Ultrasound Diagnosis of Fetal Anomalies
Authors
INTRODUCTION
The recent development of high-resolution ultrasound equipment has markedly improved the diagnostic accuracy of ultrasound. In particular, the introduction of high-frequency vaginal probes has enabled early diagnosis of certain fetal abnormalities from the 12th to 14th week of pregnancy. Such early testing is of special importance for women with a history of pregnancies associated with birth defects. The frequency with which different organ systems can be imaged is shown in Table 1.1 The sequential appearance of fetal neural structures during the first trimester of pregnancy is shown in Table 2.2
TABLE 1. Frequency in Which Specific Fetal Anatomic Structures Are Visualized by Transvaginal Sonography from Approximately 10 to 14 Weeks' Gestation*
Gestation (weeks) | <10 | 10–12 | 12–14 | >14 |
Patients | 19 | 34 | 65 | 26 |
Four chamber | - | 13 (37) | 44 (68) | 21 (81) |
Head | - | 13 (37) | 43 (66) | 16 (62) |
Spine | - | 5 (15) | 28 (43) | 8 (31) |
Stomach | 2 (10) | 19 (56) | 62 (95) | 24 (92) |
Kidneys | - | 5 (15) | 38 (58) | 14 (54) |
Bladder | 3 (6) | 14 (42) | 52 (80) | 23 (88) |
*Percentages are shown in parentheses. Study included 150 low-risk pregnancies and utilized Acuson equipment and a 5-MHz transducer.
(Adapted from Johnson P, Sharland G. Maxwell D, Allan L: The role of transvaginal sonography in the early detection of congenital heart disease. Ultrasound Obstet Gynecol 2:2481, 1992).
TABLE 2. Sequential Appearance of Fetal Neural Structures During the First Trimester of Pregnancy
| Mentstrual Age (Weeks) | |||||||
Structure | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
Cephalic pole |
| ________________________________→ |
|
| ||||
Univentricular system |
| __________________→ |
|
|
| |||
Falx cerebri |
|
|
| ____________________→ |
| |||
Biventricular system |
|
|
| ______________________→ |
| |||
Choroid plexus |
|
|
| ______________________→ |
| |||
Thalamus |
|
|
|
|
| ___________→ |
| |
Third Ventricle |
|
|
|
|
| ___________→ |
| |
Corpus callosum |
|
|
|
|
| ___________→ |
| |
Cerebral peduncles |
|
|
|
|
| ___________→ |
| |
Pons |
|
|
|
|
|
| ____→ | |
Cerebellum |
|
|
|
|
|
| ____→ | |
Cerebellar tentorium |
|
|
|
|
|
| ____→ | |
Hippocampus |
|
|
|
|
|
| ____→ | |
Posterior fossa (cisterna magna) |
|
|
|
|
|
| ____→ | |
IV ventricle |
|
|
|
|
|
| ____→ | |
Cerebral arteries |
|
|
|
|
| ______→ |
| |
Corpus striatum |
|
|
|
|
|
| ____→ | |
Calvarial skeleton |
|
|
|
|
|
| ____→ | |
Spinal skeleton |
|
|
|
|
|
| _→ | |
(Achiron R, Achiron A: Transvaginal ultrasonic assessment of the early fetal brain. Ultrasound Obstet Gynecol 1:336, 1991)
The examination for the detection of congenital anomalies is referred to as either detailed ultrasound study or targeted imaging for fetal anomalies (TIFFA)3, 4 In such examinations, a variety of fetal anatomic views (“targets”') are specifically sought after and imaged by experienced ultrasonographers. The indications for TIFFA are listed in Table 3.
TABLE 3. Targeted Imaging for Fetal Anomalies: Indications
Poor obstetric history
History of previous affected fetus: multifactorial, genetic or chromosomal abnormality
Findings on standard obstetric study that increases risk for aneuploidy: choroid plexus cyst, pyelectases, hyperechogenic bowel, two vessel umbilical cord, heart defect, cleft lip, increased nuchal thickness, cystic hygroma, hydrops, diaphragmatic hernia, omphalocele, short humerus or femur bone.
Findings on standard obstetric study that increase risk for spina bifida: lemon/banana sign.
Inability to obtain a specific anatomic view on standard study.
Abnormal level of maternal serum or amniotic fluid volume alpha-fetoprotein.
Abnormal maternal triple-screen risk.
Amniotic fluid volume abnormalities.
Intrauterine growth restriction.
Breech at term.
Maternal diabetes mellitus.
Advanced maternal age.
History of adoption.
Exposure to teratogen.
Absent end diastolic velocity by umbilical Doppler study.
Other indications (maternal anxiety, thick placenta)
Despite these technical advances, ultrasound accuracy remains dependent on gestational age. For example, if only one ultrasound examination is to be performed in the second trimester of pregnancy, the best time would be between weeks 19 and 20. Accuracy also depends on the skill of the ultrasonographer in obtaining the correct anatomic views and in differentiating between normal and abnormal structures.
The ultrasonographer also plays an essential role in 1) interpretation of the pathophysiology of a discovered defect, and 2) delineating the risk involved. For example, the risk for spina bifida in a woman with elevated maternal serum alpha-fetoprotein is much lower if a detailed ultrasound study is normal (Table 4).5 Thus, the need for amniocentesis to evaluate amniotic fluid alpha-fetoprotein (which is a better predictor of spina bifida than maternal serum alpha-fetoprotein) should be reevaluated in relation to the newly assigned risk (see Table 4).5 Similarly, the risk for autosomal trisomy is lower than the age-related or maternal triple-screen risk in women with a normal second trimester fetal ultrasound examination (Table 5, Table 6, Table 7 and Table 8.)
TABLE 4.Risks of Open Spina Bifida and Ventral Wall Defect by Population Incidence and MSAFP in Patients Who Have a Negative Ultrasonographic of 95% Sensitivity*
AFP | Ventral | Prior Incidence of Open Spina Bifida per 1000 | ||||||||
Multiples | Wall Defect |
| ||||||||
of Median | Only | 0.1 | 0.2 | 0.4 | 0.8 | 1 | 2 | 3.5 | 5 | 25 |
2 | 3600 | 18000 | 9000 | 4500 | 2300 | 1800 | 900 | 510 | 360 | 71 |
| 16000 | 79000 | 39000 | 20000 | 9800 | 7900 | 3900 | 2200 | 1600 | 310 |
| 69000 | 340000 | 170000 | 86000 | 43000 | 34000 | 17000 | 9800 | 6800 | 1300 |
2.5 | 1500 | 4700 | 2400 | 1200 | 590 | 470 | 240 | 140 | 95 | 19 |
| 6300 | 21000 | 10000 | 5100 | 2600 | 2100 | 1000 | 590 | 410 | 81 |
| 28000 | 90000 | 45000 | 22000 | 11000 | 9000 | 4500 | 2600 | 1800 | 350 |
3 | 590 | 1500 | 740 | 370 | 190 | 150 | 75 | 43 | 31 | 6.8 |
| 2600 | 6500 | 3200 | 1600 | 810 | 650 | 320 | 190 | 130 | 26 |
| 11000 | 28000 | 14000 | 7000 | 3500 | 2800 | 1400 | 800 | 560 | 110 |
3.5 | 250 | 530 | 270 | 130 | 68 | 54 | 28 | 16 | 12 | 3.1 |
| 1100 | 2300 | 1200 | 580 | 290 | 230 | 120 | 67 | 47 | 10 |
| 4700 | 10000 | 5100 | 2500 | 1300 | 1000 | 510 | 290 | 200 | 41 |
4 | 110 | 210 | 110 | 54 | 28 | 22 | 12 | 7.1 | 5.2 | 1.8 |
| 470 | 930 | 460 | 230 | 120 | 94 | 47 | 27 | 19 | 4.6 |
| 2000 | 4000 | 2000 | 1000 | 510 | 400 | 200 | 120 | 81 | 17 |
4.5 | 50 | 93 | 47 | 24 | 13 | 10 | 5.6 | 3.6 | 2.8 | 1.4 |
| 210 | 400 | 200 | 100 | 51 | 41 | 21 | 12 | 9 | 2.6 |
| 930 | 1800 | 880 | 440 | 220 | 180 | 88 | 51 | 36 | 7.8 |
5 | 24 | 44 | 22 | 12 | 6.3 | 5.3 | 3.1 | 2.2 | 1.9 | 1.2 |
| 100 | 190 | 94 | 48 | 24 | 20 | 10 | 6.3 | 4.7 | 1.7 |
| 440 | 810 | 410 | 200 | 100 | 82 | 42 | 24 | 17 | 4.2 |
*Each number in the table is the denominator of the risk with a standard numerator of 1.
The left-hand column gives risk of ventral wall defects, and the remaining columns give risk of open spina bifida based on prior population incidence. In each column, the upper figure provides the risk based on prior incidence and on MSAFP.
The lower figure in each column is boldface and is the best estimate of risk combining prior population incidence, MSAFP, and normal ultrasound.
MSAFP = maternal serum alpha-fetoprotein.
(Thornton JG, Lilford RJ, Newcombe RG: Tables for estimation of individual risks of fetal neural tube and ventral wall defects, incorporating prior probability, maternal serum alpha-fetoprotein, and ultrasonographic examination results. Am J Obstet Gynecol 164:154, 1991)
TABLE 5. Ultrasound Findings Associated With Increased Risk For Aneuploidy
Women Younger than 33 Years of Age | Women Older than 33 Years of Age |
(An Abnormal Score is | (An Abnormal Score is |
2 Nuchal Fold | 2 Nuchal fold |
2 Cardiac, CNS or other anatomic defect | 2 Cardiac, CNS or other 2 anatomic defect |
1 Short FL/expected FL* | 1 Short FL/expected FL* |
1 Short HL/expected JL † | 1 Short HL/expected JL † |
1 Pyelectasis ‡ | 1 Pyelectasis ‡ |
| 1 Echogenic bowel |
| 1 Choroid plexus cyst |
Abnormal scores in both age groups increase risk for autosomal trisomy to a level greater than 1:182-the standard age related risk of a 35-year-old pregnant woman at 16 weeks' gestation. As a result, an abnormal score warrants counseling for possible assessment of fetal karyotype. Sensitivity and specificity of abnormal score are > 80%. Similarly, a normal score of 0 after 33 years of age would decrease the risk for aneuploidy (see Table 6).
*Expected femur length (mm) = -9.3105 + 0.9028 × biparietal diameter, value abnormal if measured/expected length .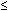 0.9.
0.9.
†Expected humeral length (mm) = -7.904 + 0.849 × biparietal diameter, value abnormal if measured/expected length < 0.9.
‡Anteroposterior diameter of renal pelvis 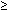 4 mm.
4 mm.
(Adapted from Benacerraf BR, Neuberg D, Bromley et al: Sonographic scoring index for prenatal detection of chromosomal abnormalities. J Ultrasound Med 11:449,1992).
TABLE 6.Risks for Autosomal Trisomy Before and After Sonographic Score of 0* When maternal Age Falls Between 33 and 46 Years of Age
|
| After Scan | Lower Limit† |
Maternal Age | Before Scan | (Score = 0) | 95% CI |
33 | 1 : 294 | 1 : 1666 | 0.9 |
34 | 1 : 232 | 1 : 1428 | 1.2 |
35 | 1 : 182 | 1 : 1111 | 1.5 |
36 | 1 : 140 | 1 : 833 | 2.0 |
37 | 1 : 112 | 1 : 666 | 2.5 |
38 | 1 : 87 | 1 : 536 | 3.2 |
39 | 1 : 68 | 1 : 400 | 4.1 |
40 | 1 : 53 | 1 : 312 | 5.3 |
41 | 1 : 42 | 1 : 244 | 6.8 |
42 | 1 : 33 | 1 : 192 | 8.7 |
44 | 1 : 20 | 1 : 116 | 14.5 |
46 | 1 : 12 | 1 : 68 | 24.3 |
*Score of 0 = normal ultrasound findings. Note that a normal score of 0 after 33 years of age would decrease the risk of aneuploidy necessitating reevaluation of need for assessment of fetal karyotype.
† Lower limit of the Confidence Interval for sensitivity and specificity.
(Adapted from Nadel AS, Bromley B, Frigoletto FD JR, Benacerraf BR: Can the presumed risk of autosomal trisomy be decreased in fetuses of older women following a normal sonogram? J Ultrasound Med 14:297, 1995).
TABLE 7.Midtrimester Risk for Trisomy 21 on Basis of Maternal Age in Structurally Normal Fetus as Modified by Ultrasonography (All Risks Are Expressed as 1/ x)
|
|
|
|
|
| Short | Pyelectasis |
|
|
|
|
|
| Short | Short | Short | Femur and | with Short |
|
|
|
Maternal | Age or Triple | Normal | Femur | Femurby | Humerus | Humerusby | Femur | Nuchal | Echogeni |
|
Age (yr) | Screen Risk | Ultrasonography | byBPD/FL | O/E | by O/E | O/E | orHumerus | Fold | cBowel | ShortEar |
20 | 1231 | 18,871 | 260 | 463 | 301 | 108 | 194 | 29 | 91 | 194 |
21 | 1145 | 17,553 | 242 | 431 | 280 | 100 | 181 | 27 | 85 | 180 |
22 | 1065 | 16,326 | 225 | 401 | 261 | 93 | 168 | 26 | 79 | 168 |
23 | 1000 | 15,330 | 212 | 376 | 245 | 88 | 158 | 24 | 74 | 157 |
24 | 942 | 14,441 | 199 | 354 | 231 | 83 | 149 | 23 | 70 | 148 |
25 | 887 | 14,598 | 188 | 334 | 217 | 78 | 140 | 21 | 66 | 140 |
26 | 842 | 12,908 | 178 | 317 | 206 | 74 | 133 | 20 | 63 | 133 |
27 | 798 | 12,233 | 169 | 300 | 196 | 70 | 126 | 19 | 59 | 126 |
28 | 755 | 11,574 | 160 | 287 | 185 | 66 | 120 | 18 | 56 | 119 |
29 | 721 | 11,053 | 153 | 271 | 177 | 64 | 114 | 18 | 54 | 114 |
30 | 685 | 10,501 | 145 | 258 | 168 | 60 | 109 | 17 | 51 | 108 |
31 | 650 | 9,964 | 138 | 245 | 159 | 57 | 103 | 16 | 49 | 103 |
32 | 563 | 8,631 | 120 | 212 | 138 | 50 | 89 | 14 | 42 | 89 |
33 | 452 | 6,929 | 96 | 170 | 111 | 40 | 72 | 11 | 34 | 72 |
34 | 352 | 5,396 | 75 | 133 | 87 | 31 | 56 | 9 | 27 | 56 |
35 | 274 | 4,200 | 59 | 104 | 68 | 25 | 44 | 7 | 21 | 44 |
36 | 213 | 3,265 | 46 | 81 | 53 | 19 | 34 | 6 | 17 | 34 |
37 | 166 | 2,545 | 36 | 63 | 41 | 15 | 27 | 5 | 13 | 27 |
38 | 129 | 1,978 | 28 | 49 | 32 | 12 | 21 | 4 | 10 | 21 |
39 | 100 | 1,533 | 22 | 38 | 25 | 10 | 17 | 3 | 8 | 17 |
40 | 78 | 1,196 | 17 | 30 | 20 | 8 | 13 | 3 | 7 | 13 |
41 | 61 | 935 | 14 | 24 | 16 | 6 | 10 | 2 | 5 | 10 |
42 | 47 | 721 | 11 | 18 | 12 | 5 | 8 | 2 | 4 | 8 |
43 | 37 | 567 | 9 | 15 | 10 | 4 | 7 | 2 | 4 | 7 |
44 | 29 | 445 | 7 | 12 | 8 | 3 | 5 | 2 | 3 | 5 |
45 | 22 | 337 | 5 | 9 | 6 | 3 | 4 | 1 | 3 | 4 |
46 | 17 | 261 | 4 | 7 | 5 | 2 | 4 | 1 | 2 | 4 |
47 | 13 | 199 | 4 | 6 | 4 | 2 | 3 | 1 | 2 | 3 |
48 | 10 | 153 | 3 | 4 | 3 | 2 | 2 | 1 | 2 | 2 |
49 | 8 | 123 | 3 | 4 | 3 | 2 | 2 | 1 | 2 | 2 |
FL, femur length: O, observed; E, expected (mean).
Note, as an example, that at maternal age of 36 years the risk for Down Syndrome is 1 : 213. However, following a normal second trimester scan the risk can be adjusted to 1 : 3265. As a result assessment of fetal karyotype would become unnecessary.
(Adapted from Vintzileos AM, Egan JFX: Adjusting the risk for trisomy 21 on the basis of second trimester ultrasonography. Am J Obstet Gynecol 172:837, 1995).
TABLE 8.Midtrimester Risk for Trisomy 21 on Basis of Triple Screen in Structurally Normal Fetus as Modified by Ultrasonography (All Risks Are Expressed as 1/ x)
|
|
|
|
|
| Pyelectasis |
|
|
|
Triple |
|
|
| Short | Short Femur | with Short |
|
|
|
Screen | Normal | Short Femur | Short Femur | Humerus | and Humerus | Femur | Nuchal | Echogeni |
|
Risk | Ultrasonography | by BPD/FL | by O/E | by O/E | by O/E | orHumerus | Fold | c Bowel | Short Ear |
15,000 | 160,000 | 3163 | 5631 | 3661 | 1303 | 2359 | 347 | 1100 | 2348 |
14,500 | 154,667 | 3057 | 5444 | 3539 | 1260 | 2281 | 335 | 1063 | 2270 |
14,000 | 149,333 | 2952 | 5256 | 3417 | 1216 | 2202 | 324 | 1026 | 2192 |
13,500 | 144,000 | 2846 | 5068 | 3295 | 1173 | 2124 | 312 | 990 | 2114 |
13,000 | 138,667 | 2741 | 4881 | 3173 | 1129 | 2045 | 300 | 953 | 2035 |
12,500 | 133,333 | 2636 | 2693 | 3051 | 1086 | 1966 | 289 | 917 | 1957 |
12,000 | 128,000 | 2530 | 4505 | 2929 | 1043 | 1888 | 277 | 880 | 1879 |
11,500 | 122,667 | 2425 | 4318 | 2807 | 999 | 1809 | 266 | 843 | 1801 |
11,000 | 117,333 | 2319 | 4130 | 2685 | 956 | 1730 | 254 | 807 | 1722 |
10,500 | 112,000 | 2214 | 3942 | 2563 | 912 | 1652 | 243 | 770 | 1644 |
10,000 | 106,667 | 2109 | 3754 | 2441 | 869 | 1573 | 231 | 733 | 1566 |
9,500 | 101,333 | 2003 | 3567 | 2319 | 826 | 1495 | 220 | 697 | 1488 |
9,000 | 96,000 | 1898 | 3379 | 2197 | 782 | 1416 | 208 | 660 | 1409 |
8,500 | 90,667 | 1792 | 3191 | 2075 | 739 | 1337 | 197 | 624 | 1331 |
8,000 | 85,333 | 1687 | 3004 | 1953 | 695 | 1259 | 185 | 587 | 1253 |
7,500 | 80,000 | 1582 | 2816 | 1831 | 652 | 1180 | 174 | 550 | 1175 |
7,000 | 74,667 | 1476 | 2628 | 1709 | 609 | 1102 | 162 | 514 | 1096 |
6,500 | 69,333 | 1371 | 2441 | 1587 | 565 | 1023 | 151 | 477 | 1018 |
6,000 | 64,000 | 1266 | 2253 | 1465 | 522 | 944 | 139 | 440 | 940 |
5,500 | 58,667 | 1160 | 2065 | 1343 | 478 | 866 | 128 | 404 | 862 |
5,000 | 53,333 | 1055 | 1878 | 1221 | 435 | 787 | 116 | 367 | 783 |
4,500 | 48,000 | 949 | 1690 | 1099 | 392 | 708 | 135 | 331 | 705 |
4,000 | 42,667 | 844 | 1502 | 977 | 348 | 630 | 93 | 294 | 627 |
3,500 | 37,333 | 739 | 1315 | 855 | 305 | 551 | 82 | 257 | 549 |
3,000 | 32,000 | 633 | 1127 | 733 | 261 | 473 | 70 | 221 | 470 |
2,500 | 26,667 | 528 | 939 | 611 | 218 | 394 | 59 | 184 | 392 |
2,000 | 21,333 | 422 | 751 | 489 | 175 | 315 | 47 | 147 | 314 |
1,500 | 16,000 | 317 | 564 | 367 | 131 | 237 | 36 | 111 | 236 |
1.000 | 10,667 | 212 | 376 | 245 | 88 | 158 | 24 | 74 | 157 |
500 | 5,333 | 106 | 188 | 123 | 44 | 80 | 13 | 38 | 79 |
FL, Femur length; O, observed; E, expected (mean)
Note: The finding of short femoral and humeral lengths, relative to expected values (see Table 5), would adjust a triple screen risk for Down syndrome from 1 : 2000 to 1 : 175. This adjustment suggests that further testing to assess fetal karyotype would now be warranted.
(Adapted from Vintzileos AM, Egan JFX: Adjusting the risk for trisomy 21 on the basis of second trimester ultrasonography. Am J Obstet Gynecol 172:837, 1995)
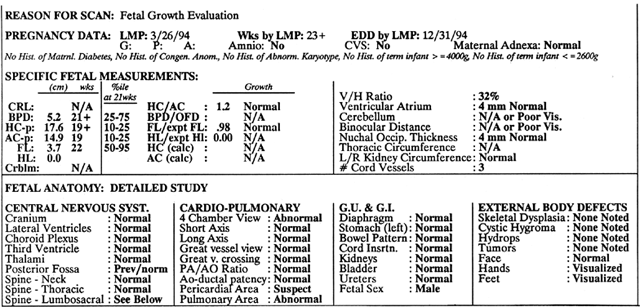
TIFFA examinations entail ultrasonic visualization of at least 34 fetal views and measurement of seven specific sites (Fig. 1). For each of these views, the ultrasonographer should indicate whether the structure is normal, previously normal, not applicable, suspect, abnormal, or poorly visualized. Poor visualization occurs when (1) fetal movement or position does not allow adequate imaging of a specific area, or (2) gestational age is less than 19 to 20 weeks.
A normal TIFFA scan takes approximately 1 hour to complete. However, the presence of an anomaly is likely to extend the study and make it necessary for the mother to undergo a combination of genetic counseling, amniocentesis, or chorionic villi sampling to define fetal karyotype.
Studies that definitively resolve any possible concern regarding the long-term safety of ultrasound as well as its cost:benefit analysis are not yet available.6
Ewigman and co-workers7 evaluated the efficacy of routine antenatal diagnostic imaging with ultrasound (RADIUS) in reducing adverse perinatal outcome. Secondary outcomes included maternal morbidity and diagnostic accuracy of ultrasound. Study subjects were recruited from 92 obstetric and 17 family medicine practices in six states. Selection was based on predefined entry criteria that placed all study patients (screened group) in a low-risk category. Ultrasound screening examinations were performed on 7812 study subjects during two pregnancy intervals: 15 to 22 weeks, and 31 to 35 weeks. The women (n = 7718) in the control group had an ultrasound examination performed only if it was clinically indicated. The sensitivity in the detection of congenital anomalies was significantly higher in the screened versus control groups (34.8% vs 11%, p < 0.01); however, the higher detection of anomalies did not result in improved perinatal outcome. Romero8 and others9, 10 attribute the lack of improvement in perinatal outcome to (1) the limited sensitivity in the detection of anomalies in the RADIUS study, and (2) the low rate of pregnancy termination in women diagnosed with a fetal anomaly.
The low sensitivity (16.6%) in the detection of anomalies in the RADIUS study, particularly before 24 weeks' gestation, is very disturbing because it does not represent the current diagnostic capability of ultrasound. Specifically, in four studies11, 12, 13, 14 published from 1990 to 1992, the sensitivity of ultrasound screening for congenital anomalies before 24 weeks' gestation was 40%, 60.7%, 74.4%, and 84.3%, respectively. The specificity and negative predictive values were greater than 98%.11,12, 13, 14 Except for the Helsinki trial,11 the positive predictive values in these studies were equal to or greater than 98%.12, 13, 14
CONGENITAL HEART DISEASE
The frequency of congenital heart disease (CHD) is approximately 6 to 8 per 1000 newborns, and the recurrence risk is 8%. In 25% of cases, the etiology can be related to familial, chromosomal, or environmental causes (Table 9).15 The prenatal risk factors for CHD are shown in Table 10.16 The overall survival rate of fetuses with CHD remains poor (17% to 24%).17, 18
TABLE 9. Major Monogenic, Chromosomal, and Environmental Etiologies of Congenital Heart Disease and Arrhythmias
Monogenic
Hypoplastic left heart syndrome (? AR)
Hypoplastic right heart syndrome (? AR)
Endocardial fibroelastosis (AR ?, XLR)
Complete heart block (AD)
Supravalvular aortic stenosis (AR)
Wolff-Parkinson-White syndrome (AD)
Apert, Noonan, Holt-Oram, Marfan, osteogenesis imperfecta, tuberous sclerosis, and Ehlers-Danlos syndromes; all AD
Ellis-Van Creveld, Carpenter, Meckel-Gruber, and Laurence-Moon-Biedl syndromes; some forms of mucopolysaccaridosis, mucolipidosis, and glycogenosis; thrombocytopenia, absent radius
Duchenne and Dreifus muscular dystrophies (XLR)
Chromosomal
Trisomy 21 (50% incidence of CHD: VSD, ASD, cushion defect)
Trisomy 18 (99% incidence of CHD: VSD, PS)
Trisomy 13 (90% incidence of CHD: VSD, dextrocardia)
Trisomy 22 (65+% incidence of CHD: VSD, ASD)
Trisomy 8 (mosaic, 50% incidence of CHD: VSD, ASD
Trisomy 9 (mosaic, 50% incidence of CHD: VSD, coarct, DORV)
+14q-(50% incidence of CHD: ASD, Tet)
18q-(50% incidence of CHD: VSD)
4p-and 5p-(incidence of CHD 40% and 20%, respectively (VSD, ASD)
XO Turner (35% incidence of CHD: coarct, ASD, AS)
Environmental
Maternal diabetes mellitus (3%–5% incidence of CHD: VSD, TGA, coarct, cardiomyopathy)
Phenylketonuria (25%–50% incidence of CHD: VSD, ASD, tet)
Rubella (35% incidence of CHD: VSD, ASD, PS)
Lithium (10% incidence of CHD: Ebstein's anomaly, tricuspid atresia, ASD)
Alcohol (25%–30% incidence of CHD: VSD, ASD)
Amphetamines (5%–10% incidence of CHD: VSD, TGA)
Hydantoin (2%–3% incidence of CHD: PS, AS, Coarct)
Trimethadione (15%–30% incidence of CHD: TGA, HLHS)
AD = autosomal dominant; AR = autosomal recessive, AS = aortic stenosis; ASD = atrial septal defect; coarct = coarctation of the aorta; DORV = double-outlet right ventricle; HLHS = hypoplastic left heart syndrome; PS = pulmonary stenosis; tet = tetralogy of Fallot; TGA = transposition of the great arteries; VSD = ventricular septal defect; XLR = X-linked recessive.
(Modified from Nora N: Genetics and Counseling in Cardiovascular Disease. Springfield, Charles C Thomas. 1978)
TABLE 10. Prenatal Risk Factors for Congenital Heart Disease
Fetal Risk Factors
Intrauterine growth retardation
Fetal cardiac dysrhythmia
Abnormal karyotype (trisomy 13–18 and 21, Turner's syndrome)
Noncardiovascular structural anomalies
Maternal Risk Factors
Heart disease
Congenital
Acquired
Drug ingestions
Alcohol
Narcotics
Amphetamines
Anticonvulsants
Lithium
Birth control pills
Other sex hormones
Polyhydramnios
Oliogohydramnios
Rh sensitization
Diabetes mellitus
Phenylketonuria
Preeclampsia
Collagen vascular disease
Familial Risk Factors
Congenital heart disease
Genetic syndromes
(DeVore GR. The prenatal diagnosis of congenital heart disease: A practical approach for the fetal sonographers. J Clin Ultrasound 13:229, 1985)
The examination of the fetal heart includes evaluation of many views, including the four chambers, the parasagittal view (also known as the left ventricular outflow tract), the short axis, the great vessels, and the aortic arch.
The Four-Chamber View
The structures that can be visualized in the four-chamber view are as follows:
- Both ventricles, including the position or' the heart in the chest and the appearance of the pericardial and pulmonary areas
- The interventricular septum (IVS)
- The atrioventricular valves
- The septum primum and secundum (Fig. 2).
The ultrasonographer should obtain a number of views of the four chambers to gain adequate visualization of all these structures (Fig. 3).
THE VENTRICLES.
Although both ventricles should be similar in thickness and size, the right ventricle is normally characterized by the appearance of the moderator band, which consists of thickened trabeculae extending from the septum to the parietal wall of the right ventricle (see Fig. 3A). Absence of the moderator band from its normal location implies corrected or L-transposition of the great arteries. In this abnormality, the right ventricle functions as the left chamber, assuming the role of pumping blood into the aorta. Thus, in contrast to D-transposition, L-transposition allows blood flow to the lungs and body to be maintained normally.
Hyperechogenicity or thickness of the ventricular walls or ventricular septum, or both, is consistent with cardiomyopathy (Fig. 4).
In dextroversion, the apex of the heart points to the right side of the chest. The main differential diagnoses of this condition are cystic adenomatoid malformation of the lungs, diaphragmatic hernia, and polysplenia. In levoversion the apex of the heart points to left side of the chest excessively; the main differential diagnosis of this condition includes left pulmonary hypoplasia and a right-sided pulmonary mass.
THE INTERVENTRICULAR SEPTUM.
Defects in the IVS constitute the most common form of congenital heart disease and can occur in various locations within the septum. Ultrasonic visualization of small ventricular septal defects, regardless of location, may not be possible in the four-chamber plane. For example, membranous or perimembranous defects (located adjacent to the aortic valve) are situated below the anterior portion of the IVS and are not visualized in the four-chamber plane (Fig. 5). Such defects usually are depicted in the parasagittal plane (Fig. 6).
THE ATRIOVENTRICULAR VALVES.
The septal insertion of the tricuspid valve should be visualized slightly below that of the mitral valve (see Fig. 2 and Fig. 3). If the insertion is lower, Ebstein anomaly should be considered. Interestingly, hyperechogenicity limited to the area of the mitral valve is usually inconsequential; that is, cardiomyopathy is not a consideration.
THE SEPTUM PRIMUM AND SECUNDUM.
The foramen ovale is the opening between both atrial septi, allowing blood to flow from the right atrium to the left. In endocardial cushion defect, a single common atrioventricular valve is noted superior to a large ventricular septal defect.
Diagnostic Utility of the Four-Chamber View
Because the four-chamber view depicts so many cardiac structures and is also the plane used to evaluate cardiac arrhythmia, it is included in all obstetric studies. Copel and associates19 showed that 92% of cardiac defects may be discovered by some abnormality in the four-chamber view and proposed routine screening for CHD. After including the four-chamber view in their examinations, Vergani and colleagues20 improved their CHD diagnosis sensitivity from 43% to 81%. Subsequently, Sharland and Allan21 showed that after routinely including the four-chamber view in obstetric studies, 53 fetuses were referred for possible CHD over a period of 32 months; previously, from 1980 to 1988, only 8 fetuses had been referred. They reported a 77% sensitivity for the four-chamber view in the diagnosis of CHD. Studies by Bromley and co-workers22 and Wigton and associates,23 however, suggest that the sensitivity of the four-chamber view in detecting CHD is even lower, ranging from 69% to 39%, respectively. This is understandable because the four-chamber view does not allow for assessment of the pulmonary and aortic arteries-outflow tracts that are evaluated best in the parasagittal, short axis, and great vessel views.
Parasagital View
The parasagittal view encompasses the left ventricle. aortic outflow, a portion of the right ventricle, and the left atrium (see Fig. 6). This plane is significant for two main reasons:
- The aorta at the level of the aortic valve can be readily assessed.
- Membranous IVS defects (located adjacent to the aortic outflow tract and below the plane of the four chamber view) stand a better chance of being visualized in this view.
Short Axis View
The short axis view (Fig. 7) encompasses the aortic and pulmonary valves as well as the ductus arteriosus and the right branch of the pulmonary artery. It is used to evaluate the size of the aorta and pulmonary arteries at the valvular level.
Great Vessel View
The great vessel view depicts the relatively parallel relationship of the superior vena cava, aorta, and pulmonary arteries after the latter two cross each other (Fig. 8). Clear visualization of this plane indicates that the crossing of the aorta and pulmonary artery is normal and virtually rules out transposition of the great vessels. Aortic or pulmonary stenosis also can be recognized in this view.
In truncus arteriosus, the pulmonary artery is not clearly depicted by ultrasound. Instead, the aortic outflow tract is large and the aorta is overriding.
The great vessel view also allows a comparative measurement of the two arteries a short distance beyond the valvular level; the measurements should be obtained from the inner aspects of these arteries. Normally the ratio of pulmonary artery: aorta has a mean value of 1.09 (range = 0.75 to 1.43) and is independent of gestational age.24
Aortic Arch
The aortic arch is depicted in Figure 9. Coarctation of the aorta most frequently occurs at the isthmus, the area of the arch at the insertion site of the ductus arteriosus. The isthmus is normally narrow in the fetus but dilates in the newborn after the ductus closes. Because narrowing in the isthmus occurs normally, in utero diagnosis of coarctation of the aorta is difficult.
ABNORMALITIES OF THE CHEST AND GASTROINTESTINAL TRACT
Esophageal Atresia
This is a very rare anomaly occurring at a rate of 1:25,000 births. In 10% of cases it can be characterized ultrasonographically by a dilated proximal esophagus, absence of stomach echo, and polyhydramnios.25 In the remaining 90% of cases, however, the defect cannot be ultrasonically recognized because of abnormal communication channels between the proximal aspects of the esophagus, trachea, and stomach, which result in free flow of amniotic fluid.
Pulmonary Cystic Adenomatoid Malformation
Three types of pulmonary cystic adenomatoid malformation have been characterized26:
Type I: The cysts are large (
Type II: The cysts are less than 1 cm in diameter, but other anomalies of the renal or gastrointestinal tract may be present.
Type III: The lesion is microcystic, brightly echogenic, and large, and its presence is associated with a poor prognosis (see Fig. 10).
A pulmonary cystic adenomatoid malformation can result in a right or left shift of the mediastinum (see Fig. 10). The differential diagnosis includes pulmonary sequestration and diaphragmatic hernia.27
Pulmonary Hypoplasia
Pulmonary hypoplasia is associated with a high mortality rate. Lung weight at autopsy is
TABLE 11. Etiology of Pulmonary Hypoplasia
Etiology | Example |
Hydrothorax | Fetal hydrops |
Pulmonary tumor | Cystic adenomatoid malformation |
Chest mass | Teratoma, anterior thoracic meningocele |
Elevation of the diaphragm | Ascites, obstructive uropathy |
Oligohydramnios | Severe obstructive uropathies, intrauterine growth |
| restriction (IUGR) |
Diaphragmatic hernia | Prolonged rupture of membranes |
Although high-resolution equipment has enabled lung tissue to be visualized adjacent to the heart, most studies dealing with antenatal diagnosis of pulmonary hypoplasia have focused on reduced chest size as a predictor for the condition.29, 30 More recently, Vintzileos and colleagues31 showed that the highest sensitivity (85%) and positive predictive value (83%) for the diagnosis of pulmonary hypoplasia could be obtained with the following ratio:
[chest area - heart area/chest area] × 100
The chest area should be traced at the midpoint of the chest wall. The ratio is independent of gestational age and is considered abnormal when it is less than or equal to 62 (5th percentile). The ratio can be used when the abdominal circumference is large (as in ascites or obstructive uropathy) or small (as in omphalocele). It is not applicable to fetuses with diaphragmatic hernia because chest size is artificially enlarged in this condition.
Diaphragmatic Hernia
Congenital defects in the diaphragm result in herniation of bowel, stomach, and possibly parts of the liver and pancreas, into the chest. The hernia displaces the heart and mediastinum, making antenatal diagnosis possible. The most common defect (1:3000 births) involves the foramen of Bochdalek, which is situated in the posterolateral aspect of the diaphragm.32 This condition may be associated with cardiac abnormalities or aneuploidy (trisomy 13, 18, and 21).
In utero corrective surgery is possible in cases of isolated diaphragmatic hernia. The outcome of open in utero surgery, however, should be weighed against that of extracorporeal oxygen membrane oxygenation in the neonate, which has been reported to carry a success rate of only 55%. Data on this subject are still evolving.33
Another rare type of diaphragmatic hernia is found through the foramen of Morgagni. This hernia is associated with a poor prognosis because of its association with other anomalies, such as hydrocephalus, encephalocele, enlarged cisterna magna, malrotation of the gut, cardiac anomalies, and trisomies.32
The differential diagnosis of diaphragmatic hernia includes (1) eventration or displacement of visceral contents into the chest secondary to a thin diaphragm; (2) chest mass; and (3) pulmonary tumor.
Ventral Wall Defects
Fetal bowel normally is located outside the abdomen in the first trimester of pregnancy. This is known as physiologic herniation, a condition that can be appreciated with the use of high-frequency vaginal transducers.34, 35 Thus, the diagnosis of omphalocele should not be made before the 14th week of pregnancy.
True ventral wall defects occur at a rate of 1:2500 births. Two main forms are described: omphalocele and gastroschisis. In omphalocele, the fetal gut (and sometimes the liver) is outside the abdomen but is covered by a sac-like cyst interface (Fig. 11). In gastroschisis, segments of intestine are noted in the peritoneal cavity without any sac-like cover. In contrast to omphalocele, the umbilical cord in gastroschisis is inserted into the abdominal wall (see Fig. 11). Gastroschisis is not associated with other fetal abnormalities, but a chromosomal abnormality may be found in approximately 50% of fetuses with omphalocele, particularly if fetal liver is not part of the hernia.36, 37 Other anomalies involving the cardiac, skeletal, renal, and central nervous systems may also be present. The Beckwith-Weidman syndrome of organomegaly and hypoglycemia has been described in association with omphalocele.
In the presence of a ventral wall defect, the mode of delivery (vaginal delivery vs cesarean section) remains controversial.38, 39 Vaginal delivery appears safe, however, when the hernia is small and the liver is not herniated.
Obstructive Gastrointestinal Lesions and Hyperechogenic Bowel
Duodenal atresia results from failure of recanalization of the upper gastrointestinal tract at 8 weeks' gestation. It may be associated with Down syndrome and other anomalies involving the gastrointestinal. cardiac, and renal systems. This condition is characterized by dilation of the stomach and proximal part of the duodenum (double-bubble sign). Occasionally the diagnosis can be made before the 20th week of pregnancy, but it is more likely to be made after the 20th week, when the quantity of swallowed amniotic fluid exceeds the resorptive capacity of the gut.40
Small bowel obstruction results from in utero avascular necrosis of segments of jejunum and ileum. Dilation of small bowel proximal to the necrotic segments is ultrasonically recognizable by the presence of valvular flaps projecting into the lumen.
The pattern of bowel dilation in other obstructive bowel abnormalities is not specific enough to allow for a precise diagnosis. These abnormalities include malrotation of the bowel, volvulus, intussusception, and Hirschprung disease (congenital intestinal aganglionosis).
Meconium within normal bowel sometimes appears bright and has been described as hyperechogenic bowel (Fig. 12). In many fetuses that exhibit this entity, the outcome may be norma141; however, it has been associated with cystic fibrosis and aneuploidy.42 Prospective couples should be counseled regarding appropriate antenatal testing.
Differentiation between hyperechogenic bowel and meconium peritonitis may be possible. In the latter condition, bowel perforation results in extravasation of meconium into the abdominal cavity. Meconium induces an inflammatory response, formation of adhesions, and calcium deposition in the area.
URINARY TRACT ABNORMALITIES
The frequency of urinary abnormalities is approximately 7:1000 newborns, and the recurrence risk is 8% to 10%.
The etiology of urinary tract abnormalities should extend beyond the apparent site of the lesion. For example, dilation of the ureteropelvic junction (UPJ) may result either from obstruction at the upper ureter or from pathology at a more caudad level. This includes increased intraluminal pressure secondary to reflux at the level of the vesicoureteral junction resulting in dilation of the UPJ. Similarly, a posterior urethral valve (PUV) will dilate the UPJ and even produce hydronephrosis. Thus, in discussing urinary tract abnormalities, it is reasonable to start with the urethra and proceed cephalad.
Posterior Urethral Valve
Most PUVs are partial, such that the resulting bladder dilation is not severe. The ureters may only be slightly dilated, and if present, hydronephrosis is moderate (Fig. 13). In the severe form, however, the pathophysiology is severe and may resemble the findings in agenesis of the urethra. In complete PUV, the bladder is markedly dilated. The ureters also are convoluted and large. Hydronephrosis is severe. Oligohydramnios prevents appropriate pulmonary development and leads to pulmonary hypoplasia.
Early treatment of this condition (less than 20 weeks' gestation) is important to prevent permanent renal damage. The indications for therapy include the following:
- Early diagnosis (18 to 20 weeks' gestation)
- A fetal urine sample showing sodium concentrations less than 100 mg/dL, chloride < 90 mg/dL, and osmolality, 210 mOsm/dL—all indirectly pointing to the ability of the kidneys to provide the function of salt conservation
- Decreasing amniotic fluid volume but not frank oligohydramnios
A number of treatment options are available:
- Open fetal surgery to marsupialize the bladder
- A two-ended curved catheter to drain urine into the amniotic cavity (catheter insertion has been difficult, however, and is frequently followed by catheter displacement and blockage)
- Frequent bladder drainage procedures.43
To prevent pulmonary hypoplasia, treatment should be initiated by the 20th pregnancy week, Although early treatment may prevent development of pulmonary hypoplasia, however, 30% to 50% of these children may subsequently develop dysplastic kidneys and require renal transplantation.44
Prune-Belly Syndrome
In prune-belly syndrome, the bladder is dilated, the abdominal wall lax, and testicles undescended (cryptorchidism). Although this condition may result from a PUV, it is also believed that it may represent a primary mesoderm defect in the abdominal wall and urinary tract muscles.45 Fortunately in most cases of PUV the urethral obstruction is incomplete, allowing for fetal urination and maintenance of near-normal amniotic fluid volume.
The Ureteropelvic Junction
Dilation of the UPJ (Fig. 14) results from (1) abnormal recanalization of the upper part of the ureter, resulting in varying degrees of obstruction; or (2) absence of the longitudinal muscle fibers in the upper ureter and renal pelvis. In the latter dysfunction, there is abnormal forward propulsion of urine, resulting in a dilation of the UPJ.46 Importantly, dilation of the UPJ may be associated with other anomalies involving the fetal cardiac, gastrointestinal, and central nervous systems.
Further, male fetuses are more frequently affected than female (5:1). The UPJ dilation also occurs more frequently on the left side and can be unilateral in 70% of cases.
Pyelectasis
The etiology of a dilated renal pelvis or pyelectasis is similar to that of a dilated UPJ. Reflux occurring at the level of the vesicoureteral valves also may result in pyelectasis (Fig. 15).
The definition of mild or minimal pyelectasis in the literature is confusing.47 Further, the outcome of fetuses with minimal pyelectasis is not all “benign” as suggested in some studies.48 In fact, many such fetuses may require subsequent medical or surgical intervention. Thus, an anteroposterior diameter equal to or greater than 4 mm or 7 mm before and after 33 weeks' gestation, respectively, warrants postnatal follow-up.48
Moreover., approximately 3.3% of fetuses with pyelectasis in the range of 3 to 5 mm may also have chromosomal abnormalities, including Down syndrome.49, 50 Interestingly, 25% of fetuses with Down syndrome have pyelectasis, whereas only 2.8% of fetuses with a normal karyotype show this condition.49
Hydronephrosis
Dilation of the renal calyceal system results from abnormalities at a lower level of the urinary tract. It is differentiated from multicystic dysplastic disease by (1) ultrasonic visualization of radially placed calyces communicating with the renal pelvis and (2) preservation of some renocortical tissue (Fig. 16).
In utero diagnosis of pyelectasis, UPJ dilation, and hydronephrosis is significant because it identifies fetuses who will require postnatal urologic evaluation. As a result, early surgical intervention or medical treatment with antibiotics will prevent irreversible renal compromise.
Renal Agenesis
The frequency of this condition is 1:4000 births, and the risk of recurrence is 3%.51 The etiology is variable and may include chromosomal or genetic disorders (recessive or dominant mode of inheritance). It may also be part of the VATER association: vertebral defects, anal atresia, tracheoesophageal fistula, and radial and renal dysplasia. The outcome of fetuses with renal agenesis is poor because of oligohydramnios and associated pulmonary hypoplasia.
Intravenous administration of furosemide (40 mg IV) has not been helpful in differentiating renal agenesis from severe intrauterine growth restriction. Further, because of associated oligohydramnios as well as an enlarged adrenal gland that mimics renal appearance, the diagnosis by gray scale ultrasound is difficult (sensitivity, 50%).51 In suspected cases, however, the use of color Doppler velocimetry may show absence of renal arteries in bilateral renal agenesis and only one renal artery in the unilateral form of this abnormality (Fig. 17).52
Infantile Polycystic Kidney Disease
This is an autosomal recessive disorder. The kidneys are enlarged (ratio of kidney:abdominal circumference greater than the norm of 30%) and show small multicystic echoes.53 Unfortunately, the diagnosis cannot always be made before 24 weeks' gestation54 because several subtypes of infantile polycystic kidney disease exist, and these vary in onset and severity.
Multicystic-Dysplastic Kidney Disease
The etiology of this condition may be secondary to a chromosomal abnormality or a mutant gene. It is rare, occurring at a rate of 1:10,000 births, and has a male-to-female ratio of 2:1. The condition is unilateral in 75% of cases.
The etiology is related to either (1) poor induction of nephron formation or (2) obstructive uropathy. Defects in nephron formation occur early in embryogenesis, thus impeding renal development. In such cases, the kidneys are very small, each weighing as little as 1 g, and cysts are evident only via microscopic examination. This defect is classified as Potter type II-B dysplastic kidney.55 If the defect develops by 9 to 13 postmenstrual weeks, the fetal kidney assumes a large multicystic appearance (Potter type II-A; Fig. 18).
Megacystis-microcolon-intestinal hypoperistalsis (MMIH) syndrome is a fatal autosomal recessive disorder believed to result from an end-organ receptor defect confined to the smooth muscle of the urinary and gastrointestinal tracts.56, 57, 58, 59 It is characterized by a large, thick-walled bladder plus dilation and distention of the small bowel, including the duodenum.57, 58 The kidneys may also appear hydronephrotic or even multicystic. Amniotic fluid volume may be either normal or excessive. The condition is differentiated from PUV by the absence of oligohydramnios and dilation of the proximal urethra. This entity has been described only recently, so it is not clear whether diagnosis by ultrasound is always possible before 24 weeks' gestation.
ANOMALIES OF THE CENTRAL NERVOUS SYSTEM
The frequency of central nervous system anomalies varies according to geographic area and race. It is approximately 2:1000 newborns, and the recurrence risk is 1% to 2%.
Anencephaly
In anencephaly, the cerebral hemispheres and overlying skull and scalp are absent. In exencephalus, the cranial bones are also absent, but a large amount of disorganized brain tissue is present. The diagnosis can now be made in the latter part of the first trimester of pregnancy with the use of high-frequency transvaginal ultrasound (Fig. 19).60
Microcephaly
The diagnosis of microcephaly is more likely to be accurate when the head circumference is
TABLE 12. Small Head Perimeter Measurements (mm), 2-and 3-SD below Mean for Fetal Age in Weeks of Value in Diagnosis of Microcephaly
Age (wk) | -2 SD | -3 SD | -4 SD | -5SD |
20 | 145 | 131 | 116 | 101 |
21 | 157 | 143 | 128 | 113 |
22 | 169 | 154 | 140 | 125 |
23 | 180 | 166 | 151 | 136 |
24 | 191 | 177 | 162 | 147 |
25 | 202 | 188 | 173 | 158 |
26 | 213 | 198 | 183 | 169 |
27 | 223 | 208 | 194 | 179 |
28 | 233 | 218 | 203 | 189 |
29 | 242 | 227 | 213 | 198 |
30 | 251 | 236 | 222 | 207 |
31 | 260 | 245 | 230 | 216 |
32 | 268 | 253 | 239 | 224 |
33 | 276 | 261 | 246 | 232 |
34 | 283 | 268 | 253 | 239 |
35 | 289 | 275 | 260 | 245 |
36 | 295 | 281 | 266 | 251 |
37 | 301 | 286 | 272 | 257 |
38 | 306 | 291 | 276 | 262 |
39 | 310 | 295 | 281 | 266 |
40 | 314 | 299 | 284 | 270 |
SD = standard deviations.
(Adapted from chervenak FA, Jeanty P, Cantraine F et al: The diagnosis of fetal microcephaly. Am J Obstet Gynecol 149:512, 1984)
TABLE 13. Selected Conditions Associated with Microcephalus with Features Potentially Diagnosable by Ultrasound
| Frequent or Occasional Features |
Syndromes |
|
Meckel-Gruber | Encephalocele, cleft palate, polycystic kidney, polydactyly, IUGR (A/R) |
Dubowitz | IUGR, hypertelorism, olinodactyly of fifth finger (A/R) |
Robert | IUGR, cleft lip, hypertelorism, hypomelia, CHD, polycystic kidneys, polyhydramnios, hydrocephalus (A/R) |
Seckel | IUGR, facial hypoplasia with prominent nose (A/R) |
De Lange | IUGR, micrognathia, micromelia, CHD, sporadic etiology |
Coffin-Siris | IUGR, CHD, Dandy-Walker anomaly, hypoplastic fifth finger (unknown etiology) |
Bloom | IUGR A/R |
Chromosomal Deviations |
|
Trisomy 13 | Holoprosencephaly, hydronephrosis, polycystic kidneys, IUGR, single umbilical artery, CHD |
Trisomy 18 | Polyhydramnios, hydronephrosis, polycystic kidneys, IUGR, single umbilical artery, CHD, omphalocele, choroid plexus cysts |
VATER associations | Esophageal atresia, radial hypoplasia, renal dysplasia |
Del (4p) and Del (5p) | Hypertelorism, IUGR, CHD |
Del (13 q) | Hypertelorism, IUGR,CHD, hydrocephalus, small thumbs |
Del (18p) | IUGR, holoprosencephaly |
Del (18q) | Cleft lip, CHD, horseshoe kidney |
Environmental Factors |
|
Alcohol | IUGR, hydrocephalus, CHD |
Amniopterin | IUGR, hydrocephalus, neural tube defect, hypoplasia/frontal bone, cleft palate, broad nasal bridge, hypertelorism, CHD |
Hydantoin | IUGR, hypertelorism, cleft lip, CHD, duodenal atresia, single umbilical artery |
Maternal phenylketonuria | IUGR, CHD |
Rubella | IUGR, CHD |
Other Associations |
|
Aniridia-Wilms | IUGR, genitourinary anomalies, |
tumor | Wilms tumor |
A/R = autosomal recessive; CHD = congenital heart disease; IUGR = intrauterine growth retardation; VATER = vertebral defects, imperforate anus, tracheoesophageal fistula, and radial and renal dysplasia.
(Adapted from Sabbagha RE, Toig RM, Tamura RK. Ultrasound examination of the fetus: Externally visible defects. In: Lin CC, Verp MS, Sabbagha RE, eds. The high risk fetus: Pathophysiology, diagnosis and management. New York: Springer-Verlag 1993:271)
Sonographic measurement of the frontal lobe (i.e., the distance from the posterior wall of the frontal horn to the inner aspect of the skull) has been proposed as a helpful diagnostic tool because the frontal aspect of the brain is the area most compromised.61, 62, 63 The normal frontal lobe values ± 2 SD from 16 to 24 weeks' gestation are shown in Table 14.
TABLE 14. Frontal Lobe Distance from Posterior Wall of Frontal Horn to Inner Aspect of Skull
Measurement (cm) (± 2 SD)* | No. of Weeks' Gestation |
1.4 ( ± 0.4 ) | 16 |
1.6 ( ± 0.2 ) | 17–18 |
1.7 ( ± 0.2 ) | 19–20 |
1.8 ( ± 0.2 ) | 21–23 |
1.9 ( ± 0.4 ) | 24 |
* Measurement used to enhance diagnosis of microcephaly (see text).
(Adapted from Goldstein I, Reece A, Pilu G et al: Sonographic assessment of the fetal frontal lobe: A potential tool for prenatal diagnosis of microcephaly. Am J Obstet Gynecol 158:1057, 1988)
Meningocele and Encephalocele
In cranial meningocele, the meninges protrude through a bony defect in the skull. Occipital presentation of meningocele is most common, occurring in 75% of cases. The differential diagnosis of cranial meningocele includes (1) scalp edema associated with fetal hydrops, (2) cystic hygroma, (3) teratoma, and (4) encephalocele (Fig. 20).64, 65
Spina bifida is frequently associated with meningocele. The latter represents extrusion of the meninges through the bony defect. Some cerebrospinal fluid (CSF) is usually contained within the meningocele. As a result, the area appears cystic, an ultrasound characteristic that enhances visibility of small spina bifida (Fig. 21).
Hydrocephaly
Figure 22 depicts the flow of CSF in hydrocephaly. Any lesion preventing normal flow of CSF from the lateral ventricles to the cisterns results in hydrocephaly.
Obstruction at the level of the aqueduct of Sylvius results in enlargement of the third and lateral ventricles (Fig. 23). The etiology of such obstruction includes (1) X-linked recessive inheritance, (2) infection with cytomegalovirus or toxoplasmosis, and (3) other lesions that impinge on the area.
Hydrocephaly plus dilation of the posterior fossa results from obstruction of CSF flow at the level of the foramina Luschka and Magendie (see Dandy-Walker syndrome, below). Table 15 lists a number of other specific syndromes that result in hydrocephaly.
TABLE 15. Skeletal Dysplasias, Chromosomal Abnormalities, and Birth Defects Associated with Hydrocephalus
Apert's syndrome
Robert's syndrome
Osteogenesis imperfecta
Thanatophoric dwarfism
Trisomies 13, 18, and 21
Triploidy
Aneurysm of the vein of Galen
Papilloma of the choroid plexus
In approximately 25% of hydrocephalic fetuses, an abnormal karyotype is found. Of these fetuses, approximately 85%, have additional anomalies. Thus, the prognosis should be individualized depending on specific etiology, but this cannot always be determined antenatally.
Hydrocephalic fetuses should not be delivered before pulmonary maturity is attained because there is a weak correlation between the extent of compression of the cerebral cortex and the severity of mental retardation.
ROUTE OF DELIVERY.
In a term fetus with normal chromosomes, isolated hydrocephalus (i..e, no other abnormality noted), and head circumference less than 90th percentile, vaginal delivery may be a reasonable option. If head circumference is equal to or greater than 90th percentile at term, cesarean section may be the least traumatic route. When hydrocephalus is associated with conditions incompatible with life, such as renal agenesis, thanatophoric dwarfism, or trisomy 18, cephalocentesis may be used to accomplish vaginal delivery. The combination of hydrocephalus with spina bifida also can determine the mode of delivery (see section on Spina Bifida. below).
In contrast to simple ventriculomegaly, hydrocephalus usually is more progressive, and it is frequently associated with compression of the choroid plexus (see Fig. 23).
The progression of hydrocephalus may be gauged by (1) the ventricular:hemispheric ratio, and (2) the diameter of the ventricular atrium (see Fig. 22 and Fig. 23).66, ,68 Dilation of the ventricular atrium is also a characteristic finding in agenesis of the corpus callosum. In such cases, the third ventricle is displaced cephalad and the frontal horns laterally.69
DIFFERENTIAL DIAGNOSES.
Differential diagnoses of hydrocephaly include the following:
- Holoprosencephaly: In this condition, the cerebral hemispheres do not separate; a single ventricle is noted, and hypotelorism is a characteristic finding. Fetal karyotype is abnormal in 50% of fetuses with holoprosencephaly, and trisomy 13 is the most frequent chromosomal abnormality. The diagnosis may be made in the latter part of the first trimester with the use of high-frequency vaginal ultrasound (Fig. 24).
- Hydranencephaly. In this condition, early bilateral vascular thrombosis of the carotid arteries bilaterally results in infarcted and atrophic cerebral hemispheres. The cerebellum, midbrain, and basal ganglia are seen, however. and are characteristically surrounded by a fluid interface.
- Porencephaly. In contrast to hydranencephaly, the condition results from localized brain atrophy secondary to a vascular accident.
- Aneurysm of the vein of Galen (Fig. 25):70 Dilation of the vein of Galen results from an arteriovenous malformation in which one or more arterioles feed the vein of Galen and distend it. The diagnosis is clarified by color Doppler velocimetry (see Fig. 25). The distension may result in hydrocephaly, and it may lead to high-output cardiac failure (see Fig. 25).
Choroid Plexus Cyst
A choroid plexus cyst is visualized in approximately 1% of fetuses scanned at 16 to 22 weeks' gestation (Fig. 26). In 1% to 2% of such fetuses, fetal trisomy 18 or 21 is noted.71, 72, 73, 74 Parents therefore should be counseled regarding the assessment of fetal karyotype. They should also be informed that trisomy 18 is a fatal condition, but that approximately 10% of infants with this defect live beyond 1 year of age. These parents should be counseled with respect to the emotional and financial cost of caring for an infant with this disorder.75
It has been postulated that the risk for aneuploidy may be small if (1) the cyst is less than 1 cm in diameter, (2) the cyst decreases in size or disappears as the pregnancy advances to 22 weeks, and (3) no other anomalies are noted. Whether there is indeed a decreased risk in such pregnancies, however, remains controversial. Kupfermink and co-workers76 showed that in a fetus with isolated choroid plexus cyst, the risk for aneuploidy is high (1:25) and thus amniocentesis is warranted.
The Posterior Fossa
The posterior fossa shows the cerebellum and the cisterna magna (Fig. 27). Normally, the cisterna magna measures 1 to 10 mm. The differential diagnoses of an enlarged cisterna magna include trisomy 18, arachnoid cyst, and obstruction of normal CSF flow at the level of the foramina Luschka and Magendie. CSF obstruction occurs in the following conditions:
- Dandy-Walker malformation
- Abnormalities of the subarachnoid space:
In Dandy-Walker malformation, dilation of the posterior fossa results from atretic foramina Luschka and Magendie, hypoplastic cerebelli, and vermis (roof of the fourth ventricle). As a result, the dilated fourth ventricle “herniates” into the area of the cisterna magna, ultrasonically appearing as symmetrically dilated cisterna magna (see Fig. 27). In contrast, an arachnoid cyst in the posterior fossa is less symmetric.77
Spina Bifida
In spina bifida, splaying of the spine is noted in the longitudinal plane. However, small spinae bifida located in the lumbosacral area are best visualized by careful cross-sectional imaging (see Fig. 21). The size of spinae bifida varies from a tiny bone defect to one involving many segments of the spine (myeloschisis).
Importantly, an Arnold-Chiari type II malformation occurs in nearly all cases of spina bifida. This is characterized by forward scalloping of the frontal aspect of the fetal head (lemon sign; see Fig. 21), and an abnormal, banana-shaped cerebellum (banana sign). The ultrasonographer should always look for these signs during the examination; if they are present, the ultrasonographer should extend the examination to search for spina bifida.80
MODE OF DELIVERY.
Currently, there are no controlled prospective studies that indicate whether a fetus with spina bifida should be delivered vaginally or by cesarean section. In a recent retrospective report, however, Luthy and associates81 showed that the mean functional level of infants delivered by cesarean section extended to a lower level than that of infants delivered vaginally (L4 vs L2). This two-segment difference may determine the prognosis as to whether an infant will be able to walk.81,82 Thus, obstetricians prefer delivery by cesarean section because it allows controlled handling of the fetal lumbosacral area.
EXTERNALLY VISIBLE BODY DEFECTS AND SKELETAL ABNORMALITIES
Cleft Lip and Palate
It is now possible to image the fetal nose area and upper lip to rule out cleft lip (Fig. 28). Cleft lip alone is genetically different from the combination of a cleft lip and palate and is more likely to occur in female infants (sex ratio, 2:1). Importantly, both conditions may be components of genetic, chromosomal, or other syndromes.
Cystic Hygroma
Cystic hygroma is a rare (1:6000 pregnancies) disorder and usually occurs as a sporadic event, although in some cases a recessive mode of inheritance is implicated. Importantly, 75% of cases have an associated chromosomal abnormality, including 45,X trisomies (two thirds of cases).13, 18, 21
In this disorder, poor development of communication channels between the jugular veins and the cervical lymph sacs (jugular lymphatic obstruction sequence) results in the formation of cystic hygromas (Fig. 29). These can vary in size depending on the degree of obstruction. In some cases, cystic hygromas have been noted to regress in utero, implying growth in the communication channels with advancing gestational age.83 In mild cases, the differential diagnosis includes thickening of the nuchal occipital fold secondary to aneuploidy.84 In severe cases, massive fetal hydrops develops and intrauterine fetal death occurs in the second trimester of pregnancy.
Fetal Hydrops
This term is used to describe accumulation of fluid in some or all serous cavities in conjunction with massive fetal edema. The etiology is immune in 13% of cases and nonimmune in the remaining 87%. In the latter group, the etiology is idiopathic in 22%, extrinsic in 1% (i.e., due to infections, such as TORCH or parvovirus B 19), and intrinsic in 64%. The most common intrinsic causes are cystic hygroma and cardiac defects. Other intrinsic etiologies include: chromosomal abnormality, arteriovenous shunts, twin-to-twin transfusion syndrome, fetomaternal hemorrhage, pulmonary masses. and inherited diseases, such as α-thalassemia, Tay-Sachs disease, and hemoglobinopathy.85
Sacrococcygeal Teratoma
Sacrococcygeal tumor appears as a predominantly echogenic mass attached to the sacrococcygeal area. It is quite rare (1:40,000 births) but has the potential to become malignant. The size is variable, but the majority of tumors are small (Fig. 30). In approximately 10% of cases, the mass is equal to or greater than 10 cm at term, requiring delivery by cesarean section.86
|
Skeletal Dysplasia
Skeletal dysplasia involves a heterogeneous group of abnormalities characterized by variability in the mode of inheritance, recurrence risk, gene penetrance, and type of limb shortening.87 It is beyond the scope of this chapter to describe each possible abnormality in detail.
In most skeletal dysplasias, the first noticeable feature is short limbs (Fig. 31. Once this is established, a detailed survey of fetal anatomy is carried out to identify the specific dysplasia. Attention to the following areas is helpful: (1) facial abnormality, (2) cephalic size, (3) shortened radius, (4) segment of bone shortened, (5) small or barrel-shaped chest, (6) cardiac defects, (7) head size, (8) protuberance of abdomen; (9) fetal edema; and (10) demineralization of bone. The abnormal ultrasound findings in various skeletal dysplasias are listed in Table 16.
TABLE 16. Abnormal Ultrasound Features in Various Skeletal Dysplasias
Abnormal Ultrasound |
|
Feature | Skeletal Dysplasias |
Amniotic fluid volume and polyhydramnios | Robert S, VATERS association |
Acromelia | Ellis-van Creveld dysplasia, Poland sequence acrodysostosis |
Bowing of long bones |
|
Tibia | Campomelic dysplasia, metaphyseal chondradysplasia |
Femur | Split-hand S |
Cardiac abnormalities | Thanatophoric dysplasia, chondroectodermal dysplasia, |
| short rib polydactyly, campomelic dwarfism, Robert S |
Cleft lip and cleft palate | All facial skeletal dysplasias, diastrophic dwarfism, |
| chondroectodermal dysplasia, short-rib |
| polydactyly, campomelic dwarfism |
Clubfoot | Osteogenesis imperfecta, arthrogryposis, Robert S, other |
Contractures | Femoral hypoplasia/unusual facies S, popliteal web S, |
| sirenomelia sequence, arthrogryposis |
Demineralization and poor calcification of bone |
|
Mainly noted in spine | Achondrogenesis |
Mainly noted in skull | Hypophophatasia |
Mainly noted in long | Osteogenesis imperfecta |
bones with fractures |
|
Dolichocephaly | Craniosynostosis, trilobed or clover-leaf skull |
Femur hypoplasia | Femoral hypoplasia/unusual facies S, ectrodactyly- |
| ectodermal dysplasia-clefing S |
Fetal motion absence | Arthrogryposis |
Finger abnormalities and | Ectrodactyly ectodermal dysplasia-clefing S, Aase S |
triphalangeal thumb |
|
Head shape abnormalities |
|
Brachycephaly | Craniosynostosis, acrodysostosis, achondroplasia, |
| (heterozygous form) |
Hydrocephaly | Thanatophoric dwarfism, osteogenesis imperfecta |
Hydropic appearance | Achondrogenesis, short rib polydactyly |
| (Saldino-Noonan type) |
Hypertelorism | Cleidocranial cleidostosis, acrodysostosis, VATERS |
Intrauterine growth | Robert S, VATERS |
retardation |
|
Kyphoscoliosis | Metatrophic dysplasia |
Limbs lower fusion | Caudal regression S |
Long bones (increased thickness) | Diastrophic dysplasia |
Macrocephaly | Achondroplasia, campomelic dysplasia |
Mesomelia | Neivergelt or Langer dysplasia |
Micromelia (severe) | Robert S, type II osteogenesis imperfecta, achondrogenesis |
Microcephalus | Robert S, thanatophoric dwarfism, De Lange S, |
| chondrodysplasia punctata |
Protuberant abdomen | Achondrogenesis, thanatophoric dwarfism, short rib |
| polydactyly (Majewski type) |
Radial bone hypoplasia | Miller S, TAR S, AASE S, VATERS, acromelia |
Renal disease | Short rib polydactyly, campomelic dwarfism, asphyxiating |
| thoracic dysplasia (Jeune type), short rib polylydactyly |
Thorax (decreased size) | Thanatophoric dwarfism, achondrogenesis, metatrophic |
| dwarfism, asphyxiating thoracic dysplasia (Jeune type), |
| short rib polydactyly, campomelic dysplasia |
| cleidocranial dysostosis |
Umbilical cord (single umbilical artery) | VATERS |
Aase S = triphalangeal thumb, ventricular septal defect, and aplastic anemia; S = syndrome; TAR = thrombocytopenia with absence of the radius; VATERS = vertebral and ventricular septal defects, anal atresia, tracheoesophageal fistula, radial and renal dysplasia, and single umbilical artery association).
REFERENCES
Johnson P, Sharland G, Maxwell D, Allan L: The role of transvaginal sonography in the early detection of congenital heart disease. Ultrasound Obstet Gynecol 2: 2481, 1992 |
|
Achiron R, Achiron A: Transvaginal ultrasonic assessment of the early fetal brain. Ultrasound Obstet Gynecol 1: 336, 1991 |
|
Sabbagha RE, Sheikh Z, Tamura RK et al: Predictive value, sensitivity, and specificity of ultrasonic targeted imaging for fetal anomalies in gravid women at high risk for birth defects. Am J Obstet Gynecol 152: 822, 1985 |
|
Sabbagha RE, DalCompo SA, Shuhaibar L: Targeted imaging for fetal anomalies. In Sabbagha RE (ed): Ultrasound Applied to Obstetrics and Gynecology, 3rd ed. Philadelphia. JB Lippincott, 1994 |
|
Thornton JG, Lilford R J, Newcombe RG: Tables for estimation of individual risks for neural tube and ventral wall defects, incorporating prior probability, maternal serum alpha-fetoprotein, and ultrasonic examination results. Am J Obstet Gynecol 164: 154, 1991 |
|
Reece RA, Assimakopoulos E, Zheng XZ et al: The safety of diagnostic ultrasonography: Concern for the fetus. Obstet Gynecol 76: 139, 1990 |
|
Ewigman BG, Crane JP, Frigoletto FD et al: A randomized trial of prenatal ultrasound screening in a low risk population: Impact on perinatal outcome. N Engl J Med 329: 821, 1993 |
|
Romero R: Editorial. Routine obstetric ultrasound. Ultrasound Obstet Gynecol 3: 303, 1993 |
|
Berkowitz RL: Editorial. Should every pregnant woman undergo ultrasonography? N Engl J Med 329: 874, 1993 |
|
Hegge FN: Letter to the editor. N Engl J Med 330: 570, 1994 |
|
Saari-Kamppainen A, Karjalainen O, Heinonen OP: Ultrasound screening and perinatal mortality: Controlled trial of systematic one-stage screening in pregnancy: The Helsinki Ultrasound Trial. Lancet 336: 387, 1990 |
|
Chitty LS, Hunt GH, Moore J, Lobb MO: Effectiveness of routine ultrasonography in detecting fetal structural abnormalities in a low risk population. Br Med J 303: 1165, 1991 |
|
Shirley IM, Bottomley F, Robinson VP: Routine radiographer screening for fetal abnormalities by ultrasound in an unselected low risk population. Br Med J 65: 564, 1992 |
|
Luck CA: Value of routine ultrasound scanning at 19 weeks: A four year study of 8849 deliveries. Br Med J 304: 1474, 1992 |
|
Nora N: Genetics and Counseling in Cardiovascular Disease. Springfield, Charles C Thomas, 1978 |
|
DeVore GR: The prenatal diagnosis of congenital heart disease—a practical approach for the fetal sonographers. J Clin Ultrasound 13: 229, 1985 |
|
Ferrazi E, Fesslova V, Bellotti M et al: Prenatal diagnosis of and management of congenital heart disease. J Reprod Med 34: 207, 1989 |
|
Crawford DC, Chita SK, Allan LD: Prenatal detection of congenital heart disease: Factors affecting obstetric management and survival. Am J Obstet Gynecol 159: 352, 1988 |
|
Copel JA, Pilu G, Green J et al: Fetal echocardiographic screening for congenital heart disease: The importance of the four-chamber view. Am J Obstet Gynecol 157: 648, 1987 |
|
Vergani P, Mariani S, Ghidini A et al: Screening for congenital heart disease with the four chamber view of the heart. Am J Obstet Gynecol 167: 1000, 1992 |
|
Sharland GK, Allan LD: Screening for congenital heart disease prenatally: Results of 2 1/2 year study in the south east Thames region. Br J Obstet Gynaecol 99: 220, 1992 |
|
Bromley B, Estroff JA, Sanders SP et al: Fetal echocardiography: Accuracy and limitations in a population at high risk for heart defects. Am J Obstet Gynecol 166: 1473, 1992 |
|
Wigton TR, Sabbagha RE, Tamura RK et al: Sonographic diagnosis of congenital heart disease: Comparison between the four chamber view and multiple cardiac views. Obstet Gynecol 82: 219, 1993 |
|
Comstock CH, Riggs T, Lee W et al: Pulmonary-to-aorta diameter ratio in the normal and abnormal fetal heart. Am J Obstet Gynecol 165: 1038, 1991 |
|
Faarant P: The antenatal diagnosis of esophageal atresia by ultrasound. Br J Radiol 53: 1202, 1988 |
|
Stocker JT, Madewell JE, Grake RM: Congenital cystic adenomatoid malformation: Classification and morphologic spectrum. Hum Pathol 8: 155, 1977 |
|
Mariona F, McAlpin G, Zador I et al: Sonographic detection of fetal extrathoracic pulmonary sequestration. J Ultrasound Med 5: 283, 1986 |
|
Thibeault DW, Beatty EC, Hall RT et al: Neonatal pulmonary hypoplasia with premature rupture of membranes and oligohydramnios. J Pediatr 107: 273, 1985 |
|
Chitkara U. Rosenburg J. Chervenak FA et al: Prenatal sonographic assessment of the fetal thorax: Normal values. Am J Obstet Gynecol 156: 1069, 1987 |
|
Fong K, Ohlsson A, Zaber A: Fetal thoracic circumference: A prospective cross sectional study with real time ultrasound. Am J Obstet Gynecol 158: 1154, 1988 |
|
Vintzileos AM, Campbell WA, Rodis JF et al: Comparison of six different ultrasonographic methods for predicting lethal fetal pulmonary hypoplasia. Am J Obstet Gynecol 161: 606, 1989 |
|
Sabbagha RE, Comstock CH: Abnormalities of the chest and gastrointestinal tract. In Sabbagha RE (ed): Ultrasound Applied to Obstetrics and Gynecology, 2nd ed. Philadelphia, JB Lippincott, 1987 |
|
Wenstrom KD, Weiner CP, Hanson J W: A five-year state-wide experience with congenital diaphragmatic hernia. Am J Obstet Gynecol 165: 838, 1991 |
|
Green JJ, Hobbins JC: Abdominal ultrasound examination of the first trimester fetus. Am J Obstet Gynecol 159: 165, 1988 |
|
Gray DL, Martin CM, Crane JP: Differential diagnosis of first trimester ventral wall defect. J Ultrasound Med 8: 255, 1989 |
|
Benacerraf BR, Saltzman DH, Esteroff JA, Frigoletto FD: Abnormal karyotype of fetuses with omphalocele: Prediction based on omphalocele contents. Obstet Gynecol 75: 317, 1990 |
|
Nyberg DAS, Fitzsimmons J, Mack LA et al: Chromosomal abnormalities in fetuses with omphalocele: Significance of omphalocele contents. J Ultrasound Med 8: 299, 1989 |
|
Kirk PE, Wah RM: Obstetric management of the fetus with omphalocele or gastroschisis: A review and report of 112 cases. Am J Obstet Gynecol 146: 512, 1983 |
|
Sipes SL, Weiner CP. Sipes DR II et al: Gastroschisis and omphalocele: Does either antenatal diagnosis or route of delivery make a difference in perinatal outcome? Obstet Gynecol 76: 195, 1990 |
|
Nelson LH, Clark CE, Fishburne JI et al: Value of serial sonography in the in utero detection of duodenal atresia. Obstet Gynecol 59: 657, 1982 |
|
Paulson EK, Hertzberg BS: Hyperechoic meconium in the third trimester fetus: An uncommon normal variant. J Ultrasound Med 10:677. 1991 |
|
Pretorius D, Budorick N, Cahill T et al: Midtrimester echogenic bowel and chromosomal abnormalities (SPO abstract 20). Am J Obstet Gynecol 166: 283, 1992 |
|
Evans MI, Sacks AJ, Johnson PM et al: Sequential invasive assessment of fetal renal function and the intrauterine treatment of fetal obstructive uropathies. Obstet Gynecol 77: 545, 1991 |
|
Estes JM, Adzick NS, Harrison MR: Antenatal open surgery for the abnormal fetus. In Sabbagha RE (ed): Ultrasound Applied to Obstetrics and Gynecology, 3rd ed. Philadelphia, JB Lippincott, 1994 |
|
Garris J, Kangarloo H, Sarti D et al: The ultrasound spectrum of prune-belly syndrome. J Clin Ultrasound 8: 117, 1980 |
|
Antonakopoulos GN, Fuggle WJ, Newman J et al: Idiopathic hydronephrosis. Arch Pathol Lab Med 109: 1097, 1985 |
|
Hoddick WK, Kogan BA, Jeffry RB et al: Minimal fetal renal pyelectasis. J Ultrasound Med 4: 85, 1985 |
|
Corteville JE, Gray DL, Crane JP: Congenital hydronephrosis: Correlation of fetal ultrasonographic findings with infant outcome. Am J Obstet Gynecol 165: 384, 1991 |
|
Benacerraf BR, Mandell J, Estroff JA et al: Fetal pyelectasis: A possible association with Down syndrome. Obstet Gynecol 76: 5855, 1990 |
|
Carter CO: The genetics of urinary tract malformations. J Genet Hum 32: 23, 1984 |
|
Romero R, Cullen M, Grannum P et al: Antenatal diagnosis of renal anomalies with ultrasound: III. Bilateral renal agenesis. Am J Obstet Gynecol 151: 38, 1985 |
|
Jeanty P, Romero R, Kepple D et al: Prenatal diagnosis in unilateral empty renal fossa. J Ultrasound Med 9: 651, 1990 |
|
Grannum P, Bracken M, Silverman R, Hobbins JC: Assessment of fetal kidney size in normal gestation by comparison of ratio of kidney circumference to abdominal circumference. Am J Obstet Gynecol 136: 249, 1980 |
|
Simpson JL, Sabbagha RE, Elias S et al: Failure to detect polycystic kidneys in utero by second trimester ultrasonography. Hum Genet 60: 295, 1982 |
|
Potter EL: Type II cystic kidney: Early ampullary inhibition in normal and abnormal development of the kidney, p 154. Chicago, Year Book Medical, 1972 |
|
Penman DG, Lilford RJ: The megacystis-microcolon-intestinal hypoperistalsis syndrome: A fatal recessive condition. J Med Genet 26: 66, 1989 |
|
Berdon WE, Baker DH, Blanc WA et al: Megacystis-microcolon-intestinal hypoperistalsis syndrome: A new cause of intestinal obstruction in the newborn. Report of radiologic findings in 5 newborn girls. AJR 126: 957, 1976 |
|
Vintzileos AM, Eisenfeld LI, Herson VC et al: Megacystis-microcolon-hypoperistalsis syndrome: Prenatal sonographic findings and review of the literature. Am J Perinatol 3: 297, 1986 |
|
Young ID, Mckeever PA, Brown LA et al: Prenatal diagnosis of the megacystis-microcolon-intestinal hypoperistalsis syndrome. J Med Genet 26: 403, 1989 |
|
Kennedy KA, Flick KJ, Thurmond AS: First trimester diagnosis of exencephaly. Am J Obstet Gynecol 162: 461, 1990 |
|
Chervenak FA, Rosenberg J, Brightman RC et al: A prospective study of the accuracy of ultrasound in predicting fetal microcephaly. Obstet Gynecol 69: 908, 1987 |
|
Chervenak FA, Jeanty P, Cantraine F et al: The diagnosis of fetal microcephaly. Am J Obstet Gynecol 149: 512, 1984 |
|
Goldstein I, Reece A, Pilu G et al: Sonographic assessment of the fetal frontal lobe: A potential tool for prenatal diagnosis of microcephaly. Am J Obstet Gynecol 158: 1057, 1988 |
|
Santolaya J, Alley D, Jaffe R, Warsof SL: Antenatal classification of hydrops fetalis. Obstet Gynecol 79: 256, 1992 |
|
Sabbagha RE, Tamura RK, DalCompo S: Fetal cranial and craniocervical masses: Ultrasound characteristics and differential diagnosis. Am J Obstet Gynecol 138: 511, 1980 |
|
Pretorius DH, Drose LA, Manco-Johnson ML: Fetal lateral ventricular ratio determination during the second trimester. J Ultrasound Med 5: 121, 1986 |
|
Pilu G, Reece A, Goldstein I et al: Sonographic evaluation of the normal developmental anatomy of the fetal cerebral ventricles: The atria. Obstet Gynecol 73: 2505, 1989 |
|
Cordoza JD, Goldstein RB, Filly RA: Exclusion of fetal ventriculomegaly with a single measurement of the width of the lateral ventricular atrium. Radiology 169: 711, 1988 |
|
Comstock C: Agenesis of the corpus callosum in the fetus: Diagnosis and significance. The Female Patient 17: 40, 1992 |
|
Jeanty P, Kepple D, Roussis P, Shah D: In utero detection of cardiac failure from aneurysm of the vein of Galen. Am J Obstet Gynecol 163:50. 1990 |
|
Chitkara U, Cogswell C, Norton K et al: Choroid plexus cysts in the fetus: A benign anatomic variant or pathologic entity ? Report of 41 cases and review of the literature. Obstet Gynecol 72: 185, 1988 |
|
Gabrielli S, Reece AE, Pilu G et al: The clinical significance of prenatally diagnosed choroid plexus cysts. Am J Obstet Gynecol 160: 1207, 1989 |
|
Rotmensch S, Luo JS, Nores JA et al: Bilateral choroid plexus cysts in trisomy 21. Am J Obstet Gynecol 166: 591, 1992 |
|
Platt LD, Carlson DE, Medearis AL et al: Fetal choroid plexus cysts in the second trimester of pregnancy: A cause for concern. Am J Obstet Gynecol 164: 1652, 1991 |
|
Hershey DW: Fetal choroid plexus cysts revisited—Is genetic amniocentesis indicated? J Ultrasound Med 11: 14775, 1992 |
|
Kupferminc M J, Tamura RK, Sabbagha RE et al: Isolated choroid plexus cyst(s): An indication for amniocentesis. Am J Obstet Gynecol 171: 1068, 1994 |
|
Sabbagha RE: Targeted imaging for fetal anomalies: Clues to reaching a correct diagnosis. Obstet Gynecol Rep 2: 142, 1990 |
|
Thurmond AS, Nelson DW, Lowensohn RI et al: Enlarged cisternal magna in trisomy 18: Prenatal ultrasonographic diagnosis. Am J Obstet Gynecol 161: 83, 1989 |
|
Ghidini A, Sirtori M, Vergani P: Fetal intracranial calcifications. Am J Obstet Gynecol 160: 8622, 1989 |
|
Van den Hof MC, Nicolaides KH, Campbell S: Evaluation of the lemon and banana signs in one hundred thirty fetuses with open spina bifida. Am J Obstet Gynecol 162: 322, 1990 |
|
Luthy DA, Wardinsky T, Shurtleff DB et al: Cesarean section before the onset of labor and subsequent motor function in infants with meningomyelocele diagnosed antenatally. N Engl J Med 162: 624, 1991 |
|
Hobbins JC: Diagnosis and management of neural tube defects to day. N Engl J Med 324: 690, 1991 |
|
Macken MB, Granmyre EB, Vincer MJ: Regression of nuchal cystic hygroma in utero. J Ultrasound Med 8: 101, 1989 |
|
Benacerraf BR, Neuberg D, Bromley B, Frigoletto FD: Sonographic scoring index for prenatal detection of chromosomal abnormalities, J Ultrasound Med 11:449, 1992 |
|
Santolaya J, Alley D, Jaffe R, Warsof SL: Antenatal classification of hydrops fetalis. Obstet Gynecol 79: 256, 1992 |
|
Salerno AP: Fetal sacrococcygeal teratoma. Perspect Probl Obstet Gynecol 1: 3, 1985 |
|
Smith DW: Recognizable patterns of human malformation. Philadelphia. WB Saunders, 1976 |