Treatment of Malignancy of the Vulva
Authors
INTRODUCTION
Carcinoma of the vulva represents 3–5% of malignancies of the female genital tract.1 In developed countries it is the fourth most common gynecological malignancy after endometrial, ovarian, and cervical cancers. The incidence of invasive vulvar cancer is 1–2/100,000 and it is ten times higher for women older than 75 years.2 Ninety-five per cent of both preinvasive and invasive lesions of the vulva are squamous. Melanoma accounts for 2.4–5% of vulvar cancer and Bartholin gland carcinoma for 1–3%. Very rare tumors are represented by basal cell carcinoma, invasive Paget’s disease or intraepithelial Paget’s disease with underlying adenocarcinoma, sarcoma, and metastatic tumors of the vulva.3 Verrucous carcinoma is a low aggressive variant of squamous cell carcinoma with very low propensity for lymphatic spread.4 There is some consensus regarding the behavior and the staging, treatment, and prognosis of squamous cancers. The experience regarding the management of less common lesions such as Paget's disease, sarcomas, adenocarcinomas (including those of Bartholin's gland), and melanomas is limited.5
EPIDEMIOLOGY
Squamous cell carcinoma of the vulva affects postmenopausal women, occurring primarily in the fifth and sixth decades. However, Carter et al. have observed the occurrence of invasive vulvar cancer also in younger premenopausal patients,with a trend for multifocal lesions associated with vulvar intraepithelial neoplasia (VIN) in immunosuppressed patients.6 These lesions are associated with human papillomavirus (HPV) infections. Most epidermoid (squamous) carcinomas of the vulva are of low histologic grade and exhibit a predictable clinical course, with progressive local extension and then extension to the regional lymph nodes. A clear understanding of the prognostic factors and natural history of this disease allows for individualization of treatment. Earlier detection of the malignant lesions of the vulva is an important factor that makes individualization of treatment possible. Statistics indicate that about half of patients presenting with vulvar carcinoma are International Federation of Gynecology and Obstetrics (FIGO) stage I. Recently less radical interventions have been proposed. Improved preoperative and postoperative management of high-risk patients has resulted in improved survival.
VIN, including dysplasia and carcinoma in situ (CIS), is seen with increasing frequency in younger women. This reflects a changing pattern of contemporary sexual behavior with increased frequency of HPV infection of the lower reproductive tract. CIS is considered a precursor lesion, but the rate of progression to invasive carcinoma is only 4–6% with a median progression time of 5 years.7, 8 Although VIN frequently is present adjacent to invasive squamous carcinoma lesions, its presence or absence has no prognostic significance. Immunosuppression related to HIV infection or transplant medications is an important factor that has increased the incidence of vulvar dysplasia.
Multiple sexual partners and previous genital warts are strongly associated with the development of CIS. Smoking is an important risk factor. Patients who smoke and have a history of genital warts exhibit a 35-fold increase in the risk of CIS and subsequent invasive carcinoma.9 Both invasive and intraepithelial carcinoma of the vulva frequently are associated with prior, concurrent, or subsequent neoplasia of the vagina and cervix. Between 13% and 40% of patients with vulvar lesions have epithelial carcinomas elsewhere in the lower reproductive tract. Complete screening of the entire anogenital tract is indicated before final staging and treatment planning, and it must be included in the follow-up of the patient treated for carcinoma of the vulva. There is no evidence for the association with hypertension, diabetes, or obesity that had been considered in the past. The association between chronic vulvar inflammatory conditions and invasive carcinoma is unclear. Lichen sclerosus areas can be described in up to 30% of invasive squamous carcinoma specimens.10 It is not known if lichen sclerosus areas are precursor lesions.
Human papillomavirus type 16 (HPV-16) has been the strain of virus most frequently associated with intraepithelial and invasive carcinoma of the lower genital tract, including the vulva. HPV-16 DNA can be isolated in 70–80% of intraepithelial lesions and in 10–50% of invasive lesions.11, 12 The differences in HPV DNA detection rates may be related to decreased retrieval of the virus in the vulvar lesions.
Vulvar intraepithelial neoplasia
As already noted, there has been a substantial increase in the prevalence of VIN in young patients. VIN usually is multifocal, and the most common sites are the hairless skin of interlabial folds, the posterior fourchette, and the perineum. Lesions may be macular or papular with a keratotic rough surface. White lesions are caused by hyperkeratosis, whereas red ones represent hypervascularity. Gray or brown lesions are produced by melanocytic overactivity and pigment incontinence. Vulvoscopy and punch biopsies are the most important tools in the diagnostic approach. The use of a colposcope is of benefit in the evaluation of these lesions, but grading of the lesion is limited because of the keratin layer. Unlike the cervix and vagina, the histologic abnormality may extend beyond the area of a lesion visible with colposcopy.
A more conservative approach for the treatment of VIN has been followed recently. Wide local excision, removing a full thickness of skin encompassing epidermis and dermis with a 2-cm lateral margin, is sufficient treatment.13 The histologic abnormality may extend beyond the clinically or colposcopically apparent lesion. Frozen-section evaluation of the margins has been advocated by some authors to trim an additional margin and achieve complete excision. Nevertheless, a more conservative approach can be followed for microscopically positive margins waiting for the development of clinical lesions before proceeding with re-excision procedures.14 The use of CO2 laser ablation is an effective nonmutilating alternative that is particularly useful for multifocal lesions. Proper application minimizes thermal damage to the underlying normal tissues. This property makes the CO2 laser an excellent tool for management of intraepithelial neoplasia of the anogenital epithelium. Super-pulse technology allows high-intensity output and short duration of the laser beam to dissect through the layers of the epithelium with extreme precision and ensures complete removal of the atypical epithelium. Use of the CO2 laser results in minimal scarring, excellent function preservation (anal sphincter), and low recurrence rates. The only disadvantage for the use of the CO2 laser is the loss of tissue for pathologic examination. Proper evaluation with biopsies to rule out the presence of invasive carcinoma before therapy is essential. CO2 laser ablation is an excellent alternative to the more traditional superficial or “skinning” vulvectomy.
INVASIVE CARCINOMA OF THE VULVA: STAGING AND PATTERN OF SPREAD
Planning of appropriate therapy for invasive carcinoma begins with clinical evaluation of the lesions and an assessment of the patient to determine her risk as an operative candidate. Vulvar carcinoma has a propensity to remain locally confined. Metastases occur by way of the lymphatics to the groin, a site amenable to clinical evaluation and successful treatment by inguinal lymphadenectomy.
Staging
Clinical criteria for staging of carcinoma of the vulva were formulated and adopted in 1970 by the FIGO. A modified surgical staging system was adopted in 1989 and revised in 1995 (Table 1).15 The definition of microinvasive carcinoma follows the recommendations of the International Society for the Study of Vulvar Disease and the International Society of Gynecologic Pathologists.16 The staging emphasizes definition of the primary tumor by size and location, including the involvement of structures contiguous with the vulva. Status of the nodes is based on surgical evaluation of the groin. The presence or absence of distant metastasis also is considered, including cystoscopic or proctoscopic evaluation. The survival results for stages I and II are similar and excellent, approximately 90% when no positive groin nodes are present.17 Stage III lesions have a 50% 5-year survival rate, although successful treatment of 60–70% of patients has been reported.
Table 1. FIGO staging of vulvar carcinoma (1995)
| Stage | Clinical Findings |
| 0 | Carcinoma in situ |
| I | Confined to vulva or perineum, <2 cm in greatest dimension; no nodal metastasis |
| IA | Stromal invasion <1.0 mm |
| IB | Stromal invasion >1.0 mm |
| II | Confined to vulva or perineum, >2 cm; nodes are negative |
| III | Any size lesion with spread to urethra, vagina, or anus or with unilateral regional lymph node metastasis |
| IVA | Invasion of upper urethra, bladder mucosa, rectal mucosa, pelvic bone, or bilateral regional node metastasis |
| IVB | Any distant metastasis, includes pelvic lymph nodes |
FIGO, International Federation of Gynecology and Obstetrics.
Pattern of spread
Squamous carcinoma of the vulva usually proceeds in a predictable fashion through its regional lymphatics, first to the inguinofemoral nodes and only then to pelvic and distant sites. Therefore, the superficial inguinal lymph nodes are the most common site for detecting metastasis. Metastasis to the contralateral nodes in the absence of involvement of the ipsilateral nodes is rare.18
Midline lesions involving the clitoris may have spread to bilateral inguinal nodes. Direct metastasis of invasive squamous carcinoma of the vulva to the pelvic lymph nodes is rare, occurring with a frequency of 5% of all the cases, 15–20% of the patients with positive groin nodes and nearly 0% of those with negative groin nodes.19 Appropriate groin dissection is the most important factor in decreasing mortality for early vulvar cancer.20 Patients who develop groin lymph nodal recurrence have a 90% mortality.21 When lymphadenectomy is performed, this should always include superficial and even deep lymph nodes and should be bilateral in presence of central location of the primary lesions or lymph node involvement. The modification of the groin dissection increases groin recurrence and mortality.22
INVASIVE CARCINOMA OF THE VULVA: SURGERY
Treatment failures primarily result from failure to ensure adequate margins during excision of the primary lesion or from recurrence within the lymphadenectomy site. The latter should not occur with proper surgical technique for removal of microscopic clinically occult metastases. It is, however, a more frequent problem when extensive metastases associated with clinically suspicious or positive nodes are present before surgery. In addition to an increased frequency of local (incisional) recurrence, positive groin nodes also are associated with an increased frequency of pelvic lymph node metastasis and progression of disease to distant sites.
Size and location of the lesion are the primary parameters in deciding what procedure to perform. Early stage lesions are treated surgically. Advanced stage lesions are treated preferentially with chemoradiation. Pelvic exenteration with radical vulvectomy and inguinal lymph node dissection is a surgical option with significant physical and psychological morbidity as well as mortality. This procedure is rarely indicated. Treatment recommendations are summarized in Table 2. Microinvasive tumors (less than 1 mm of invasion, stage IA) may be treated by wide local excision with low risk of groin lymph node or vulvar recurrence.23, 24 A 1-cm lateral margin is recommended. Lesions less than 2 cm in diameter (stage IB) may be treated successfully by wide radical excision. A 2-cm lateral margin is obtained, and dissection is carried down to the fascia of the urogenital diaphragm for the deep margin.25 When the invasion of the lesion is greater than 1 mm, ipsilateral groin lymph node dissection is recommended. This approach of radical local excision and ipsilateral node dissection works best for lesions that are lateral or posterior. Stage II lesions (greater than 2 cm in diameter) are treated by radical vulvectomy with inguinal-femoral lymph node dissection. Certain stage III lesions with extension to the lower urethra or proximal vagina may be treated the same way.
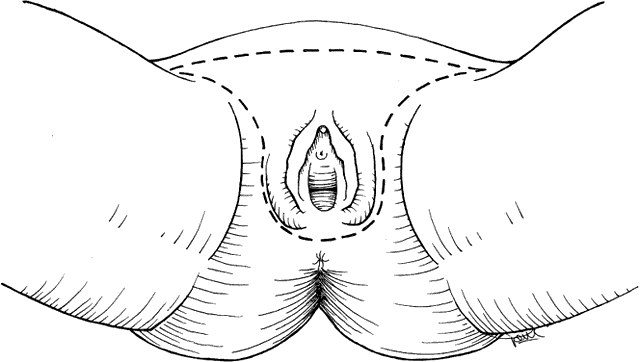
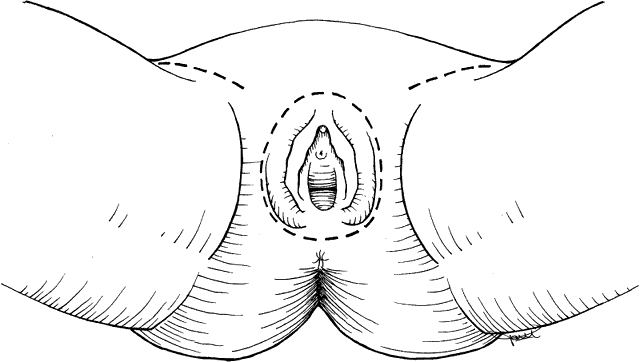
The surgical radical treatment required a triangular incision that excised the entire vulva en bloc down to the urogenital diaphragm and extended laterally to include the groin lymph nodes (Fig. 1). This traditional resection, named “butterfly” incision originally described by Taussig25 and Way,26 has recently been replaced by the “triple incision”, a more conservative technique that consists of separation of the treatment of the primary lesion from the dissection of the regional lymph nodes27 (Fig. 2). This results in a decreased rate of wound separation and infection. The cosmetic results also are satisfactory for the patient. An important issue is to consider the amount of free resection margins to obtain during the excision of primary lesion: some recent studies have demonstrated that microscopic resection margins <0.8 cm translate to an acceptable local control.28 Moreover, concerning vulvar operations, in case of substantial loss of tissue, the recent improvement in reconstructive surgery techniques has permitted a faster recovery and a better psychosexual impact on these patients.
In some selected cases of advanced and fixed disease, pelvic exenteration (anterior, posterior or total) could be proposed, even if the morbidity resulting from these procedures is high and the quality of life could be compromised.
Among early complications after radical vulvectomy wound separation is the most frequent and it is observed in up to 14% of patients and seromas of the femoral triangle are observed in 10–15% of patients.29 Other medical complications are significant and occur in up to 15% of patients. These include deep vein thrombosis, pulmonary embolism, and myocardial infarction. Urinary tract infections also are common. Late complications are less common: significant lower extremity lymphedema or recurrent cellulitis are related to inguinal lymphadenectomy (incidence 30–70%).30 Urinary incontinence and pelvic prolapse have been reported in 15–20% of women and may require further reconstructive surgery. Osteitis pubis is rare but results in significant morbidity for the patient. Associated morbidities include sexual dysfunction with dyspareunia from stenosis of the vagina (75%). Poor self-esteem should be explored because the patient is not likely to express her feelings in this area. A sense of mutilation is common. Operative mortality can be 2%.31
Table 2. Approach to the treatment of vulvar cancer
Stage | Treatment |
0 | Wide local excision |
IA | Wide local excision |
IB | Wide radical excision Ipsilateral inguinal lymphadenectomy Bilateral if midline or clitoris lesion |
II | Radical vulvectomy with bilateral inguinal Lymphadenectomy |
III | Lesions involving outside vagina or lower urethra: radical vulvectomy with bilateral inguinal lymph node dissection Lesions involving anus or rectovaginal septum: chemoradiation ± surgery |
IV | Chemoradiation ± surgery |
One of the most important recent developments in the treatment of vulvar carcinoma has been the individualization of treatment. Less radical surgery for early stage disease does not compromise survival. No lymphadenectomy is needed for patients with microinvasive tumors (stage IA). For unilateral lesions less than 2 cm in diameter, contralateral lymphadenectomy is not required unless lymph node metastasis is found in the ipsilateral side at the time of surgery. Performing separate incisions for inguinal lymph node dissection results in better wound healing with a reduced rate of infection.28
Careful evaluation of both the clinical and the pathologic status of the inguinal nodes is important. In the absence of inguinal node metastases, most patients with lesions smaller than 2 cm should be cured, particularly if their primary lesion is excised with wide margins negative for disease. A close surgical margin is a powerful predictor of local recurrence. Deep or lateral margins greater than 8 mm are associated with no recurrence in the study of Heaps and colleagues.28 The risk of regional lymph node metastasis is related to the primary lesion, depending on lymphatic and vascular invasion, clinical stage (tumor size), and depth of stromal invasion.32, 33 When the stromal invasion exceeds 2 mm, the risk of node metastasis has been reported to be greater than 20%, whereas the risk is 40% when the thickness or invasion exceeds 4 mm.34 Although it is possible to anatomically and experimentally demonstrate the presence of pathways of direct lymphatic communication from the vulva to the pelvis, particularly in the area of the clitoris, such direct pelvic metastases have not been of clinical significance, occurring in squamous carcinoma in only up to 3% of cases.29 Literature describes two surgical techniques for groin dissection in patients affected by vulvar cancer. The fundamental difference between superficial inguinal lymphadenectomy and inguinal femoral lymphadenectomy is that inguinal nodes are the primary drainage site.35 In this case, the cribriform fascia is left intact and none of the nodes below this ligament are removed. However, aberrant lymphatic drainage to contralateral or ipsilateral deep nodes or pelvic nodes may bypass the superficial sentinel group and explain the unexpected recurrences. The Gynecologic Oncology Group’s (GOG) experience with hemivulvectomy and ipsilateral superficial inguinal dissection identified a 6% incidence of ipsilateral groin failure in patients with prior negative dissection22 which is comparable to the 4% incidence identified in 1994 by Burke.36 Therefore superficial inguinal node dissection is associated with an unacceptably high prevalence of groin recurrence, even if the excised superficial nodes were negative. Groin recurrence carries an extremely high mortality rate (92%).22
Clinical evaluation of the status of inguinal nodes has been subject to widespread misunderstanding and criticism because of variability in correlation between clinical impression and histologic status of the lymph nodes. In nodes that are not clinically suspicious, the frequency of metastasis varies from 12% to 43%. This wide range is not simply a reflection of variable clinical acumen, but also a statistical function. First, there is a significant variability in the frequency of metastatic inguinal lymphadenopathy reported within any series, varying from 37% to 68%. This is an uncontrollable variable reflecting the character of the population being evaluated. From review of large reported series, between 68% and 87% of the patients with clinically positive nodes will have metastatic cancer. Conversely, two thirds to three quarters of patients who have positive nodes will have palpable suspicious or clinically positive nodes before surgery. This implies that 25–30% of the lymph node metastases are present in unsuspicious lymph nodes. During lymphadenectomy of clinically suspicious nodes, only the enlarged nodes are removed. If frozen section confirms the presence of carcinoma, it is not necessary to proceed with full dissection of the femoral triangle. These patients require postoperative radiation. The least possible disruption of the femoral triangle results in decreased radiation complications, particularly lymphedema and wound breakdown. In a reported series of 40 patients with inguinal node metastasis, 20% had thigh or groin recurrence.37 Such recurrence occurred only in patients who had clinically suspicious or positive groin nodes before surgery. No patient with microscopic node disease, that is, metastases in nonpalpable or unsuspicious nodes, experienced recurrence in the groin.
Intraoperative lymphatic mapping and sentinel node identification has the potential to improve detection of lymph node metastasis and decrease morbidity by minimizing radical lymph node dissections. The technique of sentinel node biopsy was originally developed for selecting patients with melanoma for regional node dissection. The major advantage of using the sentinel node in early vulvar cancer is to decrease the associated morbidity of complete groin dissection. Vital blue dye or a radioactive tracer is injected close to the tumor. The sentinel node can be identified by lymphoscintigraphy in the case of the radioactive tracer. Use of 99mTc-labeled sulfur colloid is technically feasible and may be sensitive for detecting subclinical metastasis in regional lymphatics.38 The blue dye is identified during dissection in surgery. The predictive value of intradermal injection of patent blue dye has been found to be low.38 In a review of the literature Makar et al.39 reported the successful identification of the sentinel node in 82.5% of patients using the blue dye technique and 100% using lymphoscintigraphy. These data need a randomized controlled trial, because false-negative sentinel nodes have been reported.40 Although pelvic lymphadenectomy formerly was emphasized as an essential component of “adequate” treatment of invasive carcinoma of the vulva, this is no longer the case. First, it has been documented that direct metastasis of squamous carcinoma of the vulva to the pelvic lymph nodes bypassing the inguinal lymphatics rarely occurs.41 Second, the clinical status of the inguinal nodes does reflect the probability of pelvic node metastasis. Pelvic node involvement is not seen in the absence of ipsilateral groin metastasis. Clinically positive groin nodes are associated with pelvic node metastases in 33% of cases. Based on collected data, 19% of patients with microscopic groin metastasis also have pelvic node metastasis. This number increases with three or more microscopically positive nodes.39, 40, 41, 42, 43 The overall risk of pelvic node metastases is between 8.5% and 16%.44 Basically, only the patient with clinically apparent metastases in the groin nodes or in Cloquet's node is at risk for pelvic metastases. The other indication for pelvic lymphadenectomy is the presence of enlarged nodes in computed tomography scan of the pelvis. An extraperitoneal approach should be used. The absolute survival rate after positive pelvic lymphadenectomy has been reported to vary from 12.5% to 25%. Patients with positive groin lymph nodes currently are treated with postoperative radiation, which includes the pelvic nodes in the radiation field.
INVASIVE CARCINOMA OF THE VULVA: RADIATION THERAPY
Although the effective use of radiation therapy for the treatment of vulvar carcinoma has been demonstrated in prospective and retrospective studies, the use of elective groin irradiation in patients with vulvar cancer is controversial. Locoregional control, survival rates, and complications may be improved by the use of radiation in advanced vulvar cancer. In particular, the need for exenterative surgery can be reduced with the utilization of preoperative radiation.
A prospective randomized study by Homesley et al., in 1986, examines the use of radiation after radical vulvectomy with inguinal and pelvic lymphadenectomy in patients who had positive groin nodes.37 For patients with clinically positive nodes, the 2-year survival rates were 59% for the group that received radiation and 31% for the group that had lymphadenectomy only. The patients with clinically positive nodes or multiple microscopically positive nodes benefited the most. Recurrence occurred in 5.1% in the radiation group versus 23.6% in patients treated with surgery alone. This study also evaluated prophylactic use of groin irradiation in preventing recurrences after surgery. For clinically negative nodes, there seems to be no significant difference in groin recurrence rates between radical lymphadenectomy and groin radiation.41
Several subsequent studies45, 46, 47 have clarified the prognostic significance of positive groin nodes and FIGO and International Gynecological Cancer Society (IGCS) recommended the guidelines of postoperative irradiation (Table 3).48
Table 3. Guidelines for postoperative irradiation
| No radiotherapy | Patients with one or two micrometastases (<5 mm)
|
| Radiotherapy | One macrometastases (>5 mm) Extracapsular spread of the groin lymph node Two or more micrometastases (<5 mm) |
In 2002, Cochrane Gynaecological Cancer Group compared surgery versus radiotherapy in vulvar carcinoma49 but none of the trials satisfied the criteria for the Cochrane reviews. Therefore the Cochrane had to be modified to allow any comment about the roles of surgery and radiotherapy. However, because of the rarity of the vulvar cancer, only one randomized trial, one case–control, and one observational study have been carried out that satisfied these modified criteria.
Manavi proposed 65 groins treated with radiotherapy in patients affected by T1 vulvar cancer with clinically uninvolved nodes. The relapse rate after radiotherapy was 4.6% (3 patients).50 Perez in a observational study51 showed a relapse rate of 10.5% in patients affected by vulvar cancer T1 or T2. The randomized controlled trial of Stehman et al.52 compared surgery versus radiotherapy in patients T1, T2, T3 with clinically involved nodes. After irradiation five recurrences occurred in 27 groins (18.5%) but no relapses occurred in 25 groins after surgery. Unfortunately this trial was stopped because of the radiotherapy doses, dose distribution, and techniques applied.
In 2003, Katz evaluated 227 vulvar cancer patients (stage I–IV) and showed that the groin recurrence was comparable after inguinofemoral lymphadenectomy alone, radiotherapy alone or a combination.53 The authors concluded that elective radiotherapy on the groins prevents most groin recurrences in patients with minimal or microscopically inguinofemoral disease. However, if compared with lymphadenectomy, radiotherapy alone resulted in a higher groin recurrence rate (16%). Therefore, radiotherapy is highly effective in the treatment of vulvar cancer but the use of irradiation as elective therapy in clinically negative nodes or in regional recurrences is not yet better defined.
Extrapolation from the results of chemoradiation in carcinoma of the anus has led investigators to explore the use of cisplatin, mitomycin C, and 5-fluorouracil (5-FU) as radiosensitizers.54, 55 Small retrospective series suggest that response rates to radiation are improved by the addition of concurrent chemotherapy. Nevertheless, the role of radiation and chemoradiation in the treatment of advanced vulvar cancer is not well defined. Patients with ulcerated or fixed groin nodes can be treated with primary radiotherapy with or without concurrent chemotherapy. In some cases, the nodes can be resected after irradiation. In a Cochrane Review by Ansink et al., data showed the efficacy of preoperative chemoradiation for advanced vulvar cancer and a high resectability rate (96%).56
INVASIVE CARCINOMA OF THE VULVA: CHEMOTHERAPY
There is no evidence that chemotherapy alone has a curative role in vulvar cancer. The possible role of chemotherapy in combination regimens is still to be defined.
Neoadjuvant chemotherapy
Based on encouraging results of neoadjuvant chemotherapy followed by radical surgery in cervical cancer a pilot study on vulvar cancer was conducted at the end of the 1980s.57 This pilot study was carried out on 21 patients with advanced squamous cell carcinoma (FIGO stage IVA, TNM stages T2N2M0, T3N2M0, T4N2M0). Patients underwent two to three cycles of cisplatin, bleomycin, and methotrexate. The tumor response rate was poor, no patients achieved complete response and less than 10% achieved partial response. Inguinal lymph node clinical response was higher (66%) and significantly related to the primary tumor responsiveness. Surgery was feasible in 19 patients, but no evidence of significant distal disease control was obtained. Overall 3-year survival was only 24%. Similarly to cervical cancer, response to chemotherapy was a prognostic factor. The only apparent benefit that these patients achieved from this treatment was a relief from the symptoms in most cases. The Gynaecological Cancer Cooperative Group (GCCG) reported a European Organization for Research and Treatment of Cancer (EORTC) trial that included 18 previously untreated patients and ten patients with recurrent disease. The response rate in primary tumors was 67% (14/20) and in patients with recurrent disease was 60% (6/10). All patients were initially judged not susceptible to surgical treatment. After chemotherapy with bleomycin, methotrexate, and lomustine, eight (29%) patients were found to have resectable disease. Similar response rates have been recently reported by the same group using a modified regimen with a reduced dose of methotrexate.55
Neoadjuvant chemoradiotherapy
This strategy should be considered only in patients with T3 and T4 vulvar carcinomas grossly involving the urethra and/or the anus or those who have fixed inguinal metastasis. In these patients, the attempt is to reduce the aggressiveness of surgery and therefore reduce complications and increase quality of life. Drugs that have been most frequently associated with radiotherapy were 5-FU, cisplatin, mitomycin, and bleomycin.56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67 The association between radiotherapy and bleomycin was disappointing.67, 68 In a study conducted on 15 inoperable patients only two became susceptible to surgery and only one patient survived more than 4 years without signs of recurrence. In another study which included 20 patients with primary disease, the clinical response rate was high but only one patient survived without signs of disease for more than 60 months.68 The association of radiotherapy with 5-FU and cisplatin or mitomycin has been more encouraging. Different studies have demonstrated the efficacy of these associations.57, 58, 61, 69 Worthy of notice for its size, is the multi-institutional GOG trial conducted by Moore on 71 evaluable patients with stage III and IV disease.69 After chemoradiotherapy with cisplatin and 5-FU, 46% of the patients had no visible disease, only 3% had unresectable disease, and in only 4% of the patients was urinary and/or gastrointestinal continence not preserved. Neoadjuvant chemoradiotherapy also appear to be effective in the treatment of lymph node metastasis. Complete pathological regression has been demonstrated in up to 55% of patients after a randomized controlled trial.58
As expected, local and systemic side effects are common. In most studies, after 3–5 weeks randomized controlled trials have to be interrupted for about 1 week. Wound complications following secondary surgery after initial randomized controlled trial are common.65 Therefore radiotherapy or chemoradiotherapy treatment without subsequent radical surgery is often preferred.
Chemoradiotherapy as single treatment
It has been proposed by different authors that patients who undergo primary chemoradiotherapy and achieve a complete response may not require surgery. Even in patients with FIGO stage III and IV disease chemoradiotherapy can lead to a complete response in a good proportion of patients. Berek et al. achieved clinical complete response in more than two thirds of these patients.67 A study was conducted on 33 patients of which nine did not receive any previous treatment.65 These patients underwent radiation therapy with concurrent infusional 5-FU with or without mitomycin C. Of these nine patients, six achieved a complete clinical response but half of them relapsed with a median follow-up of 16 months. Russell et al.66 reported the results of 18 patients affected by primary vulvar carcinoma treated with 5-FU with or without cisplatin or mitomycin C with concurrent radiotherapy. Clinical complete response was obtained in 16 patients and at the time of the report 12 patients had no evidence of disease (NED) with a median follow up of 24 months (2–52 months). Promising results have also been obtained by Wahlen et al.65 on 19 patients with FIGO stage II and III disease. Overall, clinically complete responses were obtained in ten (53%) patients. Of these ten patients only one experienced recurrent disease both locally and distantly. The other nine patients remained NED with a median follow up of 18.5 months (range 3–56 months). More recently Cunningham et al. reported a study on 14 patients in whom the location and the extent of the disease made pelvic exenteration the only surgical option.68 The regimen adopted was two cycles of chemotherapy with cisplatin (50 mg/mq) and 5-FU (1000 mg/mq/24 x 96 h) in addition to radiation therapy. Total radiation doses to the vulva and groins ranged from 50 to 65 Gy, with pelvic doses of 45–50 Gy. In this study nine (64%) patients obtained a complete clinical response and did not undergo surgery. Of these only one patient relapsed with follow-up of 7–81 months, mean 36.5. Considering these satisfactory results, this type of treatment is the most commonly used for unresectable advanced disease.
Adjuvant chemoradiotherapy
Postoperative radiotherapy has been considered for many years as the standard adjuvant treatment after surgery in patients with unfavorable prognostic factors. Postoperative combined chemoradiotherapy has been recently used in view of the excellent results obtained in patients with cervical cancer. Han et al. reported the results of six patients with vulvar cancer FIGO stage III and IV who underwent adjuvant chemoradiotherapy.55 This group of patients was compared to patients undergoing adjuvant radiotherapy. The comparison between these two groups appeared in favor of the first even if the difference was not statistically significant due to the limited number of patients.
A recent study from Bellati et al.70 reported the results of a prospective observational study on postoperative chemotherapy (cisplatin) in 14 patients treated by radical surgery with multiple lymph node metastases for advanced primary squamous cell carcinoma of the vulva. The overall survival was 86% and the disease-free survival was 71%. Only four (29%) patients suffered a recurrence and only two (14%) died of disease. Treatment was well tolerated. In no case was treatment suspension necessary, all patients who commenced treatment were assessable for toxicity. Only two patients suffered from grade 4 neutropenia during chemotherapy. Three patients suffered from long-term severe lymphedema. Disease recurred in four patients and three of these patients were subjected to surgery with no severe postoperative complications.
Therefore in patients affected by vulvar cancer with multiple lymph node metastases, radical surgery followed by adjuvant chemotherapy is a feasible strategy, with an acceptable short- and long-term complication rate. Results in terms of overall survival and disease-free survival are promising. Further studies are needed to determine the optimal combined modality treatment.
INVASIVE CARCINOMA OF THE VULVA: RECURRENCE OF DISEASE
The number of positive nodes is the most important predictor for recurrence. Patients with one microscopically positive node have an excellent prognosis, with an overall survival rate of 90%. The presence of three or more positive nodes carries a poor prognosis, with a 5-year survival rate of 50%, even after radiation to the groin and pelvis.39, 71 Recurrence localized to the vulva may be treated by local resection. Local resection may achieve disease control for an extended period or may be only palliative. Lesions limited to the vulva that can be resected with a negative margin can result in recurrence-free survival in up to 75% of cases.72 Radiation is another alternative to treat these recurrences. Central recurrence to the vagina with no evidence of inguinal node involvement may be treated by exenteration. Groin recurrence may be treated with radiation if the patient did not get radiation after the primary surgery. Groin recurrence is not associated with long-term survivors. Both local and systemic dissemination of disease occasionally have been treated successfully with chemotherapy.
CONCLUSIONS
One of the most important recent developments in the treatment of vulvar carcinoma has been the individualization of treatment.
Surgery remains the gold standard in patients with primary vulvar cancer.
Less radical surgery for early stage disease does not compromise survival. No lymphadenectomy is needed for patients with microinvasive tumors (stage IA). For unilateral lesions less than 2 cm in diameter, contralateral lymphadenectomy is not required unless lymph node metastasis is found in the ipsilateral side at the time of surgery. Performing separate incisions for inguinal lymph node dissection results in better wound healing with a reduced rate of infection.28
In recent years, physicians have understood the fundamental importance of quality of life, and have tried to reduce permanent morbidity by tailoring surgery and attempting alternative treatments. Chemotherapy alone has not yet acquired sufficient evidence to be adopted outside clinical trials. More promising appears to be the role of concomitant chemoradiation therapy in unresectable tumors. It is our opinion that in the future the trend will be to adopt more frequently such a procedure in a neoadjuvant setting in order to reduce surgical aggressiveness and therefore reduce postoperative morbility and increase the quality of life of these patients. The role of chemotherapy alone will probably remain confined to palliative treatment of metastatic and recurrent tumors.
OTHER MALIGNANCIES OF THE VULVA
Although squamous cell carcinoma of the vulva is an infrequent entity, it represents the majority of malignancies of the vulva (95%). The remaining 5% of cases include metastatic and rare primary lesions, which may arise from the accessory structures or specialized cells of the anogenital epithelium. Metastatic lesions are rare and represent hematogenous emboli or retrograde lymphatic flow. Frequent primary sites are the cervix, endometrium, and rectum, followed by the urethra, ovary, and breast. Such metastases always reflect the adverse prognosis of the disseminated primary lesion; treatment is primarily palliative and is coordinated with treatment of the primary malignancy.
Carcinoma of Bartholin's gland
The diagnosis of primary malignancy of Bartholin's gland can be made only when there is an area of apparent transition from normal Bartholin structures to a neoplastic focus or a tumor that occupies the area of Bartholin's gland with no evidence of a primary tumor arising elsewhere.
The presenting symptoms of the malignancy usually are nonspecific. The incidence increases after the age of 40 (median age, 50 years). Conservative therapy may have been instituted for a lesion thought to be cystic or inflammatory. The diagnosis usually is made when the lesion fails to respond to conservative therapy, including possible incision and drainage or marsupialization. Further delay may follow these procedures because of complications like poor healing, infection, or induration with edema. Such delay in diagnosis and institution of therapy may explain the adverse prognosis associated with these lesions. The emphasis of management of the primary lesion must be on complete excision. Therefore, patients older than 40 years of age presenting with a Bartholin's gland abscess or mass (even if cystic) should have an excision procedure. Radical local excision, hemivulvectomy, or radical vulvectomy seem to be appropriate procedures, depending on the size of the primary lesion.72 The incidence of groin node metastasis is about 30%, so lymphadenectomy should be performed. Radiation therapy may have some role in improving local control for patients with large lesions.
Verrucous carcinoma
Another infrequent malignancy occurring throughout the anogenital tract, possibly involving vulva, vagina, or cervix, is verrucous carcinoma. Verrucous carcinoma is a distinct variant of squamous cell carcinoma that occurs in wet mucosae. It is most commonly found in the oral cavity. This carcinoma also develops in anogenital mucosae with similar behavior. An association with HPV-6 has been reported. The tumor is locally aggressive and extends into the underlying tissues. Typically, the tumor is extensive with a “cauliflower-like” appearance. Metastasis to lymph nodes is rare. Radiation is contraindicated in the treatment of verrucous carcinoma. The tumor is reported to be resistant to radiation therapy and may become more aggressive and invasive after radiation therapy because of anaplastic transformation. Aggressive primary surgery to ensure adequate excision is curative.73 Radical local resection is adequate treatment because the tumor is locally destructive. Enlarged groin lymph nodes should be evaluated preoperatively by fine-needle aspiration because they usually are inflammatory.
Malignant melanoma of the vulva
The anogenital tract, particularly the vulva, is a preferred site for melanoma. These lesions represent only 0.5–5.0% of malignancy of the female genital organs. Melanoma is the second most common malignancy of the vulva. The disease presents predominantly in white postmenopausal patients. The most common location of the lesions is the clitoris and labia minora. Most vulvar melanomas arise de novo or from junctional nevi. The behavior of vulvar melanomas is similar to that of melanomas found elsewhere on the body. In contrast to the relatively predictable behavior of squamous carcinomas of the vulva, which spread in a stepwise fashion to regional lymph nodes, melanomas may present with early distant metastasis. Melanomas spread through the regional lymphatics, but they also penetrate the surrounding dermal lymphatics. This explains their greater propensity to disseminate elsewhere in the body at an early stage of disease by both hematogenous and lymphatic pathways. Early lesions with less than 1 mm of invasion have an excellent 5-year prognosis with survival rate greater than 90%. All other lesions have dismal outcomes, with 5-year survival rates between 21% and 54%.74 Depth of invasion is the most important prognostic factor. Three classification systems for depth of invasion exist (Table 4). Chung and coworkers modified Clark level definitions to account for the variation in the histologic features of the subepithelial tissue of the clitoris and labia majora and labia minora.75 They concluded that it was possible to demonstrate a prognostic value in such a classification, with a clear-cut necessity for regional lymphadenectomy for lesions with invasion for stage III or greater. The classic recommendation has been for radical vulvectomy. Recent studies indicate that a more conservative approach does not compromise survival. Lesions with less than 1 mm of invasion may be treated by radical local excision.76 For lesions with more than 1 mm of invasion, radical vulvectomy with bilateral inguinofemoral lymph node dissection is advocated. Recommendations for selection of high- and low-risk patients have been formulated with proposals for less radical surgery.77 As with the treatment of melanoma elsewhere on the body, the necessity for adjunctive therapy with chemotherapy or immunotherapy to complement surgery is critical, but it has limited success.
Table 4. Staging systems for vulvar melanomas
| Stage | Clark | Chung | Breslow |
| I | Intraepithelial | Intraepithelial | <0.76 mm |
| II | Into papillary dermis | 1 mm granular layer | 0.76–1.5 mm |
| III | Filling dermal papillae | 1.1–2 mm granular layer | 1.51–2.25 mm |
| IV | Into reticular dermis | >2 mm from granular | 2.26–3.0 mm |
| V | Into subcutaneous fat | Into subcutaneous fat | >3 mm |
Paget's disease of anogenital origin
Paget's disease occurs infrequently in the anogenital area, but when present, it is associated with distressing symptoms of itching. The origin of the lesion continues to be unclear. Paget's disease is an intraepithelial lesion with occasional in situ involvement of the skin appendages. Paget's disease is associated with synchronous and metachronous adenocarcinomas. The lesions are associated with an underlying adenocarcinoma component in 15% of cases.78 These adenocarcinomas arise from the skin appendages. Also, about 30% of patients have or develop adenocarcinomas of nonvulvar locations.79 The most common locations are breast, colon, and rectum. Screening for these cancers is important for patients with Paget's disease.
Metastasis to regional lymphatics is rare. Only lesions associated with invasive underlying adenocarcinomas may present with metastasis to inguinal lymph nodes, and the prognosis is poor. Paget's disease may be extensive. Microscopic margins commonly extend well beyond the clinical lesion. This presents a problem for adequate excision of the primary lesion. Wide local excision is adequate treatment.80 The excised primary lesion can be examined intraoperatively to determine whether there is invasion into the underlying tissue or an associated adenocarcinoma of an accessory gland. If underlying invasion is suspected, a radical local excision should be performed with deep margins extending to the urogenital diaphragm fascia. Some authors advocate review of the margins by frozen section at the time of surgery.81 Nevertheless, this approach may not avoid recurrence. Repeat local excision of a recurrence usually is effective treatment. There is a greater propensity for the malignancy to metastasize if an invasive adenocarcinoma is present. Inguinal lymphadenectomy can be performed later once such diagnosis has been established.
REFERENCES
Ansink AC, Heintz AP: Epidemiology and etiology of squamous cell carcinoma of the vulva. Eur J Obstet Gynecol Reprod Biol 48: 111–5, 1993 |
|
Landis S, Murray T, Bolden S, Wingo P: Cancer statistics, 1998. CA Cancer J Clin 48: 6–29, 1998 |
|
Beller U, Quinn MA, Benedet JL et al: Carcinoma of the vulva. FIGO 6th Annual Report on the results of treatment in Gynecological Cancer. Int J Gynaecol Obstet 95 Suppl 1: S7–27, 2006 |
|
Japaze H, Van Dinh T, Woodruf JD: Verrucous carcinoma of the vulva: study of 24 cases. Obstet Gynaecol 1982; 60: 462–6 |
|
Coulter J, Gleeson N: Local and regional recurrence of vulvar cancer: management dilemmas Best Pract Res Clin Obstet Gynaecol 17: 663–81, 2003 |
|
Carter J, Carlson J, Fowler, J et al: Invasive vulvar tumors in young women: A disease of the immunosuppressed? Gynecol Oncol 51: 307, 1993 |
|
Roy M: VIN: Latest management approaches. Contemp Obstet Gynecol 32: 170, 1988 |
|
Hording U, Junge J, Poulsen H et al: Vulvar intraepithelial neoplasia: III. A viral disease of undetermined progressive potential. Gynecol Oncol 56: 276, 1995 |
|
Brinton LA, Nasca PC, Mallin K et al: Case-control study of cancer of the vulva. Obstet Gynecol 75: 859, 1990 |
|
Hart WR, Norris HJ, Helwig EB: Relation of lichen sclerosus et atrophicus of the vulva to development of carcinoma. Obstet Gynecol 45: 369, 1975 |
|
Kurman RJ, Trimble CL, Shah KV: Human papillomavirus and the pathogenesis of vulvar carcinoma. Curr Opin Obstet Gynecol 4: 582, 1992 |
|
Ansink AC, Krul MRL, DeWeger RA et al: Human papillomavirus, lichen sclerosus, and squamous cell carcinoma of the vulva: Detection and prognostic significance. Gynecol Oncol 52: 180, 1994 |
|
Joura EA: Epidemiology, diagnosis and treatment of vulvar intraepithelial neoplasia. Curr Opin Obstet Gynecol 14(1): 39–43, 2002 |
|
Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C: Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO(2) laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol 100(2): 271–5, 2006 |
|
Creasman WT: New gynecologic cancer staging. Gynecol Oncol 58: 157, 1995 |
|
Microinvasive cancer of the vulva: Report of the ISSVD task force. J Reprod Med 29: 454, 1984 |
|
Kosary CL: FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: An analysis of 1973-87 SEER cases of cancers of the endometrium, cervix, ovary, vulva and vagina. Semin Surg Oncol 10: 31, 1994 |
|
Hoffman JS, Kumar NB, Morley GW: Prognostic significance of groin lymph node metastasis in squamous cell carcinoma of the vulva. Obstet Gynecol 66: 402, 1985 |
|
Cavanagh D: Vulvar Cancer-continuing evolution in management. Gynecol Oncol 66: 362–7, 1997 |
|
Raspagliesi F, Hanozet F, Ditto A et al: Clinical and pathological prognostic factors in squamous cell carcinoma of the vulva. Gynecol Oncol 102(2): 333–7, 2006 |
|
Marsden DE, Hacker NF: Contemporary management of primary carcinoma of the vulva. Surg Clin North Am 81(4): 799–813, 2001 |
|
Stehman FB, Bundy BN et al: Early stage I carcinoma of the vulva treated with ipsilateral superficial inguinal lymphadenectomy and modified radical hemivulvectomy: a prospective study of the GOG. Obstet Gynecol 79(4): 490–497, 1992 |
|
Kelley JL III, Burke TW, Tornos C et al: Minimally invasive vulvar carcinoma: An indication for conservative surgical therapy. Gynecol Oncol 144: 240, 1991 |
|
Magrina JF, Gonzalez-Bosquet J, Weaver AL et al: Squamous cell carcinoma of the vulva stage IA: Long-term results. Gynecol Oncol 76: 24, 2000 |
|
Taussig F: Carcinoma to the vulva: an analysis of 155 cases. Am J Obstet Gynecol 40: 764, 1940 |
|
Way S: Carcinoma to the vulva: an analysis of 155 cases. Am J Obstet Gynecol 791: 692, 1960 |
|
Hacker NF, Leuchter RS, Berek JS et al: Radical vulvectomy and bilateral inguinal lymphadenectomy through separate groin incisions. Obstet Gynecol 58: 574, 1981 |
|
Heaps JM, Fu YS, Montz FJ et al: Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol 38: 309, 1990 |
|
Figge CD, Gaudenz R: Invasive carcinoma of the vulva. Am J Obstet Gynecol 119: 382, 1974 |
|
De Hullu JA, Hollema H, Lolkema S et al: Vulvar carcinoma. The price of less radical surgery. Cancer 95(11): 2331–8, 2002 |
|
De Hullu JA, van der Zee AG: [Surgical treatment of early-stage vulva carcinoma and the complications of the operation.] Ned Tijdschr Geneeskd 149(7): 336–42, 2005 |
|
Binder SW, Huang I, Fu YS et al: Risk factors for the development of lymph node metastasis in vulvar squamous carcinoma. Gynecol Oncol 37: 9, 1990 |
|
Boyce J, Fruchter RG, Kasambilides E et al: Prognostic factors in carcinoma of the vulva. Gynecol Oncol 20: 364, 1985 |
|
Homesley HD, Bundy BN, Sedlis A et al: Clinical and surgicopathologic findings in squamous cell carcinoma of the vulva: A Gynecologic Oncology Group study. Proceedings of the Second Meeting of the International Gynecologic Cancer Society 2: 60, 1989 |
|
Parry Jones E: Lyphatics of the vulva. J Obstet Gynaecol Br Commonw 70: 751–65, 1963 |
|
Burke TW, Levenback C, Coleman RL et al: Surgical therapy of T1 and T2 vulvar carcinoma: further experience with radical wide excision and selective inguinal lymphadenectomy. Gynecol Oncol 57(2): 215–20, 1995 |
|
Homesley HD, Bundy BN, Sedlis A et al: Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol 68: 733, 1986 |
|
Terada KY, Shimizu DM, Wong JH: Sentinel node dissection and ultrastaging in squamous cell cancer of the vulva. Gynecol Oncol 76: 40, 2000 |
|
Makar AP, Scheistroen M, van den Weyngaert D, Tropé CG: Surgical management of stage I and II vulvar cancer: the role of the sentinel node biopsy. Review of literature. Int J Gynecol Cancer 11(4): 255–62, 2001 |
|
Petereit D, Mehta M, Buchler D et al: A retrospective review of nodal treatment for vulvar cancer. Am J Clin Oncol 16: 38, 1993 |
|
Figge DC, Tamimi HK, Greer BE: Lymphatic spread in carcinoma of the vulva. Am J Obstet Gynecol 152: 387, 1985 |
|
Sim FH, Taylor WF, Pritchard DJ: Lymphadenectomy in the management of stage I malignant melanoma: A prospective randomized study. Mayo Clinnic Proc 61: 697, 1986 |
|
Tasseron EWK, van der Esch EP, Hart AA et al: A clinicopathologic study of 30 melanomas of the vulva. Gynecol Oncol 46: 170, 1992 |
|
Parmley TH, Woodruff JD, Julian CG: Invasive Paget's disease. Obstet Gynecol 46: 341, 1975 |
|
Origoni M, Sideri M, Garsia S, Carinelli SG, Ferrari AG: Prognostic value of pathological patterns of lymph node positivity in squamous cell carcinoma of the vulva stage III and IVA FIGO. Gynecol Oncol 45(3): 313–6, 1992 |
|
Paladini D, Cross P, Lopes A, Monaghan JM: Prognostic significance of lymph node variables in squamous cell carcinoma of the vulva. Cancer 74(9): 2491–6, 1994 |
|
Van der Velden J, Hacker NF: Prognostic factors in squamous cell cancer of the vulva and the implications for treatment. Curr Opin Obstet Gynecol 8(1): 3–7, 1996 |
|
Beller U, Quinn MA, Benedet JL, Creasman WT et al: Carcinoma of the vulva. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 95 Suppl 1: S7–27, 2006 |
|
Van Der Velden J, Ansink A, Primary groin irradiation versus primary groin surgery for early vulvar cancer (Cochrane Review): The Cochrane Library Issue I. Oxford: Update 2002 |
|
Manavi M, Berger A, Kucera E, Vavra N, Kucera H. Does T1, N0-1 vulvar cancer treated by vulvectomy but not lymphadenectomy need inguinofemoral radiation? Int J Radiat Oncol Biol Phys 38(4): 749–53, 1997 |
|
Perez CA, Grigsby PW, Galakatos A et al: Radiation therapy in management of carcinoma of the vulva with emphasis on conservation therapy. Cancer 71(11): 3707–16, 1993 |
|
Stehman FB, Bundy BN, Thomas G et al: Groin dissection versus groin radiation in carcinoma of the vulva: a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys 24(2): 389–96, 1992 |
|
Katz A, Eifel PJ, Jhingran A, Levenback CF: The role of radiation therapy in preventing regional recurrences of invasive squamous cell carcinoma of the vulva. Int J Radiat Oncol Biol Phys 57(2): 409–18, 2003 |
|
Berek JS, Heaps JM, Fu YS et al: Concurrent cisplatin and 5-fluorouracil chemotherapy and radiation therapy for advanced-stage squamous carcinoma of the vulva. Gynecol Oncol 42: 197, 1991 |
|
Han SC, Kim DH, Higgins SA et al: Chemoradiation as primary or adjuvant treatment for locally advanced carcinoma of the vulva. Int J Radiat Oncol Biol Phys 47: 1235, 2000 |
|
Van Doorn HC, Ansink A, Verhaar-Langereis M, Stalpers L. Neoadjuvant chemoradiation for advanced primary vulvar cancer. Cochrane Database Syst Rev 3: CD003752, 2006 |
|
Benedetti-Panici P, Greggi S, Scambia G, et al. Cisplatin, bleomycin, and methotrexate preoperative chemotherapy in locally advanced vulvar carcinoma. Gynecol Oncol 150: 49, 1993 |
|
Durrant KR, Mangioni C, Lacave AJ et al: Bleomycin, methotrexate, and CCNU in advanced inoperable squamous cell carcinoma of the vulva: a phase II study of the EORTC Gynaecological Cancer Cooperative Group (GCCG). Gynecol Oncol 37: 359–62, 1990 |
|
Wagenaar HC, Colombo N, Vergote I et al: Bleomycin, methotrexate, and CCNU in locally advanced or recurrent, inoperable, squamous-cell carcinoma of the vulva: an EORTC Gynaecological Cancer Cooperative Group Study. European Organization for Research and Treatment of Cancer. Gynecol Oncol 81: 348–54, 2001 |
|
Franzone P, Bruzzone M, Amorsoso D et al: Bolus mitomycin C and 5-fluorouracil in 5 days continuous cronomodulated infusion in association with radiotherapy in advanced vulvar cancer. Proc ASCO 17: 365a, 1998 |
|
Landoni F, Maneo A, Zanetta G et al: Concurrent preoperative chemotherapy with 5-fluorouracil and mitomycin C and radiotherapy (FUMIR) followed by limited surgery in locally advanced and recurrent vulvar carcinoma. Gynecol Oncol 61: 321, 1996 |
|
Lupi G, Raspagliesi F, Zucali R et al: Combined preoperative chemoradiotherapy followed by radical surgery in locally advanced vulvar carcinoma. A pilot study. Cancer 77: 1472, 1996 |
|
Sebag-Montefiore D, McLean C, Arnott S et al: Treatment of advanced carcinoma of the vulva with chemoradiotherapy – can exenterative surgery be avoided? Int J Gynecol Cancer 4: 150–155, 1994 |
|
Thomas G, Dembo A, DePetrillo A et al: Concurrent radiation and chemotherapy in vulvar carcinoma. Gynecol Oncol 34: 263–267, 1989 |
|
Wahlen SA,Slater JD,Wagner RJ et al: Current radiation therapy and chemotherapy in the treatment of: primary squamous cell carcinoma of the vulva. Cancer 75: 2289, 1995 |
|
Russell AH, Mesic JB, Scudder SA et al: Synchronous radiation and cytotoxic chemotherapy for locally advanced orrecurrent squamous cancer of the vulva. Gynecol Oncol 47: 14–20, 1992 |
|
Berek J, Heaps J, Fu Y, Juillard G, Hacker N. Concurrent cisplatin and 5-fluorouracil chemotherapy and radiation therapy for advanced-stage squamous carcinoma of the vulva. Gynecol Oncol 42: 197–201, 1991 |
|
Cunningham MJ, Goyer RP, Gibbons SK et al: Primary radiation, cisplatin, and 5-fluorouracil for advanced squamous carcinomaof the vulva. Gynecol Oncol 66: 258–61, 1997 |
|
Moore DH, Thomas GM, Montana GS et al: Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. Int J Radiat Oncol Biol Phys 42: 79, 1998 |
|
Bellati F, Angioli R, Manci N et al: Single agent cisplatin chemotherapy in surgically resected vulvar cancer patientswith multiple inguinal lymph node metastases. Gynecol Oncol 96: 227–31, 2005 |
|
Iversen T: Irradiation and bleomycin in the treatment of inoperable vulval carcinoma. Acta Obstet Gynecol Scand 61: 195–7, 1982 |
|
Koh WJ, Wallace HJ 3rd, Greer BE et al: Combined radiotherapy and chemotherapy in the management of local-regionally advanced vulvar cancer. Int J Radiat Oncol Biol Phys 26: 809–16, 1993 |
|
Farias-Eisner R, Cirisano FD, Grouse D et al: Conservative and individualized surgery for early squamous carcinoma of the Gynecol Oncol 53: 55–8, 1994 |
|
Jaramillo BA, Ganjei P, Averette HE et al: Malignant melanoma of the vulva. Obstet Gynecol 66: 398–401, 1985 |
|
Chung AF, Woodruff JM, Lewis JL Jr: Malignant melanoma of the vulva: A report of 44 cases. Obstet Gynecol 45: 638–46, 1975 |
|
Sim FH, Taylor WF, Pritchard DJ: Lymphadenectomy in the management of stage I malignant melanoma: A prospective randomized study. Mayo Clinnic Proc 61: 697, 1986 |
|
Tasseron EW, van der Esch EP, Hart AA et al: A clinicopathological study of 30 melanomas of the vulva. Gynecol Oncol 46: 170–5, 1992 |
|
Parmley TH, Woodruff JD, Julian CG: Invasive vulvar Paget's disease. Obstet Gynecol 46: 341–6, 1975 |
|
Hart WR, Millman JB: Progression of intraepithelial Paget's disease of the vulva to invasive carcinoma. Cancer 40: 2333–7, 1977 |
|
Bergen S, DiSaia PJ, Liao SY et al: Conservative management of extramammary Paget's disease of the vulva. Gynecol Oncol 33: 151-6, 1989 |
|
Stacy D, Burrell MO, Franklin EW: Extramammary Paget's disease of the vulva and anus: Use of intraoperative frozen-section margins. Am J Obstet Gynecol 155: 519, 1986 |