Genetic Counseling
Authors
INTRODUCTION
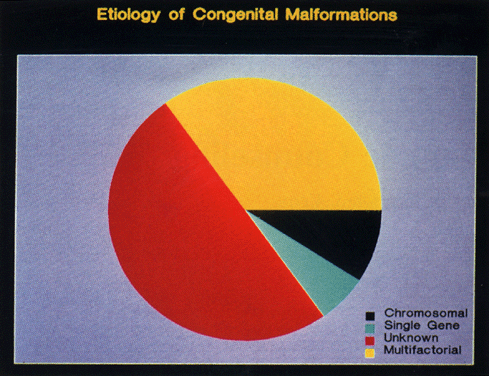
Genetic evaluation is an integral part of medical care for (1) the patient contemplating a pregnancy, (2) the prenatal patient, and (3) the patient who has had an abnormal child, anomalous stillborn, or two or more spontaneous abortions. Often, couples with normal medical histories are unaware that 5% of infants are born with a major anomaly1 (defined as a congenital malformation that requires treatment or interferes with physical well-being). In an additional 2% to 3% of offspring of such couples, a single gene mutation or a multifactorial genetic disorder is discovered in childhood or early adulthood.1 Approximately 50% of congenital defects have either a genetic etiology (i.e., a chromosomal abnormality, a single gene mutation, a multifactorial condition) or a teratogenic etiology; the remaining 50% have no identified cause. Figure 1 demonstrates the relative frequency of defects due to known and unknown causes.
Obstetrician-gynecologists—because of their knowledge of a family's medical history, and because they are often present at the time an abnormality is detected—provide initial genetic counseling for many patients. The purpose of this chapter, which updates previous communications,2,3 is to review the etiology and incidence of congenital anomalies in newborns, to outline the general principles of genetic counseling, and to identify couples who should receive formal genetic counseling.
CHROMOSOMAL DISORDERS
Mechanisms
During meiosis of germ cells, each gamete (egg or sperm) should receive 23 chromosomes from the parental stem cell. An error during meiosis (e.g., nondisjunction of chromosome pairs or sister chromatids) can lead to daughter cells with abnormal numbers of chromosomes (aneuploidy) (e.g., 22 or 24 chromosomes, rather than the expected 23). Mosaicism, the presence of more than one cell line, originates from a mitotic error in a somatic cell, rather than in a gamete. Structural chromosomal abnormalities arise after a chromosomal break in either a gamete or a somatic cell. Polyploidy (triploidy, tetraploidy) is the presence of an extra set(s) of chromosomes (i.e., 69 or 92) and can result from a meiotic or mitotic error, or from dispermy (fertilization of an egg by two sperm).
Most chromosomal abnormalities are the result of random, sporadic nondisjunction; consequently, recurrence risk for the family is low (1%). On the other hand, one in 500 phenotypically normal persons is a carrier of a balanced chromosomal rearrangement.4 These persons are at increased risk for offspring with unbalanced chromosomal abnormalities.
Incidence
One in 160 liveborn infants has a chromosomal abnormality4 (Table 1). Trisomy 21 is the most common, followed by balanced and unbalanced structural rearrangements, sex-chromosome abnormalities (47,XXX; 47,XXY; 47,XYY), trisomy 18, and trisomy 13. The incidence of autosomal trisomies and of 47,XXX and 47,XXY increases with increasing maternal age. Above age 30, the risk for a trisomy 21 newborn increases exponentially from year to year. The likelihood that a 35-year-old mother will have a child with a chromosomal abnormality is 1 in 200. By age 38, the risk increases to 1 in 100; by age 42, it is 1 in 40.5,6
TABLE 1. Frequency of Chromosomal Abnormalities in Newborns
Abnormality | Incidence |
Numerical Disorders |
|
Autosomal trisomies |
|
Group D* trisomy | 1/20,000 |
Group E* trisomy | 1/8000 |
Group G* trisomy | 1/800 |
Other | 1/57,000 |
Sex-Chromosome Disorder |
|
45,X | 1/10,000 (females) |
47,XXX | 1/1000 (females) |
Other (female) | 1/2700 (females) |
47,XXY | 1/1000 (males) |
47,XXY | 1/1000 (males) |
Other (male) | 1/1350 (males) |
Structural Disorder |
|
Balanced | 1/500 |
Unbalanced | 1/1700 |
Total | 1/160 |
*Because not all surveys employed banding techniques, individual chromosomes within the group could not always be identified. However, most group D trisomies are no.13, group E no. 18 and group G no 21.
(Hook EB, Hamerton JL: The frequency of chromosome abnormalities detected in consecutive newborn studies-results by sex and by severity of phenotypic involvement, In Hook EB, Porter IH[eds]: Population Cytogenetics: Studies in Humans, pp. 63–79, New York, Academic Press, 1977)
In contrast to liveborns, the frequency of chromosomal abnormalities in abortuses and stillborns is much higher. Nearly 50% of embryos spontaneously aborted at less than 13 weeks' gestation are chromosomally abnormal; 50% of these have autosomal trisomy, 22% polyploidy, 18% monosomy X, and 10% a translocation or other chromosomal abnormality. Of fetuses spontaneously aborted between weeks 13 and 26, approximately 20% are chromosomally abnormal,7 as are 5% of stillborns.8,9
MENDELIAN DISORDERS (SINGLE GENE MUTATIONS)
Mechanisms
Genes are segments of DNA that code for individual polypeptides. Each chromosome consists of many genes and of DNA that does not code for any protein product. Chromosomes and genes exist in pairs—one of each pair derived from the mother, the other from the father. Alleles are alternate forms of a gene that occupy the same position (locus) on homologous chromosomes (the two chromosomes of a pair). If the alleles at a given locus are identical, the individual is homozygous for that gene; if the alleles are not identical, heterozygosity exists. Mutant genes can be transmitted from a heterozygous or homozygous parent to an offspring of either sex. The exception to this statement is noted in the section on X-linked disorders.
AUTOSOMAL DOMINANT.
An allele is considered dominant if, in the heterozygous state, it results in a clinically evident altered phenotype. Transmission is from either a male or female parent to an offspring of either sex. Each offspring of an affected parent has a 50% chance of inheriting the mutant allele.
Matings between persons who are both heterozygous for the same dominant abnormal allele occur only rarely; 50% of their offspring would be heterozygous, 25% homozygous normal, and 25% homozygous abnormal. Homozygosity for severe dominant conditions, such as achondroplasia, is usually lethal. When a disorder is so deleterious that heterozygotes do not reproduce, new cases are the result of new mutations rather than familial transmission.
Occasionally, a dominant disorder will be present in a grandparent and grandchild, but will seem to have “skipped a generation.” This is an indication of incomplete penetrance; that is, some persons with the mutant allele may not manifest any of the associated findings, possibly as a result of the effects of other modifying genes. Expressivity (severity of phenotypic manifestations) also varies from person to person and family to family. Thus, a person must be carefully examined before it can be concluded that he is unaffected. The clinical significance of varied expressivity is that one affected person in a family may show only minor signs, whereas other family members may show much more severe manifestations of the same gene. For example, a person with neurofibromatosis may have café au lait spots, but not neurofibromas.
In dominantly transmitted disorders the underlying defect is frequently unknown, and therefore antenatal diagnosis may not be possible. However, as more defects are defined with DNA analysis, prenatal diagnosis of more disorders will be possible.
AUTOSOMAL RECESSIVE.
If the phenotype is significantly altered only in the homozygous state (i.e., when two copies of the identical abnormal allele are present) the disorder is termed recessive. If both parents are heterozygous, each offspring has a 25% chance of being homozygous affected, a 50% chance of being heterozygous (a carrier), and a 25% chance of being homozygous normal. These probabilities are independent of the sex of the child. If only one parent is a heterozygote, 50% of the children will be heterozygous and 50% will be homozygous normal; none will be affected.
Persons who are related (consanguineous) are more likely to have alleles in common than are unrelated persons. Therefore, homozygous affected children are more likely to result from a consanguineous mating than from a nonconsanguineous one. Most offspring of consanguineous matings, however, do not have autosomal-recessive disorders because even first cousins have only one eighth of their genes on average in common (see later discussion).
Some autosomal-recessive mutant genes code for abnormal enzyme proteins, thereby leading to errors of metabolism. Heterozygotes usually are not identified until the birth of an affected child. (The parents are obligatory heterozygotes.) Exceptions include disorders such as sickle cell disease, Tay-Sachs disease, and β-thalassemia, for which heterozygote screening can be performed. Antenatal diagnosis is possible for some autosomal-recessive disorders.
X-LINKED.
Males have only one X chromosome (hemizygous); therefore, they will manifest an abnormal phenotype whether a mutant X-linked gene is dominant or recessive. All of their daughters, and none of their sons will receive the gene. Females, on the other hand, have two X chromosomes; thus, in most cases, they will not manifest an X-linked recessive trait even when they carry the gene. However, half of the sons of heterozygous females will be affected, and half of the daughters will be heterozygotes, like the mother. In unusual situations a female may exhibit the phenotype conferred by an X-linked recessive gene. This may occur because (1) her father is affected and her mother is heterozygous; (2) she has only one X chromosome (45,X); or (3) lyonization (random inactivation of one X chromosome in females) by chance has resulted in the inactivation of the normal X chromosome in most cells, leaving only the abnormal allele active.
For some X-linked disorders, specific antenatal diagnostic testing is available; for others, affected males can be prevented only by elective abortion of all male fetuses of heterozygous females. Of course, this approach entails the loss of normal as well as abnormal males. Also an isolated case of an affected male may be due to a new mutation in a single gamete; that is, the mother of an affected male with no other affected relatives is not necessarily a heterozygote. Such mothers should be tested for heterozygosity if a reliable test is available, both to predict the recurrence risk in future pregnancies and to determine whether other relatives (e.g., sisters, aunts of the proband) are at risk for affected sons.
Very few genetic disorders are transmitted in X-linked dominant fashion. One example is vitamin D-resistant rickets with hypophosphatemia.
Y-LINKED AND MITOCHONDRIAL.
Genes associated with testicular development are located on the Y chromosome. Obviously transmission is from father to all sons. Y-linked inheritance has been proved for few other traits.
Genes can be transmitted via mitochondrial DNA (mtDNA) as well as by nuclear DNA. Mitochondria, found in the oocyte cytoplasm, are transmitted from mother to all offspring. Leber optic atrophy is an example of a disorder transmitted by mtDNA. Variability in expression among persons with abnormal mtDNA may be due to the proportion of mitochondria that have the abnormal gene (mosaicism) or to modifying nuclear genes.
Incidence
Although rare individually, mutant genes in the aggregate cause a large number of congenital disorders (Table 2).10 Estimates of the total frequency of single gene disorders in liveborns vary from 1 in 200 to 1 in 125, depending on the population, the completeness of the ascertainment, and the rigorousness of the diagnosis and classification.11
TABLE 2. Number of Mendelian Disorders
Autosomal-dominant | 4458 |
Autosomal recessive | 1730 |
X-linked | 412 |
Y-linked | 19 |
Mitochondrial | 59 |
Total | 6678 |
(McKusick VA: Mendelian Inheritance in Man,11th ed. Baltimore, Johns Hopkins University Press,1994)
In a registry of persons born in British Columbia between 1952 and 1983 and followed through 1984, the overall incidence of autosomal-dominant disorders was 1.4 in 1000.1 Late-onset dominant disorders, however, are inevitably underascertained by this method. For example, neurofibromatosis, osteodystrophies and chondrodystrophies, tuberous sclerosis, and hereditary spherocytosis were the most common dominant disorders reported to the Registry. However, more common disorders (e.g., Huntington chorea, dominant hypercholesterolemia) would not have manifested yet in persons born between 1952 and 1983 and therefore would not have been reported to the Registry.
In the same data set, the total incidence of autosomal-recessive disorders was 1.7 per thousand, with cystic fibrosis, phenylketonuria, and other amino acid disorders the most common. Thalassemias and sickle cell anemia were less common in this Canadian population than they are in some urban areas of the United States.
The incidence of X-linked disorders was 0.5 in 1000. Muscular dystrophy, color vision abnormalities, and hemophilia were the most prevalent disorders.
POLYGENIC/MULTIFACTORIAL DISORDERS
Mechanism
Most normal anatomic and physiologic variation (e.g., height, skin color) is not due to a single gene, but rather to the interaction between multiple genes (polygenic) and environmental factors (multifactorial). Most congenital anomalies restricted to a single structure or organ system (e.g., congenital heart defects, pyloric stenosis) are also ascribed to polygenic/multifactorial inheritance. This theoretical mode of inheritance postulates that liability for a disorder is determined by a person's (1) genetic inheritance and (2) environmental exposures. Fetuses whose total liability is beyond a “threshold” will manifest the disorder.12 As expected, such anomalies recur more frequently in families with an affected member than in the general population, because these families have been proved, by the birth of an affected child, to have greater than average liability for the disorder. For example, the recurrence risk for sibs of a child with pyloric stenosis is 3% to 12%13,14 The risk for male sibs of female probands is higher than for male sibs of male probands because females are less likely to have the disorder, thereby demonstrating a higher liability in families with affected females.
In any case, the relatively low recurrence risks associated with these disorders exclude a mendelian disorder, in which the sib recurrence risk is either 25% (for autosomal or X-linked recessive disorders) or 50% (for autosomal dominant disorders). Of course, one must be careful to avoid misdiagnosis. For example, if a congenital heart defect is not isolated, but rather is found in association with multiple other anomalies, the child may have a mendelian or chromosomal syndrome, and it would be misleading to counsel the family on the basis of polygenic/multifactorial recurrence risks. Some polygenic/multifactorial disorders are diagnosable prenatally (e.g., neural tube defects); others are not.
Incidence
Approximately 40% of congenital malformations are thought to be due to the interaction between multiple genes and environmental factors. These disorders affect 23 in 1000 infants.1 Additionally, many common medical disorders that first manifest in childhood or adulthood are of multifactorial inheritance. These conditions affect an additional 24 in 1000.1 The most common polygenic/multifactorial disorders are listed in Table 3.
TABLE 3. Relatively Common Disorders Usually Inherited in Polygenic/Multifactorial Fashion
Cardiac anomalies
Clubfoot
Congenital hip dislocation
Cryptorchidism
Diabetes mellitus
Diaphragmatic hernia
Epilepsy
Facial clefts
Hydrocephaly
Hypospadias and epispadias
Imperforate anus
Incomplete müllerian fusion
Inguinal hernia
Intestinal stenosis or atresia
Meckel's diverticulum
Neural tube defects
Omphalocele
Pyloric stenosis
Strabismus
Umbilical hernia
ENVIRONMENTAL FACTORS
Environmental factors are frequently implicated as the cause of congenital malformations; however, infectious diseases, maternal illness, radiation, drugs, and environmental chemicals are probably responsible for less than 10% of malformations (i.e., 1 in 350 newborns).15 Although malformations caused by environmental factors are not strictly speaking genetic in etiology, it has been shown that the effects of a teratogen can vary greatly among the offspring of a population of normal persons, probably based in large part on genetically controlled metabolic and immunologic factors.
GENETIC COUNSELING
Every physician should be able to take an appropriate history and decide whether referral for detailed genetic counseling is necessary. In this section, the types of information that should be ascertained during the evaluation are presented, with an emphasis on the specific areas of genetic counseling relevant to the patient presenting for obstetric or gynecologic care. Common indications for counseling are listed in Table 4.
TABLE 4. Common Indications for Genetic Counseling
Pregnancy in a woman age 35 or older, or whose partner is age 45 years or older
Chromosomal abnormalities in one of the parents
Genetic or congenital abnormality, or mental retardation, in a previous offspring or other close family member
Diagnosis of heterozygosity in one member (autosomal- dominant or X-linked disorders) or both members (autosomal-recessive disorders) of a couple
Exposure to a potential teratogen
Multiple spontaneous abortions, unexplained or anomalous stillbirths, or neonatal deaths
Family history of site-specific cancer
The purpose of genetic counseling is to provide couples with facts about the genetic disorder of concern to them, help them decide on the best course of action, and assist them in adjusting psychologically to their situation. The reproductive choices that are subsequently made should be the couple's, not the counselor's. The desirability of undertaking a pregnancy, use of alternative reproductive options (e.g., donor egg, sperm, or embryo), prenatal diagnosis, pregnancy termination, or sterilization must all be discussed in an informative and, optimally, nondirective fashion. Nondirective counseling implies that the counselor will try to remain impartial and objective in providing information. Counselors must try to avoid even nonverbal messages (facial expressions, ignoring certain statements of the patient) that indicate their own biases. Of course, being nondirective does not require the absence of empathy, understanding, or even suggestion of a course of action as long as the suggestion is intended to help the couple discover their optimal choices. Difficult as it may be, physicians and others undertaking genetic counseling should avoid imposing their own preferences on patients.
Before beginning genetic counseling, an accurate and specific diagnosis of the condition for which the couple is seeking counseling is necessary. If the woman is 40 years old and concerned about her chance for a child with a chromosomal abnormality, counseling can be straightforward. On the other hand, if a couple has a “retarded” relative and no additional information is available, very little in the way of helpful counseling can be offered.
Therefore, it is sometimes necessary to postpone counseling until appropriate medical records can be obtained. Such records should be perused to determine how the diagnosis was made, and how other potential diagnoses were excluded. For example, most neural tube defects are of polygenic/multifactorial inheritance, with a recurrence risk of 2% for parents with one affected child. However, in some cases an encephalocele occurs as a component of Meckel's syndrome, an autosomal-recessive disorder with a 25% recurrence risk. Neural tube defects may also be secondary to trisomy 13 and thus carry a lower recurrence risk. If secondary to ingestion of valproic acid, or if present with other anomalies consistent with amniotic band syndrome, the recurrence risk for a neural tube defect may be no greater than that of the general population. Physicians must consider all possibilities before discussing recurrence risks and potential approaches to prenatal diagnosis.
A second requirement for counseling is that the counselor have knowledge both of the disorder in question and of whether genetic heterogeneity exists (i.e., whether transmission patterns may differ among families). For example, retinitis pigmentosa can be transmitted in an autosomal dominant, autosomal recessive, or X-linked fashion. Counselors should also be familiar with the concepts of variable expression and nonpenetrance of single gene disorders and be prepared, if appropriate, to examine crucial family members.
Genetic Counseling of the Prenatal or Preconception Couple
A screening questionnaire can be useful (Fig. 2), but it must be reviewed with the patient to be sure she has understood the questions. One should inquire about the health of the couple, their first-degree relatives (sibs, parents, offspring), second-degree relatives (uncles, aunts, nephews, nieces, grandparents), and third-degree relatives (first cousins). Consanguinity of the couple (relationship by descent from a common ancestor) may not be volunteered and must be sought. Abnormal reproductive outcomes (spontaneous abortions, fetal deaths, and anomalous liveborn infants) should be explored in detail. One should determine the patient's and spouse's current exposure to alcohol, drugs, infections, or other potentially toxic agents, as well as to toxins (e.g., radiotherapy or chemotherapy) in the past.
Parental ages should be determined, for the most common indication for counseling and antenatal diagnosis is advanced maternal age. Advanced paternal age (50 years or older) confers an increased risk for a child with a new dominant mutation, such as achondroplasia or Marfan's syndrome, but probably does not increase the risk for a chromosomal abnormality.
Specific conditions for which the fetus is at increased risk should be identified and carrier or prenatal testing offered if available. Risks of prenatal diagnosis, accuracy, and limitations of the various modalities (e.g., amniocentesis, chorionic villus sampling [CVS], ultrasound) should be clearly stated.
Ethnic origin must be discussed because certain genetic disorders occur at high frequencies in particular ethnic groups. For some of these conditions, carrier (heterozygote) testing of the potential parents is available (Table 5). If both parents prove to be heterozygous, prenatal diagnosis should be offered. For example, Ashkenazi Jews are at increased risk for offspring with Tay-Sachs disease and Canavan disease. The potential parents should be screened to determine whether they are carriers of either of these genes and, if so, whether they desire prenatal diagnosis. Prenatal testing for sickle cell disease, β-thalassemia, and α-thalassemia—common disorders in the black, Mediterranean, and Asian populations, respectively—is also available. In the near future, screening for cystic fibrosis (CF) heterozygotes may be recommended in some white populations. Currently, however, the CF mutation can at best be detected in 90% of known heterozygotes even with the use of multiple probes for the most common mutations. In some ethnic groups (e.g., Latinos, blacks, Asians) the detection efficiency is significantly lower. Therefore, although carrier testing is appropriate for relatives of persons with CF, as well as for partners of CF patients who are planning a pregnancy, general population screening has not yet been accepted.
TABLE 5. Ethnic Origin and Heterozygote Screening
Ethnic Group | Genetic Disorders | Screening Test |
Ashkenazi Jews | Tay-Sachs disease | Serum or leucocyte |
|
| hexosaminidase |
| Canavan disease | DNA analysis |
Blacks | Sickle cell anemia, | Hemoglobin |
| β-thalassemia | electrophoresis |
Mediterraneans | β-Thalassemia | MCV of <80, hemoglobin |
(Greeks, Italians) |
| electrophoresis |
Southeast Asians | α-Thalassemia | MCV of <80, hemoglobin |
and Chinese |
| electrophoresis |
Northern European | Cystic fibrosis | DNA analysis (not yet |
Whites |
| generally accepted for |
|
| population screening) |
MCV = mean corpuscular volume
Finally, all couples should be informed of the general population risk for a child with a serious anomaly (5%).1 Although prenatal testing is possible for certain disorders, no test can exclude the possibility of an abnormal child.
Counseling the Consanguineous Couple
A small number of couples will consult their physician because of concerns about consanguinity. (Consanguinity is defined as relationship by descent from a common ancestor). If one of the members of the couple is the product of a consanguineous relationship or has a close relative who is, there is no concern so long as the individual in question is phenotypically normal and does not have an autosomal recessive or other genetic disorder.
A more pressing issue exists if the members of the couple are themselves closely related. Offspring of first-cousin matings have a two-fold increase in risk compared to the general population for perinatal and childhood death, malformations, and mental retardation.16 These figures assume that the couple is phenotypically normal and that there is no family history of an autosomal-recessive disorder. If a familial disorder for which carrier testing is possible does exist, or if ethnic background warrants (see Table 5), heterozygote testing should be performed. If both members are heterozygous for a recessive trait, the risk to each offspring for the disease is 25%. If an autosomal recessive disease exists in the family but testing is unavailable, the couple should be informed that the likelihood that they share a gene for a recessive familial disorder inherited from a common ancestor is 1 in 16. If they do, the chance that each child will inherit that gene from both parents is 1 in 4. The risk is greater if relatives such as parents or sibs of the couple have the gene or the disorder in question. Because of the complexity of these issues, couples who are first cousins or more closely related should be referred to a geneticist for counseling. Parenthetically, a child who is the result of a closer mating (e.g., brother-sister, father-daughter, uncle-niece) has a much higher risk of abnormality.16
Fortunately, as the genetic distance between relatives increases, the likelihood of carrying identical mutant genes decreases sharply. Consistent with this is the empiric finding that, as a group, second- and third-cousin matings are not at increased risk for abnormal offspring. However, if a genetic disorder does exist in a given family, couples should be given specific counseling as to their risk for that disorder.
Counseling the Couple with a Mentally Retarded Child
Counseling should begin with a complete history of the pregnancy and the affected child. The mother should be questioned regarding intrapartum illnesses, infection, use of prescription and illicit drugs, and alcohol ingestion. Details of the delivery, condition of the neonate at birth, presence of other anomalies in the retarded child, reports of cytogenetic studies including fragile-X testing, metabolic studies, patterns of early childhood development and hospitalizations, and the possibility of early abuse or neglect should be reviewed. If no etiology for the retardation can be determined, parents can be offered empiric recurrence risks (Table 6).17,18,19
TABLE 6. Risk for Retardation in Sibs of Retarded Probands
| Proband IQ | Proband IQ |
| 50–69(%) | <50(%) |
Bundey et al18 | 19.7 | 14.5 |
Costeff and Weller17 | 21.6 | 9.5 |
Herbst and Baird19 | 5.4 | 4.2 |
Estimates of risk vary among studies, depending in part on completeness of ascertainment of the population. For example, underascertainment is likely in a registry study because investigators do not examine sibs; reliance is placed on routine reporting to the registry. Recurrence risks are increased for families with more than one retarded child, for brothers of male index cases, and for sibs of index cases without other neurologic abnormalities.
Unfortunately, in the absence of a specific diagnosis, prenatal diagnosis is impossible. In this situation parents should be explicitly informed that amniotic fluid or CVS studies cannot exclude a recurrence.
Genetic Counseling in Spontaneous Abortions
The couple experiencing multiple spontaneous abortions will be identified in the course of the routine genetic history cited above. A detailed medical and family history should be taken, with an emphasis on information relating to pregnancy losses (e.g., gestational age, complications of pregnancy, maternal health, teratogen exposure, genotype or phenotype of abortuses, if known). Both genetic and nongenetic causes should be considered.
The first step in counseling couples experiencing fetal loss is education. Many couples are mistakenly convinced that fetal loss has occurred as a result of a preventable factor(s) for which they or their physician are responsible. One should explicitly inform patients that at least 15% of clinically recognized pregnancies result in fetal loss, and that loss rates increase with increasing maternal age. Approximately 50% of abortuses less than 13 weeks' gestational age, and 20% of abortuses at ages 13 to 26 weeks have chromosomal abnormalities7; prevention of abortion in such situations is impossible and, indeed, undesirable.
Empiric risk figures for future pregnancies should be stated. If a couple has at least one liveborn and one or more pregnancy losses at less than 13 weeks' gestation, the likelihood of another loss is 25% to 30%.20 This risk increases only slightly with increasing numbers of spontaneous abortions. If a couple has no liveborns or if their abortus is known to have been chromosomally normal, the risk rises to a maximum of 40% to 45%.21,22 No evidence supports the old concept that the recurrence risk is only increased in the face of three spontaneous abortions, or that in such cases the recurrence risk is 80% to 90%.
Although relatively infrequent causes of loss overall, structural chromosomal rearrangements nonetheless play an important role in repetitive abortions. In 2% to 5% of couples experiencing repetitive abortions, either the female or the male will show a balanced translocation or inversion. If a couple's pregnancies include not only abortions, but also fetal deaths or anomalous infants, the likelihood of a parental rearrangement is higher than if the history includes abortions only. Evaluation for repetitive abortions is indicated after two or three losses in the first 4 months of pregnancy. However, the occurrence of an unexplained fetal death or anomalous liveborn infant necessitates evaluation irrespective of the number of prior abortions. Optimally, chromosomal studies should be performed on the abnormal fetus or infant; however, if this has not been done, parental chromosomal complements should be determined. If a parental translocation or inversion is detected, referral to a geneticist is appropriate. Discussions with persons found to carry chromosomal rearrangements should include counseling regarding prenatal chromosomal studies (CVS, amniocentesis) in future pregnancies.
Other genetic causes of spontaneous abortion, namely mendelian (single gene) and multifactorial disorders, are doubtless involved in some pregnancy losses; however, the magnitude of their contribution is unknown. Luteal phase deficiency, uterine and cervical abnormalities, and maternal disease and infection are potential nongenetic causes of fetal loss and should be evaluated if genetic studies are normal. Exposure to an environmental toxin is only rarely the cause of a spontaneous abortion. The role of immunologic factors in reproductive loss is currently under investigation.
Genetic Counseling After the Birth of an Abnormal Child
CONFIRMATION OF DIAGNOSIS.
If possible, the child should be examined by an experienced physician or geneticist to confirm the diagnosis. If a chromosomal abnormality is suspected, cytogenetic studies are necessary, even if the clinical findings seem obvious. A complete family history, as described earlier, should be elicited. Examination of first-degree relatives may be helpful if an autosomal dominant disorder is suspected. In cases of an unexplained or anomalous fetal or neonatal death, the physician should attempt to obtain photographs as well as autopsy, cytogenetic, biochemical, and radiographic reports.
GENETIC COUNSELING.
Initially, the parents may experience denial, shock, bewilderment, grief, fear, or anger. Counseling at this point is best directed toward explaining the nature of the infant's disorder and the prognosis, providing answers to questions, and offering emotional support. This information should ideally be given by the primary physician, in consultation with the pediatrician and medical geneticist. The couple may insist that nothing is wrong with the child, that she looks just like baby pictures of another (unaffected) member of the family. The counselor(s) should accept emotional outbursts with sympathy and empathy, while maintaining professional objectivity. Some couples may benefit from the support of relatives, social workers, clergy, or formal psychological counseling. The initial stage of counseling is usually not the appropriate time for extensive discussion regarding recurrence risks for future offspring or the possible availability of prenatal testing. The parents should be told, however, that such information is available.
After the initial crisis has passed, more detailed genetic counseling is required. Timing of subsequent genetic counseling will vary, depending on the psychological readiness of the couple. Many couples will search for exogenous, discrete factors that might have caused the abnormal condition, in the subconscious desire to identify, understand, and control this factor and thus prevent a similar outcome in future pregnancies. In this process, there often occurs a tendency to blame the spouse or oneself. Rarely is the “blame” realistic, as would be the case, for example, for a disorder known to be associated with a teratogen or for an inherited autosomal dominant trait. Most couples can and should be reassured that nothing could have prevented the abnormal pregnancy. They should be encouraged to view the abnormality as the result of an unavoidable “accident” of which they are the victims, not the perpetrators.
Appreciation of various psychological defense mechanisms helps one understand the difficulty some intelligent couples have in comprehending and recalling genetic information. For this reason, more than one counseling session may be necessary. A letter to the couple reviewing the factual information provided during the counseling process is usually helpful. Occasionally it may be appropriate to hold a genetic counseling session which includes other members of the family.
Antenatal Diagnosis of Fetal Anomalies
With the increasing sensitivity of ultrasound diagnosis and the rise in the number of couples being screened and undergoing prenatal diagnosis, antepartum detection of anomalies is becoming more common. Once an abnormality is detected, it is important that the couple understand the immediate and long-term significance of the fetal defect. Couples often find consultation with appropriate pediatric specialists (e.g., cardiologists, neurologists, geneticists) useful. Before the stage of pregnancy at which the fetus would be viable, the option of pregnancy termination should be discussed (see later discussion). Some couples will choose to continue pregnancies with known fetal abnormalities, and they need continued support. The likelihood that a couple will choose to continue a pregnancy with a chromosomal abnormality is related to the specific diagnosis and gestational age at the time the diagnosis is made. Almost all couples with chromosomal abnormalities diagnosed by CVS choose to terminate an abnormal pregnancy, regardless of prognosis. In contrast, when a chromosomal abnormality is diagnosed by amniocentesis, pregnancies with more optimistic prognoses (i.e., sex-chromosome abnormalities) are less likely to be terminated.23 Couples continuing pregnancies with known fetal abnormalities grieve for the loss of their fantasized normal child and begin to accept and plan for the birth of a handicapped child. When the diagnosis or prognosis is uncertain, the remainder of the pregnancy may be especially difficult for the parents.
Pregnancy Termination for Genetic Indications
When faced with a prenatal diagnosis of a severe fetal abnormality, many couples elect pregnancy termination. Particularly after quickening, psychological sequelae from late-pregnancy termination for genetic indications can be as severe as those following a stillbirth.24,25 Depression and guilt may be more severe in patients terminating for a genetic abnormality because the pregnancy is wanted (as opposed to an elective termination) and the patient has made the active decision to discontinue the pregnancy (as opposed to fetal demise or stillbirth).24
Grief reactions are common immediately after termination.24,25,26 Even after 6 months, some patients or their partners have not resolved their grief; in these cases, psychiatric intervention for prolonged grief reactions may be indicated.26
Although programs providing counseling and follow-up usually exist for patients with pregnancy loss, stillbirth, or neonatal death, few are available specifically for patients undergoing pregnancy termination for genetic indications. Steps should be taken to facilitate grieving in these patients. The couple may wish to view, hold, name, or bury the fetus; mementos such as photographs, footprints, and infant clothing can be offered. Even if the couple say that they do not want such keepsakes, arrangements can be made to keep them available in case the parents change their minds.
Dilation and evacuation is the most common method of second-trimester pregnancy termination performed in the United States.27 This method can provide adequate specimens for confirmation in some cases, particularly if a definite diagnosis has already been made (e.g., chromosomal abnormality, mendelian disorder). In other cases, when an autopsy is desired, or when the parents want to see and hold the fetus, induction of labor with prostaglandins is more appropriate.
Genetic Counseling and Screening of Gamete Donors
The common practice of selecting donors because they have previously had normal children does little to ensure genetically normal offspring. Even two persons heterozygous for the same autosomal-recessive trait have a 75% likelihood that a given child will be phenotypically normal. Gamete donors should be genetically screened in a manner similar to that for partners planning a normal pregnancy. Thus, one elicits the health status of the donor and the first-, second-, and third-degree relatives; spontaneous abortions, fetal deaths, and anomalous liveborn infants are noted, as are age and exposure to drugs or toxins.
As discussed elsewhere,27,28 absolute genetic grounds for excluding a donor include:
- Presence of a disorder resulting from a single mutant gene (mendelian disorder, such as Marfan's syndrome or retinitis pigmentosa)
- Presence of certain mendelian disorders in a relative, if it is not possible to exclude the donor as a heterozygote (e.g., Huntington's disease in the donor's parent, Werdnig-Hoffmann disease in a sib)
- Presence of a chromosomal abnormality in the donor (e.g., balanced translocation or inversion)
- Previous trisomic offspring
- Presence of a serious polygenic/multifactorial disorder (e.g., spina bifida, cardiac anomaly) in the donor or a first-degree relative
- Donor blood group that is incompatible with that of the recipient (e.g., Rh-positive donor and Rh-negative recipient).
These are the same findings that, if detected in couples attempting nonassisted pregnancy, would warrant genetic counseling. Potential donors whose ethnic origin is appropriate should additionally be screened for Tay-Sachs disease, Canavan disease, α-thalassemia, β-thalassemia, sickle cell disease, or cystic fibrosis. Even if the donor is heterozygous for one of these disorders, he or she need not be rejected as a donor in every instance; however, one must be certain that the other parent is not heterozygous for the same disorder and that the couple agrees to a heterozygous donor. Screening for many other disorders is possible, but probably not cost-effective. The desirability of performing cytogenetic studies to identify donors with balanced translocations, inversions, or low-frequency aneuploidy is controversial. Such studies may not be warranted unless the potential donor or a close relative has a suspicious reproductive history, such as multiple abortions, an unexplained fetal death, or an anomalous liveborn.
In addition to these absolute indications for donor exclusion, various relative indications could be suggested:
- Serious polygenic disorders (e.g., cleft lip) in a donor's second- or third-degree relative
- Less serious polygenic disorders in the donor (e.g., mild hypertension)
- Advanced donor age (greater than 40 years for a male donor or 34 years for a female donor)
- Prior exposure to mutagens, such as radiation therapy or chemotherapy
- Unexplained stillborn or anomalous liveborn offspring born to a donor or a near relative, if one does not wish to perform cytogenetic studies on the donor.
Other reasons for exclusion might be proposed. For example, persons with human leukocyte antigen (HLA) DR3 are at increased risk for Addison's disease. Offspring of donors with HLA-DR3 have a 50% likelihood of inheriting the parental DR3 allele. However, given the number of such HLA-disease associations, if we were to exclude donors for such reasons, the supply of acceptable donors might quickly be exhausted. Thus, the goal of screening should not be to select a genetically “perfect” donor—an impossibility—but rather to eliminate donors whose likelihood of producing genetically abnormal offspring is considerably increased. Recipient couples should be counseled that despite appropriate screening of donors, it is impossible to guarantee normal offspring.
Late-Onset Disease
Some genetic disorders do not manifest until adult life, sometimes after reproduction has occurred. One example is a woman who has a mutated breast cancer gene (BRCA1 or BRCA2) and is at increased risk for breast and ovarian cancer. Because the mutation is inherited in autosomal dominant fashion, each child has a 50% chance of inheriting it.29 Presymptomatic testing and prenatal diagnosis are now possible. Persons who request such testing are best referred to centers that have experience in the issues of accuracy of the available tests and their predictive value. Careful consideration should be given to the potential benefits and liabilities for the individual patient in knowing she is a carrier of the gene in question. Medical, psychological, and economic consequences must be explored, as well as issues of confidentiality.30,31
GENETIC SCREENING
The purpose of genetic screening is to test a population in order to identify those with an increased risk for a genetic disorder (e.g., maternal serum alpha-fetoprotein to screen for neural tube defect).
Before screening is undertaken, a number of prerequisites should be met. First, the person to be screened (or their guardian in the case of neonatal screening) should understand the purpose of screening and the potential risks and benefits. Although formal written consent is not obtained in most screening programs, verbal consent should be obtained, not merely assumed. Compulsion to participate is unethical. Second, in most cases screening is not warranted if no intervention is possible (e.g., screening neonates for Tay-Sachs disease). The possibility of intervention by pregnancy termination or neonatal therapy may justify screening. Third, the screening test should be reliable, simple to perform, and inexpensive. The prevalence of the disease in the population should be sufficiently great to warrant screening. Finally, resources must be available for follow-up of cases with a positive screening result. The ability to distinguish heterozygotes from homozygotes, to deal with inconclusive results, and to communicate test results in a clear and timely fashion are necessary. Counselors must also be sensitive to the need to avoid stigmatization of persons found to carry abnormal genetic traits.
CONCLUSION
All physicians caring for women have the responsibility (1) to detect “genetic disorders or other conditions that might lead to birth defects”32; and (2) either to counsel the patient or to refer her for formal genetic consultation. We must be cognizant of and sensitive to the powerful psychological implications of the information provided during genetic counseling.
REFERENCES
Baird PA, Anderson TW, Newcombe HB, Lowry RB: Genetic disorders in children and young adults: A population study. Am J Hum Genet 42: 677, 1988 |
|
Verp MS: Genetic counseling and screening. In Lin C-C, Verp MS, Sabbagha RE (eds): The High-Risk Fetus, pp 160–171. New York, Springer-Verlag, 1993 |
|
Elias S, Simpson JL, Meyers, CM: Genetic counseling. In Sciarra JJ (ed): Gynecology and Obstetrics, Chap 3–111. Philadelphia, Lippincott-Raven, 1991 |
|
Hook EB, Hamerton JL: The frequency of chromosome abnormalities detected in consecutive newborn studies—differences between studies—results by sex and by severity of phenotypic involvement. In Hook EB, Porter IH (eds): Population Cytogenetics: Studies in Humans, pp 63–79. New York, Academic Press, 1977 |
|
Hook EB: Rates of chromosome abnormalities at different maternal ages. Obstet Gynecol 58: 282, 1981 |
|
Hook EB, Cross PK, Schreinemachers DM: Chromosomal abnormality rates at amniocentesis and in live-born infants. JAMA 249: 2034, 1983 |
|
Ruzicska P, Czeizel A: Cytogenetic studies on midtrimester abortion. Humangenetik 10: 273, 1970 |
|
Bauld R, Sutherland GR, Bain AD: Chromosome studies in investigation of stillbirths and neonatal deaths. Arch Dis Child 49: 782, 1974 |
|
Machin GA, Crolla JA: Chromosome constitution of 500 infants dying during the perinatal period. Humangenetik 23: 183, 1974 |
|
McKusick VA: Mendelian Inheritance in Man, 11th ed. Baltimore, Johns Hopkins University Press, 1994 |
|
Kalter H, Warkany J: Congenital malformations: Etiologic factors and their role in prevention. N Engl J Med 308: 424, 1983 |
|
Fraser FC: The multifactorial threshold concept: Uses and misuses. Teratology 14: 267, 1976 |
|
Carter CO, Evans KA: Inheritance of congenital pyloric stenosis. J Med Genet 6: 233, 1969 |
|
Dodge JA: Genetics of hypertrophic pyloric stenosis. Clin Gastroenterol 2: 523, 1973 |
|
Wilson JG: Embryotoxicity of drugs in man. In Wilson JG, Fraser FC (eds): Handbook of Teratology, Vol 1, pp 309–355. New York, Plenum, 1977 |
|
Vogel F, Motulsky AG: Human Genetics, 3rd ed, pp 714–715. Berlin, Springer-Verlag, 1997 |
|
Costeff H, Weller L: The risk of having a second retarded child. Am J Med Genet 27: 753, 1987 |
|
Bundey S, Thake A, Todd J: The recurrence risks for mild idiopathic mental retardation. J Med Genet 26: 260, 1989 |
|
Herbst DS, Baird PA: Sib risks for nonspecific mental retardation in British Columbia. Am J Med Genet 13: 197, 1982 |
|
Warburton D, Fraser FC: Spontaneous abortion risks in man: Data from reproductive histories collected in a medical genetics unit. Am J Hum Genet 16: 1, 1964 |
|
Poland BJ, Miller JR, Jones DC et al: Reproductive counseling in patients who have had a spontaneous abortion. Am J Obstet Gynecol 127: 685, 1977 |
|
Boué J, Boué A, Lazar P: Retrospective and prospective epidemiological studies of 1500 karyotyped spontaneous human abortions. Teratology 12: 11, 1975 |
|
Verp MS, Bombard AT, Simpson JL et al: Parental decision following prenatal diagnosis of fetal chromosome abnormality. Am J Med Genet 29: 613, 1988 |
|
Lloyd J, Laurence KM: Sequelae and support after termination of pregnancy for fetal malformation. Br Med J 290: 907, 1985 |
|
Blumberg BD, Golbus MS, Hanson KH: The psychological sequelae of abortion performed for a genetic indication. Am J Obstet Gynecol 122: 799, 1975 |
|
Black RB: A 1 and 6 month follow-up of prenatal diagnosis patients who lost pregnancies. Prenat Diagn 9: 795, 1989 |
|
Verp MS, Cohen MR, Simpson JL: Necessity of formal genetic screening in artificial insemination by donor. Obstet Gynecol 62: 474, 1983 |
|
Verp MS: Genetic issues in artificial insemination by donor. Semin Reprod Endocrinol 5: 59, 1987 |
|
American College of Obstetricians and Gynecologists: Breast-ovarian cancer screening. ACOG Committee Opinion 176, 1996 |
|
Burke W, Daly M, Garber J et al: Recommendation for follow-up care of individuals with an inherited predisposition to cancer: II. BRCA1 and BRCA2. JAMA 277: 997, 1997 |
|
Rothenberg K, Fuller B, Rothstein M et al: Genetic information and the workplace: Legislature approaches and policy challenges. Science 275: 1755, 1997 |
|
American College of Obstetricians and Gynecologists: Standards for Obstetric-Gynecologic Services, 5th ed. Washington, DC, 1982 |