Strategies for Overcoming the Barriers to Cervical Cancer Screening in Low-Resource Settings
Authors
INTRODUCTION
From a public health and epidemiologic perspective, cervical cancer has two features that make it unique. The first is that cervical cancer is caused by specific types of a sexually transmitted DNA tumor virus, human papillomavirus (HPV). A woman’s risk of developing cervical cancer is determined in part by her risk of having been exposed to HPV. The causal relationship between anogenital infections with specific high-risk types of HPV and cervical cancer also may explain the increases in cervical cancer observed among women infected with human immunodeficiency virus (HIV). Immunocompromised women are at increased risk for developing persistent HPV infections, which are a prerequisite for the development of cervical cancer precursors. The second feature that makes cervical cancer unique from a public health and epidemiologic perspective is that in contrast to most other cancers, cervical cancer can be prevented through screening programs designed to identify and treat precancerous lesions referred to as high-grade squamous intraepithelial lesions (SIL). As a result, women who have access to screening programs have much lower rates of cervical cancer than do women who do not have such access. The incidence of cervical cancer varies dramatically among regions of the world and among different sociodemographic groups of women within a given region.
This chapter reviews cervical cancer prevention programs with an emphasis on identifying barriers to the implementation of screening programs in low-resource settings. Novel strategies for cervical cancer prevention that are being developed specifically for low-resource settings are discussed.
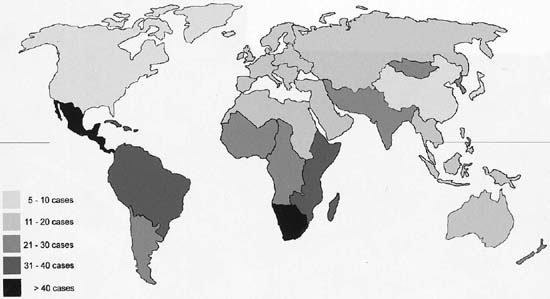
Cancer of the cervix is the second most common cancer among women worldwide, with an estimated 493 000 new cases and 274 000 deaths in 20021. Over 80% of cervical cancer cases occur in the developing world, where cervical cancer accounts for 15% of female cancers compared to 3.6% of cancers in the developed world. The highest incidence rates are observed in sub-Saharan Africa, Melanesia, Latin America and the Caribbean, South-Central Asia and Sout East Asia (Fig 1). Age standardized incidence rates (ASIRs) in those countries with cancer registries show a very wide range from less than 15 per 100 000 in Europe, North America and Japan to over 30/100 000 in sub-Saharan Africa.
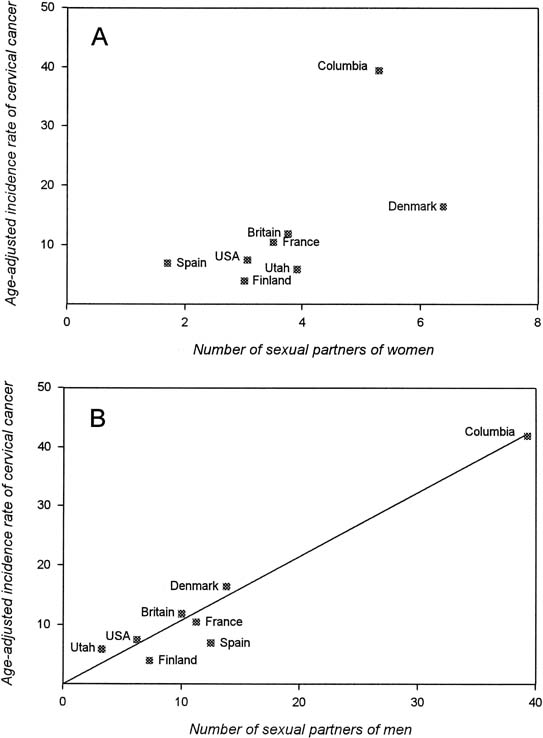
Risk factors for invasive cervical cancer and cervical cancer precursors are well characterized (Table 1) and include markers of sexual activity and of exposure to sexually transmitted diseases. These markers include the number of sexual partners that a woman has had and the number of sexual partners that a woman’s male partner has had. Bosch and colleagues2, 3 studied the relationship between sexual behavior and cervical cancer risk and found a better correlation with the number of sexual partners that a woman’s partner has had than with the number of partners that she has had (Fig. 2). Further studies from the same group on the role of male sexual behavior showed that women at greatest risk are those who initiate sexual activity at an early age with an older man who has had multiple sexual partners.
Table 1. Risk factors associated with cervical intraepithelial neoplasia in various epidemiologic studies
| Sexual activity |
| Number of sexual partners |
| Male partners with multiple sexual partners |
| Uncircumcised male partner |
| Early sexual activity (especially <16 years old) |
| Sexually transmitted diseases |
| Human papillomavirus |
| Herpes simplex virus |
| Chlamydia trachomatis |
| Early age of first pregnancy |
| Parity |
| Low socioeconomic class |
| Cigarette smoking |
| Human immunodeficiency virus |
| Immunosuppression from any cause |
| Vitamin deficiencies and nutritional factors |
| Interval since last Pap smear |
| Oral contraceptive use greater than 5 years |
| Previous history of squamous intraepithelial lesion |
Data from Bosch FX, Lorincz A, Munoz N, et al: The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 55:244, 2002.
A potential role for sexually transmitted agents in the development of cervical cancer has been suggested for many years. Almost every known sexually transmitted agent, including Neisseria gonorrheae, Treponema pallidum, Chlamydia trachomatis, Trichomonas vaginalis, herpes simplex virus, cytomegalovirus, and sperm, has been suggested as a potential causative factor. Although exposure to each of these agents has been shown in various studies to be associated with an increased risk of developing cervical cancer, in general the increased risk because of exposure was relatively low. In contrast to the relatively low and variable risk associated with exposure to most of these agents, today there are many data showing that the development of invasive cervical cancer requires infection with specific high-risk types of HPV. In 1995, the International Agency for Research on Cancer (IARC) concluded that four case–control studies supported the classification of HPV types 16 and 18 as carcinogenic to humans and HPV types 31 and 33 as probably carcinogenic to humans.4 More recent evidence, based on 11 case–control studies in nine countries involving 1918 women with histologically confirmed squamous cell cervical cancer and 1928 control women, shows that 15 HPV types have been classified as high-risk for cervical cancer: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82.5, 6 Three were classified as probably high-risk (26, 53, and 66) and 12 as low-risk types (6, 11, 140, 42, 43, 44, 54, 61, 70, 72, 81, and CP6108). Low risk types are associated with genital warts and low grade intraepithelial lesions of the anogenital tract, whereas high-risk types are consistently associated with anogenital cancers. However, infections with high-risk types of HPV are common, and most individuals infected will clear the infection without any clinical consequences. Only a small number of infected individuals will ulitmately develop cancer. High-risk and low-risk types of HPV infection are extremely common among sexually active women of reproductive age, regardless of where they live, and it is unlikely that different rates of exposure to high-risk types of HPV explain the markedly different incidence rates of invasive cervical cancer observed in different geographic areas. Instead, it seems that much of the difference in cervical cancer rates observed between developed and low-resource settings is a result of differences in the rates of cervical cancer screening.
PATHOGENESIS OF CERVICAL CANCER
Existence of precursor lesions
Understanding how cervical cancer develops is key to developing interventions to prevent death from this disease. For more than 100 years, it has been recognized that invasive cervical cancer develops from histologically well-characterized precursor lesions. Histopathologic studies done in the early 1900s showed that early invasive cervical cancers usually develop adjacent to noninvasive intraepithelial lesions that are histopathologically similar in appearance to the adjacent early invasive cancer. Because of their histologic semblance to invasive cervical cancer, these intraepithelial lesions were referred to as carcinoma in situ, and it was presumed that because of their spatial relationship with invasive cervical cancer the cancer developed from them.7 Prospective follow-up studies of women with carcinoma in situ subsequently showed that 70% of women with these lesions eventually develop invasive cervical cancer.8, 9 As a result, the concept of screening for precursor lesions and eliminating the lesions by performing a cone biopsy to prevent progression to invasive cervical cancer was developed.
With the widespread adoption of exfoliative cervical cytology as a screening method, it quickly was realized that intraepithelial cervical lesions that are cytologically and histologically less severe than carcinoma in situ are relatively common among women in the general population. These less severe intraepithelial lesions originally were referred to as dysplasia.10 Dysplasia was subdivided into three grades – mild, moderate, and severe – based on the degree of cytologic and histologic abnormality that was present.11 As with carcinoma in situ, prospective follow-up studies of women with dysplasia showed that some of these women would progress to either carcinoma in situ or invasive cervical cancer if left untreated.12 It also was later observed that dysplasia and carcinoma in situ shared many biologic features, and these lesions were grouped together as a single entity referred to as cervical intraepithelial neoplasia (CIN).13 The CIN classification divides cervical cancer precursors into three categories: CIN 1 (mild dysplasia), CIN 2 (moderate dysplasia), and CIN 3 (severe dysplasia and carcinoma in situ). Today, many laboratories use a two-tiered terminology for cytopathology and histopathology. This terminology divides cervical cancer precursor lesions into two grades, low-grade SIL (CIN 1) and high-grade SIL (CIN 2, 3).14, 15
Natural history of squamous intraepithelial lesions
Studies of the natural history or behavior of different grades of SIL (CIN) have provided widely varying estimates of the rates of regression and progression (Table 2).12 The variation in natural history observed among different studies is to be expected because various studies have used different entry criteria, different diagnostic criteria for diagnosing the specific grades of SIL (CIN), and different study designs. Some studies have used punch biopsy and endocervical curettage to establish the diagnosis, whereas other studies have used cytology. Using a cervical biopsy to obtain tissue for histopathologic diagnosis may remove (treat) lesions and may interfere with long-term analysis because it would increase the frequency of apparent spontaneous regression and decrease the frequency of observed progression.16
Table 2. Natural history of squamous intraepithelial lesions (cervical intraepithelial neoplasia) in untreated women
Grade | % Regress | % Persist | % Progress* |
CIN 1 | 57 | 32 | 11 |
CIN 2 | 43 | 35 | 22 |
CIN 3 | 32 | 56 | 12 |
CIN, cervical intraepithelial neoplasia.
*Progression to carcinoma in situ.
Data from Mitchell MF, Tortolero-Luna G, Wright T, et al: Cervical human papillomavirus infection and intraepithelial neoplasia: A review. J Natl Cancer Inst Monogr 21:17, 1996.
One of the largest long-term clinical follow-up studies of women with SIL (CIN) was by Nasiell and coworkers16, 17 in Sweden. In this study, 555 women with mild dysplasia (CIN 1) identified by cervical cytology and colposcopy and 894 women with moderate dysplasia (CIN 2) identified by cervical cytology, colposcopy, and, in some instances, cervical biopsy were followed on average for 39 and 78 months. In 62% of the women with CIN 1, regression to normal occurred; in 22%, there was persistence of CIN 1 or CIN 2; and in 16%, there was progression of CIN 1 to CIN 3. In women with CIN 2 followed without biopsy for an average of 51 months, spontaneous regression occurred in 50%, and progression to CIN 3 occurred in 35%. In women with CIN 2 who underwent biopsy, the rate of regression was 57%, and the rate of progression was 27%. Most other prospective follow-up studies of low-grade SIL (CIN 1) found similar rates of regression, progression, and persistence to those reported by Nasiell and coworkers. In aggregate, these studies indicate that approximately 11% of women with low-grade SIL (CIN 1) progress to carcinoma in situ if left untreated, and about 32% continue to have low-grade SIL (CIN 1) (persistent disease). Most low-grade SIL (CIN 1) (approximately 57%) regress spontaneously if left untreated (see Table 2).
A study evaluated the records of a large cytology laboratory in Toronto, Canada, and linked the records of the laboratory with the Ontario Tumor Registry for the years of 1962 through 1980.18 During this period, women with dysplasia who were evaluated by this laboratory usually did not undergo treatment. This study provides a unique insight into the long-term natural history of untreated SIL (CIN) (Table 3). After 10 years of follow-up, only 12% of untreated mild dysplasia and 17% of untreated moderate dysplasia progressed to carcinoma in situ. At 10 years, 88% of mild dysplasia cases and 83% of moderate dysplasia cases had regressed.18
Table 3. Long-term follow-up of abnormal cervical cytology in Toronto
| Cytologic Grade | Length of Follow-Up | |
2 Years | 10 Years | |
Regression rates* | ||
| Mild dysplasia | 44% | 88% |
| Moderate dysplasia | 33% | 83% |
Progression rates† | ||
| Mild dysplasia | 0.6% | 12% |
| Moderate dysplasia | 1.5% | 17% |
| Severe dysplasia | 2.8% | 21% |
* Regression to within normal limits.
† Progression to carcinoma in situ or worse.
Modified from Holowaty P, Miller AB, Rohan T, To T: Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst 91:252, 1999.
High-grade SIL (CIN 2,3) has a greater potential to progress than does low-grade SIL (CIN 1) (see Table 2). MacIndoe and colleagues19 performed a prospective study of untreated carcinoma in situ and found that 29% of patients followed 1–20 years progressed to invasive cancer. Progression rate increased with the length of follow-up, peaking at 34.6% in patients followed for 14 years. Kottmeier8 reported that 71% of women with carcinoma in situ subsequently developed invasive carcinoma when followed for a minimum of 12 years. Other long-term retrospective studies showed progression of carcinoma in situ to invasive cancer in 22–60% of cases.
The prevalence of low-grade SIL (CIN 1) decreases proportionally with age.20, 21 Mathematical calculations derived from incidence rates suggest that new lesions arise in the form of low-grade SIL (CIN 1) instead of high-grade SIL (CIN 2, 3). The annual incidence of high-grade SIL is 1000–2000 times higher among women with previously documented low-grade SIL than among women with previous normal cytologic findings.20, 21 It previously was thought that high-grade SIL (CIN 2, 3) always develops from low-grade SIL. The mean age at diagnosis of women with different grades of dysplasia, carcinoma in situ, and invasive cervical cancer in unscreened populations suggests a temporal association. Patten22 reported that the mean age at diagnosis was 32 years for mild dysplasia, 36 years for moderate dysplasia, 38 years for severe dysplasia, 42 years for carcinoma in situ, and 52 years for invasive cervical cancer. These numbers suggest that it takes approximately 10 years for mild dysplasia to progress to carcinoma in situ and another 10 years for carcinoma in situ to progress to invasive cervical cancer. These estimates are similar to the time required for progression calculated from mathematical models.23, 24
Several lines of evidence suggest that high-grade SIL may develop as an independent event without progressing from low-grade SIL. Burghart20 and Koss25 argued based on histopathologic mapping studies that high-grade SIL does not develop by a direct transformation from low-grade SIL. Instead, these authors suggested that high-grade SIL develops de novo from the epithelium adjacent to low-grade SIL. A similar conclusion was reached by prospective follow-up studies that suggest at least some high-grade SIL can develop independent of pre-existing low-grade SIL. In a study of women visiting a sexually transmitted disease clinic, most cases of high-grade SIL arose de novo in the absence of a cytologically detectable, low-grade SIL (CIN 1).26 In another study of women infected with high-risk HPV types, Nobbenhuis and associates27 found that 88% of the incident cases of SIL first were identified as high-grade lesions. Some authors now challenge the entire concept that low-grade SIL is a precursor to either high-grade SIL or invasive cervical cancer.28 There is no controversy, however, that high-grade SIL can act as a “true” cancer precursor that can progress to invasive cervical carcinoma.
Invasive cervical cancer develops over a long period of time from well-defined precursor lesions that can be classified into low-grade and high-grade forms (Fig. 3). The median age at presentation and rates of progression of these precursors are related to their histopathologic or cytologic grade. Low-grade SIL (CIN 1) is characterized by a relatively young age at presentation, high rates of spontaneous regression, and relatively low rates of progression to invasive cervical cancer. In contrast, high-grade SIL (CIN 2, 3) is characterized by an older age at presentation, low rates of spontaneous regression, and higher rates of progression to invasive cervical cancer (see Table 2). As a result of these marked differences, it now is generally accepted that screening programs should focus on detecting high-grade SIL (CIN 2, 3) rather than low-grade SIL (CIN 1). Another point that is crucial for developing rational screening strategies is that the average time required for SIL (CIN) of any grade to progress to invasive cervical cancer is long. Based on prospective follow-up studies, it is estimated that it takes on average 10–20 years to progress from low-grade SIL (CIN 1) to invasive cervical cancer; this allows ample time for interventions to prevent the development of cervical cancer. The first step in these interventions is for women with SIL (CIN) to be detected through a screening program. The second step is for women with SIL (CIN) who have been identified through a screening program to be treated appropriately to prevent the subsequent development of invasive cervical cancer.
CERVICAL CANCER SCREENING
Impact of screening
Although there has yet to be a randomized controlled clinical trial showing the efficacy of cytologic screening programs, there is little doubt that screening programs play a major role in reducing the incidence and the mortality of invasive cervical cancer. Data showing evidence of effectiveness come from historical studies, case–control studies of women with invasive cervical cancer, analysis of data from large regional screening programs, and mathematical modeling studies. The historical studies include data from more than a dozen areas varying in size from cities to countries that have reported declines in the incidence of invasive cervical after the introduction of cytologic cervical cancer screening programs, including Iceland, the Nordic countries, Canada, and parts of Scotland.
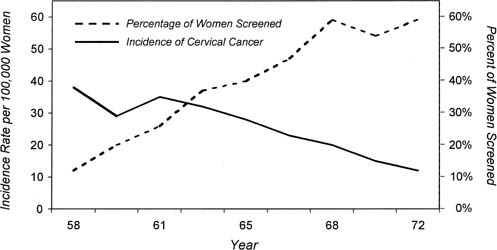
In Iceland before the introduction of cytologic screening in 1964, the mortality from cervical cancer had been increasing. By 1970, the annual mortality from invasive cervical cancer began to decline, and by 1974, the mortality rate had decreased to approximately half what it was in the late 1960s (from 23.1 per 100,000 in 1965–1970 to 14.6 per 100,000 in 1970–1975).29 Historical studies indicate that the incidence of cervical cancer in the Nordic countries between 1945 and 1980 also was correlated directly with the extent of cytologic cervical cancer screening.30 Cytologic screening was introduced into a national program in Finland in the mid-1960s and was adopted rapidly, and a marked decrease in the incidence of cervical cancer occurred coincident with its introduction. In contrast, in Denmark and Sweden, cytologic screening was introduced much more slowly and less uniformly. As a result, less of a decline in the incidence of cervical cancer was experienced in these countries. In Norway, cytologic screening was performed only sporadically, and the overall incidence of invasive cervical cancer increased over this time period. Further evidence of the impact of screening is found in Canada, where the incidence of cervical cancer decreased from 28.4 to 6.9 per 100,000, and mortality decreased from 11.4 to 3.3 per 100 000 women during the first 20 years of the screening program in British Columbia.31 Reductions in the incidence and mortality of invasive cervical cancer also have been documented in the United States after the widespread introduction of cytologic screening. The American Cancer Society estimated that only 30% of all American women had ever had a Pap smear in 1961, but this increased to 87% by 1987 (Fig. 4).32 In 1940, the incidence of invasive cervical cancer in the United States was 32.6 per 100,000 women, which is similar to that currently seen in many developing countries, whereas by 1984, the incidence had decreased to 8.3 per 100,000 women. This 75% reduction in incidence was paralleled by an equivalent reduction in mortality.33 In 2000, there were only 12,800 cases of invasive cervical cancer diagnosed in the United States and 4600 cervical cancer deaths.34
One of the earliest case–control studies showing the effectiveness of cytologic screening was by Clarke and Anderson,35 who matched by age 212 women diagnosed with invasive cervical cancer in the Toronto area in the mid-1970s with controls drawn from neighbors. The relative risk for invasive cervical cancer in women who had undergone cytologic screening was 0.37 (p <0.001) compared with women who had not been screened, and protection remained after stratification by income, smoking, age, marital history, and access to medical care. Many subsequent case–control studies from around the world, including the United States, Scotland, Italy, the Netherlands, and Denmark, all found that participation in cytologic screening reduces a woman’s risk for developing invasive cervical cancer.36, 37, 38, 39, 40
The IARC performed a comprehensive analysis of data from the largest cervical cancer screening programs conducted in the 1960s and 1970s in eight developed countries to determine the impact that screening had on reducing the incidence of invasive cervical cancer. Based on their analysis, they concluded that well-organized screening programs were effective in reducing the incidence of and mortality from cervical cancer, assuming diagnosis and treatment are available based on screening outcome.41, 42
Designing effective screening programs
Many factors are important in ensuring that cervical cancer screening programs lead to a reduction in the incidence of and mortality from cervical cancer. Some of these factors relate to the specific screening test used, including test performance characteristics such as sensitivity, specificity, and negative and positive predictive value. For cervical cytology, factors include how the sample is collected from the cervix, whether one uses the conventional Papanicolaou (Pap) test or one of the newer liquid-based cervical cytology methods, and the quality of the laboratory that interprets the cervical cytology. Test performance characteristics play a crucial role in determining programmatic issues, such as the target ages at which screening should be initiated and stopped, the interval at which screening is performed, and the minimal screening coverage required to obtain a given level of protection in the population. Programmatic factors that are unrelated to the individual screening test performance are of equal importance in ensuring that the screening program is effective and include education of the population as to the benefits of screening, training providers at all levels of the screening process, providing acceptable protocols and facilities for the treatment of cases identified through the screening process, and obtaining the resources required to sustain screening.
Screening intervals
The impact of screening interval has been evaluated in epidemiologic studies and mathematical simulation models of cervical cancer screening programs. A Chinese screening study conducted in the high-risk rural community of Jingan found a strong relationship between screening interval and risk of developing invasive cervical cancer.43 This study was initiated in 1974 and included an initial invitation to attend screening that was provided to all women who were age 25 years or older and subsequent invitations to undergo repeat screening that were provided to all women who were age 30 years or older at 2-yearly intervals. The study was conducted for 12 years, and greater than 95% of the eligible women received at least one Pap test. A case–control study was conducted of 109 cases of invasive cervical cancer identified in this community and 545 control women matched for age, area of residence, and attendance at the same round of screening as the case was detected. A strong relationship between the length of time since the last Pap test and the risk of invasive cervical cancer was observed (Table 4). Women whose last Pap test was 4–6 years earlier had a 4.22 times greater risk of having cervical cancer than women who had had a negative Pap test within the last 2 years. Another case–control study of 189 women with invasive cervical cancer and 1023 hospitalized control women from Bangkok, Thailand, evaluated the impact of screening frequency on risk of developing invasive cervical cancer.44 In this study, enrollees were interviewed with respect to their screening history. Of the women with invasive cervical cancer, 69% reported never having had a Pap test compared with 45% of the control women enrolled from nongynecologic hospital wards. After adjusting for age, age at first intercourse, number of sexual partners, educational status, and number of episodes of vaginal discharge, a strong association was observed between screening frequency and the risk of invasive cervical cancer (Table 5). Compared with women who had never been screened, the odds ratio of invasive cervical cancer in women having yearly Pap tests was 0.20. The odds ratio was 0.39 for women who had Pap tests every 2–5 years.
Table 4. Relative risk of cervical cancer in relation to screening frequency in Chinese women
Time Since Last Negative Pap Test | Cases | Controls | Odds Ratio (95% CI) |
0–2 years | 94 | 520 | 1.00 |
4–6 years | 9 | 21 | 4.22 (1.51–11.90) |
>8 years | 6 | 4 | 11.41 (2.38–63.66) |
CI, confidence interval.
Data from Zhang ZF, Yu SZ, Esteve J, Yang XZ: Risk factors for cancer of the cervix in a rural Chinese population. Int J Gynecol Cancer 43:762, 1989.
Table 5. Relative risk of cervical cancer in relation to screening frequency in Thai women
Frequency | Cases | Controls | Odds Ratio (95% CI) |
None | 130 | 464 | 1.00 |
Only 1 | 33 | 106 | 0.92 (0.58–6.46) |
Once every 2–5 years | 14 | 88 | 0.39 (0.21–0.74) |
CI, confidence interval.
Data from Wangsuphachart V, Thomas DB, Koetsawang A, Riotton G: Risk factors for invasive cervical cancer and reduction of risk by “Pap” smears in Thai women. Int J Epidemiol 16:362, 1987.
In 1986, an IARC working group used data from eight screening programs to calculate the relationship between length of time since the last cervical cytology and the relative protection against cervical cancer among women who had had two or more negative Pap tests.42 This analysis found that with increasing time since the last negative Pap test, relative protection decreases (Table 6). Mathematical modeling studies that have used these data and assumed the sensitivity of a single cervical cytology is 0.70 indicate that for women who have had a negative Pap test at 35 years of age, the relative level of protection obtained by biannual screening is as great as that obtained by annual screening and that triannual screening provides almost as much protection (Table 7).45 Even screening at 5-yearly and 10-yearly intervals seems to provide relatively high levels of protection. We performed mathematical modeling studies using data from South Africa and found that even a once-in-a-lifetime screen at age 35 years might reduce a woman’s risk of cervical cancer by 17%.46
Table 6. Relative protection against cancer in women with two or more negative Papanicolaou tests
Time Since Last Negative Pap Test (months) | Relative Protection* (95% CI) |
| 0–11 | 15.3 (10.0–22.6) |
| 12–23 | 11.9 (7.5–18.3) |
| 24–35 | 8.0 (5.2–11.8) |
| 36–47 | 5.3 (3.6–7.6) |
| 48–59 | 2.8 (1.9–4.0) |
| >120 | 1.0 |
CI, confidence interval.
*Geometric mean relative protection.
Data from IARC: Working Group on Evaluation of Cervical Cancer Screening Programmes: Screening for squamous cerical cancer: Duration of low risk after negative results of cervical cytology and its implication for screening policies. BMJ 293:659, 1986.
Table 7. Modeled impact of screening interval on incidence of cervical cancer
Screening Interval | Reduction in Cancer Incidence (%) | No. of Tests |
Yearly | 93 | 30 |
Biannual | 93 | 15 |
Triannual | 91 | 10 |
Every 5 years | 84 | 6 |
Every 10 years | 64 | 3 |
Data from Miller AB: Cervical Cancer Screening Programmes: Managerial Guidelines. Geneva, World Health Organization, 1992.
Target screening ages
Another important issue is the most appropriate age to initiate and to stop screening. This depends on many factors, including the level of resources that are available for screening and the age-specific cervical cancer incidence rate for a particular setting. The best data available to determine the most appropriate target population for screening come from mathematical modeling studies. Using data from Cali, Colombia, the 1986 IARC working group modeled the impact of initiating screening at different ages (Table 8). Initiating screening before 25 years of age had no additional benefit in this model, and initiating screening before 35 years of age had only a minimal impact on the expected reduction in cervical cancer incidence but increased the number of screening tests that were required.
Table 8. Modeled impact of target screening age on incidence of cervical cancer*
Target Age (years) | Reduction in Cancer Incidence (%) | No. of Tests |
20–64 | 84 | 9 |
25–64 | 84 | 8 |
30–64 | 81 | 7 |
35–64 | 77 | 6 |
*Modeled using a 5-year screening interval and assuming a 70% sensitivity of the first screening test.
Data from IARC: Working Group on Evaluation of Cervical Cancer Screening Programmes: Screening for squamous cerical cancer: Duration of low risk after negative results of cervical cytology and its implication for screening policies. BMJ 293:659, 1986.
Screening coverage
Screening coverage (i.e., the proportion of the target population that participates in the recommended screening program) is crucial in determining the overall impact that the screening program will have on rates of disease. Because most cervical cancers occur among women who have not participated in cervical cancer screening programs, increasing the proportion of the population that participates in a screening program generally would have a much more significant impact on reducing the incidence of invasive cervical cancer than using a slightly more sensitive screening test or changing the screening interval. Table 9 provides data from a 1986 cost-effectiveness study of different cervical cancer screening strategies for Chile.45 A screening program incorporating three Pap smears at 10-year intervals in women between ages 30 and 50 years that reached 90% of the target population would be much more effective than a program that screened the same group of women at 3-year intervals but reached only 30% of the population. Although this type of mathematical modeling provides considerable insight into different screening strategies, the modeling is based on many assumptions that may not be correct. The model that was used to generate the estimates provided in Table 8 is quite sensitive to assumptions regarding the performance of screening tests over time and assumes that the level of protection afforded by a negative screening test is independent of age. This assumption had to be made because age-specific data were unavailable for incorporation into the model. The data that are available suggest, however, that the natural history of cervical cancer precursors probably varies with age, and some studies have suggested that specific risk factors, such as early age at first intercourse, are associated with a reduction in the length of protection that a negative Pap smear provides.47, 48
Table 9. Modeled impact of compliance on performance of screening program*
Variable | Strategy 1 | Strategy 2 |
Age (years) | 30–55 | 30–55 |
Screening interval (years) | 3 | 10 |
Compliance (%) | 30 | 90 |
Reduction in mortality (%) | 15 | 44 |
*Modeled by Eddy using data from Chile.
Data from Miller AB: Cervical Cancer Screening Programmes: Managerial Guidelines. Geneva, World Health Organization, 1992.
Real-world experience has confirmed that screening coverage is crucial. Mortality from cervical cancer in the United Kingdom decreased by 30% after the introduction of screening in the 1960s. A large proportion of this decrease in mortality may have been due to falling rates in older women, however, thought to be the result of a “cohort effect” caused by changes in sexual exposure to HPV and socioeconomic status that is unrelated to the introduction of cytologic screening.49 The need for an effectively managed national program in the United Kingdom was realized by the mid-1980s, which led to the introduction of a computerized call and recall system for women aged 20–64 years. The invitation-based system, combined with target payments for general practitioners, improved population coverage from 40–60% in 1989 to 83% in 1993. An audit of this program in 24 self-selected districts estimated that the number of cases of cervical cancer in the participating districts would have been 57% (95% confidence interval 28–85%) greater if there had been no screening.49
Opportunistic versus organized programs
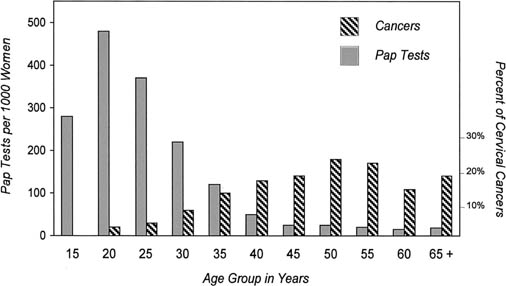
Obtaining widespread coverage of the target population at the correct screening interval is achieved most easily through organized, as opposed to opportunistic, screening programs. The contrast between Norway, where Pap smears were performed on an opportunistic basis among women undergoing gynecologic examinations for a variety of reasons, and Finland, where a similar number of Pap smears were obtained as part of an organized screening program, highlights this difference.30 The reduction in mortality was substantial for all ages in Finland; in Norway, mortality rates decreased only among women aged 30–49 years, and even for that age group, the reduction in cervical cancer mortality was only half of that obtained in Finland. These data suggest that spontaneous or opportunistic screening has less impact on incidence and mortality from cervical cancer than organized screening programs. The lower efficacy of opportunistic screening reflects the failure of screening to be directed at the most at-risk population. Most opportunistic screening occurs among young women undergoing speculum examinations for sexually transmitted disease screening or as part of routine reproductive health examinations. In contrast, women in their 30s–50s are the most appropriate target population for cytologic cervical cancer screening. Figure 5 shows the mismatch between who is screened and who should be screened, even in a country such as South Africa that has limited screening resources, when a policy of opportunistic screening is followed. During the period shown, Pap tests in Cape Town were obtained most commonly among women younger than age 30 receiving obstetric care, whereas the peak incidence of invasive cervical cancer was in women in their 50s. In addition, opportunistic screening produces less of a reduction in cervical cancer mortality because of suboptimal follow-up and management of women found to have cytologic abnormalities and the lack of a coordinated campaign of informing and educating women about cervical cancer prevention.
CERVICAL CANCER PREVENTION PROGRAMS IN LOW-RESOURCE SETTINGS
In many developing countries, screening is opportunistic, sporadic, or does not occur at all. In 1986, the World Health Organization estimated that approximately 40–50% of women in developed countries had been screened in the past 5 years. In contrast, only 5% of women in developing countries had been screened, and most women who had been screened were younger than age 35 years.50 Most screening activity in developing countries was limited to women attending primary health care, antenatal, and family planning clinics in urban areas, with no organized efforts to ensure that high-risk women attended for screening, treatment, and follow-up. A situation analysis by Chirenje and colleagues51 of cervical cancer diagnosis and treatment in east, central, and southern African countries including Kenya, Lesotho, Tanzania, Uganda, and Zimbabwe documented significant low capacity. Cytologic screening was being conducted at primary health care facilities in only two countries. In Tanzania, only one of four tertiary care hospitals performed screening. Most of the screening took place in family planning clinics (45%) or postnatal clinics (29%) where treatment facilities were minimal. As a result of minimal or nonexistent cervical cancer screening services, most women with invasive cervical cancer in developing countries present with advanced-stage disease resulting in high morbidity and mortality.52
BARRIERS TO PREVENTION PROGRAMS
Competing health needs
There are a variety of barriers to implementation of comprehensive cytologic screening programs in low-resource settings. Perhaps the most important of these barriers is competing health needs. Of female deaths in poor countries, 70% result from communicable diseases, such as tuberculosis, malaria, and HIV, or from pregnancy-related causes. Average maternal mortality in Africa in 1996 was 870 deaths per 100,000 live births compared with 12 per 100,000 live births in the United States.53 In Sierra Leone, Mozambique, Somalia, Rwanda, Ethiopia, and other African nations, women face a one in seven chance of dying from pregnancy-related complications. The UNAIDS organization estimated that as of December 1998 there were 13.8 million women living with HIV/AIDS worldwide.54 Despite the importance of cervical cancer as the most common cause of cancer-related deaths in women, other health issues frequently are given a higher priority. Additional barriers to implementing cervical cancer screening include war and civil unrest, which have been endemic in many countries for decades, and widespread poverty. For example, 25% of all medical personnel were believed to be killed during genocide in Rwanda.55 Poverty may be the greatest barrier to screening. Only 26% of families in sub-Saharan Africa have running water or proper sanitation facilities.56 In such areas, health care services are often poorly developed and focus efforts on curative, rather than preventive, health.
Screen, diagnose, and treat strategies
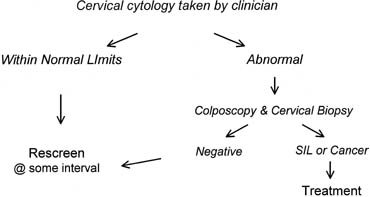
Another barrier to implementing comprehensive cytologic screening programs is the nature of the screening test itself and how women with abnormal test results are managed. The current “screen, diagnose, and treat” approach that is used in developed countries for cervical cancer prevention is shown in Figure 6. With this approach, women have a cervical cytology specimen obtained by a clinician at the point of care; this specimen is evaluated at a distant laboratory; and if an abnormality is detected, the woman is recalled for colposcopy and cervical biopsy. Only if a biopsy-confirmed precancer or cancer is identified does the woman undergo treatment.
|
For this approach to be effective, many requirements must be met. Clients and clinicians need to be aware of the availability and benefits of cervical cytologic screening. Clinicians must to be trained in performing speculum examinations and how to obtain a cytologic specimen from the cervix. Although the supplies required for obtaining a specimen are minimal (a speculum, light source, Ayres-type spatula or other sampling device, fixative, and possibly a cytobrush for sampling the endocervical canal), these supplies must be available at the point of care. Cervical cytology specimens usually are not evaluated at the clinical site where they are obtained, and a reliable transport system to transport the specimen to a cytology laboratory for evaluation must be established. Developing and maintaining a high-quality cytology service is not an easy undertaking. Interpreting cervical cytology tests is considered by many pathologists to be one of the most difficult tasks in pathology, and obtaining a high level of proficiency requires approximately 2 years of training to develop a high level of competency.57 Cytopathologists (who have a medical education and 4 or more years of additional specialty training) evaluate specimens judged abnormal by cytotechnicians. Facilities for training such professional staff are often in short supply in low-resource settings. Skill maintenance requires an ongoing continuing medical education program and access to cervical biopsy specimens from women diagnosed as having abnormal cervical cytology to allow correlations to be made. Cytology laboratories require trained laboratory managers and careful oversight to maintain quality assurance programs; this includes careful monitoring of how specimens are processed and interpreted, rescreening of a given percentage of cervical cytology specimens diagnosed as being within normal limits, workload limits to ensure that the slides are not screened too quickly, and laboratory-wide correlation between the cytologic results and the findings observed at colposcopy. Standards and guidelines for setting up and maintaining a good-quality cytology service have been discussed in depth elsewhere.45
When the Pap test has been evaluated, an infrastructure must be in place that allows laboratory results to be transmitted back to the clinical site. It often takes several weeks for cervical cytology results to become available, and the system must facilitate tracing and notification of patients with abnormal results about the need for further evaluation and treatment.
When screening has taken place, the current standard clinical practice in developed countries is to perform a diagnostic colposcopy and cervical biopsy to determine whether or not a woman with an abnormal Pap test has SIL (CIN). This requires the availability of expensive colposcopes and biopsy instruments, skilled clinicians who have undergone training in colposcopy, a pathology laboratory to process the biopsy specimen, and a pathologist to evaluate the biopsy specimen. Midlevel practitioners frequently are trained to perform colposcopy in the United States, but in most low-resource settings, only physicians perform colposcopy. Training in colposcopy requires an investment in time and resources. In the United States, many colposcopy training programs are based on a 3–4-day dedicated course, followed by a preceptorship that often requires 50 supervised colposcopic procedures before the clinician is allowed to perform unsupervised colposcopic procedures. In low-resource settings, there are often few clinicians with the clinical expertise to train other clinicians in colposcopy and limited resources for training. In the situation analysis of cervical cancer diagnosis and treatment in east, central, and southern African countries described earlier, only 31% of district and provincial hospitals had facilities to perform a cervical biopsy. Colposcopy was not available at any of the district hospitals and in only 6% of the provincial hospitals. Colposcopy services often are limited to tertiary care facilities in low-resource settings. Because it usually takes several days to process and confirm a diagnosis by cervical biopsy, women who have undergone biopsy have to return to the clinic for a second visit to learn the results of the biopsy and whether or not they require treatment. For women living in rural areas, this return visit can be too time-consuming and expensive to manage.
When a SIL (CIN) or an invasive cervical cancer is determined by biopsy, the woman requires treatment. In the developed world, most SIL (CIN) are treated using simple outpatient ablative (electrofulguration, cryosurgery, cold coagulator, and laser ablation) or excisional (loop electrosurgical excision or large loop excision of the transformation zone) modalities. Only a few women require surgical procedures, such as cold knife conization or hysterectomy. In contrast, in low-resource settings, outpatient treatment modalities are often not available. In the situation analysis of east, central, and southern African countries, only 4% of institutions had the equipment to perform cryosurgery, and almost all women with SIL (CIN) underwent a cone biopsy under general anesthesia. For women found to have invasive cervical cancer, the situation was even bleaker. Although surgical treatment services for cervical cancer were available at all tertiary institutions, functional radiotherapy units with adequate staffing were available only in Zimbabwe.
Monitoring and evaluation
It is crucial that a method be in place for evaluating and monitoring the efficiency and effectiveness of the screening program over time. This evaluation system needs to be at the level of the total target population. In developed countries, process measures, such as the total number of smears that are taken, the ages of the patients who are screened, the rate of cytologic abnormalities, the number of colposcopies that are performed, and the number of cases of precancers that are treated, usually are used to monitor the screening process. These numbers are usually available from either the laboratories or the screening and treatment centers. Cancer registries usually are used to monitor the overall effectiveness of the screening program that can be measured in terms of the incidence of invasive cervical cancer. More sophisticated evaluations of effectiveness require linkage between screening history in all women diagnosed with invasive cervical cancer. This linkage permits estimates of the relative risk in women who are screened and among women who do not participate in the screening program. Although process measures should be available in most low-resource settings, cancer registries often either are absent or capture only a few cases in most low-resource settings, and it is difficult to evaluate the efficacy of screening programs.
In many countries, the major barrier to implementation of a cervical screening program is not only the cost of providing the screening test, but also the resources and technologic requirements needed for the systems that support the test within a prevention program. The complex strategy shown in Figure 6 offers multiple opportunities for a screening program to break down and cytology specimens, results, or patients to be lost or treated inappropriately. It is not surprising that cervical cancer prevention programs that require such a complex infrastructure have proved difficult to sustain in most low-resource settings.
NEW APPROACHES TO CERVICAL CANCER PREVENTION FOR LOW-RESOURCE SETTINGS
To make cervical cancer prevention programs more accessible to women living in low-resource settings, the screening test and the screening strategy outlined in Figure 6 needs to be made more appropriate to the realities of low resource environments. A number of screening tests and protocols have been evaluated in diverse settings in the past 10 years or so. Two of the most studied alternative screening tests to cytology are Visual Inspection with Acetic Acid (VIA) and HPV DNA testing. There is now a considerable body of evidence evaluating the test characteristics in both cross-sectional and longitudinal studies of both screening tests in a range of screening protocols.
Screen and treat strategies
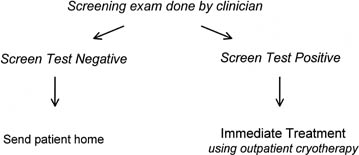
Simply changing the screening test would not make cervical cancer prevention programs more accessible to women in low-resource settings because all of the resources and technologic requirements needed for the systems that support the test within a prevention program still would be required. What is needed is a viable alternative that compares favorably in safety and effectiveness with the screen, diagnose, and treat strategy. It has been suggested that consolidating screening and diagnosis, and eliminating the requirement for colposcopy and cervical biopsy would reduce greatly the infrastructure requirements of a cervical cancer prevention program.58, 59 Such a “screen and treat” strategy would couple screening with immediate treatment using simple outpatient ablative methods, such as cryotherapy or electrofulguration, in all women found to be screen positive (Fig. 7). the outcome of two randomized controlled trials evaluating screen and treat strategies are presented below.
|
Visual screening methods
Visual screening methods involve washing the cervix with a chemical contrast solution, such as a 5% solution of acetic acid or an iodine solution, and inspecting the cervix either with the naked eye or with a handheld low-magnification device to determine whether a cervical cancer or precursor lesion is present. This approach is identical to the Schiller (iodine) test that was introduced in the 1930s.60 The Schiller test consisted of applying an iodine solution to the cervix and inspecting the cervix with the naked eye to detect nonstaining areas. The Schiller test initially was well received by clinicians because it provided the first method that could be used to screen for cervical cancer precursors. As it became more widely used, however, the low specificity of the Schiller test was recognized as a significant disadvantage. Only a small proportion of women who were classified as positive with the Schiller test had areas of the cervix that did not stain with iodine because they had a cervical cancer precursor lesion. Most of these women had areas of cervical ectopy or immature squamous metaplasia that lacked glycogen and did not stain with iodine.61 Because the only method available for evaluating women with a positive screening test at that time was a cold knife conization, the test became unpopular and was largely discontinued after the Pap test, which was considerably more specific, was introduced in the 1950s.
The reconsideration of visual screening as a method for cervical cancer screening in the 1990s was due to several factors. One was the realization that cytologic screening is difficult, if not impossible, to implement and sustain in most low-resource settings. Another reason to reconsider visual screening was that even when practiced in developed countries, cytology has a much poorer performance than previously thought. Meta-analyses of the performance of cervical cytology found that the sensitivity for the detection of high-grade SIL (CIN 2, 3) is only 0.49–0.67 and that the specificity of cytology is only 0.62–0.77.62, 63 In addition, the availability of simple outpatient ablative treatment methods, such as cryotherapy, that were not available at the time the Schiller test was introduced in the 1930s and 1940s make low specificity less of a problem than it was in the 1940s, when the only option for screen-positive women was to undergo a cone biopsy. Ablative outpatient treatment methods have minimal complications and serious side effects.64, 65, 66, 67
The most widely tested visual inspection method consists of applying a 5% solution of acetic acid to the cervix and visualizing the cervix with the naked eye to determine whether an acetowhite “lesion” is present (Fig. 8). This approach to visual screening has been referred to by a variety of terms, including VIA, acetic acid washes, acetic acid visualization, cerviscopy, and direct visual inspection. In most studies, VIA is performed by specifically trained midlevel nurses, although in one study from India the examination was performed by cytotechnicians and in one study from China it was performed by gynecologic oncologists.
|
Many large studies have been completed that have evaluated the performance of VIA when used in the screening setting (Table 10).68, 69, 70, 71, 72 VIA has a reported sensitivity of 0.64–0.96 for the detection of high-grade SIL (CIN 2, 3) and invasive cervical cancer and a specificity of 0.65–1.0. In studies in which Pap smears73 were obtained and VIA was performed, the sensitivity of the two tests was comparable, but in most studies the specificity of VIA was significantly lower. The high sensitivities of VIA that were reported in the studies by Sankaranarayanan and associates71, 72 most likely reflect the fact that relatively few patients who were not VIA positive underwent colposcopy, and the prevalence of biopsy-confirmed high-grade SIL (CIN 2, 3) in women who were VIA negative probably was underestimated to a considerable degree.
Table 10. Comparison of visual inspection with acetic acid and cervical cytology*
VIA | Cervical Cytology | |||
Author | Sensitivity | Specificity | Sensitivity | Specificity |
Megavand et al.70 | 0.64 | 0.98 | 0.62 | 0.89 |
0.90 | 0.92 | 0.92 | 0.90 | |
Sankaranarayanan et al.74 | 0.96 | 1.00 | 1.00 | 0.63 |
Zimbabwe Project69 | 0.77 | 0.65 | NA | NA |
Denny et al.68 | 0.67 | 0.83 | 0.75 | 0.90 |
Bellinson et al.75 | 0.71 | 0.74 | 0.94 | 0.78 |
VIA, visual inspection with acetic acid; NA, not available.
*For the detection of high-grade squamous intraepithelial lesions (cervical intraepithelial neoplasia [CIN] 2, 3) and cancer.
VIA test results usually are reported as being positive, negative, or suspicious for invasive cancer, although some investigators also used a “borderline” or “indeterminant” category. Positive tests frequently are described as well-defined, dense acetowhite lesions that are adjacent to the squamocolumnar junction. Negative tests often are defined as including cervices with faint, ill-defined areas of acetowhitening and small dot-like areas of acetowhite epithelium.76 In one study, 114 cervical photographs taken after the application of a 5% acetic acid solution were scored by three clinicans experienced in colposcopy and VIA.76 The degree of intraobserver agreement was only moderate (pairwise unweighted κ statistic, 0.54–0.60). Using the cervical photographs, the performance of VIA varied considerably between the three observers. Sensitivity for high-grade SIL (CIN 2, 3) and cancer varied between the three observers from 0.87 to 0.97. Specificity also varied considerably (0.58–0.39). As would be expected, the interpreter with the most colposcopic experience had the highest sensitivity, but also the lowest specificity.
Our group evaluated different definitions of what constitutes a “positive” VIA result among 2754 South African women who were screened using a combination of HPV DNA testing for high-risk types of HPV, cervical cytology, cervicography, and VIA, with women with a positive result on any of the screening tests being referred for colposcopy (44% of all women screened).77 “Definite lesions” were acetowhite lesions with well-circumscribed borders; “ill-defined lesions” were lesions that were poorly circumscribed and faintly acetowhite; and “nonconfluent scattered lesions” were focal, small, punctated areas of acetowhitening usually involving the transformation zone. When only definite lesions were classified as a positive result, the sensitivity of VIA for high-grade SIL (CIN 2, 3) was 0.58. The sensitivity significantly increased to 0.70 when any lesion was classified as a positive result. The increase in sensitivity observed with expanding the definition of what constitutes a positive test result was accompanied by a significant decrease in test specificity from 0.84 to 0.79. For comparison, the sensitivity of conventional cervical cytology using a cutoff of low-grade SIL to define a positive test result produced a sensitivity of 0.57 and a specificity of 0.96.
In the same study, we also evaluated the impact of using a handheld 2.5× magnification device while performing VIA and the impact of age, other demographic factors, and sexually transmitted infections on the performance of VIA. Magnification resulted in a slight but nonsignificant increase in sensitivity for high-grade SIL (CIN 2, 3) from 0.70 to 0.74 when the definition of “positive result” was the presence of any acetowhite lesion. The nonsignificant increase in sensitivity was accompanied, however, by a slight but significant decrease in specificity from 0.79 to 0.77. No significant differences in the sensitivity and specificity of VIA were associated with the presence or absence of N. gonorrheae, C. trachomatis, or T. vaginalis or age, parity, contraceptive use, or across the duration of the study. VIA had a significantly lower specificity among HIV-infected women compared with uninfected women, and there was a nonsignificant trend toward greater specificity in women older than age 50 years, which became significant when women were classified as postmenopausal or not.
The previous discussion highlights several key points regarding VIA. The first is that currently most experts would place the sensitivity of VIA for biopsy-confirmed high-grade SIL (CIN 2, 3) between 60% and 80%, which is slightly greater than that reported for cervical cytology in most large meta-analyses.62, 78 The specificity of VIA appears to be lower, however, than that of cytology or HPV DNA testing (see later) in many settings. If VIA were used to screen, considerable numbers of women lacking high-grade SIL (CIN 2, 3) or cancer would be classified as being screening test positive and would require either further evaluation or treatment. The second point is that the performance of VIA depends on the definition of a positive test result. When a more restrictive definition of a positive test result is used, specificity is significantly increased, but sensitivity seems to be significantly decreased. The stringent definition should be used under conditions in which it is important to limit the number of false-positive results, but a less stringent definition should be used under conditions in which the key objective is to identify all women with high-grade SIL (CIN 2, 3), such as when women would have available only one or two opportunities to undergo screening in their lifetime. Use of a less stringent definition and not including magnification also would make the training requirements for VIA simpler and more appropriate for low-resource settings.
Human papillomavirus DNA testing
TYPES OF HUMAN PAPILLOMAVIRUS
There is now compelling scientific and epidemiologic evidence that specific high-risk types of HPV cause almost all cases of invasive cervical cancer. Although a full discussion of the role that HPV plays in the pathogenesis of cervical cancer is beyond the scope of this chapter, it is crucial that the nature of the relationship between HPV and cervical cancer be understood to understand the attractiveness of using HPV DNA testing as a screening method in low-resource settings. HPV is a double-stranded DNA virus that is a member of a family of DNA tumor viruses, Papovaviridae. This family also includes polyoma viruses and SV-40 virus. In humans, 85 types of papillomaviruses have been characterized and fully sequenced, and more than 120 putative novel types have been partially characterized.79 Approximately 40 types of HPV can infect the epithelium of the anogenital tract. Although the different types of HPV are similar structurally, there are significant differences with respect to the tissue that they infect and the type of lesions they produce.80
Based on their associations with specific types of lesions, the most common of the 40 anogenital HPVs have been divided into three oncogenic risk groups (Table 11). HPV types that usually are associated with condyloma acuminata of the anogenital tract and occasionally associated with low-grade SIL but only rarely associated with high-grade SIL (CIN 2, 3) and almost never associated with invasive squamous cell carcinomas of the cervix are referred to as low-risk viruses. HPV types 42, 43, and 44 are included in the low oncogenic risk viruses because they have a similarly low risk of being associated with cancer. In contrast, HPV types 16, 18, 45, 56, and 58 are associated most frequently with invasive carcinomas of the anogenital tract and are referred to as high-risk viruses.81, 82, 83, 84 Many other HPV types, such as types 31, 33, 35, 39, 51, 52, 59, and 68, have features of high-risk viruses and can be found in association with invasive cervical cancers, although less frequently than the typical high oncogenic risk viruses. These viruses used to be referred to as intermediate-risk viruses, but because more recent studies indicate that infection with these intermediate-risk viruses confers similar relative risks for high-grade SIL (CIN 2, 3) or cancer as does injection with the prototypical high oncogenic risk viruses, many authorities now classify types 31, 33, 35, 39, 51, 52, 59, and 68 as high-risk viruses.5
Table 11. Oncogenic risk grouping of anogenital human papillomavirus
Low oncogenic risk | 6, 11, 42, 43, 44, 53 |
High oncogenic risk | 16, 18, 45, 56, 58 |
Other high-risk types | 31, 33, 35, 39, 51, 52, 59, 68 |
The only HPV assay that is commercially available (Hybrid Capture II) combines the 13 most common intermediate-risk and high-risk viruses together into a single high-risk probe mixture. These are HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. The commercially available assay cannot distinguish lesions associated with the prototypical high-risk types of HPV (e.g., HPV 16, 18, 45, 56, and 58) from those associated with other high-risk viruses.
EVIDENCE LINKING HUMAN PAPILLOMAVIRUS INFECTION WITH CERVICAL CANCER
There is consistent and compelling epidemiologic evidence that HPV infection plays a central role in the development of high-grade cervical cancer precursors (CIN 2, 3) and invasive cervical cancers. Based on this evidence, it is accepted that infection with a high-risk type of HPV is a major risk factor for the development of cervical cancer. Epidemiologic studies have shown that the temporal sequence between infection and subsequent formation of cancer is correct; associations between infection with high-risk types of HPV and cervical cancer are relatively specific; and, perhaps most importantly, the natural history and biologic behavior of HPV infections and cervical cancer are consistent with a causal association between HPV and cervical cancer.5 Numerous studies have tested tissue from invasive cervical cancers using sensitive molecular methods, and these studies uniformly have identified high-risk types of HPV DNA in more than 93% of invasive cervical cancers.82, 84, 85 The same types of HPV are found, regardless of geographic area from which the cancers are selected (Table 12). When cervical cancer samples initially found to be HPV DNA negative were retested using more sensitive HPV DNA detection methods, HPV DNA was identified in almost all of the HPV DNA-negative samples, with an overall HPV DNA positivity rate of greater than 99%.86
Table 12. Prevalence of specific types of human papillomavirus in invasive cervical cancer by geographic region
Geographic Region | ||||||||||
Africa | Latin America | Southeast Asia | Europe | North America | ||||||
HPV Type | No. | % | No. | % | No. | % | No. | % | No. | % |
HPV 16 and associated | ||||||||||
HPV 16 | 79 | 43 | 255 | 51 | 42 | 43 | 56 | 65 | 33 | 58 |
HPV 31 | 5 | 3 | 35 | 7 | 1 | 1 | 5 | 6 | 3 | 5 |
HPV 33 | 5 | 3 | 18 | 4 | 2 | 2 | 1 | 1 | 0 |
|
HPV 35 | 4 | 2 | 10 | 2 | 1 | 1 | 1 | 1 | 0 |
|
HPV 52 | 4 | 2 | 16 | 3 | 2 | 2 | 3 | 4 | 0 |
|
HPV 58 | 5 | 3 | 11 | 2 | 2 | 2 | 1 | 1 | 0 |
|
HPV 18 and associated | ||||||||||
HPV 18 | 33 | 18 | 48 | 10 | 31 | 32 | 7 | 8 | 9 | 16 |
HPV 39 | 0 | 13 | 3 | 1 | 1 | 0 | 0 |
| ||
HPV 45 | 23 | 12 | 37 | 7 | 8 | 8 | 2 | 2 | 8 | 14 |
HPV 59 | 0 | 14 | 2.8 | 1 | 1 | 0 | 0 |
| ||
HPV 68 | ||||||||||
Other | ||||||||||
HPV 56 | 6 | 3 | 3 | 1 | 3 | 3 | 2 | 2 | 2 | 4 |
Miscellaneous | 5 | 3 | 16 | 3 | 4 | 4 | 1 | 1 | 0 |
|
Undetermined | 2 | 1 | 8 | 2 | 0 | 1 | 1 | 1 | 2 | |
HPV negative | 19 | 10 | 36 | 7 | 7 | 3 | 4 | 5 | 4 | 7 |
Total samples | 186 | 505 |
| 98 | 86 |
| 57 |
| ||
HPV, human papillomavirus.
Modified from Bosch FX, Manos MM, Munoz N, et al: Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst 87:779, 1995.
Numerous studies have shown a clear, consistent association between infection with specific high-risk types of HPV and invasive cervical cancer and its precursor lesions. They also have shown that exposure to HPV precedes the development of cervical disease. This evidence, combined with evidence identifying the molecular pathways by which HPV could cause cervical cancer, supports a causal relationship between HPV infection, acquired through sexual contact, and the development of SIL and invasive cervical cancer. Based on these data, the IARC has classified HPV 16 and 18 as carcinogens in humans.4 Newer data on the role of other types of HPV in the pathogenesis of cervical cancer were reviewed by Bosch and coworkers,5 and they concluded that HPV types 31, 33, 35, 39, 51, 52, 59, and 68 also should be classified as carcinogens in humans.
NATURAL HISTORY OF HUMAN PAPILLOMAVIRUS INFECTIONS
Much has been learned in recent years regarding the natural history of HPV infections. Anogenital HPV infections are rare in virgins but become common when young women initiate sexual intercourse. A prospective study of female college students in the United States who were followed at regular intervals over a 3-year period found that 26% of the women were HPV DNA positive at entry into the study.87 After only 3 years of follow-up, however, cervicovaginal HPV infections were identified in an additional 43% of the women. Two thirds of these young, sexually active college women were found to have anogenital HPV infection at some point in the study. Other studies have confirmed a high prevalence of anogenital HPV infections in sexually active young women. Of female college students from the University of California, 46% were found to have anogenital HPV infections using sensitive polymerase chain reaction assays.88 Even these relatively high prevalence figures may underestimate the number of HPV-infected women because shedding of sufficient quantities of HPV DNA to be detected with the then-available molecular methods occurs only transiently in many young women. When female college students were tested weekly for HPV DNA using polymerase chain reaction, only 26% of the women were HPV DNA positive at the first examination, but after 10 visits, the cumulative prevalence of HPV DNA positivity was 44%.89
In general, anogenital HPV infections tend to be transient and of relatively short duration in both young and older women. In the study by Ho and colleagues87 of college women, the median duration of HPV infections was only 8 months, and by 24 months of follow-up, 91% of HPV-infected women had become HPV DNA negative. The duration of infection was longer for women infected with high-risk types of HPV than for women infected with low-risk types of HPV. Similarly, in a study of young women from San Francisco, Moscicki and coworkers90 reported that approximately 70% of young women positive for HPV had regression of their infections by 24 months. Women with low-risk types of HPV infections also were more likely to show HPV regression than were women with high-risk types of HPV infection in this study. In another study of older women from New York City, it was found that persistent HPV infections (defined as the same type of HPV detected twice over a 12-month period) occurred in only 16% of the women who were HPV DNA positive at enrollment. In the New York City study, persistence was more common among women infected with high-risk types of HPV compared with low-risk types of HPV.91 Because most HPV-infected women have spontaneous resolution of their infections, the prevalence of HPV infections decreases with increasing age.
It seems that most sexually active young women are exposed to HPV at some point after initiating sexual activity.87, 88, 90, 92 Most women develop transient HPV infections that are of relatively short duration, and eventually most become HPV DNA negative. Only a small proportion of women exposed to HPV become persistently infected and continue to have detectable levels of HPV DNA in the genital epithelium. These persistently infected women are at risk for having persistent high-grade SIL and of developing invasive cervical cancer.93, 94, 95 Although the exact factors that regulate the clearance of an anogenital HPV infection are unknown, immunologic factors and viral type are important. The role of immunologic factors is shown by the finding that persistence of HPV infections is more common in HIV-infected compared with HIV-uninfected women and that rates of persistence in this population increase with increasing levels of immunosuppression.91 A possible role for humoral immunity in the loss of infection is suggested by the finding that 67% of women enrolled in a prospective follow-up study who developed an incident HPV 16 infection subsequently developed serum antibodies against HPV 16.96 The median time to seroconversion was 8.3 months, which is similar to the median duration of incident HPV infections in most studies.96
Evidence supporting a central role for persistence of infection with high-risk types of HPV in the pathogenesis of high-grade SIL (CIN 2, 3) and invasive cervical cancer comes from two types of studies. One is case–control studies of women developing high-grade SIL (CIN 2, 3) or invasive cervical cancer in which archived conventional Pap tests were tested for the presence of HPV using sensitive polymerase chain reaction-based methods. These case–control studies showed that women who are persistently infected with high-risk types of HPV are at least 30 times more likely to develop high-grade SIL (CIN 2, 3) than women who are high-risk HPV DNA negative. Also, women who are persistently infected with HPV DNA are 213 times more likely to develop invasive cervical cancer than are women who are not infected with HPV.93, 97, 98 Long-term prospective follow-up studies also have documented a central role for HPV persistence in the pathogenesis of SIL. Hopman and associates95 followed 68 women who were cytologically negative but HPV DNA positive, using cytology, colposcopy, and HPV DNA testing for 34 months. During follow-up, 17 (25%) of these women subsequently developed an abnormal Pap test, and eight (12%) developed biopsy-confirmed high-grade SIL. Of the women developing a cervical abnormality, 94% had a persistent high-risk HPV DNA infection.95 Similar findings were reported by Ellerbrock and coworkers,94 who found that 12% of cytologically and colposcopically normal women who had high-risk HPV infections subsequently developed biopsy-confirmed SIL during 36 months of follow-up. Women who were persistently infected with high-risk types of HPV were significantly more likely to develop incident SIL on follow-up than were women who were transiently infected with high-risk types of HPV.
HUMAN PAPILLOMAVIRUS DNA TESTING FOR CERVICAL CANCER SCREENING
Performance in clinical studies
Although none of the commercially available HPV DNA testing methods is approved by the US Food and Drug Administration for primary screening, many studies have evaluated HPV DNA testing as a cervical cancer screening method. We conducted a study of previously unscreened South African women older than age 35 years that compared the sensitivity and specificity of conventional cytology and Hybrid Capture II for the detection of high-grade SIL (CIN 2, 3) and cervical cancer (Table 13).99 HPV DNA testing had a significantly higher sensitivity but a lower specificity than cytology. A large National Cancer Institute-sponsored study conducted in Costa Rica also found that HPV DNA testing was more sensitive but less specific than cytology (see Table 13).100 In the Costa Rica study, the specificity of HPV DNA testing was better than that observed in South Africa. Other studies conducted in the United Kingdom, Canada, Germany, France, and China also found that the sensitivity of HPV DNA testing is superior to that of cytology (see Table 13).75, 101, 102, 103
Table 13. Screening for high-grade cervical neoplasia with human papillomavirus DNA testing
Pap Smear | Hybrid Capture II | ||||
Author | Country | Sensitivity* | Specificity | Sensitivity* | Specificity |
Kuhn99 | South Africa | 0.78 | 0.97 | 0.88 | 0.82 |
Schiffman100 | Costa Rica | 0.78 | 0.94 | 0.88 | 0.89 |
Cuzick102 | United Kingdom | 0.86 | 0.95 | ||
Belinson75 | China† | 0.94 | 0.78 | 0.98 | 0.85 |
Ratnam101 | Newfoundland | 0.40 | 0.92 | 0.68 | 0.91 |
Clavel103 | France† | 0.88 | 0.93 | 1.00 | 0.86 |
*For biopsy-confirmed high-grade squamous intraepithelial lesions (cervical intraepithelial neoplasia [CIN] 2, 3) and cervical cancer.
†Liquid-based cytology.
One of the most significant drawbacks to using HPV DNA testing as a screening test is the high rate of HPV DNA positivity in women lacking cervical disease (i.e., its apparent low specificity). Although these cases frequently are described as a being “false positives,” these women do not have false-positive results in the standard sense of the term. These women are truly infected with high-risk types of HPV and have a significantly elevated risk for subsequently developing SIL (CIN), even though they may not have SIL (CIN) at the time the test is obtained. There are many explanations for the relatively high rate of HPV DNA positivity in some of the published screening studies. In the South African screening study, almost 8% of the women were HIV-infected, 9% were infected with either gonorrhea or chlamydia, and 25% had culture-proven trichomonas. The studies from Costa Rica and Canada included many young women, and this may account for the high rates of HPV DNA positivity in women lacking cervical disease.
Many studies have documented the prevalence of HPV DNA positivity in women of different ages (Table 14).81, 101, 102, 104, 105, 106 In the studies from the United Kingdom, the Netherlands, Costa Rica, and Newfoundland, the prevalence of high-risk types of HPV in women age 35 years or older was 5% or less. This is not any higher than the rate of cytologic abnormalities in women undergoing cytologic screening in the United States.107 Much higher prevalences of HPV DNA positivity were identified in older women from Zimbabwe. More than 50% of the women from Zimbabwe were infected with HIV, however, and the strong associations between HIV and HPV infection explain the high rates of HPV DNA positivity observed in the older women in that population.106
Table 14. Impact of age on rates of human papillomavirus DNA positivity
% HPV DNA Positive by Age Group | |||||
Author | Country | <25 years | 25–34 years | 35–44 years | >45 years |
Jacobs et al104 | Netherlands*† | 13 | 10 | 2 | 2 |
Herrero et al81 | Costa Rica*† | 10 | 6 | 3 | 3 |
Ratnam et al101 | Newfoundlandठ| 17 | 12 | 5 | 4 |
Cuzick et al102 | United Kingdom†§ | 3 | 3 | 5 | |
Womack et al108 | Zimbabweठ| 32 | 22 | 24 | |
HPV, human papillomavirus.
*Women without squamous intraepithelial lesions.
†By polymerase chain reaction.
‡By Hybrid Capture II for high-risk types of HPV.
§All women.
POTENTIAL STRATEGIES FOR USING HUMAN PAPILLOMAVIRUS DNA TESTING FOR SCREENING
There are several ways in which HPV DNA testing could be incorporated into cervical cancer screening programs. One potential strategy would be to use HPV DNA testing as a “stand-alone” screening method, much as cytology is used today. When used as a stand-alone screening method, it is likely that HPV testing would need to be restricted to women older than age 35 years because of its low specificity in younger women. This restriction should not be a problem in low-resource settings, however, where the target age for screening typically begins at 35–40 years. Our group performed a randomized controlled trial of 6555 women aged 35–65 years in Cape Town, South Africa.109 The trial evaluated three 'screen and treat' strategies: (1) screening with VIA followed by cryotherapy if positive; (2) screening with HPV DNA testing using Hybrid Capture II followed by cryotherapy if positive; and (3) control group had delayed treatment for 6 months regardless of the result of the screening tests (VIA and HPV DNA testing). The prevalence of high grade cervical cancer precursors (defined histologically) was significantly lower in the two 'screen and treat' groups 12, 24, and 36 months postrandomization compared to the delayed evaluation group. However, HPV DNA testing followed by cryotherapy was twice as effective in reducing high-grade lesions compared to VIA and cryotherapy. The cumulative detection of CIN 2+ in women in the HPV and treat group was 1.42 %, 2.91% in the VIA and treat group, and 5.41% in the delayed treatment group. While minor complaints such as discharge and bleeding were common after cryotherapy, major complications were rare.
Sankaranarayanan et al. recently published a large randomized controlled trial of "screen and treat" using VIA, cytology, and HPV testing compared to a control group.110 A total of 131,746 healthy women between ages 30 and 59 years, were randomly assigned to one of four groups: (1) screening with HPV tesing; (2) cytology testing; (3) VIA testing; or (4) to receive standard of care, which is no screening in India. The study followed subjects for 8 years and is the first study to report on cancer as an outcome, as opposed to cervical cancer precursors. The incidence of cervical cancer in the HPV group was 127 cases, compared to 152 in the cytology group, 157 in the VIA group, and 118 in the control group. The hazard ratio for reduction in advanced cancer and death was 0.47 (0.32–0.69) and 0.52 (0.33–0.83), respectively, in the HPV group. The hazard ratios in the VIA and cytology groups were not statistically significant, therefore there was no significant reduction in rate of death in either the cytologic or VIA group compared to the HPV group. Further, the ASIR of invasive cancer in women with negative cytology and VIA tests, were four times the rate among HPV negative women, supporting the very high negative predictive value of HPV testing.
Both these studies support the use of HPV and treat as a strategy for cervical cancer control in low-resource settings. Current HPV DNA tests are prohibitively expensive, however, a new HPV test that can give a result within 2.5 hours and will cost around $5 a test is undergoing clinical trials. Called careHPV and able to detect 14 high-risk types of HPV, it has been developed in a collaboration with PATH and Qiagen. The test has recently been used in a cross-sectional study of 2530 Chinese women aged 30–54 years and was shown to have similar test characteristics to Hybrid Capture II which is an FDA approved test and costs around $30 per test.111 Once introduced into clinical practice, careHPV has the potential to provide onsite screening and treating at a primary care level.
Another strategy would be to perform HPV DNA testing and cervical cytology simultaneously. This strategy might be useful for women of all ages, especially if HPV DNA status were used to determine screening frequency, rather than to refer women to colposcopy. Another strategy would be to combine HPV DNA testing and cytology sequentially into a two-stage screening program (Fig. 9). In a two-stage strategy, all women would be screened using the first test, and only women who are positive on the first test would be screened using the second test.59 This strategy would increase specificity greatly, but also would reduce sensitivity.
ADVANTAGES OF HUMAN PAPILLOMAVIRUS DNA TESTING
HPV DNA testing has many advantages as a screening test compared with cervical cytology. Because HPV DNA testing is more sensitive than cytology, it is less likely that a high-grade SIL (CIN 2, 3) would be missed when HPV DNA testing is used. The performance of screening is improved, especially when screening is done at extended intervals, such as every 5 years. Another advantage is that HPV DNA testing can be performed on self-collected vaginal samples; this allows screening to be performed without a speculum examination.112 This may be advantageous for low-resource settings because it eliminates the need to train providers in how to collect samples for screening. Another important advantage is that in contrast to cytology, which simply determines whether or not a patient has a lesion at the time screening takes place, HPV DNA testing identifies which women are at greatest risk for developing cervical disease in the future. Screening programs might be able to be tailored to an individual’s specific risk for developing cervical disease in the future.
DESIGNING AND IMPLEMENTING A SCREENING PROGRAM
Key steps in the design and implementation of a successful cervical cancer screening program include determining goals and policy, mapping out or planning the program in complete detail, and implementing the program. Each of these steps contains many different elements (Table 15). All of the elements are important and essential for the screening program to succeed. Health care professionals often focus on the components that they understand best, such as training providers and developing treatment services for women with positive test results. Policy decisions, organization, and management are equally important features of any screening program, however, and must be given careful consideration. Failure to consider or successfully introduce a single component may result in a program failing to meet its goals.
Table 15. Steps in planning and implementing a screening program
Policy Phase |
Data collection aspects |
| Determining the prevalence of disease in the population |
| Review of current screening and treatment capacity and potential for increase |
| Review of community mobilization needs |
Analysis aspects |
| Determining who to screen and when |
| Review screening, diagnostic, and treatment options based on available resources |
| Review costs, resources, and program strategies based on expected benefits |
| Determine relative importance of screening program compared with other health priorities |
| Decide on scale and reach of program, set benchmarks |
Policy making |
| Commit or solicit resources for program |
| Develop or revise relevant health policies |
| Obtain high-level political and administrative support and support of medical community |
Planning Phase |
Build stakehold involvement and consensus |
Establish equipment and training needs |
Develop guidelines, protocols, and training curricula |
Develop a health information system for tracking patients and ensuring quality assurance |
Implementation Phase |
Train service providers, laboratory personnel, and community health workers |
Put all service delivery systems in place |
Distribute equipment and IEC materials and ensure resupply systems function |
Launch screening services in the community |
Monitor and evaluate the process and the outcomes |
IEC, information, education, and communication.
Policy Phase
The first step in developing a screening program is to determine whether it is feasible to introduce a cervical cancer screening service in a given setting and, if so, what are the goals of the program. There are several components to this decision, including determining the burden of disease in the population; identifying the technical resources that are currently available or that realistically can be developed; determining the most appropriate screening method, the target population, and the screening frequency (i.e., the screening policy); and determining the financial resources available for cervical cancer screening and tailoring the program to fit within these financial constraints. These components are interrelated because goals, in terms of overall reductions in cancer incidence and mortality, depend on how frequently the population is screened and with what method, and this in turn depends on the services and finances that are available.
Cervical cancer screening programs must provide a minimal level of services to be effective. This includes not only performing the actual screening, but also treating women with cervical cancer precursors, providing treatment or palliative care for women with invasive cervical cancer, caring for women with noncervical disease who present to the program, and having monitoring programs set up to assess the program’s overall effectiveness. Effective screening also requires developing awareness and acceptance among providers and the community as to the need for cervical cancer screening. One of the first steps in the policy phase is to determine the disease burden in the country and to survey the existing services that could be used for a screening program and the capacity for expansion of these services. This analysis needs to ascertain the prevalence of precancer and cancer in the community. These data already may be available at a community level or may be inferred from the disease burden seen in hospitals but may require a rapid survey. An assessment should be made of prevailing health service capacity for screening and for treatment: what are health care worker attitudes to screening (do they agree with the public health approach of targeted and infrequent screening); what are providers (at the clinic, laboratory, and treatment levels) currently able to do; what would be required to train them and increase their numbers; what infrastructure and equipment needs exist; what systems are in place for follow-up and referral? To be able to monitor the effectiveness of the screening program, assessments need to be made of what health information systems are in place for program monitoring. Assessments of the level of community awareness and interest in screening are needed: what do communities currently know about cervical cancer and what education efforts are needed; what would be needed to mobilize the community to use any services; what information, education, and communications systems exist, and what systems need to be developed; what is needed to help women access treatment when they need it?
When the situation analysis has been completed, realistic goals for the program with respect to reductions in cervical cancer incidence and mortality can be set. In setting these goals, policy makers need to consider the feasibility of introducing specific screening methods and the most appropriate target ages and screening frequency for a given setting. Each screening method has different requirements and performance characteristics, and a variety of factors need to be taken into consideration (Table 16), including not only test performance characteristics, but also staffing and training needs, infrastructure requirements, costs of the entire screening program, and acceptability of a given strategy in a given community. Policy makers then need to determine how often women are to be screened and what age screening should be initiated. These decisions are determined in large part by the financial resources that can be committed to the program. The World Health Organization has developed guidelines that recommend screening every woman once in her lifetime at around age 40 in countries where resources are limited.45 When the goal of screening older women has been achieved and when resources and services are available, screening should be extended to promote screening every 10 years for women 35–55 years old. When this target has been achieved, screening can be performed every 5 years, and the target age groups can be extended to include women age 25–60.
Table 16. Factors to be considered when selecting a screening method and strategy
Screening test performance in terms of sensitivity and specificity |
Personnel and equipment required for screening |
Personnel and equipment required for evaluating test positives |
Staff capacity and training needs |
Quality assurance measures required for a given strategy |
Community acceptability |
Specific community attributes with respect to service use and health-seeking behavior |
Presence of other health issues that impact screening (e.g., HIV) |
Costs of the entire strategy, including patient recruitment, training of personnel, testing, evaluating test positives, and treating women with disease |
It is important that policy makers have realistic expectations regarding the impact that the screening program will have with respect to reductions in cervical cancer incidence and mortality. The impact that can be obtained by low-frequency screening programs is often considerably less than policy makers and program planners expect. Using a comprehensive mathematical model to project the impact of different types of screening programs in South Africa, we projected that providing a single lifetime Pap test at age 35 years for all African women coupled with a standard colposcopic workup and treatment of women with abnormal Pap tests would reduce cervical cancer incidence by only 17%.113 Although screen and treat approaches using HPV DNA testing or VIA were more effective and less expensive, once-in-a-lifetime screens using these approaches also would reduce lifetime cervical cancer risk by less than a third. When policy makers have identified a screening strategy and the goals of the screening program, they need to commit or solicit resources adequate to implement the strategy; develop a formal national policy; and obtain high-level political, administrative, and technical support and support from the medical community.
Planning and implementation
The planning and implementation steps vary markedly among programs, depending on the country and its degree of administrative decentralization. The particular service delivery model used is country specific. In some settings, in which there are large numbers of (particularly older) women needing to be screened and in which a once-in-a-lifetime policy has been developed, a campaign approach might be useful at first to deal with prevalent cases. This type of approach also may be useful when screening older women because they do not use primary health care services for other reasons, such as family planning, antenatal care, or child care. Later and in other situations, it is important that screening services are incorporated into primary health care and are viewed as an essential component of well-woman services. Similarly the style of recruitment of women is culturally specific. In some countries with a tradition of village health workers and house-to-house censuses or population databases, it may be possible to construct a picture of who has been screened and who has not in any given population and possible for health care workers to follow-up on women systematically. In other settings, this type of infrastructure may not exist, and recruitment may need to be just as active but more anonymous. Relying on opportunistic screening has never been shown to be useful, particularly when focused on antenatal or family planning programs, which attract younger women. This does not mean, however, that every chance should not be taken to recruit older women opportunistically, for example, women coming to health centers for diabetic or hypertension treatment.
Several different practice guidelines and curricula need to be developed or adapted. Where the national policy provides overall guidance, there is also a need for more practical training guidelines (technical and clinical training in the specific screening, diagnostic, and treatment protocols chosen; community and peer education) and training for practitioners in their use. At the national or regional levels, plans should be in place for training of specialists, such as trainers, supervisors, cytologists clinical practitioners, and colposcopists. Planning for improving the quality of systems to support the screening program is important.
SUMMARY
There are many more options for cervical cancer screening today compared with even 5 years ago. Previously the only accepted screening strategy was to use cervical cytology for screening, followed by colposcopic assessment with biopsy of abnormal areas in women with an abnormal cervical cytology. Based on large studies from many different countries, it is now clear that HPV DNA testing for high-risk types of HPV and visual inspection methods are acceptable alternatives to cervical cytology as a screening test. There is also considerable ongoing research into new approaches for managing women with abnormal screening test results. This research is likely to provide viable options to the traditional approach of colposcopic assessment with biopsy of abnormal-appearing areas in women with an abnormal screening test result. Alternatives that are being investigated include elimination of the cervical biopsy and treatment of screen-positive women with the colposcopic impression of a SIL (CIN); follow-up of the first screening test with a second screening test in women who are positive on the first test, then treatment of all women who are positive on both screening tests; and simple screen and treat approaches in which all women with a positive result on a screening test undergo simple outpatient treatments, such as cryotherapy. Although all these alternative approaches are considered experimental at present, it is likely that some of them will be shown to be safe and efficacious in the near future and become available for use in developing countries.
Designing an effective cervical cancer prevention strategy for low-resource settings requires knowledge of the natural history of cervical cancer precursors, performance characteristics of the different screening methods, and special needs of a specific population. Whatever type of program is selected for a particular country, the fundamental tenets always must apply. The program must be well organized and managed. It needs to be seen as a public health program with attention to basic creeds of public health, paying attention to costs and benefits and targeting those most in need. It needs to coordinate not only the screening services, but also the community, treatment, and palliative care services. Only through a comprehensive approach can a screening program reach its ultimate objective of reducing the impact of cervical cancer, the leading cause of cancer-related death in many developing countries.
REFERENCES
Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002 cancer incidence, mortality and prevalence worldwide. IARC CancerBase no. 5 version 2.0. Lyon; IARC Press; 2004 |
|
Bosch FX, Castellsague X, Munoz N, et al: Male sexual behavior and human papillomavirus DNA: Key risk factors forcervical cancer in Spain. J Natl Cancer Inst 88:1060, 1996 |
|
Bosch FX, de Sanjose S, Castellsague X, Munoz N: Geographical and social patterns of cervical cancer incidence. In Franco E, Monsonego J (eds): New Developments in Cervical Cancer Screening and Prevention. pp 23, 33 London, Blackwell Science, 1997 |
|
IARC: Human Papillomaviruses. Vol 64:Lyon, IARC, 1995 |
|
Bosch FX, Lorincz A, Munoz N, et al: The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 55:244, 2002 |
|
Nubia Munoz, F. Xavier Bosch, Sivia de Sanjose, Roland Herrero, Xavier Casterrlsague, Keerti V Shah et al. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N Engl J Med 2003;348:518- 27 |
|
Broders AC: Carcinoma in situ contrasted with benign penetrating epithelium. JAMA 99:1670, 1932 |
|
Kottmeier HL: Evolution et traitment des epitheliomas. Rev Fran Gynecol Obstet 56:821, 1961 |
|
Petterson F: Annual Report on Results of Treatment in Gynecologic Cancer. Vol 20:Stockholm, FIGO, 1988 |
|
Reagan JW, Hamonic MJ: Dysplasia of the uterine cervix. Ann N Y Acad. Sci 63:662, 1956 |
|
Patten SF: Morphologic subclassification of preinvasive cervical carcinoma. In Wied GL, Koss LG, Reagan JW (eds): Compendium on Diagnostic Cytology. pp 108, 117 5th ed.. Chicago, Tutorials of Cytology, 1983 |
|
Mitchell MF, Tortolero-Luna G, Wright T, et al: Cervical human papillomavirus infection and intraepithelial neoplasia: A review. J Natl Cancer Inst Monogr 21:17, 1996 |
|
Richart RM: Natural history of cervical intraepithelial neoplasia. Clin Obstet Gynecol 10:748, 1968 |
|
Wright TC, Ferenczy AF, Kurman RJ: Precancerous lesions of the cervix. In Kurman RJ (ed): Blaustein’s Pathology of the Female Genital Tract. pp 253, 354 5th ed.. New York, Springer-Verlag, 2002 |
|
Solomon D, Davey D, Kurman R, et al: The 2001 Bethesda System: Terminology for reporting results of cervical cytology. JAMA 287:2114, 2002 |
|
Nasiell K, Nasiell M, Vaclavinkova V: Behavior of moderate cervical dysplasia during long-term follow-up. Obstet Gynecol 61:609, 1983 |
|
Nasiell K, Roger V, Nasiell M: Behavior of mild cervical dysplasia during long-term follow-up. Obstet Gynecol 67:665, 1986 |
|
Holowaty P, Miller AB, Rohan T, To T: Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst 91:252, 1999 |
|
McIndoe WA, McLean MR, Jones RW, Mullins PR: The invasive potential of carcinoma in situ of the cervix. Obstet Gynecol 64:451, 1984 |
|
Burghardt E: Early Histological Diagnosis of Cervical Cancer. Stuttgart, G Thieme, 1973 |
|
Stern E, Neely PM: Carcinoma and dysplasia of the cervix: A comparison of rates fornew and returning populations. Acta Cytol 7:357, 1963 |
|
Patten SF: Diagnostic Cytology of the Uterine Cervix.. 2nd ed.. Baltimore, Williams & Wilkins, 1969 |
|
Barron BA, Richart RM: A statistical model of the natural history of cervical carcinoma: II. Estimatesof the transition from dysplasia to carcinoma in situ J Natl Cancer Inst 45:1025, 1970 |
|
Barron BA, Richart RM: A statistical model of the natural history of cervical carcinoma based on a prospective study of 557 cases. J Natl Cancer Inst 41:1343, 1968 |
|
Koss LG: Diagnostic Cytology and Its Histopathologic Basis. Vol 1:3rd ed.. Philadelphia, JB Lippincott, 1992 |
|
Koutsky LA, Holmes KK, Critchlow CW, et al: A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 327:1272, 1992 |
|
Nobbenhuis MA, Walboomers JM, Helmerhorst TJ, et al: Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: A prospective study. Lancet 354:20, 1999 |
|
Kiviat NB, Critchlow CW, Kurman RJ: Reassessment of the morphological continuum of cervical intraepitheliallesions: Does it reflect different stages in the progression to cervical carcinoma? In Munoz N, Bosch F, Shah K, Mehens A (eds): The Epidemiology of Cervical Cancer and Human Papillomavirus. pp 59, 66 Vol 119:Lyons, IARC, 1992 |
|
Johannesson G, Geirsson G, Day N: The effect of mass screening in Iceland, 1965–1974, on the incidence andmortality of cervical carcinoma. Int J Cancer 21:418, 1978 |
|
Hakama M: Screening for cervical cancer: Experience of the Nordic countries. In Franco E, Monsonego J (eds): New Developments in Cervical Cancer Screening and Prevention. pp 190, 199 London, Blackwell Science, 1997 |
|
Boyes DA: The value of a pap smear program and suggestions for its implementation. Cancer 48:613, 1980 |
|
Pretorius R, Semrad N, Watring W, Fotheringham N: Presentation of cervical cancer. Gynecol Oncol 42:48, 1991 |
|
Devesa SS, Young JL Jr, Brinton LA, Fraumeni JF Jr: Recent trends in cervix uteri cancer. Cancer 64:2184, 1989 |
|
American Cancer Society: Cervical Cancer Facts and Figures. Available at http://www.cancer.org/cancerinfo, 2000 |
|
Clarke E, Anderson TW: Does screening by “Pap” smears help prevent cervical cancer? A case-control study Lancet 2:1, 1979 |
|
LaVecchia C, Franceschi S, Decarli A: “Pap” smear and the risk of cervical neoplasia: Quantitative estimates from a case-control study. Lancet 2:779, 1984 |
|
Shy K, Chu J, Mandelson M, et al: Papanicolaou smear screening interval and risk of cervical cancer. Obstet Gynecol 74:838, 1989 |
|
MacGregor JE, Moss SM, Parkin DM, Day NE: A case-control study of cervical cancer screening in north-east Scotland. BMJ 290:1543, 1985 |
|
van der Graaf Y, Zielhuis GA, Peer PG, Vooijs PG: The effectiveness of cervical screening: A population-based case-control study. J Clin Epidemiol 41:21, 1988 |
|
Olesen FA: A case-control study of cervical cytology before diagnosis of cervical cancer in Denmark. Int J Epidemiol 17:501, 1988 |
|
Eddy DM: Screening for cervical cancer. Ann Intern Med 113:214, 1990 |
|
IARC: Working Group on Evaluation of Cervical Cancer Screening Programmes:Screening for squamous cerical cancer: Duration of low risk after negative results ofcervical cytology and its implication for screening policies. BMJ 293:659, 1986 |
|
Zhang ZF, Yu SZ, Esteve J, Yang XZ: Risk factors for cancer of the cervix in a rural Chinese population. Int J Gynecol Cancer 43:762, 1989 |
|
Wangsuphachart V, Thomas DB, Koetsawang A, Riotton G: Risk factors for invasive cervical cancer and reduction of risk by “Pap” smears in Thai women. Int J Epidemiol 16:362, 1987 |
|
Miller AB: Cervical Cancer Screening Programmes: Managerial Guidelines. Geneva, World Health Organization, 1992 |
|
Kim JJ, Wright TC, Goldie SJ: Cost-effectiveness of alternative triage strategies for atypical squamouscells of undetermined significance. JAMA 287:2382, 2002 |
|
Klassen AC, Celentano DD, Brookmeyer R: Variation in the duration of protection given by screening using the Pap test forcervical cancer. J Clin Epidemiol 42:1003, 1989 |
|
Coppleson LW, Brown B: Observations on a model of the biology of carcinoma of the cervix. Am J Obstet Gynecol 122:127, 1975 |
|
Sasieni P: Trends in cervical cancer mortality (letter to the editor). Lancet 338:818, 1991 |
|
WHO: Control of cancer of the cervix uteri. Bull WHO 64:607, 1986 |
|
Chirenje ZM, Rusakaniko S, Kirumbi L, et al: Situation analysis for cervical cancer diagnosis and treatment in east, central and southern African countries. Bull WHO 79:127, 2001 |
|
Parkin DM, Pisani P, Ferlay J: Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer 54:594, 1993 |
|
WHO: Selected aspects of reproductive ill health: 1990–1995. Available at http://www.who.int/rht/rhtdimensions.htm1999 |
|
UNAIDS: AIDS Epidemic Update—December 1998. Geneva, UNAIDS/WHO, 1998 |
|
Denny LA: An Evaluation of Alternative Strategies for thePrevention of Cervical Cancer in Low Resource Settings. PhD Thesis. University of Cape Town 2000 |
|
UNDP: Human Development Report. United Nations Development Program New York, Oxford Press, 1994 |
|
Richart RM: Screening: The next century. Cancer 76:1919, 1995 |
|
Cullins V, Wright TC, Beattie KJ, Pollack A: Cervical cancer prevention using visual screening methods. Reprod Health Matters 7:134, 1999 |
|
Denny L, Kuhn L, Risi L, et al: Two-stage cervical cancer screening: An alternative for resource-poor settings. Am J Obstet Gynecol 183:383, 2000 |
|
Schiller WB: Early diagnosis of carcinoma of the cervix. Surg Gynecol Obstet 66:210, 1933 |
|
Younge PA, Hertig AT, Armstron D: A study of 135 cases of carcinoma in situ of the cervix at the Free Hospital for women. Am J Obstet Gynecol 58:867, 1949 |
|
Fahey MT, Irwig L, Macaskill P: Meta-analysis of Pap test accuracy. Am J Epidemiol 141:680, 1995 |
|
Mitchell MF, Cantor SB, Brookner C, et al: Screening for squamous intraepithelial lesions with fluorescence spectroscopy. Obstet Gynecol 94:889, 1999 |
|
Mitchell MF, Tortolero-Luna G, Cook E, et al: A randomized clinical trial of cryotherapy, laser vaporization, and loopelectrosurgical excision for treatment of squamous intraepithelial lesions of the cervix. Obstet Gynecol 92:737, 1998 |
|
Townsend DE, Richart RM: Cryotherapy and carbon dioxide laser management of cervical intraepithelial neoplasia: A controlled comparison. Obstet Gynecol 61:75, 1983 |
|
Richart RM, Townsend DE, Crisp W, et al: An analysis of “long-term” follow-up results in patients with cervicalintraepithelial neoplasia treated by cryotherapy. Am J Obstet Gynecol 137:823, 1980 |
|
Creasman WT, Hinshaw WM, Clarke-Pearson DL: Cryosurgery in the managment of cervical intraepithelial neoplasia. Obstet Gynecol 63:145, 1984 |
|
Denny L, Kuhn L, Pollack A, et al: Evaluation of alternative methods of cervical cancer screening for resource-poor settings. Cancer 89:826, 2000 |
|
Project Z: Visual inspection with acetic acid for cervical-cancer screening: testqualities in a primary-care setting. University of Zimbabwe/JHPIEGO Cervical Cancer Project Lancet 353:869, 1999 |
|
Megevand E, Denny L, Dehaeck K, et al: Acetic acid visualization of the cervix: An alternative to cytologic screening. Obstet Gynecol 88:383, 1996 |
|
Sankaranarayanan R, Shyamalakumary B, Wesley R, et al: Visual inspection with acetic acid in the early detection of cervical cancerand precursors. Int J Cancer 80:161, 1999 |
|
Sankaranarayanan R, Syamalakumari B, Wesley R, et al: Visual inspection as a screening test for cervical cancer control in developingcountries. In Franco E, Monsonego J (eds): New Developments in Cervical Cancer Screening andPrevention. pp 70, 83 Oxford, Blackwell Sciences, 1997 |
|
Belinson JL, Pretorius RG, Zhang WH, et al: Cervical cancer screening by simple visual inspection after acetic acid. Obstet Gynecol 98:441, 2001 |
|
Sankaranarayanan R, Wesley R, Somanathan T, et al: Visual inspection of the uterine cervix after the application of acetic acid in the detection of cervical carcinoma and its precursors. Cancer 83:2150, 1998 |
|
Belinson J, Qiao YL, Pretorius R, et al: Shanxi Province Cervical Cancer Screening Study: A cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 83:439, 2001 |
|
Sellors JW, Jeronimo J, Sankaranarayanan R, et al: Assessment of the cervix after acetic acid wash: Inter-rater agreement usingphotographs (1). Obstet Gynecol 99:635, 2002 |
|
Denny L, Kuhn L, Pollack A, Wright TC Jr: Direct visual inspection for cervical cancer screening: An analysis of factors influencing test performance. Cancer 94:1699, 2002 |
|
Nanda K, McCrory DC, Myers ER, et al: Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: A systematic review. Ann Intern Med 132:810, 2000 |
|
zur Hausen H: Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst 92:690, 2000 |
|
de Villiers EM: Hybridization methods other than PCR: An update. In Munoz N, Bosch FS, Shah KV, Meheus A (eds): The Epidemiology of Human Papillomavirus and Cervical Cancer. pp 111, 119 Vol 119:Lyon, IARC Scientific Publications, 1992 |
|
Herrero R, Hildesheim A, Bratti C, et al: Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 92:464, 2000 |
|
Chichareon S, Herrero R, Munoz N, et al: Risk factors for cervical cancer in Thailand: A case-control study. J Natl Cancer Inst 90:50, 1998 |
|
Rolon PA, Smith JS, Munoz N, et al: Human papillomavirus infection and invasive cervical cancer in Paraguay. Int J Cancer 85:486, 2000 |
|
Ngelangel C, Munoz N, Bosch FX, et al: Causes of cervical cancer in the Philippines: A case-control study. J Natl Cancer Inst 90:43, 1998 |
|
Bosch FX, Manos MM, Munoz N, et al: Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group J Natl Cancer Inst 87:779, 1995 |
|
Walboomers JM, Jacobs MV, Manos MM, et al: Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12, 1999 |
|
Ho GY, Bierman R, Beardsley L, et al: Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 338:423, 1998 |
|
Bauer HM, Ting Y, Greer CE, et al: Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA 265:472, 1991 |
|
Wheeler CM, Greer CE, Becker TM, et al: Short-term fluctuations in the detection of cervical human papillomavirus DNA. Obstet Gynecol 88:261, 1996 |
|
Moscicki AB, Shiboski S, Broering J, et al: The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 132:277, 1998 |
|
Sun XW, Kuhn L, Ellerbrock TV, et al: Human papillomavirus infection in HIV-seropositive women: Natural history and variability of detection. N Engl J Med 337:1343, 1997 |
|
Kjaer SK, Chackerian B, van den Brule AJ, et al: High-risk human papillomavirus is sexually transmitted: Evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 10:101, 2001 |
|
Wallin KL, Wiklund F, Angstrom T, et al: Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N Engl J Med 341:1633, 1999 |
|
Ellerbrock TV, Chiasson MA, Bush TJ, et al: Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA 283:1031, 2000 |
|
Hopman EH, Rozendaal L, Voorhorst FJ, et al: High risk human papillomavirus in women with normal cervical cytology prior to the development of abnormal cytology and colposcopy. BJOG 107:600, 2000 |
|
Carter JJ, Koutsky LA, Wipf GC, et al: The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis 174:927, 1996 |
|
Ylitalo N, Sorensen P, Josefsson AM, et al: Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: A nested case-control study. Lancet 355:2194, 2000 |
|
Josefsson AM, Magnusson PK, Ylitalo N, et al: Viral load of human papilloma virus 16 as a determinant for development of cervical carcinoma in situ: A nested case-control study. Lancet 355:2189, 2000 |
|
Kuhn L, Denny L, Pollack A, et al: Human papillomavirus DNA testing for cervical cancer screening in low-resource settings. J Natl Cancer Inst 92:818, 2000 |
|
Schiffman M, Herrero R, Hildeschein A, et al: HPV DNA testing in cervical cancer screening: Results from a high-risk province in Costa Rica. JAMA 283:87, 2000 |
|
Ratnam S, Franco EL, Ferenczy A: Human papillomavirus testing for primary screening of cervical cancer precursors. Cancer Epidemiol Biomarkers Prev 9:945, 2000 |
|
Cuzick J, Beverley E, Ho L, et al: HPV testing in primary screening of older women. Br J Cancer 81:554, 1999 |
|
Clavel C, Masure M, Bory JP, et al: Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: A study of 7932 women. Br J Cancer 89:1616, 2001 |
|
Jacobs MV, Walboomers JM, Snijders PJ, et al: Distribution of 37 mucosotropic HPV types in women with cytologically normal cervical smears: The age-related patterns for high-risk and low-risk types. Int J Cancer 87:221, 2000 |
|
Clavel C, Masure M, Bory JP, et al: Hybrid Capture II-based human papillomavirus detection, a sensitive test to detect in routine high-grade cervical lesions: A preliminary study on 1518 women. Br J Cancer 80:1306, 1999 |
|
Womack SD, Chirenje ZM, Blumenthal PD, et al: Evaluation of a human papillomavirus assay in cervical screening in Zimbabwe. BJOG 107:33, 2000 |
|
Jones BA, Davey DD: Quality management in gynecologic cytology using interlaboratory comparison. Arch Pathol Lab Med 124:672, 2000 |
|
Womack SD, Chirenje ZM, Gaffikin L, et al: HPV-based cervical cancer screening in a population at high risk for HIV infection. Int J Cancer 85:206, 2000 |
|
Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC. Screen and treat approaches for cervical cancer prevention in low-resource settings. A Randomized Controlled Trial. JAMA 294, 2173 - 2181 |
|
Sankaranarayanan R, Nene B, Shastri S, Jaynat K, Muwonge R, Budukh A et al. HPV Screening for cervical cancer in rural India. N Engl J Med 2009;360:1385 - 94 |
|
Qiao Y, Sellors JW, Eder PS, Bao Y, Lim JM, Zhao F et al. A new HPV-DNA test for cervical cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet 2008;9 (10): 929 - 936 |
|
Wright TC Jr, Denny L, Kuhn L, et al: HPV DNA testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA 283:81, 2000 |
|
Goldie SJ, Kuhn L, Denny L, et al: Policy analysis of cervical cancer screening strategies in low-resource settings: Clinical benefits and cost-effectiveness. JAMA 285:3107, 2001 |