Fetal Circulation
Authors
INTRODUCTION
In the adult, blood circulates from the left ventricle to the systemic circulation and is returned to the right side of the heart. From there, it circulates through the lungs for reoxygenation. This serial circulatory design is inappropriate for the fetus because oxygenation occurs in the placenta; therefore, a pair of parallel circulations is present. This is made possible by anatomical shunts, which normally close rapidly at birth when circulation independent of the mother is required.1
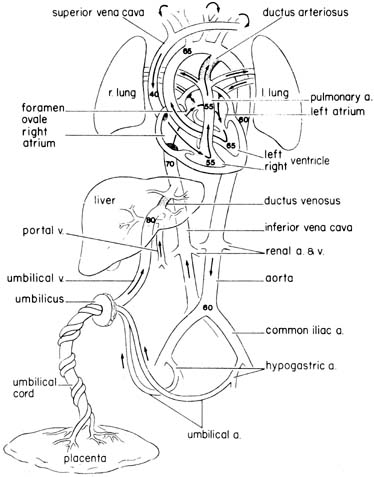
The fetal circulation is illustrated in Figure 1, which shows approximate values of the percent saturation of blood with oxygen in various areas. Most of the physiological data presented here are from chronically catheterized, unanesthetized sheep fetuses, because it is not possible to obtain such data from humans. Although species differences may occur, it is likely that the same general trends and mechanisms also apply to humans.
Well-oxygenated blood returns to the fetus from the placenta by way of the umbilical vein. The umbilical vein enters the liver, where it joins with the portal venous system. Some of this blood is shunted directly to the inferior vena cava through the ductus venosus, while some traverses the hepatic parenchyma. An average of 50% takes the latter path, but the proportion is variable.
The saturation of blood in the inferior vena cava is lower than that in the ductus venosus, because it has mixed with poorly oxygenated blood returning from the lower body. However, this mixture is not complete, and a streaming of blood within the proximal inferior vena cava has been described. The inferior vena cava blood enters the right atrium, and approximately 40% is diverted to the left atrium through the foramen ovale. Most of the blood crossing the foramen ovale corresponds to the stream of well-oxygenated blood in the inferior vena cava coming from the ductus venosus. In the left atrium, this blood mixes with a relatively small quantity of pulmonary venous blood, enters the left ventricle, and then proceeds to the coronary circulation and vessels supplying the head, neck, and upper extremities. Blood entering the right atrium from the superior vena cava joins with the remaining (60%) blood in the inferior vena cava, which corresponds mainly to the less-oxygenated bloodstream from the distal inferior vena cava (fetal lower body). This blood enters the right ventricle. From here a small proportion enters the pulmonary circulation, but most is shunted from this bed through the ductus arteriosus, which joins the descending aorta. This blood supplies the gut, kidneys, lower body, and also the umbilical circulation.2
Following the route described, the blood perfusing the fetal lower body is ejected from both the right and left ventricles, while blood perfusing the upper body is ejected from the left ventricle. The differential streaming and the fact that inferior vena cava blood crosses the foramen ovale accounts for the approximately 5% to 10% higher oxygen saturation observed in the ascending aorta (blood perfusing vital organs such as the brain and heart) than in the descending aorta.
DISTRIBUTION OF BLOOD FLOW
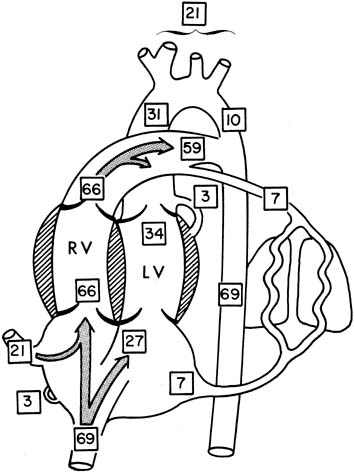
The distribution of blood flow in the fetus is generally described as a percentage of the cardiac output. This is a simple concept in the adult, who has two essentially equal serial circulations: systemic and pulmonary. In a fetus, who has two unequal parallel circulations, distribution is described as the percentage of combined ventricular output (CVO), which is the combined output of left and right ventricles.
The percentage of CVO in various areas of the heart and other vessels is shown in Figure 2.3 For obvious reasons, little of this information is available in term infants, and the values are obtained from unanesthetized chronically catheterized term sheep fetuses.
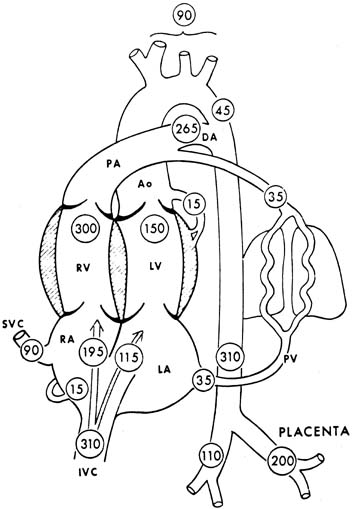
The human fetal cardiac output at term has been estimated to be approximately 500 mL per minute per kilogram, according to data obtained from noninvasive measurements.4 A term sheep fetus is similar in weight to a human fetus (approximately 7 pounds [3.2 kg]), but species differences may occur (e.g., in the proportion of blood flow to the brain). Values of central blood flow in milliliters per minute per kilogram fetal body weight are shown in Figure 3. Note that the CVO is 450 mL per minute, with twice as much from the right as the left ventricle. Approximately 45% of the CVO is umbilical blood flow (i.e., approximately 200 mL per minute per kilogram). The remaining 55% of CVO distribution to the fetal organs has been determined in fetal sheep. With minor variations among studies, typical values of organ blood flow expressed as percent of the CVO and as milliliter per minute ( 100 grams are shown in Table 1.5 Note that the major part of the CVO perfuses the brain and carcass.
The estimated cardiac output of the human fetus (553 mL/kg/min−1) is higher than that of sheep (450 mL/kg/min−1). In addition, the right and left ventricular outputs are more similar in the human compared with the sheep.5 The ratio of the right-to-left ventricular outputs decreases with advancing gestation, from 1.3 at 15 weeks to 1.1 at 40 weeks. These data are consistent with the fact that the larger human brain requires a higher left ventricular output than the brain of the sheep.
Table 1. Organ Blood Flow and Cardiac Output Distribution in the Normoxic Sheep Fetus
| Blood Flow* | %CVO |
| Combined ventricular output 456 ± 17 | 100 |
| Umbilicoplacental circulation 204 ± 9 | 43 ± 3 |
| Heart 192 ± 13 | 3 ± 1 |
| Brain 165 ± 8 | 6 ± 5 |
| Adrenals 215 ± 15 | .1 ± .01 |
| Kidneys 163 ± 9 | 3 ± .5 |
| Gut 43 ± 45 ± 1 | |
| Spleen 226 ± 25 | 1 ± .7 |
| Liver 7 ± 1 | .4 ± .1 |
| Carcass 22 ± 2 | 33 ± 3 |
*Combined cardiac output and umbilico-placental blood flow are expressed as mL/min/kg fetal body weight. Other organ blood flows are expressed as mL/min/100 g organ weight.
FETAL BLOOD PRESSURES
The fetus is surrounded by the fluid-filled amniotic cavity, so pressures must be related to that of amniotic fluid. In the absence of uterine contractions, this latter pressure is generally stable.
Fetal systemic arterial blood pressure is considerably lower than that in an adult, averaging 55 mmHg (systolic/diastolic, approximately 70/45 mmHg) at term. Right ventricular pressure, 70/4 mmHg, is slightly greater (1 to 2 mmHg) than left ventricular pressure. Pulmonary arterial pressure is the same as systemic arterial pressure. There is a slightly greater pressure in the right atrium (3 mmHg) than in the left atrium (2 mmHg), thus ensuring right-to-left blood flow across the foramen ovale. Pressure in the systemic venous bed is similar to that of the right atrium, with the exception of the umbilical vein, where pressure is approximately 8 to 10 mmHg.
Systemic blood pressures are somewhat lower earlier in gestation. This is reflected in the fact that premature newborns have lower blood pressure than term infants. Thus, at 30 weeks' gestation, the mean arterial blood pressure is only 35 mmHg.
FETAL HEART RATE
Fetal heart rate analysis is the prime means by which a fetus is evaluated for adequacy of oxygenation, so knowledge of its rate and regulation are of great importance to an obstetrician.
The average heart rate in a nonmedicated term fetus before labor is 140 beats/min. Earlier in pregnancy, it is higher than this, although not substantially so. At 20 weeks' gestation, the average fetal heart rate is 155 beats/min, and at 30 weeks' gestation, it is 144 beats/min. Variations of 20 beats/min above or below these values are encountered in normal fetuses.
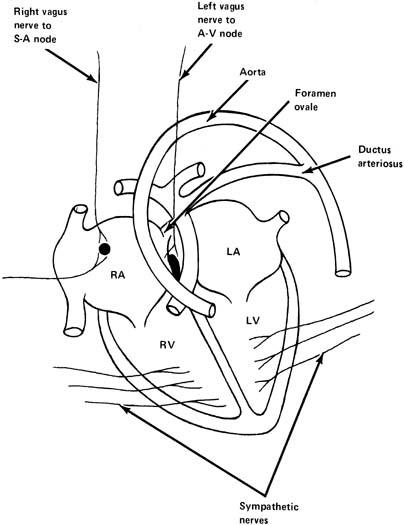
A fetal heart is similar to that of an adult in that it has its own intrinsic pacemaker activity that results in rhythmic contractions. The sinoatrial (SA) node, which is found in one wall of the right atrium, has the fastest rate of contraction, and it sets the rate in a normal heart (Fig. 4). The next fastest pacemaker rate is found in the atrium and, finally, the ventricle has a slower rate of beating than either the SA node or atrium. In various cases of complete or partial heart block in a fetus, variations in rate below normal can be encountered. A fetus with complete heart block typically has a rate of approximately 60 beats/min.
Variability of the FHR from beat to beat, and longer-term trends in heart rate over periods of less than 1 minute, are important properties. Variability has prognostic importance clinically. Valuable empiric interpretations can be made from its presence, decrease, or absence. The mean fetal heart rate is a result of many physiological factors that modulate the intrinsic rate of the heart.
CARDIAC OUTPUT REGULATION
In adults, the beat-to-beat amount of blood pumped by the heart is determined by the amount of blood returning to the heart. In accordance with the Frank-Starling mechanism, when the cardiac muscle is stretched before contraction by an increased inflow of blood, it contracts with a greater force than before and is able to pump out more blood. In other words, the heart pumps all the blood that flows into it without excessive damming of blood in the veins. This mechanism has been studied in unanesthetized fetal lambs and has been shown to be less developed than in adult sheep. The imperfect mechanism in the fetal lamb is probably caused by the fact that the heart muscle is not as well developed as in the adult sheep. It is likely that the same is true of a human fetus, as it is generally more immature than that of a fetal lamb when born. As a consequence, increases in the filling pressure or preload produce minor if any changes in CVO, suggesting that the fetal heart normally operates near the top of its function curve.
The output of the fetal heart is also related to the heart rate. Some researchers have shown that spontaneous variations of heart rate relate directly to cardiac output; that is, as rate increases, output increases. However, different responses have been observed during right or left atrial pacing studies. No changes were observed in left ventricular output when the right atrium was paced, whereas the output decreased during left atrial pacing.6 Clearly, additional factors are operating to explain such differences. The relationship between fetal heart rate and cardiac output has not been confirmed in human fetuses under physiologic conditions, because the spontaneous increase in heart rate has been found to be associated with a decrease in stroke volume, maintaining the cardiac output unchanged.7
In addition to heart rate and preload, cardiac output depends on afterload and intrinsic contractility. The fetal heart appears to be very sensitive to changes in the afterload, represented by the fetal blood pressure. In this way, increases in afterload dramatically reduce the stroke volume or cardiac output. As has already been stated, the fetal heart is incompletely developed compared with that of adults. Many ultrastructural differences between the fetal and adult heart account for a lower intrinsic capacity of the fetal heart to contract. Each of these four determinants of cardiac output does not work separately, but rather they interact dynamically to modulate the fetal cardiac output during physiologic conditions. Cardiac output responses during hypoxic bradycardia are described later.
In clinical practice, it is reasonable to assume that at modest variations of heart rate from the normal range there are relatively small effects on the cardiac output. However, at extremes (e.g., tachycardia above 240 beats/min or a bradycardia below 60 beats/min), cardiac output and umbilical blood flow are likely to be substantially decreased.
REGULATION OF FETAL CIRCULATION
Parasympathetic Nervous System
The parasympathetic nervous system consists primarily of the vagus nerve (tenth cranial nerve), which originates in the medulla oblongata. Fibers from this nerve supply the SA node and also the atrioventricular node (AV) (see Fig. 4). Stimulation of the vagus nerve or injection of its mediator, acetylcholine, results in a decrease in heart rate in a normal fetus because of vagal influence on the SA node, decreasing its rate of firing and decreasing the rate of transmission of impulses from atrium to ventricle. In a similar fashion, blocking of this nerve in a normal fetus with a substance that blocks the effects of acetylcholine (e.g., atropine) causes an increase in the fetal heart rate of approximately 20 beats/min at term. This demonstrates that there is normally a constant vagal tone on the fetal heart rate, tending to decrease it from its normal intrinsic rate.
The vagus nerve is also responsible for transmission of impulses causing beat-to-beat variability of fetal heart rate. Blocking the vagus nerve with atropine results in a disappearance of this variability. Hence, it has been postulated that two vagal influences impinge on the heart: a tonic influence tending to decrease its rate and an oscillatory influence that results in fetal heart rate variability.
The vagal tone is not necessarily constant. Its influence increases with gestational age. In fetal sheep, vagal activity increases as much as four-fold during acute hypoxia or experimentally produced fetal growth restriction.
Sympathetic Nervous System
Sympathetic nerves are widely distributed in the myocardium at term (see Fig. 4). Stimulation of the sympathetic nerves will release norepinephrine and cause an increase in fetal heart rate and also an increase in the vigor of cardiac contractions. These result in an increase in cardiac output. The sympathetic nerves are a reserve mechanism to improve the pumping activity of the heart during intermittent stressful situations. There is normally a tonic sympathetic influence on the heart.
When the β-adrenergic receptor blocker propranolol is administered to a normal fetus, the fetal heart rate will decrease approximately 10 beats/min. There is, however, only a small decrease in fetal heart rate variability after blocking the sympathetic nerves.
α-Adrenergic receptors also are found in a fetus at term. When they are stimulated with methoxamine, there is an increase in blood pressure and a decrease in the kidney and carcass blood flow. The opposite is observed after a-adrenergic blockers as phentolamine or phenoxybenzamine.8 Several factors cause the parasympathetic and sympathetic nervous systems to increase their activity.
Baroreceptors
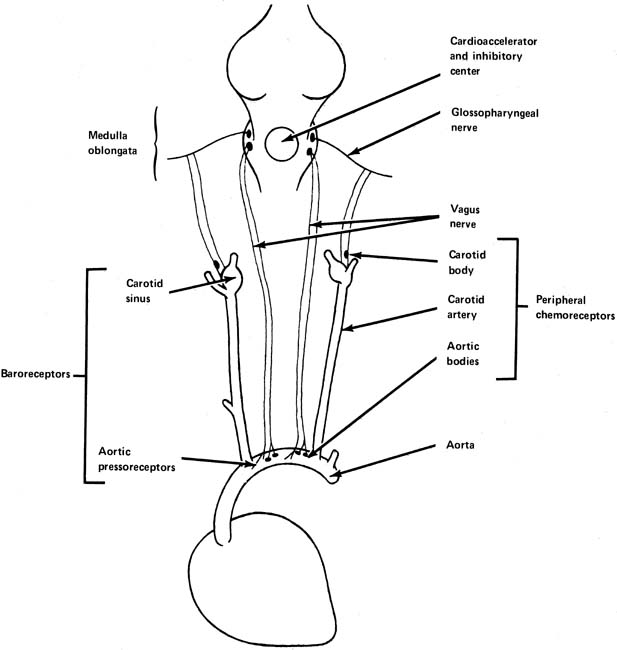
In the walls of the arch of the aorta and in the carotid sinus at the junction of the internal and external carotid arteries are found small stretch receptors that are sensitive to increases in blood pressure (Fig. 5). When blood pressure increases, impulses are sent from these receptors by way of the vagus and glossopharyngeal nerve to the midbrain, resulting in further impulses through the vagus nerve to the heart, tending to slow it. This is an extremely rapid response, being noted with almost the first increase of blood pressure. It is a protective stabilizing function by the body attempting to lower blood pressure by decreasing heart rate and cardiac output when blood pressure is increasing.
Chemoreceptors
Chemoreceptors are found in both the peripheral and central nervous systems. They have their most dramatic effects on the regulation of respiration, but they are still important in the control of the circulation. The peripheral chemoreceptors are found in the carotid and aortic bodies (see Fig. 5). Like the pressoreceptors, they are found in the arch of the aorta and in the area of the carotid sinus. The central chemoreceptors, found in the medulla oblongata, respond to changes in the oxygen and carbon dioxide tensions in blood or cerebrospinal fluid perfusing this area.
In adults, when oxygen in the arterial blood perfusing the central chemoreceptors is decreased or the carbon dioxide content is increased, there is ordinarily a reflex tachycardia. There is also a substantial arterial blood pressure increase, which is extremely pronounced with increases in carbon dioxide concentration. Both of these effects, that is, tachycardia and an increase in blood pressure, are thought to be protective in attempting to circulate more blood through the affected areas to bring about a decrease in carbon dioxide tension or an increase in oxygen-selective hypoxia or hypercapnia of the peripheral chemoreceptors alone in the adult produces a bradycardia, in contrast to the tachycardia and hypertension seen with central hypoxia or hypercapnia.
The peripheral chemoreceptors seem to be highly important in the cardiovascular responses to hypoxia. The interaction of baroreceptor and chemoreceptor systems in a fetus is not well understood, although the net result of hypoxia in a fetus is bradycardia and hypertension. During basal conditions, they seem to contribute to stabilize heart rate and blood pressure.9
Central Nervous System
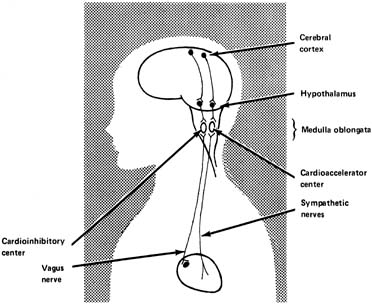
It has been established that in adults there are influences on heart rate from the higher centers of the brain (Fig. 6). Heart rate can be increased by various emotional stimuli such as fear or sexual arousal. Observations of fetal lambs and monkeys have shown that the electroencephalogram or electro-oculogram shows increased activity at times in association with variability of the heart rate and body movements. At other times, apparently when the fetus is sleeping, activity slows, and the fetal heart rate variability decreases, suggesting an association between these two factors and central nervous system activity.
|
The hypothalamus is thought to be the area of dispatch of nerve impulses produced by physical expressions of emotion, including acceleration of the heart rate and elevation of the blood pressure. It has been shown in fetal lambs that stimulating an electrode in the hypothalamus causes the fetal heart rate to increase, at least initially, followed by a decrease, probably because of the baroflex mentioned earlier. The increase in blood pressure and heart rate appears to be mediated by the sympathetic nerves.
The medulla oblongata contains the vasomotor center, an integrative center where the net input results in either cardioacceleration or cardiodeceleration. It is probably in these centers that the net result of numerous central and peripheral inputs is processed to generate irregular oscillatory vagal impulses, giving rise to FHR variability.
Humoral Regulation
ADRENAL MEDULLA
The fetal adrenal medulla produces epinephrine and norepinephrine in response to stressful situations (e.g., hypoxia). Both of these substances act on the heart and cardiovascular system in a way similar to sympathetic stimulation. That is, they produce a faster heart rate, greater force of contraction of the heart, and an increased arterial blood pressure. However, it is not clear whether catecholamines exert a regulatory function in a resting fetus, at least in sheep.10
RENIN-ANGIOTENSIN SYSTEM
Angiotensin II seems to play a role in fetal circulatory regulation at rest, but its main activity is observed during hemorrhagic stress on a fetus.
VASOPRESSIN
Vasopressin has been shown to affect the distribution of blood flow in fetal sheep. However, this is probably only operative during hypoxia and possibly other stressful situations.
PROSTAGLANDINS
Arachidonic acid metabolites are found in high concentrations in the fetal circulation and in many tissues. Their main role seems to be in the regulation of umbilical blood flow as well as in maintaining the patency of the ductus arteriosus during fetal life.
Other hormones such as a-melanocyte-stimulating hormone (a-MSH), atrial natriuretic hormone, neuropeptide Y, thyrotropin-releasing hormone (TRH), cortisol, and metabolites such as adenosine have also been described to be present in the fetus and to participate in the circulatory function regulation, but their overall quantitative importance in the human is still not determined.
Blood Volume Control
CAPILLARY FLUID SHIFT
In adults, when the blood pressure of the body is elevated by excessive blood volume, some fluid moves out of the capillaries into interstitial spaces, thereby decreasing the blood volume back toward normal. Conversely, if an adult loses blood through hemorrhage, some fluid shifts out of the interstitial spaces into the circulation, thereby increasing the blood volume back toward normal. There is normally a delicate balance between the pressure inside and outside the capillaries. This mechanism to regulate blood pressure is slower than the almost instantaneous regulation found with the reflex mechanisms discussed earlier. Its role in a fetus is imperfectly understood, but studies performed on sheep show that a fetus appears to be able to keep its blood volume closer to normal than an adult after reductions or expansions of volume.11
INTRAPLACENTAL PRESSURES
Fluid moves down hydrostatic pressure gradients and also in response to osmotic pressure gradients. The actual values of these factors within the placental site where fetal and maternal blood closely approximate are controversial. It seems likely, however, that some delicate balancing mechanisms within the placental site prevent rapid fluid shifts between mother and fetus. As noted earlier, maternal arterial blood pressure is much higher (approximately 100 mmHg) than that of a fetus (approximately 55 mmHg); hence, some compensatory mechanism must be present to equalize the effective pressures at the exchange points. Imbalances may be responsible for the hydrops encountered in some cases of Rh isoimmunization and extreme fetal tachycardia.
OXYGEN TRANSFER TO THE FETUS
It is likely that many stillbirths and cases of fetal depression are the result of inadequate exchange of the respiratory gases. Oxygen has the lowest storage-to-utilization ratio of all nutrients in a fetus. From animal experimentation it can be calculated that in a term fetus, the quantity of oxygen is approximately 50 mL and the normal oxygen consumption is approximately 21 mL per minute. This means that in theory, a fetus has a 2- to 3-minute supply of oxygen. Fetuses do not, however, consume the total quantity of oxygen in their bodies within 3 minutes, nor do they die after this time. In fact, irreversible brain damage does not occur until approximately 10 minutes have elapsed. This is because a fetus has a number of important compensatory mechanisms that enable it to survive on a lesser quantity of oxygen for longer periods. This is discussed in a later section. The clinical situations in which there is total cessation of oxygen delivery are rare. These include sudden total abruption of the placenta or complete umbilical cord compression, generally after prolapse of the cord.
It is of value to examine the factors that determine oxygen transfer from mother to fetus (Table 2).12 Because the transfer of oxygen to a fetus is dependent on rates of blood flow and not limitations of diffusion, the blood flow on each side of the placenta assumes major importance for maintenance of fetal oxygenation. Studies of animals suggest that in the normal placenta there is a safety factor of approximately 50% in the uterine blood flow. That is, the uterine blood flow can decrease to half its normal value before fetal acidosis becomes evident. This applies only to the normal situation with normal placental reserve and is unlikely to be so in pathologic situations, such as an infant of a hypertensive mother. In this situation, placental function may be adequate for oxygenation but not for fetal growth, and a growth-restricted infant may result from such a pregnancy. Furthermore, with superimposition of uterine contractions on such a fetus, there may be transient inadequacy of uterine blood flow (and hence fetal oxygenation) during the uterine contractions; this may be recognized by responses of the fetal heart rate, namely, late decelerations.
Table 2. Factors Determining Oxygen Transfer from Mother to Fetus
| Intervillous blood flow |
| Fetal placental blood flow |
| Oxygen tension in maternal arterial blood |
| Oxygen tension in fetal arterial blood |
| Oxygen affinity of maternal blood |
| Oxygen affinity of fetal blood |
| Hemoglobin concentration or oxygen capacity of maternal blood |
| Hemoglobin concentration or oxygen capacity of fetal blood |
| Maternal and fetal blood pH and carbon dioxide tension (Bohr effect) |
| Placental diffusing capacity |
| Placental vascular geometry |
| Ratio of maternal to fetal blood flow in exchange areas |
| Shunting around exchange sites |
| Placental oxygen consumption |
Additional important determinants of fetal oxygenation include oxygen tension in maternal arterial and fetal arterial blood. In general, maternal arterial oxygen tension depends on adequate ventilation and pulmonary integrity. Disruptions of this function are relatively rare in obstetrics, although they can occur with pulmonary disease such as asthma, with congestive heart failure, or in mothers with congenital cardiac defects. The oxygen affinity and oxygen capacity of maternal and fetal blood are also important determinants of fetal oxygen transfer.
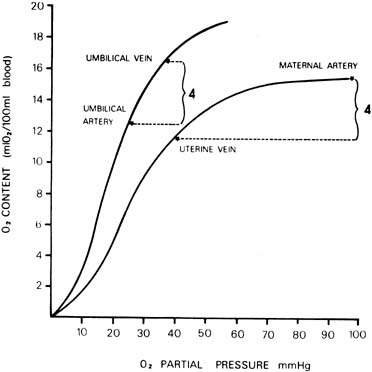
At a given oxygen tension, the quantity of oxygen carried by blood depends on the oxygen capacity (which is dependent on the hemoglobin concentration) and the oxygen affinity. The oxygen affinity of fetal blood is greater than that of maternal blood. That is, the oxygen dissociation curve of a fetus is to the left of that of the mother. In addition, the hemoglobin concentration of fetal blood is approximately 15 grams per 100 mL in a term fetus, whereas that of the mother is approximately 12 grams per 100 mL. Both of these factor, an increased oxygen affinity and higher oxygen capacity, confer advantages to a fetus for oxygen uptake across the placenta. Probable values of the oxygen content and oxygen tension in umbilical vessels and maternal uterine artery and vein are illustrated in Figure 7.
Because most measurements have been made in a human fetus during or after labor and delivery, the values of oxygen saturation and pH are generally decreased compared with those of an adult. In fact, investigations on chronically instrumented animals and data obtained from human fetuses by cordocentesis13 have shown that the oxygen saturation and content of fetal blood and acid–base status are very close to that of maternal blood. Only the oxygen tension is lower. The arteriovenous oxygen differences across each side of the placenta are also illustrated in Figure 7. Notice that the quantity of oxygen delivered or taken up by each 100 mL of circulating blood in the placenta is approximately equal in the mother and fetus. This suggests approximate equality of blood flow on each side of the placenta. A number of additional miscellaneous factors determine the rate of oxygen transfer across the placenta; they are listed in Table 2 as the last six determinants. They appear to be relatively minor compared with the major factors already outlined.
CARBON DIOXIDE AND ACID–BASE BALANCE
Carbon dioxide crosses the placenta even more readily than does oxygen. In general, the determinants for oxygen transfer also apply to carbon dioxide. It is limited by rate of blood flow and not by resistance to diffusion. The carbon dioxide tension in fetal blood in the undisturbed state is close to 40 mmHg. It is well known that maternal arterial carbon dioxide tension is approximately 34 mmHg and that a pregnant woman is in a state of compensated respiratory alkalosis. The pH of fetal blood under undisturbed conditions is probably close to 7.4, and the bicarbonate concentration is close to that in maternal blood.
Bicarbonate and the fixed acids cross the placenta much more slowly than does carbon dioxide, (i.e., equilibration takes a matter of hours rather than seconds). A situation analogous to respiratory acidosis occurs in the fetus when blood flow, either uterine or umbilical, is acutely compromised. In such cases, the pH decreases and carbon dioxide tension is elevated, but the metabolic acid–base status remains unchanged. This occurs during severe variable decelerations in association with uterine contractions, especially during the second stage of labor. These acid–base changes are generally rapidly resolved with cessation of the contraction and the deceleration. However, if significant oxygen lack is unrelieved, the fetus will decrease its oxygen consumption, redistribute blood flow, and depend partly on anaerobic metabolism to supply its energy needs, albeit with decreased efficiency. Under these conditions, lactate (an end product of anaerobic metabolism) is produced, resulting in metabolic acidosis. The acidosis may also be aggravated by a combined respiratory acidosis because of retained carbon dioxide. Unlike carbon dioxide, lactate is lost rather slowly from a fetus.
UTERINE BLOOD FLOW
Because uterine blood flow is one of the prime determinants of passage of the respiratory gases across the placenta, its characteristics and the factors affecting it are discussed.14
Uterine blood flow rises progressively throughout pregnancy and in the term fetus is approximately 700 mL per minute. This represents approximately 10% of the mother's cardiac output. Approximately 70% to 90% of the uterine blood flow passes through the intervillous space, and the remainder largely supplies the myometrium.
The uterine vascular bed is thought to be almost maximally dilated under normal conditions, with little capacity to dilate further. It is not autoregulated, so flow is proportional to the mean perfusion pressure. However, it is capable of marked vasoconstriction by α-adrenergic action. It is not responsive to changes in respiratory gas tensions. The uterine blood flow is determined by the following relationship:
uterine blood flow = (uterine artery pressure − uterine venous pressure) ÷ uterine vascular resistance.
Hence, any factor affecting any of the three values on the right side of the aforementioned equation will alter uterine blood flow. A number of causes of decreased uterine blood flow are shown in Table 3.
Table 3. Factors Causing a Decrease in Uterine Blood Flow
| Uterine contractions |
| Hypertonus |
| Abruptio placentae |
| Tetanic contraction |
| Overstimulation with oxytocin |
| Hypotension |
| Sympathetic block |
| Hypovolemic shock |
| Supine hypotensive syndrome |
| Hypertension |
| Essential |
| Preeclampsia |
| Vasoconstrictors, endogenous |
| Sympathetic discharge |
| Adrenal medullary activity |
| Vasoconstrictors, exogenous |
| Most sympathomimetics (α-adrenergic effects) |
| Exception is ephedrine (primarily β-adrenergic effect) |
| Strenuous exercise |
Uterine contractions decrease uterine blood flow as a result of increased uterine venous pressure brought about by increased intramural pressure of the uterus. There may also be a decrease in uterine arterial pressure with contractions. Uterine hypertonus causes decreased uterine blood flow through the same mechanism.
In sheep, it has been shown that if uterine arterial perfusion pressure is altered without changing the resistance of the uterine vascular bed, there is a direct relationship between uterine blood flow and the pressure. Hence, hypotension caused by any of the mechanisms noted in Table 3 will cause a decrease in blood flow.
In the case of maternal arterial hypertension, it is likely that there is a concomitant increased vascular resistance that is shared by the uterine vascular bed. This, therefore, results in a decrease in uterine blood flow. Either endogenous or exogenous vasoconstrictors result in decreased blood flow because of increased uterine vascular resistance.
There are few useful means of increasing uterine blood flow in cases in which it is known to be less than optimal. The most important clinical considerations are the avoidance or correction of factors responsible for an acute decrease in blood flow, such as excessive uterine activity or maternal hypotension.
Some of the β-mimetic agents that are used as uterine relaxants for premature labor may increase uterine blood flow; but this effect, if any, is small and may only be the result of decreased uterine tonus . There are a number of experimental means of increasing uterine blood flow, sometimes transiently, but these have no place clinically. Examples of such treatments include estrogens, acetylcholine, nitroglycerin, cyanide, ischemia, and chronic hypoxia.
Clinically, it has been accepted for many years that maternal bed rest may improve the outcome in suspected fetal growth restriction. This may be caused by an overall higher uterine blood flow while the patient is reclining, compared with the active ambulatory state.
UMBILICAL BLOOD FLOW
Umbilical blood flow measured by ultrasonographic techniques is approximately 360 mL per minute, or 120 mm per minute per kg in an undisturbed fetus15 at term. This figure is considerably less than that of a sheep, in which it is approximately 200 mm per minute per kilogram. The differences may be explained by the somewhat higher metabolic rate (body temperature 39(C) and lower hemoglobin concentration (10 g/dL) in a fetal sheep. It is important to recognize this species difference, because most of our information on fetal circulatory physiology comes from fetal sheep. The umbilical blood flow is approximately 40% of the combined ventricular output, and approximately 20% of this blood flow is shunted, that is, it does not exchange with maternal blood in sheep. It is either carried through actual vascular shunts within the fetal side of the placenta or it does not approach maternal blood closely enough to exchange with it.
Umbilical blood flow is unaffected by acute moderate hypoxia but is decreased by severe hypoxia. Innervation of the umbilical cord is not seen, but umbilical blood flow decreases with the administration of catecholamines. It is also decreased by acute cord occlusion. There are no known means of increasing umbilical flow in cases in which it is thought to be decreased chronically. However, certain fetal heart rate patterns, namely, variable decelerations, have been ascribed to transient umbilical cord compression in a fetus during labor. Manipulation of maternal position either to the lateral or Trendelenburg position can sometimes abolish these patterns, as can amniotic fluid replenishment by the technique of amnioinfusion. The implication is that cord compression has been relieved by these practices.
HYPOXIA
Fetal Responses
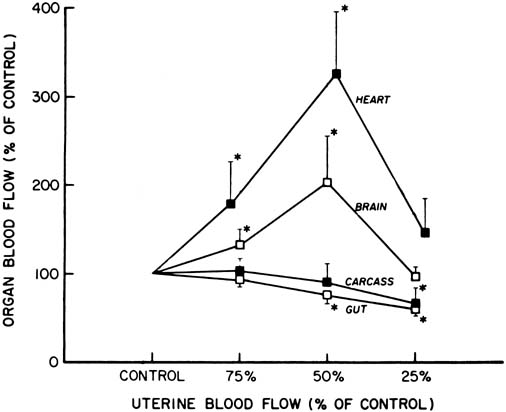
Studies of chronically prepared animals have shown that a number of responses occur during acute hypoxia or asphyxia in a previously normoxic fetus. There is little or no change in combined cardiac output and umbilical (placental) blood flow, but there is a redistribution of blood flow favoring certain vital organs, namely, heart, brain, and adrenal glands, and a decrease in the blood flow to the gut, spleen, kidneys, and carcass.16 This initial response is presumed to be advantageous to a fetus in the same way as the diving reflex is in an adult seal, in that the blood containing the available oxygen and other nutrients is supplied preferentially to vital organs.
Fetal oxygen consumption decreases to values as low as 60% of control (from approximately 8 to 5 mm per minute per kilogram) during fetal hypoxia in a chronically instrumented fetus with arterial oxygen tension of 10 mmHg. This decrease is rapidly instituted, stable for periods up to 45 minutes, proportional to the degree of hypoxia, and rapidly reversible on cessation of maternal hypoxia. It is accompanied by a fetal bradycardia of about 30 beats/min below control (approximately 170 beats/min control to 140 beats/min hypoxia in fetal sheep) and an increase in fetal arterial blood pressure (approximately 54 mmHg control to 61 mmHg hypoxia mean pressure). There is also progressive fetal acidosis during fetal hypoxia (fetal arterial pH 7.38 control to 7.33 after 25 minutes hypoxia). This is a metabolic acidosis caused by lactic acidosis accumulation as a result of anaerobic metabolism in those partially vasoconstricted beds in which oxygenation is inadequate for normal basic needs. During fetal asphyxia, the increase in carbon dioxide tension superimposes a respiratory component on the acidosis.
The series of responses just described, that is, redistribution of blood flow favoring vital organs, decreased total oxygen consumption, and anaerobic glycolysis, ay be thought of as temporary compensatory mechanisms that enable a fetus to survive moderately long periods (e.g., up to 30 minutes) of limited oxygen supply without decompensation of vital organs, particularly the brain and heart. The close matching of blood flow to oxygen availability to achieve a constancy of oxygen consumption has been demonstrated in the fetal cerebral circulation and in the fetal myocardium. In studies on hypoxic lamb fetuses, cerebral and myocardial oxygen consumption was constant over a wide range of arterial oxygen contents because the decrease in arteriovenous oxygen content accompanying hypoxia was compensated for by an increase in the respective blood flows.
However, during more severe asphyxia or sustained hypoxemia, these responses are no longer maintained, and a decrease in the cardiac output, arterial blood pressure, and blood flow to the brain and heart have been described (Fig. 8). These changes may be considered to be a stage of decompensation, after which tissue damage and even fetal death may follow.17
Metabolic Effects
It is known that a fetus depends partially on anaerobic metabolism for its energy needs during oxygen insufficiency.18 It has also been shown in experimental animals that a newborn's ability to tolerate asphyxia depends on cardiac carbohydrate reserves. Whether this also applies to a human fetus is unknown, but clinical observations support the view that carbohydrate-depleted fetuses die more readily than those with normal reserves. A nutritionally growth-restricted fetus also is more susceptible to intrauterine asphyxia and depression than a normal fetus.19
It has been stated that the prime aim of compensatory responses in hypoxia is maintenance of the circulation, and maintenance of the integrity of cardiac function is paramount in this regard. It is likely that carbohydrate availability is critical in supplying substrates for glycolysis at more severe degrees of hypoxia.
Mechanisms of Responses
The cardiovascular responses to hypoxia are instituted rapidly and are mediated by neural and hormonal mechanisms. As it has been previously mentioned, the tonic influence of the autonomic nervous system on heart rate, blood pressure, and the umbilical circulation in a normoxic fetus is quantitatively minor. This is in marked contrast to autonomic activity during hypoxia.
In studies using total pharmacologic blockade, it has been shown that parasympathetic activity is augmented three to five times and β-adrenergic activity doubles when measured by heart rate response. The net result of these changes is a decrease in fetal heart rate during hypoxia. Augmented β-adrenergic activity also may be important in maintaining cardiac output and umbilical blood flow during hypoxia, probably by increased inotropic effect on the heart.
α-Adrenergic activity is important in determining regional distribution of blood flow in hypoxic fetal sheep by selective vasoconstriction. As noted earlier, during hypoxia there is preferential blood flow to the brain, heart, and adrenals and decreased supply to the carcass, lungs, kidneys, and gut. α-Adrenergic blockade reversed the hypertension and increased peripheral resistance observed during fetal hypoxia. These changes are caused by a decrease in the resistance in the gut, spleen, lungs, and probably carcass, indicating a participation of the α-adrenergic system in their vasoconstriction.
Plasma concentrations of catecholamines, vasopressin, β-endorphin, and atrial natriuretic factor increase during hypoxia in a fetus. The contributions of catecholamines to the circulatory responses to hypoxia were described earlier. Vasopressin contributes to the increase in blood pressure observed during hypoxia by decreasing umbilical and gut blood flows. β-Endorphin and probably other endogenous opioids also participate in the response to hypoxia. The blockade of its receptors with naloxone further increases the hypertensive response by increasing the vasoconstriction in the kidneys and carcass. During hypoxia, a decrease in the fetal blood volume has been described. Atrial natriuretic factor may play a role in this response. In addition, nitric oxide, prostaglandins, cortisol, and adenosine have all been implicated in regulation of the fetal circulation during hypoxia.
Most of these results have been obtained from the chronically catheterized fetal sheep model. The relative contributions of these and other mediators to the cardiovascular response to hypoxia in a human fetus continue to be explored, because it is clear that the redistribution of blood flow is a powerful mechanism for protection of fetal organs from damage during periods of oxygen insufficiency.
REFERENCES
Dawes GS: Fetal and Neonatal Physiology. Chicago, Year Book Medical Publishers, 1968 |
|
Rudolph AM: Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res 57:811, 1985 |
|
Rudolph AM: Congenital Diseases of the Heart. Chicago, Year Book Medical Publishers, 1974 |
|
Reed KL, Meijboom EJ, Sahn DJ et al: Cardiac Doppler flow velocities in human fetuses. Circulation 73:41, 1986 |
|
De Smedt MC, Visser GH, Meijboom EJ: Fetal cardiac output estimated by Doppler echocardiography during mid- and late gestation. Am J Cardiol 60:338, 1987 |
|
Anderson PAW, Glick KL, Killam AP et al: The effect of heart rate on in utero left ventricular output in the fetal sheep. J Physiol 372:557, 1986 |
|
Kenny J, Plappert T, Doubilet P et al: Effects of heart rate on ventricular size, stroke volume, and output in the normal human fetus: A prospective Doppler echocardiographic study. Circulation 76:52, 1987 |
|
Edelstone DI, Merick RE, Caritis SN et al: Umbilical venous blood flow and its distribution before and during auto-nomic blockade in fetal lambs. Am J Obstet Gynecol 138:703, 1980 |
|
Hanson MA: The importance of baro- and chemo-reflexes in the control of the fetal cardiovascular system. J Dev Physiol 10:491, 1988 |
|
Rudolph AM, Heymann MA: Control of the foetal circulation. In: Comline RS, Dawes GS, Nathanielsz PW (eds): Foetal and Neonatal Physiology: Proceedings. Sir Joseph Barcroft Centenary Symposium. London, Cambridge University Press, 1973 |
|
Brace RA, Gold PS: Fetal whole-body interstitial compliance, vascular compliance, and capillary filtration coefficient. Am J Physiol 247:R 800, 1984 |
|
Longo LW: Respiratory gas exchange in the placenta. In: Fishman AP (ed): Handbook of Physiology, The Respiratory System, Gas Exchange. Bethesda, MD, American Physiological Society, 1987 |
|
Soothill PW, Nicolaides KH, Rodeck CH et al: Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Therapy 1:168, 1986 |
|
Greiss FC: Concepts of uterine blood flow. In: Wynn RM (ed): Obstetrics and Gynecology Annual, Vol 2. New York, Appleton-Century-Crofts, 1973 |
|
Gill RW, Trudinger BJ, Garrett WJ et al: Fetal umbilical venous flow measured in utero by pulsed Doppler and B-mode ultrasound. Am J Obstet Gynecol 139:720, 1981 |
|
Cohn HE, Sacks EJ, Heymann MA et al: Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol 120:817, 1974 |
|
Yaffe H, Parer JT, Block BS et al: Cardiorespiratory responses to graded reductions of uterine blood flow in the sheep fetus. J Dev Physiol 9:325, 1987 |
|
Low JA, Pancham SR, Worthington D et al: The acid-base and biochemical characteristics of intrapartum fetal asphyxia. Am J Obstet Gynecol 121:446, 1975 |
|
Mann LI, Tejani NA, Weiss RR: Antenatal diagnosis and management of the small-for-gestational age fetus. Am J Obstet Gynecol 120:995, 1974 |