Cordocentesis
Authors
INTRODUCTION
In 1983, a major advance in maternal-fetal medicine was achieved: the development of a safe and simple technique of obtaining fetal blood before labor. Daffos1 is credited with having performed the first cordocentesis, but many persons contributed to the evolution of this procedure, including the scientists and engineers who brought ultrasound to the forefront of imaging.
The history of cordocentesis began in 1964, when Freda and Adamsons2 described a hysterotomy with extrauterine umbilical transfusion of a hydropic fetus. Although the infant died of prematurity, this technique ultimately proved successful.3 It required a major surgical procedure, however, that placed both mother and fetus at significant risk. In 1972, Valenti4 used ultrasound guidance to obtain a fetal biopsy specimen. He used a pediatric cystoscope (18 Fr), which he inserted into the uterus after administering regional anesthesia and performing a laparotomy incision. He theorized that the technique could be useful for intrauterine blood sampling and transfusion. One year later he confirmed his theory when he diagnosed hemoglobinopathies by sampling fetal vessels on the placental surface.5 In 1974, Patrick and colleagues6 described the percutaneous placement of a 1.7-mm fetoscope. In 1980, Rodeck7 reported on 133 fetoscopic procedures performed for fetal blood sampling. Of the 108 patients who continued their pregnancy, there was a 3.7% fetal loss rate. In 1981, Rodeck reported on his experience performing fetoscopically guided intravascular transfusion at 23- to 25-weeks' gestation.8 In 1982, Bang and associates,9 recognizing the limitations of fetoscopy, performed an ultrasound-guided percutaneous transfusion into the fetal hepatic vein at 30-weeks' gestation. In 1983, Daffos and co-workers1 published a description of fetal blood sampling by ultrasound-guided puncture of the umbilical vein, near the placental insertion of the cord, with a 20-gauge needle. Centers across the world were quick to see the possible applications of this procedure, and a plethora of reports followed.
TECHNIQUE
A cordocentesis is performed by the advancement of a needle in the sonographic plane to the targeted puncture site. The technique has two variations: freehand and needle-guided.
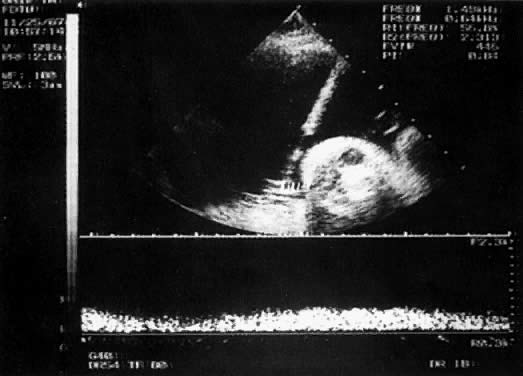
With the freehand technique, the transducer and needle are managed either by one person or by a team of two. If one is working alone, a second person is required to withdraw the sample or to inject through the needle. Because the needle direction can be readjusted laterally, many prefer the freehand to the needle-guided technique. With the needle-guided technique, the needle's travel is limited to one plane (Fig. 1). The needle tract is displayed on the ultrasound screen, permitting precise alignment of the tract with the vessel (Fig. 2).
The needles used for cordocentesis are between 20 and 25 gauge. A 20 gauge is used most often for freehand procedures because its stiffness facilitates redirection; however, the risk of bleeding and bradycardia10 can be minimized by the use of a smaller needle. Further, studies of midtrimester amniocentesis suggest that the larger the gauge of the needle, the greater the risk of membrane rupture. Generally, smaller gauge needles are used for needle-guided procedures. A 22-gauge needle is satisfactory for both diagnostic and therapeutic procedures. Choice of needle length depends on the thickness of the maternal abdominal wall, amniotic fluid volume, and sampling site. A 5-inch needle generally is adequate. If necessary, a therapeutic amniocentesis can be performed to facilitate cordocentesis.
Cordocentesis is done on an outpatient basis in a location with close proximity to the delivery suite. A relaxed, informal setting helps to alleviate the mother's anxiety, which tends to improve her cooperation. After reviewing the benefits, risks, and options, an informed written consent is obtained. If the fetus is viable, a nonstress test or ultrasound assessment is performed before the procedure to document the presence or absence of fetal well-being. The mother is placed in a recumbent position with a lateral tilt, and a suitable target site is chosen. Neither local anesthetic nor maternal sedation is needed for diagnostic procedures, but may be employed for therapeutic procedures of any length. We have used 5 to 10 mg diazepam intravenously with good results. Prophylactic antibiotics are unnecessary.
Ideally, the placental umbilical cord origin is chosen for sampling because it is fixed. Alternatively, a midsegment (free loop) may be used. The umbilical vein is preferred because of the lower associated risk of a reactive fetal bradycardia.11 Maternal repositioning may be necessary to improve access to the cord. Hepatic vein12 or cardiac sampling13 are alternatives if cordocentesis is not feasible. Although the transplacental approach may not be avoidable when the placenta is anterior, it increases the risk of fetal-maternal hemorrhage and possible sensitization.14 We specifically seek to avoid the placenta when evaluating a fetus for hemolytic anemia by seeking a free loop to puncture.
The maternal abdomen is prepared aseptically. We use acetone to remove the acoustic coupling gel (unsterile), followed by alcohol and povidone-iodine. The sterile-gloved operator prepares all necessary equipment on a sterile tray and drapes the mother. If a linear transducer or needle-guided technique is being used, an assistant also dons sterile gloves and places the transducer in a sterile sheath. Sterile acoustic coupling gel is applied to the abdomen, and the sampling site is relocated. The needle is inserted and advanced until it abuts the cord. With a controlled thrust, the vessel is punctured. If a free loop is chosen, it is imaged longitudinally and the needle advanced gradually until it pins the cord against either the uterine wall or a fetal body part. It is then punctured with a thrust. The stylet is removed; blood should be seen to fill the hub. At the placental origin, the vessel punctured is identified either by injection of a small amount of saline or blood (flush method) or by measurement of the vessel pressure. Streaming of fluid toward the fetus indicates that the vein has been punctured. With a midsegment puncture, the flush method can prove misleading. When necessary, the fetal heart rate can be monitored by placement of a Doppler gate in the umbilical artery.
If fetal movement is a concern (particularly if the placenta is posterior or whenever a free loop is targeted), immediately upon puncture, 0.3 mg/kg pancuronium (to a maximum of 0.6 mg) is given either intravascularly or intramuscularly into the fetal buttock or thigh with a 25-gauge needle. Neuromuscular blockade persists for 1 to 2 hours. Blood samples are withdrawn with 1-mL syringes because those with a larger barrel create too much negative pressure and can collapse the vein. The sample volume varies with the indication. We have routinely withdrawn 5 to 6 mL after 20-weeks' gestation without apparent sequelae. Heparinized syringes are unnecessary, except for those used for blood gas analysis. The samples are immediately transferred to a container with the appropriate anticoagulant or preservative.
Before the procedure, the operator should determine which studies are desired and should rank them in importance. The samples are taken in rank order in case the needle slips out of the lumen or a complication occurs, causing the procedure to end prematurely. We send a complete blood count at each procedure, using the elevated mean corpuscular volume as an indication that the sample is fetal in origin. We also routinely perform a Kleihauer-Betke stain of the blood smear if the placental cord origin is sampled. Other tests of purity include anti-i agglutination tests, human chorionic gonadotropin determination, and the measurement of factor V and VIII activity,15 but they rarely are necessary.
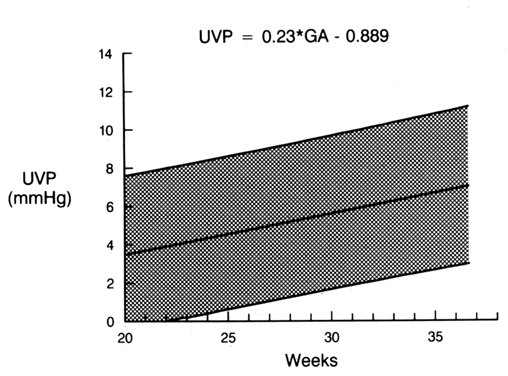
Umbilical venous pressure is measured by attachment of a pressure transducer to the needle. The value obtained should be corrected for amniotic fluid pressure (Fig. 3). This measurement is very useful in evaluating nonimmune hydrops. An elevated value suggests a cardiac etiology. After the needle is removed, the uterine and umbilical puncture sites are observed for bleeding and the duration of any loss is recorded. The fetal heart is viewed to determine whether a bradycardia is present. If the fetus is viable, external fetal heart monitoring is performed for a minimum of 1 hour. If pancuronium has been given, the tracing usually is nonreactive with a mild tachycardia. Before being discharged, the mother is counseled to call if she has contractions, leakage of fluid, bleeding, fever, or uterine tenderness. She is told that infection may present in an indolent fashion, mimicking a viral syndrome with myalgias and a low-grade fever. Activity is not restricted. Arrangements are made for the patient to be contacted with test results. If the patient is Rh-negative, the fetus should be typed and RhoGAM administered when appropriate.
The importance of a team approach cannot be stressed enough. Expert personnel are needed to support the mother through the procedure and to assist the operator (Fig. 4). The laboratories must be familiar with the analysis of small volumes of blood and must recognize the need for efficient and rapid service. Should there be a complication, the team must be familiar with resuscitative procedures and be prepared to move rapidly to perform a cesarean delivery. Likewise, the delivery suite staff should be aware that a procedure is taking place and be ready to provide anesthesia and nursing personnel as necessary.
COUNSELING: BENEFITS VERSUS RISKS
Prenatal counseling is done in a supportive and nondirective fashion, centering on an evaluation of the benefits versus the risks. The patient must be apprised of her options, which often include: (1) expectant care; (2) pregnancy termination; and (3) obtaining more information to determine either the possibility of perinatal treatment or the wisdom of options 1 and 2. Alternative techniques (i.e., amniocentesis or chorionic villi sampling) and their pros and cons should be reviewed where appropriate. Inherent to this counseling discussion is knowledge of gestational age and the likelihood of preterm labor or fetal distress. The risks quoted should reflect those associated with the indication, rather than a global risk for all procedures.
Benefits
If a karyotypic abnormality is suspected, the rapidity of the result (1 to 3 days from fetal blood vs 10 to 14 days from amniocytes) is the primary benefit of cordocentesis. This is especially important near term, where the mode of delivery or the need to intervene for fetal distress may be changed because of the presence of an abnormal karyotype. Rapid results also are important when the pregnancy is between 20 to 24 weeks, if termination of pregnancy is to remain an option. If the patient is late in the second or early in the third trimester and not considered at risk for preterm delivery, amniocentesis may be a better option if only a karyotype is desired. The patient, after counseling, may decide to accept any added risk from cordocentesis in order to minimize the waiting time. Anxiety can play a significant role in a patient's decision-making process.
Women who are red blood cell alloimmunized must be made aware of the alternative (i.e., amniocentesis). The undisputed benefit of obtaining fetal blood to determine blood type and hematocrit should be compared with amniocentesis, which is unable to identify the fetus who is not at risk, and Liley curve plots may not accurately reflect the hematocrit.
Risks
Any benefit, however, must be weighed against the risk of cordocentesis. There remains much controversy over the actual risks of the procedure. The absolute incidence of a procedure related loss is unknown because there have been no controlled studies. Some of the controversy arises from differences in the definition of a “procedure-related loss.” Should there be a duration of time after the procedure in which a complication is not considered the result of the sampling? Should all losses, including neonatal losses, be factored into the equation, or just ones “obviously” related to the procedure? Should the operator decide which loss is procedure-related? Most would agree that a bradycardia or amnionitis associated with fetal or neonatal demise would indeed be procedurerelated; but is a severely growth-restricted fetus with reversal of umbilical artery diastolic flow, who has an uneventful cordocentesis and dies 1 week later, a procedure-related loss or a loss secondary to the underlying disease? There has been no agreement in the published literature, and this issue was not adequately dealt with in a recent review of complications.16 For example, in the widely quoted series by Daffos and colleagues,17 of 359 completely documented pregnancies, there were 7 losses (1.9%). Of the seven losses, one died of abruption associated with severe preeclampsia 3 months after the procedure; one died 3 days after a cordocentesis complicated by a 6-minute bradycardia; one had IUGR and died 5 days after sampling; and one died 2 months after the procedure (at autopsy, a tight spiral of the middle part of the cord was noted). Three other losses occurred before 28 weeks: The first loss involved a diabetic pregnancy with fetal hydramnios, the second involved a twin pregnancy with suspected twin-to-twin transfusion, and the third was a postnatal death occurring at 27 weeks. The circumstances surrounding these deliveries were not given. Daffos and associates17 believed that only one of the seven deaths was truly related to the procedure. If true, this would reduce the procedure-related loss rate for low-risk procedures to 0.3%, a rate similar to that for amniocentesis.
The risk of cordocentesis varies according to the indication. For example, fetuses with nonimmune hydrops have a higher procedure-related loss (25%) compared with fetuses with severe growth deficiency (13.8%), structural anomalies (6.6%), or those where the indication for cordocentesis was prenatal diagnosis (1.3%).18 Fetuses with nonimmune hydrops, however, also have a high perinatal loss rate (70% to 90%)19 if nothing is done. Therefore, patients should be counseled regarding the background loss rate for the particular problem, as well as the additional procedural loss rate. Given the fact that most of the cases reported by Daffos and co-workers17 were low risk, a procedure-related risk of 0.3% seems realistic. Of 73 ongoing low-risk pregnancies, Maxwell18 reported 1 loss (1.3%). A recent publication20 reported a 0.33% risk for all diagnoses excluding chromosomal abnormalities. The procedure-related loss rate for hemolytic disease was 1/421 procedures (0.2%). The best statistics to quote to the patient are either those of the institution to which you refer or those of your own institution.
Counseling must include a discussion of complications (Table 1) other than fetal death, particularly when the fetus is extremely premature. Bradycardia occurs commonly (6.6%).11 The incidence of bradycardia may be related to the size of the needle; Bovicelli and associates10 reported a 0% incidence of bradycardia with the use of a 25-gauge needle. Fortunately, episodes of bradycardia usually are short and of no likely consequence. Work from the University of Iowa reveals that the incidence of bradycardia is higher if the umbilical artery is punctured, rather than the vein (21% vs 3.4%, respectively).11 Bradycardia is also more common in severely growth-restricted fetuses. Fetuses experiencing a bradycardia have as a group lower blood pH levels, lower PO2 levels, and higher PCO2 levels before the onset of the bradycardia, as well as a higher umbilical artery systolic-to-diastolic ratio than fetuses who do not experience a bradycardia.11 These findings imply that the increased procedure-related loss rate for severely growth-restricted fetuses may be related to the increased incidence of bradycardia. It has been theorized that the umbilical arterial smooth muscle is more at risk for spasm than is muscle in the vein and that, in a hypoxic environment, it has an exaggerated response to catecholamines.11
TABLE 1. Complications of Cordocentesis
Fetal death
Bradycardia
Amnionitis
Cord hematoma
Umbilical cord bleeding
Fetal maternal hemorrhage
Premature rupture of membranes
Preterm labor
Abruptio placentae
Complications secondary to premature delivery
Maternal sepsis, followed by adult respiratory distress synodrome
Risk of emergency cesarean section
Failure to obtain sample
Before the procedure, it is important to discuss with the patient whether or not she would want to proceed with an emergency cesarean delivery if a prolonged bradycardia occurs. This is especially important either at the lower limit of viability based on gestational age or when the estimated weight is less than 500 g because size is a major technical problem for the neonatologist. The risk of a bradycardia also highlights the need to perform cordocentesis in close proximity to a delivery suite if the fetus is considered viable.
No treatments for fetal bradycardia proved effective. Atropine (1 mg IV) has been given to the mother21 and to the fetus during episodes of fetal bradycardia. Bald and associates22 reported the administration of 0.3 mg epinephrine for fetal asystole after cordocentesis and noted a return to a normal heart rate. We have observed a similar response, but in association with a fetal intracranial hemorrhage. On several occasions we also have observed fetal asystole followed by the spontaneous return of a normal rate. Pharmacologic treatment of a postcordocentesis bradycardia should be considered experimental. Currently, we prefer to stimulate the fetus by manually compressing it through the maternal abdomen.
Bleeding from the umbilical cord after cordocentesis is common (23.1%, Bovicelli10; 29%, Weiner11; 41%, Daffos17), and the duration of bleeding from the artery is reported to be longer than that from the vein.11 Differences among centers with respect to bleeding may relate to the size of the needle used (25-gauge, Bovicelli; 22-gauge, Weiner; 20-gauge, Daffos) and the technique employed (needle-guided, Bovicelli and Weiner; freehand, Daffos). The needle-guided technique likely reduces trauma to the cord because of the lack of lateral mobility of the needle. In fact, there is no correlation between the fetal platelet count and the incidence or duration of bleeding from the puncture site when the needle-guided technique is used.23
The risk of amnionitis after cordocentesis should be equal to the risk of amnionitis after amniocentesis if an equal number of skin to amniotic sac punctures are made and the time and amount of manipulation required to obtain the sample is the same. The risk of amnionitis is approximately 1% when the freehand technique is used and less than 0.3% when the needle-guided technique is used.11 Rarely, chorioamnionitis can lead to the development of maternal sepsis and adult respiratory distress syndrome.24
The umbilical cord may appear hyperechoic after puncture secondary either to microscopic air bubbles or to the extravasation of blood.25 Hematomas of the cord have been observed in pathologic specimens26 with the freehand technique, although most are not associated with adverse sequelae. Using the needle guide, we have observed neither hematoma nor laceration in any of the cords after the nine emergency cesarean deliveries performed after a diagnostic cordocentesis at the University of Iowa.
Fetal-maternal transfusion has been reported after both cordocentesis and amniocentesis, especially when the placenta was anterior.14 It is imperative that Rh-negative women be given Rh immunoglobulin after a procedure unless the fetus is known to be Rh-negative or the patient is already sensitized.
The safety of cordocentesis is believed to be both technique-dependent and experience-dependent. Fewer punctures are reported with the freehand technique, but a lower fetal/neonatal loss rate is reported with the needle-guided technique. The rate of bleeding is reported to be reduced with the needle-guided technique and with the use of smaller gauge needles. Several investigators have found no relationship between success rate or complication rate and the level of experience of the operator.20,27 Boulot and colleagues,21 however, did report a definite learning curve with the freehand technique, with a greater number of complications occurring among their first 30 procedures.
Although teaching models have been described,28 the best experience probably is gained by the practice of ultrasound-guided amniocentesis or chorionic villi sampling. Once the operator is comfortable with these techniques, cordocentesis can be attempted under supervision in fetuses with lethal anomalies. This sequence is followed by supervised diagnostic and finally therapeutic procedures. There is no absolute number of supervised cordocenteses necessary before one can safely proceed alone because each operator has his or her own learning curve. Supervisors must decide when an operator is competent to carry out this invasive procedure unsupervised. Supervision includes assessing whether the procedure has been appropriately chosen and that the patient has been adequately counseled. In general, it takes at least one procedure per month to maintain skill. Cordocentesis should be performed only in centers with active fetal diagnostic and treatment units where the number of procedures allow operators to maintain their skills.
INDICATIONS FOR CORDOCENTESIS
The four most common indications for diagnostic cordocentesis are as follows:
- To obtain a rapid karyotype
- To manage fetal hemolytic disease
- To work up severe early-onset fetal growth deficiency
- To confirm suspected congenital infection.
The remaining indications for cordocentesis constitute less than 5% of the total number of procedures done (Table 2).
TABLE 2. Indications for Diagnostic Cordocentesis*
Indication | Percent |
Rapid Karyotype | 50.7 |
Hemolytic Disease | 33.7 |
Severe growth retardation | 21.7 |
Congenital infection | 16.9 |
Miscellaneous indications | 4.2 |
*There are no significant differences in the distribution of indications among institutions. Categories are not exclusive. For example, a fetus with severe growth deficiency would have a karyotype and a search for infection performed.
(Weiner CP, Okamura K: Diagnostic fetal blood sampling—technique related losses. Fetal Diagn Therapy, in press 1996)
Rapid Karyotype
The most common indication for cordocentesis is a rapid karyotype after the sonographic detection of a fetal anomaly. The risk of an abnormal fetal karyotype associated with a structural abnormality, early-onset growth deficiency, or severe oligohydramnios varies from 12% to 24%.29,30 Knowledge of an abnormal fetal karyotype has the following effects on pregnancy management:
- The selected mode of delivery may be changed if fetal distress is detected.
- The hospital of delivery may be changed if the patient has been referred from a level-one or level-two facility.
- The need for repetitive and expensive antenatal testing may be eliminated.
- Accurate counseling regarding the recurrence risk (an option that may be lost if a macerated stillborn fetus is delivered) is facilitated.
- The option of pregnancy termination is afforded.
Although other fetal samples can be used to provide a karyotype, and in some laboratories may be tested within the same time frame, cordocentesis also permits a search for other causes of the abnormality (e.g., infection causing hydrocephalus). Fluorescent in situ hybridization techniques allow for the rapid diagnosis of specific chromosomal abnormalities from uncultured amniocytes or trophoblasts. As this technique improves, the need to perform cordocentesis for a rapid karyotype likely will be reduced.
Red Blood Cell Alloimmunization
The management of fetal hemolytic disease is made easier by cordocentesis. Previously, obstetricians had to rely on amniotic fluid  OD450 measurements to predict fetuses at risk for anemia. At a given gestational age, however, there is wide variation in fetal hematocrit and optical density. It has been estimated that in 2.1%31 of cases the
OD450 measurements to predict fetuses at risk for anemia. At a given gestational age, however, there is wide variation in fetal hematocrit and optical density. It has been estimated that in 2.1%31 of cases the 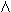 OD450 result could lead to a life-threatening error in management. A fetal blood sample is used to confirm that the fetus is antigen-positive and to obtain a fetal reticulocyte count, a direct Coombs' test, and a total bilirubin level for the assignment of risk (Table 3). In addition, cordocentesis has provided a safe and easy means of delivering red blood cells to the fetus, obviating the need for fetal intraperitoneal transfusion. This has increased the survival of the hydropic fetus from 70% to greater than 90%. Evaluation of fetal hemolytic anemia is the most common indication for cordocentesis at the University of Iowa.
OD450 result could lead to a life-threatening error in management. A fetal blood sample is used to confirm that the fetus is antigen-positive and to obtain a fetal reticulocyte count, a direct Coombs' test, and a total bilirubin level for the assignment of risk (Table 3). In addition, cordocentesis has provided a safe and easy means of delivering red blood cells to the fetus, obviating the need for fetal intraperitoneal transfusion. This has increased the survival of the hydropic fetus from 70% to greater than 90%. Evaluation of fetal hemolytic anemia is the most common indication for cordocentesis at the University of Iowa.
TABLE 3. Criteria for Repeat Cordocentesis of Affected Fetus
| Interval for |
|
| ||||
| Cordocentesis | Interval for |
| ||||
Pattern | Hematocrit | Reticulocytes | and/or | DC | (Weeks) | Scan (Weeks) | Comments |
1 | Normal | Normal |
| -/tr | — | 4 | Repeat if initial maternal |
|
|
|
|
|
|
| indirect Coombs' test |
|
|
|
|
|
|
| <128 and 2-fold |
2 | Normal | Normal or <2.5 |
| 1+/2+ | 5–6 | 2 | Do not repeat after 32 |
|
| percentile |
|
|
|
| weeks if studies unchanged. |
|
|
|
|
|
|
| Delivery at term. |
3 | Normal | >97.5 percentile |
| 3+/4+ | 2 | 1 | Continue through 34 |
|
|
|
|
|
|
| weeks if hematocrit |
|
|
|
|
|
|
| stable. Deliver at |
|
|
|
|
|
|
| 37–38 weeks if not |
|
|
|
|
|
|
| transfused. |
4 | <2.5 percentile | Any |
| Any | 1–2 | 1 | Repeat as long as |
| but >30% |
|
|
|
|
| hematocrit criteria fulfilled. |
|
|
|
|
|
|
| Deliver with pulmonary |
|
|
|
|
|
|
| maturity not transfused. |
DC = direct Coombs' test.
(Weiner CP, Williamson RA, Wenstrom KD et al: Management of fetal hemolytic disease by cordocentesis: I. Prediction of fetal anemia. Am J Obstet Gynecol 165:546, 1991)
Severe Early-Onset Growth Restriction
Cordocentesis aids in the evaluation of severe, early-onset fetal growth restriction by providing a rapid karyotype and samples to search for infection. The most common karyotype abnormalities found are triploidy, trisomy 13, trisomy 18, and trisomy 21.32 The incidence of aneuploidy with isolated early-onset growth deficiency approximates 7%.32 The incidence of documented infection is approximately 5%.33 The incidence of infected fetuses, however, is expected to increase as techniques for detection improve. Polymerase chain reaction techniques may reduce the need for cordocentesis if an amniotic fluid sample is sufficient.
Cordocentesis also provides a sample for blood gas determination. Although a normal umbilical Doppler resistance index in the setting of fetal growth restriction all but excludes fetal hypoxemia, a high index does not necessarily mean hypoxemia. Fetal acidemia would indicate the need for an immediate cesarean section.34 The disadvantage of cordocentesis in the fetus with severe earlyonset growth restriction is the increased risk of bradycardia (17%).11
To date, there is no proven therapy for the long-term treatment of fetal hypoxemia secondary to severe early-onset growth restriction. Maternal hyperoxygenation35 can provide short-term improvement. Repetitive cordocentesis to document acid-base status is not recommended because of the high risk of bradycardia. Because the fetal pH reportedly correlates with subsequent neurologic outcome,36 the finding of significant fetal hypoxemia may be an indication for delivery in an attempt to avoid damage.
Congenital Infection
Cordocentesis originally was developed by Daffos and associates17 to detect fetal toxoplasmosis. It now has been applied to the diagnosis of infections such as congenital rubella, cytomegalovirus, varicella, parvovirus, coxsackievirus, and human immunodeficiency virus (HIV).37 Viral-specific immunoglobulin M (IgM) can be detected after 20- to 22-weeks' gestation38; however, a negative IgM does not rule out infection. There is ample evidence that some infected fetuses may fail either to mount an IgM response or to sustain the response. If testing is done before 20-weeks' gestation, or if the IgM is negative, then a given virus should be sought by culture, electron microscopy, antigen detection, or DNA or RNA probes. Cordocentesis can be used to diagnose and treat human parvovirus infection with associated hydrops secondary to fetal anemia. It also can provide indirect evidence of infection through the measurement of platelet number, white blood cell count and differential, and liver enzymes. Controversy exists regarding whether or not HIV-infected persons should be tested because of the possibility of infecting the fetus; however, the transfer of other viruses from mother to fetus secondary to cordocentesis has not been reported.
Miscellaneous Indications
Cordocentesis also has been used to diagnose fetal thrombocytopenia in pregnancies complicated by immune thrombocytopenia. With increasing knowledge that the risk of an antenatal fetal hemorrhage secondary to immune thrombocytopenia is exceedingly low if not zero and that the fetal platelet count should not influence mode of delivery,39 it is clear that the risk of cordocentesis far outweighs the risk of fetal thrombocytopenia. Therefore cordocentesis is not indicated for pregnancies complicated by immune thrombocytopenia.
In instances of platelet alloimmunization or alloimmune thrombocytopenia, cordocentesis has become the mainstay for both the diagnosis and the evaluation of treatment. Nonresponders to maternally administered human immune globulin intravenous (IGIV) or corticosteroids, or both, have been treated with weekly platelet transfusions.40 Despite the lack of increase in the platelet count in approximately one third of IGIV-treated persons, antenatal fetal intracranial hemorrhage has yet to be reported.41 This suggests that the endothelial defect associated with alloimmune thrombocytopenia is somehow stabilized by IGIV with or without steroids. A normal platelet count documented by cordocentesis at term will permit the patient to undergo a trial of labor. If the platelet count is less than 50,000, a platelet transfusion may be performed, and the patient may be allowed a trial of labor.
Fetal thyroid function has been determined in women with circulating thyroid-stimulating immunoglobulin, and fetuses with hyperthyroidism have been treated successfully with maternal propylthiouracil (PTU) therapy.42 The finding of a fetal goiter may indicate hypothyroidism, which can be treated with intra-amniotic T4.43 Other forms of fetal therapy (e.g., treatment of fetal supraventricular tachycardia [SVT]) can be evaluated with cordocentesis.44
Several past indications for cordocentesis (e.g., hemoglobinopathies) now are being replaced by DNA diagnosis in the first and second trimester with the use of either chorionic villi sampling or amniocentesis. Blood analysis is reserved for families whose DNA studies are not informative or who are referred late and have not been studied previously. Because the field of molecular genetics continues to evolve rapidly, it is best to consult a geneticist for up-to-date information if a question is presented regarding the need for a fetal blood sample for the diagnosis of a genetic condition.
THE FUTURE OF CORDOCENTESIS
Will cordocentesis suffer the same fate as fetoscopy—here one day, gone another—or will the indications for cordocentesis gradually be refined, limiting its practice to situations not suitable for genetic technology or noninvasive fetal testing? The decade of cordocentesis has given us a unique opportunity to study the fetus. It has contributed to our understanding of human fetal physiology, metabolism, and disease. It has allowed the correlation of blood gas data with Doppler and fetal heart rate patterns.45 However, as molecular genetics shrinks the role of cordocentesis for prenatal diagnosis of hereditary disease and as cytogenetic techniques make inroads that supplant the need for fetal blood sampling to obtain a rapid karyotype, it is likely that there will be fewer indications for cordocentesis in the next decade. A diminished role for cordocentesis will demand further regionalization of care in order for some persons to maintain the skill and further the knowledge base of normal fetal physiology and fetal disease.
REFERENCES
Daffos F, Capella-Pavlovsky M, Forestier F: A new procedure for fetal blood sampling in utero: Preliminary results of fifty-three cases. Am J Obstet Gynecol 146: 985, 1983 |
|
Freda VJ, Adamsons K Jr: Exchange transfusion in utero. Am J Obstet Gynecol 89: 817, 1964 |
|
Asensio SH, Figueroa-Longo JG, Pelegrina IV: Intrauterine exchange transfusion. Am J Obstet Gynecol 95: 1129, 1966 |
|
Valenti C: Endoamnioscopy and fetal biopsy: A new technique. Am J Obstet Gynecol 114: 561, 1972 |
|
Valenti C: Antenatal detection of hemoglobinopathies. Am J Obstet Gynecol 115: 851, 1973 |
|
Patrick JE, Perry TB, Kinch RAH: Fetoscopy and fetal blood sampling: A percutaneous approach. Am J Obstet Gynecol 119: 539, 1974 |
|
Rodeck CH: Fetoscopy guided by real-time ultrasound for pure fetal blood samples, fetal skin samples, and examination of the fetus in utero. Br J Obstet Gynaecol 87: 449, 1980 |
|
Rodeck CH, Kemp JR, Holman CA et al: Direct intravascular fetal blood transfusion by fetoscopy in severe Rhesus isoimmunisation. Lancet 1: 625, 1981 |
|
Bang J, Bock JE, Trolle D: Ultrasound-guided fetal intravenous transfusion for severe rhesus haemolytic disease. Br Med J 284: 373, 1982 |
|
Bovicelli L, Orsini LF, Grannum PAT et al: A new funipuncture technique: Two-needle ultrasound- and needle biopsy-guided procedure. Obstet Gynecol 73: 428, 1989 |
|
Weiner CP, Wenstrom KD, Sipes SL, Williamson RA: Risk factors for cordocentesis and fetal intravascular transfusion. Am J Obstet Gynecol 165: 1020, 1991 |
|
Nicolini U, Nicolaidis P, Fisk NM et al: Fetal blood sampling from the intrahepatic vein: Analysis of safety and clinical experience with 214 procedures. Obstet Gynecol 76: 47, 1990 |
|
Westgren M, Selbing A, Stangenberg M: Fetal intracardiac transfusions in patients with severe rhesus isoimmunization. Br Med J 296: 885, 1988 |
|
Nicolini U, Kochenour NK, Greco P et al: Consequences of feto-maternal hemorrhage following intrauterine transfusion. Br Med J 297: 1379, 1988 |
|
Forestier F, Cox WL, Daffos F, Rainaut M: The assessment of fetal blood samples. Am J Obstet Gynecol 158: 1184, 1988 |
|
Ghidini A, Supulveda W, Lockwood CJ, Romero R: Complications of fetal blood sampling. Am J Obstet Gynecol 168: 1339, 1993 |
|
Daffos F, Capella-Pavlovksy M, Forestier F: Fetal blood sampling during pregnancy with use of a needle guided by ultrasound: A study of 606 consecutive cases. Am J Obstet Gynecol 153: 655, 1985 |
|
Maxwell DJ, Johnson P, Hurley P et al: Fetal blood sampling and pregnancy loss in relation to indication. Br J Obstet Gynaecol 98: 892, 1991 |
|
Romero R, Pilu G, Jeanty P et al (eds): Prenatal Diagnosis of Congenital Anomalies. Norwalk, Appleton & Lange, 1988 |
|
Weiner CP, Okamura K: Diagnostic fetal blood sampling—technique releted losses. Fetal Diagn Therapy (in press 1996) |
|
Boulot P, Deschamps F, Lefort G et al: Pure fetal blood samples obtained by cordocentesis: Technical aspects of 322 cases. Prenat Diagn 10: 93, 1990 |
|
Bald R, Chatterjee MS, Gembruch U et al: Antepartum fetal blood sampling with cordocentesis: Comparison with chorionic villus sampling and amniocentesis in diagnosing karyotype anomalies. J Reprod Med 36: 655, 1991 |
|
Weiner CP: Fetal blood sampling and fetal thrombocytopenia. Fetal Diagn Therapy 10: 173, 1995 |
|
Wilkins I, Mezrow G, Lynch L et al: Amniotic and life-threatening respiratory distress after percutaneous umbilical blood sampling. Am J Obstet Gynecol 160: 427, 1989 |
|
Jauniaux E, Nicolaides KH, Campbell S, Hustin J: Hematoma of the umbilical cord secondary to cordocentesis for intrauterine fetal transfusion. Prenat Diagn 10: 477, 1990 |
|
Janniaux E, Donner C, Simon P et al: Pathologic aspects of the umbilical cord after percutaneous umbilical blood sampling. Obstet Gynecol 73: 215, 1989 |
|
Perry KG Jr, Hess LW, Roberts WE et al: Cordocentesis (funipuncture) by maternal-fetal fellows: The learning curve. Fetal Diagn Ther 6: 87, 1991 |
|
Timor-Tritsch IE, Yeh MN: In vitro training model for diagnostic and therapeutic fetal intravascular needle puncture. Am J Obstet Gynecol 157: 858, 1987 |
|
Shah DM, Roussis P, Ulm J et al: Cordocentesis for rapid karyotyping. Am J Obstet Gynecol 162: 1548, 1990 |
|
Weiner CP: Cordocentesis for diagnostic indications: Two years' experience. Obstet Gynecol 70: 664, 1987 |
|
Bowman JM: Rh immune disease: Diagnosis, management and prevention. In Sciarra JJ (ed): Gynecology and Obstetrics, Vol 3, Chap 66. Philadelphia, JB Lippincott, 1991 |
|
Eydoux P, Choiset A, LePorri N et al: Chromosomal prenatal diagnosis: Study of 936 cases of intrauterine abnormalities after ultrasound assessment. Prenat Diagn 9: 255, 1989 |
|
Weiner CP: Fetal growth deficiency and its evaluation. In: James DK, Steer PJ, Weiner CP, Gonik B (eds): High Risk Pregnancy: Management Options, pp 757–770. Philadelphia, WB Saunders, 1994 |
|
Pardi G, Cetin I, Marconi AM et al: Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med 328: 692, 1993 |
|
Nicolaides KH, Campbell S, Bradley RJ et al: Maternal oxygen therapy for intrauterine growth retardation. Lancet 1: 942, 1987 |
|
Soothill PW, Ajayi RA, Campbell S et al: Relationship between fetal acidemia at cordocentesis and subsequent neurodevelopment. Ultrasound Obstet Gynecol 2: 80, 1992 |
|
Valente P, Sever JL: In utero diagnosis of congenital infections by direct fetal sampling. Isr J Med Sci 30: 414, 1994 |
|
Grose C, Itani O, Weiner CP: Prenatal diagnosis of fetal infection: Advances from amniocentesis to cordocentesis—congenital toxoplasmosis, rubella, cytomegalovirus, varicella virus, parvovirus and human immunodeficiency virus. Pediatr Infect Dis J 8: 459, 1989 |
|
Burrows RF, Kelton JG: Pregnancy in patients with idiopathic thrombocytopenic purpura: Assessing the risks for the infant at delivery. Obstet Gynecol Surv 48: 781, 1993 |
|
Nicolini U, Tannirandorn Y, Gonzalez P et al: Continuing controversy in alloimmune thrombocytopenia: Fetal hyperimmunoglobulinemia fails to prevent thrombocytopenia. Am J Obstet Gynecol 163: 1144, 1990 |
|
Bussel JB, McFarland JG, Berkowitz R: Antenatal management of fetal alloimmune and autoimmune thrombocytopenia. Trans Med Rev IV:149, 1990 |
|
Wenstrom KD, Weiner CP, Williamson RA, Grant SS: Prenatal diagnosis of fetal hyperthyroidism using funipuncture. Obstet Gynecol 76: 513, 1990 |
|
Perelman AH, Johnson RL, Clemons RD et al: Intrauterine diagnosis and treatment of fetal goitrous hypothyroidism. J Clin Endocrinol Metab 71: 618, 1990 |
|
Weiner CP, Thompson MIB: Direct treatment of fetal supraventricular tachycardia after failed transplacental therapy. Am J Obstet Gynecol 158: 570, 1988 |
|
Visser GHA, Sadovsky G, Nicolaides KH: Antepartum heart rate patterns in small-for-gestational-age third-trimester fetuses: Correlations with blood gas values obtained at cordocentesis. Am J Obstet Gynecol 162: 698, 1990 |