ovarian stroma. Pseudomyxoma ovarii, an appearance characterized by dissection of acellular lakes of mucin through the ovarian stroma, is present in two thirds of cases and is usually multifocal or extensive.
The ovarian tumors associated with mucinous tumors that are disseminated throughout the peritoneum are of two types. Tumors composed of pools of mucin with scanty simple or focally proliferative mucinous epithelium displaying minimal cytologic atypia and rare mitotic figures have been designated DPAM along with the abdominal disease (Fig. 12). Additional morphologic features that are characteristically seen in these secondary ovarian lesions include strips of mucinous epithelium floating in mucin pools, often with a hypersecretory or hypermucinous appearance. These are nearly always associated with appendiceal adenomas lacking significant atypia. The ovarian mucinous proliferation closely resembles the peritoneal mucinous lesions as well as the appendiceal adenoma.
In the past, ovarian mucinous tumors characterized by epithelial proliferation and moderate-to-marked atypia or signet ring cells, significant mitotic activity, and obvious stromal invasion were often included in the category of PMP because of the abundant extracellular mucin production (Fig. 13). However, because they contain overtly histologically malignant epithelium, are associated with gastrointestinal carcinomas, and have a poor prognosis, they are not diagnosed as PMP, but rather as metastatic mucinous carcinomas. They are the ovarian manifestation of PMCA and are virtually always metastatic.41 Because these tumors display obvious cytologically malignant features, they are rarely confused with ovarian APMTs.
|
Immunohistochemical and molecular biologic studies have provided confirmatory evidence that the bland-appearing mucinous tumors associated with PMP involving the ovaries are secondarily derived from the associated appendiceal mucinous tumor.43, 44
Behavior
The behavior of mucinous tumors that are disseminated throughout the peritoneal cavity depends on tumor morphology. Cases designated DPAM, which are nearly always derived from appendiceal neoplasms, constitute the classic description of PMP found in the older literature, in which the behavior is characterized as slowly progressive, with reaccumulation of mucinous ascites and compatible with prolonged survival if treated symptomatically by periodic evacuation of the ascites. The five- and 10-year survival rates are 75% and 68%, respectively.41 In contrast, those cases designated PMCA are metastatic mucin-secreting carcinomas from the appendix or colon and are associated with an aggressive clinical course, with more than 90% of patients dead within three years. It is clear, therefore, that the term pseudomyxoma peritonei, although useful as an operative and gross description, lacks sufficient specificity for use as a histologic diagnosis.
Mucinous Carcinoma
Clinical and Operative Findings
After exclusion of metastatic tumors to the ovaries, primary ovarian mucinous carcinomas are uncommon and, in fact, are much less common than metastatic mucinous carcinomas.36, 45 Because of the limitations of the published data, clinical and pathologic features including proportion of ovarian neoplasms, bilaterality rates, and stage distribution are variable and may be unreliable. Mucinous carcinomas appear to comprise fewer than 5% of ovarian epithelial neoplasms, 12% of ovarian mucinous neoplasms, and 11% of ovarian carcinomas. However, these are probably overestimates because some of the studies from which these data are derived were published before the recognition of the variety of metastatic mucinous carcinoma patterns in the ovaries. A recent large series suggests that primary mucinous carcinomas of the ovary are indeed rare and represent only about 2.4% of ovarian carcinomas.45
Mucinous carcinomas occur in women in the 4th to 7th decades. The mean age is 54 years. The clinical presentation is similar to that of benign mucinous neoplasms, inasmuch as the majority of cases are stage I. Therefore, these patients do not generally present with signs and symptoms of abdominal carcinomatosis as do the majority of women with serous ovarian carcinomas. Rather, because these tumors can be quite large, the clinical presentation is generally that of a large pelvic or abdominal mass and abdominal distention.
Nearly 60% of patients with mucinous carcinoma have FIGO stage I disease. The majority of stage I cases are unilateral. If microinvasive tumors are excluded from the carcinoma category, this figure drops to approximately 50%. The proportion of stage I cases varies widely among studies, a finding that reflects variation in criteria as well as the difficulty of distinguishing intraepithelial carcinoma from invasive carcinoma.36 Nearly all intraepithelial carcinomas have been stage I. Ascites may occur, but the gelatinous, mucinous ascites characteristic of PMP does not occur in association with primary ovarian mucinous tumors (see PMP above).
Pathology
Because mucinous carcinomas arise from atypical proliferative mucinous neoplasms, their gross appearance is similar. Mucinous carcinomas are typically large, cystic, and multiloculated. Solid areas and intracystic nodules are more common in carcinomas than in cystadenomas and APMTs. Rarely, the tumor is predominantly solid.
Invasive mucinous carcinomas resemble APMTs but display unequivocal stromal invasion measuring more than 5 mm.36 Stromal invasion in primary ovarian mucinous neoplasms is characterized by three different patterns: (1) a confluent glandular growth with crowded glands that merge together without intervening stroma, and back-to-back cribriform glands with well-defined, sharply etched rounded spaces; (2) clusters of single cells with abundant eosinophilic cytoplasm surrounded by clear spaces infiltrating the stroma; and (3) glands of varying sizes infiltrating the stroma in a haphazard pattern. A nodular pattern with glands and nests of tumor in a nodular arrangement surrounded by normal ovarian stroma is strongly suggestive of metastatic disease.
Cytologic atypia is usually marked and characterized by an increased nuclear/cytoplasm ratio, irregularly clumped chromatin, and prominent nucleoli. The cytoplasm is scant and eosinophilic with irregular borders and occasional mucin droplets. Stratification is usually prominent but exceeds three cell layers in fewer than 50% of cases. Necrosis is often present. Mitotic figures are usually prominent, with more than five per ten high-power fields in the majority of cases. Most mucinous carcinomas are architecturally well differentiated (purely glandular), but the nuclear grade is often high.
Before making a diagnosis of mucinous carcinoma of the ovary, it is imperative to exclude metastatic mucinous carcinoma. Mucinous adenocarcinomas of the pancreas, biliary tract, colon, appendix, and cervix can mimic ovarian mucinous tumors.
The distinction of APMT from mucinous carcinoma can be difficult. Stromal invasion must be identified to diagnose mucinous carcinoma; the various patterns of stromal invasion are discussed above. If cytologically malignant features are present but unequivocal stromal invasion cannot be confidently identified, a diagnosis of APMT with intraepithelial carcinoma is warranted.
Behavior
The overall prognosis of patients with mucinous carcinoma is better than that for serous carcinoma because a high proportion of patients present in stage I. Nonetheless, despite the differing clinical and pathologic features of ovarian serous and mucinous carcinomas, their behavior, when stratified by stage, is similar. Recurrence and mortality in stage I mucinous carcinoma occur in 8–12% of cases, which is similar to well-staged stage I serous carcinomas.32 Advanced stage mucinous carcinoma (FIGO III and IV) is uniformly fatal, as is serous carcinoma of advanced stage. Limited data are available on FIGO stage II tumors, as only 10% of patients are in stage II. Similarly, few data are available on high-grade stage I mucinous carcinomas. Advanced stage primary ovarian mucinous carcinoma is rare, comprising less than 1% of ovarian carcinomas.
TRANSITIONAL CELL (BRENNER) TUMORS
The preferred term by WHO for this group of tumors is transitional cell; however, the term Brenner tumor, based on one of the earliest descriptions of the tumor by Brenner, is firmly entrenched in the literature and remains an approved term for some variants in this tumor group. Transitional cell tumors comprise 3.2% of ovarian epithelial neoplasms. Nearly all of these are benign Brenner tumors; atypical proliferative and malignant forms are very uncommon. The transitional epithelial cell type, characterized by a relatively uniform population of stratified cells with ovoid nuclei displaying nuclear grooves, is named because of its resemblance to urothelium.
Benign Transitional Cell Tumors
Benign transitional cell tumors comprise approximately 3.1% of ovarian epithelial neoplasms. The mean patient age is 50 years. They are common incidental findings and are very often microscopic in size. Therefore, the presenting symptoms are usually unrelated to the ovarian tumor. Most tumors are 2 cm or smaller, but occasionally they are large and may exceed 10 cm. They are well-circumscribed, firm, and rubbery, with a smooth or slightly bosselated serosal surface. The cut surfaces are typically solid and fibrous, usually gray, white, or yellow, and may be whorled or lobulated; occasionally, a cystic component is present. Calcification is sometimes seen. Small tumors can usually be seen arising in the ovarian cortex.
Microscopically, the characteristic feature is sharply demarcated epithelial nests in a dense fibrous stroma (Figs. 14 and 15). The epithelial cells are relatively uniform in size with prominent cell borders and pale to eosinophilic cytoplasm. The nuclei are oval, often with small nucleoli, and longitudinal grooves are usually present. Atypia and mitotic activity are generally not present. The nests often become cystic and contain eosinophilic debris or mucin. Occasionally, metaplastic endocervical-like mucinous epithelium lines the cystic nests; some investigators have termed these tumors metaplastic Brenner tumor. The metaplastic mucinous component may occasionally form the dominant part of the tumor and accounts for the association of Brenner tumors with mucinous cystadenomas. About 9% of Brenner tumors are associated with mucinous cystadenomas, with the frequency ranging up to 16% in reported series. Recent data suggest that this may be an underestimate. The stroma varies from closely resembling ovarian cortical stroma to densely fibrous. Hyalinized areas are common, and dystrophic calcification is present in 50% of cases, often in the hyalinized areas. The stroma is occasionally luteinized. The clinical behavior is benign.
|
|
Atypical Proliferative Transitional Cell Tumors
These uncommon neoplasms, also termed proliferating or borderline transitional cell or Brenner tumor, or transitional cell or Brenner tumor 'of low malignant potential', present in patients whose mean age is 59 years. They are always unilateral and confined to the ovary. The tumors are larger than their benign counterparts and are usually cystic and measure 10–28 cm with a mean diameter of 18 cm. Friable papillary or polypoid masses project into the cyst lumens, and there is usually a benign Brenner component with a more solid and fibrous cut surface. Microscopically, the intracystic papillary component is composed of transitional-type epithelium resembling low-grade noninvasive papillary transitional cell neoplasms of the urinary tract. Mucinous metaplasia may be present in the epithelium lining the papillae. Underneath the papillae and within the wall there may be solid sheets of transitional epithelium with little intervening stroma. Nearly all cases display areas of benign transitional cell neoplasm, and occasionally, the proliferating component can be seen arising directly from the benign component. The cytologic features are similar to those in benign transitional cell tumors, but occasionally significant atypia and mitotic activity are present. Some authors have separately classified 'proliferative' and 'low malignant potential' transitional cell tumors, the former characterized by a resemblance to grade 1–2 urothelial papillary transitional cell carcinoma (TCC) and the latter displaying high-grade nuclear atypia resembling grade 3 urothelial papillary TCC or in situ squamous cell carcinoma. In all cases, stromal invasion is absent. Among more than 50 reported cases of atypical proliferative transitional cell tumors, there has been one local recurrence and no convincing evidence of malignant behavior.46 Accordingly, we prefer the term atypical proliferative transitional cell (Brenner) tumor.
Malignant Transitional Cell Tumors
Malignant transitional cell tumors are difficult to define clinicopathologically for several reasons. First, published data are very limited. Second, large databases including the FIGO annual report and the Surveillance, Epidemiology, and End Results (SEER) database do not separately classify ovarian TCCs; in addition, malignant Brenner tumor is erroneously classified by SEER in a miscellaneous category for noncarcinomas. Third, papillary serous carcinomas not infrequently have focal areas that display features resembling TCC. Currently, experts believe that most tumors classified as ovarian TCCs are serous carcinomas with a transitional-like pattern and that genuine TCC of the ovary is very rare.
Approximately 10–15% of advanced stage ovarian carcinomas contain a transitional cell component. Although some authors have found that a transitional cell pattern predominates in 22% of advanced stage ovarian carcinomas, others have found this pattern to be rare. Some investigators have defined two clinicopathologic types: malignant Brenner tumor, in which a benign or atypical proliferative Brenner component is identified, and TCC, in which no benign or atypical proliferative Brenner component is identified.47
Clinical and Operative Findings
The clinical presentation is nonspecific and is similar to those of the more common cell types of ovarian cancer; thus, pelvic or abdominal pain and a mass are common. Malignant Brenner tumors occur at a mean age of 63 years while TCCs present at a median age of approximately 55 years.
The size of malignant Brenner tumors ranges up to 25 cm with a mean diameter of 14 cm. Among stage I tumors, 16% are bilateral. The stage distribution is as follows: stage I, 64%; stage II, 12%; stage III, 18%; stage IV, 6%. In contrast, 69% of TCCs present in advanced stage. The median tumor size is 13 cm.
Pathology
The gross appearance is typically solid and cystic. The cysts may exhibit polypoid, friable mural nodules, and the cyst fluid is watery or mucinous. Hemorrhage and necrosis may be prominent, and half have foci of gritty calcification. In the malignant Brenner tumor, the benign Brenner component may be identifiable as a solid fibrous nodule within a cyst wall. Serous carcinomas that have a minor transitional cell component have the gross features of papillary serous carcinoma (see above, Malignant Serous Tumors).
The histologic patterns of TCC are often mixed with other types of carcinoma, most often serous. Up to 10% of ovarian carcinomas containing the TCC pattern are said to be pure. More than 50% of the tumor should display the patterns of TCC for a diagnosis of TCC. A diagnosis of malignant Brenner tumor is warranted when a benign or atypical proliferative Brenner component is identified.
The characteristic microscopic feature is thick, blunt, and often elongated papillary folds with fibrovascular cores, lined by transitional-type epithelium resembling urothelium. The papillae often appear to arise from a cyst wall with a similar lining of stratified and atypical transitional cells. Foci of squamous or glandular differentiation are common. Stromal invasion is present and characterized by haphazard infiltrative growth of epithelium at the base of the papillae into the cyst wall or extensive areas of solid epithelial proliferation with scant or no fibrovascular support. A less-common architectural pattern is characterized by solid areas resembling benign Brenner tumor, but the epithelial nests have a disorderly growth pattern that appears invasive and is sometimes associated with stromal desmoplasia. Cytologic atypia is usually prominent and corresponds to grade 2 or 3 papillary TCC of the urinary tract. High-grade pleomorphic nuclear features and bizarre giant cells may occur, further supporting the opinion that these tumors are transitional-like variants of papillary serous carcinoma.48 Mitotic activity is prominent. Stromal inflammation, necrosis, and dystrophic calcification are often present. Benign or atypical proliferative Brenner components are required for the diagnosis of a malignant Brenner tumor and are absent in TCCs (see above). In addition, malignant Brenner tumors, like their benign counterparts, often have prominent stromal calcification in contrast to TCCs, in which this type of calcification is uncommon.
TCCs must be distinguished from metastatic TCC from the urinary tract. This is rarely a problem and is usually done clinically; urinary tract TCCs that metastasize to the ovaries generally do so late in their evolution and therefore are generally large, deeply invasive, and thus clinically evident.
The presence or absence of a benign or atypical proliferative Brenner component distinguishes a TCC from a malignant Brenner tumor as discussed above. Poorly differentiated papillary serous carcinomas may have transitional-like areas in up to 22% of cases. The appearance of thick, blunt papillae of transitional cell differentiation contrasts with the papillae of serous carcinoma, which are generally thinner.
Behavior
Limited data indicate that ovarian TCC is more aggressive than malignant Brenner tumor. This is suggested by the tendency for TCC to present in more advanced stage, with most malignant Brenner tumors presenting in stage I.47 However, it is unlikely that the behavior is different when stratified by stage. The apparent behavioral difference may arguably be due to obliteration of a recognizable benign Brenner component by the more rapidly growing, clinically aggressive tumors, rendering their classification as TCC only because a benign component can no longer be identified. Alternatively, TCC may simply reflect a variant of papillary serous carcinoma; this is supported by many similar clinical and pathologic features between these two types of ovarian carcinoma, and an immunophenotype that resembles papillary serous carcinoma of the ovary rather than TCC of the urinary tract.48
Rare types of ovarian epithelial tumors including mucinous tumors with mural nodules, small cell carcinoma, squamous cell carcinoma, and carcinosarcoma are discussed in more specialized texts.
REFERENCES
Yemelyanova AV, Cosin JA, Bidus MA, Boice CR, Seidman JD. Pathology of stage I versus stage III ovarian carcinoma with implications for pathogenesis and screening. Int J Gynecol Cancer 2008; 18:465-9. |
|
Kurman RJ, Shih I-M. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol 2008;27:151-60. |
|
Seidman JD, Soslow RA, Vang R, et al. Borderline ovarian tumors: diverse contemporary viewpoints on terminology and diagnostic criteria with illustrative images. Hum Pathol 2004;35:918-33. |
|
Serov SF, Scully RE, Sobin LH: Histologic typing of ovarian tumors. In International Histological Classification and Staging of Tumors (no. 9). Geneva, Switzerland, World Health Organization, 1973 |
|
International Federation of Gynecology and Obstetrics: Classification and staging of malignant tumors in the female pelvis: Acta Obstet Gynecol Scand 50:1, 1971 |
|
Kempson RL, Hendrickson MR. Ovarian serous borderline tumors: the citadel defended. Human Pathol 2000;31:525-6. |
|
Seidman JD, Kurman RJ. Ovarian serous borderline tumors: a critical review of the lilterature with emphasis on prognostic indicators. Hum Pathol 2000;31:539-57. |
|
Longacre TA, McKenney JK, Tazelaar HD, et al. Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long term (>5 yr) follow-up. Am J Surg Pathol 2005;29:707-23. |
|
McKenney JK, Balzer Bl, Longacre TA. Patterns of stromal invasion in ovarian serous tumors of low malignant potential (borderline tumors): a re-evaluation of the concept of stromal microinvasion. Am J Surg Pathol 2006;30:1209-21. |
|
McKenney JK, Balzer BL, Longacre TA. Lymph node involvement in ovarian serous tumors of low malignant potential (borderline tumors): pathology, prognosis and proposed classification. Am J Surg Pathol 2006;30:614-24. |
|
Seidman JD, Varallo MR. Micropapillary serous carcinoma: the solution to the ovarian borderline tumor conundrum. Pathol Case Rev 2007;12:136-42. |
|
Seidman JD, Kurman RJ. Subclassification of ovarian serous borderline tumors into benign and malignant types: a clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol 1996;20:1331-45. |
|
Eichorn JH, Bell DA, Young RH, et al. Ovarian serous borderline tumors with micropapillary and cribriform patterns: a study of 40 cases and comparison with 44 cases without these patterns. Am J Surg Pathol 1999;23:397-409. |
|
Slomovitz BM, Caputo TA, Gretz HF et al. A comparative analysis of 57 serous borderline tumors with and without a noninvasive micropapillary component. Am J Surg Pathol 2002;26:592-600. |
|
Goldstein NS, Ceniza N. Ovarian micropapillary serous borderline tumors: clinicopathologic features and outcome of seven surgically staged patients. Am J Clin Pathol 2000;114:380-6. |
|
Prat J, de Nictolis M. Serous borderline tumors of the ovary: a long-term follow-up study of 137 cases including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol 2002;26:1111-28. |
|
Deavers MT, Gershenson DM, Tortolero-Luna G, et al. Micropapillary and cribriform patterns in ovarian serous tumors of low malignant potential:a study of 99 advanced stage cases. Am J Surg Pathol 2002; 26:1129-41. |
|
Smith-Sehdev AE, Sehdev PS, Kurman RJ. Noninvasive and invasive micropapillary (low grade) serous carcinoma of the ovary: a clinicopathologic analysis of 135 cases. Am J Surg Pathol 2003;27:725-36. |
|
Gershenson DM, Sun CC, Lu KH, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol 2006;108:361-8. |
|
Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol 2004;28:496-504. |
|
Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol 2006;30:230-6. |
|
Bell DA, Scully RE: Atypical and borderline endometrioid adenofibromas of the ovary: A report of 27 cases. Am J Surg Pathol 9: 205, 1985 |
|
Bell KA, Kurman RJ: A clinicopathologic analysis of atypical proliferative (borderline) tumors and well differentiated endometrioid adenocarcinomas of the ovary. Am J Surg Pathol 24: 1465, 2000 |
|
Roth LM, Emerson RE, Ulbright TM. Ovarian endometrioid tumors of low malignant potential: a clinicopathologic study of 30 cases with comparison to well-differentiated endometrioid adenocarcinoma. Am J Surg Pathol 2003;27:1253-9. |
|
Leitao MM, Boyd J, Hummer A, et al. Clinicopathologic analysis of early-stage sporadic ovarian carcinoma. Am J Surg Pathol 2004;28:147-59. |
|
Daya D, Nazerali L, Frank GL: Metastatic ovarian carcinoma of large intestinal origin simulating primary ovarian carcinoma: A clinicopathologic study of 25 cases. Am J Clin Pathol 97: 751, 1992 |
|
Lash RH, Hart WR: Intestinal adenocarcinoma metastatic to the ovaries: A clinicopathologic evaluation of 22 cases. Am J Surg Pathol 11: 114, 1987 |
|
Lewis MR, Deavers MT, Silva EG, Malpica A. Ovarian involvement by metastatic colorectal adenocarcinoma: still a diagnostic challenge. Am J Surg Pathol 2006;30:177-84. |
|
DeCostanzo DC, Elias JM, Chumas JC: Necrosis in 84 ovarian carcinomas: a morphologic study of primary versus metastatic colonic carcinoma with a selective immunohistochemical analysis of cytokeratin subtypes and carcinoembryonic antigen. Int J Gynecol Pathol 16: 245, 1997 |
|
Seidman JD. Prognostic importance of hyperplasia and atypia in endometriosis. Int J Gynecol Pathol 15:1-9, 1996. |
|
Bell DA, Scully RE: Benign and borderline clear cell adenofibromas of the ovary. Cancer 56: 2922, 1985 |
|
Pecorelli S (ed): FIGO annual report on the results of treatment in gynaecological cancer, Vol 23. J Epidemiol Biostat 3: 1, 1998 |
|
Czernobilsky B, Silverman B, Enterline HT: Clear cell carcinoma of the ovary: A clinicopathologic analysis of pure and mixed forms and comparison with endometrioid carcinoma. Cancer 25: 762, 1970 |
|
Chan JK, Tian C, Monk B, et al. Prognostic factors for high-risk early-stage epithelial ovarian cancer: a Gynecologic Oncology Group study. Cancer 2008;112:2202-10. |
|
Hart WR, Norris HJ: Borderline and malignant mucinous tumors of the ovary: Histologic criteria and clinical behavior. Cancer 31: 1031, 1973 |
|
Riopel MA, Ronnett BM, Kurman RJ: Evaluation of diagnostic criteria and behavior of ovarian intestinal-type mucinous tumors: Atypical proliferative (borderline) tumors, and intraepithelial, microinvasive, invasive, and metastatic carcinomas. Am J Surg Pathol 23: 617, 1999 |
|
Prayson RA, Hart WR, Petras RE: Pseudomyxoma peritonei: A clinicopathologic study of 19 cases with emphasis on site of origin and nature of associated ovarian tumors. Am J Surg Pathol 18: 591, 1994 |
|
Ronnett BM, Kurman RJ, Zahn CM et al: Pseudomyxoma peritonei in women: A clinicopathologic analysis of 30 cases with emphasis on site of origin, prognosis, and relationship to ovarian mucinous tumors of low malignant potential. Hum Pathol 26: 509, 1995 |
|
Seidman JD, Khedmati F. Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) tumors and their relationship with Walthard cell nests: a clinicopathologic study of 120 tumors. Arch Pathol Lab Med 2008; in press. |
|
Rutgers JL, Scully RE: Ovarian mullerian mucinous papillary cystadenomas of borderline malignancy: A clinicopathologic analysis. Cancer 61: 340, 1988 |
|
Ronnett BM, Zahn CM, Kurman RJ et al.: Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis: A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei.” Am J Surg Pathol 19: 1390, 1995 |
|
Carr NJ, McCarthy WF, Sobin LH: Epithelial noncarcinoid tumors and tumor-like lesions of the appendix: A clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer 75: 757, 1995 |
|
Cuatrecasas M, Matias-Guiu X, Prat J: Synchronous mucinous tumors of the appendix and the ovary associated with pseudomyxoma peritonei: A clinicopathologic study of six cases with comparative analysis of c-Ki-ras mutations. Am J Surg Pathol 20: 739, 1996 |
|
Szych C, Staebler A, Connolly DC et al.: Molecular genetic evidence supporting the clonality and appendiceal origin of pseudomyxoma peritonei in women. Am J Pathol 154: 1849, 1999 |
|
Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous carcinomas in the ovary: Incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol 27:985-93, 2003. |
|
Roth LM, Gersell DJ, Ulbright TM: Ovarian Brenner tumors and transitional cell carcinoma: Recent developments. Int J Gynecol Pathol 12: 128, 1993 |
|
Austin RM, Norris HJ: Malignant Brenner tumors and transitional cell carcinoma of the ovary. Int J Gynecol Pathol 6: 29, 1987 |
|
Ordonez NG: Transitional cell carcinomas of the ovary and bladder are immunophenotypically different. Histopathology 36: 433, 2000 |