The Biophysical Profile
Authors
INTRODUCTION
Much information about normal and abnormal fetal behavior has been discovered through clinical and basic science research. These observations have aided the development of antenatal tests to distinguish healthy fetuses from potentially compromised fetuses. The primary goals of antenatal surveillance programs are ultimately to decrease perinatal morbidity and mortality. In the United States, the perinatal mortality rate was 6.9 in 2001.1 According to the National Center for Health Statistics, this rate is defined as the number of late fetal deaths (after 28 weeks) plus infant deaths within 7 days of birth per 1000 total births after 28 weeks. The US stillbirth rate in 2002 was 6.4 per 1000 total births.2 The nonstress test (NST) and contraction stress test (CST) were the two primary methods available for fetal surveillance before the biophysical profile was developed. Reassuring NST and CST tests are highly predictive of a healthy, nonasphyxiated newborn.3 Unfortunately, abnormal test results are poorly predictive of an asphyxiated newborn. In fact, the false-positive rate of both tests is greater than 50%.3 Combination testing with an NST and real-time ultrasonographic assessment of certain fetal parameters has been used since the late 1970s. This combination testing is called the biophysical profile (BPP). It includes both acute markers of fetal status (fetal heart rate reactivity [FHR-R], fetal breathing [FB] movements, fetal gross body movements [FM], and fetal tone [FT]) and some chronic markers of the fetal and intrauterine condition (amniotic fluid volume [AFV] and, in one form of the BPP, placental grading, too). The BPP may be considered a type of intrauterine Apgar score, and it may predict neonatal acidosis at delivery better than the Apgar score.4 Clinical applications, efficacy, and management protocols for different clinical situations are discussed in this chapter.
THE GRADUAL HYPOXIA MODEL
Fetal biophysical activities such as FB, FM, and FHR-R are not random events but are controlled by specific centers within the central nervous system (CNS). Because normal fetal neurodevelopment requires increasing oxygen availability, the presence of such complex biophysical activities indicates a functioning, nonhypoxemic CNS.
The lack of normal biophysical activity may be due to hypoxia, reflecting a comatose state. However, an abnormal score may occur in a healthy fetus in the resting phase of a normal sleep–wake cycle. Fetal activity cycles may be short term (20–80 minutes) or long term (diurnal). Manning emphasizes that the clinical significance of an absent biopysical activity is directly related to the time the fetus is observed. The observation time must exceed the average period of a given sleep state (about 20 minutes) and ideally should be at least 1.5 times as long, or 30 minutes.5
Individual CNS centers that may regulate the biophysical activities appear to vary in their sensitivity to hypoxia. Accordingly, a “gradual hypoxia” model has been proposed in which the activities that first appear embryologically are the last to disappear with progressively worsening hypoxia.4, 6 Table 1 shows the postulated neurodevelopmental sequence of acquisition of fetal biophysical activities. For example, the first activity to appear, FT, at about 7.5–8.5 weeks, is also presumably the last activity to disappear with progressively worsening hypoxia.7, 8 FM allegedly develops after FT (at 9 weeks),8 then FB (at 20–21 weeks),8 and finally FHR-R, at approximately 28 weeks.9 According to the gradual hypoxia model, FHR-R should be the first component of the BPP to become abnormal (i.e., to be lost) in hypoxic states. The gradual hypoxia concept would help explain the increased incidence of abnormal outcome as more biophysical activities decrease in occurrence and eventually disappear. Ultimately, the persistent and true lack of FT should be associated with the highest perinatal death rate.
Table 1. Proposed fetal central nervous system centers
FT | Cortex (subcortical area?) | | | ↑ |
FM | Cortex-nuclei | Embryogenesis | Hypoxia |
FB | Ventral surface of 4th ventricle | | | | |
NST | Posterior hypothalamus, medulla | ↓ | | |
(Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ: The use of real-time scanning in antepartum fetal evaluation: The fetal biophysical profile. In Sanders RC, Hill MC (eds): Ultrasound Annual, p 254. New York, Raven Press, 1985)
Hypoxia can cause acute and chronic fetal effects. The BPP measures some acute fetal characteristics that can be affected by hypoxia (i.e., FB, FM, FT, and FHR-R) and some chronic characteristics that can show effects of prolonged hypoxia (i.e., decreased AFV and, in Vintzileos' BPP methodology, a grade 3 placenta). Presumably, progressively worsening hypoxia causes increasingly more of the measured biophysical variables to become abnormally decreased in frequency or entirely absent. The composite BPP score may thus, in many circumstances but not all, provide a noninvasive estimation of the degree of fetal acidemia.
Vintzileos and colleagues correlated BPP scores with umbilical cord blood gases in 124 nonlaboring patients, within 6 hours of cesarean delivery.10 Twenty fetuses were acidotic (cord arterial pH <7.20). They found that FHR-R and FB were the first two activities inhibited by fetal acidosis. If an abnormal BPP was equated with the absence of only these two components, the test had 100% sensitivity, 92% specificity, 71% positive predictive value (PPV), and 100% negative predictive value (NPV) for an acidotic umbilical arterial pH. FM and FT began to decrease as pH decreased to a value between 7.10 and 7.20, and these activities were absent at a pH less than 7.10.
Ribbert and co-workers studied 14 fetuses with severe intrauterine growth retardation (IUGR) from 25 to 39 weeks' gestation using BPPs followed by cordocentesis.11 They calculated the difference between the umbilical venous pH values in these fetuses and those of appropriately grown fetuses of the same gestational age. They found a significant correlation (r = 0.84, p < 0.001) between the total BPP score and this calculated umbilical venous pH difference. No correlation was noted between the BPP score and the similarly calculated differences in umbilical venous oxygen tension, carbon dioxide tension, oxygen saturation, or hemoglobin concentration. Seven of the 14 BPPs were abnormal (<8). In agreement with Vintzileos,10 Ribbert and associates11 found that FHR-R and FB were the first components to become abnormal and FM and FT were the last components to become abnormal. They postulated that the BPP score can predict fetal acidemia, but close attention should be given to the individual components of the BPP.
In contrast, Manning and collaborators were unable to find an obvious sequential loss of biophysical activities in a larger study of 525 fetuses with an abnormal BPP within 48 hours of delivery.12 The appropriate clinical interpretation of a definite hypoxic threshold for different fetal activities is controversial and requires more study.
Because the fetal behavioral characteristics measured by the BPP develop at different gestational ages, age-related interpretation of BPP results is necessary in very young fetuses. A description of each BPP component follows.
COMPONENTS OF THE BIOPHYSICAL PROFILE
Fetal heart rate reactivity
A normal fetus that is not acidotic or neurologically depressed should have a reactive fetal heart rate (FHR-R) pattern during movement.13 However, healthy fetuses may require 80 minutes to demonstrate FHR-R, presumably because of prolonged normal sleep–wake cycles.14
As noted previously, FHR-R develops at approximately 28 weeks.9 Castillo and colleagues studied 30 normal fetuses every 2 weeks from 24 to 32 weeks' gestation and found a trend of increasing reactivity with increasing gestational age.15 Defining FHR-R as three accelerations of 15 beats per minute (bpm) lasting 15 seconds in 30 minutes, they found that the incidence of nonreactive NSTs (NR-NSTs) decreased from 50% at 24 weeks' gestational age to 6.7% at 32 weeks. Other investigators have also noted increased FHR-R with increasing gestational age.16, 17, 18 Castillo and associates also found that they could decrease the incidence of NR-NSTs by increasing the testing time from 30 to 90 minutes. After 90 minutes, only 6.7% of NSTs were nonreactive at 24 weeks, whereas 50% were nonreactive after 30 minutes. Likewise, at 30 minutes, 6.7% of the NSTs were nonreactive at 32 weeks' gestation, but only 3.3% were still nonreactive after 90 minutes. Thus, it is reasonable to extend the NST testing period in fetuses less than 32 weeks' gestation.
Finally, Castillo and co-workers found that by defining FHR-R as three accelerations of 10 bpm (instead of 15 bpm), all fetuses greater than or equal to 26 weeks' gestation were reactive by the end of 60 minutes, and 87% of the 24-week fetuses were reactive. However, before this particular modified definition of FHR-R can be used routinely, more evaluation is necessary.
By provoking FHR accelerations, vibratory acoustic stimulation (VAS) may decrease the number of false-positive NST responses and decrease testing time.19, 20 VAS-induced FHR accelerations may be highly predictive of the absence of acidosis.21 Several investigators have noted predominantly nonacidotic fetal scalp or umbilical arterial cord pH values after a reactive FHR response to VAS. These studies also show that a nonreactive FHR response after VAS does not necessarily signify acidosis.
Fetal breathing movements
Fetal breathing is not reliably noted until 20 weeks with real-time ultrasonography.22 Before that time, it is difficult to distinguish FB from generalized chest movements. During a 30-minute observation period, human FB incidence ranges from 0% to 98% during the day, with a mean of 30%.22, 23, 24, 25, 26 The average rate is 30–70 times per minute.23 Normal fetuses may have prolonged apnea, up to 108 minutes.27
The incidence of human FB may be affected by certain environmental agents or certain maternal states. There is some controversy in the literature about different agents' effects on FB. Some of the data reflect studies of small numbers of fetuses (Table 2).22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41
Table 2. Incidence of fetal breathing
|
|
| Controversial or |
Increased | Decreased | No Change | Few, Small Studies |
Caffeine41 | Benzodiazepines25 Demerol25 | ||
Hypercapnia25 |
| Epidural34 | |
After amniocentesis38 |
| Oxytocin34 | |
With magnesium sulfate tocolysis39 |
|
| |
| PROM with infection40 |
|
|
ASSOCIATIONS OF FETAL BREATHING WITH PERINATAL OUTCOME
Platt and colleagues studied 136 pregnancies for the presence or absence of breathing in the last study before delivery.42 In 76% of cases, the study was conducted within 3 days of delivery. Low 5-minute Apgar (<7) scores were more frequent when FB was absent (50%) than when it was present (4%). Fetal distress in labor was significantly increased in fetuses with absent FB (60% versus 3% with FB present). Later, Manning and associates investigated the use of the NST and the FB component of the BPP in 223 high-risk pregnancies.43 Eighty-six per cent of the tests were performed within 1 week of delivery. The predictive value of FB was similar to that of the NST for low 5-minute Apgar scores and intrapartum fetal distress. Tamura and Manning recommend evaluation of FB in those fetuses with a positive (but reactive) contraction stress test (CST) to help detect the fetuses destined to develop intrapartum fetal distress.26
Fetal body movement
Hypoxic or otherwise compromised fetuses may not move as much as healthy fetuses, as confirmed by electromechanical detectors.44, 45, 46 Baskett studied fetuses at different gestational ages and found a similar incidence of FM from 28 weeks onward.18 He also noted that the number of abnormal responses on FM tests (i.e., decreased or absent FM) did not differ significantly between 26 weeks and term. Caffeine does not significantly change FM incidence.41 The effect of maternal glucose administration on FM is controversial.47, 48 Cocaine intoxication may increase FM.49
Fetal tone
Baskett also found no significant difference in the proportion of abnormal results of tests of FT (<0.9%) between 26 weeks and term.18 Manning and co-workers found that when FT was absent on the last BPP before delivery, the incidence of fetal distress in labor, low 5-minute Apgar scores, and perinatal mortality were much higher than if FT was present.50 The BPPs were performed within 1 week of delivery. According to the gradual hypoxia model, FT is the last biophysical activity to be lost and should therefore be associated with the highest perinatal death rate. In a small study of 158 patients, Schifrin and associates found that absent FT (n = nine cases) correlated highly with poor outcome (in seven cases).51 In these seven poor-outcome cases, absent FT was also associated with a NR-NST, absent FB, and absent FM. In two cases of absent FT, normal outcome occurred, so the absence of FT cannot be absolutely equated with poor outcome. In both of these cases, the NST was reactive. A NR-NST and absent FT were the most efficient pair for prediction of poor outcome.
Amniotic fluid volume
In most cases, AFV does not reflect acute fetal CNS dysfunction. Nevertheless, decreased AFV is associated with increased perinatal morbidity and mortality.52 Clinical conditions associated with decreased AFV include IUGR, premature rupture of the membranes (PROM), postdatism, some major congenital anomalies, abdominal pregnancy, and excessive use of prostaglandin synthetase inhibitors.53 Decreased AFV may sometimes be due to hypoxia-induced reflex redistribution of cardiac output to the most vital organs, the heart and brain, with shunting of flow away from the amniotic fluid-producing organs, the kidneys and lungs.54, 55
Two mechanisms help to explain the relationship of decreased AFV and perinatal morbidity and mortality. Decreased fluid may reflect utero-placental insufficiency, especially in growth-retarded and postdate fetuses.56 Second, the decreased fluid may increase the likelihood of umbilical cord compression and related sequelae.
Manning and collaborators found an increased incidence of IUGR and perinatal mortality when the largest vertical pocket of amniotic fluid measured less than 1 cm.50 In a review of 7562 high-risk pregnancies, Chamberlain and associates found a sharp rise in perinatal asphyxia and mortality when severe oligohydramnios was present (largest vertical pocket
In 336 patients, Phelan and colleagues found a statistically significant increased incidence of bradycardia, meconium, and cesarean section for fetal distress when the AFV was either “adequate but decreased” or “decreased”.57 They defined decreased fluid as a pocket of fluid 1 cm or less within the uterine cavity. Adequate but decreased fluid was judged when a pocket greater than 1 cm was present but the sonographer subjectively believed that the AFV was decreased.
In a further attempt to quantify AFV, Rutherford and co-workers developed a four-quadrant amniotic fluid index.58 The uterus is thought of as divided into four quadrants, and the largest vertical pocket of amniotic fluid in each quadrant is measured. These measurements are summed to give an amniotic fluid index (AFI). They found an increased incidence of meconium, fetal distress in labor, and low 5-minute Apgar scores in pregnancies with an AFI of 5 or less. Recent studies have shown no advantage of using the AFI instead of the single largest pocket of amniotic fluid.59 Oligohydramnios is defined as a single pocket of fluid less than or equal to 2 x 1 cm or an AFI of less than or equal to 5 cm.
DEFINITION OF THE MAIN TYPES OF BIOPHYSICAL PROFILES
Two general profiles for biophysical testing have developed. Manning and colleagues' protocol, first published in 1980, has been modified.50 Vintzileos and associates' 1983 protocol remains unchanged.6 Additionally, several modified or abbreviated versions have been developed by others and are discussed later in the chapter.
Manning has provided much of the literature on the clinical efficacy of the BPP. His testing profile involves five components: NST, FB, FM, FT, and AFV assessment. Each variable is graded 0 if absent or 2 if present, for a total score of 10. In Manning's initial test design, provision was made for an intermediate value of 1 should the data fit such a pattern. However, after further experience with the BPP, he determined that such additional descriptiveness was not justified. A normal score is 10, or 8 in the absence of oligohydramnios. An equivocal score is 6, and abnormal scores are 0, 2, or 4. In the initial study of 216 high-risk patients, Manning and collaborators found improved accuracy in predicting adverse perinatal outcome when a combination of biophysical parameters was measured instead of using only a single abnormal response.50 A BPP score of 10 (all tested parameters normal) was associated with a perinatal mortality rate of 0 per 1000, whereas a BPP score of 0 (all variables abnormal) was associated with a 600 per 1000 perinatal mortality. Table 3 summarizes Manning's criteria for scoring the biophysical profile.
Table 3. Biophysical profile scoring: technique and interpretation*
Biophysical Variable | Normal (Score = 2) | Abnormal (Score = 0) |
Fetal breathing movements | Absent or no episode of | |
Gross body movements | ||
Fetal tone | Either slow extension with return to partial flexion or movement of limb in full extension or absent fetal movement | |
Reactive fetal heart rate | <2 episodes of acceleration of fetal heart rate or acceleration of <15 bpm in 20 min | |
Qualitative amniotic fluid volume | Either no pockets or a pocket <1 cm in two perpendicular planes† |
*Current “normal” amniotic fluid volume = presence of pocket of fluid >2 cm in vertical distance52
† Current amniotic fluid volume measurement for “abnormal” score = largest vertical pocket
(Manning FA, Morrison I, Lange IR et al: Fetal assessment based on fetal biophysical profile scoring: Experience in 12,620 referred high-risk pregnancies. Am J Obstet Gynecol 151: 345, 1985)
Vintzileos and colleagues' protocol6 is quite similar to the scoring system of Manning's group (Table 4). However, Vintzileos includes the placental grade as one of the BPP's components. This inclusion is based on data relating to an increased incidence of abnormal intrapartum fetal heart patterns and abruption in patients with a grade 3 placenta.6 Scores range from 0 to 1 to 2, with a total possible score of 12. An abnormal score is 7 or less.
Table 4. Vintzileos' criteria for scoring the biophysical profile
Nonstress test
Score 2: 5 or more fetal heart rate accelerations of at least 15 bpm in amplitude and at least 15 sec duration associated with fetal movements in a 20-min period
Score 1: 2–4 accelerations of at least 15 bpm in amplitude and at least 15 s duration associated with fetal movements in a 20-min period
Score 0: 1 or fewer accelerations in a 20-min period
Fetal movements
Score 2: At least 3 gross (trunk and limbs) episodes of fetal movements within 30 min. Simultaneous limb and trunk movements were counted as a single movement
Score 1: 1 or 2 fetal movements within 30 min
Score 0: Absence of fetal movements within 30 min
Fetal breathing movements
Score 2: At least 1 episode of fetal breathing of at least 60 s duration within a 30-min observation period
Score 1: At least 1 episode of fetal breathing lasting 30–60 swithin 30 min
Score 0: Absence of fetal breathing or lasting less than 30 s within 30 min
Fetal tone
Score 2: At least 1 episode of extension of extremities with return to position of flexion, and also
1 episode of extension of spine with return to position of flexion
Score 0: Extremities in extension. Fetal movements not followed by return to flexion. Open hand
Amniotic fluid volume
Score 2: Fluid evident throughout the uterine cavity . A pocket that measures 2 cm or more in vertical diameter
Score 1: A pocket that measures less than 2 cm but more than 1 cm in vertical diameter
Score 0: Crowding of fetal small parts. Largest pocket less than 1 cm in vertical diameter
Placental grading
Score 2: Placental grading 0, 1, or 2
Score 1: Placenta posterior difficult to evaluate
Score 0: Placental grading 3
Maximal score 12; minimal score 0
(Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ: The use of real-time scanning in antepartum fetal evaluation: The fetal biophysical profile. In Sanders RC, Hill MC (eds): Ultrasound Annual, p 257. New York, Raven Press, 1985)
Manning's scoring system is generally easier than that of Vintzileos and has received wider acceptance. Three important changes in Manning's profile have occurred since 1980. First, a normal score for FB requires 30 seconds of FB instead of the original 60 seconds. Second, the definition of oligohydramnios has changed. As of 1983, oligohydramnios is present when the largest vertical amniotic fluid pocket is 2 cm or less instead of less than 1 cm.52 Third, as of 1984, the NST is performed only if one of the dynamic ultrasonographically evaluated variables is abnormal.60 This third modification is discussed later in this chapter.
Although equal weight is given to each component in Manning's BPP, in 1990, he and his coworkers reported that not all compositions of low BPP scores are clinically equivalent or equally distributed. This conclusion was made after reviewing the results of a decade of experience with the BPP.61 The PPV of a particular BPP score (such as for a score of 6) depends on the specific individual variables composing the score.
For example, Manning found that the PPV for perinatal death with a BPP of 6 is significantly higher when the NST is nonreactive and FT is absent (370:1000) than when AFV and FB are absent (0:1000). Ten possible combinations of variables can give rise to a BPP score of 6, yet the distribution of observed combinations by frequency is not uniform. A scoring system with differential component weighting, although perhaps more accurate, would require considerable additional clinical evaluation for development. Nevertheless, Manning's testing results include the lowest perinatal mortality rate ever reported in the medical literature.61
Modified protocols
A modified biophysical profile, using just an NST and an AFI, has been used by a number of groups successfully, decreasing fetal testing time. The NST serves as an indicator of the acute fetal condition, and the AFI is an indicator of long term fetal status. In the late second and third trimesters, amniotic fluid reflects fetal urine production. Placental dysfunction can cause decreased renal perfusion with subsequent decreased urine production reflecting long term placental function. A normal modified BPP is a reactive NST and an AFI >5 cm. An abnormal one is a NR-NST or an AFI of less than or equal to 5 cm.
Clark and associates (1989) emphasized clinically expedient antenatal testing.62 They advocated the use of VAS with the NST, plus a four-quadrant AFV assessment. During a 3-year period, Clark and collaborators performed 5973 such tests on 2628 singleton high-risk pregnancies. If an NST was non-reactive after 5 minutes, VAS was performed and repeated once more within 10 minutes, if necessary. Only 3.2% of the NSTs were nonreactive with this protocol, significantly lower than in other series, implying a decrease in the number of falsely positive NSTs.63, 64 The average testing time was only 10 minutes, and fewer specialized personnel were needed to perform ultrasonographic studies than were needed with other BPP protocols. No unexpected fetal deaths occurred. The 3% intervention rate was similar to that of other series.65 Clark et al performed an additional 3005 more tests with this technique. One fetus died 2 days after a reactive NST, but this was due to a cord accident. Vintzileos et al66 studied 6543 fetus with this modified BPP and found no fetal deaths from hypoxia within 1 week of an normal modified BPP. Nageote et al showed that the modified BPP's prediction of adverse fetal outcome was similar to that of a negative CST.67
Manning modified his original design to exclude the NST unless one or more of the dynamic ultrasonographic components was abnormal.60 Manning believes that the AFV (a chronic stress marker) needs to be measured because of its increased predictive value of abnormal outcome in the general high-risk population and especially in certain high-risk groups, such as those with IUGR or postdates. Manning does not base fetal assessment on the NST and AFV alone, however, because a reactive NST and a normal AFV with such testing would be considered reassuring regardless of abnormalities of the other variables. Accordingly, if the NST and AFV were the only two variables present (a rare occurrence and combination), yielding a BPP score of 4, the risk of adverse outcome would still be increased. The PPV would be similar to a score of 6, but not that of a normal BPP score.61 In 26,780 high-risk pregnancies followed with the BPP, Manning's group only did an NST if one or more ultrasound parameters was abnormal. There was less than one fetal death per 1000 patients within 1 week of a normal BPP. Ninety-seven per cent had a score of 8, so only 3% needed further evaluation. The false positive rate depended on the end point used. It was 75% for a BPP score of 6 and less than 20% for a score of 0.
Role of the nonstress test in the biophysical profile
Since the NST may be the most sensitive BPP variable to detect fetal hypoxia, some believe it should be retained.68 In addition, since FB has been noted in some diabetic and postdate pregnancies even when fetal compromise was present, FB and the NST may not be interchangeable tests of fetal well-being.69 Additionally, the NST may show unsuspected spontaneous late decelerations, signaling fetal hypoxia.
Furthermore, some investigators advocate continued use of the NST to detect variable decelerations, which may be associated with adverse perinatal outcome even with normal AFV.70, 71, 72, 73 Brioschi and colleagues reviewed 1552 NSTs performed within a week of delivery and found that even if the NST was reactive, baseline abnormalities or decelerations or both were associated with significantly increased perinatal morbidity and mortality.74
Smith and co-workers reviewed 8 years of NST experience, which included 38,645 tests in 14,028 patients.75 During this time period (January of 1978 to December of 1985), 65 fetal deaths occurred. Forty-seven of these patients had a reactive NST in the last testing session before delivery. Thirty-seven of the deaths occurred within 7 days of a reactive NST, and the other 10 occurred between 8 and 11 days after a reactive NST. Autopsies were performed on 53 of the stillborn infants, and 12 of them had abnormal umbilical cord positions (usually nuchal). Variable decelerations were noted in five of these 12 cases during the last NST.
In four of the six stillbirths in their series of 583 postdate pregnancies, Eden and associates found variable decelerations during the last NST before delivery.76 All of the stillbirths occurred within 3–5 days of a reactive NST. There were no anomalies. Miyazaki and Miyazaki also noted that variable decelerations were more common in compromised postdate fetuses, even if the NST was reactive.77 Other investigators have not noted an increased risk of fetal distress or death with variable decelerations and worry that undue attention to them will lead to inappropriate intervention.78, 79 Manning believes that the NST can be omitted if all four ultrasound components are normal without "compromising the validity of the test results".80
Data on the predictive importance of variable decelerations are conflicting. However, sufficient evidence exists to compel further investigation of this FHR finding, which is only available through testing with the NST.
In summary, several investigators have modified the original BPP protocols in order to decrease testing time or improve adverse outcome predictability.
BIOPHYSICAL PROFILE IN SPECIFIC MATERNAL CONDITIONS
Labor
In a prospective, blinded study, Sassoon and colleagues monitored 95 patients (all singleton pregnancies at 36–42 weeks) with BPPs throughout labor.81 The initial BPP was performed at admission, before epidural anesthesia or rupture of the membranes. Scored by Manning's criteria (10 possible points), 75% of the patients had an initial normal score (
Additionally, as opposed to Vintzileos and colleagues' data in nonlaboring patients,10 Sassoon and co-workers found no correlation between the last BPP and the umbilical cord pH in laboring patients. Normal BPPs (
Amon et al studied the BPP during cervical ripening with prostaglandin E2, before induction of labor. They found that FB is virtually absent and that FM decreases, although FHR-R does not change.82
In summary, there are few studies correlating the intrapartum BPP with the resultant umbilical arterial (or venous) acid–base status. In contrast to the antepartum state, the BPP is not a reliable predictive test for acid–base status during the intrapartum state, nor during cervical ripening with prostaglandin E2 immediately before oxytocin induction of labor.82
Postdate pregnancy
Johnson and associates evaluated twice-weekly BPPs in 293 postdate pregnancies (defined as >294 days from the last menstrual period).83 No perinatal deaths occurred if the BPP and AFV were normal. Additionally, fetal distress (3.3%) and 5-minute Apgar scores less than 7 (1.9%) were infrequent. However, if the BPP was abnormal or oligohydramnios (defined as the largest pocket of fluid <1 cm) was present (n = 32), a significantly increased incidence of cesarean section for fetal distress (22%), low 5-minute Apgar scores (12.5%), and neonatal morbidity (19%) occurred. Neonatal morbidity was defined as meconium aspiration resulting in neonatal intensive care unit (NICU) admission. The cesarean section rate in pregnancies “prophylactically” delivered for a gestational age of 294 days or greater (with no other indication) was 42% (n = 50), compared with a 15% rate in the 180 patients allowed to begin spontaneous labor. This percentage was similar to the 16.5% cesarean section rate of the hospital obstetric population. Johnson and colleagues postulated that the BPP might help to differentiate a normal from a compromised fetus, aid delivery decisions in a postdate pregnancy, and prevent undue maternal morbidity as a result of unnecessary cesarean delivery.
Interestingly, when compared with 6536 tests on term fetuses with normal outcomes, Baskett found that 1587 tests on postdate fetuses (42–44 weeks' gestation), which also had good outcomes, were significantly more likely to have a NR-NST, abnormal FB and FT, and decreased AFV.18
In a study of 583 postdate patients (>42 weeks), Eden and associates found less mortality and significantly decreased morbidity when a fetus was assessed with twice-weekly NSTs with modified BPPs and weekly AFV evaluations. Delivery was performed for FHR decelerations, decreased AFV, or abnormal BPP scores.76 Perinatal outcomes were similar to those recorded using weekly CSTs in postdate patients, but in both testing schemes, intervention rates exceeded 25%.
In summary, management of a postdate pregnancy in the presence of an unfavorable cervix remains controversial. Again, no definitive answers are forthcoming from the available BPP studies, but data support delivery for an abnormal BPP or oligohydramnios.
Predicting infection in patients with premature rupture of membranes
Antenatal fetal surveillance in patients with PROM has been problematic. Contraction stress tests are contraindicated, and amniotic fluid cultures, even when obtainable, are not sufficiently predictive of fetal sepsis.84, 85 Several investigators have studied patients with PROM to see if BPPs are predictive of infection. Vintzileos and associates studied 73 patients with PROM.40 Fifty-three patients received BPP profile testing within 24 hours of delivery, 15 within 24–48 hours of delivery, and the other five at least 48 hours before delivery (but the time was not specified). In these 73 patients, a normal BPP (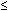 7) was associated with a 93.7% incidence of infection. The data were not analyzed separately for the different time intervals between testing and delivery. Increased fetal oxygen demands due to infection were postulated to cause adaptive (protective) CNS alterations in fetal biophysical activity, to decrease oxygen utilization. Interestingly, there was no significant difference between the umbilical cord pH values of the infected and noninfected patients with PROM.
7) was associated with a 93.7% incidence of infection. The data were not analyzed separately for the different time intervals between testing and delivery. Increased fetal oxygen demands due to infection were postulated to cause adaptive (protective) CNS alterations in fetal biophysical activity, to decrease oxygen utilization. Interestingly, there was no significant difference between the umbilical cord pH values of the infected and noninfected patients with PROM.
Vintzileos and co-workers then used the BPP to manage 73 consecutive patients with PROM.86 They then compared the outcomes (of the “study” group) with those of two historic control groups: 73 patients managed conservatively (the “control” group) and 73 patients managed with amniocentesis results (the “amniocentesis” group). Fetuses were delivered for a persistently low BPP (
Kivikoski and co-workers studied the effect of PROM in 44 pregnancies, 13 of which were eventually complicated by amnionitis (n = 11), neonatal infection (n = 1), or fetal distress (n = 1) with spontaneous variable decelerations.36 The gestational ages ranged from 28 to 41 weeks. The remaining 31 “uncomplicated” cases of PROM were matched with 31 normal antepartum patients of similar gestational age. Exclusion criteria included labor, anomalous fetuses, and initial evidence of infection or fetal distress. FB presence required at least one episode lasting at least 60 seconds during a 30-minute observation period. All patients with PROM had an intravenous infusion containing 5% dextrose, whereas none of the control patients had any infusion. Because intravenous glucose can increase FB,28, 29 the incidence of FB in the patients with PROM would have perhaps been even lower without the intravenous infusion. The incidence of FB was 90% in the 31 normal control patients and 65% in the 31 patients with uncomplicated PROM, a statistically significant difference (p <0.05). In the 13 patients with complicated PROM, FB occurred in 38% of the cases, which was not significantly different (due to small numbers) from the 65% incidence in the 31 patients with uncomplicated PROM. The investigators hypothesized that the absence of FB might be due to PROM alone. Furthermore, although the presence of FHR-R and FB may be predictive of the absence of infection in patients with PROM, the absence of FB is not necessarily predictive of the presence of infection in patients with PROM.
Goldstein and colleagues studied FB and FM in 41 cases of PROM.88 They found that 83% of the gestations with no fetal activity (no FM and no FB of >30 second duration during 30 min of observation) had a positive amniotic fluid culture. Conversely, none of the 17 gestations with an episode of fetal activity (defined in the same manner) had a positive amniotic fluid culture. When fetal activity was present, 90% (±3%) of the fetuses had negative amniotic fluid cultures for a least 48 hours. Therefore, the investigators believe that such complicated pregnancies can be monitored with biophysical testing every 48 hours when such fetal activity is present.
Conversely, Miller and co-workers were unable to find any difference between the composite BPP or any of its components in 47 patients with PROM in predicting clinical chorioamnionitis.89 None of the mothers received antibiotics or corticosteroids. None of the neonates developed sepsis, preventing comparison with Vintzileos' previously published findings of lower BPP score correlation with neonatal sepsis.86 The workers acknowledge that a type 2 error (from the low incidence of chorioamnionitis) could have prevented detection of an association between the BPP and chorioamnionitis.
Lewis et al performed a randomized trial of daily NSTs vs BPPs in the management of PPROM and found that neither test was highly sensitive in predicting infectious complications. Daily BPPs increased cost without apparent benefit.90
In summary, in patients with PROM, the presence of normal fetal biophysical activities and a reactive NST is strongly associated with the absence of fetal infection. However, it is not known if the converse is true, because no large, well-designed study has been published to answer this question. In fact, the absence of FB during BPP examination may simply be due to uncomplicated PROM.36
Intrauterine growth retardation
Ribbert and colleagues found close correlation of the BPP with umbilical venous pH from cordocentesis samples in a very small group of fetuses with severe IUGR.11 Of the 14 fetuses, seven had an abnormal BPP (
Manning and Hohler reviewed the utility of BPP testing in 960 cases of IUGR.91 The perinatal mortality rate (excluding anomalous fetuses) was only 12.5 per 1000, quite decreased from the expected rate of 60–80 deaths per 1000 in the population not receiving biophysical testing.
From a practical viewpoint, when one is confronted with a fetus with IUGR, an etiologic workup is in order, and close fetal surveillance with ultrasonography is necessary. Because continuing sonographic surveillance for fetal malformation, oligohydramnios, fetal interval growth, and possible amniocentesis is important, one can justify the primary use of the BPP. The use of umbilical artery Doppler velocimetry has decreased the fetal death rate and the overall perinatal mortality rate in IUGR gestations and pregnancies complicated by maternal hypertension. The perinatal death rate has decreased by 38%.92 As compared to abnormal umbilical artery Doppler findings, fetal heart rate and BPP abnormalities occur later in cases of fetal compromise in IUGR gestations.93
Twin gestations
Using the BPP of Vintzileos and co-workers, Lodeiro and associates monitored 49 consecutive twin pregnancies with BPPs.94 Most patients received weekly tests, and the remainder underwent twice-weekly testing. The last test occurred within 1 week of delivery. Sixty-four of the 98 NSTs (65%) were reactive, and 34.7% were nonreactive. A normal last BPP score of 8 or greater occurred in all fetuses with reactive NSTs. Sixty-two of these 64 fetuses had a good outcome. The remaining two fetuses (same pregnancy) developed chroioamnionitis and premature labor at 26 weeks' gestation, 3 days after a reactive NST and normal BPP. Both died in the early neonatal period as a result of extreme prematurity. In 28 of the 34 cases with NR-NSTs, the BPP was 8 or greater. All of these had a good outcome. Fetal distress developed in the other six, but none died. In four twin pregnancies, all eight fetuses had NR-NSTs, but only one of each pair had an abnormal BPP and only that fetus developed fetal distress. The investigators recommend use of the BPP as a secondary backup evaluation of NR-NSTs. Nonetheless, twin fetuses with reactive NSTs still require sonographic evaluation for interval growth and AFV throughout the antenatal period.
Diabetes mellitus
Johnson and colleagues used the modified BPP protocol of Manning (real-time ultrasonographic evaluation of the four sonographic components with an NST only if one of the four parameters was abnormal) to monitor 238 well-controlled diabetic patients.95 Fifty insulin-dependent diabetic women and 188 gestational diabetic women were followed with twice-weekly and weekly testing, respectively. Factors other than the BPP (not described in the article) were used to manage the patients as well. All insulin-dependent diabetic patients were delivered by 40 weeks, and all gestational diabetic patients by 42 weeks. Of the 230 patients with normal BPPs, 200 (87%) delivered at term with little maternal or fetal morbidity. Forty-five per cent of all study patients had a vaginal delivery after spontaneous labor. The induction rate for the 230 patients was 32%, and the overall cesarean section rate was 23.9% (11.7% elective + 12.1% in labor). The BPP had a low sensitivity (22%) and PPV (37.5%), but high NPV (96.5%) for neonatal morbidity. Neonatal morbidity was defined as a 5-minute Apgar score less than 7 or NICU admission for a low 5-minute Apgar score, respiratory distress, congenital anomaly, or hypoglycemia. Seven of the 200 neonates had morbidity despite normal BPP scores (false-negative rate of 3.5%). No perinatal deaths occurred in structurally normal infants. The researchers recommend the use of the BPP weekly to aid delivery decisions in pregnant women with “normalized and uncomplicated” insulin-dependent and gestational diabetes.
Dicker and collaborators monitored 98 insulin-dependent pregnant diabetic women with weekly BPPs after 28 weeks' gestation.96 Results of only 28 (2.9%) of the 978 tests were abnormal (score less than 8). When the last BPP was performed within 2 days of delivery, the NPV (94.8%) and specificity (90.2%) were relatively high but the PPV (<20%) was low for predicting intrapartum fetal distress. Similar findings for predicting a low 5-minute Apgar score were found, with a NPV of 98.7%, specificity of 87.3%, and PPV of 7.4%. For BPPs performed within 1 week of delivery, the NPVs for intrapartum fetal distress and for a low 5-minute Apgar score were similar to those obtained with twice-weekly testing. The specificities were approximately 80% for each outcome, and very low PPVs of 17% occurred for each outcome. No perinatal deaths occurred, and the investigators partially attributed this outcome to immediate intervention for abnormal scores.
In summary, there are adequate data to support the use of the BPP in the management of pregnancy in diabetic women. However, the superiority of the BPP versus the NST as the primary means of fetal surveillance has not been shown. Sonographic surveillance of the fetus of a diabetic mother is necessary to assess for malformations, interval fetal growth, macrosomia, and AFV abnormalities. In well-controlled, compliant pregnant women with uncomplicated diabetes, fetal well-being testing may be performed weekly.
Fetuses of mothers without vascular disease are at risk of lactic acidosis. NSTs and BPPs may not be predictive of fetal condition. Maternal hyperglycemia may increase FB, FM, and amniotic fluid volume. In their cordocentesis study of pregestational insulin dependent diabetic pregnancies, Salvesen et al found that 84% of fetuses had lactic acidemia.97 Only 25% of the fetuses had abnormal BPPs and only 50% had abnormal FHR variability Fifty-six per cent of the mothers were hyperglycemic at the time. They found an inverse relationship between maternal blood sugar levels and fetal pH.
Fetuses of mothers with diabetic vasculopathy are at increased risk for hypoxic acidemia and antepartum tests are predictive of the fetal condition.98 The BPP does not take hydramnios into account. Hydramnios may be associated with poor maternal glucose control which may be associated with fetal acidosis. The best way to attain fetal well-being in diabetic pregnancies may be by achieving excellent maternal blood sugar control and not by the use of currently available antepartum testing techniques.98
Magnesium sulfate tocolysis
In a study of 16 patients (six twin and ten singleton pregnancies) receiving magnesium sulfate tocolysis, Peaceman and colleagues found a significant decrease in FB and FHR-R causing a significant decrease in the BPP score.39 They suggested that these changes were due to the magnesium sulfate therapy. No significant effects on FT, FM, or AFV were found. The decreased FB was not thought to reflect changes associated with labor, because all but one fetus in premature labor exhibited FB on the initial admission BPP, before magnesium sulfate tocolysis was started. However, insufficient information about each patient's course (i.e., the rate and amount of cervical dilation, the dosage of magnesium sulfate required, and so on) was given to definitively negate the effect of labor on FB incidence.
Detecting congenital anomalies
Anomaly detection is a potential benefit of antenatal surveillance with the BPP. However, the ability to detect such anomalies is dependent on the skill of the sonographer. Anomaly detection was not successful in Platt and associates' series of 289 fetuses, because only one of five anomalous fetuses was detected before delivery with BPP testing.99 Some of these anomalies may have been difficult to detect even with specialized ultrasonography, however.
Cerebral palsy
Manning et al found an inverse, exponential, highly significant correlation between the last BPP score and the incidence of cerebral palsy (CP).100 A score of 6 or less had a sensitivity of 49%, and the more abnormal the BPP score, the higher the risk of CP. A normal BPP score was associated with CP in 0.7 per 1000 live births. A score of 6 was associated with an incidence of 13.1 per 1000, and a score of 0 was associated with 333 per 1000. They found no association of gestational age, birth weight, or the assumed timing of the injury with the incidence of CP.
Corticosteroids
Corticosteroids are often used in fetuses less than 34 weeks' gestation at risk for premature delivery to enhance fetal lung maturity. Kelly et al found decreased BPP scores in more than one-third of fetuses within 48 hours of corticosteroid treatment.101 However, within 24–48 hours of these results, the BPP scores normalized in the fetuses whose scores had decreased by 4 points. Fetal breathing and FHR-R were the parameters most often affected. Deren et al102 and Rotmensch et al103 found similar transient effects on the BPP after steroid use with restoration of normal values within 48–96 hours after steroid administration. This important information must be considered in institutions using daily NSTs or BPPs to evaluate fetuses with preterm labor or PPROM.
History of stillbirth in previous pregnancy
The risk of recurrent stillbirth is estimated to be increased up to 3 times. Therefore, a history of a previous stillbirth is an indication for antepartum fetal monitoring. No adequate study has determined the best time to begin fetal testing. A retrospective cohort study suggested beginning such testing at 32 weeks' gestation. They found no correlation with the gestational age of the previous stillbirth.104
SPECIFIC MANAGEMENT PROTOCOLS FOR BIOPHYSICAL PROFILE TESTING
After obtaining a BPP, the indications for intervention (i.e., delivery) vary only minimally between different investigators. In general, if a normal score is obtained, the testing is repeated weekly. Twice-weekly testing is recommended for complicated diabetic pregnancies, for IUGR fetuses, for pregnancies complicated by hypertension with proteinuria, and for postdate pregnancies. However, in certain high-risk situations, depending on the physician's level of concern, daily testing (or more frequently) may be helpful. Specific management guidelines used by Manning and his group and by others have been published.105, 106, 107
In general, the NST may be used as the primary test of fetal well-being. Most clinicians are capable of performing and interpreting this test, expensive testing equipment and highly specialized personnel are unnecessary, and the negative (normal) predictive value is extremely reliable. AFV should be assessed (e.g., IUGR, postdates, FHR variable decelerations). The frequency of testing depends on the degree of fetal risk and varies from daily to weekly. In addition, umbilical artery Doppler velocometry studies are recommended in fetuses with IUGR.92, 93
If the primary test (the NST) is nonreactive or unsatisfactory (i.e., from conditions such as extreme obesity), the appropriate next test is the BPP, performed according to the method of Manning. In the absence of fetal structural or alloimmunized abnormalities and in the absence of obstetric (i.e., placenta previa, prior vertical uterine incision, postdate pregnancy with a favorable Bishop score) or maternal indications (i.e., pre-eclampsia, antecedent moderate to severe medical disease) for delivery, management may proceed according to the test results. Accordingly, if the results are normal (i.e., 8/10 with normal amniotic fluid, or 8/8), then continued fetal testing is indicated. If the BPP score is 6, one should suspect chronic asphyxia and Manning et al recommend delivery if the fetus is at least 36 weeks' gestation and conditions are favorable. However, if the fetus is more than 36 weeks' gestation and lung maturity studies are immature, Manning's group recommends repeat BPP testing in 4–6 hours with delivery if oligohydramnios is present. If the BPP score is 4, chronic asphyxia should be suspected and Manning et al recommend delivery if the fetus is at least 32 weeks' gestation. If the fetus is less than 32 weeks' gestation, repeat testing is recommended. If the BPP score is 0–2, one should strongly suspect chronic asphyxia and Manning's group recommends extending the testing time to 120 minutes with delivery if the score is persistently less than or equal to 4, regardless of gestational age. Regardless of the overall BPP score, oligohydramnios always needs further evaluation. When the BPP score dictates delivery, Manning emphasizes that vaginal delivery is recommended, if there are no maternal/fetal contraindications.
The CST is rarely used. If a spontaneous CST is observed, the results are noted and interpreted in light of other fetal test results.
EFFICACY OF THE BIOPHYSICAL PROFILE
In his early work (1980), Manning found that each component of the BPP had a false-positive rate approaching 50%, which he attributed to normal periodicity in most cases.50 The combination of the five components into one score improved the PPV and NPV. A CST was required only three times (0.25%) in the first 1184 high-risk patients for further fetal evaluation.108 Additionally, 13 of 19 major congenital anomalies were detected with ultrasonography in this same group. After reviewing the results of 26,257 BPPs, Manning found only one instance when an abnormal BPP (score of 4 out of a possible 10) reverted to normal.109 This particular fetus received severe head trauma when his mother's pelvis was fractured. Over 3 weeks, the BPP score became normal. At birth, the infant was not asphyxiated and survived.
Using receiver operating characteristic (ROC) analysis, Richardson reviewed Manning and colleagues' 1980 and 1981 studies.110 The derived ROC curve indicated that no specific cutoff point for the absence of fetal asphyxia existed. Thus, any arbitrary cutoff point would miss some asphyxiated fetuses unless the false-positive rate was allowed to be excessively high. Second, analysis of the ROC at Manning's own cutoff point for intervention showed that the NST and FB alone were as efficacious as the entire profile. The additional parameters (FM, FT, AFV) did not improve discriminatory strength. Manning and his group have now used the BPP to monitor more than 28,000 high-risk pregnancies. Richardson's ROC analysis has not been applied to this much larger number of tested pregnancies. It is therefore unknown whether the original findings would be confirmed or altered.
Vintzileos and collaborators also found high individual false-positive rates for the biophysical activities but improved predictive value for adverse outcome with combination testing.6 In a small study of 150 high-risk pregnancies, the BPP was more reliable than the CST in predicting fetal hypoxia at birth.
Baskett and colleagues found increased predictability for perinatal death with the composite BPP score.111 Their prospective study analyzed the course of 4148 patients who had received a BPP within 1 week of delivery.
In 1985, Manning and co-workers109 reviewed the use of the BPP in 12,620 consecutive patients. The overall perinatal mortality rate was 7.4 per 1000, of which 50% were stillbirths and 50% were neonatal deaths. During the study interval, within the same catchment region, the perinatal mortality rate for the general population was 14.3 per 1000. Hence, a 50% decrease in perinatal mortality occurred in the tested population. Within the tested population, 75% of the perinatal deaths occurred as a direct result of either congenital malformation or severe Rh disease. Of the remaining 25% of perinatal deaths, 63% occurred as stillbirths. Unfortunately, most of these stillbirths occurred within 1 week of a normal BPP, in structurally normal fetuses. In contrast to the tested population, a much higher rate (90%) of structural normality was found among the perinatal deaths in the general population.
Later, in 1987, Manning and co-workers reviewed the efficacy of BPP testing in 19,221 consecutive high-risk pregnancies.105 The rate of fetal death after a last normal test was 0.726 per 1000 (14 deaths). Two of the 14 fetuses died within 24 hours of a normal test. The mean interval between the last test and fetal death was 4.4 days. The corrected perinatal mortality rate was 5.06 per 1000, which was lower than that expected for their low risk population. The corrected perinatal mortality rate excluded deaths due to a major anomaly or due to red blood cell alloimmunization.
Drawbacks of BPP testing include (1) the need for an ultrasound machine, (2) no permanent record of the test unless a tape is made, (3) a long time to complete if the fetus is in a quiet sleep state, and (4) no consideration of the presence of hydramnios as a parameter which can be important in diabetic pregnancies.
BIOPHYSICAL PROFILE VERSUS NONSTRESS TEST AND CONTRACTION STRESS TEST
BPP testing results in fewer equivocal findings and a higher incidence of normal test results than either the NST or CST.3, 68, 109 Manning and colleagues found a significantly higher PPV for a low 5-minute Apgar score with the BPP than with the NST in their prospective blinded study comparing the BPP (in 375 patients) with the NST (in 360 patients). In a prospective randomized study, Platt and associates99 compared the NST (in 361 patients) with the BPP (in 279 patients). An improvement in predictability, but not a statistically significant one, occurred for the NPV, sensitivity, and specificity for predicting overall abnormal outcome. Abnormal outcome was defined as fetal distress in labor, 5-minute Apgar score less than 7, small-for-gestational-age infant, or perinatal mortality. The BPP did have a significantly higher PPV for an abnormal outcome, however. Eighty-eight per cent of the pregnancies with abnormal BPPs had an abnormal outcome, compared with 33% with NR-NSTs. Both Manning and Platt and their co-workers have shown that the use of the BPP can therefore reduce the number of false-positive NSTs. Neither Manning's nor Platt's group found that either test was superior in predicting antenatal stillbirth.99, 112
In a small study of 158 predominantly high-risk patients, Schifrin and associates used real-time ultrasonography after an NST to see if a modified BPP would increase the predictability of adverse perinatal outcome.51 The interval between testing time and delivery was not noted. Although normal test results highly predicted normal outcome, regardless of testing method, abnormal test results were poor indicators of poor outcome. However, they did find the modified BPP to be more reliable than the CST in predicting an abnormal fetus after a NR-NST.
Table 5, Table 6, Table 7, Table 8, and Table 9 describe the relative advantages and disadvantages of each antenatal testing method, as well as the statistical characteristics of each.3, 62, 65, 75, 105, 109, 111, 113
Table 5. Biophysical profile
Advantages | Disadvantages |
Noninvasive | Abnormal score may reflect rest-activity cycle |
Low false-negative rate | Can be time-consuming, but average time = 18 min |
Composite score improves PPV for some poor outcomes | Requires specialized help |
May detect anomalies | More expensive than NST |
Assesses acute and chronic markers of hypoxia |
|
False-negative rate similar to CSTs |
|
May allow detection of various degrees of fetal compromise |
|
Few CSTs needed for backup evaluation |
|
Can decrease false-positive and false-negative studies associated with NST |
|
Provides information about fetal number, position, placental position, and appearance |
|
Table 6. Nonstress test
Advantages | Disadvantages |
Simple to perform | High frequency of abnormal results |
Noninvasive | Abnormal score may reflect rest-activity cycle, not hypoxia |
Outpatient setting | May be technically difficult if patient is obese, has a multiple gestation or polyhydramnios, or if fetus or mother moves |
Requires less skilled personnel | High false-positive rate |
Less expensive | Low positive predictive value |
Low false-negative rate | False-negative rate not as low as CSTs or BPPs |
Table 7. Oxytocin challenge test
Advantages | Disadvantages |
Low frequency of abnormal results | Poor PPV |
Low false-negative rate | Time-consuming: 90–120 min |
Provides a measure of fetus' reserve to tolerate labor | Hospital setting |
| Contraindicated in some cases |
| Nonphysiologic-normal fetus may not tolerate prolonged contraction, increasing false-positive results |
| Can cause fetal distress, premature labor |
Table 8. Incidence of normal studies
Table 9. Death within 1 week of normal test result
CONCLUSION
Antepartum management of high-risk pregnancies involves a balance of risks. Early delivery to minimize the morbidity and mortality associated with the high-risk condition may lead to severe morbidity or even mortality as a result of prematurity complications. Fetal surveillance tests have been developed to assess well-being at the time of the test and the statistical likelihood of future fetal well-being during a specified interval of time. In the USA the NST is widely used for primary surveillance of fetal well-being in high-risk pregnancies. Although a reactive NST is an excellent predictor of fetal well-being, most NR-NSTs are not associated with fetal compromise.3 The CST has been used as a follow-up test for NR-NSTs. Although CSTs are associated with a low false-negative rate,3 they are invasive and time-consuming, are contraindicated in certain conditions, require a hospital setting, and can precipitate premature labor or fetal distress. The BPP (with the NST) can usually be performed in less than 18 minutes in an efficient testing center.108 The BPP is noninvasive and has no contraindications. However, specialized equipment, space, and personnel are required for sonographic testing. It may be used as early as 26–28 weeks' gestation. Twice weekly BPPs are recommended in pregnancies complicated by IUGR, diabetes mellitus, prolonged gestation, and hypertension with proteinuria.
Epidemiologically, when a disease has a low prevalence (i.e., perinatal mortality in the USA), the false-negative rate of monitoring tests will be low regardless of the test's sensitivity or specificity. Additionally, the false-positive rate will be high unless the test is very specific (i.e., >99.5%).3 Both the NST and CST have high false-positive rates—greater than 50%.3
Since the NST and CST have insufficient diagnostic accuracy in regard to the abnormal fetus, multiple parameter measurement with a BPP was developed. Manning, Vintzileos, Schifrin, and Baskett and their groups each found increased positive predictability of adverse perinatal outcome when combination testing was performed.6, 50, 51, 111 Manning and colleagues found that the perinatal mortality rate in the referred high-risk population was lower than that of the low-risk population of the same area.109 The exact contribution of BPP testing to this decrease is not known. Unfortunately, there are insufficient well-designed studies comparing the efficacy of the NST, CST, and BPP.
Moreover, there will always be a small number of fetal and neonatal deaths that may not be predictable by any type of testing, such as those due to acute placental abruption, neonatal sepsis, or acute changes in maternal well-being.
More information about the relative importance of the individual BPP components is needed. It is hoped that future basic science research and clinical studies will advance the understanding of normal and abnormal fetal behavior to help optimize fetal well-being and minimize perinatal morbidity and mortality.
REFERENCES
Health, United States, 2004 with Chartbook on Trends in the Health of Americans. Hyattsville, Maryland: National Center for Health Statisitics, 2004 |
|
Martin JA, Hamilton BE, Sutton PD et al: Births: final data for 2002. Natl Vital Stat Rep 52: 1–113, 2003 |
|
Thacker SB, Berkelman RL: Assessing the diagnostic accuracy and efficacy of selected antepartum fetal surveillance techniques. Obstet Gynecol Surv 41: 121, 1986 |
|
Vintzileos AM, Campbell WA, Rodis JF: Fetal biophysical profile scoring: Current status. Clin Perinatol 16: 661, 1989 |
|
Manning FA: Fetal biophysical profile: a critical appraisal. Clin Obstet Gynecol 45: 975–985, 2002. |
|
Vintzileos AM, Campbell WA, Ingardia CJ et al: The fetal biophysical profile and its predictive value. Obstet Gynecol 62: 271, 1983 |
|
Humphrey T: Function of the nervous system during prenatal life. In Uwe S (ed): Perinatal Physiology. p 651, New York, Plenum Publishing, 1978 |
|
Ianniruberto A, Tejani E: Ultrasonographic study of fetal movements. Semin Perinatol 5: 175, 1981 |
|
Vintzileos AM, Campbell WA, Nochimson DJ et al: The use of real-time scanning in antepartum fetal evaluation: The fetal biophysical profile. In Sanders RC. Hill MC (eds): Ultrasound Annual, p 254. New York, Raven Press, 1985 |
|
Vintzileos AM, Gaffney SE, Salinger LM et al: The relationship between fetal biophysical profile and cord pH in patients undergoing cesarean section before the onset of labor. Obstet Gynecol 70: 196, 1987 |
|
Ribbert LSM, Snijders RJM, Nicolaides KH et al: Relationship of fetal biophysical profile and blood gas values at cordocentesis in severely growth retarded fetuses. Am J Obstet Gynecol 163: 569, 1990 |
|
Manning FA, Morrison I, Harman CR et al: The abnormal fetal biophysical profile score V. Predictive accuracy according to score composition. Am J Obstet Gynecol 162: 918, 1990 |
|
Jones TB, Frigoletto FD: Fetal condition and the biophysical profile. Postgrad Radiol 5: 47, 1985 |
|
Brown R, Patrick J: The nonstress test: How long is enough? Am J Obstet Gynecol 141: 646, 1981 |
|
Castillo RA, Devoe LD, Arthur M et al: The preterm nonstress test: Effects of gestational age and length of study. Am J Obstet Gynecol 160: 172, 1989 |
|
Dawes GS, Houghton CRS, Redman CWG et al: Pattern of the normal human fetal heart rate. Br J Obstet Gynaecol 89: 276, 1982 |
|
Sorokin Y, Dierker LJ, Pillay SK et al: The association between fetal heart rate patterns and fetal movements in pregnancies between 20 and 30 weeks gestation. Am J Obstet Gynecol 143: 243, 1982 |
|
Baskett TF: Gestational age and fetal biophysical assessment. Am J Obstet Gynecol 158: 332, 1988 |
|
Smith CV, Phelan JP, Platt LD et al: Fetal acoustic stimulation testing, Part fl. A randomized clinical comparison with the nonstress test. Am J Obstet Gynecol 155: 131, 1986 |
|
Smith CV, Phelan JP, Paul RH: Fetal acoustic stimulation testing: A retrospective experience with the fetal acoustic stimulation test. Am J Obstet Gynecol 153: 567, 1985 |
|
Smith CV, Nguyen HN, Phelan JP et al: Intrapartum assessment of fetal well being: A comparison of fetal acoustic stimulation with acid-base determination. Am J Obstet Gynecol 155: 726, 1986 |
|
Fox HE, Hohler CW: Fetal evaluation by real time imaging. Clin Obstet Gynecol 20: 339, 1977 |
|
Boddy K, Dawes GS: Fetal breathing. Br Med Ball 31: 3, 1975 |
|
Manning FA: Fetal breathing movements as a reflection of fetal status. Postgrad Med 61: 116, 1977 |
|
Lewis P, Boylan P: Fetal breathing: A review. Am J Obstet Gynecol 134: 587, 1979 |
|
Tamura RK, Manning FA: Fetal breathing movements. In Sciarra JJ (ed): Gynecology and Obstetrics, Vol 3. Philadelphia, Harper & Row, 1986 |
|
Patrick J, Natale R, Richardson B: Patterns of human fetal breathing activity at 34 to 35 weeks' gestational age. Am J Obstet Gynecol 132: 507, 1978 |
|
Devoe LD, Castillo RA, Searle NS: The effects of tryptophan and glucose on fetal breathing movements. Am J Obstet Gynecol 155: 135, 1986 |
|
Trudinger B J, Lewis PJ, Mangez J et al: Fetal breathing movements in high risk pregnancy. Br J Obstet Gynecol 85: 662, 1978 |
|
Fox HE, Inglis J, Steinbrecher M: Fetal breathing movements in uncomplicated pregnancies, Part I. Relationship to gestational age. Am J Obstet Gynecol 134: 544, 1979 |
|
Manning F, Wyn Pugh E, Boddy K: Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J 1: 552, 1975 |
|
Manning FA, Feyerabend C: Cigarette smoking and fetal breathing movements. Br J Obstet Gynecol 83: 262, 1976 |
|
Gennser G, Marsal K, Brantmark B: Maternal smoking and fetal breathing movements. Am J Obstet Gynecol 123: 861, 1975 |
|
Richardson B. Natale R, Patrick J: Human fetal breathing activity during electively induced labor at term. Am J Obstet Gynecol 133: 247, 1979 |
|
Boylan P, O'Donovan P, Owens OJ: Fetal breathing movements and the diagnosis of labor: A prospective analysis of 100 cases. Obstet Gynecol 66: 517, 1985 |
|
Kivikoski AI, Amon E, Vaalamo PO et al: Effect of third trimester premature rupture of membranes on fetal breathing movements: A prospective case-controlled study. Am J Obstet Gynecol 159: 1474, 1988 |
|
Vintzileos AM, Feinstein SJ, Lodeiro JG et al: Fetal biophysical profile and the effect of premature rupture of the membranes. Obstet Gynecol 67: 818, 1986 |
|
Manning FA, Platt LD, Lemay M: Effect of amniocentesis on fetal breathing movements. Br Med J 2: 1582, 1977 |
|
Peaceman AM, Meyer BA, Thorp JA et al: The effect of magnesium sulfate tocolysis of the fetal biophysical profile. Am J Obstet Gynecol 161: 771, 1989 |
|
Vintzileos AM, Campbell WA, Nochimson DJ et al: The fetal biophysical profile in patients with premature rupture of the membranes: An early predictor of fetal infection. Am J Obstet Gynecol 152:510. 1985 |
|
McGowan J, Devoe LD, Searle N et al: The effects of long- and short-term maternal caffeine ingestion on human fetal breathing and body movements in term gestations. Am J Obstet Gynecol 157: 726, 1987 |
|
Platt LD, Manning FA, Lemay M et al: Human fetal breathing: Relationship to fetal condition. Am J Obstet Gynecol 132: 514, 1978 |
|
Manning FA. Platt LD, Sipos L et al: Fetal breathing movements and the nonstress test in high risk pregnancies. Am J Obstet Gynecol 135: 511, 1979 |
|
Sadovsky E, Yaffe H: Daily fetal movement recording and fetal diagnosis. Obstet Gynecol 41: 845, 1973 |
|
Manning FA, Platt LD, Sipos L: Fetal movement in human pregnancies in the third trimester. Obstet Gynecol 54: 699, 1979 |
|
Leader LR, Baillie P. Van Schalkwyk DJ: Fetal movements and fetal outcome: A prospective study. Obstet Gynecol 57: 431, 1981 |
|
Birkenfeld A, Laufer N, Sadovsky E: Diurnal variation of fetal activity. Obstet Gynecol 55: 417, 1980 |
|
Gelman SR, Spellacy WN, Wood S: Fetal movements and ultrasound: Effect of maternal intravenous glucose administration. Am J Obstet Gynecol 137: 459, 1980 |
|
Hume RF, O'Donnell K J, Stanger CL et al: In utero cocaine exposure: Observations of fetal behavioral state may predict neonatal outcome. Am J Obstet Gynecol 161: 685, 1989 |
|
Manning FA, Platt LD, Sipos L: Antepartum fetal evaluation: Development of a fetal biophysical profile. Am J Obstet Gynecol 136: 787, 1980 |
|
Schifrin BS, Guntes V, Gergely RC et al: The role of real time scanning in antenatal fetal surveillance. Am J Obstet Gynecol 140: 525, 1981 |
|
Chamberlain PF, Manning FA, Morrison I et al: Ultrasound evaluation of amniotic fluid volume, Part I. The relationship of marginal and decreased amniotic fluid volumes to perinatal outcomes. Am J Obstet Gynecol 150: 245, 1984 |
|
Hendricks SK, Smith JR, Moore DE et al: Oligohydramnios associated with prostaglandin synthetase inhibitors in preterm labour. Br J Obstet Gynaecol 97: 312, 1990 |
|
Cohn HE, Sacks EJ, Heyman MA et al: Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol 120: 817, 1974 |
|
Seeds AE: Current concepts of amniotic fluid dynamics. Am J Obstet Gynecol 138: 575, 1980 |
|
Campbell S, Wladimiroff SW, Dewhurst CJ: The antenatal measurement of fetal urine production. Br J Obstet Gynaecol 80: 680, 1973 |
|
Phelan JP, Platt LD, Yeh SY: The role of ultrasound assessment of amniotic fluid volume in the management of the post date pregnancy. Am J Obstet Gynecol 151: 804, 1985 |
|
Rutherford SE, Phelan JP, Smith CV: The four quadrant assessment of amniotic fluid volume: An adjunct to antepartum fetal heart rate testing. Obstet Gynecol 70: 353, 1987 |
|
Chauhan SP, Doherty DD, Magann EF et al: Amniotic fluid index vs single deepest pocket technique during modified biophysical profile: a randomized clinical trial. Am J Obstet Gynecol 191: 661–667; discussion 667–668, 2004. |
|
Manning FA, Morrison I, Lange IR et al: Fetal biophysical profile scoring: Selective use of the nonstress test. Am J Obstet Gynecol 156: 709, 1987 |
|
Manning FA, Morrison MB, Harman CR et al: The abnormal fetal biophysical profile score. Am J Obstet Gynecol 162: 918, 1990 |
|
Clark SL, Sabey P, Jolley K: Nonstress testing with acoustic stimulation and amniotic fluid volume assessment: 5973 tests without unexpected fetal death. Am J Obstet Gynecol 160: 694, 1989 |
|
Phelan JP: The nonstress test: A review of 8,000 tests. Am J Obstet Gynecol 139: 7, 1981 |
|
Evertson LR, Gauthier RJ, Schifrin BS et al: Antepartum fetal heart rate testing, Part I. Evolution of the nonstress test. Am J Obstet Gynecol 133: 29, 1979 |
|
Freeman RK, Anderson G, Dorchester WA: A prospective multiinstitutional study of antepartum fetal heart rate monitoring, Part II: Contraction stress tests versus nonstress test for primary surveillance. Am J Obstet Gynecol 143: 778, 1982 |
|
Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. The use and misuse of the fetal biophysical profile. Am J Obstet Gynecol 156: 527-533, 1987. |
|
Nageotte MP, Towers CV, Asrat T, Freeman RK. Perinatal outcome with the modified biophysical profile. Am J Obstet Gynecol 170: 1672–1676, 1994. |
|
Porto M: Comparing and contrasting methods of fetal surveillance. Clin Obstet Gynecol 30: 956, 1987 |
|
Benacerraf BR, Frigoletto FD: Fetal respiratory movements: Only part of the biophysical profile. Obstet Gynecol 67: 556, 1986 |
|
Phelan JP, Lewis PE Jr: Fetal heart rate decelerations during a nonstress test. Obstet Gynecol 57: 228, 1981 |
|
Eden RD Selfert LS, Kodack LD et al: A modified biophysical profile for antenatal fetal surveillance. Obstet Gynecol 71: 365, 1988 |
|
O'Leary JA, Andrinopoulos GC, Giordano P: Variable decelerations and the nonstress test: An indication of cord compromise. Am J Obstet Gynecol 137: 704, 1980 |
|
Vintzileos AM, Campbell WA, Nochimson DJ et al: The use and misuse of the fetal biophysical profile. Am J Obstet Gynecol 156: 527, 1987 |
|
Brioschi PA, Extermann P, Terracina D et al: Antepartum nonstress fetal heart rate monitoring: Systematic analysis of baseline patterns and decelerations as an adjunct to reactivity in the prediction of fetal risks. Am J Obstet Gynecol 153: 633, 1985 |
|
Smith CV, Nguyen HN, Kovacs B et al: Fetal death following antepartum fetal heart rate testing: A review of 65 cases. Obstet Gynecol 70: 18, 1987 |
|
Eden RD, Gergely RZ, Schifrin BS et al: Comparison of antepartum testing schemes for the management of the post date pregnancy. Am J Obstet Gynecol 144: 683, 1982 |
|
Miyazaki FS, Miyazaki BA: False reactive nonstress tests in post term pregnancies. Am J Obstet Gynecol 140: 269, 1981 |
|
Anyaegbunam A, Brustman L, Divon M et al: The significance of antepartum variable decelerations. Am J Obstet Gynecol 155: 707, 1986 |
|
Meis PJ, Ureda JR, Swan M et al: Variable decelerations during nonstress tests are not a sign of fetal compromise. Am J Obstet Gynecol 154: 586, 1986 |
|
Manning FA, Morrison I, Lange IR et al: Fetal biophysical profile scoring: selective use of the nonstress test. Am J Obstet Gynecol 156: 709–712, 1987. |
|
Sassoon DA, Castro LC, Davis JL et al: The biophysical profile in labor. Obstet Gynecol 76: 360, 1990 |
|
Amon E, Fossick K, Sibai BM: The biophysical profile in patients undergoing cervical ripening by PGE2 prior to induction of labor. Am J Obstet Gynecol (suppl 1, pt 2) 164: 243, 1991 |
|
Johnson JM, Harman CR, Lange IR et al: Biophysical profile scoring in the management of the post term pregnancy: An analysis of 307 patients. Am J Obstet Gynecol 154: 269, 1986 |
|
Garite TJ, Freeman RK, Linsey M et al: The use of amniocentesis in patients with premature rupture of the membranes. Obstet Gynecol 54: 226, 1979 |
|
Cotton DB, Hill LM, Strassner HT et al: Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol 63: 38, 1984 |
|
Vintzileos AM, Bors-Koefoed R, Pelegano JF et al: The use of the fetal biophysical profile improves pregnancy outcome in premature rupture of the membranes. Am J Obstet Gynecol 157: 236, 1987 |
|
Ohlsson A, Wang E: An analysis of antenatal tests to detect infection in preterm premature rupture of membranes. Am J Obstet Gynecol 162: 809, 1990 |
|
Goldstein I, Romero R, Merrill S et al: Fetal body and breathing movements as predictors of intraamniotic infection in preterm premature rupture of membranes. Am J Obstet Gynecol 159: 363, 1988 |
|
Miller JM Jr, Kho MS, Brown HL et al: Clinical chorioamnionitis is not predicted by an ultrasonic biophysical profile in patients with premature rupture of membranes. Obstet Gynecol 76: 1051, 1990 |
|
Lewis DF, Adair CD, Weeks JW et al: A randomized clinical trial of daily nonstress testing versus biophysical profile in the management of preterm premature rupture of membranes. Am J Obstet Gynecol 181: 1495–1499, 1999. |
|
Manning FA, Hohler C: Intrauterine growth retardation: Diagnosis, prognostication, and management based on ultrasound methods. In Fleischer AC, Romero R, Manning FA et al (eds): Principles and Practice of Ultrasonography in Obstetrics and Gynecology. Norwalk, Appleton & Lange, 1991 |
|
Alfirevic Z, Neilson JP. Doppler ultrasonography in high-risk pregnancies: systematic review with meta-analysis. Am J Obstet Gynecol 172: 1379–1387, 1995. |
|
Soothill PW, Ajayi RA, Campbell S, Nicolaides KH. Prediction of morbidity in small and normally grown fetuses by fetal heart rate variability, biophysical profile score and umbilical artery Doppler studies. Br J Obstet Gynaecol 100: 742–745, 1993. |
|
Lodeiro JG, Vintzileos AM, Feinstein SJ et al: Fetal biophysical profile in twin gestations. Obstet Gynecol 67: 824, 1986 |
|
Johnson JM, Lange IR, Harman CR et al: Biophysical profile scoring in the management of the diabetic pregnancy. Obstet Gynecol 72: 841, 1988 |
|
Dicker D, Feldberg D, Yeshaya A et al: Fetal surveillance in insulin-dependent diabetic pregnancy: Predictive value of the biophysical profile. Am J Obstet Gynecol 159: 800, 1988 |
|
Salvesen DR, Freeman J, Brudenell JM, Nicolaides KH. Prediction of fetal acidaemia in pregnancies complicated by maternal diabetes mellitus by biophysical profile scoring and fetal heart rate monitoring. Br J Obstet Gynaecol 100: 227–233, 1993. |
|
Kontopoulos EV, Vintzileos AM. Condition-specific antepartum fetal testing. Am J Obstet Gynecol 191: 1546–1551, 2004. |
|
Platt LD, Walla CA, Paul RH et al: A prospective trial of the fetal biophysical profile versus the nonstress test in the management of high risk pregnancies. Am J Obstet Gynecol 153: 624, 1985 |
|
Manning FA. Fetal biophysical profile. Obstet Gynecol Clin North Am 26: 557–577, v, 1999. |
|
Kelly MK, Schneider EP, Petrikovsky BM, Lesser ML: Effect of antenatal steroid administration on the fetal biophysical profile. J Clin Ultrasound 28: 224–226, 2000. |
|
Deren O, Karaer C, Onderoglu L et al: The effect of steroids on the biophysical profile and Doppler indices of umbilical and middle cerebral arteries in healthy preterm fetuses. Eur J Obstet Gynecol Reprod Biol 99: 72–76, 2001. |
|
Rotmensch S, Liberati M, Celentano C et al: The effect of betamethasone on fetal biophysical activities and Doppler velocimetry of umbilical and middle cerebral arteries. Acta Obstet Gynecol Scand 78: 768–773, 1999. |
|
Weeks JW, Asrat T, Morgan MA et al: Antepartum surveillance for a history of stillbirth: when to begin? Am J Obstet Gynecol 172: 486–492, 1995. |
|
Manning FA, Morrison I, Harman CR et al: Fetal assessment based on fetal biophysical profile scoring: Experience in 19,221 referred high risk pregnancies. Am J Obstet Gynecol 157: 880, 1987 |
|
Manning FA Harman CR. Morrison I: Fetal assessment based on fetal biophysical profile scoring, Part IV. An analysis of perinatal morbidity and mortality. Am J Obstet Gynecol 162: 703, 1990 |
|
Druzin ML, Smith JF, Gabbe SG. Antepartum Fetal Evaluation. In: Obstetrics-Normal and Problem Pregnancies, edited by Gabbe SG, Niebyl JR, Simpson JL. Philadelphia: Churchhill Livingstone Elsevier, 2007, p. 282. |
|
Manning FA, Baskett TF, Morrison I et al: Fetal biophysical profile scoring: A prospective study in 1,184 high-risk patients. Am J Obstet Gynecol 140: 289, 1981 |
|
Manning FA, Morrison I, Lange IR et al: Fetal assessment based on fetal biophysical profile scoring: Experience in 12,620 referred high-risk pregnancies, Part 1. Perinatal mortality by frequency and etiology. Am J Obstet Gynecol 151: 343, 1985 |
|
Richardson DK, Schwartz JS, Weinbaum PJ, Gabbe SG. Diagnostic tests in obstetrics: a method for improved evaluation. Am J Obstet Gynecol 152: 613–618, 1985. |
|
Baskett TF, Allen AC, Gray JH et al: Fetal biophysical profile and perinatal death. Obstet Gynecol 70: 357, 1987 |
|
Manning FA Lange IR, Morrison I et al: Fetal biophysical profile score and the nonstress test: A comparative trial. Obstet Gynecol 64: 326, 1984 |
|
Freeman RK, Anderson G, Dorchester W: A prospective multi-institutional study of antepartum fetal heart rate monitoring, Part I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol 143: 771, 1982 |