Dermatologic Diseases in Pregnancy
Authors
INTRODUCTION
Pregnancy may result in a number of cutaneous changes, ranging from physiologic alterations in pigmentation to serious dermatologic diseases. These can be divided into physiologic changes, which include pigmentary, vascular, structural, and appendageal alterations and the specific dermatitides that may develop only during pregnancy or the postpartum period. These entities include polymorphic eruption of pregnancy, pemphigoid gestationis, impetigo herpetiformis, and cholestasis of pregnancy, plus the less well understood prurigo of pregnancy, papular dermatitis of pregnancy, and pruritic folliculitis of pregnancy.1, 2, 3, 4, 5
PHYSIOLOGIC CUTANEOUS CHANGES IN PREGNANCY
Pregnancy is characterized by significant hormonal, immunologic, and metabolic alterations. While the details of these modifications are beyond the scope of this chapter, they play an obvious role in the physiologic changes of pregnancy.6, 7
PIGMENTARY CHANGES
Hyperpigmentation occurs in at least 90% of pregnant women, being more obvious in lighter-skinned women. Pregnant women generally observe a mild generalized hyperpigmentation of the nipples, the areolae, and the vulva. The increase in the melanocyte-stimulating hormone, estrogen, and progesterone, each play a role in these changes.8
Linea nigra
Linea alba, a line extending from the xiphoid to the pubic region, hyperpigments during pregnancy to become the “linea nigra.” This darkening usually only diminishes slightly.
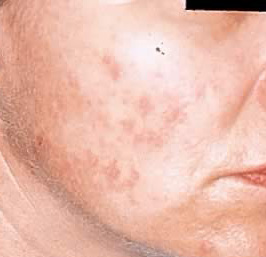
Melasma
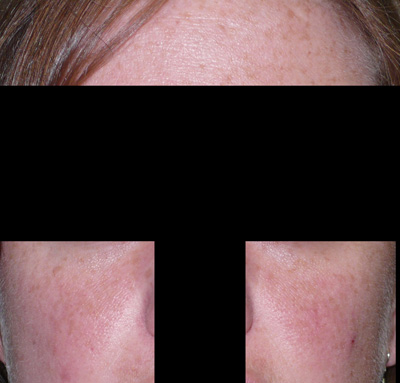
"Mask of pregnancy," or chloasma, develops in 50–70% of pregnant women during the second trimester. Macular pigmentation appears in a symmetrical pattern on the forehead, temples, cheeks, upper lip, and even the chin (Fig. 1). Similar changes can be found in about 20% of women taking oral progestational agents. While this condition may diminish within 1 year after delivery or after discontinuation of oral contraceptives, about 30% of women are bothered by this discoloration for some time. Ultraviolet (UV) exposure increases the problem of melasma. Treatment involves the use of bleaching agents such as hydroquinone 4% cream, sometimes augmented by the addition of topical tretinoin or mequinol and tretinoin solution. Such therapy needs to be continued for many months and should be used in conjunction with sunscreens to prevent further hyperpigmentation.8, 9 Tretinoin, like all retinoids, should not be prescribed during pregnancy,10 although hydroquinone is acceptable for use during pregnancy. Lasers, such as the ruby laser (694 nm) and the Q-switched alexandrite laser (755 nm), have shown unpredictable and limited benefit in the treatment of epidermal-dermal pigmented lesions such as melasma.11
VASCULAR CHANGES
The vascular changes seen in pregnancy are greatly influenced by changes in maternal hormones such as human chorionic gonadotropin (hCG), adrenocorticotrophic hormone (ACTH)-like substance, thyrotropin-releasing hormone, and estrogen. These hormones may trigger increases in cardiac output, vascular proliferation, congestion, and vasomotor instability (Table 1).7, 11, 12
Table 1. Vascular changes in pregnancy
| Spider nevi |
| Palmar erythema |
| Hemangiomas |
| Granuloma gravidarum (pyogenic granuloma) |
| Varicosities |
| Purpura |
Spider nevi
Spider nevi are bright red, blanchable, small arterioles with fine vessels radiating from the center. They are typically observed at the end of the first trimester and gradually increase in size throughout pregnancy. These are mainly seen on the skin drained by the superior vena cava, particularly around the eyes (Fig. 2). Fair-skinned individuals have a higher incidence than African-Americans (67% versus 11%, respectively). Most spider nevi regress postpartum, but a small percentage persists and requires treatment with electrocautery or laser.9
Palmar erythema
Palmar erythema is mottled erythema of the thenar and hypothenar eminences with sparing of the digits. It is seen in two-thirds of pregnant women during the first trimester and resolves in the postpartum period. Palmar erythema may also be seen in other conditions such as lupus erythematosus, hepatic cirrhosis, and hyperthyroidism, indicating a possible role of increased estrogen levels as a common cause.7
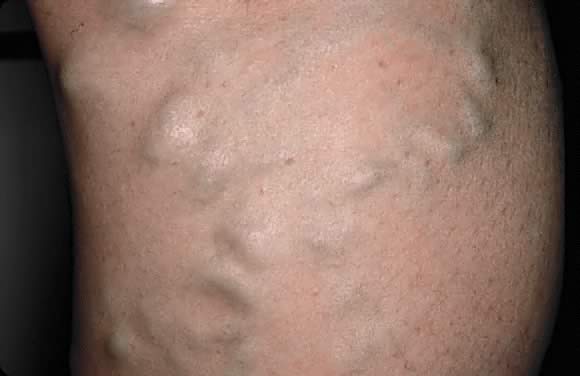
Varicosities
Increased distension in the superficial venous vasculature of legs (i.e. varicose veins), vagina, vestibule (i.e. Jacquemier–Chadwick sign), and rectum (i.e. hemorrhoids) are common in pregnancy. About 40% of pregnant women are affected. Hormonal factors and increased intra-abdominal pressure play a role. Elevation of the legs and lying-in, as in the Trendelenburg position, helps to decrease varicose veins. Varicose veins often regress in the postpartum period; however, sclerotherapy may be necessary (Fig. 3).7, 9
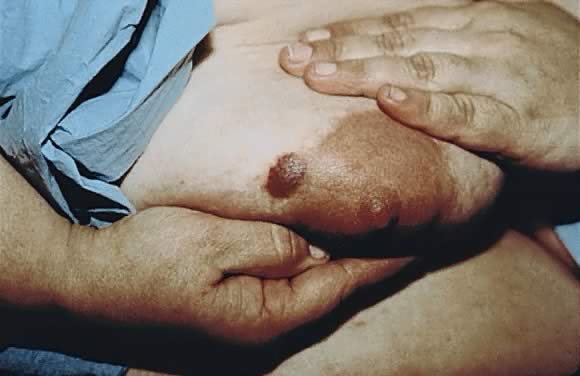
Granuloma gravidarum or pyogenic granuloma
Granuloma gravidarum is a benign, rapidly proliferating vascular lesion that commonly occurs at sites of previous trauma on the face, neck, and fingers (Fig. 4). Pregnancy, irritation, and increased estrogen levels are predisposing factors. Surgical removal can be readily accomplished.13
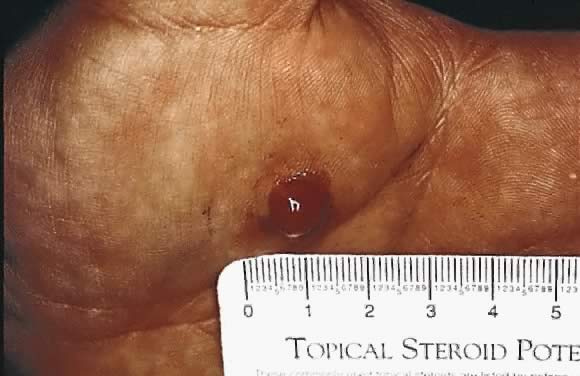
Hemangioma
Hemangiomas occur spontaneously in 5% of pregnancies during the second or third trimesters with a propensity for the hands and neck (Fig. 5).12 Nonpalpable purpura, usually seen on the lower extremities, is also a common finding during the second trimester of pregnancy.14 Both hemangiomas and purpura regress postpartum.
STRUCTURAL CHANGES
Striae gravidarum
Striae gravidarum, or “stretch marks,” occur commonly on the abdomen, thighs, and buttocks of pregnant women. These appear as atrophic, wrinkled, erythematous, purplish bands that fade only slightly over time (Fig. 6). Striae occur in 90% of fair-skinned individuals, usually in the third trimester of pregnancy. The pathogenesis of striae formation is unclear. Proposed hypotheses include increased lateral stress on connective tissue and increased glucocorticoid levels due to elevated adrenocortical activity.7, 15 Currently, there is no effective treatment for striae. Contrary to popular belief, cocoa butter, massage, shea butter, and aloe vera preparations have no efficacy.
Molluscum fibrosum gravidarum
Molluscum fibrosum gravidarum are benign, small, pedunculated, tan-to-brown, fleshy papules similar to acrochordons (skin tags) that are commonly seen on the neck, axillae, inner aspects of the thighs, and inframammary folds. They frequently appear during the second half of pregnancy and may even regress postpartum. Their etiology is unclear. Treatment options include shave excision, electrocautery, cryosurgery, and scissors removal.
APPENDAGEAL CHANGES
Eccrine sweat glands
Eccrine glands, which are involved in the regulation of body temperature through sweating, show a gradual increase in activity during pregnancy. This physiologic change, along with an increase in thyroid activity, may result in hyperhidrosis and increased miliaria.7
Apocrine glands
Apocrine glands are confined to the axillae and perineum. These glands become functional about the time of puberty. Pregnancy results in a decrease in activity of the apocrine glands, with possible improvement of such conditions as hidradenitis suppurativa, which can also be called acne inversa.7
Sebaceous glands
Sebaceous glands are sebum-producing glands associated with hair follicles. These glands demonstrate increased activity in pregnancy, resulting in new-onset or exacerbation of pre-existing acne, although a minority of patients show improvement of acne during pregnancy.9, 16 Treatment of acne during pregnancy is difficult. Most oral and some topical agents used to treat this condition are not recommended during pregnancy. Based upon the severity of the acne, agents commonly prescribed include topical benzoyl peroxide with or without antimicrobials and topical sulfur/sulfacetamide preparations. Use of a cleansing agent, such aqua glycolic astringent, and elimination of moisturizers are most helpful.
Hair
Pregnancy is associated with a decrease in the percentage of hair follicles in the telogen (resting) phase.17 Many women notice an increase in the thickness and body of the scalp hair. Hirsutism, with increased hair growth on the face, arms, legs, and back, may also be seen. Telogen effluvium, which is a delayed telogen response, results in hair loss within 1–2 months postpartum. Hair regrowth is commonly reported at approximately 6 months postpartum. No medical intervention is necessary, and treatment should consist of patient education and reassurance.18
Nails
Nail changes, such as onycholysis, transverse grooving, brittleness, and subungual keratosis, have been reported in pregnancy. The cause of these uncommon findings is unclear.7
SPECIFIC DERMATOSES OF PREGNANCY
There has been great controversy and confusion in the literature when discussing the dermatoses that are unique to pregnancy. Many different names have been used to define clinically similar disorders. Various classifications have been proposed ever since the first contribution was written by Besnier in 1904 (Table 2).19
Table 2. Dermatoses of pregnancy
Classification | Synonyms |
Herpes gestationis | Pemphigoid gestationis |
Pruritic urticarial papules and plaques of pregnancy
| Polymorphic eruption of pregnancy |
Toxemic rash of pregnancy | |
Toxic erythema of pregnancy | |
Late onset prurigo of pregnancy | |
Cholestasis of pregnancy
| Intrahepatic cholestasis of pregnancy |
Obstetric cholestasis | |
Prurigo gravidarum | |
Jaundice of pregnancy | |
Impetigo herpetiformis |
|
Prurigo of pregnancy | Early onset prurigo of pregnancy |
Papular dermatitis of pregnancy |
|
Pruritic folliculitis of pregnancy |
|
Polymorphic eruption of pregnancy
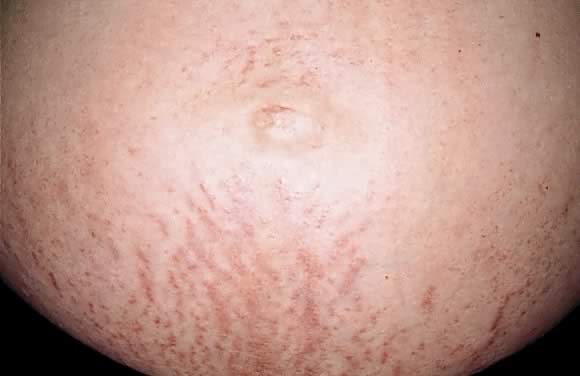
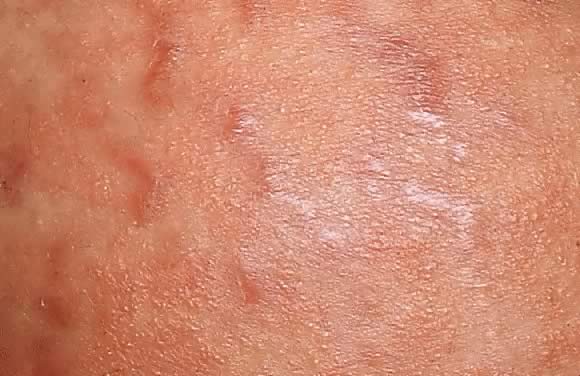
Polymorphic eruption of pregnancy (PEP) was first described by Lawley in 197920 as pruritic urticarial papules and plaques of pregnancy (PUPPP). Both PEP and PUPPP are terms that are interchangeably used, with PEP being preferred in the current literature. PEP is the most common of the dermatoses unique to pregnancy, with an incidence of 1 in 160. Seventy-five to eighty-five per cent of cases occur in primigravidas, who experience an abrupt pruritic onset in the third trimester of pregnancy, most commonly in the 35th to 39th week of gestation or immediately postpartum.21 The eruption begins on the abdomen along the striae distensae, sparing the umbilical and immediate periumbilical area (Figs. 7 and 8).22 This is in contrast to pemphigoid gestationis, in which the majority of cases arise in the umbilical area. PEP may spread to the thighs, buttocks, and extremities, but facial involvement is rare. As the name implies, the skin manifestations are quite variable. These include vesicular, target-like, annular or polycyclic papules or plaques that become confluent over time (Figs. 9 and 10). Three categories have been defined23: type I, urticarial papules and plaques; type II, nonurticarial erythema, papules, or vesicles; and type III, a combination of types I and II. The cause of PEP is unknown. One proposed theory is the rapid stretching of the skin late in pregnancy; this hypothesis is supported by the initial presentation of the eruption along the striae distensae. Increased maternal and newborn weight gain lends support to this theory; there is a higher incidence of PEP in twin pregnancies.22, 24, 25
|
|
|
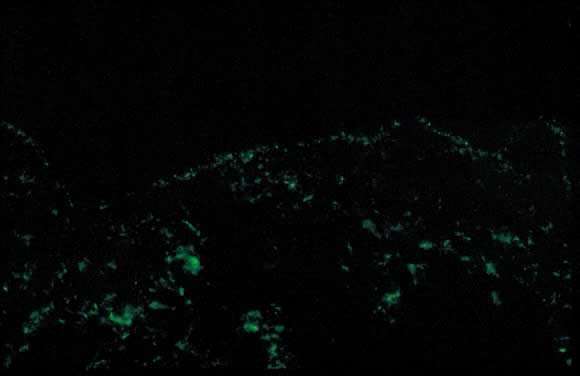
Dermatopathologic examination shows variable epidermal spongiosis with a perivascular inflammatory infiltrate in the dermis composed of lymphocytes, histiocytes, and a variable number of eosinophils. Direct immunofluorescence (DIF) is negative for a linear band of C3 or IgG along the skin dermoepidermal junction (DEJ); however, there have been reports of deposition of IgM, C3, and IgA along the DEJ and blood vessels on DIF (Fig. 11).23, 26 Differential diagnosis of PEP includes pemphigoid gestations (PG), contact dermatitis, drug eruption, and viral exanthems. DIF of skin is necessary to differentiate PEP from PG. The clinical course of PEP is usually self-limiting, with a mean duration of 6 weeks. Pruritus is most severe in the first week of onset, with spontaneous remission occurring within days of parturition. Maternal and fetal mortalities are unaffected. PEP rarely occurs in subsequent pregnancies; however, a few cases of reoccurrence are reported in the literature.21 Treatment involves symptomatic relief of pruritus with topical corticosteroids of low- to mid-potency (use of ultra-high-potency corticosteroids for an extensive period of time should be avoided) and pregnancy category B antihistamines such as loratadine and cetirizine. Hydroxyzine and diphenhydramine are pregnancy category C antihistamines that have been used to relieve pruritus. In cases of severe pruritus unresponsive to conservative measures, systemic corticosteroid administration or induced delivery is considered.
Pemphigoid gestationis
Initially described by Milton in 187222 as “herpes gestationis,” this condition was renamed pemphigoid gestationis (PG) in 1982 due to its clinical and immunofluorescence similarities with bullous pemphigoid.27 Both names continue to be used; pemphigoid gestationis is more common in the United Kingdom.28, 29
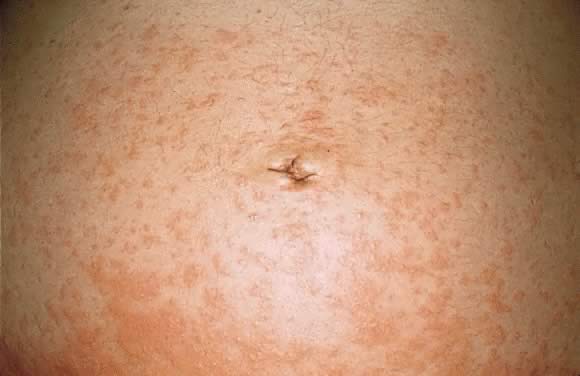
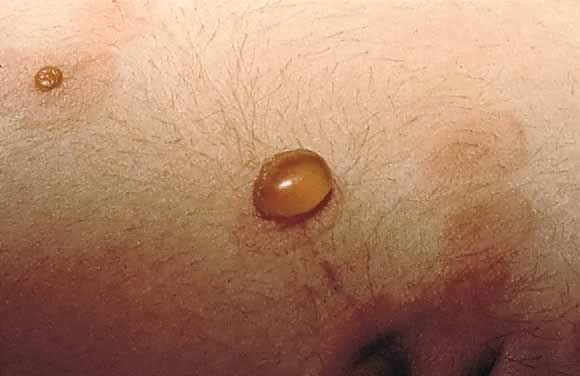
Estimated incidence of PG is 1 in 50,000 cases. Pemphigoid gestationis most commonly occurs in the second or third trimester of pregnancy; about 25% of cases may have an initial presentation immediately postpartum. Clinical presentation is an abrupt onset of an intensely pruritic, urticarial eruption on the trunk that forms tense vesicobullous lesions (Figs. 12 and 13). About 50% of cases have an initial presentation on the abdomen. Umbilical involvement accounts for a significant number of cases of PG. As in PEP, facial and mucosal membrane involvement i rare.29
Accurate diagnosis is crucial in light of the variable clinical course of this disorder. Spontaneous resolution over weeks to months postpartum is a common finding. About 75% of cases of PG present with flares immediately postpartum. Recurrence in subsequent pregnancies with an earlier onset and more severe clinical course is a common feature. Disease-free pregnancies (i.e. “skip pregnancies”) with no cutaneous involvement in patients with a history of PG have occurred. There have also been reports of PG flares developing with menstruation and the use of oral contraceptives (25% of cases).28, 29
PG is an autoimmune disorder caused by the aberrant expression of the MHC II class antigen on the chorionic villi of the placenta, which triggers an allogenic response to the placental basement membrane zone and subsequent cross-reaction with maternal skin through the maternal decidua.30, 31 There are reports of occurrence of PG in association with hydatidiform mole and choriocarcinoma.32, 33 Association of PG with other autoimmune diseases such as Graves' disease has also been reported.34 Studies also show an increased incidence in HLA DR3 and DR4. HLA DR3 occurs in the same percentage of white persons as in African American persons; however, the percentage of DR4 is lower in African-Americans, and this may explain the rarity of PG in this population.35
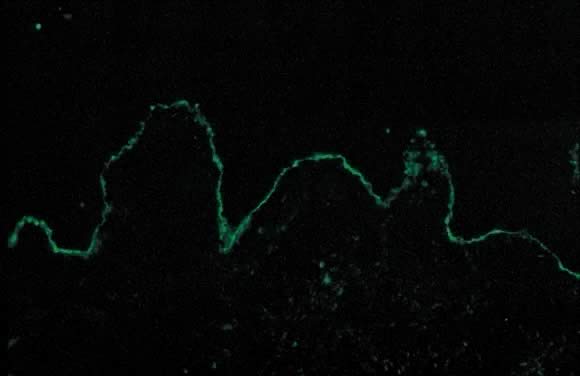
Dermatopathologic examination of PG shows subepidermal vesicle formation with focal necrosis of keratinocytes. The dermis shows papillary edema and a perivascular infiltrate consisting mainly of eosinophils and a few lymphocytes. An occasional finding on histopathology is the alignment of eosinophils along the dermoepidermal junction. DIF shows a characteristic linear band of C3 along the skin basement membrane zone of patients with PG (Fig. 14). Linear C3 deposition on DIF is diagnostic of PG in the correct clinical setting and is used to differentiate PEP from PG.36 Using this method, about 25% of cases also present with IgG deposits along the basement membrane zone. Indirect immunofluorescence (IIF) demonstrates the “PG factor,” which consists of circulating IgG complement-fixing anti-basement membrane zone antibodies in serum of patients with PG. Complement-activated IIF using monoclonal antibodies directed against IgG1 demonstrates this factor in all PG patients.37 Clinically, titers of PG factor do not correlate with disease severity. The PG factor is an IgG directed against a 180-kD hemidesmosomal (transmembrane) component of the basement membrane zone.38 Electron microscopy also demonstrates the C3 and IgG deposits in the lamina lucida.39
|
In PG, 10% of the infants born to affected mothers have skin lesions that resemble PG; this is not the case with PEP. DIF and IIF studies carried out on some of these infants are consistent with a diagnosis of PG.29 There has been considerable controversy in assessing fetal morbidity and mortality associated with PG.40 Recent consensus on infant morbidity indicates a slight increase in prematurity and small size for gestational age.41, 42 The differential diagnosis of PG includes PEP, allergic contact dermatitis, and drug eruption. A precise clinical history accompanied by diagnostic tests such as histopathology and DIF helps to distinguish among these disorders. Treatment options include oral steroids (0.5 mg/kg daily) with a possible increase in dose around the time of delivery to avoid postpartum exacerbations. Other options include plasmapheresis, topical corticosteroids, and antihistamines, all of which offer limited benefit. After delivery, depending upon breastfeeding status, alternative treatments may include dapsone, methotrexate, and cyclosporine.29
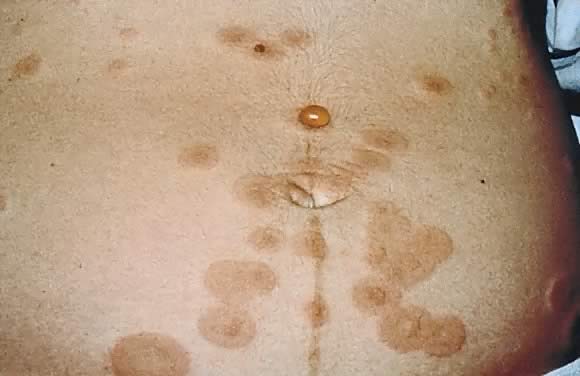
Impetigo herpetiformis
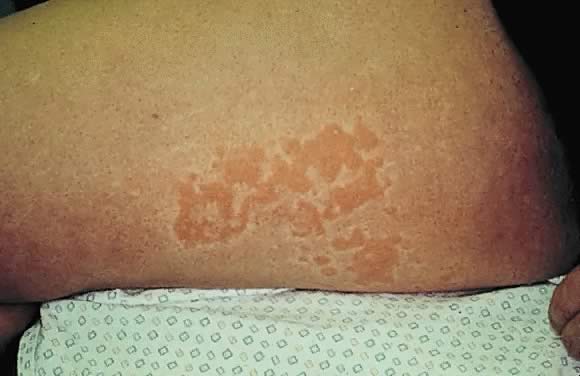
Impetigo herpetiformis is an acute eruption of pustular psoriasis during pregnancy (most often presenting in the third trimester) in individuals with no prior history of psoriasis. The first case was described by Von Hebra in 1872; since then, about 100 cases have been reported.43 Clinical presentation involves sterile pustules on an erythematous base that progressively become more confluent.44 This eruption commonly begins on the flexural and inguinal skin and gradually spreads to the trunk and involves the periumbilical skin (Figs. 15 and 16). Mucous membrane involvement of the oropharynx and the esophagus is also seen. Impetigo herpetiformis is associated with constitutional symptoms such as elevated temperature; gastrointestinal symptoms including nausea, vomiting, and diarrhea; central nervous system symptoms such as delirium; and the musculoskeletal manifestation of tetany due to hypocalcemia.8, 45 Recurrent eruptions in subsequent pregnancies usually present with an earlier onset and more severe course.45 There have also been reports of exacerbation of this condition in affected patients associated with later use of oral contraceptives.46
|
Early diagnosis and treatment is important. The few reported cases are associated with an increased risk of fetal mortality due to placental insufficiency, increased stillbirths, and fetal abnormalities.45 Laboratory findings include evidence of leukocytosis, elevated erythrocyte sedimentation rate, hypoalbuminemia, and hypocalcemia.8 Histopathologic examination of skin biopsy specimens is consistent with pustular psoriasis. The epidermis shows parakeratosis and elongation of rete ridges with spongiform pustules of Kogoj. DIF, as in psoriasis, is negative.45
Treatment involves oral corticosteroids (with limited benefit), correction of hypocalcemia, supportive measures, and antimicrobials to prevent secondary infections. Termination of pregnancy is usually curative.45, 46 Retinoids (isotretinoin) and light therapy are more effective means of treatment that can be used postpartum.
Cholestasis of pregnancy
Cholestasis of pregnancy was initially described by Svanborg47 and Thorling48 in 1954. Cholestasis of pregnancy has been referred to by many other names, including prurigo gravidarum, intrahepatic cholestasis of pregnancy, jaundice of pregnancy, and obstetric cholestasis. The etiology is believed to be multifactorial, and the condition occurs in 0.02–2.4% of pregnancies. Studies show an increased incidence among certain ethnic groups, such as some South American Indians. There is also a seasonal variation in the prevalence of this condition, with a higher incidence in the winter months. Fifty per cent of cases are believed to be familial, and a higher association has been seen in twin pregnancies.49, 50 Another possible factor that contributes to the pathogenesis of this condition is the effect of estrogen and other female hormones on the metabolism and secretion of hepatic bile.8, 49
Cholestasis of pregnancy, as the name implies, occurs only in pregnancy (most commonly during the third trimester) and resolves after delivery, with a 40–60% rate of recurrence in subsequent pregnancies. Clinical presentation includes severe generalized pruritus with no primary skin lesions. Secondary excoriations due to scratching may be the only skin findings. The extent and severity of pruritus fluctuates until the time of delivery.49, 51 Most severe pruritus occurs at night.18 About 20% of patients present with mild jaundice. This condition is the second most common cause of gestational jaundice; viral hepatitis is the most common cause.29 Laboratory values demonstrate elevated levels of bile salts, serum aminotransferases, alkaline phosphatase, and γ-glutamyl transpeptidase.49, 51 Because there are no primary skin lesions, skin biopsy results for histology and DIF are normal.
Pruritus greatly improves after delivery, and complete resolution is achieved within a few days. In cases in which symptoms continue to persist, other causes of cholestasis should be addressed.49 There have been reports of recurrence of symptoms with use of oral contraceptives.51 The differential diagnosis of pruritus in pregnancy should include parasitic infections, allergic skin reactions, and other metabolic disorders.
Fetal and maternal prognosis shows an increase in premature labor and low birth weight. The fetus and the mother are at an increased risk of intracranial and postpartum hemorrhage, respectively, due to deficiency in vitamin K, which results in cases of prolonged fat malabsorption.29, 51 Treatment options range from bed rest, a low-fat diet, and topical emollients in mild cases to the use of agents such as cholestyramine, ursodeoxycholic acid (UDCA), and S-adenosyl-L-methionine in more severe cases. Studies have shown better outcomes for both mother and infant with administration of UDCA compared with placebo. In severe cases, fetal monitoring and cesarean section may be required.51
Prurigo of pregnancy
Prurigo of pregnancy was initially described by Besnier in 190420 as prurigo gestationis. It commonly occurs in the second to third trimester of pregnancy as discrete erythematous excoriated papules on the trunk and the extensor aspect of the lower extremities (Fig. 17). The incidence is roughly 1 in 300 pregnancies. The pathogenesis of this eruption is believed to be the presence of atopy in the pregnant woman. Histolologic examination of skin biopsy specimens shows parakeratosis and mild acanthosis with a mixed inflammatory infiltrate of neutrophils and eosinophils in the perivascular area. DIF results and laboratory values are normal. There is no increased fetal or maternal risk, and treatment involves symptomatic relief with topical corticosteroids and antihistamines.8, 29
|
Papular dermatitis of pregnancy
Papular dermatitis of pregnancy was initially described by Spangler in 196252 as a generalized papular erythematous and pruritic eruption with central crust. The distribution is on the abdomen with spread to the extremities. An increased level of urine hCG and a decrease in the urinary estriol level, in combination with a significant increase in fetal morbidity and mortality, was initially described. Many believe that papular dermatitis of pregnancy and prurigo of pregnancy are similar entities. Histopathology and DIF findings are similar. The high fetal risk initially reported by Spangler52 has not been reproducible in other studies.53
Pruritic folliculitis of pregnancy
Pruritic folliculitis of pregnancy was first described by Zoberman and Farmer in 1981.54 Onset of eruption most commonly occurs in the second or third trimester of pregnancy as small erythematous papules around follicles. The eruption is typically monomorphic with distribution on the trunk and extremities (Fig. 18). Histopathologic examination shows folliculitis, and the DIF is negative. Differential diagnosis involves papular dermatitis or steroid-induced acne. The fetus is unaffected, and the treatment includes topical benzoyl peroxide.8, 54
|
OTHER DERMATOSES AND INFECTIONS AFFECTED IN PREGNANCY
In addition to the dermatoses that are specific to pregnancy, many common dermatoses and infections are affected by the hormonal and immunologic changes seen in pregnancy. These include conditions such as atopic dermatitis, seborrheic dermatitis, psoriasis, condyloma acuminata, and genital herpesvirus infection.
Atopic dermatitis
Atopic dermatitis (atopic eczema) is a chronic inflammatory skin condition with itching. There are no primary lesions, but secondarily, there is erythema, scaling, lichenification, and sometimes papules. With excoriations, there can be oozing, weeping, and secondary bacterial infection. While atopic dermatitis may be associated with hay fever and/or asthma, either in the patient or in a member of the family, there are no allergens to remove in atopic dermatitis. Atopic dermatitis may improve during pregnancy. Treatment involves the use of topical steroids. Soap should be limited to the critical areas: hands, face, axillae, and groin.
Seborrheic dermatitis
Seborrheic dermatitis is characterized by redness and scaling on the scalp, as well as the para-nasal, submental, post-auricular, sternal, inframmamary, axillary, umbilical, and inguinal areas. The cause remains elusive, although there is evidence that yeast of the Malassezia sp is involved, along with interference with the skin barrier. Treatment includes the use of topical corticosteroids, ketoconazole cream, and selenium sulfide foam. Occlusive agents, such as moisturizers and petrolatum, can set off the dermatitis.
Psoriasis
Psoriasis is a chronic inflammatory and proliferating skin condition that presents as sharply demarcated erythematous plaques with silvery scale. The effects of pregnancy on psoriasis are variable. Retrospective studies usually show improvement or no change in this skin condition with pregnancy.55, 56 Treatment options include topical corticosteroids, calcipotriol, and tar. Oral agents such as methotrexate, hydroxyurea, and retinoids are contraindicated during pregnancy. The new biologics should also be avoided during the pregnant state, although some experts have found the use of the biologics to outweigh the possible risks.
Human papillomavirus infection
The human papillomavirus (HPV) is a large virus, of which over 40 types are pathologic in humans. Warts can occur in any part of the body and may be referred to as verrucae vulgaris. In the anogenital area, these are commonly called condylomata acuminata, which often present as flesh-colored, exophytic, cauliflower-like masses. The amount of viral DNA is also greatly increased during pregnancy. Recognition of HPV infection is important because certain strains can be transmitted to the fetus through an infected birth canal, with subsequent association with juvenile respiratory papillomatosis in infants born to infected mothers. Respiratory papillomatosis in infants is rare compared with the extent of condylomas in childbearing women; thus, performance of cesarean section in this situation is controversial due to the inherent risks of the procedure itself. Treatment options during pregnancy include trichloroacetic acid or salicyclic acid topical preparations, cryotherapy, cautery, or laser ablation. Other topical agents such as podophyllin are contraindicated during pregnancy. No treatment is more than 70% effective, because the virus remains within the body for a lifetime.57
Herpes simplex virus infection
Herpes simplex virus (HSV) is a common cause of viral infections worldwide. HSV-1 and HSV-2 cause both primary and recurrent infections; primary infections are more severe. Infection clinically presents as grouped vesicles on an erythematous base that may erode and form ulcerations. Lesions frequently occur around the month, where they are referred to as cold sores, fever blister, or more properly herpes simplex labialis. Asymptomatic shedding of the herpesvirus has also been shown in the absence of any skin findings. Genital herpes infection (herpes progenitalis) at the time of delivery is associated with a high risk of neonatal infection. Even in the absence of skin lesions in infected newborns, neurologic and visceral organ damage can be severe. Recognition and treatment of herpes infection during pregnancy is very important. Patients considered high risk for HSV infection should be tested weekly with viral cultures, and if there is evidence of active infection or viral shedding, cesarean delivery should be performed.20 Acyclovir is a pregnancy category C antiviral agent that is used for primary or symptomatic infections. Valacyclovir (pregnancy category B) is also used in the treatment of HSV during pregnancy.
REFERENCES
Parish LC, Brenner S, Ramos-e-Silva M, Parish JL: Manual of Gender Dermatology, pp 1-310 Sudbury, MA, Jones and Bartlett Learning, 2010. |
|
Oumeish OY, Parish JL: Pregnancy and the skin. Clin Dermatol 2006 Jan-Feb; 24(1):78-79, 2006 |
|
Parish LC, Brenner S, Ramos-e-Silva M, Parish JL: Atlas of Women's Dermatology: from infancy to maturity, pp 123-125. New York, Taylor & Francis, 2006 |
|
Roth MM: Pregnancy dermatoses: diagnosis, management, and controversies. Am J Clin Dermatol. 2011 Feb 1;12(1):25-41. |
|
Vaughan Jones SA, Hern S, Nelson-Piercy C et al: A prospective study of 200 women with dermatoses of pregnancy correlatingclinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999 Jul;141(1):71-81. |
|
Sceppa JA, Smith BL, Marghoob AA et al: Melanosis of the areola and nipple. J Am Acad Dermatol. 2008 Aug;59(2 Suppl 1):S33-4. |
|
Elling SV, Powell FC: Physiological changes in the skin during pregnancy. Clin Dermatol. 1997;15: 35–43. |
|
Muallem MM, Rubeiz NG: Physiological and biological changes in pregnancy. Clin Dermatol 2006;24:80-84. |
|
Winton GB, Lewis CW: Dermatoses of pregnancy. J Am Acad Dermatol. 1982;6:977-998. |
|
Colley SM, Walpole I, Fabian VA, Kakulas BA: Topical tretinoin and fetal malformations. Med J Aust. 1998;168(9):467. |
|
Alster TS, Lupton JR: Lasers in dermatology. An overview of types and indications. Am J Clin Dermatol. 2001;2(5):291-303. |
|
Oumeish OY, Al-Fouzan AS: Miscellaneous diseases affected by pregnancy. Clin Dermatol. 2006;24:113-117. |
|
Vaughan Jones SA, Black MM: Pregnancy-related conditions. In Parish LC, Brenner S, Ramos-e-Silva M. Women's Dermatology: From infancy to maturity, pp 397-413. New York, Parthenon Publishing, 2001 |
|
Murray JC: Pregnancy and the skin. Dermatol Clin. 1990;8:327-334. |
|
Salter SA, Kimball AB: Striae gravidarum. Clin Dermatol. 2005;24:97-100. |
|
Brenner S, Politi Y: Dermatologic diseases and problems of women throughout the life cycle. Int J Dermatol 1995;34:369-379. |
|
Higgins CA, Westgate GE, Jahoda CA. From telogen to exogen: mechanisms underlying formation and subsequent loss of the hair club fiber. J Invest Dermatol. 2009;129:2100-2108 |
|
Millikan L. Hirsutism postpartum telogen effluvium, and male pattern alopecia. Cosmet Dermatol 2006;5:81-86 |
|
Besnier E, Brocq L, Jacquet L: La Pratique Dermatologique, p 75. Paris, Masson et Cie, 1904 |
|
Locksmith G, Duff P: Infection, antibiotics, and preterm delivery. Semin Perinatol. 2001 Oct;25(5):295-309. |
|
Matz H, Orion E, Wolf R: Pruiritic uritcarial papules and plaques of pregnancy: polymorphic eruption. Clin Dermatol 2005;24:105-108 |
|
Ghazeeri G, Kibbi AG, Abbas O. Pruritic urticarial papules and plaques of pregnancy: epidemiological, clinical, and histopathological study of 18 cases from Lebanon. Int Dermatol 2012;51:1047-1053 |
|
Aronson IK, Bond S, Fiedler VC, et al: Pruritic urticarial papules and plaques of pregnancy: Clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol 1998;39:933-939 |
|
Cohen LM, Capeless EL, Krusinski PA, Maloney ME: Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol 1989;125:1534-1536 |
|
Beckett MA, Goldberg NS: Pruritic urticarial plaques and papules of pregnancy and skin distention. Arch Dermatol 1991;127:125-126 |
|
Toussaint S, Kamino H: Noninfectious erythematous, papular, and squamous diseases. In Elder D, Elenitsas R, Jaworsky C, Johnson B (eds): Lever's Histopathology of the Skin, 8th edn, p 153. Philadelphia, Lippincott-Raven, 1997 |
|
Black MM: Refinement of the classification of polymorphic eruption of pregnancy. J Am Acad Dermatol 2008;59:722-723 |
|
Shipman AR, Reddy H, Wojnarowska F. Association between the subepidermal autoimmune blistering diseases linear IgA disease and the pemphigoid group and inflammatory bowel disease: two case reports and literature review. Clin Exp Dermatol 2012;37:461-468 |
|
Shornick JK: Dermatoses of pregnancy. Semin Cutan Med Surg 1998;17:172-181 |
|
Borthwick GM, Holmes RC, Stirrat GM: Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis: Analysis of class II MHC subregion product expression. Placenta 1988;9:81-94 |
|
Ortonne JP, Hsi BL, Verrando P, et al: Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol 1987;117:147-154 |
|
Tindall JG, Rea TH, Shulman I, Quismorio FP: Herpes gestationis in association with a hydatidiform mole. Arch Dermatol 1981;117:510-512 |
|
Slazinski L, Degefu S: Herpes gestationis associated with choriocarcinoma. Arch Dermatol 1982;118:425-428 |
|
Shornick JF, Black MM: Secondary autoimmune diseases in herpes gestationis (pemphigoid gestationis). J Am Acad Dermatol 1992;26:563-566 |
|
Shornick JK, Meek TJ, Nesbitt LT, Gilliam JN: Herpes gestationis in blacks. Arch Dermatol 1984;120:511-513 |
|
Holmes RC, Black MM, Dann J, et al: A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol 1982;106:499-510 |
|
Kelly SE, Cerio R, Bhogal BS, Black MM: The distribution of IgG subclasses in pemphigoid gestationis: PG factor is an IgG1 autoantibody. J Invest Dermatol 1989;92:695-698 |
|
Morrison LH, Labib RS, Zone JJ, et al: Herpes gestationis autoantibodies recognize a 180-kd human epidermal antigen. J Clin Invest 1988;81:2023-2036 |
|
Honigsmann H, Stingl G, Holubar K, Wolff K: Herpes gestationis: Fine structural pattern of immunoglobulin deposits in the skin in vivo. J Invest Dermatol 1977;66:389-392 |
|
Al-Fouzan AS, Galadari I, Oumeish I, Oumeish OY: Herpes gestationis (pemphigoid gestationis). Clin Dermatol 2005;24:109-112 |
|
Holmes RC, Black MM: The fetal prognosis in pemphigoid gestationis (herpes gestationis). Br J Dermatol 1984;110:67-72 |
|
Lipozencic J, Ljubojevic S, Bukvic-Mokos Z. Pemphigoid getationis. Clin Dermatol 2012;30:51-55 |
|
Breier-Maly J, Ortel B, Breier F, et al: Generalized pustular psoriasis of pregnancy (impetigo herpetiformis). Dermatology 1999;198:61-64 |
|
Oumeish OY, Parish JL: Impetigo herpetiformis. Clin Dermatol 2006;25:101-104 |
|
Lotem M, Katzenelson V, Rotem A, et al: Impetigo herpetiformis: A variant of pustular psoriasis or a separate entity? J Am Acad Dermatol 1989;20:338-341 |
|
Huang YH, Chen YP, Liang CC, Chang YL, Hsieh CC. Impetigo herpetiformis with gestational hypertension: a case repor and literature review. Dermatology 2011;222:221-224 |
|
Svanborg A: A study of recurrent jaundice in pregnancy. Acta Obstet Gynecol Scand 1954;33:434-444 |
|
Thorling L: Jaundice in pregnancy: A clinical study. Acta Med Scand 1955;151(Suppl):1-123 |
|
Reyes H: The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterol Clin North Am 1992;21:905-921 |
|
Marschall HU, Shemer EW, Ludvigsson JF, Stephenson O. Intrahepatic cholestasis of pregnancy and associated hepatobiliary disease. A population based cohrt study. Hepatology 2013; Apr 8. doi: 10.1002/hep.26444. [Epub ahead of print] |
|
Reyes H: Review: Intrahepatic cholestasis. A puzzling disorder of pregnancy. J Gastroenterol Hepatol 1997;12:211-216 |
|
Spangler AS, Reddy W, Bardawil WA, et al: Papular dermatitis of pregnancy. JAMA 1962;181:577-581 |
|
Ahmadi S, Powell FC. Pruritic urticarial papules and plaques of pregnancy: curent status. Australas J Dermatol 2005;46:53-58 |
|
Zoberman E, Farmer ER: Pruritic folliculitis of pregnancy. Arch Dermatol 1981;117:20-22 |
|
Landau JL, Moody MN, Kazakevich N, Goldberg LH. Psoriasis and the pregnant woman: what are the key considerations? Skin Therapy Lett 2011;16:1-3 |
|
Mowad CM, Margolis DJ, Halpern AC, et al: Hormonal influences on women with psoriasis. Cutis 1998;61:257-260 |
|
Hamouda T, Freij MA, Saleh M: Management of genital warts in pregnancy. Clin Exp Obstet Gynecol 2012;39:242-244 |