Small Jr, W, Diavolitsis, V, Glob. libr. women's med.,
(ISSN: 1756-2228) 2008; DOI 10.3843/GLOWM.10244
November 2008
Role of Sonography and Hysterosonography In Operative Endoscopy
Authors
INTRODUCTION
The technique of hysteroscopic resection of submucous leiomyomas was first described by Neuwirth and Amin in 1976.1 Initial laparoscopic myomectomy series were published in 1991 by Nezhat and colleagues and by Dubuisson and associates.2, 3 Surgeons have relied on physical examination, hysteroscopy, laparoscopy, and various imaging techniques, including hysterography, ultrasound with and without fluid infusion, and magnetic resonance imaging (MRI), for appropriate case selection to perform endoscopic surgery. This chapter addresses the role of transvaginal ultrasound and sonohysterography in the preoperative evaluation of candidates for endoscopic myomectomy.
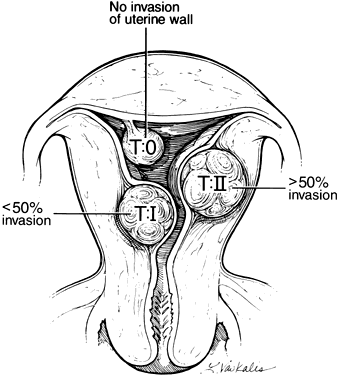
Wamstecker and deBlok, using the European Society of Hysteroscopy classification (Fig. 1), demonstrated higher endoscopic complications when submucous myomas have intramural extensions greater than 50%.4, 5 These deeply penetrating leiomyomas require greater expertise with the operative resectoscope, and in their opinion, simultaneous laparo-scopic guidance is required. Failure to appreciate the degree of uterine wall penetration can result in failure to completely resect the leiomyoma, increased rate of conversion to laparotomy, higher rates of intravascular extravasation of distending media, prolonged operating times, and increased need for repeat surgery.
A similar classification system for intramural, subserous, and pedunculated leiomyomas has not been adopted by any of the major laparoscopic societies. Standardized guidelines for appropriate lapa-roscopic myomectomy are highly desirable. Nezhat suggested that laparoscopic surgery be reserved for pedunculated or subserosal leiomyomas without significant myometrial penetration because of concerns about fistula formation and uterine rupture in subsequent pregnancies.6 Although the series and follow-up pregnancy outcomes are insufficient to extrapolate any absolute guidelines, as the degree of myometrial involvement increases, proper layered closure of the uterine defect is more difficult by laparoscopic techniques. In our opinion, endoscopic removal of intramural or submucosal leiomyomas larger than 5 cm in diameter or with myometrial involvement greater than 50% should be attempted only by very experienced endoscopists.
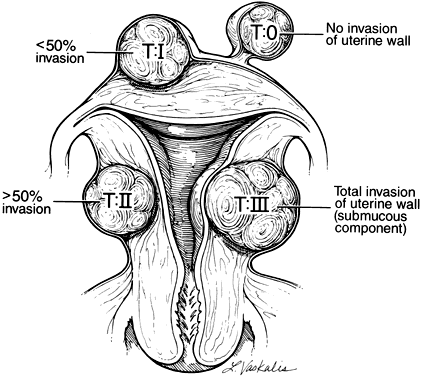
Multiple investigators with various laparoscopic suturing techniques have published series.7, 8, 9 One of these combined endoscopic myomectomy with minilaparotomy. This combination allows a smaller laparotomy incision than would normally be necessary and allows endoscopic myoma enucleation and direct uterine wall suturing through a minilaparotomy incision.9 Because of the lack of a uniform classification system in regard to myometrial involvement, it is difficult to compare these series. Given the degree of expertise required for these more complicated procedures, it is critical for the practitioner to preoperatively assess the degree of difficulty that will be encountered. A proposed classification system for laparoscopic myomectomy candidates is shown in Figure 2.
PREOPERATIVE IMAGING TECHNIQUES
Before the availability of transvaginal ultrasound, hysterography was the most widely used technique for evaluation of the uterine cavity because of the limitations of transabdominal ultrasound. Hysterography allowed for the evaluation of tubal patency.10 The major limitation of hysterography was its poor imaging of the myometrial walls and a significant number of false-positive findings of submucous leiomyomas that were intramural (Fig. 3). Saline contrast hysterosonography using transabdominal ultrasound was initially described by Nanini in 1981.11 Richman and coworkers explored the assessment of fallopian tube patency by abdominal ultrasound after saline injection in 1984.12 Because of suboptimal imaging by the transabdominal technique, hysterography remained the routine approach.
MRI is a highly accurate technique for evaluating uterine leiomyomas, adenomyosis, and uterine anomalies.13, 14, 15 T2-weighted images clearly delineate the myometrium, junctional zone, and endometrium, allowing highly accurate mapping of the size, location, and degree of myometrial involvement of uterine leiomyomas (Figs. 4 and 5). It is much more accurate in identifying and mapping adenomyosis. The major limitations of MRI imaging are cost and delays in scheduling examinations.
The advantage of transvaginal imaging of the endometrium was rapidly appreciated by the radiologic community in the mid-1980s.16 Fedele, in a frequently quoted series, found transvaginal scanning without infusion had a sensitivity of 100% and a specificity of 94% for the detection of submucous leiomyomas.17 Series by Cincinelli and colleagues and by Narayan and Goswamy found slightly lower sensitivities at 90% and 94%, respectively.18, 19 Narayan and Goswamy scanned patients in their infertility group at three points during the menstrual cycle. This allowed the investigators to take advantage of the varying endometrial echo-architecture (Figs. 6 and 7). Towbin and associates found that office hysteroscopy and transvaginal scanning without infusion were only 79% and 53% sensitive, respectively, in identifying space-occupying lesions of uterine cavity among 65 patients who underwent hysterectomy or operative hysteroscopy. The investigators thought the decreased sensitivity resulted from residents in training performing the diagnostic studies rather than experts in the field.20
The addition of saline infusion to transvaginal studies was evaluated by Bonilla-Musoles in 1992.21 He demonstrated that this method led to improved evaluation of the endometrial cavity and allowed assessment of tubal patency in some patients. The sensitivity for the detection of endometrial pathology has varied widely in the literature, which often is difficult to interpret because of the combination of premenopausal and postmenopausal women in the reported studies. Endometrial pathology such as uterine synechiae, endometrial polyps, and submucous leiomyomas are lumped together. Widrich and associates, in a series of 130 women with abnormal uterine bleeding, found a 93% sensitivity and 96% specificity for detection of hysteroscopically proved submucous leiomyomas in 13 patients.22 Bernard and coworkers found a sensitivity of 90.7% and a specificity of 95% among 162 women with abnormal uterine bleeding, of whom 30 had hysteroscopically proved submucosal leiomyomas.23
It appears that sonohysterograms are not more sensitive for the detection of submucous leiomyomas than transvaginal scans without infusion. However, this is deceptive, because the degree of myometrial involvement frequently cannot be accurately mapped without saline infusion. This problem can lead to inappropriate case selection (Figs. 8 and 9).
One of the major limitations of sonohysterography has been assessment of tubal patency. There is considerable European experience with the use of Echovist for the assessment of tubal patency. Campbell and colleagues published a review of this subject in 1994.24 In the United States, Fleischer and colleagues published their results for the use of sonohysterography and sonosalpingography using Albuminex. Among 100 infertility patients, they achieved a 98% detection rate for submucous fibroids, 96% for endometrial polyps, and 81% for intrauterine synechiae compared with hysteroscopic evaluation compared with hysterographic x-ray results. Tubal patency was successfully determined in 79% of the patients using a combination of transvaginal scanning and saline and in 92% when Albuminex was added.25
TRANSVAGINAL ULTRASOUND AND SONOHYSTEROGRAPHY
The 1990s have seen a rapid reduction in the cost of high-quality ultrasound systems. High-resolution systems with variable frequency vaginal transducers are available for $35,000–40,000, making acquisition affordable for most practitioners.
Most new transvaginal probes offer variablefrequency transducers with frequencies between 5.0 and 7.5 MHz. The higher the frequency used, the better is the resolution of the image. However, the sound attenuates more quickly at higher frequencies. As the uterus enlarges, a lower-frequency transducer must be used to visualize the organ. The 7.5-MHz vaginal probe works best with a normal or minimally enlarged uterus. The 5.0-mHz vaginal probe usually images a uterus up to the size of 12 weeks' gestation. If the uterus is larger than this, transabdominal imaging with a 2.5- or 3.5-MHz probe is required, although with multiple leiomyomas, MRI is frequently more informative.
The standard orientation in the United States is to place the front of the transducer on the left side of the ultrasound monitor. On longitudinal images, this results in the bladder being displayed on the left of the image. The vagina is seen at the top of the screen and the fundus at the base. The anteverted uterus is rotated toward the bladder and the retroverted away.
In addition to adjusting frequency and orientation, the practitioner must understand how to adjust power, overall and fine gain, depth of imaging, and magnification. These subjects and a more complete understanding of physics and ultrasound safety are beyond the scope of this chapter. On-site tutorials are available through American Institute of Medicine at various university hospitals. Examiners must not perform studies beyond the limitations of their training. Fortunately, transvaginal imaging of a minimally enlarged nonpregnant uterus can be learned quickly. Attempted visualization of ectopic pregnancy or evaluation of a complex adnexal mass takes considerably more scanning experience.
The technique of sonohysterography is relatively simple. The possibility of pregnancy must be excluded. Patients with a history of pelvic inflammatory disease should be treated prophylactically with antibiotics. A single-hinged speculum is helpful but not required. The cervix is cleaned. A 5-French sonohysterogram catheter (Ackrad Laboratories, Cranford, NJ, USA) (Fig. 10) can be inserted into the lower uterine segment or cervical canal in most menopausal patients without a tenaculum or dilators. A 7-French catheter is used for patients with a more dilated cervix to obtain better occlusion with the larger balloon size. The catheter should be flushed before insertion to avoid air bubbles during the procedure. The vaginal probe ultrasound is inserted, and the sonohysterogram is performed, usually requiring less than 10 ml of saline. The cavity should be assessed in multiple transverse and longitudinal cuts. Vagal episodes can occur, particularly in women who have not had children. Premedication in nulliparous patients with nonsteroidal antiinflammatory drugs (NSAIDs) such as ibuprofen or naproxen (Naprosyn) is recommended. The role of three-dimensional imaging is investigational26 (Figs. 11 and 12).
INTRAOPERATIVE GUIDANCE
Transabdominal ultrasound with a partially filled bladder can be helpful to the hysteroscopic surgeon. Dilation and insertion of the hysteroscope can be done under ultrasound guidance to help avoid perforation. Ultrasound can also help guide the endoscopist during resectoscopic excision of deeply invasive submucous leiomyomas, lysis of intrauterine adhesions, and division of uterine septa.27,28
CONCLUSIONS
Diagnostic transvaginal ultrasound allows for accurate mapping of the size, location, and degree of myometrial involvement of uterine leiomyomas. The addition of sonohysterography in some cases allows better determination of the degree of intramural involvement. Failure to appreciate extensive myometrial involvement can lead to inappropriate case selection. The surgeon can better assess the likelihood of complete resection of the tumor, estimate the chance of conversion to laparotomy, and anticipate the degree of blood loss. The patient can be better counseled preoperatively regarding surgical outcome, risks, and future prognosis. Ultrasound also enables assessment of the adnexa. The addition of saline or contrast agents to transvaginal ultrasound can allow assessment of tubal patency.
MRI should not be forgotten and performs well in mapping the size and location of leiomyomas and in accurately identifying adenomyosis. Other pelvic pathology such as ovarian neoplasms can be identified.
Well-controlled multicenter trials with standard classification systems are needed to evaluate the proper place of these procedures in the diagnostic and operative armamentarium. Sonography is a powerful tool that can guide the gynecologist or hysteroscopist encountering a difficult dilation caused by cervical stenosis or performing a complicated endoscopic procedure.
REFERENCES
Neuwirth RS, Amin HK: Excision of submucous fibroids with hysteroscopic control. Am J Obstet Gynecol 126: 95, 1976 |
|
Dubuisson JB, Lecuru F et al: Myomectomy by laparoscopy: A preliminary report of 43 cases. Fert Steril 56: 827, 1991 |
|
Nehzat C, Nehzat F, Silfen SL et al: Laparoscopic myo-mec-tomy. Int J Fertil 36: 275, 1991 |
|
Wamsteker K, Emanuel MH: Uterine leiomyomas. In Brosens I, Wamsteker K (eds): Diagnostic Imaging and Endoscopy in Gynecology, pp 185–198. London: WB Saunders, 1997 |
|
Wamsteker K, Emmanuel MH, deKruif JH: Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding results regarding the degree of intramural extension. Obstet Gynecol 82: 736, 1993 |
|
Nehzat C: The “cons” of laparoscopic myomectomy in women who may reproduce in the future. Int J Fertil 41: 1, 1996 |
|
Stringer NH: Laparoscopic myomectomy with the endostitch 10-mm laparoscopic suturing device. J Am Assoc Gynecol Laparosc 3: 299, 1996 |
|
Ostrzenski A: A new laparoscopic myomectomy technique for intramural fibroids penetrating the uterine cavity. Eur J Obstet Gynecol 74: 189, 1997 |
|
Nezhat C, Nezhat F, Bess O et al: Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil 39: 39, 1994 |
|
Siegler AM: Hysterosalpingography and Laparoscopy in Infertility. London: Parthenon Press, 1998 |
|
Nanini R, Chelo E, Branconi F et al: Dynamic echohysteroscopy: a new diagnostic technique in the study of female infertility. Acta Eur Fertil 12: 165, 1981 |
|
Richman TS, Viscomi GN, DeCherney A et al: Fallopian tube patency assessed by ultrasound following fluid injection. Radiology 152: 507, 1984 |
|
Hricak H, Tscholakoff D, Heinrichs L et al: Uterine leiomyomas: correlation of MR, histopathologic findings, and symptoms. Radiology 158: 385, 1986 |
|
Ascher SM, Arnold LL, Patt RH et al: Adenomyosis: Prospective comparison of MR imaging and transvaginal sonography. Radiology 190: 803, 1994 |
|
Pellerito JS, McCarthy SM, Doyle MB et al: Diagnosis of uterine anomalies: relative accuracy of MR imaging, endovaginal sonography and hysterosalpingography. Radiology 183: 795, 1992 |
|
Mendelson EB, Bohm-Velez M, Joseph N, Neiman HL: Endometrial abnormalities: Evaluation with transvaginal sonography. AJR Am J Roentgenol 150: 139, 1988 |
|
Fedele L, Bianchi S, Dorta M et al: Transvaginal sonography versus hysteroscopy in the diagnosis of uterine submucous myomas. Obstet Gynecol 77: 745, 1991 |
|
Cicinelli E, Romano F, Anastastio PS: Transabdominal sonohysterography, transvaginal sonography, and hysteroscopy in the evaluation of submucous myomas. Obstet Gynecol 85: 42, 1995 |
|
Narayan R, Goswamy RK: Transvaginal sonography of the uterine cavity with hysteroscopic correlation in the investigation of infertility. Ultrasound Obstet Gynecol 3: 129, 1993 |
|
Towbin NA, Gviazda IM, March CM: Office hysteroscopy versus transvaginal ultrasound in the evaluation of patients with excessive uterine bleeding. Am J Obstet Gynecol 174: 1678, 1996 |
|
Bonilla-Musoles F, Simon C, Serra V et al: An assessment of hysterosalpingosonography (HSSC) as a diagnostic tool for uterine cavity defects and tubal patency. J Clin Ultrasound 20: 175, 1992 |
|
Widrich T, Bradley LD, Mitchison A, Collins R: Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol 174: 1327, 1996 |
|
Bernard JP, Lecuru F, Darles C et al: Saline contrast sonohysterography as first-line investigation for women with uterine bleeding. Ultrasound Obstet Gynecol 10: 121, 1997 |
|
Campbell S, Bourne TH, Tan SL, Collins WP: Hysterosalpingo contrast sonography (HyCoSy) and its future role within the investigation of infertility in Europe. Ultrasound Obstet Gynecol 4: 244, 1994 |
|
Fleischer AC, Vasquez JM, Cullinan JA, Eisenberg E: Sonohysterography combined with sonosalpingography: Correlation with endoscopic findings in infertility patients. J Ultrasound Med 16: 384, 1997 |
|
Bonilla-Musoles F, Raga F, Blanes J et al: Three-dimensional hysterosonographic evaluation of the normal endometrium: Comparison with transvaginal sonography and three-dimensional ultrasound. J Gynecol Surg 13: 101, 1997 |
|
Weinraub Z, Maymon R, Shulman A et al: Three-dimensional saline contrast hysterosonography and surface rendering of uterine cavity pathology. Ultrasound Obstet Gynecol 8: 277, 1996 |
|
Wortman M: Hysteroscopic myomectomy: Refining skills and reducing risk. Female Patient 23: 53, 1998 |