Genetic and Nongenetic Causes of Pregnancy Loss
Authors
INTRODUCTION
A large proportion of embryos never implant, and many that do are lost without clinical recognition of pregnancy. Pregnancy losses are repetitive. Of married women in the United States, 4% have experienced two clinically recognized losses and 3% three or more losses.1 By far the most common etiology for pregnancy loss is genetic, especially cytogenetic. This chapter discusses the frequency and timing of pregnancy loss throughout gestation, the likelihood of recurrence, and the relative likelihood of pregnancy due to genetic and nongenetic causes. Additional updates on these topics are available elsewhere.2
FREQUENCY AND TIMING OF PREGNANCY LOSSES
Embryos implant 6 days after conception, but are not generally recognized clinically until 5–6 weeks after the last menstrual period. Before this time, β-chorionic gonadotropin (hCG) assays can detect preclinical pregnancies. To determine the frequency of losses before clinical recognition, Wilcox and colleagues3 performed daily urinary hCG assays beginning around the expected time of implantation (day 20 of gestation). Of pregnancies detected in this fashion, 31% (61/198) were lost; the preclinical loss rate was 22% (43/198). The clinically recognized loss rate in this cohort was 12% (19/155). These rates are consistent with data gathered by us and colleagues4 in a National Institute of Child Health and Human Development (NICHD) collaborative study using serum β-hCG assays performed 28–35 days after the previous menses. Our cohort was ascertained approximately 10 days later than the date of ascertainment in the sample of Wilcox and colleagues.4 The total loss rate (preclinical and clinical) in the NICHD cohort was lower, at 16%.
Clinically recognized first-trimester fetal loss rates of 10–12% are well documented in both retrospective and prospective cohort studies.5 Higher clinical loss rates reported in some older studies may have reflected misclassification, unwittingly including surreptitious illicit abortions. There is a tendency for recurrent losses to occur around the same time of gestation (e.g., first trimester or other).5
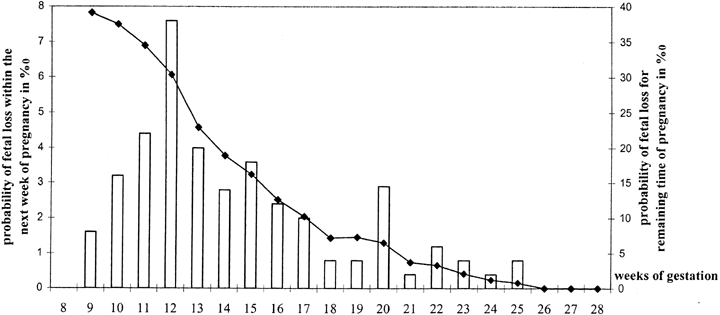
Most clinically recognized losses occur before 8–9 weeks. Information in older studies was based on clinical pregnancy losses that traditionally were not appreciated until 9–12 weeks' gestation, at which time bleeding and passage of tissue (products of conception) occurred. After ultrasonography became widely available, it was shown that fetal demise actually occurred weeks before the time overt clinical signs are manifested. This conclusion was first reached on the basis of cohort studies showing that only 3% of viable pregnancies are lost after 8 weeks' gestation.5 Studies involving obstetric registrants were very similar.6, 7, 8, 9 Given the accepted clinical loss rate of 10–12%, fetal viability must cease weeks before maternal symptoms appear; thus, most fetuses aborting clinically at 9–12 weeks have died weeks previously. That almost all losses are retained in utero for an interval before clinical recognition means most losses are “missed abortions”. This term is probably archaic.
Most pregnancy losses after 8 weeks occur in the following 2 gestational months. This can be deduced from loss rates being only 1% in women confirmed by ultrasound to have viable pregnancies at 16 weeks. An illustration of the declining loss rate with increasing week of gestation is shown in Figure 1.
LIKELIHOOD OF CLINICAL PREGNANCY LOSS
Clinical loss rates reflect many factors, but two associations are worth emphasizing. First, maternal age is positively correlated with pregnancy loss rates. A 40-year-old woman has twice the risk of a 20-year-old woman. This increase occurs in euploid as well as certain aneuploid pregnancies. Second, prior pregnancy history is important. In one study nulliparous women who had never experienced a loss, showed a low rate of 5% (4/87) in primiparas and 4% (3/73) in multiparas.10 The loss rate increases to 25–30% for women with three or more losses (Table 1). Whitley and coworkers11 derived an odds ratio of 3.19 for subsequent loss in women with two prior losses. Parazzini and colleagues12 reached similar findings. These risks apply not only to women whose losses were recognized at 9–12 weeks' gestation, but also to those whose pregnancies were ascertained in the fifth week of gestation.13 Although loss rates are increased among women who have experienced previous losses, they are not nearly as high as once thought. For decades, many obstetricians fervently believed in the concept of “habitual abortion”. Until the 1960s, the risk of subsequent losses was thought to rise sharply after three losses. Such beliefs were based on theoretical calculations made in 1938 by Malpus,14 who concluded that after three abortions the likelihood of a subsequent one was 80–90%. The occurrence of three consecutive spontaneous abortions was thus said for decades to confer on a woman the designation of “habitual aborter”. These theoretically derived risk figures not only proved incorrect, but also and unfortunately were used as the background expectation in clinical studies evaluating various treatment regimens. This practice led to unwarranted acceptance of certain interventions to prevent spontaneous abortion, the most famous of which was diethylstilbestrol (DES). Early in 1964, Warburton and Fraser15 showed that the likelihood of recurrent abortion was not nearly as high as Malpus had calculated. The risk increased to only 25–30% irrespective of whether a woman had previously experienced one, two, three, or even four spontaneous abortions. This concept has been confirmed in many subsequent studies, with the additional observation that if no previous live births have occurred, the likelihood of fetal loss is somewhat higher. Lowest risks (5%) are observed in nulliparous women with no prior losses.16
Table 1. Approximate recurrence risk figures useful for counseling women with repeated spontaneous abortions
| Prior abortions | Risk (%) |
Women with live-born infants | 0 | 5–10 |
| 1 | 20–25 |
| 2 | 25 |
| 3 | 30 |
| 4 | 30 |
Women without live-born infants | 3 | 30–40 |
Recurrence risks are slightly higher for older women, for those who smoke cigarettes or drink alcohol, and for those exposed to high levels of selected chemical toxins.
(Based on data from Warburton D, Fraser FC: Spontaneous abortion risks in man. Am J Hum Genet 16: 1, 1964; Poland BJ, Miller JP, Jones DC et al: Reproductive counseling in patients who had a spontaneous abortion. Am J Obstet Gynecol 127: 685, 1977; Regan L: A prospective study on early abortion. In Beard RW, Sharp F [eds]: Early Pregnancy Loss: Mechanisms and Treatment, p 22. London, Royal College of Obstetricians and Gynaecologists, 1988.)
Women who smoke cigarettes or drink alcohol moderately are probably at slightly higher risk.17 Recurrence risks are higher if the abortus is cytogenetically normal than cytogenetically abnormal.18 Taking all the above into account, the prognosis is reasonably good even without therapy. The predicted success rate is 70% despite two or three prior losses. This favorable success rate has been confirmed often in cohort trials. Vlaanderen and Treffers19 reported pregnancies in each of 21 women having unexplained prior repetitive losses but subjected to no intervention. Similar findings were reported by Liddell and associates20 and Houwert-de Jong and coworkers.21 Of 325 consecutive British women with idiopathic recurrent abortions followed up by Brigham and colleagues,22 70% conceived (n = 222), with 167 pregnancies persisting beyond 24 weeks. Most of the 55 losses occurred before 6–8 weeks. In a National Institutes of Health collaborative immunotherapy trial involving women with a history of losses, the success rate in 92 untreated women (placebo) was 65%.23
To be judged efficacious in preventing spontaneous abortions, therapeutic regimens must therefore show success rates significantly greater than 70%, adjusted for maternal age and other confounding variables. Essentially no therapeutic regimen can make this claim, indicating that almost never should a proposed therapy be promised as efficacious in treating women with two or three first-trimester losses. The situation could well differ if five or more losses occur, but this is unproved.
PREIMPLANTATION LOSSES DUE TO CHROMOSOMAL ABNORMALITIES
Morphologic abnormalities
Establishing an etiology for preimplantation and preclinical losses is not easy, but the one proven explanation is morphologic abnormalities in the early embryo. It is presumed that most are due to chromosomal abnormalities.
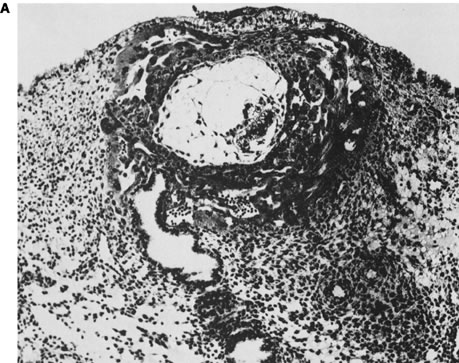
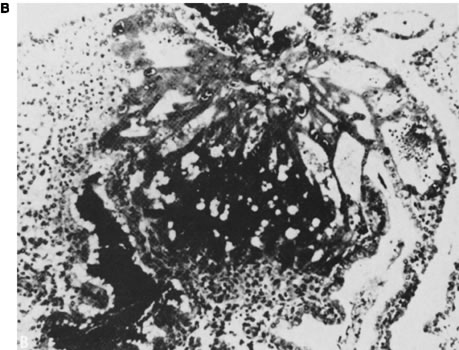
Initial advances were made decades ago by Hertig and associates24, 25, 26 who examined the fallopian tubes, uterine cavities, and endometria of women undergoing elective hysterectomy. Women studied were of proven fertility, with a mean age of 33.6 years. Coital times were recorded before hysterectomy. Eight preimplantation embryos (less than 6 days from conception) were recovered. Four of these eight embryos were morphologically abnormal. The four abnormal embryos presumably would not have implanted, or, if implanted, would not have survived long thereafter. Nine of 26 implanted embryos (6–14 embryonic days) were morphologically abnormal (Fig. 2).
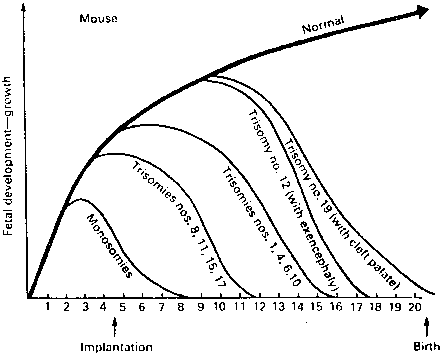
That morphologically abnormal embryos in mammals were likely to result from genetic causes was first shown in elegant mouse studies performed in the 1970s and 1980s by Gropp27 in Germany. Mice heterozygous for a variety of Robertsonian translocations were selectively mated to generate monosomies and trisomies for each chromosome. By sacrifice of pregnant animals at varying gestational ages, survival and phenotypic characteristics of the abnormal complements could be determined. In mice, as in humans, autosomal monosomy proved nonviable. Monosomes aborted around the time of implantation (4–5 days after conception) (Fig. 3). Trisomies usually survived longer but rarely to term. These findings are analogous to those observed in aneuploid human fetuses.
Chromosomal abnormalities in preimplantation embryos
In humans, a high frequency of chromosomal abnormalities began to be observed as soon as it became possible to perform cytogenetic studies on human preimplantation embryos. Embryos were initially available from in vitro fertilization programs in which embryos fertilized were neither transferred nor cryopreserved.28, 29, 30 (Successful cryopreservation techniques were not developed until several years later.) The aneuploidy rate is at least 50% in morphologically normal embryos, aneuploidy is present in 6% of sperm from ostensibly normal men31, 32 and in 20% of oocytes.33, 34 In analysis of polar bodies, the rate is age-dependent but consistent with the overall frequency in embryos.33, 34 Of cytogenetic errors 95% occur in female meiosis, similarly high percentages occur especially in older women.
Errors arise in both meiosis I and II and in contrast to traditional dogma that the basis is chromosome nondisjunction, the cytologic basis is premature chromatid separation.33 Considerable data indicate that approximately 50% of morphogically normal cleavage stage embryos of women undergoing in vitro fertilization (mean age 35–37 years) show chromosomal abnormalities based on fluorescent in situ hybridization (FISH) or, more recently, array comparative genome hybridization (array CGH).34, 35, 36, 37, 38 The frequency is predictability higher if embryos are morphologically abnormal. Absence of blastulation is a more important predictor than a mere delay.39 Overall perhaps one third of aneuploidies present at day 3 fail to persist to day 5, when implantation occurs. New 24 chromosome techniques (array CGH) confirm these data in both younger and older women.37, 38
NUMERICAL CHROMOSOMAL ABNORMALITIES: THE MOST FREQUENT CAUSES OF EARLY PREGNANCY LOSS
At least 50% of clinically recognized pregnancy losses result from a chromosomal abnormality.40 The frequency is probably higher because if one analyzes the chorionic villi recovered by chorionic villus sampling immediately after ultrasound diagnosis of fetal demise (rather than culturing spontaneously expelled products) the chromosomal abnormalities are detected 75–90%.41 Another approach is array comparative genomic hybridization (array CGH), which reveals aneuploidy for all chromosomes and does not require cultured cells.
Among second-trimester losses, one observes chromosomal abnormalities more similar in type to those observed in live-born infants: trisomies 13, 18 and 21; monosomy X; and sex chromosomal polysomies. This also holds among losses after 20 gestational weeks (stillborn infants), in which the frequency of chromosomal abnormalities is approximately 8–13%, exceeding the 20% of anatomic abnormalities.42 This frequency is less than that observed in earlier abortuses but much higher than that found among live-born infants (0.6%). Array CGH of cultured cells recovered from amniocentesis after fetal demise can identify chromosomes in stillbirths without culturing cells. Culturing fetal material derived from stillbirths carries a low rate of success. Overall, the role of genetics in stillbirth is underappreciated.43
Types of numerical chromosomal abnormalities
Autosomal trisomy
Autosomal trisomies comprise the largest (approximately 50%) single class of chromosomal complements in cytogenetically abnormal spontaneous abortions. That is, 25% of all abortuses are aneuploid given half of all abortuses having a chromosomal abnormality. Frequencies of various trisomies are listed in Table 2. Trisomy for every chromosome has been observed. The most common trisomy is trisomy 16. Most trisomies show a maternal age effect, but the effect varies among chromosomes. The increased maternal age effect is greatest for double trisomies. Correlation of placental morphologic abnormalities with specific trisomies is imprecise. By contrast, the histologic features of complete and partial hydatidiform molar gestations are so distinctive that most molar miscarriages can be correctly diagnosed by histologic examination alone.
Table 2. Chromosomal complements in spontaneous abortions recognized clinically in the first trimester
Complement | Frequency (%) | |
Normal 46,XX or 46,XY | 54.1 | |
Triploidy | 7.7 | |
69,XXX | 2.7 | |
69,XYX | 0.2 | |
69,XXY | 4.0 | |
Others | 0.8 | |
Tetraploidy | 2.6 | |
92,XXX | 1.5 | |
92,XXYY | 0.55 | |
Not stated | 0.55 | |
Monosomy X | 8.6 | |
Structural abnormalities | 1.5 | |
Sex chromosomal polysomy | 0.2 | |
47,XXX | 0.05 | |
47,XXY | 0.15 | |
Autosomal monosomy (G) | 0.1 | |
Autosomal trisomy for chromosomes | 22.3 | |
1 | 0 | |
2 | 1.11 | |
3 | 0.25 | |
4 | 0.64 | |
5 | 0.04 | |
6 | 0.14 | |
7 | 0.89 | |
8 | 0.79 | |
9 | 0.72 | |
10 | 0.36 | |
11 | 0.04 | |
12 | 0.18 | |
13 | 1.07 | |
14 | 0.82 | |
15 | 1.68 | |
16 | 7.27 | |
17 | 0.18 | |
18 | 1.15 | |
19 | 0.01 | |
20 | 0.61 | |
21 | 2.11 | |
22 | 2.26 | |
Double trisomy | 0.7 | |
Mosaic trisomy | 1.3 | |
Other abnormalities or not specified | 0.9 | |
| 100.0 |
(Pooled data from several series, as referenced by Simpson JL, Bombard AT: Chromosomal abnormalities in spontaneous abortion. In Edwards K, Benett MJ [eds]: Spontaneous Abortions, p 51. London, Blackwell, 1987.)
Trisomies incompatible with life predictably show slower growth than trisomies compatible with life (e.g., trisomies 13, 18, 21), but otherwise there are usually no features distinguishing the two groups. Abortuses from the former group may show anomalies consistent with those found in full-term live-born trisomic infants. Malformations present have been said to be more severe than those observed in induced abortuses following prenatal diagnosis.
Aneuploidy was once thought to result almost exclusively from errors at meiosis I, specifically maternal meiosis I.44 Errors at both meiosis I and II are associated with maternal age.33 Once thought to involve mostly missegregation of whole chromosomes, chromatid errors (premature chromatid separation) are as stated previously now accepted as the major cause of maternal meiotic errors.33 Irrespective, the cytologic mechanism is thought to involve decreased or absent meiotic recombination, as it also does in sperm. In trisomy 13 and trisomy 21, 90% of these maternal cases arise at meiosis I; almost all trisomy 16 cases arise in maternal meiosis I.44 An exception is trisomy 18, in which two thirds of the 90% of maternal meiotic cases arise at meiosis II.
A practical consequence of these data is that deducing chromosomal status of oocytes by analysis of polar bodies detects 95% of chromosomally abnormal embryos. This is relevant because polar body analysis is a more robust prediction of embryo status than analysis of blastomere from a 3 day embryo. In the latter mitotic nondisjunction can lead to spurious and unrepresentative results. Errors in paternal meiosis account for 10% of the acrocentric (13, 14, 15, 21, 22) trisomies44 and much less in the other trisomies. In trisomy 21, paternal meiotic errors are equally likely to arise in meiosis I or II a circumstance that contrasts with the situation in maternal meiotic errors.44 Among metacentric chromosomes, paternal contribution is uncommon. A surprising exception involves trisomy 2.
As of 2012, consensus now exists that 24 chromosome SNP or array CGH is preferable to FISH for PGD aneuploidy testing. This can be performed on polar bodies, blastomeres or blastocysts (trophectoderm biopsy).37, 38, 39, That aneuploidy is so frequent in embryos carries practical significance for assisted reproductive technologies. Testing embryos for all chromosomes and transferring only euploid embryos not only reduces pregnancy loss,45 but also improves liveborn pregnancy rates. In brief, “idiopathic” miscarriage is usually of aneuploidy explanation.46
Polyploidy
Nonmosaic triploidy (3n = 69) and tetraploidy (4n = 92) are common in abortuses. These polypoidies reflect that more than two haploid chromosomal complements are present. This phenomenon is presumably distinct from the diploid/triploid mosaicism that is found in some 30% of blastocysts.47 Triploid abortuses are usually 69,XXY or 69,XXX, resulting from dispermy. An association exists between diandric (paternally inherited) triploidy and hydatidiform mole, a "partial mole" said to exist if molar tissue and fetal parts coexist. More common is "complete" (classic) hydatidiform mole, which is 46,XX, androgenetic in origin and composed exclusively of villous tissue. Pathologic findings in diandric triploid and tetraploid placentas include a disportionately large gestational sac, focal (partial) hydropic degeneration of placental villi, and trophoblast hyperplasia. Placental hydropic changes are progressive and may be difficult to identify in early pregnancy. By contrast, placental villi often undergo hydropic degeneration after fetal demise. This can occur in all types of miscarriage; thus, histologic and cytogenetic investigations are necessary to differentiate between true and "pseudomole", because only a true mole can be associated with persistent trophoblastic disease. Fetal malformations associated with triploid miscarriage include neural tube defects and omphaloceles, anomalies reminiscent of those observed in triploid conceptuses surviving to term. Facial dysmorphia and limb abnormalities have also been reported. Tetraploidy is uncommon, rarely progressing beyond 2–3 weeks of embryonic life. Triploidy abnormality can also be associated with persistent trophoblastic disease and thus needs to be identified in order to offer hCG follow-up.
Sex chromosomal polysomy (X or Y)
The complements 47,XXY and 47,XYY each occur in about 1 per 800 live born male births; 47,XXX occurs in 1 per 800 female births. X or Y polysomies are only slightly more common in abortuses than in live borns. In pregnancies conceived by intracytoplasmic sperm injection (ICSI), the frequency of 47,XXX and 47,XXY embryos and fetuses seems to be increased.48
Monosomy X
Monosomy X is the single most common chromosomal abnormality among spontaneous abortions, accounting for 15–20% of abnormal specimens. Monosomy X embryos usually consist of only an umbilical cord stump. Anomalies characteristic of Turner syndrome may be seen, such as cystic hygromas and generalized edema (Fig. 4). Unlike live-born 45,X individuals, 45,X abortuses show germ cells; however, germ cell failure thus involves not so much failure of germ cell development as more rapid attrition in 45,X compared with 46,XX embryos.49, 50 Monosomy X usually (80%) occurs as result of parental sex chromosome loss. This observation is consistent with the lack of a maternal age effect in 45,X or possibly even an inverse age effect.
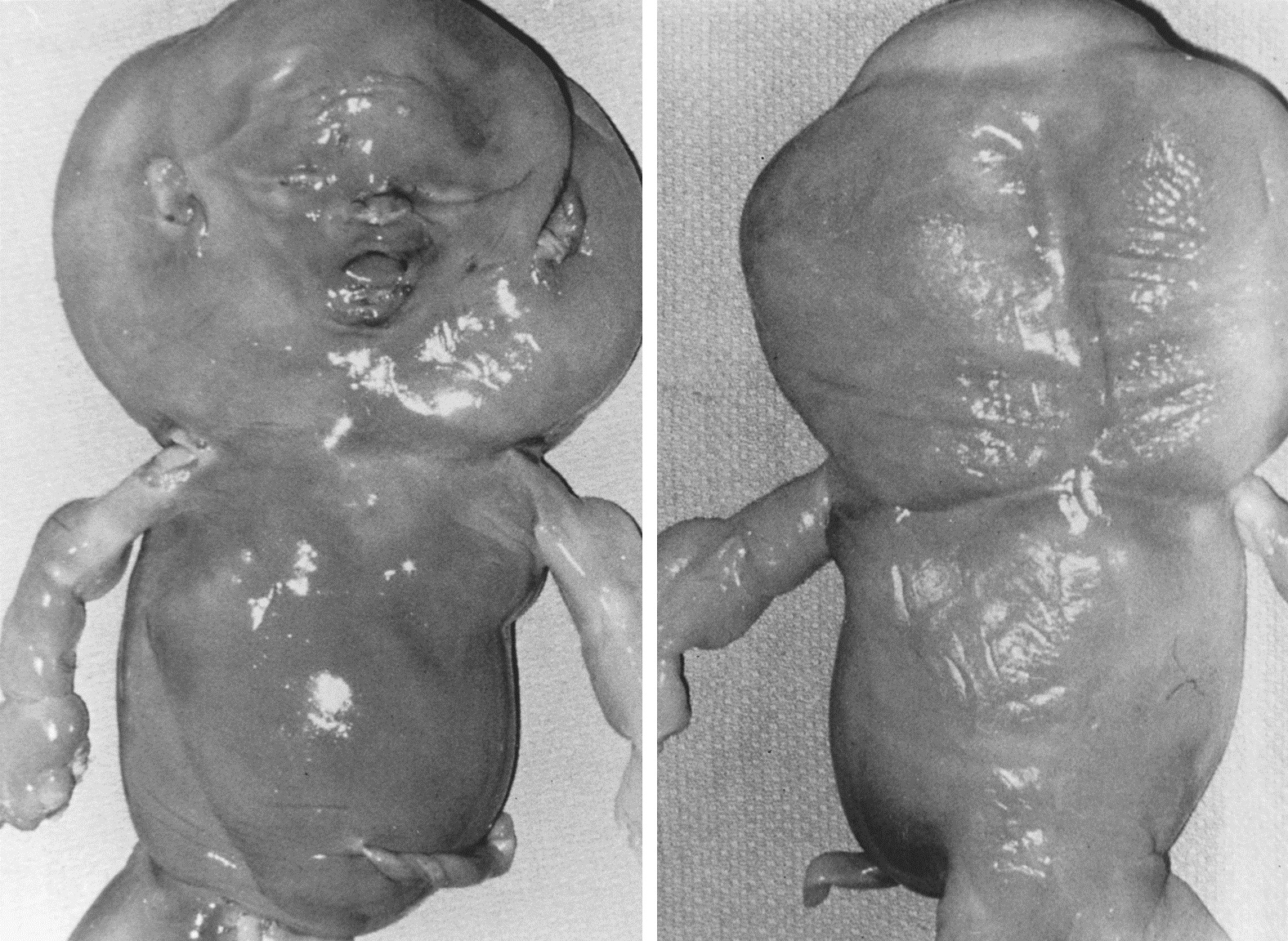 Fig. 4. Photograph of a 45,X abortus. (From Sympson JL, Bombard AT: Chromosomal abnormalities in spontaneous abortion: frequency, pathology and genetic counseling. In Edminds K, Bennett MJ [eds].: Spontaneous Abortion. London, Blackwell, 1987, p 51.)
Fig. 4. Photograph of a 45,X abortus. (From Sympson JL, Bombard AT: Chromosomal abnormalities in spontaneous abortion: frequency, pathology and genetic counseling. In Edminds K, Bennett MJ [eds].: Spontaneous Abortion. London, Blackwell, 1987, p 51.)
Relationship between recurrent losses and numerical chromosomal abnormalities
In both preimplantation and first-trimester abortions, recurrent aneuploidy occurs in some women. Recurrent aneuploidy is a frequent explanation, at least until the number of losses reaches or exceeds four. In a given family successive abortuses are likely to be either recurrently normal or recurrently abnormal. Table 3 (below) shows that if the complement of the first abortus is abnormal,51, 52 then recurrence usually involves aneuploidy, although not necessarily of the same chromosome. Further supporting aneuploidy as a genuine phenomenon is the occurrence of trisomic preimplantation embryos after aneuploid miscarriage in successive ART cycles.53, 54, 55 Munne et al.54 showed increased aneuploid embryos in couples with repeated abortions, compared with couples undergoing PGD for mendelian indications. Rates were 37% versus 21% in women under age 35 years, and 34% versus 31.5% in women over 35 years.
Table 3. Recurrent aneuploidy
| Complement of second abortus | ||||||
Complement of first abortus | Normal | Trisomy | Monosomy | Triploidy | Tetraploidy | De novo rearrangement |
Normal | ||||||
Trisomy | 142 | 18 | 5 | 7 | 3 | 2 |
Monosomy X | 31 | 30 | 1 | 4 | 3 | 1 |
Triploidy | 7 | 5 | 3 | 3 | 0 | 0 |
Tetraploidy | 7 | 4 | 1 | 4 | 0 | 0 |
De novo | 3 | 1 | 0 | 2 | 0 | 0 |
Rearrangement | 1 | 3 | 0 | 0 | 0 | 0 |
The relationship between karyotypes of successive abortuses.
(Warburton D, Kline Stein Z et al: Does the karyotype of a spontaneous abortion predict the karyotype of a subsequent abortion? Am J Hum Genet 41: 465, 1987.)
Karyotype of abortus in recurrent abortions
The concept of recurrent aneuploidy implies certain corollaries, one of which has often been the subject of controversy. Recurrent aneuploidy stratifies into recurrent losses in which couples have either experienced chromosomally abnormal abortuses repeatedly or those in which losses repeatedly show chromosomally normal abortuses. Given that 50% of all abortuses are abnormal cytogenetically, aneuploidy should be as likely to be detected in a randomly karyotyped abortus as in a sporadic abortus. Studying 420 abortuses obtained from women with repeated losses, Stephenson et al.56 found 46% chromosomal abnormalities; 31% of the original sample was trisomic. Their comparison was unselected pooled data, which showed 48% of abortuses to be abnormal; 27% of the original sample was trisomic.
The above relates to first trimester pregnancy losses. Fetal losses – recurrent or not – are more likely to be cytogenetically normal (85%) when occurring after the first trimester.42 Carp et al.57 found that among women having three or more abortuses, the likelihood that the abortus would have an abnormal karyotype was only 29%. In that series inclusion criteria extended to 20 weeks' gestation, a time at which there is less reason to expect recurrent aneuploidy than recurrence of other etiologies.
Counseling and management for recurrent aneuploidies
Couples predisposed to recurrent aneuploidy are at increased risk not only for aneuploid abortuses, but also for aneuploid live borns. The autosomal trisomy in a subsequent pregnancy might be compatible with life (e.g., trisomy 21). Indeed, the risk of live born trisomy 21 following an aneuploid abortus is about 1%. Based on detecting first-trimester trisomies, Snijders and Nicholaides58 reported a recurrence rate of 0.7% following trisomy 21 and 0.7% following trisomy 18. Bianco et al.59 provided a counseling algorithm applicable following a prior abortion of unknown karyotype. If abortions are recurrent but no information is available on the chromosomal status, the odds ratio can be used to derive a patient specific risk. For example, if the a priori Down syndrome risk is 1 in 400 and the odds ratio 1.5, a woman's calculated risk after three abortions would be 1/400 X1.5, or 1/300.
If no information exists concerning the chromosomal status of prior abortuses, paraffin blocks of products of conception can be used to detect aneuploidy, using FISH or array CGH. If results show a trisomy, likelihood of a live born trisomy is increased in subsequent pregnancies. If no information can be obtained, it is unclear whether prenatal genetic diagnosis is appropriate. The risk of an aneuploid offspring is increased, and can be calculated according to Bianco et al.59 The small but finite risk of amniocentesis or CVS is troublesome to couples who have had difficulty maintaining a pregnancy. Non-invasive approaches for aneuploidy detection are typically the chosen option. However, the sensitivity for detecting aneuploidy by noninvasive methods is not the near 100% possible with CVS or amniocentesis. Preimplantation genetic diagnosis (PGD) is another option, especially if the couple eschews clinical pregnancy termination. Selective transfer of euploid embryos clearly decreases the rate of clinical abortions in couples with repeated losses60 and live born trisomies should be decreased.
Chromosomal rearrangements
Translocations
Structural chromosomal abnormalities are an unequivocal explanation for repetitive abortions. The most common structural rearrangement encountered is a translocation, found in about 5% of couples experiencing repeated losses. Individuals with balanced translocations are phenotypically normal, but their offspring (abortuses or abnormal live-born infants) may show chromosomal duplications or deficiencies as a result of normal meiotic segregation. Among couples with repetitive abortions, about 60% of translocations are reciprocal and 40% robertsonian. Females are about twice as likely as males to show a balanced translocation.
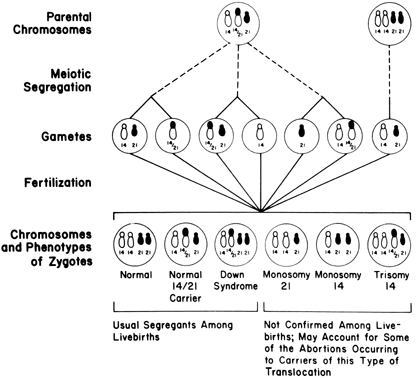
Figure 5 illustrates the clinical consequence of a balanced translocation. If a fetus or live born offspring has Down syndrome as a result of centric fusion (robertsonian) translocation, the rearrangement will have originated de novo in 50–75% of cases. The balanced translocation will not be present in either parent. The likelihood of Down syndrome recurring in subsequent offspring is minimal. On the other hand, the risk becomes significant in the 25–50% of families in which individuals have Down syndrome as result of a balanced translocation [e.g., parental complement 45,XX,-14,-21,=t(14q;21q)]. The theoretical risk of having a child with Down syndrome is 33%, but empirical risks are considerable less. The risk is only 2% if the father carries the translocation; the risk is 10% if the mother carries the translocation.61, 62 If robertsonian (centric-fusion) translocation involves other chromosomes, empirical risks are lower. In t(13q;14q), the risk for live born trisomy 13 is 1% or less.
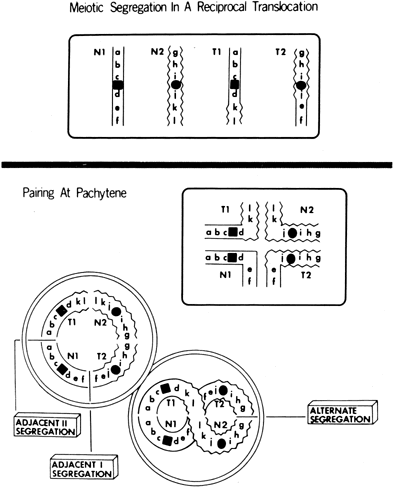
Reciprocal translocations (Fig. 6) involve interchanges between two or more chromosomes.62, 63 Empirical data for specific translocations are usually not available, but generalizations can be made on the basis of pooled data derived from many different translocations.64 Theoretical risks for abnormal offspring (unbalanced reciprocal translocations) are far greater than empirical risks. Overall, the risk is 12% for offspring of either female heterozygotes or male heterozygotes. Antenatal cytogenetic studies should be offered. The frequency of unbalanced fetuses is lower if parental balanced translocations are ascertained through repetitive abortions (3%) than through anomalous live-born infants (nearly 20%).62, 63 Presumably more unbalanced products are lethal.
Analysis of preimplantation embryos using PGD reveals that the great majority of embryos are unbalanced – 58% in Robertsonian translocations and 76% in reciprocal translocations.60 Most conceptuses would therefore be lost before clinical recognition. When a balanced translocation is detected in couples experiencing recurrent abortions, cumulative prognosis for a live-born infant is little different than if a translocation had not been detected;65, 66, 67 however, the length of time to achieve pregnancy is greatly increased.68 Thus, PGD to identify and transfer only the few balanced embryo(s) can increase the statistical likelihood of conception. This strategy is most attractive when the prospective mother is relatively older and nearly imperative in her fifth decade. Using array CGH or FISH one can readily exclude an unbalanced embryo; however, neither distinguishes a balanced (translocation heterozygote) from a (normal) embryo lacking any translocation. Novel techniques now allow a more precise diagnosis in experienced hands.69 Using PGD in couples with a translocation heterozygote, frequency of miscarriages dropped to a lower level.70 This involves treating a day 3 blastomere with caffeine and colchicine, one day later generating a metaphase that can be analyzed by fluorescent techniques (FISH) using whole chromosome painting; the "conversion" rate is about 70%.
Rarely, a translocation precludes normal live-born infants. This occurs when a translocation involves homologous, acrocentric chromosomes (e.g. t[13q13q] or t[21q21q]). If the father carries such a structural rearrangement, artificial insemination may be appropriate. If the mother carries the rearrangement, donor oocytes or donor embryos and ART should be considered.
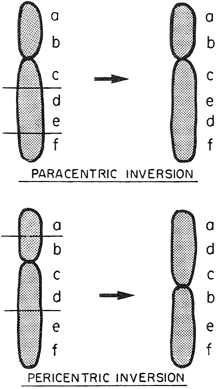
Inversions
Inversions are uncommon parental chromosomal rearrangements, but responsible for repetitive pregnancy losses analogous to translocations. In inversions, the order of genes is reversed. Individuals heterozygous for an inversion should be normal if their genes are truly just rearranged. However, individuals with inversions suffer untoward reproductive consequences as result of normal meiotic phenomena. Crossing-over involving the inverted segment yields unbalanced gametes (see Gardner et al.63 and Martin et al.71). Pericentric inversions are present in perhaps 0.1% of women and 0.1% of men experiencing repeated spontaneous abortions (Fig. 7). Paracentric inversions are even rarer.
|
Females with a pericentric inversion have a 7% risk of abnormal live-born infants; males carry a 5% risk.63 Pericentric inversions ascertained through phenotypically normal probands are less likely to result in abnormal live-born infants.
Inversions involving only a small portion of the total chromosomal length paradoxically are less significant clinically because large duplications or deficiencies arise following crossing-over, usually conferring lethality. By contrast, inversions involving only 30–60% of the total chromosomal length are relatively more likely to be characterized by duplications or deficiencies compatible with survival. Prenatal cytogenetic studies should be offered.
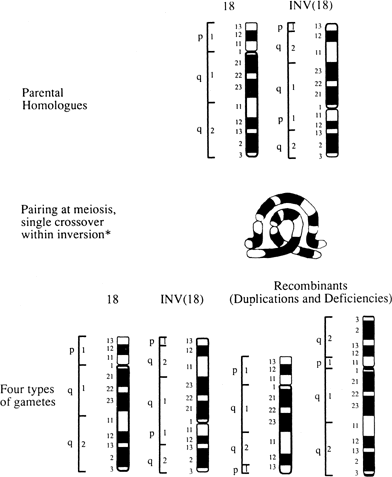
Paracentric inversions should carry less risk for unbalanced products than pericentric inversions because nearly all paracentric recombinants should in theory be lethal. However, abortions and abnormal live-born infants have been observed within the same kindred.72 The risk for unbalanced viable offspring has been tabulated at 4%.72 Prenatal cytogenetic studies should thus still be offered (Fig. 8).
MENDELIAN AND POLYGENIC FACTORS
The 30–50% of first-trimester abortuses that show no chromosomal abnormalities could still have occurred as a result of other genetic etiologies mendelian or polygenic/multifactorial. These etiologies explain far more congenital anomalies in live borns than do chromosomal abnormalities. It would thus be very naïve to assume mendelian and polygenic/multifactorial factors do not play pivotal roles in embryonic mortality. Indeed, there are innumerable candidate genes. Especially likely to be mendelian or polygenic in etiology are abortuses which demonstrate isolated structural anomalies. Cytogenetic data are often lacking on dissected specimens, making it nearly impossible to determine the relative role of cytogenetic versus mendelian or polygenic mechanisms in early embryonic maldevelopment. Philipp and Kalousek 73 correlated cytogenetic status of missed abortuses with morphologic abnormalities at embryoscopy. Embryos with chromosomal abnormalities usually showed one or more external anomalies, but some euploid embryos also showed anatomic anomalies. More recent studies are consistent.74
In addition to single gene perturbations (mendelian etiology), novel nonmendelian forms of inheritance probably play a greater role in embryonic loss than in live-born abnormalities. Mosacism may be restricted to the placenta, the embryo per se being normal. This phenomenon is termed "confined placental mosaicism". Losses caused by this mechanism may already be subsumed in extant data because most studies involved analysis only of villous material. A corollary of confined placental mosaicism is uniparental disomy, in which both homologues for a given chromosome are derived from a single parent. This presumably occurs as a result of expulsion of a chromosome from a trisomic zygote ("trisomic rescue"). Although the karyotype would appear normal (46,XX or 46,XY), the product would lack a contribution from one parent.
CLINICALLY RECOGNIZED PREGNANCY LOSSES NOT ASSOCIATED WITH CHROMOSOMAL ABNORMALITIES
Luteal phase defects
Implantation in an inhospitable endometrium has long been a popular explanation for pregnancy loss. Progesterone deficiency in particular could plausibly result in the estrogen-primed endometrium being unable to sustain implantation. Luteal phase deficiency (LPD) has specifically been hypothesized as caused by inadequate progesterone secretion by the corpus luteum.
Once almost universally accepted as a common cause of fetal wastage, LPD is now generally considered an uncommon explanation for pregnancy loss, at least outside of ART. Efficacy of treatment has also never been proved. Meta-analysis75 has shown no beneficial effect of progesterone treatment.
Luteal phase abnormalities arising during ovulation stimulation as necessitated during ART could be a different phenomenon. It is considered standard to administer progesterone until approximately 9 weeks of gestation. In this circumstance, the cells surrounding the oocyte, which would ordinarily contribute to the corpus luteum, may have been removed when the oocyte was aspirated.
Thyroid abnormalities
Decreased conception rates and increased fetal losses are accepted consequences of overt hypothyroidism or hyperthyroidism. The role of subclinical thyroid dysfunction is less clear, and not generally considered an explanation for repeated losses. Nonetheless, Negro et al.76 reported that pregnancy loss was higher in thyroid peroxidase negative women whose TSH was 2.5–4.0 mIU/L, compared to those whose TSH was <2.5 mIU/L (6.1% vs. 3.6%). Increased frequency of thyroid antibodies has in addition been observed in several series, and some consider autoimmune thyroid disease a significant cause.77 However, the value of treatment in such circumstances is unproven.
Elevations of maternal thyroid hormone per se are clearly deleterious, as shown by a family from the Azores in which a gene conferring resistance to thyroid hormone was segregating.78 Family members with an autosomal dominant mutation in the thyroid receptor β (TRβ) gene (Arg243GIn) secreted large amounts of thyroid-stimulating hormone (TSH) to compensate for end-organ resistance. During pregnancy, the fetus of such a mother becomes unavoidably exposed to high levels of maternal TSH because TSH and T4 readily cross the placenta. Loss rates were 22.8% in pregnancies of mothers who had the Arg243GIn mutation, 2.0% in those of normal mothers whose male partner had the mutation, and 4.4% in couples in which neither partner had the mutation.
Diabetes mellitus
Poorly controlled diabetes mellitus results in increased risk for fetal loss. Mills et al.4 showed that women whose glycosylated hemoglobin level was greater than 4 SD above the mean showed higher pregnancy loss rates than women with lower glycosylated hemoglobin levels. Subsequent analysis of this dataset confirmed increased loss at glycemic extremes.79 On the other hand, well-controlled or subclinical diabetes should not be considered a cause of early miscarriage. Neither Royal College of Obstetricians and Gynaecologists (RCOG) or the American Society for Reproductive Medicine (ASRM) recommend testing for occult diabetes mellitus.
Intrauterine adhesions (synechiae)
Intrauterine adhesions could interfere with implantation or early embryonic development. Adhesions may follow overzealous uterine curettage during the postpartum period, intrauterine surgery (e.g., myomectomy), or endometriosis. Curettage causing adhesions is most likely to develop when performed within 3 or 4 weeks after delivery. Individuals with uterine synechiae usually manifest hypomenorrhea or amenorrhea, but 15–30% show repeated abortions. If adhesions are detected in a woman experiencing repetitive losses, lysis under direct hyperoscopic visualization should be performed. Postoperatively, an intrauterine device or inflated foley catheter temporarily placed postoperatively in the uterus discourages reapposition of healing uterine surfaces. Estrogen administration should also be initiated. Approximately 50% of patients conceive after surgery, but the frequency of pregnancy losses remains high.
Müllerian fusion defects
Müllerian fusion defects are an accepted cause of second-trimester losses and pregnancy complications. Low birth weight, breech presentation, and uterine bleeding are consequences associated with müllerian fusion defects, when compared with women having hysterosalpingogram-proven normal uteri.
Losses seem more likely to be associated with a uterine septum than a bicornate uterus.80, 81, 82, 83 In 509 women with recurrent losses studied by three-dimensional (3D) ultrasound, Salim et al.81 found greater uterine distortion in women recounting histories of losses. However, the major problem in attributing cause and effect for second-trimester complications and uterine anomalies is that uterine anomalies are very frequent in the general population; thus, adverse outcomes could merely be coincidental. For example, in a study by Salim et al.81 23.8% of women with recurrent miscarriage had some uterine anomalies on 3D ultrasound.80 In another study, unsuspected bicornate uteri were found in 1.2% of 167 women undergoing laparoscopic sterilization; 3.6% had a severely septate uterus, whereas 15.3% had fundal anomalies.82 In another series, müllerian defects were found in 3.2% (22 of 679) of fertile women; 20 of the 22 defects were septate.83
Treatment has traditionally involved surgical correction, namely metroplasty. In order to determine if aggressive nonsurgical treatment could be just as efficacious as surgery, Ludmir et al.84 tracked 101 women with an uncorrected malformation. After first being followed without surgery and without a defined nonsurgical regimen, the same women underwent a surgically conservative but medically aggressive protocol consisting of decreased physical activity and tocolysis. Fetal survival rates in both bicornate and septate groups were, however, not significantly different before (52% and 53%, respectively) or after (58% and 65%, respectively) the change in management.
Early first-trimester abortions could be caused by müllerian fusion defects, but other explanations seem likely even when such a defect is found. Septate uteri are most plausibly causative, implantation occurring on a poorly vascularized and inhospitable surface. Abortions occurring after ultrasonographic confirmation of a viable pregnancy at, say, 8 or 9 weeks may more properly be attributed to uterine fusion defects. Women experiencing second-trimester abortions could benefit from uterine reconstruction, but reconstructive surgery is not necessarily advisable if losses are restricted to the first trimester.
Leiomyomas
Leiomyomas are very frequent, but relatively few women develop symptoms requiring medical or surgical therapy. That leiomyomas cause first- or second-trimester pregnancy wastage per se, rather than obstetric complications like preterm birth is plausible but probably uncommon. Analogous to uterine anomalies, the coexistence of two common phenomena – uterine leiomyomas and reproductive losses – need not necessarily imply a causal relationship. Hartmann et al.85 correlated ultrasonographically detected leiomyomas with pregnancy outcome in a cohort of North Carolina women. Of 1313 women ascertained early in pregnancy, the 131 ascertained by ultrasound to have leiomyomas had an increased prior spontaneous abortion rate (odds ratio 2.17). One pitfall is that uterine contractions can mimic fibroids on ultrasound.
Location of leiomyomas is probably more important than size. Submucous leiomyomas are more likely to cause abortion than subserous leiomyomas. Postulated mechanisms leading to pregnancy loss include (1) thinning of the endometrium over the surface of a submucous leiomyoma, predisposing to implantation in a poorly decidualized site; (2) rapid growth caused by the hormonal milieu of pregnancy, compromising the blood supply of the leiomyoma and resulting in necrosis ("red degeneration") that, in turn, leads to uterine contractions and eventually fetal expulsion; and (3) encroachment of leiomyomas on the space required for the developing fetus, leading to premature delivery through mechanisms presumably analogous to those operative in incomplete müllerian fusion. If pregnancies are not lost, the relative lack of space may lead to fetal deformities (i.e., positional abnormalities arising in a genetically normal fetus).
Surgical procedures to reduce leiomyomata may occasionally be warranted in women experiencing repetitive second-trimester abortions. However, leiomyomata more often probably have no etiological relationship to pregnancy loss. Surgery should be reserved for women whose abortuses were both phenotypically and karyotypically normal and in which viability until at least 9–10 weeks was documented.
Cervical insufficiency
An intact cervix is an obvious prerequisite for a successful pregnancy. Characterized by painless dilation and effacement, cervical insufficiency (preferable to the erstwhile term incompetence) usually occurs during the middle second or early third trimester. Cervical insufficiency usually follows traumatic events like cervical amputation, cervical lacerations, forceful cervical dilation, or cervical conization.86 However, etiology may be genetic, for example perturbations of a connective tissue gene (e.g., collagen, fibrillin).
Infections
Infections are known causes of late fetal losses and logical causes of early fetal losses. Microorganisms associated with spontaneous abortion include variola, vaccinia, Salmonella typhi, Vibrio fetus, malaria, cytomegalovirus, Brucella, toxoplasmosis, Mycoplasma hominis, Chlamydia trachomatis, and Ureaplasma urealyticum. Sporadic losses seem logical. However, infections as a cause of repeated losses seem less likely.
Of the many organisms implicated in repetitive abortion, U. urealyticum and M. hominis seem most plausible related to repetitive spontaneous abortions because they fulfill two important prerequisites. (1) The putative organism can persist in an asymptomatic state. (2) Virulence is not so severe as to cause infertility that leads to fallopian tube occlusion and, hence precludes the achievement of pregnancy. Some studies have suggested a relationship between bacterial vaginosis, presumed to be Gardnerella vaginalis, and abortion. However, bacterial vaginosis is typically associated with preterm delivery in the second and third trimesters.
Given lack of evidence for causality for recurrent losses, one might wonder whether the infectious agents cited cause fetal losses or merely arise after fetal demise from other causes. Cohort surveillance for infections can best shed light on the true role of infections in early pregnancy loss. The frequency of clinical infections was assessed prospectively in 386 diabetic subjects and 432 control subjects seen weekly or every other week beginning early in the first trimester.87 Infection occurred no more often in 112 subjects experiencing pregnancy loss than in 706 experiencing successful pregnancies. This held true both for the 2-week interval in which a given loss was recognized clinically as well as in the prior 2-week interval. Similar findings were observed in both control and diabetic subjects, and further were substantiated when data were stratified into ascending genital infections only versus systemic infection only.
In conclusion, infections doubtless explain some early pregnancy losses and certainly many later losses. However, in the first trimester attributable risk is low even in sporadic cases, and even less in recurrent losses.
Acquired thrombophilias
An association between second-trimester pregnancy loss and certain autoimmune diseases is accepted.42 For first-trimester losses, however, consensus is for a less significant relationship. The spectrum of antibodies found in women with pregnancy loss encompasses nonspecific antinuclear antibodies as well as antibodies against individual cellular components like phospholipids, histones, and double- or single-stranded DNA. The primary antigenic determinant is B2-glycoprotein, which has an affinity for negatively charged phospholipids.88 Antiphospholipid syndrome encompasses (1) lupus anticoagulant (LAC) antibodies, (2) anticardiolipin antibodies (aCL) or (3) anti-B2-glycoprotein. Values for the latter two should be greater than the 99th centile, of moderate or higher titers, and 12 weeks apart. Initially, descriptive studies seemed to show increased aCL antibodies in women with first-trimester pregnancy losses. A pitfall proved to be selection bias, studying couples only following spontaneous abortions. That antibodies did not arise until after the pregnancy loss was also not excluded. To address this, Simpson et al.89 analyzed sera obtained prospectively from women within 21 days of conception. A total of 93 women who later experienced pregnancy loss were matched 2:1 with 190 controls who subsequently had normal live-born offspring. No association was observed between pregnancy loss and presence of either antiphospholipid antibodies or aCL. In a recent ACOG bulletin88 three or more losses before the 10th week of pregnancy were considered to fulfill diagnostic criteria for antiphospholipid syndrome in the sense of justifying prophylactic heparin therapy. It was stated that this assumes "no maternal anatomic or hormonal abnormalities, and no paternal or maternal chromosomal are excluded". ACOG provided the caveat that such increase in antibodies neither explains losses nor confers a greatly increased risk for another loss. Given this, treatment regimens should be judicious, perhaps aspirin or heparin if at all. Control groups of fertile women showed not dissimilar frequencies.
Inherited thrombophilias
Inherited maternal hypercoagulable states are unequivocally associated with increased fetal losses in the second trimester. Postulated associations include factor V Leiden (Q1691G→A), prothrombin 2021G→A, and homozygosity for 677C→T in the methylene tetrahydrofolate reductase gene (MTHFR). Meta-analysis of 31 studies published as of 2003 revealed associations between recurrent (two or more) fetal losses less than 13 weeks and the following thrombophilias: factor V Leiden (G1691A), activated protein C resistance, prothrombin (20210A0 gene), and protein S deficiency.90 No associations were found between MTHFR, protein C, and antithrombin deficiencies and recurrent pregnancy loss. A meta-analysis of 16 studies by Kovalesky et al.91 reported an association between recurrent pregnancy loss, defined as two or more losses in the first two trimesters, and maternal heterozygosity for either factor V leiden or prothrombin 20210G7→A. Evidence is less strong for an association between inherited thrombophilias and recurrent early (<10 weeks' gestation) pregnancy loss. Most recommend testing for factor V Leiden, activated protein C resistance, fasting homocysteine, antiphospholipid antibodies, and the prothrombin gene. Pending salutary results in ramdomized clinical trials, treatment for recurrent first-trimester losses with heparin and/or other antithrombotic or anticoagulant therapies should be initiated with caution.
Exogenous agents
Numerous exogenous agents have been implicated in fetal losses but few if any studies have stratified by sporadic and recurrent losses. None have taken into account the obvious confounding variable that the loss could have involved an aneuploid embryo or fetus. Of course, every pregnant woman is exposed to low doses of ubiquitous agents. Rarely are data adequate to determine with confidence the role these exogenous factors play in early pregnancy losses.
Outcomes following exposures to exogenous agents can usually be derived only on the basis of case–control studies. In such studies, women experiencing an adverse event (e.g., abortion) recalled exposure to the agent in question more often than controls. However, case–control studies have inherent biases. The primary bias is accuracy of recall, control women having less incentive to recall antecedent events than subjects experiencing an abnormal outcome. Employers also naturally attempt to limit exposure to women of reproductive age; thus, exposures to potentially dangerous chemicals are usually unwitting, and hence, poorly documented. Pregnant women are also exposed to many agents concurrently, making it nearly impossible to attribute adverse effects to a single agent. Given these caveats, physicians should be cautious about attributing pregnancy loss to any exogenous agent. On the other hand, common sense dictates that exposure to potentially noxious agents be minimized.
X-ray irradiation and chemotherapeutic agents
Irradiation and antineoplastic agents in high doses are acknowledged abortifacients. Of course, therapeutic x-rays or chemotherapeutic drugs are administered during pregnancy only to seriously ill women whose pregnancies often must be terminated for maternal indications. Diagnostic x-rays, even pelvic x-rays, rarely result in exposures to a level that places a women at increased risk. The exposure is usually to doses that are far less (1–2 rad or 0.01–0.02 Gy), with 10 rads (0.1 Gy) considered the lowest level of significance. It is also prudent for pregnant hospital workers to avoid handling chemotherapeutic agents and to minimize exposures during diagnostic imaging.
Alcohol
Alcohol consumption should be avoided during pregnancy for many reasons. However, alcohol probably increases pregnancy loss only slightly. Some authors found only a slightly increased risk for abortion in women who drank in the first trimester, whereas others92 found alcohol consumption to be nearly identical in women who did and did not experience abortion: 13% of women who aborted and 11% of control women drank on average three to four drinks per week; other investigations have reached a similar conclusion.92 Armstrong et al.93 found the odds ratio to be 1.82 with 20 drinks or more per week.
The clinical message is that abstinence should not be expected to prevent pregnancy loss. Women should not attribute a loss to social alcohol during early gestation, evaluation for other causes is still in order.
Caffeine
In data gathered in cohort fashion, Mills et al.94 showed that the odds ratio for association between pregnancy loss and caffeine (coffee and other dietary forms) was only 1.15 (95% CI 0.89–1.49).93 Women exposed to much higher levels may, however, be at greater risk. Klebanoff et al.95 reported an association between pregnancy losses and caffeine ingestion greater than 300 mg daily (1.9-fold increase). One confounding problem is difficulty in taking into account the effects of nausea, which not only decreased caffeine ingestion but seems to be more common in successful pregnancies. In general, reassurances can be given concerning moderate caffeine exposure and pregnancy loss.
Contraceptive agents
Contraception with an intrauterine device in place increases the risk of fetal loss, and can rarely result in second trimester sepsis characterized by a flu-like syndrome. If the device is removed before pregnancy, there is no increased risk of spontaneous miscarriage. Using oral contraceptives before or during pregnancy is not associated with fetal loss. The same applies for injectable or implantable contraceptives. There is no evidence for increased pregnancy loss after spermicide exposure before or after conception.
Chemicals
Limiting exposure to potential toxins in the workplace is prudent for pregnant women. Difficulty lies in first defining the precise effect of lower exposures, and then attributing a specific risk. False alarms concerning potential toxins are frequent. Many chemical agents have been claimed to be associated with fetal losses, but only a few are accepted as potentially causative.96 These include anesthetic gases, arsenic, aniline dyes, benzene, solvents, ethylene oxide, formaldehyde, pesticides, and certain divalent cations (lead, mercury, cadmium). Workers in rubber industries, battery factories, and chemical production plans are among those at potential risk.
Cigarette smoking
Active and passive maternal smoking is damaging in every trimester of human pregnancy. Cigarette smoke contains numerous toxins which exert a direct effect on the placental and fetal cell proliferation and differentiation and can explain the increased risk of miscarriage, fetal growth restriction (FGR), stillbirth, preterm birth, and placental abruption reported by epidemiological studies and, in particular, chromosomal status of abortions.97 Smoking during pregnancy is often cited as a cause of miscarriage, but confounding variables are rarely excluded.
Increased miscarriage rates reported in smokers do, however, seem to be independent of maternal age and alcohol based on urinary cotinine levels. When 400 women with spontaneous abortions were compared with 570 who experienced ongoing pregnancies, women with urinary cotinine had an increased risk of miscarriage. The odds ratio was, however, only 1.8 (95% CI 1.3–2.6).98 However, Harlap and Shiona reported only nonsignificant risks in both first and second trimester losses (1.01 first trimester; 1.21 second trimester).99
Trauma
Women commonly attribute pregnancy losses to trauma, such as a fall or blow to the abdomen. The temptation to attribute a loss to minor traumatic events should be avoided. A nested case–control study of 392 cases and 807 controls showed no relationship between physical violence and miscarriage.100
Psychological factors
That impaired psychological well-being predisposes to early fetal losses has been claimed but never proved. Neurotic or mentally ill women experience losses, but so do normal women. Whether the frequency of losses is higher in the former is arguable, potential confounding variables not being taken into account, and no genetic factors being considered.
Investigations most frequently cites as showing a benefit of psychological well-being are those of Stray-Pedersen et al.101 Pregnant women who previously experienced repetitive abortions received increased attention but no specific medical therapy ("tender loving care"). These women (n = 16) were more likely (85%) to complete their pregnancy than women (n = 42) not offered such close attention (36%). However, only women living "close" to the university were eligible to be placed in the increased-attention group. Women living further away served as "controls". Thus, control women may have differed from the experimental group in subtle ways.
Other studies have also reported a beneficial effect of psychological well-being.101 Again, however, pitfalls exist in study design. Moreover, the biological explanation for this salutary effect remains obscure.
MANAGEMENT OF RECURRENT EARLY PREGNANCY LOSS
Faced with a couple having experienced a spontaneous abortion, the obstetrician has immediate obligations to: (1) provide the couple information on the overall frequency of fetal wastage (10–12% of clinically recognized pregnancies, and many more unrecognized) and likely etiology (genetic and especially cytogenetic), (2) provide applicable recurrence risks, and (3) determine the necessity of a formal clinical evaluation. Explicitly worth citing is the positive correlation between loss rates and both maternal age and prior losses. The maternal age effect is the result of increased trisomic abortions, but also reflective of endometrial factors.
When is formal evaluation recommended?
After three losses, couples have traditionally been directed to formal evaluation. Although lacking firm scientific basis for waiting until three losses, this is the benchmark for the Royal College of Obstetricians and Gynaecologists (RCOG) and the European Society of Human Reproduction and Embryology (ESHRE).102 The American College of Obstetricians and Gynecologists (ACOG) defines recurrent loss as either two or three consecutive losses103 and ASRM104 as two losses. Neither ACOG or ASRM consider it relevant whether losses are consecutive or not. The 2002 ACOG guideline103 requirement for consecutive losses is arguable. A couple experiencing a single loss should be counseled and provided recurrence risk rates. Infertile couples in their fourth decade may choose to be evaluated formally after only two losses. Nevertheless, evaluation is called for with repeated losses.102, 103, 104, 105
Once a couple undergoes evaluation, however, all tests employed by a given practitioner should be performed. There is little rationale for pursuing certain studies after two losses yet deferring others until three or more losses.
Any couple having a stillborn or anomalous live-born infant should undergo cytogenetic studies unless the stillborn was known to have a normal chromosomal complement. Parental chromosomal (conventional metaphase) rearrangements (i.e., translocations or inversions) should be excluded. If chromosomal studies on the stillborn were unsuccessful, common trisomies can still be ruled out by performing FISH on stored deparaffined tissue. Array CGH can also be performed without culture.
Recommended evaluation
- Couples experiencing only one first-trimester abortion should receive relevant information, but need not necessarily be evaluated formally. They should be provided with the relatively high (10–15%) pregnancy loss rate in the general population and the beneficial effects of miscarriage in eliminating an abnormal conceptus and recurrence risk of 20–25% should be offered in the presence of a prior live-born infant, and slightly higher (30–40%) in the absence of a prior live-born infant (Table 3). Risks are higher for older women than younger women. If a specific medical illness exists, treatment is necessary. If intrauterine adhesions are detected, surgical removal is necessary. Otherwise, no further evaluation need be undertaken, even if uterine anomalies or leiomyomas are present.
- Investigation may or may not be necessary after two spontaneous abortions, depending on the patient's age and personal desires. After three spontaneous abortions, evaluation is usually indicated. Occurrence of a stillborn or live-born infant with anomalies warrants genetic evaluation irrespective of the number of pregnancy losses.
- Parental chromosomal studies should be undertaken on all couples having repetitive losses. Antenatal chromosomal studies should be offered if a balanced chromosomal rearrangement or inversion is detected in either parent or if autosomal trisomy occurred in any previous abortus.
- Although not always practical, cytogenetic information on abortuses is valuable. Detection of a trisomic abortus suggests recurrent aneuploidy, justifying prenatal cytogenetic studies in future pregnancies. Performing invasive prenatal cytogenetic studies solely on the basis of repeated losses is more arguable, but not unreasonable among women aged 30 years and above. Culturing products of conception is less likely to generate results than culturing amniotic fluid cells or chorionic villi obtained after demise.
- Endocrine causes for repeated fetal losses include poorly controlled diabetes mellitus, overt thyroid dysfunction, and elevated maternal TSH levels. Subclinical diabetes or subclinical thyroid disease should not be considered firm explanations. Luteal phase defects are no longer considered a common explanation, except in pregnancies achieved with in vitro fertilization.
- Of infectious agents, only C. trachomatis and U. urealyticum seem of greatest plausibility for not only sporadic but also repetitive losses. The endometrium could be cultured for U. urealyticum or, a couple could be treated empirically with vibramycin or doxycycline.
- If an abortion occurs after 8–10 weeks' gestation, a uterine anomaly or submucous leiomyoma should be considered potentially causative. The same rationale does not necessarily apply following first-trimester losses. The uterine cavity should be explored by hysteroscopy or hysterosalpingography. Intrauterine adhesions should be lysed. If a müllerian fusion defect (septate or bicornate uterus) is detected in a woman experiencing one or more second-trimester spontaneous abortions, surgical correction may be warranted. A large submucous leiomyoma may also justify myomectomy. Cervical insufficiency should be managed by cervical cerclage during the next pregnancy.
- Women with either acquired or inherited thrombophilias appear to have a slightly increased risk for first trimester pregnancy loss. Thrombophilias explain at best only a small portion of first trimester losses. There is a much greater likelihood that thrombophilias are causative for second trimester losses.
- One should discourage exposure to cigarettes and alcohol, yet not necessarily ascribe cause and effect in an individual case. Similar counsel should apply for exposures to other potential toxins.
LATE PREGNANCY LOSS (STILLBIRTH)
Stillbirth is the term used to describe pregnancy loss at 20 weeks' gestation or greater. By weight the definition is 350 g, the 50th centile at that week gestation. In the US stillbirths occur in 1 in 160 deliveries. The incidence is increased in many conditions discussed in standard texts. Conditions include obesity, multiple gestations with or without prematurity, infections (e.g., parvovirus B19), and a host of systematic maternal diseases that include but are not limited to diabetes mellitus, chronic and gestational hypertension, autoimmune diseases, renal and thyroid diseases. Pregnancy loss after 20 weeks is higher in African Americans (11/1000) than in other ethnic groups (6/1000), including Hispanics and Native Americans.42
Recurrence
Recurrence risks are blended figures not necessarily appropriate to a given couple. However, some risk factors are broadly applicable. Maternal age is positively correlated with risk of stillborn. This reflects not only the known fetal etiologies (e.g., chromosomal), but also maternal complications (e.g., hypertension) that are also age-related. Risk of stillbirth is highest (two fold) for women delivered of a growth restricted (IUGR) infant earlier than 32 weeks.106, 107 The risk is, incidentally, independent of mode of delivery (cesarean or vaginal delivery). Using Scottish morbidity records 1981–2000 the odds ratio for stillbirth recurrence in the second pregnancy was 1.94.108
Genetic factors
Genetic factors for stillbirth are receiving increased recognition by ACOG, which has provided specific management recommendations.42 Chromosomal abnormalities are detected in 5–13% of stillbirths.42, 109, 110 Thus, special effort should be made to determine the chromosomal status of a stillborn. It is now recognized that the traditional approach of obtaining fetal tissue after a stillborn has been delivered is suboptimal. Cell culture often fails, leading to no results in perhaps 50–75% of cases. Successful culture for chromosomal analysis occurs in 80% when amniocentesis is utilized to obtain cells.42 It is tempting to eschew an invasive procedure in an already stressed patient, but this would not be in her best long-term interest.
Detecting even trisomies clinically by examination of a stillbirth is unexpectedly difficult because maceration occurs within days of fetal demise. Thus, medical records stating lack of dysmorphia should be suspect, save for obvious structural defects (e.g., cleft lip, myelomeningocele). Ultrasound results when the pregnancy was still viable are probably more reliable. If amniocentesis cannot be performed or if cultures fail, one should attempt to obtain FISH results to exclude common trisomies. This can be done on placental tissues, umbilical cord segments, or internal (non-contaminated) tissues like connective tissues.
The major yield of autopsy for a stillborn is detection of an unrecognized Mendelian explanation. This obviously alters management in subsequent pregnancies, for which reason a major effort should be exerted. Whole body photographs and whole body x-rays are recommended. Considerable success has been made, in particular, in diagnosing skeletal dysplasia, often an autosomal recessive disorder that may recur (25%) in subseqent pregnancies. Other disorders may be autosomal dominant, arising due to a de novo mutation and having negligible recurrence risk. Distinguishing between these two possibilities is important because the recurrence risks differ so much. If parents refuse autopsy, the provider should attempt to obtain as much information as possible: photographs, x-rays or MRI, exam by a geneticist, and ultrasound. A head-sparing autopsy is preferable to no autopsy, and may be acceptable to the parents.
Any isolated birth defect is more common in stillborns than neonates. This reflects adverse selection in utero, a phenomenon recognized for years in ultrasound surveillance. If an isolated, organ-specific defect occurs (e.g., cardiac defect), polygenic/multifactorial etiology and recurrence risks (2–5%) can be assumed. On the other hand, such a defect may merely be the only one evident feature, yet actually a component of a multiple malformation complex. Ability to distinguish between these possibilities is a major reason for autopsy and examination by a genetically trained provider.
Maternal evaluation
The specific maternal lab tests recommended by ACOG are listed in Table 4. In addition, a mother whose pregnancy has a medical complication has already undergone many tests, and the cause of the stillbirth may seem obvious (e.g., diabetes mellitus). It is still prudent, however, to order all the lab tests because the ostensible diagnosis may not be correct. Of note, ACOG does not recommend testing for antinuclear antibodies, for certain serologies (toxoplasmosis, rubella, cytomeglovirus, herpes simplex), nor at this time for genetic tests other than karyotype or, by extension array CGH. A panel of organ specific mutations (e.g., skeletal dysplasia) or other genetic tests can be expected to become available. On the other hand, caution is necessary before concluding that a stillbirth was caused by a condition signified by a positive lab test (e.g., thrombophilia). Such a finding does not obviate the need for fetal autopsy and fetal genetic tests.
Table 4. Maternal lab tests are recommended by ACOG42
| All mothers having stillbirths | Selected mothers having stillbirths |
| Complete blood count | Thrombophilia |
| Kleihauer-Betke or other test for fetal cells in maternal circulation | Factor V Leiden |
| Human parvovirus B-19 immunoglobulin G; immunoglobulin M antibody | Prothrombin gene mutation |
| Syphilis | Antithrombin III |
| Lupus anticoagulant | Homocysteine (fasting) |
| Anticardiolipin antibodies (aCL) | Protein S and protein C activity |
| Thyroid-stimulating hormone (TSH) | Parental karyotypes |
| Indirect Coombs test | |
| Glucose screening (oral glucose tolerance test, hemoglobin A1c) | |
| Toxicology screen |
Management in subsequent pregnancies
High-quality ultrasound and vigilant fetal surveillance is universally recommended. Induction of labor is recommended at 39 weeks, but prior to that only with demonstrated fetal lung maturity.111 Management otherwise will focus on any specific maternal factors identified (e.g., diabetes mellitus) as present. In some pregnancies, management will differ little from that of the general obstetricl patient. In others prenatal genetic diagnosis will be necessary.
PREGNANCY COMPLICATIONS AFTER THREATENED MISCARRIAGE OR LOSS OF A CO-TWIN (VANISHING TWIN)
Miscarriage, threatened miscarriage with or without an intrauterine hematoma (IUH), and the phenomenon of a vanishing twin are common in early pregnancy. What is the relationship between these complications, whether resulting in a loss or not, and subsequent pregnancies? Meta-analyses and reviews have indeed indicated an increased risk of adverse outcome in ongoing pregnancies after an early pregnancy event (e.g., threatened miscarriage). Following a single miscarriage, the risk for perinatal death is increased (OR >2.0). Following recurrent miscarriages, the OR is >2.0 for several additional adverse events: perinatal death, very preterm delivery (VPTD), placenta previa, premature preterm rupture of the membranes and low birth weight (LBW).112 Clinically relevant associations (OR >2.0) for adverse obstetric outcome in the ongoing pregnancy following complications in an index pregnancy include: preterm delivery, VPTD, placental abruption, small for gestational age (SGA), low birth weight (LBW), and very LBW (VLBW) following a threatened miscarriage; pregnancy induced hypertension, pre-eclampsia (PE), placental abruption, preterm deliver (PTD), SGA, and low 5-min Apgar score following detection of a intrauterine hematoma and VPTD, VLBW and perinatal death following a vanishing twin (VT).113 These data indicate a link between early pregnancy complications involving the placenta and subsequent adverse obstetric and perinatal outcomes.
In conclusion, despite heterogeneity among studies and failure to adjust for the many relevant confounders (e.g., age, ART, economic status, education level, ethnicity, height, marital status, parity, prolonged infertility, smoking, and maternal weight)112, 113 meta-analysis and controlled population-based prospective studies have confirmed an association between certain adverse early pregnancy events and subsequent late obstetric complications in the subsequent or ongoing pregnancy.113 In particular, the risk of preterm and very preterm delivery is increased after most first trimester complications. Early detection of these risk factors could improve the screening of women at high risk of specific obstetric complications in ongoing and subsequent pregnancies.
REFERENCES
U.S. Department of Health and Human Services: Reproductive impairments among married couples. In U.S. Vital and Health Statistics, Series 23, No.11, p 5. National Center for Health Statistics. Hyattsville, MD, 1982 |
|
Simpson JL, Jauniaux, ERM. Pregnancy loss. Obstetrics: Normal and Problem Pregnancies, 6th edition. Gable SL, Niebyl JR, Simpson JL et al Editors. Philadelphia, Elsevier Saunders; pp 592-608, 2012 |
|
Wilcox AJ, Weinberg CR, O'Connor FJ et al: Incidence of early pregnancy loss. N Engl J Med 319: 189, 1988 |
|
Mills JS, Simpson JL, Driscoll SG et al: NICHD-DIEP Study: Incidence of spontaneous abortion among normal women with insulin-dependent diabetic women whose pregnancies were identified within 21 days of conception. N Engl J Med 319: 1617, 1988 |
|
Yan J, Saravelos SH, Ma N, Ma C, Chen Z-J, Li T-C. Consecutive repeat miscarriages are likely to occur in the same gestational period. Reproductive BioMedicine; 24:634-638, 2012 |
|
Christiaens GC, Stoutenbeek P. Spontaneous abortion in proven intact pregnancies. Lancet 1984;2:571 |
|
Wilson RD, Kendrick V, Wittman BK et al: Risk of spontaneous abortion in ultrasonographically normal pregnancies. Lancet 2: 920, 1984 |
|
Gilmore DH, McNay MB: Spontaneous fetal loss rate in early pregnancy. Lancet 1: 107, 1985 |
|
Cashner KA, Christopher CR, Dysert GA: Spontaneous fetal loss after demonstration of a live fetus in the first trimester. Obstet Gynecol; 70:827-830, 1987 |
|
Regan L, Braude PR, Trembath PL: Influence of postreproductive performance on risk of spontaneous abortion. Br Med J 299: 551, 1989 |
|
Whitley E, Doyle P, Roman E et al: The effect of reproductive history on future pregnancy outcomes. Hum Reprod 14: 2863, 1999 |
|
Parazzini F, Chatenoud L, Tozzi L et al: Determinants of risk of spontaneous abortions in the first trimester of pregnancy. Epidemiology 8: 681, 1997 |
|
Simpson JL, Gray RH, Queenan JT et al: Risk of recurrent spontaneous abortion for pregnancies discovered in the fifth week of gestation. Lancet 344: 964, 1994 |
|
Malpus P: A study of abortion sequences. J Obstet Gynaecol Br Emp 45: 932, 1938 |
|
Warburton D, Fraser FC: Spontaneous abortion risks in man: Data from reproductive histories collected in a medical genetic unit. Am J Hum Genet 16: 1, 1964 |
|
Regan L: A prospective study on spontaneous abortion. In Beard RW, Sharp F (eds): Early Pregnancy Loss: Mechanisms and Treatment, p 22. London, The Royal College of Obstetricians and Gynaecologists, 1988 |
|
Kline J, Stein ZA, Susser M et al: Smoking: a risk factor for spontaneous abortion. N Engl J Med 297: 793, 1977 |
|
Boue JG, Boue A: Increased frequency of chromosomal anomalies in abortions after induced ovulation. Lancet 1: 679, 1973 |
|
Vlaanderen W, Treffers PE: Prognosis of subsequent pregnancies after recurrent spontaneous abortion in first trimester. Br Med J 295: 92, 1987 |
|
Liddell HS, Pattison NS, Zanderigo A: Recurrent miscarriage: outcome after supportive care in early pregnancy. Aust NZ J Obstet Gynaecol 31: 320, 1991 |
|
Houwert-de Jong MH, Termijtelen A et al: The natural course of habitual abortion. Eur J Obstet Gynecol Reprod Biol 33: 221, 1989 |
|
Brigham SA, Conlon C, Farquharson RG: A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod 14: 2868, 1999 |
|
Ober C, Karrison T, Odem RR et al: Mononuclear cell immunisation in prevention of recurrent miscarriages: A randomised trial. Lancet 354: 365, 1999 |
|
Hertig AT, Rock J, Adams EC: Description of human ova within the first 17 days of development. Am J Anat 98: 435, 1956 |
|
Hertig AT, Rock J, Adams EC et al: Thirty-four fertilized human ova, good, bad and indifferent, recovered from 210 women of known fertility. A study of biologic wastage in early human pregnancy. Pediatrics 25: 202, 1959 |
|
Hertig AT, Rock J: Searching for early human ova. Gynecol Invest 4: 121, 1973 |
|
Gropp A: Chromosomal animal model of human disease. Fetal trisomy and development failure. In Berry L, Poswillo DE (eds): Teratology, p 17. Berlin, Springer-Verlag, 1975 |
|
Plachot M, Junca AM, Mandelbaum J et al: Chromosome investigations in early life. Human preimplantation embryos. Hum Reprod 2: 29, 1987 |
|
Papadopoulos G, Templeton AA, Fisk N et al: The frequency of chromosome anomalies in human preimplantation embryos after in vitro fertilization. Hum Reprod 4: 91, 1989 |
|
Pellestor F, Dufour MC, Arnal F et al. Direct assessment of the rate of chromosomal abnormalities in grade IV human embryos produced by in-vitro fertilization procedure. Hum Reprod; 9:293-302, 1994 |
|
Egozcue J, Blanco J, Vidal F: Chromosome studies in human sperm nuclei using fluorescence in-situ hybridization (FISH). Hum Reprod Update 3: 441, 1997 |
|
Martin R: Chromosomal analysis of human spermatozoa. In Verlinsky Y, Kuliev A (eds): Preimplantation Genetics. New York, Plenum Press, p 91; 1991 |
|
Kuliev A, Zlatopolsky Z, Kirillova I et al. Meiosis errors in over 20,000 oocytes studied in the practice of preimplantationaneuploidy testing. Reprod Biomed Online; 22:2-8, 2011 |
|
Kuliev A., Practical Preimplantation Genetic Diagnosis, 2nd ed. Springer Verlag, London; 2012 |
|
Sandalinas M, Sadowy S, Calderon G et al. Developmental ability of chromosomally abnormal human embryos to develop to the blastocyst stage; 16:1954-1958, 2001 |
|
Schoolcraft WB, Katz-Jaffe MG, Stevens J, et al. Preimplantation aneuploidy testing for infertile patients of advanced maternal age: a randomized prospective trial. Fertil Steril; 92:157-162; 2009 |
|
Treff NR, Levy B, Su J, Northrop LE, et al. SNP microarray-based 24 chromosome aneuploidy screening is significantly more consistent than FISH. Mol Hum Reprod; 16:583-5899, 2010 |
|
Fragouli E, Katz-Jaffe M, Alfarawati S, et al. Comprehensive chromosome screening of polar bodies and blastocysts from couples experiencing repeated implantation failure. Fertil Steril;94: 875-887, 2010 |
|
Kroener L, Ambartsumyan G, Briton-Jones C, et al. The effect of embryonic progression on chromosomal abnormality. Fert Steril;98: 876-880, 2012 |
|
Simpson JL, Bombard AT: Chromosomal abnormalities in spontaneous abortion: frequency, pathology and genetic counseling. In Edmonds K (ed): Spontaneous Abortion. London, Blackwell, 1987, p 51. |
|
Sorokin Y, Johnson MP, Uhlmann WR, et al: Postmortem chorionic villus sampling: correlation of cytogenetic and ultrasound findings. Am J Med Genet 39:314, 1991. |
|
: ACOG Practice Bulletin No. 102: management of stillbirth. Obstet Gynecol. 2009 Mar;113(3):748-61. |
|
Simpson JL, Acuna JM: Genetic factors in stillbirths. Lancet. 2011 Sep 3;378(9794):878. |
|
Hassold T, Hunt P: Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr 21(6):703, 2009. |
|
Munne S, Fischer J, Warner A, et al. Preimplantation genetic diagnosis significantly reduces pregnancy loss in infertile couples: a multi-center study. Fertil Steril; 85:326-332, 2006 |
|
Hodes-Wertz B, Grifo J, Ghadir S, et al. Idiopathic recurrent miscarriage is caused mostly by aneuploid embryos. Fertil Steril; 675-680, 2012 |
|
Jacobs PA, Hunt PA, Matsuura JS, et al: Complete and partial hydatidiform mole in Hawaii: cytogenetics, morphology and epidemiology. Br J Obstet Gynaecol; 89:258-266, 1982 |
|
Bonduelle M, Liebaers I, Deketelaere V, Derde MP, Camus M, Devroey P, Van Steirteghm A: Neonatal data on a cohort of 2889 infants born after ICSI (1991-1999) and of 2995 infants born after IVF (1983-1999). Hum Reprod 17:671, 2002. |
|
Singh RP, Carr DH: The anatomy and histology of XO human embryos and fetuses. Anat Rec 155:369, 1966. |
|
Jirasek JE: Principles of reproductive embryology. In Simpson JL (ed): Disorders of Sex Differentiation: Etiology and Clinical Delineation. San Diego, Academic Press, 1976, p 51. |
|
Warburton D, Kline J, Stein Z, et al: Does the karyotype of a spontaneous abortion predict the karyotype of a subsequent abortion? Evidence from 273 women with two karyotyped spontaneous abortions. Am J Hum Genet 41:465, 1987. |
|
Warburton D, Dallaire L, Thangavelu M. et al: Trisomy recurrence: a reconsideration based on North American data. Am J Hum Genet 75:376, 2004. |
|
Al-Asmar N, Peinado V, Vera M, et al. Chromosomal abnormalities in embryos from couples with a previous aneuploidy miscarriage. Fertil Steril, 98:145-150; 2012 |
|
Munné S, Sandalinas M, Magli C, et al: Increased rate of aneuploid embryos in young women with previous aneuploid conceptions. Prenat Diagn 24:638, 2004. |
|
Lathi RB, Westphal LM, Milki AA. Aneuploidy in the miscarriages of infertile women and the potential benefit of preimplanation genetic diagnosis. Fertil Steril; 89:353-357, 2008 |
|
Stephenson MD, Awartani KA, Robinson WP: Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Hum Reprod 17:446, 2002. |
|
Carp H, Toder V, Aviram A, et al. Karyotype of the abortus in recurrent miscarriage. Fertil Steril 2001; 75;678-82. |
|
Snijders RJ, Nicolaides KH. Ultrasound markers for fetal chromosomal defects. New York: Parthenon, 1996. |
|
Bianco K, Caughey AB, Shaffer BL, et al. History of miscarriage and increased incidence of fetal aneuploidy in subsequent pregnancy. Obstet Gynecol 2006; 107:1098-102. |
|
Munne S, Escudero T, Colls P, et al. Predictability of preimplantation genetic diagnosis of aneuploidy and translocations on prospective attempts. Reprod Biomed Online 2004; 9:645-51. |
|
Boué A, Gallano P: A collaborative study of the segregation of inherited chromosome structural rearrangements in 1,356 prenatal diagnoses. Prenat Diagn 4:45, 1984. |
|
Simpson JL, Richards DS, Otano L, Driscoll DA. Prenatal genetic diagnosis. Obstetrics: Normal and problem pregnancies, 6th ed. SG Gabbe, JR Niebyl, JL Simpson (eds). Philadelphia Elsevier Saunders; pp 210-236, 2012 |
|
Gardner RJM, Sutherland GR, Shaffer LG. Chromosome abnormalities and genetic counseling,4th ed. New York, Oxford University Press,2011. |
|
Daniel A, Hook EB, Wulf G. Risks of unbalanced progeny at amniocentesis to carriers of chromosome rearrangements: data from United States and Canadian laboratories. Am J Med Genet; 33:14-53, 1989 |
|
Sugiura-Ogasawara M, Ozaki Y, Sato T, et al. Poor prognosis of recurrent aborters with either maternal or paternal reciprocal translocations. Fertil Steril; 81:367-373, 2004 |
|
Goddijn M, Joosten JH, Knegt AC, et al. Clinical relevance of diagnosing structural rearrangements in 1,356 prenatal diagnoses. Hum Reprod; 19:1013-1017, 2004 |
|
Stephenson MD, Sierra S. Reproductive outcomes in recurrent pregnancy loss associated with a parental carrier of a structural chromosome rearrangement. Hum Reprod; 21:1076-1082, 2006 |
|
Fritz MA, Schattman G. Reply of the Committee: Parental translocations and need for preimplantation genetic diagnosis? Distorting effects of ascertainment bias and the need for information-rich families. Fertil Steril; 90: 892-893, 2009 |
|
Kuliev A, Janzen JC, Zlatopolsky Z, Kirillova I, Ilkevitch Y, Verlinsky Y. Conversion and non-conversion approach to preimplantation diagnosis for chromosomal rearrangements in 475 cycles. Reproductive Biomed Online; 21:93-99, 2010 |
|
Verlinsky Y, Tur-Kaspa I, Cieslak J, et al. Preimplantation testing for chromosomal disorders improves reproductive out¬come of poor-prognosis patients. Reprod Biomed Online; 11:219-225, 2005 |
|
Martin AO, Simpson JL, Deddish RB, et al. Clinical implications of chromosomal inversions. A pericentric inversion in No. 18 segregating in a family ascertained through an abnormal proband. Am J Perinatol; 1:81-88, 1983 |
|
Pettenati MJ, Rao PN, Phelan MC, et al. Paracentric inversions in humans: a review of 446 paracentric inversions with presentation of 120 new cases. Am J Med Genet; 55:171-187, 1995 |
|
Philipp T, Kalousek DK: Generalized abnormal embryonic development in missed abortion: embryoscopic and cytogenetic findings. Am J Med Genet 111:43, 2002. |
|
Robberecht C, Pexsters A, Deprest J, et al. Cytogenetic and morphological analysis of early products of conception following hysteron-embryoscopy from couples with recurrent pregnancy loss. Prenat Diagn; 32:933-942, 2012 |
|
Karamardian LM, Grimes DA: Luteal phase deficiency: effect of treatment on pregnancy rates. Am J Obstet Gynecol 167:1391, 1992. |
|
Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A: Increased Pregnancy Loss Rate in Thyroid Antibody Negative Women with TSH Levels between 2.5 and 5.0 in the First Trimester of Pregnancy. J Clin Endocrinol Metab 95:E44, 2010. |
|
Stagnaro-Green A: Thyroid autoimmunity and the risk of miscarriage. Best Practice & Research Clinical Endocrinology & Metabolism 18:167, 2004. |
|
Anselmo J, Cao D, Karrison T et al.: Fetal loss associated with excess thyroid hormone exposure JAMA 292:691, 2004. |
|
Jovanovic L, Knopp RH, Kim H, et al. Elevated pregnancy losses at high and low extremes of maternal glucose in early normal and diabetic pregnancy: evidence for a protective adaptation in diabetes. Diabetes Care; 28:1113-1117, 2005 |
|
Ben Rafael Z, Seidman DS, Recabi K, et al: Uterine anomalies. A retrospective, matched-control study. J Reprod Med; 36:723-727, 1991 |
|
Salim R, Regan L, Woelfer B, et al. A comparative study of the morphology of congenital uterine anomalies in women with and without a history of recurrent first trimester miscarriage. Hum Reprod; 18:162-166, 2003 |
|
Proctor JA, Haney AF. Recurrent first trimester pregnancy loss is associated with uterine septum but not with bicornuate uterus. Fertil Steril; 80:1212-1215, 2003 |
|
Stampe SS: Estimated prevalence of mullerian anomalies. Acta Obstet Gynecol Scand; 67:441-445, 1988. |
|
Ludmir J, Samuels P, Brooks S, et al: Pregnancy outcome of patients with uncorrected uterine anomalies managed in a high-risk obstetric setting. Obstet Gynecol 75:906, 1990. |
|
Hartmann KE, Herring AH: Predictors of the presence of uterine fibroids in the first trimester of pregnancy: A prospective cohort study. J Soc Gynecol Investig 11[Abstract 789], 340A, 2004. |
|
Ludmir J, Owen J. Cervical Insufficiency. Gabbe: Obstetrics: Normal and problem pregnancies, 6th ed. SG Gabbe, JR Niebyl, JL Simpson (eds). Philadelphia Elsevier Saunders; pp 609-626, 2012 |
|
Simpson JL, Mills JL, Kim H, et al: Infectious process: an infrequent cause of first trimester spontaneous abortions. Hum Reprod 11:668,1996 |
|
ACOG Practice Bulletin: Antiphospholipid Syndrome. Obstetrics and Gynecology 117:192, 2011. |
|
Simpson JL, Carson SA, Chesney C, et al: Lack of association between antiphospholipid antibodies and first-trimester spontaneous abortion: prospective study of pregnancies detected within 21 days of conception. Fertil Steril 69:814, 1998. |
|
Rey E, Kahn SR, David M, et al. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet 361:901, 2003. |
|
Kovalesky G, Gracia CR, Berlin JA et al. Evaluation of the association between hereditary thromphilias and recurrent pregnancy loss. Arch Intern Med 164:558, 2004. |
|
Halmesmaki E, Valimaki M, Roine R, et al: Maternal and paternal alcohol consumption and miscarriage. Br J Obstet Gynaecol 96:188, 1989. |
|
Armstrong BG, McDonald AD, Sloan M: Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am J Public Health 82:85, 1992. |
|
Mills JL, Holmes LB, Aarons JH, et al: Moderate caffeine use and the risk of spontaneous abortion and intrauterine growth retardation. JAMA 269:593, 1993. |
|
Klebanoff MA, Levine RJ, DerSimonian R, et al: Maternal serum paraxanthine, a caffeine metabolite, and the risk of spontaneous abortion. N Engl J Med 341:1639, 1999. |
|
Savitz DA, Sonnenfeld NL, Olshan AF: Review of epidemiologic studies of paternal occupational exposure and spontaneous abortion. Am J Ind Med 25:361, 1994. |
|
Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev 83:699, 2007. |
|
Ness RB, Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med.; 340:333-339, 1999. |
|
Harlap S, Shiono PH. Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet; 2:173-176, 1980 |
|
Nelson DB, Grisso JA, Joffe MM, et al: Violence does not influence early pregnancy loss. Fertil Steril 80:1205, 2003. |
|
Stray-Pedersen B, Stray-Pedersen S: Recurrent abortion: the role of psychotherapy. In Beard RW, Sharp F (eds): Early Pregnancy Loss: Mechanism and Treatment. London, Royal College of Obstetricians and Gynecologists, 1988, p 433. |
|
Jauniaux E, Farquharson RG, Christiansen OB, Exalto N. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod 21:2216,2006. |
|
ACOG Practice Bulletin No. 24. Management of recurrent pregnancy loss. Int J Gynaecol Obstet.; 78:179-90, 2002 |
|
American Society for Reproductive Medicine (ASRM). Intravenous immunoglobin (IVIG) and recurrent spontaneous pregnancy loss. A Practice Committee Report. Birmingham, AL: ASRM, 2006 |
|
Clifford K, Rai R, Regan L. Future pregnancy outcome in unexplained recurrent first trimester miscarriage. Hum Reprod; 12:387-389, 1997 |
|
Surkan PJ, Stephansson O, Dickman PW, Cnattingius S: Previous preterm and small-for-gestational-age births and the subsequent risk of still birth. N Engl J Med 350:777, 2004. |
|
Getahum D, Ananth CV, Kinzler WL: Risk factors for antepartum and intrapartum stillbirth: a population-based study. Am J Obstet Gynecol 196:499, 2007. |
|
Bhattacharya S, Prescott GJ, Black M, Shetty A: Recurrence risk of stillbirth in a second pregnancy. BJOG Published online 24 June 2010 (DOI: 10.1111/j.1471-0528.2010.02641.x) |
|
Laury A, Sanchez-Lara PA, Pepkowitz S, Graham JM Fr.: A study of 534 fetal pathology cases from prenatal diagnosis referrals analyzed from 1989 through 2000. Am J Med Genet A 143A:3107, 2007. |
|
Korteweg FJ, Bouman K, Erwich JJ, Timmer A, Veeger NJ, Ravise JM, et al.: Cytogenetic analsysi after evaluation of 750 fetal deaths: proposal for diagnostic workup. Obstet Gynecol 111:865, 2008. |
|
Reddy UM, Wapner RJ, Rebar RW, Tasca RJ: Infertility, assisted reproductive technology, and adverse pregnancy outcomes. Obstetrics & Gynecology 109:967, 2007. |
|
van Oppenraaij RH, Jauniaux E, Christiansen OB, Horcajadas JA, Farquharson RG, Exalto N; ESHRE Special Interest Group for Early Pregnancy (SIGEP). Predicting adverse obstetric outcome after early pregnancy events and complications: a review. Hum Reprod Update. 15: 409,2009 |
|
Jauniaux E, Van Oppenraaij RH, Burton GJ. Obstetric outcome after early placental complications. Curr Opin Obstet Gynecol. 22:452, 2010. |