Myomectomy
Authors
INTRODUCTION
Menorrhagia
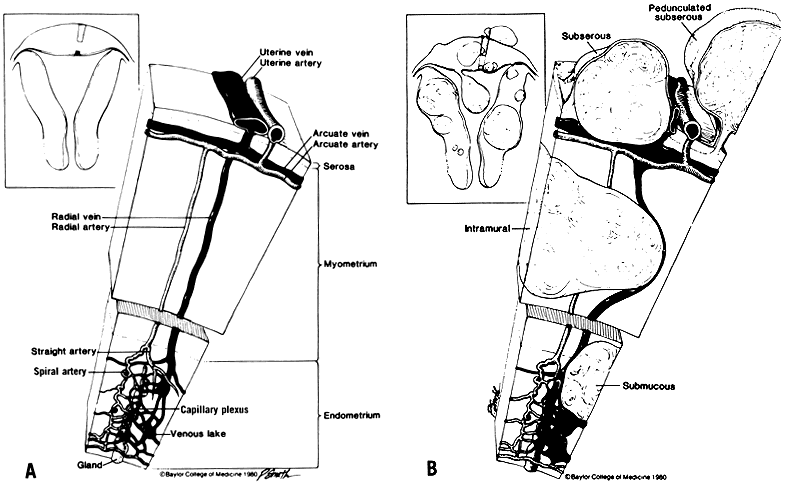
The variation on number, size, and location of myomas results in a spectrum of clinical symptoms and signs. Approximately 20–50% of myomas produce symptoms, but many patients are asymptomatic. A review of the literature by Buttram and Reiter1 reveals that 30% of patients with myomas present with menstrual abnormalities, predominantly menorrhagia. Understanding of the pathogenesis of this symptom is lacking, because only 5% of myomas are submucous and could not account directly for the menorrhagia. There are anatomic alterations by myomas in the uterine blood supply (Fig. 1). Interference of myomas with uterine contractility may also explain the occurrence of menorrhagia in the absence of submucous myomas. Recent studies contend that menorrhagia associated with myomas is related to endometrial changes caused by the myoma,2 venous congestion,3 and the increased surface area of the endometrial cavity in the presence of myomas.4
Infertility
The anatomical location of the fibroid is an important factor in causing infertility, and myomas greater than 5 cm,5 those that distort the endometrial cavity and affect endometrial development have been clearly linked to a reduction in implantation rate,6 higher miscarriage rates,7, 8 and lower overall pregnancy rates.9 Those located near the cervix or tubal ostia are also likely to pose a problem.10 Furthermore, submucous or intramural myomas can also cause dysfunctional uterine contractility, obstruct gamete transport, and alter endometrial receptivity to embryo implantation.1, 8, 11 Endometrial vascular disturbance and inflammation associated with myomas can also result in implantation failure or miscarriage.1, 2 Any or all of these findings could explain how myomas cause infertility.
Infertility patients with myomas need to be evaluated in the same fashion as any infertility patient. According to Buttram and Reiter, only 1% to 3% of patients undergoing myomectomy have no additional cause of infertility.1 However, as many as 30% of patients with causes of infertility in addition to myomas undergo myomectomy. Reports of myomectomies in patients with primary infertility and no other detectable causes of infertility, noted pregnancy rates as high as 40% after myomectomy. Gehl-bach and associates' life table analysis of patients after abdominal myomectomy demonstrated a cumulative pregnancy rate of 57% and live birth rate of 48%.12 In their analysis, the number of myomas was not associated with pregnancy rate, but the presence of adhesions at the time of myomectomy had a significant impact on reducing the likelihood of conception. Rosenfeld reported a 65% spontaneous pregnancy rate after abdominal myomectomy in patients with unexplained infertility and the diagnosis of subserosal or intramural myomas.13
Fetal Wastage
Spontaneous abortion, premature labor, outlet obstruction, and abnormal presentation all occur with increased frequency in patients with myomas. A review of 1941 cases of myomectomy by Buttram and Reiter1 revealed a 41% spontaneous abortion rate before myomectomy and 19% after myomectomy. Spontaneous abortion may result from increased uterine irritability, altered blood supply to the endometrium, and distortion of the endometrial cavity with subsequent interference with implantation, and molecular changes in the endometrium overlying the myoma.6
Pelvic Pressure and Pain
Patients with myomas are at increased risk for developing degeneration with subsequent pain. The differential diagnosis of pelvic pain in these patients includes pelvic inflammatory disease, endometriosis, torsion, aborting myoma, diverticulitis, appendicitis, or ovarian carcinoma. Ultrasound may prove helpful in the diagnosis, as may magnetic resonance imaging. This differential diagnosis is the same as for patients who do not have myomas, except for the entity of degenerating myomas. The distinguishing features of degenerating myomas include severe low abdominal and pelvic pain, uterine tenderness, low-grade fever, leukocytosis, and occasionally peritoneal signs. The location of the myoma may cause the patient to present with pelvic pressure, but this correlates more with uterine size. A fibroid that presses on the bladder may cause urgency, frequency, urinary retention, and even overflow incontinence. A tumor that impinges on the rectosigmoid area can produce constipation or rarely intestinal obstruction. Hydronephrosis and hydroureter can result if it causes pressure on the ureter.
Other Symptoms
Endometriosis is commonly found in the endometrium superficial to a submucous myoma and may manifest with all of its classic findings: pain, fever, and uterine tenderness. Patients with aborting myomas present with uterine cramps, abdominal pain, fever, and vaginal bleeding, and a mass can be seen protruding through the cervical os. Occasionally, patients with fibroids may present with a rapidly enlarging pelvic mass. Although sarcomatous malignant change in myomas is rare (probably <0.5%), it must never be overlooked in these patients. It is especially ominous in the postmenopausal patient.
SIGNS OF MYOMAS
Physical findings in patients with myomas include an enlarged, irregular uterus or pelvic mass. If the uterus is larger than the size at 12 weeks' gestation, it may be difficult to assess the ovaries and be certain that they are separate from the pelvic mass. Patients who complain of menorrhagia have various degrees of iron deficiency anemia. Occasionally, patients present with paradoxical polycythemia, which may be caused by increased erythropoietin levels produced by myomatous tissue. Parasitic myomas may obstruct omental blood vessels and result in ascites. Pedunculated submucous tumors that protrude through the cervical os may manifest with ulceration and infection. Rarely, uterine inversion has been reported with these types of myomas.
INDICATIONS FOR MYOMECTOMY
Although there are many clear-cut indications for myomectomy, therapy must be individualized according to the presentation, desires, and relative risks of a particular patient in many situations. Severe menorrhagia in a 26-year-old nulligravid patient with multiple myomas usually is managed by myomectomy, but a 42-year-old patient with the same findings has a set of choices for which data are available for guidance. The decision to perform a myomectomy versus hysterectomy should be based on the patient's strong desire to retain fecundity and the patient's desire to retain the uterus. Case-controlled studies have shown no difference in the risk of hemorrhage, febrile morbidity, blood transfusion, life-threatening events, and rehospitalization between women who undergo myomectomy versus hysterectomy, and have concluded that myomectomy is a safe alternative to hysterectomy.14 One study, even showed a possible decreased risk for visceral injury and infection with abdominal myomectomy compared to hysterectomy.15
The indications for myomectomy are severe menorrhagia in the setting of leiomyomas, protracted symptoms not responding to medical management, recurrent pregnancy loss in the presence of myomas, obstruction of pelvic organs (i.e. ureters, bowel, bladder, and fallopian tubes) by myomas, rapidly enlarging myomas, infertility resulting from myomas, and myomas of a certain size (still controversial what that size is) when the patient desires to retain fecundity or the uterus.
SURGICAL METHODS
Techniques of Abdominal Myomectomy
The general principles of sound surgical techniques are as much a part of myomectomy as for any operation. Adequate exposure can be achieved through a Pfannenstiel incision. However, if more space is needed with a transverse incision either a Cherney or Maylard procedure can be performed. However, when the uterus is greater than 16 weeks' size and cannot be delivered through the transverse incision, a vertical midline incision may be more appropriate. The surgeon must also take into account the location of the myomas before deciding on the incision. For instance, a broad ligament myoma may require dissection in the pelvic sidewall with subsequent unroofing of the ureter; this is done more easily through a vertical midline incision. Good clinical judgment rather than controlled clinical studies provides better guidance in these situations. A mild Trendelenburg position helps ensure exposure. Gentle handling of the tissue is thought to reduce the likelihood of adhesions. Although there is little that may be gentle about myomectomy, the surgeon can operate in such a manner as to minimize tissue damage.
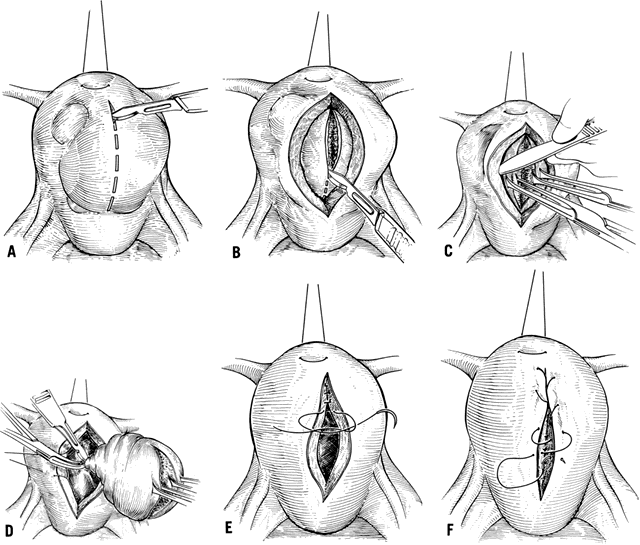
After the field is clear and exposure is adequate, the surgeon must plan the uterine incision. This decision is based on the number and location of the myomas. The most preferred incision is a vertical incision on the anterior surface of the uterus. This minimizes blood loss and keeps the ovaries from adhering to the posterior wall of the uterus postoperatively. Occasionally, the surgeon must perform “transcavity enucleation” as described by Bonney,16 to avoid a posterior incision. In this manner, posterior fibroids can be removed through an anterior uterine incision, which avoids the hazards of a posterior incision. The caveat with this method is that the surgeon must affirm that the posterior defect is adequately repaired, that the cavity must not be compromised by suture, and that, subsequently, delivery be performed by cesarean section. The surgeon often can remove multiple myomas from a single incision, whereas at other times, multiple incisions are required. Although there is no proof, it is felt that minimizing the amount of damaged peritoneal surface relates linearly to the extent of adhesion formation. Minimizing the length of the uterine incision and the number of uterine incisions is a general strategy to be employed.
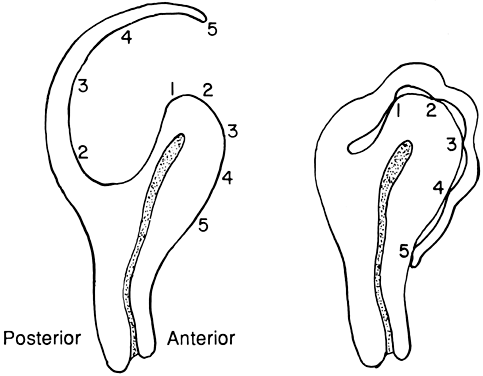
Another method for approaching the posterior myoma has been described by Bonney and is called the Bonney hood (Fig. 2). This approach uses a transverse posterior fundal incision and subsequent enucleation of the myoma. After interrupted sutures in layers are used to close the dead space, the extra serosa is sutured with fine suture to the anterior surface of the uterus, creating a functional anterior incision. This allows a posterior approach but avoids a posterior defect.
The general strategy employed for removing a myoma regardless of location involves incising through the pseudocapsule of the myoma. This maneuver exposes the tissue planes and allows isolation of the capsule with Allis clamps. Blunt and sharp dissection is used to peel the myoma out of the capsule. The surgeon can use a knife, Mayo scissors, electrocoagulation, laser, or blunt dissection to accomplish this. Complete excision of the myoma leaves an oozing cavitary defect in the uterus. If more myomas are resectable through this incision, they are removed at this point; otherwise, the wound is closed. With this method, the surgeon mindful of vital adjacent structures (e.g. ureters, uterine vessels, cornua) to avoid pelvic injuries.
Closing the resultant uterine defect involves much individualization of approach. The goal is to restore normal anatomy and to ensure adequate hemostasis, which requires attention to filling in the dead space. Despite meticulous attempts to achieve this, much area often remains open and fills in with blood. The resultant tamponade eventually aids in hemostasis, but these pockets of blood provide a rich culture medium for infection. Although some advocate the use of continuous suture in this area, interrupted sutures afford greater chance at tissue approximation, minimizing dead space. Layered interrupted sutures are time consuming but provide the best opportunity at tissue coaptation. After the myometrial layers have been adequately reapproximated, excess serosa may be trimmed and the serosal defect repaired with a fine polyglycolic suture in a running “baseball” fashion. This allows a minimum of exposed suture material and decreases adhesion formation (Fig. 3).
Techniques to Reduce Blood Loss
Myomectomy is perhaps one of the bloodiest gynecologic operations performed, and the most significant morbidity associated with it includes blood loss. A variety of methods have been described that are aimed at minimizing blood loss. The two main approaches, medical and mechanical, diminish uterine blood flow to the myoma. The blood supply to a myoma is highly variable, and it is difficult to predict the blood vessel location when dissecting the myoma. The anatomy can be significantly distorted by the myomas, such that even the major blood supply to the uterus may be altered. The optimal uterine incision to minimize blood loss is an anterior, vertical incision. This cuts across a minimum of collateral channels and a minimum number of blood vessels.
One popular medical approach to decreasing blood loss in myomectomy uses 8-L-arginine vasopressin (Pitressin). Dilute solutions of this are injected directly into the myoma, raising a circumferential wheal and causing vasoconstriction. Most investigators report using pitressin diluted from 0.2 to 1 unit per milliliter of diluent, using a maximum of 20 units total. Some feel that this is unnecessary and that it only delays bleeding. It does blanch the tissue and provide better visualization of the tissue planes. Although the drug is not approved by the US Food and Drug Administration for this use, many have used it safely for a number of years. Physicians must be cognizant of the potential side effects of this medication, as for any medication. Its use is contraindicated in patients with epilepsy, migraine, asthma, heart failure, and nephritis. In patients with documented coronary disease, angina, and even myocardial infarction has been described. Myomectomy is rarely performed in such patients. Pitressin may produce water intoxication, the early signs of which must be recognized. Headache, drowsiness, and listlessness always precedes convulsions and coma, which can result from severe overdosage of this medication.
The most direct method to minimize blood loss during myomectomy is to use mechanical methods that decrease uterine blood flow. Many methods have been described using a variety of clamps and tourniquets, but all apply the same general principle. Bonney16 first described the use of an atraumatic clamp that compresses the uterine vessels and decreases uterine blood flow. The Bonney clamp is applied from the pubic end of the abdominal wound; it must contain the round ligament in its grip, or it slips below the uterine vessels. Blood flow from the infundibulopelvic ligament is compressed using a ring forceps; the uterine blood flow is essentially stopped. The location of the ureter must be known before applying any clamps. Other variations of this method use bulldog clamps, rubber-shod clamps, or tourniquets. The disadvantage of using a tourniquet is that a small incision is often made in an avascular area or the broad ligament (although this does not have to be done).
The tourniquet is then applied around the cardinal ligaments, obstructing uterine vessel blood flow. A tourniquet can also be applied around the infundibulopelvic ligament in a similar fashion. The defect in the broad ligament must be repaired with a fine suture and therefore becomes another site for possible adhesion formation. The unknown aspect of these mechanical methods is the duration that the blood supply to the uterus can be occluded before irreversible ischemic damage occurs. Some investigators recommend that the clamps or tourniquets be released every 15 minutes to prevent this phenomenon. Ranney and Frederick17 described a histamine-like substance that accumulates in the uterus that has its blood supply obstructed. They suggest that this may lead to postoperative shock. There are no reported cases of postoperative thrombosis of the uterine vessels, necrosis of the uterus, and damage to the tubes and ovaries, but the physicians must be aware of the potential hypoxic injury to the uterus with this method. A randomized, prospective study18 comparing Pitressin to mechanical occlusion of the uterine and ovarian vessels showed that it was just as effective as mechanical occlussion in minimizing blood loss at the time of myomectomy, with no difference in operative blood loss, operating time, pre and post-operative hematocrit, transfusion rates, and length of hospital stay between groups.
An additional strategy for minimizing risk during myomectomy involves the use of autologous blood to minimize infection. Patients can store up to 1 unit every 2 weeks, and with the ability to freeze autologous blood, a large quantity of blood can be obtained. These patients should be given supplemental iron during this time. Although this is not always practical for the patient with menorrhagia, it does allow many the option. In the patient with large fibroids, gonadotropin-releasing hormone (GnRH) agonists can be used concomitantly to shrink the myomas. The GnRH agonists allow time for the patient to bank autologous blood, and they decrease the size of the myomas and thereby make myomectomy technically easier. The cell saver has also been used in myomectomies19 and avoids the risk of infection and transfusion reaction.
Techniques to Prevent Adhesion Formation
Perhaps the biggest threat that myomectomy poses to fertility is that of adhesion formation. Gehlbach and colleagues demonstrated that adhesions, even at the time of myomectomy, significantly reduces the likelihood for future conception.12 Second-look laparoscopy has demonstrated the formation of a significant degree of pelvic adhesions as early as 8 days after abdominal myomectomy.20 Higher degrees of adhesion have been demonstrated with posterior wall incisions, uterine size larger than 13 weeks' gestation, and with myomectomies involving intramural myomas. Multiple strategies have been described for minimizing this potential complication. The vertical, anterior uterine incision can minimize blood loss and prevent the formation of adhesions that involve the tubes, ovaries, or bowel.21 This incision should be employed if feasible. Other strategies utilize adhesion barriers such as Interceed, Gore-Tex and Seprafilm; and medical approaches using dextran 40, factor 13 with fibrinogen, copious irrigation, and meticulous hemostasis have also been employed. In a recent prospective study of 63 women undergoing an abdominal myomectomy that compared the effectiveness of Seprafilm to Dextran 40, factor 13 with fibrinogen, and a control group in preventing post-surgical adhesion formation, Seprafilm was more effective than the medical approaches [14.3% Seprafilm vs. 70.6% Dextran 40, 75% factor 13 with fibrinogen, and 76.9% control group] in preventing uterine adhesion formation at a second-look laparoscopy.22
Vaginal Myomectomy
Rarely, a myoma manifests as a mass protruding through the cervical os. Invariably, these tumors are pedunculated submucous myomas, which may make them amenable to removal through the vaginal approach. This form of myomectomy has the risk of increased infection, and occasionally hemostasis cannot be achieved, forcing the surgeon to proceed to abdominal hysterectomy. Despite these hazards, this approach has the advantages of not entering the peritoneal cavity with subsequent adhesion formation, producing a much faster recovery, causing generally lower blood loss, and being a less deforming procedure. Goldrath23 described the use of laminaria tents preoperatively to dilate the cervix and aid exposure of the pedicle. The surgeon can double clamp the pedicle and incise the stalk with cautery or a knife and then place Heaney-type sutures of 0-Vicryl. When this is feasible, a relatively fast operation can be done. A myomas with a thicker stalk does not always permit this approach, and the surgeon can incise the pedicle with cautery. The pedicle usually then recedes into the uterine cavity, and the physician observes for bleeding. If hemostasis cannot be achieved by conservative management, hysteroscopy can be performed to attempt hemostasis using directed cautery. If this fails, the surgeon can attempt to pack the uterus and vagina and carefully observe the patient. The last resort is to perform an abdominal hysterectomy. These patients should be counseled preoperatively about these risks and the possibility of abdominal hysterectomy. Despite the hazards, this operation usually results in a good outcome. Goldrath reports that 83 of 92 patients had successful vaginal myomectomies.23
Hysteroscopic Resection of Myomas
The patient with symptomatic submucous myomas presents the clinician with a therapeutic challenge. When the uterus is small, an abdominal myomectomy has distinct disadvantages. Hormonal therapy is often ineffective. The patient who presents with menorrhagia needs a diagnosis, and the most direct approach is to perform hysteroscopy in addition to endometrial sampling. This approach distinguishes between submucous myomas, dysfunctional uterine bleeding, endometrial polyps, and neoplastic causes. The effectiveness of hysteroscopic resection of submucosal myomas for abnormal uterine bleeding is dependent upon the degree of intramural extension. Hysteroscopic resection of submucosal myomas with more than 50% intramural extension may result in incomplete resection.24
The patient who has submucous myomas as the cause of menorrhagia has many options for treatment. For the patient who does not desire fertility, vaginal or abdominal hysterectomy is appropriate management. The patient who desires fertility has two options: hysteroscopic resection and abdominal myomectomy. Although women report a high satisfaction and improvement in symptoms and quality of life after a hysteroscopic myomectomy, long-term follow-up of 285 women with menorrhagia or metrorrhagia who had hysteroscopic resection of one or more submucosal myomas required additional surgery for 9.5% at 2 years, 20.5% at 5 years, and 26.7% at 8 years.25 Furthermore, data suggest that hysteroscopic resection provides fertility results that are comparable or perhaps superior to abdominal myomectomy while providing the added benefit of lower incidence of postoperative morbidity and shorter length of hospital stay.10 Neuwirth26 described 28 patients who underwent hysteroscopic resection of submucous myomas and had long-term follow-up. Compared to myomectomy via laparotomy, hysterosocpic myomectomy is associated with a lower risk of scar rupture during subsequent pregnancy and vaginal delivery, as any scar resulted does not involve the whole thickness of the uterine wall. Pelvic adhesions, which is a common occurrence following open myomectomy, is avoided. The patients were between 25 and 48 years of age at the time of surgery; therefore, it is difficult to make firm conclusions about postoperative fecundity. However, 17 of the 28 patients had normal menses postoperatively, 7 underwent hysterectomy, 2 were lost to follow-up, and 2 had repeat resections. Of the 17 patients, 5 became pregnant with a total of 8 pregnancies. This study suggests that hysteroscopic myomectomy is a viable treatment option for the infertile patient with symptomatic submucous myomas. However, hysteroscopy is not without its risks. Although rare, uterine perforation, distention, medium hazards, infection, and hemorrhage are serious complications. The patient must be counseled about the possibility of altered menses postoperatively and the unknown potential for Asherman's syndrome.
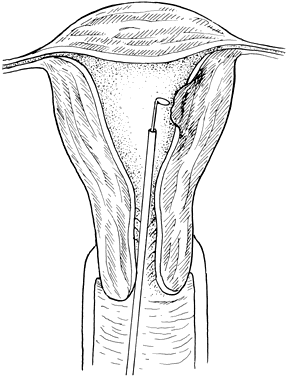
The technique of hysteroscopic resection sometimes requires laparoscopic guidance. This prevents the possibility of damage to intraabdominal structures during the procedure. The instrument generally required is a resectoscope that is designed to pass through the hysteroscope. The uterus is distended with either normal saline, lactate Ringer's solution, or a hypo-osmotic solution (i.e., sorbitol, glycine, mannitol) delivered by pump or by hand-held syringe. A cutting electrosurgical current is delivered by a cautery unit. The submucous myoma is shaved down to the surface of the surrounding myometrium, and hemostasis is ensured (Fig. 4). A sterile 12- or 14-French Foley catheter may be placed in the uterine cavity and distended to provide tamponade and further ensure hemostasis when necessary. It can be left in place for several days if an extensive intrauterine surgery is performed or if there is concern for development of Asherman's syndrome. This keeps the uterine wall from reconnecting until healing is completed. A 2-month course of high dose estrogen (Premarin 2.5 mg daily for 3 of every 4 weeks with medroxyprogesterone acetate 10 mg daily added during the 3rd week) is also given to avoid Asherman's syndrome.27 The patient's recovery is relatively quick. Some investigators have used preoperative GnRH analog to shrink the myoma, although there are no controlled studies to document the benefit.
Laparsocopic Myomectomy
The laparoscopic approach to myomectomy is becoming increasingly popular. It has been argued that, compared with the abdominal approach, this technique offers a lower degree of blood loss, a shorter length of hospital stay, and a lower overall complication rate.28, 29 However, the procedure is not without its drawbacks. Laparoscopic resection of multiple myomas is more time consuming, larger myomas are more difficult to remove from the abdomen, and adequate repair of the myometrium after removal of an intramural fibroid is often too difficult to accomplish. The procedure is limited mainly to the removal of subserosal myomas, although it has been used to remove intramural myomas. Blood loss is reportedly minimal, and hemostasis is achieved with a minimum of complications. This operation is limited to small myomas and should be performed only by the surgeon skilled in operative laparoscopy.
Based on their experience with 109 myomectomies, 70 of which were performed laparascopically, Darai and colleagues recommended that laparoscopic myomectomy be reserved for patients presenting with the maximum number of four myomas, with none surpassing a diameter of 7 cm.30 Comparable spontaneous pregnancy rates have been reported after laparoscopic and abdominal myomectomies in selected groups of patients.31 However, these results should be considered in the context of the criteria chosen for patient selection. The outcome of the method employed is closely associated with the size of the myoma, the number of myomas present, and myoma location. Inadequate uterine repair after laparoscopic myomectomy may result in grave obstetric consequences because of uterine rupture.32, 33 Women of childbearing age with symptomatic intramural fibroids should undergo abdominal myomectomy or a modified laparoscopic procedure to ensure proper closure of the myometrial defect.34 It may be more prudent to reserve the complete laparoscopic approach for the pedunculated and subserosal fibroids.
COMPLICATIONS OF MYOMECTOMY
Like any other pelvic surgery, myomectomy may be associated with hemorrhage, infection, bowel obstruction, adhesion formation, damage to bowel, bladder, fallopian tube, ureter, wound infection, and wound separation. Furthermore, roughly 20–25% of patients undergoing myomectomy require another pelvic operation, usually hysterectomy because of recurrence of symptoms. Recurrent myomas are common, especially in patients with multiple myomas. Patients with a solitary myoma have a 27% recurrence rate, and those with multiple myomas have a rate of 59%.
Patients undergoing myomectomy should be counseled pre-operatively about these risks and must understand that a myomectomy may not be possible. Although it is not common, uncontrolled intraoperative hemorrhage may require a hysterectomy. Because it is difficult to predict pre-operatively which patients will require this procedure, all patients should be warned of the risk. The patient must also be aware of the increased potential for blood transfusion intra-operatively and during recovery. Significant febrile morbidity results, and it is often difficult to distinguish infection from fever caused by the release prostaglandins during myomectomy. Nonsteroidal antiinflammatory agents are often useful in preventing this type of fever. Furthermore, patients should be counselled about cesarean section in subsequent pregnancies if the uterine cavity is entered or large intramural fibroids are removed.
A significant but not easily quantified complication of myomectomy is that of infertility caused by the procedure. Some information in the literature suggests that myomectomy may cause infertility. Adhesions that limit the mobility of the adnexa may decrease the ability of the fimbria to pick up an oocyte. They may also isolate the fimbria from the ovary. It is not clear whether peritoneal adhesions limit fertility, but they may contribute to the problem.
MEDICAL MANAGEMENT OF MYOMAS
Medical therapy, with GnRH agonists, is limited to pre-operative management since there is no definitive medical treatment to reduce myoma size or prevent their development. GnRH agonists have been shown to decrease myoma size by 46% and uterine volume by 57%.35 Maximum benefit in regard to shrinking myomas is seen after approximately 6–8 weeks of therapy. It is recommended that therapy be started during the luteal phase (day 21) to minimize the estrogen withdrawal bleeding that occurs about 10 days after commencement of therapy. Patients tend to remain amenorrheic and hypoestrogenic if properly suppressed. However, fibroids can regrow after discontinuation of therapy. Although these agents can cause a significant reduction in size of myomas, it is not clear that this offers any distinct advantage except to augment pre-operative hemoglobin levels.36
The levonorgesterol-releasing IUD, anti-progestins (i.e., RU-486), and selective receptor modulators (SPRMS), have also been shown to substantially reduce bleeding associated with myomas. In a prospective study37 to evaluate the usefulness of the levonorgesterol IUD in reducing blood loss in women with symptomatic myomas, 95% of women who were anemic at the beginning of the study had a hemoglobin of 13.6 g/dl after 1 year of use, and 40% of patients were amenorrheic at 12 months. Anti-progestins and SPRMS have also been shown to have similiar reduction in uterine and myoma size as GnRH agonists, and can cause a significant reduction in bleeding without causing a hypo-estrogenic state.38, 39 However, anti-progestins have been associated with endometrial hyperplasia because of unopposed exposure of the endometrium to estrogen.40
Pirfenidone, an anti-fibrotic agent has also been shown to inhibit new myoma growth through inhibition of TGF-beta action, but may not affect myomas already present.41, 42
ROLE OF MYOMECTOMY IN INFERTILITY SURGERY
There are numerous reports that patients with unexplained infertility and myomas have increased fecundity after myomectomy. Babaknia and colleagues43 evaluated 34 patients with primary infertility and 12 patients with secondary infertility. After myomectomy, 38% of patients with primary infertility and 50% of those with secondary infertility had term pregnancies. Many other investigators have reported an approximately 50% pregnancy rate after myomectomy, with 75% of these occurring in the first year. These findings have been confirmed by Rosenfeld,13 who evaluated 23 patients with unexplained infertility and myomas. In this study, 65% of patients conceived after myomectomy, all but one within the first year. The age of the patient, duration of infertility, size and number of fibroids, hysterosalpingography, or presence of menorrhagia did not predict pregnancy outcome.
One study suggests that myomectomy may decrease fertility, probably because of adhesion formation. In this study, 50% of the patients conceived; however, the most important correlation with subsequent fertility was surgical indication. Only 16% of patients with a normal infertility evaluation conceived, whereas 68% of patients who underwent exploration for a pelvic mass conceived. These investigators conclude that myomectomy may decrease fertility in the patient with unexplained infertility and that it may be unjustified.44 However, one review of 23 trials between 1982 and 1996 demonstrated a conception rate of more than 60% for infertile patients after myomectomy.45
Although there may be little doubt about the effectiveness of myomectomy in improving spontaneous conception rate of patients with unexplained infertility, the effect of myomas and the significance of their location in the uterus on the success of assisted reproductive technologies has been a matter of debate. Although the effect of subserosal and corporal myomas away from the uterine cavity may be minimal, submucosal and intramural myomas significantly reduce pregnancy and implantation rates, even in the absence of uterine cavity deformity.46, 47 At a minimum, surgical treatment is recommended for patients with intramural or submucosal myomas before employing one of the techniques of assisted reproduction.
MYOMECTOMY AND PREGNANCY OUTCOME
Buttram and Reiter1 reviewed 18 studies containing 1193 women who had myomectomy for infertility, 40% of whom conceived postoperatively. They also compared spontaneous abortion rates preoperatively and postoperatively and found them to drop from 41% to 19% after myomectomy. Sudik et al. 5 also reported a cumulative pregnancy rate of 58.2%, within the first year, after abdominal myomectomy. Because of the short interval between operation and conception, patients should conceive soon after myomectomy (about 3–4 months later) since recurrence is already detectable 6 months after surgery.
Myomectomy will become a more popular operation as the trend to delay childbearing continues. The popular view that unnecessary hysterectomy is performed all too often has patients requesting this more morbid procedure. The role of this procedure in infertility will increase despite or perhaps even because of the development of the new reproductive technologies. A century of experience with myomectomy has improved the technique dramatically. New technology and innovative minds will continue this trend.48, 49
REFERENCES
Buttram VC Jr, Reiter RC: Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril 36: 433, 1981 |
|
Deligdish L, Loewenthal M: Endometrial changes associated with myomata of the uterus. J Clin Pathol. 1970 Nov;23(8):676-80. |
|
Farrer-Brown G, Beilby J, Tarbit M: Venous Changes in the Endometrium of Myomatous Uteri. Obstet Gynecol. 1971 Nov;38(5):743-51. |
|
Sehgal N, Haskins AL: The mechanism of uterine bleeding in the presence of fibromyomas. Am Surg. 1960 Jan;26:21-3. |
|
Sudik R, Husch K, Steller J et al: Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996 Apr;65(2):209-14. |
|
Rackow BW, Taylor HS: Uterine leiomyomas affect endometrial HOXA10 expression. J Soc Gynecol Investig 2006; 13(2):280A |
|
Li TC, Mortimer R, Cooke ID: Myomectomy: a retrospective study to examine reproductive performance before and after surgery. Hum Reprod. 1999 Jul;14(7):1735-40. |
|
Bajekal N, Li TC: Fibroids, infertility and pregnancy wastage. Hum Reprod Update. 2000 Nov-Dec;6(6):614-20. |
|
Bulletti C, DE Ziegler D, Levi Setti P et al: Myomas, pregnancy outcome, and in vitro fertilization. Ann N Y Acad Sci. 2004 Dec;1034:84-92. |
|
Ubaldi F, Tournaye H, Camus M et al: Fertility after hysteroscopic myomectomy. Hum Reprod Update. 1995 Jan;1(1):81-90. |
|
Horne AW, Critchley HO: The effect of uterine fibroids on embryo implantation. Semin Reprod Med. 2007 Nov;25(6):483-9. |
|
Gehlbach DL, Sousa RC, Carpenter SE, Rock JA: Abdominal myomectomy in the treatment of infertility. Int J Gynecol Obstet 40: 45, 1993 |
|
Rosenfeld DL: Abdominal myomectomy for otherwise unexplained infertility. Fertil Steril 46: 328, 1986 |
|
Sawin SW, Pilevsky ND, Berlin JA et al: Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000 Dec;183(6):1448-55. |
|
Iverson RE Jr, Chelmow D, Strohbehn K et al: Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996 Sep;88(3):415-9. |
|
Bonney V: The technique and results of myomectomy. Lancet 220: 171, 1931 |
|
Ranney B, Frederick I: The occasional need for myomectomy. Obstet Gynecol 53: 437, 1979 |
|
Ginsburg ES, Benson CB, Garfield JM et al: The effect of operative technique and uterine size on blood loss during myomectomy: a prospective randomized study. Fertil Steril. 1993 Dec;60(6):956-62. |
|
West S, Ruiz R, Parker WH: Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006 Jan;85(1):36-9. |
|
Ugur M, Turan C, Mungan T et al: Laparoscopy for adhesion prevention following myomectomy. Int J Gynecol Obstet 53: 145, 1996 |
|
Tulandi T, Murray C, Guralnick M: Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993 Aug;82(2):213-5. |
|
Tsuji S, Takahashi K, Yomo H et al: Effectiveness of antiadhesion barriers in preventing adhesion after myomectomy inpatients with uterine leiomyoma. Eur J Obstet Gynecol Reprod Biol. 2005 Dec 1;123(2):244-8. Epub 2005 Jun 9. |
|
Goldrath MH: Vaginal removal of the pedunculated submucous myoma: the use of laminaria. Obstet Gynecol 70: 670, 1987 |
|
Wamsteker K, Emanuel MH, de Kruif JH: Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993 Nov;82(5):736-40. |
|
Emanuel MH, Wamsteker K, Hart AA et al: Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999 May;93(5 Pt 1):743-8. |
|
Neuwirth RS: Hysteroscopic management of symptomatic submucous fibroids. Obstet Gynecol 62: 509, 1983 |
|
Speroff L and Fritz M. Clinical Gynecologic Endocrinology and Infertility. (7th edn). Philadelphia, PA: Lippincott Williams & Wilkins, 2005 |
|
Mais V, Ajossa S, Guerriero S et al: Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996 Feb;174(2):654-8. |
|
Palomba S, Zupi E, Russo T et al: A multicenter randomized, controlled study comparing laparoscopic versus Fertil Steril. 2007 Oct;88(4):942-51. Epub 2007 Mar 8. |
|
Darai E, Deval B, Darles C et al: Myomectomy: laparoscopy or laparotomy. Contracept Fertil Sex 24: 751, 1996 |
|
Miller CE, Johnston M, Rundell M: Laparoscopic myomectomy in the infertile woman. J Am Assoc Gynecol Laparosc 3: 525, 1996 |
|
Dubuisson JB, Chavet X, Chapron C et al: Uterine rupture during pregnancy after laparoscopic myomectomy. Hum Reprod 10: 1475, 1995 |
|
Harris WJ: Uterine dehiscence following laparoscopic myomectomy. Obstet Gynecol 80: 545, 1992 |
|
Nezhat C: The “cons” of laparoscopic myomectomy in women who may reproduce in the future. Int J Fertil Menopausal Stud 41: 280, 1996 |
|
Andreyko JL, Blumfeld Z, Marshall LA et al: The use of an agonistic analog of gonadotropin releasing hormone to treat leiomyomas: assessment by magnetic resonance imaging. Am J Obstet Gynecol 158: 903, 1988 |
|
Stovall TG, Muneyyirci-Delale O, Summitt RL Jr et al: GnRH agonist and iron versus placebo and iron in the anemic patient before surgery for leiomyomas: a randomized controlled trial. Leuprolide Acetate Study Group. Obstet Gynecol. 1995 Jul;86(1):65-71. |
|
Grigorieva V, Chen-Mok M, Tarasova M et al: Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003 May;79(5):1194-8. |
|
Murphy AA, Morales AJ, Kettel LM et al: Regression of uterine leiomyomata to the antiprogesterone RU486: dose-response effect. Fertil Steril. 1995 Jul;64(1):187-90. |
|
Chwalisz K, DeManno D, Garg R et al: Therapeutic potential for the selective progesterone receptor modulator asoprisnil in the treatment of leiomyomata. Semin Reprod Med. 2004 May;22(2):113-9. |
|
Steinauer J, Pritts EA, Jackson R et al: Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet Gynecol. 2004 Jun;103(6):1331-6. |
|
Lee BS, Margolin SB, Nowak RA: Pirfenidone: a novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production. J Clin Endocrinol Metab. 1998 Jan;83(1):219-23. |
|
Young SL, Al-Hendy A, Copland JA: Potential nonhormonal therapeutics for medical treatment of leiomyomas. Semin Reprod Med. 2004 May;22(2):121-30. |
|
Babaknia A, Rock JA, Jones HW: Pregnancy success following abdominal myomectomy for infertility. Fertil Steril 30: 644, 1978 |
|
Berkeley AS, DeCherney AH, Polan ML: Abdominal myomectomy and subsequent fertility. Surg Gynecol Obstet 156: 319, 1983 |
|
Vercellini P, Maddalena S, De Giorgi O et al: Abdominal myomectomy for infertility: a comprehensive review. Hum Reprod 13: 873, 1998 |
|
Eldar-Geva T, Meagher S, Healy DL et al: Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril 70: 687, 1998 |
|
Ramzy AM, Sattar M, Amin Y et al: Uterine myomata and outcome of assisted reproduction. Hum Reprod 13: 198, 1998 |
|
Wallach EE: Myomectomy: a guide to indications and technique. Contemp Obstet Gynecol 31: 74, 1988 |
|
Semm K: New methods of pelviscopy (gynecologic laparoscopy) for myomectomy, ovariectomy, tubectomy, and adenectomy. Endoscopy 11: 85, 1979 |