Surgical Procedures for Tubal Sterilization
Authors
INTRODUCTION
Surgical sterilization is a relatively simple, safe, and extremely effective method for preventing future pregnancy. The procedure may be performed on an outpatient or inpatient basis, in the postpartum or postabortal period or as an interval operation. The surgical approach can be via a laparotomy or laparoscopy to access the entirety of the fallopian tube. Transvaginal approaches to tubal sterilization are also a viable option. Hysteroscopic methods to occlude the tubal lumen from within have become popular since 2002 with only the Essure device (Conceptus, San Carlos CA) still available in the United States. The timing and choice of a specific procedure, the surgical approach, and the type of anesthesia depends on the facilities available and the background and experience of the surgeon as well as the patient’s history and preference. This chapter focuses on traditional methods of surgical sterilization. Subsequent chapters discuss the details of laparoscopic and hysteroscopic sterilization techniques.
The 2002 US National Survey of Family Growth noted that tubal sterilization is the most commonly used method of contraception for women over age 35. The same publication noted an increasing number of women undergoing tubal sterilization with a decreasing number of women relying on their partner’s vasectomy between 1982 and 2002.1 Female sterilization is one of the most frequently performed surgeries in the US with over 600,000 performed annually.2, 3 Many epidemiologic studies of tubal sterilization failure rates have been conducted. The most often cited and largest is known as the CREST study, short for the US Collaborative Review of Sterilization, originally published in 1996.4 This multicenter, prospective cohort study, which followed 10,685 women who had tubal sterilizations performed between 1978 and 1986 by all methods available at the time, noted a cumulative lifetime failure rate of 1.85% or 18.5 pregnancies per 1000 procedures. There were considerable differences in failure rates between methods, however. The lowest failure rates were noted among women who had a postpartum partial salpingectomy and unipolar coagulation.4 The data could not be analyzed for the specific method of partial salpingectomy. It is important to note that age is the single most important factor impacting failure rates such that patients who were younger at the time of their sterilization procedure were more likely to experience a subsequent pregnancy.4
PATIENT SELECTION, COUNSELING, AND PREOPERATIVE CONSIDERATIONS
Because tubal sterilization procedures are designed to be permanent, proper patient counseling and informed consent is of paramount importance preoperatively in preparing the patient for a sterilization procedure. There must be no contraindications to elective surgery. The decision for sterilization should be made on an entirely voluntary basis following appropriate discussion regarding risks, benefits, and alternatives. Patients should understand that tubal sterilization is intended to be permanent and if they are not sure of their decision, there are effective long-acting reversible contraceptive methods (such as implants and intrauterine contraceptives) that have failure rates as good as or better than permanent surgical sterilization.5
Various materials are available to assist the physician in patient counseling and in obtaining informed consent. In discussing tubal sterilization, it is appropriate to discuss with the patient the benefits of this procedure, as well as the potential risks. The benefits are substantial. All methods of tubal sterilization are extremely effective with low failure rates. Tubal sterilization involves a one-time surgical procedure that is immediately effective and does not require the continued use of other contraceptive methods unless hysteroscopic approach is used, and another contraceptive method is required until tubal occlusion can be verified. The failure rate of a properly performed sterilization operation is less than 1% and there are no consistent differences in efficacy documented among the standard techniques used today.3, 4, 6 Analysis of the CREST data demonstrated a 5-year cumulative failure rate of 13 per 1000 women undergoing sterilization (all methods aggregated).4 The data regarding cumulative failure rates are best described in Table 1 from Practice Bulletin number 133 of the American College of Obstetricians and Gynecologists:3
Table 1. Pregnancy rates by sterilization method
| Method | 5-year (per 1000 procedures) | 10-year (per 1000 procedures) | Ectopic (per 1000 procedures) |
| Postpartum partial salpingectomy | 6.3 | 7.5 | 1.5 |
| Bipolar coagulation* | 16.5 | 24.8 | 17.1 |
| Silicone band methods | 10.0 | 17.7 | 7.3 |
| Spring clip | 31.7 | 36.5 | 8.5 |
| Hysteroscopy (Essure)† | 1.64 | — | — |
| Vasectomy | 11.3 | No association |
*Secondary analysis of 5-year failure rates with bipolar coagulation performed in different decades found that failure was significantly lower in later periods,reflecting improved technique with the methods: 19.5 per 1000 procedures for 1978–1982 versus 6.3 per 1000 procedures for 1985–1987. Peterson et al. Pregnancy after tubal sterilization with bipolar electrocoagulation. U.S. Collaborative Review of Sterilization Working Group. Obstet Gynecol 1999;94:163–7.
†US Food and Drug Administration recommended projections based on Bayesian statistical analysis (Baskinski 2010). Data from Basinski. A review of clinical data for currently approved hysteroscopic sterilization procedures. Rev Obstet Gynecol 2010;3:101–10; Jamieson et al. The risk of pregnancy after vasectomy. Obstet Gynecol 2004;103:848–50 & Erratum 2004;104:200; Peterson et al. The risk of pregnancy after tubal sterilization.: findings from US Collartorative Reveiew of Sterilization. Am J Obstet Gynecol 1996;174:1161–70; Peterson et al. The risk of ectopic pregnancy after tubal sterilization. US Collaborative Review of Sterilization Working Group. N Engl J Med 1997;336:762–7; Peterson et al. Pregnancy after tubal sterilization with bipolar electrocoagulation. US Collaborative Review of Sterilization Working Group. Obstet Gynecol 1999;94:163–7.
Reproduced with permission from ACOG Practice Bulletin 1333
It is important to consider a woman’s past surgical and medical history in the decision regarding surgical approach. A laparoscopic approach may be best avoided in women who have a history of multiple abdominal surgeries, intra-abdominal adhesive disease, repaired abdominal wall hernias, morbid obesity or contraindications to general anesthesia. It may be better to consider a hysteroscopic approach in such a patient. However, in women who have had prior tubal surgery or known uterine anomalies a hysteroscopic approach may not be ideal. For further details on the laparoscopic and hysteroscopic procedures as well as postpartum sterilization refer to their respective chapters. This chapter focuses on open surgical techniques of interval sterilizations via a laparotomy or colpotomy to visualize the fallopian tubes.
The risks associated with voluntary sterilization, although present, are minimal. Female sterilization is more complex than male sterilization and often is performed under general anesthesia. Most complications occur secondary to general anesthesia. Thus, the risks of anesthesia, the general risks of elective surgery, and the possibility of failure, with subsequent intrauterine or ectopic pregnancy, should be explained to the patient preoperatively.7 The amount of anesthesia necessary depends on the surgical approach. General anesthesia is the most common method of anesthesia for both laparotomy and laparoscopy approaches to interval tubal sterilization. Regional, neuroaxial anesthesia can be used for laparotomy as well as colpotomy and is the most common method of anesthesia for postpartum tubal sterilization.8 Hysteroscopic procedures can be performed with minimal anesthesia and/or sedation, conduction anesthesia, or general anesthesia.6
TYPE OF INCISION: MINILAPAROTOMY OR COLPOTOMY
Elective tubal sterilization may be performed via laparoscopy, hysteroscopy, laparotomy or colpotomy. This chapter focuses on the open abdominal and vaginal approaches. In either case, the usual preoperative preparation of the abdomen or vagina should be employed.
In the United States it is rare for a patient to need a minilaparotomy for an interval tubal sterilization procedure, though most postpartum sterilizations are still approached in this method with a periumbilical laparotomy incision. (For a complete discussion of postpartum sterilization procedures, refer to the appropriate chapter.) For interval tubal sterilization via a minilaparotomy, either a vertical or a transverse incision is satisfactory, both incisions provide easy access to the fallopian tubes. In many instances, a Pfannenstiel-type of transverse incision made approximately 2–3 cm above the symphysis pubis and approximately 2–3 cm in length is satisfactory. The fascia is divided transversely, the rectus muscles are retracted laterally, and the transversalis fascia and the underlying parietal peritoneum is incised in a vertical fashion. To avoid bladder injury, the bladder must be adequately emptied immediately before surgery or an indwelling Foley catheter can be placed. A device to elevate the uterus to the level of the anterior abdominal wall is sometimes helpful and allows the incision size to be kept to a minimum. Several different types of uterine elevators are available, and all are satisfactory. Following the sterilization procedure, the incision is closed in the customary fashion. A subcuticular absorbable suture is often useful and has excellent patient acceptance. One of the advantages of an interval tubal sterilization via a minilaparotomy is that it does not require more than basic surgical instruments and training.8, 9, 10
Although less common today than minilaparotomy, and generally abandoned since the development of laparoscopic and hysteroscopic sterilization, colpotomy is included in this chapter for historical significance and because it may still be an acceptable approach in selected patients such as those who are very obese, have a history of abdominal wall (i.e. umbilical) hernia repairs and contraindications to hysteroscopic sterilization. In this technique, the peritoneal cavity is entered through an incision made in the posterior vaginal fornix between the uterosacral ligaments. The incision may be either transverse or vertical and is made directly through the vagina into the cul-de-sac. The oviducts are then drawn into the surgical field and the selected sterilization procedure is performed. The colpotomy incision is usually closed in one layer with absorbable suture material. Since infection is a concern following colpotomy, the use of prophylactic antibiotics should be considered to minimize this risk.11 In some instances, the mobility of the fallopian tubes is limited by the pelvic anatomy or uterine anomalies such as fibroids, scarring from prior pelvic surgery or infection, and the size of the colpotomy incision. The patient must be able to tolerate lithotomy position and the surgeon must have adequate vaginal surgical experience to perform a vaginal approach for the chosen tubal sterilization procedure without excessive risk. These factors and the advancements in laparoscopic and hysteroscopic sterilization techniques limit the vaginal approach and likely contribute to the rare use of this technique.8
HISTORY AND METHODS OF TUBAL STERILIZATION
Tubal sterilization has been performed for over 100 years. A review of the historic milestones and of the most significant operative techniques in this field is presented in Table 2. Many modifications of the following techniques have periodically been introduced and all seem to be effective as long as the basic principles are followed.
Table 2. Selected chronology of tubal sterilization
Year | Scientist | Event |
1834 | Blundell | First recommendation in the US for incision and removal of a portion of fallopian tube for sterilization |
1881 | Lundgren | First report of tubal sterilization by simple ligation at time of cesarean delivery described by Samuel Smith Lungren of Toledo, Ohio in 1880 |
1910 | Madlener | Technique for crushing and ligation of fallopian tube; 89 procedures, 3 deaths, 0 pregnancies by 1919 |
1924 | Irving | Procedure of ligation, division, and burial of proximal stump in myometrium; modified technique described; 814 procedures, 0 failures by 1950 |
1930 | Bishop and Nelms | Procedure for ligation and resection devised by their late associate, Pomeroy; 60 sterilizations |
1934 | Aldridge | Technique for temporary sterilization; 1 successful reversal and pregnancy |
1935 | Kroener | Fimbriectomy procedure; 200 fimbriectomies, 0 failures by 1969 |
1946 | Uchida | Technique for tubal ligation, resection, and burial; 5000 sterilizations, 0 failures by 1961; 20,000 sterilizations, 0 failures by 1975 |
| 1960s | Parkland | Method popularized at Parkland Memorial Hospital |
Since 1972, when US federal courts removed legal restrictions limiting the use of tubal sterilization for nonmedical reasons it has become the second most commonly used method of contraception in the US. The increase in prevalence of permanent surgical sterilization among American women is due to many factors, including advances in laparoscopic and hysteroscopic techniques. Globally, surgical sterilization is the most commonly used method of contraception. An estimated 180 million women rely solely on tubal sterilization to prevent pregnancy.12
THE POMEROY TECHNIQUE
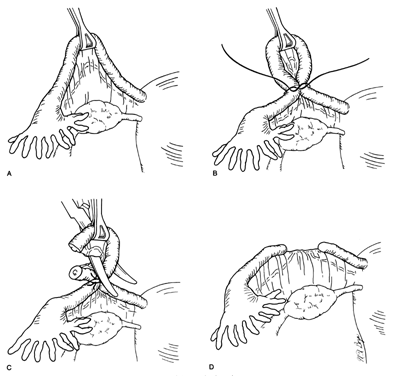
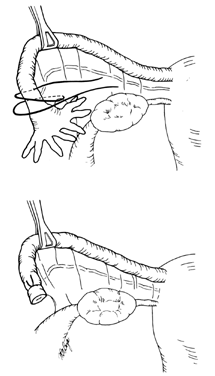
The Pomeroy, or "modified" Pomeroy, technique for bilateral partial salpingectomy is the most common method for interval surgical female sterilization (Fig. 1) via partial salpingectomy. The popularity of this technique is based on its inherent simplicity and its long-established efficacy. Following accurate identification of the fallopian tube, a Babcock clamp is placed around the proximal portion of the tubal ampulla and the tube is elevated to reveal the vascular supply of the mesosalpinx (see Fig. 1A). It is important to follow the tube distally to its fimbriated end to ensure that it is the fallopian tube and not the round ligament. A single strand of rapidly absorbable suture material (1-0 or 0 plain catgut) is placed around the elevated loop of tube and firmly tied. The fallopian tube is thus ligated and the blood supply is occluded simultaneously (see Fig. 1B). A hemostat may now be placed on the suture strands immediately distal to the knot, and the excess suture may be excised. The hemostat now becomes a useful holder for the next step in the procedure. At this point, a second tie of the same suture material may be applied at the discretion of the surgeon, but this is not usually necessary. While gentle traction is maintained on the elevated section of tube, the open blade of the Metzenbaum scissors is used to pierce the mesosalpinx and approximately 1 cm of tube is excised (see Fig. 1C). The excised tube should be appropriately labeled and sent to the pathology laboratory for documentation. With the contraction of the muscularis, the white avascular endosalpinx appears as an elevated area in the center of each cut segment. The proximal and distal ends of the divided and ligated oviduct are now examined for bleeding and then the tube is returned to the abdominal cavity and the procedure is repeated on the opposite tube.
The end result following dissolution of the absorbable suture material and return of the proximal and distal portions of the tube to their normal anatomic positions is shown in Figure 1D. The use of absorbable suture material allows this separation to occur and is probably a critical factor in the development of the anatomic discontinuity. This factor undoubtedly is related to the low failure rate reported for this procedure. Accordingly, the newer synthetic absorbable suture materials with longer dissolution times are probably less desirable than simple plain catgut.
The major advantages of the Pomeroy technique are that it is easily taught, is simple to perform, and is highly-effective. There has been some evidence that the effectiveness is related to the length of tubal segment removed.13 Its acceptance for both postpartum and interval sterilization is quite high. It can be performed abdominally, vaginally, or laparoscopically and the complications are minimal. It has no major disadvantages; however, it can be difficult to perform in the setting of tubal adhesive disease due to the inability to elevate a knuckle of tube. The reported pregnancy rate is less than 1 per 1000 procedures in the first year, but up to 7.5 per 1000 procedures 10 years since sterilization.4
THE PARKLAND PROCEDURE
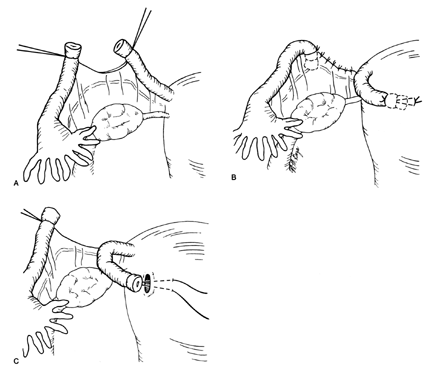
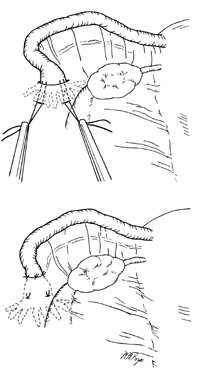
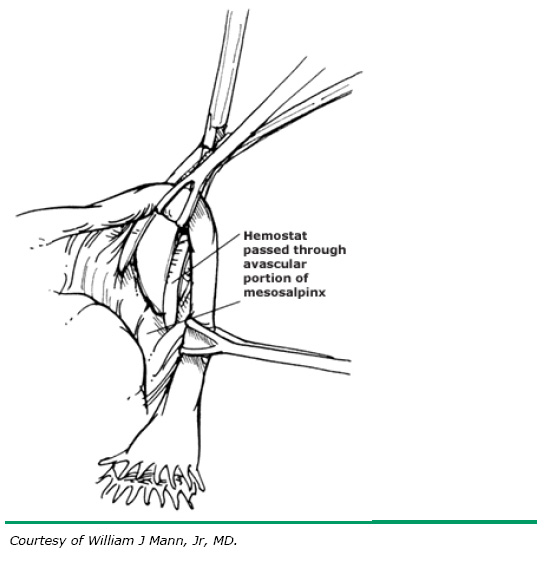
Similar to the Pomeroy technique, this is a partial salpingectomy procedure. However, rather than ligation of a knuckle of tube followed by creation of a window in the mesosalpinx, the window is created first. The Parkland procedure is performed by identifying an avascular section of the mesosalpinx (see Fig. 2A). A window is created in this region (see Fig. 2B), below the tube, with Metzenbaum scissors or a hemostat while elevating the tube with Babcock clamps. By opening the hemostat or scissors within the window it can be stretched in parallel with the tubal lumen. A 2-cm segment of the mid-portion of the tube is then ligated proximally and distally with separate 0 chromic, or plain gut, sutures (see Fig. 2C). The segment between the suture ligatures is then excised (see Fig. 2D). The Parkland method provides for immediate anatomic separation of the disconnected tubal segments unlike the Pomeroy technique.6, 14 The failure rate of this method is similar to the Pomeroy or “modified Pomeroy.”
Fig. 2. A. Identification of avascular region of mid-portion of tube.
 B. Window through mesosalpinx is created below the tube.
B. Window through mesosalpinx is created below the tube.
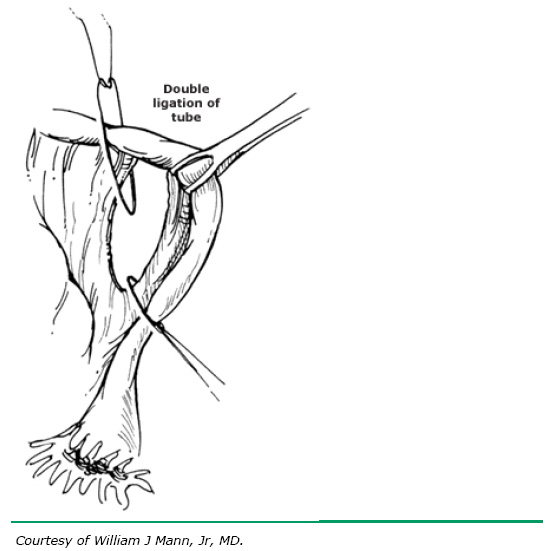 C. Rapidly absorbable (0 chromic or plain gut) sutures placed proximally and distally.
C. Rapidly absorbable (0 chromic or plain gut) sutures placed proximally and distally.
THE MADLENER TECHNIQUE
The Madlener technique is only of historic significance as it should be abandoned due to high failure rates.14 A loop of tube is elevated and crushed before ligation with permanent suture. While no tube is excised, the ligated segment undergoes aseptic necrosis. The end result is similar to the laparoscopic sterilization procedure employing a Silastic band for occlusion as described elsewhere in this text, but with much higher failure rates likely due fistula formation beneath the permanent suture allowing the ligated segments of tube to reconnect.
THE IRVING PROCEDURE
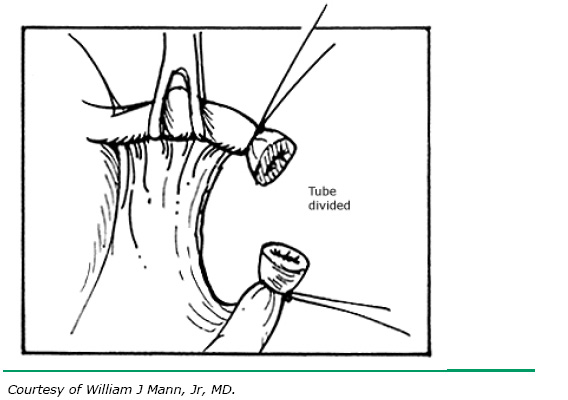
The Irving procedure was introduced as a technique for ligation and division of the oviduct at the time of cesarean delivery (Fig. 4). This technique was developed due to the perceived higher failure rates for traditional tubal sterilization when performed at cesarean, possibly caused by increased hypertrophy and vascularity of the oviducts in the immediate postpartum period. In the technique as originally described, the tube is divided at approximately the ampullary–isthmic junction. With the ends of the suture left long, the proximal tube is buried within the substance of the myometrium on the anterior uterine surface just above the insertion of the round ligament. However, the tube may be buried posteriorly if this is more convenient (see Fig. 4B). The end of the distal portion of the tube is buried between leaves of the broad ligament. When the procedure is performed at the time of cesarean, as the uterus undergoes involution, the buried proximal ends of the tubes become more and more compressed and eventually become obliterated. The Irving procedure is not recommended as an interval procedure, although when performed in the puerperal period, it is highly effective and has a low failure rate.14
THE UCHIDA TECHNIQUE
The Uchida technique for tubal sterilization is shown in Figure 5. A saline-epinephrine solution is injected into the subserosal area of the tube, causing the muscular tube to separate from the serosa. The ballooned serosa is incised, and the muscular tube is withdrawn. A 5-cm section of the tube is then excised and the proximal end ligated. A purse-string suture is applied. The procedure may be extended so as to include fimbriectomy and removal of the isthmus and ampulla with another suture placed around the mesosalpinx.6, 14
The Uchida technique is more complex than the other procedures. Nevertheless, the technique is associated with relatively few failures.
THE KROENER FIMBRIECTOMY
The technique of fimbriectomy as described by Kroener employs the ligation of the distal ampulla of the tube with two permanent sutures and then division and removal of the infundibulum of the tube (Fig. 6). Ligation and hemostasis are accomplished simultaneously. The simplicity with which this excisional procedure is performed on the distal portion of the tube accounted for its early popularity, especially when the sterilization was being performed through a colpotomy incision. While this method is generally used if a vaginal approach is attempted, the Kroener fimbriectomy should be not be used regardless of surgical approach due to high failure rates and more effective alternatives.
THE ALDRIDGE PROCEDURE
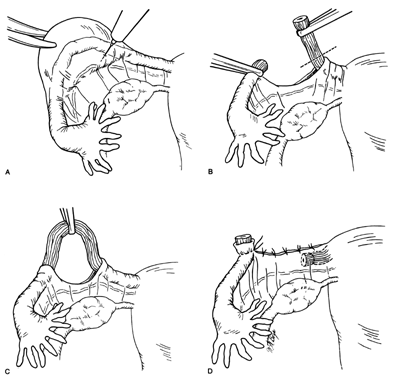
The Aldridge procedure is of interest because the fallopian tube remains intact (Fig. 7). The fimbrial end of the fallopian tube is drawn into a pocket beneath the peritoneum of the broad ligament. The buried fimbrial end is then secured in place by several sutures of nonabsorbable suture material. This circle of sutures incorporates the serosal and muscular layers of the tube in the peritoneum of the broad ligament. Following the introduction of this procedure, numerous failures were reported and the procedure has not been popular in recent years.
MECHANICAL OCCLUSIVE DEVICES AND ELECTROCOAGULATION
In addition to the above surgical procedures for tubal occlusion, both Silastic bands and occlusive clips may be employed by either a minilaparotomy or a colpotomy incision. Unipolar or bipolar electrosurgical instruments can also be used to disrupt the tubal lumen and provide for tubal sterilization. These mechanical occlusive devices and electrocoagulation techniques when used directly are applied by methods that are similar to the laparoscopic applications discussed in the chapter on laparoscopic sterilization. There appears to be little advantage to the use of these techniques at the time of either minilaparotomy or colpotomy. Such devices are preferentially best reserved for endoscopic application. Their use is detailed in the chapter on laparoscopic sterilization techniques.
SHORT-TERM AND LONG-TERM COMPLICATIONS
Immediate surgical complications of elective interval tubal sterilization procedures include hemorrhage, infection, or damage to nearby viscera. Incisional or vaginal bleeding is usually easily controlled with either pressure or additional sutures. The rare instance of intra-abdominal bleeding may necessitate a repeat laparotomy or laparoscopy. Superficial or deep infection may require antibiotic therapy. The use of prophylactic antibiotics for routine postpartum tubal ligation or laparoscopic tubal ligation is not indicated.15 However, if colpotomy is made to perform sterilization, antibiotics should be considered.
All tubal sterilization procedures presently employed are effective but pregnancies do occur. The failure rate was traditionally quoted as two to four pregnancies per 1000 operations, but the CREST data demonstrated a 5-year cumulative failure rate of 13 per 1000 women undergoing sterilization (all methods aggregated).4 In many reported series, luteal phase pregnancies have occurred, indicating that the patient was already pregnant when the procedure was performed. This can be minimized by scheduling the procedure during the proliferative phase of the menstrual cycle or by testing for pregnancy before surgery using a high-sensitivity hCG test. The literature indicates that sterilization procedures have a biologic failure rate. Studies have also indicated failures may be due to operator error, poor execution of surgical technique, and other reasons.13, 16 Intrauterine pregnancies tend to occur early, often during the first year or two following the sterilization procedure. Later pregnancies have a higher chance of presenting as tubal ectopic pregnancies. One report indicates that approximately 7% of total pregnancies following tubal sterilization are ectopic.17 The CREST study indicates a 10-year cumulative probability of ectopic pregnancy for all methods of tubal sterilization combined of 7.3 per 1000 procedures. Again, women who underwent tubal sterilization at a younger age were found to be at higher risk of subsequent ectopic pregnancy than older women.18 Careful follow-up is mandatory following female sterilization. Unexplained pelvic pain in a sterilized patient should suggest the possibility of ectopic pregnancy. Any woman who has undergone a tubal sterilization procedure should be counseled to seek immediate medical care if she experiences symptoms of pregnancy.
The debate over whether tubal sterilization procedures cause menstrual abnormalities also benefited from the CREST study. This study and many others have demonstrated that after tubal sterilization there does not appear to be any substantial change in menstrual cycles, duration of menstrual flow, and menstrual pain. In fact, there may be a decrease in these symptoms after tubal sterilization according to the CREST cohorts. This wealth of evidence from epidemiologic investigations in the published medical literature has not found any support for the idea of a "post-tubal ligation syndrome".3, 17, 19
Accordingly, the weight of the evidence at the present time indicates that tubal sterilization does not significantly increase the risk to the patient for the development of subsequent gynecologic abnormalities. In fact there appears to be a protective effect with regards to a reduced risk of ovarian cancer and need for hospitalization due to pelvic inflammatory disease.20 The odds ratios for women at high risk of ovarian cancer who have undergone tubal sterilization developing it is 0.3–0.9.8, 21 Though tubal sterilization does not protect against sexually transmitted infections, it is postulated that by occluding the lumen to transit of sperm and egg there is also occlusion to transit of ascending bacterial infections into the upper genital tract.20
REVERSIBILITY
All of the procedures described in this chapter are designed as irreversible procedures to be offered to patients seeking permanent sterilization. These procedures are not intended to be reversed. As stated previously in this chapter, it is extremely important that patients understand that tubal sterilization is intended to be permanent and if they are not sure of their decision, there are effective long-acting reversible contraceptive methods (such as implants and intrauterine contraceptives) that have failure rates as good or better than permanent surgical sterilization. Studies have demonstrated that rates of regret after tubal sterilization range from 0.9 to 26% and that these rates are highest in women who are younger at the time of the sterilization procedure.22 The CREST study indicated that there was a 14-year cumulative rate of 12.7% of all women expressing regret, but 20.3% in women under the age of 30 at the time of sterilization.23
Nevertheless, there are occasions when due to a change in personal life or social situation the patient may request reversal of a tubal sterilization procedure. In general, the potential for reversal is directly proportional to the amount of normal tube remaining. Thus, the amount of tube either removed or destroyed at the original procedure will, in large part, determine the success of a reversal operation, should one be attempted. This fact, at times, may be a consideration for the surgeon in the choice of a surgical procedure. The use of microsurgery for the reversal of tubal sterilization is presented elsewhere in this volume. The probability of successful pregnancy after tubal reversal is low and pregnancy can be a costly and risky undertaking.
Several techniques of female sterilization are summarized in Table 3, with regard to the degree of tubal destruction, the failure rate (pregnancy), and the potential for reversal.
Table 3. Techniques of female sterilization
| Technique | Popularity* | Tubal Destruction | Failure and/or Pregnancy Rate | Reversal Potential |
Uchida | 1+ | 50% | Rare | Very poor |
Fimbriectomy | 1–2+ | 40% | Poor | |
Irving | 1+ | 30% | Poor | |
Pomeroy | 5+ | 3–4 cm | 2–4:1000 women | Good |
Aldridge | Rarely done | None | Significant | Excellent |
*Arbitrary scale of 1 (least popular procedure) to 5 (most common procedure).
(Sciarra JJ: Survey of tubal sterilization procedures. In Sciarra JJ, Zatuchni GI, Speidel JJ [eds]: Reversal of Sterilization, p 129. Hagerstown, MD, Harper & Row, 1978)
REFERENCES
Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36. |
|
Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6. |
|
American College of O, Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 Pt 1):392-404. |
|
Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Am J Obstet Gynecol. 1996;174(4):1161-8; discussion 8-70. |
|
Hatcher RA, Trussell J, Nelson AL, Cates W. Contraceptive Technology Revised 20th Edition. New York, NY: Ardent Media Inc, Bridging the Gap Foundation; 2011. |
|
Peterson HB. Sterilization. Obstet Gynecol. 2008;111(1):189-203. |
|
Peterson HB, DeStefano F, Rubin GL, Greenspan JR, Lee NC, Ory HW. Deaths attributable to tubal sterilization in the United States, 1977 to 1981. Am J Obstet Gynecol. 1983;146(2):131-6. |
|
Pollack A. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 46, September 2003. (Replaces technical bulletin number 222, April 1996). Obstet Gynecol. 2003;102(3):647-58. |
|
Penfield AJ. Minilaparotomy for female sterilization. Obstet Gynecol. 1979;54(2):184-8. |
|
Lee RB, Boyd JA. Minilaparotomy under local anesthesia for outpatient sterilization: a preliminary report. Fertil Steril. 1980;33(2):129-34. |
|
Miesfeld RR, Giarratano RC, Moyers TG. Vaginal tubal ligation--is infection a significant risk? Am J Obstet Gynecol. 1980;137(2):183-8. |
|
EngenderHealth (Firm), Ross JA. Contraceptive sterilization : global issues and trends. New York, NY: EngenderHealth; 2002. xv, 204 p. p. |
|
Robinson DC, Stewart SK, Reitan RE, Gist RS, Jones GN. Laparoscopic pomeroy tubal ligation: a comparison with tubal cauterization in a teaching hospital. J Reprod Med. 2004;49(9):717-20. |
|
Surgical sterilization of women [Internet]. Wolters Kluwer Health. 2013 [cited January 26, 2014]. Available from: http://www.uptodate.com/contents/surgical-sterilization-of-women?source=search_result&search=surgical+sterilization&selectedTitle=1~150#H1. |
|
Bulletins--Gynecology ACoP. ACOG practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113(5):1180-9. |
|
Chi IC, Gardner SD, Laufe LE. The history of pregnancies that occur following female sterilization. Int J Gynaecol Obstet. 1979;17(3):265-7. |
|
Wolf GC, Thompson NJ. Female sterilization and subsequent ectopic pregnancy. Obstet Gynecol. 1980;55(1):17-9. |
|
Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of ectopic pregnancy after tubal sterilization. U.S. Collaborative Review of Sterilization Working Group. N Engl J Med. 1997;336(11):762-7. |
|
Peterson HB, Jeng G, Folger SG, Hillis SA, Marchbanks PA, Wilcox LS, et al. The risk of menstrual abnormalities after tubal sterilization. U.S. Collaborative Review of Sterilization Working Group. N Engl J Med. 2000;343(23):1681-7. |
|
Abbuhl SB, Muskin EB, Shofer FS. Pelvic inflammatory disease in patients with bilateral tubal ligation. Am J Emerg Med. 1997;15(3):271-4. |
|
Narod SA, Sun P, Ghadirian P, Lynch H, Isaacs C, Garber J, et al. Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet. 2001;357(9267):1467-70. |
|
Hillis SD, Marchbanks PA, Tylor LR, Peterson HB. Tubal sterilization and long-term risk of hysterectomy: findings from the United States collaborative review of sterilization. The U.S. Collaborative Review of Sterilization Working Group. Obstet Gynecol. 1997;89(4):609-14. |
|
Curtis KM, Mohllajee AP, Peterson HB. Regret following female sterilization at a young age: a systematic review. Contraception. 2006;73(2):205-10. |